Abstract
Background:
Animal and epidemiologic studies suggest that exposure to light at night (LAN) may disrupt circadian patterns and decrease nocturnal secretion of melatonin, which may disturb estrogen regulation, leading to increased breast cancer risk.
Objectives:
We examined the association between residential outdoor LAN and breast cancer incidence using data from the nationwide U.S.-based Nurses’ Health Study II cohort.
Methods:
We followed 109,672 women from 1989 through 2013. Cumulative LAN exposure was estimated using time-varying satellite data for a composite of persistent nighttime illumination at scale for each residence during follow-up. Incident invasive breast cancer cases were confirmed by medical record review. We used Cox proportional hazard models to calculate hazard ratios (HRs) and 95% confidence intervals (CIs), adjusting for anthropometric, reproductive, lifestyle, and socioeconomic risk factors.
Results:
Over 2,187,425 person-years, we identified 3,549 incident breast cancer cases. Based on a fully adjusted model, the estimated HR for incident breast cancer with an interquartile range (IQR) () increase in cumulative average outdoor LAN was 1.05 (95% CI: 1.00, 1.11). An association between LAN and breast cancer appeared to be limited to women who were premenopausal at the time of a case [ (95% CI: 1.01, 1.14) based on 1,973 cases vs. (95% CI: 0.91, 1.09) based on 1,172 cases in postmenopausal women; ]. The LAN–breast cancer association was observed only in past and current smokers at the end of follow-up [ (95% CI: 0.94, 1.07) based on 2,215 cases in never smokers; (95% CI: 1.01, 1.19) based on 1,034 cases in past smokers vs. (95% CI: 1.07, 1.37) for 300 cases in current smokers; ].
Conclusions:
Although further work is required to confirm our results and to clarify potential mechanisms, our findings suggest that exposure to residential outdoor light at night may contribute to invasive breast cancer risk. https://doi.org/10.1289/EHP935
Introduction
International differences in breast cancer incidence rates and increases in rates among populations that migrate from low- to high-risk areas support a role of environmental determinants of breast cancer (Reynolds et al. 2004b; Willett 2001). In addition, twin studies show that inherited genetic factors explain only a minor proportion of susceptibility to breast cancer, which further implicates the environment in driving breast cancer risk (Lichtenstein et al. 2000). Recent evidence has demonstrated associations between night-shift work and invasive breast cancer (Schernhammer et al. 2006b), and shift work is currently classified as a 2A “probable human carcinogen” by the International Agency for Research on Cancer (IARC) (Straif et al. 2007). It has been hypothesized that the relationship between night shift work and invasive breast cancer is mediated by exposure to light at night (LAN) (Schernhammer et al. 2006b). Evidence points to the potential role of exposure to LAN in contributing to breast cancer risk (Blask et al. 2011; Stevens et al. 2007; Stevens 2009), and animal and epidemiologic data suggest that exposure to LAN can modulate pineal gland function to decrease melatonin secretion (Haim and Zubidat 2015; Jasser et al. 2006) and disrupt circadian patterns and sleep (Blask et al. 2014; Stevens et al. 2007), which may increase breast cancer risk (Blask 2009). Mechanistically, light falling onto specific retinal ganglion cells at night triggers the pineal gland to stop the release of melatonin and disrupts the circadian system (Blask et al. 2014). Outdoor LAN has been used as a surrogate for greater total evening and nighttime circadian-effective light exposure because people living in communities with higher outdoor LAN likely drive on roads that are lit by street lighting, experience higher levels of light exposure during evening outdoor activities, and have more outdoor light intrusion into their bedrooms in the evening (Stevens 2011), although it is unclear how well outdoor LAN captures personal LAN exposure (Rea et al. 2011).
With the above biological mechanisms as a foundation, recent studies have examined links between outdoor LAN and breast cancer in six ecological analyses (Keshet-Sitton et al. 2016a; Kim et al. 2015; Kloog et al. 2008; Kloog et al. 2010; Portnov et al. 2016; Rybnikova et al. 2015), two case–control studies (Bauer et al. 2013; Keshet-Sitton et al. 2016b), and one prospective cohort in California (Hurley et al. 2014). These studies have all reported associations between outdoor LAN and breast cancer; however, to our knowledge, no prior study has participants living throughout the continental United States; time-varying, residence-level exposure data; and individual-level information on anthropometric, reproductive, lifestyle, and sociodemographic risk factors.
Previous studies have reported that the association between LAN and breast cancer may be modified by body mass index (BMI), menopausal status, and urbanicity (Hurley et al. 2014; Keshet-Sitton et al. 2016c; Portnov et al. 2016). In addition, race and socioeconomic status (SES) (Palmer et al. 2012), as well as air pollution (Parikh and Wei 2016; Wong et al. 2016), and smoking (Cui et al. 2006; Gaudet et al. 2013; Gaudet et al. 2016; Gram et al. 2015; Reynolds et al. 2004a) have been associated with breast cancer risk in past analyses, and these exposures could have possible synergistic effects on breast cancer risk in concert with LAN if these factors increase susceptibility to breast cancer. Analyses within the Nurses’ Health Study cohorts have shown some regional differences in breast cancer risk (Laden et al. 1997), and differing underlying susceptibility may lead associations between LAN and breast cancer risk to vary across regions. Night-shift work has been consistently associated with breast cancer within the Nurses’ Health Study cohorts, and it has been proposed that this association may be mediated by circadian disruption (Schernhammer et al. 2001; Schernhammer et al. 2006b). Because both LAN exposure and night-shift work are hypothesized to increase breast cancer risk through the same pathway of circadian disruption, it is possible that these two factors may by synergistic. For instance, for women working rotating shifts, their circadian patterns may be disrupted on the days they work night shifts, and LAN exposure may additionally disrupt their circadian patterns on days that they are working day shifts or not working.
Using data from the nationwide U.S.-based Nurses’ Health Study II (NHSII) from 1989 through 2013, we aimed to examine the association between outdoor LAN and invasive breast cancer incidence. We hypothesized that there would be a positive association between LAN and invasive breast cancer incidence. A priori, we also aimed to examine whether this association differed by menopausal status, tumor estrogen receptor status, race, smoking, night-shift work, census-tract SES, air pollution exposure, region, or urban/nonurban residence status.
Methods
Population
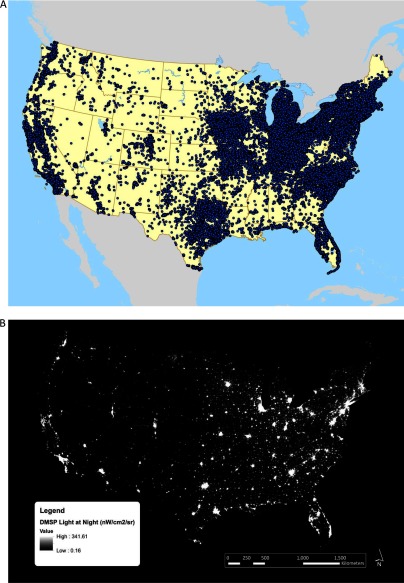
NHSII is an ongoing prospective cohort study of 116,430 women who were registered nurses, 25–42 y of age, and living in 14 U.S. states when enrolled in 1989. Participants complete biennial questionnaires on lifestyle factors, health behaviors, medical history, and incident disease. Response rates at each questionnaire cycle have consistently been . All mailing addresses over follow-up were geocoded to the street or ZIP code centroid level to obtain latitude and longitude (Figure 1A). NHSII participants have changed residences since baseline, and currently there are nurses in each of the 48 U.S. continental states. We excluded women who did not have a geocoded address at the street segment level ( at baseline), who had missing LAN exposure information ( at baseline), who had missing information on menopausal status ( at baseline), or who had a prior history of breast cancer at baseline ( at baseline).
