Abstract
Objective
Total bilirubin (TB) was recently recognized as an endogenous anti-inflammatory and anti-oxidant molecule. Uric acid (UA) takes part in cardiovascular diseases by inducing oxidative stress, inflammation, and endothelial dysfunction. We assessed the relationship between serum TB levels, serum UA levels, and inflammatory status assessed by neutrophil-to-lymphocyte ratio (N/L) and arterial stiffness and arterial wave reflection in patients with a clinical diagnosis of coronary artery disease (CAD).
Methods
We included 145 consecutive patients admitted with stable angina pectoris (SAP) or acute coronary syndrome (ACS). Blood samples were drawn at admission for complete blood count and biochemistry. Non-invasive pulse waveform analysis for the determination of augmentation index (AIx) and carotid-femoral pulse wave velocity (PWV) measurements were performed with the commercially available SphygmoCor system.
Results
When patients were divided into tertiles of PWV and AIx, median N/L and median serum UA levels were the highest and mean TB levels were the lowest in the third tertile (p<0.001 for all). AIx and PWV were positively associated with serum UA and N/L and negatively associated with serum TB levels (p<0.001 for all). After adjustments for age, gender, heart rate, systolic blood pressure, and presence of diabetes, significant correlations persisted for N/L, UA, and TB in ACS patients (p<0.05). In the SAP group, TB was significantly negatively correlated with AIx and PWV, and UA was significantly positively correlated with PWV (p<0.05).
Conclusion
N/L ratio and serum UA and TB levels might be used to risk-stratify patients with respect to arterial stiffness in CAD patients, especially in the presence of ACS.
Keywords: arterial stiffness, arterial wave reflection, neutrophil-to-lymphocyte ratio, uric acid, total bilirubin, coronary artery diasease
Introduction
“Arterial stiffness” collectively describes distensibility, compliance, and elasticity of the arterial vasculature (1). Carotid-to- femoral pulse wave velocity (PWV) is considered the gold standard method because of its relative ease in determination and reliability (2). Central aortic pressure wave is composed of a forward-traveling incident wave created by ventricular systole and a subsequent reflected wave from the periphery. Increased aortic stiffness leads to faster transmission of both waves; so, a reflected wave arrives earlier and causes a disproportionate increase in systolic pressure in late systole (3). Augmentation of the central aortic pressure wave is expressed as augmentation pressure (AP) in absolute terms. Augmentation index (AIx), which is an indirect measure of arterial stiffness, is the AP as a percentage of central pulse pressure (PP) (4).
Bilirubin, the end product of heme catabolism, was recently recognized as an endogenous anti-inflammatory and anti-oxidant molecule. It has been revealed that bilirubin is a potent scavenger of reactive oxygen species, and it suppresses the oxidation of lipids and lipoproteins (5). Uric acid, which is generated as the end product of purine metabolism in humans, has also been shown to play a key role in cardiovascular diseases, hypertension, and related conditions by inducing oxidative stress, inflammation, and endothelial dysfunction (6).
We aimed to investigate if there is any association between serum total bilirubin levels, serum uric acid levels, neutrophil-to- lymphocyte ratio (N/L), and arterial stiffness measured by PWV and arterial wave reflection assessed by AIx in patients with a clinical diagnosis of coronary artery disease (CAD).
Methods
This cross-sectional study consisted of 145 consecutive patients who were admitted to our clinic with stable angina pectoris (SAP) or acute coronary syndrome (ACS) and decided to undergo coronary angiography with a clinical diagnosis of CAD between October 2012 and May 2013. Eighty-three patients had SAP, which was defined as angina pectoris and/or angina equivalent symptoms suggestive of CAD, and they had either positive stress test results or other indications for coronary angiography. Twenty-nine patients had an unstable pattern of chest pain suggesting unstable angina pectoris (USAP) with or without ischemic electrocardiographic findings, and 33 patients were admitted with acute myocardial infarction (AMI). AMI was defined according to the latest universal definition of myocardial infarction (7).
Anthropometric parameters; past medical history; presence of hypertension, diabetes, and hyperlipidemia; smoking habits; family history of CAD; and medications were recorded for each patient. Blood samples were drawn from the antecubital vein at admission for complete blood count (Cell-Dyn 3700, Abbott, USA) and biochemistry analysis UniCell DxC 800, Beckman Coulter USA). Total white blood cell (WBC) count and differential leukocyte count were determined using an automated blood cell counter.
Selective left and right coronary angiography was performed through the femoral artery by standard Judkins technique with 6 Fr catheters (MediCath, Barcelona, Spain) using a GE Innova 4100 (GE Healthcare, Milwaukee, WI, USA).
Non-invasive pulse waveform analysis was performed on the day following coronary angiography with the commercially available SphygmoCor system (AtCor Medical, Sydney, Australia). Peripheral pressure waveforms were recorded from the radial artery, using applanation tonometry with a high-fidelity micromanometer. After the acquisition of 20 sequential waveforms, a validated (8) generalized transfer function was used to generate the corresponding central aortic pressure waveform. Only high-quality recordings, defined as an in-device quality index of 80% and visually acceptable curves by the investigator, were included in the analysis. AP AIx, and PP were recorded. Entire pulse wave analysis was performed in the sitting position under standardized conditions in the morning hours. Peripheral blood pressure measurements were performed with a validated automated arm blood pressure monitor (Omron M3W, Omron Healthcare), keeping the radial artery at the level of the heart. Blood pressure recordings were taken immediately prior to tonometric measurements. The average of two measurements taken at 5-minute intervals was recorded.
