ABSTRACT
Repair of damaged DNA requires the activation of kinases, which in turn phosphorylate diverse proteins including histone H2A.X, an event conserved from yeast to human. By combining genetics, biochemical, and cytological approaches, we recently reported that, in addition to H2A.X, phosphorylation of histone variant H2A.W.7 is required for DNA damage response in Arabidopsis. This work provides direct evidence for the functional diversification of plant-specific H2A.W histone variants, which are tightly associated with heterochromatin. We place our findings in perspective of other recent reports and discuss how DNA damage is being recognized and repaired in heterochromatin.
KEYWORDS: histone variant, H2A.W, DNA damage response, heterochromatin, phosphorylation
Introduction
Genome integrity is constantly challenged by endogenous and exogenous sources of genotoxic stress, which can result in DNA damage. In eukaryotes, DNA damage repair operates in the context of chromatin, which is built of repeated nucleosomes each of which consists of DNA wrapped around four dimers of the histone proteins H3, H4, H2A, and H2B. Histone protein families comprise classes of non-allelic variants, characterized by primary sequence, expression profile, post-translational modifications, and associated deposition machineries. Together, these features of histones have a strong impact on nucleosome stability and the binding of chromatin interacting and regulatory factors, and influence chromatin organization in response to DNA damage.1–4
A network of conserved multiprotein complexes assembles at DNA double-strand breaks (DSBs) and recruits and activates members of the phosphatidylinositol 3-kinase-related kinase (PIKK) family. In mammals and plants, the ataxia telangiectasia mutated (ATM) kinase is the primary kinase activated in response to DSBs. H2A.X is specifically phosphorylated by ATM at the conserved SQ motif at the C-terminus of the protein (known as γH2A.X). One of the earliest events induced by DSBs, phosphorylation of H2A.X, initiates the DNA damage response (DDR). A positive feed-back loop leads to spreading of the region marked by γH2A.X around the DSBs, up to 100 kb or 1 Mbp in yeast and mammals, respectively. These regions can be visualized as discrete γH2A.X foci throughout the nucleoplasm in metazoan and plant nuclei.1–6 In plants, phosphorylation of H2A.X and formation of γH2A.X foci depend on ATM, primarily. The activity of another PIKK kinase, ATM and Rad3-related (ATR) that responds to single-stranded DNA and stalled replication forks, accounts for 10% of γH2A.X foci.4,5
A phosphoproteomic screen aimed at identifying proteins phosphorylated in response to DNA damage in Arabidopsis revealed an enrichment of peptides corresponding to the C-terminal tail of the heterochromatin associated histone variant H2A.W.7 and containing the SQ phosphorylation site characteristic for ATM/ATR kinases.7 Following these initial results, we analyzed the dynamics of H2A.W.7 phosphorylation after induction of DSBs.8 Together with the analysis of the sensitivities of h2a.w.7 and h2a.x mutants to DSBs our data demonstrate that ATM-dependent phosphorylation of both H2A.X and H2A.W.7 is required for proper DDR in Arabidopsis. In contrast to H2A.X, which acts primarily in euchromatin, H2A.W.7 is required for DDR in heterochromatin. Our study suggests that plants evolved a distinct pathway that is dependent on the histone variant H2A.W.7 to facilitate repair of DSBs in heterochromatin (Fig. 1). In this context, we discuss recently published papers in the context of DNA damage recognition and repair in heterochromatin.
Figure 1.
