Abstract
Histone deacetylase inhibitors are promising agents for various T-cell lymphomas, including cutaneous T-cell lymphoma, peripheral T-cell lymphoma, and adult T-cell lymphoma/leukemia. CCR4 is an important therapeutic target molecule because mogamulizumab, an anti-CCR4 antibody, has shown promising efficacy against various T-cell lymphomas. In this study, we examined the in vitro synergistic effects of mogamulizumab and histone deacetylase inhibitors against various T-cell lymphomas. First, we examined the expression of CCR4 mRNA and surface CCR4 in various T-cell lymphoma cell lines and found that it was downregulated upon treatment with vorinostat, a pan-histone deacetylase inhibitor. Next, we used isoform-specific histone deacetylase inhibitors and short-interfering RNA to determine the histone deacetylase isoform involved in the regulation of CCR4, and demonstrated that romidepsin, a class I selective histone deacetylase inhibitor, reduced CCR4 most efficiently. Moreover, among class I histone deacetylases, histone deacetylase 2 knockdown led to a reduction of CCR4 in lymphoma cells, suggesting that CCR4 expression is mainly regulated by histone deacetylase 2. When we examined the CCR4 expression in skin samples from primary cutaneous T-cell lymphoma, obtained from the same patients before and after vorinostat treatment, we found that CCR4 expression was greatly reduced after treatment. Finally, when we conducted an antibody-dependent cell-mediated cytotoxicity assay with mogamulizumab by using various lymphoma cells, we found that the efficacy of mogamulizumab was significantly reduced by pretreatment with vorinostat. Altogether, our results suggest that the primary use of histone deacetylase inhibitors before treatment with mogamulizumab might not be suitable to obtain synergistic effects. Moreover, these results have potential implications for optimal therapeutic sequences in various CCR4-positive T-cell lymphomas.
Introduction
Mature T-cell neoplasms comprise approximately 20 sub-classified categories of non-Hodgkin lymphomas and are broadly divided into cutaneous T-cell lymphomas (CTCL) and peripheral T-cell lymphomas (PTCL).1–3 For instance, according to the World Health Organization (WHO) classification, PTCL includes peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma, anaplastic large cell lymphoma (ALCL), adult T-cell leukemia/lymphoma (ATLL), and others. CTCL mainly consist of mycosis fungoides and Sézary syndrome.1–3 In addition, the main mature natural killer (NK)-cell neoplasms include extranodal NK/T-cell lymphoma, nasal type and NK-cell leukemia.1–3
Combination chemotherapy, including cyclophosphamide, hydroxydoxorubicin, vincristine, and prednisone (CHOP) as well as CHOP-like regimens, has usually been the standard first-line treatment for patients with PTCL and advanced CTCL.4 Except for anaplastic lymphoma kinase-positive ALCL, however, the efficacy of these combination therapies is unsatisfactory, and most patients have a poor prognosis.5 Improvement of the survival of patients with malignant lymphoma has recently been expected on the basis of the appearance of various molecular targeted therapeutic drugs. Novel molecular targeted therapies have also been developed against T-cell and NK-cell neoplasms. Two particularly noteworthy therapies are mogamulizumab, an anti-CCR4 antibody, and histone deacetylase (HDAC) inhibitors, including vorinostat and romidepsin. These two promising therapies are currently being applied separately for the treatment of T-cell and NK-cell lymphomas.
Mogamulizumab is a humanized anti-CCR4 antibody developed against ATLL that highly expresses CCR4, a chemokine receptor. Mogamulizumab prompts potent antibody-dependent cellular cytotoxicity (ADCC) activity against malignant cells.6–8 CCR4 is expressed in ATLL and in approximately 38% of PTCL.9 In addition, expression of CCR4 is promoted in CTCL with the progression of the disease.10 In recent years, mogamulizumab has been shown to be clinically effective against CCR4-positive CTCL and PTCL.11 Moreover, mogamulizumab has been shown to be effective against T-cell and NK-cell lymphomas in preclinical studies.12 Mogamulizumab is, therefore, a promising agent for the treatment of CCR4-positive T-cell and NK-cell lymphomas.
Eighteen isoforms of HDAC are known.13,14 In particular, class I HDAC (HDAC1, HDAC2, and HDAC3) are considered to inhibit the transcription of tumor-suppressor genes and additional related genes (e.g., p21, miR-16).14–17 The inhibition of class I HDAC could, therefore, restore the expression of tumor suppressor genes and exert an anti-tumor effect.17,18 HDAC inhibitors can be classified into two types, namely pan-HDAC inhibitors and isoform-specific HDAC inhibitors. While pan-HDAC inhibitors broadly inhibit multiple HDAC, isoform-specific HDAC inhibitors target specific HDAC. The pan–HDAC inhibitor, vorinostat/suberoylanilide hydroxamic acid (SAHA), is a first-line therapy against advanced CTCL19 and HBI-8000, a new pan-HDAC inhibitor, has also been suggested from preclinical studies to be active against ATLL.20 The class I-specific HDAC inhibitor, romidepsin, has shown promising efficacy against PTCL.21 In addition, a novel pan-HDAC inhibitor, belinostat was recently approved for use in relapsed or refractory PTCL in the USA.22 As described above, we can expect the clinical application of HDAC inhibitors in various T-cell lymphomas.
The synergistic effects of molecular targeted drugs could be studied for future therapeutic strategies. The efficacy of HDAC inhibition in combination with anti-PD-1 antibodies,23 bortezomib,24 DNA methyltransferase inhibitors,25 a Bruton tyrosine kinase inhibitor,26 and other treatments has been suggested. These are expected to have a synergistic effect through the combined use of molecular targeted drugs. However, there is a risk that molecular targeted drugs with different modes of action may adversely affect each other. Among these combinations, in this study, we clarify the effect of the combined use of mogamulizumab and HDAC inhibitors on various T-cell and NK-cell lymphomas. Based on our findings, we discuss what benefits or adverse effects might be assumed for patients if these molecular targeting agents are used in clinical practice.
