Abstract
The kinase MEKK2 (MAP3K2) activates the MEK5/ERK5 cell signaling pathway and may play an important role in tumor growth and metastasis. Thus, MEKK2 may represent a novel kinase target for cancer. In order to identify inhibitors of MEKK2, we screened a library of compounds using a high throughput MEKK2 intrinsic ATPase enzyme assay. We identified two hits with validated structures and confirmed activity in the primary assay (IC50 values = 322 nM and 7.7 μM) and two orthogonal MEKK2 biochemical assays. Compound 1, the more potent hit, was the subject of further investigation. Limited structure-activity relationship (SAR) studies were performed on this iminocoumarin hit which resulted in ≥20-fold more potent analogs (e.g. 8 and 16 nM IC50). Two analogs had improved selectivity in a 50-member kinase profiling panel compared to the hit. These studies suggested that substitutions around the phenoxy ring of this scaffold can impart improved potency and selectivity for MEKK2. Analog Compound 1s (16 nM IC50) was further verified by external testing to inhibit MEKK2 and MEKK3 with similar potencies. Compound 1s displayed activity in cell-based assays in which it inhibited ERK5 pathway activation in cells and inhibited cell migration in a scratch assay. Thus, we have identified a scaffold that has promising potential to be developed into a highly selective and potent inhibitor of MEKK2. Information from these SAR studies provides specific guidance for the future design of MEKK2 inhibitor probes.
Keywords: MEKK2, MAP3K2, kinase, inhibitor
Introduction
The ERK5 signaling pathway is one of the mitogen-activated protein kinase (MAPK) signaling pathways that alter gene expression in response to extracellular stimuli. [1,2]. The ERK5 pathway is activated by oxidative stress, hyperosmolarity and growth factors such as epidermal growth factor (EGF) [3]. ERK5 is activated through phosphorylation by the MAPK kinase MEK5 (MAP2K5). MEK5 in turn is activated through phosphorylation by the MAPK kinase kinases MEKK2 (MAP3K2) and MEKK3 (MAP3K3). The mechanism leading to MEKK2 activation is still not fully understood. However, it is known that MEKK2 autophosphorylates in response to stimuli and this autophosphorylation is required for activation [4]. In addition to the ERK5 pathway, MEKK2 also activates the JNK MAPK pathway by phosphorylation of the MAP2K MEK7 which in turn activates JNK [5,6]. Mouse gene knock-out studies have provided clues to the biological function of the MEKK2/3-MEK5-ERK5 pathway. ERK5 knock-out in mice resulted in embryonic lethality due to defects in cardiac development and angiogenesis [7]. ERK5 appears to protect many cell types from stress-induced apoptosis [3,8,9]. MEK5 gene knock out in mice resulted in lethality apparently due to abnormal cardiac development, similar to the ERK5 knockout mouse [10]. MEKK2 knock-out mice displayed normal development and fertility [5,11]. However, knock out of the MEKK3 gene in mice resulted in embryonic lethality due to cardiac development defects [12]. These animal knock out studies indicate that MEKK2 and MEKK3 signal in response to different stimuli. MEKK2 is activated by cell attachment to fibronectin and then localizes to focal adhesions. MEKK2 knockdown by RNAi in breast cancer cell lines was shown to stabilize focal adhesions and inhibit cell migration in scratch assays [13,14].
