Abstract
Heart valve diseases (HVDs) arise from a number of different processes that affect both the structure and function of the valve apparatus. Despite diverse aetiologies, treatments for HVDs are limited to percutaneous or surgical interventions. The search for medical therapies to prevent or slow the progression of HVDs has been hampered by our poor understanding of the progression from subclinical to symptomatic phases, and our limited knowledge of the molecular signals that control the susceptibility of valve interstitial cells to pathological remodeling. Clinical evidence has suggested a link between certain neurotransmitters and valvular diseases of the heart. The fenfluramine–phentermine appetite suppressants popular in the 1980s were linked to mitral valve dysfunction, and ergot-derived dopamine agonists for Parkinson’s disease have been associated with an increased risk of mitral and aortic valve regurgitation. The effect does not appear to be limited to medications, as valvular pathologies have also been observed in patients with carcinoid tumours of serotonin-producing enterochromaffin cells. The role of neurotransmitter molecules in valve pathology has not been adequately characterized and may represent a target for future medical therapies. Here we present current evidence from both clinical and basic science suggesting a link between neurotransmitters and HVDs, opening the door to future research in this area.
Keywords: Neurotransmitters, Serotonin, Heart valve disease, Valve interstitial cells, Remodeling
1. Introduction
Heart valve diseases (HVDs) are a group of progressive multifactorial disorders that result from genetic or environmental disruption of leaflet function. Human heart valves are microstructurally complex anisotropic organs that maintain unidirectional blood flow and dynamically respond to cardiac remodeling. These pathologies generally present as either stenosis, a pathological leaflet thickening and narrowing of the valve opening, or regurgitation, the improper coaptation of valve leaflets that allows for hemodynamic backflow. Through molecular and cellular mechanisms, these valvular pathologies progress from mild and undetectable subclinical stages to symptomatic diseases that require aggressive medical and surgical intervention.1
Anatomically, the two atrioventricular valves and the two semilunar valves share common features. Atrioventricular valves are in-flow valves, which allow blood to enter the ventricles and prevent blood backflow during systole. The semilunar valves are out-flow valves that allow emptying of the ventricle and prevent backflow during diastole. The atrioventricular valves are inlet valves, and display trileaflet anatomy on the right side (tricuspid) and bileaflet anatomy on the left side (mitral).2 Although anatomically different, they share the same fundamental features, which are large annuli and multiple connections between the leaflets and the ventricle in the form of chordae tendinae, creating what are known as the mitral or triscuspid ventricular complex. The two semilunar valves, on the other hand, share a more consistent anatomy in the majority of the population, with three leaflets and three commissures. They reside within the aortic or pulmonic root, and are inserted into a virtual annulus called the basal ring, creating what is known as a functional aortic or pulmonic annulus. The two ventricles form a circuit in series which must function in unison to maintain normal physiologic conditions.2 Despite the anatomical similarities between the valves of each type, they are inevitably affected by the differential pressures on the left and the right side of the heart. The mitral and aortic valves on the systemic side experience nearly three times the force and pressure as the tricuspid and pulmonic valves on the pulmonary side, which may explain why degeneration of the left-sided valves is more prevalent.3
Many of the catalysts of valvular pathologies have been identified and are well described. These range from inflammatory processes to genetic factors to immunologically-mediated conditions to degenerative pathologies, among others.3 Recently, the feedback from fields such as endocrinology, psychiatry, neurology and bariatrics strongly suggests that diseases and medical treatments that affect systemic levels of certain neurotransmitters may unexpectedly affect the heart (Table 1). Approximately half of the patients with carcinoid tumours, for example, also present with valvular disease. Carcinoid tumours arise from serotonin-synthesizing enterochromaffin cells, and the metastatic proliferation of these cells elevates circulating serotonin levels, which contributes to the development of fibrous plaques on the heart valves.4,5 This same aetiology is seen in patients who were prescribed the ‘fen-phen’ serotonergic appetite suppressants, a combination popular in the 1980s.6 Patients taking ergot-derived dopamine agonists for the treatment of Parkinson’s disease have also been shown to have significantly higher rates of valvular disease.7 This would suggest that certain neurotransmitters may play a role in the development and progression of certain types of HVDs. We have to keep in mind that these molecules can exert their effects using two different networks, the neuronal and the vascular, and thus have two potential pathways through which to affect sites such as heart valves. What remains unclear is the precise mechanism and the pathways involved with these new forms of valvular diseases.
Table 1.
Medications for which there is evidence of serotonin dysregulation and valvulopathy
| Drug | Indications | Mechanism of valvulopathy |
|---|---|---|
| Fenfluramine | Anorectic—obesity | Metabolite norfenfluramine activates 5HT2B receptors |
| Dexfenfluramine | Anorectic—obesity | Metabolite norfenfluramine activates 5HT2B receptors |
| Benfluorex | Anorectic—obesity | Metabolite norfenfluramine activates 5HT2B receptors |
| Hyperlipidemia | ||
| Pergolide | Parkinson’s disease | Activates 5HT2A and 5HT2B receptors |
| Cabergoline | Hyperprolactinemia | Activates 5HT2A and 5HT2B receptors |
| Parkinson’s disease | ||
| Methysergide | Migraine | Likely serotonergic |
| Ergotamine | Migraine | Likely serotonergic |
This review attempts to collect, organize and unify the current state of knowledge on the topic of neurotransmitters and HVDs at the cellular level, and suggest areas for future research. There is presently no unified literature on this subject, and we draw most of our evidence from small case series, in vitro work and animal studies. A greater awareness and understanding of the current state of the literature regarding neuronal and chemical regulation of heart valves may reveal future directions for research and expand our understanding of HVD.
2. Valvular development and the relevance to clinical valvular disease
At the embryologic stage, the atrioventricular and semilunar valves share a common development pathway before their anatomical divergence. The origins of what will become valve leaflets begin with an epithelial to mesenchymal transition (EMT) during early cardiac development. EMTs and the reverse MET process (mesenchymal-to-endothelial transformation) occur a number of times during heart development.2,8 The precursors to all valves is an EMT that leads to the development of a cushion of mesenchymal cells. This cushion of mesenchymal cells eventually remodels when the transdifferentiation of those cells into interstitial fibroblasts generates collagen fibres. After further remodeling, these cells will become mature valve tissue. This transition and transdifferentiation process is not cell-autonomous, and requires external activating stimuli.9 This common developmental pathway indicates that the fundamental difference between atrioventricular and semilunar valves is anatomic: the semilunar valves remain attached to the endocardial cushion at one level only, whereas the atrioventricular valves keep two attachments, one to the annulus and the other to the ventricle. The end-result of this complex developmental process, incompletely described above, is four mature and structurally distinct heart valves.
