1. Introduction
The passenger-carrying paddle steamer Princess Alice was sunk in a collision on the River Thames in 1878 with the loss of over 640 lives and, curiously, may represent one of the largest mass poisoning episodes in history [1, 2]. Raw discharge from the London sewers had been released into the Thames (standard practice at the time) and some survivors reported the unusually foul nature of the water. The extraordinarily high death toll of the Princess Alice accident (> 80%) is in stark contrast to the similarly violent sinking of the pleasure craft Marchioness on the Thames a century later in 1989, where only 51 of 130 people on board were lost (< 40% fatalities) [3, 4]. The Board of Trade inquiry and the Coroner’s inquest at the time of the Princess Alice disaster were primarily concerned with establishing blame for the collision and determining if there were any criminal charges to be filed. That there may have been significant deaths caused by hydrogen sulfide (H2S) inhalation, possibly accelerated by the victims thrashing at the surface, was not given much serious consideration [1, 2]. Nevertheless, it was only nine years after the Princess Alice disaster occurred that the necessary investment was made to treat and separate the sewage before releasing it into the Thames [5]. It has since become entrenched in sanitary engineering lore that many of the Princess Alice deaths were due to poisoning, probably by H2S [6].
While it is now widely accepted that H2S acts as a signaling molecule and exhibits some potentially beneficial therapeutic effects [7–15] at elevated levels H2S is highly toxic. Gaseous H2S continues to be one of the most common hazardous substances attributed to acute poisoning deaths in occupational settings [16]. However, there are still facets of H2S toxicity that remain elusive – including, but not necessarily limited to, a comprehensive estimate of the prevalence of this poisonous gas emitted into the environment and any effects associated with such exposures, the mechanism(s) of its cellular toxicity, and lack of effective antidotes. Herein, we consider some of the knowledge gaps associated with H2S in the environment and its toxic effects on the human body when inhaled.
2. Some Physical Properties of Hydrogen Sulfide
Hydrogen sulfide, H2S (also known as hydrosulfuric acid, hydrogen sulfide, sewer gas, and stink damp, dihydrogen monosulfide, dihydrogen sulfide, sulfane, sulfurated hydrogen, and sulfur hydride) possessing the characteristic smell of rotten eggs. The gas is slightly heavier than air with a specific gravity of 1.19 at 15° C (the mean environmental temperature) and a molar mass of 34.08 g/mol. Among its hazardous traits, H2S is corrosive, explosive (at 4.3–45% by volume in air) and flammable (260° C ignition temperature).
Although not as polar as water, the structure of H2S, is similar to that of water, and it is also moderately soluble in water [17]. The pKa for the reaction H2S ⇆ H+ + HS− is 7.04, with the second pKa being unaccessible in water [18, 19]. Except where otherwise stated, data are provided for H2S in the standard state (25°C, 101 kPa). Consequently, in aqueous media at pH 7.4 (prior to any biochemical modification and irrespective of the exposure route in vivo) hydrogen sulfide is ~30% H2S and ~70% HS− (hydrosulfide). Where any greater precision is unnecessary and in keeping with common practice in the biochemical/toxicological literature, this mixture in aqueous media and the form bound to metal ions is referred to as “sulfide” throughout.
2.1 Brief Environmental Chemistry of Hydrogen Sulfide
If released as a gas, hydrogen sulfide remains in the atmosphere for approximately one day in the summer and 42 days in winter, becoming converted to sulfur dioxide and sulfuric acid [20] in a reaction catalyzed by hydroxyl radical:
Equations 1. Conversion of hydrogen sulfide to sulfur dioxide in air by hydroxyl radical
H2S + HO• ➔ HS• + H2O
HS• + O2 ➔ HO• + SO
SO + O2 ➔ SO2 + O
H2S can also be intentionally removed from the air by combustion, producing elemental sulfur and/or SO2 [21]:
Equations 2. Conversion of hydrogen sulfide to elemental sulfur and sulfur dioxide in air by combustion
2 H2S(g) + O2(g) ➔ 2 H2O(l) + 2 S(s)
2 H2S(g) + 3 O2(g) ➔ 2 H2O(l) + 2 SO2(g)
In the presence of metal ions, hydrogen sulfide tends to react and form metal sulfides, the salts of hydrogen sulfide[18, 19]. Such a reaction is the basis for the lead(II) acetate paper test used to detect H2S, with the moistened paper turning black due to formation of a PbS precipitate [21]:
Equation 3. Detection of hydrogen sulfide using lead acetate paper
Pb(CH3CO2)2(s) + H2S(g) ➔ PbS(s) + 2 CH3CO2H(g)
H2S partitions into water, forming solutions of hydrosulfuric acid (or sulfhydric acid). If sufficiently aerated, H2S can be oxidized, forming elemental sulfur and water. Additional biological methods of H2S removal have been explored to manage large-scale anthropogenic sources of H2S in liquid form [22]. H2S also enters soil when deposited from the air, due to surface spills, or other natural events [23]. Air, however, is the medium where most H2S is found, and where it poses the greatest risk to people. These interconnected chemical processes can be thought of as a simplified form (or subset) of the global sulfur cycle (Figure 1).
Figure 1.

Participation of hydrogen sulfide in the global sulfur cycle.
2.2 Detection and Quantitative Analysis
While the human nose remains a more sensitive detector than most other equipment (see section 6) it is not an adequate monitoring system, since it can become desensitized and is not useful for assessment of accumulated doses. Many devices suitable for personnel exposure monitoring have and continue to be described (much of this in trade publications rather than peer-reviewed literature) but a survey of these is outside the scope of this review. Such devices, either individually worn, or mounted in work areas, tend to be based on principles of optical spectrometry, electrochemistry, or conductivity changes in films/nanomaterials [24–27]. Multiple colorimetric tests for H2S have been known for some considerable time [25], but the lead acetate method (see section 2.1) with the reagent typically impregnated into tape within a cartridge, remains popular due to its sensitivity.
Methods suitable for analysis of environmentally-relevant samples began to be developed about half a century ago [28]. Nowadays, for many forensic purposes, straightforward gas chromatography-mass spectrometry (GC-MS) is quite adequate [29], but GC coupled to chemiluminescent detection of sulfur appears to offer some advantages for the analysis of biological materials [30]. There are many methods available for the quantitative determination of H2S in fuel gases, but fewer in the case of air samples. Essentially dedicated instruments based on pulsed fluorescence detection of SO2 generated from H2S are commercially available and have found application to quantitation of emissions from point sources [31, 32]. Gas chromatography with flame photometric detection (GC/FPD) is the U.S. Environmental Protection Agency (EPA) recommended test method for H2S in air [33]. Approaches to the analysis of water samples are more numerous and include chromatographic, iodometric, photometric and potentiometric procedures. In comparison, however, inductively coupled plasma-atomic emission spectrometry (ICP-AES) in conjunction with vapor generation techniques has been shown to be advantageous in terms of detection limit, throughput and lower reagent consumption/waste generation [34, 35].
All the above analytical methods are reliable, each with reasonably well-documented detection limit, linear range, reproducibility and known interferences. Where possible, they have been extensively checked against each other. As experimental sensitivity can always be increased by the collection and processing of larger sample volumes, selection of a particular method for any situation is largely a matter of cost, convenience, availability of equipment and practical experience of the personnel involved. The uncertainties evident in the analysis presented below (sections 3 and 4) are, in all probability, essentially independent of the analytical instrumentation, but instead reside in the sampling protocols and incomplete data sets. Accurate identification and documentation of all potential sources, plus determination of the invariably temporal nature of their emissions, remain challenging hurdles to entirely reliable estimations of net environmental H2S fluxes.
3. Emissions & Environmental Sources
As a key component of the sulfur cycle (Figure 1) H2S can be produced naturally in the environment through the anaerobic breakdown of sulfate by bacteria, anthropogenically by way of a variety of industrial practices (see below) and by degradation of sulfur-containing protein in mammals [23, 36, 37]. Although not the focus of this particular review, H2S is a member of the small group of currently recognized gasotransmitters (along with nitric oxide and carbon monoxide)[9, 11, 13, 23, 38].
Background H2S air concentrations typically range between 0.11 ppb and 0.33 ppb, although concentrations in urban areas can be as high as 1 ppb. As expected, the closer one lives to sources of H2S emissions, the higher the background levels tend to be (and can exceed 90 ppb) [23]. According to the literature reviewed by the World Health Organization several years ago [39], yearly H2S emissions from all sources on land are from 53 to 100 million metric tons of sulfur, while oceanic emissions are between 27 and 150 million metric tons. Here we have also summarized source-based emission estimates for hydrogen sulfide as reported in the literature over the 10-year period 2004–14 (Table 1).
Table 1.
Meta-analysis of hydrogen sulfide source categories with maximum measurements collected by studies conducted between 2004–14, shown in descending order by study incidence and broken down by maximum measurement types (concentration, flux, flux density).
| Source Categories (# of studies) | Maximum Measurements Reporteda | ||
|---|---|---|---|
| Concentration (mg/m3) |
Flux (mg/hour) |
Flux Density (mg/m2/hour)b |
|
| AFO (n=31) | 8.66E+03 | 6.30E+07 | 2.12E+04 |
| Wastewater (n=20) | 1.31E+03 | 8.91E+06 | 1.07E+01 |
| Decomposition (n=10) | 3.13E+03 | 1.44E+05 | 8.97E−03 |
| Geothermal (n=9) | 9.45E+01 | 3.78E+08 | 9.95E+03 |
| Energy Production (n=7) | 5.18E+02 | 2.57E+09 | – |
| Other (n=2) | 4.50E+03c | – | – |
(−) no measurements reported
Due to significant variance between data collection methods and data reported across the studies, only the measured maxima are used for comparison purposes.
Additional flux densities were reported on animal feeding operation (AFO) sources using variable units (e.g. pigs or birds, but not by area), so their measurements have not been included in this comparison table.
Total sulfur in water, plant emissions and sulfur-bituminous concrete emissions.
The results of this literature review (Table 1) indicate that out of the 79 studies in the assessment, and the 132 results included [40], animal feeding operations (AFOs) were the most commonly studied sources (n = 31). This data reflects the propensity for H2S poisonings relative to AFOs and the consequent regulatory activities. While typically the maximal concentration measurements taken within AFO operations were the highest among the studies reviewed (8.66E+03 mg/m3), AFOs did not always contribute the highest fluxes into the air (6.30E+07 mg/hour); natural geothermal activity and anthropogenic energy production were one to two orders of magnitude greater. This observation is likely due to the indoor nature of AFO sources, which can tend to accumulate H2S rather than emit the gas at rates comparable to less enclosed sources. As of 2016, there were in the region of a quarter of a million AFOs in the U.S. and less than one thousand fossil fuel burning power plants (50 MW capacity, or greater) suggesting that the net fluxes of H2S from all AFO and all energy production sources might be roughly comparable. Given the smaller number of geothermally active sites, the net contribution from these is likely lower. Only the two studies in the “other” category described releases into water, although it should be noted that the search terms used to conduct the review could have given preferential treatment to air monitoring over water measurements (for instance, “releases” was not a search term). The apparent lack of any studies assessing H2S levels from a variety of other types of sources, such as fires, paper mills and tanneries, is noteworthy.
3.1 Naturally Produced H2S
In the environment, H2S is often produced by sulfate-reducing bacteria through the anaerobic digestion of organic material. It is also expected that some plants may use and emit H2S [41, 42]. Significant environmental sources of the gas include places where the breakdown of organic matter coupled with a lack of oxygen occurs, including swamps, hydrocarbon deposits, volcanoes, undersea vents, sulfur springs, and stagnant bodies of water. H2S may also be present naturally in well-water [23, 39, 43].
