Abstract
The continuing HIV/AIDS epidemic and the spread of multi-drug resistant Mycobacterium tuberculosis has led to the perpetuation of the worldwide tuberculosis epidemic. While M. bovis BCG is widely used as a vaccine, it lacks efficacy in preventing pulmonary tuberculosis in adults [1]. To combat this ongoing scourge, vaccine development for tuberculosis is a global priority. Most infected individuals develop long-lived protective immunity, which controls and contains M. tuberculosis in a T cell-dependent manner. An effective T cells response determines whether the infection resolves or develops into clinically evident disease. Consequently, there is great interest in determining which T cells subsets mediate anti-mycobacterial immunity, delineating their effector functions, and evaluating whether vaccination can elicit these T cells subsets and induce protective immunity. CD4+ T cells are critical for resistance to M. tuberculosis in both humans and rodent models. CD4+ T cells are required to control the initial infection as well as to prevent recrudescence in both humans and mice [2]. While it is generally accepted that class II MHC-restricted CD4+ T cells are essential for immunity to tuberculosis, M. tuberculosis infection elicits CD8+ T cells responses in both people and in experimental animals. CD8+ T cells are also recruited to the lung during M. tuberculosis infection and are found in the granulomas of infected people. Thus, how CD8+ T cells contribute to overall immunity to tuberculosis and whether antigens recognized by CD8+ T cells would enhance the efficacy of vaccine strategies continue to be important questions.
1 Introduction
The continuing HIV/AIDS epidemic and the spread of multi-drug resistant Mycobacterium tuberculosis has led to the perpetuation of the worldwide tuberculosis epidemic. While M. bovis BCG is widely used as a vaccine, it lacks efficacy in preventing pulmonary tuberculosis in adults [1]. To combat this ongoing scourge, vaccine development for tuberculosis is a global priority. Most infected individuals develop long-lived protective immunity, which controls and contains M. tuberculosis in a T cell-dependent manner. An effective T cells response determines whether the infection resolves or develops into clinically evident disease. Consequently, there is great interest in determining which T cells subsets mediate anti-mycobacterial immunity, delineating their effector functions, and evaluating whether vaccination can elicit these T cells subsets and induce protective immunity. CD4+ T cells are critical for resistance to M. tuberculosis in both humans and rodent models. CD4+ T cells are required to control the initial infection as well as to prevent recrudescence in both humans and mice [2]. While it is generally accepted that class II MHC-restricted CD4+ T cells are essential for immunity to tuberculosis, M. tuberculosis infection elicits CD8+ T cells responses in both people and in experimental animals. CD8+ T cells are also recruited to the lung during M. tuberculosis infection and are found in the granulomas of infected people. Thus, how CD8+ T cells contribute to overall immunity to tuberculosis and whether antigens recognized by CD8+ T cells would enhance the efficacy of vaccine strategies continue to be important questions.
2 Do CD8+ T Cells Contribute to Immunity Against Tuberculosis?
In 1992, Flynn and colleagues showed that mice lacking β2-microglobulin (β2m) succumb rapidly following IV infection [3]. Because β2m is required for assembly and trafficking of the class I heavy chain, no class I MHC is expressed on the cell surface in the absence of β2m. Consequently, class I MHC-restricted CD8+ T cells fail to be positively selected during thymic development, leading to a developmental deficiency of CD8+ T cells. These data were the first truly convincing evidence that CD8+ T cells were required for optimum resistance to tuberculosis. However, this interpretation was confounded by the fact that other β2m-associated proteins exist and some function as antigen presenting molecules including CD1, H2-M3, and class Ib MHC proteins [4, 5]. Subsequently, a comparison of transporter associated with antigen processing (TAP)-1 knockout (−/−) mice (defective in class I MHC processing) and CD1d−/− mice, demonstrated a requirement for an intact class I MHC antigen processing pathway, and confirmed the requirement for CD8+ T cells in resistance to M. tuberculosis [6]. Mice with disruptions in the β2m or TAP1 genes are unable to control M. tuberculosis replication in the lung and die prematurely compared to normal mice following infection via the intravenous or aerosol route [3, 6, 7]. The increased susceptibility of CD8−/− mice and the class I MHC heavy chain knockout (KbDb−/−) further corroborated the requirement for CD8+ T cells following primary infection [8, 9]. In addition to these genetic models, a variety of other experimental approaches confirm that CD8+ T cells mediate protection against tuberculosis [reviewed in [10]]. These include CD8+ T cells deletion, adoptive transfer of CD8+ T cells, and vaccination to elicit CD8+ T cells, all which show that CD8+ T cells are required for optimal immunity against virulent M. tuberculosis. Thus, antibody-mediated depletion of CD8+ T cells in vivo increases host susceptibility, while adoptive transfer of purified CD8+ T cells enhances host resistance [11–13]. While the above-cited studies using the mouse model show that CD8+ T cells play a crucial role in immunity to M. tuberculosis, the relative contribution of CD8+ T cells still needs to be more clearly defined. For example, it is not known when during the natural history of tuberculosis CD8+ T cells are important, how CD8+ T cells mediate their protective effect, and whether CD8+ T cells perform any unique effector functions. The possibility that CD8+ T cells adversely affect host immunity to tuberculosis and worsen disease under some circumstances has not been ruled out.
Furthermore, several misconceptions exist concerning the role of CD8+ T cells during infection. For example, CD4+ T cells are perceived to be more important than CD8+ T cells in host immunity. This is based in part on studies showing that mice lacking CD4+ T cells are more susceptible than mice lacking CD8+ T cells [11]. However, it is now understood that CD4+ T cells are required for the development of CD8+ memory T cells. Therefore, mice lacking class II MHC are more susceptible to tuberculosis because they lack CD4+ T cells, but also because they mount a suboptimal CD8+ T cell response. On the other hand, the ability of DNA vaccination to prolong survival and decrease bacterial load after aerosol infection in CD4−/− mice supports a protective role for class I MHC-restricted CD8+ T cells [14, 15]. However, these data need to be re-examined. It has been known for some time that CD4−/− mice have class II MHC-restricted T cells [16]. Now there is evidence that these class II MHC-restricted T cells make up a large fraction of the residual T cells. Furthermore, many of these class II MHC-restricted T cells express CD8 [17]. Thus, depletion of CD8+ T cells in the CD4−/− model does not prove that class I MHC-restricted T cells mediate immunity following vaccination and CD4−/− mice are unlikely to reveal the full potential of CD8+ T cells to protect mice from tuberculosis. Finally, class I MHC has other functions distinct from antigen presentation such as acting as an inhibitory ligand for NK cell receptors [18]. Thus, the exact contribution of CD8+ T cells in host defense against M. tuberculosis remains to be delineated.
Perhaps the most important issue is whether CD8+ T cells mediate immunity against M. tuberculosis in people. Although at this time, we cannot definitively answer this question, data that CD8+ T cells are crucial for immunity to M. tuberculosis in non-human primates [19] and cattle [20, 21] bolster the argument that CD8+ T cells are likely to be relevant to mycobacterial infection in general. The results of ongoing vaccination studies have the greatest potential to determine their true relevance in people. However, there is abundant circumstantial data that infected people generate CD8+ T cells and those CD8+ T cells express effector functions that can suppress bacterial growth [22–24]. The study of human CD8+ T cells has also identified T cells distinct from class Ia MHC-restricted CD8+ T cells—such as γδ-TCR+ T cells, CD1-restricted T cells, and MAIT cells—which also are activated and specifically recognize antigens produced by M. tuberculosis. These additional subsets of CD8+ T cells have been reviewed elsewhere [25–27].
3 Which Mycobacterial Antigens are Recognized by CD8+ T Cells?
While CD4+ T cells primarily recognize antigens that enter the endocytic pathway and are presented by class II MHC, most CD8+ T cells recognize short peptides of 8–10 amino acids derived from cytosolic proteins that are presented by class I MHC molecules. Under certain conditions, class IMHC can present antigens that enter the endocytic pathway, a process known as cross-presentation. Both self and foreign proteins in the cytosol are cleaved by the proteosome and the resulting peptides are translocated from the cytosol into the endoplasmic reticulum (ER) by the TAP1/TAP2 heterodimer. Once in the ER, the peptides assemble with the class I MHC heavy chain and β2m to form a trimeric complex, which is then transported to the cell surface. The sampling of the cytosol by class I MHC explains why CD8+ T cells are critical for host resistance to viral infections and certain intracellular bacterial infections. One impediment to understanding the role of class I MHC-restricted CD8+ T cells in immunity to tuberculosis is the paucity of mycobacterial antigens that are known to be recognized by CD8+ T cells. Following macrophage infection, M. tuberculosis survives and replicates in the phagosome. Just how bacterial antigens traffic from the phagosome to the cytoplasm where they can enter the class I MHC processing pathway is a matter of controversy and several mechanisms have been proposed [28, 29]. Ultimately, mycobacterial antigens do enter the class IMHC pathway, since class I MHC-restricted CD8+ T cells are elicited by infection in both people and experimental animals.
