Abstract
Background
Red blood cell-derived microparticles are biologically active, submicron vesicles shed by erythrocytes during storage. Recent clinical studies have linked the duration of red blood cell storage with thromboembolic events in critically ill transfusion recipients. In the present study, we hypothesized that microparticles from aged packed red blood cell units promote a hypercoagulable state in a murine model of transfusion.
Methods
Microparticles (MPs) were isolated from aged, murine packed red blood cell (RBC) units via serial centrifugation. Healthy male C57BL/6 mice were transfused with MPs or an equivalent volume of vehicle, and whole blood was harvested for analysis via rotational thromboelastometry. Serum was harvested from a separate set of mice following MP or saline injection, and analyzed for fibrinogen levels. RBC-derived MPs were analyzed for their ability to convert prothrombin to thrombin. Finally, mice were transfused with either RBC MPs or saline vehicle, and a tail bleeding time (TBT) assay was performed following an equilibration period of 2, 6, 12, or 24 hours.
Results
Mice injected with RBC-derived MPs demonstrated an accelerated clot formation time (109.3±26.9 vs 141.6±28.2 sec) and increased alpha angle (68.8±5.0° vs 62.8±4.7°) compared with control (each p<0.05). Clotting time and maximum clot firmness were not significantly different between the two groups. RBC-derived MPs exhibited a hundredfold greater conversion of prothrombin substrate to its active thrombin form (66.60±0.03 vs 0.70±0.01 peak OD; p<0.0001). Additionally, serum fibrinogen levels were lower in MP-injected mice compared with saline vehicle, suggesting thrombin-mediated conversion to insoluble fibrin (14.0 vs 16.5 μg/mL, p<0.05). In the TBT model, there was a more rapid cessation of bleeding at 2 hours post-transfusion (90.6 vs 123.7 sec) and 6 hours post-transfusion (87.1 vs 141.4 sec) in MP-injected mice as compared with saline vehicle (each p<0.05). There was no difference in TBT at 12 or 24 hours.
Conclusion
Red blood cell-derived microparticles induce a transient hypercoagulable state through accelerated activation of clotting factors.
Keywords: RBC microparticles, storage lesion, hypercoagulability, thromboelastometry
INTRODUCTION
Traumatic injury is the number one cause of death in individuals under 46 years old.1 Over 40 million people are injured each year in the United States (US), and 192,000 die as a result of these injuries.2 Hemorrhagic shock accounts for a third of these deaths3–5, with the majority of these deaths being potentially preventable.6, 7 While resuscitative strategies are an area of ongoing research8, 9, blood transfusion has remained the standard of care throughout the modern medical era.10
Current US Food and Drug Administration (FDA) regulations allow the storage of packed red blood cells (pRBC) for up to 42 days.11 Throughout the storage period, erythrocytes demonstrate a progressive series of biochemical and morphological changes collectively termed the RBC “storage lesion”.12 Microparticles (MPs) are submicron vesicles released from aging RBCs as a key element in the storage lesion.12–14 Previously dismissed as nonfunctional cellular debris, MPs have recently been shown to play integral roles in intracellular signaling and various disease states.15–17 While the exact functions of RBC-derived MPs are an area of ongoing study, their biochemical composition has been well-characterized. Specifically, these spherical, subcellular fragments contain residual hemoglobin and RBC proteins encompassed by a phosphatidylserine-rich phospholipid membrane.18
Given the possible role of phosphatidylserine in regulating coagulation19, preliminary studies have investigated the effect of RBC-derived MPs on individual coagulation factors. Both procoagulant (e.g. thrombin generation) and anticoagulant properties (e.g. activated protein C regulation) of RBC-derived MPs have been observed in an in vitro setting.20 The aggregate effect of MPs on recipient coagulation parameters, however, remains poorly understood. In the present study, we hypothesized that RBC-derived MPs promote an overall hypercoagulable state in the transfusion recipient.
MATERIALS AND METHODS
Animal model
Male C57BL/6 mice aged 8 to 10 weeks were obtained from Jackson Laboratories (Bar Harbor, ME) and used for all experiments. They were fed water and a standard laboratory diet ad libitum, and acclimated for two weeks in a climate-controlled environment with a daily light-dark cycle. All mice weighed 21 to 30 grams prior to experimentation. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Cincinnati.
Murine blood harvesting protocol
Packed RBC units were prepared using a modification of a previously described technique.21 Briefly, donor mice were anesthetized with intraperitoneal pentobarbital at 0.1 mg/g body weight, then whole blood was harvested via open cardiac puncture. Fresh whole blood was immediately mixed with citrate phosphate double dextrose (CP2D) anticoagulant in a 1:7 ratio to prevent inadvertent clotting. Anticoagulated whole blood was centrifuged, after which platelet-rich plasma and leukocyte-rich buffy coat were removed. The remnant erythrocyte pellet was washed twice with a 10:1 ratio of phosphate-buffered saline (PBS) in order to purify the RBC unit of any platelet contamination. The RBC pellet was then resuspended in additive solution-3 (AS-3) storage solution in a 2:9 ratio.
Packed RBC units were stored for 14 days at 4°C in light-protected conditions. Previous studies have demonstrated that murine erythrocytes accumulate age-related changes at an accelerated rate as compared to human pRBCs.21 Current US FDA regulations allow human pRBC units to be stored up to 42 days under similar conditions. Therefore, the 14-day storage duration was chosen as it reflects the murine equivalent of 42-day storage in human pRBC units. Following the storage period, serial centrifugation was used to remove any cellular components in the pRBC unit, and RBC-derived MPs were isolated via ultracentrifugation as previously described.13
Microparticle characterization
MPs isolated from one mL of mouse pRBCs were labelled with conjugated fluorescent antibodies (Affymetrix, Santa Clara, CA) for Ter119 (murine erythrocyte marker), CD41 (murine platelet marker), and CD45 (murine leukocyte marker). The final MP concentration was determined via dual-laser flow cytometry, which was gated for events ranging from 0.1 to 1.0 μm. Using separate gates for each fluorophore, the quantity of Ter119-positive events was compared to CD41-positive and CD45-positive events.
