Abstract
Background and aims
No prospective study has investigated whether elevated lipoprotein(a) concentrations are associated with an increased risk of abdominal aortic aneurysm (AAA). We aimed to prospectively investigate this association.
Methods
In 1987–1989, the Atherosclerosis Risk in Communities study measured plasma lipoprotein(a) in 13,683 participants aged 45–64 years, without a history of AAA surgery. We followed them for incident, clinical AAA events through 2011.
Results
During the 272,914 person-years of follow-up, over a median of 22.6 years, we documented 505 incident AAA events. The age-, sex-, and race-adjusted model showed that individuals in the highest quintile of plasma lipoprotein(a) had an increased risk of AAA. Further adjustment for the other potential confounding factors, including other plasma lipids (high- and low-density lipoprotein cholesterol and triglyceride concentrations), attenuated the association, but individuals in the highest versus lowest quintile of plasma lipoprotein(a) still had a significantly increased risk of AAA [hazard ratio (95% confidence interval): 1.57 (1.19–2.08)]. Interaction testing suggested no difference in the associations for whites and African Americans (p for interaction=0.96). A restricted cubic spline analysis demonstrated a positive dose-response relation of plasma lipoprotein(a) with AAA, with a steep increase in AAA risk above the 75th percentile (p for overall association=0.0086, p for non-linear association=0.097).
Conclusions
In this population-based cohort study, elevated lipoprotein(a) concentrations were independently associated with an increased risk of AAA. The association reflected a threshold of increased AAA risk at high lipoprotein(a) concentrations, rather than a steady monotonic association.
Keywords: abdominal aortic aneurysm, lipoprotein(a), prospective study, population-based study
INTRODUCTION
Abdominal aortic aneurysm (AAA) is a relatively common disease in Western populations (1). With societal aging, the number of AAA cases is growing (2). Although AAA is usually asymptomatic, ruptured AAA is often fatal (3). Thus, it is very important to identify populations at high risk of AAA to prevent AAA events. Some risk factors for AAA, like male sex and cigarette smoking, are clear AAA risk factors, but whether low-density lipoprotein (LDL) cholesterol is a risk factor for AAA is disputed (4).
Lipoprotein(a), consisting of an LDL-like lipoprotein and apolipoprotein(a), is a risk factor for several cardiovascular diseases, including coronary heart disease (5–8) and stroke (9–11). Several case-control studies have suggested that lipoprotein(a) is also associated positively with the prevalence of AAA (12, 13). However, to the best of our knowledge, there is no prospective study investigating the association between lipoprotein(a) concentrations and AAA risk in the general population.
Using hospitalized AAA data through 2011 from the Atherosclerosis Risk in Communities study (ARIC), we tested the hypothesis that lipoprotein(a) is positively associated with the incidence of AAA, independent of other AAA risk factors, including other lipid profiles, in a population-based prospective study in the U.S.
Materials and methods
Study population
The ARIC study is a community-based cohort study to identify the determinants of atherosclerosis and cardiovascular diseases. Details of the study are available elsewhere (14). Briefly, ARIC recruited 15,792 mostly White or African American men and women aged 45 to 64 years, between 1987 and 1989, from Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi (African Americans only); and suburbs of Minneapolis, Minnesota. Follow-up is ongoing for several cardiovascular diseases, including AAA.
The institutional review boards at each site approved the study protocol, and all participants provided written informed consent.
Abdominal aortic aneurysm risk factors
The main exposure of interest was the plasma lipoprotein(a) concentration at baseline. Each ARIC center obtained blood from seated participants, processed it immediately, and frozen samples, including EDTA plasma at −70 °C. Within 6 weeks, lipoprotein(a) was measured in a central laboratory as the total protein component [apolipoprotein(a) + apolipoprotein B] using a double-antibody enzyme-linked immunosorbent assay technique (15). This protein moiety represents approximately one-third of total lipoprotein(a) mass (16). Thus, a value of 100 μg/mL lipoprotein(a) protein is comparable to a total lipoprotein(a) value of 300 μg/mL. The assay reliability (between-person variance divided by the total variance) was 0.90, with essentially no within-person variability (indicative of a largely genetic predisposition), in a 40-person subsample (16).
