Keywords: nerve regeneration, ischemic cerebral infarction, ischemia/reperfusion, electroacupuncture, Shuigou (GV26), collateral circulation, angiogenesis, neural regeneration
Abstract
Acupuncture at Shuigou (GV26) shows good clinical efficacy for treating stroke, but its mechanism remains poorly understood. In this study, a cerebral infarction model of ischemia/reperfusion injury received electroacupuncture at GV26 (15 Hz and 1 mA, continuous wave [biphasic pulses], for 5 minutes). Electroacupuncture effectively promoted regional cerebral blood flow on the infarct and non-infarct sides, increased infarct lesions, lectin, and number of blood vessels, upregulated von Willebrand factor and cell proliferation marker Ki67 expression, and diminished neurological severity score. These findings confirm that electroacupuncture at GV26 promotes establishment of collateral circulation and angiogenesis, and improves neurological function.
Introduction
Since the 1990s, many clinical studies on the treatment of cerebral infarction have shown that neuroprotection alone does not lead to satisfactory clinical benefits (Ginsberg, 2008). This has led researchers to consider neurons and microvessels together as a functional entity, and consequently, the concept of a neurovascular unit was proposed (Iadecola, 2004). Although thrombolytic therapy and embolectomy have benefited a small number of patients on specific current treatments, its time window and complications have limited the number of patients that receive this therapy (Görner et al., 2007; Singh et al., 2013). There are other treatments such as improving cerebral blood circulation, nerve protection, and hyperbaric oxygen, yet except for early application of antiplatelet drugs, other drugs do not currently show stable effects (Hennerici et al., 2006; Zhou et al., 2007; Sandercock et al., 2008; Levy et al., 2009; Hao et al., 2012; Chang and Jensen, 2014). In clinical practice, we have found that acupuncture has a good effect on patients with acute ischemic stroke (Park et al., 2014; Lim et al., 2015). Shuigou (GV26) is an important acupoint for treatment of cerebrovascular disease. Many studies have found that acupuncture at Shuigou inhibits neuronal apoptosis (Chen et al., 2008), alters nerve function (Ren and Ma, 2010; Ma et al., 2011; Zhong et al., 2013), alleviates pathological damage (Wei et al., 2010), reduces infarct size (Huang, 2012), inhibits expression of inflammatory factors (Ding et al., 2007), and reduces calcium overload and free radical damage (Yan, 2007).
Our previous studies have indicated that electroacupuncture (EA) strongly regulates expression of vascular endothelial growth factor (VEGF)/vascular endothelial growth factor receptor 1 (Li et al., 2014), apelin/putative receptor protein related to AT1 mRNA and relative proteins (Yang et al., 2017), and promotes vascular endothelial cell proliferation (Du et al., 2011). EA also reduces expression of caldesmon, calponin, and protein kinase C in vessels, which may relieve smooth muscle vasospasm in blood vessels (Xu et al., 2012; Lv et al., 2015). Previous research has been based on permanent models of middle cerebral artery occlusion (MCAO), which do not remove the intraluminal thread. The hypothalamus is visibly impaired when blocked by intraluminal thread for more than 60 minutes (Du et al., 2011; Xu et al., 2012; Li et al., 2014). However, in clinical practice, hypothalamic infarction is unusual and human stroke involves a substantial degree of reperfusion (Carmichael, 2005).
Our hypothesis is that EA at GV26 may also be a significant contributor to angiogenesis in reperfused MCAO. Hence, these experiments were performed to comprehensively analyze the role of EA in reconstruction of the neurovascular unit.
Materials and Methods
Animals
In total, 128 male specific-pathogen-free Wistar rats aged 6–8 weeks old and weighing 200 ± 20 g were purchased from the Department of Laboratory Animal Science, Peking University Health Science Center, China (SCXK (Jing) 2014-0004). All rats were housed under environmentally controlled conditions (temperature 22 ± 3°C under 12-hour light/dark cycles with lights on at 6:00), with free access to food and water. All experiments were approved by the Laboratory Animal Ethics Committee at the Tianjin University of Chinese Medicine, China (approval number: TJAB-TJU20160024). The rats were randomly divided into a MCAO group (n = 64, MCAO only) and EA group (n = 64, MCAO + EA).