Thromboelastometry
Rotational thromboelastometry (ROTEM; TEM Systems Inc, Durham, NC) analysis was performed to assess and quantify changes in coagulation parameters in accordance with manufacturer instructions. For ex vivo experiments, fresh whole blood was harvested from healthy male C57BL/6 as previously described, then anticoagulated with sterile 0.1 M citrate solution. Citrated blood was treated with RBC-derived MPs or saline vehicle in a 1:5 ratio, then analyzed within 1 min of whole blood collection. Non-activated TEM (NATEM) analysis was used to determine overall coagulation; extrinsic pathway TEM (EXTEM) was used to determine coagulation via the extrinsic pathway; and fibrin TEM (FIBTEM) was used to quantify the fibrin contribution to clot. EXTEM and FIBTEM were performed via addition of 20 μL of thromboplastin or cytochalasin D, respectively, to 300 μL of citrated blood prior to thromboelastometric analysis per manufacturer protocol. Maximum clot firmness (MCF), alpha angle, clotting time (CT), and clot formation time (CFT) were determined for each sample. In vivo experiments were performed in a similar fashion, with fresh whole blood harvested from mice injected with RBC-derived MPs or saline vehicle.
On ROTEM, the X-axis represents time and Y-axis measures clot formation and strength of the thrombus.22 Clotting time (CT) reflects the activation and propagation of the clotting cascade. Clot formation time (CFT) and alpha angle, which are derived from the same variables, reflect the cleavage and activation of fibrinogen. Maximum clot formation (MCF) measures the contribution of both fibrin and platelets to the strength of the thrombus, and is defined as the maximal Y-axis amplitude.
Thrombus characterization
Percent contribution of platelets (%MCFPLT) to the overall clot was calculated with the following equation: (EXTEMMCF – FIBTEMMCF)/EXTEMMCF, as previously described by Midura et al.23 Fibrinogen contribution to thrombus was quantified via FIBTEMMCF, using cytochalasin D to impair platelet activation prior to thromboelastometry.
Clotting factor analysis
Healthy male C57BL/6 mice were intravenously transfused with RBC-derived MPs or an equivalent volume (200 μl) of saline vehicle. This volume is approximately that which a 70 kg human would receive with a transfusion of 2 units of pRBCs. At intervals, whole blood was collected via open cardiac puncture, anticoagulated, then centrifuged to isolate the plasma fraction. Fibrinogen concentration of plasma samples was measured via ELISA kit (MyBioSource, San Diego, CA) according to the manufacturer’s instructions. In separate experiments, whole blood was separated into cellular and serum fractions using serum separator tubes, then serum was analyzed for D-dimer and fibrinogen degradation product (FDP) levels via ELISA kits (MyBioSource, San Diego, CA) according to the manufacturer’s instructions.
Thrombin activation was determined using Zymuphen MP-Activity functional assay (Aniara Diagnostica, West Chester, OH). Briefly, RBC-derived MPs were diluted 1:20 and added to microplate wells supplemented with Factor Xa-Va mixture, calcium, thrombin inhibitors, and purified prothrombin. The activity of the generated thrombin substrate was measured via spectrophotometry. An equivalent volume of saline vehicle served as negative control. The assay was performed per manufacturer protocol.
Tail bleeding time assay
Healthy male C57BL/6 mice aged 8 to 10 weeks were anesthetized under 2% inhaled isofluorane. MPs isolated from 1 mL of murine pRBC were resuspended in 200 μL of PBS and intravenously transfused via penile vein. An equivalent volume of PBS vehicle served as a control. A tail bleeding time (TBT) assay was then performed at 2, 6, 12, and 24 hours after treatment.24, 25 Mice were anesthetized with intraperitoneal pentobarbital at 0.1 mg/g body weight, then placed on a heating pad to preclude hypothermia as a confounding factor. Mouse tails were cleansed with 140-proof ethanol. A 10-blade Bard-Parker surgical scalpel was used to resect exactly 5 mm of mouse tail measured from the tip (Aspen Surgical, Caledonia, MI).25 New scalpel blades were used for each experiment to prevent inadvertent crush injury to the tail vein. The freshly cut tail was immediately immersed in normal saline (NS) warmed to 37°C. Tail bleeding time was measured from time of incision to time of cessation of bleeding. After cessation of bleeding, tails were observed for an additional 60 seconds to ensure that re-bleeding did not occur.
Statistical analysis
Mouse experiments were conducted in triplicate to ensure repeatability of findings. Results are reported in means and standard deviation where applicable. Two-tailed student t-tests were used to compare treatment groups. Analysis of variance (ANOVA) tests were used to compare differences between three or more groups. A p-value of less than 0.05 was considered statistically significant.
RESULTS
In initial experiments, we characterized the MPs isolated from stored pRBC units. Utilizing flow cytometry, we determined that MPs derived from murine pRBC units stored for 14 days yielded a concentration of 10.7±1.1 × 106 MPs/mL of murine pRBCs, consistent with our previous data13. Flow cytometry using antibodies to Ter119 (RBC-specific antigen), and CD41 (platelet-specific) and CD45 (leukocyte-specific) indicated that the majority of MPs recovered from stored pRBC units using our isolation protocol were erythrocyte-derived (Figure 1). These data are consistent with previously published results from our laboratory using similar methods.13
FIGURE 1.
