Abstract
Recent breakthroughs in organ-on-a-chip and related technologies have highlighted the extraordinary potential for microfluidics to not only make lasting impacts in the understanding of biological systems but also to create new and important in vitro culture platforms. Adipose tissue (fat), in particular, is one that should be amenable to microfluidic mimics of its microenvironment. While the tissue was traditionally considered important only for energy storage, it is now understood to be an integral part of the endocrine system that secretes hormones and responds to various stimuli. As such, adipocyte function is central to the understanding of pathological conditions such as obesity, diabetes, and metabolic syndrome. Despite the importance of the tissue, only recently have significant strides been made in studying dynamic function of adipocytes or adipose tissues on microfluidic devices. In this critical review, we highlight new developments in the special class of microfluidic systems aimed at culture and interrogation of adipose tissue, a subfield of microfluidics that we contend is only in its infancy. We close by reflecting on these studies as we forecast a promising future, where microfluidic technologies should be capable of mimicking the adipose tissue microenvironment and provide novel insights into its physiological roles in the normal and diseased states.
Keywords: Microfluidics microfabrication, Adipocyte, Adipokine, Batokine, Hormone, Bioanalytical methods
Introduction
The global epidemic of obesity has brought increasing attention to research for understanding the biology of adipocytes (fat cells). Adipose tissue or fat tissue, which accounts for 5% to 50% of human body weight, was traditionally thought to be a passive reservoir for long-term energy storage, but now it is known to be a complex, essential, and highly active metabolic and endocrine organ with meaningful roles in the integration of systemic metabolism [1]. There are two major types of mammalian adipose tissues, white adipose tissue (WAT) and brown adipose tissue (BAT) [2], although intermediate (e.g., “brite” or “beige” cells) and locale-specific subtypes (visceral, omental, etc..) do exist.
WAT is the main type of adipose tissue in the adult human and is mainly found in subcutaneous regions and visceral depots. WAT consists of adipocytes (the main cellular component of WAT that are crucial for both energy storage and endocrine activity), precursor cells, fibroblasts, vascular cells, macrophages, smooth muscle, and immune cells. WAT is a critical regulator of system energy homeostasis and acts both for energy storage and as an endocrine organ. Under the appropriate hormone levels (e.g., high insulin), extra energy obtained from food or from the bloodstream, including glucose and fatty acids, is absorbed by adipocytes and transformed into triacylglycerols during lipogenesis for long-term storage. Upon energy deficiency or during other hormonal signaling events (e.g., low insulin, high glucagon), triacylglycerols are hydrolyzed (lipolysis), and the non-esterified fatty acid (NEFA) and glycerol components are released into circulation for delivery to other organs. The processes of glucose and fatty acid uptake, lipogenesis, and lipolysis are precisely controlled by a complex regulatory network, a topic which is reviewed elsewhere [3, 4].
WAT also secretes numerous hormones that regulate the metabolic functions of nearly all other organs; these hormones are referred to as “adipokines.” A detailed list of key adipokines and their known sources and functions are summarized in Table 1. More importantly, under the condition of obesity, WAT not only accumulates and expands throughout the body but also recruits immune cells that infiltrate into adipose tissue and change the adipokine secretion profile. Although this process is not fully understood, it is related to systemic inflammation and insulin resistance [20].
Table 1.
List of important adipokines in WAT*
| Adipokine | Function | Regulation in the obese | Year | Ref. |
|---|---|---|---|---|
| TNF-α | Inflammation, antagonism of insulin signaling | Upregulated | 1993 | [5] |
| Leptin | Appetite control through central nervous system | Upregulated | 1994 | [6] |
| Adiponectin | Insulin sensitizer, anti-inflammatory action | Downregulated | 1995 | [7] |
| IL-6 | Variable with origin and targeted tissues | Upregulated | 1998 | [8] |
| RBP4 | Role in systemic insulin resistance | Upregulated | 1999 | [9] |
| Resistin | Promotes insulin resistance and inflammation through macrophages | Upregulated | 2001 | [10] |
| IL-18 | General inflammation | Upregulated | 2005 | [11] |
| CCL2 | Monocyte recruitment | Upregulated | 2006 | [12] |
| Lipocalin 2 | Promotes insulin resistance and inflammation through adipocytes | Upregulated | 2007 | [13] |
| Nampt | Monocyte chemotactic activity | Upregulated | 2007 | [14] |
| ANGPTL2 | Local and vascular inflammation | Upregulated | 2009 | [15] |
| CXCL5 | Antagonism of insulin signaling through the JAK–STAT pathway | Upregulated | 2009 | [16] |
| SFRP5 | Suppression of pro-inflammatory WNT signaling | Downregulated | 2010 | [17] |
| Asprosin | induces hepatic glucose production | Upregulated | 2016 | [18] |
TNF-α tumor necrosis factor alpha, IL-6 interleukin 6, IL-18 interleukin 18, RBP4 retinol binding protein 4, CCL2 CC-chemokine ligand 2, Nampt nicotinamide phosphoribosyltransferase (also called pre-B-cell colony-enhancing factor 1, PBEF1, or visfatin), ANGPTL2 angiopoietin Like Protein 2, CXCL5 signifies one of the cytokine-like proteins with a highly conserved ELR motif preceding the N-terminal cysteine, SFRP5 secreted frizzled-related protein 5
List modified from Ouchi et al. [19]
Distinctly differing from WAT, the BAT derives its signature brown color from the high number of mitochondria, and the tissue mainly participates in thermogenesis. BAT helps maintain normal body temperature in newborn infants and hibernating mammals, and while its percentage of total body mass decreases with age, it is still present in the normal adult human [21]. This tissue is also known to have systemic effects by secreting regulatory molecules, referred to as batokines [22], and the specific batokine profile is distinct from adipokines. Batokines with autocrine, paracrine, and endocrine effects are listed in Table 2.
Table 2.
