Abstract
The burden of cancer in the United States is unevenly spread across its different populations, with stark differences in both disease prevalence and outcome on the basis of race and ethnicity. Although a large portion of these differences can be explained by a variety of sociobehavioral and socioeconomic factors, even after these exposures are taken into consideration, considerable disparities persist. In this review, we explore a conceptual framework of biological theories and unifying concepts, based on an evolutionary perspective, that may help better define common guiding principles for exploration of underlying causes of cancer health disparities. The ultimate goal of this conceptual perspective is to outline approaches that may aid in establishing integrated pathway and processes analyses to provide useful insights to guide the development of future interventions. These interventions will improve outcome, increase prevention, and ultimately eliminate all disparities.
The main indicators of health disparities, when comparing different groups or populations for any given disease or condition, include i) increased prevalence or frequency, ii) earlier onset, iii) faster progression, and iv) poorer outcome in terms of survival, morbidity, and disability. Together they provide a useful means of comparing or determining the difference in the overall impact that a certain disease or condition has on the quality of life in specific underserved or disadvantaged populations. The over-arching goal of health disparities research is to elucidate the cause and mechanism of these differences and to define means of intervention that will alleviate and eventually eliminate the disparity. Because the different influences on health and disease are multifactorial, involving diverse aspects of biology, behavior, physical environment, sociocultural factors, and influences of socioeconomic status (including access to health care, the science of health disparities requires a transdisciplinary approach. Moreover, a full appreciation of how gene–environment interactions converge to influence health outcomes demands a broader appreciation of the diverse ways that molecular pathways combine and coalesce to influence changes in biological programs and processes that influence health. Central goals that will lead to more effective intervention require identifying and defining how these biological programs and processes are influenced by differential exposures of environment, behavior, and lifestyle across the life-course in different populations.
Racial/Ethnic Differences in Cancer Outcomes in the United States
Few medical conditions or diseases have a greater impact on the quality of life across different racial, sociocultural, and ethnic populations than cancer. According to the Office of Management and Budget standard classifications for Racial/Ethnic groups in the United States, cancer is the second leading cause of death of individuals of European American or white ancestry, the second leading cause of death of individuals of African American or black ancestry, the first leading cause of death in individuals of Hispanic ancestry, the leading cause of death for individuals of American Indian or Alaskan native ancestry, and the leading cause of death of individuals of Asian or Pacific Islander ancestry1 (Tables 1 and 2). Trends for cancer are heterogenous across different racial and ethnic groups in the United States. For men, the incidence rates of all cancers are 12% higher for men of African descent than for men of European ancestry. In contrast, women of African descent show a 7% lower incidence. These rates are much lower (18%–40%) in men and women of Hispanic, Asian/Pacific Islander, and American Indian/Alaska Native descent1 (Tables 1 and 2).
Table 1.
Age-Adjusted Cancer Incidence Rates (Number of Cases per 100,000 People) across Race and Ethnicity in the United States from 2009 to 20131
| Variable | All races | White | Black | Asian/Pacific Islander | American Indian/Alaska native | Hispanic |
|---|---|---|---|---|---|---|
| All sites | ||||||
| Male | 512.1 | 519.3 | 577.3 | 310.2 | 426.7 | 398.1 |
| Female | 418.5 | 436.0 | 408.5 | 287.1 | 387.3 | 329.6 |
| Lung | ||||||
| Male | 75.0 | 77.7 | 90.8 | 46.6 | 71.3 | 42.2 |
| Female | 53.5 | 58.2 | 51.0 | 28.3 | 56.2 | 25.6 |
| Liver | ||||||
| Male | 11.8 | 9.7 | 16.9 | 20.4 | 18.5 | 19.4 |
| Female | 4.0 | 3.3 | 5.0 | 7.6 | 8.9 | 7.5 |
| Breast | ||||||
| Male | NA | NA | NA | NA | NA | NA |
| Female | 123.3 | 128.3 | 125.1 | 89.3 | 98.1 | 91.7 |
| Prostate | ||||||
| Male | 123.2 | 114.8 | 198.4 | 63.5 | 85.1 | 104.9 |
| Female | NA | NA | NA | NA | NA | NA |
| Colorectum | ||||||
| Male | 46.9 | 46.1 | 58.3 | 37.8 | 51.4 | 42.8 |
| Female | 35.6 | 35.2 | 42.7 | 27.8 | 41.2 | 29.8 |
NA, not available/applicable.
Table 2.
Age-Adjusted Cancer Mortality Rates (Number of Cases per 100,000 People) across Race and Ethnicity in the United States from 2010 to 20141
| Variable | All races | White | Black | Asian/Pacific Islander | American Indian/Alaska native | Hispanic |
|---|---|---|---|---|---|---|
| All sites | ||||||
| Male | 200.4 | 204.0 | 253.4 | 122.7 | 183.6 | 142.5 |
| Female | 141.5 | 145.5 | 165.9 | 88.8 | 129.1 | 97.7 |
| Lung | ||||||
| Male | 55.9 | 58.3 | 69.8 | 31.7 | 46.2 | 27.3 |
| Female | 36.3 | 39.8 | 35.5 | 18.0 | 30.8 | 13.4 |
| Liver | ||||||
| Male | 9.2 | 8.0 | 13.3 | 14.3 | 14.9 | 13.1 |
| Female | 3.7 | 3.3 | 4.6 | 6.1 | 6.8 | 5.8 |
| Breast | ||||||
| Male | NA | NA | NA | NA | NA | NA |
| Female | 21.2 | 21.1 | 30.0 | 11.3 | 14.1 | 14.4 |
| Prostate | ||||||
| Male | 20.0 | 18.7 | 42.8 | 8.8 | 19.4 | 16.5 |
| Female | NA | NA | NA | NA | NA | NA |
| Colorectum | ||||||
| Male | 17.7 | 17.3 | 25.9 | 12.4 | 19.5 | 15.0 |
| Female | 12.4 | 12.3 | 16.9 | 8.8 | 14.0 | 9.2 |
NA, not available/applicable.
The disparities in cancer incidence and mortality rate are much more significant when examined by organ site. Rates of lung cancer is close to twofold higher in men than in women. Lung cancer incidence in men of African descent is nearly 17% higher than men of European ancestry1 (Table 2). Women of African descent show 12% lower frequency and 11% lower lung cancer mortality rate than white women; however, men of African descent die at a 20% higher rate from lung cancer than men of European ancestry. Although the incidence of breast cancer is nearly equivalent between women of African and European ancestry in the United States, women of African descent nonetheless experience a >40% higher mortality rate.1 The rate of liver cancer has nearly tripled in the United States over the past 30 years, and, by the year 2030, Hispanic men are projected to have the highest age-adjusted rate at nearly 2.3 times the current rate.2, 3 Currently, the incidence of liver cancer in men of African ancestry is >74% higher than men of European descent, and liver cancer rates in men of Hispanic ancestry are greater than twofold higher than white men. Both Hispanic and black men experience higher (both >66%) mortality rates from liver cancer than white men.1 Prostate cancer is by far one of the greatest cancer health disparities. Compared with men of European descent, men of African descent have a >72% higher frequency of prostate cancer and experience a stunning 2.3-fold higher mortality rate1 (Tables 1 and 2).
These striking and provocative differences in cancer prevalence, onset, progression, and survival across multiple racial and ethnic populations result from the convergence and interdependent influences at the biological, behavioral, environmental, and socioeconomic level. Deconvolving this degree of complexity will require both multidisciplinary and trans-disciplinary approaches and perspectives to define interactions, at the gene–environment interface, that will allow us to make causal inferences about the relationship between exposures and biological susceptibility that will ultimately guide effective intervention. Therefore, developing unifying perspectives that interrogate the cause of disease at the biological level (including pathways, programs, and physiological processes) and defining how they respond or adapt to differing environmental and behavioral exposures are imperative to uncover common associations and to reveal causal intersections. In this review, with the use of a pathway- and process-oriented approach, we explore the causal relationships between exposures and biologically differing outcomes of disease from an evolutionary perspective to illustrate that adaptations that evolve to become genetically hard-wired, at first to increase fitness within a particular environmental context, can become maladaptive and confer predisposition to pathologic processes, on transition to a different environment. We then speculate that similar forces may be at play in the evolution of cancer health disparities.
