Abstract
Syd-1 proteins are required for presynaptic development in worm, fly, and mouse. Syd-1 proteins in all three species contain a Rho GTPase activating protein (GAP)-like domain of unclear significance: invertebrate Syd-1s are thought to lack GAP activity, and mouse mSYD1A has GAP activity that is thought to be dispensable for its function. Here, we show that Drosophila melanogaster Syd-1 can interact with all six fly Rhos and has GAP activity toward Rac1 and Cdc42. During development, fly Syd-1 clusters multiple presynaptic proteins at the neuromuscular junction (NMJ), including the cell adhesion molecule Neurexin (Nrx-1) and the active zone (AZ) component Bruchpilot (Brp), both of which Syd-1 binds directly. We show that a mutant form of Syd-1 that specifically lacks GAP activity localizes normally to presynaptic sites and is sufficient to recruit Nrx-1 but fails to cluster Brp normally. We provide evidence that Syd-1 participates with Rac1 in two separate functions: (1) together with the Rac guanine exchange factor (RacGEF) Trio, GAP-active Syd-1 is required to regulate the nucleotide-bound state of Rac1, thereby promoting Brp clustering; and (2) Syd-1, independent of its GAP activity, is required for the recruitment of Nrx-1 to boutons, including the recruitment of Nrx-1 that is promoted by GTP-bound Rac1. We conclude that, contrary to current models, the GAP domain of fly Syd-1 is active and required for presynaptic development; we suggest that the same may be true of vertebrate Syd-1 proteins. In addition, our data provide new molecular insight into the ability of Rac1 to promote presynaptic development.
Keywords: Syd-1, Rac, Bruchpilot, Neurexin, Trio
THE assembly of synaptic connections between neurons and their targets is initiated by cell–cell adhesion and culminates in the clustering of presynaptic and postsynaptic components at apposing sites (Melom and Littleton 2011; Chia et al. 2013). Forward genetic screens in worm and fly have identified the presynaptic cytosolic protein Syd-1 as a critical regulator of presynaptic assembly (Hallam et al. 2002; Owald et al. 2010; Holbrook et al. 2012). In both species, Syd-1 is among the first proteins to arrive at nascent presynaptic sites and is required for the subsequent recruitment of multiple presynaptic components, including scaffolding proteins, active zone (AZ) proteins, synaptic vesicles (SVs), and mitochondria (Hallam et al. 2002; Dai et al. 2006; Patel et al. 2006; Owald et al. 2010, 2012; Holbrook et al. 2012). Fly Syd-1 has been shown to directly bind and cluster the AZ ELKS family protein Bruchpilot (Brp; Owald et al. 2010) and the presynaptic adhesion molecule Neurexin (Nrx-1; Owald et al. 2012); presynaptic loss of fly Syd-1 disrupts Brp and Nrx-1 localization within neuromuscular junction (NMJ) boutons, decreasing the number of boutons that form (Owald et al. 2010, 2012). More recently, a mouse Syd-1 ortholog, mSYD1A, was identified and shown to be required for the localization of SVs to presynaptic sites (Wentzel et al. 2013), suggesting that at least some aspects of Syd-1 function are conserved between invertebrates and vertebrates.
Syd-1 proteins in both invertebrates and vertebrates contain a Rho GTPase activating protein (GAP)-like domain; however, the importance of this domain to the ability of Syd-1 to promote presynaptic development is unclear. Classically, RhoGAPs increase the GTPase activity of one or more Rho family proteins—Rac, Rho, and Cdc42—accelerating their switch from an active, GTP-bound state to a conformationally distinct inactive, GDP-bound state (Scheffzek and Ahmadian 2005; Tcherkezian and Lamarche-Vane 2007). Rho GTPases are also regulated by guanine exchange factors (GEFs) that accelerate their switch back from the GDP- to the GTP-bound state (Rossman et al. 2005). Rho GTPases and their regulators have been implicated in multiple aspects of neural development, including presynaptic assembly, but the molecular mechanism(s) by which they regulate the latter are unknown (Ball et al. 2010; Nahm et al. 2010; Tolias et al. 2011). The RhoGAP domains of the worm and fly Syd-1 proteins lack key conserved amino acids and so have been thought to lack GAP activity (Hallam et al. 2002; Wentzel et al. 2013); indeed, while worm Syd-1 was recently found to bind GTP-bound MIG-2/Rac, no GAP activity has ever been detected (Hallam et al. 2002; Xu et al. 2015). By contrast, mouse mSYD1A does have GAP activity toward RhoA (Wentzel et al. 2013), but whether this activity is required for mSYD1A function is not clear. Mutant forms of mSYD1A that lack the RhoGAP domain are sufficient to cluster SVs at presynaptic sites in cultured neurons, but their ability to rescue the SV docking defect in mSYD1AKO mutant mice has not been tested, and the mouse genome contains a second Syd-1 homolog (Wentzel et al. 2013), whose presence complicates the interpretation of these experiments (see Discussion).
Here, we show that, contrary to previous evidence (Wentzel et al. 2013), fly Syd-1 does have RhoGAP activity. We use a mutant form of Syd-1 that specifically lacks GAP activity to show that Syd-1 has both GAP-dependent and GAP-independent functions in presynaptic assembly. And, finally, we identify Rac1 as a Rho GTPase that is regulated by Syd-1 and provide evidence that Rac1 promotes presynaptic development by promoting clustering of both Nrx-1 and Brp.
Materials and Methods
DNA constructs
New syd-1 transgenes were created using a UAS-syd-1-1xFLAG DNA construct previously shown to rescue loss of syd-1 from R7 photoreceptor neurons (Holbrook et al. 2012). Two new UAS full-length syd-1 transgenes were created: (1) UAS-syd-1wt-3xFLAG in which two additional FLAG tags were added to facilitate detection in pull-down experiments; and (2) UAS-syd-1RA-3xFLAG, which is identical to UAS-syd-1wt-3xFLAG but has a substitution at codon 979 (numbered according to the fly Syd-1 isoform C; GenBank accession: AAF57207.2) causing it to specify alanine instead of arginine (R979A). DNA encoding the region from Q643 to M1164 (again numbered according to the isoform C sequence), which includes the predicted RhoGAP domain but lacks the C2 domain, was subcloned from each of the two new constructs into the pGEX-4T1 vector in such a way as to be in-frame with the N-terminal glutathione S-transferase (GST) open reading frame and an added C-terminal HA epitope tag, resulting in two new GST transgenes: GST-syd1wt GAP and GST-syd-1RA GAP.
Biochemistry
Wild-type and constitutively-active mutant GST-Rac1, Rac2, Mtl, RhoA, RhoL, and Cdc42 fusion constructs were obtained from the Drosophila Genomics Resource Center. GST-fusion proteins, including GST-Syd-1wt GAP and GST-Syd-1RA GAP, were expressed, in and purified from, Escherichia coli by standard methods (Amersham Pharmacia Biotech; Garcia-Mata et al. 2006). Total protein concentrations were determined by the Bradford protein assay, and protein purity by Coomassie Blue staining of SDS-polyacrylamide gels. Flies containing actin (act)-Gal4 [from the Bloomington Drosophila Stock Center (BDSC)] were crossed to flies containing the UAS-syd-1wt-3xFLAG or UAS-syd-1RA-3xFLAG transgene. Lysates from the heads of adult progeny of both sexes were prepared as described in Emery (2007) and incubated with GST alone or a constitutively-active mutant GST-Rho GTPase bound to glutathione-Sepharose 4B resin (Amersham Pharmacia Biotech). After 1 hr on ice, samples were washed and analyzed by western blots probed with anti-FLAG antibody. GTPase activity assays were performed with EnzChek Phosphate Assay Kit (E-6646; Life Technologies).
Genetics and fly husbandry
Flies were grown on standard food at 25°. BG380-Gal4 (Budnik et al. 1996) was used to drive expression of UAS-Rac1wt (Luo et al. 1994), UAS-Rac1V12 (Luo et al. 1994), UAS-syd-1wt-1xFLAG (Holbrook et al. 2012), or UAS-syd-1RA-3xFLAG (see above) in motorneurons; wild-type animals contained BG380-Gal4 alone, syd-1 mutant animals were syd-1w46/syd-1CDtransheterozygotes that contained BG380-Gal4 alone (Holbrook et al. 2012), and trio mutant animals were trioS6A/trioS137203 transheterozygotes (Ball et al. 2010). In all cases, we analyzed females only.
NMJ dissections and staining
Late third-instar larval NMJs were dissected in Schneider’s insect medium (Sigma-Aldrich) and fixed with Bouin’s solution (Sigma-Aldrich) for 15 min. All samples, except those stained with anti-Nrx-1 (see below), were then stained by standard methods: washed 3× in PBS containing 0.3% Triton-X 100 (PBT), blocked for 30 min in 5% normal goat serum, incubated with primary antibodies at 4° overnight, washed 3× with PBT, incubated overnight with secondary antibodies at 4° overnight, washed 3× with PBT, and mounted in Vectashield (Vector Laboratories). We used the following antibodies: fluorescence-conjugated anti-HRP (1:250; Jackson Immuno Labs); mouse anti-Brp (nc82, 1:100; Developmental Studies Hybridoma Bank); rabbit anti-FLAG (1:250; Sigma-Aldrich); mouse anti-Dlg (4F3, 1:250; Developmental Studies Hybridoma Bank); guinea pig anti-Nrx-1 (1:500; Li et al. 2007); rabbit anti-GluRIIC (1:2500; Marrus et al. 2004); all secondary antibodies (goat IgG coupled to Alexa Fluor 488, Alexa Fluor 555, or AlexFluor 633; 1:500) were from Life Technologies.
