Abstract
Members of the Navajo Nation, who possess a high prevalence of cardiometabolic disease, reside near hundreds of local abandoned uranium mines (AUM), which contribute uranium, arsenic and other metals to the soil, water and air. We recently reported that hypertension is associated with mine waste exposures in this population. Inflammation is a major player in the development of numerous vascular ailments. Our previous work establishing that specific transcriptional responses of cultured endothelial cells treated with human serum can reveal relative circulating inflammatory potential in a manner responsive to pollutant exposures, providing a model to assess responses associated with exposure to these waste materials in this population. To investigate a potential link between exposures to AUM and serum inflammatory potential in affected communities, primary human coronary artery endothelial cells were treated for 4 h with serum provided by Navajo study participants (n = 145). Endothelial transcriptional responses of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and chemokine ligand 2 (CCL2) were measured. These transcriptional responses were then linked to AUM exposure metrics, including surface area-weighted AUM proximity and estimated oral intake of metals. AUM proximity strongly predicted endothelial transcriptional responses to serum including CCL2, VCAM-1 and ICAM-1 (P < 0.0001 for each), whereas annual water intakes of arsenic and uranium did not, even after controlling for all major effect modifiers. Inflammatory potential associated with proximity to AUMs, but not oral intake of specific metals, additionally suggests a role for inhalation exposure as a contributor to cardiovascular disease.
Keywords: arsenic, cardiovascular disease, CCL2, ICAM-1, endothelium, inflammation, uranium, VCAM-1
INTRODUCTION
One of the richest uranium (U) ore deposits in the U.S., located in the Four Corners region of the Southwestern U.S., was actively mined on the Navajo Nation between the 1940s and 1980s.1 Today, there are more than 500 abandoned uranium mines (AUM) and 4 abandoned mill sites that continue to pose a risk to these communities.2 Uranium, arsenic (As), nickel (Ni), vanadium (V) and copper (Cu) are among the major contaminants still found in the soil, air and ground water3 to which this population may be chronically exposed.4 The impact of such exposures on cardiovascular health remains poorly researched.
American Indians, including Navajo, are known to have a high prevalence of chronic health conditions including cardiovascular disease, hypertension, diabetes and obesity.5 As such, there is concern that this population may be vulnerable to added cardiovascular stressors including environmental contaminant exposure. We recently reported that uranium mining exposure was associated with hypertension in the Navajo population.6 A handful of studies found links between arsenic exposure and circulating biomarkers of inflammation (e.g., intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), C-reactive protein (CRP)).7,8 However, assessment of single circulating factors (e.g., cytokines, etc.) may fail to capture the overall inflammatory and pathological influence that all circulating components transmit to the endothelium. Endothelial cells respond to cumulative circulating pro- and anti-inflammatory mediators in a manner that reveals the balance of inflammation, that is, inflammatory potential, which we define as the transcriptional responses of inflammatory markers from primary endothelial cells treated in vitro with serum derived from human subjects.9 Endothelial cell expression of adhesion molecules and chemokines (e.g., chemokine (C–C motif) ligand, CCL2) is a principal activity in the pathogenesis of chronic atherosclerotic vascular disease.10,11
In humans, serum inflammatory potential, as determined by serum-stimulated responses of human primary coronary artery endothelial cells, has been linked directly to cardiovascular disease12 as well as to inhalation exposures to diesel emissions.13,14 Furthermore, it has been reported that polyphenols (namely resveratrol), as a nutraceutical treatment, led to reduced serum inflammatory potential in healthy subjects, as compared with placebo.15 Such studies are coherent with animal toxicological research showing that the inhalation of various toxicants, including gases and particles, leads to inflammatory and antidilatory alterations in the serum composition.16–18
In the present study, we assessed the inflammatory potential of serum from a population of Navajo community members living in varied proximity to AUMs. We hypothesized that serum inflammatory potential would be adversely influenced by exposures to AUMs in the Southeastern corner of the Navajo Nation as indicated by increased expression of ICAM-1, VCAM-1 and CCL2 from serum-treated endothelial cells.
MATERIALS AND METHODS
Study Population
Demographic data, water use and clinical data were obtained in partnership with the Diné Network for Environmental Health (DiNEH) Project2 using surveys which were administered in interview fashion to a cohort of 1304 participants from 20 Chapters of the Navajo Nation between 2005 and 2010. Power calculations were based on primary end points; a volunteer subset of 145 subjects provided serum samples from 2010–2011. All blood samples were drawn by trained clinical staff, either in hospital settings or in mobile clinics provided by Indian Health Services, in morning to afternoon sessions upon subject arrival without restrictions on diet or activity. Height and weight were also measured during this time to determine body mass index (BMI). All participants provided informed consent, with oversight and approval from the University of New Mexico Human Research Review Committee and the Navajo Nation Human Research Review Board.
Geospatial data
The locations of the homes of the survey participants were determined by using a hand-held Global Positioning System instrument. The mean duration of residence was determined from survey responses. The locations and surface areas (in m2) of the AUMs and mills within the study area were obtained from U.S. Army Corps of Engineers documentation compiled for the US Environmental Protection Agency.