Allostasis as an Evolutionary Concept of Conditional Adaptive Selection
The term allostasis is an evolutionary term first coined by the neuroscientist Sterling and Eyer4 and later popularized by McEwen5 to describe predictive physiological adaptation to stress. It provides an evolutionary perspective on changing cellular programs and physiological processes that adapt to re-establish homeostasis in response to changes or challenges in the environment. Although used originally by McEwen5 to describe the role of the hypothalamic–pituitary–adrenal axis in providing immediate adaptive response to stress through the orchestrated systemic release of glucocorticoids and catecholamines in response to physiological and psychogenic stressors, this concept has broader implications, as discussed below. The central concept in allostasis proports that any physiological response that may have been adaptive and under positive selection to favor homeostasis can lead to pathologic processes if it is overused, overactive, or if there is a change in the environment that renders that adaption less fit. The change in state that results in the pathologic process is referred to as the allostatic load. The allostatic load is the pathologic process, disease, or physiological burden associated with the adaptive response. Ultimately, the actual pathologic process is defined by the mediators of the adaptive response. For example, in the hypothalamic–pituitary–adrenal axis, the primary mediators of the allostasis are the typical fight or flight response mediators released from the adrenal gland, including norepinephrine, epinephrine, cortisol, and dihydroepiandosterone sulfate in addition to other cytokine mediators of a global stress response.5, 6, 7 These mediators induce changes in the cellular physiological programs that control the sympathetic nervous system, the cardiovascular system, and metabolic processes. The cumulative results of the chronic use or overuse of these pathways are the gradual progression of cellular and physiological dysfunction, which are demonstrated by increased hypertension, elevated cholesterol, high glycated hemoglobin, and increased hip-to-waist ratio. The increased wear and tear on the body or weathering, as a result of these exposures, is what defines allostatic load.4, 6, 7, 8
The concept of allostasis provides a theoretical framework to understand the role of biological processes in adaption and how their overuse can lead to disease. It also provides insights that help clarify aspects of normal physiological functions through a better understanding of how their overuse results in disease. Further, these processes can be biologically and genetically shaped by interactions with the environment, as is the case for persistence of the sickle cell variant, hemoglobin S (HbS) gene in populations of African descent. In the United States, the frequency of the HbS allele is 8% to 10%, but in certain populations in Africa, the prevalence is as high as 30%. Those individuals who are homozygous for the disease are symptomatic. The high frequency of this allele in these populations suggests the role of a significant Darwinian selection that has maintained its prevalence. The underlying reason for this selective pressure stems from the protection that heterozygous expression of HbS provides against severe parasitic infection with Plasmodium falciparum malaria, endemic in these regions. The red cells of heterozygous carriers of HbS undergo sickling under the conditions of reduced oxygen and decreased intracellular pH in parasite-infected cells.9 These cells are rapidly removed by the phagocytic reticuloendothelial system of the liver and spleen. In addition, sickled red cells have impaired formation of parasite-derived membrane structures, containing the Plasmodium falciparum erythrocyte membrane protein 1, which plays a major role in disease processes.9, 10 Similarly, other malaria-protective genetic polymorphisms, including HbC and glucose-6-phosphate dehydrogenase (G6PD) deficiency, have shown an increased frequency in areas of Africa and Southeast Asia where malaria is endemic.9, 10, 11 In each of these cases, the allostatic load associated with adaptation under selection is hemolytic anemia.9, 10, 11 These provide classic examples of how allostatic processes are genetically and biologically shaped by interactions with the environment.12
Such mechanisms of adaptive selection need not occur exclusively through Mendelian inheritance but instead are much more likely to be broadly polygenetic. An illustration of this point is the evolution of the adaptive capability of subcutaneous and visceral adipose tissue to store energy in anticipation of periods of food and nutrient deprivations. As suggested by Power and Schulkin,13, 14 although this process originally evolved as an adaptive response when food availability was limited, the chronic overuse of this adaptation, when food is readily available, has led to an allostatic load that is reflected in the growing worldwide epidemics of obesity, diabetes, heart disease, other conditions of metabolic imbalance, and stroke.13 A third example of allostasis is the differential expression of the apolipoprotein L1 variant (APOL1) in individuals of African descent.15, 16, 17, 18, 19, 20 Variants of APOL1 have been shown to provide a protective influence against trypanosomiasis (African sleeping sickness); however, this adaptive trait results in an allostasis that contributes to the nearly threefold to fourfold higher risk of end-stage renal disease (ESRD) experienced by individuals of African descent compared with other populations.15, 16, 17, 18, 20, 21 This type of allostasis is likely to be the result of traits that are transmitted through a combination of Mendelian and polygenic mechanisms. Moreover, it provides a further example of how genetic and biological properties are shaped by interactions with the environment and highlights how infectious disease has been, by far, one of the most influential forces in this process.
Allostasis in Tissue Repair: A Common Underlying Theme in Diverse Disease
Since the time of Virchow in the 19th century pathologists have observed the similarities between the tumor stroma or the tumor microenvironment and the processes of a healing wound.22, 23, 24, 25, 26, 27 This observation is best known in the writings of Dvorak28, 29 who referred to malignant tumors as wounds that do not heal. The juxtaposition of these observations in the context of allostasis allows one to posit, from an evolutionary perspective, that altered regulatory control of tissue repair results in a more robust repair or over-healing that could conceivably provide an adaptive survival advantage in response to wounds, tissue damage, or both encountered in battle, while hunting, or by accident. One example of an allostatic load that results from this altered capacity for tissue repair is reflected in the occurrence of profibrotic conditions, including keloid scarring,30, 31, 32, 33 uterine leiomyoma,34, 35 the glomerulosclerotic conditions associated with ESRD,36 and sarcoidosis.37, 38, 39, 40, 41 Most notably, these are conditions that occur at a considerably higher frequency in individuals of African descent.30, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 In the ensuing sections, the common mechanisms underlying some of these conditions are emphasized, and examples are described of how dysregulation of specific features of these shared mechanisms contribute to cancer incidence and progression.
Interpreting Keloid Scarring: A Wound Healing Allostatic Load
Keloid scarring is a polygenic condition that demonstrates autosomal dominant inheritance with variable penetrance.32, 33 Keloids represent heterogeneous fibroproliferative disorders characterized by cellular proliferation of fibroblast, macrophages, and other immune cells, including lymphocytes and mast cells, that are often in close contact with one another and spread into adjacent tissues.31, 32, 33, 46 Keloid fibrosis occurs 20 times more frequently in individuals of African descent than in those of European ancestry.32, 33 Several studies that involve either candidate approaches are genome-wide association studies in a specific population have identified a number of polymorphism in genes associated with the profibrotic processes during keloid formation.33 Many of them are linked to transforming growth factor (TGF)-β–dependent signaling in addition to other pathways involved in growth, differentiation, cell migration, inflammation, and the epithelial reprogramming that plays a central role in wound healing and tumor invasion known as epithelial-to-mesenchymal transition (EMT).33, 47, 48, 49 They include TGF-β1, TGF-β2, TGF-β3, TGF-βR1, TGF-βR2, TGF-βR3, SMAD3, SMAD6, SMAD7, epidermal growth factor receptor (EGFR), tumor necrosis factor (TNF)-α induced protein 6, p53, and multiple human leukocyte antigen alleles.33 Many of these factors are involved in pathways that promote the growth and phenotypic plasticity of both fibroblasts and macrophages, the two master regulators of the tissue repair and wound healing responses in all tissues.33, 42
Cellular Dynamics of Profibrotic Processes and Disease
As master regulators of tissue repair, fibroblasts and macrophages play a collaborative role in almost every type of inflammatory or autoimmune profibrotic condition in humans, including pulmonary fibrosis,50 liver fibrosis,51 renal fibrosis,52 and systemic fibrosis and cancer.53 This role involves an active collusion between various phenotypic states of macrophages and fibroblasts through dynamic changes during normal tissue repair within the tumor stroma. In chronic kidney disease, focal or diffuse fibrosis within the renal glomeruli is a central feature of diabetic and nondiabetic nephropathy that plays a major role in the progression to ESRD. ESRD occurs at a fourfold higher frequency in individuals of African ancestry.18, 36, 54 Uterine leiomyomas or fibroids are benign proliferative lesions of smooth muscle cells observed eight times more frequently in women of African descent than in other populations.34, 35, 45 Sarcoidosis is an idiopathic noncaseating granulomatous inflammatory disease that often affects the lung, skin, bone marrow, and eyes. It is characterized by infiltration with well-formed coalescing clusters of histiocytes often rimmed by a fibrous layer of lamellar hyaline collage or edematous myofibroblastic tissue.55 Sarcoidosis occurs at nearly a fourfold higher rate in individuals of African ancestry than in other populations39 with a mortality rate in some regions that is 12 times higher than for those of European ancestry.38, 40 The cellular components and processes held in common by all of these conditions play central roles in wound healing.
