Abstract
Aflatoxin B1 shows potent hepatotoxic, carcinogenic, genotoxic, immunotoxic potential in humans and many species of animals. The aim of this study was to clarify the underlying mechanism of G0G1 phase and G2M phase arrest of cell cycle in the bursa of Fabricius in broilers exposed to dietary AFB1. 144 one-day-old healthy Cobb broilers were randomly divided into two groups and fed on control diet and 0.6 mg·Kg−1 AFB1 diet for 3 weeks. Histological observation showed that AFB1 induced the increase of nuclear debris and vacuoles in lymphoid follicle of BF. Results of flow cytometry studies showed that bursal cells arrested in G2M phase at 7 days of age and blocked in G0G1 phase at 14 and 21 days of age following exposure to AFB1. The qRT-PCR analysis indicated that cell cycle arrested in G2M phase via ATM-Chk2-cdc25-cyclin B/cdc2 pathway, and blocked in G0G1 phase through ATM-Chk2-cdc25-cyclin D/CDK6 pathway and ATM-Chk2-p21-cyclin D/CDK6 route. In a word, our results provided new insights that AFB1 diet induced G2M and G0G1 phase blockage of BF cells in different periods, and different pathways were activated in different arrested cell cycle phase.
Introduction
Aflatoxin B1 (AFB1), secondary metabolites generated by the fungi Aspergillus flavus and Aspergillus parasiticus, usually can contaminate agricultural products and threaten food safety1. AFB1 also presents potent hepatotoxic, carcinogenic, genotoxic, immunotoxic potential2,3 and other adverse effects in many species of animals, including rodents, fish, humans and non-human primates4. Immunosuppression is a major effect of AFB1, which is characterized by injuries of mucosal immunity, cellular immunity and humoral immunity. These injuries include alteration of organ morphology and immune organ weights, reduction of T or B lymphocytes number, inhibition of lymphocyte activity5,6, decrease of antibody production7, changes of T lymphocyte subsets of peripheral blood8,9, and increased sensitivity of poultry to bacterial10, viral and protozoan diseases11.
In order to clarify the mechanisms of AFB1-induced toxicity, many researches have been focused on the mechanism of cell cycle arrest in different cells. Previous studies have shown that AFB1 could induce G2M phase arrest in broiler’s jejunum in vivo12,13; S-phase accumulation in human bronchial epithelial cell in vitro14; G0G1 phase blockage in hepatocytes of rat in vivo15. Moreover, AFB1 can arrest immune cells particularly lymphocytes growth at G2M phase in vivo16. Further researches indicated that AFB1 can affect cell cycle through different signaling pathways. For example, AFB1-induced S-phase arrest might be mediated via inhibiting Wnt/β-catenin signaling route in HepG2 cells in vitro17, however, it may be triggered by activating ATM/ATR, Chk2, and p53 signaling pathways in human bronchial epithelial cells14. Yin et al.’s study indicated that AFB1 induced G2M phase arrest via ATM-Chk2-cdc25-cyclin B/cdc2 route in jejunum of broilers in vivo13. In rat models15, upregulation of miR-34a-5p led to cell cycle arrest at G0G1 phases via inhibiting cell cycle-related genes (CCND1, CCNE2 and MET) after exposed to AFB1. Although previous studies have shown that aflatoxin-contaminated corn induced G2M phase blockage in bursal cells18 and splenocytes8 of chickens, and G0G1 phase arrest in thymocytes18 of broilers, the molecular mechanisms and signaling pathways of cell cycle arrest of bursal cells have not been mentioned.
Bursa of Fabricius (BF), peculiar central lymphoid organ of birds, has major roles in establishment and maintenance of B cell compartment and humoral immunity19. In the present investigation, a broiler model was used to clarify the signaling pathway related molecular mechanisms involved in the cell cycle arrest of BF cells after dietary AFB1 treatement. We analyzed the histological lesions of BF, cell cycle phase distribution of bursal cells, mRNA expression levels of regulatory molecules involved in G0G1, S and G2M transitions, and the protein expression level of proliferating cell nuclear antigen (PCNA).
Results
Histopathological lesions of BF
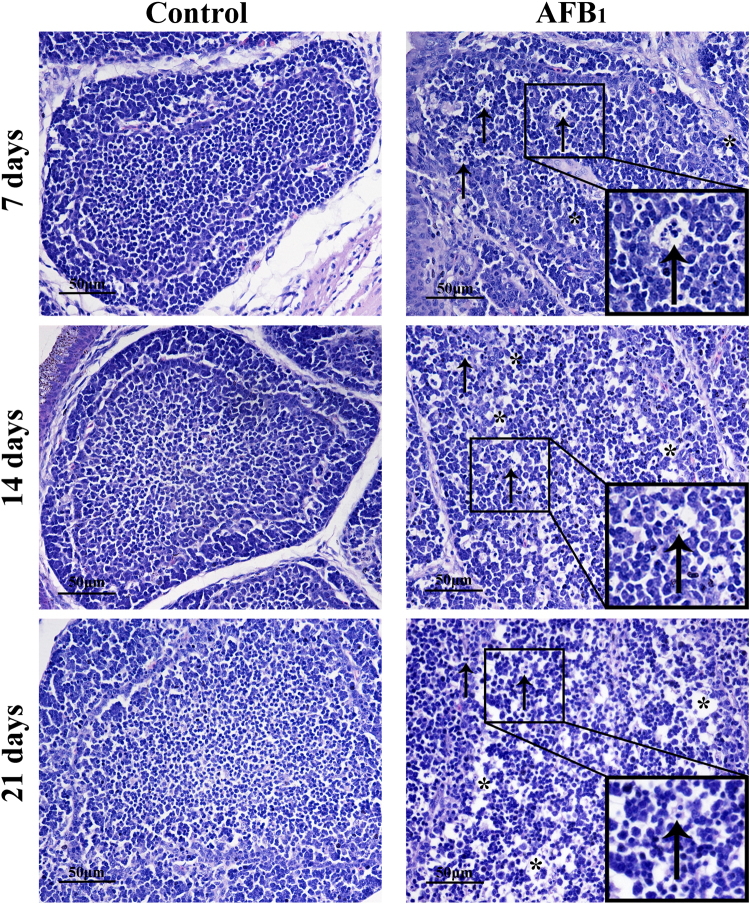
Histopathologically, there were much more nuclear debris and vacuoles in cortical and medullary areas of bursal follicles in the AFB1 group when compared with those of the control group at 7 and 14 days of age. At 21 days of age, the population of lymphocytes was decreased, and tissue cells in the medulla loosely arranged, but there were not so much nuclear debris as those at 7 days of age (see Fig. 1).
