Abstract
Objective
Sjögren’s Syndrome (SS) patients are prone to develop malignant lymphomas and a correlation has been established between lymphoproliferations and the presence in patients’ blood of an unusual B cell population that downregulated complement receptor 2/CD21. We sought to determine from which B cell compartment lymphoproliferations in SS patients emerge and what are the mechanisms that promote these clonal B cell expansions.
Methods
Using a PCR-based approach that allows us to clone and express, in vitro, recombinant antibodies produced by single B cells, we tested the reactivity of antibodies expressed by CD19+CD10−CD27−IgM+CD21−/low cells from SS patients.
Results
We identified clonal expansions in CD21−/low B cells isolated from the blood of three SS patients. All three lymphoproliferations expressed B cell receptors (BCRs) that displayed somatic hypermutation lineage trees characteristic of strong selection by antigens, one of which was identified as a ribosomal self-antigen. When mutated BCR sequences expressed by SS expanded clones were reverted, in vitro, to their germline counterparts, one remained autoreactive.
Conclusion
Clonal lymphoproliferations in SS patients preferentially accumulate in the autoreactive CD21−/low B cell compartment, which is often amplified in these subjects, and (self)-antigen recognition may drive expansion while further refining BCR (self)-reactivity.
Sjögren’s syndrome (SS) is an autoimmune disease characterized by lymphocytic infiltration of exocrine glands. The frequency of non-Hodgkin’s B cell lymphoma is 15–20 fold higher in SS patients than in the general population1. The appearance of lymphoma correlates with an increased proportion of circulating CD19+CD10−CD27−IgM+CD21−/low referred to henceforth as CD21−/low B cells, suggesting that these B cells may represent the initial reservoir for transformed clones2. In line with this hypothesis, increased numbers of circulating CD21−/low B cells are observed in patients with other autoimmune diseases including rheumatoid arthritis who are also prone to develop lymphomas, although at a lower frequency than in SS, further supporting a correlation between CD21−/low B cells and the emergence of transformed clones3. However, monoclonal expansions in the CD21−/low B cell compartment of SS patients have not yet been reported.
Here, we identified three SS patients who presented a monoclonal expansion in their CD21−/low B cells and two of these lymphoproliferations expressed autoreactive antibodies.
Methods
Patients
We recruited 8 patients with primary SS according to the American–European Consensus Group criteria4 (Table 1). All samples were collected after patients signed informed consent in accordance with protocols reviewed by the institutional review board.
Table 1.
Patients’ characteristics
| Name | SS01 | SS1003 | SS201 | SS202 | SS53 | SS03 | SS204 | SS59 |
|---|---|---|---|---|---|---|---|---|
| Gender | F | F | F | F | F | F | F | M |
| Age (yo) | 28 | 69 | 45 | 67 | 56 | 71 | 57 | 76 |
| PTPN22 genotype | CC | CC | CT | CT | CC | CC | CC | CC |
| Antinuclear | 1/1280 | + | + | + | + | + | + | 1/120, nuclear speckled |
| Anti-Ro/anti-La | Both | anti-Ro | − | anti-Ro | anti-Ro | Both | anti-Ro | − |
| Rheumatoid factor | + | + | − | − | − | + | + | − |
| Monoclonal Ig | + | + | − | − | NA | + | + | NA |
| Cryoglobulin | + | + | − | − | NA | + | + | NA |
| Lymphocytic infiltrate | Chisholm stade IV | Chisholm stade IV | Chisholm stade IV | Chisholm stade IV | Focus score per mm−1: 4.0; focal lymphocytic sialadenitis | Chisholm stade IV | Chisholm stade IV | Focus score per mm−1: 1.1; focal lymphocytic sialadenitis |
| Lymphoma | − | MALT lymphoma | MALT lymphoma | − | − | MZ Lymphoma | Lymphocytic lymphoma | − |
| Site of Lymphoma | Parotid gland | Orbit | Bone marrow | Lymph node | ||||
| Medication | steroids, HCQ | rituxan | rituxan | none | none | HCQ | rituxan | none |
F:Female; M:Male; yo: years old, CC: SS patient who did not carry the PTPN22 risk allele, CT: SS patient who carried one PTPN22 risk allele; NA: Not available, MALT: mucosa-associated lymphoid tissue, MZ: Marginal zone, HCQ: Hydroxychloroquine
Single-cell sorting
Mononuclear cells from healthy donors and SS patients were enriched for B cells by magnetic separation with CD20 microbeads (Miltenyi Biotech) and stained with anti-human CD19-Pacific Blue, anti-human CD27-PerCP Cy5.5, anti-human CD10-PE-Cy7, anti-human CD21-APC, anti-human IgM-FITC (Biolegend) prior to purification. Single CD19+CD21lowCD10+IgMhiCD27− new emigrant/transitional, CD19+CD21+CD10−IgM+CD27− mature naïve and CD19hiCD21−/lowCD10−CD27− referred to henceforth as CD21−/low B cells were sorted on a FACSAria (BD Biosciences) into 96-well PCR plates and immediately frozen on dry ice.
cDNA synthesis, Ig genes amplification, antibody production, and purification
RNA from single cells was reverse-transcribed in the original 96 well plate in 12.5μL reactions containing 100U of Superscript II RT (Gibco BRL) for 45 min at 42°C. RT-PCR reactions, primer sequences, cloning strategy, expression vectors, in vitro antibody production and purification were as described5.
