Abstract
Here we show that the most venomous spiders in the world are phylogenetically misplaced. Australian atracine spiders (family Hexathelidae), including the notorious Sydney funnel-web spider Atrax robustus, produce venom peptides that can kill people. Intriguingly, eastern Australian mouse spiders (family Actinopodidae) are also medically dangerous, possessing venom peptides strikingly similar to Atrax hexatoxins. Based on the standing morphology-based classification, mouse spiders are hypothesized distant relatives of atracines, having diverged over 200 million years ago. Using sequence-capture phylogenomics, we instead show convincingly that hexathelids are non-monophyletic, and that atracines are sister to actinopodids. Three new mygalomorph lineages are elevated to the family level, and a revised circumscription of Hexathelidae is presented. Re-writing this phylogenetic story has major implications for how we study venom evolution in these spiders, and potentially genuine consequences for antivenom development and bite treatment research. More generally, our research provides a textbook example of the applied importance of modern phylogenomic research.
Introduction
Atrax robustus, the Sydney funnel-web spider, is often considered the world’s most venomous spider species1. The neurotoxic bite of a male A. robustus causes a life-threatening envenomation syndrome in humans. Although antivenoms have now largely mitigated human deaths, bites remain potentially life-threatening2. Atrax is a member of a larger clade of 34 described species, the mygalomorph subfamily Atracinae, at least six of which (A. robustus and five Hadronyche species) cause severe envenomation in humans3. The venoms of a handful of assayed atracines include a δ-hexatoxin that induces delayed inactivation of voltage-gated sodium channels in primates4,5. Atracine venoms also include insect-specific inhibitor cystine knot (ICK) neurotoxins6 that have been proposed as natural bioinsecticides5,7,8. Chassagnon et al.9 recently showed that a unique Hadronyche double-knot venom peptide shows therapeutic potential in protecting the human brain from damage after stroke events.
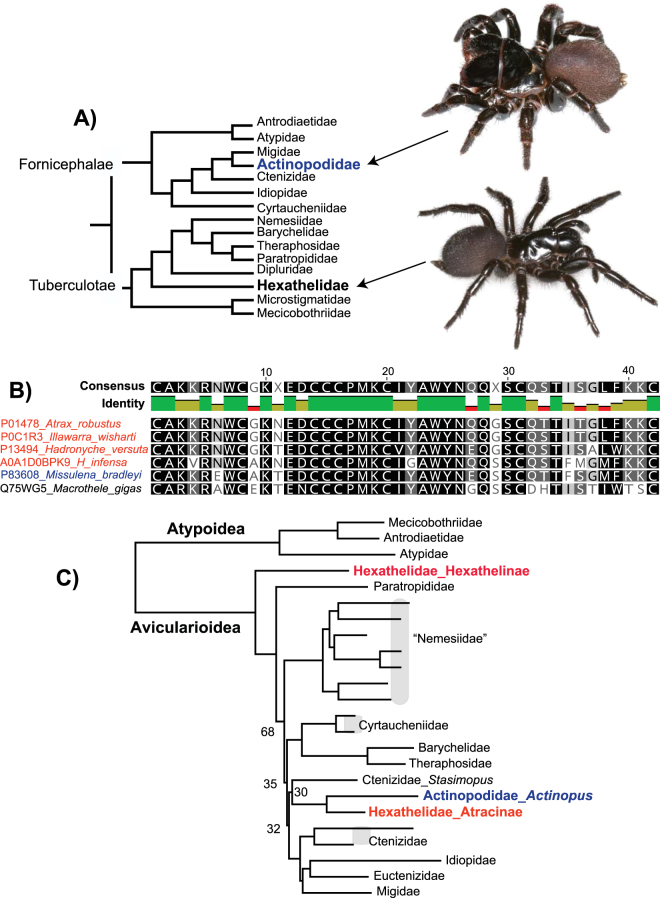
Atracinae includes three genera (Atrax, Illawarra, and Hadronyche10,11), found in eastern and southern Australia, currently placed in the family Hexathelidae. Based on the currently accepted classification [following refs12,13], hexathelids are distantly related to actinopodid mygalomorphs (Fig. 1A), an austral family that includes Australian mouse spiders (Missulena). Significantly, Missulena venom has a mode of action similar to that of Atrax, includes peptides clearly homologous to δ-hexatoxins (Fig. 1B), and Missulena bites are treated effectively using Atrax antivenoms14–16. Gunning et al.15 proposed that the similarities observed between Atrax and Missulena venoms “provides evidence of a highly conserved spider N-toxin from a phylogenetically distinct spider family that has not undergone significant modification”. This “ancient conservation” hypothesis implies a broad phylogenetic distribution of potentially dangerous venom proteins in mygalomorph spiders (Fig. 1A), although an alternative is convergent evolution at the protein level in distant relatives.