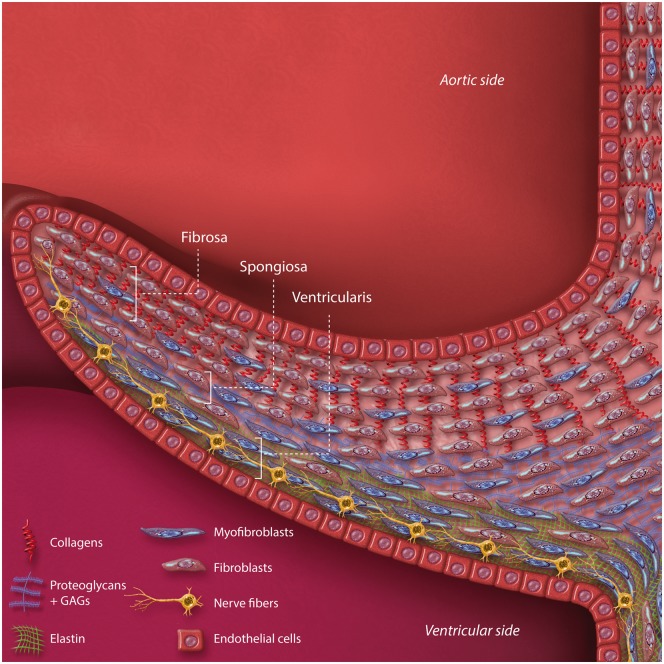
The function of each heart valve depends on a complex microstructure in a fine balance between the cellular and the extracellular matrix components.10 Leaflets are bound by an outer layer of valve endothelial cells (VECs), which form a barrier between the blood and the interstitial space. Within the leaflet interstitium, organized layers of collagen, proteoglycans and elastin comprise an extracellular matrix (ECM), which is regulated by a population of fibroblast-like, highly heterogeneous valve interstitial cells (VICs). While VICs generally exist in a quiescent state and maintain a homeostatic level of ECM remodeling, they are also capable of phenotypic transition in response to chemical and mechanical stimuli.11,12 Activation of VICs to a synthetic phenotype can allow synthesis of matrix components and the deformation of valve leaflets. An illustrated example of the valve microstructure, in this case showing a leaflet of the aortic valve, can be seen in Figure 1.
Figure 1.
Microarchitecture of the aortic valve cusp, as an example of overall valvular leaflet structure. The principal structural component of extracellular matrix is depicted and varies by layer. Prominent cell types are also depicted. GAGs, glycosaminoglycans.
Traditionally, valve leaflets have been considered passive flaps that open and shut in response to blood flow. Recent theories have begun to reject this passive-flap paradigm in favour of actively contractile valves,13 that respond to both electrical and chemical signals,14 including, potentially, neurotransmitters.15,16 Although research into the active contractility and responsiveness of valves is presently focused on mitral valves, the common developmental pathways and histological features described above would suggest that other valves may behave in a similar manner.
2.1 Serotonin
The strongest evidence of a connection between neurotransmitters and HVDs is the association between serotonin and carcinoid valve disease. The process starts with gradual thickening of the leaflets, and progresses through further fibrosis and retraction of the valve. In the case of the atrioventricular valves, this extends into the subvalvular apparatus.17 The valve dysfunction that results from the affectation is normally dual: stenosis and insufficiency.
Serotonin or 5-hydroxytryptamine (5HT) is a pleiotropic monoamine neurotransmitter that has been the subject of intense study since its discovery in 1948.18 It is formed when the essential amino acid tryptophan is hydroxylated by tryptophan hydroxlase (TPH), which is the rate limiting step, and then decarboxylated to form 5HT.19 Despite being found in highest concentrations in enterochromaffin cells and blood platelets, 5HT has diverse physiological roles in a number of organ systems.20 In the brain, 5HT is principally released from neurons of the Raphe nuclei, which connect throughout the central nervous system (CNS). 5HT thereby operates in various neurological scenes including mood, sleep, appetite, and perception.21–23 Outside the CNS, 5HT is associated with several physiological processes including gastrointestinal peristalsis, vasoconstriction, blood coagulation, insulin secretion, and cardiac development24–27. Platelets carry 5HT and release it during clotting, and are believed to be the most likely source of the neurotransmitter on the leaflets. However, VICs also express the TPH enzyme necessary to synthesize serotonin, indicating their capacity for local production of this molecule. Serotonin directs cellular signalling by binding to 5HT receptors in the plasma membrane or by covalent fixture to peptide-bound glutamine residues.26,28
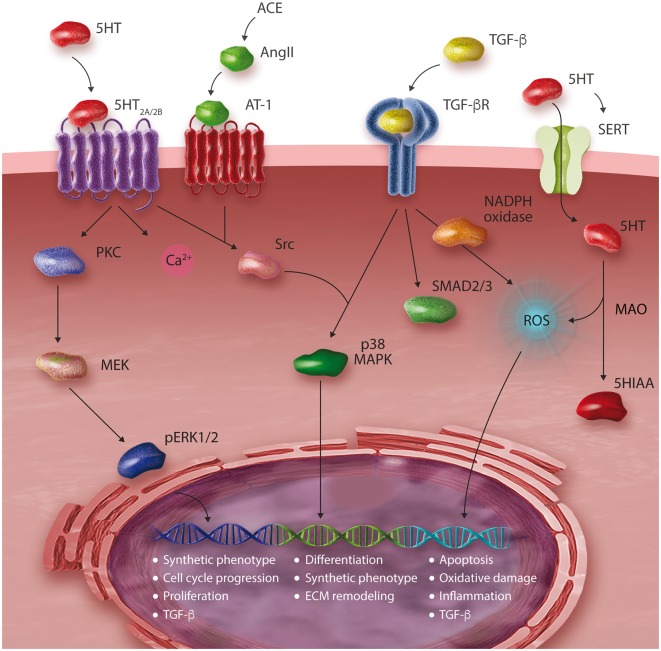
There are at least 14 subtypes of 5HT receptor, most of which are G-protein coupled receptors (GPCRs) with the exception of the cation channel 5HT3.23,29 5HT2 receptors, a group of serotonin receptors (5HT2A, 5HT2B, 5HT2C) implicated in heart disease, are canonically coupled to Gαq pathways.30–32 5HT2 signalling is complex: Gαq activates phospholipase C to produce the secondary messengers inositol phosphate and diacylglycerol, which elevate intracellular calcium and activate protein kinase C, respectively. In addition to canonical Gαq signalling, 5HT2B activation induces rapid Ras activation and Src phosphorylation, stimulating the tyrosine kinase signalling pathways of cell cycle progression and mitogenesis through ERK1/2.33–36
5HT acts as a powerful mitogen to VICs by activating 5HT2 receptors, stimulating DNA synthesis and cell-cycle progression (Figure 2).37 The 5HT2B receptor is expressed throughout cardiac tissues and is believed to play an important role in development of the heart. In mice designed by homologous recombination to lack the 5HT2B receptor, cardiac development was disrupted by absence of trabeculae and intercalated discs, leading to decreased ventricular muscle mass and mid-gestation lethality.38,39 Avian studies suggest that 5HT may induce these modelling effects through a TGF-β-dependent mechanism.27 The fibrogenic cytokine TGF-β has also been associated with pathologic ECM remodeling in numerous organ systems.40,41 In the heart, TGF-β activates VICs by promoting differentiation, migration, matrix condensation, angiogenesis and NADPH oxidase activity.19,40,42,43 Reactive oxygen species (ROS) derived from NADPH oxidase encourage synthesis of TGF-β, initiating a positive feedback loop of VIC activation.40,44 TGF-β is characteristically present in calcified valve leaflets and its signalling promotes apoptotic calcification of VICs.41
Figure 2.