List of important batokines in BAT
| Batokine | Functions or Effects | Year | Ref. |
|---|---|---|---|
| NGF | Sympathetic innervation | 1994 | [23] |
| IL-6 | Increased thermogenesis, improved insulin sensitivity | 1997 | [24] |
| VEGFA | Increased vascularization of BAT | 1999 | [25] |
| BMP8B | Increased thermogenesis, increased sympathetic activation of BAT | 2012 | [26] |
| FGF21 | Increased thermogenesis, improved insulin sensitivity, WAT “browning,” cardioprotective | 2012 | [27–29] |
| L-PGDS | Increased thermogenesis | 2012 | [30] |
| IGF-1 | Insulin mimicking effects, regulates cell growth and development | 2012 | [31] |
| IGFBP-2 | Increased bone formation | 2013 | [32] |
| Metrnl | Increased IL-4 expression, macrophage activation | 2014 | [33] |
| NRG4 | Decreased hepatic lipogenic signaling, increased energy expenditure | 2014 | [34] |
| sLR11 | Decreased thermogenesis | 2015 | [35] |
| Endothelin-1 | Increased thermogenesis | 2016 | [36] |
| Slit2-C | Improved glucose homeostasis, increased energy expenditure | 2016 | [37] |
| PM20D1 | Improved glucose homeostasis, in-creased energy expenditure | 2016 | [38] |
NGF nerve growth factor, VEGFA vascular endothelial growth factor A, BMP8B Bone morphogenic protein 8b, FGF21 fibroblast growth factor 21, L-PGDS Lipocalin-type prostaglandin D synthase, IGF-1 insulin-like growth factor 1, IGFBP-2 insulin-like growth factor binding protein 2, Metrnl meteorin-like, NRG4 neuregulin 4, sLR11 soluble form of the low-density lipoprotein receptor relative, Slit2-C slit homolog 2 protein, C-fragment, PM20D1 peptidase M20 domain containing 1
Although our understanding of adipose tissue biology has greatly improved over the last few decades, the level of established detail generally lags behind that of other endocrine systems. Consequently, researchers continue to actively experiment on the tissue and its constituent cells in attempts to further elucidate its functions and mechanisms, to better understand the pathogenesis of obesity-related disorders, and to develop therapeutic strategies for these disorders. This heightened interest has catalyzed a demand for higher performance bioanalytical methodology, and several groups—including our own—have recently shown microfluidics to offer unique opportunities for studies on adipose tissue [39–52]. Microfluidic analysis systems offer a host of well-established benefits, including decreases in experiment cost and reagent volume, increased temporal and spatial resolution in cellular function assays, and in vivo mimics of vasculature and micro-environments within channels and chambers on the devices [53]. However, when applying microfluidics to studying adipose tissue, unique challenges such as cell buoyancy give rise to specialized countermeasures that arguably position such devices into their own subcategory. In this review, we highlight recent developments in the special class of bioanalytical microfluidic devices designed to study the dynamics of adipocytes and adipose tissue toward a better understanding of the biology related to obesity, diabetes, and metabolic disorders.
Microfluidic platforms to study adipocytes
In-vitro cell models integrated onto microfluidic devices
Historically, most adipocyte-related studies—including adipocyte differentiation, lipogenesis, adipokine secretion, and regulation—have been carried out with immortalized cell lines, such as mouse 3T3-L1 [54], 3T3-F442A [55], or human Simpson-Golabi-Behmel syndrome (SGBS) cells [56]. Differentiation of these cells into adipocytes can be achieved in vitro by treatment with adipogenic stimuli, including cAMP agonists, insulin, and glucocorticoids [57]. These cell lines share many similarities with primary adipocytes such as fat storage in lipid droplets, insulin sensitivity, expression of adipocyte-specific genes, and adipokine secretion [2]. However, there are some important differences. The primary white adipocyte from a host mammal contains a single large lipid droplet, but the cell lines exhibit clustering of many smaller droplets. Also, the cell line’s adipokine secretome differs from that of primary adipocytes; for example, leptin expression is at much lower levels in 3T3-L1 and 3T3-F442A cells compared with primary tissue [58].
Recently, the development of tissue engineering technology has allowed assembly of functional tissues with multiple cells and IGF13D extracellular matrix materials to recapitulate many of the key features of both normal and pathological human organs in vitro. Researchers have successfully constructed those organ-on-a-chip models for the study of liver, kidney, intestine, lung, heart, smooth and striated muscle, fat, bone, marrow, cornea, skin, blood vessels, nerve, and blood-brain barrier over the past decade [59]. Various types of engineered adipose tissues were fabricated by incorporation of adipose cell lines into extracellular matrix materials including collagen [41, 60], silk fibroin [61], sponge-like porous polyurethane [62], decellularized extracellular matrices [35], and hyaluronic acid-based hydrogels [36]. These novel in vitro models showed better simulation of functions of in vivo adipose tissue than cell lines cultured with traditional methods. Engineered adipose tissues were also used as a simplified model to address several challenges in organ-on-a-chip engineering. For example, the importance of the “right size” of the tissue for maintaining in vivo basal metabolic rates was studied by measurement of insulin-induced glucose uptake by collagen confined adipose spheroids with various sizes [41]. Transport processes by diffusion through porous membranes within multilayer microfluidic devices, providing shear stress protection to cells or tissues cultured on-chip, were also visualized by fluorescently labeled fatty acid analog uptake by on-chip differentiated 3T3-L1 adipocytes [40]. The Meier group used a microfluidic large-scale integration (mLSI) culture platform with integrated assays to study important factors in stem cell differentiation into adipoocytes [63]. However, since this review is focused on micro-analytical systems that study the dynamics of adipose tissue metabolism, hormone secretion, and other functions, approaches using such bottom-up adipocyte-on-a-chip engineering, which has been reviewed elsewhere [64], is only covered where appropriate.
