Abstract
BRAF V600E mutations occur frequently in malignant melanoma, but are rare in most malignant glioma subtypes. Besides, more benign brain tumors such as ganglioglioma, dysembryoblastic neuroepithelial tumours and supratentorial pilocytic astrocytomas, only pleomorphic xanthoastrocytomas (50–78%) and epitheloid glioblastoma (50%) regularly exhibit BRAF mutations. In the present study, we report on three patients with recurrent malignant gliomas harbouring a BRAF V600E mutation. All patients presented with markedly disseminated leptomeningeal disease at recurrence and had progressed after radiotherapy and alkylating chemotherapy. Therefore, estimated life expectancy at recurrence was a few weeks. All three patients received dabrafenib as a single agent and all showed a complete or nearly complete response. Treatment is ongoing and patients are stable for 27 months, 7 months and 3 months, respectively. One patient showed a dramatic radiologic and clinical response after one week of treatment. We were able to generate an ex vivo tumor cell culture from CSF in one patient. Treatment of this cell culture with dabrafenib resulted in reduced cell density and inhibition of ERK phosphorylation in vitro. To date, this is the first series on adult patients with BRAF-mutated malignant glioma and leptomeningeal dissemination treated with dabrafenib monotherapy. All patients showed a dramatic response with one patient showing an ongoing response for more than two years.
Keywords: dabrafenib, BRAF, BRAF V600E, pleomorphic xanthoastrocytoma, PXA, glioma, glioblastoma, leptomeningeal disease
Introduction
Approximately 50% of all patients with malignant melanoma harbor an activating mutation (V600E) in the BRAF kinase (1,2). This leads to an activation of the MAPK pathway and thereby to uncontrolled tumor growth (3). Vemurafenib and dabrafenib are two approved inhibitors of mutated BRAF and have shown efficacy in patients with BRAF V600E mutated melanoma (4,5).
In brain tumors BRAF V600E mutations have only been reported for rare entities and at low frequency (6). Gangliogliomas, dysembryoblastic neuroepithelial tumours (DNT) and supratentorial pilocytic astrocytomas are mostly benign tumors that harbor BRAF V600E mutations in 20–60, 30 and 5% of the examined cases, respectively (7–12). Clinically relevant are BRAF V600E mutations in pleomorphic xanthoastrocytomas (PXA) (including anaplastic variants) with a frequency of 50–78% and epitheloid glioblastomas with 50% (8,9,13–18). Due to the low incidence of these tumors BRAF V600E mutations are rare in everyday clinical practice (6,19).
Based on the results for BRAF inhibition in malignant melanoma, vemurafenib and dabrafenib have been used on an individual treatment basis in a few reported brain tumor patients. These are predominantly pediatric patients with ganglioglioma treated with vemurafenib (20–23). Furthermore, there are two case reports on children treated with vemurafenib for glioblastoma and pilomyxoid astrocytoma (24,25). There are three reports on pediatric patients with BRAF mutated brain tumors treated with dabrafenib. Two children suffered from a ganglioglioma and one from a low-grade glioma (26–28). Finally, there are three reports on adult patients treated with vemurafenib for pleomorphic xanthoastrocytoma (29–31). The largest case series was reported by Chamberlain with 4 patients receiving vemurafenib for pleomorphic xanthoastrocytoma (31). Radiographic response assessment showed progressive disease in one, stable disease in two and partial response in one patient. Median progression-free survival was reported with 5 months (range, 2 10 months) and median overall survival with 8 months (range, 4–14 months). We are not aware of reports on dabrafenib in adult patients.
In this study, we report on the first case series of 3 patients with BRAF V600E mutated, malignant glioma and leptomeningeal disease successfully treated with dabrafenib.
Materials and methods
Patients
We included three consecutive patients with malignant glioma and known BRAF V600E mutation treated with dabrafenib for progressive disease. All patients have been diagnosed and treated at the Brain Tumor Center of the University Hospital Frankfurt.
Ethics and approval
The study was performed in accordance with all ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patient data were anonymized prior to analysis. Approval of our local ethics committee was obtained for this study (No. 4/09, University Hospital Frankfurt, Germany). All patients in this study gave their written informed consent for scientific evaluation.
Generation and treatment of ex vivo cell culture
After centrifugation of 2 ml of cerebrospinal fluid (CSF), the resulting pellet was resuspended in Dulbeccos modified Eagles medium (DMEM) containing 10% fetal calf serum (FCS), 100 IU/ml penicillin and 100 mg/ml streptomycin. Subsequently, the primary cell cultures were passaged for three passages; 100,000 cells/well of 24-well plates were incubated in the presence of vehicle dimethyl sulfoxide (DMSO) or 100 nM dabrafenib for 72 h. Life cell microscopy was done with a Biozero Keyence microscope.
