Abstract
Introduction
Fluorine-18 fluorodexyglucose positron emission tomography with CT attenuation correction (18F-FDG PET/CT) is useful in the detection and enumeration of focal lesions and in semi-quantitative characterization of metabolic activity (glycolytic phenotype) by calculation of glucose uptake. Total lesion glycolysis (TLG) and metabolic tumor volume (MTV) have the potential to improve the value of this approach and enhance the prognostic value of disease burden measures. This study aims to determine whether TLG and MTV are associated with PFS and OS, and whether they improve risk assessments such as ISS stage and GEP70 risk.
Materials and Methods
192 patients underwent whole body PET/CT in the TT3A trial and were evaluated using 3 dimensional region of interest analysis with TLG, MTV, and standard measurement parameters derived for all focal lesions with peak SUV above the background red marrow signal.
Results
In multivariate analysis, baseline TLG >620g and MTV >210cm3 remained a significant factor of poor PFS and OS after adjusting for baseline myeloma variables. Combined with the GEP70 risk score, TLG >205g identifies a high risk-behaving subgroup with poor expected survival. In addition, TLG >205g accurately divides ISS Stage II patients into two subgroups with similar outcomes to ISS Stage I and ISS Stage III, respectively.
Conclusion
TLG and MTV have significant survival implications at baseline and offer a more precise quantitation of the glycolytic phenotype of active disease. These measures can be assessed more readily than before using FDA approved software and should be standardized and incorporated into clinical trials moving forward.
Keywords: 18F-FDG PET/CT, myeloma, prognosis, total lesion glycolysis (TLG), metabolic tumor volume (MTV)
Introduction
Focal lesions, consisting of collections of abnormal plasma cells, develop first in the bone marrow, and remain confined there until subsequently expanding beyond bone by direct extension or development of independent, extramedullary foci. Lessons can be detected using magnetic resonance imaging (MRI) and Fluroine-18 fluorodexyglucose positron emission tomography with CT attenuation correction (18F-FDG PET/CT), and are detectable by these advanced imaging techniques in advance of the appearance of lytic lesions of bone on radiography or CT. A complex relationship exists between myeloma cells and the stroma, osteoblasts, and osteoclasts in the focal lesions which favors proliferation and survival of the myeloma clone. Importantly, bone-signaling networks are disturbed and, as the lesions progress, osteolysis occurs leading to the development of the lytic lesions typical of myeloma. As well as contributing to the overall clinical symptoms of myeloma patients, focal lesions act as reservoirs of drug resistant cells favoring disease progression and relapse. Understanding the nature of focal lesions and their value as a predictive test of clinical outcome is an important area of research.
To date clinical reporting of focal lesions has almost exclusively taken account of their number and uptake intensity1. The prognostic value of such measurements have been documented in MGUS, smoldering myeloma and myeloma, where the number of lesions and their maximum standardized uptake values (SUVmax) have been shown to correlate with progression free (PFS) and overall survival (OS)2-6. In this context we have shown that, in newly diagnosed patients, the number of PET lesions (0, 1-3 and >3) correlates with clinical outcome, with patients with >3 lesions having a worse complete remission duration (CRD), PFS and OS1,2. Having more than 3 focal lesions has also been linked to GEP70 positive high risk disease as well as to higher proliferation3. The SUVmax of focal lesions is also important with values exceeding 3.9 correlating with impaired CRD, PFS and OS2, as well as with higher numbers of lesions and GEP70 high risk status3. We and others have also shown that the persistence of PET uptake after therapy is linked to impaired clinical outcome1,2,4 with the persistence of >3 lesions at day 7 post treatment and/or before stem cell transplantation being linked to inferior PFS and OS1,2.
The number and maximum intensity of lesions are makers of disease burden and glycolytic activity, respectively. Total lesion glycolysis (TLG) is theoretically superior to these measurements because it incorporates both parameters while taking into account the level of glucose accumulation within the total volume of all the regions of interest. Metabolic tumor volume (MTV) quantifies the total metabolic tumor burden. Commercially available algorithms assisting in measurement of both of these variables are now available which facilitate standardization of the methods.
There is increasing evidence for the prognostic value of quantitative parameters obtained from initial staging using 18FDG PET/CT in patients with many solid tumors, lymphoma, and myeloma7-11. To date the SUVmax has been the most widely studied parameter, with higher levels of glucose accumulation serving as a proxy for glycolytic phenotype correlating with tumor grade in solid tumors12. More recent studies include the volume-based metabolic assessments such as MTV and TLG7. The focus of this study was to examine the role of TLG and MTV in myeloma and to determine whether these variables have increased clinical utility compared to the previously used variables which were based on measuring the number of lesions and determination of SUVmax. The analysis considers the ability of TLG, in combination with previously defined risk scores such as the GEP70 risk score and International Staging System (ISS) stage, to identify patients with high risk disease features.