Abandoned uranium mine proximity
AUM proximity was calculated as the square root of the sum of the inverse distances of participant reported household geographical location to all AUM features (portals, prospects, rim strips, pits, vertical shafts or waste piles) in the study area, weighted by the surface area of each feature. The formula is noted below:
parti denotes the geographical position of participant i. mj denotes the geographical position of abandoned mining site j. N is the number of abandoned mining sites used in this formula. areaj is the area of mining site j in square meters. The formula for AUM for participant i is then:
where the weights {wj} are defined by:
Water Analysis and Human Exposure Assessments
Study participants reported obtaining water from a total of 178 distinct sources, including 122 unregulated sources such as wells, springs or livestock watering stations; 42 public water supply sources; and 14 sources that could not be classified. To evaluate metal exposure via drinking water, DiNEH staff collected water samples for 124 water sources (101 unregulated, 23 regulated) from 2003 to 2010 following US Environmental Protection Agency analysis methods as described in Hoover et al.3 A small subset of samples was analyzed at the Carlsbad Environmental Monitoring and Research Center or at Stanford University. Navajo Nation EPA provided public water quality data. The compiled information resulted in As measurements for 113 sources (15 public water sources and 98 unregulated sources) with U measurements for 108 of those sources (16 public water sources and 92 unregulated sources). Unregulated sources without water quality information were excluded from the analyses. Public, regulated water system sources, store-purchased and bottled water without As or U measurements were assumed to be in compliance with Safe Drinking Water Act regulations for As (< 10 µg/l) and U (< 30 µg/l) (USEPA 2013).
Annual cumulative consumption of As and U was estimated based on participant self-reported volume of water consumed and the metal concentration for each water source. Participants indicated how frequently they visited the source, the typical volume of hauled water and the percentage used for cooking or drinking. This information was used to calculate the annual volume of water each individual consumed from each water source. An individual annual intake of As or U (mg year−1) from drinking water was then calculated using the associated As or U concentration for each source.
Once the cumulative annual intake of water As and U from water was determined for each participant, the continuous variable was translated to a binary variable, that is, low or high metal consumption based on a natural break in the data, at 1.4 mg year−1 for both metals. This intake point resulted in all individuals who consumed only regulated water being classified as “low” intake for both metals.
Clinical Assessments
Clinical analyses were performed by a reference laboratory (LabCorp, Phoenix, AZ, USA). Total serum cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL), and triglycerides were measured by conventional clinical analytical methods. CRP was assessed quantitatively by latex immunoturbidimetry. Interleukin 6 (IL-6) was determined by MILLIPLEX ultrasensitive human magnetic bead set (Millipore Inc; Billerica, MA, USA) and multiplexing technology to obtain serum cytokine concentration measures. Magnetic bead detection was carried out on a MAGPIX machine and multiplexing platform capable of performing quantitative analysis of low concentration of protein markers (Luminex Corporation, Austin, TX, USA). Analytical values were determined by xPONENT 4.2 Software (Luminex Corporation) by standards provided in the assay kit. Glycated hemoglobin (HbA1c) was determined by the Roche Tina-quant assay.
Inflammatory Potential Assay and Transcriptional Responses
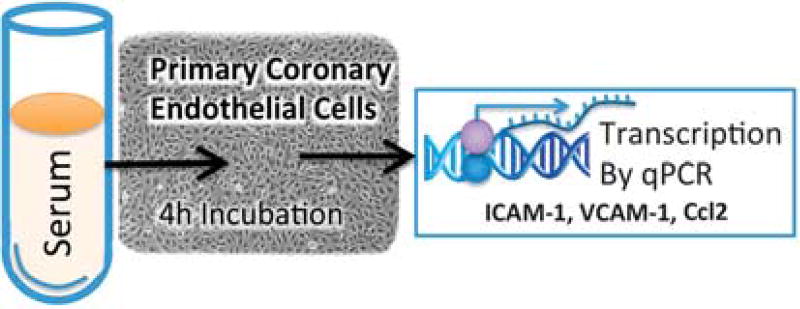
The inflammatory potential assay (Figure 1) was conducted as previously described.12,15 Primary human coronary artery endothelial cells were purchased from Lonza (Allendale, NJ, USA), which isolated and authenticated the cells; cells tested negative for mycoplasma contamination. Briefly, human primary coronary artery endothelial cells were grown to confluence in complete media on a series of 24-well plates. For 24 h prior to serum treatment, the cells were serum-starved with basal media (Lonza). Confluent human primary coronary artery endothelial cells were then incubated with 10% serum (vol:vol) obtained from DiNEH volunteers (n = 145). The samples were incubated for 4 h at 37 °C. Human CAECs were washed with PBS, lysed and cell lysates were immediately collected for RNA purification. Assays were performed in duplicate. Total RNA was isolated from samples using RNeasy Mini Prep Kits (Qiagen, Valencia, CA, USA). RNA was reverse transcribed using High Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Carlsbad, CA, USA). Quantitative determination of gene expression levels was performed on a 7900HT sequence detection system (Applied Biosystems) using TaqMan universal master mix (Applied Biosystems) according to manufacturer’s recommended conditions for intercellular adhesion molecule-1 (ICAM-1; Hs00164932_m1), vascular cell adhesion molecule-1 (VCAM-1; Hs01003372_m1) and chemokine (C–C motif) ligand 2 (CCL2; Hs00234140_m1). TATA box–binding protein (TBP; Hs00427620_m1) was used as an endogenous reference gene (Applied Biosystems). Relative gene expression was analyzed by the 2− ΔΔCT method using a relative amount of mRNA for each sample normalized to TATA box–binding protein.19
Figure 1.