Quantifying NMJ bouton number
Each bar graph with this quantification (Figure 2, G and H, Figure 3F, and Figure 4, E and F) contains the results of a single experiment in which all genotypes were grown, dissected, and stained in parallel. Confocal images were collected on a Leica SP2 microscope (with a 63× 1.4 NA oil immersion objective) and analyzed with ImageJ software (Schindelin et al. 2012; http://fiji.sc/) (n = animals). Within each experiment, the images were randomized, and quantifications were performed blind to genotype. As is standard in the field, for each animal we counted the number of boutons (identified based on shape, as visualized by anti-HRP and anti-Dlg staining) on muscle 4 or on muscles 6/7 on both sides of segment A3 (e.g., Ball et al. 2010). As is also standard, we divided bouton number by muscle area (measured in ImageJ by tracing the muscle perimeter visualized by anti-Dlg) to compensate for potential variations in animal size (Schuster et al. 1996; Ball et al. 2010). Note that we found no significant difference in muscle size among the genotypes tested (Supplemental Material, Figure S1).
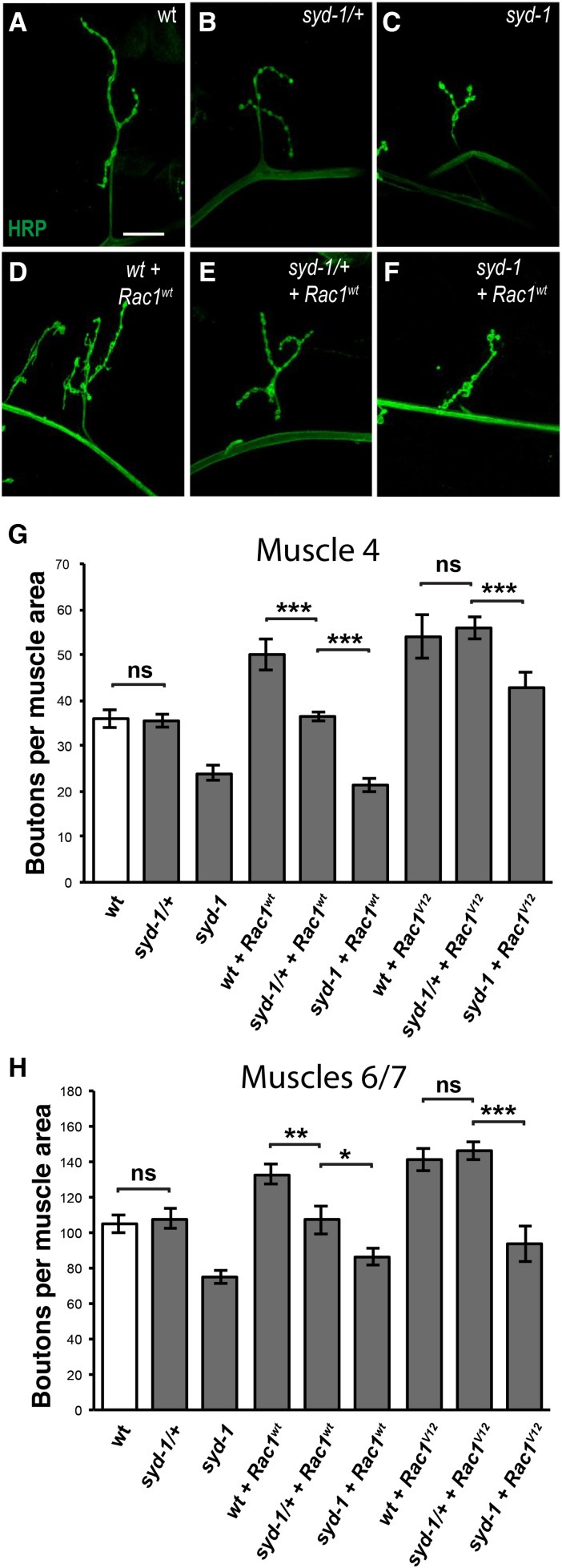
Figure 2.
Wild-type Rac1, unlike Rac1V12, requires wild-type Syd-1 levels in order to promote NMJ growth. (A–F) Third-instar larval NMJs at muscle 4 in abdominal segment 3 stained with anti-HRP (green). Bar, 25 µm. All genotypes also contain the motorneuron driver BG380-Gal4 and were costained with anti-Dlg. (A) Wild type. (B) syd-1CD/+ heterozygote. (C) syd-1CD/syd-1w46 mutant. (D) Wild-type animal in which motorneurons express wild-type Rac1 (Rac1wt). (E) syd-1CD/+ heterozygote in which motorneurons express Rac1wt. (F) syd-1CD/syd-1w46 mutant in which motorneurons express Rac1wt. (G) Quantification of the number of boutons on muscle 4 in abdominal segment 3 per muscle area. Loss of one copy of syd-1 does not cause a decrease in bouton number (36.3 ± 1.3, n = 14) compared to wild type (BG380-Gal4 alone: 35.7 ± 1.9, n = 12). By contrast, while overexpressing wild-type Rac1 (49.5 ± 3.4, n = 12) significantly increases bouton number compared to the wild-type control (P = 0.0011), loss of one copy of syd-1 prevents this increase (36.8 ± 1.0, n = 17, P = 1.9 × 10−8 compared to wild-type Rac1 in wild type). By contrast, loss of one copy of syd-1 has no effect on the increase in bouton number caused by overexpression of a constitutively-active form of Rac1, Rac1V12 (Rac1V12 alone: 58.5 ± 3.5, n = 8 (P = 4.8 × 10−5 compared to wild type); Rac1V12 in syd-1/+: 56.0 ± 2.5, n = 12). Complete loss of syd-1 impairs the ability of wild-type Rac1 (21.4 ± 1.6, n = 14, P = 1.9 × 10−8) or Rac1V12 (36.6 ± 4.2, n = 11, P = 1.4 × 10−4) to increase bouton number. Error bars represent SEM. n = animals. ns, not significant; *** P < 0.001, based on two-tailed t-tests. (H) Quantification of the number of boutons on muscles 6/7 in abdominal segment 3 per muscle area. We observed the same effects on bouton number as at both muscle 4. Wild type (105.4 ± 3.1, n = 14) and syd-1/+ heterozygotes (108.0 ± 5.7, n = 14) have similar numbers of boutons. Overexpressing either wild-type Rac1 (133.0 ± 5.7, n = 14, P = 1.3 × 10−4) or Rac1V12 (141.0 ± 6.5, n = 8, P = 2.6 × 10−5) causes an increase in bouton number. Removing one copy of syd-1 prevents wild-type Rac1 from increasing bouton number (107.4 ± 8.1, n = 14, P = 0.0015 compared to wild-type Rac1 in wild type) but has no effect on Rac1V12 (146.0 ± 5.2, n = 17). Complete loss of syd-1 impairs the ability of wild-type Rac1 (86.8 ± 5.0, n = 15, P = 1.5 × 10−6) or Rac1V12 (93.5 ± 7.8, n = 14, P = 5.3 × 10−4) to increase bouton number. Error bars represent SEM. n = animals. ns, not significant; * P < 0.05, * P < 0.01, *** P < 0.001, based on two-tailed t-tests.
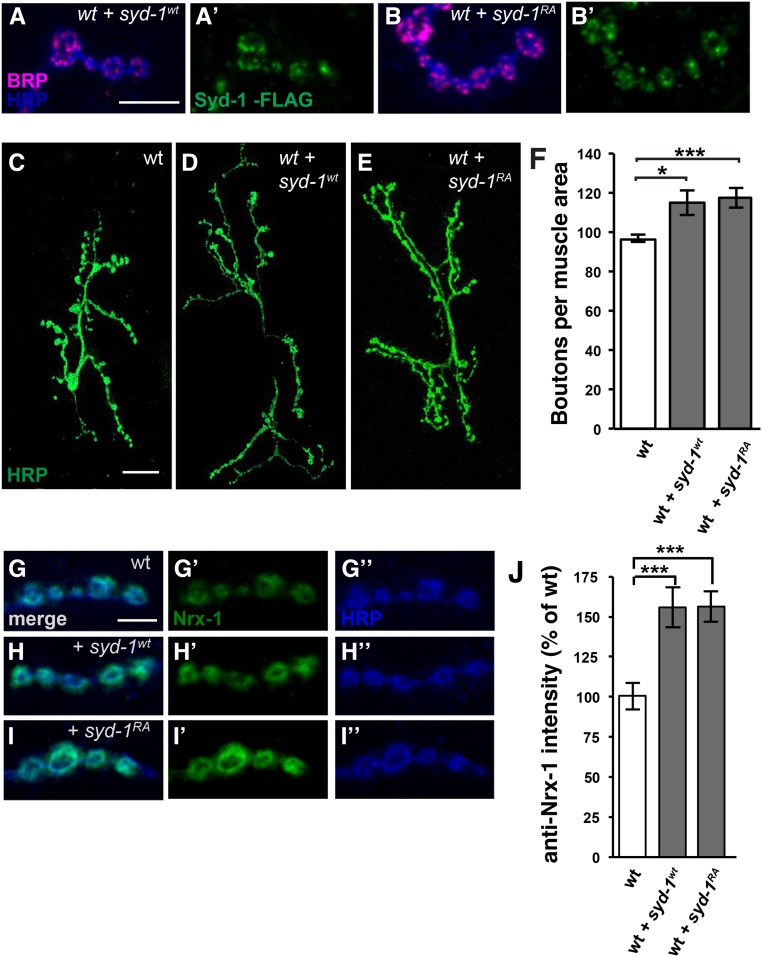
Figure 3.
