In December 2015, a 66-year-old man was referred to our university hospital with a recent diagnosis of histiocytic sarcoma. The patient had presented recurrent abdominal pain for two months without B symptoms. CT scan evidenced an ileal mass, multiple hepatic lesions, and diffuse abdominal and mediastinal adenopathies. Coronal section of PET-CT is shown in Figure 1A. After two liver biopsies, that were initially not conclusive, and a negative bone marrow biopsy, a surgical resection of the terminal ileum was performed, showing a transmural tumor interpreted as histiocytic sarcoma. Histopathological review confirmed an infiltrate of large, pleomorphic and atypical histiocytoid cells with abundant cytoplasm (Figure 2). Immunohistochemistry showed that the cells were CD3–, CD68+, CD163+, HLA-DR+, muramidase (lysozyme)+/−, S100−/+, CD1a– and langerin-. Ki67 proliferation fraction was heterogeneous, but overall low (about 10%). No mutations were detected in BRAF exon 15 and 11, while fluorescence in situ hybridization (FISH) study demonstrated the absence of PDGFRA, PDGFRB, and FGFR1 rearrangements but a copy number gain (mostly 2–5 fold, but up to 9 fold) in the 3 studied loci. PDL-1 expression was heterogeneous in 15–20% of the tumour cells. Retrospectively, the two liver biopsies were revised and tested positive for the same pathology with presence of an important T-cell reactive population.
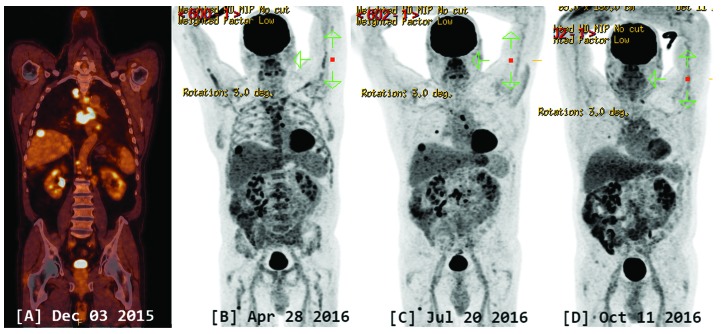
Figure 1.
PET-CT scan. A: initial coronal section. B: after conventional chemotherapy (CHOP and ICE). C: after alemtuzumab, and D: after two months of treatment with trametinib.
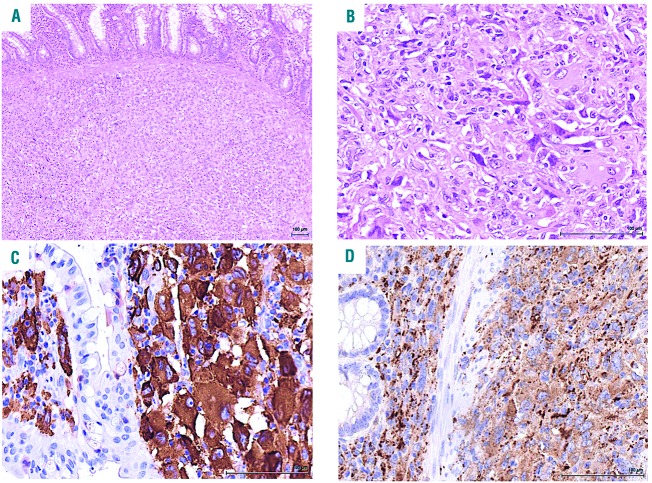
Figure 2.
Pathology of the tumor. (A) low magnification showing diffuse involvement of the intestinal wall (mucosa at the top) (hematoxylin and eosin, original magnification ×40); (B) high magnification of the tumor composed of large cells with vesicular to hyper chromatic nuclei and abundant eosinophilic cytoplasm (hematoxylin and eosin, original magnification ×200); (C-D) immunohistochemical staining showing positivity for CD163 (C) and (CD68) (D) (immunoperoxidase, original magnification x200).
An allogeneic hematopoietic stem cell transplantation (HSCT) was considered indicated, but only after at least a good response of the tumor burden. The patient was initially treated with 2 cycles of CHOP therapy (cyclophosphamide, 750 mg/m2 i.v. on day 1; doxorubicin, 50 mg/m2 i.v. on day 1; vincristine, 1.4 mg/m2 i.v. on day 1, and prednisone, 100 mg p.o. on day 1 through 5 every 21 days). A mixed response was observed on the PET-scan. Two hepatic lesions and mediastinal, subclavicular and abdominal adenopathies disappeared, while other sites (lung, liver, gut and femur) showed progression with new lesions. A salvage therapy with ICE (ifosfamide 5000 mg/m2 i.v. fractionated into three equally divided doses over 3 days, carboplatin [mg dose = 5 × area under the curve (AUC)] i.v. on day 1 and etoposide 100 mg/m2 i.v. daily for 3 consecutive days, every 21 days) was conducted for 2 cycles. Again, PET-CT (figure 1B) showed a mixed response with progressive disease in the liver, stability in the bone, and response in all adenopathies and in the lung lesion. A liver biopsy was performed and showed involvement by histiocytic sarcoma with no evidence of necrosis. A third-line therapy with alemtuzumab (30 mg subcutaneous three times a week) was initiated, but the PET-CT showed progressive disease after two months (figure 1C).
