Abstract
Maintaining normoglycaemia not only reduces the risk of diabetic microvascular complications but also corrects the metabolic abnormalities that contribute to the development and progression of hyperglycaemia (i.e. insulin resistance and beta-cell dysfunction). Progressive beta-cell failure, in addition to the multiple side effects associated with many current antihyperglycaemic agents (e.g., hypoglycaemia and weight gain) presents major obstacle to the achievement of the recommended goal of glycaemic control in patients with diabetes mellitus (DM). Thus, novel effective therapies are needed for optimal glucose control in subjects with DM. Recently, specific inhibitors of renal sodium glucose cotransporter 2 (SGLT2) have been developed to produce glucosuria and lower the plasma glucose concentration. Because of their unique mechanism of action (which is independent of the secretion and action of insulin), these agents are effective in lowering the plasma glucose concentration in all stages of DM and can be combined with all other antidiabetic agents. In this review, we summarize the available data concerning the mechanism of action, efficacy and safety of this novel class of antidiabetic agent.
Keywords: Type-2 diabetes mellitus, Kidney, Sodium–glucose co-transport, SGLT2 inhibition
Introduction
Hyperglycaemia is the principal risk factor for the microvascular complications (i.e., retinopathy, nephropathy and neuropathy) of diabetes mellitus (DM). Results from the United Kingdom Prospective Diabetes Study (UKPDS) and the Diabetes Control and Complications Trial (DCCT) have demonstrated that every 1% decrease in haemoglobin A1c (HbA1c) is associated with a ≈35% reduction in the risk of microvascular complications [1, 2]. Hyperglycaemia also plays an important part in the pathogenesis of insulin resistance and beta-cell failure (i.e., glucotoxicity [3, 4]). Studies in experimental animals and in humans have demonstrated that hyperglycaemia worsens insulin resistance and markedly impairs beta-cell function [3, 4], thereby further exacerbating hyperglycaemia and producing a vicious cycle that perpetuates and aggravates hyperglycaemia. Thus, appropriate glycaemic control in DM subjects would be anticipated not only to reduce the risk of microvascular complications, but also to ameliorate the metabolic abnormalities that contribute to the development and progression of hyperglycaemia. Despite the irrefutable evidence for the importance of maintaining optimal glycaemic control (HbA1c <6.5–7%) ≈50% of people with DM in the USA/worldwide fail to achieve this target of glycaemic control and manifest HbA1c >7% [5, 6].
Progressive beta-cell failure, weight gain and hypoglycaemia represent major obstacles to the achievement of tight glycaemic control, HbA1c ≤6.5–7%, in patients with DM [3]. Therefore, development of novel therapies that effectively lower the plasma glucose concentration and produce durable glycaemic control, while avoiding hypoglycaemia and weight gain, is needed for the optimal management of DM. Recently, specific inhibitors of renal sodium–glucose cotransporter 2 (SGLT2) have been developed that produce glucosuria and lower the plasma glucose concentration [7]. In this review, we summarize the available data concerning the mechanism of action, efficacy and safety of this novel therapeutic approach against DM.
Renal handling of glucose
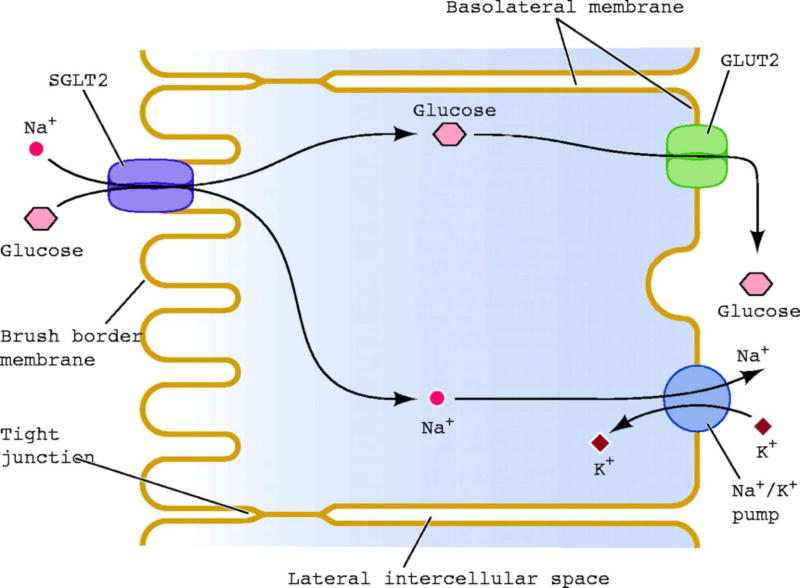
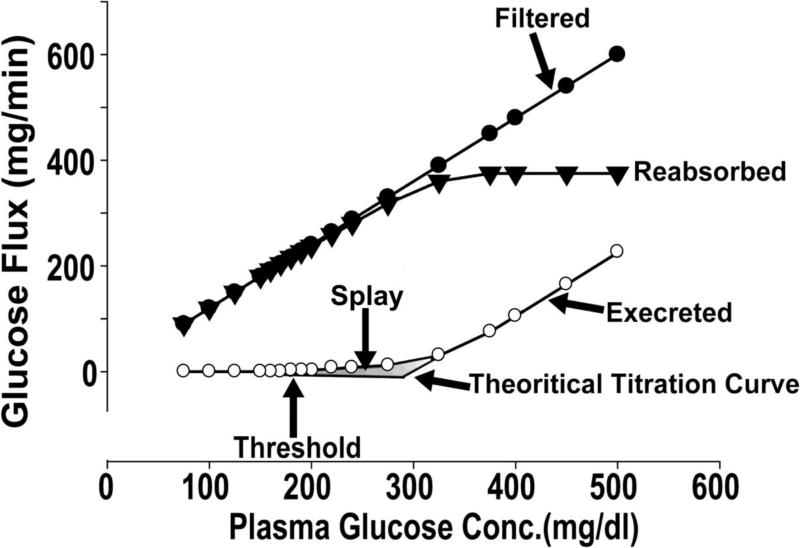
The kidney filters ≈180 L of plasma each day. In a normal healthy individual with plasma glucose concentration ≈90–100 mg/dL, this filtrate contains ≈162–180 g of glucose. In healthy individuals, virtually all of this glucose is reabsorbed in the proximal tubule and glucose is not excreted in the urine. The maximum capacity of glucose transport (Tm) of the proximal tubule, on average, is ≈375 mg/min. Renal glucose reabsorption takes place in the proximal tubule primarily in the S1 and S3 segments, and is mediated by sodium-glucose cotransporters (SGLTs), which couple glucose reabsorption to sodium reabsorption. The sodium electrochemical gradient generated by active transport of sodium provides the energy required for glucose transport (Fig. 1). To date, seven SGLTs have been identified [8]. However, only two transporters are responsible for renal glucose reabsorption. SGLT2 is expressed exclusively in the kidney and has a low affinity and high capacity for glucose transport. It is located primarily in the S1 segment of the proximal tubule and absorbs ≈80–90% of filtered glucose. SGLT1 is expressed in the kidney and gut. It transports glucose and galactose, and is responsible for most of the uptake of glucose and galactose in the gut. SGLT1 is located in the S3 segment of the proximal tubule in the kidney, and is responsible for the reabsorption of the remaining 10–20% of filtered glucose. In normal individuals, the filtered glucose load is less than the Tm (375 mg/min). Thus, all of the filtered glucose is reabsorbed and returned to the circulation. However, if the filtered glucose load is >375 mg/min (as often occurs in individuals with poorly controlled DM) the Tm is exceeded, and all of the glucose in excess of the Tm is excreted in the urine. The plasma glucose concentration at which the filtered glucose load reaches the Tm (375 mg/min) is called the ‘threshold’, above which the rate of glucose excretion increases linearly and parallels the filtered load. However, the reabsorption and excretion curves display a non-linear transition as the Tm for glucose is approached. This ‘rounding’ of the curves is termed ‘splay’ (Fig. 2) and has been explained by heterogeneity in the Tm of individual nephrons and/or glomerulotubular imbalance [8]. In healthy individuals, the plasma glucose threshold above which glucosuria begins is ≈180 mg/dL.
