Abstract
Antisense therapeutics are a biotechnological form of antibiotic therapy using chemical analogues of short single-stranded nucleic acid sequences modified to form stable oligomers. These molecules are termed antisense oligonucleotides (ASOs) because their sequence is complementary, via Watson-Crick specific base pairing, to their target messenger RNA (mRNA). ASOs modify gene expression in this sequence-dependent manner by binding to its complementary mRNA and inhibiting its translation into protein through steric blockage and/or through RNase degradation of the ASO/RNA duplex. The widespread use of conventional antibiotics has led to the increasing emergence of multiple drug-resistant pathogenic bacteria. There is an urgent need to develop alternative therapeutic strategies to reduce the morbidity and mortality associated with bacterial infections, and until recently, the use of ASOs as therapeutic agents has been essentially limited to eukaryotic cells, with ASOs as antibacterials having been largely unexplored primarily due to the poor uptake efficiency of antisense molecules by bacteria. There are conceptual advantages to bacterial antisense antibiotic therapies, including a sequence-dependent approach that allows for a rational design to multiple specific molecular targets. This review summarizes the current knowledge of antisense bacterial biotechnology and highlights the recent progress and the current obstacles in their development for therapeutic applications.
Keywords: antisense, bacteria, biotechnology, therapeutic
Introduction
Antisense biotechnology involves the steric hindrance of ribosome progression and/or transcript cleavage of a target duplex of mRNA/ASO [Fig. 1]. Transcript cleavage is mediated by activation of RNase H that degrades the RNA strand of an RNA-DNA duplex. The field of antisense therapeutics commenced with the finding that a tridecamer oligodeoxynucleotide complementary to 13 nucleotides of the Rous sarcoma virus 35S RNA inhibited viral mRNA translation and subsequent virus production (Zamecnik and Stephenson 1978). Unlike traditional antibiotics that are limited to targeting a single cellular process, ASOs (antisense antibiotics) can target any gene through sequence complementation, thus significantly enlarging the available selection of potential therapeutic targets. This theoretically allows not only for killing of the target bacteria, but also modification of its virulence functions, including mitigating toxin production and interrupting the sporulation pathway, while minimizing gut ecological disturbances. Further, ASOs can be utilized to suppress bacterial mechanisms of antibiotic resistance allowing facile modification to adjust to genetic variants of resistant strains. Aside from intracellular concentration, ASO efficiency is dependent upon oligomer length, nuclease resistance, and binding kinetics. Specific hurdles that need to be overcome include protection from enzymatic degradation, transport across the physical barrier of the bacterial outer envelope (plasma membrane and cell wall), and electrostatic interactions with the cell surface and the target RNA.
Fig. 1.
a. Steric hindrance. An ASO fully substituted with 2′OMe residues are not recognized RNase H and do not lead to RNA degradation; however, they can downregulate gene expression by steric blockade of ribosome subunit assembly and access to mRNA binding near the Shine-Dalgarno (SD) and start codon (AUG) site and the progression of protein translation. b. Degradation. An ASO gapmer consists of a PS backbone with flanking 2′OMe modified moieties that increase resistance to nuclease degradation and enhance binding to target mRNA. The unmodified “gap” in an ASO gapmer/mRNA duplex is recognized and degraded by RNase H.
Mechanistic and pharmacological considerations for antisense biotechnological applications have been previously reviewed (Geary et al. 2015; Crooke 2017). Modifications to the ASO backbone and its sugar components have been developed (Lennox et al. 2017) leading to improvements in nuclease stability, thermodynamic binding stability and binding specificity, increasing their suitability for therapeutic application. The most common ASO chemistries to be discussed infra are depicted in Table 1 and in Fig. 2. Using this table and a figure as a reference, we provide a review of the different ASOs tested while providing an overview of the applications of these oligomers as antibiotics.
Table 1.
ASO chemical modification characteristics
| Modification | Name | Chemistry | Characteristics | Mechanism |
|---|---|---|---|---|
| Phosphate | Phosphodiester (PO) | Natural DNA linkage. | Poor nuclease resistance. | Steric hindrance RNase H cleavage |
| Phosphorothioate (PS) | A non-bridging oxygen atom in the phosphate backbone replaced by a sulfur atom. | Improved nuclease resistance. | Steric hindrance RNase H cleavage |
|
|
| ||||
| Sugar | Locked/Blocked nucleic acid (LNA/BNA) | Rotationally constrained RNA analogues having an ethylene or methylene bridge between the 2′ and 4′ positions in the ribose ring. | Strong binding affinity & nuclease resistance. Not RNase H susceptible. |
Steric hindrance |
| 2′-O-Methyl RNA (2′-OMe) | A hydrogen atom in the 2′-hydroxyl group in the ribose ring of RNA replaced by a methyl group. | Improved binding affinity & nuclease resistance. Not RNase H susceptible. |
Steric hindrance | |
|
| ||||
| Other | Peptide nucleic acid (PNA) | Sugar-phosphate backbone replaced by N-2- aminoethylglycine units linked by amide bonds | Uncharged. High binding affinity. High nuclease resistance. |
Steric hindrance |
| Phosphorodiamidate morpholino oligomer (PMO) | Ribose replaced by a morpholino moiety and phosphodiester linkage replaced by an uncharged phosphorodiamidate backbone. | Uncharged. Improved binding affinity. Excellent nuclease resistance. Not RNase H susceptible. |
Steric hindrance | |
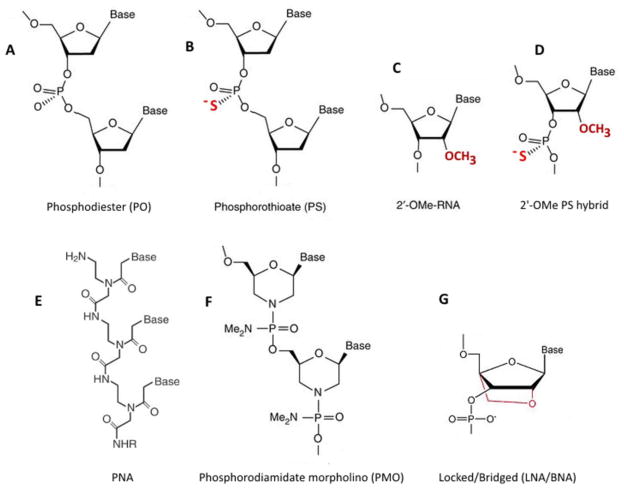
Fig. 2.
a Natural phosphodiester DNA oligomer (PO); b Phosphorothioate oligomer (PS); c)2′-O-Methyl oligonucleotide (2′-OMe); d ′-O-Methyl phosphorothioate oligomer (2′-OMe-PS; gapmer); e Peptide nucleic acid (PNA), f Phosphorodiamidate morpholino oligonucleotide (PMO) g Locked (Bridged) nucleic acid (LNA/BNA).
Methods
A narrative review highlighting biotechnological advances in the development of bacterial antisense therapeutics was prepared by synthesizing recent relevant entries of peer-reviewed texts within the PubMed literature database using combinations of the following search terms: antisense, bacteria, oligonucleotide, PNA, phosphorothioate, morpholino, PPMO, LNA, BNA and biotechnology.