Schematic of serum inflammatory potential assay. Primary human coronary artery endothelial cells were incubated with complete serum from study participants for 4 h, after which endothelial cell inflammatory responses were assessed by real time (RT)-qPCR for intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and chemokine ligand 2 (CCL2). Assays were performed in duplicate.
Statistical Analysis
Descriptive statistics were reported as median and interquartile range (IQR) for continuous variables, unless otherwise indicated. Non-Gaussian distributions were normalized using a logarithmic transformation. Tests for linear associations between dependent and independent variables were conducted, first, as simple linear regression, then including multiple variables to ensure an independent effect. Pearson correlations were performed to assess the relationship of the endothelial mRNA transcriptional responses (ICAM-1, VCAM-1 and CCL2) to demographic (age, gender) and clinical risk factors (BMI, lipids, blood pressure, CRP, IL-6 and HbA1c) and exposure metrics (annual mean intake of As and U from water; AUM proximity).
Linear regression models were constructed for ICAM-1, VCAM-1 and CCL2. Each model used demographic (age, gender) and physiologic (BMI, HbA1c) variables, and exposure metrics (annual mean intake of As and U from water; AUM proximity) as predictors. Estimated annual intake of As and U from drinking water sources were binary variables as previously described. Reduced models were derived by model selection using the Akaike Information Criterion with stepwise selection; models were successively revised so as to reduce Akaike Information Criterion. A value of P < 0.05 was considered statistically significant for models and their component variables. Statistical analyses were performed using R version 2.12.1 (The R Foundation for Statistical Computing, 2010, 64-bit) and GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA).
Code Availability
The statistical code that supports the findings of this study are available upon reasonable request from the corresponding author and Navajo Nation Human Research Review Board following Navajo Nation Human Research Policy. The process can be accessed at http://www.nnhrrb.navajonsn.gov/index.htm.
Data Availability
The US EPA GIS data for AUMs can be accessed at https://yosemite.epa.gov/r9/sfund/r9sfdocw.nsf/cf0bac722e32d408882574260073faed/8868aaa6f5752fd688257dd3007e6e6f!OpenDocument. All Navajo-specific data are the property of Navajo Nation per Navajo Nation Human Research Policy. Access to the data must be requested through application to the Navajo Nation Human Research Review Board, which requires specific guidelines for research on the Navajo Nation. The Navajo Nation Human Research Review Board operates under a Federal-Wide Assurance Number as a recognized Institutional Review Board. The process can be accessed at http://www.nnhrrb.navajo-nsn.gov/index.htm.
RESULTS
Demographic and clinical characteristics of the DiNEH subset are shown in Table 1. The mean age was 56 years (SD ± 14.8), and 38% of participants were female. Half of the participants exhibited a median BMI ≥ 30 kg/m2. Median total cholesterol and LDL were within recommended guidelines based on American Heart Association guidelines.20 HDL cholesterol was somewhat low, and triglycerides were elevated. Median systolic blood pressure was pre-hypertensive, and median diastolic blood pressure was normal.21 Median HbA1c was in the pre-diabetic range as defined by the American Diabetes Association.22 Over 80% of this subset was either pre-diabetic or diabetic based on HbA1c.
Table 1.
Characteristics of DiNEH participants.
| Variable | n | |
|---|---|---|
| Age, years | 56.1 (SD ± 14.8) | 145 |
| Female, % | 38 | 145 |
| BMI, kg/m2 | 30 (26.8–34.0) | 142 |
| Underweight (BMI ≤ 18.4), % | 0.7 | |
| Normal (BMI = 18.5–24.9), % | 14.8 | |
| Overweight (BMI = 25.0–29.9), % | 34.5 | |
| Obese (BMI ≥ 30), % | 50 | |
| TC, mg/dl | 185 (164–204) | 127 |
| LDL, mg/dl | 105 (91.5–121.5) | 127 |
| HDL, mg/dl | 45 (38–55) | 127 |
| TG, mg/dl | 184 (130–263) | 125 |
| SBP, mmHg | 129 (116–144) | 143 |
| DBP, mmHg | 78 (72–87) | 143 |
| HbA1c, % | 6.2 (5.8–7.6) | 143 |
| % Normal (HbA1c ≤ 5.6%) | 16 | |
| % Pre-diabetes (HbA1c 5.7–6.4%) | 42.7 | |
| % Diabetes (HbA1c ≥ 6.5%) | 41.3 | |
| Estimated mean annual intake from water: | ||
| Arsenic, mg year− 1 | 0.83 (0–1.15) | 144 |
| Uranium, mg year−1 | 0.82 (0–1.14) | 144 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; DiNEH, Diné Network for Environmental Health; HDL, high-density lipoprotein; HbA1c, glycated hemoglobin; IQR, interquartile range; LDL, low-density lipoprotein; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
Data are presented as median (IQR) or %, except where noted.