Figure 1.
(A) Summary of Raven12 phylogeny and currently accepted family-level classification of mygalomorph spiders (except for new family Euctenizidae13), with distant placement of hexathelids and actinopodids highlighted. Taxonomic names follow Raven12. Images of live female Missulena sp. and Atrax robustus. (B) Missulena and Atrax δ-hexatoxin homology. Results based on UniProt BLASTP search of mature δ-hexatoxin-Ar1a. (C) Summary of Hamilton et al.19 phylogeny, based on concatenated RAxML analysis of 327 anchored hybrid enrichment loci. Bootstrap = 100 if not shown.
We instead hypothesize that Atrax and Missulena venom similarities reflect homology from more recent shared ancestry (“recent homology” hypothesis). An atracine plus actinopodid relationship has been suggested in multiple molecular phylogenetic studies [refs13,17–20; Fig. 1C], all of which were hindered by a small and incomplete sample of hexathelids and actinopodids. Here we test the recent homology hypothesis using phylogenomic analyses of ultraconserved element (UCE) sequences for a taxon sample that includes all described hexathelid and actinopodid genera, and a relevant sample of other mygalomorph genera. We show convincingly that hexathelids are not monophyletic, and that atracines are sister to austral actinopodids. This result has significant implications for mygalomorph family-level classification, and for the study of venom evolution in these medically and economically important spiders.
Results and Discussion
We sampled all described hexathelid and actinopodid genera21. Many of these genera are geographically restricted and rare (e.g., Plesiothele from isolated highlands in Tasmania, Plesiolena from a handful of specimens from remote Chile), thus requiring the use of standard museum specimens for DNA extraction from some taxa (see Methods). In addition, we sampled atypoids as outgroups, and multiple diplurid genera, following hypothesized affinities of hexathelids with diplurids12,13,18,22,23. Although we did not generate UCE data for representatives of all mygalomorph families, there are no genera missing from our sample that are clear close atracine or actinopodid relatives, as suggested by recent molecular phylogenetic studies13,19,20.
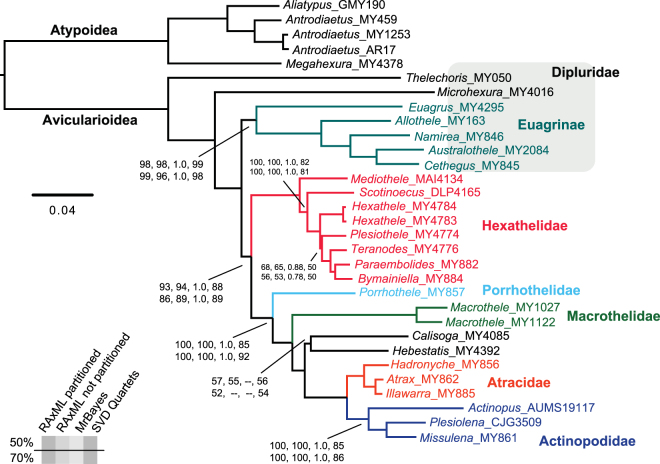
We analyzed both 50% (514 loci, 101652 basepairs) and 70% occupancy (381 loci, 78103 basepairs) UCE matrices (Supplemental Table 1). The following pertinent clades were recovered with full support (bootstrap = 100, posterior probability = 1.0) in all phylogenomic analyses, regardless of method or model used: Avicularioidea (non-atypoids with male bulb sclerites fused, lacking abdominal sclerites, etc.), Hexathelinae (including Plesiothele), Atracinae, and an atracine plus actinopodid clade (Fig. 2, Supplemental Fig. 1). Hexathelids are always fragmented into four distinct lineages, below reclassified as four separate families. As such, austral biogeographic patterns (southern South America + Australia/New Zealand) are independently replicated in the hexatheline and actinopodid lineages. Concatenated and coalescent methods are consistent in the recovery of major clades. One notable difference is the ASTRAL placement of the Porrhothele plus relatives clade, but this placement is weakly supported in ASTRAL analyses (Fig. 2, Supplemental Fig. 1).
Figure 2.
Partitioned RAxML concatenated phylogeny, based on 70% occupancy matrix. Support values from other analyses shown. If support values not shown, support = 100 or 1.0. Calisoga plus Hebestatis sister relationship is poorly supported, in some phylogenies recovered as (Hebestatis, (Calisoga, (atracids + actinopodids))).