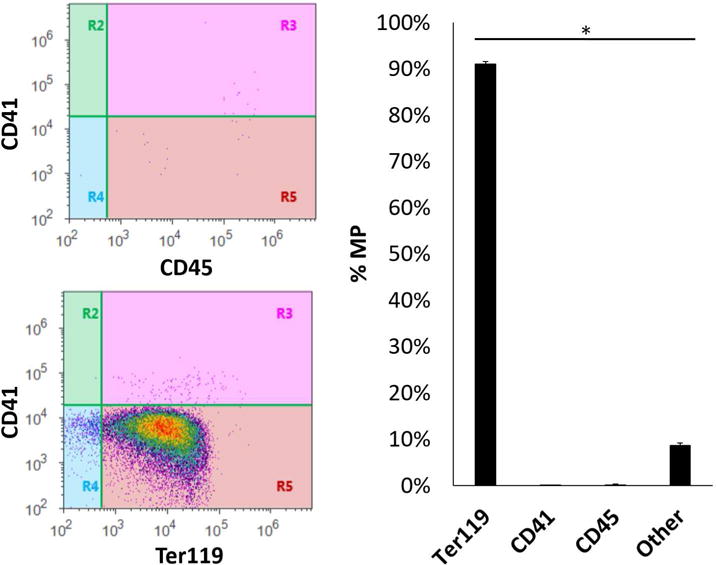
Microparticles derived from murine pRBC units stored for 14 days yields a concentration of 10.7±1.1 MP/mL of murine pRBCs. These microparticles are 91.08±0.51% derived from erythrocyte origin, as determined by flow cytometry. Other cellular origins included platelets (0.08±0.03%) and leukocytes (0.16±0.60%, p<0.05 each).
We next quantified viscoelastic coagulation parameters utilizing ROTEM, which is frequently utilized to identify hypercoagulability in the clinical setting.26–28 NATEM was used to analyze the ex vivo interaction between MPs and whole blood. As compared to whole blood samples treated with a similar volume of PBS as a dilutional control, exposure of fresh whole blood to RBC-derived MPs did not significantly alter clotting time (CT; 148.7±16.5 vs 212.5±28.2 seconds, p=0.06; Figure 2A) or mean clot formation (MCF; 65.6±1.0 vs 65.0±1.5 mm, p=0.72; Figure 2B). Clot formation time (CFT; 51.7±7.1 vs 73.2±6.7 sec; Figure 2C) and alpha angle (80.0±1.2° vs 75.5±1.1°; Figure 2D), however, were affected by MP treatment as compared with vehicle (each p<0.05). These data suggest that MP treatment alters aspects of the clotting cascade.
FIGURE 2.
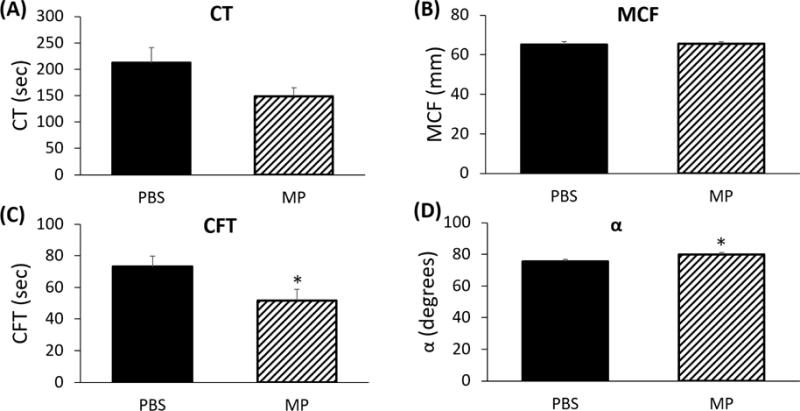
(A) RBC-derived microparticles affect clotting parameters ex vivo. Clotting time (CT) is not significantly affected by RBC-derived microparticles (212.5±28.2 vs 148.7±16.5 sec, p=NS). (B) Maximum clot formation (MCF) is not affected by RBC-derived microparticles (65.0±1.5 mm vs 65.6±1.0, p=NS). (C) Clot formation time (CFT) is decreased after addition of RBC microparticles (73.2±6.7 vs 51.7±7.1 sec, p<0.05). (E) Alpha angle is increased following addition of RBC-derived microparticles (75.5±1.1° vs 80.0±1.2°, p<0.05).
In order to determine if microparticles isolated from stored pRBC units affected NATEM parameters in vivo, we injected mice with identical volumes of either PBS or microparticles. CT (307.2±120.7 vs 334.8±62.7 sec; Figure 3A) and MCF (61.0±3.0 vs 57.8±2.8 mm; Figure 3B) were unchanged in MP-injected mice as compared with vehicle (each p=NS). Mice injected with MPs demonstrated decreased CFT (109.3±26.9 vs 141.6±28.2 sec; Figure 3C) and increased alpha angle (68.8±5.0° vs 62.8±4.7°; Figure 3D). Taken together, these data indicate accelerated fibrinogen activation in mice treated with RBC-derived MPs (each p<0.05). Both ex vivo and in vivo changes induced by MP exposure were similar amongst ROTEM parameters.
FIGURE 3.