Allostasis in Tissue Repair as a Driver That Links Tumor Stroma to Tumor Progression
The tumor stroma represents a dynamic interface that is intimately and immediately influenced by long-term influences or rapid changes in the environment. Changes and alterations at the level of the tumor microenvironment govern multiple processes, including angiogenesis, tumor migration, and invasion; tumor spread to distance sites; colonization at metastatic loci; sanctuary from immune attack; and resistance to therapy.56 Each of these properties is linked to cellular components and processes that play important roles in wound healing and tissue repair. To better appreciate the potential allostatic link between dysregulated processes of wound healing and cancer, the salient features and components that are held in common between normal tissue repair and tumor stroma must first be examined. The cellular and histologic programs of wound healing can be generally divided into the following three distinguishable phases: i) the inflammatory phase, ii) the proliferative phase, and iii) the reparative phase.26, 28, 29 The initiation of the inflammatory phase is marked by an increase in vascular permeability. This permeability is initiated either through injury-induced mechanical disruption or by the action of secreted histamine (mast cells) and vascular endothelial growth factors (VEGFs) provided by local epithelial (or tumor) cells, fibroblasts, and macrophages. Leakage of serum components, red blood cells, and platelets initiate the clotting cascade in the extra-vascular space. Complement activation by the clotting cascade serves to attract numerous inflammatory cells, including neutrophils, monocytes, mast cells, and fibroblasts to the disrupted tissue stroma. Leaked platelets, now activated through interactions with the extracellular matrix (ECM), begin to release multiple different vasoactive agents and growth factors, including platelet-derived growth factor (PDGF) and TGF-β, and additional fibrinogen. In addition to clearing dead cells and cellular debris, the incoming macrophages begin to secrete multiple cytokines, growth factors, and chemokines, including IL-1, TNF-α, IL-6, and VEGF. These factors assist in increasing the fibroblasts and endothelial cell populations during angiogenesis and the entry of bone marrow-derived mesenchymal stems cells or mesenchymal stroma cells into the tissue microenvironment. As the rapidly growing granulation tissue begins to mature fibroblasts continue to add to the matrix by the secretion of collagen and fibronectin in support of entry into the proliferative phase.
Macrophages, Myofibroblasts, and Mesenchymal Stems Cells: Cellular Plasticity as a Major Driver of Tissue Repair
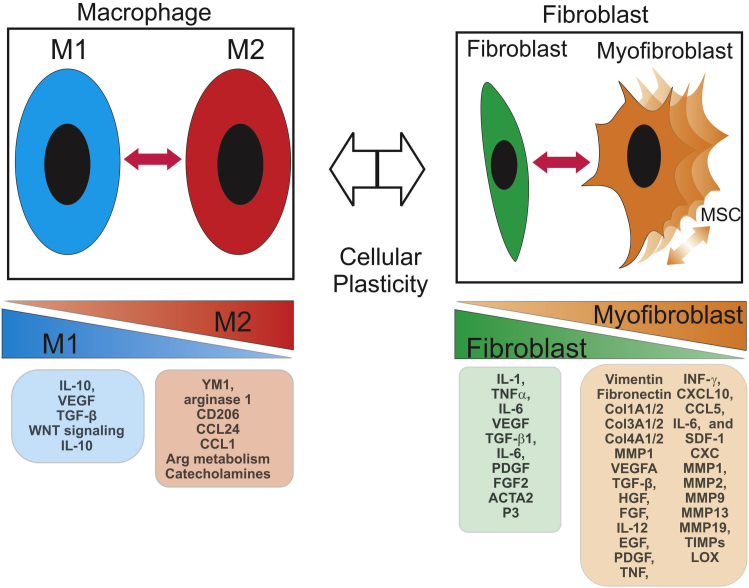
During the proliferative phase, new vessel formation continues, driven by VEGF- and PDGF-mediated increases in monocytes, fibroblast, pericytes, and endothelial cell proliferation and migration into the wound/tumor stroma. Monocytes differentiate into macrophages and become a dominant cell type in the wound/tumor stroma. The fibroblast is the second dominant component of the stroma that undergoes significant phenotypic transition. The extensive plasticity of these two components and their close interactions are the major drivers during normal tissue repair and within the tumor microenvironment57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67 (Figure 1).
Figure 1.
Schematic illustration of cellular plasticity in the macrophage and fibroblast in the healing wound and the secreted growth factors, cytokines, and other factors that drive the phenotypic transitions. ACTA2, smooth muscle α-2 actin; Arg, arginine; CCL, C-C motif chemokine ligand; CD206, cluster of differentiation; Col1A, collagen type I α; CXCL, C-X-C motif chemokine; EGF, epidermal growth factor; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; IFN, interferon; LOX, lysyl oxidase; MMP, matrix metalloproteinase; P3, forkhead box P3; PDGF, platelet-derived growth factor; SDF, stromal cell-derived factor; TGF, transforming growth factor; TIMP, tissue inhibitor of metalloproteinase; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor; YM1, β-N-acetylhexosaminidase Ym1.
Macrophages
The phenotypic distribution of macrophages plays a central role in the transition from the inflammatory to the proliferative stage. Macrophages are a heterogeneous class of highly plastic cells that span a phenotypic continuum that has been traditionally defined as two polarized states referred to as M1 and M2. The M1 end of the phenotypic spectrum is the classically activated macrophage that is induced by interferon (INF)-γ, toll receptor ligands (eg, lipopolysaccharide), TNF-α, and granulocyte-macrophage colony-stimulating factor. They chiefly produce reactive oxygen species and proinflammatory cytokines, including IL-1β, TNFα, and IL-6, and are shifted to a metabolic response that relies on anaerobic glycolytic pathways and metabolism. Their major response provides protection from microbial and/or tumor invasion. At the other end of the spectrum are the M2 macrophages that encompasses a variety of M2-like phenotypes. M2 polarized cells are induced by IL-4, IL-13, and IL-21 with variable induction by chemokine (C-C motif) ligand (CCL)2 and CXCL4, and have a heterogeneous capacity to produce IL-10, VEGF, and TGF-β. Stereotypical markers of M2 macrophages are the expression of β-N-acetylhexosaminidase Ym1, arginase 1, mannose receptor (CD206), CCL24, and CCL17, as well as a shift in arginine metabolism to ornithine and polyamines (agents that support cellular proliferation and tissue repair). Other variants of M2 macrophages have been shown to influence tissue metabolic thermogenesis by secretion of catecholamines.68, 69 The M2 phenotypic spectrum plays broad roles in repressing inflammation, promoting tissue remodeling, and driving angiogenesis. These properties provide the greatest collusion with invading tumors cells. Additional macrophage phenotypes that possess M2-like features can be induced by a diverse array of stimuli, including combinations of immune complexes with lipopolysacchairde or IL-1 (M2b), or TGF-β, WNT signaling (WNT5a), and IL-10 (M2c). Other macrophages with M2-like features are known to be induced in tissue microenvironments associated with placental and embryonic tissues, obesity, and cancer.70 M2-polarized cells maintain a high synthetic capacity to sustain the tissue remodeling, so they must rely on the more energy efficient oxidative glucose metabolism associated with fatty acid oxidation.