MCAO reperfusion model preparation
The intraluminal suture method was used for the MCAO reperfusion model (Carmichael, 2005). Rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (0.3 mL/100 g), and an incision was made on the left side of the mid-neck. The left common carotid artery, external carotid artery, and internal carotid artery were isolated. The proximal end of the common carotid artery and distal end of the external carotid artery were occluded. The distal external carotid artery was cut from the proximal side next to the ligated site and folded straight through the internal carotid artery. A nylon suture (diameter 0.205 mm) was introduced from the external carotid artery into the internal carotid artery. The length entered was 18–22 mm, with advancement into the artery stopped when resistance was felt. The suture was tied at the beginning of the external carotid artery, and the proximal end of the common carotid artery was opened. The suture was removed after 60 minutes and the external carotid artery stump ligated. The wound was cleaned and sutured. Rats were kept warm during surgery. The study process is shown in Figure 1.
Figure 1.
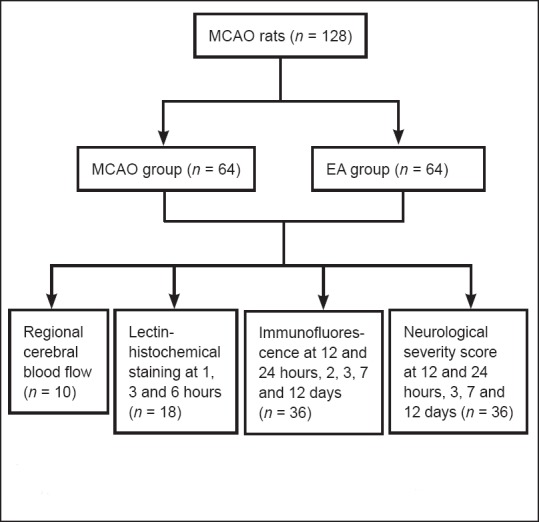
Study flow chart.
MCAO: Middle cerebral artery occlusion; EA: electroacupuncture.
EA intervention
The EA group was given EA at GV26 (lies in the center of the mentolabial groove, 1 mm interior to the nose). A half-inch needle (0.5 cun, 10 × 40 mm; Suzhou Medical Sino-foreign Joint Venture Suzhou Hua Tuo Medical Instruments Co., Ltd., Suzhou, China) was inserted obliquely upwards 2 mm below the rat nasal septum, with another needle inserted approximately 2 mm below the first needle as the reference electrode. The needle at GV26 was connected to the positive electrode of the electro-acupuncture device (Nanjing Gensun Medical Technology Co., Ltd., Beijing, China). The reference electrode was connected to the negative electrode. Stimulation administered was 15 Hz, 1 mA, continuous wave (biphasic pulses) for 5 minutes. Rats in the EA group were treated with the above intervention once per day until the day before they were euthanized. Rats in the MCAO group were similarly immobilized, but no treatment was given.
Regional cerebral blood flow (rCBF) measurement with laser Doppler
Cerebral blood flow was measured before MCAO, and immediately, 30 minutes, 1, 2, 3, 4, 5, and 6 hours after MCAO. A 2-cm longitudinal incision was made along the midline of the scalp to expose the coronal and sagittal sutures. At 4 mm towards the back of the skull from the coronal suture, and 3 mm on both sides from the sagittal suture, the skull was thinly ablated with a dental drill. A laser Doppler probe (Moor Instruments, Beijing, China) was positioned to record baseline bilateral rCBF values. The infarction model was considered successful when rCBF was 30% or less of baseline rCBF (Cuccione et al., 2017).
Lectin staining
Rats were anesthetized at pre-designated time points after infarction: 1, 3, and 6 hours after MCAO. Biotinylated tomato lectin (100 μg/100 μL, dissolved in saline; Vector Laboratories, Burlingame, CA, USA) was injected through the femoral vein. Two minutes later, the chest cavity was opened to perform cardiac perfusion with 4% paraformaldehyde. A 3-mm-thick brain slice from the cerebral artery and anterior cerebral artery bifurcation towards the back was removed, dehydrated in 30% sucrose, and cut into 35 μm thick cryosections. Sections were fixed in acetone at 4°C for 10 minutes and then dried. Sections were washed twice with 0.01 M PBS for 5 minutes each, and then incubated at 37°C for 4 hours in 0.01 M PBS. Avidin-biotin complex (VECTORSTAIN ABC kit; Vector Laboratories) was added to sections and incubated at 37°C for 1 hour. Sections were rinsed for 5 minutes, 2 times. 3,3′-Diaminobenzidine (DAB) (ZLI-9032; ZSGB Co., Ltd., Beijing, China) staining reagent was added and incubated for 15 minutes, then sections were thoroughly washed with water to terminate the reaction, followed by dehydration through a graded alcohol series and clearance with xylene. Sections were mounted with neutral resin. The Image Pro-Plus image analysis system (Media Cybernetics, Rockville, MD, USA) was used for analysis, with eight fields randomly chosen from infarcts and surrounding areas to reflect collateral circulatory blood vessels (Mancuso et al., 2006).