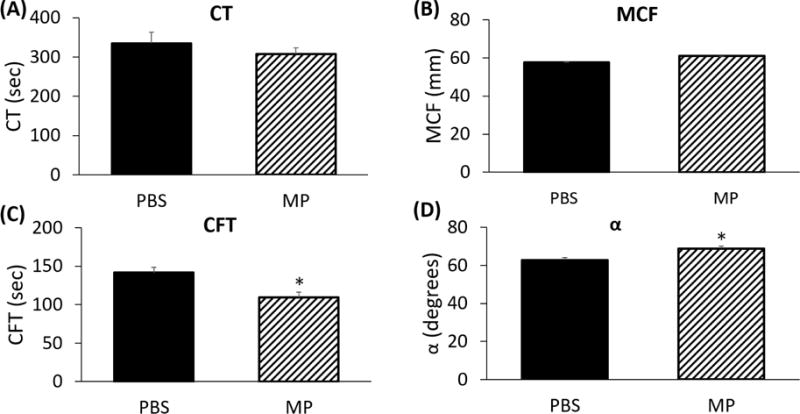
RBC-derived microparticles affect clotting parameters in vivo. (A) Clotting time (CT) is not significantly affected by RBC-derived microparticles (334.8±62.7 vs 307.2±120.7 sec, p=NS). (B) Maximum clot formation (MCF) is not affected by RBC-derived microparticles (57.8±2.8 vs 61.0±3.0 mm, p=NS). (C) Clot formation time (CFT) is decreased after addition of RBC microparticles (141.6±28.2 vs 109.3±26.9 sec, p<0.05). (D) Alpha angle is increased following addition of RBC-derived microparticles (62.8±4.7° vs 68.8±5.0°, p<0.05).
To determine whether thrombus composition was affected by MP transfusion, we used FIBTEM and EXTEM-derived parameters to calculate its individual components. Fibrinogen content, as estimated by the FIBTEMMCF, was not affected by RBC-derived MPs (16.6±7.1 vs 15.4±3.1 mm, p=NS). Platelet contribution to the clot matrix was similarly unaffected (72.6±7.1% vs 73.6±4.1%, p=NS, data not shown).
Based on this data, we hypothesized that RBC-derived MPs affect thrombin function, since thrombin activation (factor II) is directly responsible for conversion of fibrinogen (factor I) into fibrin (factor Ia).29 Utilizing a thrombin generation assay ex vivo¸ we compared thrombin activation between RBC-derived MPs as compared with saline vehicle. As compared to controls, RBC-derived MPs produced a nearly hundredfold greater activation of thrombin substrate (66.60±0.03 vs 0.70±0.01 peak OD; p<0.0001; Figure 4A). Additionally, mice injected with MPs had lower circulating fibrinogen levels at 1 hour after injection, compared with PBS-injected controls (14.0 vs 16.5 μg/mL, p<0.05; Figure 4B), further suggesting thrombin-mediated cleavage of circulating fibrinogen into insoluble fibrin strands. The presence of activated fibrin was confirmed via increased circulating D-dimer levels (1.6±0.6 vs 0.9±0.3 ng/mL; Figure 4C) and FDP levels (1.7±0.3 vs 1.3±0.2 mg/mL; Figure 4D) in MP-injected mice compared with controls (p<0.005 each).
FIGURE 4.
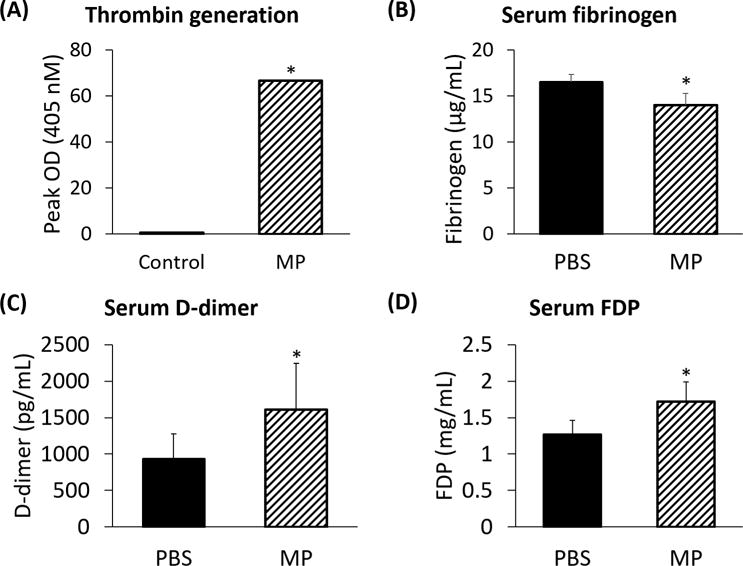
(A) Erythrocyte-derived microparticles demonstrate a nearly hundredfold greater conversion of prothrombin to thrombin than controls (66.60±0.03 vs 0.70±0.01 peak OD; p<0.05). Transfusion of microparticles from pRBC units causes a decrease in (B) circulating fibrinogen levels (16.5 vs 14.0 μg/mL), (C) D-dimer levels (0.9±0.3 vs 1.6±0.6 ng/mL), and (D) fibrin degradation product levels (1.3±0.2 vs 1.7±0.3 mg/mL) as compared with control (each p<0.05).
To confirm the functional presence of a clinically relevant hypercoagulable state, we performed a tail bleeding assay on mice treated with saline vehicle or RBC-derived MPs (Figure 5). Mice injected with RBC-derived MPs demonstrated a shorter interval to hemostasis at 2 hours (90.6±10.8 vs 123.7±5.0 sec) and 6 hours (87.1±12.9 vs 141.4±11.1 sec) following tail bleed, compared with PBS (each p<0.05). No difference was observed at 12 or 24 hours. Furthermore, whereas PBS vehicle did not alter TBT over time (ANOVA p=NS), injection of MPs resulted in a shorter TBT that increased to match PBS controls over time (ANOVA p=0.02).
FIGURE 5.