Schematic representation of serotonergic signalling in valve interstitial cells (VICs). 5HIAA, 5-hydroxyindoleacetic acid; 5HT, serotonin; ACE, angiotensin converting enzyme; AngII, angiotensin II; AT-1, angiotensin receptor 1; MAPK, mitogen activated protein kinase; MEK, mitogen activated protein kinase kinase; pERK1/2, phosphorylated p42/44 mitogen activated protein kinases; PKC, protein kinase C; ROS, reactive oxygen species; SERT, serotonin transporter; MAO, monoamine oxidase.
Though essential for proper modelling during development, 5HT has been associated with pathological remodeling effects on mature heart valves. Several experiments with excess serotonin have demonstrated this. Both intravenous and oral administrations of 5HT have been shown to induce HVD in adult mammals.45,46 In Sprague–Dawley rats given daily 5HT injections for three months, 6 out of 10 rats developed carcinoid-like valvular thickening due to increased number of interstitial cells and ECM synthesis.45 In a replicate study, these effects were preventable by coadministration of the 5HT2 receptor antagonist terguride.47
Interestingly, upregulation of 5HT2 receptor mRNA has been repeatedly observed in diseased heart valve leaflets, a process that may increase the sensitivity of these tissues to 5HT by increasing the number of available receptors.12,32,45,48 Porcine leaflets incubated with 5HT have been observed to significantly stiffen in a time-dependent manner, favouring a significantly less organized and contractile valve microstructure.49 It is notable that cyclic stretch alone in porcine aortic valves increases expression of 5HT2 receptor genes, increasing the leaflets’ sensitivity to 5HT signalling.12 Pathological stiffening and consequent mechanical stress may induce a sinister positive feedback loop of increasing 5HT synthesis and sensitivity that propagates valvular remodeling.
5HT signalling in VICs has been linked to the renin–angiotensin system, particularly through the peptide hormone angiotensin II (AngII). Angiotensin receptor I (AT-1) is a major receptor to AngII that couples the Gαq/11 protein: its activation has been shown to promote inflammation, cardiac hypertrophy, collagen synthesis, oxidative stress and valve thickening in mice and humans.37,50–52 Recently, the AT-1 and 5HT2B receptors have been shown to work in concert in cardiac fibroblasts, such that both are required for the activity of either one.53 AngII and its immediate source, angiotensin converting enzyme (ACE), are upregulated and colocalize with lesions in diseased aortic valves.54,55 AT-1 is generally absent from healthy valve tissues but is upregulated in αSMA-expressing VICs, implying that AT-1 expression may be a characteristic of pathologically differentiated fibroblasts.54
5HT can also directly enter the cell via the serotonin transporter (SERT), a sodium-dependent transporter protein, which diminishes its function at plasma membrane receptors by reducing the amount of circulating 5HT.19 SERT is expressed throughout the foetal and mature heart, including VICs.56,57 SERT-deficient mice generated by homologous recombination rapidly developed fibrotic valves, likely as a result of deficient 5HT metabolism.58 It is notable that in late stage HVD, VICs appear to downregulate SERT.48,59 The mechanism of this downregulation remains unclear, but may take place through an increase in Akt phosphorylation.60 The effects of serotonin are mediated through many different types of receptors and membrane transporters that can affect multiple different pathways in the heart valve tissue, as described above.
2.2 Catecholamines
The catecholamine family includes many different neurotransmitters, including L-DOPA, dopamine and norepinephrine. There have been reports in the literature of multiple valves affected by these neurotransmitters, with echocardiographic features similar to those described for serotonin. Patients taking ergot-derived dopamine receptor agonists such as pergolide and cabergoline, for example, have been shown to be significantly more likely to develop clinically important valve regurgitation.7
Catecholamines are formed from hydroxylation of the essential amino acid tyrosine to L-DOPA by tyrosine hydroxylase, the rate limiting step in catecholamine biosynthesis.61,62 Decarboxylation of L-DOPA yields the neurotransmitter dopamine, a monoamine neurotransmitter that regulates mood, attention, motivation, sleep-wake cycles and cognition.63–65 Dopamine signals within the CNS act by binding to its GPCRs (D1, D2, D3, D4, D5) or undergoing hydroxylation to produce norepinephrine (NE), the major neurotransmitter of the sympathetic nervous system.66 NE can be synthesized in postganglionic synaptic terminals throughout all major organs for neuronal transmission and in the chromaffin cells of the adrenal medulla for systemic transmission.67 Methylation of the primary amine of NE produces epinephrine and takes place to varying degrees in the adrenal medulla, heart, and brain.68 In the body, NE is associated with a ‘fight or flight’ response to stress, as its release increases heart rate, blood pressure, blood flow to skeletal muscle and triggers glycogenolysis.69
The signalling functions of NE result from its activity at cellular adrenergic receptors, all of which are GPCRs. Two groups of NE receptors have been characterized, known as α- and β-adrenergic receptors. The α-adrenoreceptors are divided into α1 and α2 families and β-adrenoreceptors are divided into β1, β2 and β3. The α1 family are believed to preferentially couple Gαq/11 and act as stimulatory receptors to induce smooth muscle contraction and vasoconstriction.70 The α2 family canonically couple the Gαi protein to decrease levels of cAMP, and α2-receptor activation has been implicated in the prevention of heart failure.71,72 All of the β-adrenoreceptors canonically couple Gαs to increase production of cAMP by adenylyl cyclase, and exert multiple stimulatory effects on the heart.67,73 Chronic activation of β-adrenoreceptors has been associated with heart failure.73 Thus, NE exerts seemingly paradoxical cardiovascular effects via heterogeneous adrenoreceptors. Extracellular NE can also be transported into the cell by the norepinephrine transporter (NET) for catabolism by MAO-A.74
NE has been shown directly affect VICs, enhancing mitral valve contractility and leaflet tension by sympathetic neurotransmission.14,15 As noted previously, the close proximity of sympathetic nerve terminals and contractile aortic and mitral VICs indicates at least some role for the sympathetic nervous system in the regulation of valve leaflets. Immunohistochemical localization of sympathetic nerve terminals in close proximity to contractile VICs complements the determination that VICs express α1-, α2-, β1- and β2-adrenoreceptors.16,75,76
Valvular nervous systems have been reported to degenerate with age.77 HVD is associated with aging as well as increased sympathetic activity.78 Age-related changes in the autonomic nervous system may help to explain this phenomenon.79–81 Diseased mitral valves analyzed by immunohistochemical staining have been shown to significantly upregulate β1- and β2-adrenoreceptor expression, suggesting that NE may plan an as-yet undescribed role in the regulation of mitral valve prolapse.82 It is possible that degeneration of valvular nervous systems and altered adrenoreceptor expression may drive age-related HVD. Overactivation of β-adrenoreceptors in cardiac fibroblasts has been shown to induce proliferation, inflammatory cytokines, and fibrotic processes.83–85 Mice deficient in the dopamine hydroxylase enzyme cannot synthesize NE and have exhibited impaired proliferation during vascular remodeling.86 NE may serve as a mitogen to valve tissues as well. In VICs, norepinephrine has been shown to stimulate expression of the marker of differentiation αSMA, while downregulating TGF-β through β-adrenergic signaling.82 The significance of NE signalling on TGF-β-mediated valvulopathy is a topic in need of further study. In the presence of NE, interstitial cells have also been shown to adapt their relative expression of MMPs, collagens, and elastin, which may indicate that adrenergic neurotransmission modulates the ECM.82 While these data suggest a significant role for norepinephrine, there is a clear need for more work in vitro to understand the cellular effects of valvular sympathetic neurotransmission in HVDs.