Based on the previous report (17), we selected a number of factors that potentially might confound the association of lipoprotein(a) with AAA, including age, sex, race (white or African American), height (cm), weight (kg), smoking status (current, former or never), pack-years of smoking, hypertension (systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg or hypertension medication use) (18), diabetes mellitus (a fasting blood glucose ≥126 mg/dl, non-fasting blood glucose ≥200 mg/dl, a self-reported physician diagnosis of diabetes, or use of antidiabetic medication in the past 2 weeks) (19), high-density lipoprotein (HDL) cholesterol (mmol/L), LDL cholesterol (mmol/L), and triglycerides (mmol/L). In addition, current use of female hormone replacement therapy (estrogen and/or progesterone) was included in potential confounding factors.
Identification of abdominal aortic aneurysm
Several strategies were used to identify AAA events (17, 20). ARIC interviewers annually called participants to ask about their interim hospitalizations and identify deaths. ARIC also identified additional hospitalizations or deaths by surveying local hospitals. Moreover, participant identifiers were linked with fee-for-service Medicare data for 1991–2011 to identify additional events for participants who were over 65 years. Incident clinical AAAs were defined on the basis of a single hospital discharge or two outpatient encounters having International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes of 441.3 or 441.4, or procedure codes of 38.44 or 39.71, which were required to be verified by diagnosis codes, or death codes of ICD-9 441.3 or 441.4 or ICD-10 code I71.3 or I71.4. Thoracic, thoracoabdominal, or unspecified aortic aneurysms (n=7) were classified as AAA non-events because of possibly different pathophysiology.
Statistical analysis
SAS version 9.4 software (SAS Institute Inc., Cary, NC) was used for statistical analyses. All statistical tests were two-tailed and p values < 0.05 were regarded as significant.
We excluded participants meeting any of the following hierarchical criteria at baseline: prior AAA surgery (n=11), uncertain AAA status during follow-up (i.e. loss to follow-up, n=30), non-white participants in Washington County or Minneapolis or non-white/black participants in Forsyth County (n=48) to allow for multivariable adjustment for race and study site (21), and missing data on lipoprotein(a) (n=571) or any covariates (n=1,063). In addition, we excluded participants using cholesterol-lowering drugs (n=386). Ultimately, 13,683 participants were included in the present analyses.
We used a naturally log-transformed continuous measure of plasma lipoprotein(a) because of skewness (Supplemental Fig.). We firstly calculated mean levels or percentages of potential confounding factors at baseline according to quintiles of plasma lipoprotein(a) level. We calculated person-years of follow-up from baseline to the first endpoint: AAA, death, loss to follow-up, or administrative censoring at December 31, 2011. We computed hazard ratios (HRs) and their 95% confidence intervals (CIs) of clinical AAA using Cox’s proportional hazard regression models. We tested the proportional hazards assumption in the Cox’s proportional hazard regression models using risk factor-by-time interactions and found it was not violated. We conducted the pooled analysis across sex because we found no statistical interaction between sex and lipoprotein(a) in relation to AAA risk. Model 1 adjusted for age, sex, and race/ARIC field center; and Model 2 additionally for height, weight, smoking status, pack-years smoking, hypertension, diabetes mellitus, LDL and HDL cholesterol, triglycerides, and estrogen and/or progesterone use. We also conducted a race-specific analysis because of known differences in lipoprotein(a) concentrations between whites and African Americans. Since there were few AAA events in African American within the first quintile of plasma lipoprotein(a), we compared sex-specific AAA risk for the fifth quintile of lipoprotein(a) compared with the first through fourth quintiles. We constructed a cubic spline graph with 5 knots at the 5, 25, 50, 75 and 95th percentiles in order to examine a dose-response relationship in detail between a naturally log-transformed continuous measure of plasma lipoprotein(a) and AAA risk (22). The p-value for the association was based on a chi-square test for whether all terms in the cubic spline are equal to zero.