A potential role of MEKK2 in cancer has been explored to a limited extent. In a study linking MEKK2 to prostate cancer, MEKK2 was expressed at 4.4-fold higher levels in prostate cancer tissue compared to benign tissue [15]. Prostate cancer cell lines expressed even higher levels of MEKK2. The microRNA miR-520b has been shown to suppress tumor growth in breast cancer and hepatocellular carcinoma cells via suppressing expression of MEKK2 and Cyclin D1 [16]. Importantly, knock-down of just the MEKK2 expression by siRNA was able to reduce the growth of hepatocarcinoma cells in vitro and in vivo. Furthermore, restoration of the expression of another tumor suppressor, miR17/20a, enhanced tumor cell sensitivity to lysis by natural killer cells via suppressing MEKK2 [17]. MEKK2 has been shown to be highly expressed in primary colorectal cancer compared to normal mucosa [18]. An in vivo mouse xenograft model of breast cancer was used to assess the role of MEKK2 in tumor growth and metastasis [19]. shRNA-mediated knockdown of MEKK2 inhibited activation of ERK5 in response to EGF using the breast cancer cell line MDA-MB-231. Surprisingly, MEKK2 knock down had no observable impact on cell growth in vitro, but strongly inhibited both tumor growth and metastasis in vivo. The smaller tumors resulting from the MEKK2 knock-down cells displayed increased apoptosis over size-matched control tumors. Knockdown of MEKK2 in the BT474 breast cancer cell line also resulted in inhibited tumor growth in vivo. In contrast, the knockdown of ERK5 inhibited metastasis without significantly impacting tumor growth. In this animal model, activation of ERK5 by MEKK2 appeared to regulate metastasis, while another MEKK2-dependent pathway, potentially the JNK pathway, was important for tumor growth. In addition, the downstream components activated by MEKK2, MEK5 and ERK5, have also been implicated in cancer progression by numerous studies [20]. An inhibitor of MEKK2 may reduce activation of both the ERK5 and JNK pathways leading to improved anti-tumor efficacy.
Thus, the current literature data supports the concept of MEKK2 as a novel drug target. However, no specific and potent small molecule inhibitors of MEKK2 have been reported to date. We have previously reported the discovery of approved drugs (e.g. ponatinib), clinical trial drugs and other research tool compounds that potently inhibit MEKK2 activity in biochemical assays [21,22]. However, all of these lack selectivity for MEKK2 and it is unknown if any of them inhibit MEKK2 activity in cells.
Materials and Methods
Materials
Common reagents such as HEPES, dithiothreitol (DTT), MgCl2 and dimethyl sulfoxide (DMSO) were reagent grade quality purchased from Thermo Fisher Scientific (Waltham, MA) or Sigma-Aldrich (St. Louis, MO). Organic synthesis and structure confirmation of Compound 1 and its analogs 1b – 1s are described in Supplementary Material. Compounds 1, 1a and 2 were purchased from Life Chemicals (Niagara-on-the-Lake ON, Canada) and Enamine (Monmouth Jct., NJ). All synthesized and purchased compounds were verified for purity (≥95%) and mass by LC/MS. The structures of all synthesized compounds were confirmed by 1H and 13C NMR. Compounds were dissolved in DMSO and DMSO was used as solvent control and concentration of DMSO was kept constant under all experimental conditions.
Cell culture
MDA-MB-231 cell line (ATCC, Manassas, VA) was cultured at 37°C in an atmosphere of 5% CO2 and 95% humidity in DMEM-high glucose media supplemented with 10% FBS, 200 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin and 15 mM HEPES. All reagents and plasticware for cell culture were obtained from ThermoFisher Scientific.
MEKK2 Biochemical Assays
The MEKK2 intrinsic ATPase activity assay, MEKK2 TR-FRET binding assay, MEKK2 transphosphorylation activity assay and IC50 determinations were performed as previously described [21]. MEKK2 activity assays were performed using ATP concentrations at the apparent Km of the assay format. For all MEKK2 assay formats, compound concentration response curves were generated using data points that represent the average of 2 or 3 determinations per concentration and 8–10 compound concentrations tested.
Kinase Profiling
Kinase profiling of a serine/threonine and tyrosine kinase panel was performed by Carna Biosciences, Inc. (Kobe, Japan) using a mobility shift assay format as previously reported [23]. In this 50-member kinase assay panel, compounds were tested at 1 μM concentration in duplicate and average percent inhibition reported. The IC50 data generated by Carna Biosciences was performed using target enzyme and another kinase as a substrate in an enzyme-linked immunosorbent assay (ELISA)-based format to detect product. In these assays, each concentration was tested in duplicate and data averaged before calculating the IC50 values. Carna Biosciences kinase assays were performed using ATP concentrations at the apparent Km for ATP in each kinase assay.