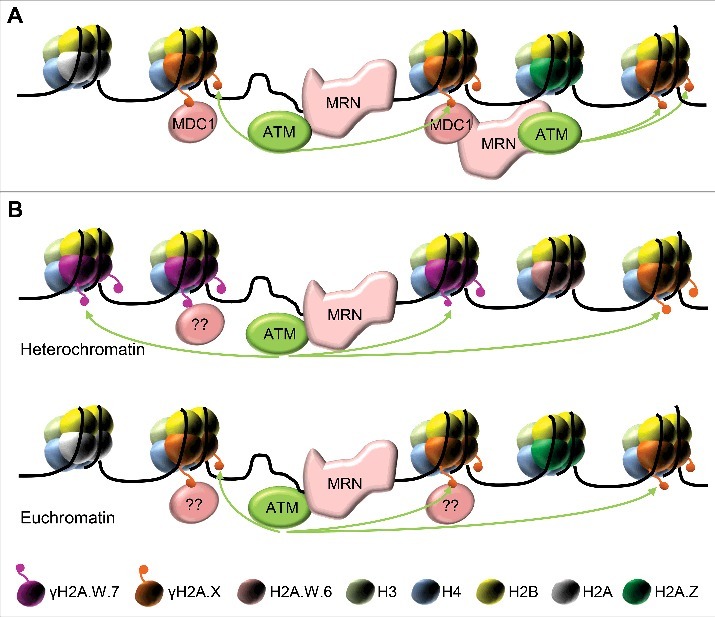
Recognition of DSB in animals (A) and plants (B). The conserved MRN complex binds to DNA double strand breaks and recruits the ATM kinase, which phosphorylates H2A.X. In plants, ATM-dependent phosphorylation of H2A.W.7 (but not of H2A.W.6) and H2A.X is required for heterochromatic and euchromatic DNA damage response, respectively. In animals, MDC1 protein binds to γH2A.X and enables its spreading around DSB by recruiting more MRN and ATM (A), a mechanism that is not conserved in plants (B). The nature of putative plant-specific protein(s) that recognize γH2A.W.7 and γH2A.X (ovals with question tags) and the mechanisms controlling the early events in DDR remain elusive.
Histone variants in DNA damage response
The importance of the phosphorylation of the histone variant H2A.X for DDR in plants has been well established4,5 and our study further emphasized the conservation of the function of H2A.X in DDR in Arabidopsis. Our work unambiguously demonstrates that phosphorylation of another H2A histone variant, H2A.W.7, is involved in sensing DNA damage in plants. Histone H2A.W variants are found only in land plants and are characterized by the conserved KSPKK motif at the end of a C-terminal tail that is longer than other H2A variants.9 In Arabidopsis, H2A.W variants share the same sequence features, localization, genome-wide distribution patterns, and role in chromatin condensation but only H2A.W.7 acquired features to function in DDR.8–10 Surprisingly, H2A.W variants containing the SQ motif are only found in some species of gymnosperms and angiosperms. This could be related to substantial differences in heterochromatin organization between plant species.11 In many species, heterochromatin is not organized into prominent chromocenters but is instead dispersed. This is the case for grass species and may be related to the absence of H2A.W.7 in the grass genomes sequenced so far.
In unicellular eukaryotes, such as S. cerevisiae, S. pombe, G. lamblia, and some protists, H2A.X features are merged into the canonical H2A so that the canonical H2A acts as the H2A.X variant.12,13 Similarly, features of H2A.Z and H2A.X are combined in a single variant, H2A.v, in Drosophila.14 In the ancestors of modern land plants, H2A and H2A.X diverged early during evolution and we could not find any species where the features of these two variants are fused into one.10
In addition to H2A.X phosphorylation, DDR dependent modifications also occur on histones H2A, H3, and linker histone H1 in yeast and metazoa.2,15 In plants, indirect evidence for the involvement of H2A.Z and H3 comes from the DNA damage sensitivity of mutants in their respective deposition machineries.4 However, DDR induced modifications on other histone variants and their significance in DSB repair remain elusive in plants.
Heterochromatin-specific DNA damage response pathways
Heterochromatin is mainly composed of repetitive DNA sequences and transposons. This special compartment of the nucleus possesses specific properties necessary not only to keep transposons silent but also to maintain genome integrity by preventing potentially deleterious illegitimate recombination between repetitive sequences and ensuring the proper definition of the centromere. However, the compact nature of heterochromatin poses an obstacle to DDR and repair; it has been noted that heterochromatin accumulates more mutations than less condensed euchromatin.3,29 It also appears that DDR in heterochromatin is facilitated by distinct mechanisms.1,3,16 In mammals, a specific DDR pathway involving HP1 and KAP1 proteins that are phosphorylated by ATM facilitates DSB repair in heterochromatin. As a consequence, HP1 is released from chromatin leading to heterochromatin relaxation.1,17–19 In plants however, KAP1 is not conserved and the HP1 homolog LHP1 is not located in constitutive heterochromatin.20 Thus, in analogy to the KAP1/HP1 mechanism in mammals, it is likely that generation of γH2A.W.7 in response to DNA damage is required to make heterochromatin more accessible to DNA repair machinery and/or to attract repair machinery to damage sites. Hence, plants evolved an alternative way to modulate DDR in the absence of the KAP1/HP1 heterochromatin-specialized DNA repair pathway, suggesting that other mechanisms exist in other species.
