Abstract
Background
Storage of platelets results in a progressive defect termed Platelet Storage Lesion (PSL). The PSL is characterized by poor platelet quality on a variety of assays. Metabolic defects are thought to underlie the PSL, thus this study was designed to quantitatively probe specific metabolic pathways over platelet storage.
Study Design and Methods
Relative incorporation of stable isotope labeled substrates was quantified by isotopologue analysis of key acyl-coenzyme A thioester products for fresh, viable (post-collection, day 2–5), and expired platelets (after day 5). We examined the incorporation of acetate, glucose and palmitate into acetyl- and succinyl-CoA via liquid chromatography-tandem mass spectrometry.
Results
Storage related defects in the incorporation of acetyl-CoA derived from acetate and palmitate were observed. Carbon derived from palmitate and acetate in succinyl-CoA was reduced over storage time. Glucose incorporation into succinyl-CoA increased in viable platelets, then decreased in expired platelets. Carbon derived from octanoate and pyruvate remained partially able to incorporate into acetyl- and succinyl-CoA in expired platelets, with high variability in pyruvate incorporation.
Conclusion
Isotopologue analysis is useful in probing substrate specific defects in the PSL.
Keywords: Platelet concentrate, platelet storage lesion, mass spectrometry, stable isotope labeling
Introduction
Traumatic injury is the leading cause of death in the United States for patients between the age of 1 to 40 and a major cause of mortality in civilian1 and military patients2. Notably, 16% of the preventable deaths from injury are due to hemorrhage, highlighting the importance of achieving hemostasis and stabilization. In the Emergency department, patients are frequently coagulopathic3–4, and death by acute blood loss typically occurs within a 6-hour window from injury. Transfusion of platelets, plasma, and red blood cells (RBCs), with some controversy surrounding the ideal ratio, has demonstrated efficacy in restoring hemostasis5, but issues with supply, efficacy, and storage of platelets are a major challenge. The national blood supply has been under increasing stress due to increased medical demand, decreased donation, and increasingly stringent regulation to protect patients receiving transfusions6. Platelets are especially critical to meeting the demands of trauma medicine and are also increasingly subject to supply constraints due to their unique storage needs.
Collection and storage of platelets for use in hospital settings requires 1–2 days for microbiological testing before use. During storage, platelets intended for transfusion are kept at room temperature in gas permeable bags with constant agitation7. After 5–7 days platelets are no longer considered viable for human transfusion due to the risk of bacterial contamination8 leaving only a 3-day window for their use in some regulatory environments. This is considerably shorter than the 8–10 day normal in vivo turnover of platelets. In addition, even before the 5-day regulatory cut-off, platelet storage induces a time-dependent “platelet storage lesion” (PSL) which reduces the effectiveness of stored and transfused platelets9.
Importantly, the three functions -activation, aggregation, degranulation- whose deficiency defines the PSL, are all energetically demanding. The PSL has been shown to derive from dysfunctional metabolism, oxidative stress, and damage to the platelet during storage10. A challenge in addressing the PSL is that the metabolism of platelets is only partially understood11. Importantly, platelets require tight homeostatic control of calcium, adenosine (ADP/ATP) and lipid metabolism and signaling to allow appropriate clotting responses upon activation. Platelets are anuclear but contain mitochondria and are capable of mitochondrial protein synthesis. A set of studies on platelet metabolism, utilizing radioactive tracers, have shown that platelets are capable of utilizing a number of substrates to maintain critical homeostatic processes including maintenance of lipid membrane components14–15. Modern liquid chromatography –mass spectrometry (LC-MS) now offers a highly sensitive and specific platform for performing metabolic studies with more throughput, sensitivity, and specificity than previous techniques16. Recently, Paglia, et al., deployed sophisticated LC-MS based assays to interrogate the PSL, and observed a shift in platelet metabolism over days of storage, characterized by downregulation of the Krebs cycle, which was hypothesized to lead to eventual depletion of critical intermediate metabolites, membrane lipids, and a loss of function17. However, these previous studies examined the metabolites that build up during storage, and while absolute quantification of metabolites may provide insight into the metabolic state of a system, it is possible for compensatory metabolic actions to occur that maintain a metabolite pool by altering the utilization of other precursors18–19. Therefore, in this study, we aimed to expand the previous work on platelet metabolism by using stable isotope labeling and mass isotopomer distribution analysis to probe the active metabolism of fresh, viable, and expired platelets. This provides orthogonal and additive information to existing studies using absolute quantification20. Quantifying the relative enrichment of isotopologues enables evaluation of metabolic pathway activity, as isotopic labeling of a metabolite pool reveals the relative contribution of specific enzymes and pathways from a substrate to product pair21. Here, we have tested a panel of three isotopic tracers ([13C2]-acetate, [13C6]-glucose, and [13C16]-palmitate) to examine metabolism of key carbon pathways by analysis of isotopic enrichment in various acyl-CoenzymeA (CoA) species.