Western blot analysis
Glioma cells derived from CSF of patient 3 were incubated for 1 h in DMEM containing 10% FCS in the presence of vehicle or 100 nM dabrafenib. After the incubation cells were washed with ice-cold phosphate-buffered saline (PBS) and immediately frozen by placing the dishes in fluid nitrogen. Lysates were prepared as described using lysis buffer P and subjected to SDS-PAGE analysis (32). Membranes were probed with antibodies for P-AKT (Ser473), P-Erk1/2 (Thr202/Tyr204), P-S6RP (Ser235/235) or Rab11 (#7100; Cell Signaling Technology, Danvers, MA, USA). The secondary anti-rabbit antibody [Peroxidase AffiniPure Goat Anti-Rabbit IgG (H+L)] was purchased from Jackson ImmunoReseach Laboratories (West Grove, PA, USA). Chemiluminescence solution was used for detection.
Results
Histopathology and molecular markers
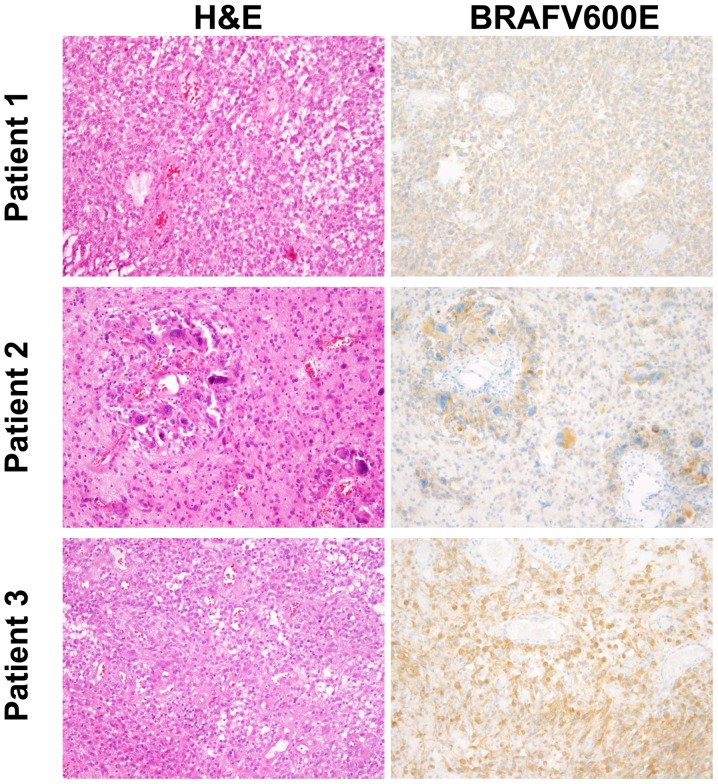
All 3 patients presented with highly pleomorphic astroglial tumors (Fig. 1). Besides anaplasia, the tumors showed vascular proliferations and partly necroses, as well as a strong immunohistochemical expression of BRAF V600E (Fig. 1). In all patients we performed sequencing of the BRAF gene and confirmed the BRAF V600E mutation. We profiled all three tumors for DNA methylation patterns using the Illumina HumanMethylation450 BeadChip. Methylation patterns did not reveal a precise diagnosis. Therefore, diagnoses were based on morphological characteristics. Patients 1 and 2 showed an anaplastic pleomorphic xanthoastrocytoma, while the tumor in patient 3 fulfilled the criteria for glioblastoma, but without signs for epithelioid differentiation.
Figure 1.
Neuropathology. Histological (left column, H&E) and immunohistochemical (BRAF V600E) staining of the initial tumor tissue is shown for all three patients.
Previous treatment and response to dabrafenib
Patient 1
The first patient (male) presented at the age of 24 with blurred vision, headache and papilledema. MRI scan showed a large, contrast enhancing tumor (maximum diameter of 6 cm) with minor hemorrhage in the right temporal lobe. A gross total resection was feasible and histopathology showed an anaplastic pleomorphic xanthoastrocytoma. The MGMT promotor was unmethylated and IDH1 R132H was not detectable. Immunohistochemistry as well as DNA-sequencing revealed BRAF V600E mutation. After gross total resection of the right temporal tumor all symptoms resolved and he was treated with combined radiochemotherapy according to the EORTC 22981/26981 study (33,34). After 2 cycles of temozolomide chemotherapy he developed lower back pain and sciatica in both legs. Furthermore, he reported on incontinence and constipation. MRI showed disseminated leptomeningeal spreading with several contrast enhancing lesions in the brain and major contrast enhancement mainly around the lumbar spinal cord and the nerve fibers. CSF analysis showed a cell count of 28/µl, elevated lactate (5.12 mmol/l), elevated albumin (3960 mg/l) and clearly detectable tumor cells. He then received palliative radiotherapy for the lumbar spine as this was the symptomatic region. Despite radiotherapy, all symptoms and all MRI lesions progressed shortly after radiotherapy. Two months after radiotherapy we initiated oral dabrafenib treatment with 150 mg twice daily. Dabrafenib was well tolerated, but the patient developed acne inversa as a side-effect of dabrafenib. MRI scans and clinical symptoms improved markedly. After 2 months of treatment all symptoms had resolved completely. Fig. 2 shows brain MRI and lumbar MRI before and one year after the initiation of dabrafenib treatment. All lesions fully regressed and all CSF parameters normalized. To date, he is on dabrafenib for 27 months and MRI still shows complete remission without contrast enhancing lesions.