Mesenchymal Stem/Stromal Cells
Macrophages exhibit a strong bi-directional communication with tissue and myeloid progenitors. M2-polarized macrophages have been found to interact with mammary gland stem cells and to influence mesenchymal stem/stromal cell (MSC) growth and motility.61, 71 In turn MSCs have been shown to induce M2-like phenotypic transitions in macrophages.62, 71, 72 MSCs are a multipotent class of cell typically capable of differentiation into chondrocytes, osteoblasts, and adipocytes. More recent studies indicate an expanded plasticity that include myofibroblast, myocytes, and neurons.71, 73, 74, 75 Although this population of cells is more abundant in the bone marrow and adipose tissue, several studies have shown that there is a significant pool of circulating MSCs that home to sites of tissue damage and repair.71, 73, 75 On arrival, MSCs participate in extensive functional interactions with both the tumor and surrounding stromal components. The capacity to selectively migrate to damaged or injured tissue has suggested a reparative function for the MSC; however, evidence suggests that this potentially reparative role may be commandeered by the deregulated repair in the tumor stroma in support of tumor progression.71, 73 This level of influence occurs through both direct contact and paracrine mechanisms. At each stage within this process the MSCs have the capacity to change and be changed by the tumor and stromal components. An important outcome of these interactions is the programming or reprograming of metastatic potential in both the tumor and tumor stroma through the promotion of EMT within the cellular components of the stroma and tumor.71, 73, 74, 75 This observation stands in stark contrast to the potential therapeutic role that MSCs may have in promoting repair of damaged or degenerating tissue.73 This tumor–host interaction is one of the most important yet enigmatic processes in the succession of events from tumor initiation, growth, invasion, and metastasis.71, 73, 75
Myofibroblasts
The myofibroblast is one of the most dominant components of the proliferative phase of wound healing.57, 58, 76 Like the macrophage, it is a highly plastic and heterogeneous component of the stroma that has multiple origins and exists along a spectrum of phenotypic forms. Myofibroblasts are considered activated forms of the fibroblast. Although it is not known which cell type contributes the most to the activated population in each tissue, they are thought to have several potential origins. These include mesenchymal stem cells, of monocyte-derived precursor or (fibrocytes) origin, or resident populations of activated fibroblasts that can be found in different tissues prone to profibrotic disease, for example, the stellate cells of the liver and pancreas and the renal mesangial cells.57, 58, 76, 77 Myofibroblasts are activated and recruited to damaged tissue by a variety of stimuli, many of which are provided by the infiltrating macrophages and other components of the inflammatory and proliferative phases, including TGF-β1, IL-6, PDGF, and fibroblast growth factor (FGF)2. In the evolving tumor stroma, these signals are also provided by both invading and in situ tumors.57, 58, 76 Fibroblast activation is characterized by increased expression of α-smooth muscle actin and vimentin and by increased synthesis of a variety of components of the ECM, including laminins, fibronectin, and type I, type III, and type IV collagen. The synthesis of these components is coupled to and coordinated with the secretion of various enzymes that remodel the ECM, including matrix metalloproteinases MMP1 and MMP3 (stromolysin). At approximately the same time, myofibroblasts also initiate a secretory program with elaboration of an array of growth factors and cytokines, including VEGFA, TGF-β, hepatocyte growth factor, FGF, EGF, PDGF, TNF, INF-γ, CXCL10, CCL5, IL-6, and stromal derived factor-1 (/CXCL12). Under normal conditions, the proliferative phase progresses to the remodeling phase that is marked by significant regression of the activated macrophage and fibroblast components, mostly by apoptosis, resulting in the formation of an indolent scar.26, 58, 76 However, if the repeated injury to the tissue continues unabated, as in the case of tumor stroma, the myofibroblasts enter into a more extensive program of secretion, synthesis, and deposition of components that dramatically change the nature of the ECM, thereby producing an environment that supports new vessel formation, invasion, and metastasis. This includes an expanded array of MMPs, including MMP1, MMP2, MMP3, MMP9, MMP13, and MMP19, tissue inhibitors of metalloproteinases, and lyslyl oxidase, all of which have a dramatic effect on the tumor stroma. The resulting remodeling and increased stiffness of the matrix unmasks growth factors and chemokines and provides a mechanochemical signal that promotes migration and, together with the action of tumor-associated macrophages and MSCs, provide an environment that favors invasion and metastasis.25, 27, 58, 61, 67
Epithelial and Mesenchymal Plasticity Collude with Inflammation in the Tumor Stroma to Enhance Tumor Invasion and Metastasis
The orchestrated interaction between components of innate immunity, myeloid-derived stromal/stem cells, and myofibroblast has its greatest effects on the emerging malignant epithelial cells, which in addition to promoting plasticity in the macrophage and myofibroblast compartment are also responding to similar agents produced by these cell types to generate a self-perpetuating dynamic that maintains and sustains the plasticity.26, 47, 49 The program of epithelial plasticity referred to as EMT is a dynamic process that occurs during normal wound healing.48, 49, 78, 79 Cells that have undergone EMT acquire traits that promote tumor progression, including increased migration, invasion, resistance to anoikis, resistance to chemotherapy, and the acquisition of stem cell-like tumor-initiating cell-like qualities, including self-renewal.47, 48, 78, 80 The major drivers of this process include Wnt and Notch ligands in addition to TGF-β, all of which are constantly supplied by the transitioning myofibroblasts and polarized macrophages in the tumor stroma.47, 48, 78, 80 Like the macrophage and fibroblast phenotypes, the process of EMT in tumor cells exists along a continuous spectrum between epithelial and mesenchymal properties along which tumors may reside in states of partial EMT.47, 48, 78, 81 This level of plasticity provides an adaptive advantage for the tumor cell, enabling it to revert from a mesenchymal to a more epithelial state that allows it to colonize the metastatic niche. Depending on the tumor and stromal context, multiple other different ligands also play a role in driving and maintaining EMT, including FGF, EGF, insulin-like growth factor, hepatocyte growth factor, and PDGF. In addition, inflammatory signaling controlled by IL-1β and TNFα and other pathways driven through NF-κB–activated transcriptional pathways have significant influence. During this process, a dynamic balance is established between pro-epithelial master regulators (eg, FOXA1, GATA3) and pro-EMT master regulators (eg, SNAIL, SLUG, ZEB, and Twist1). A delicate balance exists between these cellular pathways where transition from one state to the other depends on the interaction between processes that antagonize versus processes that favor transcriptional activation of opposing poles of differentiation.47, 48, 80, 82 Many of these regulatory circuits appear to be stably regulated at the level of chromatin, defining a critical role for epigenetic regulation during these phenotypic transitions.48, 81
Inflammation, Epithelial and Mesenchymal Plasticity Converge in Epithelial Cancers
There are many examples of the role of inflammation and cellular phenotypic plasticity in the evolution and progression of epithelial cancers. The microenvironment of the involuting breast undergoes a well-recognized stromal wound healing process that contributes to the risk, incidence, and progression of breast cancer.83, 84, 85, 86, 87, 88, 89 The poorer outcome in women who are diagnosed within 5 years of full-term pregnancy has been attributed to the dynamic plasticity of the polarizing stromal components, including macrophages and fibroblasts, in the involuting mammary gland.90, 91 Several studies have suggested that racial differences in the frequency of more aggressive forms of breast cancer such as triple-negative breast cancer (a subtype characterized by more mesenchymal features) may be attributed to features associated with a protracted wound healing response after pregnancy. Thus, the higher incidence of early-onset breast cancers in women of African ancestry is associated with higher parity, older age at first birth, and early weaning or never-having breast fed.90, 92, 93 It is tempting to speculate that an underlying higher level of epithelial and mesenchymal plasticity may contribute to these differing attributes and outcome. In addition to the role of mesenchymal elements in the tumor stromal response, several reports have implicated specific immune inflammatory signatures more prevalent in breast cancers of women of African ancestry as an additional contributing factor in poorer outcome.94 Moreover, recent studies suggest the white adipose tissue inflammation may also be a risk factor that contributes to the incidence and progression of breast cancer associated with obesity and/or metabolic imbalance and that women of African ancestry show higher amounts of pro-tumorigenic M2-polarized macrophages.95, 96, 97 Similarly, the inflammation associated with metabolic imbalance is heavily regulated by TGF-β,84 and recent studies have demonstrated that genetic variation in the TGF-β signaling pathway may influence breast cancer survival.98, 99, 100 In addition, the observation that vitamin D impedes that action of TGF-β in the stroma provides an underlying explanation for the association of reduced vitamin D responses and higher incidence and worse breast cancer outcome in women of African ancestry.101, 102, 103 Similar associations with vitamin D and mutations in the TGF-β signaling pathway have been observed in colon cancer.104, 105 The role of inflammatory processes in colon cancer has been well studied. These observations have served as the underlying conceptual basis for the advocated use and efficacy of aspirin in decreasing colorectal cancer risk and progression.106 Myofibroblasts play an extensive role in the biology of colon cancer.107 Recent studies are beginning to suggest possible differences in the activation of stem cell–like self-renewal pathways in colon cancer based on race with a focus on the role of specific microRNAs.108, 109, 110, 111 Like breast cancer, recent studies are beginning to reveal differential signatures and influences of inflammatory pathways in prostate cancer112, 113, 114 and the role of EMT in the generation and support of the stem cell niche.115, 116, 117 As in colon cancer, a role of aspirin in the reduction of risk and progression of prostate cancer, particularly in African American men, has been proposed.113 Each of these observations provide an example of cellular processes (eg, EMT) and signaling pathways (eg, TGF-β) that are influenced by behavioral, environmental, and genetic determinants (eg, reproductive history, obesity, medication, and vitamin metabolism).
Non-alcoholic fatty liver disease (NAFLD) has become one of the most common diseases in Western countries, and new projections predict a significant increase in hepatocellular carcinoma (HCC) in both the Hispanic and African American communities which parallels the prevalence of obesity in these populations.2, 118 The resident myofibroblast of the liver are the hepatic stellate cells. As in breast, colon, and prostate cancers, activated hepatic stellate cells respond to hepatic tissue damage signals. In the case of NAFLD, hepatocellular damage incurred by mechanisms of fatty acid lipotoxicity collude with the resident hepatic macrophages, the Kupffer cell, in the inflammatory and fibrogenic evolution characteristic of the progression of NAFLD to non-alcoholic steatohepatitis and ultimately to HCC.119, 120 The precise mechanism that leads to HCC from NAFLD will require further elucidation, but, as in the stroma of breast, colon, and prostate, inflammatory activation of Kupffer cells leads to the generation of superoxides, TGF-β1, and PDGF that is linked to activation of hepatic stellate cells which, in turn, secrete increased levels of CCL2 and VEGF that promote and sustain the inflammatory response by the recruitment of additional inflammatory cells.119, 121 Thus, like breast, prostate, and colon cancers, ancestrally programmed differences in pathways, linked to wound healing quality and capacity, could influence the onset and progression of HCC.