Selection of antisense target
A salient objective in ASO design is the achievement of high specificity with minimal off-target effects. In order to predict the efficacy of candidate antisense therapeutics, a step-wise evaluation should be performed of several parameters that can affect the specificity and potency of ASOs for their complimentary mRNA target.
i) Target gene
A list of candidate bacterial genes should be compiled by selecting among those relevant to cellular functions of particular interest for the target organism. Commonly targeted bacterial gene functions and pathways are those essential for growth such as fatty acid biosynthesis, cell division, and RNA polymerases (Rasmussen et al. 2007; Clatworthy et al. 2007; Bai et al. 2011; Dinan and Loftus 2013; Ji and Lei 2013). Other attractive targets include those that bypass intrinsic or acquired bacterial mechanisms for development of antibiotic resistance, toxin/spore production, or the formation of biofilms (Wang et al. 2010; Meng et al. 2015; Sully and Geller 2016). A protein of interest can offer multiple targets such as structural subunits, interacting proteins, and regulators of expression (Oh et al. 2014).
ii) Target location and local structure
A target sequence within an mRNA molecule has an in vivo secondary structure that significantly affects its hybridization affinity to an ASO, a critical step in the antisense mechanism. While there is currently an incomplete understanding of all factors that influence ASO/RNA hybridization, the major determinant is local RNA structure (Crooke 2017). Disruption of a target secondary structure incurs a thermodynamic cost during ASO binding, therefore targeting of unstructured, open single-stranded regions are generally preferential. In particular, it is suggested that stacked residues within the 5′ end of mRNA loop structures are conformationally favorable targets for ASO binding (Lima et al. 1992; Lima et al. 2014). A commonly successful target region for genes of interest is near the translational initiation region (start codon and Shine-Dalgarno sequences) as this area is accessible for ribosome assembly and sensitive to steric inhibition. In silico analysis of RNA secondary structure, minimum free energy, and mRNA accessible site tagging (MAST) prediction informatics (Zuker 2003; Bo and Wang 2005; Reuter and Mathews 2010; Hamada et al. 2016; Wu et al. 2016; Shi et al. 2017) can be used to predict thermodynamic qualities of a list of candidate sequences. The predicted hybridization affinity of an ASO to its cognate sequence correlates with the activity observed in both binding assays and in vivo (Monia et al. 1993; Monia et al. 1996). Candidate sequences with good predicted values can then be accessed by in vitro testing of ASO efficiency within a suitable transcription/translation system (Castro-Roa and Zenkin 2015).
iii) Predicting off-target effects
Off-target ASO hybridization within a cell typically occurs only at highly accessible unstructured sites in the mRNA with less than 2 mismatches as these interactions have been found to be inhibited by higher order structure and RNA-binding proteins (Lima et al. 2014). ASO specificity can be empirically evaluated using quantitative measurement methods such as qPCR of predicted off-target genes and cellular transcriptomics (Hagedorn et al. 2017).
ASO chemistries
ASOs are typically 10–30 nucleotides in length. Unmodified phosphodiester ASOs are rapidly attacked by cellular nucleases [Fig. 2A]. Increased oligonucleotide target affinity is correlated with increased cellular antisense activity. To achieve appreciable clinical application, an ASO chemical structure needs to be optimized by a combination of sugar, backbone, nucleobase, and 3′- and 5′-terminal modifications (Khvorova and Watts 2017). Numerous chemical modifications [Table 1] have been described to confer resistance against nucleases, to improve stability of ASO/hybrid formation, and/or to preserve target specificity [Fig. 2].
Phosphorothioate (PS) linkages [Table 1; Fig. 2B] were the first chemical modification applied to ASO antisense biotechnology to improve ASO stability to nucleases (Eckstein 2014). Aside from stability, the binding affinity for an ASO to a target RNA is also dependent on its chemistry (locked/bridged nucleic acids > 2′-O-methyl RNA > DNA > phosphorothioate DNA). While PS-ASO are compatible with ASO/RNA cleavage by RNase H, they can exhibit a reduced binding affinity toward its RNA target. This can be counteracted through the incorporation of flanking 2′-O-methylated (2′-OMe) sugar-modified nucleotides [Table 1; Fig. 2C]; however, a limitation of ribose sugar modifications such as 2′-OMe is that they are unable to activate RNase H cleavage of the target mRNA. To achieve optimal activity, a combination of advantageous modifications to an ASO is often required to circumvent individual modification limitations. For example, chimeric gapmers consisting of PS-ASO with 2′-OMe modifications added to the flanking nucleotides [Fig. 2D] increase the stability of the ASO/RNA duplex while retaining susceptibility to RNase cleavage (Choung et al. 2006). To activate RNase H activity, a 2′-OMe-PS gapmer should contain a core of at least six 2′-deoxyribose nucleosides with 2′-OMe nucleotides flanking its 5′ and 3′ ends (Monia et al. 1993; Monia et al. 1996; Beaman et al. 2014). Similarly, three LNA residues at each end of an ASO are sufficient to increase stability 10-fold compared to an unmodified oligodeoxynucleotide (Kurreck et al. 2002).
The first “proof of principle” application of PS-ASO to a bacterium (Harth et al. 2000) demonstrated decreased expression of the targeted glutamine synthetase gene couple along with a moderate reduction in the growth of M. tuberculosis. Representative examples of additional PS-ASO minimum inhibitory concentrations (MIC) for various mRNA targets and bacteria are summarized in Table 2.
Table 2.
Phosphorothioate (PS) - mRNA target, organism and MIC
| PS | Gapmer | Carrier | Sequence (5′-3′) | mRNA | Organism | MIC | Reference |
|---|---|---|---|---|---|---|---|
| HP2/HP1 | No | Hairpin extensions | GCGCATATGCGCAATCTTTCGGCTCACGTCTGTCATGCGCGCGC | mycolyl transferase | M. tuberculosis | 10 μM | (Harth et al. 2007) |
| PS-833 | No | PEI | CGAGTCCCTTTTTACCAA | mecA | S. aureus | 8 μM | (Meng et al. 2009) |
| rpoD | No | (RXR)4XB | TTTGCTCCAT | rpoD | S. flexneri | 5 μM | (Bai et al. 2012) |
| rpoD | No | LF2000 cationic lipid | N/A | rpoD | MRSA | N/A | (Chen et al. 2015) |
| rpoB | 2′-OMe | Bolasome complex | CATTCACTTTTCACCTCTCAATAAT | rpoB | C. difficile | 0.2 μM | (Hegarty et al. 2016) |
Peptide nucleic acid (PNA) is an oligonucleotide analogue in which the nucleic acid sugar backbone is replaced by an uncharged backbone of achiral N-(2-aminoethyl) glycine units. PNA exhibit resistance to proteases and nucleases and form strong complexes with complementary strands of nucleic acids; however their antisense efficacy is hampered by poor intracellular delivery. The application of antisense inhibition to bacteria was first demonstrated using a PNA [Table 1; Fig. 2E], which decreased expression of the E. coli beta-lactamase gene (Good and Nielsen 1998). Of note, PNAs were found to be most effective when targeting the 5′-UTR of a gene, with optimal inhibition associated with nucleotide lengths of 15 to 18 bases (Doyle et al. 2001). The antibacterial effect of PNAs was extended to a mouse model of E. coli infection, though this investigation necessitated the use of a bacterial strain with a defective outer membrane (Tan et al. 2005), as cellular entry of naive PNA oligomers into a cell is limited by an intact bacterial outer-membrane (Wahlestedt et al. 2000). To overcome this cell membrane barrier problem, many groups have chemically conjugated various cell-penetrating peptides (CPPs), such as (KFF3)K, to PNA oligomers in order to enhance cellular delivery (Patenge et al. 2013; Hansen et al. 2016). Representative examples of (KFF)3K-conjugated PNA MIC for various mRNA targets and bacteria are summarized in Table 3.