Figure 1.
The impact of AFB1 exposure on BF’s histopathological (nuclear debris and vacuoles). Histological assessment of H&E-stained BF tissues of broilers at 7, 14 and 21 days of age in the control group and AFB1 group. ↑Marks nuclear debris in BF, *increased number of vacuoles.
Cell cycle phase distribution of bursal cells
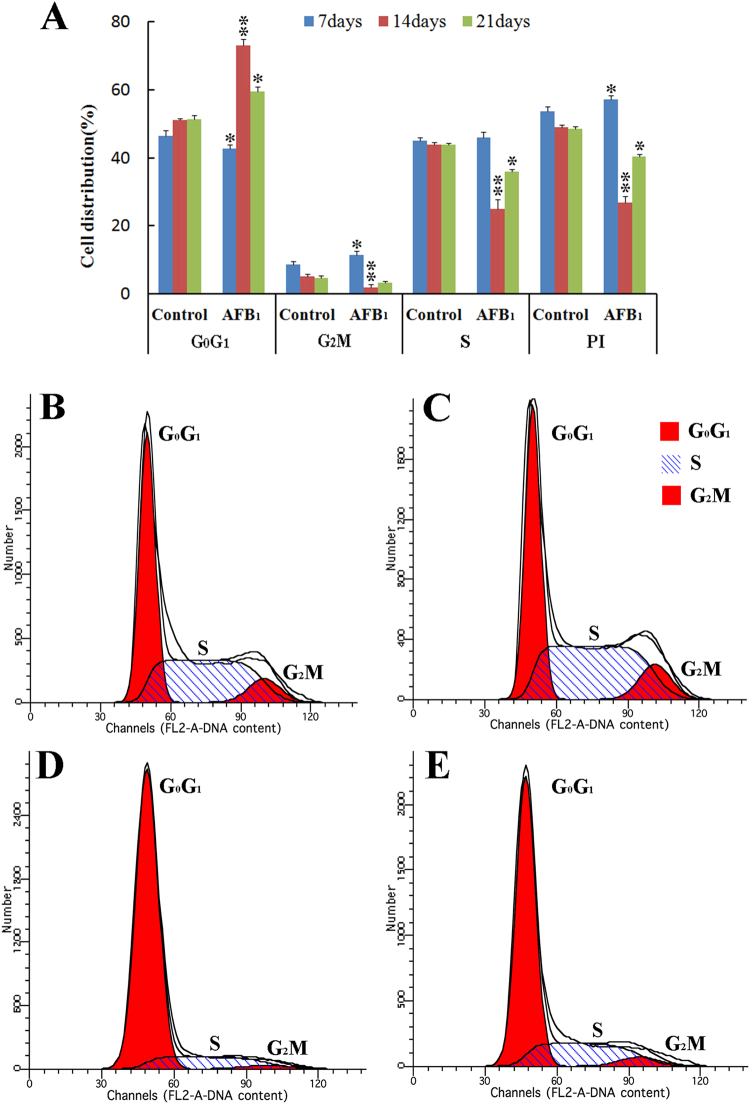
In the AFB1 group, the percentage of cells in G0G1 phase was lower than that of the control group at 7 days of age (p < 0.05), but was higher at 14 and 21 days of age (p < 0.05 or 0.01). The percentage of bursal cells in G2M phase was increased when compared with that of the control group at 7 days of age (p < 0.05), but was significantly decreased at 14 days of age (p < 0.01). In comparison to the control group, the percentage of bursal cells in S phase were decreased at 14 and 21 days of age (p < 0.05 or 0.01), but the change was not obvious at 7 days of age (p > 0.05). There were decreased tendency on PI values between the control group and AFB1 group at 14 and 21 days of age (p < 0.05 or 0.01), but the PI value was increased at 7 days of age (p > 0.05). These results showed that G2M phase was arrested at 7 days of age and G0G1 phase was blocked at 14 and 21 days of age in chicken’s BF. Histograms of cell cycle distribution by flow cytometer were shown in Fig. 2.
Figure 2.
Effect of AFB1 on cell cycle phase distribution of BF in chickens. Bar graph (A) shows the change of percentages of G0G1, S and G2M phase distribution of BF cells. Data are presented as means ± standard deviation (n = 6). Letters *mean p < 0.05 and **mean p < 0.01 between the AFB1 group and control group, respectively. Histograms by flow cytometry show that cell cycle arrest in G2M phase at 7 days of age (C) and blockage in G0G1 phase at 14 and 21 days of age (D and E) in the AFB1 group when compared with those of the control group (B).