Many mygalomorph species are short-range endemics, known from few locations, often from very few (<10) specimens. In addition, these spiders often live notoriously cryptic lifestyles, residing in difficult-to-find subterranean burrows, concealed by hidden trapdoors or other entrance constructs. Basically all previous molecular phylogenetic studies have been somewhat hindered by this rareness and/or cryptic biology, with taxa excluded because “DNA-preserved” samples were unavailable. Here we were able to generate 100 s of UCE loci from specimens collected over 30 years ago, and subsequently preserved in low percentage alcohol at room temperatures (i.e., “standard” museum specimens). It is important to note that only museum specimens extracted using phenol/chloroform resulted in useable data; four older specimens extracted using Qiagen all failed (Supplemental Table 1). UCE-based phylogenomics from museum specimens has been demonstrated for other animal taxa [e.g., bees24, birds25, snakes26,]. Our study extends this utility to arachnids, and demonstrates the potential effectiveness of the UCE method for thousands of rare taxa currently residing in museums worldwide.
Detailed comparisons to earlier studies of mygalomorph phylogeny are provided in the Supplemental Text. Here we make three general claims that are supported by this and prior studies. First, hexathelids, defined by a single morphological synapomorphy (possession of numerous labial cuspules12,27), are not monophyletic and require re-classification. Second, hexathelines (with numerous labial cuspules and six spinnerets) are relatively early-diverging avicularioids, along with multiple non-diplurine diplurid lineages (e.g., Ischnothelinae, Euagrinae, etc.). Third, atracine hexathelids are monophyletic, and sister to a monophyletic Actinopodidae. This combined lineage occupies a relatively derived position in mygalomorph phylogeny [see also ref.19]. A much larger phylogenomic sample including multiple representatives of all mygalomorph families will be required to solidify this placement.
Like other spiders, atracine venoms are complex chemical cocktails, including a very large number of peptides and other molecules7. For example, Palagi et al.28 used modern mass spectrometry methods to survey venoms of multiple atracine taxa and found a large number of peptides (800 peptides in female venoms, ~400 in male venoms), marked sexual differences, and clear species-level differences. Despite this peptide diversity, primate-targeting δ-hexatoxins are a primary component of the atracine venom peptidome4, with some species possessing multiple δ-hexatoxin in-paralogs28. Even with minor differences at the protein level (Fig. 1B), bites of all atracines with these δ-hexatoxins cause a superficially similar envenomation syndrome in humans4,29. Among known spider venom peptides, the δ-actinopoditoxin of male Missulena bradleyi is most similar to atracine δ-hexatoxins (Fig. 1B), and M. bradleyi venoms have a similarly selective mode of action on vertebrate sodium channels14,15. Furthermore, Missulena bites are sometimes of medical concern16,30, and such bites are effectively treated using antivenoms developed for atracines14. Our phylogenomic results (Fig. 2) indicate that all of these biological similarities reflect recent shared common ancestry of these spider lineages, rather than the alternatives of convergence or ancient phylogenetic conservation of venom composition.
Our phylogenomic hypothesis (Fig. 2) provides a robust comparative framework for addressing the evolutionary assembly of venoms, including the medically important δ-hexatoxins, in the atracine plus actinopodids clade. We make the following general predictions. First, we hypothesize that both Actinopus and Plesiolena possess homologs of δ-hexatoxins. Characterization of Actinopus venom peptides in particular would allow reconstruction of ancestral proteins for the entire clade. Our prediction also implies that both Actinopus and Plesiolena, like Missulena, have the potential to cause dangerous bites. We note however that Mullen and Vetter31 state that Actinopus bites in southern South America “produce only local pain and transient muscle contractions”. Our phylogenomic hypothesis indicates that the most relevant taxon for understanding Atrax venom evolution is the little-studied sister genus Illawarra, as also reflected by very high δ-hexatoxin similarity (Fig. 1B). Finally, we note that the species tree framework specified here provides a basis for the study of all venom molecules in these spiders, such as the insect-specific ICK neurotoxins in the Shiva superfamily6. A comprehensive study of the venom peptidome in all atracine plus actinopodid genera would provide considerable insight into molecular evolution in these important spiders.
