Abstract
Functional neuroimaging evidence suggests that there are differences in the neural correlates of episodic memory for laboratory stimuli (laboratory memory) and for events from one’s own life (autobiographical memory). However, this evidence is scarce and often confounded with differences in memory testing procedures. Here, we directly compared the neural mechanisms underlying the search and recovery of autobiographical and laboratory memories while minimizing testing differences. Before scanning, participants completed a laboratory memory encoding task in which they studied four-word “chains” spread across three word pairs. During scanning, participants completed a laboratory memory retrieval task, in which they recalled the word chains, and an autobiographical memory retrieval task, in which they recalled specific personal events associated with word cues. Importantly, response times were similar in the two tasks, allowing for a direct comparison of the activation time courses. We found that during memory search (searching for the memory target), similar brain regions were activated during both the autobiographical and laboratory tasks, whereas during memory recovery (accessing the memory traces; i.e., ecphory), clear differences emerged: regions of the default mode network (DMN) were activated greater during autobiographical than laboratory memory, whereas the bilateral superior parietal lobules were activated greater during laboratory than autobiographical memory. Also, multivariate functional connectivity analyses revealed that regardless of memory stage, the DMN and ventral attention network exhibited a more integrated topology in the functional network underlying autobiographical (vs. laboratory) memory retrieval, whereas the fronto-parietal task control network exhibited a more integrated topology in the functional network underlying laboratory (vs. autobiographical) memory retrieval. These findings further characterize the shared and distinct neural components underlying autobiographical and laboratory memories, and suggest that differences in autobiographical vs. laboratory memory brain activation previously reported in the literature reflect memory recovery rather than search differences.
Keywords: autobiographical memory, default mode network, episodic memory, functional connectivity, functional MRI, graph theory
Graphical Abstract
1.0 INTRODUCTION
Episodic memory retrieval requires the engagement of numerous processes, including elaborating the retrieval cue, searching for memories, and monitoring recovered memories according to retrieval goals (Burgess & Shallice, 1996; Conway, 2001, 2005; Conway & Pleydell-Pearce, 2000; Conway & Rubin, 1993; Gilboa, et al., 2006; Norman & Bobrow, 1979). These retrieval processes are typically studied in laboratory settings, where various parameters can be controlled to help reduce variability associated with the encoding condition, retention interval, retrieval demand, etc. Although it is generally assumed that the basic mechanisms in operation during laboratory memory (LM) studies also support memory in more naturalistic contexts, such as autobiographical memory (AM), past research has also identified important differences, which underscore the need to explore memory function in both controlled as well as naturalistic settings.
Behavioral research on AM, using techniques like journal entry or open-ended memory cues, has found that AMs compared to LMs typically place more emphasis on the self and are more vivid. AMs also tend to be more remote than LMs, and less frequently scrutinized (Conway, 2001, 2005; Conway & Pleydell-Pearce, 2000). These differences suggest the possibility that AMs and LMs may differ in certain aspects of retrieval processes. For example, AM retrieval may be more dependent on self-referential processing, whereas LM retrieval may be more dependent on control and monitoring processes (Cabeza & St Jacques, 2007; Conway, 2005; Gilboa, 2004; Moscovitch & Winocur, 2002).
Consistent with these differences, reviews of the functional neuroimaging literature have noted that during AM vs. LM retrieval, certain regions are more frequently activated (Cabeza & St Jacques, 2007; Gilboa, 2004; Svoboda, McKinnon, & Levine, 2006), perhaps reflecting the recruitment of processes more specific to the memory retrieval type. For example, compared to LM studies, AM studies more often show greater activation in regions associated with (a) self-referential processing, such as the medial prefrontal cortex (mPFC), (b) visuo-spatial processing, such as visual cortex and the posterior midline cortex (PMC: retrosplenial and posterior cingulate cortices), (c) recollection and retrieval confidence, such as the angular gyrus (AG), and (d) vividness and recollection, such as the hippocampus (Cabeza, et al., 2004; Daselaar, et al., 2008; Gilboa, Winocur, Grady, Hevenor, & Moscovitch, 2004; Greenberg, et al., 2005; Holland, Addis, & Kensinger, 2011; Lin, Horner, & Burgess, 2016; Nyberg, et al., 1995; St. Jacques, Rubin, LaBar, & Cabeza, 2008; Summerfield, Hassabis, & Maguire, 2009). Consistent with the behavioral reports that AMs are more vivid and self-oriented, most of the regions differentially recruited by AM are part of the default mode network (DMN; Buckner, Andrews-Hanna, & Schacter, 2008; Laird, et al., 2011), which has been linked to a range of internally oriented cognitive processes, such as mind-wandering, mental imagery, scene construction, and future thinking (Buckner, et al., 2008; Dixon, Fox, & Christoff, 2014; Hebscher, Levine, & Gilboa, in press; Smallwood & Schooler, 2015). Indeed, a quantitative meta-analysis of AM and LM functional neuroimaging studies found that activations in the DMN were more frequent in AM than LM studies (McDermott, Szpunar, & Christ, 2009). Conversely, consistent with the behavioral reports that LMs are more dependent on control and monitoring processes, activations in regions associated with cognitive control and “retrieval effort,” such as the lateral PFC (LatPFC) and superior parietal lobule (SPL), were more frequent in LM studies.
Although qualitative and quantitative reviews of the functional neuroimaging literature suggest differences between the regions supporting AM and LM, these reviews were mostly based on separate AM and LM studies. Only a handful of functional neuroimaging studies have directly compared AM and LM within-subjects (Cabeza, et al., 2004; Chen, Gilmore, Nelson, & McDermott, 2017; Fink, et al., 1996; Nyberg, Forkstam, Petersson, Cabeza, & Ingvar, 2002; Summerfield, et al., 2009). Consistent with the conclusions of the reviews, these direct-contrast studies found that the MTL, medial PFC, PMC, and visual cortex showed greater activity for AMs than LMs (Cabeza, et al., 2004; Chen, et al., 2017; Fink, et al., 1996; Nyberg, et al., 2002; Summerfield, et al., 2009), whereas LatPFC and SPL showed the converse pattern (Chen, et al., 2017; Fink, et al., 1996; Nyberg, et al., 2002).
However, previous functional neuroimaging studies directly comparing AM and LM retrieval are limited for a couple of reasons. First, they did not attempt to differentiate between activity associated with different retrieval stages. Although various terms have been used (Cabeza, 2008; Cabeza, et al., 2011; Cabeza & St Jacques, 2007; Ciaramelli, Grady, Levine, Ween, & Moscovitch, 2010; Nyberg, et al., 1995), here, we refer to the sequential phases as (a) search (searching for the memory target), (b) recovery (accessing the memory traces; i.e., ecphory), and (c) elaboration (maintaining recovered memories in working memory while adding additional details). Not differentiating between these stages makes differences between AM and LM activations difficult to interpret. For example, during both AM (Addis, Wong, & Schacter, 2007; Daselaar, et al., 2008; St. Jacques, Rubin, & Cabeza, 2012) and LM (Cabeza, et al., 2011; Ciaramelli, et al., 2010; Polyn, Natu, Cohen, & Norman, 2005), different regions are activated at different retrieval stages. Thus, it is possible that that greater engagement of DMN regions during AM (e.g., Cabeza, et al., 2004; Chen, et al., 2017; Summerfield, et al., 2009) and LatPFC and SPL during LM (e.g., Chen, et al., 2017; Fink, et al., 1996; Nyberg, et al., 2002) occur at different stages of retrieval. To investigate this issue, in the current study we compared AM and LM activity during memory search vs. recovery (we did not investigate elaboration).
Another general limitation of previous AM-LM studies is that they mostly focused on the role of individual regions (i.e., they examined univariate activation) and did not examine during AM vs. LM retrieval functional interactions between regions (i.e., functional connectivity). However, a previous AM study demonstrated that DMN-related regions (e.g., hippocampus, mPFC) are more functionally connected during AM than semantic memory (Greenberg, et al., 2005) and others have shown increased DMN functionally connectivity during AM retrieval (McCormick, St-Laurent, Ty, Valiante, & McAndrews, 2015; Muscatell, Addis, & Kensinger, 2010), but these studies only examined bivariate functional connectivity (i.e., seed-to-voxel or seed-to-seed connectivity). Although these bivariate functional connectivity analyses provide vital information, they do not investigate the topology of subnetworks (e.g., the DMN) within the context of whole-brain networks. Here, we used graph theory-based, multivariate functional connectivity analyses (Bullmore & Sporns, 2009; van den Heuvel & Sporns, 2013) to characterize connectivity patterns in the context of whole-brain networks. The investigation of the functional network topology underlying AM vs. LM may provide further insight into mechanisms underlying these two types of memory.
