Abstract
Objectives
Metastatic melanoma patients with leptomeningeal disease (LMD) have an extremely poor prognosis, with a median survival measured in weeks, and few treatment options. Outcomes of a retrospective cohort of patients with LMD that were treated with intrathecal interleukin-2 (IT IL-2) were reviewed to assess the long-term efficacy of this therapy.
Methods
The records of metastatic melanoma patients with LMD who were treated with IT IL-2 from 2006 to 2014 in a Compassionate Investigational New Drug study were reviewed. IL-2 (1.2 mIU) was administered intrathecally via Ommaya reservoir up to five times per week in the inpatient setting for 4 weeks; patients with good tolerance and clinical benefit received maintenance IT IL-2 every 1–3 months thereafter.
Results
The cohort included 43 patients. The median age of the patients was 47 years (range 18–71), and 32 (74%) were male. 23 patients (53%) had positive cerebrospinal fluid (CSF) cytology and radiographic evidence of LMD, 8 (19%) had positive CSF cytology only, 9 (21%) had radiographic evidence only and 3 (7%) were diagnosed based on pathology review after craniotomy. The median overall survival (OS) from initiation of IT IL-2 was 7.8 months (range, 0.4–90.8 months), with 1-year, 2-year and 5-year OS rates of 36%, 26% and 13%. The presence of neurological symptoms (HR 2.1, P=0.03), positive baseline CSF cytology (HR 4.1, P=0.001) and concomitant use of targeted therapy (HR 3.0, P=0.02) was associated with shorter OS on univariate analysis. All patients developed symptoms due to increased intracranial pressure which was managed with supportive medications and/or CSF removal, and there were no treatment-related deaths.
Conclusion
These results demonstrate that despite their historically dismal prognosis a subset of metastatic melanoma patients with LMD treated with IT IL-2 can achieve long-term survival, but these data need to be verified in a prospective trial setting.
Keywords: intrathecal therapy, interleukin-2, leptomeningeal disease, melanoma
Key questions.
What is already known about this subject?
Development of leptomeningeal disease (LMD) in patients with melanoma has a poor prognosis (with a median survival of only weeks) and has no consistently effective therapeutic options.
T cells are present in small amounts in cerebrospinal fluid (CSF), and if expanded may provide a way of mounting an immune response against immunogenic melanoma cells in the intrathecal compartment.
Our prior study has shown dramatic increase in immune cells in human CSF after intrathecal injection of interleukin-2, (IL-2) a known immunostimulant.
What does this study add?
This review reports the clinical outcomes of intrathecal delivery of IL-2 in a large and rigorously analysed series of patients with melanoma and LMD.
It describes the methodology of such treatment, including management of increased intracranial pressure, which is a consistent side effect of both the treatment and the disease.
The results show increased survival measured in months or years, rather than weeks in a subgroup of patients, with 36% surviving a year or more.
No such data have previously been reported.
How might this impact on clinical practice?
The gain in survival associated with intrathecal IL-2 should prompt extension of immunotherapy for use not only systemically, but also within the intrathecal space when LMD is present in patients with melanoma.
These data emphasise that LMD is not untreatable, and that although treatment with intrathecal IL-2 is associated with significant toxicity, it potentially offers significant extension of life expectancy that may be enhanced by refining other immunotherapeutic strategies for use in melanoma LMD.
Introduction
One of the most devastating complications of metastatic melanoma (MM) is the development of leptomeningeal disease (LMD).1 Our previous analysis of 330 MM patients with central nervous system (CNS) involvement demonstrated that patients with LMD have a median overall survival (OS) of only 1.8 months.2 While several treatment options are available for melanoma patients with parenchymal brain metastases, few options are available for patients with LMD, and there is limited evidence of long-term clinical benefit from them.3
The systemic administration of immunotherapy can achieve durable survival in patients with MM, but it appears that the immunoregulatory components of T cells vary between the systemic and intrathecal compartments.4–7 Initial studies with intrathecal interleukin-2 (IT IL-2) have shown it dramatically increases the number of leucocytes, specifically lymphocytes, in the cerebrospinal fluid (CSF), therefore overcoming the paucity of immune cells typically observed in the CSF.8 Although systemically administered IL-2 can be found in the CSF, these levels appear not sufficient to cause a robust IT immune response; thus IT administration was chosen as the preferred route.9 10 However, survival data on the efficacy of IT immunotherapy, specifically with IL-2, remain sparse in patients with LMD, mainly due to the fact that these patients are excluded from most trials, including trials using immunotherapy for parenchymal brain metastases.11 Importantly, most of the patients described here developed LMD at a point in time when no other systemic treatment options had gained regulatory approval yet and were excluded from ongoing clinical trials using checkpoint inhibitors. The purpose of our retrospective review was to evaluate the long-term outcomes of MM patients with LMD treated with IT administration of IL-2 at our institution.
