Summary
Human astrocytes network with neurons in dynamic ways that are still poorly defined. Our ability to model this relationship is hampered by the lack of relevant and convenient tools to recapitulate this complex interaction. To address this barrier, we have devised efficient coculture systems utilizing 3D organoid-like spheres, termed asteroids, containing pre-differentiated human pluripotent stem cell (hPSC)-derived astrocytes (hAstros) combined with neurons generated from hPSC-derived neural stem cells (hNeurons) or directly induced via Neurogenin 2 overexpression (iNeurons). Our systematic methods rapidly produce structurally complex hAstros and synapses in high-density coculture with iNeurons in precise numbers, allowing for improved studies of neural circuit function, disease modeling, and drug screening. We conclude that these bioengineered neural circuit model systems are reliable and scalable tools to accurately study aspects of human astrocyte-neuron functional properties while being easily accessible for cell-type-specific manipulations and observations.
Keywords: human pluripotent stem cells, neurons, astrocytes, synapses, coculture, three-dimensional spheres, organoids, disease modeling
Highlights
-
•
Novel three-dimensional “asteroid” coculture system is described
-
•
Complex human astrocyte-specific morphology is generated in vitro
-
•
This system produces rapid and tight association of astrocytes with synapses
In this article, Krencik and colleagues show that high-density cocultures of pre-differentiated human astrocytes with induced neurons, from pluripotent stem cells, elicit mature characteristics by 3–5 weeks. This provides a faster and more defined alternative method to organoid cultures for investigating human neural circuit function.
Introduction
Astrocytes have numerous dynamic synapse-modulating and homeostatic functions during development, maturation, and disease (Blanco-Suarez et al., 2016, Verkhratsky and Nedergaard, 2016). While most investigations into these cellular functions have been conducted in rodent models due to ease of experimental access, human astrocytes are known to have unique gene expression, morphological characteristics (Colombo et al., 1997b), and functionality (Oberheim et al., 2009, Zhang et al., 2016) that may have consequences for higher brain circuit processing in healthy and disease conditions (Krencik et al., 2016, Oberheim Bush and Nedergaard, 2017). One strategy to examine human cellular function is to study the interplay between human pluripotent stem cell (hPSC)-derived astrocytes (hAstros) and hPSC-derived neurons (hNeurons). We previously reported a long-term protocol consisting of weekly dissociation of three-dimensional (3D) spheres of hAstros, termed astrospheres, to induce developmental maturation and purity followed by attachment onto two-dimensional (2D) substrates for coculture studies (Krencik et al., 2015, Krencik and Ullian, 2013, Krencik and Zhang, 2011). More recently, organoid approaches have been reported to mature and maintain long-term glial-neuronal interactions over many months within intact 3D spheres (Pasca et al., 2015, Qian et al., 2016, Sloan et al., 2017). Here, we detail an alternative fast and reliable protocol to recapitulate these 3D interactions mirroring the complexity found in human brain. This method has the ease and advantage of allowing precise control of both the numbers and types of human neural cells using identical or different genetic backgrounds, as well as rapidly inducing mature synapse formation.
To investigate human astroglial activity in a more native-like environment, several innovative methods have been recently devised. One approach was to engraft human fetal- or hPSC-derived glial progenitors and hAstros into immunodeficient rodents followed by in vivo and ex vivo analysis (Chen et al., 2015, Han et al., 2013, Krencik et al., 2011, Li et al., 2015). While this technique is advantageous for being able to integrate cells into intact neural networks with measurable behavioral output, there are at least two major limitations. First, the human cellular response in a xeno-species environment may be altered compared with integration within matched species (Goldman, 2016). Second, scalability and optimization of conditions is impractical in chimeric mice due to time, cost, and space limitations. To overcome these issues, many human culture studies have been recently conducted with 3D spheroids or organoids, as an improved technique over 2D monolayers, to maintain structural integrity and tight cellular interactions (Kelava and Lancaster, 2016, Simian and Bissell, 2017).
Typically in 3D cultures of human neural cells, hAstros appear gradually over a long time period with no precise experimental control over the number or maturity of cells (Pasca et al., 2015, Qian et al., 2016, Sloan et al., 2017). The neuronal population is also not well specified as they contain a mixture of progenitor and mature neuronal subtypes. In an ideal experimental neural coculture system, the ratio of glial to neuronal cells as well as the extracellular milieu should be stable over time to better mimic the long-term nervous system environment during normal and disease conditions. Therefore, we aimed to develop such systematic and repeatable coculture methods to rapidly mimic many aspects of the endogenous cell-cell interactions. This system described here can be applied for studying cell-autonomous versus cell non-autonomous effects by combining cells from different genetic backgrounds or treatment conditions. Furthermore, it can be used as a surrogate for monitoring cellular engraftment of human neural types as a model for cellular replacement therapy.