How does H2A.W.7 confer specific properties to heterochromatin that enable a more efficient response to DNA damage? The proximity of the KSPKK motif to the SQ motif in H2A.W.7 may indicate that SQ phosphorylation changes the ability of the C-terminal tail of H2A.W to promote chromatin fiber to fiber interaction,9 leading to a less compact heterochromatin. Chromatin folding is also regulated by the linker histone H1 and its phosphorylation causes chromatin relaxation, presumably by reducing its affinity for DNA.15,21 H1 depletion causes enhanced sensitivity to genotoxic stress in chicken cells,22 Drosophila,23,24 and plants (our unpublished data). Effects of the loss of Drosophila H1 on genome integrity are mainly confined to heterochromatin.23,24 It is tempting to speculate that binding of the linker histone H1 to linker DNA might be regulated by the C-terminal tail of H2A.W, which is positioned at the DNA entry/exit site of the nucleosome. Thus, an alternative but not mutually exclusive hypothesis is that SQ phosphorylation of H2A.W.7 antagonizes chromatin condensation mediated by linker histone H1, thereby making heterochromatin more accessible to DNA repair machinery.
In Arabidopsis, pericentromeric heterochromatin is defined by repressive histone H3 modifications (K9me1, K9me2, and K27me1), DNA methylation, and the histone H2A.W variant. Several pathways contribute to the formation of the silent and compact heterochromatin state, which are likely to interact and complement each other.25 Lysine K27 monomethylation is deposited by histone methyltransferases ATXR5 and 6 specifically on the histone variant H3.1.26 ATXR5/6 double mutants display a heterochromatin over-replication phenotype, which results in constitutive activation of DDR, particularly in highly endoreduplicated nuclei.27–29 In a recent paper, Feng et al. reported that this leads to a large-scale heterochromatin re-organization and formation of overreplication associated centers which accumulate γH2A.X and the DNA repair protein RAD51 in their inner layer.29 Because this phenotype is caused by replication stress, which was not assessed in our work on H2A.W.7, it is currently not possible to make a parallel between the roles of H2A.W.7 phosphorylation and ATXR5/6-mediated H3.1 K27me1 in protecting heterochromatin from DNA damage. As both pathways operate predominantly in heterochromatin, it will be of interest to analyze possible cross-talk between H2A.W.7 and ATXR5/6 in DDR.
Repair of DSBs in heterochromatin
Several pathways are available for the repair of DSBs, including canonical non-homologous end-joining (c-NHEJ), alternative non-homologous end-joining (alt-NHEJ), and homologous recombination (HR). The repair pathway that is used depends on the nature of the break, its location in the genome, and the cell cycle stage.1,3–5,16 Due to the highly repetitive nature of heterochromatin, one would expect that HR is not the first choice for repair of heterochromatic DSBs. However, it has been found that heterochromatic DSBs are repaired by HR.30 In yeast and metazoa, DNA DSB repair does not take place simultaneously across the genome and is notably delayed in the compact and transcriptionally silent constitutive heterochromatin.31,32 This is not caused by either delayed recognition of DSBs or repair initiation.30 This delayed repair is accompanied by the translocation of heterochromatic DSBs to the nuclear periphery where repair by HR takes place, thereby physically separating homologous sequences and preventing illegitimate recombination between repetitive sequences which could lead to chromosomal translocations.1,16,30,33–35
In our work, we did not address DNA repair in relation to γH2A.W.7 directly. However, we showed that γH2A.W.7 mediated DDR, as revealed by a time-dependent increase of γH2A.W.7 foci in heterochromatin and total levels of γH2A.W.7, is delayed compared to γH2A.X. Furthermore, we observed slow kinetics of γH2A.W.7 dephosphorylation during recovery of DNA damage, suggesting that repair of γH2A.W.7 marked DSBs is delayed as well. This seems to be important considering the large number of γH2A.W.7 foci outside of the pericentromeric heterochromatin, presumably originating from H2A.W.7 present over transposable elements along chromosome arms.8 In the absence of evidence for the relocation of DSBs to the nuclear periphery in plants, it is possible that the slower dynamics of γH2A.W.7 repair keeps these genomic regions in a conformation that inhibits illegitimate recombination of heterochromatic repeats, thereby preventing deleterious chromosomal rearrangements. This would be an alternative to the slow DSB repair in metazoa which depends on 53BP1.36,37 To analyze this more directly, it will be necessary to compare the dynamics of γH2A.W.7 foci localized inside and outside heterochromatic chromocenters. It also remains to be determined which repair pathway is involved in repair of DSBs marked by γH2A.W.7, and whether γH2A.W.7 and γH2A.X marked DSBs influence each other.