ELISAs and IFAs
Antibody reactivity analysis was performed as described previously with the highly polyreactive ED38 antibody as positive control for HEp-2 reactivity and polyreactivity assays, whereas mnUNGdef05 λ50 and mnUNGdef05 κ69 previously cloned from single mature naïve B cells from UNG-deficient patient 5 were used as weak HEp-2-reactive and polyreactive control antibodies 5,6. Antibodies were considered polyreactive when they recognized all 3 distinct antigens: double-stranded (ds) DNA, insulin, and lipopolysaccharide (LPS). Ro52 and rheumatoid factor reactivity was also assessed by ELISA after coating plates with 1μg/mL Ro52/SSA (Sigma-Aldrich) or 1μg/mL rabbit IgG revealed by peroxidase-conjugated rabbit anti-human IgG (Jackson Immuno Research Laboratories) For indirect immunofluorescence assays, HEp-2 cell-coated slides (Bion Enterprises Ltd.) were incubated in a moist chamber at room temperature with purified recombinant antibodies at 50–100 μg/mL according to the manufacturer’s instructions.
Strategy to revert mutated antibodies to unmutated counterpart
Using a multi-template PCR strategy, germline VH and JH templates were first amplified with overlapping reverted CDR3 primers combined with primers annealing with either upstream germline VH genes or downstream JH segments. A second PCR reaction fused the 2 overlapping VH and JH PCR fragments generating germline revertant antibody sequences7.
Immunoprecipitation and end labeling of associated RNAs
Five micrograms of each monoclonal antibody was coupled to SureBeads protein G magnetic beads (Bio-Rad) for 30 minutes in PBS at room temperature with rotation. Beads were washed once in PBS pH 7.4 and then equilibrated in NET-2 lysis buffer [50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM MgCl2, 0.5% Nonidet P-40]. Cell lysates, prepared in NET-2 containing 1× complete protease inhibitors (Roche), 100 μM PMSF, 200 U/mL RNase OUT (Invitrogen), were added to the beads and incubated for 30 minutes. The beads were then washed three times in 750 μL of NET-2, transferred to new microcentrifuge tubes, and washed an additional three times. Beads were then resuspended in 350 μL NET-2, 2 μL glycogen, 5 μL 20% SDS, 40 μL NaOAc. The resuspended beads were then extracted twice with acid phenol:chloroform:isoamyl alcohol (25:24:1, v/v), and the nucleic acids precipitated from the aqueous phase with 2.5 volumes ethanol. T4 RNA ligase was used to end-label the precipitated RNA with [32P]pCp overnight at 4° C. Labeled RNAs were then resolved on a 5% polyacrylamide, 7 M urea gel. Gels were dried, exposed on Fujifilm imaging plates, and visualized with the Typhoon imager system (GE healthcare).
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version 5.0; GraphPad, San Diego, CA). Differences between groups of research subjects were analyzed for statistical significance with unparametric Mann Whitney tests. A p-value ≤ 0.05 was considered significant.
Results
Sjögren’s syndrome monoclonal expansions accumulate in CD21−/low B cells
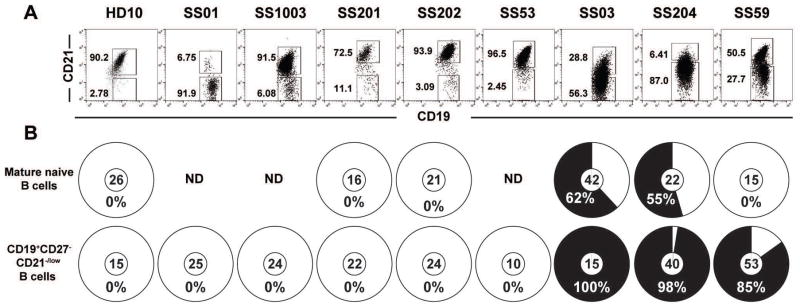
To determine the B cell compartment from which clonal lymphoproliferations originate or colonize in SS, we analyzed CD21−/low B cells and CD19+CD10−CD27−IgM+CD21+ mature naïve B cells to amplify their immunoglobulin genes from four SS patients in addition to four previously reported patients2,5 (Table 1). Patients SS01, SS201, SS03, SS204 and SS59 showed enhanced frequencies of peripheral CD21−/low B cells compared to only a few percent in healthy donors3 (Figure 1A). Immunoglobulin sequence analyses revealed monoclonal expansions in three of these patients; SS204 and SS59 in which clonal sequences accounted for 98% and 85% of isolated CD21−/low B cells, respectively, whereas all CD21−/low B cells from patient SS03 corresponded to an expanded clone (Figure 1B and Supplementary Tables 1–3). The frequencies of clonal expansions in mature naïve B cells from patients SS03 and SS204 were lower than in their CD21−/low B cells and not detected in CD19+CD10+CD27−IgMhiCD21−/low new emigrants/transitional B cells from patient SS03, thereby excluding a bone marrow origin for SS lymphoproliferation (Figure 1B and Supplementary Tables 4–6). The monoclonal expansion in patient SS59 who did not develop lymphoma was not identified in mature naïve B cells, suggesting that the clonal expansion may originate from CD21−/low B cells (Figure 1B and Supplementary Table 7).