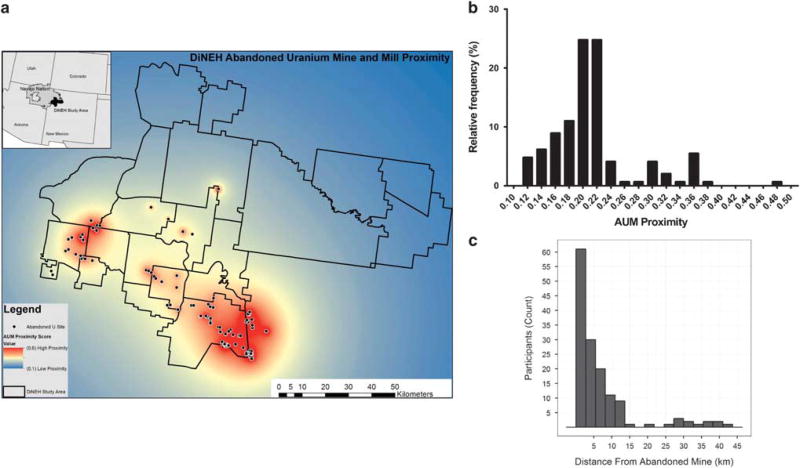
Figure 2a shows a heat map of the study area based on the surface area-weighted proximity to abandoned U mine and mill sites. The distribution of weighted AUM proximity scores (Figure 2b) shows that the majority of participants were living in regions of low AUM proximity (median 0.207; IQR 0.179 – 0.224; Figure 2a). Median residential linear actual distance from AUM was 3.54 km (IQR 1.81–8.0) (Figure 2c). The mean duration of residence in a current home for this cohort was 36.5 years (SD ± 23).
Figure 2.
(a) Study area and heat map of area-weighted proximity to abandoned uranium mine (AUM) and mill sites. The Southeastern region of the Navajo Nation sits within the Northwestern portion of New Mexico and contains ~ 100 mine sites. (b) Distribution of Navajo participants’ household weighted proximities (based on linear distance and size of mine site) to AUM, with higher numbers reflecting a greater net exposure. (c) Distribution of Navajo participant residence linear distances (in km) from AUM.
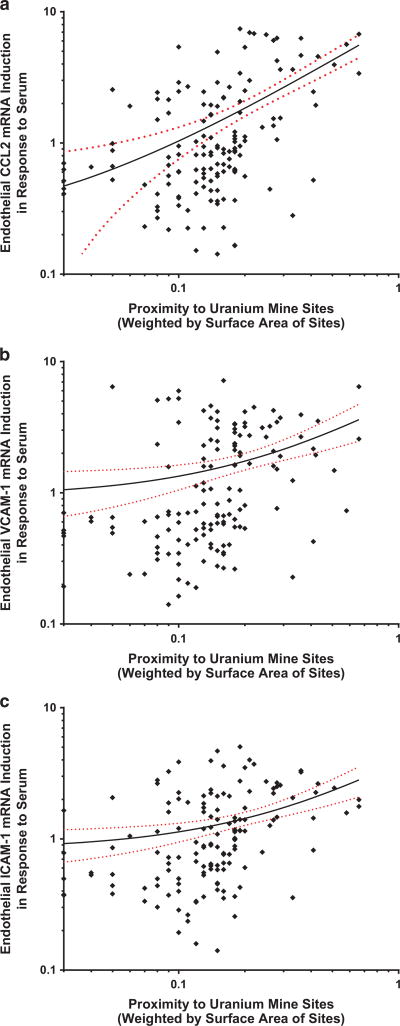
The Pearson correlation matrix (Table 2) showed that the surface area-weighted proximity to AUM was significantly correlated with all endothelial transcriptional inflammatory markers (Figure 3). CCL2, ICAM-1 and VCAM-1 mRNA were lognormally distributed (Supplementary Figure 1), positively and significantly correlated with each other (data not shown), which is consistent with their roles as inflammatory response mediators. CRP was negatively correlated with ICAM-1. Age, gender, BMI, HbA1c, lipids, and systolic or diastolic blood pressures were not correlated with any of the transcriptional responses.
Table 2.