Since the long time lag between baseline and end of follow up (in this study more than 20 years) may generate confounding, we reran models using early follow-up data only (within 10 years of baseline) for a sensitivity analysis. We also ran models after excluding early AAA events (within 10 years of baseline) and/or participants who were 60 to 64 years old to reduce the influence of unidentified AAA at baseline.
RESULTS
Baseline characteristics according to quintiles of plasma lipoprotein(a) concentration
Among the 13,683 ARIC participants included in the analysis, the mean age was 54.1 years, 55.3% were female, and 25.7% were African American. Median values of plasma lipoprotein(a) for each quintile were 11, 29, 62, 125 and 246 μg/mL. As shown in Table 1, compared with individuals in the lowest quintile of plasma lipoprotein(a), those in higher quintiles tended to be female, African American, shorter in stature, and current smokers, to have higher LDL cholesterol levels, lower triglycerides levels and use hormone replacement therapy.
Table 1.
Baseline characteristics of participants according to quintiles of plasma lipoprotein(a) concentration, ARIC, 1987–89 (n=13,683).
| Quintiles of plasma lipoprotein(a) concentration (median, range μg/mL)
|
|||||
|---|---|---|---|---|---|
| 11 (1–19) | 29 (20–43) | 62 (44–86) | 125 (87–173) | 246 (174–817) | |
| Participants, n | 2,813 | 2,659 | 2,735 | 2,731 | 2,745 |
| Age, y | 54.0±5.7 | 54.2±5.7 | 54.2±5.8 | 54.0±5.8 | 54.1±5.7 |
| Female, % | 50.0 | 52.3 | 55.6 | 56.2 | 62.3 |
| African American, % | 5.7 | 10.3 | 25.7 | 40.8 | 46.3 |
| Height, cm | 169.2±9.4 | 168.9±9.4 | 168.5±9.2 | 168.6±9.5 | 167.6±9.0 |
| Weight, kg | 77.8±16.6 | 77.2±16.1 | 78.7±16.5 | 79.6±16.9 | 79.0±17.2 |
| Current smoker, % | 25.5 | 25.8 | 26.0 | 25.4 | 26.0 |
| Pack-years of smoking | 17.4±22.6 | 17.4±22.0 | 15.5±20.9 | 14.9±21.5 | 14.2±21.4 |
| Hypertension, % | 29.3 | 27.7 | 31.7 | 38.1 | 40.4 |
| Diabetes mellitus, % | 10.1 | 8.6 | 10.2 | 12.6 | 12.8 |
| LDL cholesterol, mmol/L | 3.3±1.0 | 3.4±0.9 | 3.5±1.0 | 3.6±1.0 | 3.9±1.0 |
| HDL cholesterol, mmol/L | 1.3±0.5 | 1.3±0.4 | 1.3±0.4 | 1.4±0.4 | 1.4±0.5 |
| Triglycerides, mmol/L | 1.5±0.8 | 1.4±0.7 | 1.4±0.7 | 1.3±0.7 | 1.3±0.6 |
| Hormone replacement therapy use (women only), % | 11.3 | 10.8 | 10.2 | 9.1 | 9.0 |
ARIC, Atherosclerosis Risk in Communities study.
Values are mean ± standard deviation for continuous variables and % for categorical variables.
Associations of plasma lipoprotein(a) with abdominal aortic aneurysm
During the 272,914 person-years of follow-up, over a median of 22.6 years, a total of 505 participants developed incident, clinical AAA (Table 2). The crude incidence rates (per 1,000 person-years) were 1.9 for overall (3.1 for men and 0.9 for women, respectively). The age-, sex-, and race-adjusted model (Model 1) showed that individuals in the highest quintile of plasma lipoprotein(a) had a 1.63-fold greater risk of AAA than those in the lowest quintile. Further adjustment for the other potential confounding factors, including other lipid profiles, attenuated the association (Model 2), but individuals in the highest quintile of plasma lipoprotein(a) still had a significantly increased risk of AAA [HR (95% CI): 1.57 (1.19–2.08), p for trend=0.004]. There was no significant interaction between race and lipoprotein(a) in relation to AAA (p for interaction=0.96).
Table 2.