Integrating primary tissue or pre-differentiated adipocytes onto microfluidic systems
While the cell lines and engineered adipose tissue remain as valuable in vitro models, much of the adipocyte’s in vivo function can only be recapitulated through primary cultures. Primary adipose tissues can be isolated from lab animals and cultured according to well established protocols [42]. The adipose tissue pad or explant can be taken directly from a donor and directly integrated onto microfluidic devices, or it is often beneficial to digest the matrix tissue within the explant and isolate constituent primary adipocytes, to avoid the interference from other cell components.
Owing to the inherent positive buoyancy of the lipid droplets already present in primary tissue or pre-differentiated adipocytes, dispersed cells or tissue explants must be properly trapped or anchored within microfluidic devices. This approach prevents floating of the cells in aqueous media, allows controlled fluidic access to and from the tissue, and avoids possible clogging of microfluidic channels. This buoyancy issue has been addressed in several ways by our research group recently, including trapping cells with metallic mesh [39], 3D-printed accessories [44] (Fig. 1-C), and collagen matrix casting [45]. The issue has been addressed by others using a culture chamber with a micro-post array [52], integrating cell-laden coverslips onto multilayer devices [46, 47], or by other methods as shown in Table 3. As shown in the table, within the past several years, researchers have devised a number of trapping methods that can be chosen by those interested in using microfluidics to study adipose tissue dynamics.
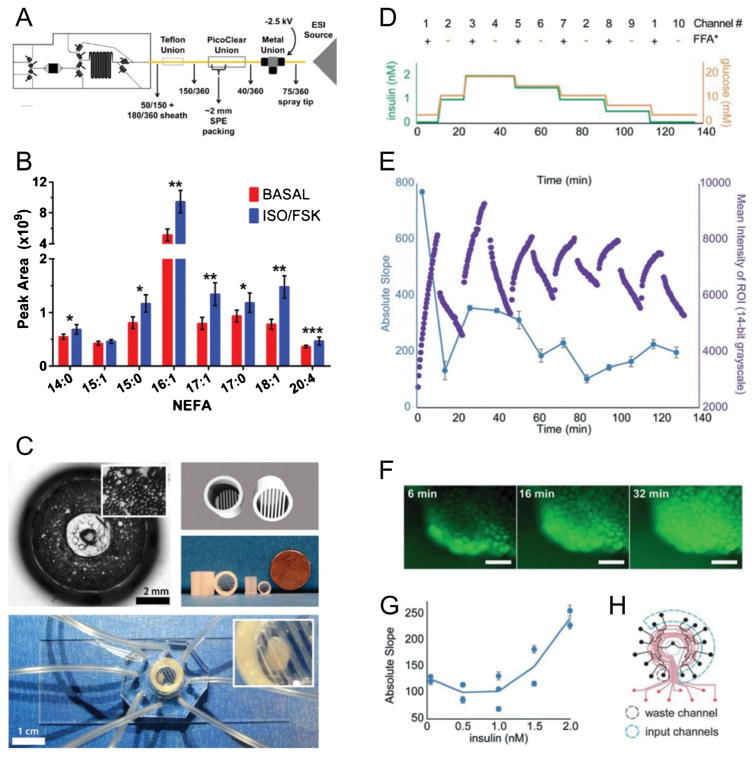
Fig. 1.
Recent examples of microfluidic systems for studying dynamic function of adipocytes and adipose tissue. (A) Schematic of microfluidic device for 3T3-L1 adipocyte stimulation and sampling, coupled to solid phase extraction and mass spectrometry (SPE-MS), for monitoring non-esterified fatty acid (NEFA) secretion profiles from adipocytes [49]. (B) Average NEFA secretion profiles during basal and isoproterenol/forskolin stimulation of adipocytes using the chip in (A) for stimulation and sampling. (C) On-chip culture systems for primary adipose tissue [42]. Primary mouse adipocytes were cultured in a 3D collagen matrix (top left) on an 8-channel microfluidic sampling device. Alternatively, explants were trapped using customized traps. 3D CAD rendering (top right) is shown with 3D-printed explant traps (middle right). (D) Temporal program of combined insulin, glucose, and fatty acid inputs to adipose explants using a microfluidic multiplexer (μMUX) device [39]. (E) Real-time fatty acid uptake responses from treatments in (D) showed insulin-dependent exchange rates. (F) Representative fluorescent images of on-chip explants used to generate data in (E). (G) Compiled data from (E) showed insulin-dependent fatty acid exchange dynamics. (H) Assignment map of the programmable, automated μMUX device, showing the 10 required input channels and one output waste channel used for (D)–(G). Reproduced from references 39, 42, and 49 with permission from the Royal Society of Chemistry and from Springer Publishing Company
Table 3.