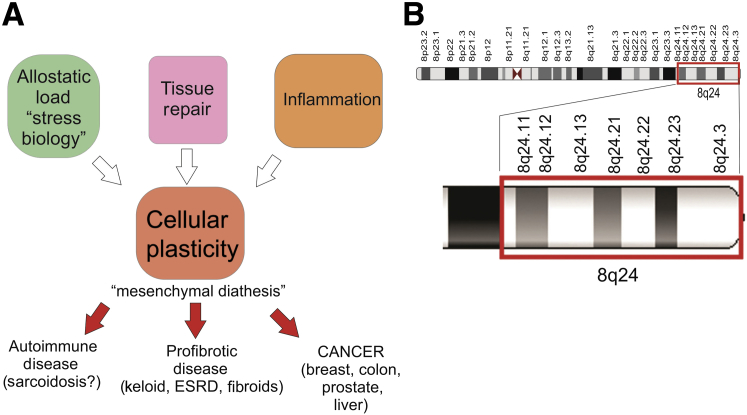
In summary, cancers of highly differentiated epithelial tissues such as breast, prostate, colon, and liver may all be associated with ancestrally programed mesenchymal diathesis that may favor more aggressive tumor phenotypes and poorer outcome in men and women of African ancestry (Figure 2A). How and if such a complex trait could have evolved under selective pressure remains an open question. Understanding how coding and regulatory loci contribute to these various pathways and processes, including how they could have co-evolved, will require extensive molecular and phenotypic correlation that must incorporate information from individual and population-specific exposures. Hints that pathway-associated collections of genes may co-evolve has come from the emergence of the chromosome 8q24 region as an important genetic susceptibility region linked to race in multiple cancers, including prostate and colon.122, 123 Several genes contained in this region are associated with cellular plasticity, EMT, and the acquisition of stem cell traits (Figure 2B). Extended efforts and approaches will be needed to profile and identify variations that alter the activity and regulation of the genes described in this review (Figures 1 and 2). Identifying and understanding differences in disease outcome will require expanded efforts focused on accruing and analyzing more genomic data from more diverse populations.124
Figure 2.
A: Flowchart of association of influences and properties of tissue repair and cellular plasticity that are causative drivers of cancer and other diseases in which mesenchymal transitions play a major role. B: Ideogram of chromosome 8q24 associated genes that have roles in epithelial-to-mesenchymal transition and stem cell-like properties. ESRD, end-stage renal disease.
Genome-wide association studies have provided a wealth of information on the role of genetic variation in the contribution to cancer risk and outcome and how it may modify disease susceptibility and risk in response to environmental and sociobehavioral exposures.125 Nonetheless, most current findings have only explained a fraction of risk and, unlike rare and highly penetrant Mendelian diseases, these variants will tend to be more common and less penetrant. The prevalence of genetic studies from men and women of African ancestry remain quite sparse, and the observation that haplotype blocks of African ancestry are significantly smaller than other populations is a strong indication of significant diversity in the genome of individuals of African descent.126 This observation only emphasizes the need for greater genomic profiling from more diverse populations with emphasis on how different polymorphisms or variants may be clustered within the different pathways and cellular processes described above. The existence of noncoding germline variants that could be determinants of gene expression in tumors has been understudied. Such expression quantitative trait-loci studies could have profound influences on pathways and cellular processes associated with wound healing and tissue repair that may enhance tumor invasion and metastasis.127 Finally, although we have focused on a broadened meaning of allostasis and allostatic load in this review, there is likely to be a strong role for the influence of bio-behavioral stress-related exposure in wound healing.128, 129, 130 This is revealed by studies that show that chronic stress promotes tumorigenesis and metastasis, as implicated by the observed efficacy of the use of β-blockers in patients with triple-negative breast cancer.130, 131, 132 Central to these observations is that the sympathetic nervous system has been shown to increase macrophage infiltration, EMT, angiogenesis, and tumor invasion.130 Once again, these studies and the others cited above provide examples of how behavioral–social determinants within the environment can influence biological responses in a manner that may reflect aspects of genetic differences.
Conclusion
In this perspective, we have outlined a conceptual platform and supporting observations that point to the process of wound healing and the cellular plasticity of tissue repair as a complex physiological response whose multiple differing gene-regulated pathways may contribute to racial differences in epithelial cancer outcomes. Approaches to validating this hypothesis will require a broad exploration of the genome, transcriptome, proteome, metabolome, and the exposome to better understand the interactions of the different genetic and gene-regulatory elements in the wound healing and tissue repair pathways and their specific contribution to different cancers. Important resources that will have to be developed to support this effort will require i) increasing the availability of genomic data from more diverse populations, ii) developing more racially diverse patient-derived xenograft cell lines, and iii) establishing of bio-specimen banks for donor-derived fibroblasts for use in the generation of inducible pluripotent stem cell lines.133
Footnotes
Race in Cancer Health Disparities Theme Issue
Supported by the Intramural Research Programs of the NIH, National Cancer Institute, Center for Cancer Research grant ZIA-BC010847 (J.S.B., S.P., and K.G.), and National Institute on Minority Health and Health Disparities grant ZIA-MD000005 (J.S.B., A.C., A.J., and K.G.).
Disclosures: None declared.
This article is part of a review series on understanding the complex role of race in cancer health disparities.
Current address of K.G., Columbia University Medical Center, New York, NY.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Petrick J.L., Kelly S.P., Altekruse S.F., McGlynn K.A., Rosenberg P.S. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol. 2016;34:1787–1794. doi: 10.1200/JCO.2015.64.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altekruse S.F., McGlynn K.A., Reichman M.E. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sterling P., Eyer J. Biological basis of stress-related mortality. Soc Sci Med E. 1981;15:3–42. doi: 10.1016/0271-5384(81)90061-2. [DOI] [PubMed] [Google Scholar]
- 5.McEwen B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 6.Seeman T.E., McEwen B.S., Rowe J.W., Singer B.H. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A. 2001;98:4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chrousos G.P., Gold P.W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 8.Geronimus A.T. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis. 1992;2:207–221. [PubMed] [Google Scholar]
- 9.Miller L.H., Baruch D.I., Marsh K., Doumbo O.K. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 10.Fairhurst R.M., Baruch D.I., Brittain N.J., Ostera G.R., Wallach J.S., Hoang H.L., Hayton K., Guindo A., Makobongo M.O., Schwartz O.M., Tounkara A., Doumbo O.K., Diallo D.A., Fujioka H., Ho M., Wellems T.E. Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature. 2005;435:1117–1121. doi: 10.1038/nature03631. [DOI] [PubMed] [Google Scholar]
- 11.Ruwende C., Khoo S.C., Snow R.W., Yates S.N.R., Kwiatkowski D., Gupta S., Warn P., Allsopp C.E.M., Gilbert S.C., Peschu N., Newbold C.I., Greenwood B.M., Marsh K., Hill A.V.S. Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature. 1995;376:246–249. doi: 10.1038/376246a0. [DOI] [PubMed] [Google Scholar]
- 12.Kwiatkowski D.P. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet. 2005;77:171–192. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Power M.L., Schulkin J. Maternal obesity, metabolic disease, and allostatic load. Physiol Behav. 2012;106:22–28. doi: 10.1016/j.physbeh.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Power M.L., Schulkin J. Johns Hopkins University Press; Baltimore: 2009. The Evolution of Obesity. [Google Scholar]
- 15.Olabisi O.A., Zhang J.Y., VerPlank L., Zahler N., DiBartolo S., 3rd, Heneghan J.F., Schlondorff J.S., Suh J.H., Yan P., Alper S.L., Friedman D.J., Pollak M.R. APOL1 kidney disease risk variants cause cytotoxicity by depleting cellular potassium and inducing stress-activated protein kinases. Proc Natl Acad Sci U S A. 2016;113:830–837. doi: 10.1073/pnas.1522913113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosset S., Tzur S., Behar D.M., Wasser W.G., Skorecki K. The population genetics of chronic kidney disease: insights from the MYH9-APOL1 locus. Nat Rev Nephrol. 2011;7:313–326. doi: 10.1038/nrneph.2011.52. [DOI] [PubMed] [Google Scholar]
- 17.Beckerman P., Bi-Karchin J., Park A.S., Qiu C., Dummer P.D., Soomro I., Boustany-Kari C.M., Pullen S.S., Miner J.H., Hu C.A., Rohacs T., Inoue K., Ishibe S., Saleem M.A., Palmer M.B., Cuervo A.M., Kopp J.B., Susztak K. Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat Med. 2017;23:429–438. doi: 10.1038/nm.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Relman A.S. Race and end-stage renal disease. N Engl J Med. 1982;306:1290–1291. doi: 10.1056/NEJM198205273062109. [DOI] [PubMed] [Google Scholar]