Methods
Patients
The outcomes of MM patients with LMD treated with IT IL-2 under an ongoing Compassionate Investigational New Drug (CIND) study at The University of Texas MD Anderson Cancer Center between August 2006 and July 2014 were reviewed under an institutional review board-approved protocol. The data cut-off point was November 15, 2015. The diagnosis of LMD was established by CSF analysis, MRI and/or surgical/pathology report. All patients were required to have a baseline MRI of the brain and/or spine, and placement of an Ommaya reservoir. Patients who had received previous systemic therapies, including chemotherapy, immunotherapy or radiation therapy to the brain or spine, were included. Informed consent for treatment was obtained from all patients.
IT IL-2 treatment
The initial case reports used IT IL-2 doses ranging from using 2.5×105 IU to 6×106 IU.8–10 12–14 However, toxicities at the highest dose level were significant, and taking into account the half-life of IT IL-2, researchers subsequently developed the treatment schedule of using 1.2×106 IU IT IL-2 over 5 days, for up to 4 weeks, called the induction period. This regimen was also used in a large cohort of patients, reported by Papadopoulos et al in 2002. Since this time, our institution has continued with the same dosing regimen.15
Treatment with IT IL-2 consisted of an induction phase, followed by a maintenance phase, as also recently reported.16 The induction phase was defined as a treatment period of 4 weeks from the first IT IL-2 injection. Recombinant IL-2 (1.2 mIU in preservative-free water (volume ~0.3 mL)) was injected over less than 1 min via Ommaya reservoir. Treatments were administered daily for up to 5 days during the first week, and 2–3 times per week as tolerated for the next three weeks. Patients remained hospitalised throughout the induction phase. If side effects consistent with increased intracranial pressure (ICP) (eg, severe headache, uncontrolled nausea or vomiting, confusion or other change in mental status) developed that were not relieved with supportive medications, CSF was removed via the Ommaya reservoir for symptomatic relief. IT IL-2 treatments were delayed or omitted if clinically indicated (uncontrolled symptoms or significant decline in performance status). In such cases, subsequent IT IL-2 doses were reduced to 1.0 or 0.6 mIU. Patients with stabilisation or improvement of symptoms after induction therapy with tolerable toxicities continued with maintenance treatment, which consisted of a single dose of IT IL-2 initially administered weekly, then biweekly and eventually extended to every 4–12 weeks. Patients were admitted for 24 hours for maintenance treatments for monitoring and management of side effects.
Assessments
A comprehensive neurological examination was conducted prior to each administration of IT IL-2. MRI of the brain and/or the entire spine was required every 4–8 weeks. Positron emission tomography using [18F]-fluorodeoxyglucose and/or CT scans of the chest, abdomen and pelvis were used to evaluate extracranial disease. All baseline and follow-up MRIs of the brain/spine were assessed by a board-certified neuroradiologist for leptomeningeal enhancement on postcontrast T1-weighted images. Improved LMD was defined as decreased contrast enhancement of the leptomeninges, stable disease as no change from baseline imaging and progressive LMD as increased contrast enhancement.
CSF samples were evaluated for malignant cells, cell counts, glucose and protein levels. If suspicious cells were seen on cytopathology review, immunocytochemistry was used to confirm the presence of melanoma cells.
Statistical methods and analysis
Demographics, treatment and CSF characteristics, radiographic responses, extracranial disease status and OS were collected for each patient. Kaplan-Meier method was used to estimate the distribution of OS duration from the first IT IL-2 treatment, and log-rank test was used to compare distributions. Cox proportional hazards regression was used to assess the association between OS and covariates of interest.
Results
Patient characteristics
The characteristics and outcomes of 43 consecutive MM patients with LMD treated with IT IL-2 were reviewed (table 1). Median age was 47 years (range, 18.8–71.0), and most were male (74%). The median follow-up time from the first dose of IL-2 was 7.2 months, with a range of 0.4–90.8 months. Thirty-one (73%) patients had positive CSF cytology; 23 (53%) patients had positive cytology and radiographic evidence of LMD. Also, 9 of the 12 patients with negative CSF cytology at baseline had MRI findings consistent with LMD, and 3 patients had evidence of LMD on pathology review of surgical specimens. Most (77%) patients had an Eastern Cooperative Oncology Group performance status of 0–1. Neurological deficits attributable to LMD were present in 21 patients (49%), and 17 patients (40%) were on steroids (median daily dose 8 mg, range, 1–24 mg). Four patients (9%) had LMD but no history of systemic melanoma, and eight patients (19%) had no evidence of active systemic disease at the time of IT IL-2 induction. Previously treated or concomitant parenchymal brain metastases were present in 34 (79%) patients, including 17 with progressing parenchymal metastases. Serum lactate dehydrogenase (LDH) was elevated in 28 (65%).
