Abstract
Hepatocellular carcinoma (HCC) is one of the most common cancers with a high mortality rate. Late diagnosis and poor prognosis are still a major drawback since curative therapies such as liver resection and liver transplantation are effective only for an early stage HCC. Development of novel molecular targeting therapies against HCC may provide new options that will improve the efficiency of the diagnosis and the success of the therapy, thus ameliorating the life expectancy of the patients. The aptamer is an oligonucleotide nanomedicine that has high binding affinity and specificity to small and large target molecules in the intracellular and extracellular environment with agonist or antagonist function. Currently, several aptamers for diagnostic and therapeutic purposes are under development to recognize different molecules of HCC. In in vitro models, the aptamer has been shown to be able to reduce the growth of HCC cells and increase the sensitivity to conventional chemotherapies. In in vivo mouse models, aptamer could induce cell apoptosis with antitumor activity. Overall data had shown that aptamer has limited toxicity and might be safe in clinical application. This review summarizes recent information of aptamer as a potential oligonucleotide nanomedicine tool, in diagnostics, targeted therapy, and as drug delivery nano-vehicles.
Keywords: hepatocellular carcinoma, aptamer, oligonucleotide nanomedicine, future therapy
HEPATOCELLULAR CARCINOMA: CURRENT TREATMENTS AND OBSTACLES
Hepatocellular carcinoma (HCC) is one of most common cancers and the second leading cause of cancer-related death worldwide [1]. The incidence of HCC is expected to increase in the future, especially in America and northern and central Europe where diabetes, obesity, and alcohol abuse represent the major risk factors [2–4].
The success of HCC treatment primarily depends on the time of diagnosis. Early diagnosis is crucial for a favorable prognosis since curative therapies options, such as local radiofrequency ablation and surgical intervention (liver transplantation and liver resection), have a much higher efficacy in the very early and early-stage HCC as compared to later stages [4, 5]. Liver transplantation can be the best treatment for HCC with low risk of recurrence. However, due to the disparity of liver donor resources and the increasing number of patients, it is suggested as a second line treatment only in case of relapse or liver failure after liver resection and ablation therapy [6]. Patients in later stages HCC (intermediate and advanced), can receive palliative treatments such as chemoembolization and kinase inhibitors, while for patients in the terminal stage can only receive best supportive care [7].
Nevertheless, tumor recurrence after percutaneous ablation or liver resection treatment can be a problem also in the early stages HCC. The probability of 5 years HCC recurrence is around 80% after liver resection [8] and 62% after liver ablation [9]. Furthermore, palliative treatments for intermediate and advanced stages often have an unfavorable outcome due either to drug side effects or drug resistance. A recent study done by Njei et al. showed that only 46.2% of HCC cases are diagnosed at an early stage where most of the cases do not receive curative therapy [10].
Based on this evidence, HCC treatment options are still hampered by many obstacles. Therefore, the development of early diagnostic tools and new therapeutic approaches will be crucial to improving survival rate and life quality of the patient.
APTAMER: OLIGONUCLEOTIDES NANOMEDICINE
The use of antibodies has been extensively studied both in research and in clinical application [11]. Although they have the ability to selectively recognize and bind to various biological molecules, the clinical application is still limited by the high immunogenicity, high production cost, and low stability [12, 13].
In recent years, nanomedicine technology represents a promising bench-to-bedside strategy in medicine. The oligonucleotide nanomedicine has been widely studied starting from anti-sense oligonucleotides, aptamers, to small-interference RNA (siRNA). Oligonucleotide nanomedicine has been demonstrated to be a powerful tool both for cancer diagnostic and for cancer therapy. In HCC, oligonucleotide nanomedicine therapy is predicted to achieve a better result than antibody-based therapy due to the non-effective treatment of the tested drug, codrituzumab (antibody-based therapy) against HCC [14, 15].
In January 2017, after the evaluation of strictly controlled trials, the Food and Drug Administration approved the application of six oligonucleotides for therapy [16]. This breakthrough is a very promising prospect for various oligonucleotides nanomedicine, including for the aptamer.
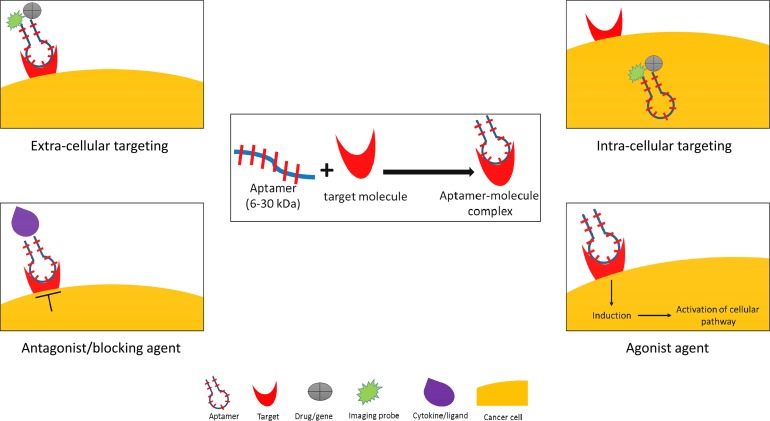
Aptamers are single-stranded RNA or DNA oligonucleotides with low molecular weight (6-30 kDa) that specifically and efficiently bind to a target molecule [17, 18]. This characteristic makes them suitable for a targeted therapy because of their ability to reach the core of the cancer cells and to internalize through endosomal pathway. Aptamers have a flexible configuration that recognizes and binds to the related target in a specific and high binding affinity via an adaptive recognition manner [19]. The aptamer-target complex has very low dissociation constants ranging from picomolar to nanomolar due to the specific hydrogen bonding [20–22]. The aptamers have a unique niche compared to other oligonucleotides. They can be developed to bind an intracellular or extracellular target, and they can be functioned as an agonist or antagonist (Figure 1).
Figure 1. Aptamer applications in cancer medicine.
Oligonucleotide aptamer can function as both extra- and intra-cellular targeting molecule, and as antagonist and agonist activating molecule. By using conjugation with imaging probe, drug, or gene-therapy, aptamer can be used as a nano-delivery agent in a direct or indirect system.
The principle of the aptamer molecular binding is based on its capability to spontaneously fold into a unique three-dimensional (3D) structure without the involvement of covalent bonds [19, 23]. As expected, the effect of aptamer-target interaction depends on the molecular function and cellular localization of the target molecule. In some cases, the aptamer-target complex can block the interaction between a ligand and its receptors that subsequently stimulates the cellular response [24] as for example, an immune response against viral infection [25, 26]. Some aptamers also have agonist-like activities that can enhance and induce protein synthesis [27, 28].