Recent approaches for integrating adipose tissue or pre-differentiated cells onto microfluidic devices for dynamic functional studies
| Entry | Cell type | Microfluidic device details | Cell trapping mechanism | Analytical method(s) | Temporal resolution | Notes | Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 3T3-L1 adipocytes | etched glass substrate, reversibly sealed 2-layer design | cover slip with differentiated cells sealed into on-chip chamber | fluorescence on-chip; coupled enzyme assay for glycerol | 1.5 min | First example of temporally-resolved, microfluidic sampling of adipocytes; on-chip, continuous flow enzyme assays quantified glycerol secretion. | 2009 | [46] |
| 2 | 3T3-L1 adipocytes | etched glass substrate and PTFE sheet, reversibly sealed 3-layer design | cover slip with differentiated cells sealed into on-chip chamber | fluorescence on-chip; coupled enzyme assay for NEFA | 1.4 min | First example of temporally-resolved, microfluidic sampling of NEFA secretion from adipocytes. | 2010 | [47] |
| 3 | 3T3-L1 adipocytes (spheroids) | PDMS substrate | trapped in collagen gel (20 μL) in microdevice outlet reservoir | fluorescence off-chip; coupled enzyme assay for glucose | 25 min(single treatment) | First microfluidic system evaluating insulin-dependent glucose uptake in adipocytes; first study on the impor- tance of tissue scaling in organs-on--a-chip. | 2013 | [41] |
| 4 | 3T3-L1 adipocytes | PDMS substrate, reversibly sealed 3-layer design, on-chip valves | cover slip with differentiated cells sealed into on-chip chamber | fluorescence on-chip; coupled enzyme assays for both NEFA and glycerol | 2.3 min | First example of multiplexed assays for quantifying lipolysis products secreted by adipocytes on-chip; first example of on-chip valving for adipocyte perfusion. | 2014 | [48] |
| 5 | primary mouse adipocytes (dispersed) | PDMS substrate, customized primary tissue culture reservoir | collagen | ELISA | 60 min(single treatment) | First example of adipokine secretion by adipocytes on microfluidic devices. | 2015 | [45] |
| 6 | primary human adipose tissue (explants) | PDMS substrate, on-chip valves | capped tissue culture chamber above a micropost array | glucose meter | ~2 min(not explicitly stated) | First comparison of adipose tissue glucose uptake by diabetic and nondiabetic human adipose tissue. | 2015 | [52] |
| 7 | primary mouse adipose tissue (explants) | PDMS substrate, custom 3D printer templated culture reservoir | 3D-printed explant trap accessory | fluorescence off-chip; coupled enzyme assay for glycerol | 10 min | First example of temporally-resolved microfluidic sampling of primary murine WAT explants; first report of 3D printer templated culture reser- voir and explant traps. | 2016 | [44] |
| 8 | primary brown adipocytes | etched glass and silicon substrates, on-chip silicon resonators | single cells trapped in microchannels | silicon resonator based thermal measurement | real-time(sub-second capable) | First example of microfluidic interrogation of brown adipocytes; first measurement of heat generation from single adipocytes. | 2016 | [51] |
| 9 | 3T3-L1 adipocytes | PDMS substrate, reversibly sealed 3-layer design, on-chip valves | cover slip with differentiated cells sealed into on-chip chamber | solid-phase extraction, mass spectrometry (SPE-MS) | 30 min | First integration of microfluidics and mass spectrometry for detection of NEFA profile secreted by adipocytes. | 2017 | [49] |
| 10 | primary mouse adipose tissue (explants) | PDMS substrate, on-chip valves, custom 3D printer templated culture reservoir | platinum mesh explant trap | fluorescence imaging of adipocytes on-chip | real-time(sub-second capable) | First observation of real-time free fatty acid exchange as a function of dy- namic glucose and insulin inputs | 2017 | [39] |
Dynamic adipose tissue function evaluated through microfluidics
Insulin-induced glucose uptake
Blood glucose levels are precisely regulated by the hormones insulin and glucagon within a narrow, normal range via strong endocrine signaling throughout the body. During feeding, insulin (secreted by pancreatic islets) increases glucose uptake into adipose and muscle tissues through glucose transporter type 4 (GLUT4) [65], a high affinity glucose transporter mostly expressed in these tissues. In the absence of insulin, only 5% of the GLUT4 is found at the cell surfaces. Insulin recruits GLUT4 to translocate to the plasma membrane from specialized GLUT4 storage vesicles, leading to a concomitant increase in cellular glucose uptake [66]. Although adipose tissue only accounts for about 10% of the insulin-stimulated glucose uptake (the other 90% occur in skeletal muscle) [67], this process is very important for energy homeostasis, as the secreted adipokines from WAT also regulate whole-body metabolism as stated earlier. Therefore, understanding the dynamics of regulated glucose transport in adipocytes using microfluidic methods should provide better opportunities for elucidating the physiological and pathophysiological mechanism of energy homeostasis.
The Elvassore group reported the first microfluidic human adipose tissue glucose uptake study [52]. Adipose tissue was trapped on an automated microfluidic injection system, which allows time resolved insulin stimulation. By comparing tissues from Type 2 diabetic and non-diabetic patients, insulin-resistance effects were observed in diabetic tissues when biopsies were treated with an insulin and glucose step, mimicking the postprandial phase. The healthy tissue showed a significant increase in glucose uptake upon insulin stimulation, whereas the diabetic tissue had no evidence of a difference in glucose uptake rate. However, the authors applied only one treatment to each of the precious tissue samples, even though the device was said to allow 1 wk of on-chip tissue culturing. Furthermore, the glucose concentration in the outflow was only measured every 10 min even though their system should be capable of ~2 min interrogation.
In a particularly valuable in vitro model for metabolic study, 3T3-L1 adipocyte spheroids were used by the Takayama group to study the scaling effects in a human-on-a-chip system [41]. They highlight the fact that allometry—the relationship between body size and shape, including anatomy and physiology—is a significant factor in organ-on-a-chip system design that should be considered when estimating the appropriate size of the “chip organism” for maintaining a similar basal metabolism rate (BMR) as in vivo. The insulin-induced glucose uptake rates by intact 3T3-L1 spheroids or mechanically dispersed spheroids within collagen were compared. This work demonstrated a significant glucose uptake difference between intact and mechanically dispersed spheroids, establishing that tissue architecture can significantly affect scaling relationships in microfabricated devices.
Fatty acid uptake
The energy reserves in adipose tissues are stored as triacylglycerol (TAG) molecules, which are mainly obtained from two routes: de novo lipogenesis from non-lipid precursors such as glucose, or uptake of free fatty acid and TAG from circulating blood. As the solubilities of free fatty acids and TAG in aqueous solution are extremely low [68], fatty acids or TAG molecules in blood are carried by lipoproteins including chylomicrons (CM), very low-density lipoproteins (VLDL), and serum albumin. Lipoprotein lipase (LPL), which is a glycoprotein secreted by adipocytes and translocated to the lumen of endothelial cells, digests TAG bound to lipoproteins thereby releasing free fatty acid for uptake by adipocytes [69]. The transport of non-esterified fatty acids (NEFAs) across the cell membranes is promoted by CD36/SR-B2, which is currently known as the predominant membrane protein facilitating fatty acid transport in adipocytes, enterocytes, cardiac myocytes, and skeletal myocytes [3]. Insulin-induced CD36/SR-B2 translocation from endosomal compartments to the cell membrane results in upregulated fatty acid uptake [3], which is a mechanism very similar to the GLUT4 regulation discussed above.