Taxonomy
Here we summarize the revised taxonomy of the Hexathelidae and related new familial rank taxa; all nomenclatural changes proposed are to be attributed to Hedin and Bond. The subfamily Atracinae is removed from Hexathelidae and elevated to the rank of family (NEW RANK); it includes the genera listed below. The subfamily Macrothelinae (Simon, 1892) is removed from Hexathelidae and elevated to the rank of family (NEW RANK). The genus Porrhothele is removed from Hexathelidae (subfamily Macrothelinae) and designated as a family (NEW FAMILY). The revised circumscription of the family Hexathelidae is documented below.
Family Atracidae Hogg, 1901 (NEW RANK)
Type genus
Atrax O. Pickard-Cambridge, 1877 (type species Atrax robustus O. Pickard-Cambridge, 1877).
Remarks
Atraceae, originally described by Hogg32, comprised the two genera Atrax and Hadronyche. The group was subsequently formally designated as a subfamily by Gray10 and diagnosed on the basis of taxa having “a broad embolic shaft” (males) and having two rows of large cheliceral teeth along with distinctive leg spination (spines on the tarsi), numerous labial cuspules, and a “coniform” anterior endite lobe. Gray11 provided a more thorough diagnosis and description of the subfamily along with a new circumscription to include the genus Illawarra. Atracidae are found in Australia (Queensland, New South Wales, Australian Capital Territory, Victoria, South Australia and Tasmania).
List of included genera
Atrax O. Pickard-Cambridge, 1877 [urn:lsid:nmbe.ch:spidergen:00013]
Hadronyche L. Koch, 1873 [urn:lsid:nmbe.ch:spidergen:00015]
Illawarra Gray, 2010 [urn:lsid:nmbe.ch:spidergen:03995].
Family Macrothelidae (Simon, 1892) (NEW RANK)
Type genus
Macrothele Ausserer, 1871 [urn:lsid:nmbe.ch:spidergen:00017] (type species Macrothele calpeiana Walkenaer, 1805).
Remarks
As a consequence of its monotypy, characters used to diagnose the family Macrothelidae are those characters attributed to the type genus. Per Raven27 macrothelids can be diagnosed from other mygalomorph taxa by having much larger posterior sternal sigilla and a single row of larger teeth on the cheliceral promargin with smaller teeth on the retromargin. As with other taxa discussed in this study, a more careful examination of morphology is warranted in light of these changes. Although monogeneric families are not ideal, it should be noted that members of the genus Macrothele are consistently recovered as a distinct, relatively early-diverging avicularioid lineage (e.g., see18; Supplemental Text). This monotypic family is known from Africa, Asia and parts of Europe (Spain, Italy, Greece).
Family Porrhothelidae (NEW FAMILY)
Type genus
Porrhothele Simon, 1892 [urn:lsid:nmbe.ch:spidergen:00021] (type species Porrothele antipodiana (Walkenaer, 1837).
Diagnosis
As a consequence of its monotypy, characters used to diagnose the family Porrhothelidae are those characters attributed to the type genus. Porrhothele was thoroughly diagnosed and described by Forster33 with additions by Raven27. Members of this family can be diagnosed on the basis of the following unique combination of characters: 1) small posterior sternal sigilla; 2) single row of promarginal cheliceral teeth; 3) male tibia swollen with dense pattern of strong promarginal spines (illustrated in Forster;33 e.g., p. 170, figs. 543–548). This monotypic family is found in New Zealand.
Family Hexathelidae (Simon, 1892) (new circumscription)
Type genus
Hexathele Ausserer, 1871 [urn:lsid:nmbe.ch:spidergen:00016] (type species Hexathele hochstetteri Ausserer, 1871)
Subfamily Hexathelinae (Simon, 1892)
Plesiothelinae Raven 1980. Type genus Plesiothele Raven, 1978 (type species Plesiothele fentoni (Hickman, 1936)). New synonymy.
Remarks
Hexathelidae is found in Australia (Queensland, New South Wales, Australian Capital Territory, Victoria, Tasmania), New Zealand, Chile and Argentina.
