Abstract
Eosinophils are a prominent cell type in particular host responses such as the response to helminth infection and allergic disease. Their effector functions have been attributed to their capacity to release cationic proteins stored in cytoplasmic granules by degranulation. However, eosinophils are now being recognized for more varied functions in previously underappreciated diverse tissue sites, based on the ability of eosinophils to release cytokines (often preformed) that mediate a broad range of activities into the local environment. In this Review, we consider evolving insights into the tissue distribution of eosinophils and their functional immunobiology, which enable eosinophils to secrete in a selective manner cytokines and other mediators that have diverse, ‘non-effector’ functions in health and disease.
This Review is dedicated to the memory of Jamie (James) Lee, whose untimely death has deprived us of his breadth of knowledge of eosinophil immunobiology and his cutting-edge hypotheses regarding the functions of eosinophils. Jamie created genetically unique mice to investigate eosinophil functions, as well as antibodies specific for eosinophil granule proteins; he shared these resources, together with his wisdom and advice, to benefit and advance investigations into the roles of eosinophils in health and disease.
Eosinophils are terminally differentiated, bone marrow-derived, granule-containing leukocytes, as are neutrophils, basophils and mast cells. These granule-containing cells can be categorized based on common precursors; eosinophils, basophils and mast cells develop from a GATA1+ granulocyte–monocyte precursor (GMP) that is distinct from the GATA1− GMP that gives rise to neutrophils, and monocytes and macrophages1. Moreover, the granule populations and their contents differ substantially between cell types. Human eosinophils contain a single population of granules that has long been known to be rich in four cationic proteins: major basic protein 1 (MBP1; also known as MBP and PRG2), eosinophil cationic protein (ECP; also known as RNase3), eosinophil-derived neurotoxin (EDN; also known as RNase2) and eosinophil peroxidase (EPX; also known as EPO). The properties and biological activities of these cationic proteins (reviewed in REF. 2) have been a focus for studying the roles of eosinophils as effector cells in host defence and, in particular, in mediating inflammatory responses in human diseases. Mouse eosinophils contain orthologues of MBP1 and EPX, but ECP and EDN are absent (although other granule proteins with RNase activities are present)3. On the basis of their cationic proteins, eosinophils were first identified more than a century ago by staining with acidic dyes, such as eosin. Subsequently, transmission electron microscopy has revealed an additional signature feature of eosinophils in that eosinophil granules, uniquely among the granule-containing leukocytes, contain a crystalline core.
Eosinophils are cells of the innate immune system that are found in evolutionary history from early vertebrates onwards3. Based on cytochemical staining, eosinophils can be readily counted in the blood, where they are a minor (<5%) component of circulating leukocytes; larger numbers of tissue-dwelling eosinophils are present outside of the vasculature. The number of eosinophils in the blood and some tissues is known to increase during specific immune responses, including host responses to helminth parasite infections, and in allergic diseases, including forms of asthma4. This eosinophilia is associated with T helper 2 (TH2) cell-mediated immune responses, including the production of IL-5, which enhances eosinophilopoiesis and eosinophil activation. Therefore, based on their ability to degranulate and release their cationic granule proteins (which have been shown to kill helminths in vitro), eosinophils were postulated to have evolved to exert host-protective, helminthotoxic functions. However, multiple studies, including those in eosinophil-deficient or eosinophil-enriched (Il5-transgenic) mice, have shown more nuanced roles for eosinophils5,6. In many experimental models of helminth infection, eosinophils were not shown to have any protective effects. Multicellular helminth parasites, having co-evolved with their hosts, may even benefit from the presence of eosinophils; for example, in the case of muscle-encysting Trichinella larvae, eosinophil-derived cytokines (including IL-10 and IL-4) suppress host responses that are otherwise toxic to the larvae4,7. Thus, the views that eosinophil-mediated immune responses to parasites are beneficial to the host and that eosinophil-associated allergic diseases are an unwanted side effect have been challenged.
It is now clear that eosinophils, which are mainly tissue-dwelling leukocytes, have a broader tissue distribution than previously appreciated and are more than just terminally differentiated effector cells. Rather, as cells of the innate immune system, eosinophils are sources of a wide variety of cytokines, and their functions include more than exocytotic degranulation. As such, eosinophils are increasingly recognized to participate in both immune homeostasis and immunity (FIG. 1). In this Review, we consider the evolving knowledge of eosinophils from mice and humans that is relevant to the functions of eosinophils as distinct sources of cytokines in varied tissue sites that are not involved in host defence against parasites or allergic disease. We consider the limitations of and improvements in detecting eosinophils in tissue sites; the composition of eosinophils, including their preformed stores of cytokines; the increasing understanding of the ultrastructural and molecular mechanisms that control selective secretion from human eosinophils; the cellular sources of eosinophil-activating IL-5; and the wide-ranging roles that eosinophils have in homeostatic and immunological processes in addition to and distinct from their terminal effector functions.
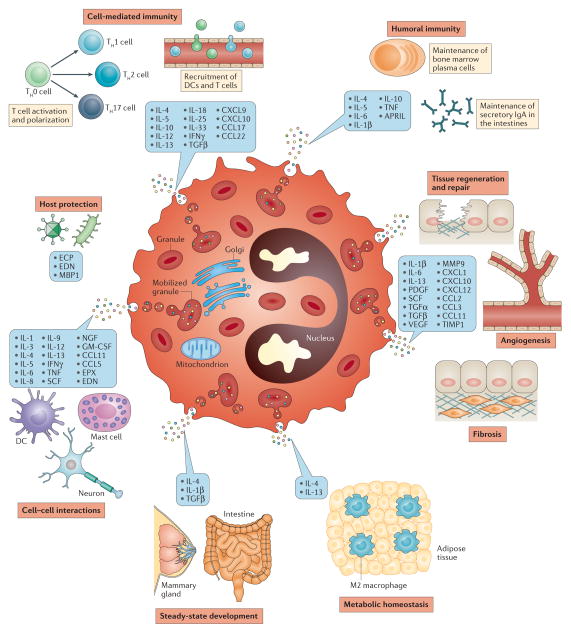
Figure 1. Eosinophil-derived mediators and their functions.
Eosinophils are a source of lipid mediators, granule-derived cationic proteins and a large number of chemokines and cytokines (many of which are stored preformed within eosinophil intracellular granules) that have wide-ranging effects in health and disease. APRIL, a proliferation-inducing ligand; CCL, CC-chemokine ligand; CXCL, CXC-chemokine ligand; DC, dendritic cell; ECP, eosinophil cationic protein; EDN, eosinophil-derived neurotoxin; EPX, eosinophil peroxidase; GM-CSF, granulocyte–macrophage colony-stimulating factor; IFNγ, interferon-γ; MBP1, major basic protein 1; MMP9, matrix metalloproteinase 9; NGF, nerve growth factor; PDGF, platelet-derived growth factor; SCF, stem cell factor; TGF, transforming growth factor; TH, T helper; TIMP1, tissue inhibitor of metalloproteinases 1; TNF, tumour necrosis factor; VEGF, vascular endothelial cell growth factor.
Detecting tissue-resident eosinophils
In contrast to the increased numbers of recruited eosinophils in associated diseases, recognizing the normal presence of eosinophils within tissue sites has been more difficult. Complementary experimental approaches are now able to detect and evaluate eosinophils present in tissue sites more sensitively.
In situ tissue analyses
Conventional detection of eosinophils in tissues based on light microscopy is limited by the use of 5–10 μm thick tissue sections, which enable only partial sampling of the tissue, and the indistinct histological resolution of common stains, which often do not detect all tissue-resident eosinophils. Moreover, as shown in various allergic and other eosinophil-enriched diseases, some tissues may lack detectable intact eosinophils because these cells have already undergone cytolysis or degranulation; the prior presence of these cells is evidenced by extracellular eosinophil granules and/or granule-derived proteins, such as MBP1. Although electron microscopy is limited to an even greater extent by the small fields that are amenable to visualization, this technique has been used to detect extracellular, core-containing granules in tissues that could not be detected by conventional histological staining8, which has provided strong evidence of an association between eosinophil cell-free granules and disease pathology. Moreover, the generation of monoclonal antibodies raised against eosinophil granule proteins (such as MBP1 and EPX) has greatly enhanced the sensitivity of detecting tissue eosinophils by immunohistochemistry and immunofluorescence. However, whereas immunofluorescence staining of eosinophil granule proteins has markedly improved the detection of eosinophils, the presence of low-abundance eosinophils in most normal tissues was not appreciated historically. For example, in a study using anti-MBP1 immunofluorescence staining, eosinophil infiltration was not detectable in human tissues, except in the lymph nodes, spleen, thymus and small intestine9.
Digesting tissues to isolate eosinophils
Newer, complementary approaches that robustly investigate low-abundance tissue eosinophils use methods for tissue digestion to release resident cells as single-cell suspensions that are amenable to flow cytometric analyses. A recent flow cytometry study of immune cells isolated from normal non-lymphoid tissues in mice showed that eosinophils are indeed normally present in many organs10. Eosinophils constituted 5% of the total myeloid cells in the lungs, 1% in the heart, liver and kidneys, and 6% in the skin10. Thus, eosinophils are now being assayed in tissue sites where they were not previously well documented.
Comprehensive analyses of tissue eosinophils by flow cytometry rely on the identification of surface markers and the high granularity of eosinophils, which is revealed by the side scatter (SSC) parameter11. Surface expression of IL-5 receptor subunit α (IL-5Rα), CC-chemokine receptor 3 (CCR3) or sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8) (in humans) or Siglec-F (also known as Siglec-5) (in mice) in the absence of other lineage-specific markers and/or in combination with an SSChi gating strategy can be used to identify eosinophils. (However, it should be noted that CCR3 is not expressed by immature eosinophils from fetal liver12.) Importantly, the choice of an appropriate eosinophil gating strategy depends upon the particular tissue and disease state that is being analysed, as certain markers that are often used to identify non-eosinophils (such as CD11C, GR1, F4/80 and MHC class II molecules) are expressed by eosinophils recruited into some tissue sites13–19. Flow cytometric analyses of eosinophils recovered from digested tissues have further aided in identifying distinct subpopulations of eosinophils, as demonstrated by the recognition of a lung-resident eosinophil population (Siglec-FmidCD62L+CD101low) that is distinct from airway-recruited eosinophils (Siglec-FhiCD62L−CD101hi)20. Phenotypic marker combinations that have been used to delineate eosinophils by flow cytometry from various tissues are presented in TABLE 1.
Table 1.