Table 3.
(KFF)3K conjugated peptide nucleic acid (PNAs) - MIC
| PS | Sequence (N-C) | mRNA | Organism | MIC | Reference |
|---|---|---|---|---|---|
| Spp4 | CTCATACTCT | acpP | E. coli | 0.6 μM | (Good et al. 2000) |
| Sau101 | CCATGATTTA | fmhB | S. aureus | 10 μM | (Nekhotiaeva et al. 2004) |
| Ec109 | GTCATGTTTT | fabD | E. coli | 2.5 μM | (Dryselius et al. 2005) |
| Ms101 | GTCATTTGGT | inhA | M. smegmatis | <5 μM | (Kulyté et al. 2005) |
| ompA | ATACCAGGTGTTATCT | ompA | K. pneumoniae | 40 μM | (Kurupati et al. 2007) |
| polA | TTCATGCCTGT | polA | B. suis | 10 μM | (Rajasekaran et al. 2013) |
| acpP-CPP1 | CTCATACTAT | acpP | E. amylovora | 2.5 μM | (Patel et al. 2017) |
Phosphorodiamidate morpholino oligomers (PMO) [Table 1; Fig. 2F] are composed of standard DNA bases in which the ribose sugar is replaced with 6-sided morpholine rings linked by neutral phosphorodiamidate, rather than anionic phosphate groups, thereby remaining uncharged under physiological conditions. PMO exhibit improved binding kinetics, are completely resistant to nuclease digestion, and can withstand autoclaving without degradation. Pioneering work with PMO in bacteria (Geller et al. 2003) demonstrated that only a surprisingly short 11-mer PMO was required to downregulate the expression of a targeted acyl carrier protein (acpP) gene in a leaky-membrane strain of E. coli. However, efficient cellular delivery to E. coli with a fully functional cell membrane necessitated conjugation of a CPP to the PMO termini, yielding a peptide-conjugated PMO (PPMO). An acpP-PMO conjugated to (KFF)3K allowed entry to wild-type E. coli in a dose-responsive manner and the sequence-specific effects of this PMO was confirmed by rescue of the growth of an E. coli strain that expressed an acpP with silent mutations within the targeted region (Tilley et al. 2006). Efficacy both in vitro and in vivo was found to be influenced by the amino acid sequence of the CPP (Mellbye et al. 2009). PPMOs targeting the acpP gene of Burkholderia cepacia complex (Bcc) were bactericidal (> 4 log reduction) against clinical isolates of Bcc (Greenberg et al, 2010). In addition they demonstrated that AcpP PPMO treatment enhanced killing by neutrophils of B. multivorans in an ex vivo model, and had a ~80% reduction in the risk of dying by day 30, with relatively little pathology, in a mouse B. multivorans infection model. A CPP-PMO targeted to DNA gyrase subunit A reduced viability of S. aureus in a skin wound model of infection, improving healing time (Sawyer et al. 2013). It is notable that PPMOs appear to be active across multiple gram-negative pathogens and are active even in the setting of antibiotic resistance to traditional antibiotics. Importantly, treatment of E. coli with the acpP PPMO was found to be more effective on a molar basis than treatment with ampicillin (Mellbye et al. 2010). Similarly, a PPMO targeted to essential genes of Acinetobacter exhibited MICs within a clinically relevant range (Geller et al. 2013).
PPMOs have also been used as external guided sequences (EGS) to inhibit the growth of E. coli and B. subtilis cultures (Shen et al. 2009). When bound to a targeted mRNA, EGS are designed to form a complex resembling tRNA. This structure is recognized in by a tRNA-processing ribozyme, RNase P, which cleaves the first base-pair of the EGS/mRNA duplex (Davies-Sala et al. 2015). Treatment of chloramphenicol-resistant E. coli with a mixture of two PPMO EGS reduced bacterial survival by 99.9% in the presence of the antibiotic compared to chloramphenicol-only treated bacteria (Wesolowski et al. 2011).
Locked (Bridged) Nucleic Acids (LNA/BNA) are ribonucleotides containing a methylene bridge connecting the 2′-oxygen of ribose with the 4′-carbon [Table 1; Fig. 1G], that “lock” oligonucleotides into conformationally-constrained structures resulting in increased stability and binding affinities. Of note, chimeric LNA oligonucleotides duplexes have been reported to be both more stable and efficient for activation of RNase H than isosequential phosphorothioates and 2′-O-methyl gapmers (Kurreck et al. 2002). A single dose 3 mg/kg dose of an (KFF)3K conjugated LNA to the cell-division gene ftsZ in a mouse S. aureus model of sepsis was found to cause a 4-log reduction in tissue bacterial burden and increase survival by 60% (Meng et al. 2015). A LNA gapmer conjugated to (RXR)4XB inhibited the growth of A. baumannii cultures and reduced the levels amikacin required to treat of A. baumannii infected cells (Lopez et al. 2015; Jackson et al. 2016).
Delivery of ASOs
Numerous ASO delivery strategies to overcome cellular boundaries are available for eukaryotic cells (Juliano 2016; Dowdy 2017; Stein and Castanotto 2017); in contrast, the development of efficient delivery vehicles for bacteria has received less attention. One major challenge in the development of efficient antisense therapeutics targeting bacterial infections is that free ASOs are poorly taken up by bacteria due to electrostatic charge or the size barrier imposed by the cell envelope. Bacterial antisense therapeutics have relied largely upon the use of peptides previously demonstrated to deliver ASO into eukaryotic cells, rather than the design of specific modalities for optimized ASO translocation across unique bacterial plasma membranes and cell walls. Potential ASO delivery systems should be evaluated in vitro for efficacious cellular uptake in a bacterial species by using a fluorescently labeled ASO delivered at 1 to 50 micromolar concentrations. Treated cells can then be fixed and viewed by a fluorescence confocal microscope. A diffuse fluorescence located throughout the bacterial cell cytoplasm is indicative of effective uptake.
Cell-penetrating peptides (CPPs) are short (<30 amino acids) peptides that facilitate translocation of bioactive molecule cargo across a cell membrane without causing significant membrane damage. The first CPP described was derived from the trans-activating transcriptional activator (TAT) protein of human immunodeficiency virus 1. CPP-ASO conjugates and complexes have been synthesized through different chemistries including cleavable disulfide linkages, and stable amide, thiazolidine, oxime and hydrazine linkages. ASO cargo can be associated with a CPP either through covalent or non-covalent interactions. CPPs usually have an amino acid composition that contains either a relative abundance of positively charged amino acids such as lysine or arginine (polycationic) or contain an alternating pattern (amphipathic) of polar/charged amino acids and non-polar, hydrophobic amino acids. A third class of CPPs are the hydrophobic peptides, containing either a-polar residues with low net charge, or hydrophobic amino acid groups crucial for cellular uptake.
Bacterial antisense studies have principally relied upon covalent conjugation of ASO oligomers to CPPs in order to achieve entry into the bacterial cell, and improved uptake has been well documented (Morris et al. 2008). The peptides (KFF)3K, (RFF)3RXB, and (RXR)4XB (where K, F, R,X and B represent lysine, phenylalanine, arginine, 6-aminohexanoic acid and β-alanine, respectively) have been the most extensively utilized for the delivery of PNA-ASO into bacteria, including K. pneumonia, S. aureus, and S. enterica serovar Typhimurium, P. aeruginosa, A. baumannii, and S. flexneri (Kurupati et al. 2007). This delivery approach appears more effective for Gram negative species, with fewer reports of successful treatment of Gram positive organisms (Wang et al. 2010; Järver et al. 2012; Patenge et al. 2013; Meng et al. 2015; Hansen et al. 2016). Gram-positive bacteria have been reported to exhibit increased susceptibility to peptide-PNAs compared to Gram-negative bacteria. While PNA exhibit excellent target specificities (Mondhe et al. 2014; Abushahba et al. 2016), the necessary inclusion of a carrier peptide may be problematic in application to complex microbiomes due to non-specific carrier peptide effects (Hatamoto et al. 2010; Ghosal and Nielsen 2012).