Carotid-femoral PWV was also assessed using the SphygmoCor device (AtCor Medical, Sydney, Australia) as previously described (9). A measuring tape was used to measure the distance between the carotid and femoral artery recording sites. PWV was calculated automatically by dividing this distance by the time interval between the rapid upstroke in the pulse wave at the carotid and femoral arteries using the peak of the R wave on electrocardiography as a reference point.
Exclusion criteria were prior percutaneous or surgical revascularization; active infectious disease; inflammatory, autoimmune, or connective tissue disease; severe renal disease; any hepatic disease; and rhythm other than sinus.
This cross-sectional study was conducted according to the recommendations of Declaration of Helsinki on Biomedical Research involving human subjects and was approved by the institutional ethics committee. Written informed consent was obtained from each participant.
Statistical analyses
SPSS statistical software (SPSS 15.0 for Windows, Inc., Chicago, IL, USA) was used for all statistical calculations. Kolmogorov-Smirnov test was used to test for normal distribution. Continuous variables were given as mean±standard deviation and medians (interquartiles); categorical variables were defined as percentages. Continuous variables were compared by ANOVA and Kruskal-Wallis tests. Spearman’s correlation coefficient was used for the analysis of the correlation between AIx, PWV, N/L ratio, serum uric acid, and total bilirubin levels. Partial rank correlation coefficients were calculated using SPSS Syntax coding, adjusting for age, gender, height, heart rate, systolic blood pressure, and diabetes mellitus. For post hoc multiple comparisons where Bonferroni’s correction was applied, statistical significance was defined as p<0.017. Otherwise, p<0.05 was considered significant.
Results
Baseline characteristics of the study population, including demographics, cardiovascular risk factors, medications, and hemodynamic data, are provided in Table 1. Mean age of the study population was 60.1±13.4 and the ratio of females was 46.2%. Seventy-two patients had significant CAD, defined as at least 50% stenosis in at least one epicardial coronary artery; 33 patients had minimal CAD, which meant CAD not fulfilling the criteria for significant CAD. Forty patients were found to have normal epicardial coronary arteries.
Table 1.
Baseline characteristics of the study population
| Age, years | 60.1±13.4 |
|---|---|
| Sex, female, % | 46.2 |
| BMI, kg/m2 | 27.3±2.9 |
| HT, % | 56.6 |
| DM, % | 33.8 |
| HPL, % | 58.6 |
| FH,% | 31.0 |
| Smoker, % | 51.7 |
| Anti-HT, % | 58.6 |
| Statin, % | 35.9 |
| Hb, g/dL | 13.6±1.8 |
| WBC, 103/mm3 | 7.5 (6.25-8.9) |
| Plt, 103/mm3 | 238 (205-291.5) |
| Neutrophil,103/mm3 | 4.6 (3.7-5.5) |
| Lymphocyte,103/mm3 | 2.2±0.7 |
| N/L | 2.0 (1.6-2.7) |
| BUN, mg/dL | 15.0 (12.0-19.0) |
| Creatinine, mg/dL | 0.8 (0.7-0.9) |
| Uric acid, mg/dL | 5.0 (4.3-6.4) |
| T. bilirubin, mg/dL | 0.7±0.3 |
| AST, U/L | 18.0 (15.9-23.0) |
| ALT, U/L | 20.0 (15.5-27.0) |
| GGT, U/L | 30.0 (21.5-37.5) |
| LDL, mg/dL | 127.1±35.7 |
| HDL, mg/dL | 44.4±15.0 |
| TG, mg/dL | 147.0 (93.0-187.5) |
| Brachial SBP mm Hg | 137.8±12.2 |
| Brachial DBP mm Hg | 85.1±9.2 |
| Central SBP mm Hg | 126.0±12.3 |
| Central DBP mm Hg | 85.4±9.2 |
| Central PP, mm Hg | 40.6±5.9 |
| HR, beats/min | 70.6±6.8 |
| AP mm Hg | 9.2±2.3 |
| AIx, mm Hg | 22.5±3.8 |
| PWV, m/s | 11.0±3.3 |
ALT - alanine amino transferase; AIx - augmentation index; Anti-HT - anti hypertension; AP - augmentation pressure; AST - aspartate amino transferase; BMI - body mass index; BUN - blood urea nitrogen; DBP - diastolic blood pressure; DM - diabetes mellitus; FH - family history; Hb - hemoglobin; HDL - high-density lipoprotein; HPL - hyperlipidemia; HR - heart rate; HT - hypertension; GGT - gamma-glutamyl transferase; LDL - low-density lipoprotein; MPV - mean platelet volume; N/L - neutrophil-to-lymphocyte ratio; PP - pulse pressure; PWV - pulse wave velocity; SBP - systolic blood pressure; TG - triglyceride; WBC - white blood cell count
Tables 2 and 3 demonstrate the serum neutrophil, lymphocyte counts, N/L ratio, serum uric acid levels, and total serum bilirubin levels detected by routine complete blood count and biochemistry analysis separately in SAP and ACS patients with respect to tertiles of PWV and Aix, respectively. Central and peripheral hemodynamic data are also given in these tables. N/L ratio and mean serum uric acid levels were the highest in the 3rd tertile of PWV and AIx, both in SAP and ACS patients, whereas mean serum total bilirubin levels were the lowest. Normal reference values were 1.8-7 mg/dL for serum uric acid and 0-1.4 mg/ dL for total serum bilirubin in our laboratory. Only two patients had out-of-range serum total bilirubin levels just above the upper limit. Eighteen patients had high serum uric acid levels; 10 of them had values between 7-8 mg/dL, 5 patients had values between 8-9 mg/dL, and 3 patients had values between 9-10 mg/dL.