Fig. 1.
Sodium–glucose reuptake in renal proximal tubule epithelial cells.
Fig. 2.
Kinetics of the renal handling of glucose.
Subjects with normal glucose tolerance have a Tm for glucose that is well above the filtered glucose load. This has major survival benefits because it allows the kidneys to conserve this critical energy source for the brain, which (with the exception of prolonged fasting) utilizes only glucose to generate energy for neuronal function. Studies in experimental animals have demonstrated that the increase in plasma glucose concentration is associated with up-regulation of SGLT2 expression in the kidney [9]. Consistent with this observation, patients with DM have increased renal Tm for glucose [10]. It has been postulated that, because hyperglycaemia results in a glucose filtration load in excess of the Tm, the increase in Tm during hyperglycaemia represents an adaptive mechanism to prevent glucosuria and preserve energy during conditions when food was sparse. Today, when food is abundant yet DM has reached epidemic proportions, this adaptive mechanism has become maladaptive. In patients with DM, it would be desirable for the kidney to excrete the excess filtered glucose load and restore normoglycaemia. In contrast, the increased Tm for glucose ‘minimizes’ glucosuria and exacerbates hyperglycaemia. Thus, the increase in renal Tm in DM patients contributes to the retention of hyperglycaemia, and the kidney can be viewed to contribute to the pathogenesis of hyperglycaemia in DM.
Increased glucose uptake in the proximal tubule in DM patients is expected to be accompanied by increased sodium reabsorption. One can speculate that the increased sodium reabsorption can lead to expansion in extracellular volume and an increase in blood pressure. Although this hypothesis has never been tested in humans, a recent micropuncture study in experimental animals demonstrated that acute inhibition of SGLT2 causes a two- to threefold increase in sodium excretion in single nephrons. However, the increased sodium excretion waned after 2 weeks, suggesting an important role of tubuloglomerular feedback in the long-term balance of fluids and salt [11].
Based upon these pathophysiologic considerations, it follows that inhibition of renal glucose reabsorption is a rational and novel approach for the treatment of DM. This approach will not only decrease plasma glucose concentration, but can be expected to have additional metabolic benefits such as lowering blood pressure and promoting weight loss. The specificity of drugs that inhibit SGLT2 over SGLT1 (which is present in the gut and kidney) avoids impaired intestinal glucose absorption and diarrhoea.
Familial renal glucosuria is a rare genetic disease in which subjects carry a mutation in the gene that encodes the SGLT2 transporter and results in massive glucosuria of 60–120 g/day [8]. However, subjects with familial renal glucosuria are asymptomatic, with normal kidney function. Persistent glucosuria potentially could cause an increase in urinary-tract infections and impair the kidney’s ability to concentrate urine. However, the fact that subjects with familial glucosuria are asymptomatic suggests that if these side effects occur in DM patients, they are likely to be mild.
Pharmacological inhibition of renal glucose uptake
Phlorizin, a natural compound isolated from the bark of apple trees, was the first SGLT inhibitor to be identified [12, 13]. It comprises two main moieties: a glucose ring connected via an oxygen atom (O-glucoside) to two phenol rings. Phlorizin competitively inhibits SGLT1 and SGLT2 in the proximal tubule, with a tenfold higher affinity for SGLT2 versus SGLT1 transporter. In healthy individuals, intravenous administration of phlorizin produces glucosuria that resembles familial renal glucosuria [13] whereas, in DM individuals, it produces massive glucosuria and normalizes the plasma glucose concentration. Despite the efficacy of phlorizin in inhibiting SGLT2 activity and normalizing the plasma glucose concentration in DM animals, low bioavailability (≈15%) after oral administration and inhibition of SGLT1 in the gastrointestinal tract negate its usefulness in humans with DM [14].
Based upon the structure of phlorizin, several other compounds with greater bioavailability after oral administration and higher selectivity for SGLT2 compared with SGLT1 have been developed (Table 1) and are in varying stages of development for clinical use. A second family of non-glucoside SGLT2 inhibitors with even greater selectivity for SGLT2 has been identified [15], but none of its members have reached clinical development.
Table 1.
SGLT2 inhibitors under development
Inhibition of renal glucose transport corrects hyperglycaemia: proof of concept
Studies undertaken with phlorizin in DM rats have provided proof of concept for the efficacy of sodium glucose cotransport inhibition in lowering the plasma glucose concentration. Studies in experimental animals have demonstrated that chronic phlorizin administration induces glucosuria and normalizes the fasting and fed plasma glucose levels. Phlorizin also completely reversed insulin resistance and corrected defects in first- and second-phase insulin secretion in DM rats [16–20]. The results of these studies demonstrate that inducing glucosuria by inhibiting renal SGLT2 is a promising strategy to restore normoglycaemia in DM individuals.
Specific SGLT2 inhibitors in DM subjects
O-glucosides, the first specific SGLT2 inhibitors
The early SGLT2 inhibitors with high specificity for SGLT2 over SGLT1 were derivatives of phlorizin. T-1095 was the first agent to be developed [21], and was followed by sergliflozin and remogliflozin [22]. Although these compounds produced a dose-dependent glucosuria with a significant reduction in plasma glucose concentration in animal models and DM patients, their bioavailability was not satisfactory and, therefore, they were not developed further [23–26].
C-glucoside inhibitors
Substitution of the O-link between the glucose and phenol moieties with a C-link provides greater resistance to beta-glucosidase and hence greater bioavailability. This has led to development of a second-generation of longer-acting specific SGLT2 inhibitors that are appropriate for single-daily dosing. Many members of this group are in varying stages of clinical development.
Dapagliflozin
Phase III trials have been completed, reviewed and approved by the US Food and Drug Administration (FDA), and by the European Medicines Agency (EMA) for the treatment of DM subjects in Europe [27]. The half-life of dapagliflozin in humans is ≈17–18 hours, making it suitable for once-daily administration [28, 29]. Dapagliflozin has high bioavailability and is absorbed rapidly after oral administration, achieving maximal plasma concentrations within 1–2 hours.