Markers used to identify mature blood and tissue eosinophils by flow cytometry
| Tissue | Eosinophil phenotype | Refs |
|---|---|---|
| Blood (human) | * FSChiSSChiAutofluorescence+ | 150,151 |
| FSChiSSChiAutofluorescence+CCR3+ | 150,151 | |
| CD45+SSChiCD24+CD16− | 152 | |
| Blood (mouse) | SSChiSiglec-F+ | 153 |
| SSChiSiglec-F+ | 21,154 | |
| CCR3+Siglec-F+ | 21,154 | |
| Bone marrow (mouse) | SSChiSiglec-F+ | 153 |
| SSChiSiglec-F+ | 21,154 | |
| CCR3+Siglec-F+ | 21,154 | |
| CD45+DAPIlowSSChiCD11B+Siglec-F+ | 75 | |
| SSChiSiglec-F+ | 155 | |
| SSChiSiglec-F+IL-5Rα+CD11B+ | 155 | |
| Spleen (mouse) | SSChiSiglec-F+ | 21,154 |
| CCR3+Siglec-F+ | 21,154 | |
| CD45+DAPIlowSSChiCD11B+Siglec-F+ | 75 | |
| SSChiSiglec-F+CCR3+ | 156 | |
| Siglec-F+F4/80low | 156 | |
| Uterus (mouse) | DAPI−CD45+SSChiCD11B+F4/80+MHC class II− | 11 |
| Adipose tissue (mouse) | CD45+DAPIlowSSChiCD11B+Siglec-F+ | 75 |
| CD45+DAPIlowCD11BhiF4/80+Siglec-F+ | 118 | |
| Small intestine (mouse) | DAPI−CD45+SSChiCCR3+ | 157 |
| SSChiCD11BhiCD11Cmid | 111 | |
| ‡ LiveCD45+SSChiSiglec-Fhi | 158 | |
| Skin (mouse) | DAPI−FSClowSSChiCD11B+Siglec-F+ | 159 |
| Bronchoalveolar lavage fluid (human) | SSChiCD16−CD14− | 160 |
| Bronchoalveolar lavage fluid (mouse) | CD45+Siglec-F+CD11C/low | 161 |
| CCR3+ | 162 | |
| Lung parenchyma (mouse) | CD45+Siglec-F+CD11C/low | 161 |
| Thymus (human) | § CCR3+MBP1+ | 132 |
| Thymus (mouse) | CD11BhiF4/80low | 134 |
| CD11BhiF4/80lowCD11Clow/midSiglec-F+CCR3+ | 134 | |
| || CD11CmidMHC class II/lowCD11B+CD8α− | 13 | |
| Fetal liver (mouse) | ¶ Siglec-F+FIRE+CR3− | 12 |
| Other (non-lymphoid) tissues (for example, heart, liver, kidney) (mouse) | ‡ Live+CD45+SSChiCD64−CD24+MHC class II−CD11B+Siglec-F+ | 10 |
CCR3, CC-chemokine receptor 3; DAPI, 4′6-diamidino-2-phenylindole; FIRE, F4/80-like receptor; FSC, forward scatter; MBP1, major basic protein 1; Siglec-F, sialic acid-binding immunoglobulin-like lectin F; SSC, side scatter.
Pretreatment of whole blood with fluorescence-activated cell sorting (FACS) lysing solution (Becton Dickinson) was necessary for the separation of neutrophils and eosinophils based on FSC and SSC properties.
Viability determined by exclusion of a Live/Dead dye.
Human thymocytes were fixed with 4% paraformaldehyde and permeabilized with 0.1% saponin to enable detection of intracellular MBP1.
Low-density mouse thymocytes collected from collagenase-digested tissue underwent depletion of CD62L-, CD3- and B220-expressing cells followed by positive selection of CD11C-expressing cells before flow cytometry analysis.
Analysis was carried out in IL-4 reporter mice, where expression of enhanced green fluorescent protein aided in the identification of eosinophils.
Eosinophil granule contents
Crystalline core-containing granules are central to the formation and functions of eosinophils. As indicated by three experimental approaches, impaired granule biogenesis during eosinophilopoiesis is lethal for developing eosinophils21–23. In the first study, combined ablation of the two main granule-derived cationic proteins (EPX and MBP1) resulted in the selective loss of eosinophil lineage-committed progenitors23. Subsequent studies showed that inhibition of the cysteine protease inhibitor cystatin F22 or of the stress-response transcription factor X-box-binding protein 1 (XBP1)21 markedly decreased or completely abolished, respectively, eosinophil differentiation. Taken together, these studies suggest that the generation and safe packaging of cationic granule proteins into intracellular granules are essential for eosinophil development and subsequent survival. The granules of mature eosinophils, as best studied for human eosinophils, contain — in addition to their cationic proteins — preformed stores of many cytokines, chemokines and growth factors (collectively referred to here as cytokines). That cytokines are stored as preformed proteins within human eosinophil granules was first indicated by the microscopic immunolocalization of cytokines, including tumour necrosis factor (TNF), IL-4, IL-6 and IL-13, to granules24–26. In a quantitative assessment, eosinophils from six human donors all contained preformed interferon-γ (IFNγ), IL-4, IL-6, TNF, IL-10, IL-12 (p70) and IL-13, each of which was predominantly present as preformed protein within eosinophil granules24. Thus, at least for human eosinophils, eosinophil-derived cytokines that are preformed proteins and do not require de novo transcription are stored within eosinophil granules. Human eosinophils can also generate cytokines based on transcriptional activation and de novo protein synthesis27, and they further regulate cytokine protein expression through the stabilization of mRNA transcripts28. Deciphering the relative contributions of de novo transcription, mRNA stabilization and mobilization of cytokines from preformed granule stores remains an important unanswered question in eosinophil biology (BOX 1).
Box 1. Experimental caveats regarding studies of eosinophils.
Cytokine mRNA and protein assays
Eosinophils respond rapidly to external stimuli by the differential release of numerous cytokines and chemokines. Both human and mouse eosinophils undergo stimulus-induced cytokine gene transcription and also can contain stabilized cytokine mRNA transcripts28 to enable de novo cytokine synthesis32. Moreover, eosinophils store preformed cytokines and chemokines within their intracellular granules, which are available for rapid stimulus-induced mobilization and release through piecemeal degranulation (PMD). For cytokine proteins that have already been synthesized and packaged within eosinophil granules, the levels of cytokine-specific mRNAs may reflect neither the intracellular content nor the levels of secreted cytokines30; therefore, studying the eosinophil transcriptome may not be sufficient to monitor the levels of preformed cytokine proteins within eosinophils that are available for regulated secretion145. The relative contributions of newly synthesized cytokines or stabilized mRNA transcripts versus preformed cytokines that are readily releasable through PMD from granule stores have not been studied.
Detecting intracellular cytokines
Cell-permeabilization protocols are commonly used to study eosinophils, often following stimulation in the presence of brefeldin A to inhibit Golgi-derived secretion. From our studies, such eosinophil permeabilization strategies can enable the intracellular detection of cytokines, but this detection is mainly restricted to those cytokines in the secretory vesicular pool. Intracellular eosinophil granules are not permeabilized by these common protocols to enable detection of their preformed cytokine stores. Indeed, increased detergent concentrations fail to detect granule-contained cytokine proteins and — probably by dissolving the cytoplasmic vesicles that may contain cytokines — lead to a decrease in detectable levels of intracellular eosinophil cytokines. Of note, brefeldin A also functions within eosinophil granules to collapse the membrano-vesicular secretory apparatus65. Immunolocalization of cytokines to eosinophil granules and more quantitatively direct assays on eosinophil granules isolated by subcellular fractionation are needed to ascertain the cytokine content of eosinophils, including those stored in granules and being mobilized into cytoplasmic secretory vesicles.
Cytokine reporter mice
Although cytokine reporter mice can indicate which cells transcribe specific cytokines — for example, 4get mice identify cells that are capable of IL-4 synthesis — the local secretion of eosinophil-derived cytokine proteins needs to be determined, including in tissue sites. In 4get mice, the secretion of IL-4 protein from these cells requires a second activating process97.
In vitro derivation of eosinophils
Studies using primary eosinophils are hindered by the small numbers of eosinophils that can be recovered from accessible compartments such as blood and the short ex vivo lifespan of isolated eosinophils. Mature eosinophils are not transfectable, nor are they amenable to RNA interference methods. To overcome these technical challenges, studies have used eosinophil-like cell lines, and protocols have been developed to derive eosinophils from human and mouse bone marrow146. Although cell lines and bone marrow-derived eosinophils are of value in understanding eosinophil development, it remains to be determined whether these cells have fully matured eosinophil granules capable of differential secretion of granule-derived cytokines and other proteins.
Electron microscopy
Whereas early electron microscopy studies could be used to evaluate eosinophil granules, the cytoplasmic secretory vesicles of eosinophils were usually not preserved, and the precise subcellular localization of cytokines was difficult to determine using cytokine-specific antibodies conjugated to large gold particles. With refinements in fixation methods and improvements in immunonanogold localization at the ultrastructural level64–66, these features can now be better studied in mouse and human eosinophils.
Mouse eosinophils are also a source of many cytokines3. For example, mouse eosinophils contain mRNA and protein for a proliferation-inducing ligand (APRIL; also known as TNFSF13), IL-4, IL-6, IL-10 and TNF29, as well as for a large number of chemokines30. Mouse eosinophils are a source of both type 1 cytokines31 and type 2 cytokines. Mouse eosinophils acquire transcripts for IL-4 and IL-13 during differentiation32, but it is not yet clear to what extent mouse eosinophils contain preformed cytokine proteins and whether these cytokines are localized to eosinophil granules, as has been found for human eosinophils.
Modes of eosinophil secretion
Human eosinophil granules, in addition to their crystalline core and surrounding matrix, are now recognized to contain an intragranular membrano-vesicular network that is thought to be involved in the selective secretion of granule-stored proteins33–37. Preformed, granule-stored cytokines can be released by three main secretory processes: classical exocytosis, cytolysis with granule release and piecemeal degranulation (PMD), of which cytolysis and PMD are more relevant to eosinophils (FIG. 2).
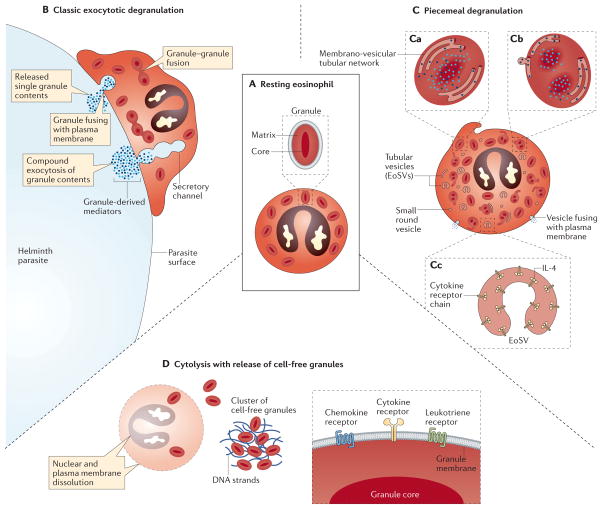
Figure 2. Modes of eosinophil secretion.
A | Intracellular granules within non-activated, resting eosinophils have a well-defined, electron-dense crystalline core and an electron-lucent outer matrix, surrounded by a trilaminar membrane. B | Eosinophils adherent to the surface of a large multicellular parasite have been shown to undergo classic exocytotic degranulation, wherein intracellular granules fuse with the plasma membrane, creating a secretory pore through which the entire granule contents are released. In compound exocytosis, granule–granule fusions occur within the cytoplasm, forming secretory channels that enable the wholesale degranulation of the combined content of multiple granules. C | By contrast, piecemeal degranulation (PMD) differentially releases granule-derived proteins, including cytokines, as discrete packets. As shown in panel Ca, granules within cells undergoing PMD exhibit varying degrees of ultrastructural alteration, including an apparent reorganization of electron-dense contents and the appearance of a membranous network of tubules within granules. As shown in panel Cb, granule-derived proteins are differentially mobilized into small round vesicles and tubular structures, the latter termed eosinophil sombrero vesicles (EoSVs), that emerge from mobilized granules and seem to derive directly from the intragranular membrano-vesicular network of tubules. As shown for eotaxin-elicited PMD of IL-4 in panel Cc, tubular EoSVs express lumen-oriented receptor chains that are bound by their cognate cytokine ligand, which indicates that a mechanism of receptor-mediated chaperoning may contribute to differential cytokine secretion. After emerging from granules, cytoplasmic EoSVs and small vesicles traverse the cytoplasm and fuse with the plasma membrane to release their granule-derived cargo. D | Eosinophils may also be induced to undergo a cytolytic cell death pathway characterized by dissolution of the nuclear and plasma membranes, extrusion of DNA nets and expulsion of intact granules that are observed individually and as clusters of cell-free extracellular granules within tissues. A portion of cell-free, extracellularly deposited eosinophil granules retain an intact trilaminar outer membrane, express outwardly oriented functional receptors on their outer membranes as shown in the right panel, and remain competent to undergo stimulus-dependent secretion within tissues.