Pearson correlations between mRNA transcriptional responses and demographic and clinical risk factors and exposure metrics.
| Variable | VCAM-1 | ICAM-1 | CCL2 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| r | P | r | P | r | P | |
| Age | − 0.047 | 0.572 | − 0.037 | 0.657 | 0.007 | 0.93 |
| Female | 0.072 | 0.39 | 0.066 | 0.429 | 0.02 | 0.813 |
| BMI | − 0.07 | 0.409 | − 0.082 | 0.333 | − 0.102 | 0.229 |
| TC (mg/dl) | 0.096 | 0.285 | 0.125 | 0.161 | 0.034 | 0.703 |
| LDL (mg/dl) | 0.098 | 0.277 | 0.091 | 0.311 | − 0.027 | 0.766 |
| HDL (mg/dl) | 0.135 | 0.13 | 0.162 | 0.068 | − 0.0001 | 0.999 |
| TG (mg/dl) | − 0.013 | 0.888 | 0.033 | 0.715 | 0.135 | 0.131 |
| SBP (mmHg) | − 0.026 | 0.756 | − 0.071 | 0.397 | − 0.02 | 0.813 |
| DBP (mmHg) | 0.001 | 0.989 | − 0.005 | 0.952 | − 0.009 | 0.917 |
| HbA1C (%) | − 0.019 | 0.825 | 0.003 | 0.968 | 0.065 | 0.441 |
| IL-6 (pg/ml) | − 0.108 | 0.213 | − 0.117 | 0.176 | − 0.134 | 0.122 |
| CRP (mg/l) | − 0.099 | 0.239 | − 0.182 | 0.029 | −0.103 | 0.221 |
| Water Ua | 0.087 | 0.298 | 0.127 | 0.129 | 0.157 | 0.059 |
| Water Asa | 0.084 | 0.317 | 0.075 | 0.37 | 0.111 | 0.185 |
| AUM proximity | 0.226 | 0.006 | 0.378 | < 0.0001 | 0.471 | < 0.0001 |
Abbreviations: AUM, abandoned U mine; As, Arsenic; BMI, body mass index; CRP, C-reactive protein; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; IL-6, interleukin 6; LDL, low-density lipoprotein; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; U, Uranium.
Pearson correlation coefficients (r). P < 0.05 was considered statistically significant.
Continuous variable. Non-Gaussian distributions were log transformed. Italic values indicate significant values.
Figure 3.
Linear regression plots of weighted abandoned uranium mine (AUM) proximity associations with (a) chemokine ligand 2 (CCL2), (b) vascular cell adhesion molecule-1 (VCAM-1) and (c) intercellular adhesion molecule-1 (ICAM-1) mRNA from endothelial cell responses to participant serum.
Results of final reduced regression models for CCL2, ICAM-1 and VCAM-1 are shown in Table 3. Although drinking water was also considered as an exposure route, multivariate regression models showed that only household proximity to AUM had a strong and independent influence on the overall serum inflammatory potential. The regression models based on the demographic, physiologic variables and exposure metrics explain ~ 20 percent of the total variability for CCL2 (R2 = 0.200), 17 percent for ICAM-1 (R2 = 0.171) and 11 percent for VCAM-1 (R2 = 0.107). Stepwise regression analysis removed all other variables (Age, gender, BMI, HbA1c and estimated annual water intakes of As and U) from the models except AUM proximity.
Table 3.
Reduced regression model results showing AUM proximity is the sole predictor of endothelial mRNA responses to serum from Navajo participants.
| Endothelial response variable | Coefficient estimate of AUM proximity | SE | P-value | Adjusted R2 | P-value of model |
|---|---|---|---|---|---|
| VCAM-1 | 5.582 | 1.304 | 3.41E−05 | 0.107 | < 0.0001 |
| ICAM-1 | 5.335 | 0.964 | 1.45E−07 | 0.171 | < 0.0001 |
| CCL2 | 6.954 | 1.142 | 9.99E−09 | 0.200 | < 0.0001 |
Abbreviations: AUM, abandoned uranium mine; BMI, body mass index; CCL2, chemokine (C–C motif) ligand 2; HbA1c, glycated hemoglobin; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1.
Results of reduced regression models are shown. Age, gender, BMI, HbA1c and water intake variables did not appear as predictors in any of the models after model selection.