Materials and Methods
Sample collection
Viable platelets were gather from remnants of unused platelet concentrate (PC) (3 remnant bags across different days) used for transfusions at the University of Pennsylvania Trauma Center. Expired platelets (9 bags across different days) were obtained from the Blood Bank of the Hospital of the University of Pennsylvania. Fresh platelets (10 unique donors) were taken from the platelet rich plasma (PRP) of healthy donors of mixed sex, age, and blood type as previously described22.
Chemicals and reagents
Glucose, [13C6]-glucose, sodium chloride (NaCl), potassium chloride (KCl), sodium bicarbonate (NaHCO3), calcium chloride (CaCl2), magnesium chloride (MgCl2), 5-sulfosalacylic acid (SSA), ammonium acetate, and trichloroacetic acid (TCA) were purchased from Sigma (MO, USA). [13C2]-acetate and [13C16]-palmitate were purchased from Cambridge Isotopes (MA, USA). 8.5 mL acid-citrate-dextrose Vacutainer® tubes (Sol A) were purchased from BD Biosciences (NJ, USA). All solvents and additives used including water, acetonitrile, methanol, and formic acid were Optima LC-MS grade purchased from Fisher Scientific (PA, USA).
Stable isotope tracing
Platelets were purified from blood or concentrate media by centrifugation with no brakes at 200 × g for 10 min and the supernatant was discarded. The platelet pellet was resuspended in 1 mL pre-warmed Tyrode’s solution (139 mM NaCl, 3 mM KCl, 17 mM NaHCO3, 3 mM CaCl2, and 1 mM MgCl2) at 37°C containing 5 mM [13C6]-glucose or 5 mM glucose in combination with either 1 mM [13C2]-acetate or 100 µM [13C16]-palmitate and transferred to a 1.5 mL microcentrifuge tube. Samples used for isotopic correction were treated with Tyrode’s containing only 5 mM glucose. Suspended platelets were incubated with tracers for 1 hour at 37°C prior to quenching and acyl-CoA extraction.
Extraction and analysis of acyl-CoA thioesters
The extraction and LC-MS analysis have been described in detail previously18. Briefly, treated platelets were pelleted by centrifugation at 1,000 × g for 2 min and resuspended in 1 mL of ice-cold 10% TCA followed by pulse-sonication for 30 s on ice using a sonic dismembranator (Fisher), followed by a 10 min centrifugation at 15,000 × g. The supernatant was transferred to a fresh tube, and the pellet was discarded. The supernatant was purified by solid-phase extraction using Oasis HLB 1 cm3 (30 mg) SPE columns (Waters) as follows: tubes were conditioned with 1 mL of methanol followed by 1 mL of water. The collected supernatant was applied, washed with 1 mL of water, and eluted using 1 mL of methanol containing 25 mM ammonium acetate. Eluted compounds were evaporated to dryness under nitrogen and resuspended in 50 µL of 5% SSA in water.
