Abstract
Tremendous progress has been made in the identification of rheumatoid arthritis (RA) risk factors in 2017. The results of epidemiologic studies highlighted dietary factors and hormones that are associated with slowing the transition from one preclinical phase of RA to another, potentially protecting individuals from developing RA.
Subject ontology terms: Health sciences/Diseases/Rheumatic diseases/Rheumatoid arthritis [URI/692/699/1670/498], Health sciences/Health care/Public health/Epidemiology [URI/692/700/478/174], Health sciences/Risk factors [URI/692/499]
The development of anti-citrullinated protein antibodies (ACPAs) and the subsequent onset of ACPA+ rheumatoid arthritis (RA) are central to the pathogenesis of seropositive RA. Some individuals with an increased genetic susceptibility to RA will develop ACPA, of whom a proportion will go on to present symptomatically with arthralgia and early inflammatory arthritis, before being diagnosed with ACPA+ RA1. A growing list of environmental factors, including cigarette smoke, inhaled particulates (such as silica), diet, hormones, medication use and infections can affect the transitions between these preclinical phases in the pathogenesis of RA2 (Figure 1). Previous research has identified factors, in particular smoking, that accelerate these transitions and increase the risk of developing ACPA+ RA2. Although the identification of smoking as an ACPA+ RA risk factor is of crucial importance, most patients with RA are non-smokers, and the incidence of RA seems to be stable despite a decrease in the number of individuals who smoke. Other environmental factors are therefore likely to be important contributors to RA risk. The epidemiologic observations that consumption of omega-3 fatty acids3, a long-term healthy diet4, and use of oral contraceptives5 might protect against RA offer potential strategies to lower RA risk and to generate hypotheses concerning the biologic mechanisms of these environmental factors.
Figure 1. Preclinical phases of ACPA+ rheumatoid arthritis.
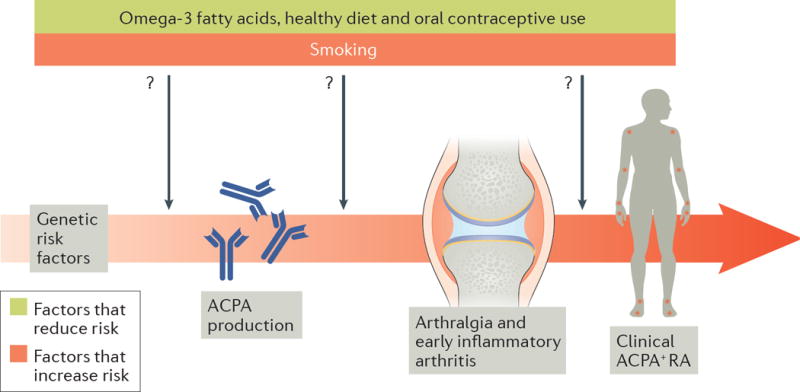
Individuals at increased genetic risk of rheumatoid arthritis (RA) may develop anti-citrullinated protein antibodies (ACPAs). Environmental factors such as smoking increase the risk of ACPA production, whereas other environmental factors, such as omega-3 fatty acid intake, might decrease the risk of ACPA production. ACPA+ individuals may then go on to develop arthralgias and undifferentiated inflammatory arthritis, which can progress to clinically-apparent ACPA+ RA. Similar to the risk of ACPA production, smoking increases the risk of progression to inflammatory arthritis, whereas intake of omega-3 fatty acids might decrease this risk. A healthy diet and use of oral contraceptives may decrease the overall risk of RA, but the mechanisms and particular preclinical phases of RA affected by these risk factors are unclear.
In 2017, several studies have established the role of dietary factors in the pathogenesis of RA. Gan et al.3 investigated whether omega-3 fatty acids are important in the preclinical phases of RA on the basis of previous studies that associated fish6 or fish oil7 intake with a decreased risk of RA and favourable outcomes among patients with RA. Gan et al. measured the composition of omega-3 fatty acids in erythrocyte membranes in 47 ACPA+ individuals who did not have diagnosed RA, ten of whom had undifferentiated inflammatory arthritis3. Higher levels of total erythrocyte-bound omega-3 fatty acids were strongly associated with lower odds of having undifferentiated inflammatory arthritis (OR 0.09, 95%CI 0.01-0.85 per increasing standard deviation)3. The authors then followed 35 ACPA+ individuals who did not have inflammatory arthritis and found that higher levels of docosapentaenoic acid at baseline protected against developing inflammatory arthritis (HR 0.52, 95%CI 0.27-0.98) during a mean follow-up of 2.6 years3.
