SUMMARY
Many animals, ranging from vinegar flies to humans discriminate a wide range of tastants, including sugars, bitter compounds, NaCl and sour. However, the taste of Ca2+ is poorly understood, and it is unclear if animals such as Drosophila melanogaster are endowed with this sense. Here, we examined Ca2+ taste in Drosophila and showed that high levels of Ca2+ are aversive. The repulsion was mediated by two mechanisms—activation of a specific class of gustatory receptor neurons (GRNs) that suppresses feeding, and inhibition of sugar-activated GRNs, which normally stimulates feeding. The distaste for Ca2+, and Ca2+-activated action potentials required several members of the variant ionotropic receptor (IR) family (IR25a, IR62a, and IR76b). Consistent with the Ca2+ rejection, we found that high concentrations of Ca2+ decreased survival. We conclude that gustatory detection of Ca2+ represents an additional sense of taste in Drosophila, and is required for avoiding toxic levels of this mineral.
INTRODUCTION
The ability to discriminate the chemical composition of food is used by most animals to identify calorie-rich foods, and to consume the appropriate concentrations of minerals. Many animals ranging from flies to humans, perceive sugars as attractive, and bitter compounds as aversive (Liman et al., 2014). This differs from the behavioral responses to Na+, which reverse depending on the concentration (Liman et al., 2014). Na+ is required for survival, and low levels are attractive, whereas high concentrations can be toxic and are repulsive to the taste. Similar to Na+, low or modest levels of Ca2+ promote survival, and high concentrations can lead to adverse effects. In humans, hypercalcemia is associated with many common diseases, and can be life-threatening (reviewed in Žofková, 2016).
In contrast to the extensive behavioral and mechanistic studies focusing on other forms of taste, especially sweet and bitter (Liman et al., 2014), far less is known about Ca2+ taste. While some studies support the concept that humans perceive Ca2+ in food (Tordoff, 2001), the sensitivity of humans to Ca2+ is unresolved. One study suggests that a human taste receptor contributes to sensing Ca2+ (T1R3) (Tordoff et al., 2012). However, the mechanism is unclear because this receptor also functions in the gustatory detection of sweet and umami in mammals (Max et al., 2001; Nelson et al., 2002; Nelson et al., 2001). Nevertheless, due to the positive and negative effects of Ca2+ on health, in principle, detection of this mineral could be attractive or repulsive depending on its concentration.
To address the fundamental questions concerning the behavioral, molecular and cellular mechanisms through which an animal tastes Ca2+, we focused on the vinegar fly. We found that flies are indifferent to modest levels of Ca2+ in food. However, they show strong repulsion to high Ca2+, indicating that they possess the sense of Ca2+ taste. Ca2+ avoidance was mediated by neurons distinct from those that respond to bitter compounds. A diet containing high levels of Ca2+ diminished viability. We found that three members of family of variant ionotropic receptors (Benton et al., 2009) (IR25a, IR62a, and IR76b) are required for the behavioral and electrophysiological responses to Ca2+, indicating that they are critical molecular components that enable flies to avoid ingesting excessive levels of Ca2+.
RESULTS
Flies taste Ca2+ in food, and high levels are aversive
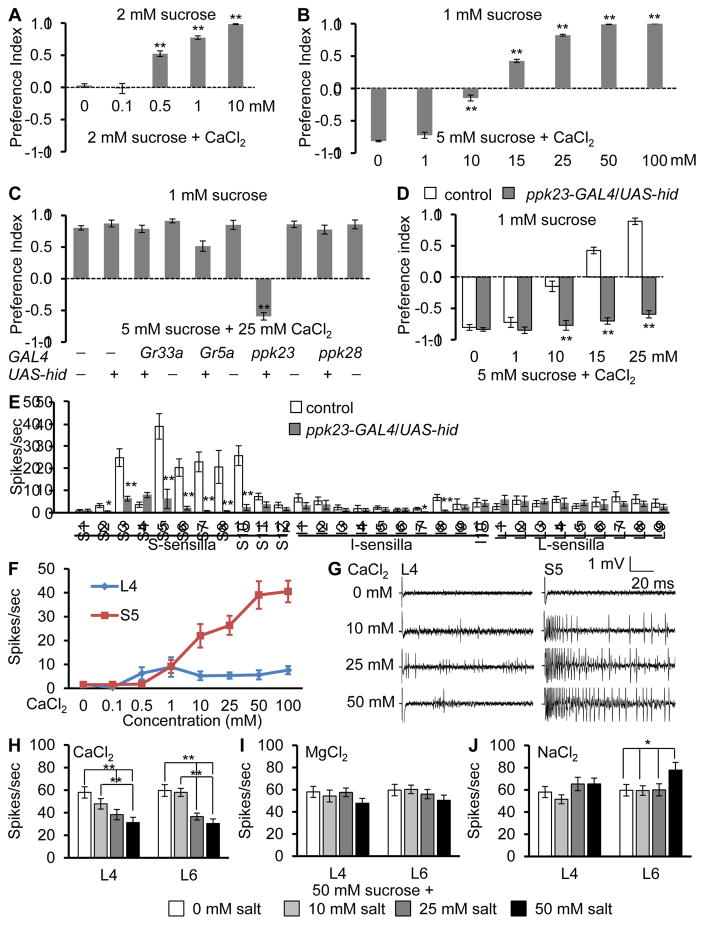
To determine the behavioral response of Drosophila to Ca2+, we employed a binary food choice assay (Meunier et al., 2003; Moon et al., 2006). We mixed 2 mM sucrose, or 2 mM sucrose plus various concentrations of CaCl2 with either blue or red food dye, and determined the feeding preferences by inspection of the colors of the abdomens. Complete preference for 2 mM sucrose or 2 mM sucrose and CaCl2 results in preference indexes (P.I.) of 1.0 and −1.0, respectively, whereas no taste bias results in a P.I. of 0. In the absence of CaCl2, the flies show no discrimination (Figure 1A), demonstrating that the red and blue dyes do not affect this feeding behavior. 0.1 mM CaCl2 had no impact on their taste preference, demonstrating that it was neither attractive nor aversive (Figure 1A). However, higher concentrations of Ca2+ mixed with sucrose (0.5—10 mM CaCl2) induced progressively stronger avoidance (Figure 1A). The Cl2 was not responsible for this repulsion since flies show no distaste for 10—100 mM MgCl2 (Figure S1A), while 1 mM and 10 mM levels of other Ca2+-containing compounds were also aversive (Figure S1B). Thus, flies have a sense of Ca2+ taste.
Figure 1. Flies avoid Ca2+ via ppk23 GRNs.