Cell-Based Assays
For ERK5 pathway inhibition analysis, MDA-MB-231 cells were incubated in serum-free media overnight and then treated with compound (or DMSO as solvent control) for 1.5 h prior to stimulating cells for 30 min with 0.2 M sorbitol to activate the ERK5 pathway. Cell lysates were made and standard western blot analysis was performed as previously reported [24], using anti-phosphoERK5 antibody (Cell Signaling, Danvers, MA, cat# 3371) and then the membrane was stripped and re-probed with anti-ERK5 recognizing total ERK5 (Cell Signaling). The scratch cell migration assay was performed according to published protocol [25]. After a confluent cell monolayer was reached and the scratch in the cell monolayer created, compound or 1% DMSO was added to the normal growth media and the same area of the scratch photographed at 0 and 18–24 hr later.
Results and Discussion
We previously reported the development and validation of a novel intrinsic ATPase activity assay for MEKK2 and demonstrated its utility as a high throughput assay for the discovery of small molecule inhibitors of MEKK2 [21]. We used this assay to screen a collection of approximately 15,000 commercially available compounds ([21] and data not shown). Herein, we report the structures and activities of two confirmed hits from this screen. Hit compound 1 generated IC50 values in the MEKK2 ATPase activity assay of 322 ± 161 nM (Table 1). Compound 1 was further confirmed as a hit by re-synthesis. Two completely different MEKK2 assay formats were used to confirm the activity observed in the ATPase assay. The MEKK2 TR-FRET binding assay measured binding to MEKK2 via competitive displacement of a labeled ATP-site binding small molecule. An additional MEKK2 activity assay was employed that detects the native transphosphorylation activity of MEKK2 using kinase-inactive MKK6 as a substrate. Phosphorylation of MKK6 was detected by western-style quantitative slot blot using a phospho-MKK6 specific antibody [21]. Compound 1 produced average potencies of 355 ± 134 nM in the binding assay and 261 ± 14 nM in the transphosphorylation assay format (Table 1). Thus, compound 1 confirmed activity in all three MEKK2 assay formats. Compound 2 (hit #2) generated IC50 values in the MEKK2 ATPase activity assay of 7.7 ± 1.6 uM and similar potency in the MEKK2 binding assay with an ave. IC50 of 7.9 ± 3.6 uM (Table 1). Thus, both hits were confirmed as MEKK2 inhibitors with multiple assay formats. Since compound 1 was significantly more potent, we focused further work on this scaffold.
Table 1.
Structures and activities of screening hits in multiple MEKK2 assay formats
| Compound | Structure | IC50 ± SD (nM) for MEKK2a | ||
|---|---|---|---|---|
|
| ||||
| Intrinsic ATPase Assay | Binding Assay | Transphos. Assay | ||
| 1 |
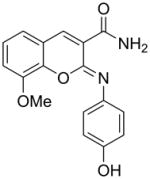
|
322 ± 161 | 355 ± 134b | 261 ± 14b |
| 2 |
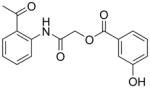
|
7,710 ± 1,610 | 7,930 ± 3,600 | NDc |
IC50 values are the mean ± SD of at least three independent determinations using the indicated assay.
Values as reported in [21].
Not determined
We sought to perform limited SAR on compound 1 to explore the potential of this iminocoumarin scaffold for improved potency and selectivity. We focused our efforts on substitution around the phenol ring and modifying the methoxy group on the chromene ring and assessing activity using the ATPase assay (Table 2). Replacing the hydroxy group of the phenol ring with a methoxy group (Table 2, compound 1a) resulted in complete loss in inhibitory activity. Many other substitutions were tested (data not shown), but all resulted in a complete loss in activity, indicating that the hydroxy group is absolutely required for activity. This finding may be analogous to a reported LRRK2 inhibitor in which modeling and SAR suggested that a critical 4-hydroxy on a phenol group may act as donor and acceptor with conserved residues [26]. At the R′2 position of the phenol ring, we substituted with a chlorine, methyl, hydroxy and a fluorine group (Table 2, compounds 1b–e). The presence of a methyl or hydroxy group had little impact on the potency. In contrast, the chlorine or fluorine group resulted in enhanced potencies with IC50s of 60 and 115 nM. At the R′1 position on the phenol group, we substituted with CF3, methyl and fluorine groups (compounds 1f–h) which resulted in dramatic enhancement of MEKK2 potency with IC50 values of 38, 28 and 39 nM, respectively.
Table 2.