Relative expressions of cell cycle-related genes
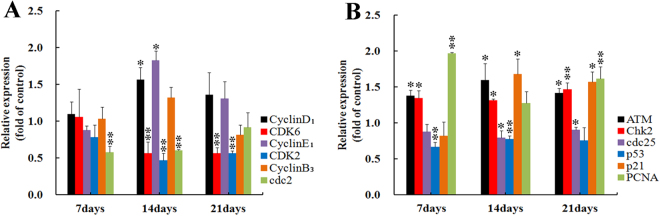
At 7 days of age, compared with the control group, the mRNA level of cdc2 was obviously decreased (p < 0.01), however, there were not significantly changes on the mRNA expressions of cyclin D1, cyclin E1, cyclin B3, CDK6 and CDK2 in the AFB1 group (p > 0.05). At 14 days of age, the mRNA contents of cyclin D1 and cyclin E1 were increased (p < 0.05), nevertheless, the expressions of CDK6, CDK2 and cdc2 were obviously decreased when compared with those of the control group (p < 0.01). In the AFB1 group, the mRNA expressions of CDK6 and CDK2 were obviously decreased when compared with those of the control group at 21 days of age (p < 0.01), but there were no significant changes for cyclin D1, cyclin E1, cyclin B3 and cdc2 (p > 0.05).
Compared with those of the control group, the mRNA levels of PCNA were significantly increased at 7 and 21 days of age (p < 0.01), the mRNA levels of p53 were obviously decreased at 7 and 14 days of age (p < 0.01). However, the expressions of p21 were increased at 14 and 21 days of age (p < 0.05), but there was no obvious change at 7 days of age. Furthermore, the mRNA expressions of ATM and Chk2 were higher at 7, 14 and 21 days of age than those in the control group (p < 0.05 or 0.01), and the mRNA levels of cdc25 were lower at 14 and 21 days of age (p > 0.05). The results were shown in Fig. 3 and Supplementary Fig. S1. The representatives of amplification curves of aforementioned 12 genes at 14 days of age were displayed in Supplementary Fig. S2.
Figure 3.
The relative expressions of mRNA in bursal cells from the broilers in the AFB1 group. Bar graph (A). The mRNA expressions of cyclin D1, CDK6, cyclin E1, CDK2, cyclin B3 and cdc2 in the bursal cells of the AFB1-fed broilers are expressed as fold change relative to the control-fed broilers. Bar graph (B). The mRNA levels of ATM, Chk2, cdc25, p53, p21 and PCNA in the bursal cells of the AFB1-fed broilers are expressed as fold change relative to the control-fed broilers. All data are expressed as means ± standard deviation. *p < 0.05, **p < 0.01 vs control, n = 6 for each group.
PCNA Expression
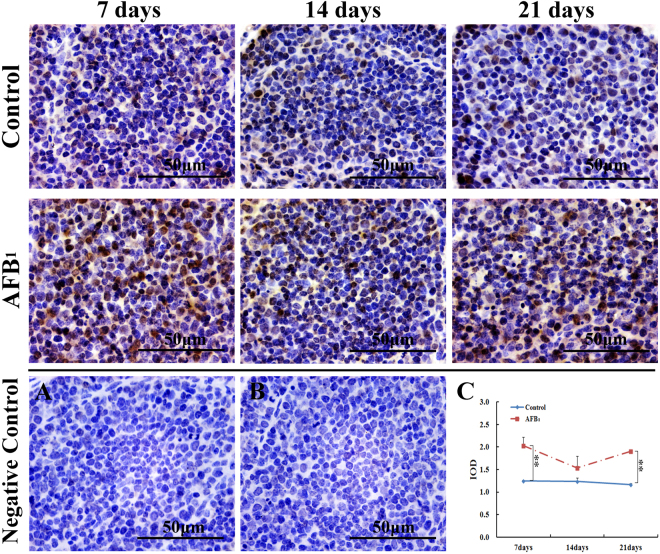
As shown in Fig. 4, the nuclei of PCNA-positive cells were stained with brown color by immunohistochemical method. Compared with the control group, the numbers of PCNA-positive cells were increased in the AFB1 group. According to Fig. 4C, the IOD of PCNA-positive cells were found to be significantly increased in the AFB1 group at 7 and 21 days of age.
Figure 4.
Protein expression of PCNA in bursal cells from the broilers in the control group and AFB1 group. Image (A and B) are representatives of the negative control staining in the control group and AFB1 group at 7 days of age, respectively. Line chart (C). shows the change of integrated optical density (IOD) of PCNA-positive cells by immunohistochemical method. All data are expressed as means ± standard deviation. **p < 0.01 vs control, n = 6 for each group.
Discussion
BF is primary central lymphoid organ of chicken20, which is related to diversification of B cells19. Histopathologically, consistent with our earlier studies8,18,21, obvious lymphocyte depletion, more vacuoles and debris were observed in the BF in the AFB1 group. It has been reported that chickens with poorly developed bursal follicles are expected to be very sensitive to bacterial22 and viral23 diseases, because lymphoid follicles of BF have a crucial roles in humoral immune reactions24. So these histological lesions could finally impaire the humoral immune function in chickens after exposed to AFB1.
Cell cycle is divided into different phases, including G0, G1, S, G2 and M phases. By FCM method, different phases of cell cycle are normally determined based on DNA content25. Many studies showed that AFB1 induce cell cycle arrest at different phases depending on the cell types, such as the accumulation of G2M phase cell in thymocytes26 and broiler’s jejunal epithelia13, the increase of S-phase cell population in human bronchial epithelial cells14, and the arrest at G0G1 phases in lymphocytes16 and liver cells27. Our previous study have shown that the percentage of bursal cells in the G2M phase was increased after treated with AFB-contaminated corn18. However, the present study showed that consumption of 0.6 mg·kg−1 AFB1 diet induced the arrest at G2M phase in bursal cells of broilers at 7 days of age and the blockage at G0G1 phases at 14 and 21 days of age. The results showed an interesting finding that the characteristics of cell cycle arrest could be changeable in the same kinds of cells with the increase of the AFB1 exposure time. Similar results have also been reported in splenocytes and thymocytes8,18. Why were the bursal cells arrested in different cell cycle phase at different period after exposed to AFB1? The transcription and translation of different cyclin genes may be related to the mechanisms of different cell cycle phase arrest. To further investigate the possible mechanism, the expressions of some cyclin genes were detected with qRT-PCR method.