Injections of 20 µL of the sample maintained at 4 °C were made from a Leap CTC autosampler (CTC Analytics, Switzerland) for LC-MS/MS analysis. Acyl-CoAs were separated using a reversed-phase HPLC Phenomenex Luna C18 column (2.0 mm × 150 mm, pore size 5 µm) with 5 mM ammonium acetate in water as solvent A, 5 mM ammonium acetate in 95:5 ACN/water (v/v) as solvent B, and 80:20:0.1 (v/v/v) ACN/water/formic acid as solvent C as previously described19. Gradient conditions were as follows: 2% B for 1.5 min, increased to 25% over 3.5 min, increased to 100% B in 0.5 min and held for 8.5 min, washed with 100% C for 5 min, before equilibration for 5 min. The flow rate was 200 µL/min. Samples were analyzed using an API 4000 triple-quadrupole mass spectrometer (Sciex, Foster City, CA) in the positive electrospray (ESI) mode. The column effluent was diverted to the mass spectrometer from 8 to 18 min and to waste for the remainder of the run. The MS operating conditions were: ion spray voltage (5.0 kV), nitrogen as curtain gas (15 units), ion source gas 1 (8 units), gas 2 (15 units), and collision-induced dissociation (CID) gas (5 units). The ESI probe temperature was 450 °C, the declustering potential was 105 V, the entrance potential was 10 V, the collision energy was 45 eV, and the collision exit potential was 15 V. A loss of 507 amu was monitored for each acyl-CoA, with a mass increase of 1 amu in both precursor and product ion for each increasing isotopologue as previously described23.
Isotopologue and Statistical Analysis
Peak areas for acetyl-CoA (M+0−M+2) and succinyl-CoA (M+0−M+4) were integrated using Analyst 1.4.1 software to quantify 13C labelling of the acyl moiety by determining the mass isotopologue distribution. An isotopologue array generated for each acyl-CoA from each sample was multiplied by the inverse of the correction matrix determined experimentally from the platelets incubated with unlabelled glucose to determine the absolute enrichment of each isotopologue as described by Fernandez et al.24 and implemented via a free on-line tool25. This approach reduces the underestimation of isotopic labelling in highly labelled substrates. Therefore, results are presented as a percentage of the unlabelled isotopologue for each acyl-CoA, so that increasing percentages of enrichment in non-M+0 isotopologues indicates the presence of 13C derived from the labelled substrate investigated.
Results
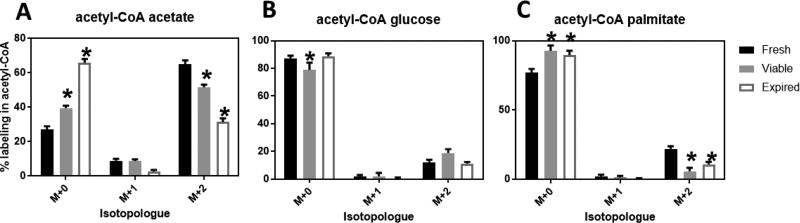
Analysis of acetyl-CoA labeling patterns revealed that platelets incorporate into acetyl-CoA variable amounts of carbon derived from acetate, glucose and palmitate (Fig. 1). The utilization of acetate to produce acetyl-CoA was remarkably high (mean, SD; 64.9, 7.2% in M+2% labeling) and had a strong reduction upon storage (Fig. 1A), with a decrease in the % labeling in acetyl-CoA from fresh to viable (51.7, 2.6%) to expired (31.4, 6.5%) platelets with a p-value < 0.01 by 2-way ANOVA with Dunnett’s multiple comparisons test comparing fresh versus viable and expired platelets. A relatively smaller change in glucose utilization was observed over storage (M+2 % labeling for fresh 12.3,5.5%; viable 18.87,4.9%; expired 11.3,3.4%), and this change was only statistically significant in the labeling pattern observed for viable platelets compared to fresh (Fig. 1B). Acetyl-CoA produced from palmitate was moderate in fresh platelets (M+2 %labeling of 22.0, 5.8%) but markedly decreased in viable and expired plates (5.8, 4.3% and 10.9, 5.2%, respectively) (Fig. 1C).