3. Discussion
From endothelial cells to the mesencymal cushion to interstitial fibroblasts, the cellular transitions and transdifferentiations involved in the development of heart valves are not autonomous. External stimuli are responsible for activating EMT in the embryo, and it is plausible to suggest that this EMT could be reactived in adulthood. The addition of substances that have the ability to act through different axes (i.e. neuronal and systemic) may represent the catalyst needed for a secondary remodeling of the valve, changing the cellular homeostasis and thus the valve function. Neurotransmitters have the ability to impact the valve systemically and neuronally, and they may be uniquely suited to trigger significant pathological valve remodeling.
This review draws attention to an issue that, up to this point, has been under-described in the literature: a clinically important association between neurotransmitters and valvular diseases. This association has implications for two distinct but overlapping groups. The first group is patients prescribed medications that affect systemic levels of neurotransmitters and the clinicians that prescribe them, and the second is clinicians and scientists investigating potential drug therapies to prevent or manage HVDs. For the former group, a greater understanding of the potential valvular implications of medications that treat psychiatric, neurologic and endocrine disorders provides a more complete picture of the risks and benefits of different pharmacologic treatments. For the latter group, HVDs have a significant window period when clinical signs and symptoms are not present, a paradigm that supports the development of medical treatments to delay or prevent the need for surgical or percutaneous valvular intervention. An examination of the neurochemical basis of HVD reveals several clinically relevant perspectives for both of these groups.
Serotonergic 5HT2 receptors, particularly 5HT2B, have been shown to induce pathological remodeling of heart valves through a variety of mechanisms.39,40,50,52 5HT2B activation is a common feature of valvulopathic drugs, and 5HT2B agonism is a characteristic screened by the US National Institute of Mental Health Psychoactive Drug Screening Program to identify drug compounds with potential adverse effects on valves.87 Research studies have been done in an attempt to block these receptors when using serotonin. 5HT2B antagonists in vitro have been used to inhibit 5HT-induced valve remodeling and reduce intracellular oxidative stress.27,88 Antagonism of 5HT2B receptors has been shown to attenuate p38 MAPK activation by avoiding pathological TGF-β signalling, possibly by receptor internalization of Src within the antagonized GPCRs.89In vivo murine studies support this work, and have found that terguride, a dopamine receptor agonist and a 5HT2A and 5HT2B antagonist, protects heart valves from fibrosis and heart failure during long-term 5HT administration and post-banding of the pulmonary artery, respectively.47,90 Rats given pergolide, a valvulopathic ergoline derivative, developed severe aortic and mitral regurgitation secondary to valvular thickening, but administration of the 5HT2B receptor antagonist cyproheptadine prevented these effects.91
The serotonin-norepinephrine reuptake inhibitor (SNRI) sibutramine has been repeatedly shown to exempt heart valves from the damage observed with other serotonergic drugs, and in some studies has even been shown to attenuate pathological cardiac remodeling in obese patients.92–93 However, it is unclear how much of this cardioprotective effect is due to weight loss and caloric restriction and how much is due to cellular signalling mechanisms. Because sibutramine blocks reuptake of a spectrum of monoamines, it remains to be conclusively determined whether pure SSRIs infer a greater risk for HVD (see Table 2). An early case control study examining large cohorts of patients exposed to SSRI before hospitalization found no association between SSRI use and relative risk of developing HVD.94 It is notable that the patients in this study were exposed to SSRIs for unknown amounts of time. A recent study that accounted for dose and treatment duration, albeit in smaller cohorts, established a dose-dependent relationship between SSRI use and HVD with a significant odds ratio of 3.08.95 In a progressive disorder such as HVD, duration of SSRI exposure is likely an important factor in considering associations. Another study evaluated SSRI use among patients who had taken benfluorex, an anorexic with 5HT2B agonist activity, and found that SSRI use did not correlate with an increased risk of valvular regurgitation.96 However, because benfluorex has been shown to bind 5HT2 receptors, these results may not effectively estimate the risk associated with SSRIs alone.92 Further studies must be performed which account for the dose and duration of SSRI exposure, exclusive of other valvulopathic agents, in order to properly estimate the safety of these drugs.
Table 2.
Studies of heart valve disease associated with exposure to selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs)
| Author/study | Active drug | Controls | Relevant findings |
|---|---|---|---|
| Multicenter prospective cohort study of obese type II diabetic patients92 | 15 mg sibutramine for 12 months (n=72), followed by 12 months open label extension (n=133) | Placebo (n=77) | Incidence of left-sided valvular disease: 2.6% sibutramine, 3.7% placebo (OR = 0.693; 90% CI, 0.131–3.681) |
| Retrospective cohort study of patients who underwent transthoracic echo during hospitalization at Duke University Medical Center, USA93 | SSRI exposure before admission (n=292) | No exposure to SSRI before hospitalization (n=5145) | Incidence of mild or greater aortic regurgitation: 15.8% SSRI, 18.9% no SSRI (P = 0.20) |
| Incidence of moderate or greater mitral regurgitation: 16.8% SSRI, 17.2% no SSRI (P = 0.87) | |||
| Incidence of FDA grade mitral or aortic regurgitation: 26.7% SSRI, 30.4% no SSRI (P = 0.19) | |||
| Prospective cohort study of obese outpatients at Jeanne d’Arc Hospital, France94 | 10 mg (n=64) or 20 mg (n=60) sibutramine | Placebo (n=60) | Incidence of mitral valve abnormalities: 5% 20 mg, 11% 10 mg, 5% placebo (NS) |
| Incidence of aortic valve abnormalities: 7% 20 mg, 5% 10 mg, 4% placebo (NS) | |||
| Prospective cohort study of obese patients at Federico II University Hospital, Italy95 | 15 mg sibutramine for 3 months with caloric restriction (n=11) | Placebo with caloric restriction (n=9) | No evidence of valvular lesions found in this study |
| Multicenter retrospective cohort study of patients who had previously been exposed to benfluorex for 3+ months97 | SSRI exposure for 3+ months (n=90) | No history of SSRI exposure (n=742) | Incidence of mild or greater aortic regurgitation: 22.2% SSRI, 19.5% no SSRI (P = 0.58) |
| Incidence of mild or greater mitral regurgitation: 15.6% SSRI, 15.9% no SSRI (P = 0.63) | |||
| Multicenter retrospective case-control study98 | Valvular regurgitation determined by echo (n=206) | Age matched controls (n=195) | Exposure to SSRI/SNRIs: 11.6% of diagnosed valvular regurgitation cases, 4.1% of controls (OR = 3.08; 95% CI, 1.35–7.04) |
This body of evidence would suggest an increasing role for multidisciplinary teams when selecting treatments for patients with psychiatric or neurologic disorders. Psychiatrists and neurologists must work closely with cardiac specialists to ensure that treatments for psychiatric disorders do not unintentionally contribute to the development and progression of valvular diseases. These patients are at significant risk to go un- or under-monitored if what we have learned from past clinical experiences is not kept in mind. Further study of these medications will provide a clearer and more comprehensive picture of the potential valvular implications of medications that affect neurotransmitters, which will facilitate a more accurate assessment of their risks and benefits.