Secreted M. tuberculosis protein antigens have been extensively studied in part because they are targets of T cell-mediated immunity [30]. Antigens such as Antigen 85 (Ag85), early secretory antigen target-6 (ESAT6) (esxA; Rv3875), culture filtrate protein-10 (CFP10) (esxB; Rv3874), and others elicit strong CD4+ T cells responses in both mice and humans [31, 32]. In contrast, fewer M. tuberculosis antigen epitopes have been identified that are recognized by CD8+ T cells. In 2000, Lewinsohn et al. cloned CD8+ T cells from a PPD+ individual using autologous M. tuberculosis infected DC [24]. Four of the CD8+ T cells clones were class I MHC-restricted and two recognized distinct peptide epitopes derived from the CFP10 protein [8]. These data are significant for several reasons. First, it demonstrates that CFP10 has access to the class I MHC processing pathway and shows that CFP10-specific CD8+ T cells are primed following infection. Second, CFP10-specific CD8+ T cells clones recognize infected cells, which indicates their potential to act as effector cells [8, 33]. Finally, CFP10-specific CD8+ T cells were detected at frequencies as high as 1/700 total CD8+ T cells, suggesting that CFP10 can be an immunodominant antigen in people. A study by Shams et al. defined a 15mer peptide (CFP1071–85), which contains at least two distinct epitopes, each one that can be presented by class I and II MHC to both CD8+ and CD4+ T cells, respectively [33]. The promiscuity of these epitopes for different alleles of class I and class II MHC proteins may explain why nearly all of people with latent M. tuberculosis infection recognize CFP10.
CFP10 is encoded within the RD1 locus of the M. tuberculosis genome, a region of DNA that is present in M. tuberculosis and pathogenic M. bovis strains, but is deleted from all BCG strains [34–39]. RD1 appears to be the original deletion that resulted in the attenuation of M. bovis BCG, the widely used vaccine strain. Through targeted mutation, it is now confirmed that the RD1 locus is critical for the virulence of M. tuberculosis. The RD1 locus encodes several proteins that by definition are implicated in virulence. Two of the proteins encoded within the RD1 locus are CFP10 and ESAT6, which are secreted as a heterodimer [40]. The cfp10 gene is in the same operon as the esat6 gene, and the two genes share 40 % sequence homology and belong to the ESAT6 family of small proteins [36, 39, 41]. In addition to RD1, M. tuberculosis H37Rv has 11 distinct loci that encode 23 ESAT6-related proteins (reviewed in [42]). This extended family of ESAT6-related proteins has been given the name EsxA through EsxW, with the best characterized ESAT6 and CFP10 proteins named EsxA and EsxB. Other proteins in the RD1 locus, adjacent to ESAT6 and CFP10, are required for the secretion of the CFP10 and ESAT6, and together, the locus appears to encode a specialized secretory apparatus, which is referred to as ESX-1.
Just as CFP10 elicits antigen-specific CD8+ T cells following M. tuberculosis infection, specific epitopes within the ESAT6 protein are recognized by CD8+ T cells obtained from the blood of infected individuals. Lalvani et al. identified two class I MHC-restricted epitopes from the ESAT6 protein in an individual with active tuberculosis [43]. In a second study, CD8+ T cells specific for ESAT6 were detected in two unrelated individuals; one a PPD+ household contact and a second with untreated healed tuberculosis [44]. In both cases the CD8+ T cells recognized ESAT621–29 presented by HLA-A68.02 and these T cells were present at a frequency of up to 1/2500 peripheral blood lymphocytes. The frequency of T cells recognizing ESAT621–29 was similar to the frequency of T cells specific for PPD. Furthermore, the frequency of ESAT621–29-specific CD8+ T cells was stable during 21 months of follow-up. Pathan et al. argue that the persistent high frequency of these T cells suggest that ESAT6-specific CD8+ T cells have a role in protection against tuberculosis [44].
These observations prompted my own lab to ascertain whether members of the ESAT6-related family of proteins are targets of the CD8+ T cells response in the mouse model. We identified two epitopes from the CFP10 protein: CFP1011–25 and CFP1032–39, which are recognized by CD4+ and CD8+ T cells, respectively. The CFP1032–39-specific CD8+ T cells were H-2 Kk-restricted and were elicited following infection with virulent M. tuberculosis (Erdman and H37Rv) but not H37Ra or BCG. CFP1032–39-loaded Kk-tetramers identified CFP10-specific CD8+ T cells in the lung, spleen and LN. Surprisingly, nearly 30 % of pulmonary CD8+ T cells recognize CFP1032–39, demonstrating the CFP10 is an immunodominant antigen in mice of the H-2 k haplotype [45]. We also studied other family members including TB10.3 (esxR; Rv3019c) and TB10.4 (esxH; Rv0288) [46–48]. These two proteins both contain an identical epitope (TB10.3/10.420–28), which is recognized by H-2 Kd-restricted CD8+ T cells following respiratory M. tuberculosis infection [45, 49]. As many as 30–40 % of the CD8+ T cells in the lungs of BALB/c mice recognize this epitope and these CD8+ T cells have cytolytic activity both in vitro and in vivo [45, 50]. The identification of immunodominant epitopes such as CFP1032–39 and TB10.3/10.420–28 that are recognized by CD8+ T cells following infection is critical for studies elucidating the key elements of protective immunity. In contrast to CD8+ T cells that recognize subdominant epitopes, the high frequency of CD8+ T cells that are specific for dominant epitopes can facilitate studies on the activation, differentiation, trafficking, and effector functions of these T cells.
There now exist five well-characterized epitopes that are recognized by murine CD8+ T cells elicited by infection. Three of these, TB104–11, TB1020–28, and CFP1032–29 are immunodominant in H-2b, 2d, or 2k mice, respectively, and represent 20–50 % of the CD8+ T cells in the lungs of chronically infected mice [45, 50]. The other two (32C, EspA) account for 1–10 % of CD8+ T cells [51–53]. It is quite remarkable that a number of ESAT6-related proteins including ESAT6 (EsxA), CFP10 (EsxB), and TB10.3/10.4 (EsxR/H) generate CD8+ T cells responses in infected people and mice. Why this group of proteins should be so frequently recognized by T cells is unknown. However, as they are all small secreted proteins, they may efficiently enter the class I and class II MHC antigen processing pathways (see below).
4 What is the Basis for the Extreme Immunodominance of Certain Mycobacterial Antigens Recognized by CD8+ T Cells Following M. tuberculosis Infection?
During infection, pulmonary CD8+ T cells responses are dominated by large expansions of T cells that recognize a limited number of antigens. While the identity of the immunodominant bacterial epitopes varies from person to person, people infected with M. tuberculosis also mount CD8+ T cells responses that preferentially recognize a limited number of bacterial epitopes [22]. The person-to-person variation in which epitopes are immunodominant presumably reflects the HLA diversity of people. Why there should be such extreme immunodominance in a complex microorganism with ~4,000 genes is unknown. Some possibilities include the kinetics of the response (do early responses become immunodominant?), the amount of antigen produced by the bacteria (do abundant proteins drive immunodominance), or T cells recognizing immunodominant epitopes are overrepresented in the naive T cells repertoire. An important question is whether the immunodominance of certain antigens is beneficial or detrimental to the host. Investigators studying the CD8+ T cells response to HIV debate a similar issue [54]. Some believe that a more diverse T cells response is associated with better host protection. Based on paradigms established studying other pathogens, immunodominant antigens could serve as decoys that focus the host response on antigens that do not lead to protective immunity. Such questions have been difficult to address to date, since bacterial genes that are required for bacterial virulence encode nearly all of the epitopes that have been defined (including TB10.4 and CFP10).