Figure 1.
Locations of Nurses’ Health Study II (NHSII) addresses from 1989–2011 (A) and 2010 U.S. Defense Meteorological Satellite Program’s (DMSP’s) Operational Linescan System (OLS) light at night data in nanowatts per centimeter squared per steradian (B).
Informed consent was implied by return of the baseline questionnaire. The institutional review boards of Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health approved the original NHSII as well as this analysis.
Outcome
We identified incident invasive breast cancer cases by self-report on biennial questionnaires from 1989 through 2013. A study physician performed medical record review to confirm cases (ICD-8 code 174.0) (WHO 1965) and to abstract information on invasiveness. Over 82% of cases were confirmed after medical record review; the remaining cases were confirmed by state cancer registries, death records, contact with participants, or contact with next of kin. Tissue block collection and tissue microarray (TMA) construction were described in detail previously (Sisti et al. 2016; Tamimi et al. 2008). Immunohistochemical (IHC) analysis was performed according to a standard protocol (Sisti et al. 2016; Tamimi et al. 2008). Cases were considered estrogen receptor (ER) positive if any tissue core showed any nuclear staining for ER. Cases with complete absence of staining for ER were considered ER negative. For cases missing ER status by IHC (77% of all cases), ER status was based on the pathology report or on the medical record. We could not determine ER status for of cases, primarily in the later years, because study staff are still working to collect data. For the years 2011–2013, we are actively collecting tissue, and the majority of data on tumor ER status for these years are based on pathology reports. We have previously demonstrated a very high concordance between pathology reports and TMA (Collins et al. 2008). Additionally, we have shown in previous analyses that cases missing tumor marker information were not significantly different from cases with tumor marker information in terms of characteristics and accepted breast cancer risk factors (Wang et al. 2015).
Exposure
Data on annual outdoor LAN were derived from satellite imagery data from the U.S. Defense Meteorological Satellite Program’s (DMSP’s) Operational Linescan System, maintained by the National Oceanic and Atmospheric Administration’s (NOAA’s) Earth Observation Group (NOAA National Geophysical Data Center 2015). This database contains annual composites made after excluding the outer quarters of the satellite swath, sun and moon luminance, glare, clouds, atmospheric lightning, and ephemeral events such as fires. Although these images capture only a fraction of the light originating from the Earth’s surface, they represent the relative levels of nighttime illumination at ground level (Hsu et al. 2015). The processed imagery data are georectified to a 30 arc-second grid equivalent to . Previous studies have shown that the low-dynamic range 6-bit DMSP data do not vary within urban areas, where levels of LAN are high (nearly every residence in an urban or suburban area was assigned the maximum value of 63) (Hurley et al. 2013). Therefore, we used the DMSP Global Radiance Calibrated Nighttime Lights high-dynamic range data, which can be transformed into units of radiance () (Figure 1B) (Hsu et al. 2015). High-dynamic range data were available for 1996, 1999, 2000, 2002, 2004, 2005, and 2010. To ensure comparability across years and satellites, we used interannual calibration coefficients provided by NOAA to derive exposure estimates (NOAA 2017). We assigned a nighttime radiance value for each geocoded address in each questionnaire year from 1989–2011 using ArcGIS (ESRI, Redlands, CA). If a participant changed addresses during follow-up, their estimated exposure was updated at the date of the new questionnaire in which they indicated their new address. For addresses before 1997, exposure was assigned based on the 1996 LAN data. For addresses after 1997, exposure was assigned based on the most recent past LAN measure. We calculated cumulative average outdoor LAN for each participant at each questionnaire response, which accounted for changes in LAN over time as well as for participant changes of address.
Statistical Analysis
Person-years of follow-up were accrued from the return date of the 1989 questionnaire until the participant became a case or died, or until the end of follow-up (31 May 2013). Person-time was skipped if a participant missed a questionnaire or temporarily moved outside of the contiguous United States, but these participants could contribute person-time to the analysis if they filled out a subsequent questionnaire or moved back to the contiguous United States. We used the date of the last questionnaire completed as the end of follow-up. Deaths were usually reported by families, and deaths among nonrespondents were identified by searching the National Death Index, which has been validated in prior studies in this cohort (Rich-Edwards et al. 1994). We fit time-varying Cox proportional hazards regression models to calculate the hazard ratio (HR) for developing breast cancer (overall and by ER status) associated with cumulative average outdoor LAN, using both continuous LAN based on an interquartile range (IQR) increase () as well as quintiles of LAN with a test for trend based on the median value for each quintile. We used likelihood ratio tests to compare the fit of models that included cubic splines with models having linear terms only to test for statistically significant departures from linearity. To test for violations of the proportional hazards assumption, we included interaction terms of LAN exposure and calendar time and performed likelihood ratio tests.
Analyses were stratified by age at follow-up and by calendar year, and we adjusted for all of the following covariates a priori as potential confounders based on questionnaire data because they are potential breast cancer risk factors and may be correlated with LAN. Time-invariant factors included race (white/nonwhite), benign breast disease history at baseline (yes/no), family history of breast cancer at baseline (yes/no), age at menarche (years), height at baseline (inches), BMI at age 18 (kilograms per meter squared), and personal income assessed in 2001 (thousands of U.S. dollars per year; ). Time-varying factors included parity and age (years) at first birth [nulliparous/1–2 children (before 25/25–29/)/3–4 children (before 25/25–29/)/5–8 children (before 25/ ], BMI (kilograms per meter squared; based on self-reported weight at each questionnaire and height at baseline), menopausal status (yes/no), oral contraceptive use (yes/no), mammography screening (yes/no), smoking status (current/past/never), marital status (married/nonmarried), living alone (yes/no), night-shift work (never/ever performing shift work after 1989), alternative healthy eating index (AHEI) (continuous) (Chiuve et al. 2012), and physical activity [total metabolic equivalent of task hours per week (MET hrs/wk)]. Family history of breast cancer was defined as a mother, sister, or grandmother with any type of breast cancer. Menopausal status was assessed every two years based on self-report and was used as time-varying in analyses. Physical activity was evaluated every 2–6 y based on a validated measure of self-reported total physical activity in the past year (Wolf et al. 1994). Values were carried forward for years between physical activity questionnaires. The AHEI, an overall diet quality measure based on alcohol consumption, foods, and nutrients predictive of chronic disease risk, was calculated from food frequency questionnaires asking about typical consumption in the past year (Willett et al. 1985) that were completed every 4 y. Values were carried forward for years between food frequency questionnaires. We accounted for area-level SES by including census-tract median home value and income, and we also adjusted for census-tract population density and for region of United States based on geocoded address. Census data came from the 2000 U.S. Census (U.S. Census Bureau 2000). To reduce potential confounding by air pollution exposures that have been associated with breast cancer in previous studies (Parikh and Wei 2016; Wong et al. 2016), we adjusted for modeled 24-mo average particulate matter () exposure, which was predicted at each geocoded address using a generalized additive mixed model (Yanosky et al. 2014). The model extended from 1989–2006; values from 2007–2013 were carried forward. We used indicator variables in models to account for missing values in covariates, which has been shown to be a valid approach for dealing with missing data without losing power (Song 2016).
