Abstract
The calcium-activated protein phosphatase, calcineurin, lies at the intersection of protein phosphorylation and calcium signaling cascades, where it provides an essential nodal point for coordination between these two fundamental modes of intracellular communication. In excitatory cells, such as neurons and cardiomyocytes, that experience rapid and frequent changes in cytoplasmic calcium, calcineurin protein levels are exceptionally high, suggesting that these cells require high levels of calcineurin activity. Yet, it is widely recognized that excessive activation of calcineurin in the heart contributes to pathological hypertrophic remodeling and the progression to failure. How does a calcium activated enzyme function in the calcium-rich environment of the continuously contracting heart without pathological consequences? This review will discuss the wide range of calcineurin substrates relevant to cardiovascular health and the mechanisms calcineurin uses to find and act on appropriate substrates in the appropriate location while potentially avoiding others. Fundamental differences in calcineurin signaling in neonatal verses adult cardiomyocytes will be addressed as well as the importance of maintaining heterogeneity in calcineurin activity across the myocardium. Finally, we will discuss how circadian oscillations in calcineurin activity may facilitate integration with other essential but conflicting processes, allowing a healthy heart to reap the benefits of calcineurin signaling while avoiding the detrimental consequences of sustained calcineurin activity that can culminate in heart failure.
Keywords: Calcineurin, Heart failure, Microdomains, Transmural gradient, Circadian rhythms
1. Introduction
It has been forty years since Claude Klee began her seminal work deciphering the structure and regulation of the calcium-activated protein phosphatase calcineurin [1–3] and almost twenty years since Jeff Molkentin and Eric Olson demonstrated that sustained activation of calcineurin in cardiomyocytes is sufficient to promote hypertrophic remodeling, decompensated failure, and arrhythmogenic death [4]. Numerous studies have subsequently verified that inhibition of calcineurin is an effective method for blunting hypertrophic growth and protecting the heart from both initial oxidative damage and subsequent pathological remodeling in response to a variety of insults [5–10]. Hundreds, if not thousands of papers have been published citing a role for calcineurin in cardiovascular disease, yet there is much we still do not understand regarding control and specificity of this enzyme that must function in the calcium-rich environment of a continually contracting heart without initiating a pathological signaling cascade. Here, we will review regulation of calcineurin and mechanisms it uses to target specific substrates within the myocardium. Key features unique to calcineurin signaling in the heart will be addressed including: fundamental differences in calcineurin signaling in neonatal verses adult cardiomyocytes and the importance of maintaining heterogeneity in calcineurin activity across the myocardium, particularly as it relates to ion channel activity and arrhythmogenesis. Finally, we will discuss how a circadian pattern of calcineurin activity may allow a healthy heart to reap the benefits of calcineurin signaling while avoiding the detrimental consequences of sustained calcineurin activity that can culminate in heart failure.
2. Calcineurin structure and regulation
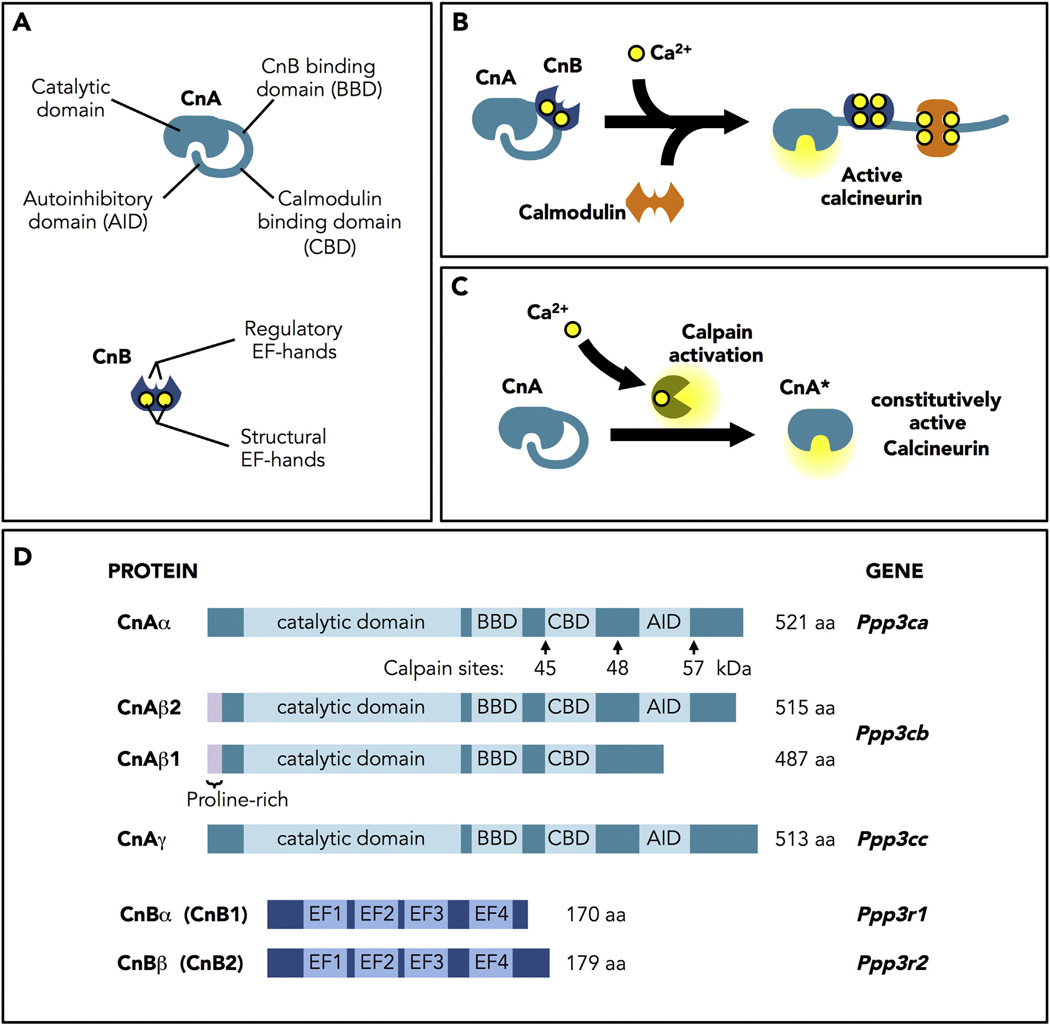
Calcineurin is a heterodimer composed of a 60-kDa catalytic subunit (CnA) and a 19-kDa regulatory subunit (CnB). CnA contains an N-terminal catalytic domain, a CnB binding domain, a calmodulin binding domain, and a C-terminal autoinhibitory domain (AID). CnB has four EF-hand Ca2+-binding sites: two structural sites that bind Ca2+ with high affinity in the nM range, that are always occupied [11, 12], and two regulatory sites that bind in the µM range (Fig. 1A). Binding of Ca2+ to the regulatory sites initiates a series of conformational changes that allow binding of a calmodulin/Ca2+ complex and a change in the orientation of the AID to expose the active site [13, 14] (Fig. 1B). Truncation of CnA to remove the AID yields a constitutively active phosphatase (CnA*) that no longer responds to Ca2+/calmodulin (Fig. 1C). It is this truncated form, under the control of the alpha myosin heavy chain (αMHC-CnA*) that has now been used by many investigators to study the effects of calcineurin activity in the myocardium [4].
Fig. 1.
Calcineurin structure, regulation and activation. A. Structure of the catalytic (CnA) and regulatory (CnB) subunits of calcineurin. CnA contains an N-terminal catalytic domain, a CnB binding domain, a calmodulin binding domain, and a C-terminal autoinhibitory domain (AID). CnB has four EF-hand Ca2+-binding sites: two structural sites that bind Ca2+ with high affinity in the ηM range, that are always occupied, and two regulatory sites that bind in the µM range. B. Model of calcineurin activation. Binding of Ca2+ to the regulatory sites initiates a series of conformational changes that allow binding of a calmodulin/Ca2+ complex and a change in the orientation of the AID to expose the active site. C. Constitutively active calcineurin. Truncation of CnA to remove the AID yields a constitutively active phosphatase (CnA*) that no longer responds to Ca2+/calmodulin. D. Calcineurin proteins and genes. In mammals, three genes encode CnA (α, β, and γ). Only CnAα (PPP3CA) and CnAβ (PPP3CB) are expressed in the heart. Of the two genes encoding the CnB regulatory subunit, only CnBα (PPP3R1) is expressed in the heart.
During the cardiac cycle, cytosolic Ca2+ raises from 0.1 µM to 1 µM [15], reaching well within the range to activate calcineurin, which requires only 0.6 µMfor half maximal activity in the presence of 20 µMcalmodulin [16]. It is not completely understood how calcineurin avoids beat-to-beat activation. However, many factors are likely involved, including the rate of association with calmodulin and regional differences in availability of this accessory protein [17]. An important concept that has immerged is the role of signaling microdomains that are define by the local concentrations of Ca2+, calmodulin, and target substrate [18–20]. Free calmodulin in an adult cardiomyocyte is estimated in the range of 50 to 100 nM [21]. This is low enough to impact calcineurin's threshold for activation by Ca2+. For instance, at 30 nM calmodulin, half maximal activation of calcineurin requires 1.3 µMCa2+ [16]. Therefore, in a subcellular domain with limited free calmodulin, calcineurin might only be activated when there is an unusually high systolic Ca2+ transient, such as those following adrenergic input, whereas, calcineurin in a domain with free access to calmodulin would be able to respond to a lower Ca2+ input.
In mammals, three genes encode CnA (α, β, and γ). Only CnAα (PPP3CA) and CnAβ (PPP3CB) are expressed in the heart. Of the two genes encoding the CnB regulatory subunit, only CnBα/CnB1 (PPP3R1) is expressed in the heart (Fig. 1D). Transcript and protein levels for CnAβ, but not CnAα, increase in response to mechanical stress or hypertrophic agonists in a calcineurin-dependent fashion [10, 22, 23]. Mice lacking CnAα have a severely shortened life span, therefore, a rigorous analysis of their cardiovascular phenotype has not been undertaken [24–26]. The hearts of mice lacking CnAβ are smaller than wild type littermates and show reduced hypertrophy in response to pressure overload [27]. Taken together, these findings suggest that CnAβ is the dominant isoform in the heart and that it may participate in a feed-forward amplification loop.
2.1. Cardiac expressed isoforms
Although CnAα and CnAβ isoforms are structurally similar, there are some notable differences. Purified enzyme composed of CnAβ is less sensitive to inhibition by FK506 [28]. CnAβ also contains a unique proline-rich N-terminal domain that increases its affinity for certain substrates [29]. Of particular note, the bHLH transcription factor ATOH8 was recently shown to interact specifically with CnAβ but not CnAα [30]. ATOH is involved in skeletal muscle and cardiac development [31, 32], however its role in heart disease has not yet been explored.
A unique splice variant of CnAβ was recently identified, CnAβ1, that lacks the AID [33]. Similar to the C-terminal truncated CnA* proteins, the CnAβ1 variant is constitutively active. Remarkably, however, a cardiomyocyte-specific αMHC-Cnβ1 transgene does not provoke hypertrophy but exerts beneficial effects following myocardial infarction by promoting vascularization [34, 35]. How this is accomplished is not yet understood, however, interesting data is emerging that suggests CnAβ1 may be important for proper localization and signaling of mTORC2 complexes [34, 36].
Cleavage of either CnAα or CnAβ by the Ca2+-activated protease, calpain, generates constitutively active forms of calcineurin that lack the AID (Fig. 1C) [37–39]. Calpain activation following ischemia reperfusion (I/R) damages the myocardium by cleaving an array of proteins involved in contraction and its regulation [40–42]. Elevated levels of calpain-cleaved calcineurin are found in the hearts of patients with congestive heart failure [43, 44]. Once cleaved by calpain, calcineurin would remain active until removed by proteolysis or suppressed by interaction with an inhibitory protein.
Because CnBα is the only calcineurin regulatory subunit expressed in the heart, several groups have used tissue-specific deletion of CnBα to examine the consequences of eliminating calcineurin activity in the myocardium [45, 46]. Cardiac metabolism and function decline in hearts lacking CnBα. The mice began to die of arrhythmias around 3months of age, consistent with calcineurin playing a role in supporting cardiac electrophysiology.
Finally, the Fe—Zn active site of calcineurin is susceptible to reversible, Ca2+-dependent oxidative inactivation [47, 48]. This property may help limit unrestrained calcineurin activity in the setting of oxidative stress, which often occurs in conjunction with Ca2+ overload.
2.2. Targeting of substrates to calcineurin
The catalytic cleft of calcineurin is relatively shallow and can accommodate a wide range of phospho-serine,-threonine and -tyrosine substrates [49, 50]. There is no conserved recognition motif surrounding the phosphorylated residue. Instead, targeting of most substrates relies on two docking motifs, PxIxIT and LxVP, found elsewhere in the target protein, that were initially identified in the well-characterized calcineurin substrate, Nuclear Factor of Activated T-cells (NFAT) [51, 52]. The PxIxIT domain binds to the catalytic domain of CnA, regardless of whether the enzyme is active or inactive, thereby increasing the effective local concentration of the substrate [53–55]. PxIxIT domains vary in their binding affinities, allowing for substrate selection based both on concentration and binding strength. The second docking motif, LxVP, binds to a hydrophobic pocket at the CnA/CnB interface, which is only accessible when calcineurin is active [14, 56]. An alternative model for LxVP binding has been proposed in which the LxVP docking site overlaps with the PxIxIT docking site [57]. Whether this mode of docking of substrates to CnA alone occurs when CnB is not present remains to be determined, but could impact interpretation of studies in which CnB has been deleted as a method of eliminating calcineurin activity.
2.3. Pharmacological inhibition of calcineurin
Calcineurin is the target of the major immunosuppressive drugs Cyclosporin A (CsA) and FK506 which form complexes with two different classes of immunophilins: cyclophilins and FK506 binding proteins respectively [58, 59]. The drug-immunophilin complexes bind in the same hydrophobic CnA/CnB grove used for docking of LxVP [56, 60–62], thereby blocking access of substrate proteins. The drug/immunophilin complexes do not actually block the catalytic domain, as the enzyme can still readily dephosphorylate p-nitrophenyl phosphate (pNPP), a non-proteinaceous, small molecule substrate often used in calcineurin assays [59]. Paradoxically, activity of calcineurin toward pNPP is stimulated as much as 4-fold by the presence of the drug complexes [59, 63, 64].
A number of small peptide inhibitors have been developed based on the PxIxIT, LxVP, and AID motifs (VIVIT) [65, 66]. A cell permeable derivative of PxIxIT (VIVIT) has been used successfully to inhibit pressure overload hypertrophy in vivo [67]. Because this targeted approach avoids the off-target side effects of CsA and FK506, it has been proposed as the basis for developing new therapeutic approaches aimed at cardiovascular disease [9]. For additional information Seiber and Baumgrass provide a comprehensive review of pharmacological calcineurin inhibitors [68].
2.4. Calcineurin inhibition by endogenous proteins
2.4.1. CABIN-1/CAIN (calcineurin binding protein 1)
CABIN-1 interacts with calcineurin via a PxIxIT-like domain [69, 70] as well as acting directly as a co-repressor of the transcription factor MEF2 [71, 72]. Although cardiomyocyte-specific over expression of CABIN-1 inhibits hypertrophy in vivo [8], it is uncertain whether the endogenous protein acts in this capacity, as calcineurin activity and NFAT translocation are unaltered in transgenic mice in which the calcineurin and MEF2-interacting domains of CABIN-1 have been deleted [73]. CABIN-1 is a very large, multidomain protein (N200-kDa), and therefore, may primarily function as a scaffold for calcineurin, facilitating its interactionwith other proteins, rather than acting to inhibit calcineurin.
2.4.2. Carabin (TBC1 domain family member 10C, TBC1D10C)
Carabin is a bi-functional protein inhibiting both calcineurin and the small GTPase RAS [74, 75]. Carabin mRNA and protein levels decline in models of pressure overload and in human heart failure [76]. Loss of Carabin in vivo exacerbates pressure-overload hypertrophy and failure, whereas, cardiomyocyte-specific overexpression is protective [76, 77].
2.4.3. Calcineurin homologous proteins (CHP1, 2 and 3)
CHPs are structurally related to CnB and carry out a variety of cellular functions apparently unrelated to calcineurin [78], however, when over expressed, they are capable of both negatively [79] and positively [80] influencing calcineurin activity. The role of these proteins in cardiac function has not been explored.
2.4.4. Regulators of calcineurin (RCAN1, 2 and 3)
RCANs, also known as ADAPT78,DSCR1,MCIP1, and calcipressin [81] are relatively small proteins that inhibit calcineurin [82–84]. In addition to PxIxIT and LxVP motifs, RCANs contain a C-terminal domain unique to the RCAN family that is sufficient for calcineurin binding and inhibition [85–87]. Remarkably, in purified, in vitro assays, recombinant RCAN1 is capable of competitively inhibiting calcineurin-mediated dephosphorylation of both peptide substrates and pNPP [85, 87]. The ability to inhibit pNPP dephosphorylation makes them unique among calcineurin inhibitors, including CsA and FK506. Expression of the Rcan1.4 isoform is under calcineurin/NFAT control and thus functions as a feedback inhibitor of calcineurin [88]. Expression of a cardiomyocyte-specific αMHC-Rcan1 transgene protects mice from a diversity of pathological stresses including pressure overload, damage from ischemia-reperfusion (I/R) and pathological remodeling following a myocardial infarction [5, 6, 89]. The hearts of animals deficient for RCAN1 (Rcan1−/−) are more susceptible to damage from I/R [89, 90]. Surprisingly, the hearts of Rcan1−/− mice are smaller and have a blunted hypertrophic response to pressure overload [90, 91]. This may be the result of physiological compensation or reflect RCAN1's impact on other pathways [92–94]. It has been suggested that RCAN1 may act as a chaperone or targeting protein that facilitates calcineurin signaling in vivo, although, rigorous biochemical evidence for this is currently limited [95, 96].
In addition, there are a wide variety of microRNAs relevant to cardiac remodeling and heart failure that have been proposed to act upon or be regulated by calcineurin that are beyond the scope of this review. Their importance to control and integration of calcineurin signaling in cardiac health and disease should not be overlooked [97].
3. Methods for measuring calcineurin activity
Standard biochemical assays of calcineurin activity in tissue extracts are carried out under conditions of excess Ca2+ and calmodulin [98]. They therefore do not reflect actual in vivo activity but simply provide a measure of the total potential calcineurin activity available if all the calcineurin in the cell were activated. As an in vivo alternative, many groups have used quantification of Rcan 1.4 transcript levels, as its expression is directly under the control of calcineurin/NFAT [88]. However, this approach also has limitations, as it only assesses calcineurin activity relative to NFAT-mediated transcription and therefore may not reflect the action of calcineurin on other substrates.