While providing new insights into RA pathogenesis, these results also offer individuals at high risk of developing RA owing to ACPA positivity a dietary modification that might help to lower that risk. These findings complement those of a previous study that showed that increasing levels of erythrocyte-bound total omega-3 fatty acids lowered the risk of ACPA positivity (OR 0.44, 95%CI 0.21-0.93) in 136 unaffected individuals at high risk of RA due to seropositivity or positivity for the HLA shared epitope3. Together, these studies demonstrate that omega-3 fatty acids might have dual actions: lowering the risk of developing ACPAs and preventing the onset of inflammatory arthritis once ACPAs are present. By extension, these studies suggest that omega-3 fatty acids might decrease RA risk, although this hypothesis has yet to be proven. These small but provocative studies provide the rationale for prospective studies and clinical trials of omega-3 fatty acids in preventing RA.
In an investigation of dietary quality and RA risk, Hu et al. examined whether dietary quality was associated with risk of RA in American women using the prospective Nurses’ Health Studies cohorts4. The Alternative Healthy Eating Index (AHEI) was developed using expert opinion of foods and nutrients related to the risk of chronic diseases8. The AHEI uses 11 food and nutrient categories covering healthy (fruits, vegetables, whole grains, nuts, omega-3 fatty acids, polyunsaturated fatty acids and moderate amounts of alcohol) and unhealthy (sugar-sweetened beverages, red or processed meat, trans fats and sodium) foods to calculate an overall score8. Hu et al. analysed the long-term dietary quality of the participants as a cumulative average AHEI score using repeated food frequency questionnaires and categorized women into AHEI quartiles. Those in the highest AHEI quartile were considered to have highest dietary quality and those in the lowest AHEI quartile were considered to have the lowest dietary quality4. In all, data from 169,989 women were analysed, of whom 1,007 developed RA during the 3.7 million person-years of follow-up4. Among women ≤55 years of age, the healthiest AHEI quartile had an HR of 0.67 (95%CI 0.51-0.88) for RA compared to those with lowest dietary quality(P=0.002 for trends across quartiles)4. This protective effect was most pronounced for seropositive RA among those aged ≤55 years (HR 0.60, 95%CI 0.42-0.86, P=0.003 for trend)4. However, the healthiest diet by AHEI was not associated with RA among those >55 years of age4. Two AHEI components were particularly associated with a lower risk of RA among those ≤55 years4: reduced intake of red or processed meat (HR 0.58, 95%CI 0.43-0.79) and moderate alcohol intake (HR 0.67, 95%CI 0.51-0.89)4. However, intake of omega-3 fatty acids was not associated with RA risk in this study4. Overall, these results provide further evidence that diet is an important risk factor for RA, and provide insight into potential dietary guidance for individuals at risk of developing RA.
The hormonal and metabolic changes that occur during menopause might explain why diet was only associated with RA that develops before 55 years of age4, but this association has not been firmly established9. However, hormones themselves may be associated with the risk of RA.
In 2017, Orellana et al. investigated the associations between oral contraceptive use and RA risk in women in a large Swedish case-control study5. Women who had ever used oral contraceptives had a modestly decreased RA risk (OR 0.87, 95%CI 0.78-0.97) compared to those who had never used oral contraceptive5. This protective effect was present for ACPA+ RA (OR 0.84, 95%CI 0.74-0.95), but not for ACPA− RA5. A long duration (≥7 years) of oral contraceptive use further lowered the risk of developing ACPA+ RA (OR 0.80, 95%CI 0.69-0.93)5. Ever smokers who had never used oral contraceptives had a particularly increased risk of developing ACPA+ RA (OR 2.34, 95%CI 1.95-2.82) compared to those who had never smokers but had used oral contraceptives5. The hormonal components and doses of the oral contraceptives used varied during the study period, so the importance of particular hormones and their relative doses important for RA risk are unclear5. Given the study design, it is uncertain at which phase of RA development hormones exert their protective effect. However, this study provides further evidence that hormones are important in the pathogenesis of ACPA+ RA, which is of particular interest given the predominance of RA in women and the differences in risk related to age and menopause4, 9.