Geothermal activity produces H2S, along with other toxic compounds, when the gases within magma (CO2, SO2, N, H, CO, S, Ar, Cl, and F) combine with hydrogen and water [44]. Geothermal activity has been less studied than man-made and agricultural sources of H2S in recent years. Yearly H2S emissions from all sources on land and oceanic emissions are comparable, 53–100 million metric tons and 27–150 million metric tons of sulfur, respectively [39]. Thus, it seems reasonable to infer that geothermal emissions must represent the largest source of environmental H2S as AFOs and other anthropogenic sources do not contribute to the oceanic emissions. This suggestion is supported by observations from many other studies [23]. Interestingly, high levels of H2S in the atmosphere likely due to volcanic eruptions has been implicated in several mass extinctions throughout Earth’s history [45, 46]. Small blooms of H2S have also recently been detected in the Dead Sea and off of the coast of Namibia in the Atlantic Ocean [47].
3.2 Anthropogenic Sources of H2S
In recent years, anthropogenic sources of H2S emissions into the air have been studied significantly more often than natural sources (n = 69 vs. n = 10)[40], despite the propensity for natural sources to emit H2S at high rates (Table 1). Among anthropogenic sources, H2S can be found at elevated levels in or near sewage systems, animal containment, and slaughterhouses (generally categorized as AFOs). Industrial sources where H2S can be present include oil and gas processing sites, geothermal power plants, coke ovens, food processing facilities, tanneries, and pulp/paper mills. While H2S is primarily released in gaseous form, it can also be found in liquid waste related to industrialization [23, 39].
The amount of H2S emitted into the atmosphere from human activity is difficult to quantify worldwide due to a lack of comprehensive data and/or reporting. For example, H2S emissions in the U.S. were exempt from reporting to the EPA’s Toxic Release Inventory (TRI) between 1991 and 2011. According to the TRI, in 2012 most hydrogen sulfide air releases in the U.S. were the result of three industrial sectors: pulp and paper (64% by weight), chemicals (17%), and petroleum refining (8%)[48]. Contrastingly, the most significant source of H2S emissions in western Canada is the oil and gas industry, due to geologic formations that are naturally high in H2S [49]. Overall, total known hydrogen sulfide releases in the U.S. (into air, water, and through underground injection) range between 26 and 27 million pounds per year (Table 2).
Table 2.
| 2012 (% of total) | 2013 (% of total) | 2014 Emissions (% of total) | |
|---|---|---|---|
| Total On-site Disposal or Other Releases | 26,175,250 (99.96%) | 26,920,643 (99.8%) | 25,965,719 (99.8%) |
| Fugitive Air Emissions | 9,815,319 (37%) | 9,958,673 (37%) | 9,083,805 (35%) |
| Point Source Air Emissions | 10,754,996 (41%) | 11,931,036 (44%) | 11,486,797 (44%) |
| Surface Water Discharges | 497,709 (2%) | 513,188 (2%) | 543,028 (2%) |
| Underground Injection Class I Wells | 4,700,126 (18%) | 4,153,417 (15%) | 4,490,400 (17%) |
| Total Off-site Disposal or Other Releases | 11,631 (0.04%) | 46,021 (0.2%) | 54,339 (0.2%) |
| Off-site RCRA Subtitle C Landfills and Other Landfills | 3,834 (0.01%) | 13,136 (0.05%) | 9,078 (0.03%) |
| Total On- and Off-site Disposal or Other Releases | 26,186,881 (100%) | 26,966,663 (100%) | 26,020,057 (100%) |
Animal feeding operations (AFOs) are agricultural enterprises where animals are kept and raised in confined situations resulting in high concentrations of H2S in the air. While AFOs do not emit H2S at rates on par with geothermal activity, with approximately 257,000 AFOs in the US alone, their sheer numbers can still contribute significant amounts into the air[51]. The substantial influx of AFO studies in recent years is likely due to that fact and the prioritization of quantification of air emissions from AFOs by the United States Department of Agriculture’s Initiative for Future Agriculture and Food System Program and, consequently, funding from the National Research Initiative Program [52]. In order to accurately quantify total H2S contributions to the atmosphere from AFOs, all AFO operations and their H2S management methods need to be tracked and monitored over time.
Energy production was another area identified[40] that exhibits the potential to emit H2S at high rates, although monitoring results were highly variable within this sector. H2S forms naturally within geologic formations that support oil and gas production as high-sulfur kerogens decay. When sulfur (and H2S) content are high in wells, they are referred to as “sour gas” wells. While this gas may not pose an issue at all drilling sites, where it does can have serious consequences. In Kaixian County, China, for example, 64,000 residents had to be evacuated and 243 were killed when an accidental sour gas well blowout occurred in 2003 [53]. The Saskatchewan government recently tested 43 facilities in southeast Saskatchewan that were leaking sour gas, finding average concentrations of 30,000 ppm, well above levels that can kill livestock and people [54]. Wells and refineries where H2S may be present also exist in the U.S. Out of Michigan’s 10,652 producible oil wells, for example, 1,360 saw H2S levels exceeding 300 ppm [55]. The hazards posed by high emission rates from oil and gas sites is compounded by the fact that, in the U.S., no OSHA monitoring program exists at this time, though such programs have been proposed in the past. Skrtic [43] discusses these continuing gaps in much further detail.
3.3 Major Commercial Uses
For commercial purposes, H2S is used to produce sulfur through the Claus process (Equations 4) and, subsequently, sulfuric acid (H2SO4) by the Contact Process (Equations 5). Sulfuric acid is one of the most highly traded chemical commodities in the world due to its role in producing phosphate (60% of worldwide demand) and other fertilizers (10%) [56].
Equations 4. Claus process (industrial production of elemental sulfur from H2S).
Overall: 2H2S + SO2 = 3S + 2H2O
-
Thermal Step:
-
○
2 H2S + 3 O2 → 2 SO2 + 2 H2O (ΔH = −4147.2 kJ mol−1)
-
○
-
Catalytic Step:
-
○
2 H2S + SO2 → 3 S + 2 H2O (ΔH = −1165.6 kJ mol−1)
-
○
Equations 5. Contact process (industrial production of sulfuric acid).
S(s) + O2 ➔ SO2(g)
2SO2(g) + O2(g) ⇌ 2SO3(g) (ΔH = −196 kJ mol−1)
H2SO4(l) + SO3(g) ➔ H2S2O7(l)
H2S2O7(l) + H2O(l) ➔ 2H2SO4(l)
H2S is beneficial in a variety of other sectors. It is used to make sodium sulfide and sodium hydrosulfide. These compounds are then used in the production of dyes, pesticides, and even pharmaceuticals. H2S also has roles to play in metallurgy, laboratory settings, and agriculture [23]. The nuclear energy sector utilizes H2S in large quantities to separate “heavy water,” containing the hydrogen isotope deuterium, from regular water [21].
Natural gas purification and petroleum refining supply approximately 60% of the sulfur and SO2 used in the production of sulfuric acid [56]. In petroleum refining, H2S is a result of the hydrotreating process, where sulfur compounds found in the crude oil are removed by reaction with hydrogen gas [48, 50].
3.4 H2S Suicides
On a smaller scale, emergency medical personnel may be exposed to high levels of the gas when responding to H2S suicides, also called “detergent suicides”[57, 58]. The procedure involves mixing hydrochloric acid (found in commercial pool and toilet bowl cleaners) with either lime sulfur (found in common pesticides) or bath sulfur (available in Japan) in an enclosed space to generate toxic levels of H2S gas [57, 59, 60].
The trend started in Japan in 2007 and has since moved abroad, as methods for generating H2S from household chemicals were publicized on the Internet; in 2008 alone, 500 men, women, and children committed suicide in Japan using this method. Increasingly, more people in the U.S. have followed suit. Prior to 2008, there were no records of Americans committing suicide using intentionally-generated H2S gas. Between 2008 and 2010, however, 30 such suicides occurred. Even if alerted to the presence of toxic gas, the temptation for would-be rescuers to assist collapsed individuals is great and five emergency responders were injured during rescue efforts in that period [61]. There also has been a report of suicide by H2S inhalation in an apartment building where residents not in the immediate vicinity of the release site were affected [58, 61].
These and other developments have recently led to a growing concern that H2S might find application as a terrorist weapon [59]. Disturbingly, despite this known health risk to the public, emergency personnel and certain other workers, there is no FDA-approved antidote and/or reliable protocol for treating acute hydrogen sulfide poisoning currently available.
4. H2S Regulation in the U.S
In the United States, hydrogen sulfide is regulated in a variety of ways by the U.S. Environmental Protection Agency (EPA), the Food and Drug Administration (FDA) and the Occupational Safety and Health Administration (OSHA). The exposure limits recommended by OSHA are enforceable by law, but only in the workplace. OSHA sets limits in industries where H2S is found over the threshold quantity of 1,500 pounds (680.38 kg) [62]. Additional national organizations such as the Agency for Toxic Substances and Disease Registry (ATSDR), National Institute for Occupational Safety and Health (NIOSH), and the American Conference of Governmental Industrial Hygienists (ACGIH) also provide recommended exposure limits. Data regarding occupational and ambient exposures are fraught with complications and contradictions, however, since actual H2S emission levels and concentrations within a mixture of sulfurcontaining gases are usually unknown [23, 39]. Acute exposure guidelines (Table 3) have been developed by several regulatory and non-governmental organizations primarily based on experimental animal studies.
Table 3.
Airborne hydrogen sulfide exposure limits established by various U.S. and international public safety organizations.
| Agency | Exposure Level Types | REL (ppm) | Reference |
|---|---|---|---|
| ACGIH | TLV-TWA | 1 | OSHA [63] |
| TLV-STEL | 5 | ||
| AIHA | ERPG 1a | 0.1 | AIHA [64] |
| ERPG 2 | 30 | ||
| ERPG 3 | 100 | ||
| ATSDR | MRL-Acute | 0.07 | ATSDR [23] |
| MRL-Intermediate | 0.02 | ||
| MRL-Chronic | n/a | ||
| DOE | PAC-1 | 0.51 | DOE [65] |
| PAC-2 | 27 | ||
| PAC-3 | 50 | ||
| EPA | RfC | 0.001 | EPA [51] |
| AEGL-1: 10 min | 0.75 | NRC [66] | |
| 30 min | 0.60 | ||
| 60 min | 0.51 | ||
| 4 hr | 0.36 | ||
| 8 hr | 0.33 | ||
| AEGL-2: 10 min | 41 | ||
| 30 min | 32 | ||
| 60 min | 27 | ||
| 4 hr | 20 | ||
| 8 hr | 17 | ||
| AEGL-3: 10 min | 76 | ||
| 30 min | 59 | ||
| 60 min | 50 | ||
| 4 hr | 37 | ||
| 8 hr | 31 | ||
| DFG | MAK | 5 | DFG [67] |
| IARC | Carcinogenicity classification | n/a | IARC [68] |
| NIOSH | IDLH | 100 | NIOSH [69] |
| REL: 10-min | 10 | ||
| OSHA | PEL (8-hour TWA) – general industry | n/a | OSHA [63] |
| PEL Ceiling | 20 | ||
| PEL Peak: 10 min | 50 | ||
| WHO | TWA: 24 hr | 0.10 | WHO [70] b |
| Range: 1 ppb – 100 ppm | |||
ERPGs estimate the concentrations at which most people will begin to experience health effects if they are exposed to a hazardous airborne chemical for 1 hour. (Sensitive members of the public are not covered by these guidelines; they may experience adverse effects at concentrations below the ERPG values.) A chemical may have up to three ERPG values, each of which corresponds to a specific tier of health effects:
ERPG-3 is the maximum airborne concentration below which it is believed that nearly all individuals could be exposed for up to 1 hour without experiencing or developing life-threatening health effects.