Figure 2.
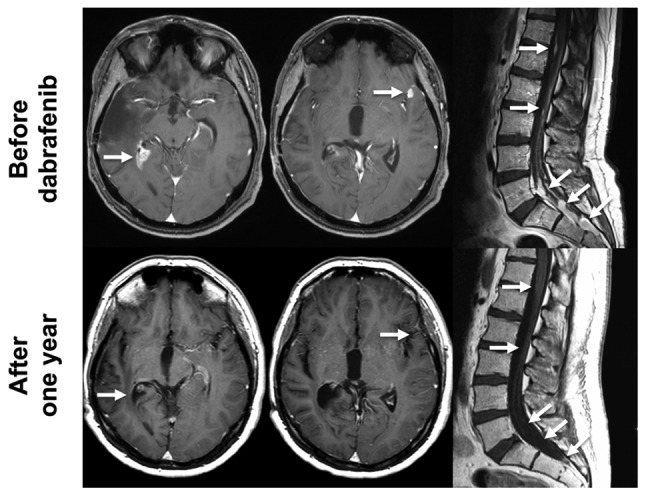
MRI of patient 1. MRI scans (T1+Gadolinium) of patient no. 1 before (upper panel) and one year after the initiation of the treatment with dabrafenib (lower panel) is shown. The lesion in the right temporal lobe (left column, white arrows), the lesion in the left temporal lobe (middle column, white arrows) and the multiple lesions in the lumbar spine (left column, white arrows) have disappeared.
Patient 2
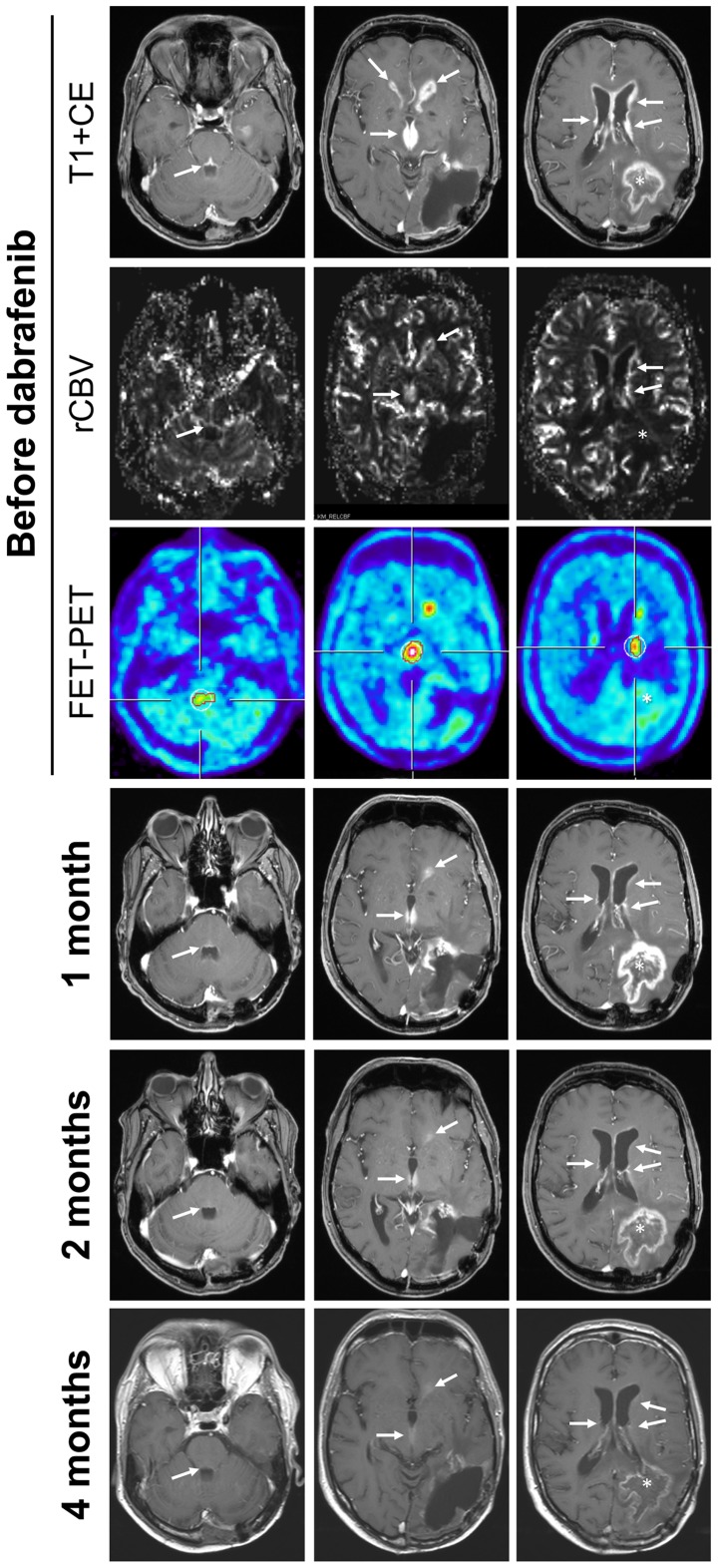
The second patient (male) was diagnosed at the age of 50. Notably, an MRI scan showed changes on T2 sequences in the left temporal lobe 6 years before (incidental finding). Because of a first epileptic seizure and progressive MRI (maximum diameter of 4 cm) with faint contrast enhancement, a gross total resection was performed at another University hospital. Histopathology suggested a glioblastoma with methylated MGMT promoter and wild-type IDH. Retrospective analyses of the initial tumor tissue at our institution and by an external reference neuropathologist revealed an anaplastic pleomorphic xanthoastrocytoma. Immunohistochemistry as well as DNA-sequencing revealed BRAF V600E mutation. After gross total resection of the left temporal tumor he was treated with combined radiochemotherapy according to the EORTC 22981/26981 study (Stupp regimen) with 6 cycles of temozolomide chemotherapy (33,34). Three and a half years after the end of the treatment MRI showed progressive tumor and the patient, suffering mild aphasia, underwent another gross total resection. Thereafter, he received 6 cycles of chemotherapy with lomustine and procarbazine. Six months after the end of the last cycle MRI again showed progressive disease and the patient suffered minimal aphasia and minimal visual deficit. Another gross total resection was achieved. Afterwards, he received a second radiotherapy with 10 × 3.5 Gy. MRI again showed progressive disease 5 months later with