In our recent study, fatty acid uptake and release was monitored by fluorescence microscopy imaging of adipose tissue explants confined within our novel 16-channel microfluidic multiplexer (μMUX) [39]. Adipose tissue explants were exposed to temporal mimics of post-prandial insulin and glucose levels while simultaneously switching between fluorescently labeled and unlabeled free fatty acids. This unique experiment, enabled by the microfluidic platform, permitted fluorescence imaging of fatty acid uptake dynamics in real time (Fig. 1D–H). Surprisingly, both the initial rates of fatty acid uptake and the release rates were observed to follow the pattern of glucose and insulin. Moreover, treatment with a CD36/SR-B2 inhibitor [70] suppressed both the fatty acid uptake and release [43]. These results suggest the current understanding of fatty acid uptake and regulation is incomplete. It is likely that CD36/SR-B2 is not only involved in fatty acid uptake but plays a key role in fatty acid release. Based on our results [39, 43], this fatty acid exchange equilibrium between uptake and release is likely regulated by insulin induced CD36/SR-B2 translocation as well as by the extracellular fatty acid concentration. This hypothesis also agrees with our observation of fatty acid transportation between adipocytes, where adipocytes along the edges of the explants absorbed fatty acids more rapidly than inner cells. Over time, fatty acid could be seen permeating into centrally positioned adipocytes [43]. Further experiments to test this hypothesis are ongoing in our group, where the microfluidic platforms shown in Fig. 1C–H are key components that provide unique experimental capabilities to ask these questions.
Secretion of non-esterified fatty acids and glycerol
In the starving or fasting states, TAGs are hydrolyzed into NEFAs and glycerol by the lipase process, and the products are secreted into blood circulation by adipose tissues as an energy source for other tissues. The hydrolysis of stored TAG involves several lipases, including hormone-sensitive lipase (HSL), monoacylglycerol lipase (MGL), and adipose triglyceride lipase (ATGL) [71]. TAG is hydrolyzed by ATGL to diacylglycerol (DAG) and one molecule of fatty acid. HSL then converts DAG to a second fatty acid and monoacylglycerol (MAG). In the last step, MGL hydrolyzes MAG to glycerol and the third fatty acid molecule. The NEFAs and glycerol are not only used as nutrients for the rest of the body, but they also have important signaling effects on energy homeostasis. For example, circulating NEFAs have been shown to reduce glucose uptake by adipocytes and muscle and increase hepatic gluconeogenesis [72], and chronic exposure to elevated level of NEFAs was shown to decrease beta cell insulin secretion [73].
Since their 2009 publication of the seminal work in the area [46], the Kennedy group has reported several microfluidic studies where NEFA and glycerol secretion was quantified from 3T3-L1 adipocytes. The 3T3-L1 adipocytes were pre-differentiated onto cover slips and integrated into a reversibly sealed multilayer microfluidics system. The constant flow containing the secretomes was mixed with NEFA or glycerol enzyme probes, and the concentrations of NEFAs and/or glycerol were detected by fluorescence imaging on-chip [46–48, 50]. Upon treatment with a beta-adrenergic agonist, up to 6-fold, sustained increases in NEFA and glycerol secretions were observed. The latest version of their microfluidic device (Fig. 1A, B) has combined solid phase extraction and mass spectrometry, providing unique dynamic information about complex NEFA secretion profiles from adipocytes [49]. Use of such integrated microsystems—combining adipocyte culture, sample preparation, microfluidic sampling, and mass spectrometry—has the potential to make significant impacts to our understanding of adipose tissue biology.
Our group also has studied glycerol secretion from primary adipose tissue explants [44]. In order to counteract the adipose pad buoyancy, in this work we used custom 3D-printed accessories to trap the tissues into on-chip reservoirs. After 30 min of high-insulin/high-glucose treatments, the explants were switched to low-glucose/low-insulin solution, and increased glycerol secretion rates were observed.
Adipokine or batokine secretion
As stated earlier, adipose tissue plays a significant role in the endocrine system for maintaining whole body energy homeostasis. However, to the best of our knowledge, our group has conducted the only adipokine secretion study using microfluidics [45]. In this design, adipocytes from dispersed primary mouse tissue were cultured in collagen within a customized culture reservoir using a raised island to interface to the microchannel. Secreted adiponectin, which is a large multimeric protein hormone with the main understood function of improving insulin sensitivity, was quantified after sampling from this device. Insulin and niacin treatments induced increased adiponectin secretion by 2.6- and 4.4-fold, respectively.
Considering the importance of adipokine and batokine secretion in physiology, we assert that microfluidics should be an ideal tool for future studies on the dynamics of adipose-derived hormone secretion and its downstream systemic effects. As such, the lists of adipokines in Table 1 and batokines in Table 2 are provided also as a reference for those interested in applying microfluidic systems to important problems in the area of adipose tissue biology.
Thermogenesis
Heat generation is one of the necessities for endotherms to maintain normal body temperature and routine metabolism. BAT is known as the main site of non-shivering thermogenesis, which enables animals to adapt to a cold environment. Uncoupling protein 1 (UCP1) is a transmembrane protein only expressed in brown adipocytes. UCP1 is located on the inner membrane of mitochondria, where it increases the inner membrane proton permeability, decreases the proton gradient generated in oxidative phosphorylation, uncouples the respiratory chain, and yields a higher oxidation rate for heat generation [22]. UCP1-mediated proton leakage is regulated by the sympathetic nervous system through β-adrenergic receptors, and binding of catecholamines (such as norepinephrine) to β-adrenergic receptors in BAT trigger thermogenesis. UCP1 is also activated by fatty acids and inhibited by purine nucleotide di- and triphosphates (e.g., ATP, ADP, GTP, and GDP).