In initial dose-ranging studies (14-day duration) in DM patients, dapagliflozin (5, 25 and 100 mg/day) produced dose-dependent glucosuria (37, 62 and 80 g/24 h, respectively) and significantly decreased the fasting plasma glucose concentration (by 19, 29, and 39 mg/dL, respectively) and the incremental area under the plasma glucose concentration curve during an oral glucose tolerance test [28]. It does not inhibit or induce P450 enzymes. Dapagliflozin is highly protein bound (97–98%) and renal excretion is low (2–4%). An inert glucuronide conjugate (M15) of dapagliflozin is the major metabolite of the drug in vivo.
In more prolonged treatment (12 weeks) in DM patients (n = 389), dapagliflozin reduced the HbA1c by ≈0.7% (from a baseline HbA1c of 7.8–8.0%) without apparent dose-dependency [30]. The reduction in HbA1c was similar in magnitude to that observed with metformin, and the reductions in fasting and post-prandial plasma glucose concentrations accounted approximately equally for the decline in HbA1c [30]. Dapagliflozin also caused weight loss of 2.2–3.1 kg and produced a modest reduction in systolic and diastolic blood pressure. The amount of glucosuria observed with dapagliflozin (50–60 g/day) is equivalent to a daily caloric loss of ≈200–240 cal/day that, over 12 weeks, could explain a weight loss of 2–3 kg.
All Phase III trials with dapagliflozin have been completed and involved 6798 DM patients randomized to dapagliflozin and placebo in a 2:1 ratio [27]. Treatment with dapagliflozin (5 and 10 mg/day) consistently caused a significant decrease in HbA1c (>0.5%) compared with placebo independent of background therapy. A comparable decrease in HbA1c was observed when dapagliflozin was given to drug-naïve DM patients or when added to metformin, sulfonylurea, thiazolidinedione or insulin. A decrease in fasting and post-prandial plasma glucose concentrations contributed equally to the decrease in HbA1c. The decrease in fasting and post-prandial plasma glucose concentrations with 10 mg/day dapagliflozin was ≈25 mg/dL and ≈55 mg/dL, respectively. The decrease in HbA1c caused by dapagliflozin was independent of sex, ethnicity, race, the body mass index or duration of DM. As expected, dapagliflozin produced a greater reduction in HbA1c in patients with higher baseline HbA1c. In a subgroup (n = 78) of patients with baseline HbA1c of 10.1–12.0%, dapagliflozin (5 and 10 mg/day) reduced the HbA1c by 2.88% and 2.66%, respectively, over 24 weeks [31].
The mechanism of action of dapagliflozin is independent of the secretion and action of insulin, so the efficacy of dapagliflozin is independent of beta-cell function or DM duration. Thus, dapagliflozin is also effective in reducing the HbA1c in patients undergoing insulin therapy. Wilding et al. [32], randomized 71 insulin-treated (≥50 units/day) DM patients who were also receiving an insulin sensitizer (metformin and/or thiazolidinedione) to add-on therapy with dapagliflozin (5 and 10 mg/day) or placebo. The insulin dose was reduced by 50% at the start of therapy, whereas the dose of the insulin sensitizer was unchanged. After 12 weeks, the placebo-subtracted declines in HbA1c were 0.70% and 0.78%, respectively (P < 0.01 vs. placebo) despite the 50% reduction in insulin dose. The placebo-subtracted reductions in body weight were 2.6 kg and 2.4 kg, respectively (P < 0.01 vs. placebo), reflecting the loss of glucose in urine and reduction in insulin dose. In a larger study (n = 800), addition of dapagliflozin (2.5, 5 and 10 mg/day) to insulin-treated DM individuals receiving ≈70–80 units/day for a mean of ≈6 years caused a dose-dependent decrease in HbA1c (−0.40, −0.49 and −0.57%, respectively) compared with placebo over 24 weeks of treatment, and the decrease in HbA1c was maintained at 48 weeks [33]. Moreover, dapagliflozin reduced the HbA1c independent of DM duration. Thus, in a 12-week study, 151 patients with new-onset diabetes (<1 year) and 58 patients with long-standing (11 years) DM were assigned randomly to 10 or 20 mg/day of dapagliflozin [34]. Although patients with long-standing DM had poor glycaemic control (HbA1c = 8.4%) despite a large dose of insulin (>50 units/day) plus metformin and a thiazolidinedione, dapagliflozin was effective in decreasing HbA1c such that the decrease in HbA1c was comparable in both groups.
In a head-to-head comparison of dapagliflozin with sulfonylurea as add-on therapy in poorly controlled DM patients on metformin therapy [35], both groups exhibited the same decline in mean HbA1c (−0.52%) over 52 weeks. Two studies with longer duration of therapy with dapagliflozin (2 years) have been reported recently [36, 37]. In one study, dapagliflozin was added to metformin, and the decrease from baseline in HbA1c was −0.54% at 1 year and −0.80% at 2 years. Similarly, in a head-to-head study of dapagliflozin versus glipizide, an additional −0.34% decrease in HbA1c was observed in the second year in subjects treated with dapagliflozin compared with a −0.12% decrease in subjects treated with glipizide [37], suggesting a more durable decrease in HbA1c with dapagliflozin compared with glipizide. Only ≈70% and ≈90% of subjects in these two studies [36, 37], respectively, entered the second-year extension study. Thus, more data are required before making definitive conclusions about the durability of SGLT2 inhibitors because it is difficult to determine whether the further decrease in HbA1c during the second year was due to the action of dapagliflozin or due to selection bias in subjects entering the second-year extension. Nevertheless, the results of both studies demonstrate that the glucose-lowering effect of dapagliflozin does not wane with time for ≤2 years.
Canagliflozin
Canagliflozin has recently been approved for by the FDA and EMA for the treatment of DM patients in combination with oral antidiabetic therapy (Table 2) as well as with insulin. Canagliflozin produced dose-dependent glucosuria in DM individuals with a maximal effect at 400 mg/day. The glucosuria was accompanied by a dose-dependent decrease in the incremental area under the plasma glucose and insulin concentration curves during a mixed meal [38]. Canagliflozin was effective in lowering the plasma glucose concentration in drug-naïve DM patients and as add-on therapy in patients treated with metformin, insulin and a combination of metformin plus sulfonylurea (Table 2). In a 12-week, placebo-controlled trial in drug-naïve DM patients, canagliflozin produced a dose-dependent decrease in HbA1c with a maximal placebo-subtracted decrease in HbA1c of 1.03% with a daily dose of 300 mg [39]. In a double-blind, placebo-controlled, dose-ranging study in metformin-treated DM patients (n = 451), canagliflozin at 50, 100, 200, and 300 mg/day reduced HbA1c by 0.7–0.9% from baseline and by 0.5–0.7% versus placebo over 12 weeks, and caused a weight loss of 1.3–2.3 kg [40]. Addition of canagliflozin (100 and 300 mg/day) to insulin-treated DM patients (n = 29) caused placebo-subtracted decreases in HbA1c after 28 days of treatment of −0.54% and −0.73%, respectively [41]. The 300-mg/day dose appeared to be slightly more effective than the 100-mg/day dose. In a 16-day trial, canagliflozin was shown to improve beta-cell function in DM patients using a model-based method to calculate insulin secretion [42].
Table 2.
