ABSTRACT
Ceftazidime-avibactam and ceftolozane-tazobactam are newly approved agents for the treatment of infections caused by multidrug-resistant Gram-negative bacteria. Resistance to both agents has been described clinically. Susceptibility testing on automated systems is unavailable for either agent. Our objective was to compare the disk diffusion and Etest methods to standard broth microdilution (BMD) methods for testing ceftazidime-avibactam and ceftolozane-tazobactam against a diverse collection of carbapenem-resistant Enterobacteriaceae (CRE) and carbapenem-resistant Pseudomonas aeruginosa (CRP) isolates, respectively. Among 74 ceftazidime-avibactam-susceptible and -resistant CRE isolates, BMD categorical agreement was higher with Etest (96%) than with disk diffusion (72%; P = 0.0003). Twenty-eight percent of ceftazidime-avibactam-susceptible CRE isolates were classified as resistant by disk diffusion. Results were comparable to those obtained with resistance defined genotypically. Among 72 ceftolozane-tazobactam-susceptible and -resistant CRP isolates, the levels of BMD categorical agreement with disk diffusion and Etest were 94% and 96%, respectively; the only errors identified were minor. Our findings demonstrate that Etest measurements of ceftazidime-avibactam and ceftolozane-tazobactam susceptibility correlate closely with standard BMD methods, suggesting a useful role clinically. On the other hand, disk diffusion measurements overcalled CRE resistance to ceftazidime-avibactam. A better understanding of ceftazidime-avibactam interpretive breakpoints is needed before disk diffusion is used routinely in the clinic. Until clinicians and microbiologists understand Etest and disk diffusion performance at their centers, test results should be interpreted cautiously.
KEYWORDS: carbapenem-resistant Enterobacteriaceae, carbapenem-resistant Pseudomonas, ceftazidime-avibactam resistance, ceftolozane-tazobactam resistance, Etest, disk diffusion
INTRODUCTION
Emergence of resistance to the newly approved antibiotics ceftazidime-avibactam (ceftaz-avi) and ceftolozane-tazobactam (ceftol-taz) is increasingly recognized among carbapenem-resistant Enterobacteriaceae (CRE) and carbapenem-resistant Pseudomonas aeruginosa (CRP) isolates, respectively (1–3). Accurate susceptibility testing is critical for identifying resistance and optimizing the use of these agents. The U.S. Food and Drug Administration (FDA) has cleared disk diffusion susceptibility testing for ceftazidime-avibactam and ceftolozane-tazobactam. Research-use-only (RUO) Etests are also available for both agents. Our objective was to compare the disk diffusion and Etest methods to standard broth microdilution (BMD) methods for a diverse collection of CRE and CRP isolates.
MATERIALS AND METHODS
Seventy-four CRE and 72 CRP clinical isolates from unique patients were selected from University of Pittsburgh Medical Center (UPMC) biorepositories. All isolates were stored at −80°C and subcultured twice on Mueller-Hinton agar (Becton, Dickinson and Company, Sparks, MD) prior to testing. PCR and DNA sequencing were used to detect resistant determinants of CRE isolates, as described previously (4–7). CRE isolates harboring metallo-β-lactamases (MBL), KPC-8, or KPC-3 Ω-loop mutations were considered to be genotypically resistant to ceftazidime-avibactam (1, 8).
MICs were determined in triplicate by BMD according to Clinical Laboratory Standard Institutes (CLSI) guidelines (9); the median MIC was used for analysis. Avibactam (kindly provided by AztraZeneca, Wilmington, DE) and tazobactam (kindly provided by Merck & Co., Kenilworth, NJ) were tested at fixed concentrations of 4 μg/ml (10), in combination with ceftazidime (purchased from the UPMC pharmacy) and ceftolozane (provided by Merck & Co., Kenilworth, NJ), respectively. Ceftazidime-avibactam disks (30 μg/20 μg) and ceftolozane-tazobactam disks (30 μg/10 μg) were purchased from Hardy Diagnostics (Santa Maria, CA). Disk diffusion testing was conducted in accordance with CLSI guidelines (9). Etest testing was performed according to the manufacturer's recommendations (bioMérieux, Marcy-I'Etoile, France). For each set of experiments, the same inoculum was used to test isolates by BMD, disk diffusion, and Etest. For all experiments, Mueller-Hinton agar and broth were purchased from Becton, Dickinson and Company (Sparks, MD). FDA criteria were used to interpret MICs and disk diffusion zone diameters (Table 1). MICs against quality control strains Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were within acceptable ranges (CLSI).