A widely used approach has been the use of luciferase or β-galactosidase reporters under the control of NFAT sites, delivered by transient transfection or adenoviral infection. Similarly, other groups have also used fluorescent proteins to monitor transcriptional activity of NFAT in the physiological context of living cells. The main advantages of the fluorescence-based assays over other traditional and classical luciferase assays are their application to individual living cells, as they do not require cell lysates for quantification. Based on the expression of GFP or RFP under the control of an NFAT-sensitive promoter, this assay displays a dose-sensitivity to different levels of NFAT and calcineurin activation [99]. To monitor activity in vivo a number of groups have developed transgenic mice carrying an NFAT-luciferase or β-galactosidase reporter [100, 101]. Even with these in vivo techniques it is important to remain aware of the potential impact of chromatin context as different reporters show different patterns of response dependent upon the location of the reporter within the genome.
A number of genetically-encoded, fluorescence resonance energy transfer (FRET) reporters have been developed recently that allow calcineurin activity to be assessed in live cells. The CaNAR reporter is based on the regulatory domain of NFAT tagged on opposing ends with a cyan and a yellow fluorescent protein. Dephosphorylation by calcineurin causes a conformational change that increases FRET transfer between the two ends [17]. This can be combined with a Ca2+ sensor to integrate Ca2+ dynamics with processing of a specific calcineurin substrate. Targeting this reporter to the cytosol, plasma membrane, mitochondria, or the ER, has been used to demonstrate that activation of the reporter in response to the same Ca2+ signal can be very different in different regions of a cell [17]. For instance, in a pancreatic β cell line, the cytoplasmic and plasma membrane reporter displayed a single, integrated response to an oscillatory Ca2+ input. In contrast, the ER and mitochondrial reporter oscillated directly in concert with the Ca2+ transient due to limited calmodulin availability proximal to the ER and rapid reversal of the reporter by cAMP activated protein kinase A (PKA) phosphorylation [17].
A new generation of FRET reporters, DuoCaN and UniCaN, has been tested in neonatal and adult cardiomyocytes and provides a direct readout of the calcineurin holoenzyme itself rather than its action on a particular substrate [102]. These reporters reveal important, fundamental differences in the pattern of calcineurin localization and activation at these two stages of development. In neonatal cardiomyocytes the reporters were homogeneous throughout the cell and activated in response to a single Ca2+ pulse. Whereas, in isolated adult myocytes the reporters localized to T-tubules, primary sites Ca2+ release during contraction, consistent with the pattern described for endogenous calcineurin [103, 104]. Furthermore, in adult myocytes the reporters did not respond to either single Ca2+ transients or low frequency pacing, but required pacing frequencies sufficient to raise diastolic Ca2+ levels between beats. Thus, during early development of the heart, Ca2+ released during contraction may be sufficient to cause sustained activation of calcineurin, thereby promoting hypertrophic growth. Whereas, calcineurin in the adult heart remains unresponsive to normal, contraction coupled Ca2+ transients. This also means that mechanistic studies carried out in neonatal cardiomyocytes may not always be directly applicable to calcineurin signaling in the adult heart. It is important to note, however, that during failure, cardiomyocytes are thought to develop characteristics typical of immature cardiomyocytes. It is not known whether the pattern of calcineurin activation likewise reverts to a fetal pattern, or if this occurs in isolated adult cardiomyocytes that are maintained in culture over time.
4. Calcineurin substrates relevant to cardiovascular health
Calcineurin has both immediate and long-term effects on cardiac function and adaptation. Rapid responses include direct changes in the function, activity, and/or localization of target proteins, changes that in general can be reversed rapidly by rephosphorylation. In contrast, calcineurin-dependent changes in transcription have longer-lasting consequences. First, we will discuss transcription factors controlled by calcineurin that have particular relevance to cardiovascular health. Then, cytoplasmic substrates not related to transcription will be discussed in the context of the specific subcellular domains in which they reside and the proteins that help organize these domains.
4.1. Transcription factors controlled by calcineurin
4.1.1. NFAT
NFATs are the most fully characterized calcineurin substrates. They include: NFATc1 (a.k.a. NFAT2), NFATc2 (NFAT1), NFATc3 (NFAT4) and NFATc4 (NFAT3). The regulatory domains of NFATs are highly phosphorylated in resting cells and flanked by a PxIxIT and LxVP motif at either end [56, 105]. Dephosphorylation by calcineurin causes translocation to the nucleus to activate target genes. NFATs tend to function as heterodimers in cooperation with other transcription factors. Of particular note relative to cardiac growth and remodeling areMEF2 and GATA, which can also be directly activated by calcineurin-mediated dephosphorylation. Cardiac-specific over expression of an activated NFATc4 is sufficient to drive hypertrophy [4], whereas, forced expression of a dominant-negative NFATc4 will blunt calcineurin-dependent hypertrophy [106], demonstrating that calcineurin-mediated remodeling acts, at least in part, via NFAT-dependent transcriptional control. At the transcript level, NFATc2 is the most abundant NFAT isoform in the heart and indeed, pathological growth in response to pressure overload or angiotensin II (AngII) infusion is markedly blunted in Nfatc2−/− mice [107]. Physiological hypertrophy in response to voluntary exercise is unaltered in these mice, suggesting that either NFATc2 is not involved in this mode of hypertrophic growth or that the other NFATs are sufficient to compensate. Pathological hypertrophy is also reduced in Nfatc3−/− mice but is unaltered in Nfatc4−/− mice [108]. Deficiency for Nfatc1 is embryonic lethal due to defects in cardiogenesis [109] and as yet, a cardiomyocyte-specific deletion has not been studied in the context of hypertrophy. Although both NFATc2 and NFATc3 appear to mediate pathological hypertrophy, they differ in their affinity for calcineurin as well as in the dynamics of their response. For instance, in HEK293 cells, NFATc2 translocates to the nucleus in response to Ca2+ released from plasma membrane channels and its subsequent exit from the nucleus is relatively slow. In contrast, translocation of NFATc3 to the nucleus requires an increase in both nuclear and cytoplasmic Ca2+, whereas, it exits from the nucleus rapidly [110]. Using NFAT-GFP fusion proteins in isolated adult atrial and ventricular cardiomyocytes, NFATc1 and NFATc3 showed different responses to AngII or endothelin-1 that depended upon both cell type and NFAT isoform [111]. Isoform-specific NFAT activation has also been reported in skeletal muscle [112, 113]. Although it is clear that NFAT-dependent changes in transcription are an important aspect of calcineurin-mediated cardiac remodeling, the identity of the target genes that are most relevant is less clear.
Calcineurin can regulate transcription through a number of other transcription factors in addition to NFAT. Of particular note, relative to cardiac remodeling, are the cAMP response element binding protein (CREB)-regulated transcriptional coactivators (CRTCs), the forkhead box protein 01 (FOXO1) and the transcription factor EB (TFEB).
4.1.2. CRTC
Similar to NFATs, dephosphorylation of CRTCs by calcineurin promotes translocation to the nucleus where they act in conjunction with CREB to promote gene expression. Although the role of CRTCs is yet to be studied in the heart, in other tissues they act to integrate hormonal and metabolic signals [114–116] and promote mitochondrial biogenesis [117–119]. The ability of CRTCs to integrate Ca2+ and cAMP signals makes them ideal candidates for metabolic regulators, particularly in the setting of increased adrenergic stress.
4.1.3. FOXO1
FOXO1 is a key factor in metabolic remodeling of the heart in diabetic cardiomyopathy and post-ischemic heart failure [120–123]. In the ischemic brain, calcineurin has been shown to dephosphorylate FOXO1 forming a complex that translocates along with FOXO1 into the nucleus acting in conjunction with NFAT to drive expression of Fas-Ligand [124], which is also released by the ischemic heart [125]. In turn, there is evidence that FOXO1 activity can provide feedback inhibition of calcineurin [126–128], affording additional integration of these two pathways.
4.1.4. TFEB
TFEB is a basic-helix-loop-helix leucine-zipper transcription factor that is considered a master regulator of autophagic and lysosomal biogenesis [129, 130]. Dephosphorylation of TFEB by calcineurin causes activation and translocation to the nucleus to promote coordinate expression of target genes. This interaction may help integrate calcineurin-dependent hypertrophic growth with the degradative processes carried out by autophagy during structural and metabolic remodeling of the myocardium [131, 132]
It is important to note that calcineurin control of transcription factor activity is not necessarily always due to direct changes in phosphorylation of the transcription factor. For instance, in response to hypertrophic agonists, there is a calcineurin-dependent increase in MEF2A and MEF2D protein levels due to an increase in the abundance of Polypyrimidine Tract Binding Protein (PTB) which then acts to increase translation of MEF2 proteins without altering transcript abundance [133]. It is not known whether PTB increases translation of other proteins with cardiovascular relevance.
4.2. Targeting calcineurin to cytoplasmic domains
Both direct immunohistochemistry and the localization of FRET reporters indicate that in adult cardiomyocytes the majority of calcineurin protein is found in the vicinity of the T-tubules, which are deep invaginations of the sarcolemma allowing for the close association between voltage-activated L-type calcium channels (LTCC) in the sarcolemma and ryanodine receptors (RyR2) in the sarcoplasmic reticulum (SR). They form the fundamental dyad for Ca2+-induced Ca2+-release involved in excitation-contraction coupling. Many other membrane channels and receptors are also found in T-tubules, which directly overlie the Z-line, or Z-disc of the sarcomere. Thus, calcineurin is in close proximity to many of the proteins involved in excitation-contraction coupling and mechanotransduction. This does not preclude the possibility of other pools of calcineurin, either free in the cytoplasm or tethered elsewhere.
Intracellular Ca2+ release from the SR is required for cardiac muscle contraction. Indeed, it is postulated that during heart failure impaired Ca2+ release results in decreased muscle contraction (systolic dysfunction) and defective Ca2+ removal hampers relaxation (diastolic dysfunction). There are numerous examples of animal models with diastolic dysfunction and hypertrophy associated with elevated calcineurin activity, [4, 134, 135]. Calcineurin specifically responds to sustained, low-amplitude Ca2+ but can ignore transient, high amplitude Ca2+ spikes [136], thus, the kind of sustained elevation in diastolic Ca2+ that occurs with diastolic dysfunction is ideally poised to support calcineurin activation and subsequent hypertrophic remodeling [137]. Diastolic Ca2+ levels depend upon the interplay between RyR release and reuptake by SERCA [138]. Normal Ca2+ handling is disrupted in patients with heart failure due to a chronic hyper adrenergic state that hyper activates then subsequently desensitizes beta-adrenergic receptors, which control RyR and SERCA function via PKA [139].
In the heart, calcineurin is often found in close association with PKA. A kinase anchor proteins (AKAPs) are scaffolding proteins that help to form multimolecular signaling complexes that contain PKA. Calcineurin binds to several AKAPs that play a prominent role in its subcellular targeting. In the heart these include: the beta isoform of AKAP6 (AKAP6β/mAKAPβ), localized to the nuclear envelope; AKAP5 (AKAP79/AKAP150), AKAP12 (Gravin), and AKAP7 (AKAP15/AKAP18), localized to the plasma membrane, T-tubules, and ER/SR; and AKAP1 (AKAP121/AKAP84), localized to ER/SR and outer membrane of mitochondria (OMM) [140].
4.2.1. AKAP6 (AKAP6β/mAKAPβ)
Located at the nuclear envelope, AKAP6 does not use a PxIxIT motif to tether calcineurin [141]. As such, the interaction neither competes with substrates for binding nor inhibits catalytic activity. In neonatal cardiomyocytes and C2C12myoblasts, AKAP6 is essential for agonist induced NFAT translocation [142] and organization of a calcineurin/MEF2 regulatory complex [143]. AKAP6 also acts as a scaffold for HDAC4 and kinases that phosphorylate it, thereby releasing HDAC suppression of MEF2-dependent transcription [144]. AKAP6 has binding domains for both RYR2 [145] and the sodium calcium exchanger (NCX1) [146], a subset of which localize to the nuclear envelope. Mice with a cardiomyocyte-specific deletion of AKAP6 are resistant to pressure overload or agonist induced heart failure [144]. AKAP6 binds a wide array of other proteins and signaling molecules important in cardiac stress responses [147], therefore, AKAP6 provides a platform near the nucleus for integrating calcineurin signaling with a diversity of cytoplasmic signals known to mediate transcriptional responses to cardiac stress.
The AKAP6/PKA/calcineurin complex also mediates control over nuclear cytoplasmic shuttling of myopodin (synaptipodin 2), an actin-bundling protein that relocates from the nucleus to the Z-disc of myocytes upon differentiation but returns to the nucleus in response to stress [148]. Phosphorylation by PKA promotes release from the Z-disc and dephosphorylation by calcineurin prevents nuclear translocation [149]. Myopodin's role in heart failure is unknown.
4.2.2. AKAP5 (AKAP79/AKAP150)
AKAP5mediates calcineurin association with several channels in the sarcolemma, including the LTCC. Quantitative analysis of a purified complex indicates that four calcineurin heterodimers bind per AKAP5 dimer [150]. Therefore, although the two PxIxIT motifs in the AKAP5 dimer would occupy two of the PxIxIT docking domains on calcineurin, two docking domains remain available for binding of calcineurin substrates, such as NFAT or nearby channel proteins. In adult cardiomyocytes there are two distinct pools of LTCCs, both of which are associated with AKAP5/calcineurin complexes [151]. The majority of LTCCs localize to T-tubules where the majority of calcineurin protein is also located. A small fraction of LTCCs are also found associated with AKAP5/calcineurin in caveolae, a specialized lipid raft domain defined by the presence of caveolin. Caveolae function both in endocytosis and as platforms for signalosomes [152, 153]. Inhibition of LTCC activity specifically within these Cav-3 containing domains has minimal impact on total LTCC current (ICa,L) or myocyte contractility, but eliminates Ca2+ influx-induced nuclear translocation of NFATc3 [154]. Thus, input from LTCC localized to caveolae plays an important role in specifying LTCC/calcineurin/NFATc3 signaling. This is supported by studies demonstrating that cardiomyocytes isolated from mice lacking AKAP5 or expressing a mutant form of AKAP5 deleted for the calcineurin PxIxIT binding motif (AKAP5-ΔPIX) are unable to sustain nuclear translocation of NFATc3 in response to adrenergic stimulation [155].
CIB1 (Ca2+-and integrin-binding protein-1) is a small EF-hand protein similar in structure to CnB that is also involved in mediating an interaction between calcineurin and LTCC [156, 157]. CIB1 expression is elevated in the atria of patients with atrial fibrillation where it can be co-immunoprecipitated with CnB, LTCC, and NCX1 [158]. CIB1 levels increase in the setting of pathological hypertrophy, but not physiological hypertrophy [157]. Loss of CIB1 blunts, whereas over expression increases, the hypertrophic response to pressure overload [157].
In heart failure there is a loss of T-tubule structure [159, 160] and a decrease in the fraction of LTCC and NCX channels associated with T-tubule domains without a loss of total channel activity in individual myocytes [161–164]. This shift in LTCC pools during failure may increase the pool of LTCC available for flux through calcineurin/NFATc3 signaling in a feed-forward process. In turn, calcineurin increases LTCC activity in failing heart [165]. It is generally accepted that adrenergic stimuli increases ICa,L via PKA phosphorylation of the LTCC [166]. Yet, peak ICa,L is increased in αMHC-CnA* transgenic mice as well as in those subjected to pressure overload failure. Furthermore, this increase is can be reversed by calcineurin inhibition but not PKA inhibition [167, 168]. The finding that PKA activation of ICa,L is specific to T-tubules and is lost upon detubulation might explain this discrepancy [164, 169, 170]. One possibility is that modification of ICa,L by calcineurin occurs throughout the cell but is masked by the opposing action of PKA in T-tubules. Alternatively, calcineurin control of ICa,L may only occur outside of T-tubule domains.
AKAP5/calcineurin is also involved in the decline in fast transient outward K+ current (Ito) that is associated with heart failure and pathological hypertrophy [171]. The αMHC-CnA* transgenic mice have a significant reduction in Ito density that can be reversed by treatment with CsA [172]. In isolated adult cardiomyocytes and intact hearts calcineurin decreases Ito, in part through AKAP5-dependent activation of NFATc3 leading to transcriptional repression of the expression of several K+ channel subunits including Kv4.3, Kv4.2, and KChIP2 [155, 173, 174]. The mechanism of NFATc3-dependent repression is not fully understood and could be secondary to expression of an unidentified repressor. It is likely that calcineurin can also affect Ito independent of transcription. In neurons, AKAP5/calcineurin has been shown to form a complex with Kv channels where the opposing forces of phosphorylation by PKA and dephosphorylation by calcineurin regulate activity-dependent trafficking of Kv4.2 [175], with calcineurin promoting surface expression. This has not yet been demonstrated in cardiomyocytes, but changes in the ability to recycle channels appropriately could theoretically contribute to the calcineurin-dependent decline in Ito function. In cardiomyocytes the AKAP5/PKA/calcineurin complex has been shown to be required for internalization and recycling of β1-adrenergic receptors (β1-AR) [176]. Calcineurin's role in turnover of the many other ion channels found in lipid rafts and how this impacts cardiac function is an important avenue for future investigation.
4.2.3. AKAP12 (Gravin/AKAP250)
AKAP12 targets PKA, PKC and calcineurin to β2ARs localized primarily to caveolae in the plasma membrane. The complex mediates desensitization and recycling of the receptor by controling its phosphorylation [177]. Inhibition of calcineurin blocks resensitization following agonist removal [178]. In contrast, the interaction of AKAP5 with β2ARs is thought to mediate the switch from stimulatory Gs to inhibitory Gi mediated signaling [179]. Whereas AKAP5 is absolutely required for the recycling of B1ARs, which couple only via Gs, and is localized throughout the T-tubules and plasma membrane [176].
4.2.4. AKAP7 (AKAP15/AKAP18)
Differential spicing generates a variety of AKAP7 isoforms [180]. Although AKAP7 does not bind calcineurin directly, it can compete with AKAP5/calcineurin for binding LTCC [181]. However, mice lacking AKAP7 respond normally to adrenergic stimulation [182], suggesting that the AKAP5 complex plays the primary role in adrenergic responses of the LTCC.
AKAP7 interacts indirectly with calcineurin by providing a scaffold for the protein phosphatase inhibitor 1 (I-1), a calcineurin substrate that controls the activity of the protein phosphatase 1 (PP1) [183]. Phosphorylation of I-1 at Thr35 by PKA induces selective inhibition of PP1 [184]. AKAP7/PKA/I-1 complexes are found associated with the SR and phospholamban (PLN) where PKA-mediated phosphorylation of I-1 helps prolong adrenergic responses at the SR by preventing their reversal by PP1. Perturbations in PP1 regulation by I-1 have been implicated in heart failure [185]. Calcineurin dephosphorylates I-1 at Thr35, releasing inhibition of PP1 [186], and providing a mechanism through which calcineurin impacts a wider range of cellular phosphatase actives in the heart. Because calcineurin has not been identified as a component of AKAP7 complexes, it may be that tethering I-1 to the SR via an AKAP7 scaffold helps to sequester this specific pool of I-1 so as to actually minimize reversal by calcineurin. Consistent with this model, PLN phosphorylation is significantly lower in cardiomyocytes deleted for CnB [46], suggesting that on the whole, calcineurin may act to promote PLN phosphorylation rather than decreasing it.