Wild-type and R979A mutant Syd-1 colocalize with Brp and cause similar increases in NMJ bouton number and Nrx-1 levels when expressed in wild-type motorneurons. (A–B’) Third-instar larval NMJ boutons on muscle 4 stained with anti-HRP (blue), anti-Brp (magenta), and anti-FLAG (green). Bar, 5 µm. (A and A’) A wild-type animal in which motorneurons express wild-type full-length FLAG-tagged Syd-1 driven by BG380-Gal4. Like endogenous Syd-1, tagged wild-type Syd-1 forms puncta that are adjacent to, and partly overlapping with, the AZ protein Brp. (B and B’) A wild-type animal in which motorneurons express R979A mutant full-length FLAG-tagged Syd-1 (Syd-1RA) driven by BG380-Gal4. The punctate pattern and levels of Syd-1RA resemble those of Syd-1wt. (C–E) Third-instar larval NMJs at muscles 6/7 in abdominal segment 3 stained with anti-HRP (green). Bar, 25 µm. All genotypes also contain the motorneuron driver BG380-Gal4, and were costained with anti-Dlg. (C) Wild type. (D) Wild type in which motorneurons express wild-type full-length Syd-1. (E) Wild type in which motorneurons express R979A mutant full-length Syd-1. (F) Quantification of the number of boutons on muscles 6/7 in abdominal segment 3 per muscle area. Overexpressing wild-type (114.9 ± 6.4, n = 10) or R979A mutant Syd-1 (117.8 ± 5.3, n = 7) causes similar, significant increases in bouton number (P = 0.02 and 5.9 × 10−4, respectively) compared to the wild-type control (BG380-Gal4 alone: 96.9 ± 1.8, n = 9). Error bars represent SEM. n = animals. * P < 0.05, *** P < 0.001, based on two-tailed t-tests. (G–I”) Third-instar larval NMJ boutons on muscle 4 stained with anti-HRP (blue) and anti-Nrx-1 (green). Bar, 5 µm. All genotypes also contain the motorneuron driver BG380-Gal4. (G–G”) Wild type. (H–H”) Wild type in which motorneurons express wild-type Syd-1. (I–I”) Wild type in which motorneurons express R979A mutant Syd-1. (J) Quantification of anti-Nrx-1 staining. Overexpressing wild-type (155 ± 12.4%, n = 14) or R979A mutant Syd-1 (156 ± 9.4%, n = 13) significantly increases Nrx-1 levels in NMJ boutons (P = p = 9.2 × 10−4 and 3.1 × 10−4, respectively) compared to the wild-type control (BG380-Gal4 alone: 100 ± 8.4% n = 15). Error bars represent SEM. n = animals. ns, not significant; * P < 0.05, ** P < 0.01, based on one-tailed t-tests.
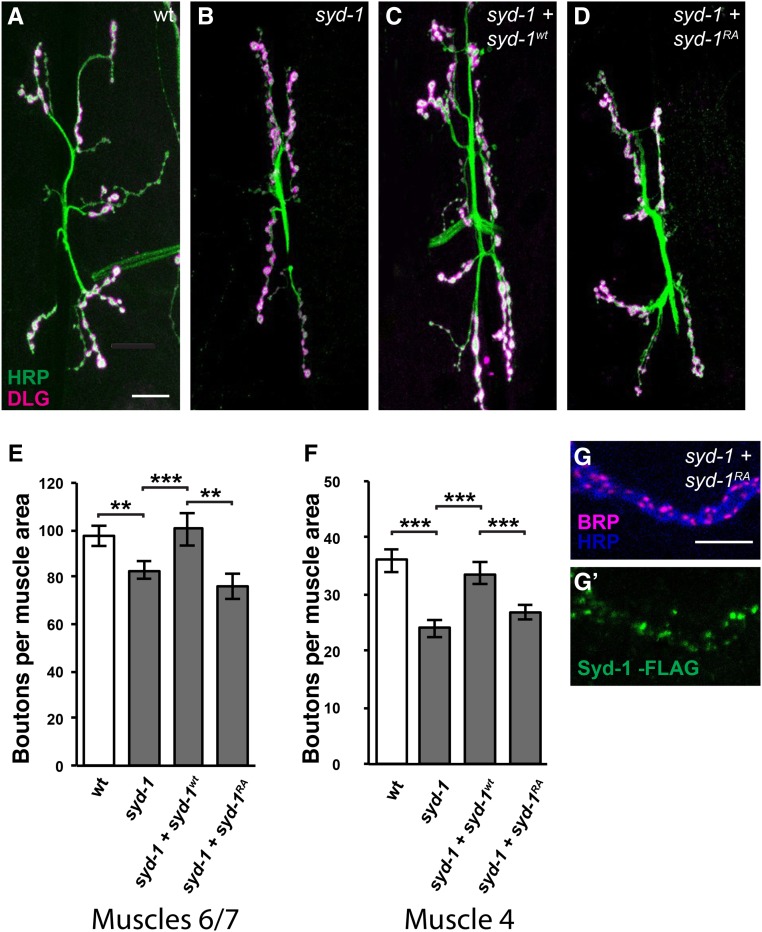
Figure 4.
The decrease in NMJ bouton number in syd-1 mutants is fully rescued by wild-type Syd-1 but unaltered by R979A mutant Syd-1. (A–D) Third-instar larval NMJs at muscles 6/7 in abdominal segment 3 stained with anti-HRP (green) and anti-Dlg (magenta). Bar, 25 µm. All genotypes also contain the motorneuron driver BG380-Gal4. (A) Wild type. (B) syd-1CD/syd-1w46 mutant. (C) syd-1CD/syd-1w46 mutant in which motorneurons express wild-type Syd-1. (D) syd-1CD/syd-1w46 mutant in which motorneurons express R979A mutant Syd-1. (E) Quantification of the number of boutons on muscles 6/7 in abdominal segment 3 per muscle area. As previously reported, loss of syd-1 causes a modest but significant decrease in NMJ bouton number (BG380-Gal4 alone: 97.6 ± 4.5, n = 14; syd-1 mutant: 82.6 ± 3.7, n = 10, P = 3 × 10−3). Overexpressing Syd-1wt in the motorneurons of syd-1 mutants rescues this defect (100.4 ± 7.0, n = 10, P = 0.0018). Overexpressing Syd-1RA does not (76.0 ± 5.2, n = 16, P = 0.0044 compared to syd-1 mutant expressing Syd-1wt). Error bars represent SEM. n = animals. ns, not significant; * P < 0.05, ** P < 0.01, based on one-tailed t-tests. (F) Quantification of the number of boutons on muscle 4 in abdominal segment 3 per muscle area. Again, loss of syd-1 decreases NMJ bouton number (BG380-Gal4 alone: 36.0 ± 2.1, n = 12; syd-1 mutant: 24.0 ± 1.6, n = 10, P = 5.5 × 10−5), and presynaptically expressed Syd-1wt rescues this defect (33.7 ± 1.9, n = 12, P = 9.9 × 10−5), but Syd-1RA does not (26.8 ± 1.2, n = 13, P = 7.9 × 10−4 compared to syd-1 mutant expressing Syd-1wt). Error bars represent SEM. n = animals. ns, not significant; *** P < 0.001, based on two-tailed t-tests. (G and G’) Third-instar larval NMJ boutons on muscle 4 of syd-1CD/syd-1w46 mutant animals in which motorneurons express R979A mutant full-length Syd-1 driven by BG380-Gal4, stained with anti-HRP (blue), anti-Brp (magenta), and anti-FLAG (green) as in Figure 3, A–B’. Bar, 5 µm. As in wild type, tagged R979A mutant Syd-1 expressed in syd-1 mutant motorneurons forms puncta that are adjacent to and partly overlapping with Brp. Note, however, that the endogenous Brp puncta appear somewhat abnormal (see Figure 6).
Quantifying Nrx-1 levels
Each bar graph with this quantification (Figure 3J, Figure 5E, and Figure 7A) contains the results of a single experiment in which all genotypes were grown and dissected in parallel. Dissected pelts were notched in a genotype-specific pattern, and then all pelts were stained and washed in a single tube.
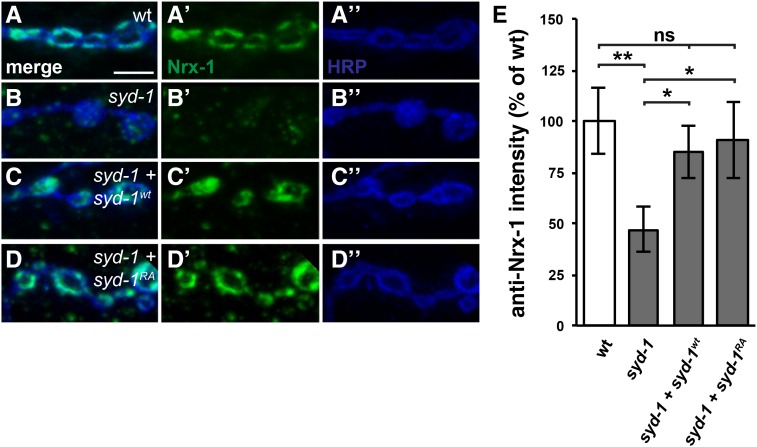
Figure 5.