contrast enhancement along all ventricles (Fig. 3). Aphasia had slightly progressed and he now showed a latent hemiparesis and mild cognitive impairment. Furthermore, MRI showed a large contrast enhancing mass in the left parietal lobe (Fig. 3). Notably, this mass did not show elevated relative cerebral blood volume (rCBV) and no relevant O-(2-(18F)Fluoroethyl)-L-tyrosine (FET) uptake in PET (35). In contrast, the disseminated enhancement in the ventricles showed both, increased rCBV (Fig. 3, second row) and marked FET uptake (Fig. 3, third row), suggesting tumor recurrence. After one month of treatment with dabrafenib at 150 mg twice daily (Fig. 3, fourth row) the disseminated tumor markedly decreased (white arrows) while the radiation necrosis increased (white star). During further follow-up (2 and 4 months) the tumor further regressed and the radiation necrosis resolved as well. All symptoms improved and 8 months after the initiation of dabrafenib MRI was stable and patient doing well. Dabrafenib was well tolerated but the patient developed several small fibroepithelial polyps.
Figure 3.
MRI and FET-PET of patient 2. MRI scans (T1+gadolinium), MR perfusion and FET-PET is shown for patient 2. Before the initiation of dabrafenib MRI scans showed a contrast enhancing mass in the left parietal lobe (white star) and disseminated contrast enhancement along all ventricles (top panel). The larger lesion in the left parietal lobe did not show increased rCBV (second row) and no FET uptake (third row) suggesting radiation necrosis. Disseminated enhancement in the ventricles showed both, increased rCBV (second row) and marked FET uptake (third row) suggesting tumor recurrence. After one month of treatment with dabrafenib (fourth row) the disseminated tumor decreased (white arrows) while the radiation necrosis increased (white star). During further follow-up (2 and 4 months) the tumor further regressed and the radiation necrosis resolved as well.