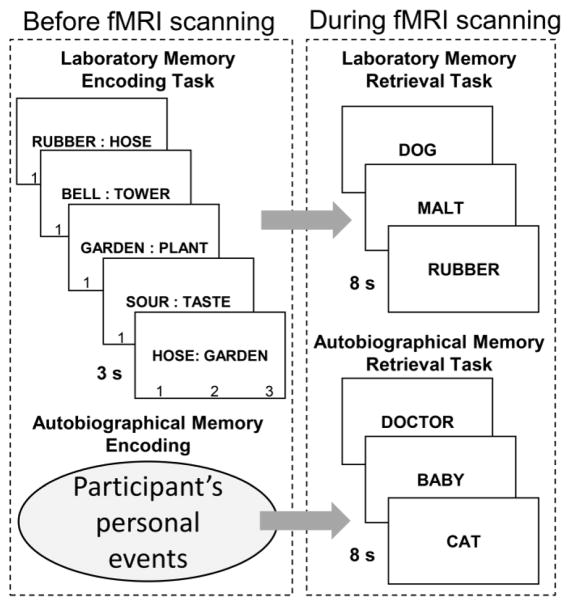
To address these limitations, we directly compared AM and LM in the same participants using tasks that were closely matched (Figure 1). Before scanning, participants completed a LM encoding task, in which participants encoded four-word “chains” spread across three semantically-related word pairs (e.g., dog-cat, cat-tiger, tiger-stripe). For AM, the encoding phase was, of course, participants’ personal experiences before the experiment. During scanning, they performed AM and LM retrieval tasks. The AM task was a cue-word Galton-Crovitz test (Crovitz & Schiffman, 1974). In each trial, a word cue was presented (e.g., baby) and participants recalled an AM of an associated unique, personal event, pressing a key when they recalled it. The LM task was a word cue-recall task. In each trial, the first word of a studied four-word chain (e.g., dog) was presented, and participants covertly recalled the other three words in the chain (e.g., dog→cat→tiger→stripe), pressing a key when they recalled the last word. We used 4-word chains instead of the typical word-pairs to lengthen the memory search time in the LM task, so that average RTs of LM target recovery matched those of the AM task (~4 s). Thus, the demands and RTs of the two tasks were very similar: in both tasks participants read a word, searched their memory for a target memory, and pressed a key upon memory recovery at about the same time.
Fig 1.
Laboratory and autobiographical memory tasks. During the LM encoding task, participants studied 60 semantically related word pairs (e.g., rubber-hose; hose-garden; garden-plant). Groups of three word pairs formed four-word “chains” (e.g., rubber-hose-garden-plant). Each word pair was presented for 3 s while participants rated the relatedness between the words (1 = medium, 2 = strong, 3 = very strong). During the LM task, participants were presented with the first word of a chain (e.g., rubber) and covertly recalled the three overlapping pairs sequentially (e.g., rubber→hose→garden→plant), pressing a button when they recalled the last word in the chain (e.g., plant). During the AM task, participants completed a Galton-Crovitz (Crovitz & Schiffman, 1974; Galton, 1879) word cue paradigm, in which participants were presented a word (e.g., dog) and covertly recalled a specific AM, pressing a button when they recalled the memory. AM = autobiographical memory; LM = laboratory memory.
The current study had two main aims. The first aim was to identify similarities and differences between AM and LM in the search-recovery dimension. Based on literature reviews/meta-analyses (Cabeza & St Jacques, 2007; Gilboa, 2004; McDermott, et al., 2009; Svoboda, et al., 2006) and the few studies with direct contrasts (Cabeza, et al., 2004; Chen, et al., 2017; Fink, et al., 1996; Nyberg, et al., 2002; Summerfield, et al., 2009), we predicted that DMN regions, including mPFC, PMC, HC/PHC and AG, would be activated greater during AM than LM, whereas LatPFC and SPL would be activated during both AM and LM, but even more so during LM. Unlike previous direct-contrast studies, we predicted that these differences would be reflected during recovery rather than search, as brain activation closer to recovery within other memory paradigms has previously been shown to be sensitive to task-specific demands/content (Polyn, et al., 2005). Also, greater fronto-parietal activity for LM than AM is likely to reflect greater monitoring in LM tasks, which typically have correct/incorrect answers, than in AM tasks, which typically do not test the accuracy of responses (Cabeza & St Jacques, 2007).
The second aim was to investigate the topology of the functional networks underlying AM and LM using graph theory-based, multivariate functional connectivity analyses (Bullmore & Sporns, 2009; van den Heuvel & Sporns, 2013). In particular, we examined the graph metric participation coefficient (Guimera & Amaral, 2005) to assess whether brain subnetworks (e.g., the DMN) during retrieval became more integrated (i.e., stronger between- / weaker within-subnetwork connections) or segregated (i.e., weaker between- / stronger within-subnetwork connections). Several recent studies have demonstrated that the degree to which a subnetwork’s topology becomes more integrated or segregated changes in response to cognitive demands of the environment (e.g., Cohen & D’Esposito, 2016; Gallen, Turner, Adnan, & D’Esposito, 2016; Geib, Stanley, Dennis, Woldorff, & Cabeza, 2017; Monge, et al., 2017). Therefore, in the current study, the investigation of the degree of subnetwork integration and segregation may provide further insight into neural mechanisms underlying AM vs. LM retrieval. Previous work has demonstrated that as memory search progresses (i.e., closer to recovery) the DMN becomes more functionally connected (Kragel & Polyn, 2015) and strongly activated (Shapira-Lichter, et al., 2012). Also, we and others have previously found that successful memory retrieval is associated with greater network integration, particularly with the hippocampus (a node of the DMN; Geib, et al., 2017; Geib, Stanley, Wing, Laurienti, & Cabeza, 2015; King, de Chastelaine, Elward, Wang, & Rugg, 2015; Schedlbauer, Copara, Watrous, & Ekstrom, 2014). Based upon these findings and the studies demonstrating greater DMN activation during AM, we predicted that the DMN would be more integrated (i.e., have higher participation coefficients) during AM than LM recovery. Also, since we predicted that fronto-parietal regions would be more important during LM than AM (e.g., Chen, et al., 2017; Fink, et al., 1996; Nyberg, et al., 2002), we also predicted that a fronto-parietal subnetwork (e.g., the fronto-parietal task control network) would more integrated during LM than AM recovery.
2.0 METHODS
2.1 Study Participants
Eighteen healthy adults (10 females) with an average age of 22.5 years (SD = 2.8 years) participated in this study. The Duke University Institutional Review Board approved all experimental procedures, and participants provided informed consent prior to testing. After study completion, participants either received class credit or were monetarily compensated for their time.
2.2 Memory Tasks
Participants were scanned while completing LM and AM tasks (Figure 1). After an LM encoding phase, participants completed 6 alternating LM and AM runs (LM-AM-LM-AM-LM-AM). Each retrieval run contained 20 trials; therefore, there were a total of 60 LM trials and 60 AM trials. Each trial was followed by a jittered interstimulus interval ranging from 2–8 s (M = 4.4 s). Participants also completed a perception task (previously reported in Cabeza et al. [2011]), which will not be discussed here.
2.2.1 Laboratory Memory Encoding/Retrieval Tasks
During LM encoding, participants studied 60 word pairs (e.g., rubber-hose; garden-plant). The word pairs were selected from free association norms (Nelson, McEvoy, & Schreiber, 2004), so that groups of three word pairs formed four-word “chains” (e.g., rubber-hose-garden-plant; dog-cat-tiger-stripe; malt-shake-rattle-snake). Each word pair was presented for 3 s while participants rated the relatedness between the words (medium, strong, very strong); participants were aware of the chains and instructed to memorize the chains. The pairs of one chain were intermixed with the pairs of other chains (e.g., rubber-hose, dog-cat, malt-shake), and each pair was presented twice; the average number of trials between word pairs belonging to the same chain was 17.8 (SD = 3.4) trials. Words were chosen such that only the presented pairs (e.g., rubber-hose) had listed association strengths (M = 0.14, SD = 0.17) in order to promote sequential recall within each chain. No normed association strength was found between noncontiguous words of the same chain (e.g., rubber-plant) or between words in different chains (e.g., rubber-tiger). In order to ensure participants understood the task, before LM encoding, participants completed a practice trial in which they rated word pairs and overtly recalled the respective word chain.
During LM retrieval, for each trial, participants were presented with the first word of a chain (e.g., rubber), which remained on the screen for 8 s. During the 8 s block, participants were asked to covertly recall the three overlapping pairs sequentially (e.g., rubber→hose→garden→plant) and press a button when they recalled the last word in the chain (e.g., plant). The use of word chains (as opposed to single words or word pairs) allowed LM to occur at a similar duration and within a similar manner as AM. Prior to scanning, participants practiced this task by overtly recalling the word pairs. After scanning, participants completed an overt version of this task to confirm successful recall of the words in each chain (see Cabeza et al. [2011] for more details on the post-scan task).