Hazard ratios and 95% confidence intervals for incident, clinical abdominal aortic
| Quintiles of plasma lipoprotein(a) level (median, range μg/mL)
|
p for trend | |||||
|---|---|---|---|---|---|---|
| 11 (1–19) | 29 (20–43) | 62 (44–86) | 125 (87–173) | 246 (174–817) | ||
| No. at risk | 2,813 | 2,659 | 2,735 | 2,731 | 2,745 | |
| Person-years | 56,518 | 53,977 | 55,205 | 54,032 | 53,184 | |
| Cases | 96 | 100 | 103 | 87 | 119 | |
| Model 1 | 1 | 1.10 (0.83–1.45) | 1.19 (0.90–1.57) | 1.06 (0.79–1.42) | 1.63 (1.24–2.13) | 0.002 |
| Model 2 | 1 | 1.04 (0.79–1.38) | 1.15 (0.87–1.52) | 1.04 (0.78–1.40) | 1.57 (1.19–2.08) | 0.004 |
ARIC, Atherosclerosis Risk in Communities study.
Model 1: adjusted for age, sex, and race/ARIC field center.
Model 2: adjusted for Model 1 factors + height, weight, smoking status, pack-years of smoking, hypertension, diabetes mellitus, LDL cholesterol, HDL cholesterol, triglycerides, and hormone replacement therapy use.
The continuous relation of plasma lipoprotein(a) with AAA incidence was assessed further using a restricted cubic spline (Fig. 1). Plasma lipoprotein(a) showed a positive dose-response relation with risk of AAA (p for overall association=0.0086), with a steep increase in AAA risk above the 75th percentile [natural log-transformed lipoprotein(a) ≈4.2]. The p for non-linear association was 0.097.
Fig. 1.
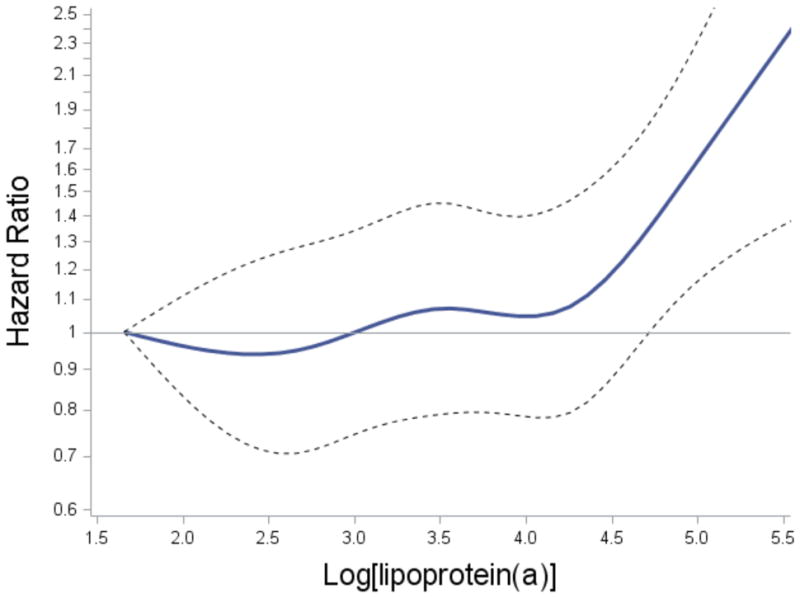
Multivariably adjusted (Model 2) association of natural log-transformed lipoprotein(a) with AAA.
Solid and dashed lines represent hazard ratios and 95% confidence interval, respectively. The reference value was 5th percentile of lipoprotein(a).
Both sensitivity analyses produced similar results to main results, although the analyses were less likely to be statistically significant, due to the lower statistical power (data not shown).
DISCUSSION
In the ARIC cohort, individuals with higher plasma lipoprotein(a) had an increased risk of incident AAA, independent of other AAA risk factors, including other plasma lipids such as LDL cholesterol and triglycerides. The association reflected a threshold of increased AAA risk at high lipoprotein(a) concentrations, rather than a steady monotonic association. To the best of our knowledge, this is the first prospective population-based cohort study to investigate the association of plasma lipoprotein(a) with AAA events, although several previous case-control studies have suggested a positive association of lipoprotein(a) with the presence of AAA (12, 13).