Classical exocytosis
Unlike the exocytotic degranulation of mast cells and basophils that is mediated by ligation of the IgE receptor FcεRI, a physiological mechanism to elicit comparable acute degranulation of eosinophils (that is, the release of entire granule contents) has rarely been observed in vivo, although eosinophils can be observed to degranulate on the surface of large multicellular helminths in vitro38. Rather, ultrastructural studies show that in vivo, the majority of tissue eosinophils release their granule-derived contents secondary to cytolysis, with extracellular expulsion of intact granules, or through vesicle-mediated PMD.
Cytolysis with granule release
Human eosinophils can undergo cytolysis in vivo39, which is characterized by chromatin de-condensation, dissolution of nuclear and plasma membranes, formation of extruded nuclear DNA nets and extracellular expulsion of membrane-bound granules40,41. Using electron microscopy, the presence of extracellular, membrane-bound granules with the signature crystalline core of eosinophils has been documented in numerous tissues and secretions, including in multiple diseases of the respiratory tract, such as asthma42–45, allergic rhinitis46–48, nasal polyps49, eosinophilic pneumonia50–52 and other eosinophil-associated disorders53–58. For example, in human eosinophilic oesophagitis, cell-free eosinophil granules were present in 70% of the electron microscopy images of oesophageal tissue sections59. In mice, extracellular eosinophil granules have been detected in gastrointestinal tissues60 but have not yet been examined more widely. Although the mechanisms mediating eosinophil cytolysis in vivo remain to be defined, the presence of cell-free, intact, membrane-bound eosinophil granules that retain their content of preformed cytokines and cationic proteins is well recognized in diverse human diseases42–58,61.
These extracellular eosinophil granules are thought to have functional roles, as they remain ligand-responsive, secretion-competent structures8,62. Isolated human eosinophil granules express membrane receptors, including for a chemokine (CCR3), a cytokine (IFNγ receptor α-chain, also known as IFNGR1) and cysteinyl leukotrienes (CysLTR1, CysLTR2 and the purinergic receptor P2Y12); mouse eosinophil granules express CCR3, but not IFNγ receptor α-chain60,63. These receptors are expressed on the external membrane surfaces of eosinophil granules and so are topologically oriented to engage their ligands in the extracellular environment60,62,63. Ligand engagement of these receptors can activate signal transduction pathways within granules, including kinase-mediated signalling (for example by p38 MAP kinase)62. Moreover, ligand-stimulated cell-free granules can differentially secrete their content of cationic proteins (ECP and EPX) and cytokine proteins62. For example, human eosinophil granules stimulated with IFNγ release IL-4 and IL-6, but not IL-8, IL-10, IL-12 (p70) or IL-13 (REF. 62). Thus, cytolytic cell death of eosinophils can provide a means whereby eosinophil granule-derived proteins may continue to be released selectively from granules into tissues in the absence of intact eosinophils.
Piecemeal degranulation
In PMD, granule-derived proteins are differentially packaged into secretory vesicles that traverse the cytoplasm and fuse with the plasma membrane to deliver specific proteins to the extracellular space while leaving intracellular granules intact and available for repetitive rounds of PMD. Improvements in techniques for electron microscopy fixation and immunonanogold localization at the ultrastructural level have enabled structural insights into the mechanisms by which granule constituents can be mobilized into the secretory apparatus64–66. Eosinophil granules contain an intricate membrano-vesicular network of tubules that are collapsible by treatment with the vesicular transport inhibitor brefeldin A, concurrent with the loss of cytoplasmic small vesicles and eosinophil sombrero vesicles (EoSVs)65, and that therefore are likely to contribute directly to the formation of cytoplasmic secretory vesicles (FIG. 3A). Using immuno-electron microscopy localization, granule-derived, MBP1-containing vesicles have been shown in close proximity to mobilized granules, trafficking throughout the cytoplasm and fusing with the plasma membrane33,35 (FIG. 3B,C). Electron tomography has enabled a 3D view of intracellular granules undergoing PMD and has confirmed that small vesicles and tubular secretory vesicles emerge out of mobilized granules as apparent extensions of the intragranular vesicular network34,65. EoSVs36 are morphological hallmarks of enhanced PMD in human eosinophils, as exemplified in an electron microscopy study of biopsy tissue from individuals with eosinophilic oesophagitis59.
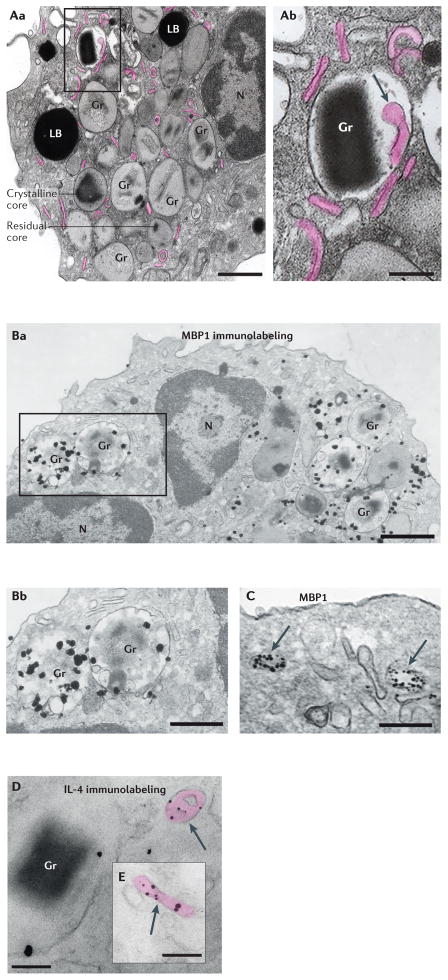
Figure 3. Ultrastructure of activated human eosinophils and immunolocalization of mobilized MBP1 and IL-4.
A | Conventional transmission electron microscopy of an activated eosinophil. In response to stimulation of eosinophils, their secretory granules exhibit structural changes, including the loss of a well-defined crystalline core and the reorganization of electron-dense materials, which is indicative of granule emptying. Aa | Representative granules exhibiting a well-defined electron-dense core (crystalline core) or progressive loss of core (residual core) are indicated. Activated eosinophils contain increased numbers of large tubular carriers (known as eosinophil sombrero vesicles (EoSVs)) that derive from mobilized granules and are seen as elongated, curved or folded circumferential structures (highlighted in pink). Ab | Intragranular membrano-vesicular domains (arrow) give rise to granule-derived small vesicles and EoSVs and are associated with the release of granule-stored products. B,C | Pre-embedding immunonanogold electron microscopy of an eotaxin 1-stimulated eosinophil, showing secretory granules containing major basic protein 1 (MBP1) in progressive stages of emptying (panel Ba). Note the structural disarrangement of the granules in panel Bb and vesicular trafficking of MBP1 in the lumen of large carriers (panel C, arrows). D,E | These carriers (arrows) were labelled for IL-4. Note that IL-4 mobilization is linked to granule and vesicle membranes, which is indicative of binding to cognate IL-4 receptor α-chain. Cells were prepared as described in REFS 39,40. LB, lipid body; Gr, granule; N, nucleus. Scale bars, 900 nm (panel Aa), 500 nm (panel Ab), 700 nm (panel Ba), 400 nm (panel Bb), 300 nm (panel C), and 200 nm (panels D and E). Electron microscopy images courtesy of R. C. N. Melo, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
In mouse eosinophils, PMD has been inferred by decreased intracellular chemokine contents30 and demonstrated by electron microscopy studies of activated mouse eosinophils that exhibited structural changes within granules consistent with content emptying60. However, mouse eosinophils do not seem to exhibit the morphologically distinct EoSVs that are notable in human eosinophils.
Despite the fact that granules in human eosinophils contain numerous and diverse preformed cytokines, different stimuli uniformly elicit distinct and selective patterns of secretion of nominal type 1, type 2 and regulatory cytokines24,27. Thus, based on studies of human eosinophils, the secretion of granule-stored cytokines by PMD is rapid, stimulus-dependent and highly differential in terms of which cytokines are secreted. However, it remains unclear how extracellular stimuli induce eosinophil granule secretion and how specific cytokines are selected from a heterogeneous cytokine pool within granules for packaging into secretory vesicles37. We have documented robust expression within eosinophil granules of the receptors for several cytokines that are stored preformed within granules67. The best studied of these is IL-4 receptor subunit α (IL-4Rα), which is more densely expressed within eosinophil granules than on the cell surface33,67. When eosinophils were stimulated with eotaxin 1 (also known as CCL11) to secrete IL-4, there was a rapid increase in the level of intracellular vesicle-associated IL-4Rα accompanied by the translocation of IL-4 from granule stores into cytoplasmic secretory vesicles67. Immuno-electron microscopy showed that IL-4 within secretory vesicles was proximate to the vesicle membrane (FIG. 3D,E). Furthermore, an IL-4Rα-binding antibody that competitively targets the IL-4-binding site on the receptor chain failed to detect the vesicle-associated IL-4Rα, which indicates that IL-4 is transported bound to its cognate receptor chain within secretory vesicles67. Of note, owing to the high surface membrane area to volume ratio of EoSVs that is inherent in their curved, elongated tubular structure, they are particularly well suited to a membrane receptor-mediated mechanism of cytokine transport. Taken together, these data indicate a potential role for cytokine receptors in the sorting and transport of granule-stored cytokines.
Regulating tissue eosinophils
IL-5 and the eotaxin chemokines have long been recognized as central regulators of eosinophil proliferation and tissue accumulation, respectively. There are three human eotaxins, eotaxin 1, eotaxin 2 (also known as CCL24) and eotaxin 3 (also known as CCL26), whereas mice only express eotaxin 1 and eotaxin 2 (REF. 3). IL-5 promotes the proliferation of eosinophil precursors in the bone marrow and the activation and survival of mature eosinophils in the periphery, whereas local eotaxin expression drives eosinophil recruitment into tissues (reviewed in REF. 3) The important role of IL-5 in mediating eosinophilia is the basis for anti-IL-5 therapies that are currently emerging as candidates for the treatment of eosinophilic diseases, including eosinophil-associated asthma68.