ERPG-2 is the maximum airborne concentration below which it is believed that nearly all individuals could be exposed for up to 1 hour without experiencing or developing irreversible or other serious health effects or symptoms which could impair an individual’s ability to take protective action.
ERPG-1 is the maximum airborne concentration below which it is believed that nearly all individuals could be exposed for up to 1 hour without experiencing other than mild transient health effects or perceiving a clearly defined, objectionable odor.
While not discussed in WHO’s 2010 report on select air pollutants, the World Health Organization did publish air quality guidelines on H2S in this report from 2000.
Abbreviations & Definitions (alphabetical): ACGIH = American Conference of Governmental Industrial Hygienists; AEGL = acute exposure guideline level; AEGL-1 = nondisabling threshold limit; AEGL-2: disabling threshold limit; AEGL-3: lethality threshold limit; AIHA = American Industrial Hygiene Association; ATSDR = Agency for Toxic Substances and Disease Registry; DFG = Deutsche Forschungsgemeinschaft; DOE = U.S. Department of Energy; ERPG = emergency response planning guideline; IDLH = immediately dangerous to life and health; IARC = International Agency for Research on Cancer; MAK = maximum workplace concentration across an 8-hour day, 40- hour work week; MRL = minimum risk level (inhalation factors, not oral, have been derived); MRL-Acute = MRL for acute-duration inhalation exposure (≤14 days); MRL-Chronic = MRL for chronic-duration inhalation; MRLIntermediate = MRL for intermediate-duration inhalation exposure (15–364 days); NAS = National Academy of Sciences; NIOSH = National Institute for Occupational Safety and Health; NRC = National Research Council; OSHA = Occupational Safety and Health Administration; PAC-1 = All protective action criteria correspond to 60-minute AEGL values. PAC-1 is for mild, transient health effects; PAC-2 = irreversible or other serious health effects that could impair the ability to take protective action; PAC-3 = life-threatening health effects; PEL = permissible exposure limit; PEL Peak: 10 min = acceptable maximum peak above ceiling over an 8-hour shift for 10 minutes once only if no other measured exposure occurs; PPM = parts per million; REL = recommended exposure limit; RfC = daily inhalation exposure limit over a lifetime that does not present risk of deleterious effects; TLV-STEL = threshold limit value – short-term exposure limit; TLV-TWA = threshold limit value – time weighted average.
At a national level, H2S was originally on the proposed hazardous air pollutants (HAPs) list of the Clean Air Act Amendments of 1990 with 188 other pollutants that are known, or suspected, to cause serious adverse health and/or environmental effects. Instead of ambient air quality standards, HAPs are regulated at the source nationally by limiting industrial emissions. The levels permitted are driven by the most effective technological controls available. Successful petitioning resulted in the removal of H2S from the HAPs list in 1991 [71]. Hydrogen sulfide is, however, found on the U.S. EPA’s list of Extremely Hazardous Substances [43] as determined by the Emergency Planning and Community Right-To-Know Act in the event of accidental releases [72]. Additionally, starting in 2011, U.S. companies were required to report their emissions of H2S to the Toxic Release Inventory (TRI) a system for tracking toxic chemicals that may pose environmental and health risks. There had previously been a TRI reporting stay for hydrogen sulfide enacted in 1995 that was then lifted in 2011 [73].
H2S does not fall under the regulatory authority of the EPA for National Ambient Air Quality Standards, but the EPA does have a reference concentration for chronic inhalation (RfC) at 2 × 10−3 mg/m3 (1.4 ppb). The EPA also has the regulatory authority to institute regulations on specific H2S sources if it so chooses, such as oil and gas wells. It is assumed that daily exposures of H2S above this level over a lifetime will have deleterious effects. No parallel reference dose for chronic oral exposure (RfD) exists at this time [51]. In lieu of national limits on H2S, individual states in the U.S. can choose to limit exposures, although their standards vary significantly. See Appendix B in Skrtic 2006 [43] for state ambient hydrogen sulfide standards.
5. Exposure Pathways
Inhalation is the main route of exposure for hydrogen sulfide, with dermal/eye contact, injection, or ingestion being plausible but less likely routes. Humans can typically smell H2S at low concentrations in the air, between 0.0005 and 0.3 ppm, lower than our most sensitive H2S monitoring equipment [23]. Because H2S in gaseous form is heavier than air, the highest risk of exposure for people is in enclosed spaces at, or just below, ground level. In common with many toxicants, the most well-documented arena for understanding hydrogen sulfide risks is in the occupational setting. According to available data from OSHA and the Bureau of Labor Statistics (BLS), H2S is one of the most dangerous gases in the workplace, second only among toxic gases to carbon monoxide. From 2004–14, approximately 83 workers lost their lives, and 120 were sickened and missed work due to exposure to H2S while on the job. The majority of both fatal and nonfatal workplace incidents involved exposures to males, not females [62, 74–79]. Beyond summary statistics, it is difficult to interpret trends from the (incomplete) U.S. data available on occupational fatalities and injuries due to H2S exposures. However, workers in industries such as petroleum production and refining, sewer and wastewater treatment, agricultural silos and pits, textile manufacturing, pulp and paper processing, food processing, hot asphalt paving, and mining are considered those most at-risk [78–80].
Favorable conditions for high H2S production and accumulation — such as hot weather, confined spaces, and low wind — are likely better indicators than one’s job, especially for assessing risk outside of occupational settings. For the American public also, hydrogen sulfide remains a significant inhalation hazard. In 2012, there were an estimated 809 non-occupational exposures resulting in 5 deaths as logged in the National Poison Data System [81]. In 2013 there was an increase in both exposures and deaths (855 and 10 respectively) second only to carbon monoxide with regard to deaths (n = 60)[82]. The elderly, asthma sufferers and children with otherwise compromised respiratory systems are at higher risk of the compound’s negative effects since H2S targets the respiratory tract [23, 39].
It is not reliably known what fraction of H2S to which a person may be exposed can be absorbed into the body and become systemically distributed as H2S/HS− in the bloodstream [83–85]. H2S/HS− appears to be primarily detoxified through oxidation in the liver, and also by methylation [86]. However, urinary thiosulfate, the last product of mammalian mitochondrial sulfide oxidation, is the most commonly used biomarker for H2S exposure [87].
The toxic effects of H2S can vary greatly based on the level, route and duration of the exposure. For discussion purposes, we find it convenient to consider three main types of exposure: acute, post-acute, and chronic (Figure 2) – based on descriptions in the peer-reviewed literature and lower-range regulatory limits (Table 3). These exposure characteristics and their effects are neither rigorously delimited, nor generally accepted, as most authors do not distinguish between acute and what we shall call “post-acute” effects. In our view, the distinction is important because “post-acute” effects occur more than about 10–15 min after sub-lethal acute exposure, once H2S/HS− has already disappeared from the circulation, but in the time frame during which victims of H2S poisonings present at the emergency clinic (Frawley et al., 2017, manuscript in revision).
Figure 2.
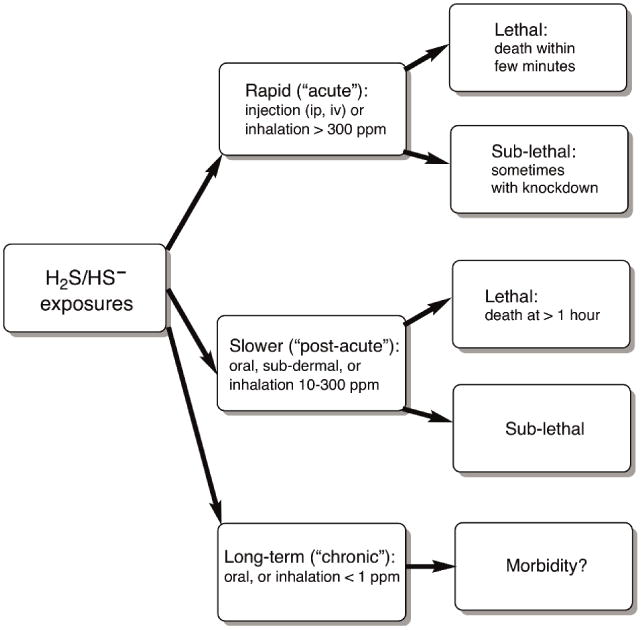
Categories of Lethal and Sub - lethal H2S Poisonings
6. Human Health Effects
There is, of course, a body of work describing H2S as a modulator of physiological functions [38, 39, 88, 89]. It has been implicated in the regulation of blood pressure, neurotransmission, antiinflammatory action, aiding digestion, and other activities [7, 12, 14, 15, 23, 90]. Investigations looking into the role that H2S may play in suspended animation [91, 92] may have experienced a loss of interest as the effect in question appears diminished in larger animals [93]. To date, however, the majority of well-documented health effects arising from exposure to hydrogen sulfide remain negative, especially at levels above 1 ppm in air. Most hydrogen sulfide toxicity studies have either involved retrospective analysis of acute, uncontrolled incidents where the exact concentration and any pre-existing conditions of the victims were not known or more controlled research studies with animals, whose results were then extrapolated to humans. The consensual viewpoint is that the respiratory tract and nervous system are especially sensitive to the effects of H2S exposure [23, 94]. The general symptoms exhibited by individuals exposed to varying levels of gaseous H2S are summarized below (Table 4).
Table 4.
Pathophysiological responses to hydrogen sulfide at various concentrations in air.
| Concentrations (ppm) | Expected Effects/Symptoms |
|---|---|
| 0.00011–0.00033 | Typical background concentrations (OSHA) |
| 0.0005 | Lowest concentration detectable by human olfactory senses (ATSDR) |
| 0.01–1.5 | Odor threshold (when rotten egg smell is first noticeable to some). Odor becomes more offensive at 3–5 ppm. Above 30 ppm, odor described as sweet or sickeningly sweet (OSHA) |
| 2–5 | Prolonged exposure may cause nausea, tearing of the eyes, headaches or loss of sleep. Airway problems (bronchial constriction) in some asthma patients (OSHA) |
| 20 | Possible f atigue, loss of appetite, headache, irritability, poor memory, dizziness (OSHA) |
| 50 – 100 | Slight conjunctivitis (“gas eye”) and respiratory tract irritation after 1 cause digestive upset and loss of appetite (ANSI and OSHA)-hour exposure. May |
| 100 | Coughing, eye irritation, loss of sense of smell after 2–15 minutes. Altered respiration, pain in the eyes and drowsiness after 15–30 minutes followed by throat irritation after 1 hour. Several hours of exposure results in gradual increase in severity of these symptoms and death may occur within the next 48 hours (ANSI and OSHA) |
| 100 – 150 | Loss of smell (olfactory fatigue or paralysis) (OSHA) |
| 200 – 300 | Marked conjunctivitis and respiratory tract irritation after 1 hour of exposure (ANSI and OSHA). Pulmonary edema may occur from prolonged exposure (OSHA) |
| 500 – 700 | Staggering, collapse in 5 minutes (OSHA). Serious damage to the eyes. Loss of consciousness and possibly death in 30 minutes - 1 hour (ANSI and OSHA) |
| 700 – 1000 | Rapid unconsciousness, “knockd own” or immediate collapse within 1 to 2 breaths, cessation of respiration and death within minutes (ANSI, ATSDR, and OSHA) |
| 1000 – 2000 | Unconsciousness at once, with early cessation of respiration and death in a few minutes. Death may occur even if individual is removed to fresh air at once (ANSI and OSHA) |
At all levels – acute, post-acute, and chronic – the effects of H2S inhalation still presents many unknowns, although markedly more gaps exist within post-acute and chronic exposures. Effects following dermal exposure and ingestion, as well as genotoxicity and reproductive effects, are even less well understood [23, 94].
