Abstract
For many years primary Sjögren’s syndrome (pSS) has been considered an orphan disease, since no specific therapies were recognized as being capable of contrasting the development and progression of this disorder. The treatment of oral and ocular features, as well as of the systemic organ involvement, has been entrusted to the joint management of different subspecialty physicians, like ophthalmologists, otolaryngologists, dentists and rheumatologists. These latter subspecialty doctors are usually more involved in the treatment of systemic extraglandular involvement and, to do it, they have long been using the conventional therapies borrowed by the treatment schedules adopted in other systemic autoimmune diseases. The increasing knowledge of the biological pathways that are operative in patients with pSS, and the parallel development of molecular biology technology, have allowed the production and availability of a number of biological agents able to positively act on different disease mechanisms, and thus are candidates for testing in therapeutic trials. Meanwhile, the scientific community has made a great effort to develop new accurate and validated classification criteria and outcome measures to be applied in the selection of patients to be included and monitored in therapeutic studies.
Some of the new-generation biotechnological agents have been tested in a number of open-label and randomized controlled trials that have produced in many cases inconclusive or contradictory results. Behind the differences in trial protocols, adopted outcome measures and predefined endpoints, reasons for such unsatisfactory results can be found in the large heterogeneity of clinical subtypes in the examined cohorts. The future challenge for a substantial advancement in the therapeutic approach to pSS could be to identify the pathologic mechanisms, outcome tools and biomarkers that characterize the different subsets of the disease in order to test carefully selected target therapies with the highest probability of success in each different clinical phenotype.
Keywords: Sjögren’s syndrome, clinical subsetting, classification criteria, outcome measures, pathogenetic mechanisms, biological therapies
Introduction
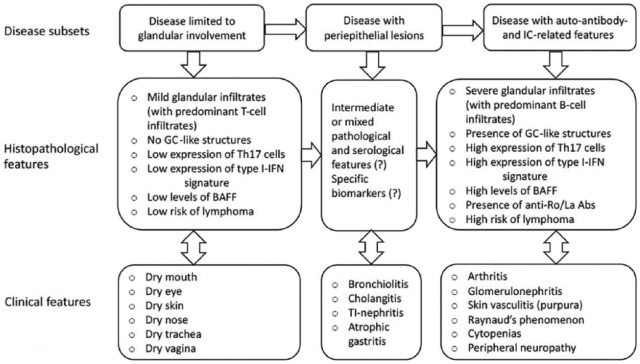
Sjögren’s syndrome (SS) is a chronic autoimmune disorder whose typical manifestations are oral and ocular dryness. SS is defined as primary (p) when it presents alone and secondary when associated with other diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE).1,2 In both variants of the disease, the sicca complaints are the result of mononuclear infiltration of the salivary and lacrimal glands. The clinical spectrum of pSS may vary from signs and symptoms arising from the isolated involvement of salivary and lachrymal glands, or more rarely of other exocrine glands, to manifestations derived from the involvement of different organs or systems. The ‘so-called’ systemic extraglandular involvement is present in approximately 60% of patients with pSS. The extraglandular features can be classified as periepithelial when driven by lymphocytic infiltration around epithelial tissues of parenchymal organs such as lung, kidney and liver, or mediated by the microvascular deposition of immune complexes that may cause purpura, glomerulonephritis and peripheral neuropathy.2 Similarly to what happens in other autoimmune systemic disorders, in a relevant number of cases, pSS is characterized by B-cell hyperactivity and consequently by hypergammaglobulinaemia and production of autoantibodies, namely rheumatoid factor (RF) and anti-Ro and anti-La antibodies (see Figure 1).1,2
Figure 1.
Schematic representation of the heterogeneity of the clinical, serological and histopathological features in patients with Sjögren’s syndrome (pSS). The pattern of expression in the subgroups with limited glandular manifestations (on the left) and immune-complex-mediated extra-glandular features (on the right) are certainly better known than those of patients with periepithelial lesions (in the middle). It may be supposed that this latter subgroup may have intermediate clinical and pathologic features and present specific serological markers.2 BAFF, B-cell activating factor; GC, germinal centre; IC, immune complex; IFN, interferon; Th17, T helper 17; TI, tubulo-interstitial; Abs, antibodies.
The fact that SS can present such a wide spectrum of clinical features suggests that variable pathogenetic mechanisms may be operative in the different subsets of patients. This hypothesis is supported by the findings that mononuclear cell amount and organization as well as T/B-cell ratio in the infiltrates of target tissues, gammaglobulin and antibody levels in the serum, cytokine expression in both peripheral blood and glands, can be completely different in patients with the disease limited to exocrine gland aggression or characterized by extraglandular features (see Figure 1). Even the impact of fatigue, which represents one of the major contributors to the impaired quality of life in patients with pSS, is variable in different subsets of patients with this disorder. The pathogenetic mechanisms underlying this feature are still far from being completely understood.3 Severe fatigue has been described in approximately one third of the patients and found to be closely associated with arthralgias/myalgias, widespread pain assuming the clinical aspect of fibromyalgia, anxiety, depression and impaired sleep patterns.3 Unexpectedly, the levels of proinflammatory cytokines are inversely related to patient-reported levels of fatigue.4 These data may help to explain why treating inflammation does not necessarily improve fatigue in patients with pSS and may raise doubts about the previously formulated hypothesis that proinflammatory cytokines directly mediate fatigue in chronic immunological conditions. In addition, some data suggest that widespread pain, which is closely related to fatigue, is predominant in seronegative patients without extraglandular involvement.5
On the opposite site, the presence of immune-complex-mediated features, together with salivary gland enlargement, cryoglobulinemia, hypocomplementemia, lymphopenia, germinal centre-like organization of mononuclear infiltrates in the salivary glands, RF positivity, hypergammaglobulinemia with monoclonal component characterize the patients with more severe extraglandular manifestations, highest levels of disease activity and high risk to develop lymphoma in the course of pSS.6–8
Substantial progress has been made in recent years in the knowledge of the main biological pathways that are activated in the disease course. Additionally, the advances in molecular biology have opened up the possibility of developing specific target therapies that are potentially able to interfere with or block the main cells or biological molecules that are believed to play fundamental roles in SS pathogenesis. This has enhanced international efforts to conduct randomized controlled studies in the setting of pSS. To do so, reliable and accurate classification criteria have been developed to select homogeneous groups of patient to be enrolled in clinical trials. Moreover, validated and sensitive outcome measures have been constructed with the purpose of accurately assessing the results of any innovative therapeutic intervention.