Results
High-Density Neuronal Coculture Induces Complex Human Astrocyte Morphology
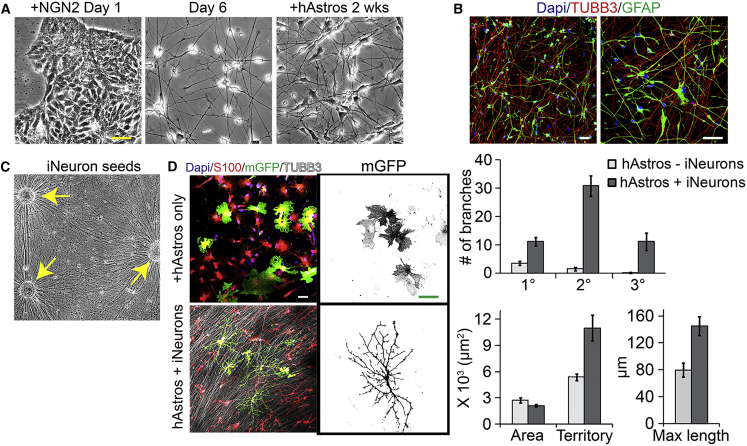
In order to better recapitulate endogenous human astrocyte complexity, we compared the morphology of hAstros in the absence or presence of neurons. One particular disadvantage in using hNeurons for coculture studies is that the population will also contain a mixture of undifferentiated neuroepithelial cells and gliogenic progenitors, typically in rosette structures, that generate immature synaptogenic hAstros over time (Johnson et al., 2007). These hPSC-derived “endogenous” glial cells will confound any phenotypes observed in neuronal studies. To overcome this, we utilized a clonal hPSC line with inducible expression of a transgene to generate Neurogenin 2 (NGN2) protein, which rapidly generates homogeneous cortical iNeurons after induction in a neural supportive medium (Wang et al., 2017) (Figure 1A). We determined whether pre-differentiated hAstros (>6 months, from embryonic stem cell line WA09 and from previously characterized induced pluripotent stem cell lines; Krencik et al., 2015) were able to induce maturation of iNeurons in a 2-week conventional 2D coculture as previously reported in hNeurons (Krencik et al., 2011). As expected, hAstros induced expression of a synapse-related gene, as measured by a Synapsin 1-promoter-driven GFP reporter, and the hAstros themselves become elongated and branched (Figures S1A and 1B). However, this culture paradigm did not induce the astrocytes to exhibit the morphological complexity of astrocytes within the human brain.
Figure 1.
2D Coculture of iNeurons and Pre-matured hAstros
(A) Example images showing the continued hPSC colony structure in the iNeuron line within 1 day after induction of NGN2, and the subsequent drastic change toward neuronal morphology by day 6. hAstros could be added to the iNeurons to be used for coculture studies.
(B) Cocultures of iNeurons (TUBB3-positive, nuclei are DAPI positive) with pre-matured hAstros (GFAP positive) are stable at 2 weeks. hAstro morphology becomes branched and elongated after 2 weeks of coculture, although not similar to that of astrocytes in the brain.
(C) Example of high-density neuronal fibers projecting out of iNeuron seeds (yellow arrows).
(D) hAstros (S100- and mGFP-positive) on high-density neuronal fibers induces morphological rearrangement that increases the number of branches and size compared with dissociated hAstros without iNeurons. n = 10 technical replicates for hAstros only, n = 11 for cocultures.
Error bars represent SEM. Scale bars are 50 μm.
We next examined if hAstro morphological complexity could be enhanced in higher density cultures that are more similar to endogenous conditions. During coculture studies, we observed that hAstros tend to extend upon neuronal processes (Movies S1 and S2). This surprisingly also occurred, although to a lesser extent, when hAstros were cultured on parallel carbon nanofibers without neurons (Figure S1B). We therefore examined the morphological response of hAstros to high densities of parallel-orientated neuronal fibers using a membrane-targeted GFP reporter (mGFP). To mimic neuronal tracts, we generated organoid spheres of hPSC-derived neural progenitors from the iNeuron line without induction of NGN2, seeded spheres separated by a few hundred micrometers onto Matrigel-coated glass, and then induced NGN2 expression for 2 weeks to promote maturation and neuronal process extension toward neighboring seeds (Figure 1C). Next, we cultured pre-differentiated (>6 months) dissociated hAstros on these tracts, or on Matrigel without neurons as a control, for an additional 2 weeks. In comparison with cultures in the absence of iNeurons, the coculture system led to a dramatic restructuring of hAstros with increased number of branches (N = 10 versus 11 technical replicates for each, branches of WA09-derived hAstros only versus hAstros + iNeurons; primary = 3.6 ± 0.7 versus 11.2 ± 1.4; secondary = 1.6 ± 0.6 versus 30.9 ± 3.6; tertiary = 0.2 ± 0.2 versus 11.2 ± 3.0). The overall area of total membrane per cell was not different, yet total territory occupied per hAstro was increased (5,379.6 ± 1,226.6 versus 10,994.7 ± 4,714.2 μm2) indicating membrane restructuring with no change in total membrane content. In support of this, the length of the maximum process was also increased (79.5 ± 35.1 versus 145.2 ± 44.2 μm) (Figure 1D). These data suggest that high-density neuronal axons are sufficient to induce profound changes in hAstro morphology and yield hAstros morphologically similar to endogenous human white-matter-tract astrocytes.