2.2.2 Autobiographical Memory Task
For the AM task, we used a Galton-Crovitz (Crovitz & Schiffman, 1974; Galton, 1879) word cue paradigm to evoke AMs; the word cues were chosen from a subset of Paivio, Yuille, and Madigan (1968)’s nouns, which we found to evoke AMs. For each trial, participants were shown a word (e.g., dog) on the screen for 8 s. Participants were instructed to covertly recall a specific AM involving the presented word and, within the 8 s time period, press a button when they recovered the memory. The words presented during the AM task and LM encoding/retrieval tasks were different, and we attempted to reduce the possibility of participants associating an AM word with a LM word by choosing AM and LM words that were not highly semantically related (i.e., low cue-to-target strength; Nelson, et al., 2004). As AMs are hypothesized to be retrieved by recalling chains of stimuli that end with a specific AM (Conway, 2001), the AM and LM retrieval tasks were well matched.
2.3 MRI Data Acquisition
A 4T GE scanner (GE Healthcare, Waukesha, WI, USA) was used to collect all functional and anatomical images. Participants wore earplugs to reduce scanner noise, and foam pads were used to reduce head motion. Scanning started with a T1-weighted sagittal localizer series (anterior [AC] and posterior [PC] commissures were identified in the midsagittal slice, and 34 contiguous oblique slices were prescribed parallel to the AC-PC plane) followed by a high-resolution T1-weighted structural image (repetition time = 450 ms, echo time = 9 ms, field of view [FOV] = 24 cm, 2562 matrix, slice thickness = 1.9 mm). The functional images were collected with an inverse spiral sequence (repetition time = 2 s, echo time = 27 ms, FOV = 24 cm, 642 image matrix, flip angle = 60 ); thirty-four contiguous slices were acquired with the same slice prescription as the anatomical images (slice thickness = 3.75 mm).
2.4 Functional MRI Analysis
2.4.1 Preprocessing
The first six functional images were discarded to allow for scanner equilibrium. All functional images were preprocessed in a standard SPM12 (London, United Kingdom; http://www.fil.ion.ucl.ac.uk/spm/) pipeline. Briefly, functional images were slice timing corrected (reference slice = first slice), realigned to the first scan in the first session, and subsequently unwarped. Following, the functional images were coregistered to the skull-stripped T1 image (skull-stripped by segmenting the T1 image and only including the gray matter, white matter, and cerebrospinal fluid segments) and subsequently normalized to MNI space (voxel size was maintained at 3.75x3.75x3.8 mm3). Lastly, the functional images were spatially smoothed using a 5 mm Gaussian kernel.
2.4.2 Search and Recovery Model
In order to explore our first aim, we modeled the functional imaging data to examine search and recovery separately for the AM and LM tasks; it should be noted that within the current study we did not examine post-recovery activation. For the search regressor, a stick function was placed at stimulus onset, and for the recovery regressor, a stick function was placed 1 s before the button press; these functions were convolved with a standard hemodynamic response function (HRF). To examine if the search and recovery regressors were collinear, for each regressor (search and recovery) for each participant, we estimated variance inflation factors. All variance inflation factors were under 4.0, which indicates that the search and recovery regressors were not collinear (Mumford, Poline, & Poldrack, 2015).
To further examine the time courses of regions identified from the whole-brain analyses, we used a finite impulse response (FIR) function (Henson, Cansino, Herron, Robb, & Rugg, 2003). We examined six 2 s time bins starting from stimulus onset (i.e., 0 s to 12 s from stimulus onset). Select significant clusters from the search and recovery model analyses were used as regions of interest (ROIs) within the FIR function analysis.
For all analyses, trials in which the participant did not respond or responded in less than 2 s were excluded from analysis; participants did not respond for more trials during the LM (M = 18.7, SD = 9.9 trials) than AM (M = 5.7, SD = 5.7 trials) task, t(17) = 6.37, p < .0001, but responded in less than 2 s for more trials during the AM (M = 6.2, SD = 8.8 trials) than LM (M = 1.8, SD = 2.9 trials) task, t(17) = 2.52, p < .05. Also, for all functional MRI general linear models, the six rigid-body motion parameters were included as nuisance variables and we implemented a high-pass temporal filter with a cutoff of 128 s.
2.4.3 Functional Network Construction/Analysis
For our second aim, we conducted graph theoretical, multivariate functional connectivity analyses (Bullmore & Sporns, 2009; van den Heuvel & Sporns, 2013). This approach allowed us to examine the functional networks underlying AM and LM search and recovery. A graph theoretical framework views the brain as a network consisting of nodes (brain regions) and edges (functional connections between brain regions). The analysis of the edges allows for the characterization of various properties of the functional network underlying a cognitive state of interest. To examine the functional networks underlying AM/LM search and recovery, we used a beta time series approach (Rissman, Gazzaley, & D’Esposito, 2004), which considers two regions to be functionally connected if the beta time series of the regions are correlated (Fornito, Yoon, Zalesky, Bullmore, & Carter, 2011; Geib, et al., 2017; Geib, et al., 2015; Monge, et al., 2017; Schedlbauer, et al., 2014). We chose to use a beta time series approach, rather than a psychophysiological-interaction approach, because beta-series connectivity has been demonstrated to be more sensitive to the variability in the shape of the hemodynamic response (Cisler, Bush, & Steele, 2014). We conducted a single trial model analysis to obtain for each trial the beta estimates. To reduce the possible influence of collinearity, the beta estimates were calculated using a method developed by Mumford, Turner, Ashby, and Poldrack (2012). This method estimates a first-level model in which one regressor models a specific trial of interest and another regressor models all the other trials (each run included a regressor modeling these other trials). To model the stages of interest for each trial (i.e., search and recovery), one stick function was placed at stimulus onset (for search) and, another was placed 1 s before the button press (for recovery); each function was convolved with a standard HRF. Each model also included the six motion regressors and six run constant regressors; we also implemented a 128 s cutoff high-pass temporal filter. This analysis yielded beta estimates for the four trial types of interest – AM search, AM recovery, LM search, and LM recovery.
For this study, to construct the AM/LM search and recovery networks, we used spherical ROIs (radius = 3 mm) and corresponding subnetwork partitions derived from Power, et al. (2011). We specifically chose nodes from four subnetwork partitions of interest – the default mode, fronto-parietal task control, dorsal attention, and ventral attention networks (see Supplementary Table 1 for a full list of ROIs). These subnetworks were chosen because we were interested in the DMN and other fronto-parietal networks; even though the combination of these four subnetworks do not cover the whole brain, here, we refer to this combination as a whole-brain network. For each ROI, for each trial type of interest, we used custom MATLAB scripts (Geib, et al., 2015) to extract the beta time series from each ROI, and correlated the beta time series of each ROI with every other ROI. This resulted in two undirected, weighted connectivity matrices for each trial type of interest (AM search network, AM recovery network, LM search network, and LM recovery network) representing the pairwise Pearson correlations between the mean beta time series of each ROI with every other ROI within the network (85 x 85 matrices). The correlation values between each ROI represent the functional connectivity strength between ROIs. Eighteen ROIs were excluded from analysis due to poor coverage in some participants (Supplementary Table 2); an additional eight ROIs were excluded from the analysis extracting the beta values derived from the univariate activation contrasts when examining the relation between univariate activation and participation coefficients due to poor coverage in some participants (Supplementary Table 3).
Within the second aim, we were specifically interested in differences in the topology of subnetworks (e.g., the DMN) contained within the AM and LM search/recovery networks. Therefore, using the Brain Connectivity Toolbox (Rubinov & Sporns, 2010; https://sites.google.com/site/bctnet/), we estimated participation coefficients (Guimera & Amaral, 2005), which reflect the relative strength of between- to within-subnetwork connections (higher participation coefficients indicate stronger between-subnetwork connections), for each node within each weighted full-brain network (AM and LM search/recovery networks). Within each full-brain network, the participation coefficients were averaged within the four subnetworks of interest (e.g., a DMN-participation coefficient within the AM recovery network). These subnetwork-averaged participation coefficients were used for analysis. For visualization purposes, the connectivity matrices corresponding to each retrieval type (AM and LM – search and recovery combined) were averaged across participants, and for each subnetwork within the averaged AM and LM networks, between-subnetwork strength was estimated. Between-subnetwork strength was only estimated for visualization purposes; statistical task differences were only estimated on participation coefficients.