Main pathogenetic mechanisms of SS
Innate immunity mechanisms
Interferons
Several studies in both peripheral blood9 and minor salivary gland (MSG) tissues10,11 from patients with pSS have shown an upregulation of genes induced by type I interferon (IFNα and β), type II IFN (IFNγ) or both. This phenomenon has been defined as the IFN signature and it is shared by other systemic autoimmune disorders like SLE. Plasmacytoid dendritic cells are considered the main source of IFNα,11 while T helper 1 (Th1) CD4+ cells and natural killer (NK) cells are believed to be the main IFNγ producers in pSS salivary gland tissues.10 Type I IFN signature seems to be prevalent in the peripheral blood of patients with SS whereas type II IFN signature appears to be predominant in SS salivary gland biopsies.12
Once produced, probably after a local infection of still unidentified viruses and the consequent activation of some toll-like receptors, type I IFNs induce apoptosis of salivary epithelial cells, exposure of endogenous autoantigens to the immune system, upregulation of B-cell activating factor (BAFF) expression, increased B-cell survival and differentiation, and ultimately autoantibody production and immune complex formation.13
Proinflammatory cytokines
It has been widely documented that some proinflammatory cytokines, such as interleukin (IL)-1, tumour necrosis factor (TNF)-α and IL-6 are upregulated in salivary gland tissue obtained from patients with pSS.14 IL-6 certainly plays an important role in the differentiation of extrafollicular Th cells, germinal centre B cells and plasma cells. IL-6 levels were also found to be increased in serum, saliva and tears of patients with pSS. Salivary gland epithelial cells from patients with this disorder have been shown to produce IL-6.15 The mechanisms of action of IL-6 are now better known and may open up new therapeutic possibilities for the treatment of pSS. IL-6 binding to its specific receptor leads to homodimerization of the receptor component gp130, which mainly results in the activation of the Janus kinase 1 (JAK1), and the subsequent phosphorylation of the signal transducer and activator of transcription 3 (STAT3).16 STAT3 plays a central role in transmitting cytokine signals to the nucleus and promoting cell proliferation and anti-apoptotic signals.17,18 Furthermore, the JAK1/STAT3 pathway might be involved in other functions, such as increased expression of IFNγ in Th1 cells, and of IL-17 in Th17 cells, respectively.19
Adaptive immunity mechanisms
Antigen presentation
Cathepsin S is an endoprotease located in the lysosomes of antigen-presenting cells. It is involved in controlling autoantigen presentation to CD4+ T cells by interfering with the binding of major histocompatibility complex II molecules.20 Increased levels of cathepsin S have been found in the tears of patients with SS.21
Costimulation
Costimulation is a central event in the perpetuation and amplification of autoimmune response; B7 (CD80 and CD86) molecules expressed on classic antigen-presenting cells play a critical role in enhancing immune responses by providing activation signals to T cells, through their link to the CD28 receptor. The cytotoxic T lymphocyte antigen 4 (CTLA4) molecule is the counterpart of this system, competing with CD28 for the link with B7 molecules, and thus has an inhibitory effect on the costimulatory system. The second type of costimulatory molecule is represented by the interaction of the CD40 molecule on B cells with the CD40 ligand on antigen-presenting cells, T cells, endothelial and epithelial cells, and B cells. In pSS, CD80, CD86 and CD40 molecules were found to be expressed on salivary gland epithelial cells, indicating that these costimulatory systems are locally activated.22 The inhibition of the costimulatory signals is considered crucial for the treatment of autoimmune diseases.
B-cell activation
B-cell activation is considered a key pathogenetic mechanism of SS. Hypergammaglobulinemia, formation of germinal centre-like structures in the infiltrated salivary gland tissue, production of RF and autoantibody directed against ribonucleoproteins are the biological phenomena related to the increased B-cell presence and activation.23 Although the pathways influencing B-cell activation in pSS remain partially unknown, many studies have indicated that a number of genes are involved in B-cell survival, proliferation and signalling.24 The increased number of B cells among the mononuclear cells infiltrating salivary gland tissue, namely in the germinal centre-like structures, suggests the antigen-driven nature of the local immune response.
BAFF/BAFF receptor interaction
The B-cell activating factor (BAFF) axis comprises two ligands, which are BAFF itself (named also Blys) and the ‘a proliferating-inducing ligand’ (APRIL), and three related receptors (BCMA, TACI, BR3) with different affinity for the two ligands.25 BAFF is a member of the TNF family that acts by prolonging the survival of circulating B cells. Its binding to the corresponding receptors inhibits intracellular apoptotic pathways and provides survival signals for B cells.26 High BAFF levels have been found in patients with pSS, and have been shown to be associated with the specific immunological markers of the disease, systemic disease activity and lymphoproliferative features, such as monoclonal lymphocytic selection, myoepithelial sialadenitis and lymphoma. In addition, it has been suggested that the abnormal BAFF signal found in B cells infiltrating the salivary glands can play a key role in the formation of ectopic germinal centres,14 survival of self-reactive B cells, and their localization to follicle/marginal zone niches.27
B-cell receptor (BCR) signalling
BCR activation is another mechanism that is able to induce B-cell survival, proliferation, functional differentiation and migration. These findings may suggest new rationale ways for targeting B-cell signalling in pSS. Some kinases, such as phosphatydil-inositol 3-kinase delta isoform (PI3Kδ), and Bruton’s tyrosine kinase (BTK), are crucial in B-cell receptor (BCR) signal transduction. The blockade of these kinases has recently been licensed for the treatment of chronic lymphocytic leukemia.28 Recent data support activation of the PI3Kδ pathway in affected salivary gland tissues from an animal model of SS.29 Moreover, BTK levels were shown to be increased in circulating B cells of a significant percentage of patients with pSS, in association with high serum RF levels.30 Even spleen tyrosine kinase (Syk) seems to have an important role in inducing B-cell differentiation and proliferation after the engagement of BCR in lymphoma and autoimmune disease such as systemic lupus and pSS.31
CD22 is a type I transmembrane protein expressed on most mature B lymphocytes. It acts as a coreceptor of BCR and its engagement by specific reactants, such as high-affinity monoclonal antibodies, may induce an intensification of the normal inhibitory role of CD22 on the BCR, leading to reduced signalling and diminished activation of B cells.32
Ectopic germinal centre formation
The formation of ectopic germinal centres is increasingly recognized as a crucial mechanism in the pathogenesis of SS and other systemic autoimmune diseases. Germinal centre formation in the salivary glands of patients with pSS is associated with higher focus scores, RF positivity and anti-Ro/La positivity, the presence of extraglandular manifestations, and it is considered as a marker of lymphoma development.33,34 An increased number of CD4-T follicular helper cells and follicular dendritic cells have been found in the salivary glands of patients with SS. These cells probably participate in the germinal-like centre organization and may drive B-cell differentiation towards memory B cells and plasma cells.35,36
Finally, it has recently been demonstrated that a large number of biological effector molecules may take part in the formation and maintenance of germinal-centre-like structures. These biological mediators include some cytokines, such as IL-21 and IL-22, chemokines CCL19 and CCL21, and chemokine receptors CXCL12 and CXCL13.37,38 The fact that Th17 cells (a CD4+ Th subtype implicated in the pathogenesis of SS) are a major source of IL-21 and IL-22 seems to provide a rationale for targeting these cells in future therapeutic interventions.39 Some data suggest that a synergistic role in this pathologic network may be played by lymphotoxin β. Its receptor blockade in the NOD mouse model of SS led to reduction of B-cell accumulation in lacrimal glands.40
Classification criteria and outcome measure for SS
Given the advances in the knowledge of the pathogenetic mechanisms of SS, and the increasing availability of biological agents that are able to target the activated pathways in the disorder, a growing interest has arisen in assessing the most effective way of conducting clinical trials. The 2016 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria for pSS represent an important advancement in reaching an agreement on the way to select patients to be recruited for clinical studies.41,42
These criteria are the last step of a lengthy scientific process that started a long time ago and has produced a plethora of different classification criteria sets.43 The new ACR/EULAR classification criteria are applicable either to patients with at least one symptom of ocular or oral dryness [verified by using the validated questionnaire previously included in the American European Consensus Group (AECG) classification criteria],44 or to patients with at least one of the systemic features listed in ESSDAI (EULAR SS Disease Activity Index).45 The criteria are based on five objective items and the individuals are classified as having pSS if they have a total score of at least 4, derived from the weighted sum of the five items. Anti-SSA/Ro antibody positivity and focal lymphocytic sialadenitis with a focus score of at least 1 focus/4 mm2 of tissue have the highest weight in the criteria set, each scoring three points (Table 1).
Table 1.