TABLE 1.
Interpretative criteria for ceftazidime-avibactam and ceftolozane-tazobactama
| Agent | MIC (mg/liter) |
Disk diffusion zone diam (mm) |
||||
|---|---|---|---|---|---|---|
| S | I | R | S | I | R | |
| Ceftazidime-avibactam | ≤8/4 | ≥16/4 | ≥21 | ≤20 | ||
| Ceftolozane-tazobactam | ≤4/4 | 8/4 | ≥16/4 | ≥21 | 17–20 | ≤16 |
S, susceptible; I, intermediate; R, resistant.
Categorical agreement (CA) and essential agreement (EA) were defined using standardized criteria (11). Specifically, CA was defined as agreement in interpretive results (i.e., calling an isolate susceptible or resistant) between reference BMD and disk diffusion or Etest. Intermediate interpretations were applied for ceftolozane-tazobactam. EA was defined as agreement within one 2-fold dilution between BMD and Etest MICs. FDA criteria were used to define minor errors, major errors (ME), and very major errors (VME) (Table 2) (11).
TABLE 2.
Essential and categorical agreement between BMD and Etest or disk diffusion for testing susceptibility to ceftazidime-avibactam and ceftolozane-tazobactama
| Drug, pathogen (no. of isolates) | BMD |
Etest |
Disk diffusion |
|||||
|---|---|---|---|---|---|---|---|---|
| Median MIC (μg/ml)b | Range of MIC (μg/ml)b | No. (%) of resistant isolates | No. (%) of isolates with EA | No. (%) of isolates with CA | No. of errors | No. (%) of isolate with CA | No. of errors | |
| Ceftazidime-avibactam, CRE (n = 74) | 2 | 0.25–512 | 13 (18) | 66 (89) | 72 (97) | 2 (VME) | 56 (76) | 18 (ME) |
| Ceftolozane-tazobactam, CRP (n = 72) | 1 | 0.5–256 | 6 (8) | 57 (79) | 69 (96) | 3 (minor) | 68 (94) | 4 (minor) |
BMD, broth microdilution; CA, categorical agreement; CRE, carbapenem-resistant Enterobacteriaceae; CRP, carbapenem-resistant Pseudomonas aeruginosa; EA, essential agreement; ME, major error; VME, very major error. Minor errors were identified as BMD results that were categorized as resistant or susceptible and Etest/disk diffusion results that were categorized as intermediate. Major errors were identified as BMD results that were categorized as susceptible and Etest/disk diffusion results that were categorized as resistant. Very major errors were identified as BMD results that were categorized as resistant and Etest/disk diffusion results that were categorized as susceptible.
The median ceftazidime-avibactam MIC for E. coli ATCC 25922 was 0.25 μg/ml (CLSI reference range, 0.06 to 0.5 μg/ml), and the median ceftolozane-tazobactam MIC for P. aeruginosa ATCC 27853 was 0.5 μg/ml (CLSI reference range, 0.25 to 1 μg/ml).
RESULTS
Ceftazidime-avibactam against CRE.
Ceftazidime-avibactam was tested against 74 CRE isolates, which included 59 Klebsiella pneumoniae, 9 Escherichia coli, and 6 Enterobacter cloacae isolates. Ninety-six percent (71/74) of CRE harbored carbapenemases, including KPC-3 (n = 27), KPC-2 (n = 25), KPC-3 variants with Ω-loop mutations (n = 6), NDM-1 (n = 5), OXA-48 (n = 2), and KPC-4, KPC-8, OXA-181, OXA-232, KPC-2/VIM-1, and KPC-3/NDM-1 (n = 1 each). CRE that were genotypically resistant to ceftazidime-avibactam harbored MBL (n = 7), KPC-3 Ω-loop mutations (n = 6), or KPC-8 (n = 1).