Placebo-subtracted decrease in HbA1c (%) with different SGLT2 inhibitors in DM subjects receiving various types of background therapy
| Background therapy |
Ipragliflozin | Canagliflozin | Dapagliflozin | Empagliflozin |
|---|---|---|---|---|
| Drug-naïve | −1.23 | −1.03 | −0.66 | −0.69 |
| Metformin | −1.29 | −0.93 | −0.54 | −0.71 |
| Sulphonylurea | −1.14 | −1.03 | −0.59 | −0.60 |
| Pioglitazone | −0.88 | NT | −0.55 | −0.61 |
| Insulin | NT | −0.73 | −0.55 | −0.62 |
NT, not yet tested/reported.
Empagliflozin
Similar to other SGLT2 inhibitors, empagliflozin produced dose-dependent glucosuria in normal and DM subjects [43, 44], and the 24-hour urinary glucose excretion with 100 mg/day was 74 g [45]. In a placebo-controlled, 12-week study in 495 metformin-treated DM subjects with poor glycaemic control, empagliflozin caused a dose-dependent decrease in the fasting plasma glucose (FPG) concentration and HbA1c with a placebo-subtracted decrease in FPG and HbA1c of 27 mg/dL and 0.7%, respectively, with a dose of 25 mg/day [46]. Empagliflozin also was effective in lowering HbA1c as add-on to metformin plus sulfonylurea and metformin plus pioglitazone. In one 24-week study (46A), 10 mg (n=225) and 25 mg (n=216) empagliflozin or placebo (n=225) were added to DM patients treated with metformin plus sulfonylurea. The placebo-subtracted decrease in HbA1c was −0.65 and −0.60%, respectively. In a 12-week study (46B), 10 mg (n=165) and 25 mg (n=168) empagliflozin was added to DM patients treated with pioglitazone with or without metformin. The placebo-subtracted HbA1c after 12 weeks was –0.48 and 0.61%, respectively.
Ipragliflozin
In a 12-week, double-blind study, 361 Japanese DM patients treated with ipragliflozin at doses ranging from 12.5 to 100 mg/day experienced a 0.9% reduction in HbA1c at the two highest doses (50 and 100 mg/day) [47]. Body weight also was dose-dependently reduced by ≤2 kg with the 100-mg/day dose. In a 16-week study, ipragliflozin monotherapy (50 mg/day) caused a 1.2% decrease from baseline (8.3%) in HbA1c in 62 DM patients [48]. A 100-mg ipragliflozin dose significantly decreased the HbA1c as add-on therapy in DM patients treated with metformin, sulfonylurea and pioglitazone (Table 2).
LX4211
In a Phase IIA study, LX4211 (which inhibits SGLT2 and to a lesser extent than SGLT1) at doses of 150 and 300 mg/day reduced the HbA1c by 1.2%. However, baseline HbA1c (8.2–8.5%) was higher than in most other studies, and placebo decreased HbA1c by 0.5% [49].
TS-071
In a 12-week, Phase II, placebo-controlled study, TS-071 caused a dose-dependent decrease in HbA1c in 236 Japanese DM patients. The placebo-corrected decrease in HbA1c was 0.82% at a 5 mg/day dose [50].
PF04971729
In a 12-week, double-blind, placebo-controlled Phase II study in 328 patients with DM, PF04971729 caused a dose-dependent decrease in HbA1c. The placebo-subtracted decrease in HbA1c with the highest dose (25 mg/day) was 0.72%, comparable with the placebo-subtracted decrease in HbA1c caused by sitagliptin (0.76%). The decrease in HbA1c produced by PF04971729 was accompanied with a ≈30 mg/dL decrease in the FPG concentration compared with a 17-mg/dL decrease in the sitagliptin-treated group [51].
SGLT2 inhibitors and renal function
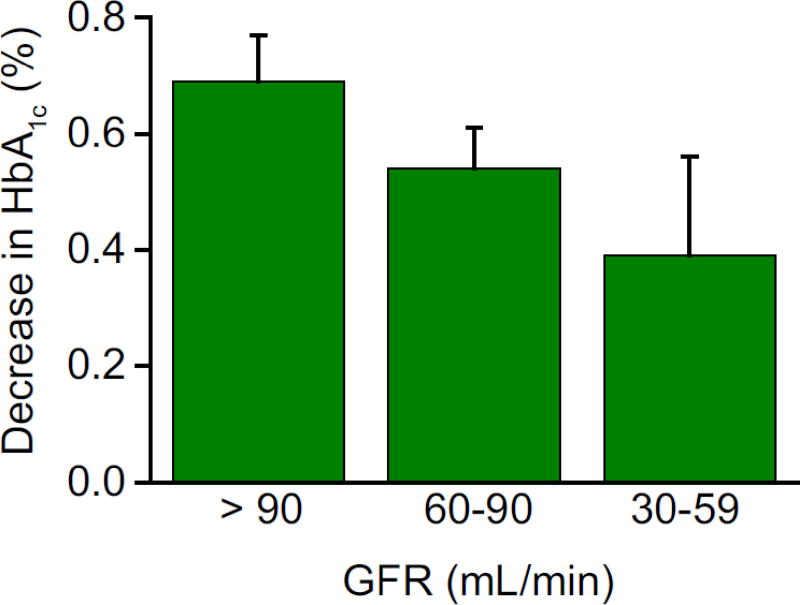
Studies with dapagliflozin have demonstrated that treatment with SGLT2 inhibitors has no significant effect on renal function. Moreover, because most of the drug clearance is via the liver, no dose adjustment is necessary in patients with renal impairment. Because of their mechanism of action, the efficacy of SGLT2 inhibitors to reduce plasma glucose concentrations is highly dependent upon renal function. As the glomerular filtration rate (GFR) decreases, there is a decrease in glucose filtration load and a progressive decrease in the glucose-lowering ability of the drug. In subjects with a mild decrease in renal function (GFR = 60–90 mL/min), the glucosuria produced by dapagliflozin [27] is decreased by 40% and the reduction in HbA1c was decreased by ≈22% (Fig. 3). Interestingly, among subjects with similarly impaired renal function, ipragliflozin was reported to produce comparable glucosuria in subjects with GFR >90 mL/min [52]; however, the decrease in FPG concentration was decreased by one-half (12.9 mg/dL compared with 24.5 mg/dL, respectively). In subjects with moderately impaired renal function (GFR = 30–59 mL/min), the glucosuria produced by ipragliflozin and dapagliflozin was reduced markedly (≈80%) and the decrease in FPG and HbA1c was clinically insignificant (4 mg/dL and −0.11%, respectively). In subjects with an estimated glomerular filtration rate (eGFR) of 30–50 mL/min, canagliflozin (100 and 300 mg/day) produced a significant decrease in HbA1c compared with placebo [53]
Fig. 3.
Impact of reduced renal function on the glucose-lowering efficiency of dapagliflozin. Adapted from Bristol Myers-Squibb NDA [27].
Mechanism of glucosuria
Glucosuria can be promoted by lowering the Tm or increasing the glucose splay. A study in rodents with sergliflozin demonstrated that SGLT2 inhibition reduces the Tm markedly without a significant change in the glucose splay [22]. Dapagliflozin produced a marked decrease in the Tm and splay in DM and non-DM individuals. However, the decrease in Tm caused by dapagliflozin was not sufficient to explain the glucosuria produced by dapagliflozin at the FPG concentration. This could be explained by decreasing the threshold of plasma glucose concentration during treatment with dapagliflozin from ≈180 mg/dL to ≈40 mg/dL.