The 2016 ACR/EULAR classification criteria for pSS.20
| Consensus criteria items for the classification of pSS: | |
| Abnormal unstimulated salivary flow rate* (⩽0.1 ml/min) | (1 point) |
| Abnormal Schirmer’s test (<5 mm in 5 min) | (1 point) |
| Abnormal findings with lissamine green or fluorescein staining | |
| ⩾5 in Ocular Staining Score or ⩾4 in Van Bijsterveld Score | (1 point) |
| Presence of anti-Ro/SSA antibodies | (3 points) |
| Histological evidence of focal lymphocytic sialadenitis,$ with a focus score ⩾1 focus/4 mm2 (1 focus = 50 lymphocytes/4 mm2) | (3 points) |
| Diagnosis is considered established if score ⩾4 points, after application of inclusion and exclusion criteria | |
| Inclusion criteria: | |
| Dryness of eyes or mouth for at least 3 months, not explained otherwise (e.g. medications, infection) | |
| Exclusion criteria: | |
| Status post head/neck radiation | |
| HIV/AIDS | |
| Sarcoidosis | |
| Active infection with hepatitis C virus (PCR replication rate) | |
| Amyloidosis, graft versus host disease, IgG4-related disease | |
| The lack of any other potentially associated disease is the key requirement for classification as pSS |
The patient is asked to sit still, not to speak or to chew for 5–15 min; the saliva produced during this time is transferred to a test tube and weighed.
Biopsy: removal of 3–5 labial minor salivary glands from the lower lip under local anaesthesia; fixation of biopsy material in formalin and HE staining.
ACR/EULAR, American College of Rheumatology/European League Against Rheumatism; AIDS, acquired immunodeficiency syndrome; HE, hematoxylin and eosin stain; HIV, human immunodeficiency virus; IgG, immunoglobulin G; PCR, polymerase chain reaction; pSS, primary Sjögren’s syndrome.
The inclusion of the salivary gland ultrasonographic (US) examination has been proposed among the classification items. However, consensual US procedures and validated US scoring systems are needed before considering this possibility.46
With respect to the 2002 AECG criteria that have represented the most used classification criteria in the last 15 years,47 the exclusion criteria of the ACR/EULAR criteria have also been updated. Immunoglobulin G4 (IgG4)-related disease has been added and it has been established that hepatitis C infection has to be confirmed by evidence of viral genome in peripheral blood. Finally, preexisting lymphoma has been removed from the exclusion criteria.41,42
The 2016 ACR/EULAR classification criteria have again pointed out the central role of MSG biopsy and specific autoantibodies for pSS classification. Recently, consensus guidelines on how to correctly perform a salivary gland biopsy and the related histopathologic examination have been released by an expert group for application in clinical research and in clinical trials.48
In the last 5 years the EULAR task force on pSS has proposed and validated two new tools for assessing disease activity and patient-reported outcomes. The ESSDAI45 is a multidomain scoring system in which each domain (constitutional, lymphadenopathy, glandular, articular, cutaneous, respiratory, renal, muscular, peripheral nervous system, central nervous system, haematological and biological) can be separately scored. Each domain score is also stratified according to the severity of the manifestations. The ESSDAI has been validated and the minimal clinically significant difference was defined as a reduction of the activity score of at least three points.49
A second tool, the EULAR Sjögren’s syndrome patient-reported index (ESSPRI), has been created with the purpose of assessing the subjective symptomatology of patients. The total score of ESSPRI is defined as the mean score of three Lykert’s numerical scales for dryness, pain and fatigue.50 The minimal clinically significant difference has also been defined as an improvement of at least one point or 15% of the baseline value.49
The ESSDAI and ESSPRI are increasingly being used as inclusion criteria and endpoints in clinical trials evaluating innovative therapies for pSS. Moreover, their use in clinical practice could also be recommended.
It has also been suggested that, in trials aimed at evaluating the effectiveness of any experimental drug on patients with systemic features, those with at least moderate activity (ESSDAI ⩾5) are the best candidates to be included.49 However, it has been pointed out that patients with an ESSDAI greater than 13, and so with a well known increased mortality risk, should be excluded. A ESSDAI greater than 13 has also been suggested as an additional risk factor for the development of lymphoma in the following course of the disease.49,51
Similarly, in trials devoted to evaluating the efficacy of any drug on subjective symptoms, it has been suggested that patients who have an unsatisfactory symptom state corresponding to a ESSPRI of at least 5 should be selected.49
A modified ESSDAI scoring system, the so-called ClinESSDAI, has been derived from the ESSDAI by exclusion of the biological domain.52 This new scale for systemic disease activity was built to eliminate problems of collinearity in trials in which biomarkers included in the biological domain of the original ESSDAI were analysed as separate outcome measures. The psychometric properties of ClinESSDAI, including reliability and sensitivity to change, are similar to those of ESSDAI.52
Other disease-specific indexes have been developed for evaluation of subjective features of the patients, such as the Profile of Fatigue and Discomfort (PROFAD) and Sicca Symptoms Inventory (SSI). In addition, nondisease-specific indices have been proposed and used in clinical trials to assess the health-related quality of life and functional impairment (Short Form 36), as well as other aspects of disease burden such as anxiety, depression or sleep disorder.53
Management of sicca symptoms
Therapy of sicca complaints is crucial to improve the quality of life of patients with SS (see Table 2). At the same time, this is a very difficult challenge for physicians since evidence-based treatment options are few and most therapeutic procedures are empirically based.
Table 2.
Some recommended treatments for sicca symptoms in patients with primary Sjögren’s syndrome.
| Sicca manifestation | Therapeutic measure (grade of recommendation) |
|---|---|
| Keratoconjunctivitis sicca | Tear substitutes (A) |
| Secretagogues: pilocarpine, cevimeline (A) |
|
| Cyclosporine A eye drops 0.1% (B) |
|
| Short-term topical corticosteroids (C) |
|
| Punctal plugs (C) | |
| Oral dryness | Patient education |
| Avoid drugs that promote xerostomia (A) |
|
| Topical fluorides for caries prevention (A) |
|
| Secretagogues: pilocarpine, cevimeline (A) |
|
| Saliva substitutes, sugar-free chewing gum and electrostimulation of salivary glands (C) |
Grades of Recommendation according to Centre for Evidence-based Medicine in Oxford:
(A) Evidence obtained from meta-analyses or at least one randomized controlled trial.
(B) Evidence from at least one well designed experimental study.
(C) Evidence from at least one well designed descriptive study or case–control studies.
General measures such as air humidification of the environment, namely of the bedroom, caries prevention, and smoking cessation play an important role. Treatment of dry eye requires a close and constant collaboration with an ophthalmologist. Various tear substitutes are available to treat keratoconjunctivitis sicca. The composition of the tear substitutes varies in accordance with the complex physiology of the pathogenesis of dry eye which can be limited to lachrymal glands or may sometimes even compromise the meibomian glands. Generally, the use of preservative-free eye drops is preferred to avoid the development of local hypersensitivity phenomena. A randomized controlled trial (RCT) has shown that local anti-inflammatory treatment with cyclosporine A eye drops can be effective.54 Pilocarpine and cemiveline, which are effective in stimulating salivary flow, have also been proven to improve subjective complaints and objective signs of dry eye.55,56 The use of punctal plugs to block the drainage system is effective in increasing the residence time of both residual and instilled tears on the ocular surface.57
Treatment of oral dryness and related manifestations is usually conducted with the essential contribution of dentists and otolaryngologists. In patients with pSS the decrease in saliva production causes difficulty in swallowing dry foods, chewing and speaking, and increases susceptibility to multiple caries and oral infections such as dental caries, periodontal disease and oral candidiasis.58 Patients with pSS should be educated about the need for regular oral-health monitoring and care to prevent these infective processes.
Saliva substitutes, lubricating agents and mechanical stimulation by chewing sugar-free gum are usually employed in patients with mild hyposialia.59 Topical fluoride and fluoride toothpaste to prevent caries are also strongly recommended. A multicentre RCT has shown that mild intraoral electrostimulation can alleviate the oral dryness and had no adverse effects.60
In patients with moderate to severe oral dryness and with residual salivary-gland function, oral muscarinic agonists, such as pilocarpine or cevimeline, are the treatment of choice in the absence of contraindications.55,56 Commonly reported adverse effects include sweating, warmth and flushing sensation, increased urinary frequency, headache and abdominal discomfort.