2.5 Statistical Analysis
For the univariate activation analyses, to correct for multiple comparisons at p < .05, unless otherwise stated, all voxel-wise results were thresholded at p < .001 with a cluster extent threshold of 35 voxels, as determined by Monte Carlo simulations implemented in 3dClust-Sim in AFNI version 17.0 (the most updated version; Cox & Hyde, 1997). For the conjunction analysis, we used a more liberal threshold of p < .05 with a cluster extent threshold of 35 voxels, which is a common method for conjunction analyses (e.g., Bowman & Dennis, 2016; Dennis, Bowman, & Peterson, 2014) because the probability of activations passing in multiple contrasts is extremely low (for example, the p value for quadruple conjunction with each contrast at < .05 is < .00001). For the 2 (Task: AM, LM) x 2 (Stage: search, recovery) repeated measures, full factorial ANOVA (described below), the Task x Stage interaction contrast was used as an exclusive mask (thresholded at p < .10) within the main effects contrasts, as main effects are difficult to interpret in the presence of an interaction. To determine if a significant Task x Stage interaction reflects a difference during search or recovery, the interaction analyses were followed by separate paired t-tests for each stage. For the examination of task differences within each subnetwork in participation coefficients, we used a linear mixed effects model and corrected for multiple comparisons (a correction of four comparisons for each subnetwork) using a false discovery rate procedure (Benjamini & Hochberg, 1995). All analyses not conducted within SPM were conducted within Statsmodels 0.6.1 (Seabold & Perktold, 2010) ran in Python 3.4.5 (Python Software Foundation, https://www.python.org/) and SAS 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
3.0 RESULTS
3.1 Behavioral Performance
Participants exhibited similar RTs for the AM (M = 4.06, SD = 0.99 s) and LM (M = 4.13, SD = 0.53 s) tasks, t(17) = 0.32, p = .76, indicating that the tasks were matched in level of difficulty; the similar RTs allowed for a direct comparison of the AM and LM neural correlates. Regarding the LM post-scan task, LM performance during the scan and post-scan overt task were similar (percentage of same as scan performance: M = 76.9%, SE = 3.0 %), indicating that trials included in the fMRI analysis were trials in which the chain was recalled successfully.
3.2 Similarities and Differences between AM and LM Brain Activations
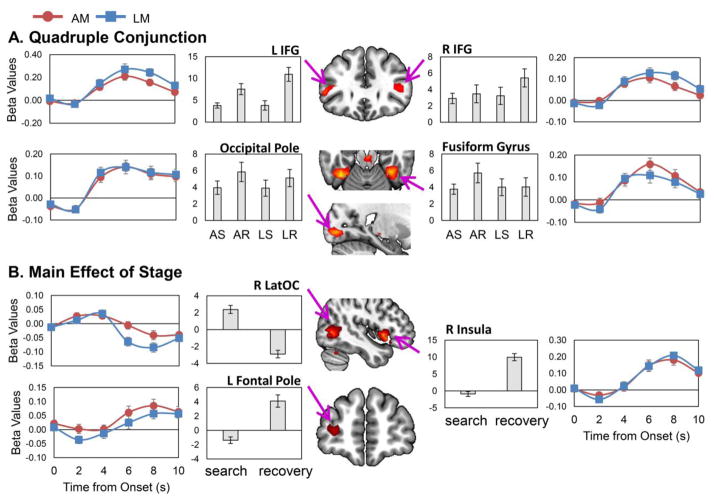
Our first aim was to identify similar and different brain activation patterns between AM and LM in the search-recovery dimension. First, we identified regions that exhibited shared activation during AM/LM search and recovery. To do this, we conducted a quadruple conjunction analysis: AM-search ∩ AM-recovery ∩ LM-search ∩ LM-recovery. We found shared activation for AM and LM in bilateral inferior frontal gyrus, bilateral inferior temporal/fusiform gyri, and right occipital pole (Figure 2-A), as well as in supplementary motor and superior lateral occipital cortices (Table 1).
Fig 2.
Similarities between autobiographical and laboratory brain activations. Panel A shows the quadruple conjunction, which shows regions that exhibited shared activation during AM/LM search and recovery: AM-search ∩ AM-recovery ∩ LM-search ∩ LM-recovery. We found that bilateral IFG, fusiform gyrus, and the occipital pole exhibited shared activation during AM/LM search and recovery. Panel B shows the main effect of stage from the 2 (Task: AM, LM) x 2 (Stage: search, recovery) full factorial ANOVA. We found that the right LatOC was activated greater during search than recovery, and left frontal pole and right insula were activated greater during recovery than search. Results are shown overlaid on a standard MNI template (neurological convention). The line graphs represent the finite impulse response beta plots and the bar graphs represent the beta values for each trial condition of interest; error bars represent the standard error of the mean. AM = autobiographical memory; LM = laboratory memory; L = left; R = right; IFG = inferior frontal gyrus; LatOC = lateral occipital cortex; AS = autobiographical search; AR = autobiographical recovery; LS = laboratory search; LR = laboratory recovery.
Table 1.
Shared Activations for Autobiographical and Laboratory Memory (Quadruple Conjunction).
| Cluster | Hem | BA | MNI Coord (mm) | t-value | Size (voxels) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
| Positive (Task > Baseline) | |||||||
| L | - | 30 | 6 | 166 | |||
| Bilateral Inferior Frontal Gyrus (IFG) | 45 | 53 | 3.24 | ||||
| R | 45 | 44 | 30 | 10 | 2.60 | 52 | |
| Bilateral Inferior Temporal/Fusiform | L | - | - | - | 419 | ||
| Gyri | 37 | 38 | 48 | 21 | 4.71 | ||
| R | 37 | 37 | −44 | −21 | 4.27 | 43 | |
| Occipital Pole | R | 18 | 22 | −86 | −2 | 4.07 | 71 |
| Supplementary Motor Cortex | M | 6 | −8 | 8 | 52 | 3.98 | 55 |
| Superior Lateral Occipital Cortex | L | 19 | −31 | −74 | 29 | 2.81 | 72 |
Note. BA = Brodmann area; Hem = Hemisphere; L = Left; M = Midline; R = Right; MNI Coord = Montreal Neurological Institute Coordinates.
We next conducted a 2 (Task: AM, LM) x 2 (Stage: search, recovery) repeated measures, full factorial ANOVA to examine (a) regions that were differentially activated during search vs. recovery; (b) regions that were differentially activated during AM vs. LM; and (c) regions that exhibited interactions between task and memory stage (Table 2).
Table 2.
Brain Activations of Task x Stage ANOVA.
| Cluster | Hem | BA | MNI Coord (mm) | F-value | Size (voxels) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
| Main Effect of Stage: Search > Recovery | |||||||
| Lateral Occipital Cortex | R | 37 | 48 | −63 | −2 | 58.03 | 132 |
| Main Effect of Stage: Recovery > Search | |||||||
| Precentral Gyrus | M | 6 | −4 | −22 | 55 | 34.84 | 44 |
| Paracingulate Gyrus | M | 32 | −1 | 27 | 33 | 93.56 | 1805 |
| Insula | R | 13 | 40 | 12 | −2 | 72.03 | |
| Cerebellum | R | 37 | −67 | −24 | 71.59 | 753 | |
| Frontal Pole | L | 10 | −31 | 57 | 6 | 27.31 | 55 |
| Postcentral Gyrus | R | 1 | 26 | −33 | 48 | 25.14 | 58 |
| Interaction: AM > LM during Recovery | |||||||
| DMN: Posterior Midline Cortex (PMC) | M | 23 | −12 | −52 | 10 | 60.40 | 379 |
| DMN: Medial PFC | M | 10 | 3 | 57 | −5 | 43.65 | 336 |
| DMN: Angular Gyrus | L | 39 | −50 | −63 | 17 | 24.18 | 35 |
| DMN: MTL – Hippocampus | R | 36 | 22 | −14 | −21 | 22.9 | 81 |
| DMN: MTL – Bilat. Post. Parahipp. G. | R | 36 | 22 | −30 | −17 | 27.43 | |
| L | 37 | −23 | −41 | −13 | 32.23 | 55 | |
| Superior Frontal Gyrus | L | 8 | −20 | 23 | 40 | 27.19 | 49 |
| Interaction: LM > AM during Recovery | |||||||
| Bilateral Superior Parietal Lobule | R | 7 | 29 | −67 | 55 | 36.25 | 154 |
| L | 7 | −34 | −56 | 55 | 24.60 | 126 | |
Note. Bilat. Post. Parahipp. G. = Bilateral Posterior Parahippocampal Gyrus; DMN = Default Mode Network; BA = Brodmann area; Hem = Hemisphere; L = Left; M = Midline; R = Right; MNI Coord = Montreal Neurological Institute Coordinates.
We first examined the main effect of stage to identify regions showing differences between search and recovery for both AM and LM (Table 2-upper panel). Given that these regions showed the same pattern for AM and LM, they are displayed below the regions identified within the quadruple conjunction (Figure 2-B). One region that exhibited a main effect of stage was the right lateral occipital cortex, which showed (during both AM and LM) greater activity during search than recovery. In the reverse direction, several regions, including the frontal pole and insula (Figure 2-B), were activated greater during recovery than search.
Then, we turned to the main effect of task in order to investigate brain regions that were differentially activated during AM and LM regardless of stage. To ensure that these differences were truly stage-independent, we masked out all voxels showing a Task x Stage interaction at a lenient threshold (see Statistical Analyses in Methods). We did not find any regions that exhibited a main effect of task.