Methods
Primary lymphoma and control samples
We collected samples from patients with ATLL (n=1), PTCL-NOS (n=2), and advanced CTCL (n=6). Six samples were obtained before and after treatment of SAHA/vorinostat. For quantitative reverse transcriptase polymerase chain reaction (RT-PCR) analysis of normal control cells, CD4 positive cells were collected from healthy donors using a magnetic cell sorting system (Miltenyi Biotec, Bergisch Gladbach, Germany) or cell sorter (Dako Cytomation MoFlo, Tokyo, Japan). Samples were collected under a protocol approved by the Institutional Review Boards of Akita University.
Cell lines
We used the following 15 cell lines for this study: My-La, HH, MJ, and HUT78 (CTCL cell lines), MT-1, MT-2, MT-4, and TL-Su (ATLL cell lines), SR786 and Karpass299 (K299) (ALCL cell lines) and Kai3, SNK6, HANK1, SNK10 and KHYG1 (NK-cell lymphoma/leukemia cell lines). HH, HUT78, and MJ cell lines were purchased from the American Type Tissue Collection. My-La was from the European Collection of Cell Cultures. K299 and SR786 were purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen. KHYG1, MT-1, MT-2, MT-4, TL-Su, and Kai3 were bought from the Japanese Collection of Research Bioresources Cell Bank. HANK1 was a gift from Dr Yoshitoyo Kagami (Toyota Kosei Hospital, Japan). Cells were cultured in Arteimis-1 medium (with or without 2% inactivated human serum), which is a chemically defined, serum-free medium purchased from NihonTechno Service Co. Ltd. (Ibaraki, Japan). It contains recombinant human insulin (5.0 μg/L), recombinant human interleukin-2 (250 IU/mL), and human serum albumin (2 g/L). It does not contain any other cytokines or growth factors.
Real-time quantitative reverse transcriptase polymerase chain reaction analysis
Real-time quantitative RT-PCR was performed with the Taqman method (Life Technology) using the Light Cycler 480 probe master (Roche Diagnostics, Basel, Switzerland). The Taqman probes for CCR4 (Hs00747615_s1) and GAPDH (Hs02758991_g1) were purchased from Applied Biosystems (Foster City, USA). mRNA levels were normalized to those of GAPDH and the relative level of expression of specific mRNA was presented by 2−ΔCt or 2−ΔΔCt. Quantitative stem-loop reverse transcription was then performed using a First-Strand cDNA Synthesis Kit (GE Healthcare, Buckinghamshire, UK).
Flow cytometric analyses
For flow cytometric analysis, cells were stained at 4°C with fluorescein isothiocyanate-conjugated anti-human CCR4 (R&D Systems, Minneapolis, USA). The appropriate isotype controls used were unlabeled mouse IgG1 antibody (R&D Systems). After washing, cells were analyzed using a FACS Canto flow cytometer (BD Biosciences, San Jose, CA, USA).
Immunohistochemical analysis
Immunostaining for CD4 and CCR4 in paraffin-embedded blocks of primary T-cell lymphoma samples was conducted according to the protocols of the kits’ manufacturers. CONFIRM anti-CD4 (SP35) was purchased from Roche Diagnostics. The CCR4 staining kit was bought from Kyowa Medex (Tokyo, Japan).
Chemicals
Vorinostat was purchased from R&D Systems. Romidepsin, CI994, RGFP966, and ricolinostat were bought from Cosmo Bio Co., Ltd. (Tokyo, Japan). PCI-34051 was purchased from Santa Cruz Biotechnology (Santa Cruz, USA).
Transient short interfering RNA transfection
For transient knockdown of HDAC1 (product n. L-003493-00), HDAC2 (product n. L-003495-02), and HDAC3 (product n. L-003496-00), we used Dharmacon ON-TARGET plus SMART pool short interfering (si) RNA. For a non-targeting control, we used Dharmacon ON-TARGET plus Non-targeting pool (product n. D-001810-10). These were purchased from GE Health Care Japan (Tokyo, Japan). siRNA transfection was performed using the Amaxa cell optimization kit V (Amaxa, Koeln, Germany) according to Amaxa guidelines.
Antibody-dependent cell-mediated cytotoxicity assay
Cell lines were used as target cells. Human peripheral blood mononuclear cells from healthy donors were used as effector cells. Target cells (2.5×103) and effector cells (1.25×105) were co-cultured in 96-well plates with mogamulizumab or solvent alone (control) for 4 h in Artemis-1 medium. After incubation, the supernatant of each well was obtained, and percentage cell death was calculated by measuring the lactate dehydrogenase concentration in the supernatant using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, USA).
Cell migration assay
In vitro cell migration was assayed using a CytoSelect 96-Well Cell Migration Assay kit (5 μm, Fluorometric Format) (Cell Biolabs, Inc. San Diego, CA, USA) according to the manufacturer’s protocol. Migration was stimulated by CCL22 (500 ng/mL) in the lower chamber; no serum was added to the upper chamber. The incubation period was 16 h and the cell lysis buffer was transferred to a 96-well plate. Relative fluorescence units were measured by a plate reader at 480 nm/520 nm. The migration index was calculated as the number of cells migrating toward the concentration gradient of chemokines divided by the number of cells migrating toward medium only.
Western blot analysis
HDAC1 (#5356), HDAC2 (#5113) and HDAC3 (#3949) were purchased from Cell Signaling (Danvers, USA). Tubulin (MS-581-P0) was acquired from NeoMarkers (Fremont, CA, USA).
Statistical analysis
A Student t-test was used to examine the statistical significance of the findings.