4.2.5. AKAP1 (AKAP121/AKAP84) and calcineurin's impact on mitochondrial dynamics
Mitochondrial dysfunction has emerged as a critical factor in the progression of heart failure [187–190]. Impaired mitochondrial biogenesis and loss of mitochondrial content is associated with the transition from compensated hypertrophy to failure in patients with heart disease [191]. Mitochondrial electron transport is impaired in αMHC-CnA* transgenic mice and superoxide production is increased both before and after I/R [192]. Mitochondrial changes reported in mice with a cardiomyocyte-specific deletion of CnB range from deficiencies in the ability to oxidize fatty acids [46] to aberrant mitochondrial architecture [45]. Taken together, these findings suggest that either sustained activation of calcineurin or insufficient calcineurin activity has a profound negative effect on cardiac mitochondrial function. Diverse mechanisms are likely involved, however, calcineurin's influence on mitochondrial dynamics is emerging as an important aspect.
Mitochondria are dynamic organelles that undergo a continuous process of fission and fusion that is essential for their repair and regeneration. Calcineurin promotes fission by dephosphorylating Dynamin Related Protein 1 (DRP1), which contains an LxVP calcineurin-docking motif [193], thereby activating it to initiate fission at the OMM [194, 195]. PKA rephosphorylates DRP1, thus, promoting fusion. Intermyofibrillar mitochondria in the ventricular wall are often described as spanning from Z-band to Z-band, thereby placing sites of fission in alignment with the primary pool of calcineurin in adult cardiomyocytes. AKAP1 interacts with DRP1, PKA and calcineurin [196–198]. Although the nature of these interactions is not yet well defined. The extent to which intermyofibrillar mitochondria undergo fission and fusion is currently debated [199] and may be limited due to the spatial constraints imposed by close proximity to the sarcomere. However, genetic data indicate that fission and fusion are essential to cardiovascular health as mice with cardiomyocyte-specific disruption of mitofusions 1 and 2 (Mfn1/Mfn2) (to prevent fusion) or Drp1 (to prevent fission) progress to heart failure [200–203].
4.2.5.1. Calcineurin-dependent mitochondrial fission is required for hypertrophy
Mitochondrial fission is required for hypertrophic growth of both neonatal [204, 205] and adult cardiomyocytes [206]. Expression of a dominant-negative DRP1 or treatment with mdivi-1, a DRP1 inhibitor, is sufficient to block induction of hypertrophic growth by norepinephrine. Conversely, depletion of the fusion protein MFN2 is sufficient to stimulate hypertrophic growth without agonist treatment [205]. On a certain level it is intuitive that fission must occur during maturation of neonatal cardiomyocytes to convert the continuous reticulum found in embryonic cells into an ordered intermyofibrillar array, however, treatment with mdivi-1 also blunts hypertrophic growth and preserves cardiac function in the adult heart in the setting of pressure overload induced heart failure [206]. Furthermore, mdivi-1 reduces infarct size and preserves cardiac function following ischemia reperfusion [207, 208]. The extent to which calcineurin-mediated mitochondrial fission contributes to other forms of ischemic and non-ischemic heart failure, and the potential for therapeutic targeting remains a promising avenue of research [209].
4.2.5.2. Additional mitochondrial targets
BAD, a pro-apoptotic Bcl2 protein, is dephosphorylated by calcineurin promoting its translocation to the OMM [210] where it initiates opening of the mitochondrial permeability transition pore (MPTP), release of cytochrome c and activation of a caspase-3 apoptotic cascade [211, 212]. This mechanism contributes to neuronal death in the hippocampus following ischemia [213], but its role in triggering death of cardiomyocytes is less well understood.
Cofilin is an actin-binding protein that translocates to mitochondria and induces cell death in response to oxidative stress [214, 215]. In cardiomyocytes, the cofilin phosphatase Slingshot 1L promotes cofilin translocation to mitochondria and increases damage from ischemia reperfusion [216]. Calcineurin activates Slingshot making cofilin-mediated damage an indirect target of calcineurin [217, 218]. Hyperphosphorylated cytoplasmic cofilin aggregates have been found in the hearts of patients with idiopathic cardiomyopathy [219]. Therefore, whether calcineurin's impact on cofilin is protective or pathological may depend on context.
4.2.6. Calcineurin's impact on the AKAP/PKA interaction
A varied range of signaling molecules are assembled by each AKAP. All AKAPs bind PKA, whereas only a subset of AKAP complexes also contain calcineurin. The catalytic domains of PKA (PKAcat) are bound to AKAPs in an inactive form by their regulatory submits (either RI or RII). Binding of cAMP to the R subunits releases activated PKAcat from the complex. Phosphorylation of RII at Ser-96 can both increase affinity for AKAP [220] and decrease its affinity for PKAcat [221, 222]. The RI subunit is pseudo-phosphorylated at the corresponding residue and is therefore not regulated by changes in phosphorylation. RII Ser-96 is a calcineurin substrate and contains an LxVP targeting motif [56]. In fact, a phospho-peptide from the RII subunit is the standard peptide substrate used to assay calcineurin activity in vitro. Dissociation of PKAcat from RII upon activation exposes the phospho-Ser-96 site allowing access by calcineurin or other phosphatases. Thus, in AKAP complexes containing RII, calcineurin is posed to facilitate reassociation of PKAcat with RII in the inactive form [223]. In heart extracts RII is primarily found in the phosphorylated form [224] and is decreased in human hearts with dilated cardiomyopathy [220].
4.3. Calcineurin and mechanotransduction
4.3.1. Mechanotransduction at the plasma membrane
Calcineurin activation in response mechanical stress lies downstream of Ca2+ entry through transient receptor potential canonical (TRPC) channels composed of homo or heterotetramers of either TRPC1/4/5 (stretch activated) or TRPC3/6/7 (activated by diacylglycerol downstream of G protein coupled receptors). Over expression of TRPC3/ 6/7 members promotes calcineurin/NFAT activation and hypertrophy [225, 226], whereas inhibition of either subtype blunts hypertrophy and failure in response to sustained agonist or pressure overload [227]. Expression of TRPC6 increases in response to pressure overload under the control of calcineurin/NFAT, thereby creating another feedforward amplification loop driving pathological hypertrophy [228]. The anti-hypertrophic effect of natriuretic peptides acts in part by inhibiting TRPC6 channel activity [229]. TRPC1/3/6 activation of calcineurin/NFAT signaling also occurs during stretch activation of myofibroblasts [230].
Polycystin-1 (PC-1), another member of the TRPC family of Ca2+ transporters, can act as a mechanosensor outside of its role as a component of primary cilia. In cardiomyocytes, PC-1 activates calcineurin/NFAT signaling in part by increasing LTCC stability [231]. Pressure overload hypertrophy and calcineurin activation are blunted in PC-1−/− mice.
Syndacan-4 is a transmembrane heparin sulfate proteoglycan that increases in the left ventricle following pressure overload [232, 233]. Syndacan-4 is required for activation of calcineurin/NFAT signaling in response to mechanical stress through TRPC6 in cardiac fibroblasts, promoting production of extracellular matrix and differentiation into activated myofibroblasts thereby increasing myocardial stiffness [233–235].
4.3.2. Mechanotransduction at the Z-disc
The Z-disc is emerging as a nodal point for cardiomyocyte signal transduction [236]. Calcineurin is tethered to the Z-disc via interactions with muscle LIM protein (MLP) [237] and calsarcin [104, 238]. Deficiency for MLP reduces activation of calcineurin following an adrenergic stimulus or biomechanical stress, whereas loss of calsarcin-1 increases mechanical stress activation of calcineurin, but has no effect on adrenergic activation of calcineurin.
PICOT (protein kinase C-interacting cousin of thioredoxin) interacts directly with MLP and can disrupt its interaction with calcineurin [239]. Over expression of PICOT inhibits calcineurin activation and blunts pressure overload-induced hypertrophy, whereas, mice heterozygous for a PICOT disruption have an exacerbated response to pressure overload [240].
LMCD1 (LIM and cysteine-rich domains 1) is another Z-disc protein that facilitates calcineurin activation. LMCD1 levels increase in pressure overload, following MI and in response to AngII infusion [241, 242]. Transgenic over-expression of LMCD1 increases calcineurin activation in response to pressure overload and accentuates pathological remodeling in vivo, whereas depletion of LMCD1 blunts NFAT activation in vitro [242].
Active mechanisms for controlling calcineurin protein levels are also present at the Z-disc. The muscle-specific RING finger 1 (MuRF1) is an E3 ubiquitin ligase that targets CnA for degradation via the proteasome [243]. Murf1−/− mice exhibit enhanced fibrosis and hypertrophy in response to pressure overload that can be normalized by calcineurin inhibition. For an expanded discussion of mechanotransduction at the Z-disc and the integration of calcineurin at this signaling juncture, several excellent reviews are available [236, 244–247].
4.4. Targeting calcineurin to the Na+/H+ exchanger 1 (NHE1)
Calcineurin binds directly to NHE1 in the plasma membrane through a PxIxIT motif [248]. Over expression of NHE1 in neonatal cardiomyocytes increased calcineurin activity by increasing intracellular pH. Moreover, this interaction required clustering of NHE1 into lipid rafts, creating a microdomain with higher pH. Transgenic over expression of NHE1 is sufficient to induce nuclear translocation of NFAT and cardiac hypertrophy [249]. NHE1 is found exclusively with caveolae in adult myocardium [152], whereas only a small portion of NCX1 is found in caveolae [250, 251]. Calcineurin can inhibit NCX1 activity [252, 253], thus the colocalization of NHE1 with NCX1 in lipid rafts provides a microdomain platform for calcineurin coupling to coordinate regulation of these exchangers.
The calcineurin targets highlighted here are by no means exhaustive of all known calcineurin substrates and targeting molecules that may be relevant to cardiovascular biology, however, we have tried to cover the majority of those for which molecular studies have been carried out in the context of cardiomyocytes. Given the broad-spectrum nature of phosphatase substrate selection in general, it is quite likely that some of targets most relevant to cardiovascular health are yet to be identified.
5. Not all calcineurin activity is pathological
Research on calcineurin's function in the diverse contexts described above has focused primarily on disease progression and pathological outcomes, however, the abundance of calcineurin in heart tissue suggests that it carries out fundamental processes needed to maintain normal function and health. This is supported by the observation that cardiac metabolism and function are severely compromised in mice with a cardiomyocyte-specific deletion of the regulatory subunit PPP3r1 [45, 46]. Although a multitude of studies have demonstrated calcineurin's involvement in pathological hypertrophy, its role during physiological hypertrophy is not as clear. Cardiomyocyte-specific overexpression of an Rcan1 transgene to inhibit calcineurin blunts exercise-induced physiological hypertrophy [5], yet exercise failed to activate a genetically encoded NFAT-Luciferase transgene reporter [100]. It may be that NFAT is not the pertinent calcineurin substrate during physiological hypertrophy. This would be consistent with the observation that pathological but not physiological hypertrophy is blunted in Nfatc2−/− mice [107]. Even in the context of pathological hypertrophy, some calcineurin-dependent processes may be beneficial. For instance, cardiomyocyte-specific overexpression of a Rcan2 transgene inhibited hypertrophy in the setting of pressure overload but impaired relaxation and increased stiffness [254]. Pharmacological inhibition with CsA increased mortality in a model of severe pressure overload [255]. Whether this was due to off target effects of the drug or inhibition of a beneficial calcineurin-dependent adaptation requires further study.
A recent study demonstrates that calcineurin is activated in models of both hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM) [256]. The HCM phenotype was caused by a mutation in cardiac troponin C (cTnC) that increased both Ca2+ affinity and tension, whereas the DCM cTnC mutation decreased Ca2+ affinity and myofilament tension. Although both models displayed calcineurin-dependent hypertrophic growth, the former manifest as concentric hypertrophy and the later as eccentric hypertrophy, suggesting that increased and decreased tension set in motion a different array of calcineurin-dependent responses. This study is an elegant illustration that pathological activation of calcineurin can derive from varied inputs and have diverse outcomes. Similarly, we propose that, although there are certain to be some aspects of similarity, the behavior and targets of calcineurin signaling in a healthy heart may be distinct from those set in motion by pathological stresses.
6. Maintaining the transmural gradient
Healthy hearts display variation in the morphology of the action potential across the ventricular wall such that the action potential duration (APD) is shorter in the sub-epicardial cardiomyocytes (EPI) than in the sub-endocardial cardiomyocytes (ENDO). This allows ventricular repolarization to proceed in a synchronized wave from EPI to ENDO, supporting efficient pump function and preventing arrhythmias. These transmural differences are due to gradients in the functional distribution of several membrane currents including the transient outward K+ current (Ito), the LTCC (ICa,L), and the Na/K ATPase pump current (Ip) [257–259]. Models based on these known differences predict that both diastolic and systolic Ca2+ is higher in ENDO than in EPI [260] and indeed, actual measures of calcineurin and NFAT indicate higher activity in ENDO compared to in EPI [174]. Mechanical stress is likewise greater in ENDO than in EPI [261] and therefore represents a potential underlying mechanism for establishing a gradient in calcineurin activity across the ventricular wall.
In NFATc3-null mice the transmural gradient in Ito is lost due to an increase in Kv4.2 protein and Ito in ENDO, thereby shortening APD in ENDO to match that of EPI [174]. Chronic infusion of the β adrenergic agonist isoproterenol also leads to a loss of the in Ito gradient by reducing Kv4.2 and Ito in EPI, thus increasing APD in EPI to match ENDO [262]. This change requires both β1-ARs and NFATc3. It therefore is likely mediated through AKAP5/calcineurin repression of Ito described above. Interestingly, loss of NFATc3 does not alter transmural expression of the transcription factor Irx5 that has been linked to establishing the Ito gradient during development [263].
Calcineurin can also influence the gradient in ICa,L, which is likewise higher in ENDO than in EPI. In a model of compensated pressure overload hypertrophy, where the Ito gradient was not yet effected, there was a calcineurin-dependent increase in peak ICa,L across the ventricular wall that was greatest in ENDO [264]. Thus, in contrast to Ito, and its gradient, which are suppressed by calcineurin, the gradient in peak ICa,L is accentuated by calcineurin activity. One important difference in mechanism of control is that calcineurin interacts directly with both the N and C termini of the LTCC [165, 167] whereas its control of Ito is indirect. Relative to the gradient in Ip, calcineurin activation increases expression of the Na/K ATPase in the heart [265, 266] and studies from brain demonstrate direct activation of the channel by calcineurin-mediated dephosphorylation [267]. Therefore, calcineurin activity may also control the transmural gradient in Ip, however, no studies have addressed this.
Also of note, miR-21 is among a limited number of microRNAs that display a transmural pattern of expression that peaks in the ENDO [268]. MiR-21 is also found upregulated in the hearts of young αMHC-CnA* mice before the onset of increased fibrosis, suggesting that MiR-21 expression is calcineurin-dependent [269]. Thus, calcineurin impacts transmural gradients through multiple mechanisms, some of which may remain to be identified.
Taken together, calcineurin activity is essential for establishing and maintaining an appropriate transmural gradient in ion channel activity and APD in a healthy heart. Whereas, sustained activation of calcineurin across the myocardium contributes to the collapse of the APD gradient increasing arrhythmogenic potential. It is relevant to note that αMHC-CnA* mice die of arrhythmias rather than overt pump failure.
7. Circadian activation of calcineurin in the heart
Timing of calcineurin activation may be as critical as location. The cardiovascular system displays significant circadian rhythms in many physiological processes and continual disruption of normal circadian rhythms predisposes humans to cardiovascular disease [270–274]. In a healthy rodent heart there is a circadian pattern of calcineurin activity that peaks at the end of the animal's active period and is at its lowest at the end of its rest period [275]. As much as a 20-fold change can be seen in nuclear translocation of NFAT, binding of NFAT to chromatin, and expression of the calcineurin/NFAT controlled gene, Rcan1.4, over the course of twenty-four hours. Therefore, although both sustained activation of calcineurin [4] and its complete absence [45] have pathological consequences, a diurnal pattern of calcineurin activation is compatible with heart health. The peak in calcineurin activity immediately precedes a time of day when the heart is less susceptible to damage from I/R [275, 276]. These diurnal changes in susceptibility appear to be mediated by changes in RCAN1 levels, as Rcan1−/− mice no longer display this temporal window of protection [89].
Circadian activation of calcineurin is not unique to the heart and has also been documented in skeletal muscle [277], as evidenced by changes in Rcan1.4 transcript levels and activity of NFAT reporters. In a series of elegant experiments these investigators demonstrated that circadian activation of the calcineurin/NFAT pathway was independent of the transcriptional circadian clock mechanism, and was driven instead by muscle innervation and contractile activity. Related mechanisms are likely involved in circadian activation of calcineurin in the heart.
Sleep deprivation studies in rat indicate a prolongation in action potential duration (APD) attributable to a decrease in Ito [278]. These changes were significant after only one day demonstrating the immediate impact of proper circadian entrainment on fundamental electrical properties of the heart. Whether the decline in Ito is directly attributable to disruption of a normal circadian pattern of signaling through AKAP5/ calcineurin/NFATc3 remains to be determined. It is interesting to note that several of the genes displaying transmural expression across the ventricular wall [279] also display a circadian pattern of expression the heart.
Circadian changes in signaling pathways controlled by protein phosphorylation are very often overlooked but are not unique to calcineurin. Some are directly under circadian entrainment, such as changes in adrenergic activity, while others may respond directly to feeding patterns, such as insulin release. Corresponding changes in the activity of PKA and protein kinase B (AKT) provide important signals regulating cardiac function, metabolism, and remodeling [280]. The circadian oscillations in calcineurin activity and other kinase/phosphorylation cascades may allow temporal separation of processes that are incompatible but essential.
8. Calcineurin in neonatal versus adult cardiomyocytes
Isolated neonatal cardiomyocytes have proven an invaluable tool for studying the role of calcineurin in the heart. However, not all findings from neonatal cells may be directly translated to the adult heart as there are fundamental structural, metabolic, and functional differences [281, 282]. Of particular note are differences in Ca2+ handling, release, and storage. All of which can have a direct impact on the character and duration of the Ca2+ signal perceived by calcineurin. In early embryonic cardiomyocytes Ca2+ is released primarily from the perinuclear SR through the cooperative action of inositol-3-phosphate receptors (IP3Rs) and RyRs to drive contraction [283]. At the time of birth, some junctional Ca2+ release units or “dyads” have formed that position RyRs in close apposition to Ca2+ channels in the sarcolemma, however, these are primarily located in the periphery of the cell, as T-tubules do not form until 10 days after birth, simultaneous with the appearance of the protein junctophilin-2 [284]. At this point in development the majority of RyRs are internal and not associated with a dyad. These “orphaned” RyRs are triggered consecutively via a “fire-diffuse-fire” mechanism to propagate the Ca2+ wave [285]. This results in a slower time to peak and decay of both the action potential and the Ca2+ transient compared to in adult myocytes. In addition, after birth, there is a dramatic increase in the capacity for buffering cytosolic Ca2+. This substantially slows the diffusion of Ca2+ relative to other ions, thus increasing the potential for establishing confined Ca2+ microdomains that may act to shield specific pools of calcineurin from activation. It is important to note that neonatal cardiomyocytes will spontaneously contract in culture whereas isolated adult cardiomyocytes do not, reflecting fundamental differences in Ca2+ handling. An excellent review provides details of the changes that occur during maturation from embryonic to adult cardiomyocytes [285] as well as discussing the consequences of T-tubule loss and increase in caveolae density in heart failure.