Figure 1. SS monoclonal expansions accumulate in CD21−/low B cells.
(A) Dot plots represent CD19 and CD21 expression on CD19+CD27− gated B cells from a representative healthy donor (Tables S3–43) and SS patients. (B) The frequencies of monoclonal expansion (in black) in CD19+CD27−CD21−/low and mature naïve B cells are summarized in pie charts with the number of sequences analyzed indicated in the centers. ND, not done.
Sequences of monoclonal expansions reveal strong antigen-specific selection
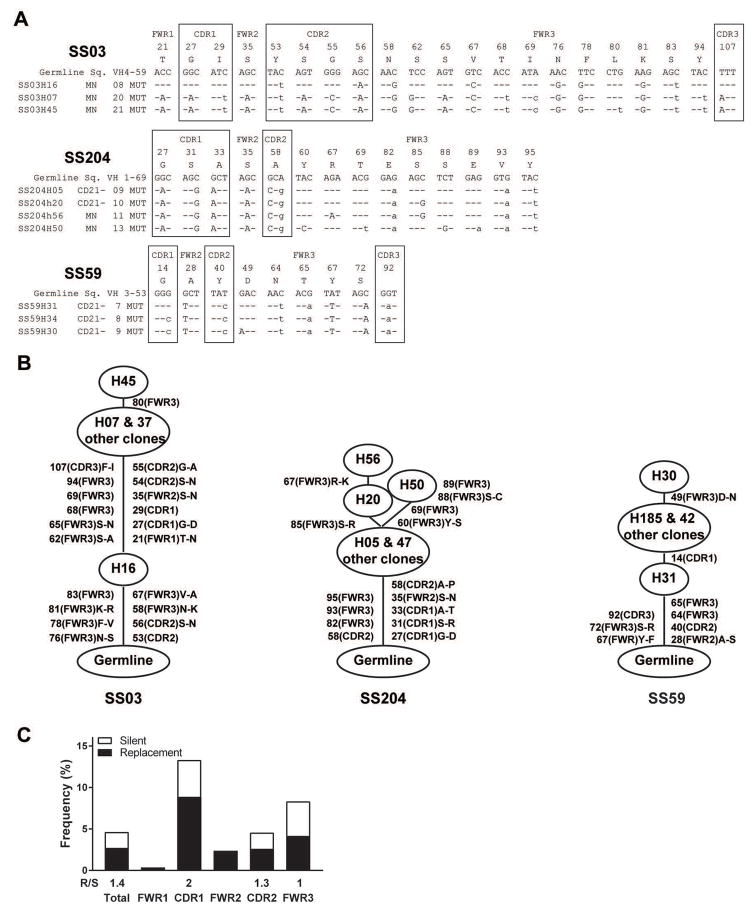
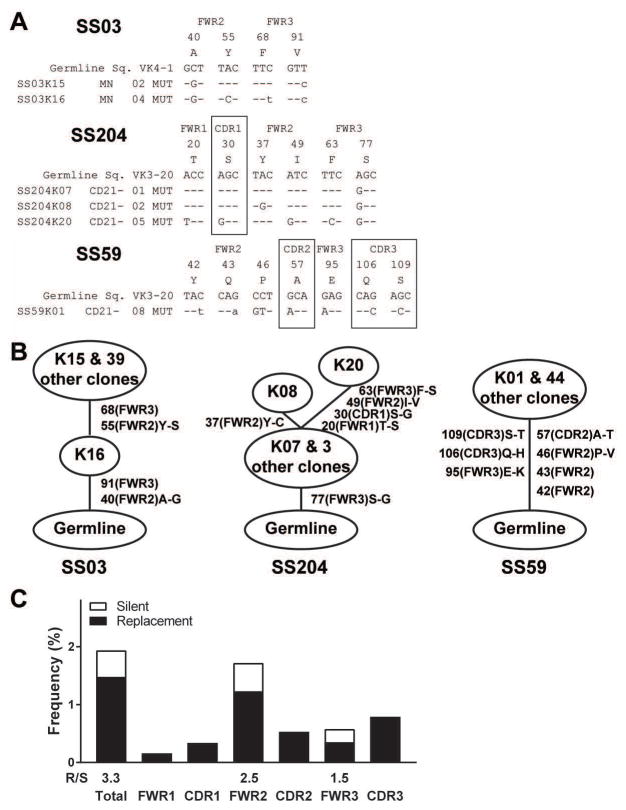
We then analyzed the characteristics of antibodies expressed by SS monoclonal expansions. The three expanded clones expressed antibodies with mutated heavy and light chain genes, revealing that they originate from antigen-stimulated B cells (Figure 2 and 3). The preferential accumulation of replacement mutations in the heavy chain complementarity determining region 2 (CDR2) and CDR1 of clonal expansion SS03 and SS204, respectively, further support antigenic selection pressure (Figure 2A and B). In addition, the identification of related heavy chain gene sequences with different numbers of mutations in the three clonal expansions also suggests ongoing antigenic selection. (Figure 2A and B). This was especially evident in the clonal expansion of patient SS03, in which clone H16 from the mature naïve B cell compartment only displayed 8 mutations in its heavy chain that were all shared by the related clones that contained 12 additional mutations (Figure 2A and B). Of note, the expanded clone from patient SS59 contained the lowest numbers of mutations in both heavy and light chain genes and correlated with a modestly expanded CD21−/low B cell population, suggesting that lymphoproliferation and expansion have begun more recently than for the other two SS clonal expansions (Figure 2A and B). Lineage tree analysis for heavy chains expressed by SS clonal expansions revealed linear and poorly branched mutation acquisition patterns, suggesting a strong and antigen-specific selection8 (Figure 2B). Kappa chains from expanded clones SS03 and SS204 also displayed intraclonal diversification with poorly branched lineage trees (Figure 3). Strong antigenic selection was further evidenced by elevated replacement (R) to silence (S) mutation ratios in complementary determining regions (CDRs) and sometimes framework regions (FWRs) of heavy and kappa chains expressed by SS lymphoproliferations (Figure 2C and 3C). We conclude that clonal expansions in Sjögren’s syndrome patients appear to be driven by strong antigenic stimulation.
Figure 2. Clonal expansions in SS patients are driven by antigenic stimulation.
(A) Sequence alignment for Ig heavy chains of the monoclonal expansions found in three SS patients. Replacements are in uppercase and silent mutations are in lowercase. Codon positions within framework regions (FWRs) and complementary determining regions (CDRs) are indicated. (B) Clonal trees of the monoclonal expansions found in the three SS patients are represented. Mutations and their codon position are written on the sides of branches, with affected regions in parenthesis. Amino acid changes are indicated. (C) Frequencies of mutations in VH genes from combined lymphoproliferations were calculated from the number of replacement and silent nucleotide exchanges per base pair in FWRs and CDRs. The replacement versus silent (R/S) mutation ratio for each region is indicated when the number of silent mutation is not zero.
Figure 3. Kappa chains from SS expanded clones also displayed intraclonal diversification with poorly branched lineage trees.
(A) Sequence alignment for Ig kappa chains of the monoclonal expansions found in three SS patients. Replacements are in uppercase and silent mutations are in lowercase. Codon positions within FWRs and CDRs are indicated. (B) Clonal trees of the monoclonal expansions found in the three SS patients are represented. Mutations and their codon position are written on the sides of branches, with affected regions in parenthesis. Amino acid changes are indicated. (C) Frequencies of mutations in Vκ genes from combined lymphoproliferations were calculated from the number of replacement and silent nucleotide exchanges per base pair in FWRs and CDRs. The replacement versus silent (R/S) mutation ratio for each region is indicated when the number of silent mutation is not zero.