Table 1.
Baseline characteristics of study patients
| Variable | N (%) |
| Gender | |
| Male | 32 (74%) |
| Female | 11 (26%) |
| Basis for LMD diagnosis | |
| CSF and radiology positive | 23 (53%) |
| CSF positive only | 8 (19%) |
| Radiology positive only | 9 (21%) |
| Surgical pathology positive only | 3 (7%) |
| Mutation status | |
| BRAF mutant | 21 (49%) |
| NRAS mutant | 9 (21%) |
| BRAF/NRAS wild-type | 3 (7%) |
| Other (KIT, ABL1, CDKN2A) |
3 (7%) |
| Unknown | 7 (16%) |
| Prior systemic therapy | |
| No | 11 (26%) |
| Yes | 32 (74%) |
| Temozolomide | |
| No | 29 (67%) |
| Yes | 14 (33%) |
| Immunotherapy | |
| No | 23 (53%) |
| Yes | 20 (47%) |
| Prior immunotherapy received | |
| Biochemotherapy (containing IL-2 and interferon regimen) |
9 (21%) |
| High-dose IL-2 | 4 (9%) |
| Adoptive cell therapy | 3 (7%) |
| Ipilimumab | 4 (9%) |
| Anti-PD1 | 3 (7%) |
| BRAF or BRAF/MEK inhibitors | |
| No | 32 (74%) |
| Prior radiation therapy | |
| No | 16 (37%) |
| Yes | 27 (63%) |
| Steroids | |
| No | 26 (60%) |
| Yes | 17 (40%) |
| Previous parenchymal brain metastases | |
| No | 9 (21%) |
| Yes | 34 (79%) |
| LDH > ULN | |
| No | 15 (35%) |
| Yes | 28 (65%) |
| Neurological symptoms present | |
| No | 22 (51%) |
| Yes | 21 (49%) |
| Extracranial disease | |
| None/LMD only | 12 (28%) |
| Systemic controlled | 20 (47%) |
| Systemic uncontrolled | 11 (26%) |
| Concomitant therapy | |
| None | 27 (63%) |
| Immunotherapy | 2 (5%) |
| Targeted* | 7 (16%) |
| Temozolomide | 2 (5%) |
| Radiation | 5 (12%) |
Definition of extracranial disease: none = no concurrent systemic disease; LMD only = LMD with no prior or concurrent systemic disease, and patient never diagnosed with parenchymal brain metastases; systemic controlled = concurrent but controlled systemic disease; systemic uncontrolled = progressive systemic disease.
*BRAF inhibitor ± MEK inhibitor.
CSF, cerebrospinal fluid; IL, interleukin; LDH, lactic dehydrogenase; LMD, leptomeningeal disease; ULN, upper limit of normal.
The majority (74%) of patients had received prior systemic therapy (table 1). During the induction phase, 27 patients did not receive any concomitant anticancer therapy. Six patients received radiation during induction (stereotactic radiosurgery (SRS, n=1), focal sinonasal radiation (n=1), whole-brain radiation (WBXRT, n=4)). Some patients continued systemic therapy that had been initiated prior to the first dose of IT IL-2 (ipilimumab (n=2); BRAF inhibitor (BRAFi) alone (n=3) or in combination with MEK inhibitor (n=1); temozolomide alone (n=3) or in combination with WBXRT (n=3); biochemotherapy (n=1)). These patients had developed LMD while receiving these systemic therapies and were continued on this treatment for extracranial disease control at the treating physicians’ discretion.
Overall survival
The median OS from the start of IT IL-2 was 7.8 months (range, 0.4–90.8 months; figure 1A). The 1-year, 2-year and 5-year OS rates were 36%, 26%, and 13%, respectively.
Figure 1.