Conclusions and perspective
Heterochromatin organizes as a distinct nuclear compartment in many organisms and this organization appears to influence DSB repair. We and others have shown that specific mechanisms evolved to facilitate DNA repair in heterochromatin. In plants, this mechanism depends on the subfunctionalization of one member of the heterochromatic histone variant H2A.W. How phosphorylation of the SQ motif of H2A.W.7 impacts its function in heterochromatin organization remains to be understood (Fig. 1).
In contrast to the majority of the factors in the DDR and DSB repair pathways, several signal transduction components acting at the early stages of DDR are not encoded in plant genomes.4,5,38 For example, in addition to KAP1, plants also lack 53BP1 and p53, and the function of p53 in plants seems to be mediated by the unrelated, plant specific SOG1 protein.38,39 It is conceivable that delayed DDR and DSB repair mediated by γH2A.W.7 involves other plant specific factors which remain to be identified. Furthermore, MDC1, which binds to γH2A.X and interacts with the MRN complex to initiate DNA repair,2,6 is also not found in plants, leaving open the question of how γH2A.W.7 and γH2A.X contribute to the recruitment of the repair machinery to DSBs. DDR in plants is less well understood than in animals or yeast and more effort will be necessary to elucidate the early steps in DDR and repair, which are obviously different in plants compared to metazoa.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Mattia Dona for comments on the manuscript and James Matthew Watson for editing.
Funding
Research in Berger group is funded by Austrian Academy of Sciences and grants from the Austrian Science Fund (FWF; P26887 and P28320).
References
- 1.Dabin J, Fortuny A, Polo SE. Epigenome maintenance in response to DNA damage. Mol Cell. 2016;62:712–27. doi: 10.1016/j.molcel.2016.04.006. PMID:27259203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dantuma NP, van Attikum H. Spatiotemporal regulation of posttranslational modifications in the DNA damage response. EMBO J. 2016;35:6–23. doi: 10.15252/embj.201592595. PMID:26628622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watts FZ. Repair of DNA double-strand breaks in Heterochromatin. Biomolecules. 2016;6. doi: 10.3390/biom6040047. PMID:27999260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dona M, Mittelsten Scheid O. DNA damage repair in the context of plant Chromatin. Plant Physiol. 2015;168:1206–18. doi: 10.1104/pp.15.00538. PMID:26089404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amiard S, Gallego ME, White CI. Signaling of double strand breaks and deprotected telomeres in Arabidopsis. Front Plant Sci. 2013;4:405. doi: 10.3389/fpls.2013.00405. PMID:24137170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turinetto V, Giachino C. Multiple facets of histone variant H2AX: a DNA double-strand-break marker with several biological functions. Nucleic Acids Res. 2015;43:2489–98. doi: 10.1093/nar/gkv061. PMID:25712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roitinger E, Hofer M, Kocher T, Pichler P, Novatchkova M, Yang J, Schlogelhofer P, Mechtler K. Quantitative phosphoproteomics of the ataxia telangiectasia-mutated (ATM) and ataxia telangiectasia-mutated and rad3-related (ATR) dependent DNA damage response in Arabidopsis thaliana. Mol Cell Proteomics. 2015;14:556–71. doi: 10.1074/mcp.M114.040352. PMID:25561503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorkovic ZJ, Park C, Goiser M, Jiang D, Kurzbauer MT, Schlogelhofer P, Berger F. Compartmentalization of DNA damage response between Heterochromatin and Euchromatin is mediated by distinct H2A Histone Variants. Curr Biol. 2017;27:1192–9. doi: 10.1016/j.cub.2017.03.002. PMID:28392109. [DOI] [PubMed] [Google Scholar]