Aptamers are also known as “chemical antibodies” since they can bind specifically to target molecules either in the intracellular or extracellular environment [20, 29]. Aptamers have high versatility in targeting different molecules of different nature, size, and complexity, ranging from ions to whole cell, antibiotic, protein, bacteria, and virus [30]. However, compared to conventional antibodies, aptamers exhibit significant advantages. Aptamer commonly sustains its specificity and sensitivity by binding their ligands via adaptive recognition involving conformational alteration and molecular shape complementary [31]. They are stable at room temperature and in non-physiological conditions, non-immunogenic, non-toxic, and suitable for long-term repeated administration [32–34]. They also have high bio-distribution in biological fluids [35, 36] and high capacity to penetrate and remain in the tumor site [37]. The low-cost and well-standardized chemical synthesis, which is 1000 times cheaper than the antibodies production [38, 39], are also important aspects.
The aptamer can be generated by using Systematic Evolution of Ligands by Exponential Enrichment (SELEX), first described by Tuerk and Ellington [40, 41]. SELEX method is based on chemical process consisting of selection (binding phase, partitioning phase, and elution phase), amplification, and conditioning [42]. In the binding phase, a random nucleic acid library is incubated with the target molecule, followed by the partitioning phase that separates target-bound oligos from the remaining unbound library. The bound oligos are then eluted and are amplified to generate an enriched pool of aptamers candidates. The process can be repeated for 8-20 cycles to obtain candidates with the highest affinity to the target molecule. Finally, the sequences of chosen aptamers are characterized by sequencing [43].
Since its launch in 1990, conventional SELEX has been progressively modified to improve aptamer specificity and affinity, to simplify the process and to increase time and cost efficiency [44–46]. The modifications in the SELEX method depend on the purpose and target molecules. For example, in vivo SELEX, cell-SELEX, one-round SELEX, in silico SELEX, capillary electrophoresis-SELEX, magnetic bead-based SELEX, and high-throughput sequencing-SELEX are several novel SELEX technologies that are already well established [47]. Several SELEX methods are listed in Table 1.
Table 1. Modified SELEX methods.
| SELEX methods | Principle | Ref |
|---|---|---|
| In vivo | Localizing target molecules inside a living cell | [90] |
| Cell-based | Targeting the whole live cell | [91] |
| One-round step | One selection rounds of aptamer generation | [92] |
| In silico | Computational docking technology | [93] |
| Capillary electrophoresis | Electrophoretic mobility based separation | [94] |
| Magnetic bead-based | Magnetic beads immobilization | [95] |
| High-throughput sequencing-based | High-throughput sequencing and bioinformatics analysis | [96] |
| Ligand-guided selection | Using specific antibody to compete with the target molecule | [97] |
| Isogenic cell | Isogenic cell line application in counter selection step | [98] |
| Quantitative parallel aptamer selection system | Combination of microfluidic, next generation sequencing, and in situ-synthesized arrays | [99] |
DEVELOPMENT OF APTAMER AGAINST HCC
As mentioned above, the aptamer is a potent tool in basic and clinical biomedicine. Until now, numerous aptamers for different biomedical applications as biosensor and imaging nanoparticle for diagnostic [48–50], drug delivery agent [51–53], and theranostic (therapy and diagnostic) [54–56] had been discovered. Since Pegaptanib, an aptamer targeting vascular endothelial growth factor (VEGF), had been approved for age-related macular degeneration treatment [57], several aptamers had been shown to be a promising tool in clinical applications. Aptamer AS1411 (for acute myeloid leukemia and renal cell carcinoma) and NOX-A12 (for chronic lymphocytic leukemia and refractory multiple myeloma) are currently used in clinical trials [58, 59]. AS1411 was shown to selectively recognize cancer cells in vivo without any major side effects and toxicity [59]. Meanwhile, several aptamers are still under preclinical trials.
The increasing number of cases and poor prognosis of HCC highlight the need for a significant, appropriate, and efficient management of the disease. The screening and verification of potential aptamers as molecular probes against HCC will be needed to discover novel biomarkers in diagnostic and therapeutic implications [60]. An aptamer against Lipocalin-2 (a 24kDa secretory glycoprotein) had been proposed as an effective biomarker in HCC that may improve the diagnosis [61]. It had been reported that aptamers could also be used to monitor the progression of HCC by detecting metastatic cells [62] and circulating tumor cells [63]. The current evidence on the development of aptamers for HCC diagnosis, targeted therapy, and theranostic approach are listed in Table 2.
Table 2. Aptamers development against HCC.
| Aptamer (Oligotype) | Target | Principle | Function | Ref. |
|---|---|---|---|---|
| LNC2_apta2&4, (DNA) | Lipocalin-2 | Sandwich-based assay | Detection | [61] |
| Aptamer C-2 (DNA) | HepG2 | Cell-SELEX | Detection | [100] |
| TLS11a (DNA) | HepG2 | Cell-SELEX | Detection | [64] |
| TLS11a (DNA) | HepG2 | Dual recognition and signal amplification | Cytosensor | [67] |
| TLS11a (DNA) | HepG2 | Voltammetric based | Cytosensor | [68] |
| LY-1 (DNA) | HCCLM9 | Quantum dots and magnetic particles | Prognostic probe | [62] |
| SLeX-AP (DNA) | Circulating tumor cells | Biocompatible transparent nanostructured substrates | Controlling personalized treatment | [63] |
| TLS11a (DNA) | HepG2 | ‘Activatable’ aptamer-based fluorescence probe | Detection and imaging | [71] |
| Bio-TLS11a (DNA) | HepG2 | Streptavidin-fluorescent silica nanoparticles combination | Detection and imaging | [70] |
| TLS11a (DNA) | HepG2 | Aptamer- based electrochemical biosensors | Detection and imaging | [65] |
| TLS11a (DNA) | HepG2 | Label-free microcantilever array | Detection | [66] |
| AS1411 (DNA) | Nucleolin | AS1411-Dox adduct | Drug delivery | [76] |
| TLS11a-GC (DNA) | LH86 | SELEX | Drug delivery | [77] |
| TLS11a (DNA) | MEAR | Aptamer-biodegradable polymer | Drug delivery | [79] |
| EPAP (RNA) | EpCAM | Aptamer-gene therapy | Therapy | [81] |
| OPN-R3 (RNA) | Osteopontin | SELEX | Therapy | [74] |
| GT75 (DNA) | Elongation factor 1A | Liposome-aptamer | Drug delivery | [78] |
| AFP (RNA) | Alpha-fetoprotein | SELEX | Detection and therapy | [83] |
| Ep-MNPs (DNA) | EpCAM | Magnetic nanoparticle-aptamer | Imaging and therapy | [86] |
| AP273 | Alpha-fetoprotein | CE-SELEX | Imaging and therapy | [84] |
Aptamer for HCC diagnosis
The starting point of aptamer research in HCC was the development of a diagnostic tool. By using an aptamer generated by a cell-SELEX method, whole live HCC cells can be recognized. Aptamer that specifically binds to HCC cells in tissue samples and cell lines may facilitate the discovery of novel biomarker and ideal nanoparticle for HCC early diagnosis. TSL11a is one of the most studied aptamers, also in combination with other molecular probes to improve performance [64]. The TLS11a-based electrochemical biosensor (aptasensor) had been proposed as a simple, selective, and label-free diagnostic tool for the detection of the HepG2 cells. This conjugated aptamer could detect cancer cells at a very low concentration (2 cells/mL) with a wide linear dynamic range [65]. The TLS11a aptamer-based microcantilever biosensor with similar fundamental principle and function could also detect HepG2 cells, even though its sensitivity was less than the previous method (300 cells/mL) [66]. Another type of aptamer diagnostic tool using biosensor technology is a cytosensor aptamer that was developed based on a dual recognition and signals amplification strategy. In this method, TLS11a was covalently conjugated to a gold nanoparticle and horseradish peroxidase (HRP) for a sensitive detection of 30 cells/mL [67]. In a sequent development, this aptamer together with an indium tin oxide electrode assay and multifunctional nanoprobes improved the detection limit to 10 cells/mL [68]. These cytosensors have a great potential for the development of HCC diagnostic tools.