A robust rational basis for CPP design is still lacking, indicating a need for further knowledge regarding cell wall and membrane structure and chemistry. Limitations of CPPs include low cell selectivity and the necessity for formation of conjugates or noncovalent complexes to the ASO cargo. In addition to covalent conjugation of ASOs with CPPs via a stable or cleavable linker, other promising peptide-base strategies for ASO delivery into bacteria have been described, including noncovalent electrostatic or hydrophobic ASO interactions with a CPP, complex formation between ASO and a lipid-conjugated peptide, and ASO condensation by peptide-functionalized cationic polymers. Noncovalent complexes require a strong molar excess of CPPs and extensive experimentation is required to determine the most optimal CPP for any given cargo and cell type.
Nanomaterials including polymers, lipids, and inorganic compounds have been utilized for the delivery of ASOs (Ahmed 2017). Nanomaterials can promote ASO cellular delivery by forming complexes with the oligomer, condensing their chiral secondary structure, thereby protecting it from enzymatic degradation and improving cellular entry (Zhao et al. 2009; Chen et al. 2015). Their most serious drawback problem is that in order to achieve adequate complexation with negatively charged ASO cargo, the cationic nature of many carrier compounds often exhibits cellular toxicity. Polyethylenimine (PEI) is a cationic polymer with repeating unit composed of the amine group and two aliphatic CH2CH2 spacers. PEI and anionic liposomes have been used to encapsulate PS-ASO targeting the oprM (multidrug efflux pump duct) gene, resulting in a significant dose-dependent reduction of oprM protein expression and inhibition of the growth of multidrug-resistant P. aeruginosa in the presence of piperacillin (Wang et al. 2010). The PS-ASO nanocomplexes were formed at a molar ratio of PEI nitrogen to ASO-ODN phosphate (N/P) of 8 and an optimum charge ratio of 8.0. The first successful application of cationic bolasome (CAB) ASO nanocomplexes composed of cyclohexyl-dequalinium chloride as a bacterial delivery system for 2′-O-methyl phosphorothioate gapmers achieved nanomolar MICs in treated C. difficile broth cultures (Hegarty et al. 2016). ASO transport by CAB nanocomplexes is attributed to interactions with bacterial membrane cardiolipin (Weissig et al. 2000; Marín-Menéndez et al. 2017). Cardiolipin is an anionic glycerophospholipid with an essential role in outer membrane remodeling in bacteria (Dong et al. 2016). Decoration of palmitoyl oleyl phosphatidyl choline/dioleyl phosphatidylethanolamine liposomes with CAB nanocomplexes has been reported to increase their stability in physiological solutions (Mamusa et al. 2017a; Mamusa et al. 2017b).
Targeting antibiotic resistance
Specific antibiotic resistance determinants can be targeted to increase the susceptibility of resistant bacteria. A combination of three hairpin-modified PS-ASOs targeting mycolyl transferase transcripts was reported to induce a 90% reduction in mycolyl transferase expression with an 8-fold increased sensitivity to isoniazid (Harth et al. 2007). Methicillin resistance in S. aureus (MRSA) is caused by production of a lactamase penicillin binding proteins encoded by the mecA and blaZ genes. PS-ASOs designed to block expression mecA and blaZ were used simultaneously in vivo to restore methicillin susceptibility to MRSA. Similarly, PNAs targeted to mecA and ftsZ (cell division) of MRSA significantly increased susceptibility to oxacillin (Goh et al. 2014; Goh et al. 2015), while a phosphorothioate ASO targeted to mecA delivered using an anionic liposome resulted in increased susceptibility to oxacillin both in vitro and in a MRSA mouse model of sepsis (Meng et al. 2015). A (KFF)3K-conjugated 25-mer PPMO and 13-mer PNA were reported to significantly increase E. coli sensitivity to cefotaxime in a dose dependent manner (0–40 nM) (Readman et al. 2016). Two recent papers (Daly et al. 2017; Sully et al. 2017) demonstrate how PPMO targeting a single enzyme can restore activity to specific traditional antibiotics. The combined treatment of tobramycin with a PPMO targeting the 30S ribosomal subunit gene, rpsJ, was found to exhibit synergy in the inhibition of P. aeruginosa growth (Howard et al. 2017). PPMOs targeting acpP, lpxC, and rpsJ of P. aeruginosa inhibited growth in many clinical strains. This PPMO activity could be enhanced up to 8-fold by the addition of polymyxin B nonapeptide at sub-inhibitory concentrations. Importantly, treatment with various combinations of a PPMO and a traditional antibiotic demonstrated synergistic growth inhibition. Treatment of P. aeruginosa PA103-infected mice with PPMOs targeting acpP, lpxC, or rpsJ significantly reduced the bacterial burden in the lungs at 24 h by almost 3 logs.
Extracellular matrix biofilms formed by many bacteria can enhance virulence by increasing the establishment of infection and survival in tissues. Biofilms also typically necessitate the use of much larger treatment dose of antibiotics. A (KFF)3K-PNA targeting the motility gene, motA, was found to inhibit P. aeruginosa PAO1 biofilm formation in in a dose-dependent manner (Hu et al. 2011). PS-ASO simultaneously targeting two glucosyltransferases inhibited Streptococcus mutans biofilm formation in vitro (Kalesinskas et al. 2015). A (KFF)3K-PNA targeting acpP had significant activity against biofilm forms of non-typeable Haemophilus influenzae (Otsuka et al. 2017). PPMO targeting acpP was effective at both preventing P. aeruginosa PAO1 biofilm formation and at reducing existing biofilms (Howard et al. 2017). In addition, mechanisms that can restore activity to antibiotics can be targeted. PNAs targeting a multidrug efflux pump of Campylobacter jejuni increased the susceptibility to ciprofloxacin and erythromycin. Similarly, a PPMO to the acrA gene of the E. coli efflux system increased antibiotic efficacy with successful inhibition of bacterial growth at up to 40-fold lower antibiotic doses (Ayhan et al. 2016).
Future challenges and prospects
Conventional antibiotics can non-selectively kill beneficial commensal bacteria and the resulting dysbiosis of the microbiome can lead to medical complications. Antisense antibiotics represent a paradigm shift for medicine. Following 40 years of development, ASO bacterial therapeutics are nearing clinical utility. Many desirable properties such as uptake by the cells, stability against nucleases, and a strong affinity for target mRNA have been demonstrated. It is encouraging that the first PPMO modality has recently been approved by the US-FDA for clinical trials for treatment of Duchenne’s muscular dystrophy. Adjuvant use of antisense ASO can potentially allow for use lower doses of traditional antibiotics, that would otherwise be sun-inhibitory, to minimize the impact to the host microbiome and the chance of incurring resistance. In addition to antibiotic resistance, attention has recently turned to the targeting of bacterial virulence functions controlled by canonical regulators of bacterial quorum sensing systems. Quorum sensing is a process of cell communication that allows bacteria to respond to cell density and adjust gene expression (Rutherford and Bassler 2012). CPP-conjugated LNA to the agr quorum sensing gene of community-associated MRSA inhibited the expression of agr, several agr-regulated virulence genes, and afforded high levels of protection in a mouse skin infection model (Da et al. 2017). Of particular future interest will be the application of ASO to interrupt bacterial toxin production and sporulation pathways to decrease virulence and recrudescence of disease.