Although IL-5 is a signature product of TH2 cells, and prior dogma assumed that IL-5-driven eosinophilia was solely an effector arm of adaptive TH2 cell-mediated immunity, an additional source of IL-5 has now been established69,70. A non-TH2 cell source of IL-5 had been indicated by several observations. First, in mouse models of allergic lung inflammation, eosinophils participate in the recruitment of dendritic cells (DCs) and TH2 cells into draining lymph nodes71 and affected lung tissue72, which places eosinophils upstream of TH2 cell-mediated effector responses. Second, recombination activating gene 2 (Rag2)-knockout mice mount an eosinophil-rich inflammatory response to inhaled IL-33 despite the absence of lymphocytes73. The biologically relevant non-T cell source of IL-5, as confirmed by IL-5 reporter mice69, is group 2 innate lymphoid cells (ILC2s), which are major IL-5-expressing cells within tissues69,74. ILC2-derived IL-5 is implicated in the homeostatic homing of eosinophils into the small intestine69, eosinophil-dependent metabolic homeostasis in tissues75 and fostering an eosinophil-rich inflammatory exudate into the lungs during an allergic response76 (including in humans77,78). The epithelial cell-derived alarmins, IL-25, IL-33 and thymic stromal lymphopoietin, promote eosinophilia in part by eliciting IL-5 production by ILC2s79,80.
Although they are less specific than IL-5 for eosinophils, the cytokines IL-3 and granulocyte–macrophage colony-stimulating factor (GM-CSF) are also implicated in the priming, activation and survival of tissue eosinophils. Similarly to the receptor for IL-5, receptors for IL-3 and GM-CSF use the common β-chain, and their respective signalling pathways elicit both overlapping and distinct downstream effects in vivo (reviewed in REF. 81). GM-CSF is constitutively secreted by intestinal epithelial cells82 and probably maintains intestinal eosinophils in a unique activation state83, such that it may be particularly important to the homeostatic functions of intestinal tissue eosinophils. In vivo, the tissue accumulation of eosinophils is ultimately determined by a complex balance between signalling induced by pro-survival factors such as IL-3, IL-5 and GM-CSF and signalling through eosinophil surface-expressed inhibitory receptors (reviewed in REF. 84), such as Siglec-8 (REF. 85), paired immunoglobulin-like receptor B (PIRB)86, leukocyte immunoglobulin-like receptor 3 (LIR3)87 and IRp60 (also known as CD300A)88. Moreover, the manner whereby eosinophils die within tissues (apoptosis versus cytolysis) has important downstream implications for the tissue microenvironment, as cell-free granules released from cytolytic eosinophils can remain secretion-competent within tissues (see earlier). Delineating the tissue expression patterns of IL-3, IL-5 and GM-CSF and determining their differential effects on eosinophil activities in vitro and in vivo are active areas of research. It will also be important to determine the outcomes of combinatorial signalling downstream of the common β-chain and individual inhibitory receptors, which will have potential implications for the development of combinatorial therapies for the treatment of eosinophilic diseases.
Steady-state functions of tissue eosinophils
Elucidating the functions of eosinophils in tissues has been advanced by generating mouse strains that transgenically overexpress IL-5 and by developing mice deficient in eosinophils (reviewed in REF. 89). The latter include ΔblGATA mice, in which deletion of a high-affinity GATA-binding site in the promoter region of the transcription factor Gata1 ablates the eosinophil lineage90; PHIL mice, in which diphtheria toxin is expressed under the control of the eosinophil-specific Epx promoter91; inducible eosinophil-deficient (iPHIL) mice, in which the human diphtheria toxin receptor has been inserted into the Epx genomic locus, enabling the temporal depletion of eosinophils by addition of exogenous diphtheria toxin92; and eosinophil-targeting Cre recombinase-expressing (EoCre) mice, which enable targeted deletion of specific genes in eosinophils93. In parallel, the central role of eosinophil-derived cytokines in promoting eosinophil functions, particularly in tissue sites, has been supported by the generation of cytokine reporter mice that can sensitively detect cells transcribing specific cytokines in vivo94. Of note, IL-4, which is a canonical cytokine marker of TH2 cell-mediated immunity, has been studied in 4get mice95 in response to helminth infection, showing that eosinophils — and, to a lesser extent, basophils and TH2 cells — are sources of transcribed Il4 (REF. 96). Moreover, as discussed below, 4get mice have been particularly revealing of the roles of tissue-resident eosinophils as distinct sources of IL-4. These mice can be used to identify cells that have transcribed Il4, but the release of IL-4 protein from these cells is likely to require a second activating process97 that has not been defined for mouse eosinophils in vivo.
Collectively, these approaches indicate that, in addition to their involvement in allergic inflammatory diseases and helminth parasite infections, eosinophils residing within specific tissue niches function to maintain tissue, metabolic and immune homeostasis in the steady state.
Steady-state development
Mammary gland and uterine eosinophils
Puberty hormones and growth factors initiate early development within the mammary glands of females, characterized by bifurcation, elongation and branching of the prenatal rudimentary ductal tree. Branching of the maturing ductal tree is shaped by soluble mediators and cell–cell interactions provided by the surrounding stroma, which positively or negatively regulate the growth and shape of proliferating ducts. Alternatively activated M2 macrophages and eosinophils are prominent cellular constituents of the mammary gland stroma and are implicated in regulating elongation and branching, respectively, of the ductal tree98,99. The influx of eosinophils into the mammary gland depends on the local production of eotaxins100,101, and eotaxin 1-deficient mice (which have decreased numbers of mammary gland eosinophils) exhibit defective ductal branching99. Eosinophils are also recruited to the mammary glands during pregnancy, when further development occurs. Although the mechanism(s) by which eosinophils regulate ductal branching remain undefined, eosinophil-derived transforming growth factor-β (TGFβ) and CC-chemokine ligand 6 (CCL6) have been implicated in the negative regulation of ductal branching and in promoting macrophage recruitment, respectively99,102.
Eosinophil recruitment to the healthy cycling uterus is well described, particularly in rodents. Eosinophils are the most prevalent type of innate immune cell within the uterus throughout the murine oestrous cycle, with numbers reaching a maximum at oestrus and metoestrus11. Eosinophil infiltration of the uterus is induced by oestradiol-driven local expression of eotaxin 1 (REF. 101). Similarly, eotaxin 1 and its receptor are detected within the endometrium throughout the human menstrual cycle103. Studies in humans and rodents have shown that eosinophils accumulate and degranulate in the uterus in association with the tissue-degradative processes that prepare the cervix for delivery104 and during tissue-regenerative phases post-partum105. However, despite the cyclical appearance of uterine eosinophils and their apparent association with tissue degradation and subsequent uterine repair and remodelling processes, mice deficient in IL-5 or eotaxin 1, or genetically devoid of eosinophils, exhibit near normal pregnancy and parturition101,106. Thus, specific, non-redundant roles for uterine eosinophils remain unclear.
Intestinal eosinophils
Eosinophils constitutively home to all regions of the gastrointestinal tract (with the exception of the oesophagus)107–109, driven by an eotaxin 1 gradient that is probably formed downstream of the actions of ILC2-derived cytokines on epithelial cells69. Intestinal eosinophils participate in mucosal immune homeostasis. For example, the absence of eosinophils is associated with decreased production of secretory IgA at the intestinal mucosa, alterations in the intestinal micro-biome and dysregulated mucosal barrier integrity110,111. Moreover, eosinophil-deficient mice exhibit alterations in the gut-associated lymphoid tissue — specifically, Peyer’s patches are smaller and contain fewer cells, and decreased numbers of CD103+ T cells and DCs are present in the lamina propria110. Specific mechanisms of eosinophil-dependent effects on intestinal immune homeostasis have yet to be delineated, although eosinophil-derived TGFβ110 and IL-1β111 have been hypothesized to be involved in supporting the production of IgA.
Tissue regeneration
Eosinophils in regenerating muscle
Eosinophils are also associated with IL-4-driven regenerative responses to tissue injury112,113. Efficient tissue regeneration in response to muscle injury probably involves the activation and proliferation of fibrocyte–adipocyte progenitors (FAPs) to promote a stromal environment that is favourable to myogenic differentiation114. FAPs have the dual potential to promote the differentiation of myogenic progenitors114 or to themselves undergo adipogenesis115. The pivotal decision of FAPs to provide signals that enhance myogenic differentiation during muscle repair is driven by IL-4- and/or IL-13-induced signalling through FAP-expressed IL-4Rα. IL-4Rα-dependent functions of FAPs are required for both regenerating muscle fibres and the phagocytic removal of necrotic fibres. In a model of cardiotoxin-elicited muscle injury, 4get mice were used to show that eosinophils are the main IL-4-expressing cells that infiltrate injured skeletal muscle, and the regenerative response was severely compromised in ΔblGATA (eosinophil-deficient) mice113.
Eosinophils in regenerating liver
Similarly, liver regeneration requires IL-4Rα expression by hepatocytes, and exogenous IL-4 is sufficient to promote hepatocyte proliferation in the absence of injury, which indicates that direct IL-4-induced signalling of hepatocytes drives liver regeneration112. Liver injury in mice causes an increase in the local production of eotaxin 1, and similar to injured skeletal muscles, recruited eosinophils are the main IL-4-expressing cells within injured livers. Eosinophil-deficient mice have an impaired regenerative response to partial hepatectomy or carbon tetrachloride-induced liver injury, which directly implicates recruited eosinophils in liver regeneration112.
These observations provide evidence that eosinophils and the IL-4 they produce contribute to tissue repair in two highly regenerative organs (skeletal muscle and liver). In each case, eosinophil-derived IL-4 mediates its effects on resident or recruited non-immune cells.
Metabolic homeostasis
Adipose tissue eosinophils
In lean mice, the majority of macrophages within visceral adipose tissue (VAT) are of the alternatively activated M2 phenotype; these cells express anti-inflammatory genes such as Il10 and arginase 1 (Arg1) and secrete products that support the insulin sensitivity of adipocytes116. By contrast, monocytes recruited into the VAT of obese mice predominantly develop characteristics of classically activated M1 macrophages and secrete pro-inflammatory cytokines, including TNF, that promote low-grade inflammation, leading to insulin resistance116,117. The type 2 cytokines IL-4 and IL-13 drive macrophages towards an M2-like phenotype. Maintaining an anti-inflammatory milieu (and by extension glucose homeostasis) within adipose tissue thus requires a continual supply of IL-4, of which adipose tissue eosinophils are a crucial source75,118. The number of adipose tissue eosinophils inversely correlates with the level of obesity in mice118,119, and eosinophil-deficient mice fed a high-fat diet had increased adiposity and compromised tolerance to insulin and glucose118. Importantly, this phenotype was reversed upon reintroduction of eosinophils118. Upstream of eosinophils, ILC2s are the main source of IL-5 within VAT, and the number of ILC2s correlates with the number of eosinophils (for example, mice that are devoid of ILC2s have a concomitant reduction in the number of VAT eosinophils)75. Therefore, an emerging paradigm suggests that the coordinated actions of ILC2s, eosinophils and M2 macrophages regulate metabolic homeostasis in VAT.
Thermoregulation
The participation of eosinophils in metabolic processes includes a role in thermo-regulation120,121. In response to cold or shivering, M2 macrophage-derived catecholamines induce a pro-thermogenic phenotype in adipocyte precursors, characterized by overexpression of mitochondrial brown fat uncoupling protein 1. The resulting uncoupling of mitochondrial proton gradients in beige adipocytes increases energy expenditure, which is dissipated as heat, thereby promoting thermoregulation. Within poorly innervated white adipose tissue, adipocyte beiging is driven by eosinophil-derived IL-4. IL-4 activates IL-4Rα–STAT6 signalling pathways in M2 macrophages, which promote the expression of tyrosine hydroxylase, the rate-limiting enzyme in the production of catecholamines. Thermogenic demands are thereby met by increasing the mass of beige fat within white adipose tissue121.