Patient 3
The third patient (male) was diagnosed at the age of 25. He first noticed headache and then developed mild aphasia and impairment of fine motor skills of his right hand. MRI scan showed a contrast enhancing, cystic mass in the left temporal lobe with a maximum diameter of 5 cm. First, the tumor was biopsied. As histopathology did reveal a malignant astrocytic tumor and because of the tumor size a gross total resection was done. Histopathology showed a glioblastoma without epithelioid cells. Analysis of MGMT promoter status was inconclusive, the tumor showed regular expression of ATRX and was negative for IDH1 R132H on immunohistochemistry. Immunohistochemistry as well as DNA-sequencing revealed BRAF V600E mutation.
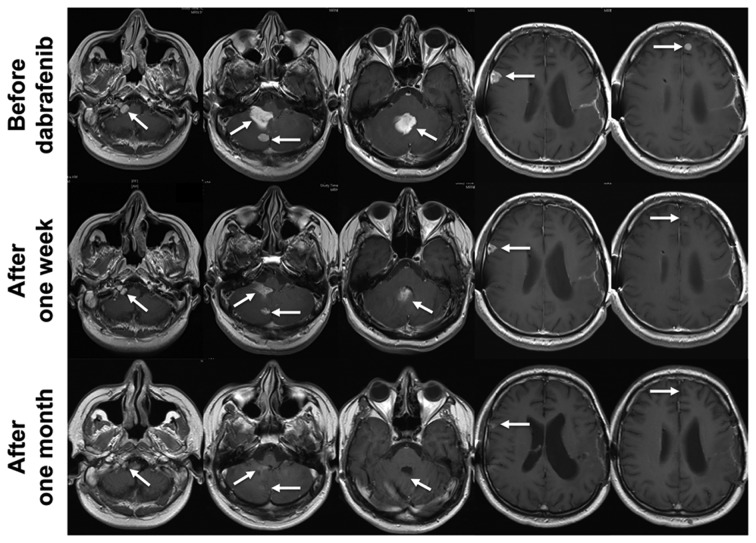
After gross total resection he still suffered mild aphasia and latent hemiparesis and was treated with combined radiochemotherapy according to the EORTC 22981/26981 study (Stupp regimen) with 6 cycles of temozolomide chemotherapy (33,34). MRI follow-up two months after the last cycle showed local tumor progression and another gross total resection was performed. He then received another irradiation with 10 × 3.5 Gy. Second follow-up MRI showed disseminated tumor spreading suggestive for leptomeningeal gliomatosis. At that time-point, the health insurance refused our application for treatment with dabrafenib and chemotherapy with lomustine was initiated. After two cycles all lesions had progressed. The patient now suffered massive headache, nausea and vomiting due to hydrocephalus. Therefore, he received a ventriculoperitoneal shunt. The first row of Fig. 4 shows contrast enhancing lesions in the right foramen jugulare, the fourth ventricle, the right cerebellum, the right frontal lobe and the left frontal lobe. Now the health insurance decided to cover the costs for dabrafenib. Dabrafenib was started with 150 mg twice daily, was well tolerated and no drug related side-effects have been reported. As early as one week after the initiation of dabrafenib MRI showed a partial remission and the patient improved dramatically (Fig. 4). After one month he reached a nearly complete response (Fig. 4). All symptoms had resolved completely. Three months after the initiation of dabrafenib MRI was stable.
Figure 4.
MRI of patient 3. MRI scans (T1+gadolinium) are shown before (upper panel), one week after (middle panel) and one month after (lower panel) the initiation of dabrafenib. MRI one week after the initiation of the treatment shows a dramatic response for all lesions. After one month MRI scans show an almost complete remission.
Ex vivo tumor cell culture
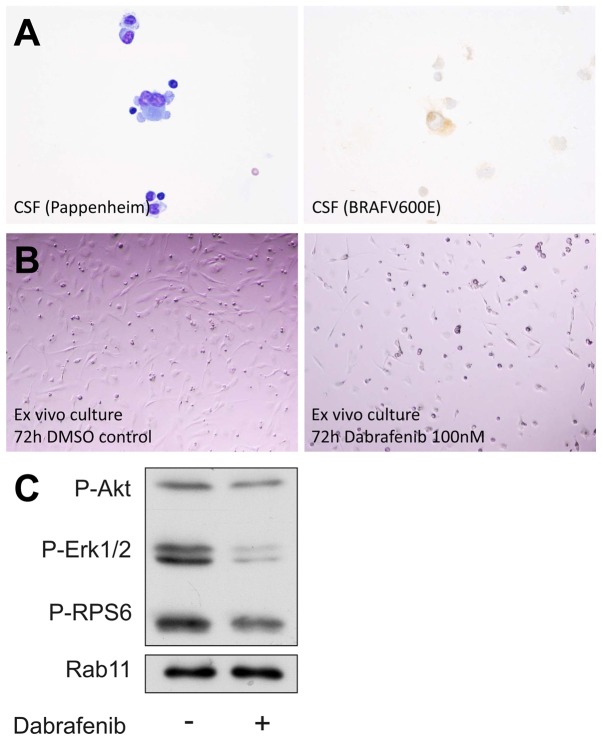
Fig. 5A shows CSF of our first patient with microscopic CSF analysis after Pappenheim staining (left) and immunohistochemistry for mutant (V600E) BRAF (right). CSF showed pleomorphic, partly multinucleated tumor cells with basophilic cytoplasms, cytoplasmic blebbing (Fig. 5A, left) and an expression of BRAF V600E (Fig. 5A, right). We were able to generate an ex vivo tumor cell culture as described in Materials and methods. During culture the cells remained positive for BRAF V600E but exhibited slow growth kinetics. Cells were treated with dabrafenib, survival was analyzed by light microscopy and protein lysates were obtained for western blotting. Fig. 5B shows microscopic photographs of the cells 72 h after treatment with DMSO control (left) and after treatment with dabrafenib at a concentration of 100 nM (right). This nicely shows that these cells not only harbor a BRAF V600E mutation, but also are susceptible to kinase inhibition with dabrafenib. Furthermore, our western blot analysis shows that phosphorylation of ERK, a major downstream target of BRAF, is inhibited in these cells by dabrafenib.