Results
Vorinostat downregulates the expression of the CCR4 chemokine receptor in various T-cell lymphoma cell lines
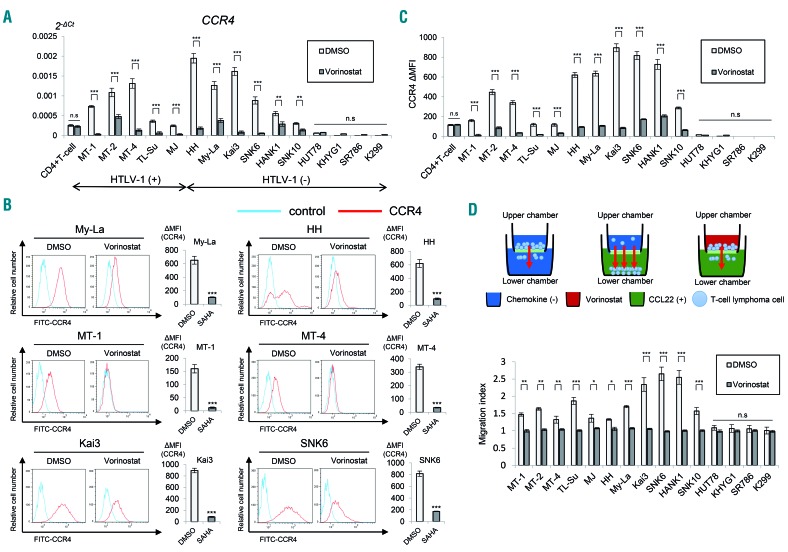
We first examined the expression of CCR4 in 15 T-cell and NK-cell lymphoma cell lines and a sample of peripheral blood mononuclear cells to investigate the effect of vorinostat, a pan-HDAC inhibitor, on CCR4 expression. The expression of CCR4 was analyzed by quantitative RT-PCR using the Taqman method. The chemokine receptor was expressed in most (11 out of 15) cell lines, including ATLL (MT-1, MT-2, MT-4, and TL-SU), CTCL (My-La, HH, and MJ), and NK/T-cell lymphoma cell lines (Kai3, SNK6, HANK1, and SNK10) (1.0- to 7.6-fold change). However, as previously reported, anaplastic lymphoma kinase-positive ALCL cell lines (SR786 and K299) had lower expression compared with CD4-positive T cells.27 Next, we investigated the effect of vorinostat on CCR4 expression in T-cell and NK-cell lymphoma cell lines. Since the IC50 (24 h) of vorinostat in T-cell lymphomas is 5 μM, as previously reported,16 an exposure experiment at this concentration was conducted. When quantitative RT-PCR was performed with the vorinostat-treated cells, the mRNA levels of CCR4 were markedly reduced, except for those in the cell lines HUT78, KHYG1, SR786, and K299, which did not express CCR4 (Figure 1A). This result was independent of the presence or absence of a human T-cell leukemia virus type-1 infection.
Figure 1.
The pan-histone deacetylase inhibitor, vorinostat reduces CCR4 expression in various T-cell and natural killer-cell lymphoma cell lines. Bars indicate the mean ± standard error of the mean (SEM) of three independent experiments. Asterisks (*) indicate statistical significance: *0.01 ≤ P < 0.05, **0.001 ≤ P < 0.01, ***P < 0.001, n.s: not siginificant. (A) Quantitative RT-PCR analysis of CCR4 in vorinostat-treated T- and NK-cell lymphoma cell lines (5 μM for 24 h). The Student t-test was used to examine statistical significance. x-axis: cell lines. y-axis: 2−ΔCt values for microRNA expression. (B) Flow cytometry analysis of CCR4 in indicated T- and NK-cell lymphoma cells treated with vorinostat. Cells were stained with CCR4-FITC 24 h after vorinostat treatment (5 μM). Representative flow cytometry histograms are shown. ΔMFI (mean fluorescence intensity) values were obtained after subtraction of the isotype control, MFI from CCR4 MFI. SAHA: suberoylanilide hydroxamic acid. (C) ΔMFI of CCR4 in vorinostat-treated T- and NK-cell lymphoma cell lines (5 μM for 24 h). x-axis: cell lines. y-axis: ΔMFI of CCR4 in dimethylsulfoxide (DMSO) or vorinostat-treated T- and NK-cell lymphoma cell lines. (D) Migration assay of lymphoma cells treated with 5 μM vorinostat for 24 h. A schematic illustration of the migration assay is also shown. RFU: relative fluorescence units.
Next, we examined the changes in the expression of CCR4 protein using flow cytometry. Similar to the change in CCR4 mRNA, the CCR4 mean fluorescence intensity decreased markedly in the 11 CCR4-expressing cell lines after vorinostat treatment (Figure 1B,C). To investigate whether vorinostat affects the function of CCR4, we carried out in vitro CCL22-dependent chemotaxis assays. In this experiment, cell lines were plated onto the upper chamber of transwell plates, and the cell migration capability from the upper chamber to the lower chamber, where CCL22 was bound, was examined (Figure 1D). There was no fetal calf serum in either chamber. The migration was quantified on the basis of relative fluorescence units, as previously described.28 Control cells migrated from the upper to the lower chamber, but the migration was significantly suppressed by vorinostat (Figure 1D). From the foregoing outcomes, we concluded that vorinostat decreases mRNA expression and surface expression of CCR4, and as a result, suppresses the function and migration of T-cell lymphoma cells.
CCR4 expression in cutaneous T-cell lymphoma and adult T-cell leukemia/lymphoma cells is regulated by HDAC2
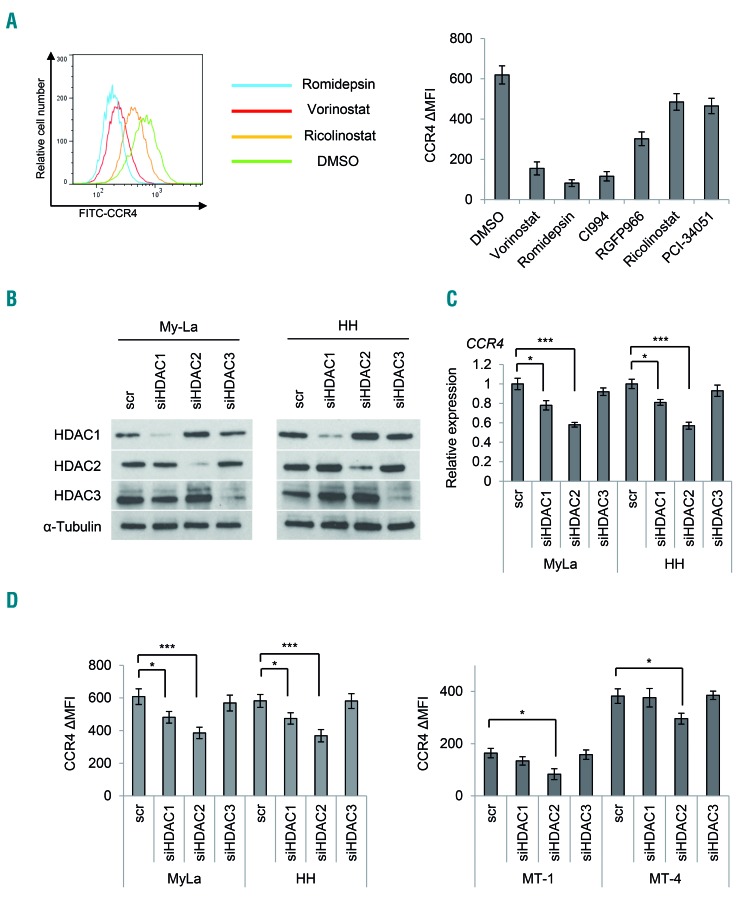
Given the results shown in Figure 1, we speculated that the expression of CCR4 is regulated by HDAC. Among the known 18 HDAC, class I HDAC (HDAC1, HDAC2, and HDAC3) are involved in gene transcription within the cell nucleus.29 In order to narrow down which HDAC could control CCR4 expression, we used various isoform- or class-selective HDAC inhibitors. In detail, we used the following class-specific HDAC inhibitors: romidepsin as a class I-selective HDAC inhibitor,30 CI-994 as an HDAC1/HDAC2-selective inhibitor,31 and RGFP966 as an HDAC3-selective inhibitor.32 We also studied HDAC6 and HDAC8 inhibitors, which are expected to have therapeutic effects on hematopoietic tumors:33,34 ricolinostat as an HDAC6-specific inhibitor and PCI-34051 as an HDAC8-specific inhibitor.35,36 When these CTCL cells were exposed to these drugs at the IC50, romidepsin and CI-994 strongly suppressed CCR4 expression (Figure 2A). These results suggest that class I HDAC might control CCR4 expression.