- 19.Kopp J.B., Smith M.W., Nelson G.W., Johnson R.C., Freedman B.I., Bowden D.W., Oleksyk T., McKenzie L.M., Kajiyama H., Ahuja T.S., Berns J.S., Briggs W., Cho M.E., Dart R.A., Kimmel P.L., Korbet S.M., Michel D.M., Mokrzycki M.H., Schelling J.R., Simon E., Trachtman H., Vlahov D., Winkler C.A. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsa A., Kao W.H., Xie D., Astor B.C., Li M., Hsu C.Y., Feldman H.I., Parekh R.S., Kusek J.W., Greene T.H., Fink J.C., Anderson A.H., Choi M.J., Wright J.T., Jr., Lash J.P., Freedman B.I., Ojo A., Winkler C.A., Raj D.S., Kopp J.B., He J., Jensvold N.G., Tao K., Lipkowitz M.S., Appel L.J., AASK Study Investigators. CRIC Study Investigators APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan M., Ma J., Keaton J.M., Dimitrov L., Mudgal P., Stromberg M., Bonomo J.A., Hicks P.J., Freedman B.I., Bowden D.W., Ng M.C. Association of kidney structure-related gene variants with type 2 diabetes-attributed end-stage kidney disease in African Americans. Hum Genet. 2016;135:1251–1262. doi: 10.1007/s00439-016-1714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virchow R. A. Hirschwald; Berlin: 1858. Die cellularpathologie in ihrer begründung auf physiologische und pathologische gewebelehre. [PubMed] [Google Scholar]
- 23.Akaiwa H. A quantitative study of wound healing in the rat: I. cell movements and cell layers during wound healing. J Med Res. 1919;40:311–351. [PMC free article] [PubMed] [Google Scholar]
- 24.Loeb L. A comparative study of the mechanism of wound healing. J Med Res. 1920;41:247–281. [PMC free article] [PubMed] [Google Scholar]
- 25.Alter N.M. Mechanical irritation as etiologic factor of cancer: clinical observation. Am J Pathol. 1925;1:511–518.3. [PMC free article] [PubMed] [Google Scholar]
- 26.Byun J.S., Gardner K. Wounds that will not heal: pervasive cellular reprogramming in cancer. Am J Pathol. 2013;182:1055–1064. doi: 10.1016/j.ajpath.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haddow A. Molecular repair, wound healing, and carcinogenesis: tumor production a possible overhealing? Adv Cancer Res. 1972;16:181–234. doi: 10.1016/s0065-230x(08)60341-3. [DOI] [PubMed] [Google Scholar]
- 28.Dvorak H.F. Tumors: wounds that do not heal-redux. Cancer Immunol Res. 2015;3:1–11. doi: 10.1158/2326-6066.CIR-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dvorak H.F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 30.Velez Edwards D.R., Tsosie K.S., Williams S.M., Edwards T.L., Russell S.B. Admixture mapping identifies a locus at 15q21.2-22.3 associated with keloid formation in African Americans. Hum Genet. 2014;133:1513–1523. doi: 10.1007/s00439-014-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jumper N., Paus R., Bayat A. Functional histopathology of keloid disease. Histol Histopathol. 2015;30:1033–1057. doi: 10.14670/HH-11-624. [DOI] [PubMed] [Google Scholar]
- 32.Mari W., Alsabri S.G., Tabal N., Younes S., Sherif A., Simman R. Novel insights on understanding of keloid scar: article review. J Am Coll Clin Wound Spec. 2015;7:1–7. doi: 10.1016/j.jccw.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He Y., Deng Z., Alghamdi M., Lu L., Fear M.W., He L. From genetics to epigenetics: new insights into keloid scarring. Cell Prolif. 2017;50 doi: 10.1111/cpr.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catherino W.H., Eltoukhi H.M., Al-Hendy A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin Reprod Med. 2013;31:370–379. doi: 10.1055/s-0033-1348896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marshall L.M., Spiegelman D., Barbieri R.L., Goldman M.B., Manson J.E., Colditz G.A., Willett W.C., Hunter D.J. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967–973. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 36.Keeling B.H., Taylor B.R. Keloids and non-diabetic kidney disease: similarities and the APOL1-MYH9 haplotype as a possible genetic link. Med Hypotheses. 2013;81:908–910. doi: 10.1016/j.mehy.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Iannuzzi M.C., Iyengar S.K., Gray-McGuire C., Elston R.C., Baughman R.P., Donohue J.F., Hirst K., Judson M.A., Kavuru M.S., Maliarik M.J., Moller D.R., Newman L.S., Rabin D.L., Rose C.S., Rossman M.D., Teirstein A.S., Rybicki B.A. Genome-wide search for sarcoidosis susceptibility genes in African Americans. Genes Immun. 2005;6:509–518. doi: 10.1038/sj.gene.6364235. [DOI] [PubMed] [Google Scholar]
- 38.Mirsaeidi M., Machado R.F., Schraufnagel D., Sweiss N.J., Baughman R.P. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438–449. doi: 10.1378/chest.14-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rybicki B.A., Major M., Popovich J., Jr., Maliarik M.J., Iannuzzi M.C. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145:234–241. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 40.Sartwell P.E. Racial differences in sarcoidosis. Ann N Y Acad Sci. 1976;278:368–370. doi: 10.1111/j.1749-6632.1976.tb47047.x. [DOI] [PubMed] [Google Scholar]
- 41.Westney G.E., Judson M.A. Racial and ethnic disparities in sarcoidosis: from genetics to socioeconomics. Clin Chest Med. 2006;27:453–462. doi: 10.1016/j.ccm.2006.04.002. vi. [DOI] [PubMed] [Google Scholar]
- 42.Shih B., Bayat A. Genetics of keloid scarring. Arch Dermatol Res. 2010;302:319–339. doi: 10.1007/s00403-009-1014-y. [DOI] [PubMed] [Google Scholar]
- 43.Satish L., Babu M., Tran K.T., Hebda P.A., Wells A. Keloid fibroblast responsiveness to epidermal growth factor and activation of downstream intracellular signaling pathways. Wound Repair Regen. 2004;12:183–192. doi: 10.1111/j.1067-1927.2004.012111.x. [DOI] [PubMed] [Google Scholar]
- 44.Dustan H.P. Does keloid pathogenesis hold the key to understanding black/white differences in hypertension severity? Hypertension. 1995;26:858–862. doi: 10.1161/01.hyp.26.6.858. [DOI] [PubMed] [Google Scholar]
- 45.Taran F.A., Brown H.L., Stewart E.A. Racial diversity in uterine leiomyoma clinical studies. Fertil Steril. 2010;94:1500–1503. doi: 10.1016/j.fertnstert.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 46.Shaker S.A., Ayuob N.N., Hajrah N.H. Cell talk: a phenomenon observed in the keloid scar by immunohistochemical study. Appl Immunohistochem Mol Morphol. 2011;19:153–159. doi: 10.1097/PAI.0b013e3181efa2ef. [DOI] [PubMed] [Google Scholar]
- 47.Chaffer C.L., San Juan B.P., Lim E., Weinberg R.A. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35:645–654. doi: 10.1007/s10555-016-9648-7. [DOI] [PubMed] [Google Scholar]
- 48.Ye X., Tam W.L., Shibue T., Kaygusuz Y., Reinhardt F., Ng Eaton E., Weinberg R.A. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525:256–260. doi: 10.1038/nature14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheel C., Weinberg R.A. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012;22:396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tzouvelekis A., Kaminski N. Epigenetics in idiopathic pulmonary fibrosis. Biochem Cell Biol. 2015;93:159–170. doi: 10.1139/bcb-2014-0126. [DOI] [PubMed] [Google Scholar]
- 51.Mann D.A. Epigenetics in liver disease. Hepatology. 2014;60:1418–1425. doi: 10.1002/hep.27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tampe B., Zeisberg M. Contribution of genetics and epigenetics to progression of kidney fibrosis. Nephrol Dial Transplant. 2014;29 Suppl 4:iv72–iv79. doi: 10.1093/ndt/gft025. [DOI] [PubMed] [Google Scholar]
- 53.Lopez-Novoa J.M., Nieto M.A. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cowie C.C., Port F.K., Wolfe R.A., Savage P.J., Moll P.P., Hawthorne V.M. Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. N Engl J Med. 1989;321:1074–1079. doi: 10.1056/NEJM198910193211603. [DOI] [PubMed] [Google Scholar]
- 55.Cavazza A., Harari S., Caminati A., Barbareschi M., Carbonelli C., Spaggiari L., Paci M., Rossi G. The histology of pulmonary sarcoidosis: a review with particular emphasis on unusual and underrecognized features. Int J Surg Pathol. 2009;17:219–230. doi: 10.1177/1066896909333748. [DOI] [PubMed] [Google Scholar]