SGLT2 is responsible for the reabsorption of ≈80% of the glucose filtration load, but the increase in urinary glucose excretion (60–80 g/day) observed with maximal doses of SGLT2 inhibitors currently in clinical trials represents inhibition of <50% of the filtered glucose load. Under physiologic conditions, SGLT1 is located in the distal part of the proximal tubule, so only small amount of glucose reaches that part of the proximal tubule is reabsorbed by SGLT1. Thus, SGLT1 operates at submaximal transport capacity. Under conditions of complete inhibition of SGLT2, all of the filtered glucose reaches the distal part of the proximal nephron and forces SGLT1 to reabsorb glucose at full capacity and, therefore, only the fraction of filtered glucose that escapes SGLT1 is excreted in urine [54].
Non-glycaemic benefits of SGLT2 inhibitors
In addition to the beneficial effects related to improved glycaemic control, SGLT2 inhibitors have several non-glycaemic effects that make them desirable agents in monotherapy and combination treatment.
Weight loss: Weight gain is a major problem with currently available antidiabetic medications, including sulfonylureas, thiazolidinediones, and insulin. A urinary loss of 60–80 g/day of glucose equates to 240–320 cal/day or 2–3 lb per month, if this caloric deficit is not offset by an increase in caloric intake, it will produce negative energy balance and promotes weight loss. Consistent with this hypothesis, 2–3 kg weight loss has been observed in DM subjects treated with SGLT2 inhibitors in all clinical studies.
Effect on blood pressure: A consistent finding in all studies with SGLT2 inhibitors has been a reduction in systolic/diastolic blood pressure of 4–5/2–3 mmHg [30]. Although this has been attributed to the mild fluid/sodium deficit that occurs during the first several days of SGLT2 inhibition [28, 30], an alternative explanation is local inhibition of the renin–angiotensin system secondary to enhanced sodium delivery to the juxtaglomerular apparatus [55, 56]. In one study, the decrease in blood pressure produced by PF04791729 treatment was comparable with that observed with thiazide diuretics. Reabsorption of uric acid and sodium in the proximal tubule are coupled, so it is not surprising that a decrease in the serum concentration of uric acid has been observed in DM patients treated with dapagliflozin [56].
SGLT2 inhibitors and diabetic nephropathy: Hyperglycaemia is the principal risk factor for microvascular complications (retinopathy, nephropathy and neuropathy), and improved glycaemic control – no matter how achieved – would be expected to reduce the risk of microvascular complications in subjects with DM [1, 2]. Because of the important role of enhanced sodium–glucose reabsorption in the proximal tubule in the development of diabetic nephropathy [55, 56], SGLT2 inhibitors might be expected to have an additional beneficial renoprotective action above and beyond their glucose-lowering effect [55, 56]. The increased filtered glucose load in DM subjects results in increased glucose and sodium reabsorption by SGLT2 in the proximal tubule [57, 58] and decreased sodium delivery to the juxtaglomerular apparatus. This activates the renin–angiotensin system, resulting in elevated intraglomerular pressure and increased GFR [59]. By inhibiting sodium transport in the proximal tubule and increasing sodium delivery to the juxtaglomerular apparatus, secretion of renin and angiotensin are inhibited, leading to a reduction in the glomerular pressure and hyperfiltration. Consistent with this hypothesis, hyperfiltration and increased kidney size can be reversed by 6 weeks of intensive insulin therapy that normalizes the plasma glucose concentration [60]. With regard to this, chronic administration of T-1095 was found to decrease HbA1c in DM mice and stopped progression of diabetic nephropathy, with prevention of proteinuria and expansion of glomerular mesangial area [61].
Safety
The pharmacological properties of SGLT2 inhibitors suggest that they should have a good safety profile. Because of their high selectivity for SGLT2 over SGLT1, inhibition of transport of glucose or galactose in the intestinal mucosa is not anticipated and, therefore, unlike phlorizin, gastrointestinal side effects are not anticipated. Furthermore, because subjects with homozygous mutations in the SGLT2 gene are asymptomatic and have normal kidney function despite considerable glucosuria (>50–100 g/24 h), pharmacological inhibition of SGLT2 would not be expected to affect kidney function or cause volume depletion. Indeed, none of those adverse events have been observed with SGLT2 inhibitors in clinical trials. SGLT2 inhibition caused only a modest increase in urine volume with no increase in salt wasting. With dapagliflozin, 12–24 weeks of treatment caused 200–400 mL/day increases in urine volume during the first 2–3 days after initiation of therapy without excessive urine losses of sodium, potassium or other electrolytes [30]. Consistent with mild volume contraction, a small rise in haematocrit (1–2 vol %) and plasma urea nitrogen-to-creatinine ratio have been observed. Plasma electrolyte concentrations did not change after dapagliflozin treatment [28, 30]. No increase in the incidence of hypoglycaemia was observed with SGLT2 inhibitors. This can be explained by the lack of effect of SGLT2 inhibitors on glucose counter-regulatory mechanisms. Although SGLT2 inhibitors promote glucosuria, which could result in an increased incidence of urinary tract infections (UTIs), patients with uncontrolled blood sugar already have significant glucosuria. Moreover, DM individuals may have increased susceptibility to infections compared with non-DM individuals (62). It remains to be determined if additional glucosuria promotes bacterial growth. In one study in which mid-stream urine was collected, SGLT2 treatment did not increase the prevalence of urinary bacteriuria [63]. However, in DM subjects receiving dapagliflozin at doses of 5 mg/day (n = 1145) and 10 mg/day (n = 1193), a ≈50% increase in the incidence of UTIs has been observed [27]. The incidence of vulvovaginitis and balanitis are also increased by approximately two-fold with therapy using SGLT2 inhibitors [27, 30–32]. As stated above, SGLT2 inhibitors do not affect renal function as manifested by a change in serum creatinine or development of albuminuria or tubular proteinuria in subjects with DM with normal GFR [27].
In Phase III trials, canagliflozin produced a significant increase in the plasma concentration of high-density lipoprotein (HDL)-cholesterol and a significant decrease in plasma triglyceride (TG) concentration in drug-naive subjects and in DM patients whose disease was poorly controlled with metformin [64]. Furthermore, canagliflozin caused a ≈5% increase in plasma levels of low-density lipoprotein (LDL)-cholesterol, and this increase in plasma LDL-C was independent of background therapy with statins. Although the increase in plasma HDL-C and decrease in TG concentration could be explained by the weight loss caused by the drug, the cause for the increase in LDL-C is not known. Also, the impact of this change in lipid profile on cardiovascular events is not known. The incidence of total cardiovascular events in canagliflozin-treated subjects in Phase III trials was similar to that in subjects receiving other antidiabetic agents. However, there was a sign of increased incidence of cerebrovascular accident (CVA) in canagliflozin-treated subjects shortly after starting therapy. However, the increased incidence of CVA events was due primarily to a decreased number of events in placebo-treated subjects rather than due to an increased number of events in canaglifloazin-treated subjects. Nonetheless, this sign warrants further investigation, and the ongoing CANVAS study with 4330 subjects randomized to receive 100, 300 mg canagliflozin or placebo could provide definitive information about the cardiovascular safety/benefit of canagliflozin (NCT-01032629) [65]. Similarly, a 4-year duration study on the cardiovascular safety of empagliflozin (NCT-01131676) and dapagliflozin (NCT 01730534) are also ongoing [66].