After failure of these three treatment lines, the initial ileal resection piece was further analyzed by NGS (Next Generation Sequencing) using a panel of 52 genes (Ion AmpliSeqTM) covering 218 hotspot regions in search of potentially targetable genomic alterations. This analysis revealed the presence of a potential oncogenic mutation c.226G>A (p.E72K) in the PTPN11 gene, exon 3 (allele frequency 29.2%) as well as a one-copy gain of STK11, GNA11 and JAK3 loci (19p13).
The patient was discussed at our institutional molecular tumor-board. PTPN11 encodes for SHP2, which is a negative regulator of the Ras/MAPK pathway. Mutations in PTPN11, for example in Noonan syndrome, result in activated MAPK signaling.1 GNA11, is a G protein coupled receptor and also activates the MAPK pathway. Based on the PTPN11 mutation and copy number gain of GNA11, a hyperactivation of the MAPK pathway was predicted. The patient was treated with the MEK inhibitor trametinib 2 mg daily to block the RAS/MAPK pathway. Excellent partial remission was observed after 2 months of treatment: only 2 lymph nodes and 1 small pulmonary lesion persisted, with clear diminution of the size and hypermetabolic capture (Figure 1D). Unfortunately, the patient suffered from several adverse events while on trametinib treatment: he developed a grade 3 acneiform rash and a grade 3 QTc interval prolongation on ECG requiring dose reduction and several treatment interruptions. Throughout the five months of trametinib treatment, he received 10 days at 2 mg, 21 days at 1.5 mg, and 46 days at 1 mg. Despite very low dose medication, the excellent response described above persisted until progressive disease was documented on PET-CT scan five months after trametinib initiation, while still on treatment (lymph nodes, lung, liver and bone).
Due to the copy number gain of PDGFRA and PDGFRB in FISH analysis, the patient was subsequently treated with imatinib (400 mg daily for 4 weeks followed by 800 mg daily) as next clinical option. We performed NGS for 52 genes and PDGFRA and PDGFRB FISH almost simultaneously. Since the NGS found both a PTPN11 mutation and gain of copies of GNA11 but did not confirm the FISH gain of copies results of PDGFRA, we considered the NGS as higher level of evidence than the FISH analysis alone, hence our first choice was trametinib.
Three months after imatinib introduction, a mixed response could again be demonstrated on PET-scan with partial response on the supra-diaphragmatic lymph nodes, complete disappearance of the liver lesions, but increased gut and lung FDG-uptake, as well as progressive abdominal lymph nodes, together with a dissociated response in bone infiltrates. The patient was still in good performance status (ECOG 1) without specific complaints apart from grade 1 fatigue. Currently, the patient has been initiated on another experimental treatment line by checkpoint inhibitor anti-PD1 nivolumab.
Histiocytic sarcoma is an extremely rare malignant disease that has an aggressive clinical course with a dismal outcome, most patients dying within 2 years after diagnosis.2,3 Currently, no standard treatment approach is defined due to the low frequency of the disease and consequent lack of clinical studies. Despite absence of prospective clinical data, non-Hodgkin lymphoma treatment approaches are often used for multifocal disease, but results are rather disappointing.4 Few data exist for autologous and even less for allogeneic stem cell transplantation in the adult population, but some patients have shown favorable outcomes.2,5,6 Thalidomide and alemtuzumab have been reported as potential therapies in single case reports, both in the pediatric or transplant setting.6–10 Furthermore, since BRAF mutations (V600E, G464V and others)11,12 are frequently detected in histiocytic sarcoma, vemurafenib, dabrafenib and trametinib are potential targeted drugs for patients in which one of these mutations has been detected. These studies did not demonstrate an influence of the BRAF mutational status on the natural course of the disease. Whether the treatment with targeted drugs can influence the outcome remains to be determined. Also, PD-L1 overexpression and PTEN mutations have been described and can be targeted nowadays.13 However, as the disease is very rare, no clinical data of larger studies are available to date.