Figure 2.
Effect of isoform-specific histone deacetylase inhibitors and histone deacetylase knockdown on CCR4 expression. Bars indicate the mean ± standard error of the mean (SEM) of three independent experiments. Asterisks (*) indicate statistical significance: *0.01 ≤ P < 0.05, ***P < 0.001. (A) Flow cytometry analysis of CCR4 in CTCL cells (My-La) treated with various HDAC inhibitors. Cells were stained with CCR4-FITC 24 h after treatment (5 μM vorinostat, 10 nM romidepsin, 50 μM CI994, 20 μM RGFP966, 10 μM ricolinostat, and 75 μM PCI-34051). Left panel: representative flow cytometry histograms are shown. Right panel: ΔMFI of CCR4 in My-La cells treated with HDAC inhibitors. (B) Transient knockdown of HDAC (HDAC1, HDAC2, and HDAC3) in CTCL (My-La and HH) cells. Western blot analysis of HDAC1, HDAC2, and HDAC3 in cells after transient transduction of siHDAC or a scrambled control (scr). Tubulin: protein positive control. (C) Quantitative RT-PCR analysis of CCR4 after transient transfection of siHDAC and a scr in CTCL cells. x-axis: cell lines; y-axis: expression relative to control cells that were assigned a value of 1.0. (D) ΔMFI of CCR4 after transient transfection of siHDAC and scr in CTCL (My-La and HH) and ATLL (MT-1 and MT- 4) cells. x-axis: cell lines; y-axis: ΔMFI of CCR4. MFI: mean fluorescence intensity.
We further performed knockdown experiments using siRNA against HDAC1, HDAC2, and HDAC3. We confirmed the suppression of expression of these proteins by western blot analysis (Figure 2B). When we compared the change in expression of CCR4 mRNA in HDAC-knockdown cells, HDAC2 knockdown cells showed the most significantly decreased expression of CCR4 mRNA (Figure 2C). Moreover, when we examined the surface expression of CCR4 in HDAC-knockdown cells by flow cytometry, HDAC2-knockdown cells showed the greatest decrease in CCR4 mean fluorescence intensity (Figure 2D). This knockdown experiment was also performed on ATLL cell lines (MT-1 and MT-4), and confirmed a significant decrease in CCR4 mean fluorescence intensity (Figure 2D). These results suggest that class I HDAC, especially HDAC2, might be deeply involved in the regulation of CCR4 expression.
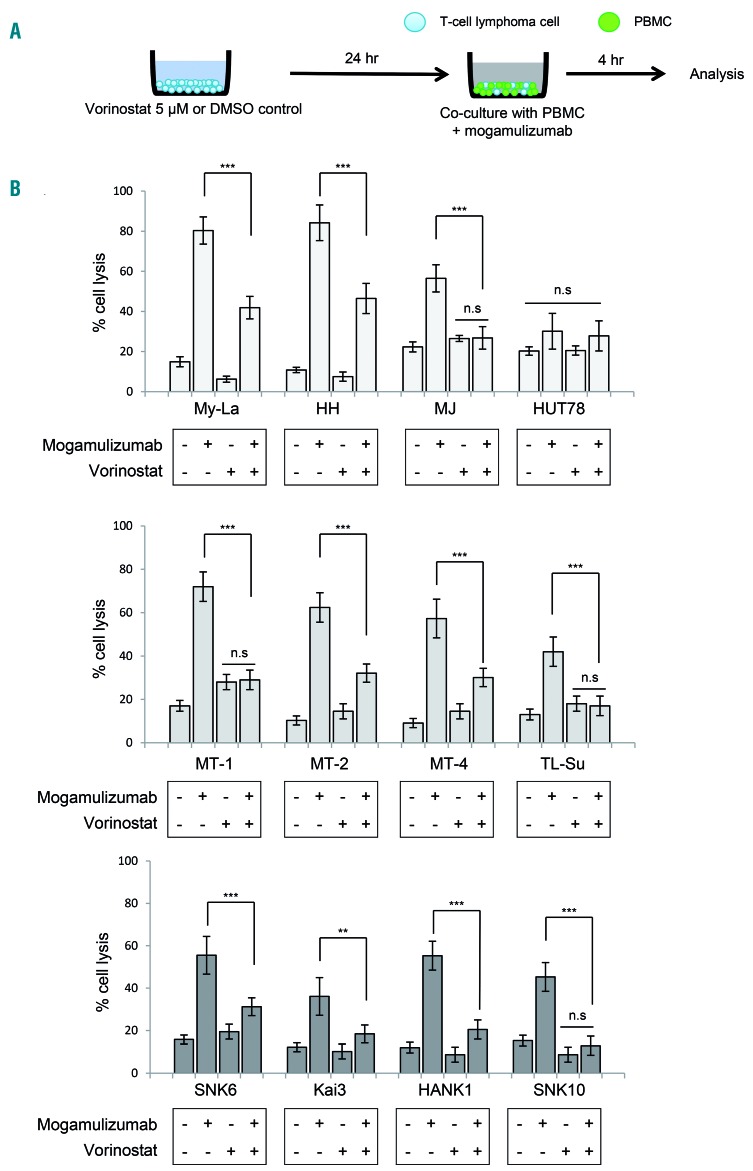
Pretreatment with histone deacetylase inhibitors significantly decreased mogamulizumab-induced antibody-dependent cell-mediated cytotoxicity