Both wild-type and R979A mutant Syd-1 fully restore NMJ Nrx-1 levels in syd-1 mutants. (A–D”) Third-instar larval NMJ boutons on muscle 4 stained with anti-HRP (blue) and anti-Nrx-1 (green). Bar, 5 µm. All genotypes also contain the motorneuron driver BG380-Gal4. (A–A”) Wild type. (B–B”) syd-1CD/syd-1w46 mutant. (C–C”) syd-1CD/syd-1w46 mutant in which motorneurons express wild-type Syd-1. (D–D”) syd-1CD/syd-1w46 mutant in which motorneurons express R979A mutant Syd-1. (E) Quantification of anti-Nrx-1 staining. As previously reported, loss of syd-1 decreases Nrx-1 levels in presynaptic boutons (BG380-Gal4 alone: 100 ± 16%, n = 13; syd-1 mutant: 47 ± 11%, n = 12, P = 0.0043). Overexpressing either wild-type or R979A mutant Syd-1 in the motorneurons of syd-1 mutants rescues this defect (85 ± 13% (n = 12, P = 0.013) and 91 ± 19% (n = 9, P = 0.021), respectively). Error bars represent SEM. n = animals. ns, not significant; * P < 0.05, ** P < 0.01, based on one-tailed t-tests.
Figure 7.
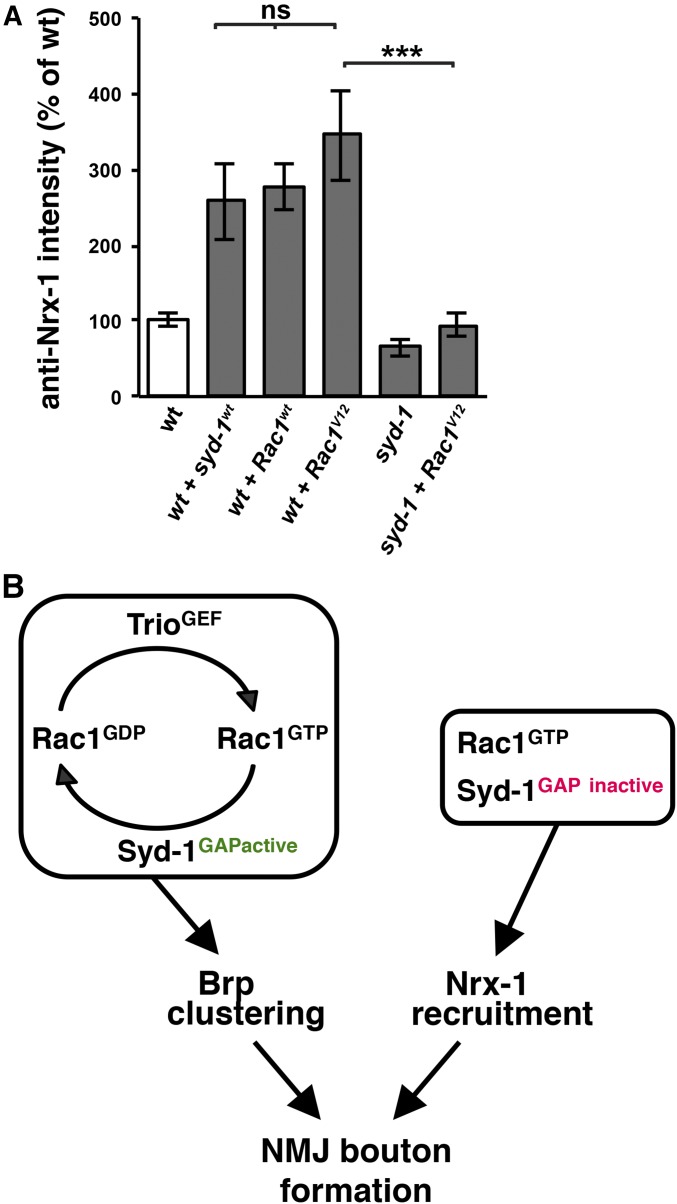
Rac1V12 requires Syd-1 in order to increase Nrx-1 levels at NMJ. (A) Quantification of anti-Nrx-1 staining. Overexpressing either Rac1wt or Rac1V12 in the motorneurons of wild-type animals increases Nrx-1 levels in presynaptic boutons [to 278 ± 31%, n = 14, P = 4.3 × 10−6, or 345 ± 60%, n = 16, P = 4.7 × 10−4, of wild-type levels, respectively (BG380-Gal4 alone: 100 ± 9%, n = 15)]. Loss of syd-1 significantly impairs the ablility of Rac1V12 to increase Nrx-1 levels (94 ± 16%, n = 8, P = 1.2 × 10−4 compared to RacV12 in wild type). Error bars represent SEM. n = animals. ns, not significant; * P < 0.05, ** P < 0.01, *** P < 0.001, based on one-tailed t-tests. (B) Model. Previous work has shown that Syd-1 is required presynaptically for the recruitment of Nrx-1 and clustering of Brp at NMJ, and that each of these processes is required for the formation of a wild-type number of NMJ boutons. In this paper, we provide evidence that Syd-1's GAP activity is required together with the RacGEF Trio to promote proper Brp puncta size by regulating the nucleotide-bound state of Rac1. The similarity between the Brp defects caused by syd-1 or trio loss suggests that Rac1 cycling between GTP- and GDP-bound states is required for normal Brp clustering (see Discussion). By contrast, we find that Syd-1 does not require RacGAP activity to recruit Nrx-1 to boutons and that GTP-bound Rac1 is sufficient for this process but that Rac1 cannot increase Nrx-1 levels in the absence of Syd-1. See Discussion for more details.
Staining for Nrx-1 was conducted as previously described (Li et al. 2007) using 0.05% Triton-X 100 and the Vectastain ABC system (Vector Laboratories) followed by Tyramide Signal Amplification (TSA; Life Technologies); TSA-treated NMJs were then incubated with fluorescence-conjugated anti-HRP (1:250; Jackson Immuno Labs) for 30 min at room temperature followed by washes with PBS.
Confocal images were collected on a Leica SP2 microscope and analyzed with ImageJ software (Schindelin et al. 2012; http://fiji.sc/) (n = animals). For each bar graph, all samples were imaged on the same day using the same laser settings and exposure, the images were randomized, and quantifications were performed blind to genotype (n = animals). For each animal, Nrx-1 levels were quantified within boutons on muscle 4 in segment A3. To do so, we used the anti-HRP channel to trace 4–10 presynaptic boutons while remaining blind to the anti-Nrx-1 channel; we duplicated this trace and moved it to a region within the sample that did not contain anti-HRP staining; we then measured the average fluorescence intensities in the anti-Nrx-1 channel within each of the traces and subtracted the measurement outside the boutons from the measurement within boutons to obtain a final fluorescence value.
Quantifying Brp puncta size
The bar graph with this quantification (Figure 6F) contains the results of two experiments: one with syd-1 genotypes and the other with trio mutants; wild-type animals were analyzed in each experiment (hence the two separate measurements of wild type shown). In each of the two experiments, all genotypes were grown and dissected in parallel. Dissected pelts were notched in a genotype-specific pattern, and then all pelts were stained and washed in a single tube. Images were collected using three-dimensional (3D) structured illumination microscopy (SIM) with three grid rotations and five phases on a Zeiss Elyra S.1 inverted microscope with a Plan-Apochromat 63×/1.4 oil immersion lens. All samples were imaged on the same day using the same laser settings and exposure. Raw images were then batch-processed under identical settings using Zen software (Carl Zeiss). The reconstructed images were then analyzed by taking the maximum z-projections, and puncta sizes were quantified automatically by the “analyze particle” feature in ImageJ (http://fiji.sc/) (n = hemisegments).
Figure 6.