Elevated lipoprotein(a) has been suggested to play several important roles in atherosclerosis in ways similar to elevated LDL, such as causing endothelial dysfunction through monocyte chemoattractant protein 1 (23), activating proinflammatory genes through oxidized phospholipids (24), and promoting smooth muscle cell proliferation (25). These pathophysiological processes are all associated with AAA as well as coronary heart disease and stroke (20, 26, 27).
African Americans have higher concentration of plasma lipoprotein(a) compared to whites, so we also conducted race-specific analyses. In both whites and African Americans, elevated plasma lipoprotein(a) was associated with increased risk of AAA. In addition, the lack of any significant interaction between race and lipoprotein(a) suggests that there is no difference in the association of lipoprotein(a) with AAA risk between whites and African Americans. However, our statistical power to detect race differences was somewhat limited by the small number of African Americans and few incident AAAs among African Americans who had low lipoprotein(a) concentrations.
Strengths of our study may include the following: ARIC measured plasma lipoprotein(a) concentrations within 6 weeks of collection. A previous study reported that lipoprotein(a) significantly decreased when measured after 3 and 28 months of collection, particularly in subjects with more small apolipoprotein(a) isoforms, which are more frequent in patients with atherosclerotic disease than control subjects (28, 29). Another study also reported that after 5 years of storage, lipoprotein(a) levels decreased significantly in myocardial infarction cases, but not in controls (30). Thus, case-control study designs may be more subject to misclassification than our design. Another strength is that we included African Americans as well as whites, and observed similar associations for both.
Several limitations should be mentioned. Firstly, we measured lipoprotein(a) only at baseline, and thus, we cannot the negate the possibility of misclassification of participants’ level during the follow-up. However, circulating lipoprotein(a) level is largely genetically determined, and thus, is little affected by age or lifestyle (30). Secondly, the assay predated contemporary lipoprotein(a) assays, and apolipoprotein(a) isoform information also was not available for our study. Small apolipoprotein(a) isoforms has been suggested to be more atherogenic than large apolipoprtotein(a) isoforms as well as be associated with higher plasma lipoprotein(a) levels (29). Thirdly, we did not perform a baseline ultrasound to rule out prevalent subclinical AAAs. However, ARIC participants were under 65 years old at baseline and AAA is unusual at that age, as screening for AAA is recommended for those aged over 65 years (31). Thus, we assume that the number of prevalent AAAs mistakenly included should have been low. Fourthly, we assessed clinical AAA using ICD codes and did not directly validate ICD codes for AAA (we did not perform imaging tests for all participants). AAA codes seem quite specific, but are probably insensitive for capturing AAA events. Thus, we might have underestimated AAA events. However, we assume that this underestimation should lead to non-differential misclassification. Finally, we excluded a relatively large number of participants with missing data on exposure or covariates. Thus, we cannot deny the possibility of bias due to these exclusions.
In conclusion, in the prospective population-based ARIC cohort, elevated lipoprotein(a) concentrations were associated with an increased risk of AAA, independent of other AAA risk factors including other plasma lipids.
Supplementary Material
No prospective study has examined the association between Lp(a) and abdominal aortic aneurysm (AAA).
Elevated Lp(a) levels are independently associated with an increased risk of AAA.
This positive association is observed in both whites and African Americans.
The association reflects a threshold of AAA risk at high Lp(a) concentrations.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
FINANCIAL SUPPORT
The Nippon Foundation provided grants to support Dr. Kubota’s fellowship at School of Public Health, University of Minnesota. The National Heart, Lung, and Blood Institute (NHLBI) supported ARIC via contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, HHSN268201100012C, and the collection of AAA data via R01 HL103695.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
AUTHOR CONTRIBUTIONS
Authors YK and AF contributed to the conception and design of the work. Authors AF and WT acquired the data, and YK performed the analysis. All authors were involved in the interpretation of data. YK and AF drafted the work, which was critically revised by all other authors. All authors approved the final version of the manuscript.