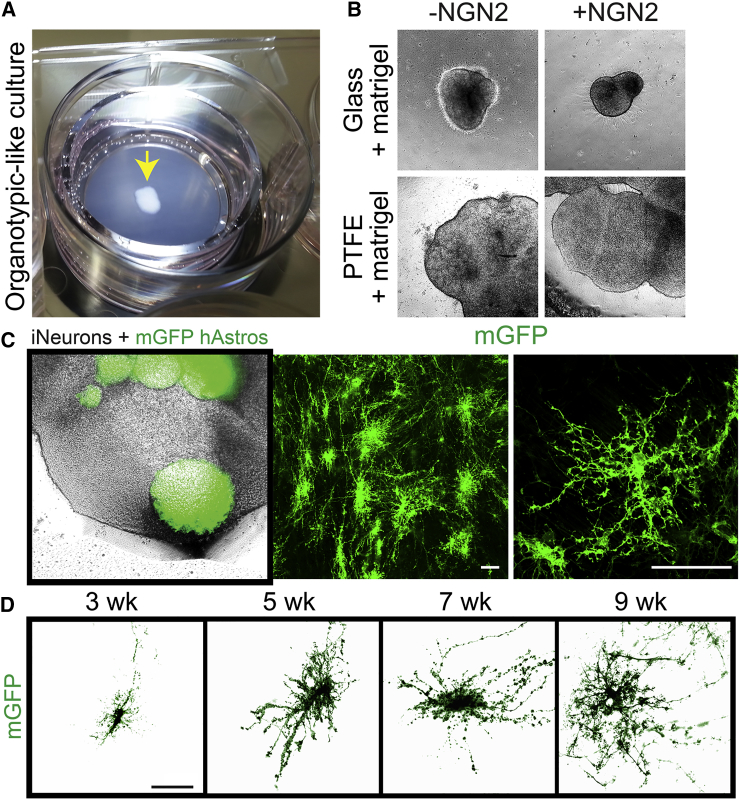
Enhanced Astrocyte Complexity in a 3D Organotypic-like Coculture Environment
Since increased neuronal process density appears to be crucial to induce complex astrocyte morphology, we next examined the effect of coculturing hAstros with a high density of neurons in 3D tissue. We turned to the method commonly utilized to maintain neural networks of rodent or human tissue, termed organotypic cultures (Humpel, 2015). We cultured neurospheres from the iNeuron line for 2 weeks, and then seeded spheres upon polytetrafluoroethylene (PTFE) membranes to generate healthy organotypic-like cultures (with medium only in contact with the lower surface for better gas exchange) (Figure 2A). Compared with being submerged in medium on glass coverslips coated with Matrigel, spheres readily flattened as thick layers on membranes, and both conditions led to complete neuronal differentiation with a lack of rosette structures after NGN2 induction (Figure 2B). To determine whether this environment would improve the complexity of hAstros over that of the high-density 2D culture described above, we seeded mGFP-labeled astrospheres, containing solely hAstros, onto the organotypic-like iNeurons for a duration of 4 weeks. Surprisingly, this culture system resulted in the presence of fine membranous processes and dramatic branching reminiscent of astrocytes within the human brain and not previously reported in vitro (Figure 2C). This morphological complexity was observed to continuously increase between 3 and 9 weeks post engraftment, and fine leaflet-like structures (reminiscent of perisynaptic astrocyte processes) (Khakh and Sofroniew, 2015) could gradually be observed (Figure 2D). Furthermore, hAstros distributed into territories, displayed club-like bulbous endings and produced long-projecting varicose processes through the territories (Figures S1C and S1D), similar to unique features observed within the human brain (Colombo et al., 1997a, Oberheim et al., 2009). These data suggest that in contrast to axons only, neuron cell bodies and dendrites may promote a more “gray-matter”-like appearance of hAstros.
Figure 2.
Organotypic-like Coculture Recapitulates Human Astrocyte Complexity
(A) Image of cultured tissue (yellow arrow) in a well of a six-well plate on a PTFE membrane insert.
(B) Comparison of organoid spheres plated on either Matrigel or PTFE, with or without NGN2 induction, demonstrating the presence or absence of rosette structures, respectively, at the same image magnification.
(C) (Left) Merge of bright-field and GFP fluorescence of mGFP astrospheres plated on top of organotypic-like iNeurons. (Middle) A single-plane confocal image of hAstros after 4 weeks of coculture. (Right) Flattened z series of a single hAstro.