Traditionally, as we know that the ATM-Chk2-cdc25 route and ATM-p21-p53-dependent pathway are the two classical pathways in cell cycle progression. Briefly, Ataxia telangiectasia mutated (ATM) is the primary kinase activated by DNA double-strand breaks (DSBs), which mediates the downstream signal cascade leading to cell cycle slowdown, DNA repair, and chromatin remodeling28. At the same time, in the process of DNA-damage, Chk2 is phosphorylated by phospho-ATM29, and then the phospho-Chk2 inhibits the activity of cdc2530,31. On the other hand, cyclin/CDK complexes are able to activate cdc25. More importantly, AFB1 can generally be metabolized by cytochrome P450 (CYP450) enzymes to generate active AFB1-8,9-epoxide (AFBO)32, which can react with DNA to form AFB1-FAPY adducts after forming AFB1-N7-Gua adducts4. The accumulation of AFB1-DNA adducts often induce DNA damage14. In the present study, the increase of ATM and Chk2 mRNA expressions, and the decrease of cdc25 mRNA level were observed at 7 days of age. Notably, the cyclin B3/cdc2 complexes were maintained at a relatively low level when compared with those of the control group. These results suggested that AFB1 activated ATM-Chk2-cdc25 pathway to inhibit cyclin B3/cdc2 expression in G2M phase. Similar results from other researchers were reported. Yin. et al.13 and Yang et al.14 showed that AFB1 induced accumulation of ATM and Chk2 in broiler’s jejunal epithelia and human bronchial epithelia in vivo and vitro, respectively.
p53, a tumor suppressor gene, has been shown to mediate cell cycle arrest, apoptosis and senescence in response to DNA damage33,34. p21 gene expression is generally under the transcriptional control of p53 protein35. However, the accumulated evidence indicates that p21-mediated cell cycle arrest can be either p53-dependent or p53-independent pathway36–38. Recent studies suggested that other unconventional cell cycle pathways are also involved in cell cycle progression. Namely, The full activation of Chk2 kinase can induce Chk2-dependent accumulation of p2139, and then induce cell cycle arrest at G1 phase through activating ATM-Chk2-p21 pathway in HaCaT cells in vitro40. In the current study, we found that the mRNA expressions of ATM, Chk2 and p21 were increased, and the expressions of p53 and cyclin D1/CDK6 complexes were decreased in the AFB1 group at 14 and 21 days of age. Interestingly, the expressions of cdc25 were also decreased when compared with that of the control group. These results indicated that AFB1 induce cell cycle arrest in G0G1 phase via two different routes at 14 and 21 days of age, namely ATM-Chk2-cdc25 pathway and ATM-Chk2-p21-p53-independent route.
p21 is a cyclin-dependent kinase inhibitor (CKI), which possesses the highest binding affinity among all PCNA-interacting partners41. Protracted p21 expression abrogated the property of PCNA to up-regulate CDKs and suppressed the PCNA mono-ubiquitination. Our results showed that AFB1 induced the increase of PCNA proteins and mRNA expressions. Because PCNA needs to be ubiquitinated for performing its biological function, these up-regulations of PCNA could not be certainly related to its promoted functions. On the other hand, p21 induces cell cycle arrest at G142 and G2M43 phases by inhibiting the activity of CDKs such as CDK6, CDK2 and cdc242,44. In this study, we found that the expressions of cyclin B3/cdc2 and cyclin D1/CDK6 complexs were decreased in the AFB1 group during the experiment. Therefore, maintaining a relatively low amounts of active cyclin/CDK complexes appears to be a major factor to regulate cell cycle progression45.
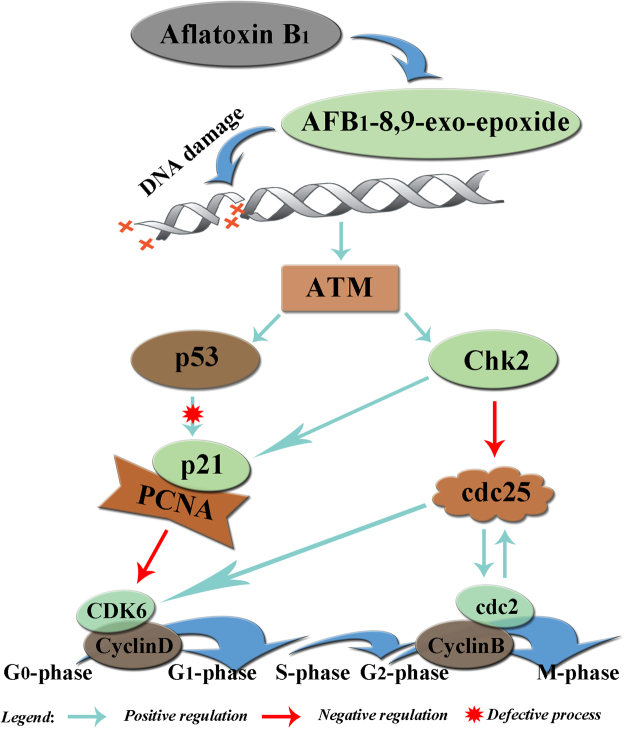
In summary, our results showed that 0.6 mg·kg−1 dietary AFB1 can induce histopathological lesions of bursa of Fabricius and cell cycle arrest in the bursal cells of broilers. Briefly, AFB1 induced G2M phase arrest via ATM-Chk2-cdc25-cyclin B/cdc2 pathway at 7 days of age, and G0G1 phase blockage through ATM-Chk2-cdc25-cyclin D/CDK6 pathway and ATM-Chk2-p21-cyclin D/CDK6 route at 14 and 21 days of age (see Fig. 5). Our results suggested that different mechanisms of G0G1 and G2M cell cycle might be involved in different stages of the development of BF, and could provide reference for deeper understanding of the mechanism of AFB1 induced immunosuppression for the same or similar studies in both human and other animals in the future.