Barriers in the further development of antisense therapies include creation of carrier compounds to deliver ASOs to the bacterial cytoplasm in an effective dose, while avoiding toxicity to both commensal microorganisms and eukaryotic host cells. Further advancements are required to improve uptake of ASOs, especially in complex microbial communities, and to establish formal ASO design application rules (Hatamoto et al. 2010). Predicting which CPP is optimal for a given organism remains a complex question and development of peptides remains an active area of research (Dias and Stein 2002; Reissmann 2014; Shi et al. 2014; Boisguérin et al. 2015). Studies on peptide composition may be useful in terms of improving the efficiency of the ASO conjugate penetration of cell envelopes of different bacteria. Incorporation of D form (or other non-natural) amino acid residues in CPP design may be beneficial to prevent deleterious proteolytic digestion. One future approach could involve the identification of bacteria-specific penetrating peptides by screening of large peptide phage display libraries in conjunction with bioinformatic selection. Screening of further bacterial mRNA virulence targets and assessment of optimal ASO lengths to balance specificity and bacterial cell entry are also additional areas for study. Recently the fact that some bacteria are capable of vitamin B12 uptake was exploited to achieve enhanced PNA delivery into S. typhimurium and E. coli by a novel conjugation of PNA antisense oligomers to vitamin B12 (Pieńko et al. 2017; Równicki et al. 2017). These PNA-B12 conjugates were synthesized by a Cu-catalyzed 1,3-dipolar cycloaddition of the vitamin B12 azide to PNAs possessing a terminal alkyne group. Importantly, this conjugation with vitamin B12 was found to transport PNA into E. coli cells more efficiently than the commonly used CPP, (KFF)3K.
ASO specificity and delivery has improved to the point where many are now as potent as conventional antibiotics in vitro. PNA, PS-ASO gapmers, PMO, and LNA oligomers have an exciting potential for inhibiting the growth of specific microbes in a complex microbial community. One promising strategy is the combined application of ASOs to synergistically target both growth and virulence. Future ASO chemical modifications and delivery systems hold promise to further enhance the efficacy of ASO to enable not only clinical applications, but also in potential application to complex microbial consortia in nonclinical biotechnology fields such as industrial and environmental microbiology.
Acknowledgments
Funding: This work was supported by a grant from the National Institutes of Health, grant # R21AI132353 (Stewart- PI).
Footnotes
Ethical Statements
Conflict of Interest: Both authors declare no conflict of interest.
Ethical Approval: This article does not contain any studies with animal or human participants performed by any of the authors.
References
- Abushahba MFN, Mohammad H, Thangamani S, Hussein AAA, Seleem MN. Impact of different cell penetrating peptides on the efficacy of antisense therapeutics for targeting intracellular pathogens. Sci Rep. 2016;6:20832. doi: 10.1038/srep20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M. Peptides, polypeptides and peptide-polymer hybrids as nucleic acid carriers. Biomater Sci. 2017 Sep 7; doi: 10.1039/c7bm00584a. [DOI] [PubMed] [Google Scholar]
- Ayhan DH, Tamer YT, Akbar M, Bailey SM, Wong M, Daly SM, Greenberg DE, Toprak E. Sequence-specific targeting of bacterial resistance genes increases antibiotic efficacy. PLoS Biol. 2016;14:e1002552. doi: 10.1371/journal.pbio.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Sang G, You Y, Xue X, Zhou Y, Hou Z, Meng J, Luo X. Targeting RNA polymerase primary σ 70 as a therapeutic strategy against methicillin-resistant Staphylococcus aureus by antisense peptide nucleic acid. PLoS One. 2012;7:e29886. doi: 10.1371/journal.pone.0029886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Zhou Y, Hou Z, Xue X, Meng J, Luo X. Targeting bacterial RNA polymerase: Promises for future antisense antibiotics development. Infect Disord Drug Targets. 2011;11:175–187. doi: 10.2174/187152611795589708. [DOI] [PubMed] [Google Scholar]
- Beaman GM, Dennison SR, Phoenix DA. Novel Antimicrobial Agents and Strategies. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2014. Antimicrobial Therapy Based on Antisense Agents; pp. 357–386. [Google Scholar]
- Bo X, Wang S. TargetFinder: a software for antisense oligonucleotide target site selection based on MAST and secondary structures of target mRNA. Bioinformatics. 2005;21:1401–1402. doi: 10.1093/bioinformatics/bti211. [DOI] [PubMed] [Google Scholar]
- Boisguérin P, Deshayes S, Gait MJ, O’Donovan L, Godfrey C, Betts CA, Wood MJA, Lebleu B. Delivery of therapeutic oligonucleotides with cell penetrating peptides. Adv Drug Deliv Rev. 2015;87:52–67. doi: 10.1016/j.addr.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Roa D, Zenkin N. Methodology for the analysis of transcription and translation in transcription-coupled-to-translation systems in vitro. Methods. 2015;86:51–59. doi: 10.1016/j.ymeth.2015.05.029. [DOI] [PubMed] [Google Scholar]
- Chen Z, Hu Y, Meng J, Li M, Hou Z, Zhou Y, Luo X, Xue X. Efficient transfection of phosphorothioate oligodeoxyribonucleotides by lipofectamine 2000 into different bacteria. Curr Drug Deliv. 2015;12:1–8. doi: 10.2174/1567201812666150817123528. [DOI] [PubMed] [Google Scholar]
- Choung S, Kim YJ, Kim S, Park H-O, Choi Y-C. Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochem Biophys Res Commun. 2006;342:919–927. doi: 10.1016/j.bbrc.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Clatworthy AE, Pierson E, Hung DT. Targeting virulence: A new paradigm for antimicrobial therapy. Nat Chem Biol. 2007;3:541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- Crooke ST. Molecular Mechanisms of Antisense Oligonucleotides. Nucleic Acid Ther. 2017;27:70–77. doi: 10.1089/nat.2016.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da F, Yao L, Su Z, Hou Z, Li Z, Xue X, Meng J, Luo X. Antisense locked nucleic acids targeting agrA inhibit quorum sensing and pathogenesis of community-associated methicillin-resistant Staphylococcus aureus. J Appl Microbiol. 2017;122:257–267. doi: 10.1111/jam.13321. [DOI] [PubMed] [Google Scholar]
- Davies-Sala C, Soler-Bistué A, Bonomo RA, Zorreguieta A, Tolmasky ME. External guide sequence technology: a path to development of novel antimicrobial therapeutics. Ann N Y Acad Sci. 2015;1354:98–110. doi: 10.1111/nyas.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly SM, Sturge CR, Felder-Scott CF, Geller BL, Greenberg DE. MCR-1 Inhibition with Peptide-Conjugated Phosphorodiamidate morpholino oligomers restores sensitivity to polymyxinEscherichia coli. MBio. 2017;8(6) doi: 10.1128/mBio.01315-17. pii: e01315–01317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias N, Stein CA. Antisense oligonucleotides: basic concepts and mechanisms. Mol Cancer Ther. 2002;1:347–355. [PubMed] [Google Scholar]