In Phase III studies of dapagliflozin, an increased incidence of bladder cancer and breast cancer was observed. However, the total number of cases was small (10 for each type of cancer), and this finding was surprising because neither breast tissue nor bladder tissue express SGLT2. In addition, rigorous 2-year carcinogenic studies in animals failed to demonstrate preneoplastic or neoplastic activity. Breast cancer and especially bladder cancer can take many years to develop so, whereas exposure to dapagliflozin was short (generally <1 year), the significance of the increased incidence of these two tumours remains to be determined. Moreover, there could have been detection bias for bladder cancer due to the frequent testing for UTIs, which could have led to the discovery of microscopic haematuria.
Place of SGLT2 inhibitors in the management of DM
All major organizations (American Diabetes Association/European Association for the Study of Diabetes, American Association of Clinical Endocrinologists) recommend metformin as first-line therapy in individuals with new-onset DM. However, metformin does not affect beta-cell function, so metformin-treated individuals experience a progressive increase in HbA1c. SGLT2 inhibitors can provide a good therapeutic option in metformin-failing DM individuals. An increase in the plasma concentration of glucagon-like peptide-1 has been observed in some clinical studies after initiation of therapy with SGLT2 inhibitors. This observation makes the combination of SGLT2 inhibitors with dipeptidyl peptidase-IV (DPP-IV) inhibitors an attractive therapeutic option in metformin-failing DM individuals. In newly diagnosed DM individuals with very high HbA1c (e.g., >9.0%), metformin alone will not lower HbA1c below the treatment goal (<7.0%). Thus, SGLT2 in combination with metformin is attractive therapy. In one study, initiating therapy with metformin plus dapagliflozin in subjects with new-onset DM produced an additive decrease in HbA1c versus either therapy alone, and more subjects (≈60%) with the combination therapy achieved the target glycaemic therapy (HbA1c <7.0%) than with either therapy alone. About 10–20% of subjects with new-onset DM cannot tolerate metformin because of gastrointestinal side effects or due to mild renal failure. Thus, SGLT2 inhibitors, as monotherapy or in combination with DPP-IV inhibitors, can provide effective initial therapy in metformin-intolerant individuals. Lastly, because SGLT2 inhibitors are effective in lowering HbA1c at all stages of DM, they can be added for subjects who fail multiple oral therapy, or in insulin-treated individuals who do not reach target glycaemic control.
Summary and conclusion
Current data in experimental animals and humans indicate that inhibition of renal glucose reabsorption with SGLT2 inhibitors is an effective novel strategy to reduce the plasma glucose concentration in DM subjects. SGLT2 inhibitors have demonstrated good efficacy, with a reduction in HbA1c of 0.7–0.8% from a starting HbA1c of ≈8.0%, and have a good safety profile. In addition, SGLT2 inhibitors produce additional non-glycaemic benefits: modest weight loss and a decrease in blood pressure. Because SGLT2 inhibitors have a mechanism of action that is independent of the secretion or action of insulin, the efficacy of this class of drug is not anticipated to decline with progressive beta-cell failure or severe insulin resistance. Thereby, this class of drug can be administered in combination with all other antidiabetic agents with anticipated additive efficacy on glycaemic control at all stages of DM. Although initial studies have demonstrated a durable decrease in HbA1c over 52–102 weeks compared with placebo and DPP-IV inhibitors, longer follow-up is warranted for conclusive decisions upon the durability of HbA1c reduction by this class of drug.
Because of their mechanism of action, the efficacy of SGLT2 inhibitors declines with decreased renal function such that in subjects with GFR <45–60 mL/min, the decrease in HbA1c produced by SGLT2 inhibitors is not clinically meaningful. SGLT2 inhibitors ae also are effective as monotherapy in newly diagnosed DM patients. To the extent that glucotoxicity contributes to the demise in beta-cell function in subjects with impaired glucose tolerance and impaired fasting glucose, these drugs may also prove useful in the treatment of ‘prediabetes.’ Currently available data indicate that SGLT2 inhibitors have a good safety profile.
Acknowledgments
Ralph Defronzo (5R01DK240923) and Muhammad Abdul-Ghani (R01 DK097554-01) receive NIH grant support.
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors received no direct compensation related to development of the manuscript. Proofreading and manuscript formatting assistance was provided by Geraldine Thompson of Envision Scientific Solutions, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
RAD has grant support from Takeda, Amylin, and BMS.
Dr. DeFronzo reports receiving grants from Amylin, Takeda, and Bristol Myers Squibb and serves on the advisory board for Amylin, Takeda, BMS, Novo Nordisk, Janssen, and Boehringer Ingelheim, and is on the Speakers Bureau for Novo Nordisk, BMS, and Janssen
Footnotes
Conflict of interest statement
RAD is a member of the Advisory Board of Takeda, Bristol Myers Squibb, Janssen, Boehringer Ingelheim, Novo Nordisk, Lexicon and Amylin. RAD is a member of the Speaker Bureau of Novo Nordisk, Amylin, BMS, and Janssen.