Figure 5.
Schematic diagram of the proposed mechanisms of AFB1 induced cell cycle arrest in BF.
Materials and Methods
Animals and diets
One hundred and forty-four one-day-old healthy Cobb broilers from Chia Tai Group (Wenjiang, Sichuan, China), weighed 40 ± 5 g, were randomly divided into two groups, namely control group (0 mg·kg−1 AFB1) and AFB1 group (0.6 mg·kg−1 AFB1). Each group consisted of three replications with 24 birds per replication. The control group received corn-soybean basal diet, which was formulated according to the National Research Council (NRC, 1994)46 and the Chinese Feeding Standard of Chicken (NY/T33-2004) recommendations, and the AFB1 group was fed with AFB1 diet. The AFB1 diet was made according to the method described by Kaoud et al.47. Briefly, 27 mg AFB1 (A6636, Sigma-Aldrich, USA) farinose solid was dissolved into 30 ml methanol completely, and then the mixture was mixed into 45 kg basal diet to formulate the AFB1 diet. The equivalent methanol was mixed into the basal diet to produce control diet. Afterward, the methanol of diets was evaporated at 98 °F (37 °C). The AFB1 concentrations were analyzed by HPLC with fluorescence detection (Waters Model 2475), and the AFB1 concentrations were determined as <0.01 mg·kg−1 for the control diet and 0.601 mg·kg−1 for the AFB1 diet. Broilers were housed in cages with electrically heated units and provided with water as well as aforementioned diet ad libitum for 21 days. Previous studies indicated that deficient or a complete lack of a functional glutathione-S-transferase (GST) with affinity toward AFBO appears to be a major reason that poultry are extremely susceptible to AFB148. Additionally, 0.6 mg·kg−1 AFB1 in diet had obvious adverse effects on the immune organs of broilers, such as spleen49, thymus and BF50. Based on these information, experimental model (broiler) and toxin concentrations (0.6 mg·kg−1 AFB1) were chosen.
All study procedures followed the medical ethics according to national and international guidelines and has been approved by Sichuan Agricultural University Animal Care and Use Committee (Approval No: 2012-024).
Histopathological examination
At 7, 14, and 21 days of age during the experiment, six broiler chickens were randomly selected from each group, and euthanized. The BF were immediately removed and fixed in 4% paraformaldehyde (PFA), embedded in paraffin, and sectioned at 5 μm. Some sections were prepared for immunohistochemistry, and some were stained with hematoxylin and eosin Y (H.E) for light microscopic observations. Typical histological changes were photographed with a digital camera (Nikon DS-Ril, Japan).
Flow cytometry assay
At 7, 14 and 21 days of age during the experiment, six broilers in each group were sampled to determine the cell cycle phase distribution of BF cells by Flow Cytometry, with a similar method described by Chen et al.51. Single cell suspension of BF was harvested by dissecting each sample into small pieces and filtered through 300-mesh nylon gauze. Then, the cells were washed and suspended in phosphate buffered saline (PBS, pH 7.2~7.4) at a concentration of 1 × 106 cells/mL. A total of 1 mL cell suspension was transferred into 5 mL culture tube and centrifuged at 800 × g for 5 min. Afterward, the cell suspension was permeabilized with 500 µL 0.25% Tritonx-100 at 4 °C for 20 min and centrifuged at 800 × g for 5 min. The supernatant was discarded, and then 1.5 µL of staining solution (Propidium iodide, BD Pharmingen, USA, 51-66211E) were added and incubated for 30 min at 4 °C in a dark room. Finally 400 µL of PBS was added and the cell cycle phase distribution was assayed by flow cytometry (BD FACSCalibur) within 45 minutes and analyzed by Mod Fit LT software for Mac V3.0 computer program.
qRT-PCR
BF from six birds in each group was removed and immediately stored in liquid nitrogen at 7, 14, and 21 days of age. Then, these BF samples were homogenized by crushing with a pestle and mortar until powdery. The powdered tissues were collected into eppendorf tubes and stored at −80 °C. As previously described13, total RNA was extracted from the powdery using TriPure Isolation Reagent (Cat No. 11667165001, Roche Applied Science, Germany) following the manufacturer’s instruction. Next, 1 µg of total RNA was used for reverse transcription using Transcriptor First Strand cDNA Synthesis Kit (Cat No. 04897030001, Roche Applied Science, Germany). For qRT-PCR reactions, cDNA was amplified using Bestar® SybrGreen qPCR mastermix (Cat No. DBI-2043, DBI® Bioscience, Germany) according to the manufacturer’s instruction for 40 cycles on a Bio-Rad C1000 Thermal Cycler (Step One Plus, Applied Biosystems, USA). Chicken β-actin was used as an reference gene52. Sequences for target genes (Table 1) were obtained from GenBank of NCBI and synthesized by Sangon Biotech (Shanghai, China). qRT-PCR data were analyzed by using the 2−∆∆Ct calculation method53 and hierarchical cluster of gene expression data were analyzed by using HemI 1.0 software (Heatmap Illustrator, China).
Table 1.