There are number of other noteable differences between neonatal, adult, and failing cardiomyocytes that impact calcineurin activity. These include changes in the expression of calcineurin isoforms [10, 22, 23] and redistribution within the cell from cytosolic to colocalization with T-tubules [102–104]. In addition, compared to adult, the neonatal heart has lower enzymatic activities for a number of antioxidant pathways [286] and is more susceptible to ischemic injury [287]. Taken together, changes that occur during maturation to an adult cardiomyocyte increase the total potential capacity for calcineurin activity while establishing environments that restrain activation and protect against uncontrolled signaling. During failure, loss of the T-tubule system and a shift toward caveolae, may release these domain constraints on calcineurin, allowing a pathological feed-forward signaling cascade.
Recent advances allowing differentiation of cardiomyocytes from embryonic stem cells or induced pluripotent stem cells (iPSCs) have provided powerful new tools for studying heart disease. While these models have the advantage of providing the genomic environment of a specific patient, it is important to note that, relative to Ca2+ release and handling, they may be more akin to embryonic cardiomyocytes than to adult. Thus, study of calcineurin dependent processes in these cells may be difficult to extrapolate to the adult heart [285].
9. Conclusion
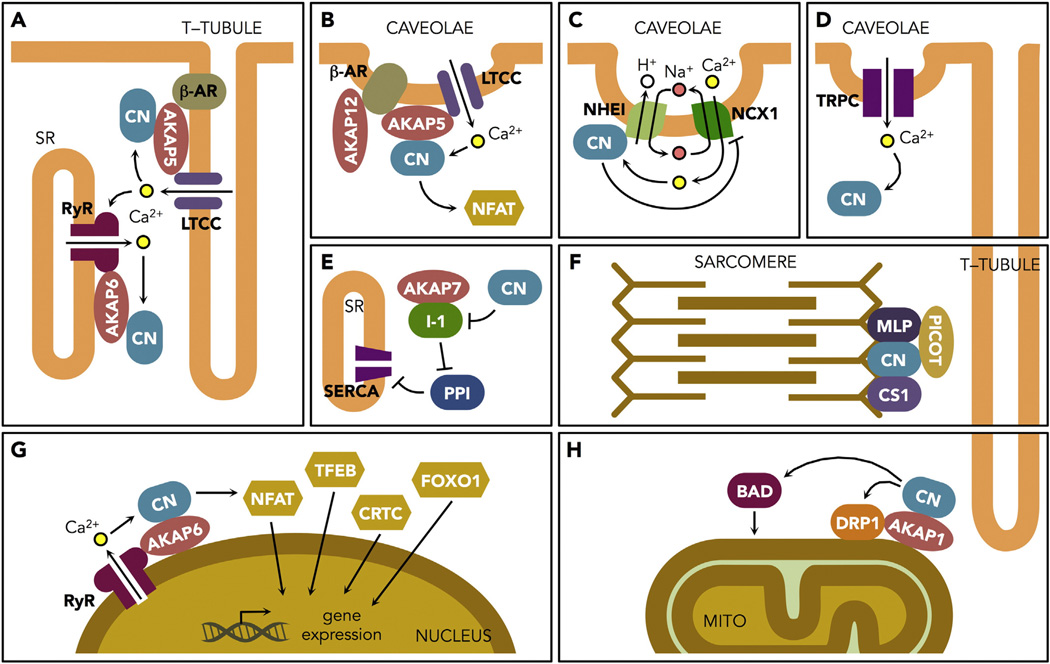
The impact of calcineurin signaling on cardiac health and disease is far more complex than simple activation of NFAT-dependent hypertrophic gene expression. Here, we have focused primarily on activity localized to cardiomyocytes (Fig. 2). However, calcineurin mediates process in all cell types and tissues of the cardiovascular system. Many of these may be equally important to maintaining a healthy heart. Key points to keep in mind are: (1) The nature of calcineurin signaling in neonatal cardiomyocytes is profoundly different than that found in adult cardiomyocytes. (2) The responses of calcineurin and its substrates depend upon the nature and composition of specific subcellular domains. (3) Not all calcineurin activity is pathological. (4) Calcineurin activity in a healthy heart is heterogeneous relative to location and timing. (5) Sustained activation of calcineurin leads to fundamental changes in a number of substrates that often act in a feed-forward fashion that accelerates cardiac decline.
Fig. 2.
Targeting of calcineurin to specific domains and substrates in adult cardiomyocytes. Key points to note include the close association of calcineurin (CN) and many of its interacting partners with the T-tubule system. Dyads formed by RyR/LTCC at SR/T-tubule junctions (A), Z-band localized mechano-sensing complexes of the sarcomere (F), and prominent sites of mitochondrial fission (H) each contain specific tethering points for CN and sit in close proximity to the T-tubule system. These major pools of CN do not appear to couple to NFAT activation during Ca2+-induced Ca2+ release associated with contraction. In contrast, CN more readily couples to NFAT in response to Ca2+ release events in caveolae-associated microdomains (B, C, D). CN associated directly with the nucleus also directly couples to transcriptional regulation (G). Some mechanisms of CN targeting are yet to be defined, such as the pool that acts on I-1 (E). See text for further details and the abbreviations list for full definitions of symbols.
Acknowledgments
This work was supported by the National Institutes of Health (grant numbers U54 HD087351 and HL098051) HL098051 to BAR); by Fondo Nacional de Desarrollo Científico y Tecnológico, FONDECYT, CONICYT, Chile (grant number 11150282 to VP); and PAI Insertion Program, CONICYT, Chile (grant 79150007 to VP). We also thank Ingenio Bravo for the final crafting of the figures.
Abbreviations
- AID
auto inhibitory domain of calcineurin
- AKAP
A kinase anchor protein
- AngII
angiotensin II
- APD
action potential duration
- β1-AR
β1-adrenergic receptors
- CABIN-1
calcineurin binding protein 1
- Carabin
TBC1 domain family member 10C
- CHP
calcineurin homologous proteins
- CIB1
Ca2+-and integrin-binding protein-1
- CN
calcineurin holoenzyme
- CnA
catalytic subunit of calcineurin
- CnB
regulatory subunit of calcineurin
- CnA*
constitutively active calcineurin
- CRTC
CREB-regulated transcriptional coactivators
- CS1
calsarcin 1
- CsA
cyclosporin A
- DRP1
dynamin related protein 1
- ENDO
endocardium
- EPI
epicardium
- FOXO1
forkhead box protein 01
- FRET
fluorescence resonance energy transfer
- I-1
protein phosphatase inhibitor 1
- ICa,L
LTCC current
- Ip
Na/K ATPase pump current
- Ito
fast transient outward K+ current
- LMCD1
LIM and cysteine-rich domains 1
- LTCC
voltage-activated L-type calcium channel
- MLP
muscle LIM protein
- MuRF1
muscle-specific RING finger 1
- NCX1
sodium calcium exchanger 1
- NFAT
nuclear factor of activated T cells
- NHE1
Na+/H+ exchanger 1
- OMM
outer membrane of mitochondria
- PC-1
polycystin-1 PC-1
- PICOT
protein kinase C-interacting cousin of thioredoxin
- PKA
protein kinase A
- pNPP
p-nitrophenyl phosphate
- PP1
protein phosphatase 1
- PLN
phospholamban
- RCAN
regulator of calcineurin
- Rcan1.4
exon four isoform of RCAN1
- RyR2
ryanodine receptor 2
- SR
sarcoplasmic reticulum
- TFEB
transcription factor EB
- TRPC
transient receptor potential canonical channel.
Footnotes
Disclosures
None.
References
- 1.Klee CB, Crouch TH, Krinks MH. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc. Natl. Acad. Sci. U. S. A. 1979;76:6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manalan AS, Klee CB. Affinity selection of chemically modified proteins: role of lysyl residues in the binding of calmodulin to calcineurin. Biochemistry. 1987;26:1382–1390. doi: 10.1021/bi00379a026. [DOI] [PubMed] [Google Scholar]
- 3.Aramburu J, Rao A, Klee CB. Calcineurin: from structure to function. Curr. Top. Cell. Regul. 2000;36:237–295. doi: 10.1016/s0070-2137(01)80011-x. [DOI] [PubMed] [Google Scholar]
- 4.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothermel BA, McKinsey TA, Vega RB, Nicol RL, Mammen P, Yang J, et al. Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3328–3333. doi: 10.1073/pnas.041614798. http://dx.doi.org/10.1073/pnas.041614798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Rooij E, Doevendans PA, Crijns HJGM, Heeneman S, Lips DJ, van Bilsen M, et al. MCIP1 overexpression suppresses left ventricular remodeling and sustains cardiac function after myocardial infarction. Circ. Res. 2004;94:e18–e26. doi: 10.1161/01.RES.0000118597.54416.00. http://dx.doi.org/10.1161/01.RES.0000118597.54416.00. [DOI] [PubMed] [Google Scholar]
- 7.Hill JA, Rothermel B, Yoo K-D, Cabuay B, Demetroulis E, Weiss RM, et al. Targeted inhibition of calcineurin in pressure-overload cardiac hypertrophy. Preservation of systolic function. J. Biol. Chem. 2002;277:10251–10255. doi: 10.1074/jbc.M110722200. http://dx.doi.org/10.1074/jbc.M110722200. [DOI] [PubMed] [Google Scholar]
- 8.DeWindt LJ, Lim HW, Bueno OF, Liang Q, Delling U, Braz JC, et al. Targeted inhibition of calcineurin attenuates cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3322–3327. doi: 10.1073/pnas.031371998. http://dx.doi.org/10.1073/pnas.031371998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu H, van Berkel TJC, Biessen EAL, et al. Therapeutic potential of VIVIT, a selective peptide inhibitor of nuclear factor of activated T cells, in cardiovascular disorders. Cardiovasc. Drug Rev. 2007;25:175–187. doi: 10.1111/j.1527-3466.2007.00011.x. http://dx.doi.org/10.1111/j.1527-3466.2007.00011.x. [DOI] [PubMed] [Google Scholar]
- 10.Taigen T, DeWindt LJ, Lim HW, Molkentin JD. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1196–1201. doi: 10.1073/pnas.97.3.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallagher SC, Gao Z-H, Li S, Dyer RB, Trewhella J, Klee CB. There is communication between all four Ca2+-bindings sites of calcineurin B †. Biochemistry. 2001;40:12094–12102. doi: 10.1021/bi0025060. http://dx.doi.org/10.1021/bi0025060. [DOI] [PubMed] [Google Scholar]
- 12.Kakalis LT, Kennedy M, Sikkink R, Rusnak F, Armitage IM. Characterization of the calcium-binding sites of calcineurin B. FEBS Lett. 1995;362:55–58. doi: 10.1016/0014-5793(95)00207-p. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Rao A, Hogan PG. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 2011;21:91–103. doi: 10.1016/j.tcb.2010.09.011. http://dx.doi.org/10.1016/j.tcb.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S-J, Wang J, Ma L, Lu C, Wang J, Wu J-W, et al. Cooperative autoinhibition and multi-level activation mechanisms of calcineurin. Nat. Publ. Group. 2016;26:336–349. doi: 10.1038/cr.2016.14. http://dx.doi.org/10.1038/cr.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. http://dx.doi.org/10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 16.Stemmer PM, Klee CB. Dual calcium ion regulation of calcineurin by calmodulin and calcineurin B. Biochemistry. 1994;33:6859–6866. doi: 10.1021/bi00188a015. [DOI] [PubMed] [Google Scholar]
- 17.Mehta S, Aye-Han N-N, Ganesan A, Oldach L, Gorshkov K, Zhang J. Calmodulin-controlled spatial decoding of oscillatory Ca2+ signals by calcineurin. eLife. 2014;3:e03765. doi: 10.7554/eLife.03765. http://dx.doi.org/10.7554/eLife.03765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heineke J, Ritter O. Cardiomyocyte calcineurin signaling in subcellular domains: from the sarcolemma to the nucleus and beyond. J. Mol. Cell. Cardiol. 2012;52:62–73. doi: 10.1016/j.yjmcc.2011.10.018. http://dx.doi.org/10.1016/j.yjmcc.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Fiedler B, Wollert KC. Targeting calcineurin and associated pathways in cardiac hypertrophy and failure. Expert Opin. Ther. Targets. 2005;9:963–973. doi: 10.1517/14728222.9.5.963. http://dx.doi.org/10.1517/14728222.9.5.963. [DOI] [PubMed] [Google Scholar]
- 20.Despa S, Shui B, Bossuyt J, Lang D, Kotlikoff MI, Bers DM. Junctional cleft [Ca2+]i measurements using novel cleft-targeted Ca2+ sensors. Circ. Res. 2014;115:339–347. doi: 10.1161/CIRCRESAHA.115.303582. http://dx.doi.org/10.1161/CIRCRESAHA.115.303582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X, Bers DM. Free and bound intracellular calmodulin measurements in cardiac myocytes. Cell Calcium. 2007;41:353–364. doi: 10.1016/j.ceca.2006.07.011. http://dx.doi.org/10.1016/j.ceca.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, et al. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- 23.Oka T, Dai Y-S, Molkentin JD. Regulation of calcineurin through transcriptional induction of the calcineurin A beta promoter in vitro and in vivo. Mol. Cell. Biol. 2005;25:6649–6659. doi: 10.1128/MCB.25.15.6649-6659.2005. http://dx.doi.org/10.1128/MCB.25.15.6649-6659.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy RN, Pena JA, Roberts BR, Williams SR, Price SR, Gooch JL. Rescue of calcineurin Aα(−/−)mice reveals a novel role for the α isoformin the salivary gland. Am. J. Pathol. 2011;178:1605–1613. doi: 10.1016/j.ajpath.2010.12.054. http://dx.doi.org/10.1016/j.ajpath.2010.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gooch JL, Toro JJ, Guler RL, Barnes JL. Calcineurin A-alpha but not A-beta is required for normal kidney development and function. AJPA. 2004;165:1755–1765. doi: 10.1016/s0002-9440(10)63430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parsons SA, Wilkins BJ, Bueno OF, Molkentin JD. Altered skeletal muscle phenotypes in calcineurin Aalpha and Abeta gene-targeted mice. Mol. Cell. Biol. 2003;23:4331–4343. doi: 10.1128/MCB.23.12.4331-4343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, et al. Impaired cardiac hypertrophic response in calcineurin Abeta-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:4586–4591. doi: 10.1073/pnas.072647999. http://dx.doi.org/10.1073/pnas.072647999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perrino BA, Wilson AJ, Ellison P, Clapp LH. Substrate selectivity and sensitivity to inhibition by FK506 and cyclosporin A of calcineurin heterodimers composed of the alpha or beta catalytic subunit. Eur. J. Biochem. 2002;269:3540–3548. doi: 10.1046/j.1432-1033.2002.03040.x. [DOI] [PubMed] [Google Scholar]
- 29.Kilka S, Erdmann F, Migdoll A, Fischer G, Weiwad M. The proline-rich N-terminal sequence of calcineurin Abeta determines substrate binding. Biochemistry. 2009;48:1900–1910. doi: 10.1021/bi8019355. http://dx.doi.org/10.1021/bi8019355. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Balakrishnan-Renuka A, Hagemann N, Theiss C, Chankiewitz V, Chen J, et al. A novel interaction between ATOH8 and PPP3CB. Histochem. Cell Biol. 2015;145:5–16. doi: 10.1007/s00418-015-1368-5. http://dx.doi.org/10.1007/s00418-015-1368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balakrishnan-Renuka A, Morosan-Puopolo G, Yusuf F, Abduelmula A, Chen J, Zoidl G, et al. ATOH8, a regulator of skeletal myogenesis in the hypaxial myotome of the trunk. Histochem. Cell Biol. 2014;141:289–300. doi: 10.1007/s00418-013-1155-0. http://dx.doi.org/10.1007/s00418-013-1155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rawnsley DR, Xiao J, Lee JS, Liu X, Mericko-Ishizuka P, Kumar V, et al. The transcription factor Atonal homolog 8 regulates Gata4 and Friend of Gata-2 during vertebrate development. J. Biol. Chem. 2013;288:24429–24440. doi: 10.1074/jbc.M113.463083. http://dx.doi.org/10.1074/jbc.M113.463083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lara-Pezzi E, Winn N, Paul A, McCullagh K, Slominsky E, Santini MP, et al. A naturally occurring calcineurin variant inhibits FoxO activity and enhances skeletal muscle regeneration. J. Cell Biol. 2007;179:1205–1218. doi: 10.1083/jcb.200704179. http://dx.doi.org/10.1074/jbc.M002417200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felkin LE, Narita T, Germack R, Shintani Y, Takahashi K, Sarathchandra P, et al. Calcineurin splicing variant calcineurin A1 improves cardiac function after myocardial infarction without inducing hypertrophy. Circulation. 2011;123:2838–2847. doi: 10.1161/CIRCULATIONAHA.110.012211. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.012211. [DOI] [PubMed] [Google Scholar]
- 35.López-Olañeta MM, Villalba M, Gómez-Salinero JM, Jiménez-Borreguero LJ, Breckenridge R, Ortiz-Sánchez P, et al. Induction of the calcineurin variant CnAβ1 after myocardial infarction reduces post-infarction ventricular remodelling by promoting infarct vascularization. Cardiovasc. Res. 2014;102:396–406. doi: 10.1093/cvr/cvu068. http://dx.doi.org/10.1093/cvr/cvu068. [DOI] [PubMed] [Google Scholar]
- 36.Gómez-Salinero JM, López-Olañeta MM, Ortiz-Sánchez P, Larrasa-Alonso J, Gatto A, Felkin LE, et al. The calcineurin variant CnAb1 controls mouse embryonic stem cell differentiation by directing mTORC2 membrane localization and activation. Cell Chem. Biol. 2016;23:1372–1382. doi: 10.1016/j.chembiol.2016.09.010. http://dx.doi.org/10.1016/j.chembiol.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Shioda N, Moriguchi S, Shirasaki Y, Fukunaga K. Generation of constitutively active calcineurin by calpain contributes to delayed neuronal death following mouse brain ischemia. J. Neurochem. 2006;98:310–320. doi: 10.1111/j.1471-4159.2006.03874.x. http://dx.doi.org/10.1111/j.1471-4159.2006.03874.x. [DOI] [PubMed] [Google Scholar]
- 38.Wu HY. Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration. J. Biol. Chem. 2003;279:4929–4940. doi: 10.1074/jbc.M309767200. http://dx.doi.org/10.1074/jbc.M309767200. [DOI] [PubMed] [Google Scholar]
- 39.Liu F, Grundke-Iqbal I, Iqbal K, Oda Y, Tomizawa K, Gong C-X. Truncation and activation of calcineurin A by calpain I in Alzheimer disease brain. J. Biol. Chem. 2005;280:37755–37762. doi: 10.1074/jbc.M507475200. http://dx.doi.org/10.1074/jbc.M507475200. [DOI] [PubMed] [Google Scholar]
- 40.Smith MA, Schnellmann RG. Calpains, mitochondria, and apoptosis. Cardiovasc. Res. 2012;96:32–37. doi: 10.1093/cvr/cvs163. http://dx.doi.org/10.1093/cvr/cvs163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Letavernier E, Zafrani L, Perez J, Letavernier B, Haymann J-P, Baud L. The role of calpains in myocardial remodelling and heart failure. Cardiovasc. Res. 2012;96:38–45. doi: 10.1093/cvr/cvs099. http://dx.doi.org/10.1093/cvr/cvs099. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Dorado D, Ruiz-Meana M, Inserte J, Rodriguez-Sinovas A, Piper HM. Calcium-mediated cell death during myocardial reperfusion. Cardiovasc. Res. 2012;94:168–180. doi: 10.1093/cvr/cvs116. http://dx.doi.org/10.1093/cvr/cvs116. [DOI] [PubMed] [Google Scholar]
- 43.Yang, et al. Increased expression of calpain and elevated activity of calcineurin in the myocardium of patients with congestive heart failure. Int. J. Mol. Med. 2010;26 doi: 10.3892/ijmm_00000448. http://dx.doi.org/10.3892/ijmm_00000448. [DOI] [PubMed] [Google Scholar]