Dr. Abdul-Ghani has no conflicts of interest
References
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 3.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13:610–30. doi: 10.2337/diacare.13.6.610. [DOI] [PubMed] [Google Scholar]
- 5.Standards of medical care in diabetes-2007. Diabetes Care. 2007;30(Suppl 1):S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 6.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care. 2008;31:81–6. doi: 10.2337/dc07-1572. [DOI] [PubMed] [Google Scholar]
- 7.Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32:515–31. doi: 10.1210/er.2010-0029. [DOI] [PubMed] [Google Scholar]
- 8.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–94. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 9.Kamran M, Peterson RG, Dominguez JH. Overexpression of GLUT2 gene in renal proximal tubules of diabetic Zucker rats. J Am Soc Nephrol. 1997;8:943–8. doi: 10.1681/ASN.V86943. [DOI] [PubMed] [Google Scholar]
- 10.Farber SJ, Berger EY, Earle DP. Effect of diabetes and insulin of the maximum capacity of the renal tubules to reabsorb glucose. J Clin Invest. 1951;30:125–9. doi: 10.1172/JCI102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302:R75–83. doi: 10.1152/ajpregu.00357.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chassis H, Jolliffe N, Smith H. The action of phlorizin on the excretion of glucose, xylose, sucrose, creatinine, and urea by man. J Clin Invest. 1933;12:1083–9. doi: 10.1172/JCI100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vick H, Diedrich DF, Baumann K. Reevaluation of renal tubular glucose transport inhibition by phlorizin analogs. Am J Physiol. 1973;224:552–7. doi: 10.1152/ajplegacy.1973.224.3.552. [DOI] [PubMed] [Google Scholar]
- 14.Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21:31–8. doi: 10.1002/dmrr.532. [DOI] [PubMed] [Google Scholar]
- 15.Li AR, Zhang J, Greenberg J, Lee T, Liu J. Discovery of non-glucoside SGLT2 inhibitors. Bioorg Med Chem Lett. 2011;21:2472–5. doi: 10.1016/j.bmcl.2011.02.056. [DOI] [PubMed] [Google Scholar]
- 16.Kahn BB, Shulman GI, DeFronzo RA, Cushman SW, Rossetti L. Normalization of blood glucose in diabetic rats with phlorizin treatment reverses insulin-resistant glucose transport in adipose cells without restoring glucose transporter gene expression. J Clin Invest. 1991;87:561–70. doi: 10.1172/JCI115031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossetti L, Shulman GI, Zawalich W, DeFronzo RA. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest. 1987;80:1037–44. doi: 10.1172/JCI113157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79:1510–5. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhanot S, Murray SF, Booten SL, et al. ISIS 388626, an SGLT2 antisense drug, causes robust and sustained glucosuria in multiple species and is safe and well-tolerated. Diabetes. 2009;58(suppl 1):A328. [Google Scholar]
- 20.Santer R, Kinner M, Lassen CL, et al. Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol. 2003;14:2873–82. doi: 10.1097/01.asn.0000092790.89332.d2. [DOI] [PubMed] [Google Scholar]
- 21.Oku A, Ueta K, Nawano M, et al. Antidiabetic effect of T-1095, an inhibitor of Na(+)-glucose cotransporter, in neonatally streptozotocin-treated rats. Eur J Pharmacol. 2000;391:183–92. doi: 10.1016/s0014-2999(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 22.Katsuno K, Fujimori Y, Takemura Y, et al. Sergliflozin, a novel selective inhibitor of low-affinity sodium glucose cotransporter (SGLT2), validates the critical role of SGLT2 in renal glucose reabsorption and modulates plasma glucose level. J Pharmacol Exp Ther. 2007;320:323–30. doi: 10.1124/jpet.106.110296. [DOI] [PubMed] [Google Scholar]
- 23.Dobbins RL, Kapur A, Kapitza C, O'Connor-Semmes RL, Tao W, Hussey EK. Remogliflozin etabonate, a selective inhibitor of the sodium-glucose transporter 2 (SGLT2) reduces serum glucose in type 2 diabetes mellitus (DM) patients. Diabetes. 2009;58(suppl 1):A573. doi: 10.1111/j.1463-1326.2011.01462.x. [DOI] [PubMed] [Google Scholar]
- 24.Hussey E, Clark R, Amin D, et al. Early clinical studies to assess safety, tolerability, pharmacokinetics and pharmacodynamics of single dose of sergliflozin, a novel inhibitor of renal glucose reabsorption in healthy volunteers and subjects with type 2 diabetes mellitus. Diabetes. 2007;56(suppl 1):A189. doi: 10.1177/0091270009351879. [DOI] [PubMed] [Google Scholar]
- 25.Hussey E, Dobbins R, Stolz R, Stockman N, Semmes R, Murray S, Nunez D. A double-blind randomized repeat dose study to assess safety, tolerability, pharmacokineticks and pharmacodynamics of three times daily dosing of sergliflozin, a novel inhibitor of renal glucose reabsorption in healthy overweight and obese subjects. Diabetes. 2007;56(suppl 1):A491. [Google Scholar]
- 26.Fujimori Y, Katsuno K, Nakashima I, Ishikawa-Takemura Y, Fujikura H, Isaji M. Remogliflozin etabonate, in a novel category of selective low-affinity sodium glucose cotransporter (SGLT2) inhibitors, exhibits antidiabetic efficacy in rodent models. J Pharmacol Exp Ther. 2008;327:268–76. doi: 10.1124/jpet.108.140210. [DOI] [PubMed] [Google Scholar]
- 27.Bristol Myers-Squibb. Princeton, NJ: Bristol-Myers Squibb; 2011. AstraZeneca. U.S. Food and Drug Administration Endocrinologic & Metabolic Advisory Committee Background Document: Dapagliflozin, BMS-512148, NDA 202293. Accessed at www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/ucm262993.htm. [Google Scholar]
- 28.Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009;85:513–9. doi: 10.1038/clpt.2008.250. [DOI] [PubMed] [Google Scholar]
- 29.Obermeier M, Yao M, Khanna A, et al. In vitro characterization and pharmacokinetics of dapagliflozin (BMS-512148), a potent sodium-glucose cotransporter type II inhibitor, in animals and humans. Drug Metab Dispos. 2010;38:405–14. doi: 10.1124/dmd.109.029165. [DOI] [PubMed] [Google Scholar]
- 30.List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650–7. doi: 10.2337/dc08-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33:2217–24. doi: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilding JP, Norwood P, T'Joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care. 2009;32:1656–62. doi: 10.2337/dc09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, Parikh S. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405–15. doi: 10.7326/0003-4819-156-6-201203200-00003. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Feng Y, List J, Kasichayanula S, Pfister M. Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: effects on glycaemic control and body weight. Diabetes Obes Metab. 2010;12:510–6. doi: 10.1111/j.1463-1326.2010.01216.x. [DOI] [PubMed] [Google Scholar]
- 35.Nauck MA, Del Prato S, Meier JJ, Duran-Garcia S, Rohwedder K, Elze M, Parikh SJ. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34:2015–22. doi: 10.2337/dc11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baily CJ, Gross JI, Yadav M, Iqbal N, Mansfield TA, List JF. Sustained efficacy of dapagliflozin when added to metformin in type 2 diabetes inadequately controlled by metformin monotherapy. Diabetologia. 2011;54(suppl 1):A146. [Google Scholar]
- 37.Del Prato S, Nauck M, Rohwedder K, Theuerkauf A, Langkilde AM, Parikh S. Long term efficacy and safety of add-on dapagliflozin vs add-on glipizide in patients with type 2 diabetes mellitus inadequately controlled with metformin: 2 year results. Diabetologia. 2011;54(suppl 1):A852. [Google Scholar]