Systemic conventional treatment
The decision to adopt systemic treatment, and the choice of the specific treatment in pSS, is often driven by the level of disease activity and by the specifically involved organ system. The few RCTs evaluating the use of conventional disease-modifying antirheumatic drugs (DMARDs) in patients with pSS did not provide conclusive evidence supporting their efficacy.61,62 Thus, treatment strategies are frequently based on experiences acquired in other autoimmune rheumatic diseases, such as SLE or RA. Following the indications given for other connective tissue disorders and in view of its favourable side-effect profile, hydroxychloroquine (HCQ) is the drug of choice for various mild to moderate systemic manifestations, such as arthralgia, arthritis and cutaneous lesions. Unfortunately, a RCT on pSS failed to demonstrate any difference between HCQ and placebo treated patients regarding sicca complaints, pain and fatigue.63 However, this study had significant limitations, such as the low disease activity of patients, the shortness of follow-up time, and the use of nonvalidated endpoints. Treatment recommendations concerning corticosteroid use and immunosuppressive drugs are summarized in Table 3. Briefly, arthralgia and arthritis are usually treated, as happens in other inflammatory arthritides, with methotrexate or leflunomide, whereas severe organ manifestations can be successfully treated with high-dose methylprednisolone and cyclophosphamide. Severe cryoglobulinaemic vasculitis may favourably respond to plasmapheresis. Finally, patients with B-cell lymphoma should be referred to experienced haematologists for a correct therapeutic approach that is usually based on lymphoma subtype and stage, according to current guidelines on the treatment of hemato-oncological disorders.64
Table 3.
Some recommended systemic traditional treatment in patients with primary Sjögren’s syndrome.
| Parotid swelling | Short-term oral corticosteroids (D) Antibiotic treatment, if required (D) |
| Arthritis | Hydroxychloroquine (C) NSAIDs Short-term oral/intraarticular corticosteroids (C) Other DMARDs as with rheumatoid arthritis (C) |
| Interstitial lung disease | Corticosteroids, oral or intravenous (C) Cyclophosphamide for active alveolitis (C) Pirfenidone, nintedanib (C) |
| Tubulointerstitial nephritis | Potassium and bicarbonate replacement (D) |
| Glomerulonephritis | Corticosteroids, oral or intravenous (C) Cyclophosphamide Mycofenolate mofetil (according to specific nephrologic guidelines) |
| Peripheral neuropathy | Gabapentinoids (D) Corticosteroids IVIg (D) |
| Cryoglobulinemic vasculitis | Corticosteroids, plasmapheresis (C) |
Grades of Recommendation according to Centre for Evidence-based Medicine in Oxford:
(A) Evidence obtained from meta-analyses or at least one randomized controlled trial.
(B) Evidence from at least one well designed experimental study.
(C) Evidence from at least one well designed descriptive study or case–control studies.
(D) Evidence from expert opinion.
DMARD, disease-modifying antirheumatic drug; IVIg, intravenous immunoglobulins; NSAID, nonsteroidal anti-inflammatory drug.
Biological target therapies: present data and future perspectives
B-cell targeted agents
Targeting B cells is certainly a rationale therapeutic approach in diseases like SS, where B-cell hyperactivity is believed to be an essential pathogenetic moment. Rituximab was the first anti-B-cell receptor monoclonal antibody approved to treat B-cell lymphomas. It acts by blocking the CD20 B-lymphocytic receptor leading to B-cell depletion in peripheral blood by antibody- and complement-dependent cytotoxicity, and by the activation of apoptotic pathways.65 In pSS B cells infiltrate the glandular tissue around the ductal epithelium with the cooperation of stromal cells and follicular dendritic cells that are essential for the organization of the germinal-centre-like structures, mono-oligoclonal selection and possibly lymphoproliferation. It has been clearly shown that rituximab therapy induces a partial depletion of B cells in the salivary glands.66 Contemporaneous reduction of T-follicular helper cells and salivary gland expression of IL-17 has also been reported.67 This could be ascribed to the fact that B-cell reduction in the glands induces a reduction of the B-cell production of some cytokines that drive the differentiation and proliferation of Th17 cell subtype.
A number of open-label prospective studies have evaluated rituximab effectiveness in patients with pSS, while only a few RCTs have been completed so far. A wide range of outcomes were assessed in these studies, including sicca features, fatigue, pain, systemic features, quality of life and lymphoma development (Table 4).
Table 4.
Open-label and randomized controlled trials with rituximab in pSS.
| Authors, years (Pts features) | Study design | Pts (No.) RTX/C | RTX regimen | Follow-up weeks | Salivary flow USF/SSF | Ocular tests Sch/ODT° | Dryness VAS oral/ocular | Fatigue VAS/Scale | Pain VAS | ESSDAI | ESSPRI | SF36 domains |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pijpe68 (early group) | Open label | 8 8/0 |
A | 12 | =/+ | =/+ | +/= | NA/+* | NA | NA | NA | + (BP/PF/Vit) |
| Pijpe68 (with MALT-L) | Open label | 7 7/0 |
A | 12 | =/= | =/= | =/= | NA/= | NA | NA | NA | + (all) |
| Devauchelle-Pensec69 | Open label | 16 16/0 |
B | 36 | =/NA | =/NA | + (general dryness) | +/NA | + | NA | NA | + (all) but PF= |
| Meiners70 | Open label | 28 28/0 |
C | 60 | NA/+ | NA/NA | +/NA | NA/+* | NA | + | + | + (PF) |
| Carubbi71 | Open label | 41 19/22 |
C +DMARDS |
120 | +/NA | +/NA | +/NA | +/NA | + | + | + | NA |
| St Clair71 | Open label | 12 12/0 |
C | 52 | =/= | =/NA | +/= | +/NA | + | NA | NA | + (Vit) |
| Dass72 | RCT (pilot) | 30 20/10 |
C | 26 | =/NA | =/NA | NA | +/+$ | = | NA | NA | + (SF) |
| Meijer73 | RCT | 30 20/10 |
C | 48 | =/+ | =/+ | +/+ | NA/+$ | NA | NA | NA | + |
| Devauchelle-Pensec74 | RCT | 120 63/57 |
C | 24 | =/NA | =/NA | + (general dryness) | + | = | NA | NA | = |
| Bowman75 | RCT | 133 67/66 |
C | 48 | =/NA | =/NA | = (general dryness) | =/=$ | = | = | = | = |
Regimen A, 375 mg/m2 at day 0, 7, 14, 21; B, 375 mg/m2 at day 0, 15; C, 1 g at day 0, 15.
MFI, Multidimensional Fatigue Index.
PROFAD-SSI, Profile of Fatigue and Discomfort, Sicca Symptoms Inventory.
=, unchanged; +, significantly improved; BP, bodily pain domain; C, controls; ESSDAI, EULAR SS disease activity index; ESSPRI, EULAR SS patient reported index; EULAR, European League Against Rheumatism; NA, not available; ODT, Ocular dye test; PF, physical functioning domain; pSS, primary Sjögren’s syndrome; Pts, patients; RTX, rituximab; Sch, Schirmer’s test; SF, social functioning domain; SF36, Short Form 36; SSF, stimulated salivary flow; USF, unstimulated whole salivary flow; VAS, visual analogue scale; Vit, vitality domain.
