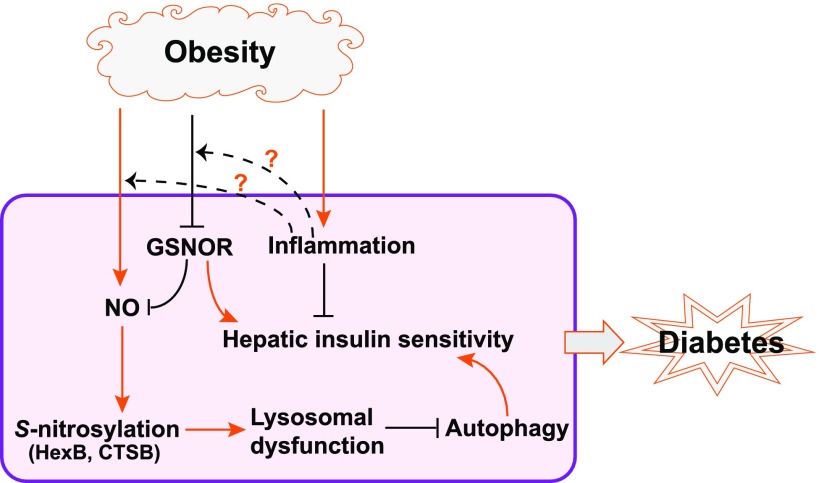
During the progression from obesity to diabetes, a critical attribute is inability to maintain metabolic homeostasis under excessive energy and nutrient exposure, which triggers insulin resistance. Intensive research efforts have linked chronic inflammation, redox changes, and intracellular stress responses to dysregulated energy metabolism, particularly insulin resistance (1). Autophagy, a lysosomal degradation pathway for damaged organelles or long-lived proteins, was recently identified as a key regulatory pathway to preserve lipid homeostasis and insulin sensitivity under metabolic stress conditions (2–4). Dysfunction of autophagy is associated with obesity and type 2 diabetes. However, the mechanisms underlying the cause of autophagic defect in metabolic disorders remain elusive. In this issue of Diabetes, a study conducted by Qian et al. (5) revealed a mechanism by which obesity cripples autophagy in the liver through S-nitrosylation, an inhibitory protein modification process induced by nitric oxide (NO). Their data argue that in obese animals, NO-induced repression of hepatic autophagy causes hepatic steatosis, impairs hepatic insulin signaling, and eventually contributes to the progression of type 2 diabetes (Fig. 1).
Figure 1.
Proposed model of impairment of hepatic autophagy and insulin action by GSNOR deficiency and nitrosative stress in obesity. Obesity decreases the activity of the denitrosylation enzyme GSNOR, causing nitosative stress and S-nitrosylation of the lysosomal enzymes HexB and CTSB. This leads to hepatic autophagic defect and insulin resistance. The “?” markers indicate the uncharacterized links between metabolic inflammation and GSNOR deficiency or NO elevation in obesity.
Autophagy is an adaptive, catabolic process that generates energy for cells under nutrient-starvation conditions and helps maintain cellular homeostasis in nutrient-rich environments through its constitutive activity (2). In the liver, autophagy participates in the basal turnover of lipids by engulfing and degrading lipid droplets (3). Declined autophagic activity is associated with hepatic steatosis and insulin resistance in obesity, where, on one hand, insulin resistance downregulates expression of the genes encoding major autophagy components (6) while, on the other hand, impaired autophagy promotes insulin resistance by exacerbating hepatic steatosis (4). The study by Qian et al. (5) confirmed that hepatic autophagic insufficiency compromises the liver’s ability to adapt to metabolic stress and thereby cripples hepatic insulin action. Compared with the earlier studies, Qian et al. provided novel insights into the mechanistic underpinning of impaired hepatic autophagy in obesity. Their study shows that diet-induced obesity triggers a global increase in protein S-nitrosylation in mouse livers. In particular, obesity increased S-nitrosylation of two lysosomal enzymes, cathepsin B (CTSB) and hexosaminidase subunit β (HexB), thus impairing lysosomal function (Fig. 1). Indeed, hyper–NO modification of HexB and CTSB was detected in livers of human patients with diabetes or patients with advanced hepatic steatosis (5).