To investigate the heat generation property of BAT, Inomata et al. [51] fabricated a microfluidic system with silicon resonator-based thermal sensors, facilitating thermal detection of single cells. Heat generated from single brown adipocyte cells was measured by resonant frequency changes in the resonators. With their devices, two types of heat emissions from single brown fat cells were detected. Continuous heat generation during norepinephrine stimulation was quantified, and pulsed heat generation was quantified without any stimulation. Cells treated with sodium azide, a respiratory inhibitor, did not show any thermal response. This study represents the only report, to our knowledge, that has assayed dynamic function of BAT using microfluidics.
Conclusions and future outlook
Herein, we have reviewed some of the recent microfluidic systems applied to the study of adipocytes, or fat cells. As summarized in Table 3, these studies include adipocyte nutrient uptake, fatty acid and glycerol secretion, hormone secretion, and thermogenesis. From these reports, we have observed that microfluidic platforms can provide high precision analytical tools to promote improved understanding of adipocyte biology. Several new microfluidics-enabled findings—particularly the time resolved NEFA secretion profile, adiponectin secretion, and the first real time observation of insulin regulated fatty acid exchange—have provided new information on the dynamics of adipose tissue function. Future work in this new sub-field of microfluidics has the potential to inform better treatments for obesity, diabetes, and metabolic disorders.
Compared to the relatively effective applications of microfluidic systems to other endocrine tissues or cell types in recent years, such as islets of Langerhans [74, 75], far fewer studies have been conducted on adipose tissue using microfluidics thus far—especially on brown adipose tissue. We surmise that there are several reasons for this deficiency in the literature. First, the importance of adipocytes in energy homeostasis, and particularly the endocrine functions of adipose tissue, has been less explored, thereby reducing exposure of the concepts to the microfluidics community. In fact, a chief purpose of providing this critical review is to address this issue. Secondly, the buoyancy of adipocytes introduces difficulties to incorporate these cells onto microfluidic devices. To prevent cell loss and channel clogging, device engineers must use creative ways to trap or anchor the cells on chip, such as the 3D-printing technology used by our group for cell-driven macro-to-micro interfacing. Thirdly, the fundamental understanding of adipocytes is still relatively underdeveloped. For example, several new hormones were identified as recently as 2016 [76], namely a new adipokine (asprosin) and two new batokines (Slit2-C and PM20D1). These latest findings, along with the more complete lists of adipose-derived hormones (Table 1 and Table 2) represent great opportunities for bioengineers and bioanalytical chemists to make contributions to the adipose biology field.
Further innovations in microfluidics should continue to provide a unique toolset that allows biologists to advance their research in ways that were not previously possible. Conversely, bioengineers and bioanalytical scientists are also seeking unique problems in medicine and biology to validate and improve their microfluidic technologies. As stated in this review, the relatively understudied adipose tissue could be an important target tissue for the microfluidics community. Concurrently, further improvements to conform microfluidics into adipose tissue biology should provide unique opportunities toward better preventions and treatments, meeting urgent needs in the global epidemics of obesity, diabetes, and metabolic disorders.
Acknowledgments
Support for this work was provided by the National Institutes of Health (R01 DK093810) and the Department of Chemistry and Biochemistry at Auburn University.
Footnotes
Compliance with ethical standards The authors have no conflicts of interest to declare in relation to this work.
References
- 1.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocr Metab. 2004;89(6):2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 2.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Bio. 2011;12(11):722–34. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glatz JF, Luiken JJ. From fat to FAT (CD36/SR-B2): Understanding the regulation of cellular fatty acid uptake. Biochimie. 2017;136:21–6. doi: 10.1016/j.biochi.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y, Burn P. Lipid metabolic enzymes: emerging drug targets for the treatment of obesity. Nat Rev Drug Discov. 2004;3(8):695–710. doi: 10.1038/nrd1469. [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 7.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270(45):26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 8.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83(3):847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 9.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, et al. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 1999;18(17):4633–44. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 11.Wood IS, Wang B, Jenkins JR, Trayhurn P. The pro-inflammatory cytokine IL-18 is expressed in human adipose tissue and strongly upregulated by TNFalpha in human adipocytes. Biochem Biophys Res Commun. 2005;337(2):422–9. doi: 10.1016/j.bbrc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 12.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, Xu Z, et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56(10):2533–40. doi: 10.2337/db07-0007. [DOI] [PubMed] [Google Scholar]