Double immunofluorescent staining
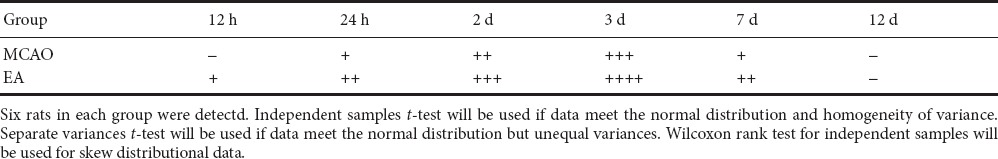
Rats were anesthetized at pre-designated time points (12, 24 hours, 2, 3, 7, and 12 days after MCAO), and the chest cavity was opened to perform cardiac perfusion. A 3-mm-thick brain slice from the cerebral artery and anterior cerebral artery bifurcation towards the back was removed, dehydrated in 30% sucrose, and cut into 14 μm thick cryosections. Sections were air-dried for 1 hour, rinsed with running water for 5 minutes, and immersed in double-distilled water for 5 minutes. Sections were then treated with the following reagents: 0.3% hydrogen peroxide for 5 minutes, 0.5% Triton X-100 for 10 minutes, digestion buffer for 10 minutes, and normal goat serum for 10 minutes. The above steps were performed at room temperature, with the sections washed three times with 0.01 M PBS for 5 minutes each between each step. Polyclonal rabbit anti-human von Willebrand factor-related antibody (vWF; 1:50; DAKO, Beijing, China) was used as a maker for endothelial cells. Polyclonal rabbit anti-mouse Ki67 antibody (1:50; Abcam, Shanghai, China) was used as a cell proliferation maker. The two primary antibodies were mixed together, added to sections (50 µL/slide), and incubated overnight at 4°C. Sections were washed three times with 0.01 M PBS (5 minutes each), incubated with goat-anti-rabbit IgG (H+L)-FITC (1:50; Invitrogen, Beijing, China) and goat-anti-mouse IgG (H+L)-TRITC (1:50; Invitrogen) mixture (50 µL) for 3 hours at room temperature, and then washed again with 0.01 M PBS three times (10 minutes each). Sections were mounted with 50% glycerol. Staining was observed and photographed with a confocal microscope (Leica Microsystems, Buffalo Grove, IL, USA). The Image Pro-Plus image analysis system (Media Cybernetics) was used to calculate the number of proliferating endothelial cells, which are vWF and Ki67-double positive cells. The number of Ki67-positive nuclei was counted to semi-quantitatively measure endothelial cell proliferation and assess the effect of EA intervention. Stained endothelial cell proliferation was classified as follows: −, no positive staining; +, 1–3 stained endothelial cells; ++, 4–6 stained endothelial cells; +++, 7–9 stained endothelial cells; ++++, more than 9 stained endothelial cells (Du et al., 2011).
Neurological severity score (NSS)
NSS was used to evaluate neurological function in the MCAO group and EA group before MCAO, and 12, 24 hours, 3, 7, and 12 days after MCAO. The assessor was blinded to the experimental group. Total NSS score was 18 points: normal score, 0; mild neurological impairment, 1–6; moderate neurological impairment, 7–12; severe neurological damage, 13–18) (Du et al., 2011).
Statistical analysis
Results of rCBF measurement, lectin staining, and neurological severity score were expressed as the mean ± SEM. All results were analyzed using SPSS 24.0 software (IBM, Armonk, NY, USA). Independent samples t-test was used if data met the normal distribution and homogeneity of variance. Separate variances t-test was used if data met the normal distribution but unequal variance. Wilcoxon rank test for independent samples was used for skewed distribution data. Results of double immunofluorescent staining were expressed as categorical data and reported descriptively.