(D) Time series representing the dynamic morphological complexity of cocultured hAstros.
Scale bars, 50 μm.
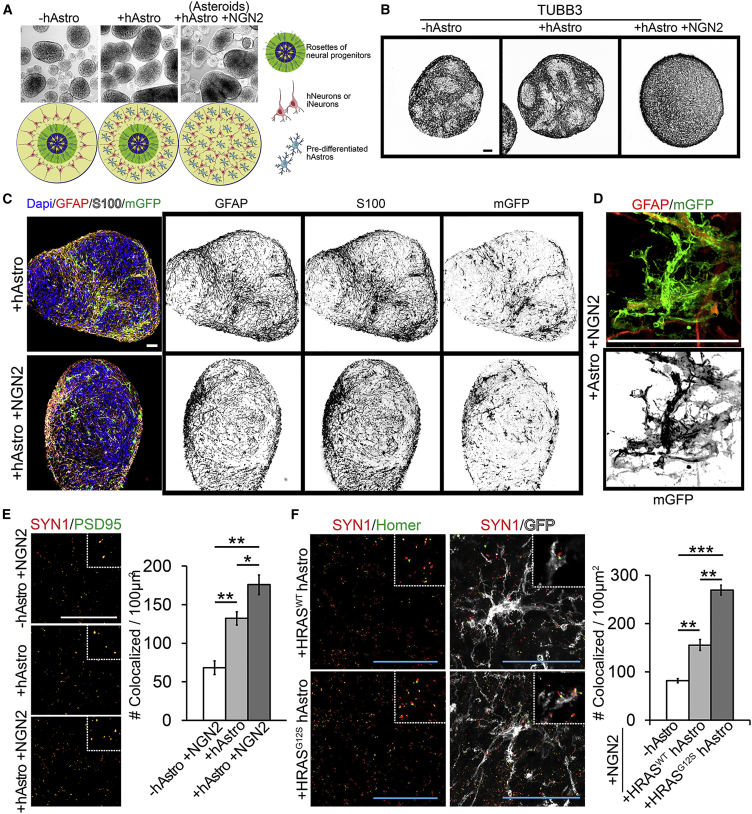
Asteroid Coculture System for Disease Modeling
We sought to optimize the emerging long-term 3D organoid methods by coculturing a defined number of human astrocytes and neurons while maintaining 3D structure and extracellular components. In order to achieve this system (which we termed asteroids), we combined dissociated neural progenitor cells and pre-matured hAstros at a 1:1 cell ratio. The presence of ROCK inhibitor (Y-27632) for 24 hr promoted sphere formation and survival (Figure 3A). To control cell numbers, we stopped growth factor treatment of hAstros before coculturing and induced neuronal progenitors to convert to a pure population of iNeurons as observed via TUBB3 (Figure 3B). After 35 days of coculture, immunostaining and 3D imaging revealed the continued presence of hAstros evenly, yet randomly, throughout the asteroids, dispersed into distinct territories among the iNeurons (Figure 3C). In all cases, sparse mGFP labeling revealed complex morphology, including fine-membrane processes projecting from individual GFAP-positive branches (Figure 3D). To determine if this 3D environment enhances maturation of astrocytes, we compared protein expression of a well-known astrocyte maturation marker in astrospheres and asteroids. Glutamate transporter GLT1 (also known as EAAT2), which has been reported to upregulate in hAstros post-rodent engraftment (Haidet-Phillips et al., 2014), had increased labeling within the asteroids (Figure S1E).
Figure 3.
Analysis of 3D Asteroid Cocultures
(A) Bright-field examples and illustration of asteroids in the absence (left panel) or presence of hAstros with (right panel) or without induction of NGN2.
(B) Progenitor cells, as rosette structures, are not present with NGN2 induction (right panel).
(C) After 35 days, hAstros are dispersed throughout the asteroids.
(D) mGFP reveals the fine-membrane processes emanating from GFAP-positive hAstro branches.
(E) The addition of hAstros significantly increases the number of opposed pre- and post-synaptic markers compared with iNeurons without hAstros; the combination of iNeurons with hAstros increases synaptic density to a further extent. ∗p < 0.05; ∗∗p < 0.01.
(F) Costello syndrome-model hAstros (HRASG12S) significantly increase synaptic density within asteroids compared with wild-type control (HRASWT) and no hAstro addition. Insets are magnified examples of synapses, including perisynaptic astrocyte processes. ∗∗p < 0.01; ∗∗∗p < 0.001.
(E and F) Data obtained by random sampling of 3–4 different spheres from each of 3 different independent biological replicates.
Error bars represent SEM. Scale bar, 50 μm.