Immune homeostasis
Early B cell activation
In T cell-dependent immune responses, B cells acquire cognate antigens through surface-expressed B cell receptors (BCRs). Antigen acquired through BCRs is processed, and antigenic peptides are presented on surface-expressed MHC class II molecules. Crosslinking of MHC class II molecules on IL-4-primed B cells leads to B cell activation122,123, which is characterized by an intracellular Ca2+ flux, upregulation of IgM production and proliferation124–126. Vaccine adjuvants such as alum facilitate early B cell activation at least in part by eliciting the infiltration of IL-4-expressing eosinophils into bone marrow and spleen compartments122,127. Adjuvant-elicited eosinophils express IL-4, IL-6, IL-10, TNF and the plasma cell survival factor APRIL128 and are required for early alum-induced activation of B cells and the production of antigen-specific IgM127. In both mice and humans, eosinophils also support increased numbers of peripheral B cells129; and in mice, eosinophils promote B cell survival, proliferation and immunoglobulin secretion by a contact-independent mechanism129.
Maintenance of bone marrow plasma cells
Downstream of acute B cell activation, long-term humoral immunity is provided by antibody-secreting plasma cells. Plasma cells develop primarily within germinal centres of lymphoid tissues before migrating into specialized bone marrow niches where the cytokine microenvironment (including APRIL, IL-4, IL-6, IL-10 and TNF) is crucial in promoting their maturation and long-term survival. Eosinophils colocalize with plasma cells within bone marrow compartments and are a predominant source of IL-6 and APRIL29,130. Ex vivo co-culture of eosinophils with plasma cells promoted plasma cell survival130, and eosinophil-deficient mice had decreased numbers of bone marrow plasma cells compared with wild-type mice despite the normal development, differentiation and antibody-affinity maturation of B cells. These results directly implicate eosinophils and their products in maintaining the long-term survival of bone marrow plasma cells128.
Regulation of mucosal IgA
Unlike the dominant role of IgG in systemic humoral immune responses, humoral immunity at mucosal surfaces is mediated mainly by IgA antibodies. Most secretory IgA is produced by plasma cells that reside within the intestinal lamina propria. Mucosal IgA has crucial roles in the front-line defence of mucosal surfaces interfacing with the environment and exerts a strong influence on the composition of the microbiota131. Driven by the constitutive expression of eotaxin 1, eosinophils home to all regions of the gastrointestinal tract (except the oesophagus), and intestinal eosinophils, similarly to bone marrow eosinophils, express plasma cell survival factors including APRIL, IL-6 and TGFβ1. Mice in which eosinophils were either temporarily depleted (using Siglec-F-specific antibodies) or genetically ablated (for example, in ΔdblGATA or PHIL mice) had decreased numbers of IgA-secreting lamina propria B cells, and IgA levels were restored upon reintroduction of eosinophils through adoptive transfer of lamina propria cells from wild-type mice. These results implicate eosinophils in the maintenance of intestinal mucosal IgA levels110,111. As might be expected, the loss of IgA-secreting plasma cells in eosinophil-deficient mice was accompanied by alterations in the mucous layer and the microbial composition of the intestinal tract (reviewed in REF. 128).
Central T cell tolerance
In the steady state, local expression of eotaxin 1 attracts eosinophils into the neonatal mouse thymus, where they localize primarily within the cortico-medullary region13. Eosinophils are similarly observed within medullary regions in thymi of young children132. A main function of the thymus is to negatively select (in other words, delete) T cells that are reactive to self-antigens, and the cortico-medullary region where eosinophils accumulate is an active site of self-reactive T cell deletion. A mouse model of MHC class I-restricted thymic T cell deletion showed eosinophil infiltration in close proximity to extensive thymocyte apoptosis, which led to the hypothesis that thymic eosinophils might function in T cell negative selection13. Further supporting a potential immunomodulatory function for thymic eosinophils, human blood and thymic eosinophils express indoleamine 2,3-dioxygenase (IDO), which catalyses the rate-limiting step in the catabolism of tryptophan to kynurenines132,133. IDO-catalysed pathways thus deplete local tryptophan levels (which are necessary for cell proliferation and protein biosynthesis) and contribute kynurenines (which inhibit cell proliferation and promote the apoptosis, specifically, of TH1 cells, but not TH2 cells). In addition, non-specific apoptosis of thymic T cells induced by gamma irradiation leads to the infiltration of eosinophils in the vicinity of apoptotic T cells. Deletion of eosinophils or neutrophils impaired the clearance of apoptotic debris in the thymus, which suggests that eosinophils support macrophage-mediated phagocytosis of apoptotic cells134. Taken together, these data suggest that eosinophils have immunomodulatory roles in defining the T cell repertoire and/or more generalized participation in innate immune responses to apoptotic cell death.
Eosinophils in other host responses
The increasing recognition of tissue eosinophils as sources of cytokines, as well as other protein, oxidative and lipid mediators, is providing evolving insights into the roles of eosinophils in diverse host responses that have often not previously been linked to eosinophil function. Eosinophils contribute to adaptive immunity through the production of DC and effector TH cell chemoattractants such as EDN, CCL17, CCL22, CXC-chemokine ligand 9 (CXCL9) and CXCL10 (REFS 27,71,72,135,136); and by functioning as antigen-presenting cells137,138. Eosinophils can be involved in tumour rejection by normalizing tumour vessels and enhancing the infiltration of CD8+ T cells, owing to eosinophil secretion of IFNγ, TNF, CCL5, CXCL9 and CXCL10 (REF. 139). For host defence responses to non-classical microbial targets, eosinophil-derived IL-25 helps to mediate protection from protozoan parasite-elicited amoebic colitis140, and eosinophils, as sources of IL-23 and IL-17, are immunomodulatory in fungal aspergillosis141. In response to systemic IL-33 administration, ILC2-derived IL-5 and the associated eosinophil recruitment have both been shown to promote increased pulmonary artery hypertrophy, which is a previously unrecognized role for eosinophils142. Moreover, eosinophils have roles in regulating perivascular adipose tissue and vascular functionality through the production of adiponectin and nitric oxide143. This last finding is a reminder that eosinophils are sources not only of conventional cytokines but also of a diversity of other mediators that contribute to their roles in homeostasis and immunity.
Conclusions and perspectives
Our evolving understanding of the varied functional capacities of eosinophils as cells of the innate immune system in steady-state tissues provides insights into how we can further explore the roles of eosinophils in tissues and elsewhere and better define the cellular and molecular mechanisms that control their varied responses. Early studies of the roles of eosinophils in host responses, immunity and allergic inflammation focused on the potential effects of eosinophil degranulation, with release of the cardinal cationic proteins that are uniquely packaged within eosinophil granules. However, eosinophils are also both sources of and responders to diverse cytokines, as well as many other mediators, including lipids144. As discussed above, recent insights into the roles of tissue-dwelling eosinophils in the steady state are mainly based on eosinophil-derived cytokines. There remain many other basic mechanisms that could underlie the functioning of mouse and human eosinophils and remain to be delineated (BOX 2).
Box 2. Unresolved issues in comparing mouse and human eosinophils.
Studies of mouse and human eosinophils have provided complementary findings that establish eosinophils as sources of cytokines. It remains to be clarified whether eosinophils from these two species share common mechanisms regulating the formation and secretion of cytokines.
Cytokine reporter mice have shown that tissue eosinophils are a source of IL-4; the use of these mice in many studies may bias the results towards recognizing eosinophils in tissue responses on the basis of their IL-4 production. Other cytokines can also be produced by mouse eosinophils, but whether new cytokine reporter mice can reveal these and under what situations is not clear.
Human eosinophils have clearly been documented to contain substantial levels of preformed cytokines within granules, a finding that has not yet been replicated with mouse eosinophils.
Secretory vesicles that arise from eosinophil granules and contain both cationic granule proteins and cytokines have been documented in human eosinophils but not yet in mouse eosinophils.
The capacity to differentially secrete a limited and selected repertoire of preformed cytokines is established for human eosinophils but not yet mouse eosinophils. The signalling mechanisms of differential secretion have not yet been determined.
It is not known whether mouse eosinophils, similarly to human eosinophils, contain stores of cytokine receptor proteins within their granules or have the intragranular membrano-vesicular network of tubules that mediates selective piecemeal degranulation (PMD) from within granules.
Are cytokine receptor proteins, if they function as transporters during PMD, recycled back from the cell surface to function at eosinophil granules?
What are the pathways that lead to eosinophil granules being populated with stores of cytokine proteins; how are cytokines processed to be stored within granules; and might cytokines even be synthesized locally within granules given that RNA has been detected within granules147,148?
Do the various chemokine, cytokine and lipid receptors that have been shown on isolated human eosinophil granules have a function when these granules are intracellular, as has been indicated by the intracrine signalling function of leukotriene C4 in regulating IL-4 secretion from human eosinophils149?
In recognition of the common lineages of eosinophils, basophils and mast cells and their functions within tissue sites as innate granule-containing leukocytes, the mechanisms that have been identified in eosinophils for the secretion of granule-derived proteins, including cytokines, may also be relevant to these other cell types. Moreover, for each of these cell types localized within tissues, it remains to be determined what local stimuli function to enable their secretion of protein, oxidative and lipid mediators. It is plausible that a large number of stimuli, including locally released mediators from other cells and cell–cell and cell–matrix interactions, may function together to elicit specific regulated responses, such as the differential secretion of preformed cytokines from within eosinophils. Although there is considerable redundancy within immune responses, eosinophils in tissue sites are being recognized for their distinct roles as sources of diverse cytokines and other mediators.
Acknowledgments
We acknowledge the many investigators who have provided contributions to understanding the immunobiology of eosinophils, and we note that space constraints limited our citations. For electron microscopy studies specifically, the expertise of A. M. Dvorak and R. C. Melo has been crucial in revealing ultrastructure-based insights into eosinophil secretion mechanisms. Our studies have been supported by US National Institutes of Health grants R37AI020241, R01AI022571 and R01HL051645 (to P.F.W.), and R01HL095699 and R01AI121186 (to L.A.S.).
Glossary
- Side scatter (SSC) parameter
In flow cytometry, the SSC parameter is a measurement of light scatter taken at a ninety-degree angle relative to the laser. As cellular components such as granules increase the light refraction, SSC is a useful parameter to distinguish cell populations on the basis of their cellular complexity.
- Eosinophil-like cell lines
The cell lines HL-60 clone 15 and EoL-1 were derived from the blood of patients with acute promyelocytic or eosinophilic leukaemia, respectively. When cultured under specific conditions, these cell lines can be differentiated into cells with cytological and functional features of eosinophils.
- Type 1 cytokines
Cytokines typically produced by T helper 1 cells, including IL-2, interferon-γ and IL-12.
- Type 2 cytokines
Cytokines typically produced by T helper 2 cells, including IL-4, IL-5, IL-6, IL-10 and IL-13.
- Intragranular membrano-vesicular network
Intricate network of interconnected vesicular tubules that is evident within eosinophil granules undergoing piecemeal degranulation. These tubules are thought to give rise to granule-derived secretory vesicles.