Figure 1.
Relative enrichment as % labeling into acetyl-CoA from 13C-labeled A) acetate, B) glucose, or C) palmitate. Fresh (n=10), viable (n=3), expired (n=9) platelets from different donors were compared using standardized treatments for 1 hour in Tyrode’s buffer in the presence of the labeled substrate. * p < 0.05 for 2-way ANOVA with Dunnett’s multiple comparison test against fresh platelets.
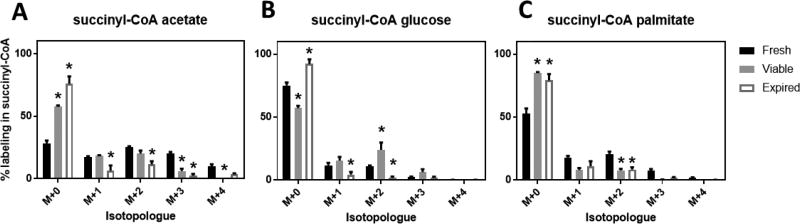
Succinyl-CoA labeling provided complimentary information to the labeling observed in acetyl-CoA. The labeling patterns of succinyl-CoA were more starkly contrasting than for acetyl-CoA, possibly since succinyl-CoA is generally a smaller pool within cells. A similar decrease in succinyl-CoA labeling was observed from labeled acetate over storage. Interesting, the labeling for fresh platelets included high amounts of M+1, M+2, M+3 and M+4 labeling, indicating that carbon from acetate in succinyl-CoA was cycled around the Krebs cycle. Labeling of succinyl-CoA from glucose also agreed with the acetyl-CoA results, with a high level of M+2 labeling in viable platelets (24.0, 10%). Surprisingly, palmitate labeling into succinyl-CoA was higher than glucose in fresh (but not viable or expired platelets), suggesting that β-oxidation of fatty acids in fresh platelets contributed to carbon within the Krebs Cycle.
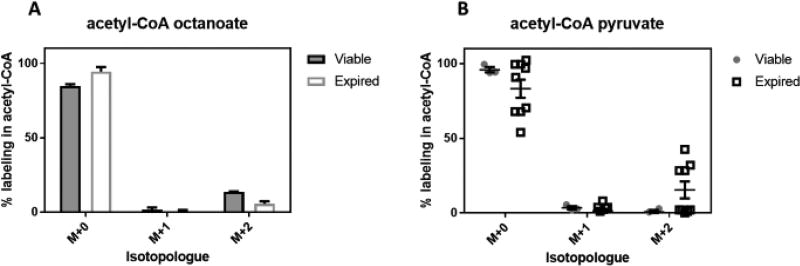
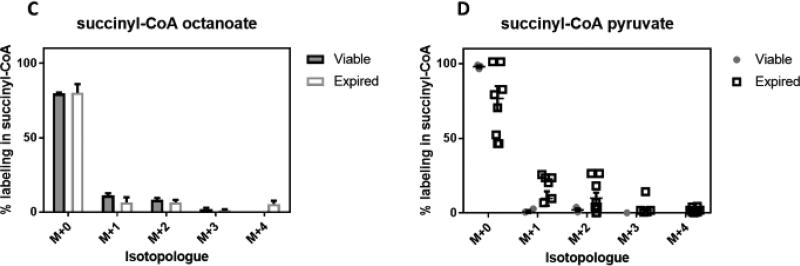
More recent work by Paglia, et al. suggested that the mode of platelet collection may affect their metabolism26, so we explored the potential of using additional tracers to elucidate metabolic consequences of the PSL using the uniformly collected viable and expired platelets. Due to the results with palmitate, we further probed β-oxidation in viable and expired platelets via incubation with [13C8]-octanoate (C8:0). This tracer allows circumventing the carnitine transport system for mitochondrial import. Fairly similar results were obtained using this tracer as compared to palmitate, with relatively minor labeling observed in succinyl-CoA. Expired platelets incorporated slightly less carbon from octanoate into acetyl-CoA than viable platelets, but not statistically so adjusting for the multiple comparisons used in this study. Using [13C3]-pyruvate, we examined the ability of viable and expired platelets incorporate the tracer into acetyl-CoA without glycolysis as required for glucose. Quite interestingly, in expired platelets, pyruvate was able to partially but quite inconsistently label acetyl-CoA (viable vs expired 0.71, 2.12% vs 15.4, 17.0%). Labeling into succinyl-CoA with pyruvate confirms this observation, showed the same across-bag inconsistency, and demonstrates that the carbon derived from pyruvate transits the Krebs Cycle. Again, after adjusting for multiple comparisons, this relation was not statistically significant, but may warrant future study as this degree of variability was not observed with any other tracer. Regardless, pyruvate as a tracer was unique in its ability to incorporate into any metabolite in the expired platelets more so than viable.