- 44.Wang J-C, Zhao Y, Li X-D, Zhou N-N, Sun H, Sun Y-Y, et al. Proteolysis by endogenous calpain I leads to the activation of calcineurin in human heart. Clin. Lab. 2012;58:1145–1152. [PubMed] [Google Scholar]
- 45.Maillet M, Davis J, Auger-Messier M, York A, Osinska H, Piquereau J, et al. Heart-specific deletion of CnB1 reveals multiple mechanisms whereby calcineurin regulates cardiac growth and function. J. Biol. Chem. 2010;285:6716–6724. doi: 10.1074/jbc.M109.056143. http://dx.doi.org/10.1074/jbc.M109.056143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaeffer PJ, Desantiago J, Yang J, Flagg TP, Kovacs A, Weinheimer CJ, et al. Impaired contractile function and calcium handling in hearts of cardiac-specific calcineurin b1-deficient mice. AJP: Heart Circ. Physiol. 2009;297:H1263–H1273. doi: 10.1152/ajpheart.00152.2009. http://dx.doi.org/10.1152/ajpheart.00152.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Culotta VC, Klee CB. Superoxide dismutase protects calcineurin from inactivation. Nature. 1996;383:434–437. doi: 10.1038/383434a0. http://dx.doi.org/10.1038/383434a0. [DOI] [PubMed] [Google Scholar]
- 48.Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J. Biol. Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- 49.Griffith JP, Kim JL, Kim EE, Sintchak MD, Thomson JA, Fitzgibbon MJ, et al. X-ray structure of calcineurin inhibited by the immunophilin-immunosuppressant FKBP12-FK506 complex. Cell. 1995;82:507–522. doi: 10.1016/0092-8674(95)90439-5. [DOI] [PubMed] [Google Scholar]
- 50.Donella-Deana A, Krinks MH, Ruzzene M, Klee C, Pinna LA. Dephosphorylation of phosphopeptides by calcineurin (protein phosphatase 2B) Eur. J. Biochem. 1994;219:109–117. doi: 10.1111/j.1432-1033.1994.tb19920.x. [DOI] [PubMed] [Google Scholar]
- 51.Liu JO, Nacev BA, Xu J, Bhat S. It takes two binding sites for calcineurin and NFAT to tango. Mol. Cell. 2009;33:676–678. doi: 10.1016/j.molcel.2009.03.005. http://dx.doi.org/10.1016/j.molcel.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Rodríguez A, Roy J, Martínez-Martínez S, López-Maderuelo MD, Niño-Moreno P, Ortí L, et al. A conserved docking surface on calcineurin mediates interaction with substrates and immunosuppressants. Mol. Cell. 2009;33:616–626. doi: 10.1016/j.molcel.2009.01.030. http://dx.doi.org/10.1016/j.molcel.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aramburu J, Garcia-Cózar F, Raghavan A, Okamura H, Rao A, Hogan PG. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol. Cell. 1998;1:627–637. doi: 10.1016/s1097-2765(00)80063-5. [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Cozar FJ, Okamura H, Aramburu JF, Shaw KT, Pelletier L, Showalter R, et al. Two-site interaction of nuclear factor of activated T cells with activated calcineurin. J. Biol. Chem. 1998;273:23877–23883. doi: 10.1074/jbc.273.37.23877. [DOI] [PubMed] [Google Scholar]
- 55.Li H, Rao A, Hogan PG. Structural delineation of the calcineurin-NFAT interaction and its parallels to PP1 targeting interactions. J. Mol. Biol. 2004;342:1659–1674. doi: 10.1016/j.jmb.2004.07.068. http://dx.doi.org/10.1016/j.jmb.2004.07.068. [DOI] [PubMed] [Google Scholar]
- 56.Grigoriu S, Bond R, Cossio P, Chen JA, Ly N, Hummer G, et al. The molecular mechanism of substrate engagement and immunosuppressant inhibition of calcineurin. PLoS Biol. 2013;11:e1001492. doi: 10.1371/journal.pbio.1001492. http://dx.doi.org/10.1371/journal.pbio.1001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gal M, Li S, Luna RE, Takeuchi K, Wagner G. The LxVP and PxIxIT NFAT motifs bind Jointlyto overlapping epitopes on calcineurin's catalytic domain distant to the regulatory domain, structure. Fold. Des. 2014;22:1016–1027. doi: 10.1016/j.str.2014.05.006. http://dx.doi.org/10.1016/j.str.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cardenas ME, Muir RS, Breuder T, Heitman J. Targets of immunophilin-immunosuppressant complexes are distinct highly conserved regions of calcineurin a. EMBO J. 1995;14:2772–2783. doi: 10.1002/j.1460-2075.1995.tb07277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 60.Kissinger CR, Parge HE, Knighton DR, Lewis CT, Pelletier LA, Tempczyk A, et al. Crystal structures of human calcineurin and the human FKBP12-FK506-calcineurin complex. Nature. 1995;378:641–644. doi: 10.1038/378641a0. http://dx.doi.org/10.1038/378641a0. [DOI] [PubMed] [Google Scholar]
- 61.Huai Q, Kim H-Y, Liu Y, Zhao Y, Mondragon A, Liu JO, et al. Crystal structure of calcineurin-cyclophilin-cyclosporin shows common but distinct recognition of immunophilin-drug complexes. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12037–12042. doi: 10.1073/pnas.192206699. http://dx.doi.org/10.1073/pnas.192206699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin L, Harrison SC. Crystal structure of human calcineurin complexed with cyclosporin A and human cyclophilin. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13522–13526. doi: 10.1073/pnas.212504399. http://dx.doi.org/10.1073/pnas.212504399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin M, Ochs RS. Mechanism for the paradoxical inhibition and stimulation of calcineurin by the immunosuppresive drug tacrolimus (FK506) Arch. Biochem. Biophys. 2003;419:207–213. doi: 10.1016/j.abb.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Swanson SK, Born T, Zydowsky LD, Cho H, Chang HY, Walsh CT, et al. Cyclosporin- mediated inhibition of bovine calcineurin by cyclophilins A and B. Proc. Natl. Acad. Sci. U. S. A. 1992;89:3741–3745. doi: 10.1073/pnas.89.9.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aramburu J, Yaffe MB, López-Rodríguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 66.Martínez-Martínez S, Rodríguez A, López-Maderuelo MD, Ortega-Pérez I, Vázquez J, Redondo JM. Blockade of NFAT activation by the second calcineurin binding site. J. Biol. Chem. 2006;281:6227–6235. doi: 10.1074/jbc.M513885200. http://dx.doi.org/10.1074/jbc.M513885200. [DOI] [PubMed] [Google Scholar]
- 67.Kuriyama M, Matsushita M, Tateishi A, Moriwaki A, Tomizawa K, Ishino K, et al. A cell-permeable NFAT inhibitor peptide prevents pressure-overload cardiac hypertrophy. Chem. Biol. Drug Des. 2006;67:238–243. doi: 10.1111/j.1747-0285.2006.00360.x. http://dx.doi.org/10.1111/j.1747-0285.2006.00360.x. [DOI] [PubMed] [Google Scholar]
- 68.Sieber M, Baumgrass R. Novel inhibitors of the calcineurin/NFATc hub — alternatives to CsA and FK506? Cell Commun. Signal. 2009;7:25. doi: 10.1186/1478-811X-7-25. http://dx.doi.org/10.1186/1478-811X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lai MM, Burnett PE, Wolosker H, Blackshaw S, Snyder SH. Cain, a novel physiologic protein inhibitor of calcineurin. J. Biol. Chem. 1998;273:18325–18331. doi: 10.1074/jbc.273.29.18325. [DOI] [PubMed] [Google Scholar]
- 70.Sun L, Youn HD, Loh C, Stolow M, He W, Liu JO. Cabin 1, a negative regulator for calcineurin signaling in T lymphocytes. Immunity. 1998;8:703–711. doi: 10.1016/s1074-7613(00)80575-0. [DOI] [PubMed] [Google Scholar]
- 71.Han A, Pan F, Stroud JC, Youn H-D, Liu JO, Chen L. Sequence-specific recruitment of transcriptional co-repressor Cabin1 by myocyte enhancer factor-2. Nature. 2003;422:730–734. doi: 10.1038/nature01555. http://dx.doi.org/10.1038/nature01555. [DOI] [PubMed] [Google Scholar]
- 72.Youn HD, Sun L, Prywes R, Liu JO. Apoptosis of T cells mediated by Ca2+-induced release of the transcription factor MEF2. Science. 1999;286:790–793. doi: 10.1126/science.286.5440.790. [DOI] [PubMed] [Google Scholar]
- 73.Esau C, Boes M, Youn HD, Tatterson L, Liu JO, Chen J. Deletion of calcineurin and myocyte enhancer factor 2 (MEF2) binding domain of Cabin1 results in enhanced cytokine gene expression in T cells. J. Exp. Med. 2001;194:1449–1459. doi: 10.1084/jem.194.10.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pan F, Sun L, Kardian DB, Whartenby KA, Pardoll DM, Liu JO. Feedback inhibition of calcineurin and Ras by a dual inhibitory protein Carabin. Nature. 2007;445:433–436. doi: 10.1038/nature05476. http://dx.doi.org/10.1038/nature05476. [DOI] [PubMed] [Google Scholar]
- 75.Nagai H, Yasuda S, Ohba Y, Fukuda M, Nakamura T. All members of the EPI64 subfamily of TBC/RabGAPs also have GAP activities towards Ras. J. Biochem. 2013;153:283–288. doi: 10.1093/jb/mvs147. http://dx.doi.org/10.1093/jb/mvs147. [DOI] [PubMed] [Google Scholar]
- 76.Bisserier M, Berthouze-Duquesnes M, Breckler M, Tortosa F, Fazal L, de Régibus A, et al. Carabin protects against cardiac hypertrophy by blocking calcineurin, Ras, and Ca2+/calmodulin-dependent protein kinase II signaling. Circulation. 2015;131:390–400. doi: 10.1161/CIRCULATIONAHA.114.010686. http://dx.doi.org/10.1161/CIRCULATIONAHA.114.010686 (discussion 400) [DOI] [PubMed] [Google Scholar]
- 77.Zhu X, Fang J, Gong J, Guo J-H, Zhao G-N, Ji Y-X, et al. Cardiac-specific EPI64C blunts pressure overload-induced cardiac hypertrophy. Hypertension. 2016;67:866–877. doi: 10.1161/HYPERTENSIONAHA.115.07042. http://dx.doi.org/10.1161/HYPERTENSIONAHA.115.07042. [DOI] [PubMed] [Google Scholar]
- 78.Di Sole F, Vadnagara K, Moe OW, Babich V. Calcineurin homologous protein: a multifunctional Ca2+-binding protein family. Am. J. Physiol. Ren. Physiol. 2012;303:F165–F179. doi: 10.1152/ajprenal.00628.2011. http://dx.doi.org/10.1152/ajprenal.00628.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin X, Sikkink RA, Rusnak F, Barber DL. Inhibition of calcineurin phosphatase activity by a calcineurin B homologous protein. J. Biol. Chem. 1999;274:36125–36131. doi: 10.1074/jbc.274.51.36125. [DOI] [PubMed] [Google Scholar]
- 80.Li G-D, Zhang X, Li R, Wang Y-D, Wang Y-L, Han K-J, et al. CHP2 activates the calcineurin/nuclear factor of activated T cells signaling pathway and enhances the oncogenic potential of HEK293 cells. J. Biol. Chem. 2008;283:32660–32668. doi: 10.1074/jbc.M806684200. http://dx.doi.org/10.1074/jbc.M806684200. [DOI] [PubMed] [Google Scholar]
- 81.Davies KJA, Ermak G, Rothermel BA, Pritchard M, Heitman J, Ahnn J, et al. Renaming the DSCR1/Adapt78 gene family as RCAN: regulators of calcineurin. FASEB J. 2007;21:3023–3028. doi: 10.1096/fj.06-7246com. http://dx.doi.org/10.1096/fj.06-7246com. [DOI] [PubMed] [Google Scholar]
- 82.Kingsbury TJ, Cunningham KW. A conserved family of calcineurin regulators. Genes Dev. 2000;14:1595–1604. [PMC free article] [PubMed] [Google Scholar]
- 83.Strippoli P, Lenzi L, Petrini M, Carinci P, Zannotti M, et al. A new gene family including DSCR1 (Down Syndrome Candidate Region 1) and ZAKI-4: characterization from yeast to human and identification of DSCR1-like 2, a novel human member (DSCR1L2) Genomics. 2000;64:252–263. doi: 10.1006/geno.2000.6127. http://dx.doi.org/10.1006/geno.2000.6127. [DOI] [PubMed] [Google Scholar]
- 84.Rothermel B, Vega RB, Yang J, Wu H, Bassel-Duby R, Williams RS. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J. Biol. Chem. 2000;275:8719–8725. doi: 10.1074/jbc.275.12.8719. [DOI] [PubMed] [Google Scholar]
- 85.Chan B, Greenan G, McKeon F, Ellenberger T. Identification of a peptide fragment of DSCR1 that competitively inhibits calcineurin activity in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13075–13080. doi: 10.1073/pnas.0503846102. http://dx.doi.org/10.1073/pnas.0503846102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aubareda A, Mulero MC, Pérez-Riba M, et al. Functional characterization of the calcipressin 1 motif that suppresses calcineurin-mediated NFAT-dependent cytokine gene expression in human T cells. Cell. Signal. 2006;18:1430–1438. doi: 10.1016/j.cellsig.2005.11.006. http://dx.doi.org/10.1016/j.cellsig.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 87.Martínez-Martínez S, Genescà L, Rodríguez A, Raya A, Salichs E, Were F, et al. The RCAN carboxyl end mediates calcineurin docking-dependent inhibition via a site that dictates binding to substrates and regulators. Proc. Natl. Acad. Sci. 2009;106:6117–6122. doi: 10.1073/pnas.0812544106. http://dx.doi.org/10.1073/pnas.0812544106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, et al. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ. Res. 2000;87:E61–E68. doi: 10.1161/01.res.87.12.e61. [DOI] [PubMed] [Google Scholar]
- 89.Rotter D, Grinsfelder DB, Parra V, Pedrozo Z, Singh S, Sachan N, et al. Calcineurin and its regulator, RCAN1, confer time-of-day changes in susceptibility of the heart to ischemia/reperfusion. J. Mol. Cell. Cardiol. 2014;74C:103–111. doi: 10.1016/j.yjmcc.2014.05.004. http://dx.doi.org/10.1016/j.yjmcc.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sanna B, Brandt EB, Kaiser RA, Pfluger P, Witt SA, Kimball TR, et al. Modulatory calcineurin-interacting proteins 1 and 2 function as calcineurin facilitators in vivo. Proc. Natl. Acad. Sci. U. S. A. 2006;103:7327–7332. doi: 10.1073/pnas.0509340103. http://dx.doi.org/10.1073/pnas.0509340103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vega RB, Rothermel BA, Weinheimer CJ, Kovacs A, Naseem RH, Bassel-Duby R, et al. Dual roles of modulatory calcineurin-interacting protein 1 in cardiac hypertrophy. Proc. Natl. Acad. Sci. U. S. A. 2003;100:669–674. doi: 10.1073/pnas.0237225100. http://dx.doi.org/10.1073/pnas.0237225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cho Y-J, Abe M, Kim SY, Sato Y. Raf-1 is a binding partner of DSCR1. Arch. Biochem. Biophys. 2005;439:121–128. doi: 10.1016/j.abb.2005.05.002. http://dx.doi.org/10.1016/j.abb.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 93.Lee HJ, Kim YS, Sato Y, Cho Y-J, et al. RCAN1-4 knockdown attenuates cell growth through the inhibition of Ras signaling. FEBS Lett. 2009;583:2557–2564. doi: 10.1016/j.febslet.2009.07.023. http://dx.doi.org/10.1016/j.febslet.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 94.Liu Q, Busby JC, Molkentin JD. Interaction between TAK1-TAB1-TAB2 and RCAN1-calcineurin defines a signalling nodal control point. Nat. Cell Biol. 2009;11:154–161. doi: 10.1038/ncb1823. http://dx.doi.org/10.1038/ncb1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mehta S, Li H, Hogan PG, Cunningham KW. Domain architecture of the regulators of calcineurin (RCANs) and identification of a divergent RCAN in yeast. Mol. Cell. Biol. 2009;29:2777–2793. doi: 10.1128/MCB.01197-08. http://dx.doi.org/10.1128/MCB.01197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shin S-Y, Yang HW, Kim J-R, Heo WD, Cho K-H, et al. A hidden incoherent switch regulates RCAN1 in the calcineurin-NFAT signaling network. J. Cell Sci. 2011;124:82–90. doi: 10.1242/jcs.076034. http://dx.doi.org/10.1242/jcs.076034. [DOI] [PubMed] [Google Scholar]
- 97.Vickers KC, Rye K-A, Tabet F. MicroRNAs in the onset and development of cardiovascular disease. Clin. Sci. 2014;126:183–194. doi: 10.1042/CS20130203. http://dx.doi.org/10.1042/CS20130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fruman D, Pai S, Klee C, Burakoff S, Bierer B. Measurement of calcineurin phosphatase activity in cell extracts. Methods. 1996;9:146–154. doi: 10.1006/meth.1996.0020. [DOI] [PubMed] [Google Scholar]
- 99.Rinne A, Blatter LA. Techniques for physiology: a fluorescence-based assay to monitor transcriptional activity of NFAT in living cells. J. Physiol. 2010;588:3211–3216. doi: 10.1113/jphysiol.2010.192419. http://dx.doi.org/10.1113/jphysiol.2010.192419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wilkins BJ, Dai Y-S, Bueno OF, Parsons SA, Xu J, Plank DM, et al. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ. Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. http://dx.doi.org/10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 101.Minami T, Yano K, Miura M, Kobayashi M, Suehiro J-I, Reid PC, et al. The Down syndrome critical region gene 1 short variant promoters direct vascular bed-specific gene expression during inflammation in mice. J. Clin. Invest. 2009;119:2257–2270. doi: 10.1172/JCI35738. http://dx.doi.org/10.1172/JCI35738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bazzazi H, Sang L, Dick IE, Joshi-Mukherjee R, Yang W, Yue DT. Novel fluorescence resonance energy transfer-based reporter reveals differential calcineurin activation in neonatal and adult cardiomyocytes. J. Physiol. 2015;593:3865–3884. doi: 10.1113/JP270510. http://dx.doi.org/10.1113/JP270510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santana LF, Chase EG, Votaw VS, Nelson MT, Greven R. Functional coupling of calcineurin and protein kinase A in mouse ventricular myocytes. J. Physiol. 2002;544:57–69. doi: 10.1113/jphysiol.2002.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Frey N, Richardson JA, Olson EN. Calsarcins, a novel family of sarcomeric calcineurin-binding proteins. Proc. Natl. Acad. Sci. U. S. A. 2000;97:14632–14637. doi: 10.1073/pnas.260501097. http://dx.doi.org/10.1073/pnas.260501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mognol GP, Carneiro FRG, Robbs BK, Faget DV, Viola JPB, et al. Cell cycle and apoptosis regulation by NFAT transcription factors: new roles for an old player. Cell Death Dis. 2016;7:e2199. doi: 10.1038/cddis.2016.97. http://dx.doi.org/10.1038/cddis.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van Rooij E, Doevendans PA, de Theije CC, Babiker FA, Molkentin JD, De Windt LJ, et al. Requirement of nuclear factor of activated T-cells in calcineurin-mediated cardiomyocyte hypertrophy. J. Biol. Chem. 2002;277:48617–48626. doi: 10.1074/jbc.M206532200. http://dx.doi.org/10.1074/jbc.M206532200. [DOI] [PubMed] [Google Scholar]