- 9.Yelagandula R, Stroud H, Holec S, Zhou K, Feng S, Zhong X, Muthurajan UM, Nie X, Kawashima T, Groth M, et al. . The histone variant H2A.W defines heterochromatin and promotes chromatin condensation in Arabidopsis. Cell. 2014;158:98–109. doi: 10.1016/j.cell.2014.06.006. PMID:24995981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawashima T, Lorkovic ZJ, Nishihama R, Ishizaki K, Axelsson E, Yelagandula R, Kohchi T, Berger F. Diversification of histone H2A variants during plant evolution. Trends Plant Sci. 2015;20:419–25. doi: 10.1016/j.tplants.2015.04.005. PMID:25983206. [DOI] [PubMed] [Google Scholar]
- 11.Feng W, Michaels SD. Accessing the inaccessible: The Organization, Transcription, Replication, and Repair of Heterochromatin in plants. Annu Rev Genet. 2015;49:439–59. doi: 10.1146/annurev-genet-112414-055048. PMID:26631514. [DOI] [PubMed] [Google Scholar]
- 12.Malik HS, Henikoff S. Phylogenomics of the nucleosome. Nat Struct Biol. 2003;10:882–91. doi: 10.1038/nsb996. PMID:14583738. [DOI] [PubMed] [Google Scholar]
- 13.Pinto DM, Flaus A. Structure and function of histone H2AX. Subcell Biochem. 2010;50:55–78. doi: 10.1007/978-90-481-3471-7_4. PMID:20012577. [DOI] [PubMed] [Google Scholar]
- 14.Madigan JP, Chotkowski HL, Glaser RL. DNA double-strand break-induced phosphorylation of Drosophila histone variant H2Av helps prevent radiation-induced apoptosis. Nucleic Acids Res. 2002;30:3698–705. doi: 10.1093/nar/gkf496. PMID:12202754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hergeth SP, Schneider R. The H1 linker histones: multifunctional proteins beyond the nucleosomal core particle. EMBO Rep. 2015;16:1439–53. doi: 10.15252/embr.201540749. PMID:26474902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amaral N, Ryu T, Li X, Chiolo I. Nuclear dynamics of Heterochromatin repair. Trends Genet. 2017;33:86–100. doi: 10.1016/j.tig.2016.12.004. PMID:28104289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodarzi AA, Noon AT, Deckbar D, Ziv Y, Shiloh Y, Lobrich M, Jeggo PA. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell. 2008;31:167–77. doi: 10.1016/j.molcel.2008.05.017. PMID:18657500. [DOI] [PubMed] [Google Scholar]
- 18.Ziv Y, Bielopolski D, Galanty Y, Lukas C, Taya Y, Schultz DC, Lukas J, Bekker-Jensen S, Bartek J, Shiloh Y. Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nat Cell Biol. 2006;8:870–6. doi: 10.1038/ncb1446. PMID:16862143. [DOI] [PubMed] [Google Scholar]
- 19.White D, Rafalska-Metcalf IU, Ivanov AV, Corsinotti A, Peng H, Lee SC, Trono D, Janicki SM, Rauscher FJ. The ATM substrate KAP1 controls DNA repair in heterochromatin: regulation by HP1 proteins and serine 473/824 phosphorylation. Mol Cancer Res. 2012;10:401–14. doi: 10.1158/1541-7786.MCR-11-0134. PMID:22205726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libault M, Tessadori F, Germann S, Snijder B, Fransz P, Gaudin V. The Arabidopsis LHP1 protein is a component of euchromatin. Planta. 2005;222:910–25. doi: 10.1007/s00425-005-0129-4. PMID:16244868. [DOI] [PubMed] [Google Scholar]
- 21.Lopez R, Sarg B, Lindner H, Bartolome S, Ponte I, Suau P, Roque A. Linker histone partial phosphorylation: effects on secondary structure and chromatin condensation. Nucleic Acids Res. 2015;43:4463–76. doi: 10.1093/nar/gkv304. PMID:25870416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto H, Sonoda E, Takami Y, Kimura H, Nakayama T, Tachibana M, Takeda S, Shinkai Y. Histone H1 variant, H1R is involved in DNA damage response. DNA Repair (Amst). 2007;6:1584–95. doi: 10.1016/j.dnarep.2007.05.003. PMID:17613284. [DOI] [PubMed] [Google Scholar]
- 23.Bayona-Feliu A, Casas-Lamesa A, Reina O, Bernues J, Azorin F. Linker histone H1 prevents R-loop accumulation and genome instability in heterochromatin. Nat Commun. 2017;8:283. doi: 10.1038/s41467-017-00338-5. PMID:28819201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vujatovic O, Zaragoza K, Vaquero A, Reina O, Bernues J, Azorin F. Drosophila melanogaster linker histone dH1 is required for transposon silencing and to preserve genome integrity. Nucleic Acids Res. 2012;40:5402–14. doi: 10.1093/nar/gks224. PMID:22406835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorkovic ZJ. MORC proteins and epigenetic regulation. Plant Signal Behav. 