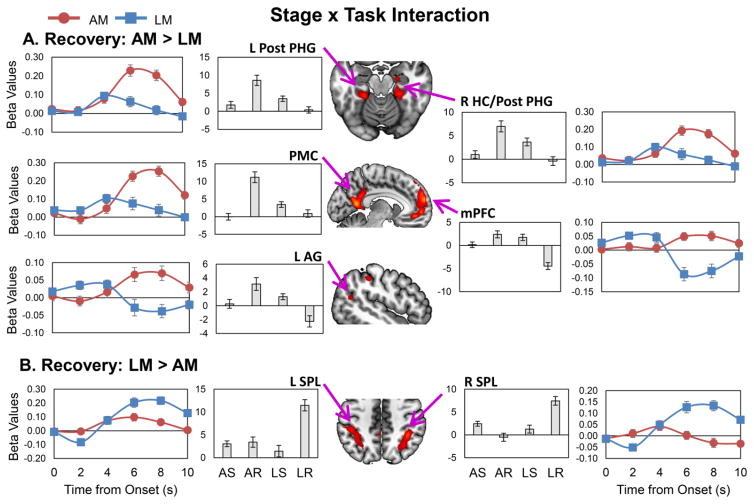
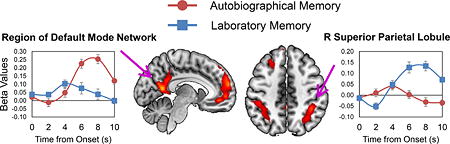
However, the Task (AM, LM) x Stage (search, recovery) interaction contrast (Figure 3; Table 2-bottom panel) identified several regions showing activation differences between AM and LM depending on memory stage. Paired t-tests indicated that these interactions occurred because there were no differences in AM vs. LM activation during memory search, but there were differences during recovery. Specifically, during AM vs. LM recovery, the DMN was activated greater (Buckner, et al., 2008; Laird, et al., 2011), including the PMC, medial PFC, AG, and MTL (right hippocampus, bilateral posterior parahippocampal gyri; Figure 3-A), whereas during LM vs. AM recovery, bilateral SPL was activated greater (Figure 3-B). In a supplementary analysis, we confirmed that these activation patterns were not the result of post-recovery (i.e., after the button press) activation (see Supplementary Results). Thus, although AM and LM did not differ in activity during search, they differed in activity during recovery, with AM differentially engaging the DMN and LM differentially recruiting bilateral SPL.
Fig 3.
Differences between autobiographical and laboratory memory brain activations as a function of memory stage. We examined the Task x Stage interaction contrast within the 2 (Task: AM, LM) x 2 (Stage: search, recovery) full factorial ANOVA. AM and LM differences were apparent only during recovery. Panel A shows regions that were activated greater during AM than LM recovery, which included bilateral posterior parahippocampal gyri, right hippocampus, PMC, mPFC, and left AG. Panel B shows regions that were activated greater during LM than AM recovery, which included bilateral SPL. Results are shown overlaid on a standard MNI template (neurological convention). The line graphs represent the finite impulse response beta plots and the bar graphs represent the beta values for each trial condition of interest; error bars represent the standard error of the mean. AM = autobiographical memory; LM = laboratory memory; L = left; R = right; AG = angular gyrus; HC = hippocampus; mPFC = medial prefrontal cortex; PMC = posterior midline cortex; Post PHG = posterior parahippocampal gyrus; SPL = superior parietal lobule; AS = autobiographical search; AR = autobiographical recovery; LS = laboratory search; LR = laboratory recovery.
3.3 Functional Networks Underlying Autobiographical and Laboratory Search and Recovery
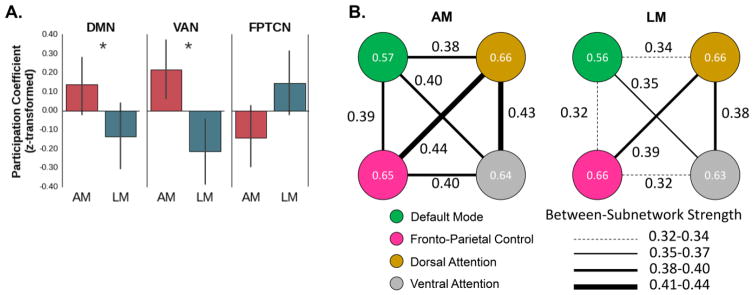
Our second aim was to investigate the topology of the functional networks underlying AM and LM search/recovery using graph theory-based, multivariate functional connectivity analyses (Bullmore & Sporns, 2009; van den Heuvel & Sporns, 2013). In particular, during AM vs. LM search and recovery, we examined whether certain subnetworks (e.g., the DMN) were more integrated with other subnetworks, as opposed to being more segregated from other subnetworks. Therefore, we estimated participation coefficients for each subnetwork. Partially consistent with our hypothesis, we found that DMN participation coefficients exhibited a main effect of task, β = −.01, z(16) = 2.02, p < .05 (uncorrected), in which the DMN exhibited a more integrated topology (i.e., exhibited higher participation coefficients) during AM than LM. Also partially consistent without our hypothesis, we found that fronto-parietal task control network (FPTCN) participants coefficients were trending toward exhibiting a main effect of task, β = .004, z(16) = 1.83, p = .067 (uncorrected), in which the FPTCN exhibited a more integrated topology during LM than AM (Figure 4-A). However, for both DMN and FPTCN participation coefficients, the Task x Stage interaction was not significant, all absolute values were β < .01, z(14) > 0.64, p > .47 (uncorrected). Intriguingly, we also found that ventral attention network (VAN) participant coefficients exhibited a main effect of task, β = −.009, z(16) = 2.44, p < .05 (uncorrected), in which the VAN exhibited a more integrated topology during AM than LM (Figure 4-A), but the Task x Stage interaction was not significant, β = −.002, z(14) = 0.24, p = .81 (uncorrected). No other comparisons were statistically significant. The difference in between-subnetwork connections in the AM vs. LM networks is illustrated in Figure 4-B, where it can be seen that the DMN and VAN were more strongly connected with other subnetworks within the AM compared to LM network. Lastly, to ensure that these findings are independent of univariate activation, within the DMN, VAN and FPTCN, we examined the relation between participation coefficients and univariate activation. Participation coefficients and univariate activation were not related to each other, all absolute values were β ≤ .001, z(16) < 1.18, p > .23, indicating that the participation coefficient results are independent of univariate activation. In sum, the graph theory analyses revealed that the DMN and VAN exhibited a more integrated topology during AM (vs. LM), whereas the FPTCN exhibited a more integrated topology during LM (vs. AM).
Fig 4.
Functional networks underlying autobiographical and laboratory memory recovery. Panel A shows that nodes within the DMN and VAN exhibited higher participation coefficients (i.e., exhibited a more integrated topology) in the AM network than in the LM network. By contrast, nodes within the FPTCN showed an opposite trend (higher participation coefficients in the LM network); z-transformed participation coefficients are presented here so participation coefficients between subnetworks are on the same scale. Panel B shows a visualization of these results, in which the colored circles represent each of the subnetworks and lines between the circles represent between-subnetwork connections. The values within each circle are the average participation coefficient across participants for the respective subnetwork. The values between each subnetwork represent the strength of each between-subnetwork connection, which are based on the AM and LM recovery networks averaged across all participants; thicker lines represent stronger between-subnetwork connections. It can be seen that the DMN and VAN had stronger between-subnetwork connections in the AM than LM network. AM = autobiographical memory; LM = laboratory memory; DMN = default mode network; VAN = ventral attention network; FPTCN; fronto-parietal task control network; * = p < .05.
4.0 DISCUSSION
Here, we compared the neural mechanisms underlying AM vs. LM retrieval. We had three main findings. First, several regions were similarly engaged during both AM and LM, including the PFC and visual cortex. Second, although AM and LM activations did not differ during search, during recovery, DMN regions were activated greater during AM, whereas bilateral SPL were activated greater during LM. Finally, AM and LM differed in network topology, where the DMN and VAN were more integrated with other subnetworks during AM, but the FPTCN was more integrated with other subnetworks during LM. These findings are discussed in greater detail below.
4.1 Similarities between AM and LM Brain Activations
In our quadruple conjunction and main effects of stage analyses, we found several PFC and visual regions that exhibited shared activations during AM and LM. The quadruple conjunction analysis showed that both AM and LM engaged bilateral inferior frontal gyri (IFG), occipital pole, and inferior temporal/fusiform gyri (Figure 2-A). The shared bilateral IFG activation may reflect control processes (Badre & Wagner, 2007) required for memory search and monitoring (Cabeza, Locantore, & Anderson, 2003; Petrides, 2005; Svoboda, et al., 2006). IFG regions often contribute to successful LM retrieval (for meta-analyses, see Kim, 2013; Spaniol, et al., 2009), and that could also be the case in this study, although we did not isolate successful retrieval processes (i.e., hits vs. miss contrast). Shared AM-LM activations in the occipital pole likely reflect processing of the visual retrieval cue (the word), whereas those in bilateral inferior temporal/fusiform gyri could also reflect processing of visual memory details, as previously demonstrated in AM (Daselaar, et al., 2008; Gilboa, et al., 2004; St. Jacques, et al., 2008; Svoboda, et al., 2006) and LM (Danker & Anderson, 2010) studies. Again, this is difficult to determine since we did not compare hits vs. misses.