DISCUSSION
Weighted proximity of residence to AUMs was associated with the circulating inflammatory potential in Navajo community participants, as indicated by ex vivo serum-stimulated endothelial transcriptional responses of ICAM-1, VCAM-1 and CCL2. In a community with a high prevalence of health conditions associated with inflammation including diabetes, hypertension and obesity, the degree of association (adjusted R2) between physical proximity to AUM and endothelial transcriptional responses representative of inflammation in multivariate models (0.107 for VCAM-1, 0.171 for ICAM-1 and 0.200 for CCL2) is striking. Furthermore, traditional known cardiovascular risk factors (e.g., BMI, lipids, CRP) were not associated with these transcriptional responses. Similarly, in prior work, we found that traditional risk factors (lipids, diabetes, smoking) also had a negligible influence on endothelial cell responses to serum derived from patients with a history of major cardiac events (myocardial infarction or angina).12 In addition, this community has known exposures to As and U in drinking water; however, estimates of oral As or U intake were also not associated with these transcriptional responses. Thus, our data reveal a broader effect of mining waste exposure beyond water intake on what we believe to be a more sensitive and reliable metric of overall inflammation than single cytokines or acute phase protein measures. Considering that actual linear distances between residences and AUMs were less than 5 km for more than half (56%) of subjects, with the effect being largely on those individuals residing closer to AUMs, our data suggest that alternate exposure pathways or indirect health impacts are induced by the presence of the AUMs. Potentially, windblown dusts from unremediated surface mines may present a hazard of metal-rich, respirable particulate matter, which is a known driver of cardiovascular toxicity.23–25 Although the bulk of material may be larger than 10 µm, and for any given windblown event, the amount/risk of exposure is low, but the smaller particles will travel further than larger particles, and considering the mean residence time of 30 years for these residents, we think it is a realistic concern that merits follow-up. Furthermore, windblown events and Aeolian transport of such contaminated dusts have been increasing, and climate change models predict this trend to continue in the foreseeable future.26,27
We assume that changes in circulatory inflammatory potential are, in the short term, temporary and reversible.15 Factors that contribute to life-long risk of cardiovascular disease, such as smoking, poor diet, sedentary lifestyle, often are reversible in the short term,28–30 but chronic unrelieved adverse behaviors and exposures will ultimately lead to vascular inflammation and promote irreversible remodeling. In developing this serum inflammatory potential assay, we have observed that whereas controlled diesel and/or nitrogen dioxide exposures in humans may acutely increase inflammatory potential,12 sub-chronic treatment with a grape seed extract (principally resveratrol) could reduce the serum inflammatory potential,14 suggesting that negative environmental factors could be offset and reversed. On the further assumption that our findings reflect a causal relationship between exposure and circulating inflammatory potential, relocation of residence or remediation of such sites could therefore allow for reversal of this potential cardiovascular stressor. Any vascular plaque development up to that intervention, however, would not likely reverse. Importantly, this is a population with a high degree of residential stability and strong cultural ties to location, with the average participant having a residence time of greater than 30 years, thus remediation strategies may be a more feasible avenue for reducing risk.
As previous studies with this approach have been conducting in studies with tightly controlled exposure/treatment conditions,12,14 the present study is, to our knowledge, the first use of endothelial cells as a biosensor for circulating inflammatory potential in a community setting. Limitations of this novel assay and, by extension, the study conclusions relate primarily to the lack of quantitative reference data, due to the responses of different batches and passages of endothelial cells being difficult to compare across studies. Unlike measurement of circulating proteins or cholesterol, which have a clear unit of concentration, the present mRNA expression data are derived from a single origin of endothelial cells and normalized within the study population. Although quality control protocols are in place to ensure that cycle threshold values are within acceptable ranges for all studies, it would not be feasible to conduct head-to-head comparisons of the present data with previously published data, although within-study trends are certainly comparable in a broader sense. In addition, as this assay remains novel, pure risk estimates of cardiovascular disease are not available. Unlike CRP or IL-6, which have been characterized thoroughly in numerous large population studies,31–33 such extensive association to cardiovascular disease outcomes in a large cohort has yet to be conducted for the present methodology. Study limitations related to the population have been previously addressed,32 but include a potential for self-selection and the lack of a clear unexposed population, although this study of 145 participants reflects a broad continuum of exposure and includes participants from regions of the Navajo Nation with no mining history. Because the Navajo population has unique genetic and cultural characteristics, comparisons with populations much farther from the Navajo Nation region (and AUMs) would be challenging if not impossible to properly match.34
Finally, endothelium subjected to chronic exposures may not respond in kind with naïve endothelial cells in culture, however, this does not detract from the observation that detectable differences exist in endothelial responses to serum from relatively unexposed versus exposed subjects. Serum represents not only the balance of inflammation but also adaptive responses35,36 by the endothelium to chronic exposures over time that often cooccur with chronic disease progression. Endothelial activation by serum serves as an important end point that shows potential for assessing cardiovascular health beyond traditional biomarkers and risk factors. More studies are needed to assess endothelial responses to serum from larger, chronically exposed populations with and without disease at different points in time. In addition, future studies should also examine anti-inflammatory markers as well as changes in endothelial expression and function that could occur with more chronic serum treatment times.