Table 2.
Complete blood count and biochemical and hemodynamic analysis, including serum uric acid and total bilirubin levels, according to tertiles of PWV, separate for stable angina pectoris and acute coronary syndrome patients
| PWV tertile I (SAP) N:37 | PWV tertile I (ACS) N:13 | PWV tertile II (SAP) N:31 | PWV tertile II (ACS) N:17 | PWV tertile III (SAP) N:15 | PWV tertile III (ACS) N:32 | P (SAP) | P (ACS) | |
|---|---|---|---|---|---|---|---|---|
| Hb, g/dL | 13.9±1.8 | 13.8±1.7 | 13.7±1.7 | 13.7±2.0 | 13.4±2.3 | 13.0±1.7 | 0.636 | 0.338 |
| WBC, 103/mm3 | 6.7 (5.7-7.8) | 8.7 (5.8-10.5) | 7 (6.1-8.8) | 7.5 (6.4-8.1) | 7.9 (7-9.4) | 7.9 (6.5-10) | 0.085 | 0.404 |
| Neutrophil, 103/mm3 | 4 (3.3-5.1) | 4.6 (3.6-5.3) | 4.2 (3.6-5.1) | 4.3 (3.4-5) | 5.1 (4.4-5.8) | 5.6 (4.3-6.4) | 0.0433 | 0.0196 |
| Lymphocyte, 103/mm3 | 2.2±0.7 | 2.7±1.0 | 2.4±0.6 | 1.9±0.6 | 2.3±0.7 | 1.9±0.6 | 0.632 | 0.0012 |
| N/L | 1.8 (1.4-2.4) | 1.8 (1.4-2.1) | 1.9 (1.6-2.2) | 2.1 (1.8-2.8) | 2.1 (1.7-3.4) | 3.2 (2.2-3.9) | 0.314 | 0.0013 |
| Platelet, 103/mm3 | 227 (208-269) | 267(234-281) | 255(211-298) | 231 (200-254) | 243 (213-258) | 245 (182-325) | 0.512 | 0.265 |
| MPV, fL | 8.5±1.1 | 8.6±0.7 | 8.8±1.2 | 8.5±0.9 | 8.8±1.2 | 8.6±1.1 | 0.634 | 0.933 |
| BUN, mg/dL | 13 (11-15) | 14 (12-18) | 14 (13-18) | 18 (13-20) | 21 (13-28.5) | 17 (12.5-19) | 0.0113 | 0.284 |
| Creatinine, mg/dL | 0.7 (0.6-0.8) | 0.8 (0.7-0.9) | 0.7 (0.65-0.8) | 0.8 (0.7-0.8) | 0.8 (0.7-0.9) | 0.8 (0.8-1.1) | 0.534 | 0.184 |
| Uric acid, mg/dL | 4.3 (4-5) | 4.8 (3.8-5.9) | 4.7 (4-5.6) | 5.1 (4.6-6.4) | 5.8 (4.7-7.3) | 6.5 (5.9-7.3) | 0.0103 | <0.0013 |
| T. bilirubin, mg/dL | 0.9±0.2 | 0.9±0.2 | 0.7±0.2 | 0.8±0.3 | 0.5±0.2 | 0.5±0.2 | <0.0014 | <0.0011 |
| AST, U/L | 18 (16-23) | 19 (16-23) | 19 (17-22.5) | 20 (14-24) | 16 (13.5-18.5) | 18.5 (16-23.5) | 0.149 | 0.880 |
| ALT, U/L | 20(18-27) | 22 (14-35) | 20 (17-26.5) | 21 (12-32) | 18 (11.5-23) | 20.5 (13.5-27) | 0.241 | 0.928 |
| GGT, U/L | 30 (23-40) | 33 (27-35) | 25 (18-35.5) | 29 (19-37) | 29 (22-38.5) | 32.5 (21-42) | 0.567 | 0.759 |
| Brachial SBP mm Hg | 131.8±10.2 | 135.2±11.2 | 138.5±10.5 | 140.5±12.7 | 144.6±11.1 | 140.5±14.0 | <0.0012 | 0.426 |
| Brachial DBF? mm Hg | 81.1±8.5 | 84.2±8.5 | 85.5±9.7 | 88.1±7.5 | 88.2±6.8 | 86.8±10.5 | 0.0182 | 0.538 |
| Central SBP mm Hg | 119.7±10.4 | 123.9±11.0 | 126.9±10.7 | 128.2±13.6 | 132.8±11.0 | 129.1±13.5 | <0.0012 | 0.482 |
| Central DBP mm Hg | 81.3±8.4 | 84.6±8.2 | 85.7±9.7 | 88.5±7.8 | 88.6±6.7 | 87.0±10.6 | 0.0152 | 0.542 |
| Central PP, mm Hg | 38.4±5.1 | 39.3±4.7 | 41.1±5.4 | 39.8±7.6 | 44.2±6.4 | 42.1±5.4 | 0.0032 | 0.242 |
| HR, beats/min | 70.9±6.4 | 67.9±4.8 | 70.4±8.0 | 70.8±7.0 | 71.8±5.2 | 70.8±7.3 | 0.815 | 0.408 |
| AP mm Hg | 7.2±1.4 | 7.2±1.1 | 9.1±1.3 | 9.0±1.9 | 11.3±1.8 | 11.5±1.8 | <0.0014 | <0.0014 |
| AIx | 18.6±2.3 | 18.4±1.7 | 22.2±1.4 | 22.6±1.7 | 25.7±1.7 | 27.2±1.5 | <0.0014 | <0.0014 |
| Gensini | 0 (0-7) | 4.5 (0-10) | 6 (0-13.3) | 21.5 (15-28) | 19.5 (11-57) | 52 (44-91) | <0.0011 | <0.0014 |