NO regulates a range of cellular functions and signaling processes through protein S-nitrosylation, the covalent attachment of a nitrogen monoxide group to the thiol side chain of cysteine, by altering protein subcellular localization, enzyme activity, protein stability, and protein complex formation (7). Denitrosylation enzymes, particularly S-nitrosoglutathione reductase (GSNOR) and thioredoxin, are critical modulators of protein S-nitrosylation (8). One important finding by Qian et al. (5) is that obesity hampers the denitrosylation capacity of the liver, which appears to be a major cause of impaired hepatic autophagy and insulin resistance under the obese condition. In obese mice, GSNOR defect exacerbated the downregulation of hepatic autophagy and impaired hepatic insulin action. If, however, those mice expressed a GSNOR transgene in their livers, hepatic autophagy was restored and glucose homeostasis and insulin sensitivity improved significantly (5). These observations implicate GSNOR as a key regulator of hepatic autophagy and insulin action in obesity and argue that boosting GSNOR activity may exert a metabolic benefit due to its role in sustaining hepatic autophagy.
Nevertheless, an important issue concerning the potential links between metabolic inflammation, nitrosative stress, and declined hepatic autophagy remains unsolved. Chronic inflammation is a prominent feature of obesity and a major cause of insulin resistance (9). The cross-talk between autophagy and inflammatory cascades mediated through the receptors recognizing pathogen-associated molecular patterns (PAMPs) or cytokines has been observed (10,11). Toll-like receptors sense PAMPs and signal through the adaptor proteins myeloid differentiation primary response protein 88 (MYD88) or TIR-domain–containing adaptor protein inducing interferon-β (TRIF) to activate proinflammatory gene expression. Both MYD88 and TRIF signaling were found to promote autophagosome formation (11). While GSNOR deficiency was shown to drive the aberrant S-nitrosylation of the key lysosomal enzymes and hepatic autophagic defect (Fig. 1), Qian et al. (5) did not provide any evidence of links between metabolic inflammation, GSNOR deficiency, and autophagic defect under the obese condition. However, this does not exclude the possibility that multiple Toll-like receptor–mediated inflammatory pathways, originating from the resident macrophages (Kupffer cells) or adipose tissues, may be involved in protein S-nitrosylation and impaired hepatic autophagy in obese animals. It remains unclear whether chronic metabolic inflammation contributes to downregulation of hepatic GSNOR or whether inflammation-regulated inducible NO synthase expression and NO production contribute to S-nitrosylation and subsequent hepatic autophagic defect. These questions highlight the need of further experiments to determine the context-dependent roles of NO in autophagy and the regulation of hepatic autophagy by inflammatory signals in obesity.
In summary, the study by Qian et al. (5) brings us a step closer to understanding the cause of dysregulated autophagy and its pathological consequences in obesity. GSNOR, a molecule that plays a key role in reversing protein S-nitrosylation, has gained the spotlight as an important player in preventing hepatic steatosis and insulin resistance in obesity. This study points out the importance of nitrosative stress and autophagy in obesity and type 2 diabetes, but it also raises several intriguing questions. First, how does obesity cause a deficiency in GSNOR? In this regard, it will be important to test whether hepatic redox status, particularly the reduction in glutathione and NADH/NADPH levels (8), or metabolic inflammation is responsible for GSNOR inactivation in obesity. Second, what is the mechanistic link between the autophagic defect and hepatic insulin resistance? Finally, since nitrosative stress may dysregulate insulin signaling through other pathways (12), it is important to understand the extent by which NO-impaired hepatic autophagy contributes to insulin resistance and type 2 diabetes. Answering these questions will be critical for the future therapeutic designs or pharmaceutical interventions for obesity and type 2 diabetes by modulating GSNOR or autophagic activity.
Article Information
Funding. This work was partially supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grant DK090313 and National Institute of Environmental Health Sciences grant ES017829, as well as American Heart Association grants 0635423Z and 09GRNT2280479. The author apologizes to colleagues whose important work in this area could not be cited due to the space limitations.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 193.
References
- 1.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010;140:900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ 2005;12(Suppl. 2):1542–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh R, Kaushik S, Wang Y, et al. . Autophagy regulates lipid metabolism. Nature 2009;458:1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab 2010;11:467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian Q, Zhang Z, Orwig A, et al. . S-Nitrosoglutathione reductase dysfunction contributes to obesity-associated hepatic insulin resistance via regulating autophagy. Diabetes 2018;67:193–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu HY, Han J, Cao SY, et al. . Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J Biol Chem 2009;284:31484–31492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 2005;6:150–166 [DOI] [PubMed]
- 8.Benhar M, Forrester MT, Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol 2009;10:721–732 [DOI] [PubMed]
- 9.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011;121:2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cadwell K. Crosstalk between autophagy and inflammatory signalling pathways: balancing defence and homeostasis. Nat Rev Immunol 2016;16:661–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J 2008;27:1110–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneki M, Shimizu N, Yamada D, Chang K. Nitrosative stress and pathogenesis of insulin resistance. Antioxid Redox Signal 2007;9:319–329 [DOI] [PubMed]