Antigen-specific CD8+ T cells responses to three different proteins have been intensively studied in the mouse model: TB10.4, CFP10, and EspA (Rv3616c). Both CFP10 and TB10 contain epitopes that are recognized by CD4+ and CD8+ T cells. This facilitates comparison of the kinetics of the CD4+ and CD8+ T cells responses to a single protein following infection. The CD4+ and CD8+ T cells response to CFP10 and to TB10.4 develop with nearly identical kinetics [45]. These responses develop early during infection and the increase in CFP10- and TB10-specific CD4+ and CD8+ T cells closely parallel the pulmonary bacterial burden. While both CD4+ and CD8+ T cells responses are detected within 2 weeks of infection, the CD4+ T cells response generally peaks slightly earlier (4–5 weeks post infection) than the CD8+ T cells response (5–8 weeks). The frequency of antigen-specific T cells in the spleen is substantially lower although the relative hierarchy of immunodominance is similar.
We considered whether the immunodominance of these epitopes is related to their initial precursor frequency in the naïve T cells repertoire. Immunomagnetic selection of tetramer bound T cells followed by dual-color tetramer staining and analysis by flow cytometry can sensitively and specifically enumerate naïve antigen-specific T cells [55, 56]. We determined the precursor frequency of naïve CD8+ T cells that recognize the immunodominant epitope TB10 as well as a less dominant epitope, EspA, in naïve BALB/c mice. For comparison, we also determined the frequency of F226–34 a well-characterized vaccinia epitope. Relative to the frequency of F226–34-specific naïve CD8+ T cells in uninfected mice (~1:16,000), TB10.4 (~1:416,000) and EspA (~1:778,000)-specific naïve CD8+ T cells were 25–50-fold less abundant. In addition, the frequency of naïve TB10.4-specific CD8+ T cells was consistently 1.9-fold greater than EspA-specific CD8+ T cells; however, we do not believe that this two-fold difference is sufficient to explain the observed dominance of the TB10.4-specific response following M. tuberculosis infection. While the precursor frequency did not explain the extreme immunodominance of TB10-specific CD8+ T cells response, these data allow one to assess the magnitude of the expansion of antigen-specific T cells following infection. Assuming that the entire repertoire of a naïve mouse has ~50 TB10-specific CD8+ T cells, the detection of 500,000 TB10-specific CD8+ T cells in the lung at the height of the response to tuberculosis represents at least a 10,000-fold expansion.
5 Cross-Priming of CD8+ T Cells
The process by which a naïve T cells becomes activated, which allows the T cells to leave the lymph node, proliferate, and express different effector functions, is known as T cells priming. Conventional wisdom is that T cells priming occurs in the draining lymph node and the dendritic cell (DC) is the antigen-presenting cell (APC) that most efficiently is able to present microbial antigens and activate T cells. As described above, the sequence of events that lead to activation of CD4+ T cells is reasonably well understood. DC, which survey different tissues, will become activated when they encounter microbial products or become infected. DC activation allows the cell to migrate from the peripheral tissue to the draining LN where they can interact with naïve T cells. Because most such encounters with bacteria or bacterial products lead to acquisition of the microbial antigens through phagocytosis or macropinocytosis, the antigen enters the endocytic pathway, which intersects with the class II MHC presentation pathway. For example, viable M. tuberculosis survives in intracellular compartments including the phagosome. One can easily imagine how its secreted antigens are selectively presented via the class II MHC pathway to CD4+ T cells. It is considerably less clear how antigens from M. tuberculosis generate CD8+ T cell responses. How bacterial antigens traffic from the phagosome to the cytoplasm where they can enter the class I MHC processing pathway is a matter of controversy.
How do DC acquire mycobacterial antigens?
Following aerosol infection, the dogma is that M. tuberculosis preferentially infects alveolar macrophages. This view is beginning to change as we recognize (at least in the mouse model) that numerous types of myeloid cells are infected soon after infection. In addition to macrophages, there is good evidence that neutrophils and DC can both be directly infected [57–59]. Furthermore, although there is evidence that immunity can be initiated in the lung [60], most investigators have found that immunity to tuberculosis is initiated in the local draining LNs of the lung [57, 59, 61, 62]. Since the DC is specialized for migrating from tissue beds to the lymph node, this is strong circumstantial evidence that acquisition of mycobacterial antigens by DC is required for T cell priming. It should be noted that we are beginning to realize that other cell types such as neutrophils can also become infected and traffic to the LN where they can prime T cells although the physiological relevance of this process is still be worked out [58]. We have previously shown that DC are required to generate CD4+ T cells immunity following M. tuberculosis infection. By taking advantage of the CD11c/DTR Tg mouse model, which allows transient ablation of DC, we demonstrated that DC are required to prime ESAT6-specific CD4+ T cells. In addition, deletion of CD11c+ cells and the subsequent delay in adaptive immunity impairs control of M. tuberculosis replication. While these experiments show a critical role for DC in the initiation of adaptive immunity to M. tuberculosis, they do not shed light on how DC acquire mycobacterial antigen, which can occur by two different processes. Although we have known for some time that both human and murine DC can be infected experimentally in vitro, there is now good evidence that DC are directly infected in vivo. Nevertheless, it has been difficult to ascertain whether the DC that are directly infected are the same DC that traffic to the draining LN and prime T cells. A second possibility is that DC acquire mycobacterial antigen indirectly. Immature tissue DC can phagocytose dead bacteria, bacteria products, and apoptotic vesicles that contain mycobacterial antigens.
Although M. tuberculosis can grow extracellularly, it is observed to be mostly intracellular except during active disease when it can reach great numbers at the center of caseous granulomas. It is unlikely that its antigens would be found in the extracellular space in the absence of cell death. Thus, it should come as no surprise that the cell death modality of M. tuberculosis infected macrophages influences innate and adaptive immunity. During M. tuberculosis infection, cellular necrosis and loss of plasma membrane integrity occurs, which among other things allows the bacteria to infect other cells [63–66]. Apoptosis of M. tuberculosis infected cells enhances innate control of intracellular bacterial replication as well as augments T cell-mediated immunity. Previous studies delineated the importance of apoptosis in cross-presentation of M. tuberculosis antigens by human DC in vitro or by apoptotic vesicles purified from BCG infected macrophages in vivo [67, 68]. We were particularly interested in how the death modality of host macrophages infected with live M. tuberculosis in vivo affected initiation of T cells immunity. To explicitly study this question, we established a novel adoptive transfer model in which macrophages from wild-type or knockout mice are infected in vitro with M. tuberculosis and then transferred into normal recipient mice. This strategy allows one to determine how the macrophage genotype influences T cells immunity and control of infection. Transfer of M. tuberculosis infected pro-apoptotic macrophages enhanced T cells immunity and led to better protection than observed after transfer of infected pro-necrotic or wild-type macrophages [69]. The enhanced antigen-specific CD8+ T cells response requires presentation of antigen derived from apoptotic macrophages by the Detour pathway [67, 68, 70], as defined by TAP-1-dependent and class I MHC-restricted cross-presentation by DC [28, 67, 69]. Enhanced T cell-mediated immunity was not restricted to CD8+ T cells and we observed that M. tuberculosis-specific CD4+ responses were also increased after transfer of infected pro-apoptotic macrophages. Thus, while apoptosis has been directly linked to increased CD8+ T cells responses via cross-presentation, it also enhances class II MHC-restricted antigen presentation. Presumably, DC phagocytosis of apoptotic vesicles delivers their cargo of M. tuberculosis antigens to the endocytic system, which intersects with the MHC II processing pathway, ultimately increasing priming of CD4+ T cells. We speculate that the proinflammatory function of apoptosis during infection, compared to its anti-inflammatory propensity during physiological cell death, may be a consequence of TLR ligands and other microbe-derived signals that are contained in apoptotic vesicles that activate DC upon their acquisition.
Why are secreted antigens preferentially presented to CD8+ T cells?