AIx - augmentation index; ALT - alanine aminotransferase; AP - augmentation pressure; AST - aspartate aminotransferase; BUN - blood urea nitrogen; DBP - diastolic blood pressure; Hb - hemoglobin; HR - heart rate; GGT - gamma-glutamyl transferase; MPV - mean platelet volume; N/L - neutrophil-to-lymphocyte ratio; PWV - pulse wave velocity; SBP - systolic blood pressure; WBC - white blood cell count. 1- significant difference between PWV tertile I - PWV tertile III, PWV tertile II - PWV tertile III. 2- significant difference between PWV tertile I - PWV tertile II, PWV tertile I - PWV tertile III. 3: significant difference between PWV tertile I - PWV tertile III. 4: significant difference between PWV tertile I - PWV tertile II, PWV tertile I - PWV tertile III, PWV tertile II - PWV tertile III. 5: significant difference between PWV tertile I - PWV tertile II. 6: significant difference between PWV tertile II- PWV tertile III. p<0.05 is considered significant. For the parameters expressed in medians, Bonferroni’s correction is applied, and p<0.017 is considered significant
Table 3.
Complete blood count, biochemical and hemodynamic analysis, including serum uric acid and total bilirubin levels, according to tertiles of AIx, separate for stable angina pectoris and acute coronary syndrome patients
| AIx tertile I (SAP) n:35 | AIx tertile I (ACS) n:13 | AIx tertile II (SAP) n:35 | AIx tertile II (ACS) n:15 | AIx tertile III (SAP) n:13 | AIx tertile III (ACS) n:34 | P (SAP) | P (ACS) | |
|---|---|---|---|---|---|---|---|---|
| Hb, g/dL | 13.8±1.8 | 13.6±1.8 | 13.6±1.6 | 13.8±2.2 | 13.8±2.6 | 13.1±1.6 | 0.490 | 0.339 |
| WBC, 103/mm3 | 6.6 (5.7-8.3) | 8.1 (5.8-9.5) | 7.4 (6.2-8.7) | 7.4 (6.4-7.8) | 7.9 (7.0-9.5) | 8.2 (6.7-9.6) | 0.062 | 0.393 |
| Neutrophil, 103/mm3 | 3.9 (2.9-5.0) | 4.6 (3.6-5.3) | 4.4 (3.6-5.1) | 4.3 (3.4-5.2) | 5.1 (4.4-6.3) | 5.5 (4.3-6.4) | 0.0097 | 0.0346 |
| Lymphocyte, 103/mm3 | 2.3±0.6 | 2.7±1.0 | 2.2±0.7 | 2.0±0.6 | 2.4±0.6 | 1.8±0.6 | 0.737 | 0.0022 |
| N/L | 1.7 (1.4-2.0) | 1.7 (1.4-2.1) | 1.8 (1.6-2.3) | 2.1 (1.8-2.7) | 2.2 (2.0-2.5) | 3.3 (2.2-4) | 0.0223 | <0.0011 |
| Platelet, 103/mm3 | 228 (209-271) | 273 (261-281) | 233 (211-286) | 231 (206-261) | 243 (213-299) | 239 (181-320) | 0.891 | 0.273 |
| MPV, fL | 8.7±1.1 | 8.5±0.7 | 8.7±1.1 | 8.7± 1. 1 | 8.7±1.4 | 8.5±1.0 | 0.996 | 0.842 |
| BUN, mg/dL | 13 (11-15) | 15 (12-18) | 15 (13-20) | 18 (12.5-19) | 17 (13-26) | 17 (13-19) | 0.0242 | 0.627 |
| Creatinine, mg/dL | 0.7 (0.6-0.8) | 0.8 (0.7-0.8) | 0.8 (0.7-0.9) | 0.8 (0.7-0.9) | 0.7 (0.6-0.8) | 0.8 (0.7-1.1) | 0.152 | 0.110 |
| Uric acid, mg/dL | 4.3 (3.9-4.8) | 4.8 (3.8-5.6) | 4.9 (4-5.9) | 5.1 (4.8-5.9) | 5.9 (4.8-7.3) | 6.8 (5.8-7.7) | 0.0023 | <0.0011 |
| T. bilirubin, mg/dL | 0.9±0.2 | 0.9±0.2 | 0.7±0.2 | 0.8±0.3 | 0.5±0.3 | 0.5±0.2 | <0.0014 | <0.0011 |
| AST, U/L | 18 (16-23) | 19 (16-23) | 19 (16-22) | 20 (14.5-24) | 17 (13-20) | 18.5 (16-25) | 0.390 | 0.927 |
| ALT, U/L | 20 (17-28) | 22 (14-35) | 20 (16.5-26) | 21 (14-30) | 19 (13-24) | 21 (13-17) | 0.588 | 0.960 |
| GGT, U/L | 29 (21.5-36) | 30 (27-34) | 29 (19.5-37) | 26 (16.5-34) | 24 (22-39) | 34.5 (23-43) | 0.941 | 0.232 |