(A—D) Two-way choice taste assays. 50—70 flies/assay
(A) Preferences of control flies (w1118) to 2 mM sucrose alone versus 2mM sucrose and the indicated concentrations of CaCl2. n=4.
(B) Preferences of control flies to 1 mM sucrose alone versus 5 mM sucrose and the indicated concentrations of CaCl2. n=4.
(C) Two-way choice assays after ablating different GRNs by UAS-hid under control of the indicated GAL4s. n=4.
(D) Effects of ablation of ppk23 GRNs (UAS-hid/ppk23-GAL4) on Ca2+avoidance. n=4.
(E—G) Assaying Ca2+-induced action potentials using tip recordings.
(E) CaCl2 (50 mM)-induced action potential frequencies in control and UAS-hid/ppk23- GAL4 sensilla. n=10–24.
(F) Responses of L4 and S5 sensilla to different CaCl2 concentrations . n=17–22.
(G) Tip recordings from S5 and L4 sensilla using 0—50 mM CaCl2.
(H—J) Effects of different salts on sucrose-induced action potentials in L4 and L6 sensilla. The pipets contained 50 mM sucrose and 0—50 mM salts: (H) CaCl2, (I) MgCl2, (J) NaCl. n≥10. Error bars indicate SEMs. ANOVA tests with Scheffe’s post hoc analyses between control and ppk23-GRNs ablated sensilla. *P < 0.05, **P < 0.01.
We tested a range of CaCl2 levels to determine the concentration necessary to eliminate the strong preference for 5 mM sucrose over 1 mM sucrose (Figure 1B). Addition of 10 mM CaCl2 neutralized this bias, while inclusion of ≥15 mM CaCl2 to the 5 mM sucrose caused the animals to prefer the lower concentration of sugar (Figure 1B). We also applied different concentration of CaCl2 mixed with sucrose directly to the labellum, and performed the proboscis extension response (PER). 2% sucrose alone stimulated a PER in all flies, while addition of CaCl2 decreased this response in a concentration-dependent manner (Figure S1C). These data indicate that Ca2+ that is sensed by the labellum causes repulsion.
ppk23 positive GRNs are essential to avoid Ca2+
To identify the GRNs required for Ca2+ avoidance, we used the GAL4/UAS system to selectively express a pro-cell death gene (UAS-hid) under the control of GAL4s that mark different classes of GRNs. These include the Gr33a-GAL4 and the Gr5a-GAL4, which are expressed in all bitter- and sweet-sensing GRNs, respectively (Moon et al., 2009; Thorne et al., 2004). We also used the ppk28-GAL4 which is expressed in GRNs that elicit attractive responses to water (Cameron et al., 2010) and the ppk23-GAL4, which is reported to function in courtship but not feeding behavior (Lu et al., 2012; Thistle et al., 2012; Toda et al., 2012). Surprisingly, expression of UAS-hid under control of the ppk23-GAL4/+ background greatly reduced the gustatory aversion to CaCl2 in a dose dependent manner (Figures 1C, 1D and S1D). In contrast, there were no significant effects from combining UAS-hid with the other GAL4s (Figures 1C and S1D). Thus, ppk23-positive GRNs are essential for tasting Ca2+.
To test whether activation of ppk23 neurons causes aversive behavior, we activated these neurons by expressing the capsaicin receptor, TRPV1. We allowed the flies to choose between 2 mM sucrose versus 2 mM sucrose plus 100 μM capsaicin. Capsaicin alone does not cause repulsion in control flies, or in animals expressing just the ppk23-GAL4 or UAS-trpV1 (Figure S1E) (Marella et al., 2006). However, flies expressing UAS-trpV1 under the control of the ppk23-GAL4 avoided capsaicin (Figure S1E), indicating that activation of ppk23 GRNs causes gustatory avoidance.
To address whether Ca2+ induces action potentials in GRNs, we performed tip recordings. There are three size classes of taste sensilla. S- and I-type sensilla house both attractive and aversive GRNs, whereas L-type sensilla contain GRNs that respond to attractive but not aversive tastants (Freeman and Dahanukar, 2015; Weiss et al., 2011). We surveyed the taste sensilla using 50 mM CaCl2 and found that six S-type, but no I- or L-type produced robust spikes (Figure 1E). S5 bristles responded with the highest action potential frequency. Therefore, we determined the dose-dependent responses of S5, and found that the action potential frequencies saturated at 50 mM CaCl2 (Figures 1F and 1G). The frequencies of Ca2+-induced action potentials were dramatically reduced in ppk23-GAL4/UAS-hid flies (Figure 1E), demonstrating that ppk23-positive GRNs mediate Ca2+-induced action potentials.
Ca2+ suppresses sugar-induced action potentials
Bitter organic tastants such as caffeine and DEET suppress feeding through two mechanisms. They activate bitter-responsive GRNs, and they suppress sugar-activated GRNs (Jeong et al., 2013; Lee et al., 2010; Meunier et al., 2003; Moon et al., 2006; Weiss et al., 2011). To investigate whether Ca2+ inhibits sugar-responsive GRNs, we tested 10 mM and 50 mM CaCl2, and recorded from L4 and L6 sensilla. We found that CaCl2 reduced the neuronal firing activated by 50 mM sucrose (Figure 1H). In contrast, neither MgCl2 nor NaCl diminished sugar-induced action potentials (Figures 1I and 1J). Thus, high levels of Ca2+ suppress feeding through dual mechanisms: activation of ppk23-positive GRNs and inhibition of sugar-activated GRNs.