- 56.Langley R.R., Fidler I.J. The seed and soil hypothesis revisited–the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011;128:2527–2535. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hinz B. Myofibroblasts. Exp Eye Res. 2016;142:56–70. doi: 10.1016/j.exer.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 58.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 59.Nwosu Z.C., Alborzinia H., Wolfl S., Dooley S., Liu Y. Evolving insights on metabolism, autophagy, and epigenetics in liver myofibroblasts. Front Physiol. 2016;7:191. doi: 10.3389/fphys.2016.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kisseleva T. The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology. 2017;65:1039–1043. doi: 10.1002/hep.28948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Condeelis J., Pollard J.W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 62.Mantovani A., Biswas S.K., Galdiero M.R., Sica A., Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 63.Das A., Sinha M., Datta S., Abas M., Chaffee S., Sen C.K., Roy S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185:2596–2606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Novak M.L., Koh T.J. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol. 2013;183:1352–1363. doi: 10.1016/j.ajpath.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sica A., Invernizzi P., Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034–2042. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- 66.Krenkel O., Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306–321. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 67.Dovas A., Patsialou A., Harney A.S., Condeelis J., Cox D. Imaging interactions between macrophages and tumour cells that are involved in metastasis in vivo and in vitro. J Microsc. 2013;251:261–269. doi: 10.1111/j.1365-2818.2012.03667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chawla A., Nguyen K.D., Goh Y.P. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aad G., Abajyan T., Abbott B., Abdallah J., Abdel Khalek S., Abdelalim A.A. Measurement of Z boson production in Pb-Pb collisions at sqrt[s(NN)]=2.76 TeV with the ATLAS detector. Phys Rev Lett. 2013;110:022301. doi: 10.1103/PhysRevLett.110.022301. [DOI] [PubMed] [Google Scholar]
- 70.Odegaard J.I., Ricardo-Gonzalez R.R., Goforth M.H., Morel C.R., Subramanian V., Mukundan L., Red Eagle A., Vats D., Brombacher F., Ferrante A.W., Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freytes D.O., Kang J.W., Marcos-Campos I., Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114:220–229. doi: 10.1002/jcb.24357. [DOI] [PubMed] [Google Scholar]
- 72.Anton K., Banerjee D., Glod J. Macrophage-associated mesenchymal stem cells assume an activated, migratory, pro-inflammatory phenotype with increased IL-6 and CXCL10 secretion. PLoS One. 2012;7:e35036. doi: 10.1371/journal.pone.0035036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mishra P.J., Glod J.W., Banerjee D. Mesenchymal stem cells: flip side of the coin. Cancer Res. 2009;69:1255–1258. doi: 10.1158/0008-5472.CAN-08-3562. [DOI] [PubMed] [Google Scholar]
- 74.Spaeth E.L., Labaff A.M., Toole B.P., Klopp A., Andreeff M., Marini F.C. Mesenchymal CD44 expression contributes to the acquisition of an activated fibroblast phenotype via TWIST activation in the tumor microenvironment. Cancer Res. 2013;73:5347–5359. doi: 10.1158/0008-5472.CAN-13-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trivanovic D., Krstic J., Djordjevic I.O., Mojsilovic S., Santibanez J.F., Bugarski D., Jaukovic A. The roles of mesenchymal stromal/stem cells in tumor microenvironment associated with inflammation. Mediators Inflamm. 2016;2016:7314016. doi: 10.1155/2016/7314016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hinz B. The role of myofibroblasts in wound healing. Curr Res Transl Med. 2016;64:171–177. doi: 10.1016/j.retram.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 77.Andreoli S.P. Racial and ethnic differences in the incidence and progression of focal segmental glomerulosclerosis in children. Adv Ren Replace Ther. 2004;11:105–109. doi: 10.1053/j.arrt.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 78.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pradella D., Naro C., Sette C., Ghigna C. EMT and stemness: flexible processes tuned by alternative splicing in development and cancer progression. Mol Cancer. 2017;16:8. doi: 10.1186/s12943-016-0579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Polyak K., Weinberg R.A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 81.Tam W.L., Weinberg R.A. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013;19:1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo Q., Minnier J., Burchard J., Chiotti K., Spellman P., Schedin P. Physiologically activated mammary fibroblasts promote postpartum mammary cancer. JCI Insight. 2017;2:e89206. doi: 10.1172/jci.insight.89206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo Q., Betts C., Pennock N., Mitchell E., Schedin P. Mammary gland involution provides a unique model to study the TGF-beta cancer paradox. J Clin Med. 2017;6:E10. doi: 10.3390/jcm6010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Faupel-Badger J.M., Arcaro K.F., Balkam J.J., Eliassen A.H., Hassiotou F., Lebrilla C.B., Michels K.B., Palmer J.R., Schedin P., Stuebe A.M., Watson C.J., Sherman M.E. Postpartum remodeling, lactation, and breast cancer risk: summary of a National Cancer Institute-sponsored workshop. J Natl Cancer Inst. 2013;105:166–174. doi: 10.1093/jnci/djs505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schedin P., Borges V. Breaking down barriers: the importance of the stromal microenvironment in acquiring invasiveness in young women's breast cancer. Breast Cancer Res. 2009;11:102. doi: 10.1186/bcr2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O'Brien J., Schedin P. Macrophages in breast cancer: do involution macrophages account for the poor prognosis of pregnancy-associated breast cancer? J Mammary Gland Biol Neoplasia. 2009;14:145–157. doi: 10.1007/s10911-009-9118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schedin P., O'Brien J., Rudolph M., Stein T., Borges V. Microenvironment of the involuting mammary gland mediates mammary cancer progression. J Mammary Gland Biol Neoplasia. 2007;12:71–82. doi: 10.1007/s10911-007-9039-3. [DOI] [PubMed] [Google Scholar]
- 89.Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6:281–291. doi: 10.1038/nrc1839. [DOI] [PubMed] [Google Scholar]
- 90.Palmer J.R., Viscidi E., Troester M.A., Hong C.C., Schedin P., Bethea T.N., Bandera E.V., Borges V., McKinnon C., Haiman C.A., Lunetta K., Kolonel L.N., Rosenberg L., Olshan A.F., Ambrosone C.B. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER Consortium. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lyons T.R., O'Brien J., Borges V.F., Conklin M.W., Keely P.J., Eliceiri K.W., Marusyk A., Tan A.C., Schedin P. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011;17:1109–1115. doi: 10.1038/nm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bertrand K.A., Bethea T.N., Adams-Campbell L.L., Rosenberg L., Palmer J.R. Differential patterns of risk factors for early-onset breast cancer by ER status in African American women. Cancer Epidemiol Biomarkers Prev. 2017;26:270–277. doi: 10.1158/1055-9965.EPI-16-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Millikan R.C., Newman B., Tse C.K., Moorman P.G., Conway K., Dressler L.G., Smith L.V., Labbok M.H., Geradts J., Bensen J.T., Jackson S., Nyante S., Livasy C., Carey L., Earp H.S., Perou C.M. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martin D.N., Boersma B.J., Yi M., Reimers M., Howe T.M., Yfantis H.G., Tsai Y.C., Williams E.H., Lee D.H., Stephens R.M., Weissman A.M., Ambs S. Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One. 2009;4:e4531. doi: 10.1371/journal.pone.0004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iyengar N.M., Brown K.A., Zhou X.K., Gucalp A., Subbaramaiah K., Giri D.D., Zahid H., Bhardwaj P., Wendel N.K., Falcone D.J., Wang H., Williams S., Pollak M., Morrow M., Hudis C.A., Dannenberg A.J. Metabolic obesity, adipose inflammation and elevated breast aromatase in women with normal body mass index. Cancer Prev Res (Phila) 2017;10:235–243. doi: 10.1158/1940-6207.CAPR-16-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iyengar N.M., Zhou X.K., Gucalp A., Morris P.G., Howe L.R., Giri D.D., Morrow M., Wang H., Pollak M., Jones L.W., Hudis C.A., Dannenberg A.J. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin Cancer Res. 2016;22:2283–2289. doi: 10.1158/1078-0432.CCR-15-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koru-Sengul T., Santander A.M., Miao F., Sanchez L.G., Jorda M., Gluck S., Ince T.A., Nadji M., Chen Z., Penichet M.L., Cleary M.P., Torroella-Kouri M. Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians. Breast Cancer Res Treat. 2016;158:113–126. doi: 10.1007/s10549-016-3847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karan D., Holzbeierlein J., Thrasher J.B. Macrophage inhibitory cytokine-1: possible bridge molecule of inflammation and prostate cancer. Cancer Res. 2009;69:2–5. doi: 10.1158/0008-5472.CAN-08-1230. [DOI] [PubMed] [Google Scholar]
- 99.Nakazawa M., Kyprianou N. Epithelial-mesenchymal-transition regulators in prostate cancer: androgens and beyond. J Steroid Biochem Mol Biol. 2017;166:84–90. doi: 10.1016/j.jsbmb.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 100.Slattery M.L., Lundgreen A., Stern M.C., Hines L., Wolff R.K., Giuliano A.R., Baumgartner K.B., John E.M. The influence of genetic ancestry and ethnicity on breast cancer survival associated with genetic variation in the TGF-beta-signaling pathway: the Breast Cancer Health Disparities Study. Cancer Causes Control. 2014;25:293–307. doi: 10.1007/s10552-013-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shany S., Sigal-Batikoff I., Lamprecht S. Vitamin D and myofibroblasts in fibrosis and cancer: at cross-purposes with TGF-beta/SMAD signaling. Anticancer Res. 2016;36:6225–6234. doi: 10.21873/anticanres.11216. [DOI] [PubMed] [Google Scholar]