To assess whether the association between LAN and breast cancer differed across subpopulations, we examined effect modification by current menopausal status, BMI (BMI vs. BMI ), race (white vs. nonwhite), smoking (current vs. past vs. never), (quintiles), census-tract population density (quintiles), census-tract median income (quintiles), census-tract home value (quintiles), census region (northeast, midwest, west, and south), census-tract urbanicity (urban vs. nonurban), and night-shift work (no night-shift work since 1989 vs. any night-shift work since 1989). Urbanicity was determined by the participant’s residence in an urban ( people) or nonurban [urban cluster (10,000–49,999 people) or small town/rural ( people)] census tract. To evaluate whether the association between continuous LAN and breast cancer risk varied across these factors, we fit separate Cox proportional hazards models within strata of these factors and estimated stratum-specific HRs. For all time-varying factors, analyses were stratified by person-time. To test for significance of statistical interaction between LAN and each factor, we used a likelihood ratio test comparing models with cross-product terms and main-effects-only models. Observations with missing values for each factor were excluded from effect modification analyses. We examined whether there was heterogeneity in the association between LAN and breast cancer risk by tumor ER status (negative vs. positive) using the contrast test method (Wang et al. 2016). We performed sensitivity analyses restricting observations to 1997–2013 and estimating cumulative average LAN exposure starting in 1997, when concurrent LAN satellite data were available. An alpha level of 0.05 was used to define statistical significance, and all analyses were performed in SAS v.9.3 (SAS Institute Inc.).
Results
We observed 3,549 invasive breast cancer cases over 2,187,425 person-years of follow-up among the 109,672 eligible cohort members from 1989–2013. Over the person-time contributing to this analysis, participants were predominantly white and were more likely to be premenopausal and married (Table 1). The majority of person-time came from participants who lived in metropolitan areas, and two-thirds of the person-time came from participants who lived in the northeastern or midwestern United States. As expected in a population of nurses, 42% of the person-years contributed came from women who had performed night-shift work. Higher cumulative average LAN was associated with higher average values over follow-up for census-tract population density and median home value as well as with nulliparity, nonwhite race, and being nonmarried.
Table 1.
Age-adjusted participant characteristics overall and by quintile of cumulative average LAN () over 2,187,425 person-years of follow-up 1989–2013 ( or %)a.
| Characteristic | Overall | LAN quintile 1 () | LAN quintile 2 () | LAN quintile 3 () | LAN quintile 4 () | LAN quintile 5 () |
|---|---|---|---|---|---|---|
| Cumulative average light at night () | ||||||
| Age (years)b | ||||||
| Racec | ||||||
| White (%) | 95 | 98 | 98 | 97 | 95 | 90 |
| Nonwhite (%) | 5 | 2 | 2 | 3 | 5 | 10 |
| Menopausal status | ||||||
| Premenopausal (%) | 74 | 73 | 74 | 74 | 75 | 75 |
| Postmenopausal (%) | 26 | 27 | 26 | 26 | 25 | 25 |
| BMI () | ||||||
| Physical activity (MET hrs/wk) | ||||||
| Missing physical Activity (%) | 7 | 7 | 7 | 7 | 7 | 9 |
| Smoking status | ||||||
| Never smoker (%) | 65 | 67 | 65 | 65 | 65 | 62 |
| Past smoker (%) | 25 | 23 | 25 | 25 | 25 | 27 |
| Current smoker (%) | 9 | 9 | 9 | 9 | 9 | 9 |
| History of benign breast diseasec | ||||||
| Yes (%) | 44 | 45 | 45 | 45 | 44 | 43 |
| No (%) | 56 | 55 | 55 | 55 | 56 | 57 |
| Family history of breast cancerc | ||||||
| Yes (%) | 11 | 10 | 10 | 11 | 11 | 11 |
| No (%) | 89 | 90 | 90 | 89 | 89 | 89 |
| Age at menarchec | ||||||
| years old (%) | 24 | 25 | 24 | 24 | 24 | 25 |
| 12 years old (%) | 30 | 30 | 31 | 30 | 30 | 29 |
| 13 years old (%) | 27 | 28 | 28 | 27 | 28 | 27 |
| years old (%) | 18 | 17 | 18 | 18 | 18 | 18 |
| Parity and age at first birth | ||||||
| Nulliparous (%) | 17 | 12 | 13 | 16 | 19 | 24 |
| 1–2 children, old (%) | 14 | 18 | 16 | 13 | 12 | 10 |
| 1–2 children, 25-29 years old (%) | 19 | 21 | 22 | 20 | 19 | 15 |
| 1–2 children, old (%) | 13 | 10 | 12 | 14 | 14 | 15 |
| 3–4 children, old (%) | 10 | 14 | 12 | 10 | 9 | 7 |
| 3–4 children, 25-29 years old (%) | 10 | 11 | 10 | 10 | 9 | 8 |
| 3–4 children, old (%) | 2 | 2 | 2 | 3 | 3 | 3 |
| 5–9 children, old (%) | 1 | 1 | 1 | 1 | 1 | 1 |
| 5–9 children, old (%) | 1 | 1 | 1 | 1 | 1 | 1 |
| Missing parity and age at first birth | 13 | 10 | 12 | 13 | 14 | 17 |
| Height at baseline (cm)c | ||||||
| 127–155 (%) | 9 | 8 | 8 | 8 | 9 | 10 |
| 156–163 (%) | 37 | 38 | 38 | 37 | 37 | 37 |
| 164–170 (%) | 38 | 39 | 39 | 38 | 38 | 37 |
| (%) | 16 | 16 | 15 | 16 | 16 | 16 |
| Oral contraceptive use | ||||||
| Never (%) | 12 | 10 | 11 | 11 | 12 | 13 |
| Past (%) | 73 | 77 | 76 | 74 | 72 | 68 |
| Current (%) | 7 | 6 | 6 | 7 | 7 | 7 |
| Missing oral contraceptive use (%) | 9 | 7 | 7 | 8 | 9 | 12 |
| Mammography screening | ||||||
| No mammogram (%) | 21 | 22 | 21 | 21 | 21 | 21 |
| Screening mammogram (%) | 53 | 53 | 54 | 53 | 53 | 51 |
| Missing mammography (%) | 26 | 25 | 25 | 26 | 26 | 28 |
| BMI at Age 18c | ||||||
| (%) | 22 | 20 | 21 | 22 | 22 | 22 |
| 19–20.4 (%) | 26 | 26 | 26 | 26 | 26 | 25 |
| 20.5–21.9 (%) | 23 | 24 | 23 | 23 | 23 | 22 |
| 22–24.9 (%) | 19 | 19 | 18 | 18 | 18 | 19 |
| 25–29.9 (%) | 8 | 8 | 7 | 7 | 8 | 8 |
| (% | 2 | 2 | 2 | 2 | 2 | 3 |
| Missing BMI at age 18 (%) | 1 | 1 | 1 | 1 | 1 | 1 |
| Alternative healthy eating indexd | ||||||
| Missing alternative healthy eating indexd (%) | ||||||
| Living alone (%) | ||||||
| Yes | 7 | 4 | 5 | 7 | 8 | 11 |
| No | 93 | 96 | 95 | 93 | 92 | 89 |
| Personal income (USD)c | ||||||
| (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| (%) | 1 | 1 | 1 | 1 | 1 | 1 |
| (%) | 2 | 3 | 3 | 2 | 2 | 2 |
| (%) | 6 | 7 | 6 | 5 | 5 | 5 |
| (%) | 17 | 19 | 17 | 16 | 16 | 17 |
| (%) | 13 | 14 | 14 | 13 | 13 | 13 |
| (%) | 14 | 12 | 14 | 15 | 15 | 14 |
| (%) | 8 | 4 | 8 | 9 | 9 | 9 |
| Missing income (%) | 38 | 38 | 38 | 38 | 38 | 39 |
| Marital status | ||||||
| Married (%) | 56 | 62 | 59 | 57 | 55 | 49 |
| Not married (%) | 44 | 38 | 41 | 43 | 45 | 51 |
| Night shift work | ||||||
| Shift work since 1989 (%) | 42 | 43 | 42 | 42 | 41 | 42 |
| No shift work since 1989 (%) | 58 | 57 | 58 | 58 | 59 | 58 |
| () | ||||||
| Census tract population density (per square kilometer) | ||||||
| Census tract median home value () | ||||||
| Census tract median income () | ||||||
| Census region | ||||||
| Northeast (%) | 33 | 32 | 39 | 32 | 28 | 33 |
| Midwest (%) | 33 | 40 | 31 | 31 | 34 | 29 |
| West (%) | 16 | 7 | 10 | 17 | 20 | 22 |
| South (%) | 19 | 21 | 20 | 21 | 18 | 16 |
| Census tract urbanicity | ||||||
| Urban (%) | 87 | 62 | 79 | 92 | 97 | 99 |
| Nonurban (%) | 13 | 39 | 20 | 9 | 3 | 1 |
Note: BMI, body mass index; LAN, light at night; MET, metabolic equivalent of task; PM2.5, particulate matter with aerodynamic diameter ; SD, standard deviation.