Discussion
Metabolomics, and tracer studies in particular, have a particular promise in optimizing transfusion medicine. As highlighted in a recent review, the tractability of both RBCs and stored platelets to metabolomics studies indicates an open avenue of investigation into pressing questions regarding collection, inter-individual variability, storage conditions, and basic biology of RBC and platelet metabolism27. A prime example of this convergence is the application of highly specific probes to reveal and quantify biochemical pathways in transfusion products, for example, the utilization of a stable isotope labeled citrate probe to elucidate an unappreciated citrate catabolic route in RBCs28. Elucidation of these potentially extensive other pathways may provide storage additives, rescue substrates, or probes to quantify metabolic capacity.
Platelets are a particularly promising system for exploiting isotopic tracer analysis. Since they are anuclear, but contain mitochondria, it is possible to trace mitochondrial metabolism. Feasibility of ex vivo tracing is quite high, as platelets can be obtained from PRP in relatively high amounts even from pediatric blood draws29. A major benefit of the metabolic tracer system is that it is self-normalizing, as each isotopologues is normalized to the entire analyte pool. Thus, tight normalization of the number of platelets across studies is not necessary and this avoids introducing less precise methods of normalization which may affect the results23. One caveat of tracer studies is that in order to maximize reproducibility of the tracer incubation, a defined media is required. In our case, we used Tyrode’s buffer, a buffered salt solution that is commonly used in platelet experiments, and allows complete control over carbon sources23. The tight experimental control over the amount of labeled versus unlabeled carbon substrates allows a quantitative substrate to product relationship to be established, but is incompatible with the inherent variability in carbon sources in the blood metabolome. Notably, this protocol is also necessary to compare different times of platelet storage because over time in storage, substrates are metabolized.
A related limitation of our study is that we compared platelets across donors, thus introducing high inter-group variability into our comparison. This is not necessarily a flaw however, since this allows across donor and pool comparisons which were somewhat revealing in this study. Most notably, the isotopologue enrichment of pyruvate across expired pools was highly variable, with between 0–50% of both acetyl- and succinyl-CoA labeled depending on sample. This raises the question if substrate specific defects in the PSL may also be donor specific. Longitudinal studies within and across donors would be needed to address the provocative suggestion that certain platelet donors may have an ideal metabolomics wiring to accommodate storage, analogous to the possibility of variants in RBC donors with advantageous mutations in glycolytic pathways27.
The high capacity for metabolism of acetate by fresh platelets was in line with previous evidence regarding the incorporation of acetate into platelets and other elements of blood14. Interestingly and not previously observed, the stark decay in the capacity for acetate metabolism to acetyl-CoA over storage indicates that this may be a pathway highly affected by the PSL. The remainder of our findings provides orthogonal confirmation of previous studies. The utilization of glucose was in good agreement with the previous study by Paglia et al.17. Increased utilization of glucose into acetyl- and succinyl-CoA for the viable platelets corresponds to the timepoints in platelet storage where the TCA cycle becomes upregulated relative to other pathways.