In examining search vs. recovery differences shared by AM and LM (the main effect of stage), for both AM and LM, we found that the right lateral occipital cortex was more strongly activated during search than recovery. Perhaps activation of this sensory related region reflects participants recalling visual details of the memory during search. Conversely, several regions showed greater activity for recovery than search during both AM and LM. These regions included the insula and the left frontal pole (Figure 2-B). The insula has been linked to both memory retrieval and decision making (Fleck, Daselaar, Dobbins, & Cabeza, 2006), perhaps reflecting here the decision to press the key for indicating successful recovery. Hierarchical rostro-caudal models of PFC function assume that the frontopolar cortex supports high level-control operations involving abstract representations and second-order relationships (Badre, 2008). In fMRI studies of episodic retrieval, the frontopolar cortex has been linked to strategic retrieval processes (Dobbins, Foley, Schacter, & Wagner, 2002; Rugg, Henson, & Robb, 2003). In sum, despite the important differences between AM and LM discussed below, it is important to emphasize that several brain regions contribute similarly to these two forms of memory, a point we have made in the past (Cabeza, et al., 2004).
4.2 Dissociations between Memory Task and Memory Stage
We next examined differences between AM and LM regardless of stage (main effect of task with interactions masked out). We did not find any regions showing such stage-independent differences. This result suggests that AM-LM differences found in previous fMRI studies (Cabeza, et al., 2004; Chen, et al., 2017; Fink, et al., 1996; Nyberg, et al., 2002; Summerfield, et al., 2009) may not be stage-independent.
Finally, the critical Task (AM, LM) x Stage (search, recovery) interaction contrast yielded AM-LM differences during memory recovery but not search. The finding that memory search processes engage similar regions for AM and LM is consistent with evidence that the neural mechanisms of memory search can be similar even if the quality of the memory targets is very different. For example, Polyn, et al. (2005) found that remembering faces, objects, and locations primarily differed closer to recovery. It is somewhat surprising that in our paradigm searching for memories of recently-encoded visual words (LM) recruited similar regions as searching for memories of relatively older and more complex multi-modal personal events (AM). This similarity suggests that the search stage of the AM word-cue task is primarily driven by semantic factors, not unlike the semantic factors driving memory search for words. This idea is consistent with models assuming that the first phase of AM retrieval is driven by personal semantics rather than episodic-sensory information (Conway & Pleydell-Pearce, 2000). It is worth pointing out that finding similar activation patterns during AM and LM search is partly due to our paradigm that matched the complexity and duration of the search phase for the two types of memory. If instead of using word quartets, the LM task used single words or word-pairs, the search phase for AM would likely be more demanding and take longer, eliciting greater activity in multiple brain regions. Thus, when comparing AM and LM activity, it is critical to match the complexity and duration of the memory search.
During recovery, in contrast, several regions showed activation differences between AM and LM. Compared to LM recovery, AM recovery differentially engaged the core components of the DMN, including PMC, mPFC, AG, and MTL regions (Figure 3-A). Many of these regions have been previously found to show greater activity during AM than LM (e.g., Cabeza, et al., 2004; Chen, et al., 2017; Summerfield, et al., 2009). We extend these studies by demonstrating that DMN activation differences during AM (vs. LM) occur predominantly during recovery rather than search. The functional role of the DMN remains unclear but is hypothesized to be important for introspection (Andrews-Hanna, 2012; Christoff, Gordon, Smallwood, Smith, & Schooler, 2009), perhaps indicating why these regions are commonly associated with AM.
One specific region of the DMN that was more strongly activated during AM recovery was the mPFC. The mPFC has been associated with self-referential processing (Gusnard, Akbudak, Shulman, & Raichle, 2001; Kelley, et al., 2002), which, in the current study, may reflect the prominent self-related dimension of AMs. Another DMN region that was more strongly activated during AM recovery was the PMC. Daselaar, et al. (2008) also found that the PMC was strongly activated during AM retrieval. Our study extends this finding by demonstrating that during AM compared to LM recovery, the PMC is more strongly activated. The PMC has previously been shown to be important for spatial imagery (Epstein, 2008; Ino, et al., 2002; O’Craven & Kanwisher, 2000) and contextually rich events (Gilmore, Nelson, Chen, & McDermott, in press; Gilmore, Nelson, & McDermott, 2016), perhaps reflecting participants retrieving more vivid memories during AM vs. LM retrieval. Lastly, we found that the right hippocampus and bilateral parahippocampal gyri were more strongly activated during AM (vs. LM) recovery. Consistent with this finding, Daselaar, et al. (2008) also found that the hippocampus was strongly activated during AM retrieval. Current theories indicate that the MTL is essential for conscious memory processes (Eldridge, Knowlton, Furmanski, Bookheimer, & Engel, 2000; Schacter, Alpert, Savage, Rauch, & Albert, 1996) and the reactivation of memory traces (Eichenbaum, 2004; Moscovitch, 1995; Squire, Stark, & Clark, 2004). Also, recent evidence indicates that the hippocampus is more functionally interconnected within the whole-brain network underlying the retrieval of vivid vs. dim visual memories (Geib, et al., 2015). As AMs are typically more vivid than LMs (Cabeza & St Jacques, 2007; Conway & Pleydell-Pearce, 2000; Rubin, 2006), it is not surprising that we found the MTL to be more strongly activated during AM than LM.
Partially consistent with the univariate activation findings, the graph theory analysis revealed that the DMN exhibited a more integrated topology (i.e., contained higher participation coefficients) during AM than LM (Figure 4-A), but the Task by Stage interaction was not significant. This finding is consistent with previous studies demonstrating that successful memory retrieval, specifically in the hippocampus which is part of the DMN, is associated with greater network integration (Geib, et al., 2017; Geib, et al., 2015; King, et al., 2015; Schedlbauer, et al., 2014). Overall, the graph theory results compliment and extend the direct comparisons of univariate activity results. Specifically, task differences in DMN activation were only evident during recovery, while both the search and recovery stages of AM were characterized by a more integrated topology in the DMN. These differences between the univariate activation and functional connectivity results may reflect different functional roles of activation in individual regions and functional connectivity in large-scale networks. In addition, since the DMN was found to be more integrated with other subnetworks during AM, this demonstrates that even though DMN regions are important for AM, they still rely on regions within other subnetworks in service of AM.
In the other direction, the Task (AM, LM) x Stage (search, recovery) revealed that bilateral SPL was activated greater during LM than AM recovery (Figure 3-B). The SPL has previously been demonstrated to be important for controlled retrieval processes (Cabeza, Ciaramelli, Olson, & Moscovitch, 2008; Dobbins, et al., 2002). Greater activation of the SPL during LM likely reflects the LM task requiring greater monitoring, as participants were instructed to retrieve a specific set of stimuli, whereas AM was less constrained. Also, it should be noted, from the examination of the FIR plots, during LM, as the SPL became more strongly activated, DMN-related regions became deactivated. Perhaps this activation pattern reflects a shift from internal processes (dependent on DMN-related regions) to controlled processes (dependent on the SPL) as LM retrieval progressed. Lastly, we did not find LatPFC to be more strongly activated during LM than AM recovery. However, the graph theory analysis revealed that the FPTCN, which contains LatPFC (in additional to lateral parietal) regions, exhibited a more integrated topology during LM than AM (Figure 4-A), demonstrating the importance of LatPFC during LM recovery; it should be noted, however, that the FPTCN exhibited a more integrated topology during both LM search and recovery.
An intriguing double dissociation in the parietal cortex is that a ventral parietal region, AG, showed greater activity for AM than LM, whereas a dorsal parietal region, SPL, showed greater activity for LM than AM. According to the Attention to Memory (AtoM) model (Cabeza, et al., 2008; Cabeza, et al., 2011; Ciaramelli, Grady, & Moscovitch, 2008), the role of dorsal parietal cortex in episodic retrieval reflects goal-directed (top-down) attention during retrieval search and monitoring, whereas the contribution of ventral parietal cortex reflects the capture of bottom-up attention by recovered memories. Although this study was not specifically designed to test the AtoM model, the double dissociation in parietal cortex is consistent with this model: AMs are typically more salient than LMs and hence more likely to capture bottom-up attention, whereas retrieving LMs typically require greater monitoring (Cabeza & St Jacques, 2007; Conway, 2005; Gilboa, 2004; Moscovitch & Winocur, 2002) and hence is more dependent on top-down attention. Further supporting this interpretation, the graph theory analysis revealed that the VAN, typically associated with bottom-up attention (Corbetta & Shulman, 2002; Miller & Buschman, 2013; Riddoch, et al., 2010; Shipp, 2004; Shulman, Astafiev, & Corbetta, 2004), exhibited a more integrated topology during AM than LM (Figure 4-A), further indicating the increased importance of bottom-up processes during AM than LM. Interestingly, the original AtoM model (Cabeza, et al., 2008; Cabeza, et al., 2011; Ciaramelli, et al., 2008) suggested that the role of top-down attention during episodic retrieval could reflect search and/or monitoring. The present results suggest that if greater SPL activity for LM than AM reflects top-down attention, perhaps the role of SPL during retrieval is primarily related to monitoring, as the AM-LM difference occurred during recovery but not search.