Although the number of antigens that have been discovered to be recognized by CD8+ T cells elicited by M. tuberculosis is still relatively small, it is striking that proteins secreted by M. tuberculosis are overrepresented [10, 22, 71, 72]. Secreted M. tuberculosis protein antigens have been extensively studied as targets of T cell-mediated immunity in part because they are easy to obtain in purified form. These include antigens such as Ag85, ESAT6, and CFP10, which elicit strong CD4+ T cells responses in both mice and humans. Another feature is that most of the antigens are small MW proteins. In a limited number of cases, it has been shown that protein secretion is required for priming of CD8+ T cells during infection. The best characterized is the response to CFP10. As discussed above, CFP10 is secreted by the ESX-1 type 7-secretion system, and bacterial mutants that have defects in this secretion apparatus produce ESAT6 and CFP10 but do not secrete them. If M. tuberculosis does not secrete CFP10, the host does not generate a CFP10-specific CD8+ T cells response following in vivo infection [73]. As the ESX-1 locus is required for bacterial virulence, another interpretation of these data is that the capacity of the bacteria to grow and induce inflammation modulates the host CD8+ T cells response. To address this issue, we evaluated an interesting point mutant of EspA (Rv3616). The protein EspA is not encoded in the ESX-1 locus, but is required for the function of the ESX-1 complex [74]. The point mutant secretes CFP10, ESAT6, and EspA, but no longer assembles the ESX-1 complex, and consequently, is attenuated [53]. Following in vivo infection with this mutant, a CFP10-specific CD8+ T cell response is generated, showing that virulence and immunogenicity of the ESX-1 locus can be dissociated [53]. These data are consistent with the hypothesis that secretion is a prerequisite for T cells priming and occurs independently of the virulence of the bacteria.
Once the mycobacterial antigens are in the endosomal compartment of DC, how do they enter the class I MHC processing pathway?
The propensity of secreted mycobacterial antigens to be recognized by CD8+ T cells may provide some clues as to how such antigens enter the class I MHC processing pathway (Fig. 1). Our thinking has changed from the idea that M. tuberculosis is a pure phagosomal pathogen to the realization that under certain circumstances, either the bacteria or bacterial products can gain access to the cytosol. For example, van der Waal et al. showed that virulent but not avirulent M. tuberculosis can translocate from the phagosome into the cytosol [75]. This paradigm-shifting observation potentially solves the problem of how mycobacterial antigens enter the class I MHC processing pathway. If the bacteria can survive in the cytosol, there is no need for cross-presentation. The class I MHC pathway could directly sample bacterial proteins secreted into the cytosol. Despite the attractiveness of this model, there are some problems with this idea. First, the kinetics of the class I MHC antigen presentation is rapid and bacterial antigens are presented to CD8+ T cells before one can detect the first evidence of escape [76]. Second, it does not explain how the CD8+ T cells responses elicited by less virulent bacteria, which do not escape, are generated. For example, although bacteria with mutations in ESX-1 escape and do not generate CFP10-specific CD8+ T cells responses, they do generate CD8+ T cells responses to other antigens that are secreted by mechanisms that are independent of the ESX-1 type VII secretion system. An alternate view of bacterial translocation into the cytosol is that breakdown of the phagosomal membrane is associated with necrosis, and the appearance of cytosolic bacteria is a transient state before cellular necrosis occurs, allowing the bacteria to disseminate [77]. The significance of cytosolic bacteria for generating CD8+ T cells responses remains to be determined.
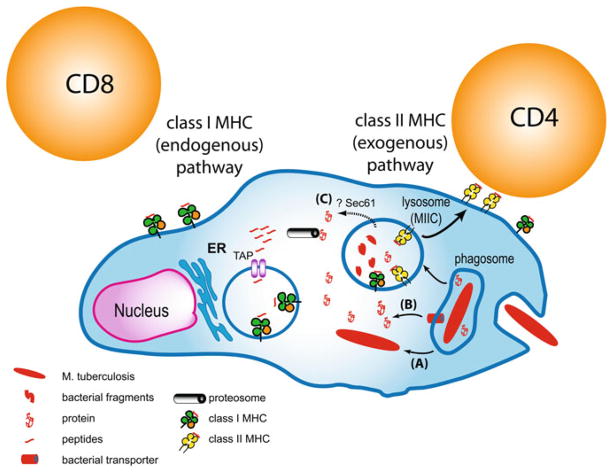
Fig. 1.
Antigen presentation pathways in infected APC. Mycobacterial peptides can be loaded onto class II MHC within the endocytic pathway. How mycobacterial peptides enter the class I MHC pathway is unknown. One possibility is that (a) the bacterium can translocate from the phagosome and enter the cytosol. Its secreted products could then be processed by the proteasome and the peptide products transported into the ER by the TAP proteins. Alternately, a bacterial secretion system, such as ESX-1, might actively transport secreted proteins across the phagosomal membrane (b). Another possibility is that host machinery, such as sec61, which performs retrograde translocation of proteins, could transport bacterial proteins into the cytosol (C)
Short of bacterial translocation, there is increasing evidence that M. tuberculosis has the capacity to damage host cells membranes and it is likely that this includes damage to the phagosomal membrane. Damage of the phagosomal membrane would allow bacterial products to leak out of the phagosome and into the cytosol. Alternately, active transport by a secreted bacterial transport apparatus (similar to the ESX-1 type VII secretion system) could translocate bacterial proteins across the phagosomal membrane. Both of these scenarios would introduce mycobacterial antigens into the cytosol of the infected cell where they could be sampled by the class I MHC processing pathway.
Finally, the finding that uninfected DC that take up apoptotic infected cells cross-present mycobacterial antigens, leading to cross-priming of CD8+ T cells, indicates that phagosomal antigens in DC have the capacity to enter the class I MHC processing pathway whether or not there are viable bacteria present [28, 67–70]. The bias towards presentation of secreted proteins could arise if soluble proteins are preferentially packaged into apoptotic blebs. How the antigens get out of the apoptotic vesicles, which are taken up by efferocytosis, and into the cytosol, still remains a formidable problem to solve. There must be host mechanisms that facilitate sampling of antigens from endocytic compartments. One possible mechanism involves the host protein Sec61, which normally mediates retrograde protein translocation as a way to transport misfolded proteins back into the cytosol where they can be targeted for degradation. Proteins secreted by M. tuberculosis into the phagosomal compartment might be translocated by Sec61 across the phagosomal membrane into the cytosol where they could enter the class I MHC processing pathway [78].
6 Effector Functions of CD8+ T Cells During M. tuberculosis Infection
T cells immunity is crucial for controlling M. tuberculosis infection and both CD4+ and CD8+ T cells play important roles in mediating host protection. The dominant paradigm of T cells immunity to tuberculosis postulates that interferon-γ (IFNγ) plays a central role in host defense. This model is supported by the finding that IFNγ is essential for immunity in both humans and in animal models [79–85]. T cells are the main producers of IFNγ and IFNγ stimulates antibacterial activity in M. tuberculosis infected macrophages. This is the basis for the (oversimplified) schema that the IFNγ production by T cells activates microbicidal activity in macrophages, which kills, or at least suppresses, the growth of M. tuberculosis. However, more and more data show that IFNγ has other important roles during M. tuberculosis infection in addition to direct activation of macrophages. It serves an important immunoregulatory role and has anti-inflammatory properties that reduces immunopathology. Conversely, T cells can mediate antimicrobial immunity independent of IFNγ. Finally, and relevant to CD8+ T cells, while IFNγ production by CD8+ T cells can suppress bacterial growth, it is not sufficient to replace IFNγ production by CD4+ T cells. This indicates that CD4+ and CD8+ T cells mediate fundamentally different functions during infection.
There are several unique roles for CD8+ T cells during the immune response to tuberculosis. Although CD8+ T cells appear to be less efficient at suppressing bacterial growth compared to CD4+ T cells, this is a complicated comparison since some of the functions expressed by CD8+ T cells are dependent on CD4+ T cells help [86, 87]. Therefore, in the absence of CD4+ T cells, CD8+ T cells may not function optimally. There is also the possibility that CD8+ T cells do not functional optimally during M. tuberculosis infection because chronic infection impairs T cells immunity. T cells immunity may be suboptimal because chronic inflammation leads to the development of T cells exhaustion or anergy. Alternately, M. tuberculosis may actively evade T cells immunity by impairing antigen presentation. There is great interest in determining whether certain vaccination strategies could enhance immunity to tuberculosis, not only by increasing the priming and expansion of M. tuberculosis-specific CD8+ T cells, but also by increasing the expression of functions that contribute to host resistance. Designing such strategies could be done more rationally if we understood the mechanisms that CD8+ T cells use to inhibit bacterial growth.