Mogamulizumab binds strongly to CCR4-positive cells and elicits powerful ADCC against tumor cells.37 We previously reported that pan-HDAC inhibitors (vorinostat and panobinostat) decreased the expression of chemokine receptor CCR6/CCR6 in CTCL.17 Together with these reports, we hypothesized that HDAC inhibitors may decrease the expression of CCR4, leading to the negative effect of mogamulizumab on ADCC activity. We therefore examined whether the combination of HDAC inhibitor and mogamulizumab might affect ADCC activity against T-cell lymphomas. We conducted a mogamulizumab-induced ADCC assay against cells pretreated with vorinostat (for 24 h) (Figure 3A). Firstly, viability with mogamulizumab was confirmed by an MTT assay and there was no change in viability of cell lines (data not shown). Compared to control cells treated with dimethylsulfoxide, we found that cytotoxicity induced by mogamulizumab was significantly reduced in vorinostat-treated cells. In particular, in the cell lines in which expression of CCR4 was weakly positive (MJ, TL-Su, and SNK10), the ADCC activity of mogamulizumab almost disappeared (Figure 3B). These results indicate that when an HDAC inhibitor and mogamulizumab are used in combination, the HDAC inhibitor downregulates CCR4 expression of lymphoma cells, resulting in a decrease of ADCC induced by mogamulizumab.
Figure 3.
Pretreatment of vorinostat significantly decreases mogamulizumab-induced antibody-dependent cell-mediated cytotoxicity. Bars indicate the mean ± standard error of the mean (SEM) of three independent experiments. Asterisks (*) indicate statistical significance: **0.001 ≤ P < 0.01, ***P < 0.001, n.s: not siginificant. (A) A schematic illustration of the ADCC assay is shown. Cells were treated with 5 μM vorinostat for 24 h or dimethylsulfoxide (DMSO) as a control before mogamulizumab treatment. Cytotoxicity was measured using the lactate dehydrogenase assay in the presence of effector cells (peripheral blood mononuclear cells. PBMC) obtained from healthy volunteers and mogamulizumab (10 mg/mL) or the same volume of solvent (control). The ratio of target:effector cells was fixed at 1:50. (B) The ADCC assay against CTCL (upper panel: MyLa, HH, MJ, and HUT78), ATLL (middle panel: MT-1, MT-2, MT-4, and TL-Su), and NK/T-cell lymphoma (lower panel: SNK6, Kai3, HANK1, and SNK10) cell lines. x-axis: cell lines; y-axis: percent cell lysis.
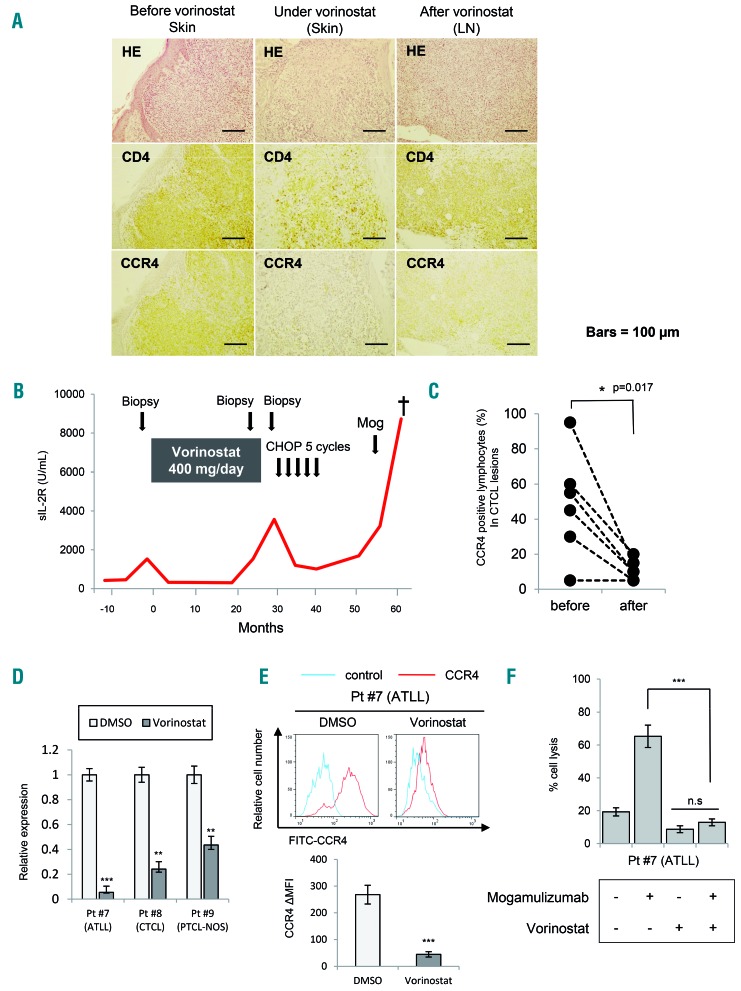
CCR4 expression was greatly reduced after vorinostat treatment in primary cutaneous T-cell lymphoma
To examine the effect of CCR4 downregulation by HDAC inhibitors in clinical cases, we examined the CCR4 expression of CTCL skin samples, which were obtained from the same patients before and after vorinostat treatment. In patient #1, we confirmed strong concordance between CCR4 positivity and CD4-positive T cells before vorinostat treatment. However, we found that CCR4 expression was greatly reduced in the specimen at the time of relapse after 2 years of vorinostat treatment (Figure 4A).
Figure 4.