Materials and Methods
192 patients enrolled in Total Therapy 3A, the details of which have been previously reported, underwent baseline whole body 18F-FDG PET/CT13,14. 108 of the 303 total multiple myeloma patients who had undergone Total Therapy 3A treatment were excluded from the study because their baseline 18F-FDG PET/CT study could not be retrieved from the data archive. 3 patients were excluded because their 18F-FDG PET/CT studies were considered non-diagnostic. As it was random which 111 patients from TT3a protocol had corrupted PET data files or were non-diagnostic, we believe omitting these patients enters no bias into the analysis, Supplementary Table 1. As of March 17, 2015, the mean follow up was 8.46 years. Fifty one patients (27%) were over the age of 65 years, 65/192 (34%) had an albumin < 3.5 g/dL, 81/192 (42%) had a B2M >= 3.5 mg/L, and 60/192 (31%) had an elevated LDH, Table 1. The GEP risk assessment and molecular subgroup distribution were typical of a group of newly diagnosed patients with 27/176 (15%) patients being high risk by GEP70. The GEP70 risk score has previously been shown to be highly predictive of survival in myeloma with low risk (LR) and high risk (HR) identified cases having disparate median overall survival times of 24 and 120+ months, respectively15. The protocol was approved by the University of Arkansas Medical Sciences, Institutional Review Board, and all patients signed informed consent in keeping with institutional, federal and international guidelines. Response was assessed using the European Bone Marrow Transplant (EBMT) criteria.
Table 1. Univariate Analysis of Baseline TLG Scores on Clinical Outcomes (OS and PFS).
| Overall Survival | Progression Free Survival | ||||
|---|---|---|---|---|---|
| Variable | n/N (%) | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age >= 65 years | 51/192 (27%) | 1.47 (0.91, 2.37) | 0.112 | 1.26 (0.82, 1.95) | 0.293 |
| Albumin < 3.5 g/dL | 55/192 (29%) | 1.16 (0.71, 1.87) | 0.557 | 1.19 (0.78, 1.83) | 0.425 |
| B2M >= 3.5 mg/L | 83/192 (43%) | 2.06 (1.31, 3.24) | 0.0013 | 2.13 (1.43, 3.16) | 0.0001 |
| B2M > 5.5 mg/L | 34/192 (18%) | 2.94 (1.80, 4.80) | <.0001 | 2.76 (1.77, 4.32) | <.0001 |
| Creatinine >= 2.0 mg/dL | 11/192 (6%) | 3.49 (1.73, 7.04) | 0.0002 | 3.49 (1.80, 6.79) | <.0001 |
| CRP >= 8 mg/L | 64/192 (33%) | 2.37 (1.51, 3.73) | 0.0001 | 1.83 (1.22, 2.73) | 0.0030 |
| LDH >= 190 U/L | 57/192 (30%) | 2.54 (1.62, 3.98) | 0.0003 | 1.88 (1.25, 2.83) | 0.0022 |
| Platelet Count < 150 × 109/L | 26/192 (14%) | 1.26 (0.80, 2.33) | 0.467 | 1.24 (0.72, 2.15) | 0.443 |
| GEP70 High Risk | 27/176 (15%) | 3.19 (1.87, 5.45) | <.0001 | 2.75 (1.61, 4.42) | <.0001 |
| GEP Proliferation Index >= 10 | 22/176 (12%) | 3.37 (1.91, 5.96) | <.0001 | 2.76 (1.62, 4.70) | 0.0002 |
| GEP Centrosome Index >= 3 | 57/176 (33%) | 2.47 (1.54, 3.97) | 0.0002 | 1.95 (1.28, 2.97) | 0.0019 |
| GEP CD-1 subgroup | 12/176 (7%) | 0.35 (0.085, 1.42) | 0.142 | 0.25 (0.06, 0.99) | 0.0487 |
| GEP CD-2 subgroup | 22/176 (12%) | 1.40 (0.33, 1.56) | 0.399 | 1.01 (0.54, 1.34) | 0.998 |
| GEP HY subgroup | 50/176 (28%) | 0.76 (0.44, 1.32) | 0.364 | 0.93 (0.59, 1.47) | 0.754 |
| GEP LB subgroup | 29/176 (16%) | 1.15 (0.64, 2.07) | 0.631 | 0.99 (0.58, 1.71) | 0.992 |
| GEP PR subgroup | 25/176 (14%) | 2.53 (1.45, 4.44) | 0.0007 | 2.06 (1.21, 3.49) | 0.0065 |