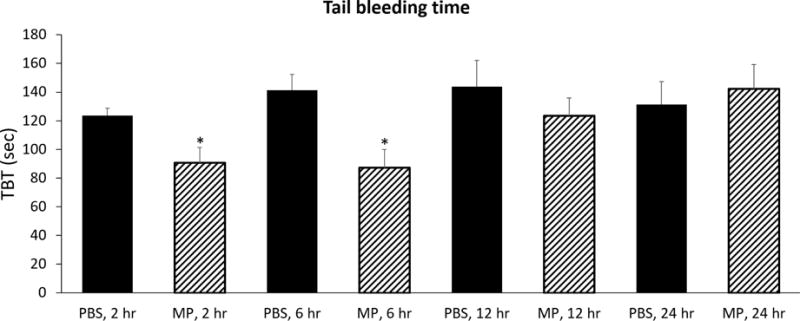
Tail bleeding time is significantly reduced at 2 hours (123.7±5.0 vs 90.6±10.8 sec) and 6 hours (141.4±11.1 vs 87.1±12.9 sec) in mice injected with RBC MP compared with PBS (each p<0.05). No difference is observed at 12 or 24 hours.
DISCUSSION
In the present study, we investigated the impact of erythrocyte-derived MPs from stored pRBC units on coagulation parameters of the transfusion recipient in a murine model. Both in vivo and ex vivo results identified a transient hypercoagulable state that persisted up to 6 hours following transfusion. Thrombophilic changes were detectable by ROTEM, as well as on a murine tail bleeding model.
Current US FDA policy allows for erythrocyte storage for up to 42 days following collection. These criteria, established in 1985, reflect the post-transfusion survival fraction and degree of hemolysis present at the end of the storage period.11 While both measures are critical to the oxygen delivery of transfused pRBC units, together, they only account for one element of the RBC storage lesion. Other elements of the storage lesion, including metabolite depletion (e.g., ATP, glutathione), loss of membrane integrity (e.g., echinocytosis, MP vesiculation), and accumulation of immunomodulatory factors (e.g., IL-1a, MIP-1a, MIP-2) play no role in current blood banking policy.12–14 Previous studies from our and other laboratories suggest that transfusion with stored pRBC units leads to a systemic inflammatory response, endothelial cell activation, and lung inflammation.13, 17, 30, 31 Likewise, from a clinical standpoint, the transfusion of aged pRBCs has been associated with myriad adverse outcomes in the transfusion recipient; including deep vein thrombosis (DVT), postoperative complications, infection, multiple organ failure, and mortality.32–38 Unfortunately, clinical studies have lacked consistency in their definition of both “fresh” and “aged” pRBCs. The definition of “fresh” most commonly refers to pRBC units less than 14 days old33, 37, 38, but this duration can range anywhere from <5 to <21 days.39–45 Other studies have defined “fresh” through the implementation of a “freshest available” protocol, in which the youngest available pRBC unit is transfused first.46–49 Likewise, the concept of “aged” pRBC units is equally inconsistent across studies, ranging from >10 to >35 days of storage.33, 37–40, 42, 45 To provide a definitive answer to this clinical quandary, Heddle et al. recently performed a large, randomized, prospective trial spanning over 30,000 patients across six hospitals.47 Aptly named the Informing Fresh versus Old Red Cell Management (INFORM) trial, the investigators found no differences in patient outcomes after transfusion of aged versus fresh blood. Rather than compare truly “fresh” and “aged” blood, however, the authors instead compared a “freshest available” transfusion policy to standard issue policy, where the oldest viable unit is transfused first. While each of these studies taken individually provides insight into the harms of prolonged pRBC storage, the lack of consistency across trials prevents these data from being directly comparable. Therefore, the potential harm to the recipient from stored pRBC units remains an area of ongoing research.
MP formation is a key metric of prolonged pRBC storage duration12–14, 18, and notably, MPs also elevated in thrombogenic disease states.50, 51 In the current study, we quantified and characterized RBC-derived MPs through common laboratory techniques.52 Previous studies from our lab have demonstrated that “fresh” pRBCs contain a relatively small number of MPs, whereas the MP concentration is significantly greater in 14-day-old murine pRBCs.13 In addition, human pRBCs have been shown to accumulate a greater concentration of MPs during storage as compared to their murine counterparts, highlighting the clinical relevance of these vesicles.13 Although the biochemical composition of these MPs has been characterized, their physiologic function remains to be fully elucidated.18 Previous studies have described both procoagulant and anticoagulant effects of these microparticles, but their overall impact on the transfusion recipient has not been described.20
Our data reveals that RBC-derived MPs promote hypercoagulable changes detectable through clinical measures of hemostasis. Ex vivo thromboelastometry illustrates the direct effect of MP exposure on whole blood, free of recipient-related factors (such as endothelial cell activation).17 We validated these findings in the in vivo environment, revealing that MP-associated hypercoagulability persists within the transfusion recipient. Both ROTEM parameters and microparticle coagulant experiments suggest that activation of thrombin and accelerated cleavage of fibrinogen may be one mechanism underlying the thrombophilic phenotype. Additionally, fibrinogen levels are lower with concurrent elevation of fibrin split products in mice transfused with MPs, indicative of consumption within the recipient.
The accelerated hemostasis in the tail wound model and procoagulant properties of the microparticles may support a potential role for RBC-derived MPs in supporting hemostasis. The clinical implications of these findings, however, are less clear. While the MPs accumulated within these units may promote improved hemostasis in actively hemorrhaging patients, transfusion of older pRBC units may also contribute to posttraumatic thromboembolic events. In addition, previous research from our laboratory has demonstrated that RBC-derived MPs potentiate a number of adverse effects within the transfusion recipient. These effects include proinflammatory changes, lung injury, and activation of leukocytes and endothelial cells.13, 17, 30, 31 With these potentially detrimental effects of RBC-derived MPs noted, any conceivable benefits of accelerated hemostasis may be outweighed by the potential ensuing harms to the acutely hemorrhaging patient.