2012;7:1561–5. doi: 10.4161/psb.22460. PMID:23072987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacob Y, Bergamin E, Donoghue MT, Mongeon V, LeBlanc C, Voigt P, Underwood CJ, Brunzelle JS, Michaels SD, Reinberg D, et al. . Selective methylation of histone H3 variant H3.1 regulates heterochromatin replication. Science. 2014;343:1249–53. doi: 10.1126/science.1248357. PMID:24626927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacob Y, Stroud H, Leblanc C, Feng S, Zhuo L, Caro E, Hassel C, Gutierrez C, Michaels SD, Jacobsen SE. Regulation of heterochromatic DNA replication by histone H3 lysine 27 methyltransferases. Nature. 2010;466:987–91. doi: 10.1038/nature09290. PMID:20631708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stroud H, Hale CJ, Feng S, Caro E, Jacob Y, Michaels SD, Jacobsen SE. DNA methyltransferases are required to induce heterochromatic re-replication in Arabidopsis. PLoS Genet. 2012;8:e1002808. doi: 10.1371/journal.pgen.1002808. PMID:22792077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng W, Hale CJ, Over RS, Cokus SJ, Jacobsen SE, Michaels SD. Large-scale heterochromatin remodeling linked to overreplication-associated DNA damage. Proc Natl Acad Sci U S A. 2017;114:406–11. doi: 10.1073/pnas.1619774114. PMID:28028228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiolo I, Minoda A, Colmenares SU, Polyzos A, Costes SV, Karpen GH. Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell. 2011;144:732–44. doi: 10.1016/j.cell.2011.02.012. PMID:21353298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cowell IG, Sunter NJ, Singh PB, Austin CA, Durkacz BW, Tilby MJ. gammaH2AX foci form preferentially in euchromatin after ionising-radiation. PLoS One. 2007;2:e1057. doi: 10.1371/journal.pone.0001057. PMID:17957241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JA, Kruhlak M, Dotiwala F, Nussenzweig A, Haber JE. Heterochromatin is refractory to gamma-H2AX modification in yeast and mammals. J Cell Biol. 2007;178:209–18. doi: 10.1083/jcb.200612031. PMID:17635934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryu T, Spatola B, Delabaere L, Bowlin K, Hopp H, Kunitake R, Karpen GH, Chiolo I. Heterochromatic breaks move to the nuclear periphery to continue recombinational repair. Nat Cell Biol. 2015;17:1401–11. doi: 10.1038/ncb3258. PMID:26502056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsouroula K, Furst A, Rogier M, Heyer V, Maglott-Roth A, Ferrand A, Reina-San-Martin B, Soutoglou E. Temporal and spatial uncoupling of DNA double strand break repair pathways within Mammalian Heterochromatin. Mol Cell. 2016;63:293–305. doi: 10.1016/j.molcel.2016.06.002. PMID:27397684. [DOI] [PubMed] [Google Scholar]
- 35.Ryu T, Bonner MR, Chiolo I. Cervantes and Quijote protect heterochromatin from aberrant recombination and lead the way to the nuclear periphery. Nucleus. 2016;7:485–97. doi: 10.1080/19491034.2016.1239683. PMID:27673416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldock RA, Day M, Wilkinson OJ, Cloney R, Jeggo PA, Oliver AW, Watts FZ, Pearl LH. ATM localization and Heterochromatin repair depend on direct interaction of the 53BP1-BRCT2 Domain with gammaH2AX. Cell Rep. 2015;13:2081–9. doi: 10.1016/j.celrep.2015.10.074. PMID:26628370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noon AT, Shibata A, Rief N, Lobrich M, Stewart GS, Jeggo PA, Goodarzi AA. 53BP1-dependent robust localized KAP-1 phosphorylation is essential for heterochromatic DNA double-strand break repair. Nat Cell Biol. 2010;12:177–84. doi: 10.1038/ncb2017. PMID:20081839. [DOI] [PubMed] [Google Scholar]
- 38.Yoshiyama KO, Kimura S, Maki H, Britt AB, Umeda M. The role of SOG1, a plant-specific transcriptional regulator, in the DNA damage response. Plant Signal Behav. 2014;9:e28889; e28889. doi: 10.4161/psb.28889. PMID:24736489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshiyama KO, Kobayashi J, Ogita N, Ueda M, Kimura S, Maki H, Umeda M. ATM-mediated phosphorylation of SOG1 is essential for the DNA damage response in Arabidopsis. EMBO Rep. 2013;14:817–22. doi: 10.1038/embor.2013.112. PMID:23907539. [DOI] [PMC free article] [PubMed] [Google Scholar]