| GEP MF subgroup | 15/176 (9%) | 1.73 (0.83, 3.62) | 0.138 | 1.49 (0.74, 2.95) | 0.2502 |
| GEP MS subgroup | 23/176 (13%) | 0.56 (0.24, 1.30) | 0.175 | 0.66 (0.33, 1.33) | 0.254 |
| Baseline PET FL >0 | 130/192 (68%) | 1.39 (0.85, 2.28) | 0.1884 | 1.32 (0.86, 2.04) | 0.205 |
| Baseline PET FL >3 | 70/192 (36%) | 1.83 (1.17, 2.86) | 0.0076 | 1.62 (1.09, 2.41) | 0.0169 |
| Baseline TLG > 205 | 34/192 (18%) | 2.99 (1.83, 4.88) | <.0001 | 2.99 (1.91, 4.67) | <.0001 |
| Baseline TLG > 620 | 14/192 (7%) | 6.90 (3.67, 12.97) | <.0001 | 9.06 (4.78, 17.18) | <.0001 |
| Baseline MTV > 210 | 14/192 (7%) | 6.20 (3.33, 11.54) | <.0001 | 5.79 (3.13, 10.66) | <.0001 |
| SUVmax > 3.9 | 69/192 (36%) | 1.59 (1.01, 2.5) | 0.0422 | 1.78 (1.20, 2.66) | 0.004 |
HR – Hazard Ratio, 95% CI- 95% Confidence Interval, p-value from Wald Test in Cox Regression
Baseline PET/CT scans were performed following 6-8 hours of fasting and after the intravenous administration of 10-15mCi (370-555Mbq) of fluorine 18 fluorodeoxyglucose (FDG). After 50-70 minutes of uptake, images were acquired on either a CTI-Reveal or a Biograph 6 PET/CT system (Siemens Medical Systems), both with full ring LSO crystal configurations. PET images were generated by 3D iterative reconstruction on a 168×168 matrix, with a zoom of 1.0, FWHM filter of either 5.0 or 6.0 mm, and 2 iterations with 8 subsets. CT data were used for localization and attenuation correction. 18F-FDG PET/CT images underwent a 3-dimensional region of interest analysis of the axial and appendicular skeleton using the commercially available, FDA-approved “Mirada Medical PET/CT XD Oncology Review” software (Mirada Medical, Oxford, U.K.).
The background red marrow of each patient was defined by using a 1cm3 diameter region of interest in the most inferior vertebral body which did not demonstrate focally increased FDG uptake or vertebroplasty material. Focal lesions for each patient were defined as focal areas, measuring at least 1cm in diameter, not otherwise demonstrated to be artefacts by comparison with co-registered CT, recognizable as discrete foci of increased 18F-FDG uptake on maximum intensity projection images (MIPs), and exhibiting a peak SUV (SUVpeak) greater than the peak SUV for the patient's background red marrow Figure 1. The volume of each lesion and its 3 dimensional margins were determined by incorporating all contiguous pixels with activity greater than 0.1 g/ml above that of the background marrow. Because of the considerable statistical variability inherent in the acquisition, reconstruction, and display of accumulations of radiopharmaceuticals in the clinical imaging setting, SUV's obtained from larger regions of interest (ROI) are more reproducible than single pixel determinations such as SUV max. For this reason, we have chosen to quantify activity by calculating the peak SUV (SUV peak) defined as the average SUVS, corrected for lean body mass, of the pixels in a sphere 1.2 cm in diameter (1 cc) centered to include the most intense pixel16. The total MTV for disease in each patient was defined as the sum of MTVs of all the individual focal lesions identified in the analysis. The TLG of each focal lesion was calculated by multiplying the MTV of that lesion with its corresponding mean SUV. The global TLG of each patient was defined as the sum of the TLGs for all the focal lesions in the analysis.
Figure 1.