SS monoclonal expansions may express autoreactive antibodies
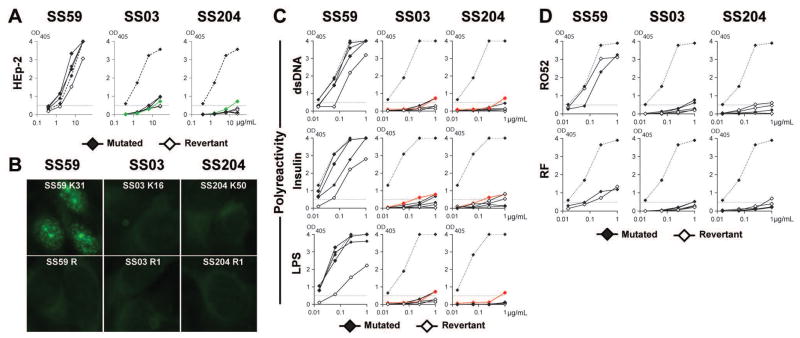
To determine the nature of the antigens that may drive lymphoproliferation, we tested the reactivity of the antibodies expressed by SS monoclonal expansions. Since CD21−/low B cells are enriched in autoreactive clones2, we assessed reactivity towards human HEp-2 cells and multispecificity/polyreactivity by ELISA5. We found that mutated recombinant antibodies cloned from two of the three SS lymphoproliferations, SS59 and SS03, were autoreactive (Figure 4A). SS59 clones were highly HEp-2 and polyreactive and recognized nuclear antigens (Figure 4A, B and C). Mutated antibodies from SS03 clonal expansion were weakly HEp-2 reactive and polyreactive, as validated by comparisons with mnUNGdef05 λ50, a low HEp-2 positive control and low polyreactive positive control antibodies, respectively6 (Figure 4A and C). SS204 antibodies were not HEp-2 reactive or polyreactive, although some weak reactivity below the low positive control was detected for double-stranded (ds) DNA and lipopolysaccharide (LPS) antigens (Figure 4A and C) Since Ro52/SSA is a major Sjögren’s self-antigen and anti-IgG rheumatoid factor reactivity has been suggested to be associated with lymphomas, we tested SS lymphoproliferations for these antigens9 (Figure 4D). Consistent with their polyreactivity, SS59 and, to a lower extent, SS03 clones displayed Ro52/SSA and rheumatoid factor reactivities, while SS204 clones did not bind these antigens (Figure 4D).
Figure 4. SS monoclonal expansions express autoreactive antibodies.
(A) Antibodies from mutated (full diamonds) and revertant (open diamonds) clones from SS patients were tested by ELISA for reactivity against HEp-2 cell lysate5. Dotted and green lines show ED38-positive and mnUNGdef05 λ50 low positive control6, respectively. Horizontal lines show cutoff OD405 for positive reactivity. (B) Recombinant mutated antibody from SS59 shows anti-nuclear reactivity. (C) Antibodies from mutated and revertant clones from SS patients were tested by ELISA for polyreactivity against dsDNA, insulin, and LPS antigens5. Dotted and red lines show ED38-positive and mnUNGdef05 κ69 low positive control6, respectively. (D) Ro52/SSA and rheumatoid factor (RF) reactivity was assessed by ELISA for mutated and revertant antibodies.
Because some prominent autoantigens in Sjögren’s syndrome patients, such as Ro60, La, Sm, and RNP, consist of proteins complexed with small noncoding RNAs10, we explored whether the self-antigens recognized by SS lymphoproliferations included these RNAs by performing immunoprecipitations on human keratinocytes with these recombinant antibodies and examining the RNAs present in immunoprecipitates. Although we did not detect the characteristic noncoding RNAs associated with these autoantigens, SS59 anti-nuclear mutated clones recognized an antigen associated with 5S and 5.8S rRNA, revealing that these antibodies may harbor anti-ribosome reactivity (Figure 5). Antibodies from SS03 and SS204 clones did not immunoprecipitate small RNAs (Figure 5). Altogether, two of the three lymphoproliferations identified in Sjögren’s syndrome patients express autoreactive antibodies that crossreact with Ro52/SSA and share rheumatoid factor reactivity and one of them recognizes a ribosomal antigen associated with 5S and 5.8S rRNA.
Figure 5. SS59 lymphoproliferation binds ribosomal components.
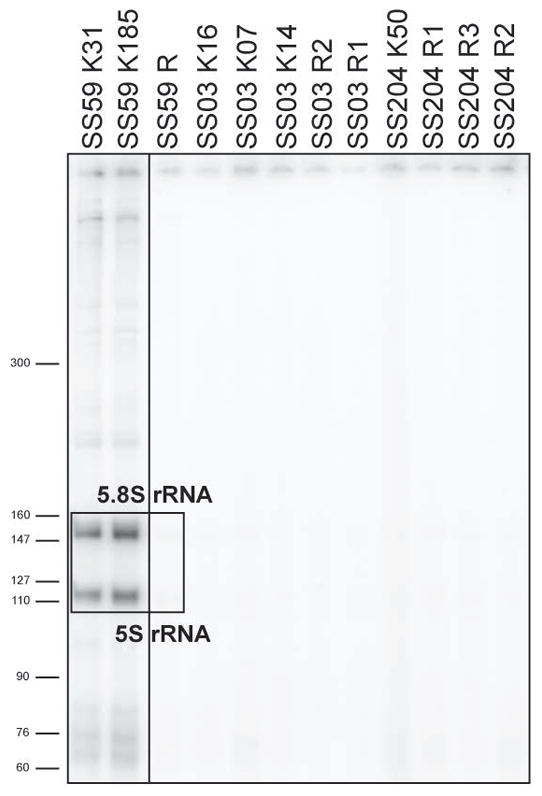
Antibodies from mutated and revertant clones from SS patients were used for immunoprecipitation from human keratinocyte lysates. RNAs within immunoprecipitates were end-labeled with [32P]pCp. The boxed region denotes the 5S and 5.8S ribosomal RNAs enriched after immunoprecipitation with recombinant mutated SS59 clones.