To demonstrate the accessibility of this system and utilization for future approaches such as imaging network communication, hAstros were pre-infected with lentivirus to stably express a genetically encoded calcium indicator, GCaMP6, and following coculture, calcium transients within hAstro processes were observed without the need for cell-type-specific promoters (Figure S1F and Movie S3). Next, as a proof of principle for synapse studies and disease modeling, we generated asteroids containing pre-matured hAstros from a Costello syndrome-specific induced hPSC line with an overactivating mutation in HRAS (HRASG12S). As previously described, HRASG12S hAstros have an increased synaptogenic potential compared with cells generated from a control line (HRASWT) (Krencik et al., 2015). First, we investigated the ability of hAstros to induce the formation of synapses within asteroids with or without induction of NGN2. We measured the density of apposed pre- and post-synaptic markers Synapsin 1 (SYN1) and PSD95, respectively, and discovered an increased density of synapses in the coculture group and furthermore with NGN2 induction at day 35 (iNeurons without hAstros, hNeurons [without NGN2 induction] with hAstros, iNeurons with hAstros, 68.3 ± 15.4, 132.1 ± 15.1, 175.8 ± 22.1 per 100 μm2, respectively; iNeurons without hAstros to hNeurons with hAstros, p = 0.007; iNeurons without hAstros to iNeurons with hAstros, p = 0.002; hNeurons with hAstros to iNeurons with hAstros, p = 0.048; n = 3 independent replicates each) (Figure 3E). Synaptic measurements were repeated with hAstros generated from three non-related wild-type hPSC biological replicate lines at 3 weeks (Figure S2A). In these cultures, we were also able to detect perisynaptic astrocyte processes utilizing the protein marker Ezrin (Lavialle et al., 2011), which surrounded synaptic structures within asteroids, but were not observed in iNeurospheres (Figure S2B). Lastly, the addition of HRASWT hAstros to the iNeurons in 3D asteroid cocultures increased apposed pre- and post-synaptic markers SYN1 and Homer, yet, similar to the values we reported previously (Krencik et al., 2015), we detected more than a 50% increase in synapse density in the presence of HRASG12S hAstros (no hAstros, HRASWT hAstros, HRASG12S hAstro per 100 μm2, 91.7 ± 5.06, 174.3 ± 12.5, 301.67 ± 11.7, respectively; no hAstros to HRASWT, p = 0.007; no hAstros to HRASG12S, p < 0.001; HRASWT to HRASG12S, p = 0.004; n = 3 independent replicates each) (Figure 3F). These data reveal the asteroid coculture system to be an efficient and innovative method to precisely investigate normal and disease-relevant roles of astrocytes upon synapse formation in a 3D setting.
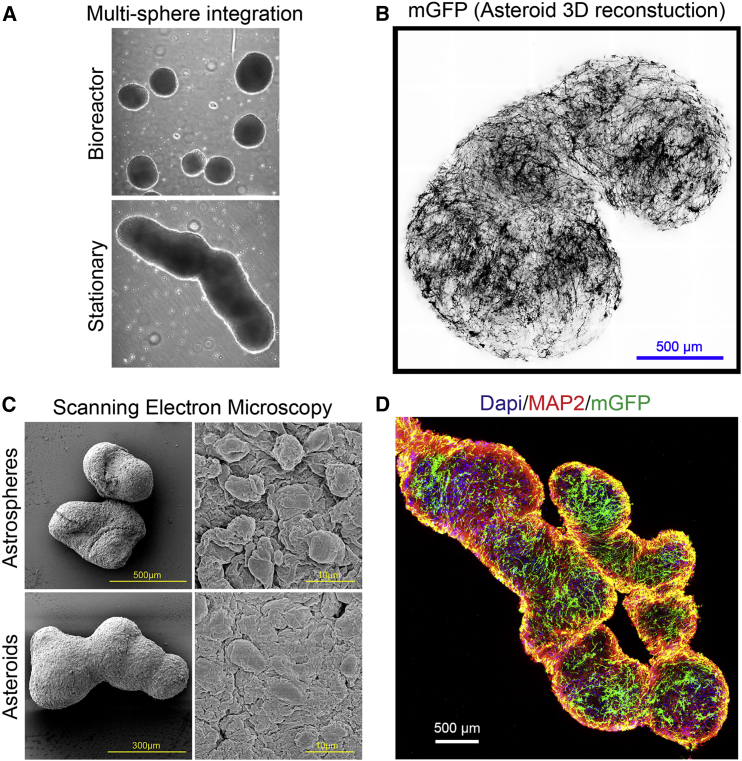
Finally, we sought to generate a more systematic and large-scale bioengineering approach for 3D sphere production rather than the random self-assembly method described above (Figure S4A). We immediately induced NGN2 directly from the hPSC line in 2D for 48 hr in neural medium and then mixed these rapidly maturing progenitors after dissociation with equal numbers of mGFP-labeled post-mitotic hAstros in microwell plates with Y-27632 to generate 3D spheres of specific and consistent sizes (Figure S4B). The ratio of neurons to astrocytes could be altered as desired before sphere formation. In addition, to regulate multiple sphere assembly, we subsequently transferred the resultant asteroids to either bioreactor spinner flasks to promote long-term separation of spheres or to stationary flasks to allow fusion through close physical contact (Figure 4A). This rapid assembly method also produced hAstros with complex morphology by 3 weeks as observed by confocal microscopy and scanning electron microscopy (Figures 4B–4D). Notably, similar to techniques reported for neurosphere organoids (Bagley et al., 2017, Birey et al., 2017, Xiang et al., 2017), this approach demonstrates the utility for investigating long-range migration or signaling of astrocyte-neuron circuits from assembled spheres that were specified to different brain regions, treated in different conditions, or generated from differing genetic backgrounds.