List of included genera
Bymainiella Raven, 1978 [urn:lsid:nmbe.ch:spidergen:00014]
Hexathele Ausserer, 1871 [urn:lsid:nmbe.ch:spidergen:00016]
Mediothele Raven & Platnick, 1978 [urn:lsid:nmbe.ch:spidergen:00018]
Paraembolides Raven, 1980 [urn:lsid:nmbe.ch:spidergen:00019]
Plesiothele Raven, 1978 [urn:lsid:nmbe.ch:spidergen:00020]
Scotinoecus Simon, 1892 [urn:lsid:nmbe.ch:spidergen:00022]
Teranodes Raven, 1985 [urn:lsid:nmbe.ch:spidergen:00023]
Methods
Specimen sampling and DNA extraction
A majority of specimens were personally identified by the authors, and are vouchered at SDSU, Auburn, and Museo Argentino de Ciencias Naturales (Supplemental Table 1). Most specimens were preserved for DNA studies (preserved in high percentage ethyl alcohol at −80 °C), and genomic DNA was extracted from leg tissue using the Qiagen DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA). Standard phenol/chloroform extractions with 24-hour incubation for lysis were used for three samples (Mediothele nahuelbuta MAI4134, Plesiolena bonnetti CJG3509, Scotinoecus sp. DLP4165; Supplemental Table 1), as tissues were preserved in 70–80% EtOH and initial Qiagen extractions produced no quantifiable DNA. All extractions were quantified using a Qubit Fluorometer (Life Technologies, Inc.) and quality was assessed via gel electrophoresis on a 1% agarose gel. Qiagen extractions resulted in >500 ng for library preparation, while phenol/chloroform extractions of older material resulted in 26–226 ng total (Supplemental Table 1). UCE data for seven taxa were generated by Starrett et al.34.
UCE data collection & matrix assembly
Sequence capture data were collected in multiple library preparation and sequencing experiments that differed mainly in sequencing platform. Up to 500 ng of genomic DNA was used in sonication, using either a Bioruptor for 7 cycles at 30 s on and 90 s off, or using a Covaris M220 Focused-ultrasonicator with treatment time of 65 s, Peak Incident Power of 50, 10% Duty Factor, and 200 cycles per burst. Samples were run out on agarose gels to verify sonication success.
Library preparation followed Starrett et al.34, with some modifications. Briefly, libraries were prepared using the KAPA Hyper Prep Kit (Kapa Biosystems), using up to 250 ng DNA (i.e., half reaction of manufacturer’s protocol) as starting material. Ampure XP beads (Beckman Coulter) were used for all cleanup steps. For samples containing <250 ng total, all DNA was used in library preparation. After end-repair and A-tailing, universal adapters were ligated onto libraries. Libraries were then amplified in a 25 μl reaction, with 15 μl adapter-ligated DNA, 25 μl 1 × HiFi HotStart ReadyMix, and 2.5 μl of each Illumina TruSeq dual-indexed primer (i5 and i7) with modified 8-bp indexes35. Amplification conditions were 98 °C for 45 s, then 16 or 18 cycles of 98 °C for 15 s, 60 °C for 30 s, and 72 °C for 60 s, followed by a final extension of 72 °C for 60 s. Samples were quantified again to ensure amplification success. Equimolar amounts of libraries were combined into 1000 ng total pools consisting of eight samples each (125 ng per sample).
Target enrichment was performed on pooled libraries using the MYbaits Arachnida 1.1 K version 1 kit (Arbor Biosciences36;) following the Target Enrichment of Illumina Libraries v. 1.5 protocol (http://ultraconserved.org/#protocols). Hybridization was conducted at 60 or 65 °C for 24 hours. Following hybridization, pools were amplified in a 50 μl reaction consisting of 15 μl of hybridized pools, 25 μl Kapa HiFi HotStart ReadyMix, 5 μl dH20, and 5 μM of each of TruSeq forward and reverse primers. Amplification conditions consisted of 98 °C for 45 s, then 16 or 18 cycles of 98 °C for 15 s, 60 °C for 30 s, and 72 °C for 60 s, followed by a final extension of 72 °C for 5 minutes. Following an additional cleanup, libraries were quantified using a Qubit fluorometer and equimolar mixes were prepared for sequencing either with an Illumina NextSeq (University of California, Riverside Institute for Integrative Genome Biology) or an Illumina HiSeq. 2500 (Brigham Young University DNA Sequencing Center).
Raw demultiplexed reads were processed with the Phyluce pipeline37. Quality control and adapter removal were conducted with the Illumiprocessor wrapper38. Assemblies were created with Velvet39 and Trinity40, both at default settings. Contigs from both assemblies were combined for probe matching, retrieving assembly-specific UCEs and overall increasing the number of UCEs per sample relative to using only a single assembly method. Contigs were matched to probes using minimum coverage and minimum identity values of 65. UCE loci were aligned with MAFFT41 and trimmed with Gblocks42,43 implemented in the Phyluce pipeline.
Individual UCE loci were imported into Geneious 10.1 (Biomatters Ltd.) and manually inspected. In particular, alignments with low % identical sites (less than 40%) were flagged for inspection. If exclusion of a single divergent sequence increased this value to >60%, the locus was retained. Subsequently, all loci were inspected - individual sequences with large internal (in conserved UCE region) gaps were excluded, and obvious alignment errors were manually adjusted. Finally, individual RAxML44 gene trees were reconstructed, with those loci not recovering a monophyletic Atypoidea excluded (taken as evidence for paralogy).