Lymphoproliferations from SS patients may originate from autoreactive B cells
To determine if autoreactive SS monoclonal expansions were derived from unmutated self-reactive clones that acquired somatic hypermutation (SHM), we reverted in vitro mutated antibody heavy- and light-chain genes to their original unmutated sequences (Supplementary Figure 1)7. Because Ig heavy chain CDR3s play an essential role in conferring antibody polyreactivity and potentially autoreactivity11, we designed primers to revert CDR3 sequences thereby considering conservative and sometimes more extended reversion scenarios for these antibodies referred to henceforth as revertants (Supplementary Figure 1). We then tested revertant reactivity by ELISAs and immunofluorescence assays and compared them to those of their mutated counterparts (Figure 4). The reverted antibody from SS59 monoclonal expansions retained HEp-2 reactivity, suggesting that this lymphoproliferation may originate from an intrinsically self-reactive B cell (Figure 4A). In line with this hypothesis, the SS59 revertant also remained polyreactive and retained Ro52/SSA and rheumatoid factor reactivity, although this unmutated antibody bound dsDNA, insulin and LPS with decreased affinity (Figure 4C and D). However, SHM was responsible for SS59 anti-nuclear reactivity because the SS59 revertant did not stain nuclear structures and did not enrich for 5S and 5.8S rRNA in immunoprecipitations (Figure 4B and 5). Revertants from SS03 and SS204 showed some weak reactivity against some tested antigens. SS03 revertants were borderline HEp-2 reactive but were not polyreactive and did not bind Ro52/SSA or IgG (Figure 4). Although SS204 revertants were not HEp-2 reactive, some of them displayed weak insulin, Ro52/SSA and rheumatoid factor reactivity (Figure 4). We conclude that B cell lymphoproliferations from Sjögren’s syndrome patients often express autoreactive antibodies and that they may originate from clones activated by self-antigens that promote their proliferation and the acquisition of SHM thereby enhancing BCR affinity for self.
Discussion
We showed that SS patients’ lymphoproliferations express autoreactive antibodies and accumulate in the CD21−low B cell compartment. The appearance of non-Hodgkin’s B cell lymphoma appears frequently in SS patients and has been reported to correlate with the proportion of CD21−/low B cells in their blood 2,12,13. In addition, patients with other autoimmune diseases including RA and SLE or chronic infections are also prone to develop lymphomas although at a lower frequency than in SS and display increased numbers of CD21−/low B cells in their blood, further supporting a correlation between CD21−/low B cells and the emergence of transformed clones 3,9,14.
By studying SS patients who presented at least 30% CD21−/low B cells in their CD19+CD27− peripheral B cell compartment, we identified three patients with monoclonal expansions in their blood. All three SS patients showed the highest frequency of expanded clones in CD21−/low B cells, suggesting that these monoclonal expansions may originate from this compartment. In line with this hypothesis, one lymphoproliferation was restricted to CD21−/low B cells and was not detected in other B cell compartments including new emigrant/transitional and mature naïve B cells. Of note, SS patients 1003 and 201 with MALT lymphoma only displayed a modest increase in CD21−/low B cells but they had been treated with rituxan that depletes these B cells and likely altered their blood frequency. Since CD21−/low B cells from SS and RA patients often expressed autoreactive antibodies, these cells may contain self-reactive precursors of these lymphoproliferations 2,3.
However, the detection of monoclonal expansions in mature naïve B cells of two SS patients may challenge this scenario and suggest that lymphoproliferations could emerge from this peripheral B cell compartment, which is also enriched in autoreactive clones15. In line with this hypothesis is the identification of a precursor clone (H16) in mature naïve B cells that displayed a heavy chain with only 8 mutations whereas all other related clones from mature naïve and CD21−/low B cells harbored 12 additional mutations. Hence, lymphoproliferative clones may originate from activated mature naïve B cells and then preferentially develop and accumulate in the CD21−/low B cell compartment. However, we cannot exclude that the detection of expanded clones in mature naïve B cells may be due to suboptimal gating strategies to distinguish CD21−/low B cells from CD21+ mature naïve B cells since all other cell surface markers to identify these two sub-populations are the same. Indeed, the overwhelming expansion of transformed clones expressing low levels of surface CD21 may contribute to their detection in CD21-expressing mature naïve B cells. Additional studies are therefore warranted to determine if B cell lymphomas in SS patients may originate from either mature naïve or CD21−/low B cells that are both enriched in autoreactive clones.