The preliminary open-label studies and two pilot controlled trials on rituximab therapy for pSS demonstrated some improvement in some subjective or objective parameters, or both.68–73,76 In spite of these encouraging results, two more recent large RCTs74,75 failed to reach the established primary endpoints, and thus did not confirm the promising previous results, although some secondary endpoints were satisfied. A composite scoring system which included dryness, pain, fatigue and global health evaluation, some objective diagnostic tests and ESSDAI score developed in a post hoc analysis of one of the RCT studies74 was found to be improved. In contrast to the poor clinical results obtained in the open-label and controlled studies, it has been clearly shown that rituximab therapy is effective in reducing the level of some biological markers of the disease as IgM-RF, gammaglobulins and IgG, autoantibody levels and B-cell number in glandular infiltrates.77
A number of reasons can be postulated to explain why rituximab treatment was ineffective in the majority of the performed studies. The heterogeneity of the populations in the studies, and of the outcome measures that were used, certainly represent critical points. Some partially successful studies were carried out in patients with a short disease duration and in patients in whom a residual glandular function was preliminarily demonstrated.68,70 To recruit patients with a long disease duration, or having a large variability of this parameter, may certainly lead to unsatisfactory results. The same may happen when patients are selected with exhausted gland function in the case that the experimental therapy is administered to demonstrate improvement of salivary flow. The effect of B-cell depletion therapy on some biological markers of B-cell activation and on the improvement of the ESSDAI score observed in the French registry study,78 where the choice of rituximab treatment was preliminarily conditioned by the presence of clear signs of systemic involvement and high disease activity at baseline, clearly indicates how this therapy can really be effective in this particular subgroup of patients. Unfortunately, the ESSDAI was not available when the oldest studies were made and no preselection of patients according to the presence of B-cell activation signals was done in many of these studies, namely in the large RCTs performed so far. Finally, the often existing discrepancies between the severity of subjective dryness complaints and the corresponding objective measurement of lachrymal and salivary flows can explain why rituximab was ineffective when only sicca symptoms were chosen to be assessed as outcome measures.
Other anti-CD20 monoclonal antibodies such as ocrelizumab and obinutuzumab (humanized B-cell-depleting agent), and ofatumumab (fully human B-cell-depleting agent) are now available and have demonstrated an acceptable safety profile in patients with B-cell lymphoma.79
Anti-BAFF therapy
Belimumab is a monoclonal antibody interfering with soluble BAFF that has been licensed for patients with SLE. A prospective 1-year open-label bicentric study (BELISS) on the effectiveness of belimumab has been carried out in 30 patients with pSS.80 The primary endpoint was a composite score which included five items: at least a 30% reduction of VAS for dryness, fatigue and pain, plus a 30% reduction of VAS of the global physician evaluation on systemic activity, plus a 25% reduction of selected biological markers. Two of the five considered items were improved in 60% of patients at the 28-week follow up. Almost 90% of the 15 patients who were responders at week 28 also responded at week 52.81 A post hoc analysis of the BELISS population revealed that high type I IFN scores at baseline were associated with better response to treatment.82
CD20 and BAFF blockade
Despite the demonstration that the BAFF/BAFF receptor axis is related to lymphoma development, some preliminary observations seem to indicate that lymphoproliferation is resistant to belimumab alone.83 These data, together with the evidence of an upregulation of BAFF levels following rituximab treatment, have suggested the potential usefulness of combination therapy of belimumab with rituximab.84 A randomized trial is currently under way in which the two target therapies are sequentially administered.
Anti-BCR signalling therapies
Small molecules capable of inhibiting transduction signals after the activation of BCR, by blocking PI3TK, BTK and Syk, are now available.28–30 RCTs have been started with these molecules in patients with pSS.
Epratuzumab is a B-cell-directed nondepleting monoclonal antibody that targets CD22.32 It is currently being evaluated in two phase III clinical trials in patients with SLE, a disease associated with abnormalities in B-cell function and activation.
In a past phase II study, the administration of epratuzumab led to a significant improvement of fatigue, decrease in patient and physician global assessments, and in composite score that also included the Schirmer-I test, unstimulated whole salivary flow, erythrocyte sedimentation rate (ESR) and IgG levels.85
Therapies against antigen-presenting cell mechanism and costimulatory molecules
Given the role of cathepsin S in antigen presentation to T cells, an inhibitor of this molecule is presently being tested in a phase II study.
Abatacept is a fusion protein composed of the Fc region of IgG1 combined with the extracellular domain of CTL4. As previously mentioned CTLA4, competing with CD28, is the natural inhibitor of the B7-CD28 costimulatory system, and so is active in negatively modulating the antigen presentation mechanism.22 Preliminary data obtained in open-label studies showed that administration of abatacept has favourable effect on systemic features, extent of glandular infiltrates and reduction of RF title and IgG levels.86,87 Starting from these data, an RCT is currently ongoing. Other monoclonal antibodies binding the CD40 molecule and interfering with CD40/CD40L linkage are presently under investigation.
Anti-cytokine therapies
In view of the demonstrated over expression of TNFα and IL-1 in pSS,14 and the good results obtained by anti-TNFα biological agents in RA, two explorative trials have been conducted in patients with pSS with two different anti-TNFα agents, and both have given negative results.88,89 Similar negative results were also obtained by using an IL-1 inhibitor.90
Considering that pSS is a disease characterized by IFN type I signature, anti-IFN therapies appear to be promising and some studies on this specific target therapy are ongoing.
Since levels of IL-6 have been found to be increased in both the blood and saliva of patients with pSS, and overproduction of this cytokine by the epithelial glandular cells has been shown,15 tocilizumab, a monoclonal antibody targeting IL-6, has been considered potentially active in this disorder. RCTs on the effectiveness of tocilizumab in patients presenting with systemic involvement and specific autoantibodies are ongoing and the results are expected relatively soon.
Because IFNs and IL-6 produce their multiple biological effects by activating JAK/STAT pathways, the increasing availability of small molecules able to modulate these transducer signals may open up new scenarios in the treatment of pSS. Phase II studies with filgotinib, a JAK1 inhibitor,91 are in progress.
Therapy against molecules favouring the lymphoid infiltrates organization and germinal-centre-like formation
Baminercept is a fusion protein that includes the lymphotoxin-β receptor. This molecular construct has been shown to be able to reduce B-cell infiltration and organization in the NOD mouse model.40 An RCT with baminercept in a series of patients with SS has been completed and has shown an improvement in ESSDAI score, but no effect on sicca features.92 The clinical application of such a treatment is presently under evaluation because of the high risk of liver damage observed in some cases.
Conclusion
In the last few years a great effort has been made by the rheumatologic scientific community to reach an agreement on classification criteria and develop validated outcome measures for pSS. The 2016 ACR/EULAR classification criteria are the result of this effort.41,42 This criteria set does not introduce new substantial elements with respect to the previous largely used AECG criteria, as demonstrated by the strong agreement between the two criteria sets.41,42 Some criticisms have been made on the new classification criteria, namely the fact these criteria appear to be less stringent with respect to previously proposed ones. This excess of sensitivity, with the consequent loss of specificity, may lead to possible erroneous selection of false-positive patients in clinical therapeutic trials.93 This risk is certainly relatively low when one considers the revised comprehensive exclusion criteria that are detailed in the criteria formulation (see Table 1).
Conversely, to have more ‘liberal’ criteria may allow the early selection of patients who may be more likely to positively respond to the innovative therapeutic regimens that are presently or will be available.94
ESSDAI and ESSPRI have been defined and validated in the last few years.45,49 Both these outcome measures are presently considered as mandatory in designing new therapeutic trials in pSS, since these indices allow the correct selection of candidate patients with a relevant level of activity and measure the effectiveness of experimental treatment. In contrast, both indices appear poorly sensitive to changes in patients with mild stable disease.95 ESSPRI can also be criticized since its extreme simplicity does not allow the capture of different components of pain and fatigue in patients with the disorder. However, more detailed validated indices are available if the achievement of more specific results is required.53
Molecular events promoting antigen presentation, costimulation, inflammatory cell homing and function, B-cell proliferation, as well as the formation of ectopic germinal centres have been the object of extensive studies in animal models of SS and in patients with pSS. The advances in molecular biology and engineering have enabled the development of promising target therapies able to interfere with or block the different pathological pathways that are active in this disorder. As demonstrated by the often unsatisfactory results of the previously conducted therapeutic trials, the possibility of successful use of these new therapeutic agents remains a major challenge. These unexpectedly negative results could be the consequence of the remarkable heterogeneity of clinical and biological features that may characterize different subsets of patients with SS. Better knowledge of the different pathways that are operative in diverse clinical phenotypes at the systemic level and in salivary gland tissue, and selection of the target therapy with the highest probability of success, appears to be the best way to reach the goal of actually improving the clinical condition and the quality of life of patients with pSS. Moreover, the identification or development of reliable and sensitive outcome measures and biomarkers specific for each of the different disease subsets is certainly the second very difficult challenge to be tackled in the near feature.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Nicoletta Del Papa, Day Hospital of Rheumatology, Department of Rheumatology, ASST G. Pini-CTO, via Pini 3, 20122 Milan, Italy.