We did not collect data on platelet function throughout this study. Thus, future studies combining substrate utilization and platelet function would be highly warranted if a substrate was found that even partially circumvented the PSL. Finally, differences in platelet metabolome by collection method has been observed26, but our study was not originally designed to address this comparison.
An additional limitation of our study was the relatively small panel of tracers used and the few analytes considered. An expanding number of stable isotope tracers have been successfully employed in metabolic studies, and this system should be amenable to a wide variety of substrates. However, our study was limited by the choice of platform and analyte coverage. We conducted these experiments on a triple quadrupole mass spectrometer, which is only capable of monitoring relatively few analytes over a given run. This limitation is more acute when performing tracing studies, which require a number of transitions for each isotopologue for each analyte. The dissemination of high resolution mass spectrometers allows additional multiplexing to cover a wider range of analytes, as well as more complicated stable isotope labeling experimental designs incorporating differential neutron coded labels (e.g. 13C vs 15N vs 2H). A number of targeted and untargeted analytical methods may be useful in probing wider acyl-CoA metabolism30, intra- vs extra-cellular metabolites31, or a much wider metabolite panel also examining glycolysis and other relevant pathways32–33.
Figure 2.
Relative enrichment as % labeling into succinyl-CoA from 13C-labeled A) acetate, B) glucose, or C) palmitate. Fresh (n=10), viable (n=3), expired (n=9) platelets from different donors were compared using standardized treatments for 1 hour in Tyrode’s buffer in the presence of the labeled substrate. * p < 0.05 for 2 way ANOVA with Dunnett’s multiple comparison test against Fresh platelets.
Figure 3.
Relative enrichment as % labeling into acetyl-CoA (A,B) and succinyl-CoA (C,D) from 13C-labeled A, C) octanoate, B, D) pyruvate. Viable (n=3) and expired (n=9) platelets from different donors were compared using standardized treatments for 1 hour in Tyrode’s buffer in the presence of the labeled substrate. Individual points in pyruvate labeling are broken out to demonstrate variability of labeling observed uniquely in pyruvate.
Acknowledgments
Support: NIH NIEHS K22ES026235, P30ES013508, NIH NCI R03CA211820
We acknowledge Drs. Skip Brass and Peisong Ma for insightful conversations contributing to this project.
Footnotes
Conflict of Interest Statement: No authors have a conflict of interest relating to this work.
References
- 1.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55(1):39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 2.Pidcoke HF, Aden JK, Mora AG, Borgman MA, Spinella PC, Dubick MA, Blackbourne LH, Cap AP. Ten-year analysis of transfusion in Operation Iraqi Freedom and Operation Enduring Freedom: increased plasma and platelet use correlates with improved survival. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S445–52. doi: 10.1097/TA.0b013e3182754796. [DOI] [PubMed] [Google Scholar]
- 3.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, Mackway-Jones K, Parr MJ, Rizoli SB, Yukioka T, Hoyt DB, Bouillon B. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65(4):748–54. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 4.Saillant NN, Sims CA. Platelet dysfunction in injured patients. Mol Cell Ther. 2014;2:37. doi: 10.1186/s40591-014-0037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, Cohen MJ, Cotton BA, Fabian TC, Inaba K, Kerby JD, Muskat P, O'Keeffe T, Rizoli S, Robinson BR, Scalea TM, Schreiber MA, Stein DM, Weinberg JA, Callum JL, Hess JR, Matijevic N, Miller CN, Pittet JF, Hoyt DB, Pearson GD, Leroux B, van Belle G Group, PS. Transfusion of plasma, platelets, and red blood cells in a1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder EL, Stramer SL, Benjamin RJ. The safety of the blood supply--time to raise the bar. N Engl J Med. 2015;372(20):1882–5. doi: 10.1056/NEJMp1500154. [DOI] [PubMed] [Google Scholar]