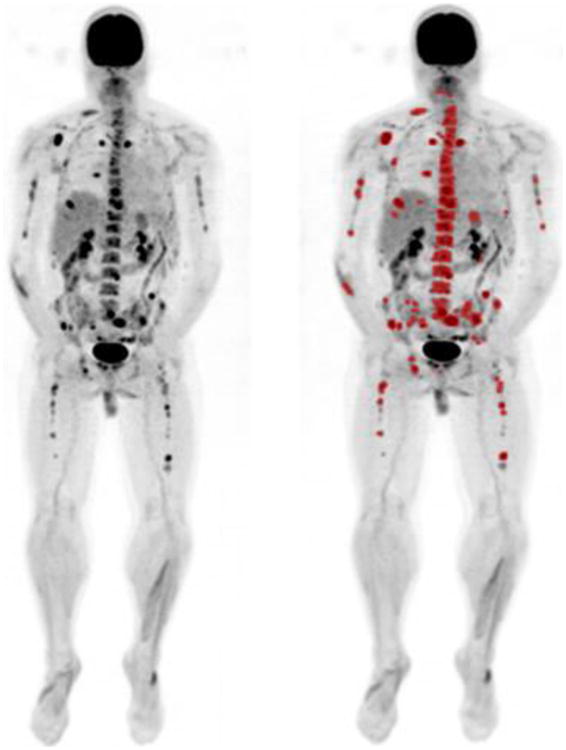
Maximum intensity projection (MIP) images from a patient with multiple focal lesions. The image on the right is following lesion segmentation for quantitation.
Statistical Analysis was performed using R 3.2.217 and SAS (SAS Institute, Cary NC) software. Univariate and multivariate analyses of clinical and imaging variables were performed using Cox proportional hazards regression. Due to high correlation, TLG and MTV scores were considered in separate analyses. A probability value of <0.05 was considered statistically significant. Survival analysis was performed using the Kaplan-Meier method and log-rank tests. Clinical endpoints included overall survival, progression free survival, and complete response duration. CRD is measured as the time from complete response onset to disease progression or death from any cause. In order to determine the cut points on the Kaplan-Meier curves for TLG and MTV, the running log-rank statistic produced by each hypothetical cut point in the data set was graphed against TLG score, and the TLG score that coincided with the highest log-rank statistic was chosen as the cut point. The cut-points for both TLG and MTV were based on progression free survival end point. 1000 random permutations of both TLG and MTV were performed and optimal cut-points determined that maximized the log-rank statistic, chosen with 80% of possible values of the covariate. Permuted log rank statistic values were determined for the 0.01 and 0.05 significance levels. The optimal binary cut points found for the true covariate values and its log-rank test statistic exceeded that of randomly permuted values at both the 0.01 and 0.05 level.
Results
Baseline characteristics show that 62/192 patients (32%) had no detectable lesions by PET. 1-3 lesions were present in 60/192 (31%) of patients and over 3 lesions in 70/192 (36%) of patients, Table 1. A baseline TLG >205g was seen in 18% (34/192) of patients, with a TLG >620g being seen in 7% (14/192). A baseline MTV >210cm3 was seen in 7% (14/192). The distribution of patients with high TLG scores between molecular subgroups was not even with patients in the PR, MF and HY subgroups having higher scores (p-value = 0.0135), Supplementary Figure 1A.
The 7 year PFS and OS for patients with a baseline TLG of less than 205g was 61.4% and 74.1% compared to patients with a baseline TLG between 205g and 620g of 40.0% and 55.0%, and to patients with a baseline TLG of greater than 620g of 0%, as no patients were progression free for 7 years, and 7.1% (p<0.0001) for OS. Figure 2. As expected survival curves for PET focal lesions also show differences in PFS and OS based on focal lesion count, Supplementary Figure 1B. A similar pattern was seen for CRD, Supplementary Figure 1C.
Figure 2.
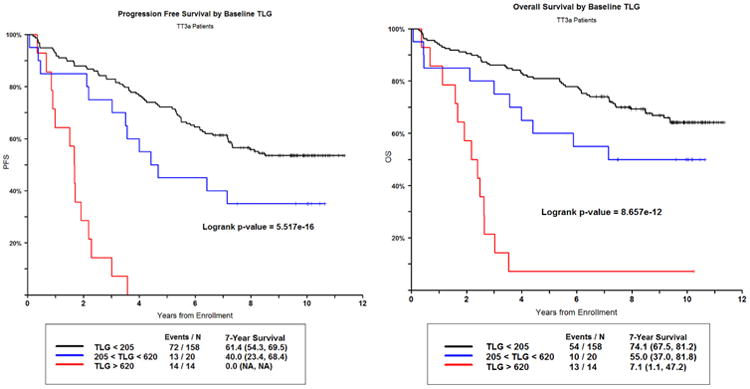
PFS (left) and OS (right) for Baseline TLG.
The MTV had prognostic importance with 7 year PFS and OS for patients with a baseline MTV of less than 55cm3 of 63.3% and 75.5% compared to patients with a baseline MTV between 55cm3 and 210cm3 of 35.5% and 54.8%, and to patients with a baseline MTV of greater than 210cm3 of 7.1% and 7.1% (p<0.0001). The Kaplan-Meier survival curves are very similar to that of the TLG Supplementary Figure 1D. Pearson Correlation Coefficient (r) between MTV and TLG was 0.94232 (p-value <.0001).