MEKK2 inhibitory activity for Compound 1 derivatives
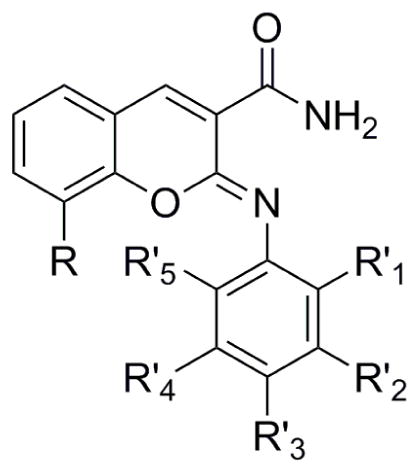
| |||||||
|---|---|---|---|---|---|---|---|
| Compound | R′1 | R′2 | R′3 | R′4 | R′5 | R | IC50 (nM)a |
| 1a | H | H | OMe | H | H | OMe | >10,000 |
| 1b | H | Cl | OH | H | H | OMe | 60b |
| 1c | H | Me | OH | H | H | OMe | 362 |
| 1d | H | OH | OH | H | H | OMe | 361 |
| 1e | H | F | OH | H | H | OMe | 115 |
| 1f | CF3 | H | OH | H | H | OMe | 38 |
| 1g | Me | H | OH | H | H | OMe | 28 |
| 1h | F | H | OH | H | H | OMe | 39 |
| 1i | F | F | OH | H | H | OMe | 36 |
| 1j | H | F | OH | F | H | OMe | 27 |
| 1k | F | H | OH | H | F | OMe | 8 |
| 1l | H | H | OH | H | H | OEt | 130b |
| 1m | H | H | OH | H | H | OPropyl | 371c |
| 1n | H | H | OH | H | H | OIsopropyl | 502b |
| 1o | H | H | OH | H | H | F | 2102 |
| 1p | H | H | OH | H | H | Br | 152b |
| 1q | H | H | OH | H | H | OH | 766 |
| 1r | CF3 | H | OH | H | H | OEt | 18c |
| 1s | Me | H | OH | H | H | OEt | 16 |
IC50 values are the mean of two to five independent determinations using the intrinsic ATPase MEKK2 activity assay.
Apparent solubility problems observed at compound concentrations >1 μM.
Apparent solubility problems observed at compound concentrations >0.3 μM.
Since fluorine at the R′1 and R′2 positions was shown to enhance the potency, we sought to determine if difluorinated analogs of compound 1 would further improve the potency. Difluorinated compounds 1i–j (Table 2,) with fluorines at the R′1/R′2 and R′2/R′4 positions generated IC50 values of 36 and 27 nM. These compounds were similar in potency as monofluorinated compound 1h. However, substitution of fluorines at the R′1 and R′5 positions of the phenolic ring generated compound 1k with an IC50 of 8 nM. The enhancement in potency of the mono and difluorinated compounds may be due to the electron withdrawing ability of this group to modulate the hydrogen bonding donor/acceptor of the phenol hydroxyl group. Limited SAR was also explored at the methoxy position of the chromene ring (Table 2). Replacement of the methoxy group with an ethoxy, propoxy, isopropoxy, fluorine, bromine and hydroxy groups resulted in compounds 1l–q which gave IC50s of 130, 371, 502, 2102, 151 and 766 nM respectfully. Thus, the ethoxy group at this position resulted in >2-fold improvement in potency which was the best among the tested substitutions. Since a methyl or CF3 group at the R′1 position on the phenol group dramatically enhanced potency of the hit compound 1, we synthesized analogs of these compounds containing an ethoxy group on the chromene ring. The methyl and CF3 substituted compounds 1r–s generated IC50s that were reduced to 16 and 18 nM, respectively (Table 2).