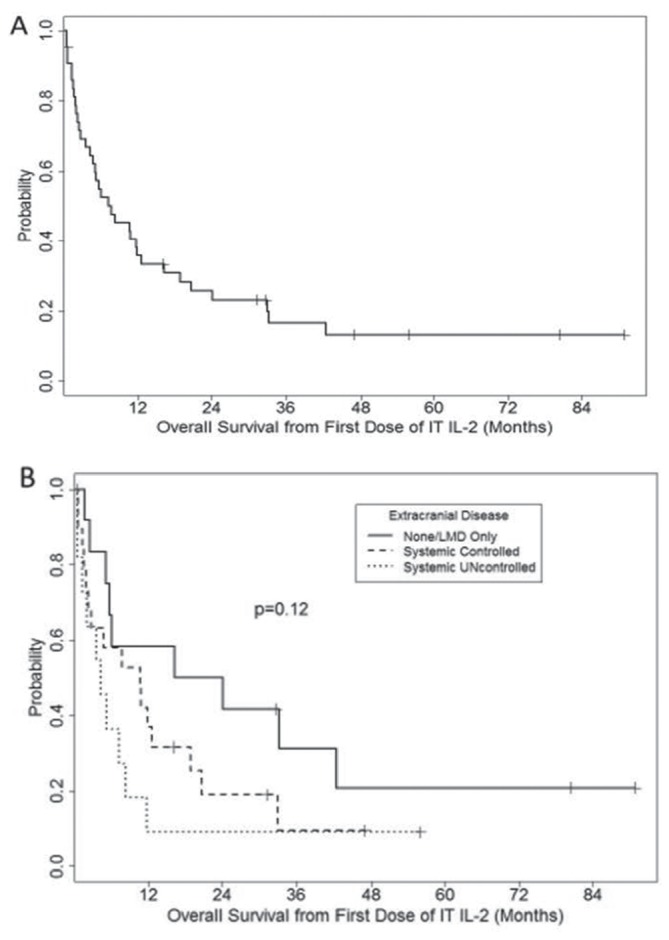
Kaplan-Meier plot of overall survival from the time of first dose of intrathecal interleukin-2 (IT IL-2) for all patients (A, n=43) and based on the extent of extracranial disease (B). Also, 12 patients had no extracranial disease at time of first IT IL-2 treatment, 20 patients had controlled disease and 11 patients had uncontrolled disease. LMD, leptomeningeal disease.
Patients with no active systemic disease at the start of treatment (n=12) had a median OS of 20.2 months (range, 1.7–90.8 months); patients with concurrent controlled systemic disease (n=20) had a median OS of 10.6 months (range, 0.5–47.0 months; HR 1.68, P=0.22 vs LMD only); and patients with progressing systemic disease (n=11) had a median OS of 4.3 months (range, 0.4–55.9 months; HR 2.57, P=0.04 vs LMD only; figure 1B). Univariate analysis of baseline clinical features identified the presence of neurological symptoms (HR 2.1, P=0.03) and concomitant use of targeted therapy (HR 3.0, P=0.02) as additional factors associated with significantly shorter OS (table 2). Concurrent use of corticosteroids, need for dose reduction and total IT IL-2 doses (table 3) during induction treatment were not significantly associated with OS.
Table 2.
Univariate Cox regression analysis for overall survival (OS) from the first dose of intrathecal interleukin-2 by baseline characteristics
| Variable | Total N | Total deaths | Median OS | HR | 95% CI | P value |
| Age | ||||||
| Per year | 43 | 35 | 7.8 | 1.001 | 0.97 to 1.03 | 0.92 |
| Sex | ||||||
| Male | 32 | 26 | 5.9 | – | – | – |
| Female | 11 | 9 | 9.6 | 0.93 | 0.43 to 1.99 | 0.85 |
| LDH | ||||||
| Normal | 28 | 24 | 10.6 | – | – | – |
| Elevated | 15 | 11 | 5.2 | 1.03 | 0.51 to 2.12 | 0.93 |
| BRAF mutation | ||||||
| No | 15 | 11 | 16.3 | – | – | – |
| Yes | 21 | 17 | 10.6 | 1.42 | 0.66 to 3.04 | 0.37 |
| Missing | 7 | 7 | * | * | * | * |
| Neuro symptoms † | ||||||
| No | 22 | 16 | 11.7 | – | – | – |
| Yes | 21 | 19 | 2.8 | 2.14 | 1.09 to 4.19 | 0.03 |
| Extracranial disease | ||||||
| None/LMD only | 12 | 9 | 20.1 | – | – | – |
| Systemic controlled | 20 | 16 | 10.6 | 1.68 | 0.73 to 3.85 | 0.22 |
| Systemic uncontrolled | 11 | 10 | 4.3 | 2.57 | 1.03 to 6.45 | 0.04 |
| Concomitant medications | ||||||
| None | 27 | 19 | 11.8 | – | – | – |
| Targeted | 7 | 7 | 4.7 | 3.02 | 1.18 to 7.74 | 0.02 |
| Other | 9 | 9 | 8.3 | 1.88 | 0.82 to 4.34 | 0.14 |
| CSF cytology | ||||||
| Negative | 13 | 7 | 33.2 | – | – | – |
| Positive | 30 | 28 | 5.1 | 4.09 | 1.72 to 9.74 | 0.001 |
*Not available.