CCR4 expression of clinical lymphoma samples before and after vorinostat treatment. Bars indicate the mean ± standard error of the mean (SEM) of three independent experiments. Asterisks (*) indicate statistical significance: *0.01 ≤ P < 0.05, **0.001 ≤ P < 0.01, ***P < 0.001, n.s: not siginificant. (A) CCR4 expression determined by immunohistochemical staining before and after vorinostat treatment. Representative results from samples (skin and lymph nodes) from patient with mycosis fungoides (MF) are shown. Specimens were stained with hematoxylin and eosin (HE), CD4, and CCR4 for a pathological diagnosis and confirmation: 200× magnification. LN: lymph node. (B) Clinical time course in patient (Pt) #1 with MF before and after vorinostat treatment. (C) CCR4-positive lymphocytes (% of total infiltrating lymphocytes) in specimens from six patients (Pt #1 – 6) before and after vorinostat treatment. The Student t-test was used to examine statistical significance. (D) Quantitative RT-PCR analysis of CCR4 in samples of primary T-cell lymphomas (ATLL, CTCL, and PTCL-NOS) (Pt #7 – 9) exposed to 5 μM vorinostat for 24 h. (E) ΔMFI of CCR4 in a sample of primary ATLL (Pt #7) treated with 5 μM vorinostat for 24 h. (F) ADCC assay against a sample of primary ATLL. Cells were treated with 5 μM vorinostat for 24 h or control cells treated with dimethylsulfoxide (DMSO) before mogamulizumab treatment. Cytotoxicity was measured using the lactate dehydrogenase assay in peripheral blood mononuclear cells obtained from healthy volunteers and mogamulizumab (10 mg/mL) or the same volume of solvent (control). The ratio of target: effector cells was fixed at 1:50.
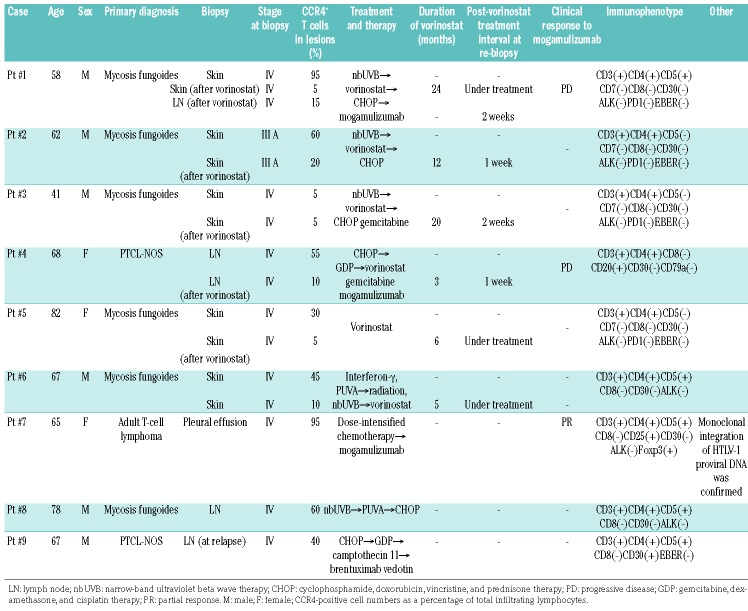
In Figure 4B, we show the clinical course of patient #1. This case showed weak positivity for CCR4 at relapse and when mogamulizumab was administered, but the response was not effective and the patient had progressive disease. Similarly, CCR4 expression was evaluated by immunohistochemical staining for six cases for which samples before and after vorinostat treatment were available (Table 1). As a result, we found that CCR4 expression decreased significantly in the specimens after vorinostat treatment (Figure 4C). Furthermore, when we examined CCR4 mRNA changes in the primary samples (patient #7: ATLL, patient #8: CTCL, and patient #9: PTCL-NOS) treated with vorinostat, we also confirmed a marked downregulation of CCR4 (Figure 4D). Significant downregulation of CCR4 was confirmed when the surface expression was analyzed in an ATLL primary sample by flow cytometry (Figure 4E). Moreover, when we conducted an ADCC assay of mogamulizumab using this primary ATLL sample, we found that the efficacy of mogamulizumab was significantly reduced by vorinostat pretreatment (Figure 4F). These results strongly suggest that ADCC of lymphoma cells could not be expected from pretreatment with HDAC inhibitors, even in the primary sample.
Table 1.
Information about the patients with T-cell lymphoma.
Discussion
HDAC inhibitors interfere with histone tail modifications, thus altering chromatin structure and epigenetically controlled pathways. Pan-HDAC inhibitors, such as vorinostat, can restore the expression of its target molecules. HDAC inhibition directly suppresses the transcription of genes regulated by HDAC, while there are genes whose expression increases with HDAC inhibition. Mediator molecules, including various microRNA which are a direct target of HDAC inhibitors, may cause enhanced gene transcription by HDAC inhibitors. The main role of HDAC inhibition in cancer treatment is the restoration of tumor suppressor gene expression, which is suppressed by HDAC. HDAC inhibitors can restore coding and non-coding genes, such as CDKN1A/p21 and miR-16.13,16 p21 is a transcriptional target molecule of tumor suppressor protein p53. miR-16 is a well-known tumor-suppressive microRNA.16 Restoration of these molecules induces cell growth arrest, cellular senescence, and apoptosis in lymphoma cells.13,16 In addition, we expect that the restoration of tumor suppressive genes downregulates oncogenes and the translation of proteins. For example, BMI1/Bmi-1, a proto-oncogene, is suppressed by the restoration of miR-16.16,38 Thus, the role of HDAC inhibitors in eradicating cancer cells involves restoring tumor suppressive genes by downregulation of oncogenes.
However, considering the effects of HDAC inhibition on chemokines, chemokine receptors, and cell surface antigens, its respective tumor-suppressive and tumor-promoting aspects must be analyzed. When considering the anti-tumor effects, enhancement of the expression of chemokine receptors or chemokines may be desired in some cases, while their suppression may be desirable in other cases. As an example of desirable enhanced expression, Zheng et al. recently showed that HDAC inhibition can enhance T-cell infiltration and T-cell dependent tumor regression by increasing the expression of CCL5, CXCL9, and CXCL10, thereby augmenting the immune effect of anti-PD-1.23 As an example of desirable reduced expression by HDAC inhibition, we recently reported that HDAC inhibition decreased CCR6 expression via upregulation of miR-150 and consequently inhibited multiple metastases of CTCL cells.17 However, as we have shown in this study, HDAC inhibitors also decrease the expression of CCR4. As a result, mogamulizumab-inducible ADCC was remarkably decreased, and a synergistic effect between mogamulizumab and HDAC inhibition could not be confirmed. A similar synergistic effect was expected from combining HDAC inhibitors with antibodies for cell surface antigens, but is not advisable. For example, Hasanali et al. previously showed that vorinostat suppresses CD30 expression and attenuates the efficacy of the anti-CD30, brentuximab vedotin, in ALCL.39 Thus, when expecting a synergistic effect from HDAC inhibition and other molecular targeted drugs, the influence of the target molecule of HDAC inhibitors must be examined sufficiently.