4. Conclusions
The regulation of valve interstitial cell phenotypic plasticity and its effect on ECM architecture remains a key unanswered question in heart valve biology. This may have significant implications for clinical practice as there are no molecular-based tools to determine the risk of HVD patients for adverse clinical outcomes. Here we present evidence of mechanisms by which neurotransmitters influence the behaviour of heart valve cells. Dysregulation of these mechanisms may initiate changes to the valve microstructure associated with HVD, of which serotonin dysregulation seems to be the most prominent. Interestingly, neurotransmitters are able to effect these changes through both neuronal and systemic pathways. Invasive structural intervention, either percutaneously or through a surgical approach, is currently the only therapy for HVD, and patients are thus forced to wait until the disease progresses to operable severity. The molecular neurotransmitter systems discussed here represent potential targets for future medical therapies for HVD. HVD is a complex disorder that progresses through many phases, and the neurotransmitters we present here may provide new insights into the pharmacodynamics and offers a variety of drug targets for different stages of the disease.
Highlights
Neurotransmitter molecules may regulate molecular mechanisms of HVD (Table 3).
Valve interstitial cells multiply and transform in response to monoamine signals.
5HT2B receptor antagonism may represent a therapy for subclinical valve disease.
Future topics of research include clearer elucidation of the mechanisms through which neurotransmitters may promote HVDs and sympathetic leaflet innervation.
Table 3.
Selected neurotransmitters shown to influence the biomechanical properties of heart valves and potentially affect the progression of HVD
| Neurotransmitter | Roles | Receptor | Proposed effects on heart valves |
|---|---|---|---|
| Serotonin | Sleep, mood, appetite, cognition, peristalsis, coagulation, insulin secretion | 5HT2 receptors | Proliferation and cell cycle progression |
| Upregulation of TGF-β cytokines | |||
| Norepinephrine | Vasoconstriction, increased heart rate, glycogenolysis, ‘fight or flight’ response | Adrenergic α- and β-receptors | Regulation of valve contractility |
Acknowledgements
This research was supported in part by the following research grants and funds: NIH R01-HL131872 (GF and RJL), R01-HL122805 (GF), AHA GRNT 24810002 (GF), The Kibel Fund for Aortic Valve Research (GF and RJL), and The Valley Hospital Foundation ‘Marjorie C Bunnel’ charitable fund (GF), and both Erin's Fund and the William J. Rashkind Endowment of the Children's Hospital of Philadelphia (RJL).
Conflict of interest: none declared.
References
- 1. Borer JS, Sharma A.. Drug therapy for heart valve diseases. Circulation 2015;132:1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tao G, Kotick JD, Lincoln J.. Heart valve development, maintenance, and disease: the role of endothelial cells. Curr Top Dev Biol 2012;100:203–232. [DOI] [PubMed] [Google Scholar]
- 3. Rajamannan NM. Cellular, molecular, and genetic mechanisms of valvular heart disease In: Otto CM, Bonow RO.. Valvular Heart Disease: A Companion to Braunwald’s Heart Disease. Philadelphia: Saunders Elsevier; 2009. p39–54. [Google Scholar]
- 4. Robiolio PA, Rigolin VH, Wilson JS, Harrison JK, Sanders LL, Bashore TM, Feldman JM.. Carcinoid heart disease. Correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation 1995;92:790–795. [DOI] [PubMed] [Google Scholar]
- 5. Gustafsson BI, Hauso O, Drozdov I, Kidd M, Modlin IM.. Carcinoid heart disease. Int J Cardiol 2008;129:318–324. [DOI] [PubMed] [Google Scholar]
- 6. Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV.. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med 1997;337:581–588. [DOI] [PubMed] [Google Scholar]
- 7. Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G.. Valvular heart disease and the use of Dopamine Agnoists for Parkinson’s disease. N Engl J Med 2007;356:39–46. [DOI] [PubMed] [Google Scholar]
- 8. Markwald RR, Norris RA, Moreno-Rodriguez R, Levine RA.. Developmental basis of adult cardiovascular diseases: valvular heart diseases. Ann N Y Acad Sci 2010;1188: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boyer B, Valles AM, Edme N.. Induction and regulation of epithelial–mesenchymal transitions. Biochem Pharmacol 2000;60:1091–1099. [DOI] [PubMed] [Google Scholar]
- 10. Zeng YI, Sun R, Li X, Liu M, Chen S, Zhang P.. Pathophysiology of valvular heart disease. Exp Ther Med 2016;11:1184–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Osman L, Yacoub MH, Latif N, Amrani M, Chester AH.. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation 2006;114: I547–I552. [DOI] [PubMed] [Google Scholar]
- 12. Balachandran K, Bakay MA, Connolly JM, Zhang X, Yoganathan AP, Levy RJ.. Aortic valve cyclic stretch causes increased remodeling activity and enhanced serotonin receptor responsiveness. Ann Thorac Surg 2011;92:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams TH, Jew JY.. Is the mitral valve passive flap theory overstated? An active valve is hypothesized. Med Hypotheses 2004;62:605–611. [DOI] [PubMed] [Google Scholar]
- 14. Itoh A, Krishnamurthy G, Swanson JC, Ennis DB, Bothe W, Kuhl E, Karlsson M, Davis LR, Miller DC, Ingels NB.. Active stiffening of mitral valve leaflets in the beating heart. Am J Physiol Heart Circ Physiol 2009;296:H1766–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curtis MB, Priola DV.. Mechanical properties of the canine mitral valve: effects of autonomic stimulation. Am J Physiol – Physiol 1992;262:H56–H62. [DOI] [PubMed] [Google Scholar]
- 16. Hu X, Zhao Q, Ye X.. Autonomic regulation of mechanical properties in porcine mitral valve cusps. Arq Bras Cardiol 2012;98:321–328. [DOI] [PubMed] [Google Scholar]
- 17. Edwards NC, Yuan M, Nolan O, Pawade TA, Oelofse T, Singh H, Mehrzad H, Zia Z, Geh JI, Palmer DH, May CJ, Ayuk J, Shah T, Rooney SJ, Steeds RP.. Effects of valvular surgery in carcinoid heart disease: an observational cohort study. J Clin Endocrinol Metab 2016;101:183–190. [DOI] [PubMed] [Google Scholar]