In univariate analysis baseline PET/CT with >3 focal lesions (HR 1.92, 95% CI 1.22-3.04, p=0.004), TLG >205g (HR 2.99, 95% CI 1.83-4.88, p<0.0001), baseline TLG > 620g (HR 6.90, 95% CI 3.67-12.97, p<0.0001), and MTV >210cm3 (HR 6.20, 95% CI 3.33-11.54, p<0.0001) were statistically significant associated with OS and PFS, Table 1.
In multivariate analysis, PFS and OS were dominantly affected by baseline TLG >620g, together with high B2M, LDH, creatinine, and GEP based centrosome index, Table 2. Importantly baseline TLG >620g and baseline MTV >210cm3 had worse PFS and OS than baseline PET with >3 focal lesions in both univariate and multivariate models. Considering OS outcome, TLG >620g and baseline MTV >210cm3 were the dominant adverse feature imparting a 4.97 and 5.49-fold higher risk of death, respectively. Similarly for PFS outcome, the most prominent adverse feature with an approximately 5.5-fold higher risk of relapse or death was observed for both TLG >620g and MTV >210cm3.
Table 2. Multivariate Analysis of Baseline TLG and MTV Scores on Clinical Outcomes (OS and PFS).
| Variable | Including TLG Scores | Including MTV Scores | ||||
|---|---|---|---|---|---|---|
| n/N (%) | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| OS | Baseline TLG > 620 | 12/176 (7%) | 4.97 (2.42, 10.20) | <.0001 | ||
| Creatinine >= 2.0 mg/dL | 11/176 (6%) | 2.30 (1.05, 5.03) | 0.0367 | |||
| LDH >= 190 U/L | 52/176 (30%) | 2.12 (1.31, 3.43) | 0.0023 | 2.22 (1.37, 3.60) | 0.0013 | |
| B2M >5.5 mg/L | 33/176 (19%) | 1.83 (1.03, 3.25) | 0.0397 | 2.914 (1.28, 3.76) | 0.0042 | |
| GEP Centrosome Index >= 3 | 57/176 (33%) | 1.99 (1.22, 3.24) | 0.0058 | |||
| Baseline MTV > 210 | 12/176 (7%) | 5.49 (2.64, 11.40) | <.0001 | |||
| GEP Proliferation Index >= 10 | 22/176 (13%) | 2.20 (1.21, 4.00) | 0.0095 | |||
| PFS | B2M > 5.5 mg/L | 33/176 (19%) | 1.76 (1.05, 2.95) | 0.0329 | 1.75 (1.05, 2.94) | 0.0334 |
| Creatinine >= 2.0 mg/dL | 11/176 (6%) | 2.47 (1.18, 5.18) | 0.0161 | 2.47 (1.18, 5.17) | 0.016 | |
| LDH >= 190 U/L | 57/176 (33%) | 1.64 (1.04, 2.56) | 0.0319 | 1.63 (1.04, 2.56) | 0.0322 | |
| GEP Centrosome Index >= 3 | 22/176 (13%) | 1.62 (1.04, 2.52) | 0.0348 | 1.61 (1.03, 2.52) | 0.0353 | |
| Baseline TLG > 620 | 12/176 (7%) | 6.33 (3.07, 13.04) | <.0001 | |||
| Baseline MTV > 210 | 12/176 (7%) | 6.38 (3.09, 13.19) | <.0001 | |||
HR- Hazard ratio, 95% CI – 95% Confidence Interval, p-value from Wald Chi-square test in Cox Regression
Usmani et al1 have previously described the prognostic value of FL number and SUVmax in the TT3 population. Using the cut-point Usmani reported as highly associated with survival outcomes, SUVmax >3.9, we found that SUVmax remained significant in the univariate analysis (HR 1.59, 95% CI 1.01 – 2.5, p=0.0422) but did not remain a significant covariate in the multivariate analysis as TLG and MTV did. The Kaplan Meier survival curves comparing SUVmax and TLG >205g show that TLG is more highly associated with PFS and OS than SUVmax, Supplementary Figure 1E.