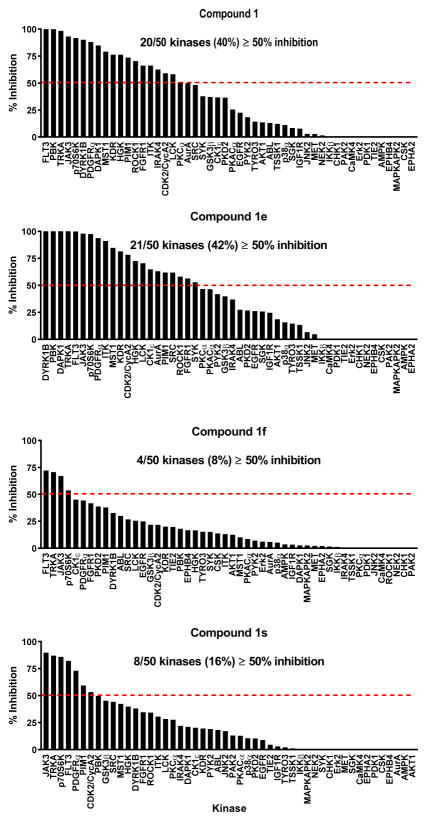
In order to assess the selectivity of this scaffold, we profiled the inhibitory activity of selected analogs against a panel of diverse kinase activity assays performed by Carna Biosciences. Selected compounds were tested at 1 μM compound against a panel of 50 serine/threonine and tyrosine kinase activity assays. Compound 1 inhibited ≥50% of the activity in 40% of the kinase panel, thus indicating low selectivity for this hit (Fig. 1). The R′2 fluoro analog of compound 1 (1e), inhibited ≥50% of the activity in 42% of the kinase panel (Fig. 1). Of note, >95% inhibition was obtained in 7 kinase assays whereas the original hit compound produced >95% inhibition in only 3 kinases. These data suggested that the 2′ F substitution resulted in reduced selectivity. In contrast, profiling of compound 1f with the R′1 CF3 substitution on the phenol group resulted in ≥50% inhibition of only 8% of the kinase panel (Fig. 1). Thus, the CF3 group appeared to impart enhanced selectivity while enhancing activity against MEKK2 by over 8-fold. Profiling of the dual modified analog compound 1s resulted in hitting 16% of the kinases in the panel (Fig. 1), but it’s also twice as potent against MEKK2 compared to compound 1f. Overall, the activity and selectivity profiles appeared similar between compounds 1f and 1s.
Figure 1. Selectivity of Compound 1 and derivatives in a panel of 50 kinase activity assays.
Kinase profiling was performed by Carna Biosciences. Compounds 1, 1e, 1f and 1s were tested at 1 μM in duplicate in each assay and the average percent inhibition was plotted for the indicated kinase. Results were sorted by activity with the highest percent inhibition on the left side of the graphs. Dashed line indicates 50% inhibition. The number and percentage of kinase assays resulting in ≥50% inhibition is provided for each compound.
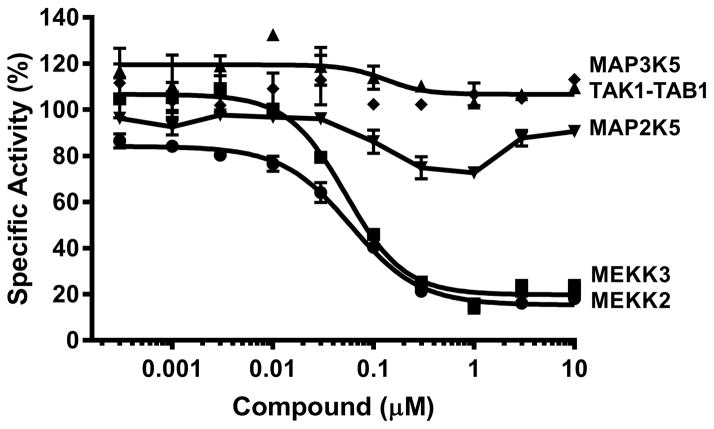
We sought to further validate the MEKK2 inhibitory activity of compound 1s using a different source of MEKK2 and a different assay format while also further assessing selectivity of other kinases in the MAPK family (Fig. 2). An MEKK2 transphosphorylation assay was performed by Carna Biosciences using MAP2K7 as a substrate and an ELISA for detection of phosphorylated product. In this assay, compound 1s generated an IC50 of 60 nM against MEKK2 which is similar (<4-fold) to the IC50 value determined by our ATPase assay format. The discrepancy in potencies could be due to the use of only the catalytic domain of MEKK2 in the CarnaBio assay, as opposed to the full-length MEKK2 in our assays. This compound also produced an IC50 of 53 nM against the closely related MEKK3, making it a dual inhibitor of MEKK2/MEKK3. This was not unexpected since the catalytic domains of MEKK2 and MEKK3 are 94% homologous [27]. Activity against MAP2K5 (MEK5) was also tested and 50% inhibition was not achieved, possibly due to solubility problems in this assay. Based on the dose response curve, we estimated an IC50 of >1 μM against MAP2K5. No activity (>10 μM IC50) was observed against MAP3K5 (ASK1) and TAK1-TAB1 (MAP3K7).