Claudio Vitali, Villa San Giuseppe, Istituto S. Stefano, Como, Italy.
References
- 1. Both T, Dalm VASH, van Hagen PM, et al. Reviewing primary Sjögren’s syndrome: beyond the dryness – from pathophysiology to diagnosis and treatment. Int J Med Sci 2017; 14: 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goules AV, Tzioufas AG. Primary Sjögren’s syndrome: clinical phenotypes, outcome and the development of biomarkers. Autoimmun Rev 2016; 15: 695–703. [DOI] [PubMed] [Google Scholar]
- 3. Karageorgas T, Fragioudakis S, Nezos A, et al. Fatigue in primary Sjögren’s syndrome: clinical, laboratory, psychometric, and biologic associations. Arthritis Care Res 2016; 68: 123–131. [DOI] [PubMed] [Google Scholar]
- 4. Howard Tripp N, Tarn J, Natasari A, et al. Fatigue in primary Sjögren’s syndrome is associated with lower levels of proinflammatory cytokines. RMD Open 2016; 2: e000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Segal BM, Pogatchnik B, Henn L, et al. Pain severity and neuropathic pain symptoms in primary Sjögren’s syndrome: a comparison study of seropositive and seronegative Sjögren’s syndrome patients. Arthritis Care Res 2013; 65: 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Skopouli FN, Dafni U, Ioannidis JP, et al. Clinical evolution, and morbidity and mortality of primary Sjögren’s syndrome. Semin Arthritis Rheum 2000; 29: 296–304. [DOI] [PubMed] [Google Scholar]
- 7. Fragkioudaki S, Mavragani CP, Moutsopoulos HM. Predicting the risk of lymphoma among patients with Sjögren’s syndrome: an easy to use clinical tool. Medicine 2016; 95: e3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nocturne G, Virone A, Ng WF, et al. Rheumatoid factor and disease activity are independent predictors of lymphoma in primary Sjögren’s syndrome. Arthritis Rheumatol 2016; 68: 977–985. [DOI] [PubMed] [Google Scholar]
- 9. Brkic Z, Maria NI, van Helden-Meeuwsen CG, et al. Prevalence of interferon type I signature in CD14 monocytes of patients with Sjögren’s syndrome and association with disease activity and BAFF gene expression. Ann Rheum Dis 2013; 72: 728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hall JC, Casciola-Rosen L, Berger AE, et al. Precise probes of type II interferon activity define the origin of interferon signatures in target tissues in rheumatic diseases. PNAS 2012; 109: 17609–17614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gottenberg JE, Cagnard N, Lucchesi C, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren’s syndrome. PNAS 2006; 103: 2770–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hall JC, Baer AN, Shah AA, et al. Molecular subsetting of interferon pathways in Sjögren’s syndrome. Arthritis Rheumatol 2015; 67: 2437–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nezos A, Gravani F, Tassidou A, et al. Type I and II interferon signatures in Sjögren’s syndrome pathogenesis: contributions in distinct clinical phenotypes and Sjögren’s related lymphomagenesis. J Autoimmun 2015; 63: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Youinou P, Pers JO. Disturbance of cytokine networks in Sjögren’s syndrome. Arthritis Res Ther 2011; 13: 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pertovaara M, Silvennoinen O, Isomäki P. Cytokine-induced STAT1 activation is increased in patients with primary Sjögren’s syndrome. Clin Immunol 2016; 165: 60–67. [DOI] [PubMed] [Google Scholar]
- 16. Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000; 19: 2548–2556. [DOI] [PubMed] [Google Scholar]
- 17. Bromberg J. Stat proteins and oncogenesis. J Clin Invest 2002; 109: 1139–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song JI, Grandis JR. STAT signaling in head and neck cancer. Oncogene 2000; 19: 2489–2495. [DOI] [PubMed] [Google Scholar]
- 19. Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev 2009; 228: 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thurmond RL, Sun S, Karlsson L, et al. Cathepsin S inhibitors as novel immunomodulators. Curr Opin Invest Drugs 2005; 6: 473–482. [PubMed] [Google Scholar]
- 21. Hamm-Alvarez SF, Janga SR, Edman MC, et al. Tear cathepsin S as a candidate biomarker for Sjögren’s syndrome. Arthritis Rheumatol 2014; 66: 1872–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kapsogeorgou EK, Moutsopoulos HM, Manoussakis MN. Functional expression of a costimulatory B7.2 (CD86) protein on human salivary gland epithelial cells that interacts with the CD28 receptor, but has reduced binding to CTLA4. J Immunol 2001; 166: 3107–3113. [DOI] [PubMed] [Google Scholar]
- 23. Kroese FG, Abdulahad WH, Haacke E, et al. B-cell hyperactivity in primary Sjögren’s syndrome. Expert Rev Clin Immunol 2014; 10: 483–499. [DOI] [PubMed] [Google Scholar]
- 24. Nezos A, Mavragani CP. Contribution of genetic factors to Sjögren’s syndrome and Sjögren’s syndrome related lymphomagenesis. J Immunol Res 2015; 754825 DOI: 10.1155/2015/754825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramos-Casals M. The B-lymphocyte stimulator connection in Sjögren’s syndrome. Rheumatology (Oxford) 2013; 52: 223–225. [DOI] [PubMed] [Google Scholar]
- 26. Cancro MP, D’Cruz DP, Khamashta MA. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J Clin Invest 2009; 119: 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Quartuccio L, Salvin S, Fabris M, et al. BLyS upregulation in Sjögren’s syndrome associated with lymphoproliferative disorders, higher ESSDAI score and B-cell clonal expansion in the salivary glands. Rheumatology (Oxford) 2013; 52: 276–281. [DOI] [PubMed] [Google Scholar]
- 28. Wiestner A. The role of B-cell receptor inhibitors in the treatment of patients with chronic lymphocytic leukemia. Haematologica 2015; 100: 1495–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nayar S, Campos J, Buckley CD, et al. Phosphatidylinositol 3-kinase delta pathway a novel therapeutic target for Sjögren’s syndrome. Ann Rheum Dis 2016; 75: A58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Corneth OBJ, Verstappen GMP, Paulissen SMJ, et al. Enhanced Bruton’s tyrosine kinase activity in peripheral blood B lymphocytes from patients with autoimmune disease. Arthritis Rheumatol 2017; 69: 1313–1324. [DOI] [PubMed] [Google Scholar]
- 31. Mócsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol 2010; 10: 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dörner T, Shock A, Goldenberg DM, et al. The mechanistic impact of CD22 engagement with epratuzumab on B cell function: implications for the treatment of systemic lupus erythematosus. Autoimmun Rev 2015; 14: 1079–1086. [DOI] [PubMed] [Google Scholar]
- 33. Risselada AP, Looije MF, Kruize AA, et al. The role of ectopic germinal centers in the immunopathology of primary Sjögren’s syndrome: a systematic review. Semin Arthritis Rheum 2013; 42: 368–376. [DOI] [PubMed] [Google Scholar]
- 34. Theander E, Vasaitis L, Baecklund E, et al. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren’s syndrome. Ann Rheum Dis 2011; 70: 1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Szabo K, Papp G, Barath S, et al. Follicular helper T cells may play an important role in the severity of primary Sjögren’s syndrome. Clin Immunol 2013; 147: 95–104. [DOI] [PubMed] [Google Scholar]