Finally, in our analysis, we explored the role of TLG, GEP70, and ISS stage in identifying patients with high risk disease. When incorporating differences in TLG scores, we find a subset of GEP70 low risk (LR) disease and high TLG (TLG >205g) patients with similar outcome to GEP70 high risk (HR) patients (Logrank p-value = 0.7013 for PFS and 0.5818 for OS) Figure 3A. These LR and TLG-high cases make up 13% of all LR, and when combined with the GEP70 HR now form a larger HR-behaving cohort comprising 27% of the total patients with a 3 year PFS and OS of 60%. Patients with both LR and low TLG have 7 year PFS and OS rates of 63.6% and 78.3%, compared to LR and high TLG of 30.0% and 40.0% (p<.0001). GEP70 risk and TLG are independent prognostic factors (Chi square = 9.82, p-value = 0.001). The additional information provided by TLG scores that relate to outcome also divides ISS stage II cases into low and high-risk behaving groups. These subgroups have similar outcomes to ISS stage I and ISS stage III, respectively Figure 3B. Patients from this cohort, with baseline ISS Stage II, have 7 year PFS and OS of 58.8% and 69.1%. When TLG is included to separate poor performing patients, ISS stage II with low TLG have 7 year PFS and OS of 68.5% and 77.8% compared to ISS stage II patients with high TLG of 21.4% and 35.7%.
Figure 3.
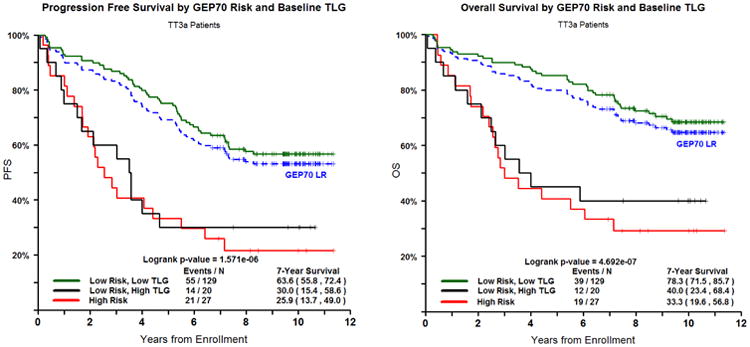
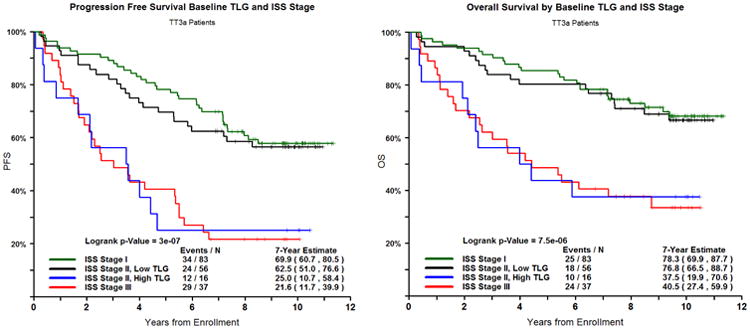
A and B. PFS (left) and OS (right) for GEP70-Risk and TLG and ISS Stage and TLG combined.
Discussion
This is among the largest series to evaluate volumetric PET measurements in oncology, and is the largest to date evaluating volumetric assessment of PET in multiple myeloma. In this work we show that baseline TLG >620g and baseline MTV >210 cm3 are significantly associated with poor PFS and OS in myeloma patients. While TLG, MTV, and the number of focal osseous lesions were all found to be statistically significant in evaluating tumor burden, TLG and MTV were found to be more highly associated with survival than the number of focal osseous lesions. In a smaller study of 47 patients, Fonti et al explored the role of TLG and MTV and prognosis in a mixed group of patients who received various therapies18. They noted that a MTV value of 77.6mL and a TLG value of 201.4g predicted patients with a good overall survival. Our study extends these findings in a clinical trial setting, comparing these functional parameters to the traditional metrics of lesion number and SUVmax, and demonstrates their clinical utility in predicting both PFS and OS.
As well as increased predictive/prognostic power, measurement of TLG and MTV has a further clinical advantage over the manual determination of the SUVmax and number of lesions. Recent improvements in commercially available software now render the process of lesion detection and delineation more practicable than with the labor-intensive techniques required in the past. These advances result in a higher degree of reproducibility and, therefore, a greater clinical accuracy and utility. Although the software for calculating TLG and MTV are FDA approved, international standards for image acquisition and lesion delineation should be developed to facilitate meaningful data comparisons in clinical research and trials.
TLG as a means of PET evaluation of neoplasms was first described by Larson et al in 19997. More than 140 studies of the value of FDG/PET TLG assessment in the evaluation of solid tumors have been published since this initial publication describing its utility in monitoring disease response to chemotherapy. In these studies a number of different methods and a wide range of threshold levels have been proposed to calculate the volume-based PET parameters. Some studies suggest that TLG may be superior to MTV, although this remains controversial. Our data would be in keeping with its superiority, as comparing our results to those of the smaller study of Fonti et al, the TLG values are similar but the MTV values are different (TLG 205g/620g vs 201.4g, and MTV 55/210mL vs 77.6mL)18.