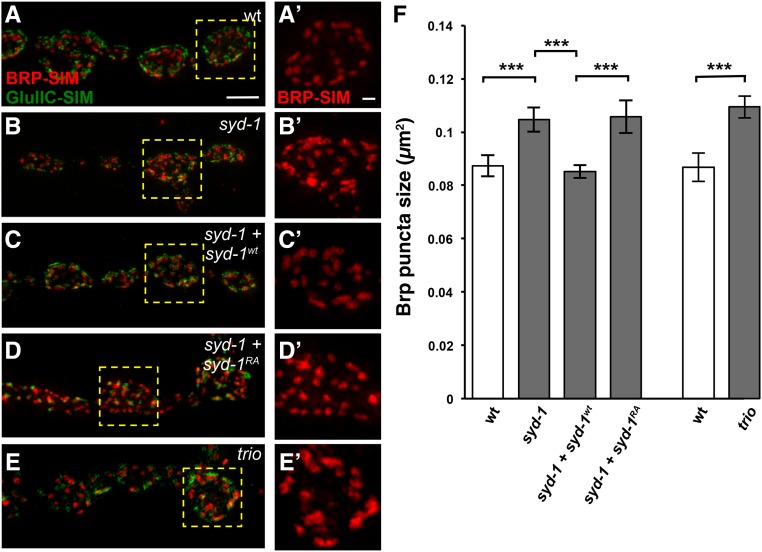
The defect in Brp clustering in syd-1 mutants is fully rescued by wild-type Syd-1, unaltered by R979A mutant Syd-1, and phenocopied by loss of the RacGEF Trio. (A–E’) SIM images of third-instar larval NMJ boutons on muscle 4 stained with anti-Brp (red) and anti-GluRIIC (green). Bar, 1 μm. Genotypes in (A–D’) also contain the motorneuron driver BG380- Gal4. (A and A’) Wild type. (B and B’) syd-1CD/syd-1w46 mutant. (C and C’) syd-1CD/syd-1w46 mutant in which motorneurons express wild-type Syd-1. (D and D’) syd-1CD/syd-1w46 mutant in which motorneurons express R979A mutant Syd-1. (E and E’) trio6A/trioS137203 mutant. (F) Quantification of the average size of anti-Brp puncta. As previously reported (Owald et al. 2010), loss of syd-1 increases the average size of anti-Brp puncta (0.105 ± 0.004 μm2, n = 17) compared to wild type (0.087 ± 0.004 μm2, n = 21, P = 3.0 × 10−4). Overexpressing Syd-1wt in the motorneurons of syd-1 mutants rescues this defect (0.085 ± 0.002 μm2, n = 14, P = 6.3 × 10−4). By contrast, overexpressing Syd-1RA in the motorneurons of syd-1 mutants does not (0.106 ± 0.006 μm2, n = 12, P = 9.7 × 10−4 compared to syd-1 mutant expressing Syd-1wt). In a separate experiment, we found that loss of Trio increases the average size of anti-Brp puncta (0.109 ± 0.004 μm2, n = 14, P = 1.2 × 10−4) to a degree similar to that caused by loss of Syd-1. Error bars represent SEM. n = hemisegments. ns, not significant; ** P < 0.01, *** P < 0.001, based on one-tailed t-tests.
Data availability and statistical analysis
Fly strains and DNA constructs are available upon request. For each experiment, genotypes and sample sizes are described in the legend of the corresponding figure. Because some transgenes were on the X chromosome, we analyzed females only for the sake of consistency. As described above, all experiments were scored blind to genotype. For each experiment a one-way ANOVA was conducted to determine if there was significant variation among genotypes. For experiments that showed a significant difference, individual post hoc pairwise comparisons were conducted between genotypes of interest by using either one- or two-tailed t-tests (see individual figure legends). For all figures: ns, not significant, * P < 0.05, ** P < 0.01, and *** P < 0.001. Exact P values are provided within the figure legends. Statistical values are presented as mean value ± SEM.
Results
Drosophila Syd-1 interacts with all six Rho GTPases, and its predicted GAP domain enhances the GTPase activities of Rac1 and Cdc42, but not RhoA
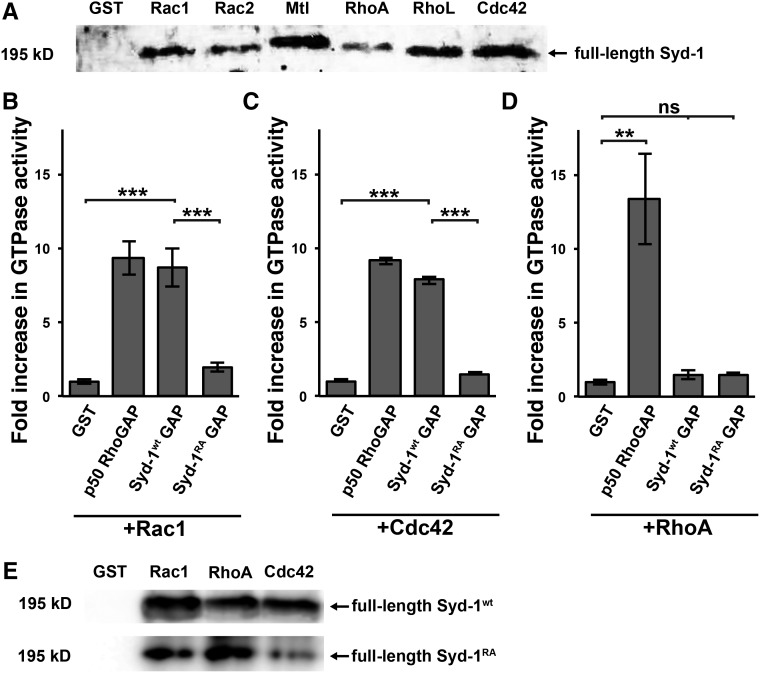
Because the sequence of the RhoGAP-like domain fly Syd-1 suggested that it might be inactive (Hallam et al. 2002; Wentzel et al. 2013), we originally hypothesized that fly Syd-1 might simply bind Rho family GTPases without increasing their GTPase activity. To test this, we used GST pull-down assays to assess the abilities of each of the six fly Rho GTPases—three Racs, two Rhos, and one Cdc42—to interact with fly Syd-1. RhoGAPs preferentially bind the GTP-bound form of Rho GTPases, a form that can be transitory. To maximize the chance of observing this binding, we therefore used a mutant form of each fly Rho GTPase that stably mimics the GTP-bound form (Garcia-Mata et al. 2006). We expressed and purified each of the six mutant fly Rho:GST fusions from E. coli and tested the ability of each one to pull down full-length FLAG-tagged fly Syd-1 from extracts of adult fly heads expressing FLAG-Syd-1 under the control of the ubiquitous actin-Gal4 driver. We found that each of the six fly Rho GTPases is able to pull down the tagged fly Syd-1 in this assay (Figure 1A), consistent with our hypothesis that fly Syd-1 can bind Rho GTPases.
Figure 1.
Drosophila Syd-1 interacts with all six Rho GTPases. Its predicted GAP domain enhances the GTPase activities of Rac1 and Cdc42 but not RhoA. (A) Western blot (probed with anti-FLAG antibodies) of protein pulled down by purified GST-Rho proteins. FLAG-tagged full-length wild-type fly Syd-1 expressed ubiquitously in adult head is pulled down by GST-tagged constitutively-active mutant forms of fly Rac1, Rac2, Mtl, RhoA, RhoL, or Cdc42, but not by GST alone. (B–D) Endpoint GTPase activity assays of wild-type fly Rac1 (B), Cdc42 (C), and RhoA (D). Each protein was assayed alone and in the presence of the negative control GST, the positive control p50 RhoGAP, the GAP domain of wild-type fly Syd-1 (Syd-1wt GAP), or the GAP domain of fly Syd-1 in which the conserved arginine of the arginine finger is replaced by alanine (Syd-1RA GAP). Activity is presented as fold-increase over the activity of each protein alone. Error bars represent SEM. n, Independent assays. As expected, GST does not increase the GTPase activity of Rac1 (1.00 ± 0.16, n = 7), Cdc42 (1.00 ± 0.09, n = 3), or RhoA (1.00 ± 0.22, n = 3), whereas p50 RhoGAP significantly increases the GTPase activity of all three (Rac1: 9.36 ± 1.16, n = 7, P = 5.9 × 10−6; Cdc42: 9.12 ± 0.22, n = 3, P = 2.2 × 10−6; RhoA: 13.32 ± 3.04, n = 3, P = 0.0077). We found that the wild-type GAP domain of Syd-1 significantly increases the GTPase activity of Rac1 (8.69 ± 1.29, n = 7, P = 3.5 × 10−5) and Cdc42 (7.83 ± 0.27, n = 3, P = 9.0 × 10−6) but not RhoA (1.48 ± 0.30, n = 3, P = 0.13). By contrast, the Syd-1RA mutant GAP domain has a severely reduced ability to increase the GTPase activity of Rac1 (1.98 ± 0.31, n = 7, P = 1.4 × 104), and Cdc42 (1.41 ± 0.08, n = 3, P = 1.1 × 10−5) and has no effect on RhoA (1.43 ± 0.18, n = 3, P = 0.10). * P < 0.05, ** P < 0.01, *** P < 0.001, based on one-tailed t-tests. (E) Like wild-type fly Syd-1 (Syd-1wt), FLAG-tagged full-length R979A mutant fly Syd-1 (Syd-1RA) expressed ubiquitously in adult head is pulled down by GST-tagged constitutively active mutant forms of Rac1, RhoA, and Cdc42 but not by GST alone.
We next assayed whether fly Syd-1 has RhoGAP activity. Because full-length fly Syd-1 could not be expressed in E. coli cells, we GST-tagged and expressed a smaller fragment that included the RhoGAP but not the PDZ or C2 domains (“GST-Syd-1wt GAP”). We purified this Syd-1 fragment and assayed its ability to increase the GTPase activities of each of the three major Rho family members in fly: Rac1, RhoA, and Cdc42. We found that the RhoGAP domain of fly Syd-1 significantly increases the GTPase activity of Rac1 and Cdc42 but not that of RhoA (Figure 1, B–D). The degree of increase is similar to that caused by the positive control p50 RhoGAP, which has previously been shown to increase the GTPase activity of all three Rho family members (Zhang et al. 1998). We conclude that, despite its noncanonical amino acid sequence, the RhoGAP-like domain of fly Syd-1 does have significant RhoGAP activity toward Rac1 and Cdc42.