Figure 4.
Bioengineering and Assembly of 3D Spheres
(A) Bioreactor spinner flasks enable long-term separation of spheres, whereas close physical contact promotes merging of multiple spheres.
(B) Morphologically complex hAstros are evenly, yet seemingly randomly, dispersed throughout the asteroids. Neurons are MAP2 positive.
(C and D) Examples of 3D imaging demonstrate the macroscale composition of integrated spheres.
Discussion
How do human astrocytes contribute to local and long-range neural circuit processing? Human explants have been used to address this question, yet the scarcity of available tissue limits the utility of such systems and has led to the emerging use of organoids and/or spheroid systems from hPSCs. Unfortunately, the techniques to date are extremely long and not yet systematic. The work presented here provides an alternative approach with a rapid and controllable technique to study the impact of human astrocyte-neuron interaction in various conditions. As 3D cultures increase background noise during fluorescent imaging compared with 2D monolayers, we plan to introduce light sheet and other emerging technologies (Bindocci et al., 2017) to achieve higher resolution during examination. With augmented complexity (e.g., addition of immune or vascular cells, oligodendrocytes, or other neuronal subtypes), these coculture systems can be further utilized for brain modeling and posing questions about precise cell-cell interactions. For example, do human astrocytes induce oligodendrocytes to myelinate or maintain blood-brain-barrier properties of endothelial cells in 3D conditions? What is the relationship between human astrocytes and microglia in synapse pruning and engulfment?
Human astrocytes have more overlap in their territorial domains, project long processes through many layers of tissue and have bulbous endings compared with their rodent counterparts. Remarkably, these distinguishing features are observed in the systems described here. Perisynaptic astrocyte processes have independent and dynamic responses to local synaptic transmission in the form of calcium fluctuations, yet the functional importance of these local phenomena is still unclear (Bazargani and Attwell, 2016). Because the coculture systems described here preserve neuronal synapses in close association with vellate astrocyte processes, this will now allow for investigation into how these long-process and fine-membrane components influence and respond to neuronal activity.
Experimental Procedures
Neural Differentiation and Processing
hAstros and hNeurons were differentiated from hPSCs (lines H9 [WA09], 13, 162D, 165D, and CS16) and characterized for cellular identity as detailed previously (Krencik et al., 2011, Krencik et al., 2015). iNeurons were produced from a transgenic hPSC line by NGN2 induction via addition of 2 μg/mL doxycycline as described previously (Wang et al., 2017). Astrospheres were supplemented with EGF and FGF2 (10 ng/mL each) before coculture experiments as detailed previously (Krencik and Zhang, 2011) and not forced to be mature or reactive by the addition of exogenous factors. All coculture experiments were conducted in neural medium consisting of DMEM/F12 containing glutamax, sodium bicarbonate, sodium pyruvate, N-2 supplement, B27 supplement (Gibco), and 1 mg/mL heparin (Sigma). For 2D culture, acid-etched glass coverslips were pre-coated with 0.1 mg/mL polyornithine (Sigma) in water and 0.08 mg/mL growth factor-reduced Matrigel matrix (Corning) in medium. Alternatively, cells were cultured on Matrigel-coated chamber slides containing carbon nanofibers (Sigma, Z694576). For organotypic-like cultures, spheres were plated on hydrophilic PTFE cell culture inserts with 30 mm diameter and 0.4 μm pore size (Millicell). For sphere cultures, cells were either self-assembled and maintained in stationary culture or assembled into specific sizes utilizing a microwell plate (Stem Cell Technologies, AggreWell-800 24-well plate) followed by optional spinning in a bioreactor (Corning Proculture Spinner Flask on Dlab MS-C-S4 Magnetic Stirrer). All cultures were maintained in 5% CO2 in 37°C in room oxygen levels.