Accumulated knowledge of the targets of HDAC inhibition could lead us to establish effective strategies for the administration order of molecular targeting agents. For example, CCR4 expression of a vorinostat-treated clinical sample from patient #1 recovered slightly 2 weeks after completion of treatment. This might be because of the recovery of epigenetically repressed CCR4 expression. Although the diminished expression of CCR4 by vorinostat was epigenetic, it appeared to be reversible.40 To test this notion, we monitored CCR4 expression of CTCL cell lines at specified time points (24, 48, and 72 h) after vorinostat treatment (5 μM, 24 h). Following the removal of vorinostat, CCR4 expression returned to pretreatment levels after 72 h (data not shown). However, it is unknown how long CCR4 expression would take to recover after long-term vorinostat exposure in vivo. The restoration of CCR4 expression to its original level after vorinostat treatment completion may require some time. For patient #1, we could not exclude the possibility that the ADCC of effector cells was attenuated by five cycles of CHOP therapy. In addition, mogamulizumab has been shown to decrease CCR4-positive regulatory T cells and increase CD8-positive T cells and NK-cell numbers.41 The effect on such tumor microenvironments is also an important factor and the positivity of CCR4 alone may not, therefore, determine the response to mogamulizumab. Nevertheless, at least, it may be beneficial for patients to use mogamulizumab followed by vorinostat as a treatment strategy for CCR4-positive T-cell lymphomas.
In this study, we showed that HDAC2 mainly regulates CCR4 expression. Avoiding unnecessary inhibition of target genes by HDAC inhibitors may be important for the reduction of side effects. The development and clinical application of isoform-specific HDAC inhibitors are progressing.42 Romidepsin, a class I-specific HDAC inhibitor has been shown to be clinically effective against PTCL.21 Currently, however, HDAC inhibitors that can be used against malignant lymphomas, including vorinostat, romdepsin, and belinostat, all suppress HDAC2. Consequently, the suppression of CCR4 by class-specific or pan-HDAC inhibitors is currently inevitable. In contrast, the HDAC6-selective inhibitor, ricolinostat, has potential for therapeutic application against multiple myeloma.43 It has also been shown that PCI-34051, an HDAC8-specific inhibitor, induces apoptosis specifically in T-cell lymphomas.36 Because these HDAC inhibitors did not suppress CCR4 expression in our study, their clinical efficacy in lymphomas should be examined for future clinical trials. In addition, because HDAC inhibitors directly restore target gene expression, they might decrease CCR4 expression by some undetermined mediating molecules such as microRNA. Alternatively, the decrease may be mediated by undetermined transcription factor(s) that regulate CCR4 expression, although we did not determine these factors. Further research is required to elucidate the specific mediator.
In summary, our results suggest that the use of HDAC inhibitors before mogamulizumab against CTCL and PTCL might reduce the benefits of mogamulizumab in patients. Conversely, mogamulizumab followed by an HDAC inhibitor may be effective. In developing a therapeutic strategy, the effect of HDAC inhibitors on combined molecular targeted drugs directly affects the benefits that patients gain.
Supplementary Material
Acknowledgments
The authors would like to thank Ms. Hiromi Kataho, Yuko Chiba, and Yukiko Abe for their outstanding technical assistance. We thank Drs K. Teshima, H. Oyagi, M. Kume (Hiraka General Hospital), Y. Abe & K. Narita (Kameda Medical Center) for collecting primary samples. This work was supported by JSPS KAKENHI [Grant-in-Aid for Scientific Research (with funds to AK & HT)].
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/1/126
References
- 1.WHO Classification of Tumours of Haematopoietic and Lymphoid tissues 2008. 4th ed. Lyon, France: International Agency for Research on Cancer (IARC). [Google Scholar]
- 2.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Leary HM, Savage KJ. Update on the World Health Organization classification of peripheral T-cell lymphomas. Curr Hematol Malig Rep. 2009;4(4): 227–235. [DOI] [PubMed] [Google Scholar]
- 4.Savage KJ. Therapies for peripheral T-cell lymphomas. Hematology Am Soc Hematol Educ Program. 2011;2011:515–524. [DOI] [PubMed] [Google Scholar]
- 5.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. [DOI] [PubMed] [Google Scholar]
- 6.Ishii T, Ishida T, Utsunomiya A, et al. Defucosylated humanized anti-CCR4 mono clonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin Cancer Res. 2010;16(5):1520–1531. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Utsunomiya A, Tobinai K, et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol. 2010;28(9):1591–1598. [DOI] [PubMed] [Google Scholar]
- 8.Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30(8):837–842. [DOI] [PubMed] [Google Scholar]
- 9.Ishida T, Inagaki H, Utsunomiya A, et al. CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T-cell and NK-cell lymphomas with special reference to clinicopathological significance for peripheral T-cell lymphoma, unspecified. Clin Cancer Res. 2004;10(16):5494–5500. [DOI] [PubMed] [Google Scholar]
- 10.Yagi H, Seo N, Ohshima A, et al. Chemokine receptor expression in cutaneous T cell and NK/T-cell lymphomas: immunohistochemical staining and in vitro chemotactic assay. Am J Surg Pathol. 2006;30(9):1111–1119. [DOI] [PubMed] [Google Scholar]
- 11.Ogura M, Ishida T, Hatake K, et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol. 2014;32(11):1157–1163. [DOI] [PubMed] [Google Scholar]
- 12.Kanazawa T, Hiramatsu Y, Iwata S, et al. Anti-CCR4 monoclonal antibody mogamulizumab for the treatment of EBV-associated T- and NK-cell lymphoproliferative diseases. Clin Cancer Res. 2014;20(19):5075–5084. [DOI] [PubMed] [Google Scholar]