- Dinan AM, Loftus BJ. (Non-)translational medicine: Targeting bacterial RNA. Front Genet. 2013;4:230. doi: 10.3389/fgene.2013.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Zhang Z, Tang X, Huang S, Li H, Peng B, Dong C. Structural insights into cardiolipin transfer from the Inner membrane to the outer membrane by PbgA in Gram-negative bacteria. Sci Rep. 2016;6:30815. doi: 10.1038/srep30815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdy SF. Overcoming cellular barriers for RNA therapeutics. Nat Biotechnol. 2017;35:222–229. doi: 10.1038/nbt.3802. [DOI] [PubMed] [Google Scholar]
- Doyle DF, Braasch DA, Simmons CG, Janowski BA, Corey DR. Inhibition of gene expression inside cells by peptide nucleic acids: effect of mRNA target sequence, mismatched bases, and PNA length. Biochemistry. 2001;40:53–64. doi: 10.1021/bi0020630. [DOI] [PubMed] [Google Scholar]
- Dryselius R, Nekhotiaeva N, Good L. Antimicrobial synergy between mRNA- and protein-level inhibitors. J Antimicrob Chemother. 2005;56:97–103. doi: 10.1093/jac/dki173. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Phosphorothioates, essential components of therapeutic oligonucleotides. Nucleic Acid Ther. 2014;24:374–387. doi: 10.1089/nat.2014.0506. [DOI] [PubMed] [Google Scholar]
- Geary RS, Norris D, Yu R, Bennett CF. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev. 2015;87:46–51. doi: 10.1016/j.addr.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Geller BL, Deere JD, Stein DA, Kroeker AD, Moulton HM, Iversen PL. Inhibition of gene expression in Escherichia coli by antisense phosphorodiamidate morpholino oligomers. Antimicrob Agents Chemother. 2003;47:3233–3239. doi: 10.1128/AAC.47.10.3233-3239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller BL, Marshall-Batty K, Schnell FJ, McKnight MM, Iversen PL, Greenberg DE. Gene-silencing antisense oligomers inhibit acinetobacter growth in vitro and in vivo. J Infect Dis. 2013a;208:1553–1560. doi: 10.1093/infdis/jit460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller BL, Marshall-Batty K, Schnell FJ, McKnight MM, Iversen PL, Greenberg DE. Gene-silencing antisense oligomers inhibit Acinetobacter growth in vitro and in vivo. J Infect Dis. 2013b;208:1553–1560. doi: 10.1093/infdis/jit460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal A, Nielsen PE. Potent antibacterial antisense peptide-peptide nucleic acid conjugates against Pseudomonas aeruginosa. Nucleic Acid Ther. 2012;22:323–334. doi: 10.1089/nat.2012.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh S, Loeffler A, Lloyd DH, Nair SP, Good L. Oxacillin sensitization of methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus pseudintermedius by antisense peptide nucleic acids in vitro. BMC Microbiol. 2015;15:262. doi: 10.1186/s12866-015-0599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh S, Stach J, Good L. Methods in Molecular Biology. Clifton, N.J: 2014. Antisense effects of PNAs in bacteria; pp. 223–236. [DOI] [PubMed] [Google Scholar]
- Good L, Nielsen PE. Antisense inhibition of gene expression in bacteria by PNA targeted to mRNA. Nat Biotechnol. 1998;16:355–358. doi: 10.1038/nbt0498-355. [DOI] [PubMed] [Google Scholar]
- Good L, Sandberg R, Larsson O, Nielsen PE, Wahlestedt C. Antisense PNA effects in Escherichia coli are limited by the outer-membrane LPS layer. Microbiology. 2000;146:2665–2670. doi: 10.1099/00221287-146-10-2665. [DOI] [PubMed] [Google Scholar]
- Greenberg DE, Marshall-Batty KR, Brinster LR, Zarember KA, Shaw PA, Mellbye BL, Iversen PL, Holland SM, Geller BL. Antisense phosphorodiamidate morpholino oligomers targeted to an essential gene inhibit Burkholderia cepacia complex. J Infect Dis. 2010;201(12):1822–1830. doi: 10.1086/652807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn PH, Hansen BR, Koch T, Lindow M. Managing the sequence-specificity of antisense oligonucleotides in drug discovery. Nucleic Acids Res. 2017;45:2262–2282. doi: 10.1093/nar/gkx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Ono Y, Kiryu H, Sato K, Kato Y, Fukunaga T, Mori R, Asai K. Rtools: a web server for various secondary structural analyses on single RNA sequences. Nucleic Acids Res. 2016;44:W302–W307. doi: 10.1093/nar/gkw337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AM, Bonke G, Larsen CJ, Yavari N, Nielsen PE, Franzyk H. Antibacterial peptide nucleic acid - antimicrobial peptide (PNA-AMP) conjugates: antisense targeting of fatty acid biosynthesis. Bioconjug Chem. 2016;27:863–867. doi: 10.1021/acs.bioconjchem.6b00013. [DOI] [PubMed] [Google Scholar]
- Harth G, Zamecnik PC, Tabatadze D, Pierson K, Horwitz MA. Hairpin extensions enhance the efficacy of mycolyl transferase-specific antisense oligonucleotides targeting Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2007;104:7199–204. doi: 10.1073/pnas.0701725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harth G, Zamecnik PC, Tang JY, Tabatadze D, Horwitz MA. Treatment of Mycobacterium tuberculosis with antisense oligonucleotides to glutamine synthetase mRNA inhibits glutamine synthetase activity, formation of the poly-L-glutamate/glutamine cell wall structure, and bacterial replication. Proc Natl Acad Sci U S A. 2000;97:418–423. doi: 10.1073/pnas.97.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatamoto M, Ohashi A, Imachi H. Peptide nucleic acids (PNAs) antisense effect to bacterial growth and their application potentiality in biotechnology. Appl Microbiol Biotechnol. 2010;86:397–402. doi: 10.1007/s00253-009-2387-8. [DOI] [PubMed] [Google Scholar]
- Hegarty JP, Krzeminski J, Sharma AK, Guzman-Villanueva D, Weissig V, Stewart DB. Bolaamphiphile-based nanocomplex delivery of phosphorothioate gapmer antisense oligonucleotides as a treatment for Clostridium difficile. Int J Nanomedicine. 2016;11:3607–3619. doi: 10.2147/IJN.S109600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JJ, Sturge CR, Moustafa DA, Daly SM, Marshall-Batty KR, Felder CF, Zamora D, Yabe-Gill M, Labandeira-Rey M, Bailey SM, Wong M, Goldberg JB, Geller BL, Greenberg DE. Inhibition of Pseudomonas aeruginosa by peptide-conjugated phosphorodiamidate morpholino oligomers. Antimicrob Agents Chemother. 2017;61:e01938–16. doi: 10.1128/AAC.01938-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Xia Y, Xiong Y, Li X, Su X. Inhibition of biofilm formation by the antisense peptide nucleic acids targeted at the motA gene in Pseudomonas aeruginosa PAO1 strain. World J Microbiol Biotechnol. 2011;27:1981–1987. [Google Scholar]