Ca2+ taste requires Ionotropic Receptors
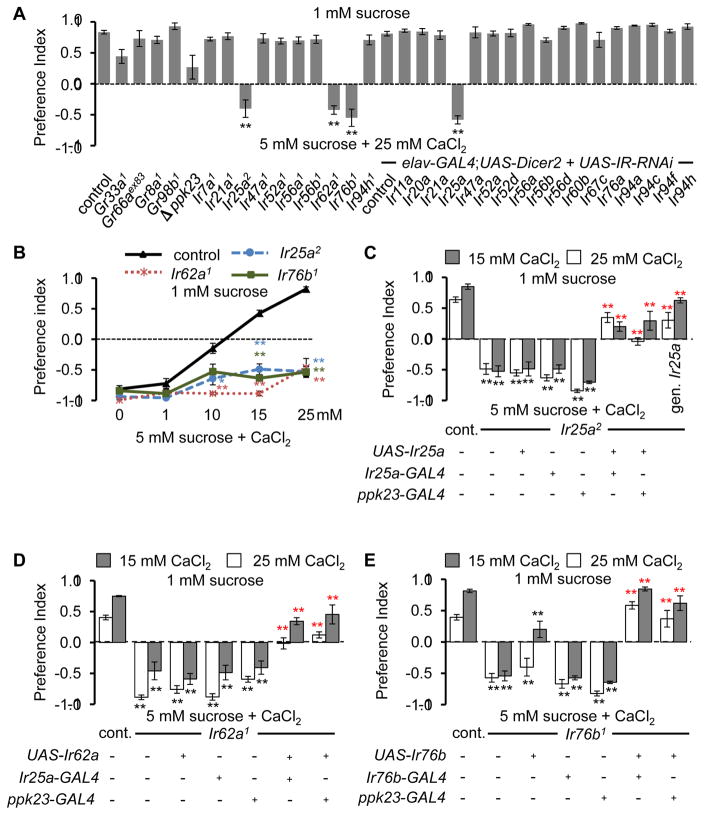
To identify candidate receptors/cation channels that are required for Ca2+ taste, we performed two-way choice assays using 1 mM sucrose alone versus 5 mM sucrose plus 25 mM CaCl2. We screened mutations disrupting two GRs (GR33a and GR66a), which are broadly required for the aversive responses to most bitter compounds and two other GRs (GR8a and GR98b), which are required for sensing the toxic amino acid, L-canavanine (Lee et al., 2012; Lee et al., 2010; Moon et al., 2009; Shim et al., 2015). None of these Gr mutations significantly impaired Ca2+ avoidance (Figure 2A), consistent with the absence of a significant impairment of Ca2+ repulsion after killing Gr33a GRNs with UAS-hid (Figure 1C). We also considered PPK23 because it is a member of the family of Degenerin/Epithelial Na+ channels, and ablation of ppk23-positive GRNs reduces Ca2+-avoidance. Although the ppk23 null mutant showed a reduced P.I., the difference from the control was not statistically significant (Figure 2A p=0.45).
Figure 2. Requirements for three Irs for rejecting Ca2+-containing food.
(A) Screening candidate chemoreceptors for defects in Ca2+ aversion. n=4—12.
(B) Dose-dependent avoidance of Ir mutants to Ca2+. n=4.
(C) Rescue of Ca2+ avoidance defects in Ir25a2 by expressing UAS-Ir25a using the indicated GAL4, or the Ir25a+ genomic transgene (gen. Ir25a). n=5—12.
(D) Rescue of Ca2+ avoidance deficits in Ir62a1 by expressing UAS-Ir62a using the indicated GAL4. n=4.
(E) Rescue of Ca2+ avoidance impairments in Ir76b1 by expressing UAS-Ir76b using the indicated GAL4. n=5—13. Error bars represent SEMs. Asterisks in A and B indicate significant differences from controls (*P < 0.05, **P<0.01) using ANOVA with Scheffe’s post hoc test. The black and red asterisks in C—E indicate significant differences from the controls and mutants, respectively (*P < 0.05, **P<0.01), using ANOVA with Scheffe’s post hoc test.
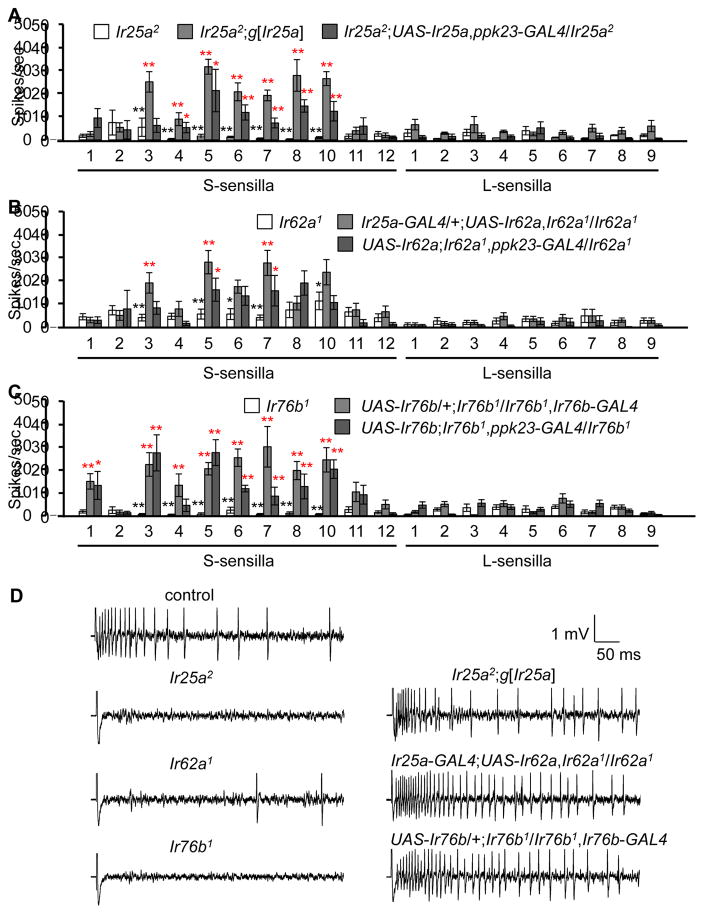
Ionotropic receptors (IRs) (Benton et al., 2009) are candidates for functioning in Ca2+ taste since multiple IRs are expressed in gustatory organs (Croset et al., 2010; Hussain et al., 2016; Koh et al., 2014; Zhang et al., 2013), and one member of this family of cation channels (IR76b) is required for tasting low levels of another mineral—Na+ (Zhang et al., 2013). To interrogate potential roles for IRs, we tested requirements for family members that are expressed in the labellum, as well as in GRNs in taste sensilla decorating the legs and wing margins. We examined the effects of mutations and RNAi lines affecting 20 Ir genes and found that the Ir25a2, Ir62a1 and Ir76b1 mutations strongly impaired Ca2+ avoidance (Figure 2A), and did so over the range of CaCl2 concentrations tested (10—25 mM; Figure 2B). In contrast, we observed normal Ca2+ responses elicited by other existing Ir mutations (Ir21a1 and Ir56b1), by flies with mutations in Ir7a, Ir47a, Ir52a, Ir56a, and Ir94h, which we generated using homologous recombination as part of a separate study, and by the RNAi lines tested (Figure 2A). The lack of phenotype using RNAi was not due to ineffectiveness of elav-GAL4;UAS-Dicer2, since we replicated the Ir25a2 phenotype by knocking down Ir25a (Figure 2A). To provide additional evidence that the deficits in Ca2+ avoidance were due to losses of Ir25a, Ir62a, or Ir76b, we conducted genetic rescue experiments. We found that the behavioral impairments exhibited by Ir25a2, Ir62a1 and Ir76b1 flies were suppressed by expression of the corresponding wild-type cDNAs using the Ir25a-GAL4, Ir76b-GAL4 or the ppk23-GAL4 (Figures 2C—2E). The deficit in Ir25a2 was also reversed using a genomic Ir25a transgene (Figure 2C). Furthermore, the profound deficits in Ca2+-activated action potentials in Ir25a2, Ir62a1, and Ir76b1 animals were significantly reversed by the wild-type transgenes (Figure 3). We conclude that these IRs are required for sensing Ca2+ in GRNs. However, they were not sufficient to confer Ca2+ sensitivity since misexpression of all three in sugar-responsive GRNs using the Gr5a-GAL4 did not confer attraction to Ca2+-containing food, or induce Ca2+-activated action potentials (Figure S2).