- 7.Ohto H, Nollet KE. Overview on platelet preservation: better controls over storage lesion. Transfus Apher Sci. 2011;44(3):321–5. doi: 10.1016/j.transci.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Goodnough LT, Shander A, Brecher ME. Transfusion medicine: looking to the future. Lancet. 2003;361(9352):161–9. doi: 10.1016/S0140-6736(03)12195-2. [DOI] [PubMed] [Google Scholar]
- 9.Inaba K, Branco BC, Rhee P, Blackbourne LH, Holcomb JB, Spinella PC, Shulman I, Nelson J, Demetriades D. Impact of the duration of platelet storage in critically ill trauma patients. J Trauma. 2011;71(6):1766–73. doi: 10.1097/TA.0b013e31823bdbf9. discussion 1773-4. [DOI] [PubMed] [Google Scholar]
- 10.Perales Villarroel JP, Figueredo R, Guan Y, Tomaiuolo M, Karamercan MA, Welsh J, Selak MA, Becker LB, Sims C. Increased platelet storage time is associated with mitochondrial dysfunction and impaired platelet function. Journal of Surgical Research. 2013;184(1):422–429. doi: 10.1016/j.jss.2013.05.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer PA, Ravi S, Chacko B, Johnson MS, Darley-Usmar VM. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol. 2014;2:206–10. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacroix J, Hebert PC, Fergusson DA, Tinmouth A, Cook DJ, Marshall JC, Clayton L, McIntyre L, Callum J, Turgeon AF, Blajchman MA, Walsh TS, Stanworth SJ, Campbell H, Capellier G, Tiberghien P, Bardiaux L, van de Watering L, van der Meer NJ, Sabri E, Vo D Investigators, A.; Canadian Critical Care Trials, G. Age of transfused blood in critically ill adults. N Engl J Med. 2015;372(15):1410–8. doi: 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]
- 13.Heddle NM, Cook RJ, Arnold DM, Liu Y, Barty R, Crowther MA, Devereaux PJ, Hirsh J, Warkentin TE, Webert KE, Roxby D, Sobieraj-Teague M, Kurz A, Sessler DI, Figueroa P, Ellis M, Eikelboom JW. Effect of Short-Term vs. Long-Term Blood Storage on Mortality after Transfusion. N Engl J Med. 2016 doi: 10.1056/NEJMoa1609014. [DOI] [PubMed] [Google Scholar]
- 14.Deykin D, Desser RK. The incorporation of acetate and palmitate into lipids by human platelets. J Clin Invest. 1968;47(7):1590–602. doi: 10.1172/JCI105851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen P, Derksen A, Van den Bosch H. Pathways of fatty acid metabolism in human platelets. J Clin Invest. 1970;49(1):128–39. doi: 10.1172/JCI106211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciccimaro E, Blair IA. Stable-isotope dilution LC-MS for quantitative biomarker analysis. Bioanalysis. 2010;2(2):311–41. doi: 10.4155/bio.09.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paglia G, Sigurjonsson OE, Rolfsson O, Valgeirsdottir S, Hansen MB, Brynjolfsson S, Gudmundsson S, Palsson BO. Comprehensive metabolomic study of platelets reveals the expression of discrete metabolic phenotypes during storage. Transfusion. 2014;54(11):2911–23. doi: 10.1111/trf.12710. [DOI] [PubMed] [Google Scholar]
- 18.Basu SS, Blair IA. SILEC: a protocol for generating and using isotopically labeled coenzyme A mass spectrometry standards. Nature protocols. 2012;7(1):1–12. doi: 10.1038/nprot.2011.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu SS, Mesaros C, Gelhaus SL, Blair IA. Stable isotope labeling by essential nutrients in cell culture for preparation of labeled coenzyme A and its thioesters. Analytical chemistry. 2011;83(4):1363–9. doi: 10.1021/ac1027353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang GF, Sadhukhan S, Tochtrop GP, Brunengraber H. Metabolomics, pathway regulation, and pathway discovery. J Biol Chem. 2011;286(27):23631–5. doi: 10.1074/jbc.R110.171405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bequette BJ, Sunny NE, El-Kadi SW, Owens SL. Application of stable isotopes and mass isotopomer distribution analysis to the study of intermediary metabolism of nutrients. J Anim Sci. 2006;84(Suppl):E50–9. doi: 10.2527/2006.8413_supple50x. [DOI] [PubMed] [Google Scholar]