While it is unknown how CD8+ T cells mediate protection against tuberculosis, there are several unique functions that CD8+ T cells have. CD8+ T cells are more likely to recognize infected cells [88], while CD4+ T cells can recognize infected cells or cells that have phagocytosed dead bacteria or their antigens. CD8+ T cells can recognize class II MHC negative cells, and while the relevance of such cells is hypothetical, class II MHC expressed by infected macrophages is resistant to upregulation by IFNγ [89, 90], which may allow the bacteria to “hide” from CD4+ T cells; however, class I MHC is not altered under these conditions. This idea is supported by a unique role for CD8+ T cells in controlling bacterial burden during latency, since depletion of CD8+ T cells, but not CD4+, leads to an increase in bacterial replication [91]. Cytokine production (IFNγ, TNF) and cell-mediated cytotoxicity (CTL activity) are the two functions most commonly described for CD8+ T cells. We will now review what is known about these two functions of CD8+ T cells during M. tuberculosis infection.
Cytokine production
Activated CD8+ T cells produce type 1 cytokines including IFNγ and TNF, which activate macrophages to produce nitrogen and oxygen radicals and LRG47 [92–94]. IFNγ production by CD8+ T cells can potentially be protective. Tascon et al. showed that the ability of adoptively transferred naïve CD8+ T cells to protect T cell-deficient recipients required IFNγ [95]. However, it is clear from others that CD8+ T cells, even ones producing IFNγ and inducing NO, cannot replace the protection mediated by CD4+ T cells [14]. Thus, the relative importance of IFNγ secretion by CD8+ T cells in hosts with intact immunity is unknown.
CTL activity
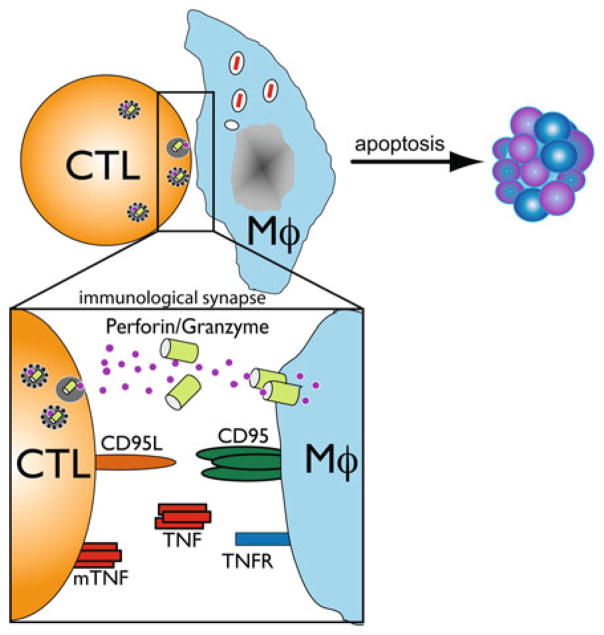
We find that CD8+ T cells elicited by M. tuberculosis infection are cytolytic in vivo [13, 45]. TB10-specific CTL activity is detected within 4 weeks of M. tuberculosis infection and persists for at least 9 months. M. tuberculosis-specific CD8+ T cells lacking perforin have reduced cytolytic activity in vivo, and in the absence of perforin, the residual cytolytic activity is FasL (CD95L) and TNFR-dependent (Fig. 2) [13]. In the lung, disruption of both perforin and FasL eliminates target cell lysis. The importance of CTL activity in mycobacterial immunity remains unknown. Although murine and human M. tuberculosis-specific CD8+ cytolytic T cell lines have been established in vitro, it has been difficult to demonstrate cytolytic activity of freshly isolated CD8+ T cells against infected macrophages [43, 96–99]. Following in vitro stimulation of pulmonary CD8+ T cells with M. tuberculosis infected DC, CD8+ T cells acquire the ability to lyse infected macrophages in vitro, and the lysis is dependent upon granule exocytosis [100].
Fig. 2.
Mechanisms of target cell lysis by CD8+ T cells
Protection
Importantly, immune CD8+ T cells isolated from WT, but not perforin-deficient mice, transfer protection against M. tuberculosis infection to recipient mice [13]. Thus, although M. tuberculosis-specific CD8+ T cells use several cytolytic pathways in a hierarchical and compensatory manner, adoptively transferred M. tuberculosis-specific CD8+ T cells require perforin to protect animals from M. tuberculosis infection. Human CD8+ T cells also require perforin to restrict intracellular M. tuberculosis growth, indicating use of a granule-dependent mechanism [101, 102]. Human CD8+ T cells, which express the antimicrobial peptide granulysin in their cytotoxic granules, also require perforin to restrict intracellular M. tuberculosis growth [101, 102]. For human CD8+ T cells, granulysin is an important granule constituent [101]; the critical effector molecules for murine CD8+ T cells are unknown.
Genetic models
Mice lacking CD8+ T cells (i.e., β2m, TAP1, and CD8 knockout mice) die more rapidly than wild-type mice lacking either CD95 or perforin [7, 9, 103, 104]. This suggests that these two major cytotoxic pathways may be functionally redundant in tuberculosis, as for certain viruses [105–107], or may be compensated by another (undefined) mechanism. On the other hand, none of these molecules are specific for CD8+ T cells and most are expressed by NK cells and some CD4+ T cells. Further complicating the interpretation of these data is the finding that both CD95 and perforin-deficient mice have increased Th1-type cytokine production that may confound a clear interpretation of the knockout results. These results highlight the role of CD95 and perforin in lymphocyte homeostasis and immunoregulation [108]. Despite the imperfect nature of these models, mice lacking perforin, generally succumb to M. tuberculosis late during infection, which suggests that CTL are more important during chronic [7] or even latent [91] infection. CD8+ T cells may be particularly important in controlling bacterial replication during latency, since depletion of CD8+ T cells, but not CD4+, leads to an increase in bacterial burden [91].
Why would killing infected macrophages be beneficial?
Lysis of M. tuberculosis infected macrophages could be beneficial if the released bacteria are subsequently taken up by activated macrophages that can mediate bacterial killing. However, most killing mechanisms used by CD8+ T cells induce target cell apoptosis, which is known to reduce intracellular bacterial viability. Furthermore, apoptosis induced by CD95 ligation on infected macrophages leads to reduced viability of M. tuberculosis and supports a possible role for the CD95/CD95L pathway in the host response [109]. In addition, CD8+ T cells have unique effector mechanisms that can kill intracellular bacteria [102, 110]. For example, human CD8+ T cells express granulysin in their cytotoxic granules that can directly kill intracellular M. tuberculosis [111, 112]. Finally, apoptotic infected cells are engulfed by uninfected macrophages, which are able to more efficiently target the efferosome containing bacteria to the lysosome where the bacteria are destroyed [113].
Are there different subsets of effector CD8+ T cells during the host response to infection?
CD4+ T cells have been divided into different subsets based on their effector functions (Th1, Th2, Th17, Treg, etc.). CD8+ T cells with different functions have been identified (Tc1, Tc2), although it is not clear whether these represent stable phenotypes. In addition, dramatic heterogeneity is observed during antigen-specific CD8+ T cell responses. Effector molecules such as IFNγ, perforin, and granzyme A, B, and C, are heterogeneously expressed at the single cell level by CD8+ T cells [114]. The early gene expression of these molecules appears stochastic, although RNA expression does not always correlate with protein expression [114]. During the acute phase of influenza infection, an infection that requires CTL to clear virus, considerable heterogeneity is evident among antigen-specific CD8+ T cells [115]. In particular, the regulation of CD8+ T cell cytotoxic activity is complex. Only a minority of responding CD8+ T cells expressed both perforin and granzymes. The co-expression of perforin and granzymes is thought to be important for target cell killing by the granule-exocytosis pathway. If the expression of granule contents is uncoordinated, CD8+ T cells may be “shooting blanks” [116]. The basis for the heterogeneity among CD8+ T cells is not understood nor whether it affects host resistance to infection. Even though CTL activity is detected early during in vivo M. tuberculosis infection [13, 45], it is unknown what percentage of the antigen-specific CD8+ T cells are competent CTL that restrict bacterial replication—it could be a small population early during infection. This may be particularly true if multiple pathways (cytotoxic granules, fas/fasL, TNF/TNFR) lead to target cell cytolysis but only a unique combination of effector molecules lead to M. tuberculosis killing (perforin + granzymes). In other words, the CTL activity may overestimate the “protective” capacity of CD8+ T cells.