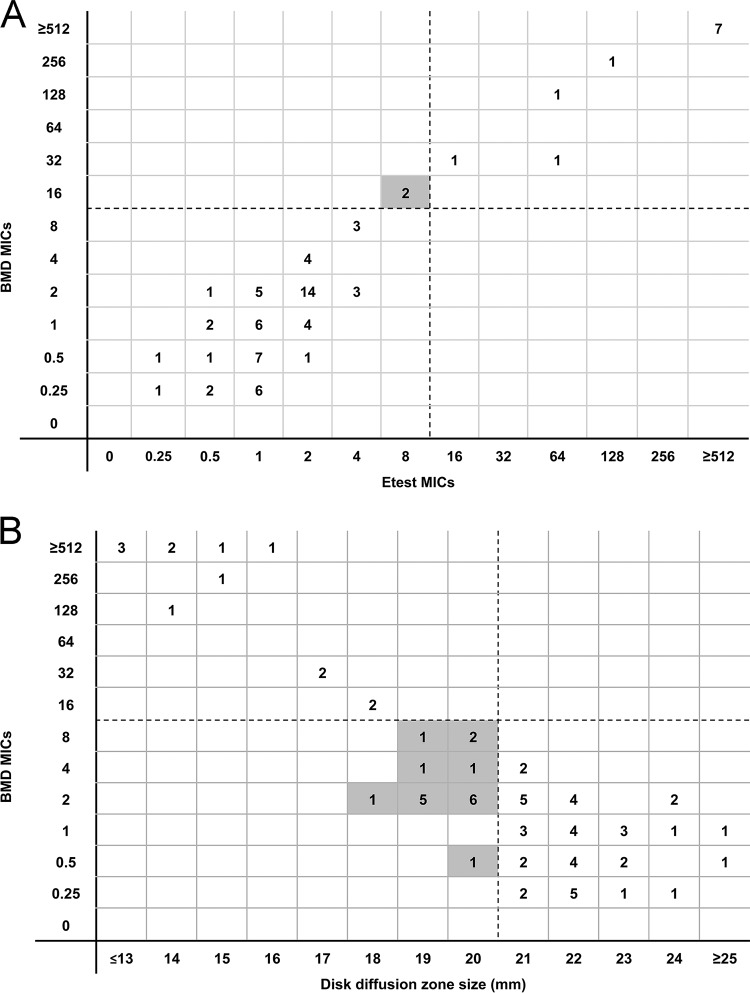
By the CLSI reference BMD method, the median ceftazidime-avibactam MIC against CRE isolates was 2 μg/ml (range, 0.25 to 512 μg/ml); 18% (13/74) of isolates were resistant (Table 2). EA between BMD and Etest was 89% (66/74) (Fig. 1A). Rates of CA with BMD were higher for Etest (97% [72/74]) than for disk diffusion (76% [56/74]; P = 0.0008). VME by Etest were noted for 2 isolates that were classified as resistant by BMD but susceptible by Etest (Table 2). ME were not noted for Etest. ME were observed for 18 isolates that were classified as resistant by disk diffusion but susceptible by BMD. No minor errors were noted for either Etest or disk diffusion given the lack of an intermediate breakpoint for ceftazidime-avibactam. Disk diffusion zones of inhibition clustered on either side of the susceptibility breakpoint; 74% (55/74) of isolates demonstrated zones between 18 and 22 mm. Results were similar when testing was repeated using Mueller-Hinton agar plates from a different manufacturer (Oxoid Ltd.; Hampshire, England). Disk diffusion tests were repeated using Mueller-Hinton agar from the disk manufacturer (Hardy Diagnostics; Santa Maria, CA) for 18 isolates associated with MEs; MEs were corroborated in 94% (17/18) of the isolates.
FIG 1.
(A) Dotted lines represent the susceptibility breakpoint for ceftazidime-avibactam. Two isolates classified as resistant by BMD and susceptible by Etest (very major errors) are identified in the shaded box. (B) Dotted lines represent the susceptibility breakpoint for ceftazidime-avibactam. Shaded boxes identify isolates classified as susceptible by BMD and resistant by disk diffusion (major errors).
Taking ceftazidime-avibactam genotypic resistance as the reference, BMD and Etest correctly categorized 99% (73/74) and 97% (72/74) of the isolates, respectively (Table 3). Using the FDA interpretive criteria for resistance, disk diffusion was inferior to both BMD (P = 0.0004) and Etest (P = 0.001), correctly categorizing only 77% (57/74) of isolates. Twenty-eight percent (17/61) of genotypically susceptible isolates were incorrectly classified as resistant by disk diffusion.
TABLE 3.
Correlation between ceftazidime-avibactam susceptibility results determined by genotype and BMD, Etest, or disk diffusion among CRE isolates
| Genotype classificationa | No. (%) of susceptible isolates |
No. (%) of resistant isolates |
||||
|---|---|---|---|---|---|---|
| BMDb | Etest | Disk diffusion | BMD | Etest | Disk diffusion | |
| Susceptible (n = 60) | 59 (98) | 60 (100) | 41 (68) | |||
| Resistant (n = 14) | 13 (93)c | 11 (79)c | 14 (100) | |||
CRE isolates were considered to be genotypically resistant to ceftazidime-avibactam if they harbored metallo-β-lactamases, KPC-8, or KPC-3 Ω-loop mutations.