What mechanisms preside over the emergence of B cell lymphomas accumulating in the CD21−/low B cell compartment? Intrinsic and extrinsic B cell mechanisms likely contribute to the development of lymphomas in SS patients. Chronic BCR stimulation by self-antigens may favor the malignant transformation of CD21−/low B cells5,8. In agreement with this hypothesis, we found that expanded B cell clones in two out of three SS patients expressed mutated autoreactive BCRs, whereas antibodies expressed by expanded B cells from patient SS204 did not appear to be autoreactive. The poorly branched trees reflecting the linear SHM acquisition history for the lymphoproliferations identified in all three SS patients suggest a strong antigen-specific selection in which rare SHM events that increase BCR (self)-reactivity are highly selected8. The putative unmutated germline clones from which the three mutated SS B cell expansions originated displayed either decreased or no self-reactivity compared to their mutated counterparts, suggesting that SHM did increase self-reactivity in both lymphoproliferations that expressed autoreactive BCRs. BCR self-reactivity seems to be a common feature shared by many B cell lymphomas including chronic lymphocytic lymphomas (B-CLL) and diffuse large B cell lymphomas (DLBCL)7,16. In DLBCL, BCRs have been reported to recognize dying cells or DNA 16–18. BCR self-reactivity is critical for the survival and maintenance of transformed B cell clones because replacement by non-autoreactive BCRs induced clonal death16. BCR self-reactivity is also associated with the emergence of B-CLL, and clones that retain strong self-reactive BCR features and are associated with worse patient prognoses7. The recognition of nuclear antigens such as ribosomal structures by SS59 B cell expansions may facilitate B cell activation and proliferation because these self-antigens contain RNAs that provide BCR co-stimulatory signals through TLR7 binding 19. Similarly, rheumatoid factor reactivity previously identified in salivary gland lymphomas or inferred due to CDR3 homology may also support TLR7-driven activation of clonally expanded B cells through their BCRs binding IgG immune complexes containing small noncoding RNAs common in the serum of Sjögren’s syndrome patients20,21. TLR7/BCR co-ligation induces the expression of AXL in B cells that may favor the formation or recruitment of these activated B cells in tertiary lymphoid structures (TLS)22. Lymphomas in SS patients usually found in mucosal tissues may initially develop in a salivary gland from a cluster of proliferating B cells that seed TLS, which offer a favorable environment for B cell expansion by providing T cell help and B cell activation factor (BAFF)9,23,24. Indeed, BAFF is important for B cell survival and its concentration is elevated in the serum and salivary glands of SS patients25. Since CD21−/low B cells are dependent on BAFF for their survival, anti-BAFF therapy may be beneficial in SS patients by affecting the survival of proliferating B cells26. Alternatively and despite the heterogeneous efficacy of anti-B cell therapy in SS, anti-CD20 therapy may be considered since it will eliminate CD21−/low B cells in Sjögren’s syndrome patients, including potential pre-malignant clonal B cell expansions that highly express CD20.
Supplementary Material
Acknowledgments
This work was supported by NIH/NIAID grants AI-061093, AI-071087 and AI-082713 (to E. M.), by NIAID/NIAMS NIH grants U19AI082714 and P30AR053483 (to K.L.S. and J.A.J), by NIGMS NIH grant R01 GM073863 (to S.L.W.) and a Ruth L. Kirschstein National Service Award from the NIH (F32ES026227 to M.B.).
We thank Dr. Nancy Ruddle for her insightful comments on the manuscript and Dr. L. Devine and C. Wang for cell sorting.
Footnotes
The authors declare no competing financial interest.
Authorship Contributions
E.M. designed research. S.G., M.B., J.B and F.R.D. performed experiments. S.G., M.B., S.L.W. and E.M. analyzed and interpreted data. D.S., P.C., J.I. and J.J. provided blood samples and clinical data. S.G. and E.M. wrote the manuscript. All authors reviewed the manuscript and provided scientific input.