| Brachial SBP mm Hg | 132±9.1 | 135.3±11.2 | 137.8±11.1 | 137.9±12 | 145.7±12.3 | 141.6±14 | <0.0014 | 0.300 |
| Brachial DBP mm Hg | 81.7±8.9 | 84.0±8.4 | 84.7±9.3 | 87.4±7.5 | 88.3±6.8 | 87.2±10.4 | 0.0643 | 0.537 |
| Central SBP mm Hg | 120.1±9.4 | 124.0±11.1 | 126.1±11.5 | 125.7±12.9 | 133.5±12.3 | 130.1±13.6 | 0.0014 | 0.274 |
| Central DBP mm Hg | 81.9±8.9 | 84.4±8.1 | 85.1±9.4 | 87.7±7.4 | 88.6±6.7 | 87.5±10.6 | 0.0583 | 0.561 |
| Central PP, mm Hg | 38.2±4.5 | 39.6±4.6 | 41.1±5.8 | 37.9±7.4 | 44.9±6.2 | 42.7±5.3 | 0.0014 | 0.0246 |
| HR, beats/m in | 70.0±6.7 | 68.7±4.2 | 71.7±7.2 | 68.8±6.9 | 71.1 ±6.1 | 71.4±7.4 | 0.585 | 0.326 |
| AP mm Hg | 6.9±1.1 | 7.3±1.1 | 9.2±1.2 | 8.4±1.5 | 11.7±1.6 | 11.6±1.6 | <0.0014 | <0.0014 |
| PWV, m/s | 7.7±1.6 | 7.2±1.1 | 10.8±1.7 | 11.4±1.8 | 14.2±2.0 | 14.6±1.6 | <0.0014 | <0.0014 |
| Gensini | 0 (0-5.5) | 4.5 (0-10) | 11 (3.3-13.8) | 18.5 (15-24) | 34 (10.5-68) | 52 (44-90) | <0.0012 | <0.0014 |
AIx - augmentation index; ALT - alanine aminotransferase; AP - augmentation pressure; AST - aspartate aminotransferase; BUN - blood urea nitrogen; DBP - diastolic blood pressure; Hb - hemoglobin; HR - heart rate; GGT - gamma-glutamyl transferase; MPV - mean platelet volume; N/L - neutrophil-to-lymphocyte ratio; PWV - pulse wave velocity; SBP - systolic blood pressure; WBC - white blood cell count 1- significant difference between AIx tertile I - AIx tertile III, AIx tertile II - AIx tertile III. 2: significant difference between AIx tertile I - AIx tertile II, AIx tertile I - AIx tertile III. 3: significant difference between AIx tertile I - AIx tertile III. 4: significant difference between AIx tertile I - AIx tertile II, AIx tertile I - AIx tertile III, AIx tertile II - AIx tertile III. 5: significant difference between AIx tertile I - AIx tertile II. 6: significant difference between AIx tertile II- AIx tertile III. 7: significant difference between AIx tertile I - AIx tertile II, AIx tertile II-AIx tertile III. p<0.05 is considered significant. For the parameters expressed in medians, Bonferroni’s correction was applied, and p<0.017 is considered significant
AIx and PWV were both positively associated with serum uric acid and N/L ratio and negatively associated with serum total bilirubin levels (Fig. 1, 2). The correlation analysis was repeated after adjustments for age, gender, heart rate, systolic blood pressure, and presence of diabetes, which could have affected the analysis. Significant associations were found to persist in the entire study population. However, when the analysis was performed separately for SAP and ACS patients, the significant correlations between PWV, Aix, and N/L disappeared in SAP patients. In addition, the relation between serum uric acid and AIx nearly lost its significance, and the association between serum total bilirubin and PWV was severely weakened in patients with SAP (Table 4a, b).
Figure 1.
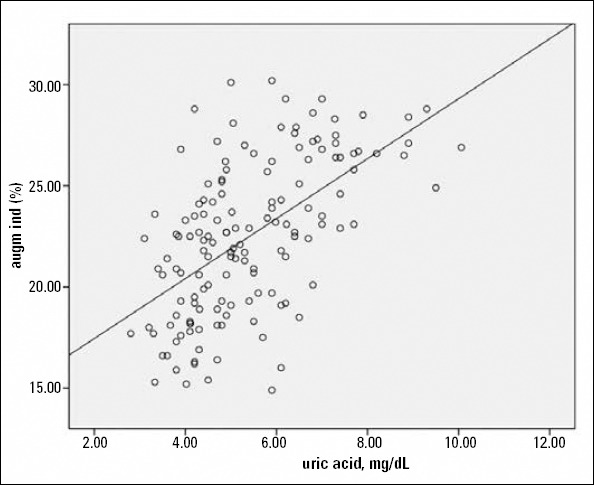
Scatterplot diagram demonstrating the relation between serum uric acid levels and augmentation index
Figure 2.