Means and percentages are based on the person-time over follow-up. All time-varying factors are updated at each questionnaire cycle.
Estimate not age-adjusted.
Not time-varying.
Based on reported consumption of foods and nutrients that have been associated consistently with lower risk of chronic disease (Chiuve et al. 2012).
Estimated associations between cumulative average outdoor LAN and breast cancer are shown in Table 2. There was an estimated 14% increased risk of breast cancer in the top quintile of outdoor LAN compared with the lowest quintile of LAN (95% CI: 1.01, 1.29) in the fully adjusted model. The association was strongest for the highest quintile but was not monotonic with increasing LAN. Cubic spline models did not significantly improve model fit relative to linear models (data not shown). Continuous analyses also showed a positive association between outdoor LAN and breast cancer, with a 5% higher rate of breast cancer (95% CI: 1.00, 1.11) in fully adjusted models for each IQR () increase in cumulative average outdoor LAN in the area around participants’ homes. The results were similar when analyses were restricted to 1997–2013, when concurrent outdoor LAN data were available [ (95% CI: 1.00, 1.13) based on 105,304 women and 2,954 breast cancer cases] (Table 3).
Table 2.
Associations between cumulative average outdoor LAN and breast cancer in the Nurses’ Health Study II () with 3,549 breast cancer cases over 2,187,425 person-years of follow-up (1989–2013).
| Exposure | Cumulative average light at night | |||
|---|---|---|---|---|
| Cases (n) | Person-years | Age-adjusted HR (95% CI) | Fully adjusted HR (95% CI)a | |
| LAN quintile 1 (Median ) | 571 | 360,609 | Reference | Reference |
| LAN quintile 2 (Median ) | 715 | 432,584 | 1.08 (0.97, 1.21) | 1.05 (0.94, 1.18) |
| LAN quintile 3 (Median ) | 710 | 459,789 | 1.05 (0.94, 1.17) | 1.01 (0.90, 1.13) |
| LAN quintile 4 (Median ) | 776 | 469,624 | 1.12 (1.01, 1.25) | 1.08 (0.97, 1.22) |
| LAN quintile 5 (Median ) | 777 | 464,820 | 1.13 (1.02, 1.26) | 1.14 (1.01, 1.29) |
| p for trendb | 0.03 | 0.02 | ||
| Continuous LAN (per IQR increasec) | 1.03 (0.99, 1.07) | 1.05 (1.00, 1.11) | ||
Note: BMI, body mass index; CI, confidence interval; HR, hazard ratio; IQR, interquartile range; LAN, light at night; PM2.5, particulate matter with aerodynamic diameter .
HRs are adjusted for benign breast disease history, family history of breast cancer, age at menarche, parity and age at first birth, height, white race, BMI, BMI at age 18, oral contraceptive use, mammography screening, menopausal status, smoking status, alternative healthy eating index, physical activity, marital status, living alone, personal income, shift work after 1989, region, , census-tract median home value, income, and population density.
Test for trend is based on the median value for each quintile.
An IQR increase in cumulative average LAN is .
Table 3.
Associations of cumulative average outdoor LAN and breast cancer risk in the Nurses’ Health Study II () with 2,954 breast cancer cases over 1,497,270 person-years of follow-up 1997–2013.
| Exposure | Cumulative average light at night | |||
|---|---|---|---|---|
| Cases (n) | Person-years | Age-adjusted HR (95% CI) | Fully adjusted HR (95% CI)a | |
| LAN quintile 1 (Median ) | 486 | 263,512 | Reference | Reference |
| LAN quintile 2 (Median ) | 611 | 298,740 | 1.09 (0.97, 1.24) | 1.07 (0.94, 1.21) |
| LAN quintile 3 (Median ) | 583 | 309,527 | 1.07 (0.95, 1.21) | 1.04 (0.92, 1.18) |
| LAN quintile 4 (Median ) | 625 | 314,141 | 1.19 (1.06, 1.34) | 1.17 (1.03, 1.33) |
| LAN quintile 5 (Median ) | 649 | 311,351 | 1.09 (0.97, 1.23) | 1.12 (0.98, 1.29) |
| p for trendb | 0.06 | 0.07 | ||
| Continuous LAN (per IQR increasec) | 1.02 (0.98, 1.07) | 1.06 (1.00, 1.13) | ||
Note: BMI, body mass index; CI, confidence interval; HR, hazard ratio; IQR, interquartile range; LAN, light at night; PM2.5, particulate matter with aerodynamic diameter .
Hazard ratios are adjusted for benign breast disease history, family history of breast cancer, age at menarche, parity and age at first birth, height, white race, BMI, BMI at age 18, oral contraceptive use, mammography screening, menopausal status, smoking status, alternative healthy eating index, physical activity, marital status, living alone, personal income, shift work after 1989, region, , census-tract median home value, income, and population density.
Test for trend is based on the median value for each quintile.
An IQR increase in cumulative average LAN is .