Heterogeneity of T cells during M. tuberculosis infection
Heterogeneity is observed for CD8+ T cells recruited to the lung during M. tuberculosis infection. Joanne Flynn’s lab reported distinct populations of IFNγ-producing and cytolytic CD8+ T cells during persistent infection (as determined by CD107 expression, a measure of cytotoxic degranulation) [117]. Our enumeration of antigen-specific CD8+ T cells from M. tuberculosis infected mice finds a discrepancy depending on whether we use tetramers (which quantify CD8+ T cells independently of function) or an elispot (which enumerates cells based on their secretion of IFNγ). We find that only 10–15 % of TB10-specific CD8+ T cells produce IFNγ [50, 52]. Thus, a substantial fraction of TB10-specific CD8+ T cells do not produce IFNγ but may express other functions. The polarization of CD8+ T cells during M. tuberculosis infection raises the question of whether these two functional subsets represent the emergence of two distinct CD8+ T cells populations or a loss of function by polyfunctional T cells and progressive T cell exhaustion.
7 Conclusions
Despite the worldwide application of BCG-vaccination and other anti-M. tuberculosis interventions, M. tuberculosis remains one of the most successful human pathogens. Eight to ten million new cases of active tuberculosis occur each year due in large part to the large reservoir of asymptomatic people chronically infected with M. tuberculosis. Estimates suggest that up to a third of the world’s population is latently infected with M. tuberculosis indicating M. tuberculosis is able to persist long term in humans. Understanding the role of CD8+ T cells in host immunity is required to design the best vaccine. Such an understanding is particularly important in the setting of HIV coinfection as HIV infects and kills CD4+ T cells, crippling one arm of T cells immunity against TB. Whether vaccine strategies can be developed to elicit CD8+ T cells that provide protection against tuberculosis remains an important question. Consequently, both commercial and academic ventures are pursuing vaccine strategies that stimulate CD8+ T cells responses. To activate CD8+ T cells, mycobacterial antigens need to enter and be processed by the MHC class I pathway, which can be targeted by DNA vaccines, recombinant viruses, and live attenuated mycobacteria. Evaluating whether or not such strategies are successful in activating CD8+ T cells has been hampered because few mycobacterial antigens have been identified that are presented by class I MHC. Thus, even basic questions concerning the function of CD8+ T cells during M. tuberculosis infection remain unanswered. These include uncertainty concerning when during infection CD8+ T cells are most important, whether CD8+ T cells elicited by vaccination mediate protection, whether CD8+ T cells perform any unique effector functions, and how CD8+ T cells mediate their protective effect. The possibility exists that CD8+ T cells effector molecules may correlate with protection. Therefore, the precise contribution of CD8+ T cells in host defense against M. tuberculosis requires elucidation. A better understanding of the effector functions used by CD8+ T cells to restrict intracellular bacterial growth would help in the development of new vaccines.
Abbreviations
- −/−
Genetically deficient (i.e., “knockout”)
- APC
Antigen presenting cell
- DC
Dendritic cell
- CFU
Colony forming unit
- IFN
Interferon
- IL
Interleukin
- LN
Lymph node
- mAb
Monoclonal antibody
- MHC
Major histocompatibility complex
- MNC
Mononuclear cells
- PLN
Pulmonary LN
- TCR
T cells receptor
- TNF
Tumor necrosis factor
- WT
Wild-type
References
- 1.Dietrich J, Lundberg CV, Andersen P. TB vaccine strategies–what is needed to solve a complex problem? Tuberculosis (Edinb) 2006;86:163–168. doi: 10.1016/j.tube.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann SHE, Cole ST, Mizrahi V, Rubin E, Nathan C. Mycobacterium tuberculosis and the host response. J Exp Med. 2005;201:1693–1697. doi: 10.1084/jem.20050842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouwer HG, Seaman MS, Forman J, Hinrichs DJ. MHC class Ib-restricted cells contribute to antilisterial immunity: evidence for Qa-1b as a key restricting element for Listeria-specific CTLs. J Immunol. 1997;159:2795–2801. [PubMed] [Google Scholar]
- 5.Lindahl KF, et al. H2–M3, a full-service class Ib histocompatibility antigen. Annu Rev Immunol. 1997;15:851–879. doi: 10.1146/annurev.immunol.15.1.851. [DOI] [PubMed] [Google Scholar]
- 6.Behar SM, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med. 1999;189:1973–1980. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sousa AO, et al. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc Natl Acad Sci U S A. 2000;97:4204–4208. doi: 10.1073/pnas.97.8.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urdahl KB, Liggitt D, Bevan MJ. CD8+ T cells accumulate in the lungs of Mycobacterium tuberculosis-infected Kb−/−Db−/− mice, but provide minimal protection. J Immunol. 2003;170:1987–1994. doi: 10.4049/jimmunol.170.4.1987. [DOI] [PubMed] [Google Scholar]
- 9.Turner J, et al. CD8- and CD95/95L-dependent mechanisms of resistance in mice with chronic pulmonary tuberculosis. Am J Respir Cell Mol Biol. 2001;24:203–209. doi: 10.1165/ajrcmb.24.2.4370. [DOI] [PubMed] [Google Scholar]
- 10.Woodworth JS, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells and their role in immunity. Crit Rev Immunol. 2006;26:317–352. doi: 10.1615/critrevimmunol.v26.i4.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.North RJ, Jung Y-J. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 13.Woodworth JS, Wu Y, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J Immunol. 2008;181:8595–8603. doi: 10.4049/jimmunol.181.12.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caruso AM, et al. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162:5407–5416. [PubMed] [Google Scholar]
- 15.D’Souza S, et al. Partial reconstitution of the CD4+-T-cell compartment in CD4 gene knockout mice restores responses to tuberculosis DNA vaccines. Infect Immun. 2006;74:2751–2759. doi: 10.1128/IAI.74.5.2751-2759.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearce EL, Shedlock DJ, Shen H. Functional characterization of MHC class II-restricted CD8+ CD4- and CD8-CD4-T cell responses to infection in CD4−/− mice. J Immunol. 2004;173:2494–2499. doi: 10.4049/jimmunol.173.4.2494. [DOI] [PubMed] [Google Scholar]
- 17.Tyznik AJ, Sun JC, Bevan MJ. The CD8 population in CD4-deficient mice is heavily contaminated with MHC class II-restricted T cells. J Exp Med. 2004;199:559–565. doi: 10.1084/jem.20031961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salcedo M, et al. Altered expression of Ly49 inhibitory receptors on natural killer cells from MHC class I-deficient mice. J Immunol. 1997;158:3174–3180. [PubMed] [Google Scholar]
- 19.Chen CY, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009;5:e1000392. doi: 10.1371/journal.ppat.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villarreal-Ramos B, et al. Investigation of the role of CD8+ T cells in bovine tuberculosis in vivo. Infect Immun. 2003;71:4297–4303. doi: 10.1128/IAI.71.8.4297-4303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer MV, Waters WR, Thacker TC. Lesion development and immunohistochemical changes in granulomas from cattle experimentally infected with Mycobacterium bovis. Vet Pathol. 2007;44:863–874. doi: 10.1354/vp.44-6-863. [DOI] [PubMed] [Google Scholar]
- 22.Lewinsohn DA, et al. Immunodominant tuberculosis CD8 antigens preferentially restricted by HLA-B. PLoS Pathog. 2007;3:e127–1249. doi: 10.1371/journal.ppat.0030127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewinsohn DM, et al. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen-presenting cells. J Exp Med. 1998;187:1633–1640. doi: 10.1084/jem.187.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewinsohn DM, Briden AL, Reed SG, Grabstein KH, Alderson MR. Mycobacterium tuberculosis-reactive CD8+ T lymphocytes: the relative contribution of classical versus nonclassical HLA restriction. J Immunol. 2000;165:925–930. doi: 10.4049/jimmunol.165.2.925. [DOI] [PubMed] [Google Scholar]
- 25.Gold MC, Lewinsohn DM. Mucosal associated invariant T cells and the immune response to infection. Microb Inf Institut Pasteur. 2011;13:742–748. doi: 10.1016/j.micinf.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behar SM, Porcelli S. CD1-restricted T cells in host defense to infectious diseases. Curr Top Microbiol Immunol. 2007;314:215–250. doi: 10.1007/978-3-540-69511-0_9. [DOI] [PubMed] [Google Scholar]