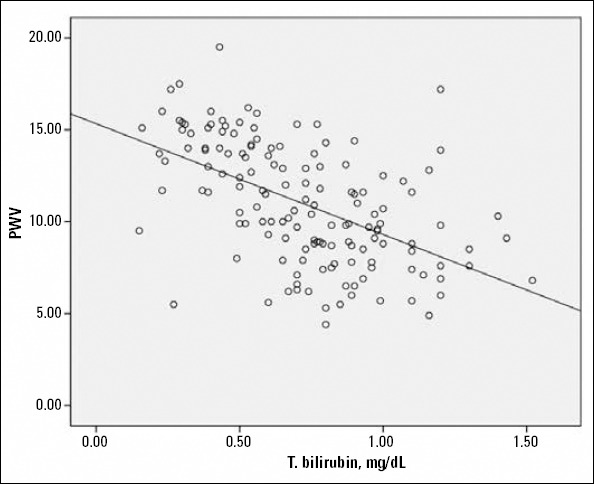
Scatterplot diagram demonstrating the relation between serum total bilirubin levels and pulse wave velocity
Table 4a.
Correlation coefficients for the association between augmentation index and neutrophil-to-lymphocyte ratio, serum uric acid, and total bilirubin levels, unadjusted and after adjustments for age, gender, systolic blood pressure, diabetes, heart rate, and height
| Entire study population | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|
| AIx | Blood analysis | r | P | r | P |
| N/L | 0.38 | <0.001 | 0.34 | <0.001 | |
| Uric acid | 0.57 | <0.001 | 0.46 | <0.001 | |
| T. bilirubin | -0.59 | <0.001 | -0.49 | <0.001 | |
| SAP | Unadjusted | Adjusted | |||
| AIx | Blood analysis | r | P | r | P |
| N/L | 0.27 | 0.014 | 0.16 | 0.187 | |
| Uric acid | 0.48 | <0.001 | 0.22 | 0.058 | |
| T. bilirubin | -0.54 | <0.001 | -0.43 | <0.001 | |
| ACS | Unadjusted | Adjusted | |||
| AIx | Blood analysis | r | P | r | P |
| N/L | 0.36 | 0.014 | 0.37 | 0.005 | |
| Uric acid | 0.53 | <0.001 | 0.25 | 0.047 | |
| T. bilirubin | -0.60 | <0.001 | -0.38 | 0.037 | |
AIx - augmentation index; N/L - neutrophil-to-lymphocyte ratio; T. bilirubin - total bilirubin. P<0.05 is considered significant
Table 4b.
Correlation coefficients for the association between pulse wave velocity and neutrophil-to-lymphocyte ratio, serum uric acid, and total bilirubin levels, unadjusted and after adjustments for age, gender, systolic blood pressure, and diabetes
| Entire study population | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|
| PWV | Blood analysis | r | P | r | P |
| N/L | 0.33 | <0.001 | 0.24 | 0.005 | |
| Uric acid | 0.50 | <0.001 | 0.34 | <0.001 | |
| T. bilirubin | -0.53 | <0.001 | -0.44 | <0.001 | |
| SAP | Unadjusted | Adjusted | |||
| PWV | Blood analysis | r | P | r | P |
| N/L | 0.23 | 0.041 | -0.03 | 0.169 | |
| Uric acid | 0.44 | <0.001 | 0.33 | 0.004 | |
| T. bilirubin | -0.48 | <0.001 | -0.11 | 0.035 | |
| ACS | Unadjusted | Adjusted | |||
| PWV | Blood analysis | r | P | r | P |
| N/L | 0.30 | 0.016 | 0.28 | 0.040 | |
| Uric acid | 0.42 | 0.001 | 0.38 | 0.004 | |
| T. bilirubin | -0.53 | <0.001 | -0.47 | <0.001 | |
N/L - neutrophil-to-lymphocyte ratio; PWV - pulse wave velocity; T. bilirubin- total bilirubin. P<0.05 is considered significant
We also searched for the correlation between Gensini score, which shows the extent of CAD, and N/L ratio, serum uric acid, and total bilirubin levels. Higher N/L ratio and serum uric acid levels and lower total bilirubin levels were associated with higher Gensini scores (Table 5).
Table 5.
Correlation coefficients for the association between Gensini score and neutrophil-to-lymphocyte ratio, serum uric acid, and total bilirubin levels
| r | P | ||
| SAP | N/L | 0.296 | 0.007 |
| Uric acid | 0.565 | <0.001 | |
| T. bilirubin | -0.327 | <0.001 | |
| r | P | ||
| ACS | N/L | 0.556 | <0.001 |
| Uric acid | 0.646 | <0.001 | |
| T. bilirubin | -0.491 | <0.001 | |
N/L - neutrophil-to-lymphocyte ratio; T. bilirubin - total bilirubin. P<0.05 is considered significant
Discussion
In this cross-sectional study, we demonstrated that patients with increased arterial stiffness and arterial wave reflection had higher N/L ratio, higher serum uric acid levels, and lower serum total bilirubin levels both in stable and unstable clinical conditions. We report significant associations between N/L ratio, serum uric acid levels, PWV, and Aix, highlighting the role of inflammation on large vasculature. We also report significant negative correlations between serum total bilirubin levels, PWV, and Aix, which points to the antioxidant protective role of bilirubin against arterial stiffening in patients with CAD. All of these associations persist in ACS patients after adjustments for possible confounders, like age, gender, height, heart rate, systolic blood pressure, and diabetes mellitus.