- 14.Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6(5):363–75. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009;10(3):178–88. doi: 10.1016/j.cmet.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Chavey C, Lazennec G, Lagarrigue S, Clape C, Iankova I, Teyssier J, et al. CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metab. 2009;9(4):339–49. doi: 10.1016/j.cmet.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329(5990):454–7. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romere C, Duerrschmid C, Bournat J, Constable P, Jain M, Xia F, et al. Asprosin, a fasting-induced glucogenic protein hormone. Cell. 2016;165(3):566–79. doi: 10.1016/j.cell.2016.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360(15):1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 22.Villarroya F, Cereijo R, Villarroya J, Giralt M. Brown adipose tissue as a secretory organ. Nat Rev Endocrinol. 2017;13(1):26–35. doi: 10.1038/nrendo.2016.136. [DOI] [PubMed] [Google Scholar]
- 23.Nechad M, Ruka E, Thibault J. Production of nerve growth factor by brown fat in culture: relation with the in vivo developmental stage of the tissue. Comp Biochem Physiol Comp Physiol. 1994;107(2):381–8. doi: 10.1016/0300-9629(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 24.Burysek L, Houstek J. beta-Adrenergic stimulation of interleukin-1alpha and interleukin-6 expression in mouse brown adipocytes. FEBS Lett. 1997;411(1):83–6. doi: 10.1016/s0014-5793(97)00671-6. [DOI] [PubMed] [Google Scholar]
- 25.Asano A, Kimura K, Saito M. Cold-induced mRNA expression of angiogenic factors in rat brown adipose tissue. J Vet Med Sci. 1999;61(4):403–9. doi: 10.1292/jvms.61.403. [DOI] [PubMed] [Google Scholar]
- 26.Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vazquez MJ, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149(4):871–85. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26(3):271–81. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, et al. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59(7):1817–24. doi: 10.2337/db09-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R, et al. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun. 2013;4:2019. doi: 10.1038/ncomms3019. [DOI] [PubMed] [Google Scholar]
- 30.Virtue S, Feldmann H, Christian M, Tan CY, Masoodi M, Dale M, et al. A new role for lipocalin prostaglandin d synthase in the regulation of brown adipose tissue substrate utilization. Diabetes. 2012;61(12):3139–47. doi: 10.2337/db12-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunawardana SC, Piston DW. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes. 2012;61(3):674–82. doi: 10.2337/db11-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahman S, Lu Y, Czernik PJ, Rosen CJ, Enerback S, Lecka-Czernik B. Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology. 2013;154(8):2687–701. doi: 10.1210/en.2012-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157(6):1279–91. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med. 2014;20(12):1436–43. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whittle AJ, Jiang M, Peirce V, Relat J, Virtue S, Ebinuma H, et al. Soluble LR11/SorLA represses thermogenesis in adipose tissue and correlates with BMI in humans. Nat Commun. 2015;6:8951. doi: 10.1038/ncomms9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klepac K, Kilic A, Gnad T, Brown LM, Herrmann B, Wilderman A, et al. The Gq signalling pathway inhibits brown and beige adipose tissue. Nat Commun. 2016;7:10895. doi: 10.1038/ncomms10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svensson KJ, Long JZ, Jedrychowski MP, Cohen P, Lo JC, Serag S, et al. A secreted Slit2 fragment regulates adipose tissue thermogenesis and metabolic function. Cell Metab. 2016;23(3):454–66. doi: 10.1016/j.cmet.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long JZ, Svensson KJ, Bateman LA, Lin H, Kamenecka T, Lokurkar IA, et al. The secreted enzyme PM20D1 regulates lipidated amino acid uncouplers of mitochondria. Cell. 2016;166(2):424–35. doi: 10.1016/j.cell.2016.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Brooks JC, Hu J, Ford KI, Easley CJ. 3D-templated, fully automated microfluidic input/output multiplexer for endocrine tissue culture and secretion sampling. Lab Chip. 2017;17(2):341–9. doi: 10.1039/c6lc01201a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loskill P, Sezhian T, Tharp KM, Lee-Montiel FT, Jeeawoody S, Reese WM, et al. WAT-on-a-chip: a physiologically relevant microfluidic system incorporating white adipose tissue. Lab Chip. 2017;17(9):1645–54. doi: 10.1039/c6lc01590e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moraes C, Labuz JM, Leung BM, Inoue M, Chun TH, Takayama S. On being the right size: scaling effects in designing a human-on-a-chip. Integr Biol (Camb) 2013;5(9):1149–61. doi: 10.1039/c3ib40040a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brooks JC, Judd RL, Easley CJ. Methods in Molecular biology. New York: Humana Press; 2017. Culture and Sampling of Primary Adipose Tissue in Practical Microfluidic Systems, vol 1566. Thermogenic Fat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brooks JC. Dissertation. Auburn University; 2016. Microfluidic interfacing for primary endocrine tissue: developing bioanalytical methodologies and novel fabrication methods for cell culture and analysis. [Google Scholar]
- 44.Brooks JC, Ford KI, Holder DH, Holtan MD, Easley CJ. Macro-to-micro interfacing to microfluidic channels using 3D-printed templates: application to time-resolved secretion sampling of endocrine tissue. Analyst. 2016;141(20):5714–21. doi: 10.1039/c6an01055e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Godwin LA, Brooks JC, Hoepfner LD, Wanders D, Judd RL, Easley CJ. A microfluidic interface for the culture and sampling of adiponectin from primary adipocytes. Analyst. 2015;140(4):1019–25. doi: 10.1039/c4an01725k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clark AM, Sousa KM, Jennings C, MacDougald OA, Kennedy RT. Continuous-flow enzyme assay on a microfluidic chip for monitoring glycerol secretion from cultured adipocytes. Anal Chem. 2009;81(6):2350–6. doi: 10.1021/ac8026965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark AM, Sousa KM, Chisolm CN, MacDougald OA, Kennedy RT. Reversibly sealed multilayer microfluidic device for integrated cell perfusion and on-line chemical analysis of cultured adipocyte secretions. Anal Bioanal Chem. 2010;397(7):2939–47. doi: 10.1007/s00216-010-3897-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dugan CE, Cawthorn WP, MacDougald OA, Kennedy RT. Multiplexed microfluidic enzyme assays for simultaneous detection of lipolysis products from adipocytes. Anal Bioanal Chem. 2014;406(20):4851–9. doi: 10.1007/s00216-014-7894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dugan CE, Grinias JP, Parlee SD, El-Azzouny M, Evans CR, Kennedy RT. Monitoring cell secretions on microfluidic chips using solid-phase extraction with mass spectrometry. Anal Bioanal Chem. 2017;409(1):169–78. doi: 10.1007/s00216-016-9983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dugan CE, Kennedy RT. Measurement of lipolysis products secreted by 3T3-L1 adipocytes using microfluidics. Methods Enzymol. 