References
- 1.U.S. Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med. 2005 Feb 1;142(3):198–202. doi: 10.7326/0003-4819-142-3-200502010-00011. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Zhao G, Zhang J, Duan Z, Xin S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population–a meta-analysis. PLoS One. 2013 Dec 2;8(12):e81260. doi: 10.1371/journal.pone.0081260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, et al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002 Nov 16;360(9345):1531–9. doi: 10.1016/s0140-6736(02)11522-4. [DOI] [PubMed] [Google Scholar]
- 4.Golledge J, Tsao PS, Dalman RL, Norman PE. Circulating markers of abdominal aortic aneurysm presence and progression. Circulation. 2008 Dec 2;118(23):2382–92. doi: 10.1161/CIRCULATIONAHA.108.802074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 2000 Sep 5;102(10):1082–5. doi: 10.1161/01.cir.102.10.1082. [DOI] [PubMed] [Google Scholar]
- 6.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009 Dec 24;361(26):2518–28. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 7.Erqou S, Thompson A, Di Angelantonio E, Saleheen D, Kaptoge S, et al. Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58,000 participants. J Am Coll Cardiol. 2010 May 11;55(19):2160–7. doi: 10.1016/j.jacc.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 8.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009 Jun 10;301(22):2331–9. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 9.Smolders B, Lemmens R, Thijs V. Lipoprotein (a) and stroke: a meta-analysis of observational studies. Stroke. 2007 Jun;38(6):1959–66. doi: 10.1161/STROKEAHA.106.480657. [DOI] [PubMed] [Google Scholar]
- 10.Milionis HJ, Filippatos TD, Loukas T, Bairaktari ET, Tselepis AD, et al. Serum lipoprotein(a) levels and apolipoprotein(a) isoform size and risk for first-ever acute ischaemic nonembolic stroke in elderly individuals. Atherosclerosis. 2006 Jul;187(1):170–6. doi: 10.1016/j.atherosclerosis.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 11.Ohira T, Schreiner PJ, Morrisett JD, Chambless LE, Rosamond WD, et al. Lipoprotein(a) and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2006 Jun;37(6):1407–12. doi: 10.1161/01.STR.0000222666.21482.b6. [DOI] [PubMed] [Google Scholar]
- 12.Takagi H, Manabe H, Kawai N, Goto SN, Umemoto T. Circulating lipoprotein(a) concentrations and abdominal aortic aneurysm presence. Interact Cardiovasc Thorac Surg. 2009 Sep;9(3):467–70. doi: 10.1510/icvts.2009.208843. [DOI] [PubMed] [Google Scholar]
- 13.Kotani K, Sahebkar A, Serban MC, Ursoniu S, Mikhailidis DP, et al. Lipoprotein(a) Levels in Patients With Abdominal Aortic Aneurysm. Angiology. 2017 Feb;68(2):99–108. doi: 10.1177/0003319716637792.8-2477. [DOI] [PubMed] [Google Scholar]
- 14.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC Investigators. Am J Epidemiol. 1989 Apr;129(4):687–702. [PubMed] [Google Scholar]
- 15.Gaubatz JW, Chari MV, Nava ML, Guyton JR, Morrisett JD. Isolation and characterization of the two major apoproteins in human lipoprotein(a) J Lipid Res. 1987 Jan;28(1):69–79. [PubMed] [Google Scholar]
- 16.Ohira T, Schreiner PJ, Morrisett JD, Chambless LE, Rosamond WD, et al. Lipoprotein(a) and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2006 Jun;37(6):1407–12. doi: 10.1161/01.STR.0000222666.21482.b6. [DOI] [PubMed] [Google Scholar]