The development of RA is complex and is likely to differ between individuals, but the epidemiologic advances made in 2017 elucidate factors that potentially protect against RA. These findings provide a rationale for RA prevention strategies using dietary and hormonal interventions. We are now closer to understanding the natural history of RA and how we might best intervene to delay or even prevent RA onset in the near future.
Jeffrey A. Sparks and Karen H. Costenbader are at the Division of Rheumatology, Immunology and Allergy, Brigham and Women’s Hospital and Harvard Medical School, 60 Fenwood Road, Boston, Massachusetts 02115, USA.
Key advances.
High levels of erythrocyte-bound omega-3 fatty acids were associated with decreased progression to inflammatory arthritis among anti-citrullinated protein antibody-positive individuals who do not have rheumatoid arthritis (RA)3.
Compared with an unhealthy diet, long-term adherence to a healthy diet was inversely related to the risk of developing RA in individuals of 55 years or younger4.
Women who had ever used oral contraceptives had a lower risk of developing RA compared with those who had never used oral contraceptives5.
Acknowledgments
The work of the authors is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), grant numbers K23 AR069688 and L30 AR066953 (to J.A.S.), R01 AR049880, R01 AR061362 and K24 AR066109 (to K.H.C.), as well as P30 AR070253 and P30 AR072577. The funding bodies had no role in the preparation of or decision to publish this manuscript. The content the sole responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Burgers LE, van Steenbergen HW, Ten Brinck RM, Huizinga TW, van der Helm-van Mil AH. Differences in the symptomatic phase preceding ACPA-positive and ACPA-negative RA: a longitudinal study in arthralgia during progression to clinical arthritis. Ann Rheum Dis. 2017;76(10):1751–4. doi: 10.1136/annrheumdis-2017-211325. [DOI] [PubMed] [Google Scholar]
- 2.Sparks JA, Karlson EW. The Roles of Cigarette Smoking and the Lung in the Transitions Between Phases of Preclinical Rheumatoid Arthritis. Curr Rheumatol Rep. 2016;18(3):15. doi: 10.1007/s11926-016-0563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gan RW, Bemis EA, Demoruelle MK, Striebich CC, Brake S, Feser ML, et al. The association between omega-3 fatty acid biomarkers and inflammatory arthritis in an anti-citrullinated protein antibody positive population. Rheumatology (Oxford) 2017 doi: 10.1093/rheumatology/kex360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Y, Sparks JA, Malspeis S, Costenbader KH, Hu FB, Karlson EW, et al. Long-term dietary quality and risk of developing rheumatoid arthritis in women. Ann Rheum Dis. 2017;76(8):1357–64. doi: 10.1136/annrheumdis-2016-210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orellana C, Saevarsdottir S, Klareskog L, Karlson EW, Alfredsson L, Bengtsson C. Oral contraceptives, breastfeeding and the risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis. 2017;76(11):1845–52. doi: 10.1136/annrheumdis-2017-211620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Giuseppe D, Crippa A, Orsini N, Wolk A. Fish consumption and risk of rheumatoid arthritis: a dose-response meta-analysis. Arthritis Res Ther. 2014;16(5):446. doi: 10.1186/s13075-014-0446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proudman SM, James MJ, Spargo LD, Metcalf RG, Sullivan TR, Rischmueller M, et al. Fish oil in recent onset rheumatoid arthritis: a randomised, double-blind controlled trial within algorithm-based drug use. Ann Rheum Dis. 2015;74(1):89–95. doi: 10.1136/annrheumdis-2013-204145. [DOI] [PubMed] [Google Scholar]
- 8.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bengtsson C, Malspeis S, Orellana C, Sparks JA, Costenbader KH, Karlson EW. Association Between Menopausal Factors and the Risk of Seronegative and Seropositive Rheumatoid Arthritis: Results From the Nurses’ Health Studies. Arthritis Care Res (Hoboken) 2017;69(11):1676–84. doi: 10.1002/acr.23194. [DOI] [PMC free article] [PubMed] [Google Scholar]