4.3 Limitations
Even though we believe the current study advances the literature examining neural mechanisms underlying AM vs. LM retrieval, it does contain a few limitations. First, in the AM task, it may be the case that participants did not recall “chains” of memories, like in the LM task, leading to a specific AM as we hypothesized (Conway, 2001); however, we do believe that participants recalled AMs as “chains” of events. Second, during the AM task, it is possible that participants did not recall specific AMs, as instructed. However, compared to other studies in which participants were instructed to retrieve specific AMs (e.g., Chen, et al., 2017; McCormick, et al., 2015), the RTs within the current study were similar or even longer, suggesting that participants were retrieving specific AMs. Third, even though we have speculated processes that may be the result of neural mechanism differences underlying AM and LM retrieval reported here, from this study, the exact reasons for these differences cannot be definitively determined. For example, the AMs vs. LMs retrieved in the current study were likely more vivid and contextually rich, which may have resulted in greater activation in regions associated with vivid memories (e.g., several regions of the DMN; for a review, see Cabeza & St Jacques, 2007). Also, the LM vs. AM task was more constrained, which may have led to neural mechanism differences, where for each trial in the LM task there was only one correct LM to be retrieved, whereas in the AM task there were possibly several AMs associated with the cue word that could have been retrieved. Future studies could further investigate the functional roles of neural mechanism differences underlying AMs vs. LMs by varying during LM retrieval the type of stimuli retrieved (e.g., varying the vividness) and the retrieval demands (e.g., varying the number of ‘correct’ LMs that could be retrieved). Fourth, the current study did not include an elaboration phase, which could differ for AMs and LMs. To address this issue, a future study could directly compare AM and LM retrieval during all three retrieval phases (search, recovery, and elaboration).
4.4 Conclusions
In sum, we examined the neural mechanisms underlying AM vs. LM search and recovery in memory tasks designed to equate the time courses of these processes within trials. Although we found differences in the neural underpinnings of AM and LM retrieval, both memory retrieval types recruited PFC and sensory-related regions, likely reflecting a common set of regions recruited during any type of episodic memory retrieval. Regarding retrieval type differences, DMN-related regions, such as the HC, mPFC and PMC, were activated greater during AM than LM, whereas the SPL showed the converse pattern. An examination of activity varying by search vs. recovery periods showed that task activation differences were driven by activity associated with memory recovery, while search-related activity was broadly similar between tasks. We further characterized the neural mechanisms underlying AM vs. LM retrieval with graph theory-based, multivariate functional connectivity analyses. While both the DMN and VAN exhibited a more integrated topology during AM (vs. LM) retrieval, the FPTCN exhibited a more integrated topology during LM (vs. AM) retrieval. These findings suggest that differences in AM vs. LM brain activation previously reported in the literature likely reflect greater recovery rather than search differences, but that functional connectivity patterns within large-scale networks differ between AM vs. LM regardless of memory stage. Perhaps the univariate activation vs. functional connectivity results reflect different functional roles of univariate activation in individual regions and functional connectivity patterns in large-scale networks.
Supplementary Material
HIGHLIGHTS.
We compared autobiographical (AM) vs. laboratory (LM) memory retrieval.
Task brain activation differences occurred during memory recovery but not search.
The default mode network was more strongly activated during AM recovery.
The superior parietal lobules were more strongly activated during LM recovery.
The default mode network exhibited a more integrated topology during AM.
Acknowledgments
This work was supported by the National Institute on Aging (R01 AG019731 to R.C.). The funding agency had no role in the decision to publish or preparation of the manuscript. The authors do not have any conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. The Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Bowman CR, Dennis NA. The neural basis of recollection rejection: increases in hippocampal–prefrontal connectivity in the absence of a shared recall-to-reject and target recollection network. J Cogn Neurosci. 2016;28:1194–1209. doi: 10.1162/jocn_a_00961. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. Response suppression, initiation and strategy use following frontal lobe lesions. Neuropsychologia. 1996;34:263–272. doi: 10.1016/0028-3932(95)00104-2. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia. 2008;46:1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: An attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Locantore JK, Anderson ND. Lateralization of prefrontal activity during episodic memory retrieval: evidence for the production-monitoring hypothesis. J Cogn Neurosci. 2003;15:249–259. doi: 10.1162/089892903321208187. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Mazuz YS, Stokes J, Kragel JE, Woldorff MG, Ciaramelli E, Olson IR, Moscovitch M. Overlapping parietal activity in memory and perception: evidence for the attention to memory model. J Cogn Neurosci. 2011;23:3209–3217. doi: 10.1162/jocn_a_00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, LaBar KS, Rubin DC. Brain activity during episodic retrieval of autobiographical and laboratory events: an fMRI study using a novel photo paradigm. J Cogn Neurosci. 2004;16:1583–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends Cogn Sci. 2007;11:219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Chen HY, Gilmore AW, Nelson SM, McDermott KB. Are there multiple kinds of episodic memory? An fMRI investigation comparing autobiographical and recognition memory tasks. J Neurosci. 2017;37:2764–2775. doi: 10.1523/JNEUROSCI.1534-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci U S A. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramelli E, Grady C, Levine B, Ween J, Moscovitch M. Top-down and bottom-up attention to memory are dissociated in posterior parietal cortex: neuroimaging and neuropsychological evidence. J Neurosci. 2010;30:4943–4956. doi: 10.1523/JNEUROSCI.1209-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: a hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Bush K, Steele JS. A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. NeuroImage. 2014;84:1042–1052. doi: 10.1016/j.neuroimage.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, D’Esposito M. The segregation and integration of distinct brain networks and their relationship to cognition. J Neurosci. 2016;36:12083–12094. doi: 10.1523/JNEUROSCI.2965-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MA. Sensory–perceptual episodic memory and its context: autobiographical memory. Philos Trans R Soc Lond B Biol Sci. 2001;356:1375–1384. doi: 10.1098/rstb.2001.0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MA. Memory and the self. J Mem Lang. 2005;53:594–628. [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychol Rev. 2000;107:261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Conway MA, Rubin DC. The structure of autobiographical memory. In: Collins AE, Gathercole MA, Conway MA, Morris PEM, editors. Theories of memory. Hove, Sussex: Lawrence Erlbaum Associates; 1993. pp. 103–137. [Google Scholar]
- Corbetta M, Shulman GL. Controls of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–214. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10:171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Crovitz HF, Schiffman H. Frequency of episodic memories as a function of their age. Bull Psychon Soc. 1974;4:517–518. [Google Scholar]
- Danker JF, Anderson JR. The ghosts of brain states past: remembering reactivates the brain regions engaged during encoding. Psychol Bull. 2010;136:87–102. doi: 10.1037/a0017937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC. The spatiotemporal dynamics of autobiographical memory: neural correlates of recall, emotional intensity, and reliving. Cereb Cortex. 2008;18:217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Bowman CR, Peterson KM. Age-related differences in the neural correlates mediating false recollection. Neurobiol Aging. 2014;35:395–407. doi: 10.1016/j.neurobiolaging.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Dixon ML, Fox KCR, Christoff K. A framework for understanding the relationship between externally and internally directed cognition. Neuropsychologia. 2014;62:321–330. doi: 10.1016/j.neuropsychologia.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: Multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn Sci. 2008;12:388–396. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD. Cerebral representation of one’s own past: neural networks involved in autobiographical memory. J Neurosci. 1996;16:4275–4282. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck MS, Daselaar SM, Dobbins IG, Cabeza R. Role of prefrontal and anterior cingulate regions in decision-making processes shared by memory and nonmemory tasks. Cereb Cortex. 2006;16:1623–1630. doi: 10.1093/cercor/bhj097. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallen CL, Turner GR, Adnan A, D’Esposito M. Reconfiguration of brain network architecture to support executive control in aging. Neurobiol Aging. 2016;44:42–52. doi: 10.1016/j.neurobiolaging.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton F. Psychometric experiments. Brain. 1879;2:149–162. [Google Scholar]