- 102.Feldman D., Krishnan A.V., Swami S., Giovannucci E., Feldman B.J. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 103.Berger U., McClelland R.A., Wilson P., Greene G.L., Haussler M.R., Pike J.W., Colston K., Easton D., Coombes R.C. Immunocytochemical determination of estrogen receptor, progesterone receptor, and 1,25-dihydroxyvitamin D3 receptor in breast cancer and relationship to prognosis. Cancer Res. 1991;51:239–244. [PubMed] [Google Scholar]
- 104.Kupfer S.S., Skol A.D., Hong E., Ludvik A., Kittles R.A., Keku T.O., Sandler R.S., Ellis N.A. Shared and independent colorectal cancer risk alleles in TGFbeta-related genes in African and European Americans. Carcinogenesis. 2014;35:2025–2030. doi: 10.1093/carcin/bgu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pibiri F., Kittles R.A., Sandler R.S., Keku T.O., Kupfer S.S., Xicola R.M., Llor X., Ellis N.A. Genetic variation in vitamin D-related genes and risk of colorectal cancer in African Americans. Cancer Causes Control. 2014;25:561–570. doi: 10.1007/s10552-014-0361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ng K., Meyerhardt J.A., Chan A.T., Sato K., Chan J.A., Niedzwiecki D., Saltz L.B., Mayer R.J., Benson A.B., 3rd, Schaefer P.L., Whittom R., Hantel A., Goldberg R.M., Venook A.P., Ogino S., Giovannucci E.L., Fuchs C.S. Aspirin and COX-2 inhibitor use in patients with stage III colon cancer. J Natl Cancer Inst. 2015;107:345. doi: 10.1093/jnci/dju345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koliaraki V., Pallangyo C.K., Greten F.R., Kollias G. Mesenchymal cells in colon cancer. Gastroenterology. 2017;152:964–979. doi: 10.1053/j.gastro.2016.11.049. [DOI] [PubMed] [Google Scholar]
- 108.Goyal S., Nangia-Makker P., Farhana L., Yu Y., Majumdar A.P. Racial disparity in colorectal cancer: gut microbiome and cancer stem cells. World J Stem Cells. 2016;8:279–287. doi: 10.4252/wjsc.v8.i9.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Farhana L., Antaki F., Anees M.R., Nangia-Makker P., Judd S., Hadden T., Levi E., Murshed F., Yu Y., Van Buren E., Ahmed K., Dyson G., Majumdar A.P. Role of cancer stem cells in racial disparity in colorectal cancer. Cancer Med. 2016;5:1268–1278. doi: 10.1002/cam4.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wahab S.M.R., Islam F., Gopalan V., Lam A.K. The identifications and clinical implications of cancer stem cells in colorectal cancer. Clin Colorectal Cancer. 2017;16:93–102. doi: 10.1016/j.clcc.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 111.Mamoori A., Gopalan V., Smith R.A., Lam A.K. Modulatory roles of microRNAs in the regulation of different signalling pathways in large bowel cancer stem cells. Biol Cell. 2016;108:51–64. doi: 10.1111/boc.201500062. [DOI] [PubMed] [Google Scholar]
- 112.Martin D.N., Starks A.M., Ambs S. Biological determinants of health disparities in prostate cancer. Curr Opin Oncol. 2013;25:235–241. doi: 10.1097/CCO.0b013e32835eb5d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smith C.J., Dorsey T.H., Tang W., Jordan S.V., Loffredo C.A., Ambs S. Aspirin use reduces the risk of aggressive prostate cancer and disease recurrence in African-American men. Cancer Epidemiol Biomarkers Prev. 2017;26:845–853. doi: 10.1158/1055-9965.EPI-16-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Prueitt R.L., Wallace T.A., Glynn S.A., Yi M., Tang W., Luo J., Dorsey T.H., Stagliano K.E., Gillespie J.W., Hudson R.S., Terunuma A., Shoe J.L., Haines D.C., Yfantis H.G., Han M., Martin D.N., Jordan S.V., Borin J.F., Naslund M.J., Alexander R.B., Stephens R.M., Loffredo C.A., Lee D.H., Putluri N., Sreekumar A., Hurwitz A.A., Ambs S. An immune-inflammation gene expression signature in prostate tumors of smokers. Cancer Res. 2016;76:1055–1065. doi: 10.1158/0008-5472.CAN-14-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Montanari M., Rossetti S., Cavaliere C., D'Aniello C., Malzone M.G., Vanacore D., Di Franco R., La Mantia E., Iovane G., Piscitelli R., Muscariello R., Berretta M., Perdona S., Muto P., Botti G., Bianchi A.A.M., Veneziani B.M., Facchini G. Epithelial-mesenchymal transition in prostate cancer: an overview. Oncotarget. 2017;8:35376–35389. doi: 10.18632/oncotarget.15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sekhon K., Bucay N., Majid S., Dahiya R., Saini S. MicroRNAs and epithelial-mesenchymal transition in prostate cancer. Oncotarget. 2016;7:67597–67611. doi: 10.18632/oncotarget.11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li P., Yang R., Gao W.Q. Contributions of epithelial-mesenchymal transition and cancer stem cells to the development of castration resistance of prostate cancer. Mol Cancer. 2014;13:55. doi: 10.1186/1476-4598-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pocha C., Kolly P., Dufour J.F. Nonalcoholic fatty liver disease-related hepatocellular carcinoma: a problem of growing magnitude. Semin Liver Dis. 2015;35:304–317. doi: 10.1055/s-0035-1562949. [DOI] [PubMed] [Google Scholar]
- 119.Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300–1312. doi: 10.1016/j.jhep.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 120.Perumpail R.B., Wong R.J., Ahmed A., Harrison S.A. Hepatocellular carcinoma in the setting of non-cirrhotic nonalcoholic fatty liver disease and the metabolic syndrome: US experience. Dig Dis Sci. 2015;60:3142–3148. doi: 10.1007/s10620-015-3821-7. [DOI] [PubMed] [Google Scholar]
- 121.Rombouts K., Marra F. Molecular mechanisms of hepatic fibrosis in non-alcoholic steatohepatitis. Dig Dis. 2010;28:229–235. doi: 10.1159/000282094. [DOI] [PubMed] [Google Scholar]
- 122.Haerian M.S., Baum L., Haerian B.S. Association of 8q24.21 loci with the risk of colorectal cancer: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2011;26:1475–1484. doi: 10.1111/j.1440-1746.2011.06831.x. [DOI] [PubMed] [Google Scholar]
- 123.Salinas C.A., Kwon E., Carlson C.S., Koopmeiners J.S., Feng Z., Karyadi D.M., Ostrander E.A., Stanford J.L. Multiple independent genetic variants in the 8q24 region are associated with prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:1203–1213. doi: 10.1158/1055-9965.EPI-07-2811. [DOI] [PubMed] [Google Scholar]
- 124.Kessler M.D., Yerges-Armstrong L., Taub M.A., Shetty A.C., Maloney K., Jeng L.J., Ruczinski I., Levin A.M., Williams L.K., Beaty T.H., Mathias R.A., Barnes K.C., Consortium on Asthma among African-ancestry Populations in the Americas (CAAPA) O'Connor T.D. Challenges and disparities in the application of personalized genomic medicine to populations with African ancestry. Nat Commun. 2016;7:12521. doi: 10.1038/ncomms12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McCarthy M.I., Abecasis G.R., Cardon L.R., Goldstein D.B., Little J., Ioannidis J.P., Hirschhorn J.N. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet. 2008;9:356–369. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- 126.Gabriel S.B., Schaffner S.F., Nguyen H., Moore J.M., Roy J., Blumenstiel B., Higgins J., DeFelice M., Lochner A., Faggart M., Liu-Cordero S.N., Rotimi C., Adeyemo A., Cooper R., Ward R., Lander E.S., Daly M.J., Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 127.Li Q., Seo J.H., Stranger B., McKenna A., Pe'er I., Laframboise T., Brown M., Tyekucheva S., Freedman M.L. Integrative eQTL-based analyses reveal the biology of breast cancer risk loci. Cell. 2013;152:633–641. doi: 10.1016/j.cell.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Antoni M.H., Lutgendorf S.K., Cole S.W., Dhabhar F.S., Sephton S.E., McDonald P.G., Stefanek M., Sood A.K. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chida Y., Hamer M., Wardle J., Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 130.Cole S.W., Nagaraja A.S., Lutgendorf S.K., Green P.A., Sood A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15:563–572. doi: 10.1038/nrc3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sloan E.K., Priceman S.J., Cox B.F., Yu S., Pimentel M.A., Tangkanangnukul V., Arevalo J.M., Morizono K., Karanikolas B.D., Wu L., Sood A.K., Cole S.W. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Melhem-Bertrandt A., Chavez-Macgregor M., Lei X., Brown E.N., Lee R.T., Meric-Bernstam F., Sood A.K., Conzen S.D., Hortobagyi G.N., Gonzalez-Angulo A.M. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2645–2652. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chang E.A., Tomov M.L., Suhr S.T., Luo J., Olmstead Z.T., Paluh J.L., Cibelli J. Derivation of ethnically diverse human induced pluripotent stem cell lines. Sci Rep. 2015;5:15234. doi: 10.1038/srep15234. [DOI] [PMC free article] [PubMed] [Google Scholar]