- Eosinophilic oesophagitis
Chronic allergic inflammatory disease characterized by the accumulation of a large number of eosinophils in the oesophagus, an organ that is normally devoid of eosinophils.
- Immunonanogold localization
Electron microscopy technique using antibodies conjugated to very small (1.4 nm) gold particles to enable subcellular localization of proteins.
- Eosinophil sombrero vesicles
(EoSVs). C-Shaped tubular vesicles that are named in recognition of their similarity in cross-sectional appearance in electron micrographs to a Mexican hat.
- Group 2 innate lymphoid cells
(ILC2s). ILCs are innate immune cells that derive from common lymphoid progenitors and are considered part of the lymphoid lineage. ILC2s produce cytokines associated with T helper 2 cells (such as IL-4, IL-5 and IL-13).
- 4get mice
Bicistronic IL-4 reporter mice were generated by the targeted addition of an internal ribosomal entry site–enhanced green fluorescent protein (IRES–eGFP) to generate IL-4–GFP–enhanced transcript (4get) mice.
- Alternatively activated M2 macrophages
Anti-inflammatory cells that function in tissue repair and remodelling. M2 macrophages are characterized by the production of IL-10 and transforming growth factor-β.
- Classically activated M1 macrophages
Macrophages that are activated by lipopolysaccharide and interferon-γ to secrete high levels of IL-12 and produce nitric oxide, promoting a pro-inflammatory antimicrobial response.
- Mitochondrial brown fat uncoupling protein 1
Also known as thermogenin. This protein spans the inner mitochondrial membrane and functions as a proton transporter, thereby uncoupling the proton gradient that is produced during oxidative phosphorylation from ATP production, causing the chemical energy to instead be dissipated as heat.
- Beige adipocytes
Adipocytes that express uncoupling protein 1 and have thermogenic capacity. They are induced in white adipose tissue by cold either directly or indirectly through activation of the β-adrenergic signalling pathway.
Footnotes
Competing interests statement
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Drissen R, et al. Distinct myeloid progenitor-differentiation pathways identified through single-cell RNA sequencing. Nat Immunol. 2016;17:666–676. doi: 10.1038/ni.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289:17406–17415. doi: 10.1074/jbc.R113.546218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JJ, et al. Human versus mouse eosinophils: “that which we call an eosinophil, by any other name would stain as red”. J Allergy Clin Immunol. 2012;130:572–584. doi: 10.1016/j.jaci.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang L, et al. Eosinophil-derived IL-10 supports chronic nematode infection. J Immunol. 2014;193:4178–4187. doi: 10.4049/jimmunol.1400852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang L, Appleton JA. Eosinophils in helminth infection: defenders and dupes. Trends Parasitol. 2016;32:798–807. doi: 10.1016/j.pt.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makepeace BL, Martin C, Turner JD, Specht S. Granulocytes in helminth infection — who is calling the shots? Curr Med Chem. 2012;19:1567–1586. doi: 10.2174/092986712799828337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang L, et al. Eosinophils and IL-4 support nematode growth coincident with an innate response to tissue injury. PLoS Pathog. 2015;11:e1005347. doi: 10.1371/journal.ppat.1005347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neves JS, Perez SA, Spencer LA, Melo RC, Weller PF. Subcellular fractionation of human eosinophils: isolation of functional specific granules on isoosmotic density gradients. J Immunol Methods. 2009;344:64–72. doi: 10.1016/j.jim.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato M, et al. Eosinophil infiltration and degranulation in normal human tissue. Anat Rec. 1998;252:418–425. doi: 10.1002/(SICI)1097-0185(199811)252:3<418::AID-AR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Yu YR, et al. A protocol for the comprehensive flow cytometric analysis of immune cells in normal and inflamed murine non-lymphoid tissues. PLoS ONE. 2016;11:e0150606. doi: 10.1371/journal.pone.0150606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diener KR, Robertson SA, Hayball JD, Lousberg EL. Multi-parameter flow cytometric analysis of uterine immune cell fluctuations over the murine estrous cycle. J Reproductive Immunol. 2016;113:61–67. doi: 10.1016/j.jri.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Voehringer D, van Rooijen N, Locksley RM. Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J Leukoc Biol. 2007;81:1434–1444. doi: 10.1189/jlb.1106686. [DOI] [PubMed] [Google Scholar]
- 13.Throsby M, Herbelin A, Pleau JM, Dardenne M. CD11c+ eosinophils in the murine thymus: developmental regulation and recruitment upon MHC class I-restricted thymocyte deletion. J Immunol. 2000;165:1965–1975. doi: 10.4049/jimmunol.165.4.1965. [DOI] [PubMed] [Google Scholar]
- 14.Jung Y, Rothenberg ME. Roles and regulation of gastrointestinal eosinophils in immunity and disease. J Immunol. 2014;193:999–1005. doi: 10.4049/jimmunol.1400413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Percopo CM, et al. SiglecF+Gr1hi eosinophils are a distinct subpopulation within the lungs of allergen-challenged mice. J Leukoc Biol. 2017;101:321–328. doi: 10.1189/jlb.3A0416-166R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le-Carlson M, et al. Markers of antigen presentation and activation on eosinophils and T cells in the esophageal tissue of patients with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2013;56:257–262. doi: 10.1097/MPG.0b013e3182758d49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel AJ, et al. Increased HLA-DR expression on tissue eosinophils in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2010;51:290–294. doi: 10.1097/MPG.0b013e3181e083e7. [DOI] [PubMed] [Google Scholar]
- 18.Sedgwick JB, et al. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149:3710–3718. [PubMed] [Google Scholar]
- 19.Cagnoni EF, et al. Bronchopulmonary lymph nodes and large airway cell trafficking in patients with fatal asthma. J Allergy Clin Immunol. 2015;135:1352–1357. e9. doi: 10.1016/j.jaci.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Mesnil C, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126:3279–3295. doi: 10.1172/JCI85664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bettigole SE, et al. The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat Immunol. 2015;16:829–837. doi: 10.1038/ni.3225. This study implicates IRE1α–XBP1 signalling, a key component of the unfolded protein response pathway, in the terminal maturation of eosinophil progenitors. These data provide a link between eosinophilopoiesis and physiological endoplasmic reticulum stress in eosinophil-committed precursors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews SP, McMillan SJ, Colbert JD, Lawrence RA, Watts C. Cystatin F ensures eosinophil survival by regulating granule biogenesis. Immunity. 2016;44:795–806. doi: 10.1016/j.immuni.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doyle AD, et al. Expression of the secondary granule proteins major basic protein 1 (MBP-1) and eosinophil peroxidase (EPX) is required for eosinophilopoiesis in mice. Blood. 2013;122:781–790. doi: 10.1182/blood-2013-01-473405. This study shows that concomitant loss of two of the main granule-derived cationic proteins (MBP1 and EPX) results in selective loss of eosinophil lineage-committed progenitors. This provides, for the first time, a link between granule protein expression and eosinophilopoiesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer LA, et al. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–123. doi: 10.1189/jlb.0108058. This study shows that blood eosinophils from healthy humans constitutively contain preformed stores of various cytokines that are rapidly and differentially released in response to specific agonists. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moqbel R, et al. Identification of messenger RNA for IL-4 in human eosinophils with granule localization and release of the translated product. J Immunol. 1995;155:4939–4947. [PubMed] [Google Scholar]
- 26.Beil WJ, Weller PF, Tzizik DM, Galli SJ, Dvorak AM. Ultrastructural immunogold localization of tumor necrosis factor-alpha to the matrix compartment of eosinophil secondary granules in patients with idiopathic hypereosinophilic syndrome. J Histochem Cytochem. 1993;41:1611–1615. doi: 10.1177/41.11.8409368. [DOI] [PubMed] [Google Scholar]
- 27.Liu LY, et al. Generation of Th1 and Th2 chemokines by human eosinophils: evidence for a critical role of TNF-alpha. J Immunol. 2007;179:4840–4848. doi: 10.4049/jimmunol.179.7.4840. [DOI] [PubMed] [Google Scholar]
- 28.Shen ZJ, Esnault S, Malter JS. The peptidyl-prolyl isomerase Pin1 regulates the stability of granulocyte-macrophage colony-stimulating factor mRNA in activated eosinophils. Nat Immunol. 2005;6:1280–1287. doi: 10.1038/ni1266. [DOI] [PubMed] [Google Scholar]
- 29.Chu VT, Berek C. Immunization induces activation of bone marrow eosinophils required for plasma cell survival. Eur J Immunol. 2012;42:130–137. doi: 10.1002/eji.201141953. This study suggests that antigen-dependent activation primes eosinophils to provide pro-survival signals to plasma cells within bone marrow niches. [DOI] [PubMed] [Google Scholar]
- 30.Rose CE, Jr, et al. Murine lung eosinophil activation and chemokine production in allergic airway inflammation. Cell Mol Immunol. 2010;7:361–374. doi: 10.1038/cmi.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanda A, et al. Th2-activated eosinophils release Th1 cytokines that modulate allergic inflammation. Allergol Int. 2015;64(Suppl):S71–S73. doi: 10.1016/j.alit.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 33.Melo RC, et al. Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic. 2005;6:1047–1057. doi: 10.1111/j.1600-0854.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melo RC, Dvorak AM, Weller PF. Electron tomography and immunonanogold electron microscopy for investigating intracellular trafficking and secretion in human eosinophils. J Cell Mol Med. 2008;12:1416–1419. doi: 10.1111/j.1582-4934.2008.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melo RC, et al. Vesicle-mediated secretion of human eosinophil granule-derived major basic protein. Lab Invest. 2009;89:769–781. doi: 10.1038/labinvest.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melo RC, Spencer LA, Dvorak AM, Weller PF. Mechanisms of eosinophil secretion: large vesiculotubular carriers mediate transport and release of granule-derived cytokines and other proteins. J Leukoc Biol. 2008;83:229–236. doi: 10.1189/jlb.0707503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melo RC, Weller PF. Vesicular trafficking of immune mediators in human eosinophils revealed by immunoelectron microscopy. Exp Cell Res. 2016;347:385–390. doi: 10.1016/j.yexcr.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scepek S, Moqbel R, Lindau M. Compound exocytosis and cumulative degranulation by eosinophils and their role in parasite killing. Parasitol Today. 1994;10:276–278. doi: 10.1016/0169-4758(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 39.Persson C, Uller L. Primary lysis of eosinophils as a major mode of activation of eosinophils in human diseased tissues. Nat Rev Immunol. 2013;13:902. doi: 10.1038/nri3341-c1. [DOI] [PubMed] [Google Scholar]
- 40.Ueki S, et al. Eosinophil extracellular trap cell death-derived DNA traps: their presence in secretions and functional attributes. J Allergy Clin Immunol. 2016;137:258–267. doi: 10.1016/j.jaci.2015.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ueki S, et al. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. 2013;121:2074–2083. doi: 10.1182/blood-2012-05-432088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Persson CG, Erjefalt JS. “Ultimate activation” of eosinophils in vivo: lysis and release of clusters of free eosinophil granules (Cfegs) Thorax. 1997;52:569–574. doi: 10.1136/thx.52.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Persson CG, Erjefalt JS. Eosinophil lysis and free granules: an in vivo paradigm for cell activation and drug development. Trends Pharmacol Sci. 1997;18:117–123. doi: 10.1016/s0165-6147(97)01042-0. [DOI] [PubMed] [Google Scholar]