Figure 2. IC50 determinations for Compound 1s in selected kinase activity assays.
IC50 determinations for Compound 1s were performed by Carna Biosciences using the indicated kinase activity assays. Biochemical kinase activity assays were performed using the indicated target kinase and another kinase as substrate in ELISA-based activity assays where the [ATP] was at or near the respective Km values for each kinase assay. Compound 1s was tested in duplicate at each of the 10 indicated concentrations for each assay and the mean ± SD percent activity was plotted. Calculated IC50 values were 60 nM for MEKK2 (●), 53 nM for MEKK3 (■), >1 μM for MAP2K5 (▼) and >10 μM for TAK1-TAB1 (◆) and MAP3K5 (▲).
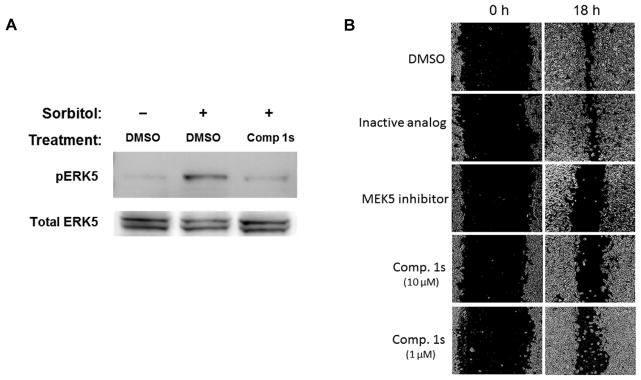
We further investigated the activity of Compound 1s in cell-based assays. Initially, we tested compound 1s for cell cytotoxicity. This compound had no effect on the viability of MDA-MB-231 cells in a 24 hr exposure resazurin cell viability assay (data not shown). We sought to determine whether Compound 1s could inhibit the ERK5 pathway using the appearance of phosphorylated ERK5 (pERK5) as an indicator of pathway activation. We treated cells with 10 μM compound for 1.5 hr and then stimulated the cells with 0.2 M sorbitol for 30 min (Fig. 3A). Western blot analysis of the cell lysates indicated that Compound 1s inhibited the phosphorylation of ERK5, an indicator of inhibition of the MEKK2/MEK5/ERK5 pathway. Furthermore, we tested Compound 1s in the scratch cell migration assay to determine if it could inhibit cell migration which has been reported to involve MEKK2 for these cells (Fig. 3B). The data indicated that an inactive analog of compound 1 had no effect compared to DMSO treated control, while the MEK5 inhibitor BIX02188 (1 μM) and Compound 1s (10 and 1 μM) showed inhibition of scratch healing. Thus, compound 1s was able to inhibit the ERK5 pathway activation in cells and cell migration in the scratch assay.
Figure 3. Activity of Compound 1s in cell-based assays.
(A) MDA-MB-231 cells were treated with DMSO or Compound 1s (10 μM) and then stimulated with sorbitol, as indicated. Cell lysates were analyzed by western blot for phosphoERK5 (pERK5) and total ERK5. Data are representative of two independent experiments. (B) A cell migration “scratch” assay was employed using MDA-MB-231 cells. After scratching the cell monolayers, cells were treated at 0 h with 1% DMSO (solvent), an inactive analog of compound 1, the MEK5 inhibitor BIX02188 or Compound 1s (10 or 1 μM). Photographs show the scratch at 0 and 18 hr as indicated. White areas are cell monolayers, while black areas are zones without cells. Each condition was performed in duplicate or singlet wells and data are representative of four independent experiments.