Phylogenomic analyses
Two datasets were assembled for phylogenomic analyses, differing in the minimum taxon coverage (50% and 70%) needed for a locus to be included in the final dataset. Concatenated and partitioned maximum likelihood and Bayesian analyses were run for each dataset. Maximum likelihood analyses were conducted using RAxML version 8.244 with the GTRGAMMA model and 200 rapid bootstrap replicates. Bayesian analyses were conducted with MrBayes 3.2.645 on the CIPRES portal46. Analyses were run for 10 million generations, logging every 1000 generations. For partitioned analyses, partitions and models were chosen using PartitionFinder 1.1.147. Two coalescent analyses were also conducted for both datasets. First, ASTRAL-II48,49 was used with individual gene trees estimated in RAxML with 500 bootstrap replicates. We also used SVDquartets50,51 with n = 500 bootstraps, as implemented in PAUP* 4.052.
Data availability
The datasets generated and analyzed during the current study are available from the NCBI Short-Read Archive (raw sequence reads, BioProject PRJNA423032) and Dryad (aligned matrices and. tre files, doi:10.5061/dryad.8d638).
Electronic supplementary material
Acknowledgements
We thank P. Cardoso, C. Grismado, C. Griswold, M. Harvey, C. Hayashi, D. Leavitt, B. McQuillan, V. Opatova, R. Raven, J. Satler, A.H. Simpson, J. Starrett and the late G. Wishart for help with specimen collection and/or specimen loans. J. Starrett was instrumental in collecting initial mygalomorph UCE data. The Rohwer lab at SDSU allowed use of a Covaris ultrasonicator. Comments of D. Leavitt helped to improve the manuscript. Permits for collection of most specimens are reported in Hedin & Bond (2006). Tasmanian Plesiothele and Teranodes were collected under permit #FA 16289, and specimens from Chile were collected under CONAF permit 027/2011. Research was funded by US National Science Foundation grants to MH (DEB 1354558) and SD (DEB 1601208).
Author Contributions
M.H. & J.B. conceptualized the research. M.H. & S.D. conducted formal analysis. All authors provided resources, and were involved in the writing, review, and editing of the manuscript. M.H. & S.D. acquired funding in support of the research.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-19946-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nicholson GM, Graudins A. Spiders of medical importance in the Asia-Pacific: Atracotoxin, latrotoxin and related spider neurotoxins. Clin. Exp. Pharm. Physio. 2002;29:785–794. doi: 10.1046/j.1440-1681.2002.03741.x. [DOI] [PubMed] [Google Scholar]
- 2.Isbister GK, et al. Catecholamine-induced cardiomyopathy resulting from life-threatening funnel-web spider envenoming. Medical J. Australia. 2015;203:302–304. doi: 10.5694/mja15.00279. [DOI] [PubMed] [Google Scholar]
- 3.Isbister GK, et al. Funnel-web spider bite: a systematic review of recorded clinical cases. Medical J. Australia. 2005;182:407–411. doi: 10.5694/j.1326-5377.2005.tb06760.x. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson GM, Little MJ, Birinyi-Strachan LC. Structure and function of δ-atracotoxins: lethal neurotoxins targeting the voltage-gated sodium channel. Toxicon. 2004;43:587–599. doi: 10.1016/j.toxicon.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Tedford H, Sollod B, Maggio F, King GF. Australian funnel-web spiders: Master insecticide chemists. Toxicon. 2004;43:601–618. doi: 10.1016/j.toxicon.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Pineda SS, et al. Diversification of a single ancestral gene into a successful toxin superfamily in highly venomous Australian funnel-web spiders. BMC Genomics. 2014;15(177):1–177.16. doi: 10.1186/1471-2164-15-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King, G. F. & Hardy, M. C. Spider-venom peptides: structure, pharmacology, and potential for control of insect pests. Ann. Rev. Ent.58, 475–496 (2013). [DOI] [PubMed]