Analyses stratified by current menopausal status at the time of breast cancer diagnosis suggested that the positive association between LAN and breast cancer was specific to premenopausal women [HR per IQR increase in LAN for premenopausal women=1.07 (95% CI: 1.01, 1.14) based on 1,973 cases; HR for postmenopausal women=1.00 (95% CI: 0.91, 1.09) based on 1,172 cases; p for interaction=0.08] (Table 4; see also Figure S1). We did not detect statistically significant heterogeneity in the HRs for outdoor LAN when comparing ER-positive versus ER-negative tumors (see Table S1; p for heterogeneity=0.33), although we did observe a positive association for ER-positive tumor types [HR per IQR increase in LAN=1.06 (95% CI: 0.99, 1.13) based on 2,137 cases], which comprised the majority of cases, and no association for ER-negative tumors [HR=0.98 (95% CI: 0.85, 1.13) based on 512 cases]. Associations were stronger in those who had worked night shifts [HR per IQR increase in LAN=1.09 (95% CI: 1.01, 1.18) based on 1,196 cases] compared with those who had never worked night shifts since 1989 [HR=1.03 (95% CI: 0.97, 1.09) based on 2,353 cases] (Table 5; see also Figure S1). The association between LAN and breast cancer was observed only in past and current smokers [HR for current smokers=1.21 (95% CI: 1.07, 1.37) based on 300 cases; HR for past smokers=1.10 (95% CI: 1.01, 1.19) based on 1,034 cases; HR for never smokers=1.00 (95% CI: 0.94, 1.07) based on 2,215 cases; p for interaction=0.008] (Table 6; see also Figure S1). There were no statistically significant differences in the association between LAN and breast cancer by BMI, race, , census-tract median income, census-tract median home value, census region, census-tract population density, or census-tract urban/nonurban status (see Figure S1).
Table 4.
Cumulative average LAN and breast cancer risk stratified by menopausal status at the time of an event in the Nurses’ Health Study II ()a.
| Exposure | Premenopausal | Postmenopausal | ||
|---|---|---|---|---|
| Cases (n) | Fully adjusted HR (95% CI)b | Cases (n) | Fully adjusted HR (95% CI)b | |
| LAN quintile 1 (Median ) | 282 | Reference | 223 | Reference |
| LAN quintile 2 (Median ) | 367 | 1.02 (0.87, 1.19) | 242 | 0.96 (0.80, 1.16) |
| LAN quintile 3 (Median ) | 415 | 1.08 (0.92, 1.26) | 229 | 0.92 (0.77, 1.11) |
| LAN quintile 4 (Median ) | 447 | 1.12 (0.96, 1.31) | 248 | 0.99 (0.82, 1.19) |
| LAN quintile 5 (Median ) | 462 | 1.20 (1.02, 1.41) | 230 | 0.95 (0.78, 1.15) |
| Continuous LAN (per IQR increasec) | 1.07 (1.01, 1.14) | 1.00 (0.91, 1.09) | ||
| p for interaction | 0.08 | |||
Note: BMI, body mass index; CI, confidence interval; HR, hazard ratio; IQR, interquartile range; LAN, light at night; PM2.5, particulate matter with aerodynamic diameter .
See Figure S1 for a graphical representation of this table.
There were 404 cases who were missing menopausal status at the time of an event. These observations are excluded from this analysis.
Hazard ratios are adjusted for benign breast disease history, family history of breast cancer, age at menarche, parity and age at first birth, height, white race, BMI, BMI at age 18, oral contraceptive use, mammography screening, smoking status, alternative healthy eating index, physical activity, marital status, living alone, personal income, shift work after 1989, region, , census-tract median home value, income, and population density.
An IQR increase in cumulative average LAN is .
Table 5.
Cumulative average LAN and breast cancer risk stratified by night shift work since 1989 in the Nurses’ Health Study II ().
| Exposure | No shift work since 1989 | Any shift work since 1989 | ||
|---|---|---|---|---|
| Cases (n) | Fully adjusted HR (95% CI)a | Cases (n) | Fully adjusted HR (95% CI)a | |
| LAN quintile 1 (Median ) | 386 | Reference | 185 | Reference |
| LAN quintile 2 (Median ) | 469 | 0.98 (0.86, 1.13) | 246 | 1.18 (0.98, 1.43) |
| LAN quintile 3 (Median ) | 472 | 0.96 (0.84, 1.10) | 238 | 1.09 (0.90, 1.32) |
| LAN quintile 4 (Median ) | 515 | 1.01 (0.88, 1.16) | 261 | 1.19 (0.98, 1.44) |
| LAN quintile 5 (Median ) | 511 | 1.04 (0.90, 1.20) | 266 | 1.29 (1.06, 1.56) |
| Continuous LAN (per IQR increaseb) | 1.03 (0.97, 1.09) | 1.09 (1.01, 1.18) | ||
| p for interaction | 0.10 | |||
Note: BMI, body mass index; CI, confidence interval; HR, hazard ratio; IQR, interquartile range; LAN, light at night; PM2.5, particulate matter with aerodynamic diameter .
See Figure S1 for a graphical representation of this table.
Hazard ratios are adjusted for benign breast disease history, family history of breast cancer, age at menarche, parity and age at first birth, height, white race, BMI, BMI at age 18, oral contraceptive use, mammography screening, menopausal status, smoking status, alternative healthy eating index, physical activity, marital status, living alone, personal income, region, , census-tract median home value, income, and population density.
An IQR increase in cumulative average LAN is .
Table 6.
Cumulative average LAN and breast cancer risk stratified by smoking status in the Nurses’ Health Study II ().
| Exposure | Never | Past | Current | |||
|---|---|---|---|---|---|---|
| Cases (n) | Fully adjusted HR (95% CI)a | Cases (n) | Fully adjusted HR (95% CI)a | Cases (n) | Fully adjusted HR (95% CI)a | |
| LAN quintile 1 (Median ) | 382 | Reference | 146 | Reference | 43 | Reference |
| LAN quintile 2 (Median ) | 460 | 1.04 (0.91, 1.20) | 189 | 1.01 (0.81, 1.25) | 66 | 1.27 (0.87, 1.87) |
| LAN quintile 3 (Median ) | 465 | 1.02 (0.89, 1.18) | 192 | 0.97 (0.78, 1.21) | 53 | 0.97 (0.65, 1.46) |
| LAN quintile 4 (Median ) | 473 | 1.02 (0.88, 1.17) | 250 | 1.23 (1.00, 1.51) | 53 | 0.98 (0.65, 1.46) |
| LAN quintile 5 (Median ) | 435 | 1.02 (0.88, 1.18) | 257 | 1.23 (0.99, 1.52) | 85 | 1.54 (1.06, 2.23) |
| Continuous LAN (per IQR increaseb) | 1.00 (0.94, 1.07) | 1.10 (1.01, 1.19) | 1.21 (1.07, 1.37) | |||
| p for interaction | 0.008 | |||||
Note: BMI, body mass index; CI, confidence interval; HR, hazard ratio; IQR, interquartile range; LAN, light at night; PM2.5, particulate matter with aerodynamic diameter .
See Figure S1 for a graphical representation of this table.
Hazard ratios are adjusted for benign breast disease history, family history of breast cancer, age at menarche, parity and age at first birth, height, white race, BMI, BMI at age 18, oral contraceptive use, mammography screening, menopausal status, alternative healthy eating index, physical activity, marital status, living alone, personal income, shift work after 1989, region, , census-tract median home value, income, and population density.
An IQR increase in cumulative average LAN is .