BMD, broth microdilution.
One K. pneumoniae isolate with a 168-to-169 EL deletion in blaKPC-3 had ceftazidime-avibactam MICs of 8 μg/ml by BMD and 4 μg/ml by Etest.
Ceftolozane-tazobactam against CRP.
Ceftolozane-tazobactam was tested against 72 CRP. The median MIC was 1 μg/ml (0.5 to 256 μg/ml) by BMD, and 8% (6/72) of isolates were resistant. EA between BMD and Etest was 79% (57/72) (Fig. 2A). Rates of BMD CA with disk diffusion and Etest were 94% (68/72) and 96% (69/72), respectively; the only errors identified were minor (Table 3 and Fig. 2). Both Etest and disk diffusion accurately identified all BMD-resistant isolates. Among susceptible isolates, Etest and disk diffusion classified 3% (2/66) and 4% (3/66), respectively, as intermediate.
FIG 2.
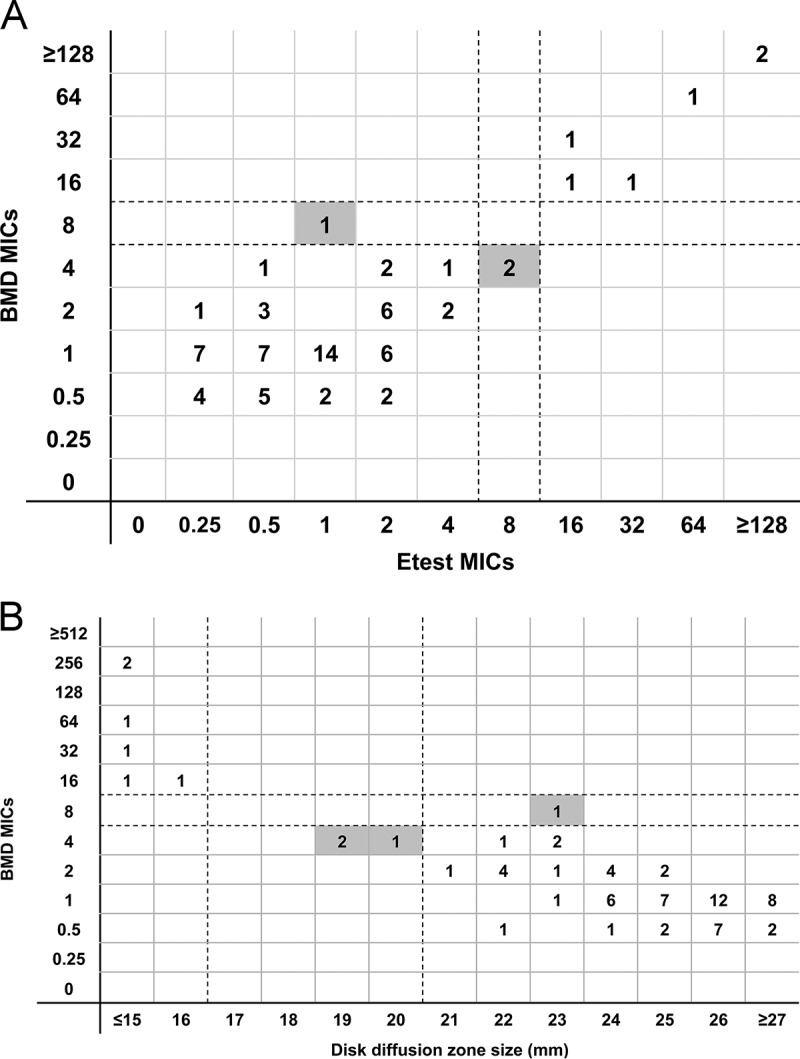
(A) The first and second dotted lines represent the susceptibility and intermediate breakpoints for ceftolozane-tazobactam, respectively. Shaded boxes identify results classified as minor errors. (B) The first and second dotted lines represent the susceptibility and intermediate breakpoints for ceftolozane-tazobactam, respectively. Shaded boxes identify results classified as minor errors.