Figure 5.
Analyses of cerebrospinal fluid (CSF) and ex vivo tumor cell culture. Pappenheim staining of CSF cells (A, left) and immunohistochemistry for BRAF V600E (A, right) is shown. The lowest panel shows light microscopic images of the ex vivo tumor cell culture after 72 h of treatment with DMSO control and the BRAF V600E inhibitor dabrafenib. (B) Microscopic photographs of the cells 72 h after treatment with DMSO control (left) and after treatment with dabrafenib at a concentration of 100 nM (right). Western blot analysis is shown in (C). Phosphorylation of ERK is inhibited by dabrafenib.
Discussion
In this study we present a retrospective series of 3 patients with BRAF V600E mutated recurrent glioma and disseminated leptomeningeal disease treated with dabrafenib at 150 mg twice daily. Despite their poor prognosis, all patients showed an impressive response and one patient is stable for as long as 27 months. Furthermore, we were able to generate an ex vivo cell culture. In these cells treatment with dabrafenib resulted in a reduced cell density and inhibition of ERK phosphorylation.
These encouraging results corroborate the need for molecular screening of rare mutations in brain tumors. Furthermore, our results add to the existing data that BRAF V600E is a driver mutation in malignant glioma. All our patients suffered from markedly disseminated leptomeningeal disease. At this stage life expectancy is usually a few weeks, further confirming the clinical relevance of our results. As all patients suffered leptomeningeal disease and responded to dabrafenib this confirms that dabrafenib crosses the blood-brain barrier and reaches effective concentrations in the CSF.
As this is a retrospective study of only 3 patients we are not able to reliably compare our results to other case reports or the case series on pleomorphic xanthoastrocytoma treated with vemurafenib by Chamberlain et al (31). Since BRAF mutations are rare in brain tumors, studies on other treatment approaches are also lacking. To the best of our knowledge this is the first series in adult patients with BRAF mutated gliomas treated with dabrafenib. Furthermore, all of our patients showed leptomeningeal disease. Whether this represents a relevant bias and might select for tumors that are particularly vulnerable to dabrafenib treatment remains unclear. Nonetheless, our results are favorable and support the use of dabrafenib in BRAF V600E mutated malignant glioma and leptomeningeal disease should not be an impediment.
In BRAF mutated malignant melanoma dabrafenib is usually combined with a MEK inhibitor such as trametinib (36). We are not aware of published cases of patients with BRAF mutated glioma that have been treated with combined BRAF and MEK inhibition. There is one study on dabrafenib and trametinib in a syngeneic murine glioma model (37). In this study, combined treatment was more effective in reducing tumor growth and extending animal survival (37). There are ongoing trials evaluating the combined treatment with dabrafenib and trametinib in patients with brain tumors.
In conclusion, this small retrospective study suggests that dabrafenib has activity in BRAF V600E mutated malignant glioma progressing after radiotherapy and chemotherapy.
Acknowledgements
The Dr. Senckenberg Institute of Neurooncology is supported by the Dr. Senckenberg Foundation.