The ability to detect focal lesions in plasma cell dyscrasias is becoming increasingly more important. As well as being able to predict prognosis in myeloma, recent studies also demonstrate that patients with MGUS and smoldering myeloma who have focal lesions on MRI have a shorter time to progression to myeloma5,6. These studies indicate that smoldering myeloma patients with more than one lesion on MRI should be considered as having a myeloma defining event and be offered therapy19.
In addition from the clinical and biological perspective, eradicating these metabolically active lesions represents a significant therapeutic challenge. A number of groups have now shown that the continuing presence of PET positive lesions after therapy either at day 7 of therapy1, before2 or after autologous transplantation4 is a poor prognostic feature. The quantitative assessment of therapeutic response using the TLG method therefore represents an opportunity to standardize and improve the early assessment of therapeutic response.
We have demonstrated previously that the expression of a series of genes (eg DKK1 and LRP8) as well as genes associated with high risk disease, are linked to PET, MRI and X-Ray changes3. In this study we extend these findings and demonstrate that patients within the PR, MF and HY subgroups have a higher TLG score suggesting they constitute a group of hyper-metabolic myelomas characterized by a more aggressive glycolytic phenotype with adverse clinical parameters and poor clinical outcome. Based on these observations we hypothesize that focal lesions act as a reservoir of cells which contribute to disease relapse. As the cells reside in a distinct but separate microenvironment, they are exposed to different selective pressures to that of the wider bone marrow resulting in the development of intraclonal heterogeneity.
In addition we identify a subset of patients with high TLG scores, classified as GEP70 LR, with adverse outcomes similar to HR patients. The GEP70 was of particular interest to us since it is a published, highly discriminatory risk assessment tool. GEP70 defined HR patients currently make up approximately 15% of the myeloma patient population, have extremely poor expected outcome, and are being specifically targeted in clinical trials. The TLG score has the potential to supplement and expand the GEP70's definition of a high risk patient. The GEP70 combined with the TLG score was able to identify an additional group of patients with high TLG scores, who are classified as GEP70 LR, but have equally poor outcomes as the GEP70 HR group. This group would not be identified by our current genetic analyses alone. Only by combining the two independent prognostic factors of imaging and genetics do we see added benefit. This could be immensely useful in enhancing the GEP70 at defining patients who have poor outcome and are in need of more aggressive treatment. Furthermore we demonstrated the ability of TLG to neatly discriminate ISS stage II patients into two groups who clinically perform more like patients in ISS stage I and ISS stage III based on low and high TLG, respectively.
In conclusion, while the number of focal lesions, SUVmax, TLG, MTV, and are clinically useful in evaluating tumor burden and glycolytic phenotype in multiple myeloma, TLG and MTV are highly associated with OS and PFS compared to the assessment of the number and SUV of focal lesions. As these volumetric measurements become more readily utilized using FDA approved software, these findings strengthen the argument for validating such measures in clinical trials as a basis for modifying therapy in the subset of patients with high TLG at baseline.
Supplementary Material
Statement of Translational Relevance.
Total lesion glycolysis (TLG) and metabolic tumor volume (MTV) are volumetric parameters applicable to 18F-FDG PET/CT scans that more accurately than conventional measurements reflect the glycolytic phenotype and overall tumor burden of focal lesions in multiple myeloma. TLG and MTV are highly associated with progression free and overall survival in addition to enhancing traditional disease burden scores such as the GEP70 risk score and ISS stage. The addition of a high TLG component to GEP70 risk identifies patients classified as low risk, but with an outcome similar to a high risk patient. Also, the additional information of TLG to the international staging system (ISS) divides ISS stage II cases into low and high-risk behaving subgroups with similar outcomes to ISS stage I and ISS stage III, respectively. Therefore, the assessment of TLG and MTV can significantly improve the prognostic value of GEP and ISS in myeloma.
Acknowledgments
We thank the patients and staff of the Myeloma Institute. This work was supported in part by PO1 CA 55819 from the National Cancer Institute.
Footnotes
Conflict of interest and disclosure: BB is a co-inventor on patents and patent applications related to use of gene expression profiling in cancer medicine that have been licensed to Myeloma Health, LLC, but has no financial interests in this company. The remaining authors declare no conflict of interest.
Supplementary information is available at CCR's website.