- 18. Rapport MM, Green AA, Page IH.. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem 1948;176:1243–1251. [PubMed] [Google Scholar]
- 19. Ni W, Watts SW.. 5-Hydroxytryptamine in the cardiovascular system: focus on the serotonin transporter (SERT). Clin Exp Pharmacol Physiol 2006;33:575–583. [DOI] [PubMed] [Google Scholar]
- 20. Hutcheson JD, Setola V, Roth BL, Merryman WD.. Serotonin receptors and heart valve disease–it was meant 2B. Pharmacol Ther 2011;132:146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daubert EA, Condron BG.. Serotonin: a regulator of neuronal morphology and circuitry. Trends Neurosci 2010;33:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hurley LM, Hall IC.. Context-dependent modulation of auditory processing by serotonin. Hear Res 2011;279:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP.. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev 1994;46:157–203. [PubMed] [Google Scholar]
- 24. Mawe GM, Hoffman JM.. Serotonin signaling in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 2013;10:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lopez-Vilchez I, Diaz-Ricart M, White JG, Escolar G, Galan AM.. Serotonin enhances platelet procoagulant properties and their activation induced during platelet tissue factor uptake. Cardiovasc Res 2009;84:309–316. [DOI] [PubMed] [Google Scholar]
- 26. Paulmann N, Grohmann M, Voigt J-P, Bert B, Vowinckel J, Bader M, Skelin M, Jevsek M, Fink H, Rupnik M, Walther DJ.. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol 2009;7:e1000229.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buskohl PR, Sun MJ, Sun ML, Thompson RP, Butcher JT.. Serotonin potentiates transforming growth factor-beta3 induced biomechanical remodeling in avian embryonic atrioventricular valves. PLoS One 2012;7:e42527.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walther DJ, Peter J-U, Winter S, Höltje M, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, Bader M.. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell 2003;115:851–862. [DOI] [PubMed] [Google Scholar]
- 29. McCorvy JD, Roth BL.. Structure and function of serotonin G protein-coupled receptors. Pharmacol Ther 2015;150:129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, Sun JH, Link JR, Abbaszade I, Hollis JM, Largent BL, Hartig PR, Hollis GF, Meunier PC, Robichaud AJ, Robertson DW.. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol 2000;57:75–81. [PubMed] [Google Scholar]
- 31. Yabanoglu S, Akkiki M, Seguelas M-H, Mialet-Perez J, Parini A, Pizzinat N.. Platelet derived serotonin drives the activation of rat cardiac fibroblasts by 5-HT2A receptors. J Mol Cell Cardiol 2009;46:518–525. [DOI] [PubMed] [Google Scholar]
- 32. Cremer SE, Moesgaard SG, Rasmussen CE, Zois NE, Falk T, Reimann MJ, Cirera S, Aupperle H, Oyama MA, Olsen LH.. Alpha-smooth muscle actin and serotonin receptors 2A and 2B in dogs with myxomatous mitral valve disease. Res Vet Sci 2015;100:197–206. [DOI] [PubMed] [Google Scholar]
- 33. Nebigil CG, Etienne N, Schaerlinger B, Hickel P, Launay J-M, Maroteaux L.. Developmentally regulated serotonin 5-HT2B receptors. Int J Dev Neurosci 2001;19:365–372. [DOI] [PubMed] [Google Scholar]
- 34. Nebigil CG, Launay JM, Hickel P, Tournois C, Maroteaux L.. 5-Hydroxytryptamine 2B receptor regulates cell-cycle progression: cross-talk with tyrosine kinase pathways. Proc Natl Acad Sci USA USA 2000;97:2591–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Launay JM, Birraux G, Bondoux D, Callebert J, Choi DS, Loric S, Maroteaux L.. Ras involvement in signal transduction by the serotonin 5-HT2B receptor. J Biol Chem 1996;271:3141–3147. [DOI] [PubMed] [Google Scholar]
- 36. Gu X, Masters KS.. Role of the MAPK/ERK pathway in valvular interstitial cell calcification. Am J Physiol Heart Circ Physiol 2009;296:H1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hafizi S, Taylor PM, Chester AH, Allen SP, Yacoub MH.. Mitogenic and secretory responses of human valve interstitial cells to vasoactive agents. J Heart Valve Dis 2000;9:454–458. [PubMed] [Google Scholar]
- 38. Nebigil CG, Hickel P, Messaddeq N, Vonesch JL, Douchet MP, Monassier L, György K, Matz R, Andriantsitohaina R, Manivet P, Launay JM, Maroteaux L.. Ablation of serotonin 5-HT(2B) receptors in mice leads to abnormal cardiac structure and function. Circulation 2001;103:2973–2979. [DOI] [PubMed] [Google Scholar]
- 39. Nebigil CG, Choi DS, Dierich A, Hickel P, Le Meur M, Messaddeq N, Launay JM, Maroteaux L.. Serotonin 2B receptor is required for heart development. Proc Natl Acad Sci USA 2000;97:9508–9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu R-M, Desai LP.. Reciprocal regulation of TGF-β and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol 2015;6:565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jian B, Narula N, Li Q, Mohler ER, Levy RJ.. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg 2003;75:457–465, 466 [DOI] [PubMed] [Google Scholar]
- 42. Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA.. Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ Res 2004;95:253–260. [DOI] [PubMed] [Google Scholar]
- 43. Chiu Y-N, Norris RA, Mahler G, Recknagel A, Butcher JT.. Transforming growth factor β, bone morphogenetic protein, and vascular endothelial growth factor mediate phenotype maturation and tissue remodeling by embryonic valve progenitor cells: relevance for heart valve tissue engineering. Tissue Eng Part A 2010;16:3375–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barcellos-Hoff MH, Dix TA.. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol Baltim Endocrinol 1996;10:1077–1083. [DOI] [PubMed] [Google Scholar]
- 45. Gustafsson BI, Tømmerås K, Nordrum I, Loennechen JP, Brunsvik A, Solligård E, Fossmark R, Bakke I, Syversen U, Waldum H.. Long-term serotonin administration induces heart valve disease in rats. Circulation 2005;111:1517–1522. [DOI] [PubMed] [Google Scholar]
- 46. Lancellotti P, Nchimi A, Hego A, Dulgheru R, Delvenne P, Drion P, Oury C.. High-dose oral intake of serotonin induces valvular heart disease in rabbits. Int J Cardiol 2015;197:72–75. [DOI] [PubMed] [Google Scholar]