- 27.Behar SM, Boom WH. Unconventional T cells. In: Kaufmann SHE, Britton WJ, editors. Handbook of tuberculosis. Vol. 1. Wiley-VCH; Weinheim: 2008. pp. 157–183. [Google Scholar]
- 28.Behar SM, Martin CJ, Nunes-Alves C, Divangahi M, Remold HG. Lipids, apoptosis, and cross-presentation: links in the chain of host defense against Mycobacterium tuberculosis. Microbes Inf Institut Pasteur. 2011;13(8–9):749–756. doi: 10.1016/j.micinf.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 30.Dillon DC, et al. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J Clin Microbiol. 2000;38:3285–3290. doi: 10.1128/jcm.38.9.3285-3290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arend SM, et al. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J Infect Dis. 2000;181:1850–1854. doi: 10.1086/315448. [DOI] [PubMed] [Google Scholar]
- 32.Smith SM, et al. Human CD8+ CTL specific for the mycobacterial major secreted antigen 85A. J Immunol. 2000;165:7088–7095. doi: 10.4049/jimmunol.165.12.7088. [DOI] [PubMed] [Google Scholar]
- 33.Caminero JA, et al. Exogenous reinfection with tuberculosis on a European island with a moderate incidence of disease. Am J Respir Crit Care Med. 2001;163:717–720. doi: 10.1164/ajrccm.163.3.2003070. [DOI] [PubMed] [Google Scholar]
- 34.Pym AS, et al. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med. 2003;9:533–539. doi: 10.1038/nm859. [DOI] [PubMed] [Google Scholar]
- 35.Brosch R, et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci U S A. 2002;99:3684–3689. doi: 10.1073/pnas.052548299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole ST, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 37.Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol. 2002;46:709–717. doi: 10.1046/j.1365-2958.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- 38.Harboe M, Oettinger T, Wiker HG, Rosenkrands I, Andersen P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorensen AL, Nagai S, Houen G, Andersen P, Andersen AB. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:1710–1717. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renshaw PS, et al. Conclusive evidence that the major T-cell antigens of the Mycobacterium tuberculosis complex ESAT-6 and CFP-10 form a tight, 1:1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6*CFP-10 complex. Implications for pathogenesis and virulence. J Biol Chem. 2002;277:21598–21603. doi: 10.1074/jbc.M201625200. [DOI] [PubMed] [Google Scholar]
- 41.Berthet FX, Rasmussen PB, Rosenkrands I, Andersen P, Gicquel B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10) Microbiology. 1998;144:3195–3203. doi: 10.1099/00221287-144-11-3195. [DOI] [PubMed] [Google Scholar]
- 42.Brodin P, Rosenkrands I, Andersen P, Cole ST, Brosch R. ESAT-6 proteins: protective antigens and virulence factors? Trends Microbiol. 2004;12:500–508. doi: 10.1016/j.tim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Lalvani A, et al. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1998;95:270–275. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pathan AA, et al. High frequencies of circulating IFN-gamma-secreting CD8 cytotoxic T cells specific for a novel MHC class I-restricted Mycobacterium tuberculosis epitope in M. tuberculosis-infected subjects without disease. Eur J Immunol. 2000;30:2713–2721. doi: 10.1002/1521-4141(200009)30:9<2713::AID-IMMU2713>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 45.Kamath AB, et al. Cytolytic CD8+ T cells recognizing CFP10 are recruited to the lung after Mycobacterium tuberculosis infection. J Exp Med. 2004;200:1479–1489. doi: 10.1084/jem.20041690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Billeskov R, et al. Difference in TB10.4 T-cell epitope recognition following immunization with recombinant TB10.4, BCG or infection with Mycobacterium tuberculosis. Eur J Immunol. 2010;40:1342–1354. doi: 10.1002/eji.200939830. [DOI] [PubMed] [Google Scholar]
- 47.Billeskov R, Vingsbo-Lundberg C, Andersen P, Dietrich J. Induction of CD8 T cells against a novel epitope in TB10.4: correlation with mycobacterial virulence and the presence of a functional region of difference-1. J Immunol. 2007;179:3973–3981. doi: 10.4049/jimmunol.179.6.3973. [DOI] [PubMed] [Google Scholar]
- 48.Skjot RL, et al. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the esat-6 gene family. Infect Immun. 2002;70:5446–5453. doi: 10.1128/IAI.70.10.5446-5453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Majlessi L, Rojas MJ, Brodin P, Leclerc C. CD8+-T-cell responses of Mycobacterium-infected mice to a newly identified major histocompatibility complex class I-restricted epitope shared by proteins of the ESAT-6 family. Infect Immun. 2003;71:7173–7177. doi: 10.1128/IAI.71.12.7173-7177.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamath A, Woodworth JSM, Behar SM. Antigen-specific CD8+ T cells and the development of central memory during Mycobacterium tuberculosis infection. J Immunol. 2006;177:6361–6369. doi: 10.4049/jimmunol.177.9.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Irwin SM, et al. Tracking antigen-specific CD8 T lymphocytes in the lungs of mice vaccinated with the Mtb72F polyprotein. Infect Immun. 2005;73:5809–5816. doi: 10.1128/IAI.73.9.5809-5816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodworth JS, et al. Mycobacterium tuberculosis directs immunofocusing of CD8+ T cell responses despite vaccination. J Immunol. 2011;186:1627–1637. doi: 10.4049/jimmunol.1002911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garces A, et al. EspA acts as a critical mediator of ESX1-dependent virulence in Mycobacterium tuberculosis by affecting bacterial cell wall integrity. PLoS Pathog. 2010;6:e1000957. doi: 10.1371/journal.ppat.1000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lichterfeld M, Yu XG, Le Gall S, Altfeld M. Immunodominance of HIV-1-specific CD8(+) T-cell responses in acute HIV-1 infection: at the crossroads of viral and host genetics. Trends Immunol. 2005;26:166–171. doi: 10.1016/j.it.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moon JJ, et al. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 2011;4:288–293. doi: 10.1038/mi.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blomgran R, Ernst J. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J Immunol. 2011;186:7110–7119. doi: 10.4049/jimmunol.1100001. (Baltimore Md: 1950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf AJ, et al. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 60.Olmos S, Stukes S, Ernst JD. Ectopic activation of Mycobacterium tuberculosis-specific CD4+ T cells in lungs of CCR7−/− mice. J Immunol. 2010;184:895–901. doi: 10.4049/jimmunol.0901230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun. 2002;70:4501–4509. doi: 10.1128/IAI.70.8.4501-4509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolf AJ, et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Behar SM, et al. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol. 2011;4(3):279–287. doi: 10.1038/mi.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Divangahi M, et al. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat Immunol. 2009;10:899–906. doi: 10.1038/ni.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen M, et al. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med. 2008;205:2791–2801. doi: 10.1084/jem.20080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gan H, et al. Mycobacterium tuberculosis blocks crosslinking of annexin-1 and apoptotic envelope formation on infected macrophages to maintain virulence. Nat Immunol. 2008;9:1189–1197. doi: 10.1038/ni.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winau F, et al. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–117. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Schaible UE, et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med. 2003;9:1039–1046. doi: 10.1038/nm906. [DOI] [PubMed] [Google Scholar]
- 69.Divangahi M, Desjardins D, Nunes-Alves C, Remold HG, Behar SM. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat Immunol. 2010;11:751–758. doi: 10.1038/ni.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winau F, Kaufmann SHE, Schaible UE. Apoptosis paves the detour path for CD8 T cell activation against intracellular bacteria. Cell Microbiol. 2004;6:599–607. doi: 10.1111/j.1462-5822.2004.00408.x. [DOI] [PubMed] [Google Scholar]