Results
EA changes rCBF in MCAO rats
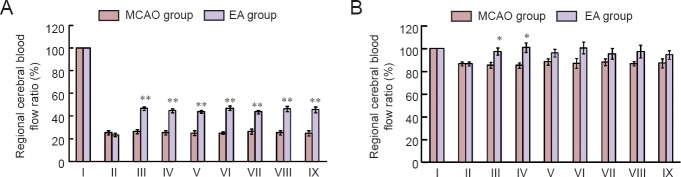
Immediately after MCAO, rCBF decreased substantially in the infarcted hemisphere. In the MCAO group, rCBF decreased rapidly to 25.30 ± 1.76% of baseline value. It remained around this level from 0.5 to 6 hours after MCAO, showing no statistically significant differences between different time points (P > 0.05). In the EA group, rCBF decreased rapidly to 23.57 ± 1.54% of baseline value immediately after MCAO. After EA intervention, rCBF increased by 20% on average to 46.84 ± 1.54% of baseline value. At 0.5, 1, 2, 3, 4, 5, and 6 hours after MCAO, rCBF was significantly different between the MCAO group and EA group (P < 0.01; Figure 2).
Figure 2.
Changes in regional cerebral blood flow ration on infarcted (A) and non-infarcted (B) sides after MCAO.
Data represent the mean ± SEM (n = 10). Independent samples t-test was used if data met the normal distribution and homogeneity of variance. Separate variances t-test was used if data met the normal distribution but unequal variance. Wilcoxon rank test for independent samples was used for skewed distribution data. *P < 0.05, **P < 0.01, vs. MCAO group. I: Before infraction; II: immediately after infarction; III: 30 minutes after infarction; IV: 1 hour after infarction; V: 2 hours after infarction; VI: 3 hours after infarction; VII: 4 hours after infarction; VIII: 5 hours after infarction; IX: 6 hours after infarction; MCAO: middle cerebral artery occlusion.
Immediately after MCAO, rCBF also decreased in the non-infarcted hemisphere. In the MCAO group, rCBF decreased to 86.66 ± 1.66% of baseline value. It remained around this level from 0.5 to 6 hours after MCAO, showing slight variations but no statistically significant differences between different time points. In the EA group, rCBF decreased rapidly to 86.66 ± 1.66% of baseline value immediately after MCAO. After EA intervention, rCBF increased to 97.46 ± 3.19% of baseline value and remained at this level. At 0.5 and 1 hour after MCAO, rCBF was significantly different between the MCAO and EA groups (P < 0.05). At 2, 3, 4, and 5 hours after MCAO, rCBF was slightly higher in the EA group than MCAO group, but the differences were not statistically significant (P > 0.05; Table 1).
Table 1.
Effect of EA on endothelial cell proliferation around infarction lesions of the MCAO rats
EA increased the quantity of blood-flowing vessels in and around infarcts in MCAO rats
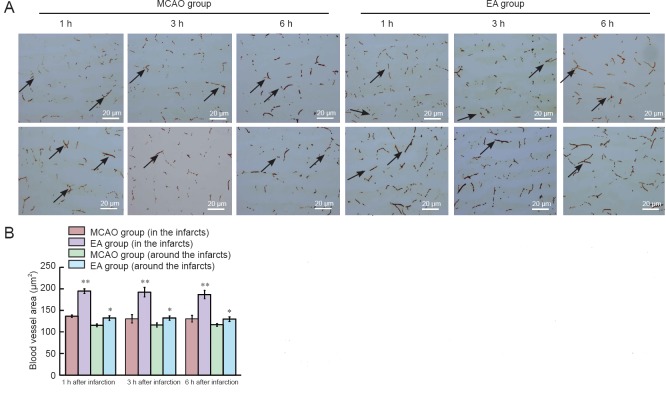
The quantity of blood-flowing vessels in the areas around/within infarcts was significantly higher in the EA group than MCAO group at every time point (P < 0.01 or P < 0.05; Figure 3).
Figure 3.
Effect of EA on quantity of blood-flowing vessels in and around cerebral infarcts in MCAO rats (lectin staining).
(A) Blood vessel staining around (upper) and within (lower) infarcts (original magnification, 100×). Arrows indicate blood vessels. Scale bars: 20 μm. (B) Blood vessel quantity in and around infarct lesions. Data represent mean ± SEM (n = 10). Independent samples t-test was used if data met the normal distribution and homogeneity of variance. Separate variances t-test was used if data met the normal distribution but unequal variance. Wilcoxon rank test for independent samples was used for skewed distribution data. *P < 0.05, **P < 0.01, vs. MCAO group. MCAO: Middle cerebral artery occlusion; EA: electroacupuncture; h: hour(s).