Sample Processing
Immunoanalysis of spheres consisted of 30 min incubation at 4°C in 4% paraformaldehyde followed by staining of intact 3D spheres or sucrose-embedded cryosectioning as 30 μm sections and attached to slides. Sections were stored at −80°C until immunostaining. For immunoanalysis, samples were blocked in 0.25% Triton X-100 with 5% goat or donkey serum and labeled with the following antibodies: S100 (Abcam, ab868), GFAP (Chemicon, MAB360), GFP (Aves, GFP-1020), TUBB3 (Covance, MMS-435P), GLT1 (a generous gift from Dr. Jeffrey Rothstein), MAP2 (Synaptic Systems, 188 004), Ezrin (Thermo Fisher, MA5-13862), SYN 1 (Synaptic Systems, 106 103), PSD95 (Abcam, ab2723), and Homer (Synaptic Systems, 160 011). hAstro morphology and synaptic colocalization was measured utilizing ImageJ software. Scanning electron microscopy was conducted at the Houston Methodist Research Institute Electron Microscopy Core. mGFP reporter was generated by infecting cells with lentiviral particles generated with a reporter construct as described previously (Addgene plasmid no. 22479; Rompani and Cepko, 2008). For calcium imaging, hAstros were infected with lentivirus generated from Addgene Plasmid no. 72303 (Dempsey et al., 2016).
Statistics
For quantification analysis, statistical tests were performed only when spheres were independently generated from separate starting hPSC cultures. SEM was calculated using averages of data from randomly selected fields throughout the spheres. All measurements are presented as means ± SEM. When appropriate, significance was tested using two-tailed unpaired t tests. In all figures, ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001.
Author Contributions
R.K. and E.M.U. designed the study; R.K., K.S., J.V.v.A., N.B., C.C., and S.B. conducted cell cultures, processing, and data analysis; R.C., C.L., C.W., M.E.W., and L.G. generated and provided the iNeuron line; P.J.H. and D.H.R. provided intellectual contributions; R.K. and E.M.U. co-wrote the paper.
Acknowledgments
R.K. is a member of the Houston Methodist Neurological Institute and the faculty of Weill Cornell Medical College. This work has been supported by the Paul G. Allen Family Foundation Award, SFARI Award 345471, NIMH (R01MH099595-01), That Man May See, NIH-NEI (EY002162) Core Grant for Vision Research, and the Research to Prevent Blindness Unrestricted Grant.
Published: November 30, 2017
Footnotes
Supplemental Information includes three figures and three movies and can be found with this article online at https://doi.org/10.1016/j.stemcr.2017.10.026.
Supplemental Information
Movie is composed of 70 frames total from images taken every 2 s as shown in Figure S1F
References
- Bagley J.A., Reumann D., Bian S., Levi-Strauss J., Knoblich J.A. Fused cerebral organoids model interactions between brain regions. Nat. Methods. 2017;14:743–751. doi: 10.1038/nmeth.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazargani N., Attwell D. Astrocyte calcium signaling: the third wave. Nat. Neurosci. 2016;19:182–189. doi: 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- Bindocci E., Savtchouk I., Liaudet N., Becker D., Carriero G., Volterra A. Three-dimensional Ca2+ imaging advances understanding of astrocyte biology. Science. 2017;356 doi: 10.1126/science.aai8185. [DOI] [PubMed] [Google Scholar]
- Birey F., Andersen J., Makinson C.D., Islam S., Wei W., Huber N., Fan H.C., Metzler K.R.C., Panagiotakos G., Thom N. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545:54–59. doi: 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Suarez E., Caldwell A.L., Allen N.J. Role of astrocyte-synapse interactions in CNS disorders. J. Physiol. 2016;595:1903–1916. doi: 10.1113/JP270988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Qian K., Chen W., Hu B., Blackbourn L.W.T., Du Z., Ma L., Liu H., Knobel K.M., Ayala M. Human-derived neural progenitors functionally replace astrocytes in adult mice. J. Clin. Invest. 2015;125:1033–1042. doi: 10.1172/JCI69097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J.A., Gayol S., Yanez A., Marco P. Immunocytochemical and electron microscope observations on astroglial interlaminar processes in the primate neocortex. J. Neurosci. Res. 1997;48:352–357. [PubMed] [Google Scholar]
- Colombo J.A., Lipina S., Yanez A., Puissant V. Postnatal development of interlaminar astroglial processes in the cerebral cortex of primates. Int. J. Dev. Neurosci. 1997;15:823–833. doi: 10.1016/s0736-5748(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Dempsey G.T., Chaudhary K.W., Atwater N., Nguyen C., Brown B.S., McNeish J.D., Cohen A.E., Kralj J.M. Cardiotoxicity screening with simultaneous optogenetic pacing, voltage imaging and calcium imaging. J. Pharmacol. Toxicol. Methods. 2016;81:240–250. doi: 10.1016/j.vascn.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Goldman S.A. Stem and progenitor cell-based therapy of the central nervous system: hopes, hype, and wishful thinking. Cell Stem Cell. 2016;18:174–188. doi: 10.1016/j.stem.