- 22.Basu SS, Deutsch EC, Schmaier AA, Lynch DR, Blair IA. Human platelets as a platform to monitor metabolic biomarkers using stable isotopes and LC-MS. Bioanalysis. 2013;5(24):3009–21. doi: 10.4155/bio.13.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worth AJ, Marchione DM, Parry RC, Wang Q, Gillespie KP, Saillant NN, Sims C, Mesaros C, Snyder NW, Blair IA. LC-MS Analysis of Human Platelets as a Platform for Studying Mitochondrial Metabolism. J Vis Exp. 2016;(110):e53941. doi: 10.3791/53941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez CA, Des Rosiers C, Previs SF, David F, Brunengraber H. Correction of 13C mass isotopomer distributions for natural stable isotope abundance. J Mass Spectrom. 1996;31(3):255–62. doi: 10.1002/(SICI)1096-9888(199603)31:3<255::AID-JMS290>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Trefely S, Ashwell P, Snyder NW. FluxFix: automatic isotopologue normalization for metabolic tracer analysis. BMC Bioinformatics. 2016;17(1):485. doi: 10.1186/s12859-016-1360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paglia G, Sigurjonsson OE, Rolfsson O, Hansen MB, Brynjolfsson S, Gudmundsson S, Palsson BO. Metabolomic analysis of platelets during storage: a comparison between apheresis- and buffy coat-derived platelet concentrates. Transfusion. 2015;55(2):301–13. doi: 10.1111/trf.12834. [DOI] [PubMed] [Google Scholar]
- 27.Nemkov T, Hansen KC, Dumont LJ, D'Alessandro A. Metabolomics in transfusion medicine. Transfusion. 2016;56(4):980–93. doi: 10.1111/trf.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Alessandro A, Nemkov T, Yoshida T, Bordbar A, Palsson BO, Hansen KC. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion. 2017;57(2):325–336. doi: 10.1111/trf.13892. [DOI] [PubMed] [Google Scholar]
- 29.Worth AJ, Basu SS, Deutsch EC, Hwang WT, Snyder NW, Lynch DR, Blair IA. Stable isotopes and LC-MS for monitoring metabolic disturbances in Friedreich's ataxia platelets. Bioanalysis. 2015;7(15):1843–55. doi: 10.4155/bio.15.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snyder NW, Basu SS, Zhou Z, Worth AJ, Blair IA. Stable isotope dilution liquid chromatography/mass spectrometry analysis of cellular and tissue medium- and long-chain acyl-coenzyme A thioesters. Rapid Commun Mass Spectrom. 2014;28(16):1840–8. doi: 10.1002/rcm.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paglia G, Magnusdottir M, Thorlacius S, Sigurjonsson OE, Guethmundsson S, Palsson BO, Thiele I. Intracellular metabolite profiling of platelets: evaluation of extraction processes and chromatographic strategies. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;898:111–20. doi: 10.1016/j.jchromb.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Ser Z, Locasale JW. Development and quantitative evaluation of a high-resolution metabolomics technology. Analytical chemistry. 2014;86(4):2175–84. doi: 10.1021/ac403845u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo L, Worth AJ, Mesaros C, Snyder NW, Glickson JD, Blair IA. Diisopropylethylamine/hexafluoroisopropanol-mediated ion-pairing ultra-high-performance liquid chromatography/mass spectrometry for phosphate and carboxylate metabolite analysis: utility for studying cellular metabolism. Rapid Commun Mass Spectrom. 2016;30(16):1835–45. doi: 10.1002/rcm.7667. [DOI] [PMC free article] [PubMed] [Google Scholar]