DISCUSSION
As new agents with activity against carbapenem-resistant Gram-negative bacteria reach the clinic, it is imperative that facile and reproducible methods for testing susceptibility become available and are validated in clinical microbiology laboratories. This report provides important insights for clinicians and microbiologists as they consider how to best determine ceftazidime-avibactam and ceftolozane-tazobactam susceptibility among CRE and CRP, respectively, at their centers and interpret the clinical significance of MICs.
Etest measurements of ceftazidime-avibactam MICs against CRE correlated closely with MICs measured by the standard BMD method, with EA of 89% and CA of 97%. Moreover, Etest was comparable to BMD in correctly identifying CRE as genotypically resistant or genotypically susceptible to ceftazidime-avibactam. Etest categorized 2 ceftazidime-avibactam-resistant CRE isolates (as determined by BMD) as ceftazidime-avibactam susceptible (VMEs). The Etest MIC against these isolates (both carrying KPC-3 Ω-loop mutations) was 8 μg/ml, which was within one 2-fold dilution of the breakpoint and in EA with BMD results. One additional isolate harboring a 168-to-169 glutamic acid-leucine (EL) deletion in KPC-3 was classified as genotypically resistant but tested susceptible by BMD (MIC = 8 μg/ml) and Etest (MIC = 4 μg/ml). Taken together, the data indicate that Etest is a suitable alternative to BMD for testing ceftazidime-avibactam against CRE. Nevertheless, Etest may misclassify some ceftazidime-avibactam-resistant isolates as susceptible. Therefore, clinicians should maintain a level of suspicion for patients who are at risk for ceftazidime-avibactam resistance. These patients include those who have failed to respond to treatment or who have developed breakthrough infections, in particular if Etest MICs are within one 2-fold dilution of the susceptibility breakpoint. In such cases, Etest MICs should be validated by BMD testing. Further refinement of the ceftazidime-avibactam interpretative criteria to include an intermediate classification may help to limit Etest VMEs; however, additional studies to validate such an approach are needed.
Using FDA interpretive criteria, disk diffusion ceftazidime-avibactam inhibition zones against CRE demonstrated low CA with BMD MICs (76%, compared to 97% CA between Etest and BMD; P = 0.0008). Disk diffusion correctly identified all isolates that were genotypically resistant to ceftazidime-avibactam but misclassified 28% of genotypically susceptible CRE isolates as resistant (MEs). A major issue with disk diffusion was that inhibition zones clustered on either side of the susceptibility breakpoint (Fig. 1B). It is unclear if this phenomenon was specific to the isolates tested, to the disks used in our experiments, or to technical issues related to the drug's distribution in agar. MEs remained when testing was conducted with Mueller-Hinton agar from different manufacturers. On the basis of these data, we cannot recommend routine disk diffusion testing of ceftazidime-avibactam against CRE. Further studies are needed to identify methodologic issues that may impact zone diameters and susceptibility breakpoints at our center and at others. Modifications to testing methods that promote a broader distribution of zone diameters will be particularly valuable.
In contrast to the results for ceftazidime-avibactam against CRE, both Etest testing and disk diffusion testing of ceftolozane-tazobactam against CRP demonstrated excellent CA with BMD MICs (96% and 94%, respectively). One CRP isolate that was ceftolozane-tazobactam resistant by BMD was identified as susceptible by Etest (VME). Otherwise, discrepancies between methods did not have major clinical implications. Our data suggest that either method is a suitable alternative to BMD, but further studies are needed to validate this conclusion. In a prior study using a ceftolozane-tazobactam Etest against 90 CRP isolates (12), CA and EA with BMD MICs were similar to results reported here (76% versus 79% and 89% versus 96%, respectively); in the prior study, however, Etest classified 50% (6/12) of ceftolozane-tazobactam-resistant CRP isolates as ceftolozane-tazobactam susceptible (VME). Future studies of methods for testing ceftolozane-tazobactam and ceftazidime-avibactam should replicate a noteworthy strength of our study design by including genetically diverse isolates that exhibit a range of MICs.
In conclusion, Etests show promise in measuring ceftazidime-avibactam and ceftolozane-tazobactam MICs against CRE and CRP isolates, respectively. These tests are currently RUO, but our data support future introduction into clinical practice. Likewise, our findings indicate that disk diffusion testing of ceftolozane-tazobactam against CRP isolates offers an accurate and less laborious alternative to BMD. Disk diffusion measurements of ceftazidime-avibactam activity against CRE isolates, on the other hand, correlated less well with BMD results and overcalled ceftazidime-avibactam resistance. Studies are needed to define zone diameter breakpoints for susceptibility and to ensure interlaboratory reproducibility before routine clinical use of disk diffusion can be considered. Until clinicians and microbiologists understand Etest and disk diffusion performance at their centers, the tests should be interpreted cautiously.