- 47. Hauso Ø, Gustafsson BI, Loennechen JP, Stunes AK, Nordrum I, Waldum HL.. Long-term serotonin effects in the rat are prevented by terguride. Regul Pept 2007;143:39–46. [DOI] [PubMed] [Google Scholar]
- 48. Cremer SE, Zois NE, Moesgaard SG, Ravn N, Cirera S, Honge JL, Smerup MH, Hasenkam JM, Sloth E, Leifsson PS, Falk T, Oyama MA, Orton C, Martinussen T, Olsen LH.. Serotonin markers show altered transcription levels in an experimental pig model of mitral regurgitation. Vet J Lond Engl 1997 2015;203:192–198. [DOI] [PubMed] [Google Scholar]
- 49. Warnock JN, Gamez CAP, Metzler SA, Chen J, Elder SH, Liao J.. Vasoactive agents alter the biomechanical properties of aortic heart valve leaflets in a time-dependent manner. J Heart Valve Dis 2010;19:86–95. discussion 96. [PubMed] [Google Scholar]
- 50. Vaughan DE, Lazos SA, Tong K.. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. J Clin Invest 1995;95:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fujisaka T, Hoshiga M, Hotchi J, Takeda Y, Jin D, Takai S, Hanafusa T, Ishizaka N.. Angiotensin II promotes aortic valve thickening independent of elevated blood pressure in apolipoprotein-E deficient mice. Atherosclerosis 2013;226:82–87. [DOI] [PubMed] [Google Scholar]
- 52. Hingtgen SD, Tian X, Yang J, Dunlay SM, Peek AS, Wu Y, Sharma RV, Engelhardt JF, Davisson RL.. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genomics 2006;26:180–191. [DOI] [PubMed] [Google Scholar]
- 53. Jaffré F, Bonnin P, Callebert J, Debbabi H, Setola V, Doly S, Monassier L, Mettauer B, Blaxall BC, Launay J-M, Maroteaux L.. Serotonin and angiotensin receptors in cardiac fibroblasts coregulate adrenergic-dependent cardiac hypertrophy. Circ Res 2009;104:113–123. [DOI] [PubMed] [Google Scholar]
- 54. O’Brien KD, Shavelle DM, Caulfield MT, McDonald TO, Olin-Lewis K, Otto CM, Probstfield JL.. Association of angiotensin-converting enzyme with low-density lipoprotein in aortic valvular lesions and in human plasma. Circulation 2002;106: 2224–2230. [DOI] [PubMed] [Google Scholar]
- 55. Helske S, Lindstedt KA, Laine M, Mäyränpää M, Werkkala K, Lommi J, Turto H, Kupari M, Kovanen PT.. Induction of local angiotensin II-producing systems in stenotic aortic valves. J Am Coll Cardiol 2004;44:1859–1866. [DOI] [PubMed] [Google Scholar]
- 56. Pavone LM, Mithbaokar P, Mastellone V, Avallone L, Gaspar P, Maharajan V, Baldini A.. Fate map of serotonin transporter-expressing cells in developing mouse heart. Genes N Y N 2000 2007;45:689–695. [DOI] [PubMed] [Google Scholar]
- 57. Pavone LM, Spina A, Lo Muto R, Santoro D, Mastellone V, Avallone L.. Heart valve cardiomyocytes of mouse embryos express the serotonin transporter SERT. Biochem Biophys Res Commun 2008;377:419–422. [DOI] [PubMed] [Google Scholar]
- 58. Mekontso-Dessap A, Brouri F, Pascal O, Lechat P, Hanoun N, Lanfumey L, Seif I, Benhaiem-Sigaux N, Kirsch M, Hamon M, Adnot S, Eddahibi S.. Deficiency of the 5-hydroxytryptamine transporter gene leads to cardiac fibrosis and valvulopathy in mice. Circulation 2006;113:81–89. [DOI] [PubMed] [Google Scholar]
- 59. Scruggs SM, Disatian S, Orton EC.. Serotonin transmembrane transporter is down-regulated in late-stage canine degenerative mitral valve disease. J Vet Cardiol Off J Eur Soc Vet Cardiol 2010;12:163–169. [DOI] [PubMed] [Google Scholar]
- 60. Rajamanickam J, Annamalai B, Rahbek-Clemmensen T, Sundaramurthy S, Gether U, Jayanthi LD, Ramamoorthy S.. Akt-mediated regulation of antidepressant-sensitive serotonin transporter function, cell-surface expression and phosphorylation. Biochem J 2015;468:177–190. [DOI] [PubMed] [Google Scholar]
- 61. Zigmond RE, Schwarzschild MA, Rittenhouse AR.. Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Annu Rev Neurosci 1989;12:415–461. [DOI] [PubMed] [Google Scholar]
- 62. Daubner SC, Le T, Wang S.. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys 2011;508:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Beaulieu J-M, Espinoza S, Gainetdinov RR.. Dopamine receptors – IUPHAR Review 13. Br J Pharmacol 2015;172:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol 2002;67:53–83. [DOI] [PubMed] [Google Scholar]
- 65. Krzymowski T, Stefanczyk-Krzymowska S.. New facts and the concept of physiological regulation of the dopaminergic system function and its disorders. J Physiol Pharmacol Off J Pol Physiol Soc 2015;66:331–341. [PubMed] [Google Scholar]
- 66. Beaulieu J-M, Gainetdinov RR.. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 2011;63:182–217. [DOI] [PubMed] [Google Scholar]
- 67. Kohm AP, Sanders VM.. Norepinephrine and β2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev 2001;53:487–525. [PubMed] [Google Scholar]
- 68. Wurtman RJ, Pohorecky LA, Baliga BS.. Adrenocortical control of the biosynthesis of epinephrine and proteins in the adrenal medulla. Pharmacol Rev 1972;24:411–426. [PubMed] [Google Scholar]
- 69. Tank AW, Lee Wong D.. Peripheral and central effects of circulating catecholamines. Compr Physiol 2015;5:1–15. [DOI] [PubMed] [Google Scholar]
- 70. O’Connell TD, Jensen BC, Baker AJ, Simpson PC.. Cardiac alpha1-adrenergic receptors: novel aspects of expression, signaling mechanisms, physiologic function, and clinical importance. Pharmacol Rev 2014;66:308–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gyires K, Zádori ZS, Török T, Mátyus P.. α2-Adrenoceptor subtypes-mediated physiological, pharmacological actions. Neurochem Int 2009;55:447–453. [DOI] [PubMed] [Google Scholar]
- 72. Brede M, Wiesmann F, Jahns R, Hadamek K, Arnolt C, Neubauer S, Lohse MJ, Hein L.. Feedback inhibition of catecholamine release by two different alpha2-adrenoceptor subtypes prevents progression of heart failure. Circulation 2002;106: 2491–2496. [DOI] [PubMed] [Google Scholar]
- 73. Najafi A, Sequeira V, Kuster DWD, Velden JVD.. β-Adrenergic receptor signalling and its functional consequences in the diseased heart. Eur J Clin Invest 2016;46:362–374. [DOI] [PubMed] [Google Scholar]