Despite some limitations, we have applied a novel and unbiased approach to assess endothelial activation by cumulative circulating inflammatory components using serum from community members overburdened by chronic cardiovascular health conditions and legacy mining exposures. This study supports the emerging idea that changes in serum composition caused by chronic toxic exposures, and transmitted by circulatory signals to the endothelium, can promote vascular inflammatory responses. Further study is needed to confirm any causal link between the exposures and health outcomes.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of Environmental Health Sciences (NIEHS P30 ES-012072, R25 ES013208, R01 ES014639 & R01 ES014565), the National Heart, Lung and Blood Institute (NHLBI T32 HL007736), the National Institute for General Medical Sciences (GM088021), the University of New Mexico General Clinical Research Center (NIH NCRR GCRC Grant # M01-RR00997), the UNM HSC Clinical & Translational Science Center (NIH NCATS Grant # 8UL1TR000041), and inkind water analyses from USEPA Region IX Laboratories. We would like to thank Deborah MacKenzie for her technical assistance. The authors wish to sincerely thank all of the Navajo chapter members who participated in this study.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Journal of Exposure Science and Environmental Epidemiology website (http://www.nature.com/jes)
References
- 1.McLemore V. The Grants uranium district: update on source, deposition, exploration. Mt Geol. 2011;48:23–44. [Google Scholar]
- 2.deLemos JL, Brugge D, Cajero M, Downs M, Durant JL, George CM, et al. Development of risk maps to minimize uranium exposures in the Navajo Churchrock mining district. Environ Health Glob Access Sci Source. 2009;8:29. doi: 10.1186/1476-069X-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoover J, Gonzales M, Shuey C, Barney Y, Lewis J. Elevated arsenic and uranium concentrations in unregulated water sources on the Navajo nation, USA. Expo Health. 2016 doi: 10.1007/s12403-016-0226-6. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blake JM, Avasarala S, Artyushkova K, Ali A-MS, Brearley AJ, Shuey C, et al. Elevated concentrations of U and co-occurring metals in abandoned mine wastes in a Northeastern Arizona native American community. Environ Sci Technol. 2015;49:8506–8514. doi: 10.1021/acs.est.5b01408. [DOI] [PubMed] [Google Scholar]
- 5.Nava LT, Zambrano JM, Arviso KP, Brochetti D, Becker KL. Nutrition-based interventions to address metabolic syndrome in the Navajo: a systematic review. J Clin Nurs. 2015;24:3024–3045. doi: 10.1111/jocn.12921. [DOI] [PubMed] [Google Scholar]
- 6.Hund L, Bedrick EJ, Miller C, Huerta G, Nez T, Ramone S, et al. A Bayesian framework for estimating disease risk due to exposure to uranium mine and mill waste on the Navajo Nation. J R Stat Soc Ser A Stat Soc. 2015;178:1069–1091. [Google Scholar]
- 7.Chen Y, Santella RM, Kibriya MG, Wang Q, Kappil M, Verret WJ, et al. Association between arsenic exposure from drinking water and plasma levels of soluble cell adhesion molecules. Environ Health Perspect. 2007;115:1415–1420. doi: 10.1289/ehp.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karim MR, Rahman M, Islam K, Mamun AA, Hossain S, Hossain E, et al. Increases in oxidized low-density lipoprotein and other inflammatory and adhesion molecules with a concomitant decrease in high-density lipoprotein in the individuals exposed to arsenic in Bangladesh. Toxicol Sci Off J Soc Toxicol. 2013;135:17–25. doi: 10.1093/toxsci/kft130. [DOI] [PubMed] [Google Scholar]
- 9.Smoliga JM, Colombo ES, Campen MJ. A healthier approach to clinical trials evaluating resveratrol for primary prevention of age-related diseases in healthy populations. Aging. 2013;5:495. doi: 10.18632/aging.100579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aird WC. Endothelium in health and disease. Pharmacol Rep PR. 2008;60:139–143. [PubMed] [Google Scholar]
- 11.Aukrust P, Halvorsen B, Yndestad A, Ueland T, Øie E, Otterdal K, et al. Chemokines and cardiovascular risk. Arterioscler Thromb Vasc Biol. 2008;28:1909–1919. doi: 10.1161/ATVBAHA.107.161240. [DOI] [PubMed] [Google Scholar]
- 12.Cung H, Aragon MJ, Zychowski K, Anderson JR, Nawarskas J, Roldan C, et al. Characterization of a novel endothelial biosensor assay reveals increased cumulative serum inflammatory potential in stabilized coronary artery disease patients. J Transl Med. 2015;13:99. doi: 10.1186/s12967-015-0457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Channell MM, Paffett ML, Devlin RB, Madden MC, Campen MJ. Circulating factors induce coronary endothelial cell activation following exposure to inhaled diesel exhaust and nitrogen dioxide in humans: evidence from a novel translational in vitro model. Toxicol Sci Off J Soc Toxicol. 2012;127:179–186. doi: 10.1093/toxsci/kfs084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schisler JC, Ronnebaum SM, Madden M, Channell M, Campen M, Willis MS. Endothelial inflammatory transcriptional responses to an altered plasma exposome following inhalation of diesel emissions. Inhal Toxicol. 2015;27:272–280. doi: 10.3109/08958378.2015.1030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal B, Campen MJ, Channell MM, Wherry SJ, Varamini B, Davis JG, et al. Resveratrol for primary prevention of atherosclerosis: clinical trial evidence for improved gene expression in vascular endothelium. Int J Cardiol. 