Bilirubin has emerged as a novel biochemical tool, the higher physiological levels of which have been shown to be protective against coronary atherosclerosis. Lower levels were associated with overt CAD and stroke (10). It was also shown that total serum bilirubin levels were associated with the presence, severity, and extent of atherosclerotic plaques detected by coronary CT angiography in patients with known CAD (11), and this finding was also supported in the present study, reporting a significant negative correlation between total serum bilirubin and Gensini score. One step further, studies investigating the association between arterial stiffness and serum bilirubin levels have been conducted recently. Zhu et al. (12) showed that bra- chial-ankle PWV (baPWV) decreased with increased levels of total bilirubin in patients with CAD, and Li et al. (13) reported that total serum bilirubin concentration was negatively associated with arterial stiffness assessed by non-invasive baPWV measurement in Chinese men.
There are possible mechanisms to explain the protective role of bilirubin against increased arterial stiffness. The bile pigments biliverdin and unconjugated and conjugated bilirubin exert their antioxidant role by the scavenging of peroxyl radicals, inhibition of membrane lipid peroxidation, and scavenging of reactive nitrogen species in vitro (14). Heme oxygenase, the catalytic enzyme of bilirubin, is the rate-limiting enzyme in the degradation of heme, which causes the formation of biliverdin, free ferrous iron, and carbon monoxide. It is suggested that heme oxygenase-1 (HO-1) and heme degradation products can attenuate complement-mediated inflammation and protect human vascular endothelium against complement-mediated injury and preserve vascular nitric oxide (15). In addition, it has been recently shown that matrix metalloproteins, the overactivity of which results in increased arterial stiffness, can be down-regulated by HO-1 (13).
There are studies that report uric acid as a determinant of arterial stiffness, partly independent from atherosclerosis risk factors (16). Although the exact mechanism has not been elucidated, pro-oxidant effects, endothelial dysfunction through a decrease in nitric oxide production, and vascular and systemic inflammation are mostly blamed (17). Hsu et al. (18) recently reported that uric acid was independently associated with arterial wave reflection and central systolic blood pressure. Our findings are in accordance with previous studies relating uric acid with increased arterial stiffness and arterial wave reflection.
We also report higher N/L ratios with increasing stiffness indices, which supports the role of inflammation on vascular stiffness. Endothelial adhesion of inflammatory cells is an early step in the development of vascular disease and causes subsequent local vascular inflammation (19). Stimulated leukocytes adhere and penetrate vascular intima more easily and release hydrolytic enzymes, growth factors, and cytokines, thus causing vascular damage (20). In addition, it was suggested that a higher N/L ratio indicated a higher ratio of sympathetic-to-parasympa- thetic activity, because adrenergic receptors are abundant on granulocytes, whereas cholinergic receptors predominate on lymphocytes (20). The sympathetic nervous system plays a major role in the vascular tone by releasing neurotransmitters on arterial smooth muscle (21), and sympathetic overactivation is associated with endothelial dysfunction (22). Renal denervation has recently been shown to cause a decrease in AIx independently of the blood pressure-lowering effects (23).
Significant associations between PWV, Aix, and N/L were found to disappear after adjustments according to possible confounders in SAP patients in contrast to ACS. Inflammation is important in all stages of coronary atherosclerosis, and N/L has recently been shown to be significantly associated with more extensive CAD both in SAP and ACS (24). In the present study, we also found a significant correlation between Gensini score and N/L, which is more powerful in ACS. In SAP, the inflammatory process is less active than in ACS, which is tentatively the reason why N/L loses its significance after correlations for PWV and AIx were adjusted for possible confounders.
Study limitations
The main limitation of this study is the cross-sectional design, which prevents us from inferring an exact mechanistic explanation for the relation between arterial stiffness and serum bilirubin, uric acid concentrations, and N/L ratio. In addition, arterial stiffness is influenced by hypertension, diabetes, and medications, which could confound the analysis. Especially, uric acid has a well-studied relation with hypertension. However, we performed the appropriate statistical adjustments, considering the possible confounders that could lead to bias. Finally, this is a single-center study including a relatively limited number of patients. We did not have the opportunity to measure serum levels of other inflammatory markers, like hs-CRP, or more investigational biomarkers of inflammation, like interleukins, adhesion molecules, selectins, and TNF-α.
Conclusion
Although this study can not provide information about causality, the hypothesis is that N/L ratio, serum uric acid, and bilirubin levels might be used to further risk-stratify patients with CAD with respect to arterial wave reflection and arterial stiffness, especially in patients with ACS. Prospective large-scale studies may show if such an approach provides any clinical benefit in terms of predicting morbidity and mortality.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept - A.T., A.F.E.; Design - A.T., A.A.; Supervision - A.T., H.F.T.; Resource - H.F.T.; Materials - A.T., A.F.E.; Data collection &/or processing - A.T., A.A.; Analysis&/or Interpretation - A.T., A.F.E.; Literature search - A.T.; Writing - A.T., A.F.E., H.F.T.; Critical review - A.T., A.F.E.; Other - A.A., H.F.T.