- 8.Herzig V, King GF. The cystine knot is responsible for the exceptional stability of the insecticidal spider toxin ω-Hexatoxin-Hv1a. Toxins. 2015;7:4366–4380. doi: 10.3390/toxins7104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chassagnon IR, et al. Potent neuroprotection after stroke afforded by a double-knot spider-venom peptide that inhibits acid-sensing ion channel 1a. Proc. Nat. Acad. Sci. 2017;114:201614728. doi: 10.1073/pnas.1614728114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray MR. Aspects of the systematics of the Australian funnel web spiders (Araneae: Hexathelidae: Atracinae) based upon morphological and electrophoretic data. Australian Ent. Soc. Misc. Pub. 1988;5:113–125. [Google Scholar]
- 11.Gray MR. A revision of the Australian funnel-web spiders (Hexathelidae: Atracinae) Records Australian Mus. 2010;62:285–392. doi: 10.3853/j.0067-1975.62.2010.1556. [DOI] [Google Scholar]
- 12.Raven RJ. The spider infraorder Mygalomorphae (Araneae): Cladistics and systematics. Bull. Amer. Mus. Nat. Hist. 1985;182:1–180. [Google Scholar]
- 13.Bond JE, Hendrixson BE, Hamilton CA, Hedin M. A reconsideration of the classification of the spider infraorder Mygalomorphae (Arachnida: Araneae) based on three nuclear genes and morphology. PLoS One. 2012;7(6):e38753. doi: 10.1371/journal.pone.0038753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rash, L. D., Birinyi-Strachan, L. C., Nicholson, G. M. & Hodgson, W. C. Neurotoxic activity of venom from the Australian Eastern mouse spider (Missulena bradleyi) involves modulation of sodium channel gating. British J. Pharm.130, 1817–1824 (2000). [DOI] [PMC free article] [PubMed]
- 15.Gunning SJ, et al. Isolation of δ-missulenatoxin-Mb1a, the major vertebrate-active spider δ-toxin from the venom of Missulena bradleyi (Actinopodidae) FEBS Letters. 2003;554(1–2):211–218. doi: 10.1016/S0014-5793(03)01175-X. [DOI] [PubMed] [Google Scholar]
- 16.Isbister GK. Mouse spider bites (Missulena spp.) and their medical importance. Medical J. Australia. 2004;180:225–227. [PubMed] [Google Scholar]
- 17.Ayoub NA, Garb JE, Hedin M, Hayashi CY. Utility of the nuclear protein-coding gene, elongation factor-1gamma (EF-1γ), for spider systematics, emphasizing family level relationships of tarantulas and their kin (Araneae: Mygalomorphae) Mol. Phylo. Evol. 2007;42:394–409. doi: 10.1016/j.ympev.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Opatova, V. & Arnedo, M. From Gondwana to Europe: Inferring the origins of Mediterranean Macrothele spiders (Araneae: Hexathelidae) and the limits of the family Hexathelidae. Invert. Syst.28, 361–374 (2014).
- 19.Hamilton CA, Lemmon AR, Lemmon EM, Bond JE. Expanding anchored hybrid enrichment to resolve both deep and shallow relationships within the spider tree of life. BMC Evol. Biol. 2016;16:212. doi: 10.1186/s12862-016-0769-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheeler WC, et al. The spider tree of life: phylogeny of Araneae based on target-gene analyses from an extensive taxon sampling. Cladistics. 2017;33:574–616. doi: 10.1111/cla.12182. [DOI] [PubMed] [Google Scholar]
- 21.World Spider Catalog. Natural History Museum Bern, version 18. 5. http://wsc.nmbe.ch. 10.24436/2 (2017).