- 71.Grotzke JE, Siler AC, Lewinsohn DA, Lewinsohn DM. Secreted immunodominant Mycobacterium tuberculosis antigens are processed by the cytosolic pathway. J Immunol. 2010;185:4336–4343. doi: 10.4049/jimmunol.1000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ernst J, et al. Meeting report: NIH workshop on the tuberculosis immune epitope database. Tuberculosis (Edinburgh, Scotland) 2008;88:366–370. doi: 10.1016/j.tube.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woodworth JS, Fortune SM, Behar SM. Bacterial protein secretion is required for priming of CD8+ T cells specific for the Mycobacterium tuberculosis antigen CFP10. Infect Immun. 2008;76:4199–4205. doi: 10.1128/IAI.00307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fortune SM, et al. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc Natl Acad Sci U S A. 2005;102:10676–10681. doi: 10.1073/pnas.0504922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van der Wel NN, et al. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 76.Grotzke JE, et al. The Mycobacterium tuberculosis phagosome is a HLA-I processing competent organelle. PLoS Pathog. 2009;5:e1000374. doi: 10.1371/journal.ppat.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Microbiol. 2010;8:668–674. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koopmann JO, et al. Export of antigenic peptides from the endoplasmic reticulum intersects with retrograde protein translocation through the Sec61p channel. Immunity. 2000;13:117–127. doi: 10.1016/s1074-7613(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 79.Jouanguy E, et al. Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guérin infection and a sibling with clinical tuberculosis. J Clin Invest. 1997;100:2658–2664. doi: 10.1172/JCI119810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Altare F, et al. Inherited interleukin 12 deficiency in a child with bacille Calmette-Guerin and Salmonella enteritidis disseminated infection. J Clin Investig. 1998;102:2035–2040. doi: 10.1172/JCI4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dorman SE, Holland SM. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J Clin Investig. 1998;101:2364–2369. doi: 10.1172/JCI2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 83.van de Vosse E, Hoeve MA, Ottenhoff THM. Human genetics of intracellular infectious diseases: molecular and cellular immunity against mycobacteria and salmonellae. Lancet Infect Dis. 2004;4:739–749. doi: 10.1016/S1473-3099(04)01203-4. [DOI] [PubMed] [Google Scholar]
- 84.Kampitak T, Suwanpimolkul G, Browne S, Suankratay C. Anti-interferon-γ autoantibody and opportunistic infections: case series and review of the literature. Infection. 2011;39:65–71. doi: 10.1007/s15010-010-0067-3. [DOI] [PubMed] [Google Scholar]
- 85.Flynn JL, et al. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bold TD, Ernst JD. CD4+ T Cell-Dependent IFN-γ production by CD8+ effector T cells in Mycobacterium tuberculosis infection. J Immunol. 2012;189(5):2530–2536. doi: 10.4049/jimmunol.1200994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Serbina NV, Lazarevic V, Flynn JL. CD4(+) T cells are required for the development of cytotoxic CD8(+) T cells during Mycobacterium tuberculosis infection. J Immunol. 2001;167:6991–7000. doi: 10.4049/jimmunol.167.12.6991. [DOI] [PubMed] [Google Scholar]
- 88.Lewinsohn DA, et al. Mycobacterium tuberculosis-specific CD8+ T cells preferentially recognize heavily infected cells. Am J Respir Crit Care Med. 2003;168:1346–1352. doi: 10.1164/rccm.200306-837OC. [DOI] [PubMed] [Google Scholar]
- 89.Noss EH, Harding CV, Boom WH. Mycobacterium tuberculosis inhibits MHC class II antigen processing in murine bone marrow macrophages. Cell Immunol. 2000;201:63–74. doi: 10.1006/cimm.2000.1633. [DOI] [PubMed] [Google Scholar]
- 90.Noss EH, et al. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J Immunol. 2001;167:910–918. doi: 10.4049/jimmunol.167.2.910. [DOI] [PubMed] [Google Scholar]
- 91.van Pinxteren LA, Cassidy JP, Smedegaard BH, Agger EM, Andersen P. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur J Immunol. 2000;30:3689–3698. doi: 10.1002/1521-4141(200012)30:12<3689::AID-IMMU3689>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 92.Chan J, Xing Y, Magliozzo RS, Bloom BR. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.MacMicking JD, et al. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science. 2003;302:654–659. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- 95.Tascon RE, Stavropoulos E, Lukacs KV, Colston MJ. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun. 1998;66:830–834. doi: 10.1128/iai.66.2.830-834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Libero G, Flesch I, Kaufmann SH. Mycobacteria-reactive Lyt-2+ T cell lines. Eur J Immunol. 1988;18:59–66. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- 97.Mohagheghpour N, et al. CTL response to Mycobacterium tuberculosis: identification of an immunogenic epitope in the 19-kDa lipoprotein. J Immunol. 1998;161:2400–2406. [PubMed] [Google Scholar]
- 98.Kaufmann SH, Vath U, Thole JE, Van Embden JD, Emmrich F. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur J Immunol. 1987;17:351–357. doi: 10.1002/eji.1830170308. [DOI] [PubMed] [Google Scholar]
- 99.Orme IM. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988;56:3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Serbina NV, Liu CC, Scanga CA, Flynn JL. CD8+ CTL from lungs of Mycobacterium tuberculosis-infected mice express perforin in vivo and lyse infected macrophages. J Immunol. 2000;165:353–363. doi: 10.4049/jimmunol.165.1.353. [DOI] [PubMed] [Google Scholar]
- 101.Stenger S, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 102.Stenger S, et al. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 103.Cooper AM, D’Souza C, Frank AA, Orme IM. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect Immun. 1997;65:1317–1320. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Laochumroonvorapong P, et al. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect Immun. 1997;65:127–132. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 106.Topham DJ, et al. Perforin and Fas in murine gammaherpesvirus-specific CD8(+) T cell control and morbidity. J Gen Virol. 2001;82:1971–1981. doi: 10.1099/0022-1317-82-8-1971. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y, Lobigs M, Lee E, Mullbacher A. Exocytosis and Fas mediated cytolytic mechanisms exert protection from West Nile virus induced encephalitis in mice. Immunol Cell Biol. 2004;82:170–173. doi: 10.1046/j.0818-9641.2004.01227.x. [DOI] [PubMed] [Google Scholar]
- 108.de Saint BG, Fischer A. The role of cytotoxicity in lymphocyte homeostasis. Curr Opin Immunol. 2001;13:549–554. doi: 10.1016/s0952-7915(00)00257-0. [DOI] [PubMed] [Google Scholar]
- 109.Oddo M, et al. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J Immunol. 1998;160:5448–5454. [PubMed] [Google Scholar]
- 110.Brookes RH, et al. CD8+ T cell-mediated suppression of intracellular Mycobacterium tuberculosis growth in activated human macrophages. Eur J Immunol. 2003;33:3293–3302. doi: 10.1002/eji.200324109. [DOI] [PubMed] [Google Scholar]
- 111.Stenger S, Niazi KR, Modlin RL. Down-regulation of CD1 on antigen-presenting cells by infection with Mycobacterium tuberculosis. J Immunol. 1998;161:3582–3588. [PubMed] [Google Scholar]
- 112.Ernst WA, et al. Granulysin, a T cell product, kills bacteria by altering membrane permeability. J Immunol. 2000;165:7102–7108. doi: 10.4049/jimmunol.165.12.7102. [DOI] [PubMed] [Google Scholar]
- 113.Martin CJ, et al. Efferocytosis Is an Innate Antibacterial Mechanism. Cell Host Microbe. 2012;12(3):289–300. doi: 10.1016/j.chom.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kelso A, et al. The genes for perforin, granzymes A-C and IFN-gamma are differentially expressed in single CD8(+) T cells during primary activation. Int Immunol. 2002;14:605–613. doi: 10.1093/intimm/dxf028. [DOI] [PubMed] [Google Scholar]
- 115.Johnson BJ, et al. Single-cell perforin and granzyme expression reveals the anatomical localization of effector CD8 + T cells in influenza virus-infected mice. Proc Natl Acad Sci U S A. 2003;100:2657–2662. doi: 10.1073/pnas.0538056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Curtsinger JM, Lins DC, Johnson CM, Mescher MF. Signal 3 tolerant CD8 T cells degranulate in response to antigen but lack granzyme B to mediate cytolysis. J Immunol. 2005;175:4392–4399. doi: 10.4049/jimmunol.175.7.4392. [DOI] [PubMed] [Google Scholar]
- 117.Einarsdottir T, Lockhart E, Flynn JL. Cytotoxicity and secretion of gamma interferon are carried out by distinct CD8 T cells during Mycobacterium tuberculosis infection. Infect Immun. 2009;77:4621–4630. doi: 10.1128/IAI.00415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]