Effect of EA on cell proliferation in blood vessels of MCAO rats
Double immunofluorescence staining of vWF and Ki67 was used to show cell proliferation in blood vessels. Co-expression of vWF and Ki67 indicated proliferating endothelial cells (Skovseth et al., 2002). The MCAO group showed endothelial cell proliferation starting from 24 hours after MCAO. Proliferation continued to increase at 48 hours, peaked at 3 days, and declined at 7 days after MCAO. The EA group showed endothelial cell proliferation starting from 12 hours after MCAO. Proliferation continued to increase at 24 and 48 hours, peaked at 3 days, and declined at 7 days after MCAO. Thus, endothelial cell proliferation showed the same trend in both groups, but appeared earlier in the EA group. Further, the EA group had more proliferating endothelial cells than the MCAO group at every time point (Figure 4 and Table 1).
Figure 4.
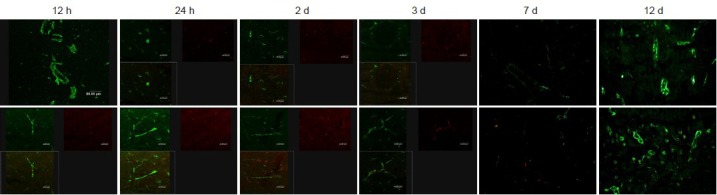
Endothelial cell proliferation around infarct lesions in the MCAO group (upper) and EA group (lower).
Green represents vWF, red represents Ki67, and yellow represents proliferating endothelial cells. In the MCAO group, proliferation of vascular endothelial cells started at 24 hours after MCAO, increased continuously for 2 days, reached a maximum at 3 days, and decreased at 7 days after MCAO. In the EA group, proliferation of vascular endothelial cells started at 12 hours after MCAO, increased continuously for 2 days, reached a maximum at 3 days, and decreased at 7 days after MCAO. MCAO: Middle cerebral artery occlusion; EA: electroacupuncture; vWF: von Willebrand factor; h: hours; d: days. Scale bars: 40 μm.
EA improved neurological function of MCAO rats
Before MCAO, NSS was 0 point in all rats. With prolonged ischemic time, NSS showed a downward trend in both MCAO and EA groups. NSS was significantly reduced in the EA group at all time points (P < 0.05), especially at 3 and 12 days after MCAO (P < 0.01; Figure 5).
Figure 5.
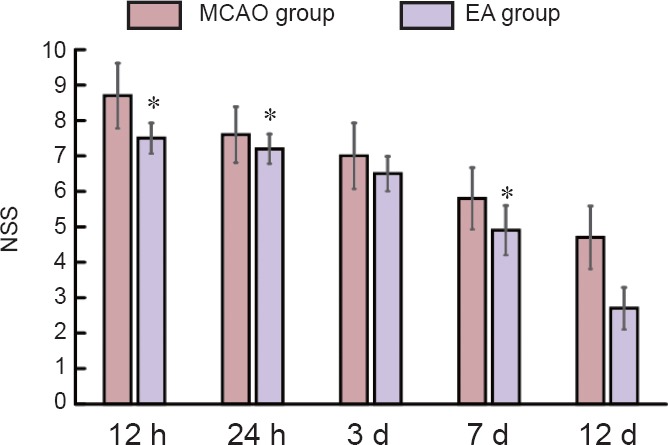
Effect of EA on NSS of MCAO rats
Data represent the mean ± SEM (n = 6). Independent samples t-test was used if data met the normal distribution and homogeneity of variance. Separate variances t-test was used if data met the normal distribution but unequal variance. Wilcoxon rank test for independent samples was used for skewed distribution data. *P < 0.05, vs. MCAO group. MCAO: Middle cerebral artery occlusion; EA: electroacupuncture; NSS: neurological severity score; h: hours; d: days.
Discussion
Permanent MCAO was used in a previous study of cerebral infarction treated with EA. The infarction area of MCAO was up to 39% of the ipsilateral hemisphere, which is close to malignant MCAO infarction. With suture occlusion of 60 minutes or longer, hypothalamic damage is robust and occurs early (Carmichael, 2005). However, hypothalamic ischemia is rarely seen in human stroke. In our study, MCAO for 60 minutes was used. Striatal infarction occurs in the core ischemic area, and the infarction area is approximately 10% of the ipsilateral hemisphere (Carmichael, 2005), which is closer to most clinical situations.
Establishment of effective collateral circulation in the early stage plays a key role in formation of infarction and the ischemic penumbra, and is a critical treatment at the early stage (ElAli, 2016; Iwasawa et al., 2016). In our study, EA effectively improved collateral circulation and increased cerebral blood perfusion within 6 hours after infarction. Starting from 12 hours after infarction, endothelial cell proliferation and NSS both decreased in the EA group compared with MCAO group. This suggests that EA provides a favorable condition for reconstruction of neurovascular units at the extremely early stage of cerebral infarction.