Primer sequence for proliferation genes.
| Gene symbol | Accession number | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Product size |
|---|---|---|---|---|
| ATM | NM001162400.1 | TTGCCACACTCTTTCCATGT | CCCACTGCATATTCCTCCAT | 110bp |
| cdc25 | NM001199572.1 | AGCGAAGATGATGACGGATT | GCAGAGATGAAGAGCCAAAGA | 163bp |
| CDK2 | NM_001199857.1 | TCCGTATCTTCCGCACGTTG | GCTTGTTGGGATCGTAGTGC | 276bp |
| CDK6 | NM_001007892.2 | CCAGACCCGCACAACCTATT | TCTTGGCTGGATTGAACGCT | 96bp |
| cdc2 | NM205314.1 | TCTGCTCTGTATTCCACTCCTG | ATTGTTGGGTGTCCCTAAAGC | 144bp |
| Chk2 | NM001080107 | AGACCAAATCACTCGTGGAGAATAC | GATGCTCTAAGGCTTCCTCTATTGT | 140bp |
| cyclin D1 | NM_205381.1 | GACTTTTGTGGCTCTGTGCG | CTGTTCTTGGCAGGCTCGTA | 202bp |
| cyclin E1 | NM_001031358.1 | CGCCACCACAAAGCAGTAAG | TCACCGGCAGCATTTCCATA | 137bp |
| cyclin B3 | NM205239.2 | ATCACCAACGCTCACAAGAAC | AGGCTCCACAGGAACATCTG | 171bp |
| p21 | AF513031.1 | TCCCTGCCCTGTACTGTCTAA | GCGTGGGCTCTTCCTATACAT | 123bp |
| p53 | NM_205264.1 | TGGAACCATTGCTGGAACCC | AGTTGCTGTGATCCTCAGGG | 127bp |
| PCNA | AB053163.1 | GATGTTCCTCTCGTTGTGGAG | CAGTGCAGTTAAGAGCCTTCC | 104bp |
| β-actin | L08165 | TGCTGTGTTCCCATCTATCG | TTGGTGACAATACCGTGTTCA | 178bp |
Immunohistochemistry (IHC)
The immunohistochemistry technique used for PCNA was performed on 5 µm thickness sections according to the report by Yu et al.54. Briefly, the sections were deparaffinized and rehydrated. After washed three times with PBS (0.1 M, pH 7.2~7.4), the sections were treated with 3.0% hydrogen peroxide in PBS at room temperature for 10 min to quench the endogenous peroxidase activity. Following washed with PBS, the sections were exposed to normal goat sera for 30 min to block nonspecific antibody binding. In a humidified chamber, the sections were incubated the rabbit anti-PCNA polyclonal antibody (bs-0754R, Bioss, Beijing, China) for 20 h at 4 °C (working dilution: 1:100). After three successive washings in PBS, secondary antibody biotinylated goat anti-rabbit IgG and streptavidin-biotin complex (SA1020, Boster, Wuhan, China) were, in turn, applied onto sections for 1 h and 30 min at 37 °C, respectively. Slides were visualized with diaminobenzidine hydrochloride (AR1022, Boster, Wuhan, China) under the microscope and stopped by immersion in distilled water, as soon as brown staining was visible. Finally, the sections were lightly counterstained with hematoxylin and placed in absolute ethylalcohol and xylene for 3 min following coverslipped. Negative controls were performed in the same way, except that PBS was used as a substitute for the primary antibody.
The stained sections were photographed using a digital camera at ×1000 magnification. For each section, six randomly selected fields were used for analyzing integrated optical density (IOD) using Image Pro Plus 5.0 software (USA)54.
Statistical Analysis
Statistical analyses were performed using SPSS 18.0 (SPSS Inc, Chicago, IL, USA). The experimental data were expressed as mean ± standard deviation ( ± SD) and independent sample test followed by post hoc t test was applied to determine the level of significance. Statistical significance was considered at p < 0.05 and markedly significant was considered at p < 0.01.
Electronic supplementary material
Acknowledgements
This work was supported by the program for Changjiang scholars and university innovative research team (IRT 0848) and the Science and Technology Department of Sichuan Province (2012FZ0066) and (2013FZ0072).
Author Contributions
P.H., H.L. and F.Y.W. carried out the majority of this work (experiment design, sample collection, statistical analysis and manuscript modification); Z.C.Z., X.P. and J.F. assisted in manuscript preparation and modification; H.M.C., C.X.G., H.T.S., Y.Z. and Z.L.C. directed research; P.H. wrote the manuscript and all the authors provided comments and feedback on the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Ping Hu, Zhicai Zuo, Hang Li and Fengyuan Wang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20164-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xi Peng, Email: pengxi197313@163.com.
Jing Fang, Email: fangjing4109@163.com.