The mechanisms of hemostasis are exceedingly complex, but can be generally categorized into three steps: (1) vasoconstriction, (2) platelet activation and aggregation, and (3) fibrin stabilization of the platelet plug.29 Whereas the biochemical reactions underlying fibrinogen activation are accelerated by exposure to RBC-derived MPs, our data suggest that neither fibrinogen content nor platelet contribution to the final thrombus are affected. Thus, MPs may play an independent role in the acceleration of thrombogenesis, rather than displacing other components within the thrombus itself.
Our exclusive use of a murine model provides several strengths to our study. Donor variability in the RBC storage lesion, including MP formation, is a well-documented phenomenon in humans and would potentially limit interpretation of the ex vivo data.53 In addition, human blood coagulation varies with diet, genetics, and a host of other factors; all of which would be difficult to control.54–56 Through the use of genetically similar mice, we were able to remove these potentially confounding factors. Furthermore, the use of a murine model for investigating the RBC storage lesion has been established in multiple investigations from our and other laboratories.13, 21
These findings must be interpreted with consideration for associated limitations. Our data demonstrates that MP transfusion resulted in decreased fibrinogen and elevated D-dimer in vivo. We have interpreted this finding as supporting data that MPs from aged pRBC units contribute to a hypercoagulable state. An alternative explanation for this data is that fibrinolysis is enhanced in vivo, but our ROTEM data indicates that murine blood does not undergo fibrinolysis as determined by the lysis index after 30 minutes on ROTEM (data not shown). In addition, during the in vivo experiments, we studied the effect of MPs from stored pRBC units as compared to mice treated with vehicle. Data from previous studies suggest that RBCs may exert a procoagulant effect.57, 58 In the present study, our goal was to understand the potential contribution of the MP component of stored pRBC units to clotting status, as harm from MPs could potentially be mitigated in the clinical setting. Future studies will focus on the effect of adding MPs to pRBCs.
CONCLUSIONS
In summary, red blood cell-derived microparticles lead to a transient hypercoagulable state in the transfusion recipient through the accelerated activation of clotting factors. Removing these potentially injurious components from aged, stored red blood cells may mitigate the development of transfusion-associated thromboembolic events.
Acknowledgments
The authors would like to thank Rebecca M. Schuster and Rosalie A. Veile for their expert technical assistance.
This work was supported by grants R01 GM107625 and T32 GM008478-23 from the National Institutes of Health.
ABBREVIATIONS
- AS-3
additive solution-3
- CFT
clot formation time
- CP2D
citrate phosphate double dextrose
- CT
clotting time
- DVT
deep vein thrombosis
- ELISA
enzyme-linked immunosorbent assay
- EXTEM
extrinsic pathway thromboelastometry
- FDA
Food and Drug Administration
- FDP
fibrinogen degradation product
- FIBTEM
fibrin thromboelastometry
- IACUC
Institutional Animal Care and Use Committee
- INFORM
Informing Fresh versus Old Red Cell Management
- MCF
maximum clot firmness
- %MCFPLT
contribution of platelets to maximum clot firmness
- MP
microparticle
- NATEM
non-activated thromboelastometry
- NS
normal saline, not significant
- OD
optical density
- PBS
phosphate-buffered saline
- pRBC
packed red blood cell
- PS
phosphatidylserine
- RBC
red blood cell
- ROTEM
rotational thromboelastometry
- TBT
tail bleeding time
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: There are no conflicts of interest to disclose.
Presentation: Portions of this work have been presented at the 39th Annual Conference on Shock in Hilton Austin, Austin, TX, on June 11–14, 2016 and at the 12th Annual Academic Surgical Congress in Las Vegas, NV, on February 7–9, 2017.
References
- 1.Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, et al. Increasing trauma deaths in the United States. Ann Surg. 2014;260:13–21. doi: 10.1097/SLA.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Web–based Injury Statistics Query and Reporting System (WISQARS) [online] Accessed October 25, 2016. http://http://www.cdc.gov/injury/wisqars/fatal.html.
- 3.Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg. 2010;34:158–63. doi: 10.1007/s00268-009-0266-1. [DOI] [PubMed] [Google Scholar]
- 4.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Stewart RM, Myers JG, Dent DL, Ermis P, Gray GA, Villarreal R, et al. Seven hundred fifty-three consecutive deaths in a level I trauma center: the argument for injury prevention. J Trauma. 2003;54:66–70. doi: 10.1097/00005373-200301000-00009. discussion -1. [DOI] [PubMed] [Google Scholar]
- 6.Gruen RL, Jurkovich GJ, McIntyre LK, Foy HM, Maier RV. Patterns of errors contributing to trauma mortality: lessons learned from 2,594 deaths. Ann Surg. 2006;244:371–80. doi: 10.1097/01.sla.0000234655.83517.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 8.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148:127–36. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Red Cross. History of Blood Transfusions [online] Accessed October 25, 2016. http://http://www.redcrossblood.org/learn-about-blood/blood-transfusions/history-blood-transfusions.