- 17.Tang W, Yao L, Roetker NS, Alonso A, Lutsey PL, et al. Lifetime Risk and Risk Factors for Abdominal Aortic Aneurysm in a 24-Year Prospective Study: The ARIC Study (Atherosclerosis Risk in Communities) Arterioscler Thromb Vasc Biol. 2016 Dec;36(12):2468–2477. doi: 10.1161/ATVBAHA.116.308147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubota Y, Evenson KR, MacLehose RF, Roetker NS, Joshu CE, et al. Physical Activity and Lifetime Risk of Cardiovascular Disease and Cancer. Med Sci Sports Exerc. 2017 Mar 27; doi: 10.1249/MSS.0000000000001274. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubota Y, Heiss G, MacLehose RF, Roetker NS, Folsom AR. Educational Attainment and Lifetime Risk of Cardiovascular Disease: the Atherosclerosis Risk in Communities Study. JAMA Intern Med. 2017 Aug 1;177(8):1165–1172. doi: 10.1001/jamainternmed.2017.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folsom AR, Yao L, Alonso A, Lutsey PL, Missov E, et al. Circulating Biomarkers and Abdominal Aortic Aneurysm Incidence: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2015 Aug 18;132(7):578–85. doi: 10.1161/CIRCULATIONAHA.115.016537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubota Y, London SJ, Cushman M, Chamberlain AM, Rosamond WD, et al. Lung function, respiratory symptoms and venous thromboembolism risk: the Atherosclerosis Risk in Communities Study. J Thromb Haemost. 2016 Dec;14(12):2394–2401. doi: 10.1111/jth.13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010 Apr 30;29(9):1037–57. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 23.Wiesner P, Tafelmeier M, Chittka D, Choi SH, Zhang L, et al. MCP-1 binds to oxidized LDL and is carried by lipoprotein(a) in human plasma. J Lipid Res. 2013 Jul;54(7):1877–83. doi: 10.1194/jlr.M036343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taleb A, Witztum JL, Tsimikas S. Oxidized phospholipids on apoB-100-containing lipoproteins: a biomarker predicting cardiovascular disease and cardiovascular events. Biomark Med. 2011 Oct;5(5):673–94. doi: 10.2217/bmm.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichikawa T, Unoki H, Sun H, Shimoyamada H, Marcovina S, et al. Lipoprotein(a) promotes smooth muscle cell proliferation and dedifferentiation in atherosclerotic lesions of human apo(a) transgenic rabbits. Am J Pathol. 2002 Jan;160(1):227–36. doi: 10.1016/S0002-9440(10)64366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siasos G, Mourouzis K, Oikonomou E, Tsalamandris S, Tsigkou V, et al. The Role of Endothelial Dysfunction in Aortic Aneurysms. Curr Pharm Des. 2015;21(28):4016–34. doi: 10.2174/1381612821666150826094156. [DOI] [PubMed] [Google Scholar]
- 27.Patel MI, Ghosh P, Melrose J, Appleberg M. Smooth muscle cell migration and proliferation is enhanced in abdominal aortic aneurysms. Aust N Z J Surg. 1996 May;66(5):305–8. doi: 10.1111/j.1445-2197.1996.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 28.Kronenberg F, Trenkwalder E, Dieplinger H, Utermann G. Lipoprotein(a) in stored plasma samples and the ravages of time. Why epidemiological studies might fail. Arterioscler Thromb Vasc Biol. 1996 Dec;16(12):1568–72. doi: 10.1161/01.atv.16.12.1568. [DOI] [PubMed] [Google Scholar]
- 29.Kronenberg F, Kronenberg MF, Kiechl S, Trenkwalder E, Santer P, et al. Role of lipoprotein(a) and apolipoprotein(a) phenotype in atherogenesis: prospective results from the Bruneck study. Circulation. 1999 Sep 14;100(11):1154–60. doi: 10.1161/01.cir.100.11.1154. [DOI] [PubMed] [Google Scholar]
- 30.Simó JM, Camps J, Vilella E, Gómez F, Paul A, et al. Instability of lipoprotein(a) in plasma stored at -70 degrees C: effects of concentration, apolipoprotein(a) genotype, and donor cardiovascular disease. Clin Chem. 2001 Sep;47(9):1673–8. [PubMed] [Google Scholar]
- 31.Milionis HJ, Winder AF, Mikhailidis DP. Lipoprotein (a) and stroke. J Clin Pathol. 2000 Jul;53(7):487–96. doi: 10.1136/jcp.53.7.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LeFevre ML, U.S. Preventive Services Task Force Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014 Aug 19;161(4):281–90. doi: 10.7326/M14-1204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