In recent years, fluorescent silica nanoparticles have been successfully used in cancer imaging due to its photostability, brightness, and high emission [69]. Biotin-labelled TLS11a combined with streptavidin-modified fluorescent silica nanoparticles showed promising results. This nanoparticle has no significant toxicity effects, both in vitro and in vivo, with stronger and more photo-stable fluorescence signal compared to conventional fluorescein isothiocyanate (FITC)-labelled aptamer [70].
The latest diagnostic approach using TLS11a aptamer is by conjugating the aptamer with fluorescence probe that can distinguish the presence of cancer and non-cancer cells. By using HCC cell lines and frozen HCC tissue section, the probe emitted a strong fluorescent signal only in the presence of the target cancer cells. This versatile technology can be adapted for other aptamer sequences targeting various cancers and diseases [71].
In a recent study, the aptamer-based chip had been developed to detect the circulating tumor cells (CTC) in blood samples of HCC patients. The SLex (aptamer for carbohydrate sialyl Lewis X) coated onto hydroxyapatite/chitosan nanofilm aptamer had clinically potential in detecting the CTCs, both for HepG2 in artificial blood and more importantly for blood from HCC patients. The detection of CTCs by using this system was significantly correlated with the tumor size, portal vein tumor thrombus, and the tumor-node-metastasis stage [63].
Aptamers for HCC-targeted therapy
HCC-targeted therapy is one of the most promising applications for aptamers. Aptamers can be utilized as a direct therapeutic agent and as a nano-delivery agent by conjugating the aptamer with an anti-cancer drug, nanoparticles, and gene therapy [72, 73]. A new RNA aptamer targeting osteopontin (OPN-R3) showed a good efficacy in down-regulating the epithelial-mesenchymal transition and the growth of HCC in a mouse model. Mouse injected with this aptamer showed significantly decreased tumor burden compared to control and mutant control aptamer group in in-vivo bioluminescence imaging [74]. This study was significant in the “proof-of-concept” study in breast cancer cells that this aptamer was relevance for modifying tumor growth and metastasis [75].
A conjugation between the aptamer and anti-cancer drug demonstrated that the aptamer was able to deliver the anticancer drug precisely into the target cell or tissue. Doxorubicin-conjugated AS1411 (AS1411-Dox) had been proposed to be a simple technique to form Drug-DNA Adduct in in vitro and in vivo models of HCC. In tissue staining, the aptamer could clearly differentiate the HCC tissue and non-HCC tissue. AS1411-aptamer were specifically bound in tumor regions compared with adjacent non-tumor tissue. Moreover, AS1411-aptamer showed the strongest staining in the most abundant area of nucleolin [76]. Doxorubicin was also conjugated with the TLS11a-GC aptamer, which specifically targeted LH86 HCC cells [77].
Conjugating an aptamer with other nanoparticles will increase effectiveness. Scaggiante et al. generated an aptamer-liposome intercalation that increases the bioavailability of the aptamer in targeting the elongation factor 1A (eEF1A) in an HCC model. The synergic effect of the aptamer-liposome and either bortezomib or idarubicin impaired the vitality of the HCC cells in a dose- and time-dependent manner [78]. Conjugation between TLS11a with a biodegradable polymer nanoparticle effectively bound to HCC cells. It showed a higher therapeutic effect when loaded with doxorubicin compared with nanoparticle without the targeting aptamer [79]. Another study in HCC showed that nanoparticles contained peptide-modified aptamer (ST21) significantly increase the cellular uptake in vitro and therapeutic efficacy in mouse in vivo model indicating a highly efficient co-delivery vehicle for tumor-specific therapy [80]. Collectively all this work indicated the advantages of aptamers as chemotherapeutic nano-vehicle to minimize the toxicity effect and to increase the drug efficacy, at least in in vitro.
The use of recombinant adenovirus carrying a tumor suppressor gene into HCC is an effective gene therapy method. However, its application is still hindered by auto-immunogenicity, low stability, and non-specific toxicity to normal cells. A novel aptamer-based gene delivery system by using RNA aptamer conjugated to tumor suppressor gene can be an effective strategy. An aptamer conjugated with Ad5-PTEN had been shown to target the epithelial cell adhesion molecule in vitro and in vivo. This method significantly inhibited cell proliferation and cell migration in HepG2 with no toxic effect observed in healthy liver cells. Moreover, it induced cell apoptosis in aggressive HepG2 xenograft in nude mice without concentration-dependent toxicity [81].
Aptamers for theranostic approach
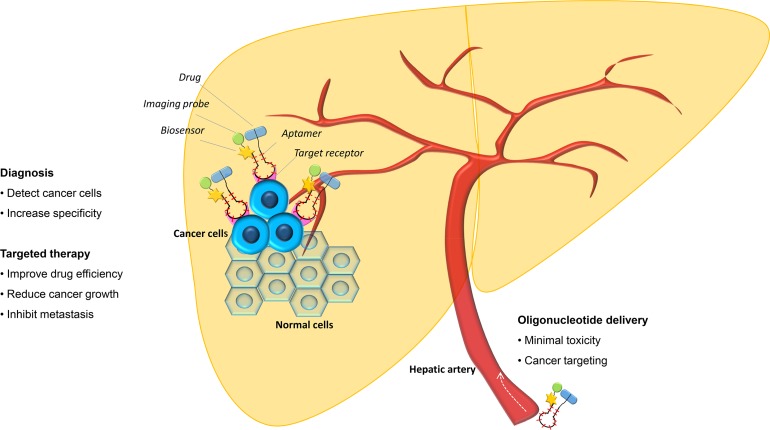
Theranostic nanoplatforms (a combination of a diagnostic and therapeutic method) is a promising approach to overcome the limitation of conventional cancer therapy and diagnosis Figure 2. Because aptamers are versatile, they can be modified and improved to obtain a specific application to treat cancer. Evaluation of aptamers, both in vitro and in vivo, had considered them as a powerful theranostic agent for their potential as dual therapeutic and diagnostic application [82].