Discussion
In this nationwide prospective analysis of female nurses, we observed a positive association between cumulative average exposure to residential outdoor LAN and breast cancer risk, which was robust to adjustment for many important breast cancer risk factors. This association was generally consistent across categories of BMI, race, , and census-tract level median income, median home value, population density, and urban/nonurban status. The association between LAN and breast cancer was observed only among current and past smokers and premenopausal women. The association between LAN and breast cancer risk was more pronounced among women who had worked night shifts and was linked most strongly to ER-positive tumor types, although differences were not statistically significant across these factors.
Studies have observed an association between exposure to LAN and cancer risk in animal models (Blask et al. 2014; Stevens et al. 2007); however, few epidemiologic studies have examined the association between outdoor LAN and breast cancer. Several ecological studies reported that satellite-based LAN was associated with breast cancer incidence at the country or community level (Keshet-Sitton et al. 2016a; Kim et al. 2015; Kloog et al. 2008, 2010; Portnov et al. 2016; Rybnikova et al. 2015). For example, one study reported a 73% higher incidence of breast cancer in communities with the highest LAN than in those with the lowest LAN across 147 communities in Israel (Kloog et al. 2008). Although these studies were consistent with our findings, they were limited by their inability to link individual exposure and outcome as well as by the lack of information on individual-level confounding. A case–control study using breast cancer cases and lung cancer controls in the U.S. state of Georgia suggested a link between higher levels of outdoor LAN exposure, assessed at the geocoded address, and breast cancer risk. The authors found that high LAN exposure () was associated with increased odds of breast cancer compared with low LAN exposure () [ (95% CI: 1.04, 1.20)] (Bauer et al. 2013). In a small case–control study in Israel, participants who reported living near “strong artificial light at night sources” had higher odds of being a breast cancer case [ (95% CI: 1.10, 2.12)] (Keshet-Sitton et al. 2016b). To the best of our knowledge, there has only been one prior prospective cohort study examining outdoor LAN and breast cancer risk. Hurley et al. (2014) examined California Teacher’s Study (CTS) data on 106,731 participants and found that women living in areas with the highest quintile of LAN (range: ) compared with the lowest quintile (range: ) had the highest risk of invasive breast cancer [HR=1.12 (95% CI: 1.00, 1.26)]. The authors assessed LAN exposure for baseline addresses (1995–96) using high-dynamic range LAN data for 2006. Consistent with our findings, the association appeared to be specific to premenopausal women [HR for the highest vs. lowest LAN quintile=1.34 (95% CI: 1.07, 1.69) vs. 1.04 (95% CI: 0.90, 1.20), respectively, for postmenopausal women], although the difference was not significant (). Because only one year of high-dynamic range data was available at the time of analysis, the CTS study did not include time-varying information on exposure. The present analysis extends that research by including time-varying exposure information at a broader range of outdoor LAN levels (ranging from ) and by expanding the geographic scope to the entire contiguous United States. Our results corroborate the findings of the CTS and provide further evidence that outdoor LAN may be associated with increased breast cancer risk.
Our analysis of effect modification of the LAN–breast cancer relationship by smoking status revealed that associations were only evident in current and past smokers. Smoking has been associated with breast cancer in some studies (Cui et al. 2006; Gaudet et al. 2013; Gaudet et al. 2016; Gram et al. 2015; Luo et al. 2011; Reynolds et al. 2004a), although other studies have not reported associations (Egan et al. 2002; Palmer and Rosenberg 1993). A prior study of 459 NHSII participants reported that creatinine-corrected melatonin concentrations in spot urine samples were significantly lower in women with pack-years of smoking than in never smokers (Schernhammer et al. 2006a), and lower melatonin has been associated with increased breast cancer risk (Schernhammer and Hankinson 2009). Our finding of increased relative risks among smokers exposed to higher LAN suggests that LAN and smoking may share similar melatonin-mediated pathways to breast cancer risk.
This study is one of the first analyses of LAN and breast cancer that incorporates information on night-shift work. In stratified analyses, the association between LAN and breast cancer was stronger among participants who worked night shifts at any time since 1989 than among those who did not, although the difference was not statistically significant (interaction ). This finding suggests that both exposure to LAN and night-shift work contribute jointly to increase breast cancer risk, possibly through mechanisms involving circadian disruption.
Although our results should be interpreted with caution given the small numbers of ER-negative cases, this analysis suggests that the association with LAN was limited to ER-positive tumors. This finding is consistent with the hypothesis that LAN acts through estrogen receptor signaling–mediated pathways to increase breast cancer risk. Hurley et al. (2014) also examined associations with LAN according to ER (and progesterone receptor) status, but they noted that numbers of cases within subgroups were small, and they did not report quantitative estimates from this analysis.
Our study has a few limitations. First, exposure misclassification could occur because of missing satellite data and data processing errors. In addition, satellite-based measures of outdoor LAN are a proxy for total personal exposure to LAN, and a study has demonstrated that outdoor LAN measures may not capture true individual exposure to LAN, including indoor sources, that is thought to drive breast cancer risk (Rea et al. 2011). Although total personal exposure to LAN may be important to discern the etiologic association between LAN and breast cancer, the association between outdoor LAN and breast cancer risk may be relevant in a policy context because city- or county-scale policies that limit outdoor nighttime lighting may affect ambient LAN levels. Alternatively, outdoor LAN could be a proxy for an unmeasured breast cancer risk factor, where policies to limit outdoor LAN would have little effect. In addition, in this analysis, LAN data were available starting in 1996, so there was a temporal mismatch between LAN measures and our breast cancer data for earlier observations. However, analyses restricted to years after 1996 showed similar results. Although we successfully geocoded 85% of addresses to the street level, residences that could not be geocoded may differ in urban/nonurban characteristics, and thus, they may differ in LAN exposure. Given the relatively low proportion of missing exposure data, any selection bias is expected to be minimal. In addition, participants were 25–42 y old at baseline in 1989, so this analysis is missing data on exposure before this period, which might be an etiologically important time window. With any study of neighborhood factors and health, there is a possibility that participants may self-select into certain neighborhoods they deem “healthier” than others. However, adjustment for established breast cancer risk factors reduces the likelihood that neighborhood self-selection explains the associations that we observed. Because of participants classified themselves as white, we were underpowered to detect differences in the association between outdoor LAN and breast cancer by race/ethnicity, and our findings may not be generalizable to women who are not white. Because all participants were nurses at enrollment, the generalizability of our findings to lower SES, nonworking groups is also potentially limited. Finally, although we adjusted for air pollution and population density, we cannot rule out the possibility that other factors that are correlated with outdoor LAN [e.g., economic activity (Rybnikova and Portnov 2015)] might explain the observed association between LAN and breast cancer risk.
This analysis has a number of strengths. First, this study was conducted over more than two decades with time-varying information about outdoor LAN recorded at the residence level. Second, this study applied high-dynamic range, objective satellite data to capture potentially important intraurban differences in outdoor LAN and included information on higher levels of LAN exposure. Third, we have time-varying information on a number of factors, including menopausal status, family history of breast cancer, parity, oral contraceptive use, smoking status, diet, physical activity, air pollution, and area-level SES. To our knowledge, no prior LAN analyses have examined effect modification by smoking status, and our findings suggest that the association between LAN and breast cancer risk existed only in current or past smokers. This is also the first LAN study of which the authors are aware to incorporate information about night-shift work into the analysis, with of the study population having worked night shifts since 1989. Thus, we were able to adjust for potential confounding by many established and suspected breast cancer risk factors. Finally, this nationwide study covers a broad geographic range of outdoor LAN levels, including participants in both urban and nonurban areas.