Our previous studies have demonstrated that EA affects expression of critical neurotransmitters, such as vasoactive intestinal peptide, calcitonin gene-related peptide, substance P, neuropeptide Y, and cyclic adenosine monophosphate (Lin and Du, 2007; Sun et al., 2008), regulates movement of vascular smooth muscle cells, and improves autonomic movement of cerebral arteries (Li et al., 2011; Shi and Du, 2012). Here, our laser Doppler experiments show that EA intervention significantly improves rCBF after cerebral infarction. Immediately after MCAO, rCBF dropped dramatically on the infarcted side to around 25% of the value before MCAO. In addition, rCBF also dropped by approximately 15% on the non-infarcted side. Afterwards, rCBF on both sides increased slightly, but was not statistically significant. After EA intervention, rCBF increased significantly on both sides. On the infarcted side, the increase was approximately 20%, with rCBF in the EA group significantly higher than the MCAO group at each time point. On the non-infarcted side, rCBF increased to nearly normal levels after EA intervention, and was higher than the infarcted side in the MCAO group.
Lectin staining showed that at 1, 3, and 6 hours after MCAO, the quantity of blood vessels decreased significantly around infarcts on the infarcted side in the MCAO group, but increased significantly in the EA group. This decrease was more pronounced in infarcts, whereas the quantity of blood vessels increased at all time points in the EA group. These results suggest that EA intervention improves collateral circulation in and around infarcts, leading to a corresponding increase in rCBF.
The degree of vascular endothelial cell proliferation after infarction determines establishment of collateral circulation. In response to vascular injury after cerebral infarction, angiogenesis is rapidly initiated to maintain integrity of the blood-brain barrier and ensure energy supply to neurons. The vascular remodeling process after cerebral infarction involves vasculogenesis and angiogenesis mechanisms (Risau, 1997), and has been confirmed in both animal models and humans (Taylor et al., 2013). Collateral vessels improve tissue perfusion around the ischemic area and local metabolism, and may promote recovery of neurological function and greater long-term benefits. Results of the MCAO group showed changes in endothelial cell proliferation after MCAO reperfusion. Endothelial cell proliferation initiated at 24 hours, peaked at 3 days, and then decreased at 7 days. Endothelial cell proliferation was higher in the EA group than the MCAO group at all time points. In our previous studies, EA significantly increased expression of VEGF/Flt-1 mRNA and their proteins, which began at 12 hours after MCAO and diminished after MCAO for 7 days (Li et al., 2014). EA significantly increased expression of apelin mRNA at 12 hours after MCAO, which diminished after MCAO for 7 days. APJ protein significantly increased after MCAO for 1 hour (Yang et al., 2017). The results of these studies correspond with our results. In addition, NSS scores in the EA group at 12, 24 hours, 3, 7, and 12 days after MCAO were significantly lower than in the MCAO group. This suggests that EA intervention effectively promotes proliferation of endothelial cells and may play a positive role in neurovascular unit reconstruction.
Nonetheless, we still lack morphological evidence for improvements in the overall structure of neurovascular units after cerebral infarction, as well as evidence of restored neuronal connections. Indeed, the following mechanisms still need to be studied: (1) those by which pathological factors affect endothelial cell proliferation and remodeling into effective vascular networks after cerebral infarction; and (2) those that guide new blood vessels to reconnect with astrocyte endfeet. In-depth studies in these areas will allow us to better understand the process of neurovascular unit reconstruction and provide a new basis for prevention and treatment of cerebral infarction.
EA at GV26 can play an intervention role in establishment of extremely early collateral circulation and improved cerebral blood flow after MCAO. Vascular endothelial cell proliferation in the EA group at 12 hours after MCAO may reflect different stages of the same process in establishment of collateral circulation. The decrease in NSS, starting at 12 hours after MCAO, suggests that proliferation of vascular endothelial cells plays a key role in reconstitution of neurovascular units. Taken together, early intervention of acupuncture can promote reconstruction of neurovascular units in cerebral infarction.
Acknowledgments
Professor Yuan-Hao Du from the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine of China is gratefully acknowledged for his advice on the design of the experiment.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 81303021.
Conflicts of interest: None declared.
Research ethics: The study protocol was approved by the Laboratory Animal Ethics Committee at the Tianjin University of Chinese Medicine of China (approval number: TJAB-TJU20160024).