Figure 2. Aptamer for HCC diagnostic and targeted therapy.
Aptamer can act both as a diagnostic tool in the conjugation with imaging probe and biosensor and a targeted therapy agent by its neutralizing effect and by its combination with antineoplastic drugs and/or gene therapy.
Theranostic aptamer was developed in many different ways due to its superior performance in solid tumor penetration over antibody [37]. Aptamer can be functional both as simultaneous diagnostic and therapeutic agent by combining its greater efficacy in targeting the cancer cells with its agonist/antagonist capability in cellular pathways. However, the information regarding theranostic approach against HCC is still limited. In 2012, a first report demonstrated that an RNA aptamer against alpha-fetoprotein (AFP) was able to detect cancer cells, inhibit the proliferation of AFP-associated HepG2 cells and decrease the gene expressions of the c-jun and c-fos oncogenes [83]. By using a CE-SELEX technology, aptamer AP273 against AFP had also been used to screen and identify HCC. AP273 had been found to competently modulate cancer cells by inhibiting the migration and invasion of HCC cells after in vivo transfection [84]. These AFP-specific aptamers could be a useful theranostic agent against AFP-related studies of HCC.
Furthermore, by integrating imaging and nano-delivery functionalities in a single agent, aptamer-based theranostic provided a novel solution for an early diagnosis that can be followed by in situ drugs released [85]. In 2014, a smart magnetic nanoparticle-aptamer probe targeting epithelial cell adhesion molecule (EpCAM) had been developed as a novel theranostic approach. Besides demonstrating an efficient in vitro magnetic resonance imaging of HCC cells, this nanoprobe also improved the delivery of the doxorubicin into the cancer cells. This nanoprobe had 98% doxorubicin entrapping efficiency and 24% doxorubicin loading efficiency which was very specific to cancer cells but not in normal cells [86].
SUMMARY AND FUTURE PERSPECTIVE
The literature reviewed above shows promising data on oligonucleotide aptamer as a considerable potential in the development of nanomedicine against HCC. In addition to its potential in situ, aptamer-based targeted diagnosis and therapy can be administered in the circulation [76]. Systemic administration not only can detect the presence of the tumor cells, but it can also exhibit potent antitumor activity and may reduce tumor metastases with limited or even no side effect [87]. Nevertheless, this technology is still relatively novel and still faces several challenges to achieve its final target in a clinical setting. Recently, only a few aptamers have reached clinical trials, and there is still no approved aptamer for HCC diagnosis and therapy. Furthermore, HCC is a highly heterogeneous disease with distinct molecular profiles related to different etiologies, subtypes, and long-term development. The main challenges of aptamer developments as a nanomedicine against HCC are represented by the selection of the efficient and specific molecular target, the chemical and biological activity of aptamers in vivo, and by the improvement of delivery method to obtain potent aptamers. It is understood that the process of aptamer selection is lengthy and the validation will be needed in different sets of samples and models. Therefore, a target molecule must be carefully selected for a specific and effective approach.
In summary, oligonucleotide aptamer is an emerging and promising nanomedicine for HCC diagnosis and therapy in the future. Aptamer can be a powerful tool with unique and distinctive characteristics that will give positive impacts, both in basic research and clinical application of HCC. However, translating the potential of oligonucleotide aptamer from pre-clinical study to clinical application is still challenging. Many aptamers with potent functions are susceptible to endogenous nuclease degradation and have short half-lives in a biological system. Nevertheless, current and upcoming technologies on aptamer modification and stabilization by using modified nucleic acids and chemical agents will enhance the function and the stability of the aptamer for clinical use [88, 89].
We predict that in a near future aptamer technology will continue to exponentially grow and to be progressively used in the development of new efficacious aptamer-based diagnostic and therapeutic agents towards cancers, including HCC. Further developments would be needed to facilitate clinical translation of the promising preclinical studies in both HCC diagnosis and targeted therapy.
ACKNOWLEDGMENTS AND FUNDING
RBL is supported by fellowships from the Lembaga Pengelola Dana Pendidikan (LPDP) of the Indonesian Ministry of Monetary. DP by NECTE project of PAR-FSC_2007-13 decree n° 3984/LAVFOR.ISTR/2014 Friuli Venezia Giulia. CHCS fellowship was partially supported by grant NIH U01AA020821. This work was supported by an in-house grant from the Italian Liver Foundation. No additional external funding was received for this study.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. https://doi.org/10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, Negri E. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67:302–9. doi: 10.1016/j.jhep.2017.03.011. https://doi.org/10.1016/j.jhep.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. https://doi.org/10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. doi: 10.1016/S0140-6736(11)61347-0. https://doi.org/10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 5.de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56:S75–87. doi: 10.1016/S0168-8278(12)60009-9. https://doi.org/10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- 6.Vitale A, Peck-Radosavljevic M, Giannini EG, Vibert E, Sieghart W, Van Poucke S, Pawlik TM. Personalized treatment of patients with very early hepatocellular carcinoma. J Hepatol. 2017;66:412–23. doi: 10.1016/j.jhep.2016.09.012. https://doi.org/10.1016/j.jhep.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 7.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. https://doi.org/10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, Makuuchi M. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–7. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 9.Facciorusso A, Del Prete V, Antonino M, Crucinio N, Neve V, Di Leo A, Carr BI, Barone M. Post-recurrence survival in hepatocellular carcinoma after percutaneous radiofrequency ablation. Dig Liver Dis. 2014;46:1014–9. doi: 10.1016/j.dld.2014.07.012. https://doi.org/10.1016/j.dld.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatol. 2015;61:191–9. doi: 10.1002/hep.27388. https://doi.org/10.1002/hep.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 12.Bradbury AR, Sidhu S, Dübel S, McCafferty J. Beyond natural antibodies: the power of in vitro display technologies. Nat Biotechnol. 2011;29:245–54. doi: 10.1038/nbt.1791. https://doi.org/10.1038/nbt.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157:220–33. doi: 10.1111/j.1476-5381.2009.00190.x. https://doi.org/10.1111/j.1476-5381.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abou-Alfa GK, Puig O, Daniele B, Kudo M, Merle P, Park JW, Ross P, Peron JM, Ebert O, Chan S, Poon TP, Colombo M, Okusaka T, et al. Randomized phase II placebo controlled study of codrituzumab in previously treated patients with advanced hepatocellular carcinoma. J Hepatol. 2016;65:289–95. doi: 10.1016/j.jhep.2016.04.004. https://doi.org/10.1016/j.jhep.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Abou-Alfa GK, Yen CJ, Hsu CH, O’Donoghue J, Beylergil V, Ruan S, Pandit-Taskar N, Gansukh B, Lyashchenko SK, Ma J, Wan P, Shao YY, Lin ZZ, et al. Phase Ib study of codrituzumab in combination with sorafenib in patients with non-curable advanced hepatocellular carcinoma (HCC) Cancer Chemother Pharmacol. 2017;79:421–9. doi: 10.1007/s00280-017-3241-9. https://doi.org/10.1007/s00280-017-3241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein CA, Castanotto D. FDA-approved oligonucleotide therapies in 2017. Mol Ther J. 2017;25:1069–1075. doi: 10.1016/j.ymthe.2017.03.023. https://doi.org/10.1016/j.ymthe.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hicke BJ, Stephens AW, Gould T, Chang YF, Lynott CK, Heil J, Borkowski S, Hilger CS, Cook G, Warren S, Schmidt PG. Tumor targeting by an aptamer. J Nucl Med. 2006;47:668–78. [PubMed] [Google Scholar]
- 18.Lundin KE, Gissberg O, Smith CI. Oligonucleotide therapies: The past and the present. Hum Gene Ther. 2015;26:475–85. doi: 10.1089/hum.2015.070. https://doi.org/10.1089/hum.2015.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermann T, Patel DJ. Adaptive recognition by nucleic acid aptamers. Science. 2000;287:820–5. doi: 10.1126/science.287.5454.820. [DOI] [PubMed] [Google Scholar]
- 20.Nimjee SM, Rusconi CP, Sullenger BA. Aptamers: an emerging class of therapeutics. Annu Rev Med. 2005;56:555–83. doi: 10.1146/annurev.med.56.062904.144915. https://doi.org/10.1146/annurev.med.56.062904.144915. [DOI] [PubMed] [Google Scholar]
- 21.Sun H, Zu Y. Aptamers and their applications in nanomedicine. Small. 2015;11:2352–64. doi: 10.1002/smll.201403073. https://doi.org/10.1002/smll.201403073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H, Zhu X, Lu PY, Rosato RR, Tan W, Zu Y. Oligonucleotide aptamers: new tools for targeted cancer therapy. Mol Ther Nucleic Acids. 2014;3:e182. doi: 10.1038/mtna.2014.32. https://doi.org/10.1038/mtna.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayer G. The chemical biology of aptamers. Angew Chem Int Ed Engl. 2009;48:2672–89. doi: 10.1002/anie.200804643. https://doi.org/10.1002/anie.200804643. [DOI] [PubMed] [Google Scholar]
- 24.Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010;9:537–50. doi: 10.1038/nrd3141. https://doi.org/10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker KC, Becker RC. Nucleic acid aptamers as adjuncts to vaccine development. Curr Opin Mol Ther. 2006;8:122–9. [PubMed] [Google Scholar]
- 26.Berezhnoy A, Castro I, Levay A, Malek TR, Gilboa E. Aptamer-targeted inhibition of mTOR in T cells enhances antitumor immunity. J Clin Invest. 2014;124:188–97. doi: 10.1172/JCI69856. https://doi.org/10.1172/JCI69856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farrow MA, Schimmel P. Editing by a tRNA synthetase: DNA aptamer-induced translocation and hydrolysis of a misactivated amino acid. Biochemistry. 2001;40:4478–83. doi: 10.1021/bi0024052. [DOI] [PubMed] [Google Scholar]
- 28.Hale SP, Schimmel P. Protein synthesis editing by a DNA aptamer. Proc Natl Acad Sci U S A. 1996;93:2755–8. doi: 10.1073/pnas.93.7.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou G, Wilson G, Hebbard L, Duan W, Liddle C, George J, Qiao L. Aptamers: A promising chemical antibody for cancer therapy. Oncotarget. 2016;7:13446–63. doi: 10.18632/oncotarget.7178. https://doi.org/10.18632/oncotarget.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero-López C, Berzal-Herranz A. Aptamers: Biomedical interest and applications. Pharm Basel. 2017;10 doi: 10.3390/ph10010032. https://doi.org/10.3390/ph10010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shigdar S, Macdonald J, O’Connor M, Wang T, Xiang D, Al Shamaileh H, Qiao L, Wei M, Zhou SF, Zhu Y, Kong L, Bhattacharya S, Li C, et al. Aptamers as theranostic agents: modifications, serum stability and functionalisation. Sensors. 2013;13:13624–37. doi: 10.3390/s131013624. https://doi.org/10.3390/s131013624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng EW, Shima DT, Calias P, Cunningham ET, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5:123–32. doi: 10.1038/nrd1955. https://doi.org/10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 33.Friberg TR, Tolentino M, Weber P, Patel S, Campbell S, Goldbaum M, LEVEL Study Group Pegaptanib sodium as maintenance therapy in neovascular age-related macular degeneration: the LEVEL study. Br J Ophthalmol. 2010;94:1611–7. doi: 10.1136/bjo.2009.174946. https://doi.org/10.1136/bjo.2009.174946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ireson CR, Kelland LR. Discovery and development of anticancer aptamers. Mol Cancer Ther. 2006;5:2957–62. doi: 10.1158/1535-7163.MCT-06-0172. https://doi.org/10.1158/1535-7163.MCT-06-0172. [DOI] [PubMed] [Google Scholar]