†At initiation of therapy.
CSF, cerebrospinal fluid; LDH, lactate dehydrogenase; LMD, leptomeningeal disease.
Table 3.
Univariate Cox regression analysis for overall survival (OS) from the first dose of intrathecal interleukin-2 (IL-2) for on-treatment characteristics
| Variable | Level | Total N | Total deaths | Median OS | HR | 95% CI | P value |
| Total IL-2 doses received during Induction | |||||||
| Per dose | 43 | 35 | 7.8 | 0.89 | 0.77 to 1.01 | 0.08 | |
| Dose reduction | |||||||
| No | 24 | 18 | 11.8 | – | – | – | |
| Yes | 19 | 17 | 5.1 | 1.73 | 0.88 to 3.41 | 0.11 | |
| CSF positive at baseline (N = 30): CSF clearing | |||||||
| No | 20 | 19 | 4.5 | – | – | – | |
| Yes | 9 | 8 | 9.3 | 0.98 | 0.42 to 2.29 | 0.96 | |
| Missing | 1 | 1 | * | * | * | * | |
| CSF negative at baseline (N = 13): cytology conversion to positive | |||||||
| No | 7 | 3 | – | – | – | ||
| Yes | 6 | 4 | 16.2 | 2.31 | 0.51 to 10.44 | 0.28 | |
*Not available.
CSF, cerebrospinal fluid.
Positive CSF cytology at baseline was associated with shorter OS (HR 4.09, P=0.001). Nine (30%) patients with positive baseline cytology converted to negative CSF cytology during induction and remained negative subsequently, consistent with a cytological response, but this was not associated significantly with OS (P=0.58). Among the 13 patients with negative cytology at baseline, 6 (50%) developed positive CSF cytology during IT IL-2, which also was not significantly associated with OS.
Radiographic evidence of LMD was apparent at baseline in 23 patients (53%) in the brain and in 19 patients (44%) in the spine (online supplementary table 1). Subsequent brain imaging demonstrated improved radiographic findings of LMD in 9 patients (39%), stable findings in 1 patient (4%) and progression in 10 patients (43%); 3 patients (13%) did not have subsequent brain imaging. Subsequent imaging of the spine showed radiographic improvement in six patients (32%), stable findings in two patients (11%) and progression in six patients (32%); five patients (26%) did not have subsequent imaging performed. Among patients with imaging evidence of LMD at baseline with subsequent imaging data, improved radiographic findings (vs stable or progressive findings) in the brain did not correlate with improved OS (P=0.33), but improved findings in the spine did (P=0.007) (online supplementary figure S1).
esmoopen-2017-000283supp001.pdf (30.4KB, pdf)
IT IL-2 toxicity
Twelve patients (28%) had elevated ICP at initiation of IT IL-2, with recorded levels of up to 40 cm H2O (normal ICP <15 cm H2O). Patients received a median of nine IT doses of IL-2 during the induction period (range, 3–14). All patients subsequently developed fever, chills and symptoms of elevated ICP, which included nausea and headache. Other symptoms included vomiting, which was managed with supportive medication, and temporary changes in mental status, which resolved when IT IL-2 dosing was delayed and/or the dose was decreased. All patients required additional CSF drainage for symptom control and ICP relief during the induction period. In eight patients, CSF drainage had to be performed thrice on the same day as IT IL-2 administration. Almost half the patients (44%) required IT IL-2 dose reduction to either 1.0 mIU or 0.6 mIU during the induction phase. Five patients had shunts in place due to increased ICP or hydrocephalus, and four patients were converted to a shunt either during induction (n=3) or while on maintenance therapy (n=1). There were no deaths attributable to IT IL-2 treatment.
Importantly, symptoms lessened significantly during the maintenance phase, and included headache, nausea, fever and chills. Typically, CSF drainage was not required and patients were discharged with 24 hours of IT IL-2 dose.
Discussion
LMD is associated with an extremely poor prognosis in virtually all tumour types, including melanoma.17 18 Notably, melanoma patients with LMD have been excluded from all large clinical trials that led to the approval of currently approved targeted and immune therapies for this disease, and from recent phase II trials assessing ipilimumab, nivolumab and dabrafenib and trametinib in patients with CNS metastases.19–22 Our analysis represents the largest cohort of melanoma patients with LMD treated with a single intervention to date, and the results demonstrate that treatment with IT IL-2 can achieve long-term survival in a subset of these patients. These findings support the need, feasibility and rationale for further investigation of intrathecal therapies for this highly aggressive disease.