2014;538:195–209. doi: 10.1016/B978-0-12-800280-3.00011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inomata N, Toda M, Ono T. Highly sensitive thermometer using a vacuum-packed Si resonator in a microfluidic chip for the thermal measurement of single cells. Lab Chip. 2016;16(18):3597–603. doi: 10.1039/c6lc00949b. [DOI] [PubMed] [Google Scholar]
- 52.Zambon A, Zoso A, Gagliano O, Magrofuoco E, Fadini GP, Avogaro A, et al. High temporal resolution detection of patient-specific glucose uptake from human ex vivo adipose tissue on-chip. Anal Chem. 2015;87(13):6535–43. doi: 10.1021/ac504730r. [DOI] [PubMed] [Google Scholar]
- 53.Roper MG. Cellular analysis using microfluidics. Anal Chem. 2016;88(1):381–94. doi: 10.1021/acs.analchem.5b04532. [DOI] [PubMed] [Google Scholar]
- 54.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5(1):19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 55.Kuri-Harcuch W, Green H. Adipose conversion of 3T3 cells depends on a serum factor. Proc Natl Acad Sci USA. 1978;75(12):6107–9. doi: 10.1073/pnas.75.12.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischer-Posovszky P, Newell FS, Wabitsch M, Tornqvist HE. Human SGBS cells – a unique tool for studies of human fat cell biology. Obes Facts. 2008;1(4):184–9. doi: 10.1159/000145784. https://doi.org/10.1159/000145784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang TH, Polakis SE. Differentiation of 3T3-L1 fibroblasts to adipocytes. Effect of insulin and indomethacin on the levels of insulin receptors. J Biol Chem. 1978;253(13):4693–6. [PubMed] [Google Scholar]
- 58.Mandrup S, Loftus TM, MacDougald OA, Kuhajda FP, Lane MD. Obese gene expression at in vivo levels by fat pads derived from s.c. implanted 3T3-F442A preadipocytes. Proc Natl Acad Sci USA. 1997;94(9):4300–5. doi: 10.1073/pnas.94.9.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32(8):760–72. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 60.Yao R, Du Y, Zhang R, Lin F, Luan J. A biomimetic physiological model for human adipose tissue by adipocytes and endothelial cell cocultures with spatially controlled distribution. Biomed Mater. 2013;8(4):045005. doi: 10.1088/1748-6041/8/4/045005. [DOI] [PubMed] [Google Scholar]
- 61.Mauney JR, Nguyen T, Gillen K, Kirker-Head C, Gimble JM, Kaplan DL. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007;28(35):5280–90. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wiggenhauser PS, Muller DF, Melchels FP, Egana JT, Storck K, Mayer H, et al. Engineering of vascularized adipose constructs. Cell Tissue Res. 2012;347(3):747–57. doi: 10.1007/s00441-011-1226-2. [DOI] [PubMed] [Google Scholar]
- 63.Wu X, Schneider N, Platen A, Mitra I, Blazek M, Zengerle R, et al. In situ characterization of the mTORC1 during adipogenesis of human adult stem cells on chip. Proc Natl Acad Sci USA. 2016;113(29):E4143–50. doi: 10.1073/pnas.1601207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanzi MC, Fare S. Adipose tissue engineering: state of the art, recent advances and innovative approaches. Expert Rev Med Devices. 2009;6(5):533–51. doi: 10.1586/erd.09.37. [DOI] [PubMed] [Google Scholar]
- 65.Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113(1):123–35. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13(6):383–96. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- 67.Kraegen EW, James DE, Jenkins AB, Chisholm DJ. Dose–response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol. 1985;248(3 Pt 1):E353–62. doi: 10.1152/ajpendo.1985.248.3.E353. [DOI] [PubMed] [Google Scholar]
- 68.Vorum H, Brodersen R, Kragh-Hansen U, Pedersen AO. Solubility of long-chain fatty acids in phosphate buffer at pH 7.4. Biochim Biophys Acta. 1992;1126(2):135–42. doi: 10.1016/0005-2760(92)90283-2. [DOI] [PubMed] [Google Scholar]
- 69.Lafontan M. Advances in adipose tissue metabolism. Int J Obes (Lond) 2008;32(Suppl 7):S39–51. doi: 10.1038/ijo.2008.237. [DOI] [PubMed] [Google Scholar]
- 70.Kuda O, Pietka TA, Demianova Z, Kudova E, Cvacka J, Kopecky J, et al. Sulfo-N-succinimidyl oleate (SSO) inhibits fatty acid uptake and signaling for intracellular calcium via binding CD36 lysine 164: SSO also inhibits oxidized low density lipoprotein uptake by macrophages. J Biol Chem. 2013;288(22):15547–55. doi: 10.1074/jbc.M113.473298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bolsoni-Lopes A, Alonso-Vale MI. Lipolysis and lipases in white adipose tissue – an update. Arch Endocrinol Metab. 2015;59(4):335–42. doi: 10.1590/2359-3997000000067. [DOI] [PubMed] [Google Scholar]
- 72.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97(12):2859–65. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eldor R, Raz I. Lipotoxicity versus adipotoxicity – the deleterious effects of adipose tissue on beta cells in the pathogenesis of Type 2 diabetes. Diabetes Res Clin Pract. 2006;74(2):S3–8. [Google Scholar]
- 74.Castiello FR, Heileman K, Tabrizian M. Microfluidic perfusion systems for secretion fingerprint analysis of pancreatic islets: applications, challenges and opportunities. Lab Chip. 2016;16(3):409–31. doi: 10.1039/c5lc01046b. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Lo JF, Mendoza-Elias JE, Adewola AF, Harvat TA, Kinzer KP, et al. Application of microfluidic technology to pancreatic islet research: first decade of endeavor. Bioanalysis. 2010;2(10):1729–44. doi: 10.4155/bio.10.131. [DOI] [PubMed] [Google Scholar]
- 76.Kajimura S. Adipose tissue in 2016: Advances in the understanding of adipose tissue biology. Nat Rev Endocrinol. 2017;13(2):69–70. doi: 10.1038/nrendo.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]