6.1 Acute Exposures (> 300 ppm, rapid onset)
Hydrogen sulfide odor becomes detectable at concentrations as low as .0005 ppm, but the sense of smell is lost after 2–15 minutes at 100 ppm [23, 94], rendering the odor of the gas an ineffective hazard warning. In situations involving extremely high H2S levels in air, potential victims run the risk of experiencing “knockdown,” or passing out in the area. Knockdown severely diminishes survival rates due to trauma sustained during the fall and/or inability to escape. Collapsed victims also endanger responders as they entice would-be rescuers into locations with dangerously high toxicant levels.
In cases of severe acute toxicity, i.e. > 500 ppm H2S, unconsciousness and death may result almost immediately [63, 94], probably through cardiopulmonary paralysis. Interestingly, it is not uncommon for individuals receiving a high dose of the toxicant for a very short duration to experience knockdown, but then spontaneously (and rapidly) recover, requiring no medical intervention and exhibiting no long-term sequelae [23]. Such observations have been well-replicated in laboratory animals [97].
6.2 Post-Acute Exposures (> 100 ppm, slower onset)
Having received lower hydrogen sulfide doses over more prolonged durations rather than acute cases, victims often present at the clinic more than 30 min after exposure, exhibiting symptoms such as difficulty in breathing, noncardiogenic pulmonary edema, cyanosis and, ultimately coma/death. In such cases, death may be delayed for hours to days and a variety of subsequent neurological disorders, if developed, require weeks to present [23, 78, 98, 99]. Levels of H2S up to approximately 10 ppm for short periods (a couple of hours) can certainly be tolerated well by healthy adults. In the 1–300 ppm range, however, there is a paucity of available quantitative data on the effects [100], but sometimes, no symptoms have been observed even during controlled exposure trials [23].
6.3 Chronic Exposures (< 1 ppm, for at least days)
The effects of low-level or long-term exposure to ambient levels of hydrogen sulfide (< 1 ppm) in air are more difficult to estimate because the mechanism(s) for chronic toxicity is(are) not well understood. At such levels and duration, based heavily on anecdotal evidence, expected symptoms of exposure could include visual complications, olfactory fatigue, nausea, respiratory irritation, and possible headaches due to the sensitivity of those systems to H2S exposure [23, 101–103].
Few places, however, provide a better natural experiment for determining health effects from chronic H2S exposure than Rotorua, New Zealand, where a population of 60,000 people lives near an active geothermal field. The most reliable background levels of H2S in this area indicate a median concentration of 30 μg/m3 (20 ppb) [104]. While initial indications were that there might be cardiovascular, nervous system and respiratory diseases stemming from this chronic low level-H2S exposure [105] some 15 years later, further studies have so far failed to identify any negative sequelae apparent in the potentially susceptible population [106–111].
7. Conflicting Observations Regarding the Chemical Toxicology of H2S
7.1 Lessons from occupational accidents
The available (anecdotal) evidence from acute human (occupational) mass exposures to H2S gas suggests approximately 20% of victims should require no treatment. But there will be ~5% fatalities and about three-quarters of the victims can be expected to arrive alive at the clinic exhibiting coma, disequilibrium, respiratory insufficiency and/or pulmonary edema [94, 112, 113]. Amongst sewer workers exposed in enclosed spaces below ground level, fatalities can be expected to be higher, but there are still survivors [114–116]. Based on their experience with workers in Canadian sour gas wells (the epicenter of H2S poisonings in North America) Burnett et al.[112] assert that “increased attention to cardiopulmonary resuscitation at the exposure site and during transportation to hospital is necessary to reduce the mortality from H2S exposure.” Neurological sequelae to sulfide poisoning have been reported [23, 94, 113, 117, 118], but these remain rare. Interestingly, discounting minor symptoms like dizziness and headaches that resolve themselves, no long-term neurological (or other) effects were evident in any of the 221 cases documented in the Canadian study[112]. A satisfactory animal model of human H2S exposure reproducibly leading to neurological sequelae following a single toxicant dose remains elusive [119]. Thus, there is a nagging suspicion that most of the reported neurological consequences of H2S exposures in humans necessitating treatment might be due to other factors such as brain anoxia, or head injury sustained during knockdown, rather than any more direct effect of the toxicant.
Where autopsies have been performed in a timely fashion, it has been noted that the internal organs of human H2S poisoning victims have been discolored – the blood and sectioned brain, in particular, appearing distinctly green. This green color is due to the formation of sulfhemoglobin [120–123] in which the porphyrin ring has been covalently modified (Figure 3) [124–126]. Significantly, at this time, these established characteristics of human poisonings have not been observed together in any of the reported animal models of which we are aware. For instance, mice given LD40 doses of NaSH by injection either die in less than 4 min or fully recover within 15 min [97]. Furthermore, purified mouse hemoglobin can readily be manipulated to undergo the same conversion to sulfhemoglobin as the human protein. However, the animals have so far never exhibited any evidence of sulfhemoglobin formation, irrespective of whether the toxicant is given by single-shot intraperitoneal injection, slow tail-vein infusion, or by inhalation (A.A. Cronican, K.L. Frawley, L.L. Pearce & J. Peterson, unpublished observations). This situation is not helpful with regard to the development of effective therapies and there are no currently approved antidotes/protocols to treat poisoning by sulfide (H2S/HS−), only suggested supportive countermeasures [23, 127, 128].
Figure 3.

Structures of Native Red Heme (Iron-Protoporphyrin IX, on left) and the Kinetically Stable Form [124] of the Green Sulfheme (on right).
Some authors in the early literature (before the structures were properly identified) have confused the terminology. For example, what is now known as either methemoglobin-sulfide, or sulfidomethemoglobin (metHbSH, where HS− is a ligand to the heme iron) some early authors [114] referred to as sulfhemoglobin (SHb). Here we reserve the latter term for the covalently modified macrocyclic structure shown in Figure 3.
7.2 H2S catabolic biochemistry
Despite some thoughtfully discussed cautionary arguments [129–131], the reader will be aware that a significant literature continues to rapidly emerge regarding the possible function of H2S as a “gasotransmitter”[10, 13, 38, 132, 133]. Taking a pragmatic stance, we may assert this body of work to be outside the scope of the present review and confounding, rather than clarifying, with regard to some important questions relevant to H2S toxicity. Any signaling functions of H2S must take place at orders of magnitude lower concentration than the relevant levels in toxicity scenarios – considerations of mass action alone probably ensuring that different small-molecule bioinorganic reactions are involved in these two circumstances. For example, there presently seems to be a concurring opinion [38, 134–139] that the catabolic elimination of H2S in mammals is catalyzed almost exclusively by the sulfide oxidase system localized within mitochondria. This may well be the case under more-or-less normal physiological circumstances, but probably not at the elevated H2S levels to be experienced during poisonings and, perhaps, some other pathological conditions. The first enzyme of the sulfide oxidase system, sulfide quinone reductase, abstracts a hydrogen atom from H2S and passes two electrons to the electron-transport system via ubiquinone. Of course, the terminal acceptor for these two electrons is oxygen at the active (ligand-binding) site of cytochrome c oxidase (complex IV). Now we have an instructive conundrum, for if the primary molecular target for the toxicant sulfide is, as widely accepted (see below) the ligand-binding site of cytochrome c oxidase, then sulfide unavoidably inhibits its own elimination (Figure 4).
Figure 4.

The Association between H2S Catabolism and the Mitochondrial Electron-Transport System.
There are, however, several lines of evidence that contradict the notion that sulfide need necessarily inhibit its own elimination completely. First, mice rendered unconscious (near death) by infusion of NaSH solutions into the tail vein over 5–10 min recover within seconds of stopping the infusion (Frawley et al., 2017, manuscript in revision) much faster than recovery from equivalently toxic levels of the similarly acting toxicant sodium cyanide. Second, the observation at autopsy of sulfhemoglobin formation in humans [120–123] is clear evidence for at least one other alternate competitive metabolic pathway for sulfide. Third, a literature has emerged describing the presence of dimethylsulfide (CH3SCH3) in exhaled breath [140, 141] another pathway for elimination of sulfide. This has been confirmed/discovered in individuals with elevated levels due to “extra-oral halitosis”; that is, not due to bacterial production of dimethylsulfide in the oral cavity, but from internal sources [142, 143]. These recent observations regarding dimethylsufide generation in humans echo the earlier literature in which the “methylation pathway” was generally recognized as a detoxification route, albeit subordinate to the sulfide oxidase system [86, 144]. Finally, it appears that sulfide (H2S/HS−) can only be detected in the bloodstream of both rats and sheep for a matter of seconds when administered intravenously at sub-lethal, but measurably toxic, levels [145–148]. In short, there are almost certainly multiple pathways through which sulfide can be eliminated from mammals, though these remain poorly delineated at this time. This ought not to be surprising as sulfide is both a good ligand and reductant [129] – that is, some of its biochemistry may not necessarily be enzyme catalyzed.
7.3 Molecular pathology
While sulfide can clearly react with multiple biomolecules and there are tissue-specific variations in the toxic response, the crucial molecular target in acute cases is generally accepted to be cytochrome c oxidase (complex IV) of the mitochondrial electron-transport system (ETS)[23, 97, 127, 128, 149–151]. Sulfide is certainly a potent inhibitor of complex IV, but it is less well known that it also reacts with the enzyme resulting in catalytic turnover [89, 149, 152, 153]. Therefore, while these reactions remain poorly understood, they do provide yet another potential route for catabolic elimination of sulfide when enzyme inhibition is sub-maximal. As molecular H2S can freely diffuse through membranes, it readily crosses the blood-brain barrier to inhibit mitochondrial ETSs within the central nervous system. In unanesthetized laboratory animals, this results in clear behavioral signs of intoxication 2 min post-injection and can lead to death from respiratory paralysis within ~3 min [23, 97], or cardiac failure after ~7 min [147, 148]. At this time, it is not clear how to reconcile the observation that free H2S/HS− seemingly only persists for a matter of seconds in the bloodstream [145–148] yet onset of symptoms associated with complex IV inhibition by H2S/HS− begins at 2 min after the toxicant dose. We remind the reader at this point that significant numbers of human victims of H2S inhalation arrive at the clinic with cardiopulmonary symptoms 30 min or more after exposure and frequently succumb hours later [51, 94, 112, 154].