Previous studies have demonstrated the safety and clinical benefit of IT treatments in patients with LMD, particularly with rituximab (anti-CD20) and trastuzumab (anti-HER2/neu) in patients with CNS lymphoma and breast cancer, respectively.23–25 However, minimal data are available about the safety and efficacy of IT immunotherapy. Two reports of a small number of patients with LMD treated with IT interferon alpha-2b reported nominal activity.26 27 We recently reported the case of a patient with melanoma who achieved LMD stabilisation following IT administration of tumour-infiltrating lymphocytes, while another case report described the safe use of IT cytotoxic T cells.28 29 Our current series of 43 patients represents the largest cohort of melanoma patients with LMD treated with any IT therapy to date. Importantly, the 5-year survival rate observed in these patients (13%) is reminiscent of long-term OS that has been observed in MM patients without CNS involvement treated with systemic IL-2 (~6%) and ipilimumab (~20%).30 31
The treatment of patients with MM has been revolutionised over the last decade by the development and approval of several immune and targeted therapies. However, the outcomes of melanoma patients with LMD remain poor. A recent retrospective review of 39 melanoma patients with LMD reported a median OS of 16.9 weeks in patients treated with targeted therapy and/or immunotherapy with or without radiotherapy.32 This represents only a small improvement compared with survival rates of ~2 months reported in previous series.2 Importantly, the long-term follow-up for our cohort is unique for LMD cohorts, and again supports that long-term disease control and survival is achievable. Despite these promising results, we acknowledge that the retrospective nature of this non-randomised study imposes some inherent limitations on their interpretation. All patients were treated under an institutional CIND, and additional treatments were administered at the discretion of the treating physician. All patients that responded to treatment with IT IL-2 and derived long-term survival remained on maintenance IT IL-2. Twenty patients received additional systemic therapy after the initial 4 weeks of induction with IT IL-2. Most common therapy given was ipilimumab (n=9) and temozolomide (n=12). Twelve patients also were treated with radiation (WBRT, SRS, spinal radiation), of which eight had not previously had any radiation to spine or brain.
None of these concomitant treatments has been shown to prolong OS in patients with LMD, but a synergism cannot be excluded. While systemically administered BRAF inhibitors (BRAFi) have been shown to achieve clinical responses in a significant subset of melanoma patients with parenchymal brain metastases, concurrent targeted therapy treatment was associated with shorter OS in our cohort of patients with LMD.20 Notably, all seven patients who received concomitant BRAFi and IT IL-2 therapy developed (progressed with) LMD while taking BRAFi. Two of these patients died within 3 weeks of IT IL-2 initiation. One of these patients had a significantly elevated LDH level (1826 IU/L; range, 265–1826, institutional upper normal limit 618 IU/L) at the time of IT IL-2 treatment initiation; both patients had markedly elevated opening pressures prior to the first dose of IT IL-2 (27 and 33 cm H2O, respectively), and both had significant LMD burden. Despite the observed association, the number of patients receiving concurrent targeted therapy was very small, and it is likely that confounding factors led to shorter OS.
The retrospective nature of this report also precluded detailed toxicity reporting for IT IL-2 therapy. While we are unable to retrospectively quantify symptoms systematically, they were likely similar in incidence and severity to those reported in the initial description of IT IL-2 for LMD from our group.15 Patients from that previous cohort were not included in the current analysis due to some patients receiving other IT chemotherapy concomitantly in addition to IL-2. However, the report for the previous cohort reported chills (100% all grades, no grade 3), fever (98% all grades, 11% grade 3), nausea (95% all grades, 30% grade 3) and headache (99% all grades, 57% grade 3) as the most common toxicities of IT IL-2. The frequent and significant toxicity incurred by this regimen will likely limit its broad dissemination to cancer centres, similar to systemic IL-2 therapy. Despite these challenges, we did not observe any treatment-related deaths with IT IL-2. In addition, we observed a significant decrease in symptom burden in patients receiving maintenance therapy, with only overnight observation required.
Our study also highlights the challenge of clinical response evaluation for patients with LMD. Almost 50% of the patients with negative cytology at baseline in this cohort converted to positive cytology during the IT IL-2 induction, and CSF cytological response did not correlate with OS. The observed frequent conversion of CSF from negative to positive with treatment could be due to the serial sampling performed as it is known that repeated cytological analysis increases the sensitivity of detection of malignant cells in CSF.17 Alternatively, it is possible that the IT IL-2 treatment could cause cells to detach from the leptomeninges, allowing improved detection. Alternative approaches for the diagnosis and monitoring of LMD, such as recently reported detection of mutations in cell-free DNA of CSF, should be evaluated in future studies.33 Notably, the recently published RANO-LM criteria for radiographic responses may also enhance treatment assessments in future clinical trials for patients with LMD.34
In conclusion, this study reports the outcomes of the largest intervention study for MM patients with LMD to date. Our results demonstrate that despite their historically dismal prognosis a subset of MM patients with LMD treated with IT IL-2 can achieve long-term survival. However, the efficacy of IT IL-2 needs to be evaluated in a prospective trial setting to validate this result. Studies are also warranted (and ongoing) to identify biomarkers that predict the clinical benefit of IT IL-2 to optimise future patient selection for this highly toxic therapy. In addition to supporting the potential clinical benefit of this treatment, these results strongly support the rationale and feasibility for additional clinical trials in melanoma patients with LMD, including the evaluation of intrathecal administration of contemporary immunotherapies (NCT03025256, NCT00338377). Despite the tremendous recent advances that have been made in the field of melanoma, our results also highlight the continued need for additional clinical trials including prospective toxicity assessment, standardised management guidelines, new treatment options and translational research efforts to further improve outcomes in these patients.