References
- 1.Trucksess MW, Stack ME, Nesheim S, Albert RH, Romer TR. Multifunctional column coupled with liquid chromatography for determination of aflatoxins B1, B2, G1, and G2 in corn, almonds, brazil nuts, peanuts, and pistachio nuts: collaborative study. Journal of Aoac International. 1994;77:1512–1521. [PubMed] [Google Scholar]
- 2.Humans IWGotEoCRt. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. Iarc Monographs on the Evaluation of Carcinogenic Risks to Humans. 2002;82:1–556. [PMC free article] [PubMed] [Google Scholar]
- 3.Yunus AW, Razzazi-Fazeli E, Bohm J. Aflatoxin B1 in Affecting Broiler’s Performance, Immunity, and Gastrointestinal Tract: A Review of History and Contemporary Issues. Toxins. 2011;3:566–590. doi: 10.3390/toxins3060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JS, Groopman JD. DNA damage by mycotoxins. Mutation Research/fundamental & Molecular Mechanisms of Mutagenesis. 1999;424:167. doi: 10.1016/S0027-5107(99)00017-2. [DOI] [PubMed] [Google Scholar]
- 5.Cooper MD, Raymond DA, Peterson RD, South MA, Good RA. The Functions of the Thymus System and The Bursa System in The Chicken. Journal of Experimental Medicine. 2006;176:6370–6404. [PubMed] [Google Scholar]
- 6.Stewart RG, et al. The effect of aflatoxin on complement activity in broiler chickens. Poultry Science. 1985;64:616–619. doi: 10.3382/ps.0640616. [DOI] [PubMed] [Google Scholar]
- 7.Giambrone JJ, Ewert DL, Wyatt RD, Eidson CS. Effect of aflatoxin on the humoral and cell-mediated immune systems of the chicken. American Journal of Veterinary Research. 1978;39:305–308. [PubMed] [Google Scholar]
- 8.Peng X, et al. Histological Lesions, Cell Cycle Arrest, Apoptosis and T Cell Subsets Changes of Spleen in Chicken Fed Aflatoxin-contaminated Corn. International Journal of Environmental Research & Public Health. 2014;11:8567–8580. doi: 10.3390/ijerph110808567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabourin PJ, et al. Evaluation of Acute Immunotoxicity of Aerosolized Aflatoxin B1 in Female C57BL/6N Mice. Journal of Immunotoxicology. 2008;3:11–20. doi: 10.1080/15476910500468635. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Horn N, Cotter PF, Applegate TJ. Growth, serum biochemistry, complement activity, and liver gene expression responses of Pekin ducklings to graded levels of cultured aflatoxin B1. Poultry Science. 2014;93:2028–2036. doi: 10.3382/ps.2014-03904. [DOI] [PubMed] [Google Scholar]
- 11.Taranu I, et al. Mycotoxin Fumonisin B1 Alters the Cytokine Profile and Decreases the Vaccinal Antibody Titer in Pigs. Toxicological Sciences. 2005;84:301–307. doi: 10.1093/toxsci/kfi086. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, et al. Effects of aflatoxin B1 exposure and sodium selenite supplementation on the histology, cell proliferation, and cell cycle of jejunum in broilers. Biological Trace Element Research. 2014;160:32–40. doi: 10.1007/s12011-014-0009-5. [DOI] [PubMed] [Google Scholar]
- 13.Yin H, et al. The molecular mechanism of G2M cell cycle arrest induced by AFB1 in the jejunum. Oncotarget. 2016;7:35592–35606. doi: 10.18632/oncotarget.9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, et al. Cytochrome P450 2A13 enhances the sensitivity of human bronchial epithelial cells to aflatoxin B1-induced DNA damage. Toxicology & Applied Pharmacology. 2013;270:114–121. doi: 10.1016/j.taap.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Liu C, et al. Upregulation of miR-34a-5p antagonizes AFB1-induced genotoxicity in F344 rat liver. Toxicon Official Journal of the International Society on Toxinology. 2015;106:46. doi: 10.1016/j.toxicon.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 16.Bahari A, Mehrzad J, Mahmoudi M, Bassami MR, Dehghani H. Cytochrome P450 isoforms are differently up-regulated in aflatoxin B 1 -exposed human lymphocytes and monocytes. Immunopharmacology and Immunotoxicology. 2014;36:1–10. doi: 10.3109/08923973.2013.850506. [DOI] [PubMed] [Google Scholar]
- 17.Zhu L, et al. miR-34a screened by miRNA profiling negatively regulates Wnt/β-catenin signaling pathway in Aflatoxin B1 induced hepatotoxicity. Scientific Reports. 2015;5:16732. doi: 10.1038/srep16732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng X, Bai S, Ding X, Zhang K. Pathological Impairment, Cell Cycle Arrest and Apoptosis of Thymus and Bursa of Fabricius Induced by Aflatoxin-Contaminated Corn in Broilers. International Journal of Environmental Research & Public Health. 2017;14:77. doi: 10.3390/ijerph14010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potworowski EFT, B lymphocytes. Organ and age distribution in the chicken. Immunology. 1972;23:199–204. [PMC free article] [PubMed] [Google Scholar]
- 20.Firth, G. A. The normal lymphatic system of the domestic fowl. Veterinary Bulletin (1977).
- 21.Yuan, S. et al. The mitochondrial and endoplasmic reticulum pathways involved in the apoptosis of bursa of Fabricius cells in broilers exposed to dietary aflatoxin B1. Oncotarget (2014). [DOI] [PMC free article] [PubMed]
- 22.Pier AC, Heddleston KL. The Effect of Aflatoxin on Immunity in Turkeys. I. Impairment of Actively Acquired Resistance to Bacterial Challenge. Avian Diseases. 1970;14:797. doi: 10.2307/1588651. [DOI] [PubMed] [Google Scholar]
- 23.Oguz H, Hadimli HH, Kurtoglu V, Erganis O. Evaluation of humoral immunity of broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Revue De Médecine Vétérinaire. 2003;154:483–486. [Google Scholar]
- 24.Sur E, Celİk İ. Effects of aflatoxin B1 on the development of the bursa of Fabricius and blood lymphocyte acid phosphatase of the chicken. British Poultry Science. 2003;44:558. doi: 10.1080/00071660310001618352. [DOI] [PubMed] [Google Scholar]
- 25.Pozarowski P, Darzynkiewicz Z. Analysis of cell cycle by flow cytometry. Methods in Molecular Biology. 2004;281:301. doi: 10.1385/1-59259-811-0:301. [DOI] [PubMed] [Google Scholar]