- 74. Schroeder C, Jordan J.. Norepinephrine transporter function and human cardiovascular disease. Am J Physiol Heart Circ Physiol 2012;303:H1273–H1282. [DOI] [PubMed] [Google Scholar]
- 75. Pinto JE, Nazarali AJ, Torda T, Saavedra JM.. Autoradiographic characterization of beta-adrenoceptors in rat heart valve leaflets. Am J Physiol – Heart Circ Physiol 1989;256:H821–H827. [DOI] [PubMed] [Google Scholar]
- 76. Chester AH, Kershaw JDB, Sarathchandra P, Yacoub MH.. Localisation and function of nerves in the aortic root. J Mol Cell Cardiol 2008;44:1045–1052. [DOI] [PubMed] [Google Scholar]
- 77. Jew JY, Williams TH.. Innervation of the mitral valve is strikingly depleted with age. Anat Rec 1999;255:252–260. [DOI] [PubMed] [Google Scholar]
- 78. Almeida-Santos MA, Barreto-Filho JA, Oliveira JLM, Reis FP, Cunha Oliveira CCD, Sousa ACS.. Aging, heart rate variability and patterns of autonomic regulation of the heart. Arch Gerontol Geriatr 2016;63:1–8. [DOI] [PubMed] [Google Scholar]
- 79. Parashar R, Amir M, Pakhare A, Rathi P, Chaudhary L.. Age related changes in autonomic functions. J Clin Diagn Res JCDR 2016;10:CC11–CC15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Moodithaya S, Avadhany ST.. Gender differences in age-related changes in cardiac autonomic nervous function. J Aging Res 2012;2012:679345.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Snyder DL, Johnson MD, Aloyo V, Eskin B, Roberts J.. Age-related changes in cardiac norepinephrine release: role of calcium movement. J Gerontol A Biol Sci Med Sci 1995;50:B358–367. [DOI] [PubMed] [Google Scholar]
- 82. Hu X, Wang H-Z, Liu J, Chen A-Q, Ye X-F, Zhao Q.. A novel role of sympathetic activity in regulating mitral valve prolapse. Circ J Off J 2014;78:1486–1493. [DOI] [PubMed] [Google Scholar]
- 83. Leicht M, Greipel N, Zimmer H.. Comitogenic effect of catecholamines on rat cardiac fibroblasts in culture. Cardiovasc Res 2000;48:274–284. [DOI] [PubMed] [Google Scholar]
- 84. Leicht M, Briest W, Zimmer H-G.. Regulation of norepinephrine-induced proliferation in cardiac fibroblasts by interleukin-6 and p42/p44 mitogen activated protein kinase. Mol Cell Biochem 2003;243:65–72. [DOI] [PubMed] [Google Scholar]
- 85. Lubahn CL, Lorton D, Schaller JA, Sweeney SJ, Bellinger DL.. Targeting α- and β-adrenergic receptors differentially shifts Th1, Th2, and inflammatory cytokine profiles in immune organs to attenuate adjuvant arthritis. Front Immunol 2014;5: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang H, Cotecchia S, Thomas SA, Tanoue A, Tsujimoto G, Faber JE.. Gene deletion of dopamine beta-hydroxylase and alpha1-adrenoceptors demonstrates involvement of catecholamines in vascular remodeling. Am J Physiol Heart Circ Physiol 2004;287: H2106–2114. [DOI] [PubMed] [Google Scholar]
- 87. Huang X-P, Setola V, Yadav PN, Allen JA, Rogan SC, Hanson BJ, Revankar C, Robers M, Doucette C, Roth BL.. Parallel functional activity profiling reveals valvulopathogens are potent 5-hydroxytryptamine(2B) receptor agonists: implications for drug safety assessment. Mol Pharmacol 2009;76:710–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Monassier L, Laplante M-A, Jaffré F, Bousquet P, Maroteaux L, Champlain JD.. Serotonin 5-HT(2B) receptor blockade prevents reactive oxygen species-induced cardiac hypertrophy in mice. Hypertension 2008;52:301–307. [DOI] [PubMed] [Google Scholar]
- 89. Hutcheson JD, Ryzhova LM, Setola V, Merryman WD.. 5-HT(2B) antagonism arrests non-canonical TGF-β1-induced valvular myofibroblast differentiation. J Mol Cell Cardiol 2012;53:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Janssen W, Schymura Y, Novoyatleva T, Kojonazarov B, Boehm M, Wietelmann A, Luitel H, Murmann K, Krompiec DR, Tretyn A, Pullamsetti SS, Weissmann N, Seeger W, Ghofrani HA, Schermuly RT.. 5-HT2B receptor antagonists inhibit fibrosis and protect from RV heart failure. BioMed Res Int 2015;2015:438403.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Droogmans S, Roosens B, Cosyns B, Degaillier C, Hernot S, Weytjens C, Garbar C, Caveliers V, Pipeleers-Marichal M, Franken PR, Lahoutte T, Schoors D, Camp GV.. Cyproheptadine prevents pergolide-induced valvulopathy in rats: an echocardiographic and histopathological study. Am J Physiol – Heart Circ Physiol 2009;296: H1940–H1948. [DOI] [PubMed] [Google Scholar]
- 92. Bach DS, Rissanen AM, Mendel CM, Shepherd G, Weinstein SP, Kelly F, Seaton TB, Patel B, Pekkarinen TA, Armstrong WF, Absence of cardiac valve dysfunction in obese patients treated with sibutramine. Obes Res 1999;7:363–369. [DOI] [PubMed] [Google Scholar]
- 93. Mast ST, Gersing KR, Anstrom KJ, Krishnan KR, Califf RM, Jollis JG.. Association between selective serotonin-reuptake inhibitor therapy and heart valve regurgitation. Am J Cardiol 2001;87:989–993. A4. [DOI] [PubMed] [Google Scholar]
- 94. De Backer T, Petrovic M, Audenaert K, Coeman M, De Bacquer D.. A happy valve in a happy patient? Serotonergic antidepressants and the risk of valvular heart disease (SERVAL). A case–control study. Acta Clin Belg 2016;71:57–62. [DOI] [PubMed] [Google Scholar]
- 95. Maréchaux S, Jeu A, Jobic Y, Ederhy S, Donal E, Réant P, Abouth S, Arnasteen E, Boulanger J, Ennezat P-V, Garban T, Szymanski C, Tribouilloy C.. Impact of selective serotonin reuptake inhibitor therapy on heart valves in patients exposed to benfluorex: a multicentre study. Arch Cardiovasc Dis 2013;106:349–356. [DOI] [PubMed] [Google Scholar]
- 96. Szymanski C, Andréjak M, Peltier M, Maréchaux S, Tribouilloy C.. Adverse effects of benfluorex on heart valves and pulmonary circulation. Pharmacoepidemiol Drug Saf 2014;23:679–686. [DOI] [PubMed] [Google Scholar]
- 97. Simone GD, Romano C, De Caprio C, Contaldo F, Salanitri T, Luzio Paparatti UD, Pasanisi F.. Effects of sibutramine-induced weight loss on cardiovascular system in obese subjects. Nutr Metab Cardiovasc Dis 2005;15:24–30. [DOI] [PubMed] [Google Scholar]
- 98. Zannad F, Gille B, Grentzinger A, Bruntz J-F, Hammadi M, Boivin J-M, Hanotin C, Igau B, Drouin P.. Effects of sibutramine on ventricular dimensions and heart valves in obese patients during weight reduction. Am Heart J 2002; 144:508–515. [DOI] [PubMed] [Google Scholar]