- 11.United States Food and Drug Administration. Workshop on Red Cell Stored in Additive Solution Systems. Bethesda, MD: Apr 25, 1985. [Google Scholar]
- 12.Hoehn RS, Jernigan PL, Chang AL, Edwards MJ, Pritts TA. Molecular mechanisms of erythrocyte aging. Biol Chem. 2015;396:621–31. doi: 10.1515/hsz-2014-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belizaire RM, Prakash PS, Richter JR, Robinson BR, Edwards MJ, Caldwell CC, et al. Microparticles from stored red blood cells activate neutrophils and cause lung injury after hemorrhage and resuscitation. J Am Coll Surg. 2012;214:648–55. doi: 10.1016/j.jamcollsurg.2011.12.032. discussion 56–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch CG, Figueroa PI, Li L, Sabik JF, 3rd, Mihaljevic T, Blackstone EH. Red blood cell storage: how long is too long? Ann Thorac Surg. 2013;96:1894–9. doi: 10.1016/j.athoracsur.2013.05.116. [DOI] [PubMed] [Google Scholar]
- 15.Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet JM, Tedgui A. Endothelial microparticles in diseases. Cell Tissue Res. 2009;335:143–51. doi: 10.1007/s00441-008-0710-9. [DOI] [PubMed] [Google Scholar]
- 16.Hargett LA, Bauer NN. On the origin of microparticles: From “platelet dust” to mediators of intercellular communication. Pulm Circ. 2013;3:329–40. doi: 10.4103/2045-8932.114760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang AL, Kim Y, Seitz AP, Schuster RM, Lentsch AB, Pritts TA. Erythrocyte Derived Microparticles Activate Pulmonary Endothelial Cells in a Murine Model of Transfusion. Shock. 2016 doi: 10.1097/SHK.0000000000000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westerman M, Porter JB. Red blood cell-derived microparticles: An overview. Blood Cells Mol Dis. 2016;59:134–9. doi: 10.1016/j.bcmd.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Lentz BR. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog Lipid Res. 2003;42:423–38. doi: 10.1016/s0163-7827(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 20.Koshiar RL, Somajo S, Norstrom E, Dahlback B. Erythrocyte-derived microparticles supporting activated protein C-mediated regulation of blood coagulation. PLoS One. 2014;9:e104200. doi: 10.1371/journal.pone.0104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makley AT, Goodman MD, Friend LA, Johannigman JA, Dorlac WC, Lentsch AB, et al. Murine blood banking: characterization and comparisons to human blood. Shock. 2010;34:40–5. doi: 10.1097/SHK.0b013e3181d494fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rugeri L, Levrat A, David JS, Delecroix E, Floccard B, Gros A, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007;5:289–95. doi: 10.1111/j.1538-7836.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- 23.Midura EF, Jernigan PL, Kuethe JW, Friend LA, Veile R, Makley AT, et al. Microparticles impact coagulation after traumatic brain injury. J Surg Res. 2015;197:25–31. doi: 10.1016/j.jss.2015.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broze GJ, Jr, Yin ZF, Lasky N. A tail vein bleeding time model and delayed bleeding in hemophiliac mice. Thromb Haemost. 2001;85:747–8. [PubMed] [Google Scholar]
- 25.Liu Y, Jennings NL, Dart AM, Du XJ. Standardizing a simpler, more sensitive and accurate tail bleeding assay in mice. World J Exp Med. 2012;2:30–6. doi: 10.5493/wjem.v2.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hincker A, Feit J, Sladen RN, Wagener G. Rotational thromboelastometry predicts thromboembolic complications after major non-cardiac surgery. Crit Care. 2014;18:549. doi: 10.1186/s13054-014-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashuk JL, Moore EE, Sabel A, Barnett C, Haenel J, Le T, et al. Rapid thrombelastography (r-TEG) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery. 2009;146:764–72. doi: 10.1016/j.surg.2009.06.054. discussion 72–4. [DOI] [PubMed] [Google Scholar]
- 28.Park MS, Martini WZ, Dubick MA, Salinas J, Butenas S, Kheirabadi BS, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67:266–75. doi: 10.1097/TA.0b013e3181ae6f1c. discussion 75–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davie EW. A brief historical review of the waterfall/cascade of blood coagulation. J Biol Chem. 2003;278:50819–32. doi: 10.1074/jbc.X300009200. [DOI] [PubMed] [Google Scholar]
- 30.Belizaire RM, Makley AT, Campion EM, Sonnier DI, Goodman MD, Dorlac WC, et al. Resuscitation with washed aged packed red blood cell units decreases the proinflammatory response in mice after hemorrhage. J Trauma Acute Care Surg. 2012;73:S128–33. doi: 10.1097/TA.0b013e3182606301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoehn RS, Jernigan PL, Japtok L, Chang AL, Midura EF, Caldwell CC, et al. Acid Sphingomyelinase Inhibition in Stored Erythrocytes Reduces Transfusion-Associated Lung Inflammation. Ann Surg. 2017;265:218–26. doi: 10.1097/SLA.0000000000001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim Y, Amini N, Gani F, Wagner D, Johnson DJ, Scott A, et al. Age of Transfused Blood Impacts Perioperative Outcomes Among Patients Who Undergo Major Gastrointestinal Surgery. Ann Surg. 2017;265:103–10. doi: 10.1097/SLA.0000000000001647. [DOI] [PubMed] [Google Scholar]