- 107.Bourajjaj M, Armand A-S, da Costa Martins PA, Weijts B, van der Nagel R, Heeneman S, et al. NFATc2 is a necessary mediator of calcineurin-dependent cardiac hypertrophy and heart failure. J. Biol. Chem. 2008;283:22295–22303. doi: 10.1074/jbc.M801296200. http://dx.doi.org/10.1074/jbc.M801296200. [DOI] [PubMed] [Google Scholar]
- 108.Wilkins BJ, De Windt LJ, Bueno OF, Braz JC, Glascock BJ, Kimball TF, et al. Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol. Cell. Biol. 2002;22:7603–7613. doi: 10.1128/MCB.22.21.7603-7613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de la Pompa JL, Timmerman LA, Takimoto H, Yoshida H, Elia AJ, Samper E, et al. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature. 1998;392:182–186. doi: 10.1038/32419. http://dx.doi.org/10.1038/32419. [DOI] [PubMed] [Google Scholar]
- 110.Kar P, Parekh AB. Distinct spatial Ca. Mol. Cell. 2015;58:232–243. doi: 10.1016/j.molcel.2015.02.027. http://dx.doi.org/10.1016/j.molcel.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rinne A, Kapur N, Molkentin JD, Pogwizd SM, Bers DM, Banach K, et al. Isoform- and tissue-specific regulation of the Ca(2+)-sensitive transcription factor NFAT in cardiac myocytes and heart failure. AJP: Heart Circ. Physiol. 2010;298:H2001–H2009. doi: 10.1152/ajpheart.01072.2009. http://dx.doi.org/10.1152/ajpheart.01072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Calabria E, Ciciliot S, Moretti I, Garcia M, Picard A, Dyar KA, et al. NFAT isoforms control activity-dependent muscle fiber type specification. Proc. Natl. Acad. Sci. 2009;106:13335–13340. doi: 10.1073/pnas.0812911106. http://dx.doi.org/10.1073/pnas.0812911106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shen T, Liu Y, Cseresnyés Z, Hawkins A, Randall WR, Schneider MF. Activity-and calcineurin-independent nuclear shuttling of NFATc1, but not NFATc3, in adult skeletal muscle fibers. Mol. Biol. Cell. 2006;17:1570–1582. doi: 10.1091/mbc.E05-08-0780. http://dx.doi.org/10.1091/mbc.E05-08-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Altarejos JY, Montminy M, et al. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 2011;12:141–151. doi: 10.1038/nrm3072. http://dx.doi.org/10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Song Y, Altarejos J, Goodarzi MO, Inoue H, Guo X, Berdeaux R, et al. CRTC3 links catecholamine signalling to energy balance. Nature. 2010;468:933–939. doi: 10.1038/nature09564. http://dx.doi.org/10.1038/nature09564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mouchiroud L, Eichner LJ, Shaw RJ, Auwerx J. Transcriptional coregulators: fine-tuning metabolism. Cell Metab. 2014;20:26–40. doi: 10.1016/j.cmet.2014.03.027. http://dx.doi.org/10.1016/j.cmet.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Than TA, Lou H, Ji C, Win S, Kaplowitz N. Role of cAMP-responsive element-binding protein (CREB)-regulated transcription coactivator 3 (CRTC3) in the initiation of mitochondrial biogenesis and stress response in liver cells. J. Biol. Chem. 2011;286:22047–22054. doi: 10.1074/jbc.M111.240481. http://dx.doi.org/10.1074/jbc.M111.240481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rahnert JA, Zheng B, Hudson MB, Woodworth-Hobbs ME, Price SR. Glucocorticoids alter CRTC-CREB signaling in muscle cells: impact on PGC-1α expression and atrophy markers. PLoS One. 2016;11:e0159181. doi: 10.1371/journal.pone.0159181. http://dx.doi.org/10.1371/journal.pone.0159181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wu Z, Huang X, Feng Y, Handschin C, Feng Y, Gullicksen PS, et al. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:14379–14384. doi: 10.1073/pnas.0606714103. http://dx.doi.org/10.1073/pnas.0606714103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, et al. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J. Clin. Invest. 2012;122:1109–1118. doi: 10.1172/JCI60329. http://dx.doi.org/10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kappel BA, Stöhr R, De Angelis L, Mavilio M, Menghini R, Federici M. Posttranslational modulation of FoxO1 contributes to cardiac remodeling in post-ischemic heart failure. Atherosclerosis. 2016;249:148–156. doi: 10.1016/j.atherosclerosis.2016.04.001. http://dx.doi.org/10.1016/j.atherosclerosis.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 122.Qi Y, Zhu Q, Zhang K, Thomas C, Wu Y, Kumar R, et al. Activation of Foxo1 by insulin resistance promotes cardiac dysfunction and β-myosin heavy chain gene expression. Circ. Heart Fail. 2015;8:198–208. doi: 10.1161/CIRCHEARTFAILURE.114.001457. http://dx.doi.org/10.1161/CIRCHEARTFAILURE.114.001457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Puthanveetil P, Wan A, Rodrigues B. FoxO1 is crucial for sustaining cardiomyocyte metabolism and cell survival. Cardiovasc. Res. 2013;97:393–403. doi: 10.1093/cvr/cvs426. http://dx.doi.org/10.1093/cvr/cvs426. [DOI] [PubMed] [Google Scholar]
- 124.Shioda N, Han F, Moriguchi S, Fukunaga K. Constitutively active calcineurin mediates delayed neuronal death through Fas-ligand expression via activation of NFAT and FKHR transcriptional activities in mouse brain ischemia. J. Neurochem. 2007;102:1506–1517. doi: 10.1111/j.1471-4159.2007.04600.x. http://dx.doi.org/10.1111/j.1471-4159.2007.04600.x. [DOI] [PubMed] [Google Scholar]
- 125.Qiu H, Liu J-Y, Wei D, Li N, Yamoah EN, Hammock BD, et al. Cardiac-generated prostanoids mediate cardiac myocyte apoptosis after myocardial ischaemia. Cardiovasc. Res. 2012;95:336–345. doi: 10.1093/cvr/cvs191. http://dx.doi.org/10.1093/cvr/cvs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, et al. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc. Natl. Acad. Sci. U. S. A. 2007;104:20517–20522. doi: 10.1073/pnas.0610290104. http://dx.doi.org/10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, et al. Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation. 2006;114:1159–1168. doi: 10.1161/CIRCULATIONAHA.106.637124. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.637124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yuan Y, Shi X-E, Liu Y-G, Yang G-S. FoxO1 regulates muscle fiber-type specification and inhibits calcineurin signaling during C2C12 myoblast differentiation. Mol. Cell. Biochem. 2011;348:77–87. doi: 10.1007/s11010-010-0640-1. http://dx.doi.org/10.1007/s11010-010-0640-1. [DOI] [PubMed] [Google Scholar]
- 129.Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. http://dx.doi.org/10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. http://dx.doi.org/10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 131.Lavandero S, Chiong M, Rothermel BA, Hill JA. Autophagy in cardiovascular biology. J. Clin. Invest. 2015;125:55–64. doi: 10.1172/JCI73943. http://dx.doi.org/10.1172/JCI73943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lavandero S, Troncoso R, Rothermel BA, Martinet W, Sadoshima J, Hill JA. Cardiovascular autophagy: concepts, controversies, and perspectives. Autophagy. 2013;9:1455–1466. doi: 10.4161/auto.25969. http://dx.doi.org/10.4161/auto.25969. [DOI] [PubMed] [Google Scholar]
- 133.Ye J, Cardona M, Llovera M, Comella JX, Sanchis D. Translation of myocyte enhancer factor-2 is induced by hypertrophic stimuli in cardiomyocytes through a calcineurin-dependent pathway. J. Mol. Cell. Cardiol. 2012;53:578–587. doi: 10.1016/j.yjmcc.2012.07.013. http://dx.doi.org/10.1016/j.yjmcc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 134.Marks AR. Calcium and the heart: a question of life and death. J. Clin. Invest. 2003;111:597–600. doi: 10.1172/JCI18067. http://dx.doi.org/10.1172/JCI18067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu W-Z, Wang S-Q, Chakir K, Yang D, Zhang T, Brown JH, et al. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J. Clin. Invest. 2003;111:617–625. doi: 10.1172/JCI16326. http://dx.doi.org/10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. http://dx.doi.org/10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 137.Goonasekera SA, Molkentin JD. J. Mol. Cell. Cardiol. 2012;52:317–322. doi: 10.1016/j.yjmcc.2011.05.001. http://dx.doi.org/10.1016/j.yjmcc.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 138.Louch WE, Stokke MK, Sjaastad I, Christensen G, Sejersted OM. No rest for the weary: diastolic calcium homeostasis in the normal and failing myocardium. Physiology. 2012;27:308–323. doi: 10.1152/physiol.00021.2012. http://dx.doi.org/10.1152/physiol.00021.2012. [DOI] [PubMed] [Google Scholar]
- 139.Marks AR. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J. Clin. Invest. 2013;123:46–52. doi: 10.1172/JCI62834. http://dx.doi.org/10.1172/JCI62834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Perino A, Ghigo A, Scott JD, Hirsch E. Anchoring proteins as regulators of signaling pathways. Circ. Res. 2012;111:482–492. doi: 10.1161/CIRCRESAHA.111.262899. http://dx.doi.org/10.1161/CIRCRESAHA.111.262899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li J, Negro A, Lopez J, Bauman AL, Henson E, Dodge-Kafka K, et al. The mAKAPβ scaffold regulates cardiac myocyte hypertrophy via recruitment of activated calcineurin. J. Mol. Cell. Cardiol. 2010;48:387–394. doi: 10.1016/j.yjmcc.2009.10.023. http://dx.doi.org/10.1016/j.yjmcc.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pare GC, Bauman AL, McHenry M, Michel JJC, Dodge-Kafka KL, Kapiloff MS. The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling. J. Cell Sci. 2005;118:5637–5646. doi: 10.1242/jcs.02675. http://dx.doi.org/10.1242/jcs.02675. [DOI] [PubMed] [Google Scholar]
- 143.Li J, Vargas MAX, Kapiloff MS, Dodge-Kafka KL, et al. Regulation of MEF2 transcriptional activity by calcineurin/mAKAP complexes. Exp. Cell Res. 2013;319:447–454. doi: 10.1016/j.yexcr.2012.12.016. http://dx.doi.org/10.1016/j.yexcr.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kritzer MD, Li J, Passariello CL, Gayanilo M, Thakur H, Dayan J, et al. The scaffold protein muscle A-kinase anchoring protein ? orchestrates cardiac myocyte hypertrophic signaling required for the development of heart failure. Circ. Heart Fail. 2014;7:663–672. doi: 10.1161/CIRCHEARTFAILURE.114.001266. http://dx.doi.org/10.1161/CIRCHEARTFAILURE.114.001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kapiloff MS, Jackson N, Airhart N. mAKAP and the ryanodine receptor are part of a multi-component signaling complex on the cardiomyocyte nuclear envelope. J. Cell Sci. 2001;114:3167–3176. doi: 10.1242/jcs.114.17.3167. [DOI] [PubMed] [Google Scholar]
- 146.Schulze DH, Muqhal M, Lederer WJ, Ruknudin AM. Sodium/calcium exchanger (NCX1) macromolecular complex. J. Biol. Chem. 2003;278:28849–28855. doi: 10.1074/jbc.M300754200. http://dx.doi.org/10.1074/jbc.M300754200. [DOI] [PubMed] [Google Scholar]
- 147.Passariello CL, Li J, Dodge-Kafka K, Kapiloff MS. mAKAP-a master scaffold for cardiac remodeling. J. Cardiovasc. Pharmacol. 2015;65:218–225. doi: 10.1097/FJC.0000000000000206. http://dx.doi.org/10.1097/FJC.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Weins A, Schwarz K, Faul C, Barisoni L, Linke WA, Mundel P. Differentiation-and stress-dependent nuclear cytoplasmic redistribution of myopodin, a novel actin-bundling protein. J. Cell Biol. 2001;155:393–404. doi: 10.1083/jcb.200012039. http://dx.doi.org/10.1083/jcb.200012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Faul C, Dhume A, Schecter AD, Mundel P. A. Protein Kinase, Ca2+/calmodulin-dependent kinase II, and calcineurin regulate the intracellular trafficking of myopodin between the Z-disc and the nucleus of cardiac myocytes. Mol. Cell. Biol. 2007;27:8215–8227. doi: 10.1128/MCB.00950-07. http://dx.doi.org/10.1128/MCB.00950-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gold MG, Stengel F, Nygren PJ, Weisbrod CR, Bruce JE, Robinson CV, et al. Architecture and dynamics of an A-kinase anchoring protein 79 (AKAP79) signaling complex. Proc. Natl. Acad. Sci. 2011;108:6426–6431. doi: 10.1073/pnas.1014400108. http://dx.doi.org/10.1073/pnas.1014400108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Best JM, Kamp TJ. Different subcellular populations of L-type Ca2+ channels exhibit unique regulation and functional roles in cardiomyocytes. J. Mol. Cell. Cardiol. 2012;52:376–387. doi: 10.1016/j.yjmcc.2011.08.014. http://dx.doi.org/10.1016/j.yjmcc.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harvey RD, Calaghan SC. Caveolae create local signalling domains through their distinct protein content, lipid profile and morphology. J. Mol. Cell. Cardiol. 2012;52:366–375. doi: 10.1016/j.yjmcc.2011.07.007. http://dx.doi.org/10.1016/j.yjmcc.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maguy A, Hebert TE, Nattel S. Involvement of lipid rafts and caveolae in cardiac ion channel function. Cardiovasc. Res. 2006;69:798–807. doi: 10.1016/j.cardiores.2005.11.013. http://dx.doi.org/10.1016/j.cardiores.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 154.Makarewich CA, Correll RN, Gao H, Zhang H, Yang B, Berretta RM, et al. A caveolae- targeted L-type Ca2+ channel antagonist inhibits hypertrophic signaling without reducing cardiac contractility. Circ. Res. 2012;110:669–674. doi: 10.1161/CIRCRESAHA.111.264028. http://dx.doi.org/10.1161/CIRCRESAHA.111.264028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nieves-Cintrón M, Hirenallur-Shanthappa D, Nygren PJ, Hinke SA, Dell'Acqua ML, Langeberg LK, et al. Cellular signalling. Cell. Signal. 2016;28:733–740. doi: 10.1016/j.cellsig.2015.12.015. http://dx.doi.org/10.1016/j.cellsig.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Leisner TM, Freeman TC, Black JL, Parise LV. CIB1: a small protein with big ambitions. FASEB J. 2016;30:2640–2650. doi: 10.1096/fj.201500073R. http://dx.doi.org/10.1096/fj.201500073R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Heineke J, Auger-Messier M, Correll RN, Xu J, Benard MJ, Yuan W, et al. CIB1 is a regulator of pathological cardiac hypertrophy. Nat. Med. 2010;16:872–879. doi: 10.1038/nm.2181. http://dx.doi.org/10.1038/nm.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhao F, Zhang S, Chen L, Wu Y, Qin J, Shao Y, et al. Calcium- and integrin-binding protein-1 and calcineurin are upregulated in the right atrial myocardium of patients with atrial fibrillation. Europace. 2012;14:1726–1733. doi: 10.1093/europace/eus149. http://dx.doi.org/10.1093/europace/eus149. [DOI] [PubMed] [Google Scholar]
- 159.Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, et al. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc. Natl. Acad. Sci. 2009;106:6854–6859. doi: 10.1073/pnas.0809777106. http://dx.doi.org/10.1073/pnas.0809777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dibb KM, Clarke JD, Horn MA, Richards MA, Graham HK, Eisner DA, et al. Characterization of an extensive transverse tubular network in sheep atrial myocytes and its depletion in heart failure. Circ. Heart Fail. 2009;2:482–489. doi: 10.1161/CIRCHEARTFAILURE.109.852228. http://dx.doi.org/10.1161/CIRCHEARTFAILURE.109.852228. [DOI] [PubMed] [Google Scholar]
- 161.Glukhov AV, Balycheva M, Sanchez-Alonso JL, Ilkan Z, Alvarez-Laviada A, Bhogal N, et al. Direct evidence for microdomain-specific localization and remodeling of functional L-type calcium channels in rat and human atrial myocytes. Circulation. 2015;132:2372–2384. doi: 10.1161/CIRCULATIONAHA.115.018131. http://dx.doi.org/10.1161/CIRCULATIONAHA.115.018131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Horiuchi-Hirose M, Kashihara T, Nakada T, Kurebayashi N, Shimojo H, Shibazaki T, et al. Decrease in the density of t-tubular L-type Ca2+ channel currents in failing ventricular myocytes. AJP: Heart Circ. Physiol. 2011;300:H978–H988. doi: 10.1152/ajpheart.00508.2010. http://dx.doi.org/10.1152/ajpheart.00508.2010. [DOI] [PubMed] [Google Scholar]