Figure 3. Dependence on Ir25a, Ir62a, and Ir76b for Ca2+-induced action potentials.
Tip recordings were performed in response to 50 mM CaCl2.
(A—C) Mean responses of sensilla from Ir25a2, Ir62a1 or Ir76b1, or the mutants expressing the genomic rescue transgene (g[Ir25a]) or a UAS-cDNA rescue transgene under control of the indicated GAL4s. n=10—26.
(D) Representative traces showing Ca2+-induced action potentials.
Error bars represent SEMs. The black and red asterisks indicate significant differences from the controls and the mutants, respectively (**P<0.01, *P<0.05), using single factor ANOVA with Scheffe’s post hoc test to compare two sets of data.
IR co-expression in the labellum
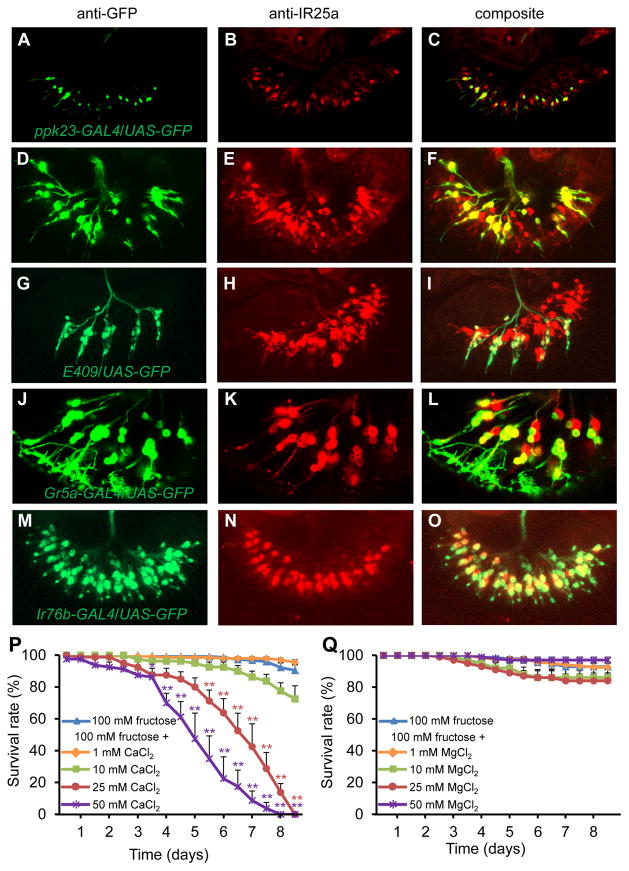
IR25a is expressed in the labellum (Croset et al., 2010) but the specific classes of GRNs expressing IR25a have not been analyzed. We first characterized the Ir25a-GAL4 reporter using UAS-mCD8::GFP to determine whether it reflected the same expression pattern as anti-IR25a. We found that anti-GFP and anti-IR25a staining overlapped extensively (Figure S3A—S3G). We introduced Ir25a-GAL4;UAS-mCD8::GFP into the Ir25a2 mutant background and found that the anti-GFP signals were indistinguishable between control and Ir25a2 flies (Figure S3H—S3J), indicating that the Ir25a2 mutation did not result in loss of IR25a-expressing GRNs.
We compared anti-IR25a staining with a variety of reporters and found that IR25a exhibited wide overlap with different classes of GRNs. Anti-IR25a staining overlapped extensively with the ppk23-GAL4 reporter (Figures 4A—4C, S3K—S3P), and anti-IR25a staining was undetectable in labella expressing UAS-hid under control of the ppk23-GAL4 (Figures S3Q and S3R). We also detected anti-IR25a staining in all bitter-sensing GRNs, which are labeled by the Gr66a reporter (Figures 4D—4F), all carbonation buffer-sensing GRNs, which are stained by the E409 GAL4 enhancer trap reporter (Figure 4G—4I), and a subset of sugar-sensing GRNs, which are marked by the Gr5a reporter (Figures 4J—4L). This indicates that IR25a is an exceptionally widely expressed chemoreceptor.
Figure 4. Labellar expression of IR25a, and toxicity of high Ca2+.
(A—O) Staining of labella from controls viewed by confocal microscopy. 3-D reconstructions generated by maximum transparency. Scale bars represent 25 μm.
(A, D, G, J, M) UAS-GFP expression driven by the indicated GAL4s. The signals were detected by anti-GFP staining (green).
(B, E, H, K, N) Anti-IR25a staining (red).
(C, F, I, L, O) Merged images of anti-GFP and anti-IR25 staining.
(P and Q) Survival of control flies fed 100 mM fructose or 100 mM fructose mixed with: (P) CaCl2, or (Q) MgCl2. n=4—7. Error bars represent SEMs. The asterisks indicate significant differences from the fructose only feeding (**P<0.01, *P<0.05.) using single factor ANOVA with Scheffe’s as a post hoc test to compare two sets of data.
To address whether the three IRs are co-expressed in the same GRNs, we performed double-labeling experiments. We found that IR76b was co-expressed extensively with IR25a (Figures 4M—O and Figures S4A—S4C). The Ir62a-GAL4 reporter is not detected in the labellum (Koh et al., 2014). However, it is expressed in the legs (Koh et al., 2014), as are the Ir25a-GAL4 and Ir76b-GAL4 reporters (Figures S4D—S4L). Therefore, to explore whether Ir62a RNA is expressed in the labellum, we performed RT-PCR using dissected labella, and found the predicted 1.8 kb band in wild-type labella (Figure S4M). However, we did not detect the band from labella from flies expressing UAS-hid under control of the Ir25a-GAL4 (Figure S4M). The combination of these data supports the proposal that Ir25a, Ir62a and Ir76b expression overlap extensively in the labellum.