Acknowledgments
The authors thank patients and their families for their participation in this study and for allowing associated research studies. They also like to acknowledge the allied health professional providers Jessie Richard, Donna Gerber, Carol Lacey, Rinata Simien, Teresa Rodgers, Masood Iqbal, Ida John and Lindsay Blair, whose dedication and outstanding care have made the safe administration of this treatment feasible for patients with LMD.
Footnotes
Contributors: All authors have contributed to this article.
Funding: Analyses for this study were supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672) and MAD acknowledges support from 1R01CA187076-02 and 5R01CA154710-04.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Le Rhun E, Zairi F, Boulanger T, et al. . [Leptomeningeal metastases from solid tumors]. Rev Prat 2014;64:15–18. [PubMed] [Google Scholar]
- 2. Davies MA, Liu P, McIntyre S, et al. . Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011;117:1687–96. 10.1002/cncr.25634 [DOI] [PubMed] [Google Scholar]
- 3. Groves MD. Leptomeningeal disease. Neurosurg Clin N Am 2011;22:67–78. 10.1016/j.nec.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 4. Robert C, Schachter J, Long GV, et al. . Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521–32. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 5. Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PubMed] [Google Scholar]
- 6. Franklin C, Livingstone E, Roesch A, et al. . Immunotherapy in melanoma: Recent advances and future directions. Eur J Surg Oncol 2017;43:604–11. 10.1016/j.ejso.2016.07.145 [DOI] [PubMed] [Google Scholar]
- 7. Freedman MS, Loertscher R, Cashman NR, et al. . Immunoregulatory properties of T-cell lines derived from the systemic and intrathecal compartments: a phenotypic and functional study. Ann Neurol 1990;27:258–65. 10.1002/ana.410270307 [DOI] [PubMed] [Google Scholar]
- 8. List J, Moser RP, Steuer M, et al. . Cytokine responses to intraventricular injection of interleukin 2 into patients with leptomeningeal carcinomatosis: rapid induction of tumor necrosis factor alpha, interleukin 1 beta, interleukin 6, gamma-interferon, and soluble interleukin 2 receptor (Mr 55,000 protein). Cancer Res 1992;52:1123–8. [PubMed] [Google Scholar]
- 9. Saris SC, Rosenberg SA, Friedman RB, et al. . Penetration of recombinant interleukin-2 across the blood-cerebrospinal fluid barrier. J Neurosurg 1988;69:29–34. 10.3171/jns.1988.69.1.0029 [DOI] [PubMed] [Google Scholar]
- 10. Heimans JJ, Wagstaff J, Schreuder WO, et al. . Treatment of leptomeningeal carcinomatosis with continuous intraventricular infusion of recombinant interleukin-2. Surg Neurol 1991;35:244–7. 10.1016/0090-3019(91)90079-O [DOI] [PubMed] [Google Scholar]
- 11. Tawbi HA FP, Algazi PA, Hamid O F, et al. . Efficacy and safety of nivolumab (NIVO) plus ipilimumab (IPI) in patients with melanoma (MEL) metastatic to the brain: Results of the phase II study CheckMate 204. J Clin Oncol 2012;35. [Google Scholar]
- 12. Papdpoulos NE MR, Grimm E, et al. . Intrathecal use of recombinant interluekin-2 (rIL-2) in the treatment of lepotmeningela disease (LMD) from metastatic melanoma. Proc Am Soc Clin Oncol 1995;1. [Google Scholar]
- 13. Moser RP BJ, Grimm EA. Biological therapy of brain tumors. Cancer Bull 1991;43:117–26. [Google Scholar]
- 14. Samlowski WE, Park KJ, Galinsky RE, et al. . Intrathecal administration of interleukin-2 for meningeal carcinomatosis due to malignant melanoma: sequential evaluation of intracranial pressure, cerebrospinal fluid cytology, and cytokine induction. J Immunother Emphasis Tumor Immunol 1993;13:49–54. [DOI] [PubMed] [Google Scholar]
- 15. Papadopoulos N, Gerber DL, Eton O, et al. . The role of intrathecal (IT) use of interleukin-2 (IL-2) in the treatment of leptomeningeal disease (LMD) in patients (pts) with melanoma. Proc. Am Soc Clin Oncol 2002;21:353a. [Google Scholar]