- 163.Gadeberg HC, Bryant SM, James AF, Orchard CH. Altered Na/Ca exchange distribution in ventricular myocytes from failing hearts. AJP: Heart Circ. Physiol. 2016;310:H262–H268. doi: 10.1152/ajpheart.00597.2015. http://dx.doi.org/10.1152/ajpheart.00597.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bryant SM, Kong CHT, Watson J, Cannell MB, James AF, Orchard CH. J. Mol. Cell. Cardiol. 2015;86:23–31. doi: 10.1016/j.yjmcc.2015.06.012. http://dx.doi.org/10.1016/j.yjmcc.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang Y, Tandan S, Hill JA. Calcineurin-dependent ion channel regulation in heart. Trends Cardiovasc. Med. 2014;24:14–22. doi: 10.1016/j.tcm.2013.05.004. http://dx.doi.org/10.1016/j.tcm.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Harvey RD, Hell JW. CaV1.2 signaling complexes in the heart. J. Mol. Cell. Cardiol. 2013;58:143–152. doi: 10.1016/j.yjmcc.2012.12.006. http://dx.doi.org/10.1016/j.yjmcc.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tandan S, Wang Y, Wang TT, Jiang N, Hall DD, Hell JW, et al. Physical and functional interaction between calcineurin and the cardiac L-type Ca2+ channel. Circ. Res. 2009;105:51–60. doi: 10.1161/CIRCRESAHA.109.199828. http://dx.doi.org/10.1161/CIRCRESAHA.109.199828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang Y, Hill JA. J. Mol. Cell. Cardiol. 2010;48:619–632. doi: 10.1016/j.yjmcc.2010.01.009. http://dx.doi.org/10.1016/j.yjmcc.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bryant S, Kimura TE, Kong CHT, Watson JJ, Chase A, Suleiman MS, et al. J. Mol. Cell. Cardiol. 2014;68:47–55. doi: 10.1016/j.yjmcc.2013.12.026. http://dx.doi.org/10.1016/j.yjmcc.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chase A, Colyer J, Orchard CH. Localised Ca channel phosphorylation modulates the distribution of L-type Ca current in cardiac myocytes. J. Mol. Cell. Cardiol. 2010;49:121–131. doi: 10.1016/j.yjmcc.2010.02.017. http://dx.doi.org/10.1016/j.yjmcc.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 171.Yang K-C, Nerbonne JM. Mechanisms contributing to myocardial potassium channel diversity, regulation and remodeling. Trends Cardiovasc. Med. 2016;26:209–218. doi: 10.1016/j.tcm.2015.07.002. http://dx.doi.org/10.1016/j.tcm.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dong D, Duan Y, Guo J, Roach DE, Swirp SL, Wang L, et al. Overexpression of calcineurin in mouse causes sudden cardiac death associated with decreased density of K+ channels. Cardiovasc. Res. 2003;57:320–332. doi: 10.1016/s0008-6363(02)00661-2. [DOI] [PubMed] [Google Scholar]
- 173.Perrier E, Perrier R, Richard S, Bénitah J-P, et al. Ca2+ controls functional expression of the cardiac K+ transient outward current via the calcineurin pathway. J. Biol. Chem. 2004;279:40634–40639. doi: 10.1074/jbc.M407470200. http://dx.doi.org/10.1074/jbc.M407470200. [DOI] [PubMed] [Google Scholar]
- 174.Rossow CF, Dilly KW, Santana LF. Differential calcineurin/NFATc3 activity contributes to the Ito transmural gradient in the mouse heart. Circ. Res. 2006;98:1306–1313. doi: 10.1161/01.RES.0000222028.92993.10. http://dx.doi.org/10.1161/01.RES.0000222028.92993.10. [DOI] [PubMed] [Google Scholar]
- 175.Lin L, Sun W, Kung F, Dell'Acqua ML, Hoffman DA. AKAP79/150 impacts intrinsic excitability of hippocampal neurons through phospho-regulation of A-type K+ channel trafficking. J. Neurosci. 2011;31:1323–1332. doi: 10.1523/JNEUROSCI.5383-10.2011. http://dx.doi.org/10.1523/JNEUROSCI.5383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li X, Nooh MM, Bahouth SW. Role of AKAP79/150 protein in β1-adrenergic receptor trafficking and signaling in mammalian cells. J. Biol. Chem. 2013;288:33797–33812. doi: 10.1074/jbc.M113.470559. http://dx.doi.org/10.1074/jbc.M113.470559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Guillory AN, Yin X, Wijaya CS, Diaz Diaz AC, Rababa'h A, Singh S, et al. Enhanced cardiac function in Gravin mutant mice involves alterations in the β-adrenergic receptor signaling cascade. PLoS One. 2013;8:e74784. doi: 10.1371/journal.pone.0074784. http://dx.doi.org/10.1371/journal.pone.0074784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shih M, Lin F, Scott JD, Wang HY, Malbon CC. Dynamic complexes of beta2-adrenergic receptors with protein kinases and phosphatases and the role of gravin. J. Biol. Chem. 1999;274:1588–1595. doi: 10.1074/jbc.274.3.1588. [DOI] [PubMed] [Google Scholar]
- 179.Tao J, Malbon CC. G-protein-coupled receptor-associated A-kinase anchoring proteins AKAP5 and AKAP12: differential signaling to MAPK and GPCR recycling. J. Mol. Signal. 2014;3:19. doi: 10.1186/1750-2187-3-19. http://dx.doi.org/10.1186/1750-2187-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Trotter KW, Fraser ID, Scott GK, Stutts MJ, Scott JD, Milgram SL. Alternative splicing regulates the subcellular localization of A-kinase anchoring protein 18 isoforms. J. Cell Biol. 1999;147:1481–1492. doi: 10.1083/jcb.147.7.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fuller MD, Fu Y, Scheuer T, Catterall WA, et al. Differential regulation of CaV1.2 channels by cAMP-dependent protein kinase bound to A-kinase anchoring proteins 15 and 79/150. J. Gen. Physiol. 2014;143:315–324. doi: 10.1085/jgp.201311075. http://dx.doi.org/10.1085/jgp.201311075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jones BW, Brunet S, Gilbert ML, Nichols CB, Su T, Westenbroek RE, et al. Cardiomyocytes from AKAP7 knockout mice respond normally to adrenergic stimulation. Proc. Natl. Acad. Sci. 2012;109:17099–17104. doi: 10.1073/pnas.1215219109. http://dx.doi.org/10.1073/pnas.1215219109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Singh A, Redden JM, Kapiloff MS, Dodge-Kafka KL, et al. The large isoforms of A-kinase anchoring protein 18 mediate the phosphorylation of inhibitor-1 by protein kinase A and the inhibition of protein phosphatase 1 activity. Mol. Pharmacol. 2011;79:533–540. doi: 10.1124/mol.110.065425. http://dx.doi.org/10.1124/mol.110.065425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Huang FL, Glinsmann WH, et al. Separation and characterization of two phosphorylase phosphatase inhibitors from rabbit skeletal muscle. Eur. J. Biochem. 1976;70:419–426. doi: 10.1111/j.1432-1033.1976.tb11032.x. [DOI] [PubMed] [Google Scholar]
- 185.Nicolaou P, Hajjar RJ, Kranias EG. Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology. J. Mol. Cell. Cardiol. 2009;47:365–371. doi: 10.1016/j.yjmcc.2009.05.010. http://dx.doi.org/10.1016/j.yjmcc.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.El-Armouche A, Bednorz A, Pamminger T, Ditz D, Didié M, Dobrev D, et al. Role of calcineurin and protein phosphatase-2A in the regulation of phosphatase inhibitor-1 in cardiac myocytes. Biochem. Biophys. Res. Commun. 2006;346:700–706. doi: 10.1016/j.bbrc.2006.05.182. http://dx.doi.org/10.1016/j.bbrc.2006.05.182. [DOI] [PubMed] [Google Scholar]
- 187.Hollander JM, Baseler WA, Dabkowski ER. Proteomic remodeling of mitochondria in heart failure. Congest. Heart Fail. 2011;17:262–268. doi: 10.1111/j.1751-7133.2011.00254.x. http://dx.doi.org/10.1111/j.1751-7133.2011.00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bayeva M, Gheorghiade M, Ardehali H. Mitochondria as a therapeutic target in heart failure. J. Am. Coll. Cardiol. 2013;61:599–610. doi: 10.1016/j.jacc.2012.08.1021. http://dx.doi.org/10.1016/j.jacc.2012.08.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rosca MG, Tandler B, Hoppel CL. Mitochondria in cardiac hypertrophy and heart failure. J. Mol. Cell. Cardiol. 2013;55:31–41. doi: 10.1016/j.yjmcc.2012.09.002. http://dx.doi.org/10.1016/j.yjmcc.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Aubert G, Vega RB, Kelly DP. Perturbations in the gene regulatory pathways controlling mitochondrial energy production in the failing heart. BBA – Mol. Cell Res. 2013;1833:840–847. doi: 10.1016/j.bbamcr.2012.08.015. http://dx.doi.org/10.1016/j.bbamcr.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Karamanlidis G, Bautista-Hernandez V, Fynn-Thompson F, Del Nido P, Tian R. Impaired mitochondrial biogenesis precedes heart failure in right ventricular hypertrophy in congenital heart disease. Circ. Heart Fail. 2011;4:707–713. doi: 10.1161/CIRCHEARTFAILURE.111.961474. http://dx.doi.org/10.1161/CIRCHEARTFAILURE.111.961474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sayen MR, Gustafsson ÅB, Sussman MA, Molkentin JD, Gottlieb RA. Calcineurin transgenic mice have mitochondrial dysfunction and elevated superoxide production. Am. J. Phys. Cell Physiol. 2003;284:C562–C570. doi: 10.1152/ajpcell.00336.2002. http://dx.doi.org/10.1152/ajpcell.00336.2002. [DOI] [PubMed] [Google Scholar]
- 193.Slupe AM, Merrill RA, Flippo KH, Lobas MA, Houtman JCD, Strack S. A calcineurin docking motif (LXVP) in dynamin-related protein 1 contributes to mitochondrial fragmentation and ischemic neuronal injury. J. Biol. Chem. 2013;288:12353–12365. doi: 10.1074/jbc.M113.459677. http://dx.doi.org/10.1074/jbc.M113.459677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. http://dx.doi.org/10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105. http://dx.doi.org/10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Merrill RA, Strack S. Mitochondria: a kinase anchoring protein 1, a signaling platform for mitochondrial form and function. Int. J. Biochem. Cell Biol. 2014;48:92–96. doi: 10.1016/j.biocel.2013.12.012. http://dx.doi.org/10.1016/j.biocel.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kim H, Scimia MC, Wilkinson D, Trelles RD, Wood MR, Bowtell D, et al. Fine-tuning of Drp1/Fis1 availability by AKAP121/Siah2 regulates mitochondrial adaptation to hypoxia. Mol. Cell. 2011;44:532–544. doi: 10.1016/j.molcel.2011.08.045. http://dx.doi.org/10.1016/j.molcel.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Abrenica B, AlShaaban M, Czubryt MP, et al. The A-kinase anchor protein AKAP121 is a negative regulator of cardiomyocyte hypertrophy. J. Mol. Cell. Cardiol. 2009;46:674–681. doi: 10.1016/j.yjmcc.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 199.Song M, Dorn GW, II, et al. Mitoconfusion: noncanonical functioning of dynamism factors in static mitochondria of the heart. Cell Metab. 2015;21:195–205. doi: 10.1016/j.cmet.2014.12.019. http://dx.doi.org/10.1016/j.cmet.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, et al. Endogenous drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ. Res. :278. doi: 10.1161/CIRCRESAHA.116.303356. http://dx.doi.org/10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 201.Chen Y, Liu Y, Dorn GW. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ. Res. 2011;109:1327–1331. doi: 10.1161/CIRCRESAHA.111.258723. http://dx.doi.org/10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, et al. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 2014;33:2798–2813. doi: 10.15252/embj.201488658. http://dx.doi.org/10.15252/embj.201488658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Papanicolaou KN, Kikuchi R, Ngoh GA, Coughlan KA, Dominguez I, Stanley WC, et al. Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circ. Res. 2012;111:1012–1026. doi: 10.1161/CIRCRESAHA.112.274142. http://dx.doi.org/10.1161/CIRCRESAHA.112.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ishihara T, Ban-Ishihara R, Maeda M, Matsunaga Y, Ichimura A, Kyogoku S, et al. Dynamics of mitochondrial DNA nucleoids regulated by mitochondrial fission is essential for maintenance of homogeneously active mitochondria during neonatal heart development. Mol. Cell. Biol. 2014;35:211–223. doi: 10.1128/MCB.01054-14. http://dx.doi.org/10.1128/MCB.01054-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pennanen C, Parra V, López-Crisosto C, Morales PE, del Campo A, Gutierrez T, et al. Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+-calcineurin signaling pathway. J. Cell Sci. 2014;127:2659–2671. doi: 10.1242/jcs.139394. http://dx.doi.org/10.1242/jcs.139394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Givvimani S, Munjal C, Tyagi N, Sen U, Metreveli N, Tyagi SC. Mitochondrial division/mitophagy inhibitor (Mdivi) ameliorates pressure overload induced heart failure. PLoS One. 2012;7:e32388. doi: 10.1371/journal.pone.0032388. http://dx.doi.org/10.1371/journal.pone.0032388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ong S-B, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 208.Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, et al. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J. 2014;28:316–326. doi: 10.1096/fj.12-226225. http://dx.doi.org/10.1096/fj.12-226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ong S-B, Kalkhoran SB, Cabrera-Fuentes HA, Hausenloy DJ. Mitochondrial fusion and fission proteins as novel therapeutic targets for treating cardiovascular disease. Eur. J. Pharmacol. 2015;763:104–114. doi: 10.1016/j.ejphar.2015.04.056. http://dx.doi.org/10.1016/j.ejphar.2015.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 211.Springer JE, Azbill RD, Nottingham SA, Kennedy SE. Calcineurin-mediated BAD dephosphorylation activates the caspase-3 apoptotic cascade in traumatic spinal cord injury. J. Neurosci. 2000;20:7246–7251. doi: 10.1523/JNEUROSCI.20-19-07246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shou Y, Li L, Prabhakaran K, Borowitz JL, Isom GE. Calcineurin-mediated Bad translocation regulates cyanide-induced neuronal apoptosis. Biochem. J. 2004;379:805–813. doi: 10.1042/BJ20031107. http://dx.doi.org/10.1042/BJ20031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Abe T, Takagi M, Nakano M, Furuya N, Takeo S. Altered Bad localization and interaction between Bad and Bcl-xL in the hippocampus after transient global ischemia. Brain Res. 2004;1009:159–168. doi: 10.1016/j.brainres.2004.03.003. http://dx.doi.org/10.1016/j.brainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 214.Klamt F, Zdanov S, Levine RL, Pariser A, Zhang Y, Zhang B, et al. Oxidant-induced apoptosis is mediated by oxidation of the actin-regulatory protein cofilin. Nat. Cell Biol. 2009;11:1241–1246. doi: 10.1038/ncb1968. http://dx.doi.org/10.1038/ncb1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wabnitz GH, Goursot C, Jahraus B, Kirchgessner H, Hellwig A, Klemke M, et al. Mitochondrial translocation of oxidized cofilin induces caspase-independent necrotic-like programmed cell death of T cells. Cell Death Dis. 2010;1:e58. doi: 10.1038/cddis.2010.36. http://dx.doi.org/10.1038/cddis.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Xiang SY, Ouyang K, Yung BS, Miyamoto S, Smrcka AV, Chen J, et al. PLCε, PKD1, and SSH1L transduce RhoA signaling to protect mitochondria from oxidative stress in the heart. Sci. Signal. 2013;6:ra108. doi: 10.1126/scisignal.2004405. http://dx.doi.org/10.1126/scisignal.2004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang Y, Shibasaki F, Mizuno K. Calcium signal-induced cofilin dephosphorylation is mediated by Slingshot via calcineurin. J. Biol. Chem. 2005;280:12683–12689. doi: 10.1074/jbc.M411494200. http://dx.doi.org/10.1074/jbc.M411494200. [DOI] [PubMed] [Google Scholar]
- 218.Zhao J-W, Gao Z-L, Ji Q-Y, Wang H, Zhang H-Y, Yang Y-D, et al. Regulation of cofilin activity by CaMKII and calcineurin. Am. J. Med. Sci. 2012;344:462–472. doi: 10.1097/MAJ.0b013e318244745b. http://dx.doi.org/10.1097/MAJ.0b013e318244745b. [DOI] [PubMed] [Google Scholar]
- 219.Subramanian K, Gianni D, Balla C, Assenza GE, Joshi M, Semigran MJ, et al. Cofilin-2 phosphorylation and sequestration in myocardial aggregates: novel pathogenetic mechanisms for idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 2015;65:1199–1214. doi: 10.1016/j.jacc.2015.01.031. http://dx.doi.org/10.1016/j.jacc.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zakhary DR, Moravec CS, Bond M. Regulation of PKA binding to AKAPs in the heart: alterations in human heart failure. Circulation. 2000;101:1459–1464. doi: 10.1161/01.cir.101.12.1459. [DOI] [PubMed] [Google Scholar]
- 221.Rangel-Aldao R, Kupiec JW, Rosen OM. Resolution of the phosphorylated and dephosphorylated cAMP-binding proteins of bovine cardiac muscle by affinity labeling and two-dimensional electrophoresis. J. Biol. Chem. 1979;254:2499–2508. [PubMed] [Google Scholar]
- 222.Granot J, Mildvan AS, Hiyama K, Kondo H, Kaiser ET. Magnetic resonance studies of the effect of the regulatory subunit on metal and substrate binding to the catalytic subunit of bovine heart protein kinase. J. Biol. Chem. 1980;255:4569–4573. [PubMed] [Google Scholar]
- 223.Manni S, Mauban JH, Ward CW, Bond M. Phosphorylation of the cAMP-dependent protein kinase (PKA) regulatory subunit modulates PKA-AKAP interaction, substrate phosphorylation, and calcium signaling in cardiac cells. J. Biol. Chem. 2008;283:24145–24154. doi: 10.1074/jbc.M802278200. http://dx.doi.org/10.1074/jbc.M802278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rangel-Aldao R, Rosen OM. Dissociation and reassociation of the phosphorylated and nonphosphorylated forms of adenosine 3″:5″-monophosphate-dependent protein kinase from bovine cardiac muscle. J. Biol. Chem. 1976;251:3375–3380. [PubMed] [Google Scholar]
- 225.Bush EW, Hood DB, Papst PJ, Chapo JA, Minobe W, Bristow MR, et al. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J. Biol. Chem. 2006;281:33487–33496. doi: 10.1074/jbc.M605536200. http://dx.doi.org/10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- 226.Nakayama H. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB J. 2006;20:1660–1670. doi: 10.1096/fj.05-5560com. http://dx.doi.org/10.1096/fj.05-5560com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wu X, Eder P, Chang B, Molkentin JD. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc. Natl. Acad. Sci. 2010;107:7000–7005. doi: 10.1073/pnas.1001825107. http://dx.doi.org/10.1073/pnas.1001825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J. Clin. Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. http://dx.doi.org/10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kinoshita H, Kuwahara K, Nishida M, Jian Z, Rong X, Kiyonaka S, et al. Inhibition of TRPC6 channel activity contributes to the antihypertrophic effects of natriuretic peptides-guanylyl cyclase-A signaling in the heart. Circ. Res. 2010;106:1849–1860. doi: 10.1161/CIRCRESAHA.109.208314. http://dx.doi.org/10.1161/CIRCRESAHA.109.208314. [DOI] [PubMed] [Google Scholar]