- 35.Zhu J, Huang H, Dong S, Ge L, Zhang Y. Progress in aptamer-mediated drug delivery vehicles for cancer targeting and its implications in addressing chemotherapeutic challenges. Theranostics. 2014;4:931–44. doi: 10.7150/thno.9663. https://doi.org/10.7150/thno.9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catuogno S, Esposito CL, de Franciscis V. Developing aptamers by cell-based SELEX. Methods Mol Biol. 2016;1380:33–46. doi: 10.1007/978-1-4939-3197-2_3. https://doi.org/10.1007/978-1-4939-3197-2_3. [DOI] [PubMed] [Google Scholar]
- 37.Xiang D, Zheng C, Zhou SF, Qiao S, Tran PH, Pu C, Li Y, Kong L, Kouzani AZ, Lin J, Liu K, Li L, Shigdar S, Dean W. Superior performance of aptamer in tumor penetration over antibody: implication of aptamer-based theranostics in solid tumors. Theranostics. 2015;5:1083–97. doi: 10.7150/thno.11711. https://doi.org/10.7150/thno.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun H, Tan W, Zu Y. Aptamers: versatile molecular recognition probes for cancer detection. The Analyst. 2016;141:403–15. doi: 10.1039/c5an01995h. https://doi.org/10.1039/c5an01995h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Germer K, Leonard M, Zhang X. RNA aptamers and their therapeutic and diagnostic applications. Int J Biochem Mol Biol. 2013;4:27–40. [PMC free article] [PubMed] [Google Scholar]
- 40.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–10. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 41.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–22. doi: 10.1038/346818a0. https://doi.org/10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 42.Stoltenburg R, Reinemann C, Strehlitz B. SELEX--a (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol Eng. 2007;24:381–403. doi: 10.1016/j.bioeng.2007.06.001. https://doi.org/10.1016/j.bioeng.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Ozer A, Pagano JM, Lis JT. New technologies provide quantum changes in the scale, speed, and success of SELEX methods and aptamer characterization. Mol Ther Nucleic Acids. 2014;3:e183. doi: 10.1038/mtna.2014.34. https://doi.org/10.1038/mtna.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darmostuk M, Rimpelova S, Gbelcova H, Ruml T. Current approaches in SELEX: An update to aptamer selection technology. Biotechnol Adv. 2015;33:1141–61. doi: 10.1016/j.biotechadv.2015.02.008. https://doi.org/10.1016/j.biotechadv.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Yu Y, Liang C, Lv Q, Li D, Xu X, Liu B, Lu A, Zhang G. Molecular selection, modification and development of therapeutic oligonucleotide aptamers. Int J Mol Sci. 2016;17:358. doi: 10.3390/ijms17030358. https://doi.org/10.3390/ijms17030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Setlem K, Mondal B, Ramlal S, Kingston J. Immuno Affinity SELEX for simple, rapid, and cost-effective aptamer enrichment and identification against Aflatoxin B1. Front Microbiol. 2016;7:1909. doi: 10.3389/fmicb.2016.01909. https://doi.org/10.3389/fmicb.2016.01909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Darmostuk M, Rimpelova S, Gbelcova H, Ruml T. Current approaches in SELEX: An update to aptamer selection technology. Biotechnol Adv. 2015;33:1141–61. doi: 10.1016/j.biotechadv.2015.02.008. https://doi.org/10.1016/j.biotechadv.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Aliakbarinodehi N, Jolly P, Bhalla N, Miodek A, De Micheli G, Estrela P, Carrara S. Aptamer-based field-effect biosensor for tenofovir detection. Sci Rep. 2017;7:44409. doi: 10.1038/srep44409. https://doi.org/10.1038/srep44409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hou Y, Liu J, Hong M, Li X, Ma Y, Yue Q, Li CZ. A reusable aptasensor of thrombin based on DNA machine employing resonance light scattering technique. Biosens Bioelectron. 2017;92:259–65. doi: 10.1016/j.bios.2017.02.024. https://doi.org/10.1016/j.bios.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 50.Ozalp VC, Kavruk M, Dilek O, Bayrac AT. Aptamers: molecular tools for medical diagnosis. Curr Top Med Chem. 2015;15:1125–37. doi: 10.2174/1568026615666150413154233. [DOI] [PubMed] [Google Scholar]
- 51.Chen K, Liu B, Yu B, Zhong W, Lu Y, Zhang J, Liao J, Liu J, Pu Y, Qiu L, Zhang L, Liu H, Tan W. Advances in the development of aptamer drug conjugates for targeted drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016:9. doi: 10.1002/wnan.1438. https://doi.org/10.1002/wnan.1438. [DOI] [PMC free article] [PubMed]
- 52.Liao J, Liu B, Liu J, Zhang J, Chen K, Liu H. Cell-specific aptamers and their conjugation with nanomaterials for targeted drug delivery. Expert Opin Drug Deliv. 2015;12:493–506. doi: 10.1517/17425247.2015.966681. https://doi.org/10.1517/17425247.2015.966681. [DOI] [PubMed] [Google Scholar]
- 53.Charoenphol P, Bermudez H. Aptamer-targeted DNA nanostructures for therapeutic delivery. Mol Pharm. 2014;11:1721–5. doi: 10.1021/mp500047b. https://doi.org/10.1021/mp500047b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sriramoju B, Kanwar R, Veedu RN, Kanwar JR. Aptamer-targeted oligonucleotide theranostics: a smarter approach for brain delivery and the treatment of neurological diseases. Curr Top Med Chem. 2015;15:1115–24. doi: 10.2174/1568026615666150413153928. [DOI] [PubMed] [Google Scholar]
- 55.Xiang D, Shigdar S, Qiao G, Wang T, Kouzani AZ, Zhou SF, Kong L, Li Y, Pu C, Duan W. Nucleic acid aptamer-guided cancer therapeutics and diagnostics: the next generation of cancer medicine. Theranostics. 2015;5:23–42. doi: 10.7150/thno.10202. https://doi.org/10.7150/thno.10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao F, Zhou J, Su X, Wang Y, Yan X, Jia S, Du B. A Smart responsive dual aptamers-targeted bubble-generating nanosystem for cancer triplex therapy and ultrasound imaging. Small. 2017;13 doi: 10.1002/smll.201603990. https://doi.org/10.1002/smll.201603990. [DOI] [PubMed] [Google Scholar]
- 57.Zhou B, Wang B. Pegaptanib for the treatment of age-related macular degeneration. Exp Eye Res. 2006;83:615–9. doi: 10.1016/j.exer.2006.02.010. https://doi.org/10.1016/j.exer.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 58.Lao YH, Phua KK, Leong KW. Aptamer nanomedicine for cancer therapeutics: barriers and potential for translation. ACS Nano. 2015;9:2235–54. doi: 10.1021/nn507494p. https://doi.org/10.1021/nn507494p. [DOI] [PubMed] [Google Scholar]