Decreasing syd-1 dosage impairs the ability of wild-type but not constitutively-active Rac1 to increase NMJ bouton number
We next wanted to test whether the GAP activity of fly Syd-1 might be required to regulate Rac or Cdc42 in vivo. Like Syd-1, both Rac1 and Cdc42 have previously been shown to act within motorneurons to regulate synaptic bouton number at fly NMJ (Rodal et al. 2008; Ball et al. 2010; Nahm et al. 2010). However, Cdc42 does so by attenuating retrograde BMP signaling, which is not known to be affected by Syd-1 loss. We therefore focused instead on testing whether Syd-1 might regulate Rac1. To do so, we took advantage of a genetic interaction test that was previously used to implicate the RacGEF Trio as a regulator of Rac1 at NMJ (Ball et al. 2010). Overexpression of Rac1 in motorneurons of wild-type animals significantly increases NMJ bouton number (Ball et al. 2010). While loss of one copy of trio has no effect on NMJ development in otherwise wild-type animals, it significantly impairs the ability of overexpressed Rac1 to increase bouton number (Ball et al. 2010). By contrast, Rac1V12, which stably mimics the GTP-bound state of Rac1 (Luo et al. 1994), increases NMJ bouton number unimpaired by loss of trio. Together, these results suggest that Trio is required to potentiate Rac1, and does so by regulating the nucleotide-bound state of Rac1.
We used the same genetic interaction test to ask whether Syd-1 might also regulate Rac1. We found that overexpression of wild-type Rac1 or the mutant Rac1V12 in motorneurons of wild-type animals significantly increases NMJ bouton number, as previously reported (Figure 2, A, D, G, and H). Loss of one copy of syd-1 from otherwise wild-type animals has no effect on NMJ development in otherwise wild-type animals (Figure 2, A, B, G, and H). However, loss of one copy of syd-1 does prevent wild-type Rac1 from increasing NMJ bouton number (Figure 2, D, E, G, and H), indicating that, like Trio, Syd-1 is required to potentiate the ability of Rac1 to promote NMJ growth. Furthermore, loss of one copy of syd-1 does not affect the ability of Rac1V12 to increase NMJ bouton number (Figure 2, G and H), indicating that, like Trio, Syd-1 is only required to potentiate Rac1 that can change its nucleotide-bound state. We note that Rac1wt and Rac1V12 increase synaptic bouton number to similar degrees at muscle 6/7 (P = 0.38), suggesting that the differential effect of syd-1 dosage on the two proteins is unlikely to be caused by a difference in the strengths of their effects. [There may be a slight difference in the strengths of their effects at muscle 4, although it is not statistically significant in the data we have (P = 0.057).] These results are consistent with a model in which the GAP activity of Syd-1 directly regulates Rac1 by altering its nucleotide-bound state, but they do not rule out the possibility that the effect of Syd-1 on Rac1 is indirect.
We did note one difference between the effects of trio and syd-1 loss on Rac1. Completely eliminating trio has no effect on the ability of Rac1V12 to increase NMJ bouton number, indicating that the only role for Trio in this process is to promote the GTP-bound state of Rac1 (Ball et al. 2010). By contrast, we found that both Rac1wt and Rac1V12 are significantly less able to increase NMJ bouton number in the complete absence of syd-1 (Figure 2, A, F, G, and H). Since the GAP activity of Syd-1 would not be predicted to affect Rac1V12, this result suggested that Syd-1 might have GAP-independent roles in promoting Rac1-induced NMJ growth, in addition to any GAP-dependent role it might have. We note that Rac1V12 can increase bouton number somewhat even in the absence of syd-1 (P = 2.8 × 10−4 at muscle 4; but P = 0.12 at muscles 6/7), indicating that Rac1V12 can act partly independently of Syd-1. To tease apart Syd-1’s potential GAP-dependent and independent functions, we decided to create a mutant version of Syd-1 that specifically lacks GAP activity.
Overexpression of wild-type or R979A mutant Syd-1 in wild-type motorneurons causes similar increases in NMJ bouton number and Nrx-1 levels
Canonical RhoGAP proteins contain a conserved arginine that is required for their GAP activity (Scheffzek and Ahmadian 2005; Tcherkezian and Lamarche-Vane 2007). To test whether the same might be true of Syd-1, we constructed a GST-tagged Syd-1 RhoGAP domain in which we replaced the codon encoding this conserved arginine (at position 979, see Materials and Methods) with a codon encoding alanine (R979A; “GST-Syd-1RA GAP”). We found that, indeed, the R979A substitution severely decreases the RhoGAP activity of Syd-1's RhoGAP domain (Figure 1, B–D). We next used the transgene encoding full-length wild-type Syd-1 (UAS-syd-1wt; Holbrook et al. 2012) to create an equivalent transgene encoding R979A mutant Syd-1 (UAS-syd-1RA). We tested whether, like other RhoGAP proteins with this R to A substitution, Syd-1RA retains the ability to interact with Rho GTPases despite losing its GAP activity (Graham et al. 1999; Scheffzek and Ahmadian 2005). Indeed, we found that FLAG-tagged R979A mutant Syd-1 is pulled down from adult fly extracts by GST-tagged constitutively-active forms of all three major Rho family members: Rac1, RhoA, and Cdc42 (Figure 1E). We conclude that the R979A substitution specifically disrupts Syd-1's GAP activity, providing us with a tool to analyze the latter’s importance for Syd-1 function.
As a first test of the abilities of Syd-1wt and Syd-1RA to promote synapse development, we overexpressed each in motorneurons of wild-type animals. We observed that, like endogenous Syd-1 (Owald et al. 2010), both Syd-1wt and Syd-1RA localize to presynaptic puncta that are adjacent to and partly overlapping with the AZ protein Brp (Figure 3, A–B’), indicating that Syd-1's GAP activity is not required for its gross subcellular localization. Presynaptic Syd-1 is necessary and sufficient to recruit Nrx-1 to AZs (Owald et al. 2012), and overexpression of Nrx-1 is sufficient to increase NMJ bouton number (Li et al. 2007). We therefore predicted that presynaptic Syd-1wt overexpression would increase both Nrx-1 levels and NMJ bouton number. Indeed, we found that overexpression of either Syd-1wt or Syd-1RA significantly increases the number of boutons in wild-type animals; the effects of the two proteins are not significantly different (Figure 3, C–F). Using anti-Nrx-1 antibodies to quantify endogenous Nrx-1 levels, we found that overexpression of either Syd-1wt or Syd-1RA also significantly increases Nrx-1 levels within NMJ boutons to similar degrees (Figure 3, G–J). We conclude that Syd-1's RhoGAP activity is not required for its ability to increase bouton number and promote Nrx-1 localization in wild-type animals.
Unlike wild-type Syd-1, R979A mutant Syd-1 does not rescue the decrease in NMJ bouton number in syd-1 mutants, despite restoring Nrx-1 levels
Loss of syd-1 causes a decrease in NMJ bouton number and a loss of Nrx-1 staining. We next wanted to compare the abilities of Syd-1wt and Syd-1RA to rescue these defects in syd-1 mutant animals. We expressed the two proteins in motorneurons of syd-1w46/syd-1CD transheterozyotes and found that Syd-1wt fully rescues the decrease in NMJ bouton number (Figure 4, A–C, E, and F) but that Syd-1RA has no effect (Figure 4, A–F). We conclude that Syd-1RA's ability to increase NMJ bouton number in wild-type animals requires the presence of endogenous wild-type Syd-1 and that Syd-1's RhoGAP activity is required for normal NMJ growth. We confirmed that, as in wild-type animals, both Syd-1wt and Syd-1RA form Brp-associated puncta in syd-1 mutant animals (compare Figure 4, G and G’ with Figure 3, A-B’), indicating that Syd-1RA's inability to rescue syd-1 loss is not caused by a defect in its gross subcellular localization. This is the first evidence in any organism that Syd-1's GAP activity is required for presynaptic development.
We next wanted to pinpoint which of Syd-1's molecular functions depends upon its GAP activity. syd-1 loss significantly decreases presynaptic Nrx-1 levels (Owald et al. 2012), and loss of nrx-1 reduces NMJ bouton number to the same degree as loss of syd-1 (Li et al. 2007; Owald et al. 2012). We therefore first tested whether the inability of Syd-1RA to rescue NMJ bouton number might be caused by an inability to recruit Nrx-1 in the absence of endogenous wild-type Syd-1. We again expressed Syd-1wt or Syd-1RA in motorneurons of syd-1 mutants and quantified anti-Nrx-1 staining within NMJ boutons. We found that, like Syd-1wt, Syd-1RA is sufficient to fully restore Nrx-1 to wild-type levels in syd-1 mutant boutons (Figure 5). We conclude that Syd-1's RhoGAP activity is not required for its ability to localize Nrx-1.
Syd-1's RhoGAP activity is required for normal Brp clustering, as is the RacGEF Trio
In addition to binding and localizing Nrx-1, Syd-1 also directly binds and clusters Brp, a major structural component of AZs that is required for normal NMJ size (Kittel et al. 2006; Owald et al. 2010). Loss of Syd-1 results in disorganized Brp puncta that are enlarged and irregularly spaced (Owald et al. 2010). We therefore next compared the abilities of Syd-1wt and Syd-1RA to promote Brp clustering. We found that expressing Syd-1wt in motorneurons fully restores the size of Brp puncta in syd-1 mutants (Figure 6, A–C’ and F), whereas expressing Syd-1RA has no effect on the Brp defect (Figure 6, D, D’, and F). We conclude that Syd-1's RhoGAP activity is required for normal Brp clustering.