- 22.Goloboff P. A reanalysis of mygalomorph spider families (Araneae) Am. Mus. Novitates. 1993;3056:1–32. [Google Scholar]
- 23.Hedin M, Bond JE. Molecular phylogenetics of the spider infraorder Mygalomorphae using nuclear rRNA genes (18S and 28S): Conflict and agreement with the current system of classification. Mol. Phylo. Evol. 2006;41:454–471. doi: 10.1016/j.ympev.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Blaimer BB, Lloyd MW, Guillory WX, Brady SG. Sequence capture and phylogenetic utility of genomic ultraconserved elements obtained from pinned insect specimens. PLoS ONE. 2016;11(8):e0161531. doi: 10.1371/journal.pone.0161531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormack JE, Tsai WLE, Faircloth BC. Sequence capture of ultraconserved elements from bird museum specimens. Mol. Ecol. Res. 2016;16:1189–1203. doi: 10.1111/1755-0998.12466. [DOI] [PubMed] [Google Scholar]
- 26. Ruane, S. & Austin, C. C. Phylogenomics using formalin-fixed and 100+ year-old intractable natural history specimens. Mol. Ecol. Res. 10.1111/1755-0998.12655 (2017). [DOI] [PubMed]
- 27.Raven RJ. The evolution and biogeography of the mygalomorph spider family Hexathelidae (Araneae, Chelicerata) J. Arachnology. 1980;8:251–266. [Google Scholar]
- 28.Palagi A, et al. Unravelling the complex venom landscapes of lethal Australian funnel-web spiders (Hexathelidae: Atracinae) using LC-MALDI-TOF mass spectrometry. J. Proteomics. 2013;80:292–310. doi: 10.1016/j.jprot.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Graudins A, Wilson D, Alewood PF, Broady KW, Nicholson GM. Cross-reactivity of Sydney funnel-web spider antivenom: neutralization of the in vitro toxicity of other Australian funnel-web (Atrax and Hadronyche) spider venoms. Toxicon. 2002;40:259–266. doi: 10.1016/S0041-0101(01)00210-0. [DOI] [PubMed] [Google Scholar]
- 30.Isbister G, Gray M. Bites by Australian mygalomorph spiders (Araneae, Mygalomorphae), including funnel-web spiders (Atracinae) and mouse spiders (Actinopodidae: Missulena spp) Toxicon. 2004;43:133–40. doi: 10.1016/j.toxicon.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Mullen, G. & Vetter, R. S. Spiders (Araneae), in Medical and Veterinary Entomology, Mullen, G., Durden, L., eds. Academic Press, New York, 413–432 (2009).
- 32.Hogg HR. On Australian and New Zealand spiders of the suborder Mygalomorphae. Proc. Zool. Soc. London. 1901;71(1):218–279. doi: 10.1111/j.1469-7998.1901.tb08176.x. [DOI] [Google Scholar]
- 33.Forster RR. The spiders of New Zealand. Part II. Ctenizidae, Dipluridae. Otago Mus. Bull. 1968;2(1–72):126–180. [Google Scholar]
- 34.Starrett J, et al. High phylogenetic utility of an Ultraconserved element probe set designed for Arachnida. Mol. Ecol. Res. 2017;17:812–823. doi: 10.1111/1755-0998.12621. [DOI] [PubMed] [Google Scholar]
- 35.Glenn, T. C. et al. Adapterama I: universal stubs and primers for thousands of dual-indexed Illumina libraries (iTru & iNext). bioRxiv. 10.1101/049114 (2016). [DOI] [PMC free article] [PubMed]
- 36.Faircloth BC. Identifying conserved genomic elements and designing universal probe sets to enrich them. Methods Ecol. Evol. 2017;8:1103–1112. doi: 10.1111/2041-210X.12754. [DOI] [Google Scholar]
- 37.Faircloth BC. PHYLUCE is a software package for the analysis of conserved genomic loci. Bioinformatics. 2016;32:786–788. doi: 10.1093/bioinformatics/btv646. [DOI] [PubMed] [Google Scholar]
- 38.Faircloth, B. C. Illumiprocessor: a trimmomatic wrapper for parallel adapter and quality trimming. http://dx.doi.org/10.6079/J9ILL (2013).
- 39.Zerbino DR, Birney E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grabherr MG, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotech. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katoh D, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- 43.Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007;56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 44.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ronquist F, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller, M. A., Pfeiffer, W. & Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees in Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, LA pp 1–8 (2010).
- 47.Lanfear R, Calcott B, Ho SY, Guindon S. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012;29:1695–1701. doi: 10.1093/molbev/mss020. [DOI] [PubMed] [Google Scholar]
- 48.Mirarab S, et al. ASTRAL: genome-scale coalescent-based species tree estimation. Bioinformatics. 2014;30:i541–i548. doi: 10.1093/bioinformatics/btu462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mirarab S, Warnow T. ASTRAL-II: coalescent-based species tree estimation with many hundreds of taxa and thousands of genes. Bioinformatics. 2015;31:i44–i52. doi: 10.1093/bioinformatics/btv234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chifman J, Kubatko L. Quartet inference from SNP data under the coalescent. Bioinformatics. 2014;30:3317–3324. doi: 10.1093/bioinformatics/btu530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chifman J, Kubatko L. Identifiability of the unrooted species tree topology under the coalescent model with time-reversible substitution processes, site-specific rate variation, and invariable sites. J. Theoret. Biol. 2015;374:35–47. doi: 10.1016/j.jtbi.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Swofford, D. L. PAUP*: phylogenetic analysis using parsimony, version 4.0 b10 (2003).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the NCBI Short-Read Archive (raw sequence reads, BioProject PRJNA423032) and Dryad (aligned matrices and. tre files, doi:10.5061/dryad.8d638).