References
- 1.Usmani SZ, Mitchell A, Waheed S, Crowley J, Hoering A, Petty N, et al. Prognostic implications of serial 18-fluoro-deoxyglucose emission tomography in multiple myeloma treated with total therapy 3. Blood. 2013 Mar 7;121(10):1819–23. doi: 10.1182/blood-2012-08-451690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartel TB, Haessler J, Brown TL, Shaughnessy JD, Jr, van Rhee F, Anaissie E, et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009 Sep 3;114(10):2068–76. doi: 10.1182/blood-2009-03-213280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waheed S, Mitchell A, Usmani S, Epstein J, Yaccoby S, Nair B, et al. Standard and novel imaging methods for multiple myeloma: correlates with prognostic laboratory variables including gene expression profiling data. Haematologica. 2013 Jan;98(1):71–8. doi: 10.3324/haematol.2012.066555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zamagni E, Patriarca F, Nanni C, Zannetti B, Englaro E, Pezzi A, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011 Dec 1;118(23):5989–95. doi: 10.1182/blood-2011-06-361386. [DOI] [PubMed] [Google Scholar]
- 5.Hillengass J, Fechtner K, Weber MA, Bäuerle T, Ayyaz S, Heiss C, et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 2010 Mar 20;28(9):1606–10. doi: 10.1200/JCO.2009.25.5356. [DOI] [PubMed] [Google Scholar]
- 6.Hillengass J, Weber MA, Kilk K, Listl K, Wagner-Gund B, Hillengass M, et al. Prognostic significance of whole-body MRI in patients with monoclonal gammopathy of undetermined significance. Leukemia. 2014 Jan;28(1):174–8. doi: 10.1038/leu.2013.244. [DOI] [PubMed] [Google Scholar]
- 7.Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor Treatment Response Based on Visual and Quantitative Changes in Global Tumor Glycolysis Using PET-FDG Imaging. The Visual Response Score and the Change in Total Lesion Glycolysis. Clin Positron Imaging. 1999 May;2(3):159–171. doi: 10.1016/s1095-0397(99)00016-3. [DOI] [PubMed] [Google Scholar]
- 8.Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014 Sep 20;32(27):3048–58. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymphoma. 2009 Aug;50(8):1257–60. doi: 10.1080/10428190903040048. [DOI] [PubMed] [Google Scholar]
- 10.Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Imaging Subcommittee of International Harmonization Project in Lymphoma.Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007 Feb 10;25(5):571–8. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 11.Ceriani L, Martelli M, Zinzani PL, Ferreri AJ, Botto B, Stelitano C, et al. Utility of baseline 18FDG PET/CT functional parameters in defining prognosis of primary mediastinal (thymic) large B-cell lymphoma. Blood. 2015 Jun 18; doi: 10.1182/blood-2014-12-616474. in press. [DOI] [PubMed] [Google Scholar]
- 12.Gerlee P, Anderson ARA. A hybrid automation model of clonal evolution in cancer: The emergence of the glycolytic phenotype. Journal of Theoretical Biology. 2008 Feb 21;250(4):705–722. doi: 10.1016/j.jtbi.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Rhee F, Szymonifka J, Anaissie E, Nair B, Waheed S, Alsayed Y, et al. Total Therapy 3 for multiple myeloma: prognostic implications of cumulative dosing and premature discontinuation of VTD maintenance components, bortezomib, thalidomide, and dexamethasone, relevant to all phases of therapy. Blood. 2010 Aug 26;116(8):1220–7. doi: 10.1182/blood-2010-01-264333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair B, van Rhee F, Shaughnessy JD, Jr, Anaissie E, Szymonifka J, Hoering A, et al. Superior results of Total Therapy 3 (2003-33) in gene expression profiling-defined low-risk multiple myeloma confirmed in subsequent trial 2006-66 with VRD maintenance. Blood. 2010 May 27;115(21):4168–73. doi: 10.1182/blood-2009-11-255620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaughnessy J, Qu P, Tian E, Nair B, Waheed S, Alsayed Y, et al. Outcome with total therapy 3 (TT3) compared to total therapy 2(TT2): Role of GEP70-defined high-risk disease with trisomy of 1q21 and activation of the proteasome gene PSMD4. J Clin Oncol. 2010 May 20;28(15):8027. [Google Scholar]
- 16.Wahl R, et al. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. The Journal of Nuclear Medicine. 2009 Mar;50(5 Suppl):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna Austria: 2015. https://www.R-project.org/ [Google Scholar]
- 18.Fonti R, Larobina M, Del Vecchio S, De Luca S, Fabbricini R, Catalano L, et al. Metabolic tumor volume assessed by 18F-FDG PET/CT for the prediction of outcome in patients with multiple myeloma. J Nucl Med. 2012 Dec;53(12):1829–35. doi: 10.2967/jnumed.112.106500. [DOI] [PubMed] [Google Scholar]
- 19.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014 Nov;15(12):e538–48. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