- 38.Sha S, Devineni D, Ghosh A, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2011;13:669–72. doi: 10.1111/j.1463-1326.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 39.Inagaki N, Kondo K, Iwasaki T, Maruyama N, Susuta Y, Sakai M, Kuki H. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2 (SGLT2) improves glycemic control and reduces body weight in Japanese type 2 diabetes mellitus (DM) Diabetes. 2011;60(suppl 1):A274. [Google Scholar]
- 40.Rosenstock J, Arbit D, Usiskin K, Capuano G, Canovatchel W. Canagliflozin an inhibitor of sodium glucose co-transporter 2 (SGLT2), improves glycemic control and lowers body weight in subjects with type 2 diabetes (T2D) on metformin. Diabetes. 2010;59(suppl 1):A21. [Google Scholar]
- 41.Devineni D, Morrow L, Hompesch M, et al. Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab. 2012;14:539–45. doi: 10.1111/j.1463-1326.2012.01558.x. [DOI] [PubMed] [Google Scholar]
- 42.Polidori D, Zhao Y, Sha S, Canovatchel W. Canagliflozin treatment improves beta cell function in subject with type 2 diabetes. Diabetes. 2010;59(suppl 1):A176. [Google Scholar]
- 43.Grempler R, Thomas L, Eckhardt M, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. 2012;14:83–90. doi: 10.1111/j.1463-1326.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 44.Koiwai K, Seman L, Yamamura N, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of BI 10773, a sodium-glucose co-transporter inhibitor (SGLT2), in Japanese healthy volunteers. Diabetes. 2010;59(suppl 1):2175P. [Google Scholar]
- 45.Heise T, Seewaldt-Becker E, Macha S, Hanter S, Huber K, Pinnetti S, Seman L. BI 10773, a sodium-glucose co-transporter inhibitor (SGLT-2), is safe and efficacious following 4-week treatment in patients with type 2 diabetes. Diabetes. 2010;59(suppl 1):629P. [Google Scholar]
- 46.Rosenstock J, Seman LJ, Jelaska A, Hantel S, Pinnetti S, Hach T, Woerle HJ. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor as add-on to metformin in type 2 diabetes with mild hyperglycemia. Diabetes Obes Metab. 2013 doi: 10.1111/dom.12185. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46A.Häring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Woerle HJ, Broedl UC on behalf of the EMPA-REG METSU™ Trial Investigators. Empagliflozin As Add-on to Metformin Plus Sulfonylurea in Patients With Type 2 Diabetes: A 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013 doi: 10.2337/dc12-2673. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46B.Kovacs CS, Seshiah V, Swallow R, Jones R, Rattunde H, Woerle HJ, Broedl UC on behalf of the EMPA-REG PIO™ trial investigators. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2013 doi: 10.1111/dom.12188. [Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Kashiwagi A, Utsuno A, Kazuta K, Yoshida S, Kageyama S. ASP1941, a novel, selective SGLT2 inhibitor, was effective and safe in Japanese healthy volunteers and patients with type 2 diabetes mellitus. Diabetes. 2010;59(suppl 1):A21. [Google Scholar]
- 48.Takinami A, Takinami Y, Kazuta K, Yoshida S, Utsuno A, Nagase I, Kashiwagi A. Ipragliflozin improved glycemic control with additional benefit of reduction of body weight and blood pressure in Japanese patients with type 2 diabetes mellitus: BRIGHTEN Study. Diabetologia. 2011;54(suppl 1):A149. [Google Scholar]
- 49.Freiman J, Ruff DA, Frazier KS, et al. LX4211, a dual SGLT2/SGLT1 inhibitor, shows rapid and significant improvements in glycemic control over 28 days in patients with type 2 diabetes (DM) Diabetes. 2010;59(suppl 1) [Google Scholar]
- 50.Seino Y, Sasaki T, Fukatsu A, Samukawa Y, Sakai S, Watanabe T. TS-071, a novel and selective SGLT2 inhibitor, improved glycemic control and decreased body weight in 12-week study of Japanese patients with type 2 diabetes mellitus. Diabetes. 2011;60(suppl):P998. [Google Scholar]
- 51.Nucci G, Amin NB, Wang X, Lee DS, Rusnak JM. The sodium glucose co-transported PF04971729, provides multifaced improvement in diabetic patients inadequately controlled on metformin. Diabetologia. 2011;54(suppl):A850. [Google Scholar]
- 52.Kadokura T, Ishikawa H, Nakajo I, Yoshida S, Utsuno A, Smulders RA, Morozumi K. The effect of renal impairment on the pharmacokinetics and urinary glucose excretion of the SGLT2 inhibitor ipragliflozin in Japanese type 2 diabetes mellitus patients. Diabetologia. 2011;54(suppl):A847. [Google Scholar]
- 53.Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, Figueroa K, Wajs E, Usiskin K, Meininger G. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463–73. doi: 10.1111/dom.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abdul-Ghani MA, DeFronzo RA, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30–50% of filtered glucose load in humans. Diabetes. 2013;62:3324–8. doi: 10.2337/db13-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10:2569–76. doi: 10.1681/ASN.V10122569. [DOI] [PubMed] [Google Scholar]
- 56.Thomson SC, Vallon V, Blantz RC. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol. 2004;286:F8–15. doi: 10.1152/ajprenal.00208.2003. [DOI] [PubMed] [Google Scholar]
- 57.Noonan WT, Shapiro VM, Banks RO. Renal glucose reabsorption during hypertonic glucose infusion in female streptozotocin-induced diabetic rats. Life Sci. 2001;68:2967–77. doi: 10.1016/s0024-3205(01)01090-6. [DOI] [PubMed] [Google Scholar]
- 58.Dominguez JH, Camp K, Maianu L, Feister H, Garvey WT. Molecular adaptations of GLUT1 and GLUT2 in renal proximal tubules of diabetic rats. Am J Physiol. 1994;266:F283–90. doi: 10.1152/ajprenal.1994.266.2.F283. [DOI] [PubMed] [Google Scholar]
- 59.Nelson RG, Bennett PH, Beck GJ, et al. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med. 1996;335:1636–42. doi: 10.1056/NEJM199611283352203. [DOI] [PubMed] [Google Scholar]
- 60.Tuttle KR, Bruton JL, Perusek MC, Lancaster JL, Kopp DT, DeFronzo RA. Effect of strict glycemic control on renal hemodynamic response to amino acids and renal enlargement in insulin-dependent diabetes mellitus. N Engl J Med. 1991;324:1626–32. doi: 10.1056/NEJM199106063242304. [DOI] [PubMed] [Google Scholar]
- 61.Arakawa K, Ishihara T, Oku A, et al. Improved diabetic syndrome in C57BL/KsJ-db/db mice by oral administration of the Na(+)-glucose cotransporter inhibitor T-1095. Br J Pharmacol. 2001;132:578–86. doi: 10.1038/sj.bjp.0703829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knapp S. Diabetes and infection: is there a link?--A mini-review. Gerontology. 2013;59:99–104. doi: 10.1159/000345107. [DOI] [PubMed] [Google Scholar]
- 63.Parikh S, Johnsson K, Ptaszynska A, Schmitz B, Sugg J, List JF. Characterization of urinary tract infection in the setting of pharmacologically induced glucosuria. Diabetologia. 2011;54(suppl):A841. [Google Scholar]
- 64.Nigro SC, Riche DM, Pheng M, Baker WL. Canagliflozin, a Novel SGLT2 Inhibitor for Treatment of Type 2 Diabetes. Ann Pharmacother. 2013;47:1301–11. doi: 10.1177/1060028013503626. [DOI] [PubMed] [Google Scholar]
- 65.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Stein P, Desai M, Shaw W, Jiang J, Vercruysse F, Meininger G, Matthews D. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS)--a randomized placebo-controlled trial. Am Heart J. 2013;166:217–223. doi: 10.1016/j.ahj.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 66.Ridderstråle M, Svaerd R, Zeller C, Kim G, Woerle HJ, Broedl UC EMPA-REG H2H-SU™ trial investigators. Rationale, design and baseline characteristics of a 4-year (208-week) phase III trial of empagliflozin, an SGLT2 inhibitor, versus glimepiride as add-on to metformin in patients with type 2 diabetes mellitus with insufficient glycemic control. Cardiovasc Diabetol. 2013;12:129–138. doi: 10.1186/1475-2840-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]