References
- 1.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 3.Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, Hughes TM, Thompson JF, Scolyer RA, Kefford RF. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239–1246. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 4.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. BRIM-3 Study Group: Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH, Jr, Kaempgen E, et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 6.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 7.Chappé C, Padovani L, Scavarda D, Forest F, Nanni-Metellus I, Loundou A, Mercurio S, Fina F, Lena G, Colin C, et al. Dysembryoplastic neuroepithelial tumors share with pleomorphic xanthoastrocytomas and gangliogliomas BRAFV600E mutation and expression. Brain Pathol. 2013;23:574–583. doi: 10.1111/bpa.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dougherty MJ, Santi M, Brose MS, Ma C, Resnick AC, Sievert AJ, Storm PB, Biegel JA. Activating mutations in BRAF characterize a spectrum of pediatric low-grade gliomas. Neuro Oncol. 2010;12:621–630. doi: 10.1093/neuonc/noq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schindler G, Capper D, Meyer J, Janzarik W, Omran H, Herold-Mende C, Schmieder K, Wesseling P, Mawrin C, Hasselblatt M, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 10.Prabowo AS, Iyer AM, Veersema TJ, Anink JJ, Schouten-van Meeteren AY, Spliet WG, van Rijen PC, Ferrier CH, Capper D, Thom M, et al. BRAF V600E mutation is associated with mTOR signaling activation in glioneuronal tumors. Brain Pathol. 2014;24:52–66. doi: 10.1111/bpa.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones DTW, Hutter B, Jäger N, Korshunov A, Kool M, Warnatz HJ, Zichner T, Lambert SR, Ryzhova M, Quang DA, et al. International Cancer Genome Consortium PedBrain Tumor Project: Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45:927–932. doi: 10.1038/ng.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, Orisme W, Punchihewa C, Parker M, Qaddoumi I, et al. St. Jude Children's Research Hospital-Washington University Pediatric Cancer Genome Project: Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45:602–612. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dias-Santagata D, Lam Q, Vernovsky K, Vena N, Lennerz JK, Borger DR, Batchelor TT, Ligon KL, Iafrate AJ, Ligon AH, et al. BRAF V600E mutations are common in pleomorphic xanthoastrocytoma: Diagnostic and therapeutic implications. PLoS One. 2011;6:e17948. doi: 10.1371/journal.pone.0017948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ida CM, Vrana JA, Rodriguez FJ, Jentoft ME, Caron AA, Jenkins SM, Giannini C. Immunohistochemistry is highly sensitive and specific for detection of BRAF V600E mutation in pleomorphic xanthoastrocytoma. Acta Neuropathol Commun. 2013;1:20. doi: 10.1186/2051-5960-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koelsche C, Sahm F, Wöhrer A, Jeibmann A, Schittenhelm J, Kohlhof P, Preusser M, Romeike B, Dohmen-Scheufler H, Hartmann C, et al. BRAF-mutated pleomorphic xanthoastrocytoma is associated with temporal location, reticulin fiber deposition and CD34 expression. Brain Pathol. 2014;24:221–229. doi: 10.1111/bpa.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiffman JD, Hodgson JG, VandenBerg SR, Flaherty P, Polley MY, Yu M, Fisher PG, Rowitch DH, Ford JM, Berger MS, et al. Oncogenic BRAF mutation with CDKN2A inactivation is characteristic of a subset of pediatric malignant astrocytomas. Cancer Res. 2010;70:512–519. doi: 10.1158/0008-5472.CAN-09-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broniscer A, Tatevossian RG, Sabin ND, Klimo P, Jr, Dalton J, Lee R, Gajjar A, Ellison DW. Clinical, radiological, histological and molecular characteristics of paediatric epithelioid glioblastoma. Neuropathol Appl Neurobiol. 2014;40:327–336. doi: 10.1111/nan.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleinschmidt-DeMasters BK, Aisner DL, Birks DK, Foreman NK. Epithelioid GBMs show a high percentage of BRAF V600E mutation. Am J Surg Pathol. 2013;37:685–698. doi: 10.1097/PAS.0b013e31827f9c5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behling F, Barrantes-Freer A, Skardelly M, Nieser M, Christians A, Stockhammer F, Rohde V, Tatagiba M, Hartmann C, Stadelmann C, et al. Frequency of BRAF V600E mutations in 969 central nervous system neoplasms. Diagn Pathol. 2016;11:55. doi: 10.1186/s13000-016-0506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguilera D, Janss A, Mazewski C, Castellino RC, Schniederjan M, Hayes L, Brahma B, Fogelgren L, MacDonald TJ. Successful retreatment of a child with a refractory brainstem ganglioglioma with vemurafenib. Pediatr Blood Cancer. 2016;63:541–543. doi: 10.1002/pbc.25787. [DOI] [PubMed] [Google Scholar]