- 59.Rosenberg JE, Bambury RM, Van Allen EM, Drabkin HA, Lara PN, Harzstark AL, Wagle N, Figlin RA, Smith GW, Garraway LA, Choueiri T, Erlandsson F, Laber DA. A phase II trial of AS1411 (a novel nucleolin-targeted DNA aptamer) in metastatic renal cell carcinoma. Invest New Drugs. 2014;32:178–87. doi: 10.1007/s10637-013-0045-6. https://doi.org/10.1007/s10637-013-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu B, Wang J, Zhang J, Zhang X, Yang D, Wu L, Luo Z, Ma Y, Zhang Q, Ma Y, Pei X, Yu H, Liu J. Screening and verification of ssDNA aptamers targeting human hepatocellular carcinoma. Acta Biochim Biophys Sin. 2014;46:128–35. doi: 10.1093/abbs/gmt130. https://doi.org/10.1093/abbs/gmt130. [DOI] [PubMed] [Google Scholar]
- 61.Lee KA, Ahn JY, Lee SH, Singh Sekhon S, Kim DG, Min J, Kim YH. Aptamer-based sandwich assay and its clinical outlooks for detecting lipocalin-2 in Hepatocellular Carcinoma (HCC) Sci Rep. 2015;5:10897. doi: 10.1038/srep10897. https://doi.org/10.1038/srep10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang FB, Rong Y, Fang M, Yuan JP, Peng CW, Liu SP, Li Y. Recognition and capture of metastatic hepatocellular carcinoma cells using aptamer-conjugated quantum dots and magnetic particles. Biomaterials. 2013;34:3816–27. doi: 10.1016/j.biomaterials.2013.02.018. https://doi.org/10.1016/j.biomaterials.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 63.Wang S, Zhang C, Wang G, Cheng B, Wang Y, Chen F, Chen Y, Feng M, Xiong B. Aptamer-mediated transparent-biocompatible nanostructured surfaces for 10.7150/thno.15284. hepotocellular circulating tumor cells enrichment. Theranostics. 2016;6:1877–86. doi: 10.7150/thno.15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shangguan D, Meng L, Cao ZC, Xiao Z, Fang X, Li Y, Cardona D, Witek RP, Liu C, Tan W. Identification of liver cancer-specific aptamers using whole live cells. Anal Chem. 2008;80:721–8. doi: 10.1021/ac701962v. https://doi.org/10.1021/ac701962v. [DOI] [PubMed] [Google Scholar]
- 65.Kashefi-Kheyrabadi L, Mehrgardi MA, Wiechec E, Turner AP, Tiwari A. Ultrasensitive detection of human liver hepatocellular carcinoma cells using a label-free aptasensor. Anal Chem. 2014;86:4956–60. doi: 10.1021/ac500375p. https://doi.org/10.1021/ac500375p. [DOI] [PubMed] [Google Scholar]
- 66.Chen X, Pan Y, Liu H, Bai X, Wang N, Zhang B. Label-free detection of liver cancer cells by aptamer-based microcantilever biosensor. Biosens Bioelectron. 2016;79:353–8. doi: 10.1016/j.bios.2015.12.060. https://doi.org/10.1016/j.bios.2015.12.060. [DOI] [PubMed] [Google Scholar]
- 67.Sun D, Lu J, Chen Z, Yu Y, Mo M. A repeatable assembling and disassembling electrochemical aptamer cytosensor for ultrasensitive and highly selective detection of human liver cancer cells. Anal Chim Acta. 2015;885:166–73. doi: 10.1016/j.aca.2015.05.027. https://doi.org/10.1016/j.aca.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 68.Sun D, Lu J, Wang X, Zhang Y, Chen Z. Voltammetric aptamer based detection of HepG2 tumor cells by using an indium tin oxide electrode array and multifunctional nanoprobes. Microchim Acta. 2017:1–10. https://doi.org/10.1007/s00604-017-2376-z.
- 69.Santra S. Fluorescent silica nanoparticles for cancer imaging. Methods Mol Biol. 2010;624:151–62. doi: 10.1007/978-1-60761-609-2_10. https://doi.org/10.1007/978-1-60761-609-2_10. [DOI] [PubMed] [Google Scholar]
- 70.Hu Z, Tan J, Lai Z, Zheng R, Zhong J, Wang Y, Li X, Yang N, Li J, Yang W, Huang Y, Zhao Y, Lu X. Aptamer combined with fluorescent silica nanoparticles for detection of hepatoma cells. Nanoscale Res Lett. 2017;12:96. doi: 10.1186/s11671-017-1890-6. https://doi.org/10.1186/s11671-017-1890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lai Z, Tan J, Wan R, Tan J, Zhang Z, Hu Z, Li J, Yang W, Wang Y, Jiang Y, He J, Yang N, Lu X, et al. An “activatable” aptamer-based fluorescence probe for the detection of HepG2 cells. Oncol Rep. 2017;37:2688–2694. doi: 10.3892/or.2017.5527. https://doi.org/10.3892/or.2017.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Farokhzad OC, Karp JM, Langer R. Nanoparticle-aptamer bioconjugates for cancer targeting. Expert Opin Drug Deliv. 2006;3:311–24. doi: 10.1517/17425247.3.3.311. https://doi.org/10.1517/17425247.3.3.311. [DOI] [PubMed] [Google Scholar]
- 73.Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103:6315–20. doi: 10.1073/pnas.0601755103. https://doi.org/10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhattacharya SD, Mi Z, Kim VM, Guo H, Talbot LJ, Kuo PC. Osteopontin regulates epithelial mesenchymal transition-associated growth of hepatocellular cancer in a mouse xenograft model. Ann Surg. 2012;255:319–25. doi: 10.1097/SLA.0b013e31823e3a1c. https://doi.org/10.1097/SLA.0b013e31823e3a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mi Z, Guo H, Russell MB, Liu Y, Sullenger BA, Kuo PC. RNA Aptamer blockade of osteopontin inhibits growth and metastasis of MDA-MB231 breast cancer cells. Mol Ther J Am Soc Gene Ther. 2009;17:153–61. doi: 10.1038/mt.2008.235. https://doi.org/10.1038/mt.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trinh TL, Zhu G, Xiao X, Puszyk W, Sefah K, Wu Q, Tan W, Liu C. A synthetic aptamer-drug adduct for targeted liver cancer therapy. PloS One. 2015;10:e0136673. doi: 10.1371/journal.pone.0136673. https://doi.org/10.1371/journal.pone.0136673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meng L, Yang L, Zhao X, Zhang L, Zhu H, Liu C, Tan W. Targeted delivery of chemotherapy agents using a liver cancer-specific aptamer. PloS One. 2012;7:e33434. doi: 10.1371/journal.pone.0033434. https://doi.org/10.1371/journal.pone.0033434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scaggiante B, Farra R, Dapas B, Baj G, Pozzato G, Grassi M, Zanconati F, Grassi G. Aptamer targeting of the elongation factor 1A impairs hepatocarcinoma cells viability and potentiates bortezomib and idarubicin effects. Int J Pharm. 2016;506:268–79. doi: 10.1016/j.ijpharm.2016.04.031. https://doi.org/10.1016/j.ijpharm.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 79.Weigum S, McIvor E, Munoz C, Feng R, Cantu T, Walsh K, Betancourt T. Targeted therapy of hepatocellular carcinoma with aptamer-functionalized biodegradable nanoparticles. J Nanoparticle Res. 2016;18:341. https://doi.org/10.1007/s11051-016-3633-5. [Google Scholar]