Toxic effect of Ca2+ in food
Because flies avoid high levels of Ca2+, we tested whether high Ca2+ is toxic to the flies. We compared the survival of flies maintained on 100 mM fructose, or on fructose mixed with different concentrations of CaCl2 (Figure 4P). There was virtually no lethality after 8.5 days in the absence of Ca2+. We found that Ca2+ decreased viability in a concentration-dependent manner. There was only slight toxicity due to 1 or 10 mM Ca2+ (Figure 4P). However, if the fructose was laced with 25 or 50 mM CaCl2, none of the flies survived after 8.5 and 8 days, respectively (Figure 4P). The times in which 50% died (LT50) were 163.5 ±9.3 hrs and 121.9 ±10.5 hrs for flies fed 25 and 50 mM Ca2+, respectively. 100 mM CaCl2 feeding affected survival rate more significantly (64.1 ±8.5 hrs). If the flies were fed MgCl2 plus fructose, there was minimal lethality even after 204 hours (Figure 4Q).
To clarify if the death is due to Ca2+ toxicity or to the detection of an aversive compound which induces stress, we tested Ca2+-insensitive mutants using a binary food-choice survival assay (Figures S4N—S4O). The flies were allowed to choose between 100 mM fructose alone, and 200 mM fructose mixed with either 50 mM Ca2+ (Figure S4N) or 100 mM Ca2+ (Figure S4O). Nearly all of the control flies survived for 10 days. However, Ir25a2, Ir62a1 and Ir76b1 flies as well as ppk23-GAL4/UAS-hid animals showed high levels of concentration-dependent lethality (Figures S4N—S4O). After 10 days on the fructose versus fructose plus 100mM Ca2+, <26.5% of the Ir25a2 or Ir62a1 flies were alive, while none of the Ir76b1 flies survived (Figure S4O). These findings support the conclusion that feeding on high Ca2+ induces lethality due to Ca2+ toxicity.
DISCUSSION
Avoiding dangerous environmental Ca2+ through dual mechanisms
Ca2+ is an essential mineral. However, at high levels it causes toxicity (Žofková, 2016). Thus, consuming the ideal Ca2+concentration is critical. Despite the fundamental importance of Ca2+ for health, behavioral studies focusing on Ca2+ attraction or avoidance have been limited, and are unexplored in Drosophila. We found that flies are endowed with the ability to taste Ca2+. Surprisingly, rather than displaying attraction and repulsion to low and high concentrations of Ca2+, respectively, which would be reminiscent of the Na+ response, there is no attraction to low Ca2+. The only reaction to Ca2+ is repulsion to high levels. The lack of Ca2+ attraction suggests that the normal environmental sources of nutrition for vinegar flies have ample Ca2+, and there is no need for selecting a taste modality for Ca2+ attraction. The avoidance behavior is due to Ca2+ rather than Cl−, because there is no avoidance to MgCl2, while other Ca2+-salts are aversive.
The question arises as to why vinegar flies have the capacity to avoid very high levels of Ca2+. Indeed, the Ca2+ concentration in the leaves of plants, such as tomatoes, eggplants and others, reaches 100 mM (Watanabe et al., 2016). Therefore, the capacity of flies to sense and avoid Ca2+ levels at least as high as 100 mM deters them from consuming high Ca2+ in their environment.
Unexpectedly, in addition to activating GRNs in S-type sensilla, Ca2+ also suppresses sugar-activated GRNs in L-type sensilla. The influence of Ca2+ on two types of taste receptor cells is reminiscent of the simultaneous effects of bitter compounds on activation of avoidance GRNs, and inhibition of sugar-activated GRNs (Jeong et al., 2013; Meunier et al., 2003). We suggest that employment of two strategies to detect high Ca2+ levels provides a double safeguard to avoid consuming excessive Ca2+, which is deleterious. Although the mechanism through which Ca2+ suppresses the sugar response remains to be defined, it might involve an odorant binding protein (OBP), as bitter compounds suppress the sugar response by binding to an OBP, which in turn associates with and inhibits sugar receptors (Jeong et al., 2013).
Composition and potential mechanism of activation of Ca2+ sensor
The taste of Ca2+ depends on three members of the variant Ir family: Ir25a, Ir62a, and Ir76b. Two of these IRs (IR25a and IR76b) are required to detect multiple types of stimuli, and this diversity appears to be defined by unique combinations of IRs, or by the IRs acting as homomeric ionotropic receptors. For example, IR25a appears to be sufficient for sensing low-amplitude temperature cycles necessary for temperature synchronization of the circadian clock (Chen et al., 2015), but collaborates with IR93a and IR21a for cool sensation (Knecht et al., 2016; Ni et al., 2016). IR25a also acts as a humidity sensor along with IR93a and IR21a. Similarly, IR76b is sufficient for sensing low salt, and acts in concert with IR41a for olfactory attraction to polyamines (Hussain et al., 2016). However, the gustatory attraction to polyamines requires just IR76b (Hussain et al., 2016). IR76b also contributes to the taste of amino acids, and in the adult, this also depends on IR20a (Croset et al., 2016; Ganguly et al., 2017). Both IR76b and IR25a are also required in leg sensilla for sensing acids (Chen and Amrein, 2017).
There are at least two models to account for the requirements for the three IRs for Ca2+ sensation. According to one model, one or both of the broadly required IRs (IR25a and IR76b) act as co-receptors and contribute to dendritic localization, in a manner similar to ORCO, which serves as a co-receptor for Odorant Receptors (ORs) (Abuin et al., 2011; Larsson et al., 2004). In this model, IR25a and IR76b only contribute to trafficking. Because no other function has been ascribed to IR62a, the specificity for Ca2+ taste would be conferred exclusively by IR62a. A second model is that IR25a and IR76b, in addition to IR62a, contribute to the response to Ca2+ independent of any contribution of their roles in trafficking. Nevertheless, as in the first model, IR62a is essential for the specificity for the Ca2+ response. We favor the second model, as IR76b is cation channel (Zhang et al., 2013), and therefore does more than contribute to subunit trafficking. However, the repertoire of IRs required to sense Ca2+ appears to be greater than Ir25a, Ir62a, and Ir76b since ectopic co-expression of these IRs in sugar-sensing GRNs was insufficient to confer a Ca2+ response to these cells. Nevertheless, it is plausible that the IR complex might be directly activated by Ca2+. The external Ca2+ concentration in the endolymph of insect chemosensory neurons is estimated to be similar to the hemolymph (~1 mM) (Kaissling and Thorson, 1980). This ionic concentration may account for the observation that GRNs are not very sensitive to Ca2+, and require mM concentrations of Ca2+ to generate action potentials.