- 44.Persson CG. Centennial notions of asthma as an eosinophilic, desquamative, exudative, and steroid-sensitive disease. Lancet. 1997;350:1021–1024. doi: 10.1016/s0140-6736(96)02335-5. [DOI] [PubMed] [Google Scholar]
- 45.Erjefalt JS, Persson CG. New aspects of degranulation and fates of airway mucosal eosinophils. Am J Respir Crit Care Med. 2000;161:2074–2085. doi: 10.1164/ajrccm.161.6.9906085. [DOI] [PubMed] [Google Scholar]
- 46.Erjefalt JS, et al. Allergen-induced eosinophil cytolysis is a primary mechanism for granule protein release in human upper airways. Am J Respir Crit Care Med. 1999;160:304–312. doi: 10.1164/ajrccm.160.1.9809048. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe K, Misu T, Inoue S, Edamatsu H. Cytolysis of eosinophils in nasal secretions. Ann Otol Rhinol Laryngol. 2003;112:169–173. doi: 10.1177/000348940311200211. [DOI] [PubMed] [Google Scholar]
- 48.Greiff L, Erjefalt JS, Andersson M, Svensson C, Persson CG. Generation of clusters of free eosinophil granules (Cfegs) in seasonal allergic rhinitis. Allergy. 1998;53:200–203. doi: 10.1111/j.1398-9995.1998.tb03871.x. [DOI] [PubMed] [Google Scholar]
- 49.Uller L, Andersson M, Greiff L, Persson CG, Erjefalt JS. Occurrence of apoptosis, secondary necrosis, and cytolysis in eosinophilic nasal polyps. Am J Respir Crit Care Med. 2004;170:742–747. doi: 10.1164/rccm.200402-240OC. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez EB, Swedo JL, Rajaraman S, Daniels JC, Grant JA. Ultrastructural and immunohistochemical evidence for release of eosinophilic granules in vivo: cytotoxic potential in chronic eosinophilic pneumonia. J Allergy Clin Immunol. 1987;79:755–762. doi: 10.1016/0091-6749(87)90207-7. [DOI] [PubMed] [Google Scholar]
- 51.Fox B, Seed WA. Chronic eosinophilic pneumonia. Thorax. 1980;35:570–580. doi: 10.1136/thx.35.8.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grantham JG, Meadows JA, 3rd, Gleich GJ. Chronic eosinophilic pneumonia. Evidence for eosinophil degranulation and release of major basic protein. Am J Med. 1986;80:89–94. doi: 10.1016/0002-9343(86)90053-7. [DOI] [PubMed] [Google Scholar]
- 53.Tajirian A, Ross R, Zeikus P, Robinson-Bostom L. Subcutaneous fat necrosis of the newborn with eosinophilic granules. J Cutan Pathol. 2007;34:588–590. doi: 10.1111/j.1600-0560.2006.00665.x. [DOI] [PubMed] [Google Scholar]
- 54.Chikwava KR, Savell VH, Jr, Boyd TK. Fatal cephalosporin-induced acute hypersensitivity myocarditis. Pediatr Cardiol. 2006;27:777–780. doi: 10.1007/s00246-006-1430-0. [DOI] [PubMed] [Google Scholar]
- 55.Gutierrez-Pena EJ, Knab J, Buttner DW. Immunoelectron microscopic evidence for release of eosinophil granule matrix protein onto microfilariae of Onchocerca volvulus in the skin after exposure to amocarzine. Parasitol Res. 1998;84:607–615. doi: 10.1007/s004360050459. [DOI] [PubMed] [Google Scholar]
- 56.Daneshpouy M, et al. Activated eosinophils in upper gastrointestinal tract of patients with graft-versus-host disease. Blood. 2002;99:3033–3040. doi: 10.1182/blood.v99.8.3033. [DOI] [PubMed] [Google Scholar]
- 57.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206–212. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 58.Mueller S, Aigner T, Neureiter D, Stolte M. Eosinophil infiltration and degranulation in oesophageal mucosa from adult patients with eosinophilic oesophagitis: a retrospective and comparative study on pathological biopsy. J Clin Pathol. 2006;59:1175–1180. doi: 10.1136/jcp.2005.031922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saffari H, et al. Electron microscopy elucidates eosinophil degranulation patterns in patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2014;133:1728–1734.e1. doi: 10.1016/j.jaci.2013.11.024. This electron microscopy study used more than 1,500 images obtained from specimens taken from nine patients with eosinophilic oesophagitis to quantitatively assess degranulation patterns in human eosinophils in vivo. It showed that more than 80% of eosinophils have signs of cytolytic release of free granules. [DOI] [PubMed] [Google Scholar]
- 60.Shamri R, et al. CCL11 elicits secretion of RNases from mouse eosinophils and their cell-free granules. FASEB J. 2012;26:2084–2093. doi: 10.1096/fj.11-200246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boyer D, Vargas SO, Slattery D, Rivera-Sanchez YM, Colin AA. Churg–Strauss syndrome in children: a clinical and pathologic review. Pediatrics. 2006;118:e914–e920. doi: 10.1542/peds.2006-0113. [DOI] [PubMed] [Google Scholar]
- 62.Neves JS, et al. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc Natl Acad Sci USA. 2008;105:18478–18483. doi: 10.1073/pnas.0804547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neves JS, Radke AL, Weller PF. Cysteinyl leukotrienes acting via granule membrane expressed receptors elicit secretion from within cell-free human eosinophil granules. J Allergy Clin Immunol. 2010;125:477–482. doi: 10.1016/j.jaci.2009.11.029. This paper provides the first demonstration that extracellular, cell-free eosinophil granules express outwardly oriented, functional cytokine receptors and G protein-coupled receptors, as well as intragranular signal transduction molecules, and are competent to undergo differential, stimulus-induced secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melo RC, Morgan E, Monahan-Earley R, Dvorak AM, Weller PF. Pre-embedding immunogold labeling to optimize protein localization at subcellular compartments and membrane microdomains of leukocytes. Nat Protoc. 2014;9:2382–2394. doi: 10.1038/nprot.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melo RC, Perez SA, Spencer LA, Dvorak AM, Weller PF. Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils. Traffic. 2005;6:866–879. doi: 10.1111/j.1600-0854.2005.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Melo RC, Dvorak AM, Weller PF. Contributions of electron microscopy to understand secretion of immune mediators by human eosinophils. Microsc Microanal. 2010;16:653–660. doi: 10.1017/S1431927610093864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spencer LA, et al. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc Natl Acad Sci USA. 2006;103:3333–3338. doi: 10.1073/pnas.0508946103. This study shows that a granule-derived cytokine can be mobilized into secretory vesicles and chaperoned through the vesicular compartment bound to its cognate receptor during eosinophil PMD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bagnasco D, et al. Targeting interleukin-5 or interleukin-5Ralpha: safety considerations. Drug Saf. 2017;40:559–570. doi: 10.1007/s40264-017-0522-5. [DOI] [PubMed] [Google Scholar]
- 69.Nussbaum JC, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diefenbach A, Colonna M, Romagnani C. The ILC world revisited. Immunity. 2017;46:327–332. doi: 10.1016/j.immuni.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 71.Jacobsen EA, Zellner KR, Colbert D, Lee NA, Lee JJ. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J Immunol. 2011;187:6059–6068. doi: 10.4049/jimmunol.1102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobsen EA, et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kondo Y, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 74.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17:765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 75.Molofsky AB, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Rijt L, von Richthofen H, van Ree R. Type 2 innate lymphoid cells: at the cross-roads in allergic asthma. Semin Immunopathol. 2016;38:483–496. doi: 10.1007/s00281-016-0556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dhariwal J, et al. Mucosal type 2 innate lymphoid cells are a key component of the allergic response to aeroallergen. Am J Respir Crit Care Med. 2017;195:1586–1596. doi: 10.1164/rccm.201609-1846OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith SG, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75–86.e8. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 79.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mitchell PD, O’Byrne PM. Epithelial-derived cytokines in asthma. Chest. 2017;151:1338–1344. doi: 10.1016/j.chest.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 81.Esnault S, Kelly EA. Essential mechanisms of differential activation of eosinophils by IL-3 compared to GM-CSF and IL-5. Crit Rev Immunol. 2016;36:429–444. doi: 10.1615/CritRevImmunol.2017020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Egea L, Hirata Y, Kagnoff MF. GM-CSF: a role in immune and inflammatory reactions in the intestine. Expert Rev Gastroenterol Hepatol. 2010;4:723–731. doi: 10.1586/egh.10.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sugawara R, et al. Small intestinal eosinophils regulate Th17 cells by producing IL-1 receptor antagonist. J Exp Med. 2016;213:555–567. doi: 10.1084/jem.20141388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Munitz A, Levi-Schaffer F. Inhibitory receptors on eosinophils: a direct hit to a possible Achilles heel? J Allergy Clin Immunol. 2007;119:1382–1387. doi: 10.1016/j.jaci.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 85.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–5020. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 86.Ben Baruch-Morgenstern N, et al. Paired immunoglobulin-like receptor A is an intrinsic, self-limiting suppressor of IL-5-induced eosinophil development. Nat Immunol. 2014;15:36–44. doi: 10.1038/ni.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tedla N, et al. Activation of human eosinophils through leukocyte immunoglobulin-like receptor 7. Proc Natl Acad Sci USA. 2003;100:1174–1179. doi: 10.1073/pnas.0337567100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Munitz A, et al. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF, and eotaxin on human peripheral blood eosinophils. Blood. 2006;107:1996–2003. doi: 10.1182/blood-2005-07-2926. [DOI] [PubMed] [Google Scholar]
- 89.Lee JJ, Rosenberg HF, editors. Eosinophils in Health and Disease. Ch. 5.6. Elsevier; 2013. pp. 111–119. [Google Scholar]
- 90.Yu C, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage. in vivo J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee JJ, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 92.Jacobsen EA, et al. Eosinophil activities modulate the immune/inflammatory character of allergic respiratory responses in mice. Allergy. 2014;69:315–327. doi: 10.1111/all.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doyle AD, et al. Homologous recombination into the eosinophil peroxidase locus generates a strain of mice expressing Cre recombinase exclusively in eosinophils. J Leukoc Biol. 2013;94:17–24. doi: 10.1189/jlb.0213089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Croxford AL, Buch T. Cytokine reporter mice in immunological research: perspectives and lessons learned. Immunology. 2011;132:1–8. doi: 10.1111/j.1365-2567.2010.03372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 96.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 97.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–429. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aupperlee MD, et al. Epidermal growth factor receptor (EGFR) signaling is a key mediator of hormone-induced leukocyte infiltration in the pubertal female mammary gland. Endocrinology. 2014;155:2301–2313. doi: 10.1210/en.2013-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gouon-Evans V, Lin EY, Pollard JW. Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002;4:155–164. doi: 10.1186/bcr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 101.Gouon-Evans V, Pollard JW. Eotaxin is required for eosinophil homing into the stroma of the pubertal and cycling uterus. Endocrinology. 2001;142:4515–4521. doi: 10.1210/endo.142.10.8459. [DOI] [PubMed] [Google Scholar]