- 26.Scott TR, Rowland SM, Rodgers RS, Bodine AB. Genetic selection for aflatoxin B 1 resistance influences chicken T-cell and thymocyte proliferation. Developmental & Comparative Immunology. 1991;15:383. doi: 10.1016/0145-305X(91)90030-3. [DOI] [PubMed] [Google Scholar]
- 27.Wang SK, Liu S, Yang LG, Shi RF, Sun GJ. Effect of fumonisin B1 on the cell cycle of normal human liver cells. Molecular Medicine Reports. 2013;7:1970–1976. doi: 10.3892/mmr.2013.1447. [DOI] [PubMed] [Google Scholar]
- 28.Mannuss A, Trapp O, Puchta H. Gene regulation in response to DNA damage. Anadolu kardiyoloji dergisi: AKD = the Anatolian journal of cardiology. 2012;3:313. [Google Scholar]
- 29.Matsuoka S, et al. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10389. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–429. doi: 10.1016/S1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 31.Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nature Reviews Molecular Cell Biology. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- 32.Wang SL. Efficient activation of aflatoxin B1 by cytochrome P450 2A13, an enzyme predominantly expressed in human respiratory tract. International Journal of Cancer. 2006;118:2665–2671. doi: 10.1002/ijc.21665. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Simpson ER, Brown KA. p53: Protection against Tumor Growth beyond Effects on Cell Cycle and Apoptosis. Cancer Research. 2015;75:5001–5007. doi: 10.1158/0008-5472.CAN-15-0563. [DOI] [PubMed] [Google Scholar]
- 34.Jiang L, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki H, Ito R, Ikeda K, Tamura TA. TATA-binding Protein (TBP)-like Protein Is Required for p53-dependent Transcriptional Activation of Upstream Promoter of p21Waf1/Cip1 Gene. Journal of Biological Chemistry. 2012;287:19792. doi: 10.1074/jbc.M112.369629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotoku, N. et al. Xylarianaphthol-1, a novel dinaphthofuran derivative, activates p21 promoter in a p53-independent manner. Bioorganic & Medicinal Chemistry Letters24, 3389–3391 (2014). [DOI] [PubMed]
- 37.Van Vleet TR, Watterson TL, Klein PJ, C. R., Jr. Aflatoxin B1 alters the expression of p53 in cytochrome P450-expressing human lung cells. Toxicological Sciences An Official Journal of the Society of Toxicology. 2006;89:399–407. doi: 10.1093/toxsci/kfj039. [DOI] [PubMed] [Google Scholar]
- 38.Yang, H., Chung, D. H., Kim, Y. B., Choi, Y. H. & Moon, Y. Ribotoxic mycotoxin deoxynivalenol induces G2/M cell cycle arrest via p21Cip/WAF1 mRNA stabilization in human epithelial cells. 243, 145–154 (2008). [DOI] [PubMed]
- 39.Buscemi G, et al. Activation of ATM and Chk2 kinases in relation to the amount of DNA strand breaks. Oncogene. 2004;23:7691. doi: 10.1038/sj.onc.1207986. [DOI] [PubMed] [Google Scholar]
- 40.Huang CY, et al. Extremely Low-Frequency Electromagnetic Fields Cause G1 Phase Arrest through the Activation of the ATM-Chk2-p21 Pathway. Plos One. 2014;9:e104732. doi: 10.1371/journal.pone.0104732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petrakis TG, et al. Exploring and exploiting the systemic effects of deregulated replication licensing. Seminars in Cancer Biology s. 2015;37–38:3–15. doi: 10.1016/j.semcancer.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Valesky EM, et al. Dimethylfumarate effectively inhibits lymphangiogenesis via p21 induction and G1 cell cycle arrest. Experimental Dermatology. 2015;25:200–205. doi: 10.1111/exd.12907. [DOI] [PubMed] [Google Scholar]
- 43.Bunz F, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 44.Quaas M, Müller GA, Engeland K. p53 can repress transcription of cell cycle genes through a p21WAF1/CIP1-dependent switch from MMB to DREAM protein complex binding at CHR promoter elements. Cell Cycle. 2012;11:4661. doi: 10.4161/cc.22917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smits VA, et al. p21 inhibits Thr161 phosphorylation of Cdc2 to enforce the G2 DNA damage checkpoint. Journal of Biological Chemistry. 2000;275:30638. doi: 10.1074/jbc.M005437200. [DOI] [PubMed] [Google Scholar]
- 46.Dale N. National Research Council Nutrient Requirements of Poultry -Ninth Revised Edition (1994) Journal of Applied Poultry Research. 1994;3:101–101. doi: 10.1093/japr/3.1.101. [DOI] [Google Scholar]
- 47.Kaoud, H. Innovative methods for the amelioration of aflatoxin (afb1) effect In broiler chicks. Sjar Net 1 (2013).
- 48.Rawal S, Kim JE, R. C., Jr. Aflatoxin B1 in poultry: toxicology, metabolism and prevention. Research in Veterinary Science. 2010;89:325–331. doi: 10.1016/j.rvsc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, et al. Effects of aflatoxin B1 on oxidative stress markers and apoptosis of spleens in broilers. Toxicology & Industrial Health. 2016;32:278. doi: 10.1177/0748233713500819. [DOI] [PubMed] [Google Scholar]
- 50.Peng X, et al. Aflatoxin B1 affects apoptosis and expression of Bax, Bcl-2, and Caspase-3 in thymus and bursa of fabricius in broiler chickens. Environmental Toxicology. 2016;31:1113. doi: 10.1002/tox.22120. [DOI] [PubMed] [Google Scholar]
- 51.Chen T, et al. Cell-cycle blockage associated with increased apoptotic cells in the thymus of chickens fed on diets high in fluorine. Human & Experimental Toxicology. 2011;30:685. doi: 10.1177/0960327110379022. [DOI] [PubMed] [Google Scholar]
- 52.Kost TA, Theodorakis N, Hughes SH. The nucleotide sequence of the chick cytoplasmic beta-actin gene. Nucleic Acids Research. 1984;11:8287–8301. doi: 10.1093/nar/11.23.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 54.Yu Z, et al. Effect of Selenium Supplementation on Apoptosis and Cell Cycle Blockage of Renal Cells in Broilers Fed a Diet Containing Aflatoxin B 1. Biological Trace Element Research. 2015;5:242. doi: 10.1007/s12011-015-0344-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.