Ca2+ sensing through ppk23 GRNs and a “mineral GRN”
The ppk23 GRNs in forelegs contribute to sensing pheromones and in courtship behavior. However, the roles of ppk23 GRNs in the labellum were unknown, but proposed to “detect a novel taste modality” (Toda et al., 2012). We conclude that ppk23 GRNs in the labellum function in detecting aversive levels of Ca2+, since killing these neurons virtually eliminates Ca2+ sensitivity. Moreover, their roles in gustatory aversion is supported by our observation that artificial gustatory stimulation of these neurons causes avoidance to a chemical (capsaicin), to which flies are normally indifferent (Marella et al., 2006). However, it seems highly unlikely that these ppk23 GRNs are tuned specifically to Ca2+.
Only six sensilla were robustly activated by high levels of Ca2+, and all were S-type. Among these sensilla is S8, which is unresponsive to bitter organic compounds (Weiss et al., 2011), but is also activated by high levels of Na+ that cause repulsion (Zhang et al., 2013). This indicates that S8 is tuned to aversive levels of multiple minerals, rather than to bitter-tasting organic chemicals. We suggest that this ppk23 GRN in S8 is a broadly tuned “mineral GRN” that senses aversive levels of these elements.
A question arises as why only six sensilla elicit robust responses to Ca2+, since Ir25a and Ir76b are expressed in GRNs in nearly all sensilla in the labellum. The Ir62a reporter is not detected in the labellum. Nevertheless, our RT-PCR results demonstrate that Ir62a RNA is expressed in the labellum, and specifically in Ir25a GRNs. Thus, Ir62a expression might be limited to the six S-type sensilla, and define the specificity. However, we suggest that this is not the explanation. Ir25a and Ir76b are widely expressed in many more than six sensilla, and expression of Ir62a in Ir25a GRNs, does not greatly extend Ca2+ responses to other sensilla, with the exception of endowing modest response to a couple of I-type sensilla. However, there were some minor differences in Ca2+-induced action potential between control flies, and mutant animals expressing wild-type transgenes. For example, while S1 sensilla are normally unresponsive to Ca2+, some Ca2+ response is induced upon expression of a Ir76b+ rescue transgene using the GAL4/UAS system, possible due to higher expression of Ir76b than in control flies. Nevertheless, our misexpression experiments with Ir62a further support the model that the IR heteromultimer required for the Ca2+ response is more complex than three subunits. Whether this involves additional IRs, or a β-subunit, remains to be determined.
Concluding remarks
Our results demonstrate that Ca2+ represents a previously unappreciated taste modality in flies, and is mediated through ppk23 GRNs in the labellum—a previously enigmatic class of GRNs. In contrast to Na+, which elicits robust attraction and avoidance at low and high concentrations respectively (Liu et al., 2003; Nakamura et al., 2002; Zhang et al., 2013), flies show Ca2+ avoidance only, and exclusively to high concentrations. Moreover, the Ca2+ GRNs are distinct from bitter GRNs, and at least one appears to be dedicated to sensing aversive levels of at least two minerals: Ca2+ and Na+. Repulsion to Ca2+ is mediated through the dual activation of avoidance GRNs and suppression of sugar-activated GRNs. The findings from this study raise the possibility that the taste of Ca2+ might be sensed exclusively as a deterrent in a host of other animals.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
The fly stocks generated in this study will be deposited with the Bloomington Stock Center for public distribution (http://flystocks.bio.indiana.edu/). Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, C.M. (craig.montell@lifesci.ucsb.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
All experiments were performed with the indicated strains of adult male and female Drosophila melanogaster. The flies were 3—6 days-old for the two-way choice assays, 3—7 days-old for the tip recordings, and 3—4 days old for the survival assays. We used w1118 as the “wild-type” control. Ir21a1, Ir56b1, Ir62a1, UAS-hid, UAS-DsRed, UAS-GFP and UAS-mCD8::GFP were from the Bloomington Stock Center. We previously described Ir76b1, Ir76b-GAL4, UAS- Ir76b (Zhang et al., 2013), Gr66aex83 (Moon et al., 2006), Gr33a1, Gr33aGAL4 (Moon et al., 2009), Gr8a1 (Lee et al., 2012), and Gr98b1 (Shim et al., 2015). Ir7a1, Ir47a1, Ir52a1, Ir56a1, and Ir94h1 mutants were generated by ends-out homologous recombination for other studies, and will be described elsewhere. The Ir RNAi lines were: Ir11a (v100422), Ir20a (v109324), Ir21a (v2471), Ir47a (v11812), Ir52a (v37173), Ir52d (v8963), Ir56a (v109691), Ir56b (v105928), Ir56d (v6112), Ir60b (v106225), Ir67c (v107921), Ir76a (v101590), Ir94a (v107734), Ir94c (v100967), Ir94f (v109702), and Ir94h (v100407) were tested after crossing elav-GAL4;UAS-Dicer2 (Bloomington Stock Center). K. Scott provided the ppk23, ppk23-GAL4 (Thistle et al., 2012), the E409-GAL4 (Hussain et al., 2016), and the ppk28-GAL4 (Cameron et al., 2010). H. Amrein provided the Gr66a-GAL4 and the Gr5a-GAL4 (Dunipace et al., 2001; Thorne et al., 2004). R. Benton provided Ir25a2 and the Ir25a-GAL4 (Abuin et al., 2011).
METHOD DETAILS
Generation of Transgenic Flies
To generate UAS-Ir25a animals, we first subcloned the full-length EST clone (IP13516) between the EcoRI/XhoI sites of the pUAST vector. The transformation vector was injected into w1118 embryos (BestGene Inc.). To obtain the Ir25a genomic transgene, P[gIr25a], we subcloned the genomic region from P[acman] CH321-90F22 (www.pacmanfly.org) into the attP154 insertion site on the 3rd chromosome (BestGene Inc.).
To generate the UAS-Ir62a transgene, we prepared RNA from labella, and amplified the full-length Ir62a cDNA by RT-PCR using the following primer pair: 5’-CAGAATTCACGAGCGAAAATGT-3’ and 5’-TACCTCGAGTGCATTAATCC-3’. We subcloned the cDNA between the EcoRI/XhoI sites of the pUAST vector, verified the cDNA by DNA sequencing, and injected the plasmid into w1118 embryos (KAIST Drosophila Research Center).
Chemicals
Sucrose and sulforhodamine B, MgCl2, KCl, CaCl2 and tricholine citrate were purchased from Sigma-Aldrich Co. Brilliant blue FCF was obtained from Wako Pure Chemical Industry Ltd.