References
- 1.Stoner L, Young JM, Fryer S. Assessments of arterial stiffness and endothelial function using pulse wave analysis. Int J Vasc Med. 2012;2012:903107. doi: 10.1155/2012/903107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31:2338–50. doi: 10.1093/eurheartj/ehq165. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fantin F, Mattocks A, Bulpitt CJ, Banya W, Rajkumar C. Is augmentation index a good measure of vascular stiffness in the elderly? Age Ageing. 2007;36:43–8. doi: 10.1093/ageing/afl115. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 4.O’Rourke MF, Pauca A, Jiang XJ. Pulse wave analysis. Br J Clin Pharmacol. 2001;51:507–22. doi: 10.1046/j.0306-5251.2001.01400.x. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu ML, Ho YC, Yet SF. A central role of heme oxygenase-1 in cardiovascular protection. Antioxid Redox Signal. 2011;15:1835–46. doi: 10.1089/ars.2010.3726. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 6.Kanbay M, Segal M, Afsar B, Kang DH, Rodriguez-Iturbe B, Johnson RJ. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart. 2013;99:759–66. doi: 10.1136/heartjnl-2012-302535. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 7.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction, Third universal definition of myocardial infarction. Circulation. 2012;126:2020–35. [CrossRef] [Google Scholar]
- 8.Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–7. doi: 10.1161/hy1001.096106. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 9.Delles C, Zimmerli LU, McGrane DJ, Koh-Tan CH, Pathi VL, McKay AJ, et al. Vascular stiffness is related to superoxide generation in the vessel wall. J Hypertens. 2008;26:946–55. doi: 10.1097/HJH.0b013e3282f7677c. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Oda E, Kawai R. A possible cross-sectional association of serum total bilirubin with coronary heart disease and stroke in a Japanese health screening population. Heart Vessels. 2012;27:29–36. doi: 10.1007/s00380-011-0123-7. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 11.Canpolat U, Aytemir K, Yorgun H, Hazırolan T, Kaya EB, Şahiner L, et al. Association of serum total bilirubin levels with the severity, extent and subtypes of coronary atherosclerotic plaques detected by coronary CT angiography. Int J Cardiovasc Imaging. 2013;29:1371–9. doi: 10.1007/s10554-013-0209-7. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 12.Zhu C, Xiong Z, Zheng Z, Chen Y, Chen X, Qian X. Association of arterial stiffness with serum bilirubin levels in established coronary artery disease. Intern Med. 2012;51:2083–9. doi: 10.2169/internalmedicine.51.7701. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Meng SY, Meng CC, Yu WG, Wang RT. Decreased serum bilirubin is associated with arterial stiffness in men. Nutr Metab Cardiovasc Dis. 2013;23:375–81. doi: 10.1016/j.numecd.2011.09.004. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 14.Basiglio CL, Arriaga SM, Pelusa F, Almará AM, Kapitulnik J, Mottino AD. Complement activation and disease:protective effects of hyperbilirubinaemia. Clin Sci (London) 2010;118:99–113. doi: 10.1042/CS20080540. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Pae HO, Son Y, Kim NH, Jeong HJ, Chang KC, Chung HT. Role of heme oxygenase in preserving vascular bioactive NO. Nitric Oxide. 2010;23:251–7. doi: 10.1016/j.niox.2010.08.002. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 16.Ishizaka N, Ishizaka Y, Toda E, Hashimoto H, Nagai R, Yamakado M. Higher serum uric acid is associated with increased arterial stiffness in Japanese individuals. Atherosclerosis. 2007;192:131–7. doi: 10.1016/j.atherosclerosis.2006.04.016. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 17.Sun N, Zhang Y, Tian JL, Wang H. Relationship between uric acid and arterial stiffness in the elderly with metabolic syndrome components. Chin Med J. 2013;126:3097–102. [PubMed] [Google Scholar]
- 18.Hsu PF, Chuang SY, Cheng HM, Sung SH, Ting CT, Lakatta EG, et al. Associations of serum uric acid levels with arterial wave reflections and central systolic blood pressure. Int J Cardiol. 2013;168:2057–63. doi: 10.1016/j.ijcard.2013.01.164. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 20.Park BJ, Shim JY, Lee HR, Lee JH, Jung DH, Kim HB, et al. Relationship of neutrophil-lymphocyte ratio with arterial stiffness and coronary calcium score. Clin Chem Acta. 2011;412:925–9. doi: 10.1016/j.cca.2011.01.021. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 21.Zacharia J, Mauban JR, Raina H, Fisher SA, Wier WG. High vascular tone of mouse femoral arteries in vivo is determined by sympathetic nerve activity via a1A- and a1D-adrenoceptor subtypes. PLoS One. 2013;8:e65969. doi: 10.1371/journal.pone.0065969. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tycinska AM, Mroczko B, Musial WJ, Sawicki R, Kaminski K, Borowska H, et al. Blood pressure in relation to neurogenic, inflammatory and endothelial dysfunction biomarkers in patients with treated essential arterial hypertension. Adv Med Sci. 2011;56:80–7. doi: 10.2478/v10039-011-0016-0. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 23.Hering D, Lambert EA, Marusic P, Ika-Sari C, Walton AS, Krum H, et al. Renal nerve ablation reduces augmentation index in patients with resistant hypertension. J Hypertens. 2013;31:1893–900. doi: 10.1097/HJH.0b013e3283622e58. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 24.Tanındı A, Erkan AF, Ekici B, Alhan A, Töre HF. Neutrophil to lymphocyte ratio is associated with more extensive, severe and complex coronary artery disease and impaired myocardial perfusion. Turk Kardiyol Dern Ars. 2014;42:125–30. doi: 10.5543/tkda.2014.18949. [CrossRef] [DOI] [PubMed] [Google Scholar]