References
- 1.Ioannidis JPA, Vassiliou VA, Moutsopoulos HM. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjögren’s syndrome. Arthritis Rheum. 2002;46(3):741–747. doi: 10.1002/art.10221. [DOI] [PubMed] [Google Scholar]
- 2.Saadoun D, Terrier B, Bannock J, et al. Expansion of autoreactive unresponsive CD21-/low B cells in Sjögren’s syndrome-associated lymphoproliferation. Arthritis Rheum. 2013;65(4):1085–1096. doi: 10.1002/art.37828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isnardi I, Ng Y-S, Menard L, et al. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood. 2010;115(24):5026–5036. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wardemann H, Yurasov S, Schaefer A, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 6.Cantaert T, Schickel J-N, Bannock JM, et al. Decreased somatic hypermutation induces an impaired peripheral B cell tolerance checkpoint. J Clin Invest. 2016;126(11):4289–4302. doi: 10.1172/JCI84645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hervé M, Xu K, Ng Y-S, et al. Unmutated and mutated chronic lymphocytic leukemias derive from self-reactive B cell precursors despite expressing different antibody reactivity. J Clin Invest. 2005;115(6):1636–1643. doi: 10.1172/JCI24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.William J, Euler C, Primarolo N, Shlomchik MJ. B cell tolerance checkpoints that restrict pathways of antigen-driven differentiation. J Immunol Baltim Md 1950. 2006;176(4):2142–2151. doi: 10.4049/jimmunol.176.4.2142. [DOI] [PubMed] [Google Scholar]
- 9.Nocturne G, Virone A, Ng W-F, et al. Rheumatoid Factor and Disease Activity Are Independent Predictors of Lymphoma in Primary Sjögren’s Syndrome. Arthritis Rheumatol Hoboken NJ. 2016;68(4):977–985. doi: 10.1002/art.39518. [DOI] [PubMed] [Google Scholar]
- 10.Hardin JA, Rahn DR, Shen C, et al. Antibodies from patients with connective tissue diseases bind specific subsets of cellular RNA-protein particles. J Clin Invest. 1982;70(1):141–147. doi: 10.1172/JCI110587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichiyoshi Y, Casali P. Analysis of the structural correlates for antibody polyreactivity by multiple reassortments of chimeric human immunoglobulin heavy and light chain V segments. J Exp Med. 1994;180(3):885–895. doi: 10.1084/jem.180.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165(20):2337–2344. doi: 10.1001/archinte.165.20.2337. [DOI] [PubMed] [Google Scholar]
- 13.Theander E, Henriksson G, Ljungberg O, et al. Lymphoma and other malignancies in primary Sjögren’s syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis. 2006;65(6):796–803. doi: 10.1136/ard.2005.041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charles ED, Brunetti C, Marukian S, et al. Clonal B cells in patients with hepatitis C virus-associated mixed cryoglobulinemia contain an expanded anergic CD21low B-cell subset. Blood. 2011;117(20):5425–5437. doi: 10.1182/blood-2010-10-312942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glauzy S, Sng J, Bannock J, et al. Defective early B cell tolerance checkpoints in Sjögren’s Syndrome patients. Arthritis Rheumatol Hoboken NJ. 2017 doi: 10.1002/art.40215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young RM, Wu T, Schmitz R, et al. Survival of human lymphoma cells requires B-cell receptor engagement by self-antigens. Proc Natl Acad Sci U S A. 2015;112(44):13447–13454. doi: 10.1073/pnas.1514944112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catera R, Silverman GJ, Hatzi K, et al. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation. Mol Med Camb Mass. 2008;14(11–12):665–674. doi: 10.2119/2008-00102.Catera. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu CC, Catera R, Zhang L, et al. Many chronic lymphocytic leukemia antibodies recognize apoptotic cells with exposed nonmuscle myosin heavy chain IIA: implications for patient outcome and cell of origin. Blood. 2010;115(19):3907–3915. doi: 10.1182/blood-2009-09-244251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau CM, Broughton C, Tabor AS, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202(9):1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin T, Weber JC, Levallois H, et al. Salivary gland lymphomas in patients with Sjögren’s syndrome may frequently develop from rheumatoid factor B cells. Arthritis Rheum. 2000;43(4):908–916. doi: 10.1002/1529-0131(200004)43:4<908::AID-ANR24>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Bende RJ, Aarts WM, Riedl RG, et al. Among B cell non-Hodgkin’s lymphomas, MALT lymphomas express a unique antibody repertoire with frequent rheumatoid factor reactivity. J Exp Med. 2005;201(8):1229–1241. doi: 10.1084/jem.20050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nündel K, Green NM, Shaffer AL, et al. Cell-intrinsic expression of TLR9 in autoreactive B cells constrains BCR/TLR7-dependent responses. J Immunol Baltim Md 1950. 2015;194(6):2504–2512. doi: 10.4049/jimmunol.1402425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mariette X. Lymphomas complicating Sjögren’s syndrome and hepatitis C virus infection may share a common pathogenesis: chronic stimulation of rheumatoid factor B cells. Ann Rheum Dis. 2001;60(11):1007–1010. doi: 10.1136/ard.60.11.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjögren’s syndrome. J Clin Invest. 1998;102(5):938–946. doi: 10.1172/JCI3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren’s syndrome. J Clin Invest. 2002;109(1):59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobi AM, Huang W, Wang T, et al. The Effect of Prolonged Treatment with Belimumab on B cells in Human SLE. Arthritis Rheum. 2010;62(1):201–210. doi: 10.1002/art.27189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.