- 13.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci USA. 2000;97(18):10014–10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009; 27(32): 5459–5468. [DOI] [PubMed] [Google Scholar]
- 15.Matthews GM, Mehdipour P, Cluse LA, et al. Functional-genetic dissection of HDAC dependencies in mouse lymphoid and myeloid malignancies. Blood. 2015;126(21): 2392–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitadate A, Ikeda S, Teshima K, et al. MicroRNA-16 mediates the regulation of a senescence-apoptosis switch in cutaneous T-cell and other non-Hodgkin lymphomas. Oncogene. 2016;35(28):3692–3704. [DOI] [PubMed] [Google Scholar]
- 17.Abe F, Kitadate A, Ikeda S, et al. Histone deacetylase inhibitors inhibit metastasis by restoring a tumor suppressive microRNA-150 in advanced cutaneous T-cell lymphoma. Oncotarget. 2017;8(5):7572–7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ju R, Muller MT. Histone deacetylase inhibitors activate p21(WAF1) expression via ATM. Cancer Res. 2003;63(11):2891–2897. [PubMed] [Google Scholar]
- 19.Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood. 2007;109(1):31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa H, Bissonnette RP, Gillings M, et al. Induction of apoptosis by HBI-8000 in adult T-cell leukemia/lymphoma is associated with activation of Bim and NLRP3. Cancer Sci. 2016;107(8):1124–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30(6):631–636. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor OA, Horwitz S, Masszi T, et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) Study. J Clin Oncol. 2015;33(23):2492–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng H, Zhao W, Yan C, et al. HDAC Inhibitors enhance T-cell chemokine expression and augment response to PD-1 immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2016;22(16):4119–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heider U, Rademacher J, Lamottke B, et al. Synergistic interaction of the histone deacetylase inhibitor SAHA with the proteasome inhibitor bortezomib in cutaneous T cell lymphoma. Eur J Haematol. 2009;82(6): 440–449. [DOI] [PubMed] [Google Scholar]
- 25.Rozati S, Cheng PF, Widmer DS, Fujii K, Levesque MP, Dummer R. Romidepsin and Azacitidine synergize in their epigenetic modulatory effects to induce apoptosis in CTCL. Clin Cancer Res. 2016;22(8):2020–2031. [DOI] [PubMed] [Google Scholar]
- 26.Mondello P, Brea EJ, De Stanchina E, et al. Panobinostat acts synergistically with ibrutinib in diffuse large B cell lymphoma cells with MyD88 L265 mutations. JCI Insight. 2017;2(6):e90196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vermeer MH, Dukers DF, ten Berge RL, et al. Differential expression of thymus and activation regulated chemokine and its receptor CCR4 in nodal and cutaneous anaplastic large-cell lymphomas and Hodgkin’s disease. Mod Pathol. 2002;15(8):838–844. [DOI] [PubMed] [Google Scholar]
- 28.Ito M, Teshima K, Ikeda S, et al. MicroRNA-150 inhibits tumor invasion and metastasis by targeting the chemokine receptor CCR6, in advanced cutaneous T-cell lymphoma. Blood. 2014;123(10):1499–1511. [DOI] [PubMed] [Google Scholar]
- 29.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furumai R, Matsuyama A, Kobashi N, et al. FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res. 2002;62(17):4916–4921. [PubMed] [Google Scholar]
- 31.Moradei OM, Mallais TC, Frechette S, et al. Novel aminophenyl benzamide-type histone deacetylase inhibitors with enhanced potency and selectivity. J Med Chem. 2007;50(23):5543–5546. [DOI] [PubMed] [Google Scholar]
- 32.Malvaez M, McQuown SC, Rogge GA, et al. HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci USA. 2013;110(7):2647–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hideshima T, Qi J, Paranal RM, et al. Discovery of selective small-molecule HDAC6 inhibitor for overcoming proteasome inhibitor resistance in multiple myeloma. Proc Natl Acad Sci USA. 2016;113(46): 13162–13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higuchi T, Nakayama T, Arao T, Nishio K, Yoshie O. SOX4 is a direct target gene of FRA-2 and induces expression of HDAC8 in adult T-cell leukemia/lymphoma. Blood. 2013;121(18):3640–3649. [DOI] [PubMed] [Google Scholar]
- 35.Santo L, Hideshima T, Kung AL, et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood. 2012;119(11):2579–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balasubramanian S, Ramos J, Luo W, Sirisawad M, Verner E, Buggy JJ. A novel histone deacetylase 8 (HDAC8)-specific inhibitor PCI-34051 induces apoptosis in T-cell lymphomas. Leukemia. 2008;22(5): 1026–1034. [DOI] [PubMed] [Google Scholar]
- 37.Ishida T, Ueda R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci. 2006;97(11):1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teshima K, Nara M, Watanabe A, et al. Dysregulation of BMI1 and microRNA-16 collaborate to enhance an anti-apoptotic potential in the side population of refractory mantle cell lymphoma. Oncogene. 2014;33(17):2191–2203. [DOI] [PubMed] [Google Scholar]
- 39.Hasanali ZS, Epner EM, Feith DJ, Loughran TP, Jr, Sample CE. Vorinostat downregulates CD30 and decreases brentuximab vedotin efficacy in human lymphocytes. Mol Cancer Ther. 2014;13(12):2784–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selcuklu SD, Spillane C. Translational epigenetics: clinical approaches to epigenome therapeutics for cancer. Epigenetics. 2008;3(2):107–112. [DOI] [PubMed] [Google Scholar]
- 41.Ni X, Jorgensen JL, Goswami M, et al. Reduction of regulatory T cells by mogamulizumab, a defucosylated anti-CC chemokine receptor 4 antibody, in patients with aggressive/refractory mycosis fungoides and Sézary syndrome. Clin Cancer Res. 2015;21(2):274–285. [DOI] [PubMed] [Google Scholar]
- 42.Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov. 2014;13(9):673–691. [DOI] [PubMed] [Google Scholar]
- 43.Yee AJ, Bensinger WI, Supko JG, et al. Ricolinostat plus lenalidomide, and dexamethasone in relapsed or refractory multiple myeloma: a multicentre phase 1b trial. Lancet Oncol. 2016;17(11):1569–1578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.