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidet-Phillips A.M., Roybon L., Gross S.K., Tuteja A., Donnelly C.J., Richard J.P., Ko M., Sherman A., Eggan K., Henderson C.E. Gene profiling of human induced pluripotent stem cell-derived astrocyte progenitors following spinal cord engraftment. Stem Cells Transl. Med. 2014;3:575–585. doi: 10.5966/sctm.2013-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Chen M., Wang F., Windrem M., Wang S., Shanz S., Xu Q., Oberheim N.A., Bekar L., Betstadt S. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpel C. Organotypic brain slice cultures: a review. Neuroscience. 2015;305:86–98. doi: 10.1016/j.neuroscience.2015.07.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.A., Weick J.P., Pearce R.A., Zhang S.C. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J. Neurosci. 2007;27:3069–3077. doi: 10.1523/JNEUROSCI.4562-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelava I., Lancaster M.A. Dishing out mini-brains: current progress and future prospects in brain organoid research. Dev. Biol. 2016;420:199–209. doi: 10.1016/j.ydbio.2016.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh B.S., Sofroniew M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R., Ullian E.M. A cellular star atlas: using astrocytes from human pluripotent stem cells for disease studies. Front. Cell. Neurosci. 2013;7:25. doi: 10.3389/fncel.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R., Weick J.P., Liu Y., Zhang Z.J., Zhang S.C. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat. Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R., Hokanson K.C., Narayan A.R., Dvornik J., Rooney G.E., Rauen K.A., Weiss L.A., Rowitch D.H., Ullian E.M. Dysregulation of astrocyte extracellular signaling in Costello syndrome. Sci. Transl. Med. 2015;7:286ra266. doi: 10.1126/scitranslmed.aaa5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R., van Asperen J.V., Ullian E.M. Human astrocytes are distinct contributors to the complexity of synaptic function. Brain Res. Bull. 2016;129:66–73. doi: 10.1016/j.brainresbull.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R., Zhang S.C. Directed differentiation of functional astroglial subtypes from human pluripotent stem cells. Nat. Protoc. 2011;6:1710–1717. doi: 10.1038/nprot.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavialle M., Aumann G., Anlauf E., Prols F., Arpin M., Derouiche A. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA. 2011;108:12915–12919. doi: 10.1073/pnas.1100957108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Javed E., Scura D., Hala T.J., Seetharam S., Falnikar A., Richard J.P., Chorath A., Maragakis N.J., Wright M.C. Human iPS cell-derived astrocyte transplants preserve respiratory function after spinal cord injury. Exp. Neurol. 2015;271:479–492. doi: 10.1016/j.expneurol.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim Bush N.A., Nedergaard M. Do evolutionary changes in astrocytes contribute to the computational power of the hominid brain? Neurochem. Res. 2017 doi: 10.1007/s11064-017-2363-0. [DOI] [PubMed] [Google Scholar]
- Oberheim N.A., Takano T., Han X., He W., Lin J.H., Wang F., Xu Q., Wyatt J.D., Pilcher W., Ojemann J.G. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca A.M., Sloan S.A., Clarke L.E., Tian Y., Makinson C.D., Huber N., Kim C.H., Park J.Y., O'Rourke N.A., Nguyen K.D. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods. 2015;12:671–678. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Nguyen H.N., Song M.M., Hadiono C., Ogden S.C., Hammack C., Yao B., Hamersky G.R., Jacob F., Zhong C. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompani S.B., Cepko C.L. Retinal progenitor cells can produce restricted subsets of horizontal cells. Proc. Natl. Acad. Sci. USA. 2008;105:192–197. doi: 10.1073/pnas.0709979104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simian M., Bissell M.J. Organoids: a historical perspective of thinking in three dimensions. J. Cell Biol. 2017;216:31–40. doi: 10.1083/jcb.201610056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan S.A., Darmanis S., Huber N., Khan T.A., Birey F., Caneda C., Reimer R., Quake S.R., Barres B.A., Pasca S.P. Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron. 2017;95:779–790.e6. doi: 10.1016/j.neuron.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A., Nedergaard M. The homeostatic astroglia emerges from evolutionary specialization of neural cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371 doi: 10.1098/rstb.2015.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Ward M.E., Chen R., Liu K., Tracy T.E., Chen X., Xie M., Sohn P.D., Ludwig C., Meyer-Franke A. Scalable production of iPSC-derived human neurons to identify tau-lowering compounds by high-content screening. Stem Cell Reports. 2017;9:1221–1233. doi: 10.1016/j.stemcr.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Tanaka Y., Patterson B., Kang Y.J., Govindaiah G., Roselaar N., Cakir B., Kim K.Y., Lombroso A.P., Hwang S.M. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell. 2017;21:383–398.e7. doi: 10.1016/j.stem.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Sloan S.A., Clarke L.E., Caneda C., Plaza C.A., Blumenthal P.D., Vogel H., Steinberg G.K., Edwards M.S., Li G. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron. 2016;89:37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie is composed of 70 frames total from images taken every 2 s as shown in Figure S1F