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M
References
- Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TS, Jensen MB. Haemodilution for acute ischaemic stroke. Cochrane Database Syst Rev. 2014:CD000103. doi: 10.1002/14651858.CD000103.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Zhang QL, Bai B. Effect of electroacupuncture on mitochondrial membrane potential and apoptosis in the cerebral cortex in rats with focal cerebral ischemia/reperfusion injury. Zhen Ci Yan Jiu. 2008;33:107–110. [PubMed] [Google Scholar]
- Cuccione E, Versace A, Cho TH, Carone D, Berner LP, Ong E, Rousseau D, Cai R, Monza L, Ferrarese C, Sganzerla EP, Berthezene Y, Nighoghossian N, Wiart M, Beretta S, Chauveau F. Multi-site laser Doppler flowmetry for assessing collateral flow in experimental ischemic stroke: Validation of outcome prediction with acute MRI. J Cereb Blood Flow Metab. 2017;37:2159–2170. doi: 10.1177/0271678X16661567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding DG, Li JK, Luo HP, Zhou ZY, Jiao Y, Wei D. The effects of electroacupuncture on the expression of TNF-α mRNA and the level of TNF-α in ischemic penumbra in cortices of rats with cerebral ischemia-reperfusion. Zhongguo Zhongyi Jizheng. 2007;16:695–697. [Google Scholar]
- Du Y, Shi L, Li J, Xiong J, Li B, Fan X. Angiogenesis and improved cerebral blood flow in the ischemic boundary area were detected after electroacupuncture treatment to rats with ischemic stroke. Neurol Res. 2011;33:101–107. doi: 10.1179/016164110X12714125204317. [DOI] [PubMed] [Google Scholar]
- ElAli A. The implication of neurovascular unit signaling in controlling the subtle balance between injury and repair following ischemic stroke. Neural Regen Res. 2016;11:914–915. doi: 10.4103/1673-5374.184485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görner A, Lemmens R, Schrooten M, Thijs V. is leukoaraiosis on CT an accurate surrogate marker for the presence of microbleeds in acute stroke patients? J Neurol. 2007;254:284–289. doi: 10.1007/s00415-006-0311-z. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Liu M, Counsell C, Wardlaw JM, Lin S, Zhao X. Fibrinogen depleting agents for acute ischaemic stroke. Cochrane Database Syst Rev. 2012:CD000091. doi: 10.1002/14651858.CD000091.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennerici MG, Kay R, Bogousslavsky J, Lenzi GL, Verstraete M, Orgogozo JM. Intravenous ancrod for acute ischaemic stroke in the European Stroke Treatment with Ancrod Trial: a randomised controlled trial. Lancet. 2006;368:1871–1878. doi: 10.1016/S0140-6736(06)69776-6. [DOI] [PubMed] [Google Scholar]
- Huang LJ. Effects of electro-acupuncture on ultrastructure of brain tissue in rats with focal cerebral ischemia. Zhongguo Zhongyi Jizheng. 2012;21:570–571. [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iwasawa E, Ichijo M, Ishibashi S, Yokota T. Acute development of collateral circulation and therapeutic prospects in ischemic stroke. Neural Regen Res. 2016;11:368–371. doi: 10.4103/1673-5374.179033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DE, del Zoppo GJ, Demaerschalk BM, Demchuk AM, Diener HC, Howard G, Kaste M, Pancioli AM, Ringelstein EB, Spatareanu C, Wasiewski WW. Ancrod in acute ischemic stroke: results of 500 subjects beginning treatment within 6 hours of stroke onset in the ancrod stroke program. Stroke. 2009;40:3796–3803. doi: 10.1161/STROKEAHA.109.565119. [DOI] [PubMed] [Google Scholar]
- Li GP, Shi L, Du YH, Li Y. Effect of electro-acupuncture and collateral circulation re-establish in early stage of cerebral infarction. Tianjin Zhongyiyao Daxue Xuebao. 2011;30:102–104. [Google Scholar]
- Li J, Ma Y, He JJ, Cui JJ, Du YH. Effects of electroacupuncture on expression of VEGF/Flt-1 mRNA and protein in rats with acute cerebral infarction. Tianjin Zhongyiyao. 2014;31:425–429. [Google Scholar]
- Lim SM, Yoo J, Lee E, Kim HJ, Shin S. Acupuncture for spasticity after stroke: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2015;2015:870398. doi: 10.1155/2015/870398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Du YH. Effects of electroacupunture on cAMP in brain arterial smooth muscle cells in acute cerebral infarction rat. Zhongguo Zhongyi Jichu Yixue Zazhi. 2007;13:860–862. [Google Scholar]