2013;166:246–248. doi: 10.1016/j.ijcard.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aragon MJ, Chrobak I, Brower J, Roldan L, Fredenburgh LE, McDonald JD, et al. Inflammatory and vasoactive effects of serum following inhalation of varied complex mixtures. Cardiovasc Toxicol. 2016;16:163–171. doi: 10.1007/s12012-015-9325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paffett ML, Zychowski KE, Sheppard L, Robertson S, Weaver JM, Lucas SN, et al. Ozone inhalation impairs coronary artery dilation via intracellular oxidative stress: evidence for serum-borne factors as drivers of systemic toxicity. Toxicol Sci Off J Soc Toxicol. 2015;146:244–253. doi: 10.1093/toxsci/kfv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson S, Colombo ES, Lucas SN, Hall PR, Febbraio M, Paffett ML, et al. CD36 mediates endothelial dysfunction downstream of circulating factors induced by O3 exposure. Toxicol Sci Off J Soc Toxicol. 2013;134:304–311. doi: 10.1093/toxsci/kft107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.NCEP. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143–3143. [PubMed] [Google Scholar]
- 21.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35:S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell ML, Ebisu K, Peng RD, Samet JM, Dominici F. Hospital admissions and chemical composition of fine particle air pollution. Am J Respir Crit Care Med. 2009;179:1115–1120. doi: 10.1164/rccm.200808-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lippmann M, Ito K, Hwang J-S, Maciejczyk P, Chen L-C. Cardiovascular effects of nickel in ambient air. Environ Health Perspect. 2006;114:1662–1669. doi: 10.1289/ehp.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niu J, Liberda EN, Qu S, Guo X, Li X, Zhang J, et al. The role of metal components in the cardiovascular effects of PM2.5. PLoS ONE. 2013;8:e83782. doi: 10.1371/journal.pone.0083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munson SM, Belnap J, Okin GS. Responses of wind erosion to climate-induced vegetation changes on the Colorado Plateau. Proc Natl Acad Sci USA. 2011;108:3854–3859. doi: 10.1073/pnas.1014947108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stovern M, Felix O, Csavina J, Rine KP, Russell MR, Jones RM, et al. Simulation of windblown dust transport from a mine tailings impoundment using a computational fluid dynamics model. Aeolian Res. 2014;14:75–83. doi: 10.1016/j.aeolia.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Ruiz-Gutiérrez V, Covas MI, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 29.Lessiani G, Santilli F, Boccatonda A, Iodice P, Liani R, Tripaldi R, et al. Arterial stiffness and sedentary lifestyle: role of oxidative stress. Vascul Pharmacol. 2016;79:1–5. doi: 10.1016/j.vph.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Xu T, Holzapfel C, Dong X, Bader E, Yu Z, Prehn C, et al. Effects of smoking and smoking cessation on human serum metabolite profile: results from the KORA cohort study. BMC Med. 2013;11:60. doi: 10.1186/1741-7015-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennet AM, Prince JA, Fei GZ, Lyrenäs L, Huang Y, Wiman B, et al. Interleukin-6 serum levels and genotypes influence the risk for myocardial infarction. Atherosclerosis. 2003;171:359–367. doi: 10.1016/j.atherosclerosis.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 32.Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB, Wilson PWF. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004;110:380–385. doi: 10.1161/01.CIR.0000136581.59584.0E. [DOI] [PubMed] [Google Scholar]
- 33.Wang TJ, Nam B-H, Wilson PWF, Wolf PA, Levy D, Polak JF, et al. Association of C-reactive protein with carotid atherosclerosis in men and women: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2002;22:1662–1667. doi: 10.1161/01.atv.0000034543.78801.69. [DOI] [PubMed] [Google Scholar]
- 34.Harmon ME, Campen MJ, Miller C, Shuey C, Cajero M, Lucas S, et al. Associations of circulating oxidized LDL and conventional biomarkers of cardiovascular disease in a cross-sectional study of the Navajo population. PLoS ONE. 2016;11:e0143102. doi: 10.1371/journal.pone.0143102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams GM, Iatropoulos MJ. Alteration of liver cell function and proliferation: differentiation between adaptation and toxicity. Toxicol Pathol. 2002;30:41–53. doi: 10.1080/01926230252824699. [DOI] [PubMed] [Google Scholar]
- 36.Yurdagul A, Finney AC, Woolard MD, Orr AW. The arterial microenvironment: the where and why of atherosclerosis. Biochem J. 2016;473:1281–1295. doi: 10.1042/BJ20150844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The US EPA GIS data for AUMs can be accessed at https://yosemite.epa.gov/r9/sfund/r9sfdocw.nsf/cf0bac722e32d408882574260073faed/8868aaa6f5752fd688257dd3007e6e6f!OpenDocument. All Navajo-specific data are the property of Navajo Nation per Navajo Nation Human Research Policy. Access to the data must be requested through application to the Navajo Nation Human Research Review Board, which requires specific guidelines for research on the Navajo Nation. The Navajo Nation Human Research Review Board operates under a Federal-Wide Assurance Number as a recognized Institutional Review Board. The process can be accessed at http://www.nnhrrb.navajo-nsn.gov/index.htm.