The obvious implication of this result is that Syd-1 regulates Brp, at least in part, by regulating the GTPase activity of one or more Rho proteins. Having already found biochemical and genetic evidence that Syd-1 regulates Rac1, we wanted to test whether Brp localization is disrupted by Rac1 loss. However, Rac1 is partly redundant with the other two fly Rac genes, and triple Rac mutant flies die early (Hakeda-Suzuki et al. 2002). We therefore chose instead to analyze Brp localization in animals lacking the Rac1 GEF Trio. Rac1 has been shown to be unable to promote NMJ bouton development in trioS6A/trioS137203 null mutants, which nonetheless survive until the end of larval development (Ball et al. 2010). We analyzed Brp puncta within the NMJ boutons of trioS6A/trioS137203 mutants and found them to be abnormally enlarged, like Brp puncta in syd-1 mutants (Figure 6, E and F). We conclude that Trio, like Syd-1, is required for proper Brp clustering, consistent with a model in which their coregulation of Rac1 and/or other Rho GTPases is required for this process.
Syd-1 is required for Rac1V12 to increase Nrx-1 levels
Finally, we wanted to follow up on our finding that complete loss of syd-1 prevents Rac1V12 from increasing NMJ bouton number. This result suggested that Syd-1 has one or more activities that are required for Rac1-induced NMJ growth but separate from Syd-1's effect on the nucleotide-bound state of Rac1. An obvious candidate for such an activity is Syd-1's recruitment of Nrx-1, since this occurs independently of Syd-1 GAP activity. If so, we would expect that: (1) Rac1V12 overexpression promotes NMJ growth, at least in part, by increasing Nrx-1 levels; and (2) eliminating Syd-1 prevents Rac1V12 from causing this Nrx-1 increase. We tested these two predictions and, indeed, found that expressing Rac1V12 in the motorneurons of wild-type animals causes a significant increase in Nrx-1 levels at NMJ (Figure 7A) and that complete loss of Syd-1 prevents this increase (Figure 7A). We conclude that Syd-1 participates with Rac1 in two separate functions that promote NMJ bouton development: (1) together with Trio, the RacGAP activity of Syd-1 is required to regulate the nucleotide-bound state of Rac1, which is required for normal Brp clustering; and (2) Syd-1, independent of its RacGAP activity, is required for the recruitment of Nrx-1 to boutons, including the recruitment of Nrx-1 that is promoted by Rac1 (Figure 7B).
Discussion
The role of Syd-1's GAP activity in clustering Brp
In this paper, we have shown that Syd-1wt and Syd-1RA promote NMJ growth to similar degrees in wild-type animals and recruit similar levels of Nrx-1 to presynaptic boutons in both wild type and syd-1 mutants. However, unlike Syd-1wt, Syd-1RA is unable to restore Brp clustering in syd-1 mutants, indicating that Syd-1's GAP activity is required for this process. Our results further suggest that Syd-1's GAP activity promotes Brp clustering by working together with the RacGEF Trio to potentiate Rac1. However, while loss of a GEF for Rac1 would be predicted to increase the proportion of GDP-bound, and therefore inactive, Rac1, we note that loss of a GAP for Rac1 should instead increase the proportion of GTP-bound, and therefore active, Rac1 (Scheffzek and Ahmadian 2005; Tcherkezian and Lamarche-Vane 2007). Why, instead, might loss of Syd-1 GAP activity impair Rac1 function at NMJ?
Given our finding that Syd-1's GAP domain also increases the GTPase activity of Cdc42, we considered the possibility that Syd-1 might potentiate Rac1 only indirectly, by acting upon Cdc42. The phenotype caused by Cdc42 loss from motorneurons—an increase in NMJ bouton number—is the same as that caused by Rac1 gain, suggesting that Cdc42 and Rac1 might antagonize one another during NMJ development. Syd-1 loss might then impair Rac1 by increasing the proportion of GTP-bound, active Cdc42. However, we do not think this model is likely for three reasons. First, presynaptic loss of cdc42 significantly enhances the formation of abnormally positioned “satellite” boutons, a hallmark of increased BMP signaling (Nahm et al. 2010), and we do not observe an increase in satellite boutons in animals overexpressing Syd-1wt (or in syd-1 mutants; Figure S2), suggesting that Syd-1 does not normally regulate Cdc42 at NMJ. Second, we directly tested whether coexpressing Cdc42 with Rac1 would impair the latter’s ability to increase NMJ bouton number and found that it does not (Figure S2). Third, a model in which decreasing syd-1 dosage potentiates a Rho GTPase with antagonistic effects on Rac1 does not explain the specific sensitivity of Rac1wt and not Rac1V12 to this manipulation. Finally, we note that Syd-1 could also theoretically potentiate Rac1 indirectly by acting upon one of the other two fly Racs, if either of the latter were antagonistic to Rac1. However, there is no evidence of such antagonism: reducing the levels of one, two, or all three fly Racs has previously been shown either to have no effect or to decrease NMJ bouton number (Ball et al. 2010), and our third point (above) applies to this model too.
Our data are therefore more consistent with Syd-1 directly regulating the nucleotide-bound state of Rac1. In previous cases in which loss of the RhoGAP or RhoGEF for a single Rho GTPase causes the same defect, the explanation has been that the ability of the GTPase to cycle between GDP- and GTP-bound states is critical (Symons and Settleman 2000; Parrini and Camonis 2011; Sharif and Maddox 2012; Fritz and Pertz 2016); loss of the regulatory RhoGAP or RhoGEF then causes the same defect because either manipulation reduces the rate of Rho cycling. While it is theoretically possible that, instead, both GDP-bound and GTP-bound forms of Rac1 are separately required to promote Brp clustering at NMJ, there is no precedent for such a model: GDP-bound Rac1 is thought to be inactive (Symons and Settleman 2000; Parrini and Camonis 2011; Sharif and Maddox 2012; Fritz and Pertz 2016). We therefore favor a model in which Rac1 cycling is important for Brp clustering at NMJ.
How might Rac1 cycling affect Brp recruitment? Brp has previously been shown to directly bind in vitro to N- and C-terminal fragments of Syd-1 that lack the RhoGAP domain (Owald et al. 2010). One possibility is that the ability of Syd-1 to bind Brp in vivo is somehow regulated intramolecularly by the process of transforming a GTP-bound Rho GTPase into a GDP-bound form (simply binding the GTP-bound form would not be sufficient, since R979A Syd-1 binds all six Rhos yet cannot cluster Brp). Alternatively, Syd-1's RhoGAP activity may have an indirect effect on Brp by, in parallel, promoting a Rho GTPase-dependent change in presynaptic structure that facilitates the ability of Syd-1 to cluster Brp properly. Rho GTPases are classically involved in regulating actin assembly (Stankiewicz and Linseman 2014), and presynaptic development is characterized by the early appearance of actin-rich structures to which other molecules, including Syd-1, are recruited (Chia et al. 2012, 2013; Nelson et al. 2013). Perhaps Rac1, regulated by Syd-1 and Trio, sculpts the local actin environment at presynaptic sites, creating a permissive environment for Syd-1 to recruit additional presynaptic components, including Brp.
The roles of Rac1 and Syd-1 in recruiting Nrx-1
In contrast to our evidence that Rac1 cycling may be important for Brp clustering, we found that Rac1V12, which stably mimics the GTP-bound state, increases Nrx-1 levels in wild type, and that Syd-1 lacking GAP activity is sufficient to increase Nrx-1 levels, even in the absence of endogenous Syd-1. These results suggest that Rac1 does not need to enter the GDP-bound state in order to promote Nrx-1 recruitment and that Rac1 cycling is therefore not required for this process. Nonetheless, complete loss of syd-1 prevents Nrx-1 recruitment, even by Rac1V12. Together, these results indicate that Syd-1 is required downstream of or in parallel to GTP-bound Rac1 to recruit Nrx-1 to boutons. Syd-1 and Nrx-1 have previously been shown to bind via an interaction between the former’s PDZ domain and the latter’s PDZ-binding domain; each protein depends on the other for its localization (Owald et al. 2012). One possibility is that Syd-1 localization or its ability to recruit Nrx-1 is potentiated by direct binding between Syd-1 and GTP-bound Rac1, an interaction of which Syd-1RA remains capable.
Fly Syd-1 compared with worm Syd-1 and mouse mSYD1A
We have shown that fly Syd-1 requires its GAP activity to cluster the ELKS protein Brp. However, worm Syd-1 apparently lacks GAP activity and yet is able to cluster ELKS (Xu et al. 2015). How might this work? Both worm and fly Syd-1 also recruit the scaffolding protein Liprin-alpha (Syd-2 in worm), which binds ELKS. Perhaps in worm this parallel mechanism of ELKS recruitment is sufficient for normal ELKS localization, alleviating the need for Rac cycling. Alternatively, some other RacGAP in worm might promote Rac cycling during synaptogenesis, creating a permissive environment for the GAP-dead worm Syd-1 to recruit ELKS.