- 36. Bombardieri M, Barone F, Humby F, et al. Activation-induced cytidine deaminase expression in follicular dendritic cell networks and interfollicular large B cells supports functionality of ectopic lymphoid neogenesis in autoimmune sialoadenitis and MALT lymphoma in Sjögren’s syndrome. J Immunol 2007; 179: 4929–4938. [DOI] [PubMed] [Google Scholar]
- 37. Barone F, Nayar S, Campos J, et al. IL-22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. PNAS 2015; 112: 11024–11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pitzalis C, Jones GW, Bombardieri M, et al. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol 2014; 14: 447–462. [DOI] [PubMed] [Google Scholar]
- 39. Alunno A, Carubbi F, Bistoni O, et al. T regulatory and T helper 17 cells in primary Sjögren’s syndrome: facts and perspectives. Mediators Inflamm 2015; 2015: 243723 DOI: 10.1155/2015/243723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fava RA, Kennedy SM, Wood SG, et al. Lymphotoxin-beta receptor blockade reduces CXCL13 in lacrimal glands and improves corneal integrity in the NOD model of Sjögren’s syndrome. Arthritis Res Ther 2011; 13: R182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shiboski CH, Shiboski SC, Seror R, et al. ; International Sjögren’s Syndrome Criteria Working Group. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 2017; 76: 9–16. [DOI] [PubMed] [Google Scholar]
- 42. Shiboski CH, Shiboski SC, Seror R, et al. ; International Sjögren’s Syndrome Criteria Working Group. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol 2017; 69: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baldini C, Talarico R, Tzioufas AG, et al. Classification criteria for Sjögren’s syndrome: a critical review. J Autoimmun 2012; 39: 9–14. [DOI] [PubMed] [Google Scholar]
- 44. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002; 61: 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seror R, Ravaud P, Bowman S, et al. EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI): development of a consensus systemic disease activity index in primary Sjögren’s syndrome. Ann Rheum Dis 2010; 69: 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jousse-Joulin S, Milic V, Jonsson MV, et al. Is salivary gland ultrasonography a useful tool in Sjögren’s syndrome? A systematic review. Rheumatology (Oxford) 2016; 55: 789–800. [DOI] [PubMed] [Google Scholar]
- 47. Vitali C, Bombardieri S, Jonsson R, et al. ; European Study Group on Classification Criteria for Sjögren’s Syndrome. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002; 61: 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fisher BA, Jonsson R, Daniels T, et al. Standardisation of labial salivary glandhistopathology in clinical trials in primary Sjögren’s syndrome. Ann Rheum Dis 2017; 76: 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seror R, Bootsma H, Saraux A, et al. Defining disease activity states and clinically meaningful improvement in primary Sjögren’s syndrome with EULAR primary Sjögren’s syndrome disease activity (ESSDAI) and patient-reported indexes (ESSPRI). Ann Rheum Dis 2016; 75: 382–389. [DOI] [PubMed] [Google Scholar]
- 50. Seror R, Ravaud P, Mariette X, et al. EULAR Sjögren’s Syndrome Patient Reported Index (ESSPRI): development of a consensus patient index for primary Sjögren’s syndrome. Ann Rheum Dis 2011; 70: 968–972. [DOI] [PubMed] [Google Scholar]
- 51. Brito-Zeron P, Kostov B, Solans R, et al. Systemic activity and mortality in primary Sjögren’s syndrome: predicting survival using the EULAR-SS Disease Activity Index (ESSDAI) in 1045 patients. Ann Rheum Dis 2016; 75: 348–555. [DOI] [PubMed] [Google Scholar]
- 52. Seror R, Meiners P, Baron G, et al. Development of the ClinESSDAI: a clinical score without biological domain. A tool for biological studies. Ann Rheum Dis 2016; 75: 1945–1950. [DOI] [PubMed] [Google Scholar]
- 53. Bowman S, Pillemer S, Jonsson R, et al. Revisiting Sjögren’s syndrome in the new millennium: perspectives on assessment and outcome measures. Report of a workshop held on 23 March 2000 at Oxford, UK. Rheumatology 2001; 40: 1180–1188. [DOI] [PubMed] [Google Scholar]
- 54. Leonardi A, Van Setten G, Amrane M, et al. Efficacy and safety of 0.1% cyclosporine A cationic emulsion in the treatment of severe dry eye disease: a multicenter randomized trial. Eur J Ophthalmol 2016; 26: 287–296. [DOI] [PubMed] [Google Scholar]
- 55. Vivino FB, et al. Pilocarpine tablets for the treatment of dry mouth and dry eye symptoms in patients with Sjögren’s syndrome: a randomized, placebo-controlled, fixed-dose, multicenter trial. P92-01 Study Group. Arch Intern Med 1999; 159: 174–181. [DOI] [PubMed] [Google Scholar]
- 56. Petrone D, Condemi JJ, Fife R, et al. A double-blind, randomized, placebo-controlled study of cevimeline in Sjögren’s syndrome patients with xerostomia and keratoconjunctivitis sicca. Arthritis Rheum 2002; 46: 748–754. [DOI] [PubMed] [Google Scholar]
- 57. Foulks GN, Forstot SL, Donshik PC, et al. Clinical guidelines for management of dry eye associated with Sjögren’s disease. Ocul Surf 2015; 13: 118–132. [DOI] [PubMed] [Google Scholar]
- 58. Le Gall M, Cornec D, Pers JO, et al. A prospective evaluation of dental and periodontal status in patients with suspected Sjögren’s syndrome. Joint Bone Spine 2016; 83: 235–236. [DOI] [PubMed] [Google Scholar]
- 59. van der Reijden WA, van der Kwaak H, Vissink A, et al. Treatment of xerostomia with polymer-based saliva substitutes in patients with Sjögren’s syndrome. Arthritis Rheum 1996; 39: 57–63. [DOI] [PubMed] [Google Scholar]
- 60. Strietzel FP, Lafaurie GI, Mendoza GR, et al. Efficacy and safety of an intraoral electrostimulation device for xerostomia relief: a multicenter, randomized trial. Arthritis Rheum 2011; 63: 180–190. [DOI] [PubMed] [Google Scholar]
- 61. Saraux A, Pers JO, Devauchelle-Pensec V, et al. Treatment of primary Sjögren’s syndrome. Nat Rev Rheumatol 2016; 12: 456–471. [DOI] [PubMed] [Google Scholar]
- 62. Carsons SE, Vivino FB, Parke A, et al. Treatment guidelines for rheumatologic manifestations of Sjögren’s: use of biologics, management of fatigue and inflammatory musculoskeletal pain. Arthritis Care Res 2017; 69: 517–527. [DOI] [PubMed] [Google Scholar]
- 63. Gottenberg JE, Ravaud P, Puechal X, et al. Effects of hydroxychloroquine on symptomatic improvement in primary Sjögren syndrome: the JOQUER randomized clinical trial. JAMA 2014; 312: 249–258. [DOI] [PubMed] [Google Scholar]
- 64. Mavragani CP, Moutsopoulos HM. Conventional therapy of Sjögren’s syndrome. Clin Rev Allergy Immunol 2007; 32: 284–291. [DOI] [PubMed] [Google Scholar]
- 65. Clark EA, Ledbetter JA. How does B cell depletion therapy work, and how can it be improved? Ann Rheum Dis 2005; 64(Suppl. 4): iv77–iv80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pijpe J, Meijer JM, Bootsma H, et al. Clinical and histologic evidence of salivary gland restoration supports the efficacy of rituximab treatment in Sjögren’s syndrome. Arthritis Rheum 2009; 60: 3251–3256. [DOI] [PubMed] [Google Scholar]