- 33.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 34.Offner PJ, Moore EE, Biffl WL, Johnson JL, Silliman CC. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137:711–6. doi: 10.1001/archsurg.137.6.711. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 35.Purdy FR, Tweeddale MG, Merrick PM. Association of mortality with age of blood transfused in septic ICU patients. Can J Anaesth. 1997;44:1256–61. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- 36.Spinella PC, Carroll CL, Staff I, Gross R, Mc Quay J, Keibel L, et al. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13:R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinberg JA, McGwin G, Jr, Vandromme MJ, Marques MB, Melton SM, Reiff DA, et al. Duration of red cell storage influences mortality after trauma. J Trauma. 2010;69:1427–31. doi: 10.1097/TA.0b013e3181fa0019. discussion 31–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriel J, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–2. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 39.Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, Ddungu H, Kyeyune D, Musisi E, et al. Effect of Transfusion of Red Blood Cells With Longer vs Shorter Storage Duration on Elevated Blood Lactate Levels in Children With Severe Anemia: The TOTAL Randomized Clinical Trial. JAMA. 2015;314:2514–23. doi: 10.1001/jama.2015.13977. [DOI] [PubMed] [Google Scholar]
- 40.Dhabangi A, Mworozi E, Lubega IR, Cserti-Gazdewich CM, Maganda A, Dzik WH. The effect of blood storage age on treatment of lactic acidosis by transfusion in children with severe malarial anaemia: a pilot, randomized, controlled trial. Malar J. 2013;12:55. doi: 10.1186/1475-2875-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fergusson DA, Hebert P, Hogan DL, LeBel L, Rouvinez-Bouali N, Smyth JA, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308:1443–51. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 42.Goel R, Johnson DJ, Scott AV, Tobian AA, Ness PM, Nagababu E, et al. Red blood cells stored 35 days or more are associated with adverse outcomes in high-risk patients. Transfusion. 2016;56:1690–8. doi: 10.1111/trf.13559. [DOI] [PubMed] [Google Scholar]
- 43.Hebert PC, Chin-Yee I, Fergusson D, Blajchman M, Martineau R, Clinch J, et al. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesth Analg. 2005;100:1433–8. doi: 10.1213/01.ANE.0000148690.48803.27. table of contents. [DOI] [PubMed] [Google Scholar]
- 44.Kor DJ, Kashyap R, Weiskopf RB, Wilson GA, van Buskirk CM, Winters JL, et al. Fresh red blood cell transfusion and short-term pulmonary, immunologic, and coagulation status: a randomized clinical trial. Am J Respir Crit Care Med. 2012;185:842–50. doi: 10.1164/rccm.201107-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steiner ME, Ness PM, Assmann SF, Triulzi DJ, Sloan SR, Delaney M, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 2015;372:1419–29. doi: 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aubron C, Syres G, Nichol A, Bailey M, Board J, Magrin G, et al. A pilot feasibility trial of allocation of freshest available red blood cells versus standard care in critically ill patients. Transfusion. 2012;52:1196–202. doi: 10.1111/j.1537-2995.2011.03437.x. [DOI] [PubMed] [Google Scholar]
- 47.Heddle NM, Cook RJ, Arnold DM, Liu Y, Barty R, Crowther MA, et al. Effect of Short-Term vs. Long-Term Blood Storage on Mortality after Transfusion. N Engl J Med. 2016 doi: 10.1056/NEJMoa1609014. [DOI] [PubMed] [Google Scholar]
- 48.Kim Y, Jung AD, Pritts TA. Age before duty: the effect of storage duration on mortality after red blood cell transfusion. J Thorac Dis. 2017;9:441–3. doi: 10.21037/jtd.2017.02.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lacroix J, Hebert PC, Fergusson DA, Tinmouth A, Cook DJ, Marshall JC, et al. Age of transfused blood in critically ill adults. N Engl J Med. 2015;372:1410–8. doi: 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]
- 50.Kasar M, Boga C, Yeral M, Asma S, Kozanoglu I, Ozdogu H. Clinical significance of circulating blood and endothelial cell microparticles in sickle-cell disease. J Thromb Thrombolysis. 2014;38:167–75. doi: 10.1007/s11239-013-1028-3. [DOI] [PubMed] [Google Scholar]
- 51.Woei AJFJ, Tesselaar ME, Garcia Rodriguez P, Romijn FP, Bertina RM, Osanto S. Tissue factor-bearing microparticles and CA19.9: two players in pancreatic cancer-associated thrombosis? Br J Cancer. 2016;115:332–8. doi: 10.1038/bjc.2016.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nielsen MH, Beck-Nielsen H, Andersen MN, Handberg A. A flow cytometric method for characterization of circulating cell-derived microparticles in plasma. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tzounakas VL, Georgatzakou HT, Kriebardis AG, Voulgaridou AI, Stamoulis KE, Foudoulaki-Paparizos LE, et al. Donor variation effect on red blood cell storage lesion: a multivariable, yet consistent, story. Transfusion. 2016;56:1274–86. doi: 10.1111/trf.13582. [DOI] [PubMed] [Google Scholar]
- 54.Couris R, Tataronis G, McCloskey W, Oertel L, Dallal G, Dwyer J, et al. Dietary vitamin K variability affects International Normalized Ratio (INR) coagulation indices. Int J Vitam Nutr Res. 2006;76:65–74. doi: 10.1024/0300-9831.76.2.65. [DOI] [PubMed] [Google Scholar]
- 55.Marckmann P, Sandstrom B, Jespersen J. The variability of and associations between measures of blood coagulation, fibrinolysis and blood lipids. Atherosclerosis. 1992;96:235–44. doi: 10.1016/0021-9150(92)90070-w. [DOI] [PubMed] [Google Scholar]
- 56.Harrison MJ, Emmons PR, Mitchell JR. The variability of human platelet aggregation. J Atheroscler Res. 1967;7:197–205. doi: 10.1016/s0368-1319(67)80081-4. [DOI] [PubMed] [Google Scholar]
- 57.Lu C, Shi J, Yu H, Hou J, Zhou J. Procoagulant activity of long-term stored red blood cells due to phosphatidylserine exposure. Transfus Med. 2011;21:150–7. doi: 10.1111/j.1365-3148.2010.01063.x. [DOI] [PubMed] [Google Scholar]
- 58.Osterud B, Unruh D, Olsen JO, Kirchhofer D, Owens AP, 3rd, Bogdanov VY. Procoagulant and proinflammatory effects of red blood cells on lipopolysaccharide-stimulated monocytes. J Thromb Haemost. 2015;13:1676–82. doi: 10.1111/jth.13041. [DOI] [PubMed] [Google Scholar]