7.4 Pulmonary considerations
Prior to the emergence of any gasotransmitter activity, there were insightful, concise reviews of H2S toxicity published [87, 154–156] that still provide an excellent entry point to this literature, as well as some lengthier scholarly documents [94, 144, 157]. A few key points worth reiterating include that while there are some relatively mild and mostly resolvable ocular conditions associated with chronic H2S exposures, the neurological sequelae reported in humans following more acute exposures may primarily be caused by brain anoxia or head trauma suffered during a collapse, both secondary to the direct toxic effects of H2S. The observed symptoms of acute gaseous exposures are hyperpnea, then unconsciousness (“knockdown”), followed by apnea and finally, death, frequently accompanied by pulmonary edema. The lung appears to be especially sensitive as hyperpnea and apnea are observed in laboratory animals administered sulfide solutions by injection [158], while edema only seems to follow H2S inhalation [87, 154, 156, 159]. Recent work with the cysteine dioxygenase knockout mouse, which accumulates sulfide, has confirmed that the lung (and pancreas) is (are) more susceptible to toxicity from endogenously elevated sulfide than liver, or kidney [160]. Also, in various other animal models, sulfide has been demonstrated to contribute to the development and progression of lung inflammation and injury [161]. Bizarrely and to the contrary, however, sulfide is apparently ameliorative in the case of lipopolysaccharide-induced acute lung-injury (ALI) in rats [162] and in burn/smoke-induced ALI in sheep[8]. Both Olson [130, 131] and Haouzi [163] have written extensively about the practicalities of manipulating sulfide in biological samples and the difficulty in distinguishing physiological from pharmacological processes, particularly at the uncertain sulfide levels obtained in tissues irrespective of the method of administration. Of course, one should expect that many of the paradoxical observations in the present literature could be resolved with improved knowledge of the underlying sulfide biochemistry. In this regard, quantitative understanding of the small molecule bioinorganic chemistry underpinning much of the field appears especially lacking. So, for example, while some authors argue that oxygen-dependent redox processes are involved in sulfide cytotoxicity observed in cultured cells [164, 165], other groups have pointed out that in the case of intact animals [97] and human patients [156] any effects of supplemental oxygen are indistinguishable from normal recovery. While less than helpfully informative, it is probably not disingenuous to describe the current status of the relevant redox biochemistry [133, 138] as complicated at best.
There is perhaps some hypersensitivity exhibited by individuals with pre-existing conditions such as asthma [23, 87], but in comparison to other common chemical reagents like ammonia and volatile organic acids, H2S is a modest lachrymator/pulmonary irritant. Accidental releases of sulfide being more likely to elicit eruptions of puerile humor from one’s laboratory colleagues than more serious consequences. Given such experiences, it is possible that the severity of inhaled H2S as an irritant has sometimes been overstated – maybe originating in attempts to explain some of the observed physiological responses to exposure predating any understanding that one or more sulfide species might be signaling molecules. During inhalation, the sulfide fluxes experienced by the lung tissues are going to be significantly greater than both the systemic levels and, also, the fluxes that the lung tissues themselves would experience following toxicant administration by alternate methods. Thus, development of pulmonary edema following H2S inhalation, the most notable issue in human fatalities [112], reflects this locally elevated exposure, but probably involves responses other than a mere reaction to an irritant. Typically, clinical presentations of pulmonary edema are secondary to either elevated pulmonary capillary pressure from left-side heart disease (cardiogenic) or injury and an increased permeability of the lung microvasculature, frequently associated with sepsis (noncardiogenic)[166, 167]. Endothelial barrier function is seemingly always compromised, while the epithelial barrier is usually, but not always affected [167]. The less-often-encountered syndromes neurogenic pulmonary edema and high-altitude pulmonary edema each show both cardiogenic and noncardiogenic features [167–169]. It has been clear for decades that H2S-induced pulmonary edema is associated with vascular permeability due to the high protein content of the extravasated fluid [84, 170, 171] – but a further similarity between this and any of the other noncardiogenic syndromes essentially remains open to question.
Multiple types of calcium and potassium ion channels (at least) are susceptible to modulation by H2S, especially within the cardiovascular system [172–174]. These emerging effects of H2S exhibit a complicated interdependence with those of nitric oxide, the relationship being demonstrably evident in endothelial and smooth muscle cells [172, 175–177]. Since the details of these interactions in physiological circumstances are still emerging, any associated pathological biochemistry is even less well delineated, but there is promising scope here for the discovery of a mechanism to explain H2S-induced pulmonary edema and, thus, potential therapeutic targets. There has been some recent focus on the lung epithelial sodium channel as a target for treating H2S-induced acute pulmonary edema [178–180]. Unfortunately, there is cause for pessimism with regard to this suggestion because multicenter clinical trials with epithelial sodium channel activators/stimulators for the treatment of patients with pulmonary edema have, thus far, proven disappointing [181]. In proof-of-concept laboratory experiments with animals, where the poisoning protocols were quite unlike human cases, it has been shown that hydroxocobalamin [182] and its biological precursor, cobinamide [183], offer some protection against injected NaSH. However, in keeping with the reported observation that free sulfide is eliminated from the bloodstream very quickly [145, 146], the hydroxocobalamin had to be given within ~2 minutes of the toxicant. Cobinamide was given during administration of the toxicant dose – neither of these protocols being of much practical value in relation to human poisonings.
8. Concluding Remarks
Hydrogen sulfide represents a public health risk worthy of continued attention, especially from an occupational perspective [16] – but the monitoring infrastructure currently in place could be improved. Incomplete databases and inadequate research regarding the health effects of H2S across the range of levels to be found in the environment, as well as the potential for the gas to be used for malicious purposes – either by individuals or on a mass scale – is concerning. Future research efforts should be focused on better monitoring of known H2S emissions, along with improving the documentation of exposures and their subsequent health impacts, identification of new potential sources and, most importantly, clarifying the mechanistic pathways by which H2S exerts its toxic effects. Currently, in the absence of any FDA-approved (or off-label) therapeutics, the only available option for treating sulfide intoxication is supportive care and speculative application of antidotes for cyanide, which have thus far exhibited only limited efficacy. The lack of a clear mechanistic understanding of acute sulfide toxicity presents a significant barrier to the rational development of effective antidotes and protocols for their therapeutic application.
Hydrogen sulfide remains a significant occupational hazard.
Major environmental sources of hydrogen sulfide in the U.S. are the result of three industrialsectors: pulp and paper (64% by weight), chemicals (17%), and petroleum refining (8%).
High emission rates from oil and gas sites remain a hazard and are compounded by the fact that, in the U.S., no OSHA monitoring program exists at this time.
Details of the mechanism(s) of hydrogen sulfide toxicity are incomplete.
There are currently no FDA-labeled antidotes and no recommended treatment options for sulfide poisoning beyond supportive care.
Acknowledgments
Acknowledgements: Supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS), grant no. NS089896 to J.P. and L.L.P.
Funding Source: CounterACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS), Grant Number NS089896 to J.P. and L.L.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lock J. The Princess Alice Disaster. Robert Hale Limited; 2013. [Google Scholar]
- 2.Thurston G. The Great Thames Disaster. London, UK: George Allen & Unwin Ltd; 1965. p. 173. [Google Scholar]
- 3.Butcher L. Shipping: Safety on the River Thames and the Marchioness disaster. House of Commons; Westminster, UK: 2010. p. 13. [Google Scholar]
- 4.Clarke LJ. In: DETR, Marchioness/Bowbelle - Formal Investigation under the Merchant Shipping Act 1995, Non-Statutory Inquiry into the identification of victims. T.a.t.R.U.K. Department of the Environment, editor. DETR; London, UK: 2001. p. 891. [Google Scholar]
- 5.Cooper PF. Historical Aspects of Wastewater Treatment. In: Lens P, Zeeman G, Lettinga G, editors. Decentralised Sanitation and Reuse: Concepts, Systems and Implementation. IWA Publishing; London, UK: 2001. [Google Scholar]
- 6.Dobraszczyk P. London’s Sewers. Oxford UK: Shire Publications Ltd; 2014. p. 64. [Google Scholar]
- 7.Dongó E, et al. The cardioprotective potential of hydrogen sulfide in myocardial ischemia/reperfusion injury (Review) Acta Physiologica Hungarica. 2011;98(4):369–381. doi: 10.1556/APhysiol.98.2011.4.1. [DOI] [PubMed] [Google Scholar]
- 8.Esechie A, et al. Beneficial effect of a hydrogen sulphide donor (sodium sulphide) in an ovine model of burn- and smoke-induced acute lung injury. Br J Pharmacol. 2009;158(6):1442–53. doi: 10.1111/j.1476-5381.2009.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancardi D, et al. Physiological and pharmacological features of the novel gasotransmitter: hydrogen sulfide. Biochim Biophys Acta. 2009;1787(7):864–72. doi: 10.1016/j.bbabio.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mancardi D, et al. Old and new gasotransmitters in the cardiovascular system: focus on the role of nitric oxide and hydrogen sulfide in endothelial cells and cardiomyocytes. Curr Pharm Biotechnol. 2011;12(9):1406–15. doi: 10.2174/138920111798281090. [DOI] [PubMed] [Google Scholar]
- 11.Paul BD, Snyder SH. H2S: A Novel Gasotransmitter that Signals by Sulfhydration. Trends Biochem Sci. 2015;40(11):687–700. doi: 10.1016/j.tibs.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6(11):917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 13.Wang R. Hydrogen sulfide: the third gasotransmitter in biology and medicine. Antioxid Redox Signal. 2010;12(9):1061–4. doi: 10.1089/ars.2009.2938. [DOI] [PubMed] [Google Scholar]
- 14.Yang G, et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine γ-lyase. Science. 2008;322(5901):587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolluru GK, et al. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frame MH, Schandl CA. A case example of asphyxia due to occupational exposure to airborne chemicals and review of workplace fatalities. J Forensic Sci. 2015;60(2):521–4. doi: 10.1111/1556-4029.12695. [DOI] [PubMed] [Google Scholar]
- 17.Oviedo ER. Civil and Environmental Engineering Department. The University of Texas at San Antonio; San Antonio, Texas: 2010. Evaluation of hydrogen sulfide concentration and control in a sewer system. [Google Scholar]
- 18.Harris DC. Quantitative Chemical Analysis. New York: W.H. Freeman and Company; 2010. [Google Scholar]