ACKNOWLEDGMENTS
We declare that we have no conflicts of interest.
This work was funded, in part, by grants from the National Institutes of Health (K08AI114883 to R.K.S., R21AI117338 to L.C., R01AI090155 to B.N.K., R21AI128338 to M.H.N., and R21AI111037 to C.J.C.).
REFERENCES
- 1.Shields RK, Chen L, Cheng S, Chavda KD, Press EG, Snyder A, Pandey R, Doi Y, Kreiswirth BN, Nguyen MH, Clancy CJ. 28 December 2016. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother doi: 10.1128/AAC.02097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, Press EG, Kreiswirth BN, Clancy CJ, Nguyen MH. 2016. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 63:1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haidar G, Phillips NJ, Shields RK, Snyder D, Cheng S, Potoski BA, Doi Y, Hao B, Press EG, Cooper VS, Clancy CJ, Nguyen MH. 25 February 2017. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: Clinical effectiveness and evolution of resistance. Clin Infect Dis doi: 10.1093/cid/cix182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Mediavilla JR, Endimiani A, Rosenthal ME, Zhao Y, Bonomo RA, Kreiswirth BN. 2011. Multiplex real-time PCR assay for detection and classification of Klebsiella pneumoniae carbapenemase gene (bla KPC) variants. J Clin Microbiol 49:579–585. doi: 10.1128/JCM.01588-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Chavda KD, Mediavilla JR, Zhao Y, Fraimow HS, Jenkins SG, Levi MH, Hong T, Rojtman AD, Ginocchio CC, Bonomo RA, Kreiswirth BN. 2012. Multiplex real-time PCR for detection of an epidemic KPC-producing Klebsiella pneumoniae ST258 clone. Antimicrob Agents Chemother 56:3444–3447. doi: 10.1128/AAC.00316-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clancy CJ, Chen L, Shields RK, Zhao Y, Cheng S, Chavda KD, Hao B, Hong JH, Doi Y, Kwak EJ, Silveira FP, Abdel-Massih R, Bogdanovich T, Humar A, Perlin DS, Kreiswirth BN, Hong Nguyen M. 2013. Epidemiology and molecular characterization of bacteremia due To carbapenem-resistant Klebsiella pneumoniae in transplant recipients. Am J Transplant 13:2619–2633. doi: 10.1111/ajt.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clancy CJ, Hao B, Shields RK, Chen L, Perlin DS, Kreiswirth BN, Nguyen MH. 2014. Doripenem, gentamicin, and colistin, alone and in combinations, against gentamicin-susceptible, KPC-producing Klebsiella pneumoniae strains with various ompK36 genotypes. Antimicrob Agents Chemother 58:3521–3525. doi: 10.1128/AAC.01949-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haidar G, Clancy CJ, Shields RK, Hao B, Cheng S, Nguyen MH. 24 April 2017. Mutations in blaKPC-3 that confer ceftazidime-avibactam resistance encode novel KPC-3 variants that function as extended-spectrum beta-lactamases. Antimicrob Agents Chemother doi: 10.1128/AAC.02534-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-10th edition: approved standard M07-A10. CLSI, Wayne, PA. [Google Scholar]
- 10.Shields RK, Clancy CJ, Hao B, Chen L, Press EG, Iovine NM, Kreiswirth BN, Nguyen MH. 13 July 2015. Effects of Klebsiella pneumoniae carbapenemase subtypes, extended-spectrum β-lactamases, and porin mutations on the in vitro activity of ceftazidime-avibactam against carbapenem-resistant K. pneumoniae. Antimicrob Agents Chemother doi: 10.1128/AAC.00548-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel JB, Novak-Weekley S. 2013. Verification of antimicrobial susceptibility testing methods: a practical approach. Clin Microbiol Newsl 35:103–109. doi: 10.1016/j.clinmicnews.2013.06.001. [DOI] [Google Scholar]
- 12.Flynt LK, Veve MP, Samuel LP, Tibbetts RJ. 2017. Comparison of Etest to broth microdilution for testing of susceptibility of Pseudomonas aeruginosa to ceftolozane-tazobactam. J Clin Microbiol 55:334–335. doi: 10.1128/JCM.01920-16. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]