- Geib BR, Stanley ML, Dennis NA, Woldorff MG, Cabeza R. From hippocampus to whole-brain: the role of integrative processing in episodic memory retrieval. Hum Brain Mapp. 2017 doi: 10.1002/hbm.23518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geib BR, Stanley ML, Wing EA, Laurienti PJ, Cabeza R. Hippocampal contributions to the large-scale episodic memory network predict vivid visual memories. Cereb Cortex. 2015:1–14. doi: 10.1093/cercor/bhv272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A. Autobiographical and episodic memory—one and the same?: evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 2004;42:1336–1349. doi: 10.1016/j.neuropsychologia.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Alain C, Stuss DT, Melo B, Miller S, Moscovitch M. Mechanisms of spontaneous confabulations: a strategic retrieval account. Brain. 2006;129:1399–1414. doi: 10.1093/brain/awl093. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cereb Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Gilmore AW, Nelson SM, Chen H-Y, McDermott KB. Task-related and resting-state fMRI identify distinct networks that preferentially support remembering the past and imagining the future. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2017.06.016. (in press) [DOI] [PubMed] [Google Scholar]
- Gilmore AW, Nelson SM, McDermott KB. The contextual association network activates more for remembered than for imagined events. Cereb Cortex. 2016;26:611–617. doi: 10.1093/cercor/bhu223. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, LaBar KS. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43:659–674. doi: 10.1016/j.neuropsychologia.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Guimera R, Amaral LAN. Functional cartography of complex metabolic networks. Nat. 2005;433:895–900. doi: 10.1038/nature03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebscher M, Levine B, Gilboa A. The precuneus and hippocampus contribute to individual differences in the unfolding of spatial representations during episodic autobiographical memory. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2017.03.029. (in press) [DOI] [PubMed] [Google Scholar]
- Henson RNA, Cansino S, Herron JE, Robb WGK, Rugg MD. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;13:301–304. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- Holland AC, Addis DR, Kensinger EA. The neural correlates of specific versus general autobiographical memory construction and elaboration. Neuropsychologia. 2011;49:3164–3177. doi: 10.1016/j.neuropsychologia.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino T, Inoue Y, Kage M, Hirose S, Kimura T, Fukuyama H. Mental navigation in humans is processed in the anterior bank of the parieto-occipital sulcus. Neurosci Lett. 2002;322:182–186. doi: 10.1016/s0304-3940(02)00019-8. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kim H. Differential neural activity in the recognition of old versus new events: An activation likelihood estimation meta-analysis. Hum Brain Mapp. 2013;34:814–836. doi: 10.1002/hbm.21474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DR, de Chastelaine M, Elward RL, Wang TH, Rugg MD. Recollection-related increases in functional connectivity predict individual differences in memory accuracy. The Journal of Neuroscience. 2015;35:1763–1772. doi: 10.1523/JNEUROSCI.3219-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel JE, Polyn SM. Functional interactions between large-scale networks during memory search. Cereb Cortex. 2015;25:667–679. doi: 10.1093/cercor/bht258. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT. Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WJ, Horner AJ, Burgess N. Ventromedial prefrontal cortex, adding value to autobiographical memories. Sci Rep. 2016;6:28630. doi: 10.1038/srep28630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C, St-Laurent M, Ty A, Valiante TA, McAndrews MP. Functional and effective hippocampal–neocortical connectivity during construction and elaboration of autobiographical memory retrieval. Cereb Cortex. 2015;25:1297–1305. doi: 10.1093/cercor/bht324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KB, Szpunar KK, Christ SE. Laboratory-based and autobiographical retrieval tasks differ substantially in their neural substrates. Neuropsychologia. 2009;47:2290–2298. doi: 10.1016/j.neuropsychologia.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Miller EK, Buschman TJ. Cortical circuits for the control of attention. Curr Opin Neurobiol. 2013;23:216–222. doi: 10.1016/j.conb.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge ZA, Geib BR, Siciliano RE, Packard LE, Tallman CW, Madden DJ. Functional modular architecture underlying attentional control in aging. NeuroImage. 2017;155:257–270. doi: 10.1016/j.neuroimage.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M. Recovered consciousness: a hypothesis concerning modularity and episodic memory. J Clin Exp Neuropsychol. 1995;17:276–290. doi: 10.1080/01688639508405123. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. The frontal cortex and working with memory. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York, New York: Oxford University Press; 2002. pp. 188–209. [Google Scholar]
- Mumford JA, Poline JB, Poldrack RA. Orthogonalization of regressors in fMRI models. PLOS ONE. 2015;10:e0126255. doi: 10.1371/journal.pone.0126255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JA, Turner BO, Ashby FG, Poldrack RA. Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. NeuroImage. 2012;59:2636–2643. doi: 10.1016/j.neuroimage.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Addis DR, Kensinger EA. Self-involvement modulates the effective connectivity of the autobiographical memory network. Soc Cogn Affect Neurosci. 2010;5:68–76. doi: 10.1093/scan/nsp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida free association, rhyme, and word fragment norms. Behav Res Methods Instrum Comput. 2004;36:402–407. doi: 10.3758/bf03195588. [DOI] [PubMed] [Google Scholar]
- Norman DA, Bobrow DG. Descriptions: an intermediate stage in memory retrieval. Cogn Psychol. 1979;11:107–123. [Google Scholar]
- Nyberg L, Forkstam C, Petersson KM, Cabeza R, Ingvar M. Brain imaging of human memory systems: between-systems similarities and within-system differences. Brain Res Cogn Brain Res. 2002;13:281–292. doi: 10.1016/s0926-6410(02)00052-6. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Tulving E, Habib R, Nilsson LG, Kapur S, Houle S, Cabeza R, McIntosh AR. Functional brain maps of retrieval mode and recovery of episodic information. NeuroReport. 1995;7:249–252. [PubMed] [Google Scholar]
- O’Craven KM, Kanwisher N. Mental imagery of faces and places activates corresponding stimulus-specific brain regions. J Cogn Neurosci. 2000;12:1013–1023. doi: 10.1162/08989290051137549. [DOI] [PubMed] [Google Scholar]
- Paivio A, Yuille JC, Madigan SA. Concreteness, imagery, and meaningfulness values for 925 nouns. J Exp Psychol. 1968;76:1–25. doi: 10.1037/h0025327. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyn SM, Natu VS, Cohen JD, Norman KA. Category-specific cortical activity precedes retrieval during memory search. Science. 2005;310:1963–1966. doi: 10.1126/science.1117645. [DOI] [PubMed] [Google Scholar]
- Power Jonathan D, Cohen Alexander L, Nelson Steven M, Wig Gagan S, Barnes Kelly A, Church Jessica A, Vogel Alecia C, Laumann Timothy O, Miezin Fran M, Schlaggar Bradley L, Petersen Steven E. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddoch MJ, Chechlacz M, Mevorach C, Mavritsaki E, Allen H, Humphreys GW. The neural mechanisms of visual selection: The view from neuropsychology. Ann N Y Acad Sci. 2010;1191:156–181. doi: 10.1111/j.1749-6632.2010.05448.x. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rubin DC. The basic-systems model of episodic memory. Perspect Psychol Sci. 2006;1:277–311. doi: 10.1111/j.1745-6916.2006.00017.x. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RNA, Robb WGK. Neural correlates of retrieval processing in the prefrontal cortex during recognition and exclusion tasks. Neuropsychologia. 2003;41:40–52. doi: 10.1016/s0028-3932(02)00129-x. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS. Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. Proc Natl Acad Sci U S A. 1996;93:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedlbauer AM, Copara MS, Watrous AJ, Ekstrom AD. Multiple interacting brain areas underlie successful spatiotemporal memory retrieval in humans. Sci Rep. 2014;4:6431. doi: 10.1038/srep06431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabold S, Perktold J. Statsmodels: econometric and statistical modeling with python. Proceedings of the 9th Python in Science Conference; 2010. p. 61. [Google Scholar]
- Shapira-Lichter I, Vakil E, Glikmann-Johnston Y, Siman-Tov T, Caspi D, Paran D, Hendler T. Inside out: a neuro-behavioral signature of free recall dynamics. Neuropsychologia. 2012;50:2245–2256. doi: 10.1016/j.neuropsychologia.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Shipp S. The brain circuitry of attention. Trends Cogn Sci. 2004;8:223–230. doi: 10.1016/j.tics.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, Corbetta M. Two cortical systems for the selection of visual stimuli. In: Posner MI, editor. Cognitive neuroscience of attention. New York, NY: The Guilford Press; 2004. pp. 114–126. [Google Scholar]
- Smallwood J, Schooler JW. The science of mind wandering: empirically navigating the stream of consciousness. Annu Rev Psychol. 2015;66:487–518. doi: 10.1146/annurev-psych-010814-015331. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- St Jacques P, Rubin DC, Cabeza R. Age-related effects on the neural correlates of autobiographical memory retrieval. Neurobiol Aging. 2012;33:1298–1310. doi: 10.1016/j.neurobiolaging.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques P, Rubin DC, LaBar KS, Cabeza R. The short and long of it: neural correlates of temporal-order memory for autobiographical events. J Cogn Neurosci. 2008;20:1327–1341. doi: 10.1162/jocn.2008.20091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield JJ, Hassabis D, Maguire EA. Cortical midline involvement in autobiographical memory. NeuroImage. 2009;44:1188–1200. doi: 10.1016/j.neuroimage.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci. 2013;17:683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.