- 80.Liu Y, Wu X, Gao Y, Zhang J, Zhang D, Gu S, Zhu G, Liu G, Li X. Aptamer-functionalized peptide H3CR5C as a novel nanovehicle for codelivery of fasudil and miRNA-195 targeting hepatocellular carcinoma. Int J Nanomedicine. 2016;11:3891–905. doi: 10.2147/IJN.S108128. https://doi.org/10.2147/IJN.S108128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiao S, Liu Z, Deng R, Li C, Fu S, Chen G, Zhang X, Ke F, Ke S, Yu X, Wang S, Zhong Z. Aptamer-mediated gene therapy enhanced antitumor activity against human hepatocellular carcinoma in vitro and in vivo. J Control Release. 2017;258:130–45. doi: 10.1016/j.jconrel.2017.05.017. https://doi.org/10.1016/j.jconrel.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 82.Mosafer J, Abnous K, Tafaghodi M, Mokhtarzadeh A, Ramezani M. In vitro and in vivo evaluation of anti-nucleolin-targeted magnetic PLGA nanoparticles loaded with doxorubicin as a theranostic agent for enhanced targeted cancer imaging and therapy. Eur J Pharm Biopharm. 2017;113:60–74. doi: 10.1016/j.ejpb.2016.12.009. https://doi.org/10.1016/j.ejpb.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 83.Lee YJ, Lee SW. Regression of hepatocarcinoma cells using RNA aptamer specific to alpha-fetoprotein. Biochem Biophys Res Commun. 2012;417:521–7. doi: 10.1016/j.bbrc.2011.11.153. https://doi.org/10.1016/j.bbrc.2011.11.153. [DOI] [PubMed] [Google Scholar]
- 84.Dong L, Tan Q, Ye W, Liu D, Chen H, Hu H, Wen D, Liu Y, Cao Y, Kang J, Fan J, Guo W, Wu W. Screening and identifying a novel ssDNA aptamer against alpha-fetoprotein using CE-SELEX. Sci Rep. 2015:5. doi: 10.1038/srep15552. https://doi.org/10.1038/srep15552. [DOI] [PMC free article] [PubMed]
- 85.Lei Y, Tang J, Shi H, Ye X, He X, Xu F, Yan L, Qiao Z, Wang K. Nature-inspired smart DNA nanodoctor for activatable in vivo cancer imaging and in situ drug release based on recognition-triggered assembly of split aptamer. Anal Chem. 2016;88:11699–706. doi: 10.1021/acs.analchem.6b03283. https://doi.org/10.1021/acs.analchem.6b03283. [DOI] [PubMed] [Google Scholar]
- 86.Pilapong C, Sitthichai S, Thongtem S, Thongtem T. Smart magnetic nanoparticle-aptamer probe for targeted imaging and treatment of hepatocellular carcinoma. Int J Pharm. 2014;473:469–74. doi: 10.1016/j.ijpharm.2014.07.036. https://doi.org/10.1016/j.ijpharm.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 87.Dassie JP, Liu XY, Thomas GS, Whitaker RM, Thiel KW, Stockdale KR, Meyerholz DK, McCaffrey AP, McNamara JO, Giangrande PH. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat Biotechnol. 2009;27:839–49. doi: 10.1038/nbt.1560. https://doi.org/10.1038/nbt.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meek KN, Rangel AE, Heemstra JM. Enhancing aptamer function and stability via in vitro selection using modified nucleic acids. Methods. 2016;106:29–36. doi: 10.1016/j.ymeth.2016.03.008. https://doi.org/10.1016/j.ymeth.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 89.Kratschmer C, Levy M. Effect of chemical modifications on aptamer stability in serum. Nucleic Acid Ther. 2017;27:335–344. doi: 10.1089/nat.2017.0680. https://doi.org/10.1089/nat.2017.0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.König J, Julius C, Baumann S, Homann M, Göringer HU, Feldbrügge M. Combining SELEX and the yeast three-hybrid system for in vivo selection and classification of RNA aptamers. RNA. 2007;13:614–22. doi: 10.1261/rna.334307. https://doi.org/10.1261/rna.334307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim JW, Kim EY, Kim SY, Byun SK, Lee D, Oh KJ, Kim WK, Han BS, Chi SW, Lee SC, Bae KH. Identification of DNA aptamers toward epithelial cell adhesion molecule via cell-SELEX. Mol Cells. 2014;37:742–6. doi: 10.14348/molcells.2014.0208. https://doi.org/10.14348/molcells.2014.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arnold S, Pampalakis G, Kantiotou K, Silva D, Cortez C, Missailidis S, Sotiropoulou G. One round of SELEX for the generation of DNA aptamers directed against KLK6. Biol Chem. 2012;393:343–53. doi: 10.1515/hsz-2011-0253. https://doi.org/10.1515/hsz-2011-0253. [DOI] [PubMed] [Google Scholar]
- 93.Chushak Y, Stone MO. In silico selection of RNA aptamers. Nucleic Acids Res. 2009;37:e87. doi: 10.1093/nar/gkp408. https://doi.org/10.1093/nar/gkp408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mendonsa SD, Bowser MT. In vitro evolution of functional DNA using capillary electrophoresis. J Am Chem Soc. 2004;126:20–1. doi: 10.1021/ja037832s. https://doi.org/10.1021/ja037832s. [DOI] [PubMed] [Google Scholar]
- 95.Duan Y, Gao Z, Wang L, Wang H, Zhang H, Li H. Selection and identification of chloramphenicol-specific DNA aptamers by Mag-SELEX. Appl Biochem Biotechnol. 2016;180:1644–56. doi: 10.1007/s12010-016-2193-6. https://doi.org/10.1007/s12010-016-2193-6. [DOI] [PubMed] [Google Scholar]
- 96.Hervas-Stubbs S, Soldevilla MM, Villanueva H, Mancheño U, Bendandi M, Pastor F. Identification of TIM3 2’-fluoro oligonucleotide aptamer by HT-SELEX for cancer immunotherapy. Oncotarget. 2016;7:4522–30. doi: 10.18632/oncotarget.6608. https://doi.org/10.18632/oncotarget.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zumrut HE, Ara MN, Fraile M, Maio G, Mallikaratchy P. Ligand-guided selection of target-specific aptamers: A screening technology for identifying specific aptamers against cell-surface proteins. Nucleic Acid Ther. 2016;26:190–8. doi: 10.1089/nat.2016.0611. https://doi.org/10.1089/nat.2016.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takahashi M, Sakota E, Nakamura Y. The efficient cell-SELEX strategy, Icell-SELEX, using isogenic cell lines for selection and counter-selection to generate RNA aptamers to cell surface proteins. Biochimie. 2016;131:77–84. doi: 10.1016/j.biochi.2016.09.018. https://doi.org/10.1016/j.biochi.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 99.Cho M, Soo Oh S, Nie J, Stewart R, Eisenstein M, Chambers J, Marth JD, Walker F, Thomson JA, Soh HT. Quantitative selection and parallel characterization of aptamers. Proc Natl Acad Sci U S A. 2013;110:18460–5. doi: 10.1073/pnas.1315866110. https://doi.org/10.1073/pnas.1315866110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ninomiya K, Kaneda K, Kawashima S, Miyachi Y, Ogino C, Shimizu N. Cell-SELEX based selection and characterization of DNA aptamer recognizing human hepatocarcinoma. Bioorg Med Chem Lett. 2013;23:1797–802. doi: 10.1016/j.bmcl.2013.01.040. https://doi.org/10.1016/j.bmcl.2013.01.040. [DOI] [PubMed] [Google Scholar]