- 67. Alunno A, Carubbi F, Bistoni O, et al. Interleukin (IL)-17-producing pathogenic T lymphocytes co-express CD20 and are depleted by rituximab in primary Sjögren’s syndrome: a pilot study. Clin Exp Immunol 2016; 184: 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pijpe J, van Imhoff GW, Spijkervet FK, et al. Rituximab treatment in patients with primary Sjögren’s syndrome: an open-label phase II study. Arthritis Rheum 2005; 52: 2740–2750. [DOI] [PubMed] [Google Scholar]
- 69. Devauchelle-Pensec V, Pennec Y, Morvan J, et al. Improvement of Sjögren’s syndrome after two infusions of rituximab (anti-CD20). Arthritis Rheum 2007; 57: 310–317. [DOI] [PubMed] [Google Scholar]
- 70. Meiners PM, Arends S, Brouwer E, et al. Responsiveness of disease activity indices ESSPRI and ESSDAI in patients with primary Sjögren’s syndrome treated with rituximab. Ann Rheum Dis 2012; 71: 1297–1302. [DOI] [PubMed] [Google Scholar]
- 71. Carubbi F, Cipriani P, Marrelli A, et al. Efficacy and safety of rituximab treatment in early primary Sjögren’s syndrome: a prospective, multi-center, follow-up study. Arthritis Res Ther 2013; 15: R172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dass S, Bowman SJ, Vital EM, et al. Reduction of fatigue in Sjögren’s syndrome with rituximab: results of a randomized double-blind, placebo-controlled pilot study. Ann Rheum Dis 2008; 67: 1541–1544. [DOI] [PubMed] [Google Scholar]
- 73. Meijer JM, Meiners PM, Vissink A, et al. Effectiveness of rituximab treatment in primary Sjögren’s syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2010; 62: 960–968. [DOI] [PubMed] [Google Scholar]
- 74. Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, et al. Treatment of primary Sjögren’s syndrome with rituximab: a randomized trial. Ann Intern Med 2014; 160: 233–242. [DOI] [PubMed] [Google Scholar]
- 75. Bowman SJ, Everett CC, O’Dwyer JL, et al. Randomized controlled trial of rituximab and cost-effectiveness analysis in treating fatigue and oral dryness in primary Sjögren’s syndrome. Arthritis Rheumatol 2017; 69: 1440–1450. [DOI] [PubMed] [Google Scholar]
- 76. St Clair EW, Levesque MC, Prak ET, et al. ; Autoimmunity Centers of Excellence. Rituximab therapy for primary Sjögren’s syndrome: an open-label clinical trial and mechanistic analysis. Arthritis Rheum 2013; 65: 1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Verstappen GM, van Nimwegen JF, Vissink A, et al. The value of rituximab treatment in primary Sjögren’s syndrome. Clin Immunol 2017; 182: 62–71. [DOI] [PubMed] [Google Scholar]
- 78. Gottenberg JE, Cinquetti G, Larroche C, et al. Efficacy of rituximab in systemic manifestations of primary Sjögren’s syndrome: results in 78 patients of the AutoImmune and Rituximab registry. Ann Rheum Dis 2013; 72: 1026–1031. [DOI] [PubMed] [Google Scholar]
- 79. Goede V, Fischer K, Engelke A, et al. Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: updated results of the CLL11 study. Leukemia 2015; 29: 1602–1604. [DOI] [PubMed] [Google Scholar]
- 80. Mariette X, Seror R, Quartuccio L, et al. Efficacy and safety of belimumab in primary Sjögren’s: results of the BELISS open-label phase II study. Ann Rheum Dis 2015; 74: 526–531. [DOI] [PubMed] [Google Scholar]
- 81. De Vita S, Quartuccio L, Seror R, et al. Efficacy and safety of belimumab given for 12 months in primary Sjögren’s syndrome: the BELISS open-label phase II study. Rheumatology 2015; 54: 2249–2256. [DOI] [PubMed] [Google Scholar]
- 82. Quartuccio L, Mavragani CP, Nezos A. Type I interferon predicts biological effect of belimumab on rheumatoid factor positive B-cells in Sjögren’s syndrome: results from the BELISS trial. Ann Rheum Dis 2016; 75(Suppl. 2): 294. [Google Scholar]
- 83. Pontarini E, Fabris M, Quartuccio L, et al. Treatment with belimumab restores B cell subsets and their expression of B cell activating factor receptor in patients with primary Sjögren’s syndrome. Rheumatology 2015; 54: 1429–1434. [DOI] [PubMed] [Google Scholar]
- 84. De Vita S, Quartuccio L, Salvin S, et al. 2014. Sequential therapy with belimumab followed by rituximab in Sjögren’s syndrome associated with B-cell lymphoproliferation and overexpression of BAFF: evidence for long-term efficacy. Clin Exp Rheumatol 32: 490–494. [PubMed] [Google Scholar]
- 85. Steinfeld SD, Tant L, Burmester GR, et al. Epratuzumab (humanised anti-CD22 antibody) in primary Sjögren’s syndrome: an open-label phase I/II study. Arthritis Res Ther 2006; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Adler S, Korner M, Forger F, et al. Evaluation of histologic, serologic, and clinical changes in response to abatacept treatment of primary Sjögren’s syndrome: a pilot study. Arthritis Care Res 2013; 65: 1862–1868. [DOI] [PubMed] [Google Scholar]
- 87. Meiners PM, Vissink A, Kroese FGM, et al. Abatacept treatment reduces disease activity in early primary Sjögren’s syndrome (open-label proof of concept ASAP study). Ann Rheum Dis 2014; 73: 1393–1396. [DOI] [PubMed] [Google Scholar]
- 88. Sankar V, Brennan MT, Kok MR, et al. Etanercept in Sjögren’s syndrome: a twelve-week randomized, double-blind, placebo controlled pilot clinical trial. Arthritis Rheum 2004; 50: 2240–2245. [DOI] [PubMed] [Google Scholar]
- 89. Mariette X, Ravaud P, Steinfeld S, et al. Inefficacy of infliximab in primary Sjögren’s syndrome: results of the randomized, controlled Trial of Remicade in Primary Sjögren’s Syndrome (TRIPSS). Arthritis Rheum 2004; 50: 1270–1276. [DOI] [PubMed] [Google Scholar]
- 90. Norheim KB, Harboe E, Goransson LG, et al. Interleukin-1 inhibition and fatigue in primary Sjögren’s syndrome: a double blind, randomised clinical trial. PLoS One 2012; 7: e30123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Norman P. Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin Invest Drugs 2014; 23: 1067–1077. [DOI] [PubMed] [Google Scholar]
- 92. St. Clair EW, Baer AN, Noaiseh G, et al. The clinical efficacy and safety of baminercept, a lymphotoxin-beta receptor fusion protein, in primary Sjögren’s syndrome: results from a randomized, double-blind, placebo-controlled phase II trial. Arthritis Rheum 2015; 67(Suppl. 10): 3844 (abstract 3203). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tsuboi H, Hagiwara S, Asashima H, et al. Comparison of performance of the 2016 ACR-EULAR classification criteria for primary Sjögren’s syndrome with other sets of criteria in Japanese patients. Ann Rheum Dis 2017; 76: 1980–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vitali C, Del Papa N. Classification and diagnostic criteria in Sjögren’s syndrome: a long-standing and still open controversy. Ann Rheum Dis 2017; 76: 1953–1954. [DOI] [PubMed] [Google Scholar]
- 95. Campar A, Isenberg DA. Primary Sjögren’s syndrome activity and damage indices comparison. Eur J Clin Invest 2010; 40: 636–644. [DOI] [PubMed] [Google Scholar]