- 230.Lighthouse JK, Small EM. J. Mol. Cell. Cardiol. 2016;91:52–60. doi: 10.1016/j.yjmcc.2015.12.016. http://dx.doi.org/10.1016/j.yjmcc.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Pedrozo Z, Criollo A, Battiprolu PK, Morales CR, Contreras-Ferrat A, Fernández C, et al. Polycystin-1 is a cardiomyocyte mechanosensor that governs L-type Ca2+ channel protein stability. Circulation. 2015;131:2131–2142. doi: 10.1161/CIRCULATIONAHA.114.013537. http://dx.doi.org/10.1161/CIRCULATIONAHA.114.013537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Finsen AV, Lunde IG, Sjaastad I, Østli EK, Lyngra M, Jarstadmarken HO, et al. Syndecan-4 is essential for development of concentric myocardial hypertrophy via stretch-induced activation of the calcineurin-NFAT pathway. PLoS One. 2011;6:e28302. doi: 10.1371/journal.pone.0028302. http://dx.doi.org/10.1371/journal.pone.0028302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Herum KM, Lunde IG, Skrbic B, Florholmen G, Behmen D, Sjaastad I, et al. Syndecan-4 signaling via NFAT regulates extracellular matrix production and cardiac myofibroblast differentiation in response to mechanical stress. J. Mol. Cell. Cardiol. 2013;54:73–81. doi: 10.1016/j.yjmcc.2012.11.006. http://dx.doi.org/10.1016/j.yjmcc.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 234.Herum KM, Lunde IG, Skrbic B, Louch WE, Hasic A, Boye S, et al. Syndecan-4 is a key determinant of collagen cross-linking and passive myocardial stiffness in the pressure-overloaded heart. Cardiovasc. Res. 2015;106:217–226. doi: 10.1093/cvr/cvv002. http://dx.doi.org/10.1093/cvr/cvv002. [DOI] [PubMed] [Google Scholar]
- 235.Kim EY, Roshanravan H, Dryer SE. Syndecan-4 ectodomain evokes mobilization of podocyte TRPC6 channels and their associated pathways: an essential role for integrin signaling. Biochim. Biophys. Acta. 2015;1853:2610–2620. doi: 10.1016/j.bbamcr.2015.07.011. http://dx.doi.org/10.1016/j.bbamcr.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 236.Frank D, Frey N. Cardiac Z-disc signaling network. J. Biol. Chem. 2011;286:9897–9904. doi: 10.1074/jbc.R110.174268. http://dx.doi.org/10.1074/jbc.R110.174268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Heineke J, Ruetten H, Willenbockel C, Gross SC, Naguib M, Schaefer A, et al. Attenuation of cardiac remodeling after myocardial infarction by muscle LIM protein-calcineurin signaling at the sarcomeric Z-disc. Proc. Natl. Acad. Sci. U. S. A. 2005;102:1655–1660. doi: 10.1073/pnas.0405488102. http://dx.doi.org/10.1073/pnas.0405488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Frey N, Barrientos T, Shelton JM, Frank D, Rütten H, Gehring D, et al. Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy in response to pathological biomechanical stress. Nat. Med. 2004;10:1336–1343. doi: 10.1038/nm1132. http://dx.doi.org/10.1038/nm1132. [DOI] [PubMed] [Google Scholar]
- 239.Jeong D, Kim JM, Cha H, Oh JG, Park J, Yun S-H, et al. PICOT attenuates cardiac hypertrophy by disrupting calcineurin-NFAT signaling. Circ. Res. 2008;102:711–719. doi: 10.1161/CIRCRESAHA.107.165985. http://dx.doi.org/10.1161/CIRCRESAHA.107.165985. [DOI] [PubMed] [Google Scholar]
- 240.Cha H, Kim JM, Oh JG, Jeong MH, Park CS, Park J, et al. PICOT is a critical regulator of cardiac hypertrophy and cardiomyocyte contractility. J. Mol. Cell. Cardiol. 2008;45:796–803. doi: 10.1016/j.yjmcc.2008.09.124. http://dx.doi.org/10.1016/j.yjmcc.2008.09.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Luosujärvi H, Aro J, Tokola H, Leskinen H, Tenhunen O, Skoumal R, et al. A novel p38 MAPK target dyxin is rapidly induced by mechanical load in the heart. Blood Press. 2010;19:54–63. doi: 10.3109/08037050903464519. http://dx.doi.org/10.3109/08037050903464519. [DOI] [PubMed] [Google Scholar]
- 242.Bian Z-Y, Huang H, Jiang H, Shen D-F, Yan L, Zhu L-H, et al. LIM and cysteine-rich domains 1 regulates cardiac hypertrophy by targeting calcineurin/nuclear factor of activated T cells signaling. Hypertension. 2010;55:257–263. doi: 10.1161/HYPERTENSIONAHA.109.135665. http://dx.doi.org/10.1161/HYPERTENSIONAHA.109.135665. [DOI] [PubMed] [Google Scholar]
- 243.Maejima Y, Usui S, Zhai P, Takamura M, Kaneko S, Zablocki D, et al. Muscle-specific RING finger 1 negatively regulates pathological cardiac hypertrophy through downregulation of calcineurin A. Circ. Heart Fail. 2014;7:479–490. doi: 10.1161/CIRCHEARTFAILURE.113.000713. http://dx.doi.org/10.1161/CIRCHEARTFAILURE.113.000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lyon RC, Zanella F, Omens JH, Sheikh F. Mechanotransduction in cardiac hypertrophy and failure. Circ. Res. 2015;116:1462–1476. doi: 10.1161/CIRCRESAHA.116.304937. http://dx.doi.org/10.1161/CIRCRESAHA.116.304937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Solaro RJ, Henze M, Kobayashi T. Integration of troponin I phosphorylation with cardiac regulatory networks. Circ. Res. 2013;112:355–366. doi: 10.1161/CIRCRESAHA.112.268672. http://dx.doi.org/10.1161/CIRCRESAHA.112.268672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. AJP: Heart Circ. Physiol. 2005;290:H1313–H1325. doi: 10.1152/ajpheart.00816.2005. http://dx.doi.org/10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Linke WA. Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc. Res. 2008;77:637–648. doi: 10.1016/j.cardiores.2007.03.029. http://dx.doi.org/10.1016/j.cardiores.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 248.Hisamitsu T, Nakamura TY, Wakabayashi S. Na+/H+ exchanger 1 directly binds to calcineurin A and activates downstream NFAT signaling, leading to cardiomyocyte hypertrophy. Mol. Cell. Biol. 2012;32:3265–3280. doi: 10.1128/MCB.00145-12. http://dx.doi.org/10.1128/MCB.00145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Nakamura TY, Iwata Y, Arai Y, Komamura K, Wakabayashi S. Activation of Na+/H+ exchanger 1 is sufficient to generate Ca2+ signals that induce cardiac hypertrophy and heart failure. Circ. Res. 2008;103:891–899. doi: 10.1161/CIRCRESAHA.108.175141. http://dx.doi.org/10.1161/CIRCRESAHA.108.175141. [DOI] [PubMed] [Google Scholar]
- 250.Cavalli A, Eghbali M, Minosyan TY, Stefani E, Philipson KD. Localization of sarcolemmal proteins to lipid rafts in themyocardium. Cell Calcium. 2007;42:313–322. doi: 10.1016/j.ceca.2007.01.003. http://dx.doi.org/10.1016/j.ceca.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Scriven DRL, Klimek A, Asghari P, Bellve K, Moore EDW, et al. Caveolin-3 is adjacent to a group of extradyadic ryanodine receptors. Biophys. J. 2005;89:1893–1901. doi: 10.1529/biophysj.105.064212. http://dx.doi.org/10.1529/biophysj.105.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Shigekawa M, Katanosaka Y, Wakabayashi S. Regulation of the cardiac Na+/Ca2+ exchanger by calcineurin and protein kinase C. Ann. N. Y. Acad. Sci. 2007;1099:53–63. doi: 10.1196/annals.1387.059. http://dx.doi.org/10.1196/annals.1387.059. [DOI] [PubMed] [Google Scholar]
- 253.Katanosaka Y, Iwata Y, Kobayashi Y, Shibasaki F, Wakabayashi S, Shigekawa M. Calcineurin inhibits Na+/Ca2+ exchange in phenylephrine-treated hypertrophic cardiomyocytes. J. Biol. Chem. 2005;280:5764–5772. doi: 10.1074/jbc.M410240200. http://dx.doi.org/10.1074/jbc.M410240200. [DOI] [PubMed] [Google Scholar]
- 254.Gelpi RJ, Gao S, Zhai P, Yan L, Hong C, Danridge LMA, et al. Genetic inhibition of calcineurin induces diastolic dysfunction in mice with chronic pressure overload. AJP: Heart Circ. Physiol. 2009;297:H1814–H1819. doi: 10.1152/ajpheart.00449.2009. http://dx.doi.org/10.1152/ajpheart.00449.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Rothermel BA, Berenji K, Tannous P, Kutschke W, Dey A, Nolan B, et al. Differential activation of stress-response signaling in load-induced cardiac hypertrophy and failure. Physiol. Genomics. 2005;23:18–27. doi: 10.1152/physiolgenomics.00061.2005. http://dx.doi.org/10.1152/physiolgenomics.00061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Davis J, Davis LC, Correll RN, Makarewich CA, Schwanekamp JA, Moussavi-Harami F, et al. A tension-based model distinguishes hypertrophic versus dilated cardiomyopathy. Cell. 2016;165:1147–1159. doi: 10.1016/j.cell.2016.04.002. http://dx.doi.org/10.1016/j.cell.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Clark RB, Bouchard RA, Salinas-Stefanon E, Sanchez-Chapula J, Giles WR. Heterogeneity of action potential waveforms and potassium currents in rat ventricle. Cardiovasc. Res. 1993;27:1795–1799. doi: 10.1093/cvr/27.10.1795. [DOI] [PubMed] [Google Scholar]
- 258.Gao J, Sun X, Potapova IA, Cohen IS, Mathias RT, Kim JH. Autocrine A2 in the T-system of ventricular myocytes creates transmural gradients in ion transport: a mechanism to match contraction with load? Biophys. J. 2014;106:2364–2374. doi: 10.1016/j.bpj.2014.04.042. http://dx.doi.org/10.1016/j.bpj.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Gaborit N, Le Bouter S, Szuts V, Varro A, Escande D, Nattel S, et al. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J. Physiol. 2007;582:675–693. doi: 10.1113/jphysiol.2006.126714. http://dx.doi.org/10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bondarenko VE, Rasmusson RL. Transmural heterogeneity of repolarization and Ca2+ handling in a model of mouse ventricular tissue. AJP: Heart Circ. Physiol. 2010;299:H454–H469. doi: 10.1152/ajpheart.00907.2009. http://dx.doi.org/10.1152/ajpheart.00907.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Thomas JD, Popović ZB, et al. Assessment of left ventricular function by cardiac ultrasound. J. Am. Coll. Cardiol. 2006;48:2012–2025. doi: 10.1016/j.jacc.2006.06.071. http://dx.doi.org/10.1016/j.jacc.2006.06.071. [DOI] [PubMed] [Google Scholar]
- 262.Rossow CF, Dilly KW, Yuan C, Nieves-Cintrón M, Cabarrus JL, Santana LF. NFATc3-dependent loss of Ito gradient across the left ventricular wall during chronic β adrenergic stimulation. J. Mol. Cell. Cardiol. 2009;46:249–256. doi: 10.1016/j.yjmcc.2008.10.016. http://dx.doi.org/10.1016/j.yjmcc.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Costantini DL, Arruda EP, Agarwal P, Kim K-H, Zhu Y, Zhu W, et al. The homeodomain transcription factor Irx5 establishes the mouse cardiac ventricular repolarization gradient. Cell. 2005;123:347–358. doi: 10.1016/j.cell.2005.08.004. http://dx.doi.org/10.1016/j.cell.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wang Z, Kutschke W, Richardson KE, Karimi M, Hill JA. Electrical remodeling in pressure-overload cardiac hypertrophy: role of calcineurin. Circulation. 2001;104:1657–1663. doi: 10.1161/hc3901.095766. [DOI] [PubMed] [Google Scholar]
- 265.Wang G, Kawakami K, Gick G. Divergent signaling pathways mediate induction of Na,K-ATPase alpha1 and beta1 subunit gene transcription by low potassium. Mol. Cell. Biochem. 2007;294:73–85. doi: 10.1007/s11010-006-9247-y. http://dx.doi.org/10.1007/s11010-006-9247-y. [DOI] [PubMed] [Google Scholar]
- 266.Li Z, Langhans SA. Transcriptional regulators of Na,K-ATPase subunits. Front. Cell Dev. Biol. 2015;3:66. doi: 10.3389/fcell.2015.00066. http://dx.doi.org/10.3389/fcell.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.de Lores Arnaiz GR, Ordieres MGL. Brain Na(+), K(+)-ATPase activity in aging and disease. Int. J. Biomed. Sci. 2014;10:85–102. [PMC free article] [PubMed] [Google Scholar]
- 268.McGahon MK, Yarham JM, Daly A, Guduric-Fuchs J, Ferguson LJ, Simpson DA, et al. Distinctive profile of IsomiR expression and novel microRNAs in rat heart left ventricle. PLoS One. 2013;8:e65809. doi: 10.1371/journal.pone.0065809. http://dx.doi.org/10.1371/journal.pone.0065809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Fontes MSC, Raaijmakers AJA, van Doorn T, Kok B, Nieuwenhuis S, van der Nagel R, et al. Changes in Cx43 and NaV1.5 expression precede the occurrence of substantial fibrosis in calcineurin-induced murine cardiac hypertrophy. PLoS One. 2014;9:e87226. doi: 10.1371/journal.pone.0087226. http://dx.doi.org/10.1371/journal.pone.0087226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.F.J.A. BSc, E.V.T. BSc, C.J.R. BSc, W.G.P. PhD, T.A.M. PhD. Consequences of circadian and sleep disturbances for the cardiovascular system. Can. J. Cardiol. 2015;31:860–872. doi: 10.1016/j.cjca.2015.01.015. http://dx.doi.org/10.1016/j.cjca.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 271.Smolensky MH, Portaluppi F, Manfredini R, Hermida RC, Tiseo R, Sackett-Lundeen LL, et al. Diurnal and twenty-four hour patterning of human diseases: cardiac, vascular, and respiratory diseases, conditions, and syndromes. Sleep Med. Rev. 2015;21:3–11. doi: 10.1016/j.smrv.2014.07.001. http://dx.doi.org/10.1016/j.smrv.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 272.Takeda N, Maemura K. The role of clock genes and circadian rhythmin the development of cardiovascular diseases. Cell. Mol. Life Sci. 2015;72:3225–3234. doi: 10.1007/s00018-015-1923-1. http://dx.doi.org/10.1007/s00018-015-1923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Virag JAI, et al. Circadian Influences on Myocardial Infarction. 2014:1–10. doi: 10.3389/fphys.2014.00422. http://dx.doi.org/10.3389/fphys.2014.00422/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Morris CJ, Purvis TE, Hu K, Scheer FAJL. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. 2016 doi: 10.1073/pnas.1516953113. http://dx.doi.org/10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sachan N, Dey A, Rotter D, Grinsfelder DB, Battiprolu PK, Sikder D, et al. Sustained hemodynamic stress disrupts normal circadian rhythms in calcineurin- dependent signaling and protein phosphorylation in the heart. Circ. Res. 2011;108:437–445. doi: 10.1161/CIRCRESAHA.110.235309. http://dx.doi.org/10.1161/CIRCRESAHA.110.235309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, et al. Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ. Res. 2010;106:546–550. doi: 10.1161/CIRCRESAHA.109.209346. http://dx.doi.org/10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Dyar KA, Ciciliot S, Tagliazucchi GM, Pallafacchina G, Tothova J, Argentini C, et al. The calcineurin-NFAT pathway controls activity-dependent circadian gene expression in slow skeletal muscle. Mol. Metab. 2015;4:823–833. doi: 10.1016/j.molmet.2015.09.004. http://dx.doi.org/10.1016/j.molmet.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Fan Y-Y. Effects of sleep deprivation on action potential and transient outward potassium current in ventricular myocytes in rats. Med. Sci. Monit. 2015;21:542–549. doi: 10.12659/MSM.893414. http://dx.doi.org/10.12659/MSM.893414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Rosati B, Grau F, McKinnon D. Regional variation in mRNA transcript abundance within the ventricular wall. J. Mol. Cell. Cardiol. 2006;40:295–302. doi: 10.1016/j.yjmcc.2005.11.002. http://dx.doi.org/10.1016/j.yjmcc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 280.Rotter D, Rothermel BA. Targets, trafficking, and timing of cardiac autophagy. Pharmacol. Res. 2012;66:494–504. doi: 10.1016/j.phrs.2012.10.001. http://dx.doi.org/10.1016/j.phrs.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Joshi-Mukherjee R, Dick IE, Liu T, O'Rourke B, Yue DT, Tung L. J. Mol. Cell. Cardiol. 2013;65:76–87. doi: 10.1016/j.yjmcc.2013.09.009. http://dx.doi.org/10.1016/j.yjmcc.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Peter AK, Bjerke MA, Leinwand LA. Biology of the cardiac myocyte in heart disease. Mol. Biol. Cell. 2016;27:2149–2160. doi: 10.1091/mbc.E16-01-0038. http://dx.doi.org/10.1091/mbc.E16-01-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Rapila R, Korhonen T, Tavi P. Excitation-contraction coupling of the mouse embryonic cardiomyocyte. J. Gen. Physiol. 2008;132:397–405. doi: 10.1085/jgp.200809960. http://dx.doi.org/10.1085/jgp.200809960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ziman AP, Gómez-Viquez NL, Bloch RJ, Lederer WJ. Excitation-contraction coupling changes during postnatal cardiac development. J. Mol. Cell. Cardiol. 2010;48:379–386. doi: 10.1016/j.yjmcc.2009.09.016. http://dx.doi.org/10.1016/j.yjmcc.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Louch WE, Koivumäki JT, Tavi P. Calcium signalling in developing cardiomyocytes: implications for model systems and disease. J. Physiol. 2015;593:1047–1063. doi: 10.1113/jphysiol.2014.274712. http://dx.doi.org/10.1113/jphysiol.2014.274712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Maulik N, Baker JE, Engelman RM, Das DK. Postnatal developmental profiles of antioxidant enzymes in heart. Ann. N. Y. Acad. Sci. 1996;793:439–448. doi: 10.1111/j.1749-6632.1996.tb33538.x. [DOI] [PubMed] [Google Scholar]
- 287.Hasegawa T, Yamaguchi M, Yoshimura N, Okita Y. The dependence of myocardial damage on age and ischemic time in pediatric cardiac surgery. J. Thorac. Cardiovasc. Surg. 2005;129:192–198. doi: 10.1016/j.jtcvs.2004.05.005. http://dx.doi.org/10.1016/j.jtcvs.2004.05.005. [DOI] [PubMed] [Google Scholar]