In our patient, we identified two alterations suggestive of MAPK activation: 1, a mutation in the PTPN11 gene and 2, a 3-fold copy gain of GNA11. MEK inhibitors, like trametinib or cobimetinib can efficiently block the MAPK. To our knowledge, this is the first case described to date with a documented response of a histiocytic sarcoma to trametinib, with another case associated with follicular lymphoma where MAPK2K1 mutation and response to MEK inhibition was documented.14 Unfortunately, due to adverse events of grade 3, the treatment had to be dose-adjusted and then definitely interrupted after five months.
Interestingly, tyrosine kinase inhibition with imatinib following trametinib discontinuation, also resulted in a documented response.
In conclusion, this case reports an excellent response to targeted MEK inhibition in a histiocytic sarcoma. Targeting the RAF/RAS/MEK pathway may be a promising therapeutic option in such patients with an otherwise dismal outcome. We could furthermore demonstrate a potential for Imatinib in the treatment of this rare disease. Further and larger clinical studies are needed, evaluating treatment responses in bigger cohorts in a controlled setting. Combining these targeted therapies could be a future treatment possibility; however, increased toxicities may be a concern. The possibility of better molecular characterisation of rare diseases raises hope for targeted therapy options in difficult to treat diseases. However, it will be difficult to obtain clinical trial data with statistical impact for all the different targeted treatment options, more so if treatment combinations are being tested.
Supplementary Material
Acknowledgments
We thank Dr méd. Edouard Stauffer, pathologist at Promed SA for his collaboration and assistance with the collection of materials.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Tidyman WE, Rauen KA. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev. 2009;19(3):230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pileri SA, Grogan TM, Harris NL, et al. Tumours of histiocytes and accessory dendritic cells: an immunohistochemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology. 2002;41(1):1–29. [DOI] [PubMed] [Google Scholar]
- 3.Hornick JL, Jaffe ES, Fletcher CD. Extranodal histiocytic sarcoma: clinicopathologic analysis of 14 cases of a rare epithelioid malignancy. Am J Surg Pathol. 2004;28(9):1133–1144. [DOI] [PubMed] [Google Scholar]
- 4.Gounder M, Desai V, Kuk D, et al. Impact of surgery, radiation and systemic therapy on the outcomes of patients with dendritic cell and histiocytic sarcomas. Eur J Cancer. 2015;51(16):2413–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsujimura H, Miyaki T, Yamada S, et al. Successful treatment of histiocytic sarcoma with induction chemotherapy consisting of dose-escalated CHOP plus etoposide and upfront consolidation auto-transplantation. Int J Hematol. 2014;100(5):507–510. [DOI] [PubMed] [Google Scholar]
- 6.Zeidan A, Bolaños-Meade J, Kasamon Y, et al. Human leukocyte antigen-haploidentical hematopoietic stem cell transplant for a patient with histiocytic sarcoma. Leuk Lymphoma. 2013;54(3):655–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shukla N, Kobos R, Renaud T, et al. Successful treatment of refractory metastatic histiocytic sarcoma with alemtuzumab. Cancer. 2012;118(15):3719–3724. [DOI] [PubMed] [Google Scholar]
- 8.Gergis U, Dax H, Ritchie E, et al. Autologous hematopoietic stem-cell transplantation in combination with thalidomide as treatment for histiocytic sarcoma: a case report and review of the literature. J Clin Oncol. 2011;29(10):e251–253 [DOI] [PubMed] [Google Scholar]
- 9.Abidi MH, Tove I, Ibrahim RB, Maria D, Peres E. Thalidomide for the treatment of histiocytic sarcoma after hematopoietic stem cell transplant. Am J Hematol. 2007;82(10)932–933. [DOI] [PubMed] [Google Scholar]
- 10.Bailey KM, Castle VP, Hummel JM, Piert M, Moyer J, McAllister-Lucas LM. Thalidomide therapy for aggressive histiocytic lesions in the pediatric population. J Pediatr Hematol Oncol. 2012;34(6):480–483. [DOI] [PubMed] [Google Scholar]
- 11.Go H, Jeon YK, Huh J, et al. Frequent detection of BRAF V600E mutations in histiocytic and dendritic cell neoplasms. Histopathology. 2014;65(2):261–272. [DOI] [PubMed] [Google Scholar]
- 12.Liu Q, Tomaszewicz K, Hutchinson L, Hornick JL, Woda B, Yu H. Somatic mutations in histiocytic sarcoma identified by next generation sequencing. Virchows Arch. 2016;469(2):233–241. [DOI] [PubMed] [Google Scholar]
- 13.Gatalica Z, Bilalovic B, Palazzo JP, et al. Disseminated histiocytosis biomarkers beyond BRAFV600E: frequent expression of PD-L1. Oncotarget. 2015:6(23):19819–19825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Facchetti F, Pileri SA, Lorenzi L, et al. Histiocytic and dendritic cell neoplasms: what have we learnt by studying 67 cases. Virchows Arch. 2017: July 10 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.