Conclusions
This prospective study, conducted over 22 y of follow-up with time-varying and objective measures of ambient LAN across the contiguous United States, provides evidence that women living in areas with high levels of outdoor LAN may be at higher risk of breast cancer even after accounting for individual and area-level risk factors for breast cancer. Athough further work is required to confirm our results and to clarify potential mechanisms, our findings suggest that exposure to outdoor light at night may contribute to breast cancer risk.
Supplemental Material
Acknowledgments
We would like to thank the participants and staff of the Nurses’ Health Study II for their valuable contributions as well as the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, MarylandD, Massachussetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, Wyoming. The authors assume full responsibility for analyses and interpretation of these data. This work was supported by a Harvard National Heart, Lung, and Blood Institute (NHLBI) Cardiovascular Epidemiology training grant (T32 HL 098048), a National Institutes of Health (NIH) National Cancer Institute grant (K99 CA201542), by NIH grant UM1 CA176726, and by a grant from the Susan G. Komen for the Cure® organization (IIR13264020).
References
- Bauer SE, Wagner SE, Burch J, Bayakly R, Vena JE. 2013. A case-referent study: Light at night and breast cancer risk in Georgia. Int J Health Geogr 12:23, PMID: 23594790, 10.1186/1476-072X-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blask DE. 2009. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev 13(4):257–264, PMID: 19095474, 10.1016/j.smrv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Blask DE, Hill SM, Dauchy RT, Xiang S, Yuan L, Duplessis T, et al. 2011. Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. J Pineal Res 51(3):259–269, PMID: 21605163, 10.1111/j.1600-079X.2011.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blask DE, Dauchy RT, Dauchy EM, Mao L, Hill SM, Greene MW, et al. 2014. Light exposure at night disrupts host/cancer circadian regulatory dynamics: Impact on the Warburg effect, lipid signaling and tumor growth prevention. PLoS ONE 9(8):e102776, PMID: 25099274, 10.1371/journal.pone.0102776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. 2012. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 142(6):1009–1018, PMID: 22513989, 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LC, Marotti JD, Baer HJ, Tamimi RM. 2008. Comparison of estrogen receptor results from pathology reports with results from central laboratory testing. J Natl Cancer Inst 100(3):218–221, PMID: 18230800, 10.1093/jnci/djm270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Miller AB, Rohan TE. 2006. Cigarette smoking and breast cancer risk: Update of a prospective cohort study. Breast Cancer Res Treat 100(3):293–299, PMID: 16773435, 10.1007/s10549-006-9255-3. [DOI] [PubMed] [Google Scholar]
- Egan KM, Stampfer MJ, Hunter D, Hankinson S, Rosner BA, Holmes M, et al. 2002. Active and passive smoking in breast cancer: Prospective results from the nurses' health study. Epidemiology 13(2):138–145, PMID: 11880753. [DOI] [PubMed] [Google Scholar]
- Gaudet MM, Gapstur SM, Sun J, Diver WR, Hannan LM, Thun MJ. 2013. Active smoking and breast cancer risk: Original cohort data and meta-analysis. J Natl Cancer Inst 105(8):515–525, PMID: 23449445, 10.1093/jnci/djt023. [DOI] [PubMed] [Google Scholar]
- Gaudet MM, Carter BD, Brinton LA, Falk RT, Gram IT, Luo J, et al. 2016. Pooled analysis of active cigarette smoking and invasive breast cancer risk in 14 cohort studies. Int J Epidemiol, PMID: 28031315, 10.1093/ije/dyw288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gram IT, Park SY, Kolonel LN, Maskarinec G, Wilkens LR, Henderson BE, et al. 2015. Smoking and risk of breast cancer in a racially/ethnically diverse population of mainly women who do not drink alcohol: The MEC Study. Am J Epidemiol 182(11):917–925, PMID: 26493265, 10.1093/aje/kwv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim A, Zubidat AE. 2015. Artificial light at night: Melatonin as a mediator between the environment and epigenome. Philos Trans R Soc Lond B Biol Sci 370(1667):pii: 20140121, PMID: 25780234, 10.1098/rstb.2014.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu F-C, Baugh KE, Ghosh T, Zhishin M, Elvidge CD. 2015. DMSP-OLS radiance calibrated nighttime lights time series with intercalibration. Remote Sens (Basel) 7(2):1855–1876, 10.3390/rs70201855. [DOI] [Google Scholar]
- Hurley S, Nelson DO, Garcia E, Gunier R, Hertz A, Reynolds P. 2013. A cross-sectional analysis of light at night, neighborhood sociodemographics and urinary 6-sulfatoxymelatonin concentrations: Implications for the conduct of health studies. Int J Health Geogr 12:39, PMID: 24127816, 10.1186/1476-072X-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley S, Goldberg D, Nelson D, Hertz A, Horn-Ross PL, Bernstein L, et al. 2014. Light at night and breast cancer risk among california teachers. Epidemiology 25(5):697–706, PMID: 25061924, 10.1097/EDE.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasser SA, Blask DE, Brainard GC. 2006. Light during darkness and cancer: Relationships in circadian photoreception and tumor biology. Cancer Causes Control 17(4):515–523, PMID: 16596305, 10.1007/s10552-005-9013-6. [DOI] [PubMed] [Google Scholar]
- Keshet-Sitton A, Or-Chen K, Huber E, Haim A. 2016a. Illuminating a risk for breast cancer: A preliminary ecological study on the association between streetlight and breast cancer. Integr Cancer Ther, PMID: 27899698, 10.1177/1534735416678983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet-Sitton A, Or-Chen K, Yitzhak S, Tzabary I, Haim A. 2016b. Can avoiding light at night reduce the risk of breast cancer?. Integr Cancer Ther 15(2):145–152, PMID: 26631258, 10.1177/1534735415618787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet-Sitton A, Or-Chen K, Yitzhak S, Tzabary I, Haim A. 2016c. Light and the city: Breast cancer risk factors differ between urban and rural women in Israel. Integr Cancer Ther 16(2):176–187, PMID: 27440788, 10.1177/1534735416660194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Lee E, Lee HS, Kim M, Park MS. 2015. High prevalence of breast cancer in light polluted areas in urban and rural regions of South Korea: An ecologic study on the treatment prevalence of female cancers based on National Health Insurance data. Chronobiol Int 32(5):657–667, PMID: 25955405, 10.3109/07420528.2015.1032413. [DOI] [PubMed] [Google Scholar]
- Kloog I, Haim A, Stevens RG, Barchana M, Portnov BA. 2008. Light at night co-distributes with incident breast but not lung cancer in the female population of Israel. Chronobiol Int 25(1):65–81, PMID: 18293150, 10.1080/07420520801921572. [DOI] [PubMed] [Google Scholar]
- Kloog I, Stevens RG, Haim A, Portnov BA. 2010. Nighttime light level co-distributes with breast cancer incidence worldwide. Cancer Causes Control 21(12):2059–2068, PMID: 20680434, 10.1007/s10552-010-9624-4. [DOI] [PubMed] [Google Scholar]
- Laden F, Spiegelman D, Neas LM, Colditz GA, Hankinson SE, Manson JE, et al. 1997. Geographic variation in breast cancer incidence rates in a cohort of U.S. Women. J Natl Cancer Inst 89(18):1373–1378, PMID: 9308708. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. 2000. Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 343(2):78–85, PMID: 10891514, 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- Luo J, Margolis KL, Wactawski-Wende J, Horn K, Messina C, Stefanick ML, et al. 2011. Association of active and passive smoking with risk of breast cancer among postmenopausal women: A prospective cohort study. BMJ 342:d1016, PMID: 21363864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOAA (National Oceanic and Atmospheric Administration). 2017. Global radiance calibrated nighttime lights. https://ngdc.noaa.gov/eog/dmsp/download_radcal.html [accessed 24 February 2017].