- 19.Housecroft CE, Sharpe AG. Inorganic Chemistry. 4th. Harlow U.K: Pearson Education Ltd; 2012. [Google Scholar]
- 20.Bottenheim JW, Strausz OP. Gas-phase chemistry of clean air at 55.degree. N latitude. Environ Sci Technol. 1980;14(6):709–18. doi: 10.1021/es60166a010. [DOI] [PubMed] [Google Scholar]
- 21.Rayner-Canham G, Overton T. Descriptive Inorganic Chemistry. W.H. Freeman and Company; New York, NY: 2009. The Group 16 Elements The Chalcogens. [Google Scholar]
- 22.Zhang L, et al. Chemical and biological technologies for hydrogen sulfide emission control in sewer systems: A review. Water Research. 2008;42(1–2):1–12. doi: 10.1016/j.watres.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 23.A.f T.S.a D.R US Department of Health and Human Services, Division of Toxicology and Environmental Medicine/Applied Toxicology Branch, editor. ATSDR. Toxicological Profile for Hydrogen Sulfide and Carbonyl Sulfide. Atlanta, Georgia: 2016. [Google Scholar]
- 24.Sherma REE, Rhodes LAE, editors. Hydrogen sulfide analyzers. Analytical Instrumentation. Instrument Society of America; Research Triangle Park: 1996. pp. 343–358. [Google Scholar]
- 25.Bethea RM. Comparison of hydrogen sulfide analysis techniques. Journal of the Air Pollution Control Association. 1973;23(8):710–713. [Google Scholar]
- 26.Moore PJ, Spitler RW. Hydrogen sulfide measurement and detection. American School of Gas Measurement Technology Proceedings; 2003. pp. 118–123. 2003 roceedings. [Google Scholar]
- 27.Pandey SK, Kim K-H, Tang K-T. A review of sensor-based methods for monitoring hydrogen sulfide. Trends in Analytical Chemistry. 2012;32:87–99. [Google Scholar]
- 28.Axelrod HD, et al. Fluorescence determinatioof sub-parts per billion hydrogen sulfide in the atmosphere. Anal Chem. 1969;41(13):1856–8. doi: 10.1021/ac60282a003. [DOI] [PubMed] [Google Scholar]
- 29.Maebashi K, et al. Toxicological analysis of 17 autopsy cases of hydrogen sulfide poisoning resulting from the inhalation of intentionally generated hydrogen sulfide gas. Forensic Sci Int. 2011;207(1–3):91–5. doi: 10.1016/j.forsciint.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Vitvitsky V, Banerjee R. H2S analysis in biological samples using gas chromatography with sulfur chemiluminescence detection. Methods Enzymol. 2015;554:111–23. doi: 10.1016/bs.mie.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blunden J, Aneja VP, Westerman PW. Measurement and analysis of ammonia and hydrogen sulfide emissions from a mechanically ventilated swine confinement building in North Carolina. Atmospheric environment. 2008;42(14):3315–3331. [Google Scholar]
- 32.Drimal M, et al. Environmental exposure to hydrogen sulfide in central Slovakia (Ruzomberok area) in context of health risk assessment. Cent Eur J Public Health. 2010;18(4):224–9. doi: 10.21101/cejph.a3610. [DOI] [PubMed] [Google Scholar]
- 33.EPA, U.S. Method 15: Determination of hydrogen sulfide, carbonyl sulfide and carbon disulfide emissions from stationary sources. Air Emissions Measurement Center; Washington DC: 2017. [Google Scholar]
- 34.Cmelik J, et al. Contribution to vapor generation-inductively coupled plasma spectrometric techniques for determination of sulfide in water samples. Talanta. 2010;80(5):1777–81. doi: 10.1016/j.talanta.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 35.Colon M, et al. Development of novel and sensitive methods for the determination of sulfide in aqueous samples by hydrogen sulfide generation-inductively coupled plasma-atomic emission spectroscopy. Anal Chim Acta. 2008;609(2):160–8. doi: 10.1016/j.aca.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Ober J. In: Sulfur, in Industrial Minerals and Rocks. Kogel J, Barker J, editors. 2006. pp. 935–70. [Google Scholar]
- 37.Sivert SM, Kiene RP, Schulz-Vogt HN. The sulfur cycle. Oceanography. 2007;20(2):117–123. [Google Scholar]
- 38.Szabo C, et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br J Pharmacol. 2014;171(8):2099–122. doi: 10.1111/bph.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO. Hydrogen Sulfide: Human Health Effects. Geneva: 2003. [Google Scholar]
- 40.Malone Rubright S. Environmental and Occupational Health. University of Pittsburgh Graduate School of Public Health; Pittsburgh: 2016. Cyanide and Hydrogen Sulfide: A Review of Two Blood Gases, Their Environmental Sources, and Potential Risks. [Google Scholar]
- 41.Jin Z, Pei Y. Physiological Implications of Hydrogen Sulfide in Plants: Pleasant Exploration behind Its Unpleasant Odour. Oxid Med Cell Longev. 2015;2015:397502. doi: 10.1155/2015/397502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wickenhauser P, et al. Ecological significance of H2S emissions by plants a literature review. Landbauforforshung Volkenrode Special issue. 2005;283:157–161. [Google Scholar]
- 43.Skrtic L. Energy and Resources Group. Universiy of California, Berkeley; Berkeley, CA: 2006. Hydrogen Sulfide, Oil and Gas, and People’s Health; p. 77. [Google Scholar]
- 44.Shinohara H, et al. Degassing activity from Iwodake rhyolitic cone, Satsuma-Iwojima volcano, Japan: Formation of a new degassing vent, 1990–1999. Earth, Planets and Space. 2002;54(3):175–185. [Google Scholar]
- 45.Knoll AH, et al. Paleophysiology and end-Permian mass extinction. Earth and Planetary Science Letters. 2007;256:295–313. [Google Scholar]
- 46.Kump LR, Pavlov A, Arthur MA. Massive release of hydrogen sulfide to the surface ocean and atmosphere during intervals of oceanic anoxia. Geology. 2005;33:397–400. [Google Scholar]
- 47.Ward P. Impact from the Deep. Scientific American. 2006 doi: 10.1038/scientificamerican1006-64. [DOI] [PubMed] [Google Scholar]
- 48.EPA, U.S. 2012 Toxic Release Inventory National Analysis Overview. Environmental Protection Agency; Washington, DC: 2014. [Google Scholar]
- 49.Hessel PA, et al. Lung health in relation to hydrogen sulfide exposure in oil and gas workers in Alberta, Canada. Am J Ind Med. 1997;31(5):554–7. doi: 10.1002/(sici)1097-0274(199705)31:5<554::aid-ajim9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 50.EPA, U.S. 2013 Toxic Release Inventory National Analysis Overview. Environmental Protection Agency; Washington, DC: 2014. [Google Scholar]
- 51.EPA, U.S. Integrated Risk Information System: Hydrogen sulfide (CASRN 7783–06–4) U.S. Environmental Protection Agency; 2003. [Google Scholar]
- 52.Li H, et al. Agricultural and Biosystems Engineering Conference - Livestock Environment VIII. Iguassu Falls, Brazil: 2008. Effects of Dietary Modification on Laying Hens in High-Rise Houses: Part I– Emissions of Ammonia, Hydrogen Sulfide and Carbon Dioxide. [Google Scholar]
- 53.Yang D, Chen G, Zhang R. Estimated public health exposure to H2S emissions from a Sour gas well blowout in Kaixian County, China. Aerosol Air Qual Res. 2006;6(4):430–443. [Google Scholar]
- 54.Leo G. Sour gas from oil wells a deadly problem in southeast Saskatchewan. CBC News: CBC News; Apr 21, 2015. 2015. [Google Scholar]
- 55.O.o.G.S. Department of Environmental Quality (DEQ), editor. DEQ. Hydrogen Sulfide (H2S) - Q & A. Michigan.gov; Michigan: [Google Scholar]
- 56.King M, Moats M, Davenport WG. Sulfuric Acid manufacture: Analysis, Control and Optimization. Burlington, MA: Elsevier; 2013. [Google Scholar]
- 57.Morii D, et al. Japanese experience of hydrogen sulfide: the suicide craze in 2008. J Occup Med Toxicol. 2010;5(28) doi: 10.1186/1745-6673-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Truscott A. Suicide fad threatens neighbours, rescuers. CMAJ. 2008;179(4):312–13. doi: 10.1503/cmaj.080878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adkins J. Hydrogen Sulfide Suicide - Latest Technique Hazardous to First Responders and the Public. Regional Organized Crime Information Center (ROCIC); 2010. [Google Scholar]
- 60.Bott E, Dodd M. Suicide by hydrogen sulfide inhalation. Am J Forensic Med Pathol. 2013;34(1):23–5. doi: 10.1097/PAF.0b013e31827ab5ad. [DOI] [PubMed] [Google Scholar]
- 61.Reedy S, Schwartz M, BW M. Suicide Fads: Frequency and Characteristics of Hydrogen Sulfide Suicides in the United States. West J Emerg Med. 2011;12(3):300–304. [PMC free article] [PubMed] [Google Scholar]
- 62.U.D.o. Labor, editor. OSHA. Occupational Safety and Health Standards: Process safety management of highly hazardous chemicals, in 1910.119(a)(1)(i) Washington, DC: [Google Scholar]
- 63.OSHA. Hydrogen Sulfide. 2012 11-5-12 12-2-13]; Available from: https://www.osha.gov/dts/chemicalsampling/data/CH_246800.html.
- 64.A.G.F.s.E.R.P. Committee, editor. AIHA. Emergency Response Planning Guidelines and Workplace Environmental Exposure Level Guides Handbook. American Industrial Hygiene Association (AIHA); Falls Church, VA: 2013. [Google Scholar]
- 65.DOE. Protective action criteria (PAC) Oak Ridge, TN: US Department of Energy and Subcommittee on Consequence Assessment and Protective Actions (SCAPA) U.S. Department of Energy: Washington, DC: 2016. [Google Scholar]
- 66.C.o.A.E.G.L.C.o.T.B.o.E.S.a.T.D.o.E.a.L.S.N.R. Council, editor. NRC. Acute Exposure Guideline Levels for Selected Airborne Chemicals. National Academies Press; Washington, DC: 2010. [Google Scholar]
- 67.Deutsche Forschungsgemeinschaft (DFG), editor. DFG. MAK- und BAT-Werte-Liste 2013, in MAK- und BAT-Werte-Liste 2013. Wiley-VCH Verlag GmbH & Co. KGaA; 2013. pp. 1–300. [Google Scholar]
- 68.IARC. Agents classified by the IARC monographs. International Agency for Research on Cancer; Lyon, France: 2013. [Google Scholar]
- 69.EDUCATION AND INFORMATION DIVISION, editor. NIOSH. Pocket Guide to Chemical Hazards: Hydrogen Sulfide. National Institute for Occupational Safety and Health; Atlanta, GA: 2016. [Google Scholar]
- 70.WHO. Air Quality Guidelines for Europe. World Health Organization; Copenhagen, Denmark: 2000. [Google Scholar]
- 71.EPA, U.S. Modifications To The 112(b)1 Hazardous Air Pollutants. n.d. 11/22/2013 [cited 2014 2/20/2014] [Google Scholar]
- 72.EPA, U.S. US Emergency Planning and Community Right-to-Know Act. 1986 [Google Scholar]
- 73.F. Register, editor. EPA, U.S. Hydrogen Sulfide; Community Right-to-Know Toxic Chemical Release Reporting, in Federal Register. 2011. [Google Scholar]
- 74.BLS. Occupational Injuries/Illnesses and Fatal Injuries Profiles: Hydrogen sulfide as primary or secondary source in fatal workplace injuries 2004–2010. In: B.o.L.S.C.o.F.O. Injuries, editor. Census of Fatal Occupational Injuries. Bureau of Labor Statistics; Washington DC: 2016. [Google Scholar]
- 75.BLS. Occupational Injuries/Illnesses and Fatal Injuries Profiles: Hydrogen sulfide as primary or secondary source in fatal workplace injuries 2011–2014. In: B.o.L.S.C.o.F.O. Injuries, editor. Census of Fatal Occupational Injuries. Bureau of Labor Statistics; Washington DC: 2016. [Google Scholar]
- 76.BLS. Occupational Injuries/Illnesses and Fatal Injuries Profiles: Number of nonfatal occupational injuries and illnesses involving days away from work by selected worker and case characteristics and source of injury/illness, All U.S., private industry, 2004–2010. In: B.o.L.S.C.o.F.O. Injuries, editor. Census of Fatal Occupational Injuries. Bureau of Labor Statistics; Washington DC: 2016. [Google Scholar]
- 77.BLS. Occupational Injuries/Illnesses and Fatal Injuries Profiles: Number of nonfatal occupational injuries and illnesses involving days away from work by selected worker and case characteristics and source of injury/illness, All U.S., private industry, 2011–2014. In: B.o.L.S.C.o.F.O. Injuries, editor. Census of Fatal Occupational Injuries. Bureau of Labor Statistics; Washington DC: 2016. [Google Scholar]
- 78.Gregorakos L, et al. Hydrogen Sulfide Poisoning: Management and Complications. Angiology. 1995;46(12):1123–1131. doi: 10.1177/000331979504601208. [DOI] [PubMed] [Google Scholar]
- 79.Praxair. Hydrogen Sulfide Safety Data Sheet. 2015 [Google Scholar]
- 80.OSHA. Hydrogen Sulfide in Workplaces. Safety and Health Topics. [cited 2016; Available from: https://www.osha.gov/SLTC/hydrogensulfide/hydrogensulfide_found.html.