We report the identification and confirmation of 2 HTS hits with in vitro MEKK2 inhibitory activity using our previously reported MEKK2 ATPase HTS assay. Initial SAR studies for one of the hits resulted in ≥20-fold more potent analogs with the most potent one having an IC50 of 8 nM for MEKK2. This small set of analogs also led to the identification of analogs with improved selectivity in a 50-member kinase profiling panel. These SAR studies suggested that substitutions around the phenoxy ring can impart improved potency and selectivity for MEKK2. We also demonstrated that one of these analogs displayed cell-based assay activity where it inhibited EKR5 pathway activation and cell migration.
Supplementary Material
Highlights.
Two novel small molecule inhibitors of MEKK2 discovered from screening
Structures validated and activities confirmed in multiple MEKK2 biochemical assays
Synthesis of analogs for iminocoumarin scaffold led to ≥20-fold more potent analogs
Kinase profiling indicated that scaffold modifications could increase selectivity
One analog inhibited ERK5 pathway activation in cells and cell migration
Acknowledgments
This work was supported by a grant from NIH/NCI (5U54CA156735, JES and GLJ) and in part by general funding from the State of North Carolina.
Abbreviations used
- DMSO
dimethyl sulfoxide
- MAPK
mitogen-activated protein kinase
- MS
mass spectrometry
- ATP
adenosine triphosphate
- ADP
adenosine diphosphate
- SAR
structure-activity relationship
- SD
standard deviation
- TR-FRET
time-resolved fluorescence resonance energy transfer
- HTS
high throughput screening
- ELISA
enzyme-linked immunosorbent assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Whitmarsh AJ. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim Biophys Acta. 2007;1773:1285–1298. doi: 10.1016/j.bbamcr.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Drew BA, Burow ME, Beckman BS. MEK5/ERK5 pathway: the first fifteen years. Biochim Biophys Acta. 2012;1825:37–48. doi: 10.1016/j.bbcan.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng J, Yu L, Zhang D, Huang Q, Spencer D, Su B. Dimerization through the catalytic domain is essential for MEKK2 activation. J Biol Chem. 2005;280:13477–13482. doi: 10.1074/jbc.M414258200. [DOI] [PubMed] [Google Scholar]
- 5.Garrington TP, Ishizuka T, Papst PJ, Chayama K, Webb S, Yujiri T, Sun W, Sather S, Russell DM, Gibson SB, Keller G, Gelfand EW, Johnson GL. MEKK2 gene disruption causes loss of cytokine production in response to IgE and c-Kit ligand stimulation of ES cell-derived mast cells. EMBO J. 2000;19:5387–5395. doi: 10.1093/emboj/19.20.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kesavan K, Lobel-Rice K, Sun W, Lapadat R, Webb S, Johnson GL, Garrington TP. MEKK2 regulates the coordinate activation of ERK5 and JNK in response to FGF-2 in fibroblasts. J Cell Physiol. 2004;199:140–148. doi: 10.1002/jcp.10457. [DOI] [PubMed] [Google Scholar]
- 7.Yan L, Carr J, Ashby PR, Murry-Tait V, Thompson C, Arthur JS. Knockout of ERK5 causes multiple defects in placental and embryonic development. BMC Dev Biol. 2003;3:11. doi: 10.1186/1471-213X-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pi X, Yan C, Berk BC. Big mitogen-activated protein kinase (BMK1)/ERK5 protects endothelial cells from apoptosis. Circ Res. 2004;94:362–369. doi: 10.1161/01.RES.0000112406.27800.6F. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Tournier C. Regulation of cellular functions by the ERK5 signalling pathway. Cell Signal. 2006;18:753–760. doi: 10.1016/j.cellsig.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Merritt AJ, Seyfried J, Guo C, Papadakis ES, Finegan KG, Kayahara M, Dixon J, Boot-Handford RP, Cartwright EJ, Mayer U, Tournier C. Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway. Mol Cell Biol. 2005;25:336–345. doi: 10.1128/MCB.25.1.336-345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo Z, Clydesdale G, Cheng J, Kim K, Gan L, McConkey DJ, Ullrich SE, Zhuang Y, Su B. Disruption of Mekk2 in mice reveals an unexpected role for MEKK2 in modulating T-cell receptor signal transduction. Mol Cell Biol. 2002;22:5761–5768. doi: 10.1128/MCB.22.16.5761-5768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Boerm M, McCarty M, Bucana C, Fidler IJ, Zhuang Y, Su B. Mekk3 is essential for early embryonic cardiovascular development. Nat Genet. 2000;24:309–313. doi: 10.1038/73550. [DOI] [PubMed] [Google Scholar]