- NOAA National Geophysical Data Center. 2015. DMSP data collected by the us air force weather agency. https://ngdc.noaa.gov/eog/dmsp.html [accessed 24 June 2017]
- Palmer JR, Rosenberg L. 1993. Cigarette smoking and the risk of breast cancer. Epidemiol Rev 15(1):145–156, PMID: 8405197. [DOI] [PubMed] [Google Scholar]
- Palmer JR, Boggs DA, Wise LA, Adams-Campbell LL, Rosenberg L. 2012. Individual and neighborhood socioeconomic status in relation to breast cancer incidence in African-American women. Am J Epidemiol 176(12):1141–1146, PMID: 23171873, 10.1093/aje/kws211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh PV, Wei Y. 2016. PAHs and PM2.5 emissions and female breast cancer incidence in metro Atlanta and rural Georgia. Int J Environ Health Res 26(4):458–466, PMID: 26983363, 10.1080/09603123.2016.1161178. [DOI] [PubMed] [Google Scholar]
- Portnov BA, Stevens RG, Samociuk H, Wakefield D, Gregorio DI. 2016. Light at night and breast cancer incidence in connecticut: An ecological study of age group effects. Sci Total Environ 572:1020–1024, PMID: 27531467, 10.1016/j.scitotenv.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Rea MS, Brons JA, Figueiro MG. 2011. Measurements of light at night (lan) for a sample of female school teachers. Chronobiol Int 28:673–680, PMID: 21867367, 10.3109/07420528.2011.602198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P, Hurley S, Goldberg DE, Anton-Culver H, Bernstein L, Deapen D, et al. 2004a. Active smoking, household passive smoking, and breast cancer: Evidence from the California Teachers Study. J Natl Cancer Inst 96(1):29–37, PMID: 14709736. [DOI] [PubMed] [Google Scholar]
- Reynolds P, Hurley S, Goldberg DE, Anton-Culver H, Bernstein L, Deapen D, et al. 2004b. Regional variations in breast cancer among California teachers. Epidemiology 15(6):746–754, PMID: 15475725. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Corsano KA, Stampfer MJ. 1994. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol 140(11):1016–1019, PMID: 7985649. [DOI] [PubMed] [Google Scholar]
- Rybnikova N, Haim A, Portnov BA. 2015. Artificial light at night (ALAN) and breast cancer incidence worldwide: A revisit of earlier findings with analysis of current trends. Chronobiol Int 32(6):757–773, PMID: 26102518, 10.3109/07420528.2015.1043369. [DOI] [PubMed] [Google Scholar]
- Rybnikova NA, Portnov BA. 2015. Using light-at-night (LAN) satellite data for identifying clusters of economic activities in Europe. Lett Spat Resour Sci 8(3):307–334, 10.1007/s12076-015-0143-5. [DOI] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. 2001. Rotating night shifts and risk of breast cancer in women participating in the nurses' health study. J Natl Cancer Inst 93(20):1563–1568, PMID: 11604480. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Kroenke CH, Dowsett M, Folkerd E, Hankinson SE. 2006a. Urinary 6-sulfatoxymelatonin levels and their correlations with lifestyle factors and steroid hormone levels. J Pineal Res 40(2):116–124, PMID: 16441548, 10.1111/j.1600-079X.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. 2006b. Night work and risk of breast cancer. Epidemiology 17(1):108–111, PMID: 16357603. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Hankinson SE. 2009. Urinary melatonin levels and postmenopausal breast cancer risk in the nurses' health study cohort. Cancer Epidemiol Biomarkers Prev 18(1):74–79, PMID: 19124483, 10.1158/1055-9965.EPI-08-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisti JS, Collins LC, Beck AH, Tamimi RM, Rosner BA, Eliassen AH. 2016. Reproductive risk factors in relation to molecular subtypes of breast cancer: Results from the nurses' health studies. Int J Cancer 138(10):2346–2356, PMID: 26684063, 10.1002/ijc.29968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M. 2016. The missing covariate indicator method is nearly valid almost always. Epidemiology Congress of the Americas, 21–24 June 2016, Miami, FL. [Google Scholar]
- Stevens RG, Blask DE, Brainard GC, Hansen J, Lockley SW, Provencio I, et al. 2007. Meeting report: The role of environmental lighting and circadian disruption in cancer and other diseases. Environ Health Perspect 115(9):1357–1362, PMID: 17805428, 10.1289/ehp.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RG. 2009. Light-at-night, circadian disruption and breast cancer: Assessment of existing evidence. Int J Epidemiol 38(4):963–970, PMID: 19380369, 10.1093/ije/dyp178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RG. 2011. Testing the light-at-night (lan) theory for breast cancer causation. Chronobiol Int 28(8):653–656, PMID: 21929297, 10.3109/07420528.2011.606945. [DOI] [PubMed] [Google Scholar]
- Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. 2007. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol 8(12):1065–1066, PMID: 19271347. [DOI] [PubMed] [Google Scholar]
- Tamimi RM, Baer HJ, Marotti J, Galan M, Galaburda L, Fu Y, et al. 2008. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res 10(4):R67, PMID: 18681955, 10.1186/bcr2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2000. U.S. Census 2000. https://www.census.gov/main/www/cen2000.html [accessed 24 June 2017].
- Wang J, et al. 2015. Alcohol consumption and risk of breast cancer by tumor receptor expression. Horm Cancer 6(5–6):237–246, PMID: 26385458, 10.1007/s12672-015-0235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhang X, Beck AH, Collins LC, Chen WY, Tamimi RM, et al. 2016. Statistical methods for studying disease subtype heterogeneity. Stat Med 35(5):782–800, PMID: 26619806, 10.1002/sim.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 1965. International Classification of Diseases, Revision 8 (ICD-8). Geneva, Switzerland:WHO. [Google Scholar]
- Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. 1985. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 122(1):51–65, PMID: 4014201. [DOI] [PubMed] [Google Scholar]
- Willett WC. 2001. Diet and breast cancer. J Intern Med 249(5):395–411, PMID: 11350564. [DOI] [PubMed] [Google Scholar]
- Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. 1994. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 23(5):991–999, PMID: 7860180. [DOI] [PubMed] [Google Scholar]
- Wong CM, Tsang H, Lai HK, Thomas GN, Lam KB, Chan KP, et al. 2016. Cancer mortality risks from long-term exposure to ambient fine particle. Cancer Epidemiol Biomarkers Prev 25(5):839–845, PMID: 27197138, 10.1158/1055-9965.EPI-15-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanosky JD, Paciorek CJ, Laden F, Hart JE, Puett RC, Liao D, et al. 2014. Spatio-temporal modeling of particulate air pollution in the conterminous United States using geographic and meteorological predictors. Environ Health 13:63, PMID: 25097007, 10.1186/1476-069X-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.