- 81.Mowry JB, et al. Annual Report of the American Association of Poison Control Centers ’ National Poison Data System (NPDS): 30th Annual Report. Clinical Toxicology. 2013;51:949–1229. doi: 10.3109/15563650.2013.863906. 2012. [DOI] [PubMed] [Google Scholar]
- 82.Mowry JB, et al. Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila) 2014;52(10):1032–283. doi: 10.3109/15563650.2014.987397. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khan AA, et al. Effects of hydrogen sulfide exposure on lung mitochondrial respiratory chain enzymes in rats. Toxicol Appl Pharmacol. 1990;103(3):482–90. doi: 10.1016/0041-008x(90)90321-k. [DOI] [PubMed] [Google Scholar]
- 84.Prior M, et al. Capsaicin pretreatment modifies hydrogen sulphide-induced pulmonary injury in rats. Toxicol Pathol. 1990;18(2):279–88. doi: 10.1177/019262339001800206. [DOI] [PubMed] [Google Scholar]
- 85.Prior MG, et al. Concentration-time interactions in hydrogen sulphide toxicity in rats. Can J Vet Res. 1988;52(3):375–9. [PMC free article] [PubMed] [Google Scholar]
- 86.Ammann HM. A New Look at Physiologic Respiratory Response to H2S Poisoning. U.S. Environmental Protection Agency, Environmental Criteria and Assessment Office (MD-52); Research Triangle Park, NC: 1987. p. 12. [Google Scholar]
- 87.Milby TH, Baselt RC. Hydrogen sulfide poisoning: clarification of some controversial issues. Am J Ind Med. 1999;35(2):192–5. doi: 10.1002/(sici)1097-0274(199902)35:2<192::aid-ajim11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 88.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. The Journal of Neuroscience. 1996;16(3):1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nicholls P, et al. Sulfide inhibition of and metabolism by cytochrome c oxidase. Biochem Soc Trans. 2013;41(5):1312–6. doi: 10.1042/BST20130070. [DOI] [PubMed] [Google Scholar]
- 90.Szabo C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal. 2012;17(1):68–80. doi: 10.1089/ars.2011.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308(5721):518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 92.Volpato GP, et al. Inhaled hydrogen sulfide: a rapidly reversible inhibitor of cardiac and metabolic function in the mouse. Anesthesiology. 2008;108(4):659–68. doi: 10.1097/ALN.0b013e318167af0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Asfar P, Calzia E, Radermacher P. Is pharmacological, H(2)S-induced ‘suspended animation’ feasible in the ICU? Crit Care. 2014;18(2):215. doi: 10.1186/cc13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.A.f T.S.a D.R. US Department of Health and Human Services Division of Toxicology and Environmental Medicine/Applied Toxicology Branch, editor. ATSDR. Toxicological Profile for Hydrogen Sulfide. Atlanta, Georgia: 2006. [Google Scholar]
- 95.ANSI. American National Standard Acceptable Concentrations of Hydrogen Sulfide. American National Standards Institute, American Industrial Hygiene Association; 1972. [Google Scholar]
- 96.OSHA. Hydrogen Sulfide: Health Hazards. n.d. [cited 2014; Available from: https://www.osha.gov/SLTC/hydrogensulfide/hazards.html.
- 97.Cronican AA, et al. Antagonism of Acute Sulfide Poisoning in Mice by Nitrite Anion without Methemoglobinemia. Chem Res Toxicol. 2015;28:1398–1408. doi: 10.1021/acs.chemrestox.5b00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haahtela T, et al. The South Karelia Air Pollution Study: acute health effects of malodorous sulfur air pollutants released by a pulp mill. Am J Public Health. 1992;82(4):603–605. doi: 10.2105/ajph.82.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hirsch A. Hydrogen sulfide exposure without loss of consciousness: chronic effects in four cases. Toxicol Ind Health. 2002;18(2):51–61. doi: 10.1191/0748233702th131oa. [DOI] [PubMed] [Google Scholar]
- 100.Cantox Environmental Inc. Health Effects Associated With Short-Term Exposure To Low Low Levels of Hydrogen Sulphide (H2S) - A Technical Review. Alberta Health and Wellness, Health Surveillance Population Health Division; Alberta, Canada: 2002. [Google Scholar]
- 101.Deng JF, Chang SC. Hydrogen sulfide poisonings in hot-spring reservoir cleaning: two case reports. Am J Ind Med. 1987;11(4):447–51. doi: 10.1002/ajim.4700110407. [DOI] [PubMed] [Google Scholar]
- 102.Jappinen P, et al. Exposure to hydrogen sulphide and respiratory function. Br J Ind Med. 1990;47(12):824–8. doi: 10.1136/oem.47.12.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thoman M. Sewer gas: Hydrogen sulfide intoxication. Clinical Toxicology. 1969;2:383–386. [Google Scholar]
- 104.Bates MN, et al. Air pollution and mortality in the Rotorua geothermal area. Aust N Z J Public Health. 1997;21(6):581–6. doi: 10.1111/j.1467-842x.1997.tb01759.x. [DOI] [PubMed] [Google Scholar]
- 105.Bates MN, Garrett N, Shoemack P. Investigation of health effects of hydrogen sulfide from a geothermal source. Arch Environ Health. 2002;57(5):405–11. doi: 10.1080/00039890209601428. [DOI] [PubMed] [Google Scholar]
- 106.Bates MN, et al. Lens Opacity and Hydrogen Sulfide in a New Zealand Geothermal Area. Optom Vis Sci. 2017;94(4):487–495. doi: 10.1097/OPX.0000000000001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bates MN, et al. Investigation of hydrogen sulfide exposure and lung function, asthma and chronic obstructive pulmonary disease in a geothermal area of New Zealand. PLoS One. 2015;10(3):e0122062. doi: 10.1371/journal.pone.0122062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bates MN, et al. Associations of ambient hydrogen sulfide exposure with self-reported asthma and asthma symptoms. Environ Res. 2013;122:81–87. doi: 10.1016/j.envres.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bates MN, et al. Associations of ambient hydrogen sulfide exposure with self-reported asthma and asthma symptoms. Environ Res. 2013;122:81–7. doi: 10.1016/j.envres.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pope K, et al. Ambient geothermal hydrogen sulfide exposure and peripheral neuropathy. Neurotoxicology. 2017;60:10–15. doi: 10.1016/j.neuro.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reed BR, et al. Chronic ambient hydrogen sulfide exposure and cognitive function. Neurotoxicol Teratol. 2014;42:68–76. doi: 10.1016/j.ntt.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Burnett WW, et al. Hydrogen sulfide poisoning: review of 5 years’ experience. Can Med Assoc J. 1977;117(11):1277–80. [PMC free article] [PubMed] [Google Scholar]
- 113.Snyder JW, et al. Occupational fatality and persistent neurological sequelae after mass exposure to hydrogen sulfide. Am J Emerg Med. 1995;13(2):199–203. doi: 10.1016/0735-6757(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 114.Adelson L, Sunshine I. Fatal hydrogen sulfide intoxication. Report of three cases occurring in a sewer. Arch Pathol. 1966;81(5):375–80. [PubMed] [Google Scholar]
- 115.Knight LD, Presnell SE. Death by sewer gas: case report of a double fatality and review of the literature. Am J Forensic Med Pathol. 2005;26(2):181–5. [PubMed] [Google Scholar]
- 116.Yalamanchili C, Smith MD. Acute hydrogen sulfide toxicity due to sewer gas exposure. Am J Emerg Med. 2008;26(4):518 e5–7. doi: 10.1016/j.ajem.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 117.Schneider JS, et al. Persistent cognitive and motor deficits following acute hydrogen sulphide poisoning. Occup Med (Lond) 1998;48(4):255–60. doi: 10.1093/occmed/48.4.255. [DOI] [PubMed] [Google Scholar]
- 118.Tvedt B, et al. Delayed neuropsychiatric sequelae after acute hydrogen sulfide poisoning: affection of motor function, memory, vision and hearing. Acta Neurol Scand. 1991;84(4):348–51. doi: 10.1111/j.1600-0404.1991.tb04967.x. [DOI] [PubMed] [Google Scholar]
- 119.Rumbeiha W, et al. Acute hydrogen sulfide-induced neuropathology and neurological sequelae: challenges for translational neuroprotective research. Ann N Y Acad Sci. 2016;1378(1):5–16. doi: 10.1111/nyas.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Adachi J, et al. Formation of sulfhemoglobin in the blood and skin caused by hydrogen sulfide poisoning and putrefaction of the cadaver. Nihon Hoigaku Zasshi. 1986;40(3):316–22. [PubMed] [Google Scholar]
- 121.Milroy C, Parai J. Hydrogen sulphide discoloration of the brain. Forensic Sci Med Pathol. 2011;7:225–226. doi: 10.1007/s12024-010-9210-9. [DOI] [PubMed] [Google Scholar]
- 122.Park SH, Zhang Y, Hwang JJ. Discolouration of the brain as the only remarkable autopsy finding in hydrogen sulphide poisoning. Forensic Sci Int. 2009;187(1–3):e19–21. doi: 10.1016/j.forsciint.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 123.Tatsuno Y, et al. Four cases of fatal poisoning by hydrogen sulfide. A study of greenish discoloration of the skin and formation of sulfhemoglobin. Nihon Hoigaku Zasshi. 1986;40(3):308–15. [PubMed] [Google Scholar]
- 124.Bondoc LL, et al. Structure of a stable form of sulfheme. Biochemistry. 1986;25(26):8458–66. doi: 10.1021/bi00374a021. [DOI] [PubMed] [Google Scholar]
- 125.Carrico RJ, Peisach J, Alben JO. The preparation and some physical properties of sulfhemoglobin. J Biol Chem. 1978;253(7):2386–91. [PubMed] [Google Scholar]
- 126.Park CM, et al. Sulfhemoglobin. Properties of partially sulfurated tetramers. J Biol Chem. 1986;261(19):8805–10. [PubMed] [Google Scholar]
- 127.ATSDR. Hydrogen Sulfide (H2S) Agency for Toxic Substances and Disease Registry, Division of Toxicology; Atlanta, GA: 2012. [Google Scholar]
- 128.ATSDR. Hydrogen Sulfide, in Medical Management Guidelines. Agency for Toxic Substances and Disease Registry; Atlanta, GA: 2014. [Google Scholar]
- 129.Li Q, Lancaster JR., Jr Chemical foundations of hydrogen sulfide biology. Nitric Oxide. 2013;35:21–34. doi: 10.1016/j.niox.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Olson KR. A practical look at the chemistry and biology of hydrogen sulfide. Antioxid Redox Signal. 2012;17(1):32–44. doi: 10.1089/ars.2011.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Olson KR, DeLeon ER, Liu F. Controversies and conundrums in hydrogen sulfide biology. Nitric Oxide. 2014;41:11–26. doi: 10.1016/j.niox.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 132.Kolluru G, Shen X, Kevil C. Detection of hydrogen sulfide in biological samples: current and future. Expert Review of Clinical Pharmacology. 2011;4(1):9–12. doi: 10.1586/ecp.10.132. [DOI] [PubMed] [Google Scholar]