- 102.Sferruzzi-Perri AN, Robertson SA, Dent LA. Interleukin-5 transgene expression and eosinophilia are associated with retarded mammary gland development in mice. Biol Reprod. 2003;69:224–233. doi: 10.1095/biolreprod.102.010611. [DOI] [PubMed] [Google Scholar]
- 103.Zhang J, Lathbury LJ, Salamonsen LA. Expression of the chemokine eotaxin and its receptor, CCR3, in human endometrium. Biol Reprod. 2000;62:404–411. doi: 10.1095/biolreprod62.2.404. [DOI] [PubMed] [Google Scholar]
- 104.Knudsen UB, Uldbjerg N, Rechberger T, Fredens K. Eosinophils in human cervical ripening. Eur J Obstetr, Gynecol, Reproductive Biol. 1997;72:165–168. doi: 10.1016/s0301-2115(96)02686-3. [DOI] [PubMed] [Google Scholar]
- 105.Timmons BC, Fairhurst AM, Mahendroo MS. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol. 2009;182:2700–2707. doi: 10.4049/jimmunol.0803138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Robertson SA, Mau VJ, Young IG, Matthaei KI. Uterine eosinophils and reproductive performance in interleukin 5-deficient mice. J Reprod Fertil. 2000;120:423–432. doi: 10.1530/jrf.0.1200423. [DOI] [PubMed] [Google Scholar]
- 107.Matthews AN, et al. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci USA. 1998;95:6273–6278. doi: 10.1073/pnas.95.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hogan SP, Mishra A, Brandt EB, Foster PS, Rothenberg ME. A critical role for eotaxin in experimental oral antigen-induced eosinophilic gastrointestinal allergy. Proc Natl Acad Sci USA. 2000;97:6681–6686. doi: 10.1073/pnas.97.12.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999;103:1719–1727. doi: 10.1172/JCI6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chu VT, et al. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 2014;40:582–593. doi: 10.1016/j.immuni.2014.02.014. One of the first papers to show alterations in intestinal immune homeostasis (including alterations in IgA production, the intestinal T cell compartment and microbiota composition) in the absence of eosinophils. [DOI] [PubMed] [Google Scholar]
- 111.Jung Y, et al. IL-1beta in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 2015;8:930–942. doi: 10.1038/mi.2014.123. This paper implicates eosinophil-derived IL-1β in promoting intestinal homeostasis, including the maintenance of intestinal IgA levels and RORγ-expressing ILCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goh YP, et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci USA. 2013;110:9914–9919. doi: 10.1073/pnas.1304046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Heredia JE, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Joe AW, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 116.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu D, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. This study implicates IL-4 and/or IL-13 derived from adipose tissue eosinophils in the maintenance of alternatively activated macrophages, thereby linking eosinophils to metabolic homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maizels RM, Allen JE. Immunology. Eosinophils forestall obesity. Science. 2011;332:186–187. doi: 10.1126/science.1205313. [DOI] [PubMed] [Google Scholar]
- 120.Rao RR, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qiu Y, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jordan MB, Mills DM, Kappler J, Marrack P, Cambier JC. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science. 2004;304:1808–1810. doi: 10.1126/science.1089926. [DOI] [PubMed] [Google Scholar]
- 123.Cambier JC, Morrison DC, Chien MM, Lehmann KR. Modeling of T cell contact-dependent B cell activation. IL-4 and antigen receptor ligation primes quiescent B cells to mobilize calcium in response to Ia cross-linking. J Immunol. 1991;146:2075–2082. [PubMed] [Google Scholar]
- 124.Lang P, et al. TCR-induced transmembrane signaling by peptide/MHC class II via associated Ig-alpha/beta dimers. Science. 2001;291:1537–1540. doi: 10.1126/science.291.5508.1537. [DOI] [PubMed] [Google Scholar]
- 125.Tabata H, Matsuoka T, Endo F, Nishimura Y, Matsushita S. Ligation of HLA-DR molecules on B cells induces enhanced expression of IgM heavy chain genes in association with Syk activation. J Biol Chem. 2000;275:34998–35005. doi: 10.1074/jbc.M002089200. [DOI] [PubMed] [Google Scholar]
- 126.Lane PJ, McConnell FM, Schieven GL, Clark EA, Ledbetter JA. The role of class II molecules in human B cell activation. Association with phosphatidyl inositol turnover, protein tyrosine phosphorylation, and proliferation. J Immunol. 1990;144:3684–3692. [PubMed] [Google Scholar]
- 127.Wang HB, Weller PF. Pivotal advance: eosinophils mediate early alum adjuvant-elicited B cell priming and IgM production. J Leukoc Biol. 2008;83:817–821. doi: 10.1189/jlb.0607392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Berek C. Eosinophils: important players in humoral immunity. Clin Exp Immunol. 2016;183:57–64. doi: 10.1111/cei.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wong TW, Doyle AD, Lee JJ, Jelinek DF. Eosinophils regulate peripheral B cell numbers in both mice and humans. J Immunol. 2014;192:3548–3558. doi: 10.4049/jimmunol.1302241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chu VT, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12:151–159. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 131.Mantis NJ, Rol N, Corthesy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tulic MK, et al. Thymic indoleamine 2,3-dioxygenase-positive eosinophils in young children: potential role in maturation of the naive immune system. Am J Pathol. 2009;175:2043–2052. doi: 10.2353/ajpath.2009.090015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Odemuyiwa SO, et al. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173:5909–5913. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]
- 134.Kim HJ, Alonzo ES, Dorothee G, Pollard JW, Sant’Angelo DB. Selective depletion of eosinophils or neutrophils in mice impacts the efficiency of apoptotic cell clearance in the thymus. PLoS ONE. 2010;5:e11439. doi: 10.1371/journal.pone.0011439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dajotoy T, et al. Human eosinophils produce the T cell-attracting chemokines MIG and IP-10 upon stimulation with IFN-gamma. J Leukoc Biol. 2004;76:685–691. doi: 10.1189/jlb.0803379. [DOI] [PubMed] [Google Scholar]
- 136.Yang D, et al. Eosinophil-derived neurotoxin (EDN), an antimicrobial protein with chemotactic activities for dendritic cells. Blood. 2003;102:3396–3403. doi: 10.1182/blood-2003-01-0151. [DOI] [PubMed] [Google Scholar]
- 137.Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol. 2014;14:719–730. doi: 10.1038/nri3754. [DOI] [PubMed] [Google Scholar]
- 138.Farhan RK, et al. Effective antigen presentation to helper T cells by human eosinophils. Immunology. 2016;149:413–422. doi: 10.1111/imm.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Carretero R, et al. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8+ T cells. Nat Immunol. 2015;16:609–617. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- 140.Noor Z, et al. Role of eosinophils and tumor necrosis factor alpha in interleukin-25-mediated protection from amebic colitis. MBio. 2017;8:e02329–e02316. doi: 10.1128/mBio.02329-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guerra ES, et al. Central role of IL-23 and IL-17 producing eosinophils as immunomodulatory effector cells in acute pulmonary aspergillosis and allergic asthma. PLoS Pathog. 2017;13:e1006175. doi: 10.1371/journal.ppat.1006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ikutani M, et al. Prolonged activation of IL-5-producing ILC2 causes pulmonary arterial hypertrophy. JCI Insight. 2017;2:e90721. doi: 10.1172/jci.insight.90721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Withers SB, et al. Eosinophils are key regulators of perivascular adipose tissue and vascular functionality. Sci Rep. 2017;7:44571. doi: 10.1038/srep44571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Luna-Gomes T, Bozza PT, Bandeira-Melo C. Eosinophil recruitment and activation: the role of lipid mediators. Front Pharmacol. 2013;4:27. doi: 10.3389/fphar.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu Y, Beyer A, Aebersold R. On the dependency of cellular protein levels on mRNA abundance. Cell. 2016;165:535–550. doi: 10.1016/j.cell.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 146.Dyer KD, Garcia-Crespo KE, Percopo CM, Sturm EM, Rosenberg HF. Protocols for identifying, enumerating, and assessing mouse eosinophils. Methods Mol Biol. 2013;1032:59–77. doi: 10.1007/978-1-62703-496-8_5. [DOI] [PubMed] [Google Scholar]
- 147.Behzad AR, et al. Localization of DNA and RNA in eosinophil secretory granules. Int Arch Allergy Immunol. 2010;152:12–27. doi: 10.1159/000260079. [DOI] [PubMed] [Google Scholar]
- 148.Wickramasinghe SN, Hughes M. High resolution autoradiographic studies of RNA, protein and DNA synthesis during human eosinophil granulocytopoiesis: evidence for the presence of RNA on or within eosinophil granules. Br J Haematol. 1978;38:179–183. doi: 10.1111/j.1365-2141.1978.tb01034.x. [DOI] [PubMed] [Google Scholar]
- 149.Bandeira-Melo C, Woods LJ, Phoofolo M, Weller PF. Intracrine cysteinyl leukotriene receptor-mediated signaling of eosinophil vesicular transport-mediated interleukin-4 secretion. J Exp Med. 2002;196:841–850. doi: 10.1084/jem.20020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Carulli G, et al. Detection of eosinophils in whole blood samples by flow cytometry. Cytometry. 1998;34:272–279. doi: 10.1002/(sici)1097-0320(19981215)34:6<272::aid-cyto5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 151.Ethier C, Lacy P, Davoine F. Identification of human eosinophils in whole blood by flow cytometry. Methods Mol Biol. 2014;1178:81–92. doi: 10.1007/978-1-4939-1016-8_8. [DOI] [PubMed] [Google Scholar]
- 152.Barnig C, et al. Circulating human eosinophils share a similar transcriptional profile in asthma and other hypereosinophilic disorders. PLoS ONE. 2015;10:e0141740. doi: 10.1371/journal.pone.0141740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur J Immunol. 2004;34:1175–1184. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]
- 154.de Bruin AM, et al. Eosinophil differentiation in the bone marrow is inhibited by T cell-derived IFN-gamma. Blood. 2010;116:2559–2569. doi: 10.1182/blood-2009-12-261339. [DOI] [PubMed] [Google Scholar]
- 155.Dyer KD, et al. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181:4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Satoh T, et al. Critical role of Trib1 in differentiation of tissue-resident M2-like macrophages. Nature. 2013;495:524–528. doi: 10.1038/nature11930. [DOI] [PubMed] [Google Scholar]
- 157.Carlens J, et al. Common gamma-chain-dependent signals confer selective survival of eosinophils in the murine small intestine. J Immunol. 2009;183:5600–5607. doi: 10.4049/jimmunol.0801581. [DOI] [PubMed] [Google Scholar]
- 158.Smith KM, Rahman RS, Spencer LA. Humoral immunity provides resident intestinal eosinophils access to luminal antigen via eosinophil-expressed low-affinity Fcgamma receptors. J Immunol. 2016;197:3716–3724. doi: 10.4049/jimmunol.1600412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cheng LE, et al. IgE-activated basophils regulate eosinophil tissue entry by modulating endothelial function. J Exp Med. 2015;212:513–524. doi: 10.1084/jem.20141671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Esnault S, et al. Semaphorin 7A is expressed on airway eosinophils and upregulated by IL-5 family cytokines. Clin Immunol. 2014;150:90–100. doi: 10.1016/j.clim.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Grimaldi JC, et al. Depletion of eosinophils in mice through the use of antibodies specific for C-C chemokine receptor 3 (CCR3) J Leukoc Biol. 1999;65:846–853. doi: 10.1002/jlb.65.6.846. [DOI] [PubMed] [Google Scholar]