- 21.Bautista F, Paci A, Minard-Colin V, Dufour C, Grill J, Lacroix L, Varlet P, Valteau-Couanet D, Geoerger B. Vemurafenib in pediatric patients with BRAFV600E mutated high-grade gliomas. Pediatr Blood Cancer. 2014;61:1101–1103. doi: 10.1002/pbc.24891. [DOI] [PubMed] [Google Scholar]
- 22.del Bufalo F, Carai A, Figà-Talamanca L, Pettorini B, Mallucci C, Giangaspero F, Antonelli M, Badiali M, Moi L, Bianco G, et al. Response of recurrent BRAFV600E mutated ganglioglioma to Vemurafenib as single agent. J Transl Med. 2014;12:356. doi: 10.1186/s12967-014-0356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rush S, Foreman N, Liu A. Brainstem ganglioglioma successfully treated with vemurafenib. J Clin Oncol. 2013;31:e159–e160. doi: 10.1200/JCO.2012.44.1568. [DOI] [PubMed] [Google Scholar]
- 24.Robinson GW, Orr BA, Gajjar A. Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy. BMC Cancer. 2014;14:258. doi: 10.1186/1471-2407-14-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skrypek M, Foreman N, Guillaume D, Moertel C. Pilomyxoid astrocytoma treated successfully with vemurafenib. Pediatr Blood Cancer. 2014;61:2099–2100. doi: 10.1002/pbc.25084. [DOI] [PubMed] [Google Scholar]
- 26.Shih KC, Shastry M, Williams JT, Jelsma PF, Abram SR, Ayyanar K, Burris HA, III, Infante JR. Successful treatment with dabrafenib (GSK2118436) in a patient with ganglioglioma. J Clin Oncol. 2014;32:e98–e100. doi: 10.1200/JCO.2013.48.6852. [DOI] [PubMed] [Google Scholar]
- 27.Lassaletta A, Guerreiro Stucklin A, Ramaswamy V, Zapotocky M, McKeown T, Hawkins C, Bouffet E, Tabori U. Profound clinical and radiological response to BRAF inhibition in a 2-month-old diencephalic child with hypothalamic/chiasmatic glioma. Pediatr Blood Cancer. 2016;63:2038. doi: 10.1002/pbc.26086. [DOI] [PubMed] [Google Scholar]
- 28.Meletath SK, Pavlick D, Brennan T, Hamilton R, Chmielecki J, Elvin JA, Palma N, Ross JS, Miller VA, Stephens PJ, et al. Personalized treatment for a patient with a BRAF V600E mutation using gabrafenib and a tumor treatment fields device in a high-grade glioma arising from ganglioglioma. J Natl Compr Canc Netw. 2016;14:1345–1350. doi: 10.6004/jnccn.2016.0145. [DOI] [PubMed] [Google Scholar]
- 29.Lee EQ, Ruland S, LeBoeuf NR, Wen PY, Santagata S. Successful treatment of a progressive BRAF V600E-mutated anaplastic pleomorphic xanthoastrocytoma with vemurafenib monotherapy. J Clin Oncol. 2016;34:e87–e89. doi: 10.1200/JCO.2013.51.1766. [DOI] [PubMed] [Google Scholar]
- 30.Usubalieva A, Pierson CR, Kavran CA, Huntoon K, Kryvenko ON, Mayer TG, Zhao W, Rock J, Ammirati M, Puduvalli VK, et al. Primary meningeal pleomorphic xanthoastrocytoma with anaplastic features: A report of 2 cases, one with BRAF(V600E) mutation and clinical response to the BRAF inhibitor dabrafenib. J Neuropathol Exp Neurol. 2015;74:960–969. doi: 10.1097/NEN.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chamberlain MC. Salvage therapy with BRAF inhibitors for recurrent pleomorphic xanthoastrocytoma: A retrospective case series. J Neurooncol. 2013;114:237–240. doi: 10.1007/s11060-013-1176-5. [DOI] [PubMed] [Google Scholar]
- 32.Steinbach JP, Wolburg H, Klumpp A, Probst H, Weller M. Hypoxia-induced cell death in human malignant glioma cells: Energy deprivation promotes decoupling of mitochondrial cytochrome c release from caspase processing and necrotic cell death. Cell Death Differ. 2003;10:823–832. doi: 10.1038/sj.cdd.4401252. [DOI] [PubMed] [Google Scholar]
- 33.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group: Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 34.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 35.Langen KJ, Galldiks N, Hattingen E, Shah NJ. Advances in neuro-oncology imaging. Nat Rev Neurol. 2017;13:279–289. doi: 10.1038/nrneurol.2017.44. [DOI] [PubMed] [Google Scholar]
- 36.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grossauer S, Koeck K, Murphy NE, Meyers ID, Daynac M, Truffaux N, Truong AY, Nicolaides TP, McMahon M, Berger MS, et al. Concurrent MEK targeted therapy prevents MAPK pathway reactivation during BRAFV600E targeted inhibition in a novel syngeneic murine glioma model. Oncotarget. 2016;7:75839–75853. doi: 10.18632/oncotarget.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]