- Lv Y, Du YH, Xu YL, Cui JJ, Yang LH, Gao L, He JJ, Li J. Effect of electroacupuncture at “Shuigou” (GV26) on immunoactivity and content of protein kinase C in the middle cerebral artery in acute cerebral infarction rats. Zhenci Yanjiu. 2015;40:219–223. [PubMed] [Google Scholar]
- Ma YY, Li GQ, Wang HP, Huang HR, Fu M, Lai ZY. Effect of electroacupuncture of different acupoints on GAP-43 expression after focal cerebral infarction in rats. Chongqing Yike Daxue Xuebao. 2011;36:38–41. [Google Scholar]
- Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, Shalinsky DR, Hu-Lowe DD, McDonald DM. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Yi SH, Lee JA, Hwang PW, Yoo HC, Kang KS. Acupuncture for the treatment of spasticity after stroke: a meta-analysis of randomized controlled trials. J Altern Complement Med. 2014;20:672–682. doi: 10.1089/acm.2014.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XJ, Ma HF. Effect of electroacupuncture on the proliferation of stem cells in the subependymal zone of the lateral ventricle of the brain in rats with hyperlipemia and cerebral ischemia. Zhen Ci Yan Jiu. 2010;35:175–181. [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Sandercock PA, Counsell C, Kamal AK. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2008:CD000024. doi: 10.1002/14651858.CD000024.pub3. [DOI] [PubMed] [Google Scholar]
- Shi L, Du YH. Effect of electroacupuncture on rCBF of rats with acute cerebral infarction. Tianjin Zhongyiyao Daxue Xuebao. 2012;31:85–87. [Google Scholar]
- Singh P, Doostkam S, Reinhard M, Ivanovas V, Taschner CA. Immunohistochemical analysis of thrombi retrieved during treatment of acute ischemic stroke: does stent-retriever cause intimal damage. Stroke. 2013;44:1720–1722. doi: 10.1161/STROKEAHA.113.000964. [DOI] [PubMed] [Google Scholar]
- Skovseth DK, Yamanaka T, Brandtzaeg P, Butcher EC, Haraldsen G. Vascular morphogenesis and differentiation after adoptive transfer of human endothelial cells to immunodeficient mice. Am J Pathol. 2002;160:1629–1637. doi: 10.1016/S0002-9440(10)61110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun DW, Du YH, Shi L. Effect of electroacupuncture on inositol triphosphate and diacylglycerol levels in cerebral arteries of cerebral ischemia rats. Zhen Ci Yan Jiu. 2008;33:392–396. [PubMed] [Google Scholar]
- Taylor CJ, Weston RM, Dusting GJ, Roulston CL. NADPH oxidase and angiogenesis following endothelin-1 induced stroke in rats: role for nox2 in brain repair. Brain sciences. 2013;3:294–317. doi: 10.3390/brainsci3010294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YY, Fan XN, Wang S, Yang S, Shi XM. Specificity effect of acupuncture at “Shuigou” (GV 26) on brain infarction area in MCAO rats and the influence of acupuncture parameter. Zhongguo Zhen Jiu. 2010;30:221–225. [PubMed] [Google Scholar]
- Xu YL, Gao L, Shi L, Li J, Liu WH, Du YL. Empirical study about the change of vascular smooth muscle's Cap and Cad expressions of cerebral infarction rats adjusted by acupuncture. Liaoning Zhongyi Zazhi. 2012;39:1864–1866. [Google Scholar]
- Yan XY. Effect of electroacupuncture on free radical content and HSP70 expression in the brain tissue in rats with cerebral ischemia-reperfusion injury. Zhen Ci Yan Jiu. 2007;32:102–104. [PubMed] [Google Scholar]
- Yang LH, Du YH, Li J. Effect of electroacupuncture on expression of apelin-apj system of cerebral vascular endothelial cell in rats with cerebral infarction. Zhenci Yanjiu. 2017;42:9–13. [PubMed] [Google Scholar]
- Zhong YH, Tang XJ, Li GQ, Huang HR. Effects of electroacupuncture at different acupoints on neuroethology and expression of neurocan after focal cerebral infarction in rats. Zhongguo Kangfu Lilun yu Shijian. 2013;19:444–447. [Google Scholar]
- Zhou HW, Zhao BD, Wen PS. Influence of nerve growth factor on the expression of nestin and neural stem cell type in rats with focal cerebral ischemia. Zhongguo Zuzhi Gongcheng Yanjiu. 2007;11:1222–1224. [Google Scholar]