Behavioral Assays
We performed two-way choice assays using 72-well plates as previously described (Meunier et al., 2003; Moon et al., 2006). We employed two paradigms. First, we used 2 mM sucrose versus 2 mM sucrose and different concentration of CaCl2, which we added to alternating wells. Second, we used 1 mM sucrose versus 5 mM sucrose mixed with different concentration of CaCl2. The two food alternatives used in each assay were added to 1% agarose, mixed with either blue (brilliant blue FCF, 0.125 mg/ml) or red food coloring (sulforhodamine B, 0.1 mg/ml).
To conduct each assay, we starved 50–70 flies (3–6 days old) for 18 hr in a humidified chamber, and introduced the animals into a dish. The dishes were kept in a dark humidified chamber, and the flies were allowed to feed for 90 min. To determine their food preferences, the flies were frozen at -20°C, the color of their abdomens were analyzed using a stereomicroscope, and the number of flies that were that were blue (NB), red (NR), or purple (NP) were counted. In cases in which there was a mixture of red and blue dyes in the abdomens, we assigned the animals as red and blue if the percentage of the blue and red colors in the abdomens, respectively, was <5%. If the other color crossed the 5% threshold, we designated the flies as purple. The preference indexes (P.I.) were calculated according to the following equation: PI = (Nred + 0.5Npurple) − (Nblue + 0.5Npurple)/(Nred + Nblue + Npurple), or PI = (Nblue + 0.5Npurple) − (Nred + 0.5Npurple)/(Nred + Nblue + Npurple), depending on the dye/tastant combinations. P.I.s of 1.0 and -1.0 indicate complete preferences for one food option or the other. A P.I. of 0 indicates no bias between the two food alternatives.
Tip Recordings
We performed tip recordings as previously described (Moon et al., 2006). Briefly, we first immobilized 3—7 days old male or female flies on ice. We then inserted a reference glass electrode filled with Ringer’s solution into the thorax of the fly, and extended the electrode toward the proboscis. We stimulated the sensilla with tastants dissolved in the buffer solution in the recording pipette (10–20 μm tip diameter). 1 mM KCl was used as the electrolyte for recording from S-type, I-type sensilla and 30 mM tricholine citrate was used for L-type sensilla. The recording electrode was connected to a preamplifier (TastePROBE, Syntech, Germany), and we collected and amplified the signals 10x using a signal connection interface box (Syntech) in conjunction with a 100–3000 Hz band-pass filter. Recordings of action potentials were made using a 12-kHz sampling rate and analyzed using Autospike 3.1 software (Syntech). Spike sorting was used as an indicator of the spike amplitudes that correspond to the action potentials of CaCl2 sensitive neurons, rather than the relatively small amplitudes of water spikes. The number of action potentials was counted from 50—550 msec after application of the CaCl2. The numbering nomenclature for the sensilla was as described (Hiroi et al., 2002).
Survival Assays
We performed survival assays using male and female control flies as previously described (Lee et al., 2010). The food consisted of 1% agarose and either 100 mM fructose alone, or 100 mM fructose plus different concentrations of CaCl2 or MgCl2 as indicated in Figures 4P, 4Q, S4N and S4O. To perform the assays, we exposed 10 male and 10 female flies (3—4 days old) to the food source at 25°. The number of viable flies were tabulated every 12 hr, and then transferred to new vials containing the same food source. The assays were terminated after 204 hrs. Each condition was tested 4—7 times.
Immunohistochemistry
The labella of the indicated flies were dissected and fixed using 4% paraformaldehyde (Electron Microscopy Sciences, Cat No 15710) with 0.2% Triton X-100 for 15 min at room temperature (Lee et al., 2012). The labella were washed ≥3 times in PBST (1x PBS and 0.2% Triton X-100), placed in blocking buffer (0.5% goat serum in PBST), cut in half with a razor blade, and incubated with blocking buffer for 30 min at room temperature. The labella were transferred to new blocking buffer containing the primary antibodies (mouse anti-GFP, Molecular Probe, 1:1000; rabbit anti-DsRed, Clontech, 1:1000; rabbit anti-IR25a, L. Vosshall, 1:1000) and incubated overnight at 4°C. The labella were washed three times with PBST and incubated with secondary antibodies (goat anti-mouse Alexa Fluor 488 or goat anti-rabbit Alexa Fluor 568) for 4 hr at 4°C. The labella were washed three times with PB ST and mounted in 1.25x PDA solution (37.5% glycerol, 187.5 mM NaCl, 62.5 mM Tris pH 8.8), and viewed by confocal microscopy (Carl Zeiss LSM510).
RT-PCR Analyses of Ir62a
20–25 proboscises from control and Ir25a-Gal4/UAS-hid flies were dissected, and RNA was extracted using TRIZOL (Invitrogen). cDNA was synthesized from the extracted RNA using AMP transcriptase (Promega). The Ir62a cDNA was amplified using the Ir62a specific primer used to generate UAS-Ir62a as described above (5’-CAGAATTCACGAGCGAAAATGT-3’ and 5’-TACCTCGAGTGCATTAATCC-3’), and the primer pair used to amplify the tubulin cDNA was: 5’-TCCTTCTCGCGTGTGAAACA- 3’ and 5’-CCGAACGAGTGGAAGATGAG-3’.
QUANTIFICATION AND STATISTICAL ANALYSIS
All error bars represent standard error of the means (SEMs). The number of times each experiment was repeated (n) is indicated in the figure legends. For the two-way choice assays, each “n” represents a single test performed with 50—70 animals. For the survival assays, each “n” includes 20 flies (10 male and 10 female flies). Each “n” for the tip recordings represents an analysis of a single, independent fly. Single factor analysis of variance (ANOVA) with Scheffe’s analysis as a post hoc test was used to compare multiple sets of data. Asterisks indicate statistical significance compared with the control (*P < 0.05, **P < 0.01).
Supplementary Material
Acknowledgments
We thank R. Benton, K. Scott and the Bloomington Stock Center for fly stocks and L. Vosshall for anti-IR25a. S.P. was supported by the Global Scholarship Program for Foreign Graduate Students at Kookmin University in Korea. This work is supported by grants to Y.L. from the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2012R1A1A2003727 and 2014R1A1A2058094), and to C.M. from the National Institute on Deafness and other Communication Disorders (DC007864).
Footnotes
CONTRIBUTIONS
Y.L. S.P., Y.K., and D.T. conducted the experiments, Y.L. and C.M. designed the experiments and wrote the paper.