- 133.Xie ZZ, Liu Y, Bian JS. Hydrogen Sulfide and Cellular Redox Homeostasis. Oxid Med Cell Longev. 2016;2016:6043038. doi: 10.1155/2016/6043038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Abou-Hamdan A, et al. Oxidation of H2S in mammalian cells and mitochondria. Methods Enzymol. 2015;554:201–28. doi: 10.1016/bs.mie.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 135.Bouillaud F, Blachier F. Mitochondria and sulfide: a very old story of poisoning, feeding, and signaling? Antioxid Redox Signal. 2011;15(2):379–91. doi: 10.1089/ars.2010.3678. [DOI] [PubMed] [Google Scholar]
- 136.Hildebrandt TM. Modulation of sulfide oxidation and toxicity in rat mitochondria by dehydroascorbic acid. Biochim Biophys Acta. 2011;1807(9):1206–13. doi: 10.1016/j.bbabio.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 137.Hildebrandt TM, Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008;275(13):3352–61. doi: 10.1111/j.1742-4658.2008.06482.x. [DOI] [PubMed] [Google Scholar]
- 138.Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285(29):21903–7. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lagoutte E, et al. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta. 2010;1797(8):1500–11. doi: 10.1016/j.bbabio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 140.Tangerman A. Measurement and biological significance of the volatile sulfur compounds hydrogen sulfide, methanethiol and dimethyl sulfide in various biological matrices. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(28):3366–77. doi: 10.1016/j.jchromb.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 141.Tangerman A, Winkel EG. The portable gas chromatograph OralChroma: a method of choice to detect oral and extra-oral halitosis. J Breath Res. 2008;2(1):017010. doi: 10.1088/1752-7155/2/1/017010. [DOI] [PubMed] [Google Scholar]
- 142.Tangerman A, Winkel EG. Intra- and extra-oral halitosis: finding of a new form of extra-oral blood-borne halitosis caused by dimethyl sulphide. J Clin Periodontol. 2007;34(9):748–55. doi: 10.1111/j.1600-051X.2007.01116.x. [DOI] [PubMed] [Google Scholar]
- 143.Tangerman A, Winkel EG. Extra-oral halitosis: an overview. J Breath Res. 2010;4(1):017003. doi: 10.1088/1752-7155/4/1/017003. [DOI] [PubMed] [Google Scholar]
- 144.Beauchamp RO, Jr, et al. A critical review of the literature on hydrogen sulfide toxicity. Crit Rev Toxicol. 1984;13(1):25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- 145.Haouzi P, et al. Are H2S-trapping compounds pertinent to the treatment of sulfide poisoning? Clin Toxicol (Phila) 2014;52(5):566. doi: 10.3109/15563650.2014.923906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haouzi P, et al. In Vivo Interactions Between Cobalt or Ferric Compounds and the Pools of Sulphide in the Blood During and After H2S Poisoning. Toxicological Sciences. 2014 doi: 10.1093/toxsci/kfu140. (On line) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sonobe T, et al. Immediate and Long-Term Outcome of Acute H2S Intoxication Induced Coma in Unanesthetized Rats: Effects of Methylene Blue. PLoS One. 2015;10(6):e0131340. doi: 10.1371/journal.pone.0131340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sonobe T, Haouzi P. H2S induced coma and cardiogenic shock in the rat: Effects of phenothiazinium chromophores. Clin Toxicol (Phila) 2015;53(6):525–39. doi: 10.3109/15563650.2015.1043440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr. 2008;40(5):533–9. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 150.Dorman D, et al. Cytochrome Oxidase Inhibition Induced by Acute Hydrogen Sulfide Inhalation: Correlation with Tissue Sulfide Concentrations in the Rat Brain, Liver, Lung, and Nasal Epithelium. Toxicol Sci. 2002;65:18–25. doi: 10.1093/toxsci/65.1.18. [DOI] [PubMed] [Google Scholar]
- 151.Guidotti T. Hydrogen sulfide: advances in understanding human toxicity. Int J Toxicol. 2010;29(6):569–81. doi: 10.1177/1091581810384882. [DOI] [PubMed] [Google Scholar]
- 152.Hill B, et al. Interactions of sulphide and other ligands with cytochrome c oxidase. An electron-paramagnetic-resonance study. Biochem J. 1984;224:591–600. doi: 10.1042/bj2240591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nicholls P, Kim JK. Sulphide as an inhibitor and electron donor for the cytochrome c oxidase system. Can J Biochem. 1982;60(6):613–23. doi: 10.1139/o82-076. [DOI] [PubMed] [Google Scholar]
- 154.Guidotti T. Hydrogen sulphide. Occup Med (Lond) 1996;46(5):361–71. doi: 10.1093/occmed/46.5.367. [DOI] [PubMed] [Google Scholar]
- 155.Haggard HW. The toxicology of hydrogen sulphide. Journal of Industrial Hygiene. 1925;7:113–121. [Google Scholar]
- 156.Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol. 1992;32:109–34. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- 157.Roth S, Goodwin V. Alberta Environment. Science and Standards Branch, Alberta Environment; Edmonton, Canada: 2003. Health Effects of Hydrogen Sulphide: Knowledge Gaps. [Google Scholar]
- 158.Almeida AF, Guidotti TL. Differential sensitivity of lung and brain to sulfide exposure: a peripheral mechanism for apnea. Toxicol Sci. 1999;50(2):287–93. doi: 10.1093/toxsci/50.2.287. [DOI] [PubMed] [Google Scholar]
- 159.Lopez A, et al. Peracute toxic effects of inhaled hydrogen sulfide and injected sodium hydrosulfide on the lungs of rats. Fundam Appl Toxicol. 1989;12(2):367–73. doi: 10.1016/0272-0590(89)90053-5. [DOI] [PubMed] [Google Scholar]
- 160.Roman HB, et al. The cysteine dioxgenase knockout mouse: altered cysteine metabolism in nonhepatic tissues leads to excess H2S/HS(-) production and evidence of pancreatic and lung toxicity. Antioxid Redox Signal. 2013;19(12):1321–36. doi: 10.1089/ars.2012.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang H, Bhatia M. Role of hydrogen sulfide in acute lung injury and acute respiratory distress syndrome. The Open Critical Care Medicine Journal. 2009;2:13–17. [Google Scholar]
- 162.Du Q, et al. In vivo study of the effects of exogenous hydrogen sulfide on lung mitochondria in acute lung injury in rats. BMC Anesthesiol. 2014;14:117. doi: 10.1186/1471-2253-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Haouzi P. Is exogenous hydrogen sulfide a relevant tool to address physiological questions on hydrogen sulfide? Respir Physiol Neurobiol. 2016 doi: 10.1016/j.resp.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Eghbal MA, Pennefather PS, O’Brien PJ. H2S cytotoxicity mechanism involves reactive oxygen species formation and mitochondrial depolarisation. Toxicology. 2004;203(1–3):69–76. doi: 10.1016/j.tox.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 165.Truong DH, et al. Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab Rev. 2006;38(4):733–44. doi: 10.1080/03602530600959607. [DOI] [PubMed] [Google Scholar]
- 166.Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353(26):2788–96. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 167.Murray JF. Pulmonary edema: pathophysiology and diagnosis. Int J Tuberc Lung Dis. 2011;15(2):155–60, i. [PubMed] [Google Scholar]
- 168.Bhagi S, Srivastava S, Singh SB. High-altitude pulmonary edema: review. J Occup Health. 2014;56(4):235–43. doi: 10.1539/joh.13-0256-ra. [DOI] [PubMed] [Google Scholar]
- 169.Sedy J, Kunes J, Zicha J. Pathogenetic Mechanisms of Neurogenic Pulmonary Edema. J Neurotrauma. 2015;32(15):1135–45. doi: 10.1089/neu.2014.3609. [DOI] [PubMed] [Google Scholar]
- 170.Lopez A, et al. Histologic and ultrastructural alterations in lungs of rats exposed to sub-lethal concentrations of hydrogen sulfide. Vet Pathol. 1988;25(5):376–84. doi: 10.1177/030098588802500507. [DOI] [PubMed] [Google Scholar]
- 171.Lopez A, et al. Biochemical and cytologic alterations in the respiratory tract of rats exposed for 4 hours to hydrogen sulfide. Fundam Appl Toxicol. 1987;9(4):753–62. doi: 10.1016/0272-0590(87)90182-5. [DOI] [PubMed] [Google Scholar]
- 172.Dunn WR, et al. Effects of hydrogen sulphide in smooth muscle. Pharmacol Ther. 2016;158:101–13. doi: 10.1016/j.pharmthera.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 173.Martelli A, et al. Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacol Res. 2013;70(1):27–34. doi: 10.1016/j.phrs.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 174.Munaron L, et al. Hydrogen sulfide as a regulator of calcium channels. Cell Calcium. 2013;53(2):77–84. doi: 10.1016/j.ceca.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 175.Altaany Z, et al. Hydrogen sulfide and endothelial dysfunction: relationship with nitric oxide. Curr Med Chem. 2014;21(32):3646–61. doi: 10.2174/0929867321666140706142930. [DOI] [PubMed] [Google Scholar]
- 176.Huang B, et al. Laminar shear flow increases hydrogen sulfide and activates a nitric oxide producing signaling cascade in endothelial cells. Biochem Biophys Res Commun. 2015;464(4):1254–9. doi: 10.1016/j.bbrc.2015.07.115. [DOI] [PubMed] [Google Scholar]
- 177.Moccia F, et al. Hydrogen sulfide regulates intracellular Ca2+ concentration in endothelial cells from excised rat aorta. Curr Pharm Biotechnol. 2011;12(9):1416–26. doi: 10.2174/138920111798281117. [DOI] [PubMed] [Google Scholar]
- 178.Jiang J, et al. Hydrogen Sulfide–Mechanisms of Toxicity and Development of an Antidote. Sci Rep. 2016;6:20831. doi: 10.1038/srep20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jiang L, et al. alpha-ENaC, a therapeutic target of dexamethasone on hydrogen sulfide induced acute pulmonary edema. Environ Toxicol Pharmacol. 2014;38(2):616–24. doi: 10.1016/j.etap.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 180.Jiang L, et al. Epithelial sodium channel is involved in H2S-induced acute pulmonary edema. Inhal Toxicol. 2015;27(12):613–20. doi: 10.3109/08958378.2015.1048909. [DOI] [PubMed] [Google Scholar]
- 181.Fronius M. Treatment of pulmonary edema by ENaC activators/stimulators. Curr Mol Pharmacol. 2013;6(1):13–27. doi: 10.2174/1874467211306010003. [DOI] [PubMed] [Google Scholar]
- 182.Truong DH, et al. Prevention of hydrogen sulfide (H2S)-induced mouse lethality and cytotoxicity by hydroxocobalamin (vitamin B12a) Toxicology. 2007;242(1–3):16–22. doi: 10.1016/j.tox.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 183.Brenner M, et al. The vitamin B12 analog cobinamide is an effective hydrogen sulfide antidote in a lethal rabbit model. Clin Toxicol (Phila) 2014;52(5):490–7. doi: 10.3109/15563650.2014.904045. [DOI] [PMC free article] [PubMed] [Google Scholar]