- 13.Ameka M, Kahle MP, Perez-Neut M, Gentile S, Mirza AA, Cuevas BD. MEKK2 regulates paxillin ubiquitylation and localization in MDA-MB 231 breast cancer cells. Biochem J. 2014;464:99–108. doi: 10.1042/BJ20140420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirza AA, Kahle MP, Ameka M, Campbell EM, Cuevas BD. MEKK2 regulates focal adhesion stability and motility in invasive breast cancer cells. Biochim Biophys Acta. 2014;1843:945–954. doi: 10.1016/j.bbamcr.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cazares LH, Troyer D, Mendrinos S, Lance RA, Nyalwidhe JO, Beydoun HA, Clements MA, Drake RR, Semmes OJ. Imaging mass spectrometry of a specific fragment of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase 2 discriminates cancer from uninvolved prostate tissue. Clin Cancer Res. 2009;15:5541–5551. doi: 10.1158/1078-0432.CCR-08-2892. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Kong G, Zhang J, Wang T, Ye L, Zhang X. MicroRNA-520b inhibits growth of hepatoma cells by targeting MEKK2 and cyclin D1. PLoS One. 2012;7:e31450. doi: 10.1371/journal.pone.0031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H, Wang P, Li X, Wang Q, Deng ZB, Zhuang X, Mu J, Zhang L, Wang B, Yan J, Miller D, Zhang HG. Restoration of miR17/20a in solid tumor cells enhances the natural killer cell antitumor activity by targeting Mekk2. Cancer Immunol Res. 2014;2:789–799. doi: 10.1158/2326-6066.CIR-13-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang L, Huang M, Wang L, Fan X, Wang P, Wang D, Fu X, Wang J. Overexpression of MEKK2 is associated with colorectal carcinogenesis. Oncol Lett. 2013;6:1333–1337. doi: 10.3892/ol.2013.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cronan MR, Nakamura K, Johnson NL, Granger DA, Cuevas BD, Wang JG, Mackman N, Scott JE, Dohlman HG, Johnson GL. Defining MAP3 kinases required for MDA-MB-231 cell tumor growth and metastasis. Oncogene. 2012;31:3889–3900. doi: 10.1038/onc.2011.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoang VT, Yan TJ, Cavanaugh JE, Flaherty PT, Beckman BS, Burow ME. Oncogenic signaling of MEK5-ERK5. Cancer Lett. 2017;392:51–59. doi: 10.1016/j.canlet.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad S, Hughes MA, Johnson GL, Scott JE. Development and validation of a high-throughput intrinsic ATPase activity assay for the discovery of MEKK2 inhibitors. J Biomol Screen. 2013;18:388–399. doi: 10.1177/1087057112466430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad S, Johnson GL, Scott JE. Identification of ponatinib and other known kinase inhibitors with potent MEKK2 inhibitory activity. Biochem Biophys Res Commun. 2015;463:888–893. doi: 10.1016/j.bbrc.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitagawa D, Yokota K, Gouda M, Narumi Y, Ohmoto H, Nishiwaki E, Akita K, Kirii Y. Activity-based kinase profiling of approved tyrosine kinase inhibitors. Genes Cells. 2013;18:110–122. doi: 10.1111/gtc.12022. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad S, Scott JE. Estradiol enhances cell-associated paraoxonase 1 (PON1) activity in vitro without altering PON1 expression. Biochem Biophys Res Commun. 2010;397:441–446. doi: 10.1016/j.bbrc.2010.05.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 26.Goring S, Taymans JM, Baekelandt V, Schmidt B. Indolinone based LRRK2 kinase inhibitors with a key hydrogen bond. Bioorg Med Chem Lett. 2014;24:4630–4637. doi: 10.1016/j.bmcl.2014.08.049. [DOI] [PubMed] [Google Scholar]
- 27.Widmann C, Sather S, Oyer R, Johnson GL, Dreskin SC. In vitro activity of MEKK2 and MEKK3 in detergents is a function of a valine to serine difference in the catalytic domain. Biochim Biophys Acta. 2001;1547:167–173. doi: 10.1016/s0167-4838(01)00183-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.