The supplementary Information includes four figures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abuin L, Bargeton B, Ulbrich MH, Isacoff EY, Kellenberger S, Benton R. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 2011;69:44–60. doi: 10.1016/j.neuron.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P, Hiroi M, Ngai J, Scott K. The molecular basis for water taste in Drosophila. Nature. 2010;465:91–95. doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Buhl E, Xu M, Croset V, Rees JS, Lilley KS, Benton R, Hodge JJ, Stanewsky R. Drosophila Ionotropic Receptor 25a mediates circadian clock resetting by temperature. Nature. 2015;527:516–520. doi: 10.1038/nature16148. [DOI] [PubMed] [Google Scholar]
- Chen Y, Amrein H. Ionotropic receptors mediate Drosophila oviposition preference through sour gustatory receptor neurons. Curr Biol. 2017;27:2741–2750. e744. doi: 10.1016/j.cub.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croset V, Rytz R, Cummins SF, Budd A, Brawand D, Kaessmann H, Gibson TJ, Benton R. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 2010;6:e1001064. doi: 10.1371/journal.pgen.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croset V, Schleyer M, Arguello JR, Gerber B, Benton R. A molecular and neuronal basis for amino acid sensing in the Drosophila larva. Sci Rep. 2016;6:34871. doi: 10.1038/srep34871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunipace L, Meister S, McNealy C, Amrein H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr Biol. 2001;11:822–835. doi: 10.1016/s0960-9822(01)00258-5. [DOI] [PubMed] [Google Scholar]
- Freeman EG, Dahanukar A. Molecular neurobiology of Drosophila taste. Curr Opin Neurobiol. 2015;34:140–148. doi: 10.1016/j.conb.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A, Pang L, Duong VK, Lee A, Schoniger H, Varady E, Dahanukar A. A molecular and cellular context-dependent role for Ir76b in detection of amino acid taste. Cell Rep. 2017;18:737–750. doi: 10.1016/j.celrep.2016.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi M, Marion-Poll F, Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoolog Sci. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- Hussain A, Zhang M, Ucpunar HK, Svensson T, Quillery E, Gompel N, Ignell R, Grunwald Kadow IC. Ionotropic chemosensory receptors mediate the taste and smell of polyamines. PLoS Biol. 2016;14:e1002454. doi: 10.1371/journal.pbio.1002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong YT, Shim J, Oh SR, Yoon HI, Kim CH, Moon SJ, Montell C. An Odorant-Binding Protein required for suppression of sweet taste by bitter chemicals. Neuron. 2013;79:725–737. doi: 10.1016/j.neuron.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaissling K-E, Thorson J. Insect olfactory sensilla: structural, chemical and electrical aspects of the functional organization. In: Sattelle DB, Hall LM, Hildebrand JG, editors. Receptors for Neurotransmitters, Hormones, and Pheromones in Insects. Amsterdam: Elsevier; 1980. pp. 261–282. [Google Scholar]
- Knecht ZA, Silbering AF, Ni L, Klein M, Budelli G, Bell R, Abuin L, Ferrer AJ, Samuel AD, Benton R, et al. Distinct combinations of variant ionotropic glutamate receptors mediate thermosensation and hygrosensation in Drosophila. Elife. 2016:5. doi: 10.7554/eLife.17879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TW, He Z, Gorur-Shandilya S, Menuz K, Larter NK, Stewart S, Carlson JR. The Drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron. 2014;83:850–865. doi: 10.1016/j.neuron.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kang MJ, Shim J, Cheong CU, Moon SJ, Montell C. Gustatory receptors required for avoiding the insecticide L-canavanine. J Neurosci. 2012;32:1429–1435. doi: 10.1523/JNEUROSCI.4630-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim SH, Montell C. Avoiding DEET through insect gustatory receptors. Neuron. 2010;67:555–561. doi: 10.1016/j.neuron.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Zhang YV, Montell C. Peripheral coding of taste. Neuron. 2014;81:984–1000. doi: 10.1016/j.neuron.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Leonard AS, Motto DG, Feller MA, Price MP, Johnson WA, Welsh MJ. Contribution of Drosophila DEG/ENaC genes to salt taste. Neuron. 2003;39:133–146. doi: 10.1016/s0896-6273(03)00394-5. [DOI] [PubMed] [Google Scholar]
- Lu B, LaMora A, Sun Y, Welsh MJ, Ben-Shahar Y. ppk23-Dependent chemosensory functions contribute to courtship behavior in Drosophila melanogaster. PLoS Genet. 2012;8:e1002587. doi: 10.1371/journal.pgen.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- Meunier N, Marion-Poll F, Rospars JP, Tanimura T. Peripheral coding of bitter taste in Drosophila. J Neurobiol. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
- Moon SJ, Köttgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr Biol. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Baldwin D, Hannaford S, Palka J, Montell C. Defective proboscis extension response (DPR), a member of the Ig superfamily required for the gustatory response to salt. J Neurosci. 2002;22:3463–3472. doi: 10.1523/JNEUROSCI.22-09-03463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Ni L, Klein M, Svec KV, Budelli G, Chang EC, Ferrer AJ, Benton R, Samuel AD, Garrity PA. The Ionotropic Receptors IR21a and IR25a mediate cool sensing in Drosophila. Elife. 2016;5:e13254. doi: 10.7554/eLife.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Lee Y, Jeong YT, Kim Y, Gee MG, Montell C, Moon SJ. The full repertoire of Drosophila gustatory receptors for detecting an aversive compound. Nat Commun. 2015;6:8867. doi: 10.1038/ncomms9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell. 2012;149:1140–1151. doi: 10.1016/j.cell.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Toda H, Zhao X, Dickson BJ. The Drosophila female aphrodisiac pheromone activates ppk23+ sensory neurons to elicit male courtship behavior. Cell Rep. 2012;1:599–607. doi: 10.1016/j.celrep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Tordoff MG. Calcium: taste, intake, and appetite. Physiol Rev. 2001;81:1567–1597. doi: 10.1152/physrev.2001.81.4.1567. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Alarcon LK, Valmeki S, Jiang P. T1R3: a human calcium taste receptor. Sci Rep. 2012;2:496. doi: 10.1038/srep00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Maejima E, Yoshimura T, Urayama M, Yamauchi A, Owadano M, Okada R, Osaki M, Kanayama Y, Shinano T. The ionomic study of vegetable crops. PLoS One. 2016;11:e0160273. doi: 10.1371/journal.pone.0160273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Ni J, Montell C. The molecular basis for attractive salt-taste coding in Drosophila. Science. 2013;340:1334–1338. doi: 10.1126/science.1234133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žofková I. Hypercalcemia. Pathophysiological aspects. Physiol Res. 2016;65:1–10. doi: 10.33549/physiolres.933059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.