- Jackson A, Jani S, Davies-Sala C, Soler-Bistué AJC, Zorreguieta A, Tolmasky ME. Assessment of configurations and chemistries of bridged nucleic acids-containing oligomers as external guide sequences: a methodology for inhibition of expression of antibiotic resistance genes. Biol Methods Protoc. 2016;1:bpw001. doi: 10.1093/biomethods/bpw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järver P, Coursindel T, Andaloussi SEL, Godfrey C, Wood MJ, Gait MJ. Peptide-mediated cell and in vivo delivery of antisense oligonucleotides and siRNA. Mol Ther Nucleic Acids. 2012;1:e27. doi: 10.1038/mtna.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Lei T. Antisense RNA regulation and application in the development of novel antibiotics to combat multidrug resistant bacteria. Sci Prog. 2013;96:43–60. doi: 10.3184/003685013X13617194309028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano RL. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016;44:6518–6548. doi: 10.1093/nar/gkw236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalesinskas P, Kačergius T, Ambrozaitis A, Jimbo R, Ericson D. Streptococcus mutans biofilm inhibition using antisense oligonucleotide to glucosyltransferases B and C. Acta Medica Litu. 2015;22:85–92. [Google Scholar]
- Khvorova A, Watts JK. The chemical evolution of oligonucleotide therapies of clinical utility. Nat Biotechnol. 2017;35:238–248. doi: 10.1038/nbt.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulyté A, Nekhotiaeva N, Awasthi SK, Good L. Inhibition of Mycobacterium smegmatis gene expression and growth using antisense peptide nucleic acids. J Mol Microbiol Biotechnol. 2005;9:101–109. doi: 10.1159/000088840. [DOI] [PubMed] [Google Scholar]
- Kurreck J, Wyszko E, Gillen C, Erdmann VA. Design of antisense oligonucleotides stabilized by locked nucleic acids. Nucleic Acids Res. 2002;30:1911–1918. doi: 10.1093/nar/30.9.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurupati P, Tan KSW, Kumarasinghe G, Poh CL. Inhibition of gene expression and growth by antisense peptide nucleic acids in a multiresistant -lactamase-producing Klebsiella pneumoniae strain. Antimicrob Agents Chemother. 2007;51:805–811. doi: 10.1128/AAC.00709-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox KA, Vakulskas CA, Behlke MA. Methods in Molecular Biology. Clifton, N.J: 2017. Non-nucleotide modification of anti-miRNA oligonucleotides; pp. 51–69. [DOI] [PubMed] [Google Scholar]
- Lima WF, Monia BP, Ecker DJ, Freier SM. Implication of RNA structure on antisense oligonucleotide hybridization kinetics. Biochemistry. 1992;31:12055–12061. doi: 10.1021/bi00163a013. [DOI] [PubMed] [Google Scholar]
- Lima WF, Vickers TA, Nichols J, Li C, Crooke ST. Defining the factors that contribute to on-target specificity of antisense oligonucleotides. PLoS One. 2014;9:e101752. doi: 10.1371/journal.pone.0101752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C, Arivett BA, Actis LA, Tolmasky ME. Inhibition of AAC(6′)-Ib-mediated resistance to amikacin in Acinetobacter baumannii by an antisense peptide-conjugated 2′,4′-bridged nucleic acid-NC-DNA hybrid oligomer. Antimicrob Agents Chemother. 2015;59:5798–5803. doi: 10.1128/AAC.01304-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamusa M, Barbero F, Montis C, Cutillo L, Gonzalez-Paredes A, Berti D. Inclusion of oligonucleotide antimicrobials in biocompatible cationic liposomes: a structural study. J Colloid Interface Sci. 2017a;508:476–487. doi: 10.1016/j.jcis.2017.08.080. [DOI] [PubMed] [Google Scholar]
- Mamusa M, Sitia L, Barbero F, Ruyra A, Calvo TD, Montis C, Gonzalez-Paredes A, Wheeler GN, Morris CJ, McArthur M, Berti D. Cationic liposomal vectors incorporating a bolaamphiphile for oligonucleotide antimicrobials. Biochim Biophys Acta Biomembr. 2017b;1859:1767–1777. doi: 10.1016/j.bbamem.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Marín-Menéndez A, Montis C, Díaz-Calvo T, Carta D, Hatzixanthis K, Morris CJ, McArthur M, Berti D. Antimicrobial Nanoplexes meet Model Bacterial Membranes: the key role of Cardiolipin. Sci Rep. 2017;7:41242. doi: 10.1038/srep41242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellbye BL, Puckett SE, Tilley LD, Iversen PL, Geller BL. Variations in amino acid composition of antisense peptide-phosphorodiamidate morpholino oligomer affect potency against Escherichia coli in vitro and in vivo. Antimicrob Agents Chemother. 2009;53:525–530. doi: 10.1128/AAC.00917-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellbye BL, Weller DD, Hassinger JN, Reeves MD, Lovejoy CE, Iversen PL, Geller BL. Cationic phosphorodiamidate morpholino oligomers efficiently prevent growth of Escherichia coli in vitro and in vivo. J Antimicrob Chemother. 2010;65:98–106. doi: 10.1093/jac/dkp392. [DOI] [PubMed] [Google Scholar]
- Meng J, Da F, Ma X, Wang N, Wang Y, Zhang H, Li M, Zhou Y, Xue X, Hou Z, Jia M, Luo X. Antisense growth inhibition of methicillin-resistant Staphylococcus aureus by locked nucleic acid conjugated with cell-penetrating peptide as a novel FtsZ inhibitor. Antimicrob Agents Chemother. 2015;59:914–922. doi: 10.1128/AAC.03781-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Wang H, Hou Z, Chen T, Fu J, Ma X, He G, Xue X, Jia M, Luo X. Novel anion liposome-encapsulated antisense oligonucleotide restores susceptibility of methicillin-resistant Staphylococcus aureus and rescues mice from lethal sepsis by targeting mecA. Antimicrob Agents Chemother. 2009;53:2871–2878. doi: 10.1128/AAC.01542-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondhe M, Chessher A, Goh S, Good L, Stach JEM. Species-selective killing of bacteria by antimicrobial peptide-PNAs. PLoS One. 2014;9:e89082. doi: 10.1371/journal.pone.0089082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monia BP, Lesnik EA, Gonzalez C, Lima WF, McGee D, Guinosso CJ, Kawasaki AM, Cook PD, Freier SM. Evaluation of 2′-modified oligonucleotides containing 2′-deoxy gaps as antisense inhibitors of gene expression. J Biol Chem. 1993;268:14514–14522. [PubMed] [Google Scholar]
- Monia BP, Sasmor H, Johnston JF, Freier SM, Lesnik EA, Muller M, Geiger T, Altmann KH, Moser H, Fabbro D. Sequence-specific antitumor activity of a phosphorothioate oligodeoxyribonucleotide targeted to human C-raf kinase supports an antisense mechanism of action in vivo. Proc Natl Acad Sci U S A. 1996;93:15481–15484. doi: 10.1073/pnas.93.26.15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Deshayes S, Heitz F, Divita G. Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol Cell. 2008;100:201–217. doi: 10.1042/BC20070116. [DOI] [PubMed] [Google Scholar]