By contrast, mouse mSYD1A does have GAP activity toward RhoA, but this activity is not required for its ability to rescue the presynaptic defects caused by mSYD1A loss (in cell culture at least; Wentzel et al. 2013). An obvious possibility is that the molecular mechanisms that promote presynaptic assembly differ substantially between vertebrates and invertebrates. However, we note that mice have a second Syd-1 homolog, mSYD1B, which has not yet been analyzed but which may have assumed some of the functions that depend on the single Syd-1 in invertebrates (Wentzel et al. 2013). Consistent with this possibility, the presynaptic defects caused by mSYD1A loss are far milder than those of the invertebrate syd-1 mutants (Wentzel et al. 2013). Because fly Syd-1RA can promote increased synaptogenesis in the presence of wild-type endogenous Syd-1, one possibility is that mouse mSYD1A lacking the RhoGAP domain can promote SV clustering in mSYD1AKO knockout cells because wild-type mSYD1B is present. It will be interesting to examine the effects of deleting both mouse Syd-1 proteins and to test the functionality of mutant versions of those proteins in the double mutant animals. Fly Syd-1 was previously thought to lack RhoGAP activity in part because mouse mSYD1A GAP activity toward RhoA is eliminated by changing a single one of its residues into the corresponding fly residue (Wentzel et al. 2013). Our results confirm that fly Syd-1's RhoGAP domain does not have GAP activity toward RhoA, but instead we found that it can regulate Rac1 and Cdc42. It will be interesting to see whether the GAP domain of mouse mSYD1B might share fly Syd-1's specificity.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.117.300538/-/DC1.
Acknowledgments
We thank A. DiAntonio, M. Bhat, and L. Luo for antibodies; C. Collins for fly stocks; and E. Heckscher for sound advice. Stocks obtained from the Bloomington Drosophila Stock Center [supported by National Institutes of Health (NIH) grant P40OD018537] were used in this study, as were DNA constructs obtained from the Drosophila Genomics Resource Center (supported by NIH grant 2P40OD010949). The nc82 antibody developed by E. Buchner and the 4F3 antibody developed by C. Goodman were obtained from the Developmental Studies Hybridoma Bank, which was created by the NICHD of the NIH and is maintained at The University of Iowa. This work was supported by NIH grants R01EY019694 (to T.G.H.) and T32GM007759 (M.A.S.).
Footnotes
Communicating editor: D. Greenstein
Literature Cited
- Ball R. W., Warren-Paquin M., Tsurudome K., Liao E. H., Elazzouzi F., et al. , 2010. Retrograde BMP signaling controls synaptic growth at the NMJ by regulating Trio expression in motor neurons. Neuron 66: 536–549. [DOI] [PubMed] [Google Scholar]
- Budnik V., Koh Y. H., Guan B., Hartmann B., Hough C., et al. , 1996. Regulation of synapse structure and function by the Drosophila tumor suppressor gene Dlg. Neuron 17: 627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P. H., Patel M. R., Shen K., 2012. NAB-1 instructs synapse assembly by linking adhesion molecules and F-actin to active zone proteins. Nat. Neurosci. 15: 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P. H., Li P., Shen K., 2013. Cellular and molecular mechanisms underlying presynapse formation. J. Cell Biol. 203: 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Taru H., Deken S. L., Grill B., Ackley B., et al. , 2006. SYD-2 Liprin-alpha organizes presynaptic active zone formation through ELKS. Nat. Neurosci. 9: 1479–1487. [DOI] [PubMed] [Google Scholar]
- Emery P., 2007. Protein extraction from Drosophila heads. Methods Mol. Biol. 362: 375–377. [DOI] [PubMed] [Google Scholar]
- Fritz R. D., Pertz O., 2016. The dynamics of spatio-temporal Rho GTPase signaling: formation of signaling patterns. F1000Res 5: F1000 Faculty Rev–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata R., Wennerberg K., Arthur W. T., Noren N. K., Ellerbroek S. M., et al. , 2006. Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol. 406: 425–437. [DOI] [PubMed] [Google Scholar]
- Graham D. L., Eccleston J. F., Lowe P. N., 1999. The conserved arginine in Rho-GTPase-activating protein is essential for efficient catalysis but not for complex formation with Rho.GDP and aluminum fluoride. Biochemistry 38: 985–991. [DOI] [PubMed] [Google Scholar]
- Hakeda-Suzuki S., Ng J., Tzu J., Dietzl G., Sun Y., et al. , 2002. Rac function and regulation during Drosophila development. Nature 416: 438–442. [DOI] [PubMed] [Google Scholar]
- Hallam S. J., Goncharov A., McEwen J., Baran R., Jin Y., 2002. SYD-1, a presynaptic protein with PDZ, C2, and RhoGAP-like domains, specifies axon identity in C. elegans. Nat. Neurosci. 5: 1137–1146. [DOI] [PubMed] [Google Scholar]
- Holbrook S., Finley J. K., Lyons E. L., Herman T. G., 2012. Loss of syd-1 from R7 neurons disrupts two distinct phases of presynaptic development. J. Neurosci. 32: 18101–18111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittel R. J., Wichmann C., Rasse T. M., Fouquet W., Schmidt M., et al. , 2006. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science 312: 1051–1054. [DOI] [PubMed] [Google Scholar]
- Li J., Ashley J., Budnik V., Bhat M. A., 2007. Crucial role of Drosophila neurexin in proper active zone apposition to postsynaptic densities, synaptic growth, and synaptic transmission. Neuron 55: 741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Liao Y. J., Jan L. Y., Jan Y. N., 1994. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 8: 1787–1802. [DOI] [PubMed] [Google Scholar]
- Marrus S. B., Portman S. L., Allen M. J., Moffat K. G., DiAntonio A., 2004. Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J. Neurosci. 24: 1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melom J. E., Littleton J. T., 2011. Synapse development in health and disease. Curr. Opin. Genet. Dev. 21: 256–261. [DOI] [PubMed] [Google Scholar]
- Nahm M., Kim S., Paik S. K., Lee M., Lee S., et al. , 2010. dCIP4 (Drosophila Cdc42-interacting protein 4) restrains synaptic growth by inhibiting the secretion of the retrograde Glass bottom boat signal. J. Neurosci. 30: 8138–8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. N., Stavoe A. K., Colon-Ramos D. A., 2013. The actin cytoskeleton in presynaptic assembly. Cell Adhes. Migr. 7: 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D., Fouquet W., Schmidt M., Wichmann C., Mertel S., et al. , 2010. A Syd-1 homologue regulates pre- and postsynaptic maturation in Drosophila. J. Cell Biol. 188: 563–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D., Khorramshahi O., Gupta V. K., Banovic D., Depner H., et al. , 2012. Cooperation of Syd-1 with Neurexin synchronizes pre- with postsynaptic assembly. Nat. Neurosci. 15: 1219–1226. [DOI] [PubMed] [Google Scholar]
- Parrini M. C., Camonis J., 2011. Cell motility: the necessity of Rac1 GDP/GTP flux. Commun. Integr. Biol. 4: 772–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M. R., Lehrman E. K., Poon V. Y., Crump J. G., Zhen M., et al. , 2006. Hierarchical assembly of presynaptic components in defined C. elegans synapses. Nat. Neurosci. 9: 1488–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal A. A., Motola-Barnes R. N., Littleton J. T., 2008. Nervous wreck and Cdc42 cooperate to regulate endocytic actin assembly during synaptic growth. J. Neurosci. 28: 8316–8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman K. L., Der C. J., Sondek J., 2005. GEF means go: turning on Rho GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6: 167–180. [DOI] [PubMed] [Google Scholar]
- Scheffzek K., Ahmadian M. R., 2005. GTPase activating proteins: structural and functional insights 18 years after discovery. Cell. Mol. Life Sci. 62: 3014–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., et al. , 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster C. M., Davis G. W., Fetter R. D., Goodman C. S., 1996. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron 17: 641–654. [DOI] [PubMed] [Google Scholar]
- Sharif B., Maddox A. S., 2012. Wound healing: GTPases flux their muscles. Dev. Cell 23: 236–238. [DOI] [PubMed] [Google Scholar]
- Stankiewicz T. R., Linseman D. A., 2014. Rho family GTPases: key players in neuronal development, neuronal survival, and neurodegeneration. Front. Cell. Neurosci. 8: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons M., Settleman J., 2000. Rho family GTPases: more than simple switches. Trends Cell Biol. 10: 415–419. [DOI] [PubMed] [Google Scholar]
- Tcherkezian J., Lamarche-Vane N., 2007. Current knowledge of the large RhoGAP family of proteins. Biol. Cell 99: 67–86. [DOI] [PubMed] [Google Scholar]
- Tolias K. F., Duman J. G., Um K., 2011. Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog. Neurobiol. 94: 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzel C., Sommer J. E., Nair R., Stiefvater A., Sibarita J. B., et al. , 2013. mSYD1A, a mammalian synapse-defective-1 protein, regulates synaptogenic signaling and vesicle docking. Neuron 78: 1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Taru H., Jin Y., Quinn C. C., 2015. SYD-1C, UNC-40 (DCC) and SAX-3 (Robo) function interdependently to promote axon guidance by regulating the MIG-2 GTPase. PLoS Genet. 11: e1005185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Chernoff J., Zheng Y., 1998. Interaction of Rac1 with GTPase-activating proteins and putative effectors: a comparison with Cdc42 and RhoA. J. Biol. Chem. 273: 8776–8782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Fly strains and DNA constructs are available upon request. For each experiment, genotypes and sample sizes are described in the legend of the corresponding figure. Because some transgenes were on the X chromosome, we analyzed females only for the sake of consistency. As described above, all experiments were scored blind to genotype. For each experiment a one-way ANOVA was conducted to determine if there was significant variation among genotypes. For experiments that showed a significant difference, individual post hoc pairwise comparisons were conducted between genotypes of interest by using either one- or two-tailed t-tests (see individual figure legends). For all figures: ns, not significant, * P < 0.05, ** P < 0.01, and *** P < 0.001. Exact P values are provided within the figure legends. Statistical values are presented as mean value ± SEM.