- 16. Shonka NA, Kessinger AM, Aizenberg MR. Intrathecal interleukin-2 for melanomatous meningitis. J Clin Oncol 2014;32:e111–e113. 10.1200/JCO.2013.49.1100 [DOI] [PubMed] [Google Scholar]
- 17. Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: Leptomeningeal metastases in solid tumors. Surg Neurol Int 2013;4:S265–88. 10.4103/2152-7806.111304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen JV, Tawbi H, Margolin KA, et al. . Melanoma central nervous system metastases: current approaches, challenges, and opportunities. Pigment Cell Melanoma Res 2016;29:627–42. 10.1111/pcmr.12538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davies MA, Saiag P, Robert C, et al. . Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol 2017;18:863–73. 10.1016/S1470-2045(17)30429-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Long GV, Trefzer U, Davies MA, et al. . Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:1087–95. 10.1016/S1470-2045(12)70431-X [DOI] [PubMed] [Google Scholar]
- 21. Goldberg SB, Gettinger SN, Mahajan A, et al. . Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016;17:976–83. 10.1016/S1470-2045(16)30053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Margolin K, Ernstoff MS, Hamid O, et al. . Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 2012;13:459–65. 10.1016/S1470-2045(12)70090-6 [DOI] [PubMed] [Google Scholar]
- 23. Perissinotti AJ, Reeves DJ. Role of intrathecal rituximab and trastuzumab in the management of leptomeningeal carcinomatosis. Ann Pharmacother 2010;44:1633–40. 10.1345/aph.1P197 [DOI] [PubMed] [Google Scholar]
- 24. Laufman LR, Forsthoefel KF. Use of intrathecal trastuzumab in a patient with carcinomatous meningitis. Clin Breast Cancer 2001;2:235 10.1016/S1526-8209(11)70419-0 [DOI] [PubMed] [Google Scholar]
- 25. Rubenstein JL, Combs D, Rosenberg J, et al. . Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood 2003;101:466–8. 10.1182/blood-2002-06-1636 [DOI] [PubMed] [Google Scholar]
- 26. Dorval T, Beuzeboc P, Garcia-Giralt E, et al. . Malignant melanoma: treatment of metastatic meningitis with intrathecal interferon alpha-2b. Eur J Cancer 1992;28:244–5. 10.1016/0959-8049(92)90420-7 [DOI] [PubMed] [Google Scholar]
- 27. Chamberlain MC. A phase II trial of intra-cerebrospinal fluid alpha interferon in the treatment of neoplastic meningitis. Cancer 2002;94:2675–80. 10.1002/cncr.10547 [DOI] [PubMed] [Google Scholar]
- 28. Glitza IC, Haymaker C, Bernatchez C, et al. . Intrathecal administration of tumor-infiltrating lymphocytes is well tolerated in a patient with leptomeningeal disease from metastatic melanoma: a case report. Cancer Immunol Res 2015;3:1201–6. 10.1158/2326-6066.CIR-15-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clemons-Miller AR, Chatta GS, Hutchins L, et al. . Intrathecal cytotoxic T-cell immunotherapy for metastatic leptomeningeal melanoma. Clin Cancer Res 2001;7:917s–24. [PubMed] [Google Scholar]
- 30. Atkins MB, Kunkel L, Sznol M, et al. . High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am 2000;6(Suppl 1):S11–14. [PubMed] [Google Scholar]
- 31. Schadendorf D, Hodi FS, Robert C, et al. . Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015;33:1889–94. 10.1200/JCO.2014.56.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Geukes Foppen MH, Brandsma D, Blank CU, et al. . Targeted treatment and immunotherapy in leptomeningeal metastases from melanoma. Ann Oncol 2016;27:1138–42. 10.1093/annonc/mdw134 [DOI] [PubMed] [Google Scholar]
- 33. Li Y, Pan W, Connolly ID, et al. . Tumor DNA in cerebral spinal fluid reflects clinical course in a patient with melanoma leptomeningeal brain metastases. J Neurooncol 2016;128:93–100. 10.1007/s11060-016-2081-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chamberlain M, Junck L, Brandsma D, et al. . Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol 2017;19:484–92. 10.1093/neuonc/now183 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2017-000283supp001.pdf (30.4KB, pdf)