- Nekhotiaeva N, Awasthi SK, Nielsen PE, Good L. Inhibition of Staphylococcus aureus gene expression and growth using antisense peptide nucleic acids. Mol Ther. 2004;10:652–659. doi: 10.1016/j.ymthe.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Oh E, Zhang Q, Jeon B. Target optimization for peptide nucleic acid (PNA)-mediated antisense inhibition of the CmeABC multidrug efflux pump in Campylobacter jejuni. J Antimicrob Chemother. 2014;69:375–380. doi: 10.1093/jac/dkt381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka T, Brauer AL, Kirkham C, Sully EK, Pettigrew MM, Kong Y, Geller BL, Murphy TF. Antimicrobial activity of antisense peptide-peptide nucleic acid conjugates against non-typeable Haemophilus influenzae in planktonic and biofilm forms. J Antimicrob Chemother. 2017;72:137–144. doi: 10.1093/jac/dkw384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RR, Sundin GW, Yang C-H, Wang J, Huntley RB, Yuan X, Zeng Q. Exploration of using antisense peptide nucleic acid (PNA)-cell penetrating peptide (CPP) as a novel bactericide against fire blight pathogen Erwinia amylovora. Front Microbiol. 2017;8:687. doi: 10.3389/fmicb.2017.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenge N, Pappesch R, Krawack F, Walda C, Mraheil MA, Jacob A, Hain T, Kreikemeyer B. Inhibition of growth and gene expression by PNA-peptide conjugates in Streptococcus pyogenes. Mol Ther Nucleic Acids. 2013;2:e132. doi: 10.1038/mtna.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieńko T, Wierzba AJ, Wojciechowska M, Gryko D, Trylska J. Conformational dynamics of cyanocobalamin and its conjugates with peptide nucleic acids. J Phys Chem B. 2017;121:2968–2979. doi: 10.1021/acs.jpcb.7b00649. [DOI] [PubMed] [Google Scholar]
- Rajasekaran P, Alexander JC, Seleem MN, Jain N, Sriranganathan N, Wattam AR, Setubal JC, Boyle SM. Peptide nucleic acids inhibit growth of Brucella suis in pure culture and in infected murine macrophages. Int J Antimicrob Agents. 2013;41:358–362. doi: 10.1016/j.ijantimicag.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen LCV, Sperling-Petersen HU, Mortensen KK. Hitting bacteria at the heart of the central dogma: sequence-specific inhibition. Microb Cell Fact. 2007;6:24. doi: 10.1186/1475-2859-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readman JB, Dickson G, Coldham NG. Translational inhibition of CTX-M extended spectrum β-lactamase in clinical strains of Escherichia coli by synthetic antisense oligonucleotides partially restores sensitivity to cefotaxime. Front Microbiol. 2016;7:373. doi: 10.3389/fmicb.2016.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissmann S. Cell penetration: scope and limitations by the application of cell-penetrating peptides. J Pept Sci. 2014;20:760–784. doi: 10.1002/psc.2672. [DOI] [PubMed] [Google Scholar]
- Reuter JS, Mathews DH. RNAstructure: software for RNA secondary structure prediction and analysis. BMC Bioinformatics. 2010;11:129. doi: 10.1186/1471-2105-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Równicki M, Wojciechowska M, Wierzba AJ, Czarnecki J, Bartosik D, Gryko D, Trylska J. Vitamin B12 as a carrier of peptide nucleic acid (PNA) into bacterial cells. Sci Rep. 2017;7:7644. doi: 10.1038/s41598-017-08032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford ST, Bassler BL. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med. 2012;2:a012427–a012427. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer AJ, Wesolowski D, Gandotra N, Stojadinovic A, Izadjoo M, Altman S, Kyriakides TR. A peptide-morpholino oligomer conjugate targeting Staphylococcus aureus gyrA mRNA improves healing in an infected mouse cutaneous wound model. Int J Pharm. 2013;453:651–655. doi: 10.1016/j.ijpharm.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen N, Ko J, Xiao G, Wesolowski D, Shan G, Geller B, Izadjoo M, Altman S. Inactivation of expression of several genes in a variety of bacterial species by EGS technology. Proc Natl Acad Sci U S A. 2009;106:8163–8168. doi: 10.1073/pnas.0903491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Li X, Dong M, Graham M, Yadav N, Liang C. JNSViewer—A JavaScript-based Nucleotide Sequence Viewer for DNA/RNA secondary structures. PLoS One. 2017;12:e0179040. doi: 10.1371/journal.pone.0179040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi N-Q, Qi X-R, Xiang B, Zhang Y. A survey on “Trojan Horse” peptides: Opportunities, issues and controlled entry to “Troy. J Control Release. 2014;194:53–70. doi: 10.1016/j.jconrel.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Stein CA, Castanotto D. FDA-approved oligonucleotide therapies in 2017. Mol Ther. 2017;25:1069–1075. doi: 10.1016/j.ymthe.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sully EK, Geller BL. Antisense antimicrobial therapeutics. Curr Opin Microbiol. 2016;33:47–55. doi: 10.1016/j.mib.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sully EK, Geller BL, Li L, Moody CM, Bailey SM, Moore AL, Wong M, Nordmann P, Daly SM, Sturge CR, Greenberg DE. Peptide-conjugated phosphorodiamidate morpholino oligomer (PPMO) restores carbapenem susceptibility to NDM-1-positive pathogens in vitro and in vivo. J Antimicrob Chemother. 2017;72(3):782–790. doi: 10.1093/jac/dkw476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X-X, Actor JK, Chen Y. Peptide nucleic acid antisense oligomer as a therapeutic strategy against bacterial infection: proof of principle using mouse intraperitoneal infection. Antimicrob Agents Chemother. 2005;49:3203–3207. doi: 10.1128/AAC.49.8.3203-3207.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley LD, Hine OS, Kellogg JA, Hassinger JN, Weller DD, Iversen PL, Geller BL. Gene-specific effects of antisense phosphorodiamidate morpholino oligomer-peptide conjugates on Escherichia coli and Salmonella enterica Serovar Typhimurium in pure culture and in tissue culture. Antimicrob Agents Chemother. 2006;50:2789–2796. doi: 10.1128/AAC.01286-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlestedt C, Nielsen PE, Larsson O, Sandberg R, Good L. Antisense PNA effects in Escherichia coli are limited by the outer-membrane LPS layer. Microbiology. 2000;146:2665–2670. doi: 10.1099/00221287-146-10-2665. [DOI] [PubMed] [Google Scholar]
- Wang H, Meng J, Jia M, Ma X, He G, Yu J, Wang R, Bai H, Hou Z, Luo X. oprM as a new target for reversion of multidrug resistance in Pseudomonas aeruginosa by antisense phosphorothioate oligodeoxynucleotides. FEMS Immunol Med Microbiol. 2010;60:275–282. doi: 10.1111/j.1574-695X.2010.00742.x. [DOI] [PubMed] [Google Scholar]
- Weissig V, Lizano C, Torchilin VP. Selective DNA release from DQAsome/DNA complexes at mitochondria-like membranes. Drug Deliv. 2000;7:1–5. doi: 10.1080/107175400266722. [DOI] [PubMed] [Google Scholar]
- Wesolowski D, Tae HS, Gandotra N, Llopis P, Shen N, Altman S. Basic peptide-morpholino oligomer conjugate that is very effective in killing bacteria by gene-specific and nonspecific modes. Proc Natl Acad Sci. 2011;108:16582–16587. doi: 10.1073/pnas.1112561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Qu R, Huang Y, Shi B, Liu M, Li Y, Lu ZJ. RNAex: an RNA secondary structure prediction server enhanced by high-throughput structure-probing data. Nucleic Acids Res. 2016;44:W294–301. doi: 10.1093/nar/gkw362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik PC, Stephenson ML. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978;75:280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q-Q, Chen J-L, Lv T-F, He C-X, Tang G-P, Liang W-Q, Tabata Y, Gao J-Q. N/P ratio significantly influences the transfection efficiency and cytotoxicity of a polyethylenimine/chitosan/DNA complex. Biol Pharm Bull. 2009;32:706–10. doi: 10.1248/bpb.32.706. [DOI] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]