Abstract
C1q/TNF-related protein 9 (CTRP9) is a paralogue of adiponectin with known favorable effects on lipid and glucose metabolism. A potential role of CTRP9 for regulation of endothelium function has been suggested by previous studies. However, no studies have examined the relation between serum CTRP9 levels and adhesion molecules in patients with type 2 diabetes mellitus (T2DM) and coronary artery disease (CAD). The present study was conducted on 337 subjects who underwent coronary angiography and were categorized into four groups according to the presence of CAD and T2DM (control, CAD, T2DM and CAD+T2DM). Serum levels of CTRP9, adiponectin, sICAM-1, sVCAM-1, sE-Selectin, IL-6 and TNF-α were measured. It was found that the circulating CTRP9 levels were independently associated with increased risk of CAD and T2DM in addition to elevated levels of serum CTRP9 in CAD, T2DM and CAD+T2DM groups. A significant association of serum CTRP9 levels with adhesion molecules in CAD and T2DM patients as well as serum TNF-α levels in CAD individuals was noted. A significant relation between the circulating levels of CTRP9 and HOMA-IR in T2DM subjects was also observed. The results revealed increased circulating levels of CTRP9 in T2DM and CAD individuals which suggests a compensatory response to insulin resistance, inflammatory milieu and endothelial dysfunction; however, more studies are needed to confirm this.
Introduction
Adipose tissue is a highly active endocrine organ that is responsible for the synthesis and secretion of several hormones, such as bioactive molecules known as adipokines [1]. In recent years, considerable research has been devoted to understanding the biology of adipokines and their potential role in obesity-related diseases such as diabetes and cardiovascular disease (CVD) [2,3]. Adipokines have been identified as having diverse functional roles in lipid and glucose metabolism and in inflammation, along with the pathogenic processes of many diseases [4,5].
Adiponectin, a well-known adipokine, exerts a positive role in regulating glucose and lipid metabolism. It has been suggested that the dysregulation of adiponectin production contributes to the development of CVD and type 2 diabetes mellitus (T2DM) [4]. The C1q TNF related protein (CTRP) family is a newly discovered paralogue of adiponectin [6]. This family has 15 members (CTRP1 to CTRP15) with related structures and diverse functions [7]. Among them, CTRP9 has the highest amino acid identity to adiponectin and is secreted as a glycoprotein from adipose tissue [8]. It has been shown that overexpression of CTRP9 decreases fasting insulin and glucose levels in mice [9]. Conversely, deletion of CTRP9 decreases insulin sensitivity and increases food intake [10]. Moreover, TNF-α inhibits CTRP9 expression in H9c2 cells [11].
Several beneficial effects of CTRP9 for the cardiovascular system have been reported. It has higher vasoactive potency than adiponectin [12], has a protective role in remodeling after acute myocardial infarction [13], decreases inflammation [14] and inhibits vascular smooth muscle cell proliferation [15]. The association of plasma CTRP9 levels with atherosclerosis has been suggested by several lines of evidence [16–18]. For instance, Chang Hee Jung et al. reported independent association between CTRP9 and arterial stiffness [19]. Jing Wang et al. showed decreased serum levels of CTRP9 in patients with coronary atherosclerosis [16].
Conflicting results have been reported in relation to CTRP9 levels and metabolic factors. Some studies have found a positive association between CTRP9 and unfavorable metabolic factors such as body mass index (BMI) and insulin resistance [19,20], while the others described inverse associations [21]. Although the effect of CTRP9 on endothelium function has been pointed out by previous studies [10,22], there is no study on the association between CTRP9 and soluble adhesion molecules as markers of endothelial function in patients with T2DM and coronary artery disease (CAD). This study aimed to evaluate the association of circulating CTRP9 levels with CAD and T2DM as well as the association between serum CTRP9 levels and soluble adhesion molecules as a marker of endothelium dysfunction in CAD and T2DM patients.
Study population, materials and methods
Study population
Participants aged 45–75 years were recruited from Rasoul-e-Akram Hospital in Tehran, Iran. This study was approved by the Ethics Committee of Iran University of Medical Sciences and was conducted in accordance with the Helsinki Declaration. Written consent was obtained from all study subjects. Patients who had at least one coronary vessel with >50% luminal stenosis were categorized as CAD patients as determined by a cardiologist. Subjects with <30% stenosis in angiography imaging were categorized as non-CAD.
American Diabetes Association (ADA) criteria were used for the diagnosis of T2DM [23]. Subjects with acute coronary syndrome and cardiovascular disease (peripheral artery, coronary artery and cerebrovascular disease) were excluded from the non-CAD group. Moreover, individuals with a history and evidence of stroke, myocardial infraction, kidney disease, cancer, autoimmune disease, chronic inflammation and those taking thiazolidinedione [11] were excluded from the study. Subjects who had smoked cigarettes in the last three months were considered to be smokers. Study participants were categorized into the following four groups according to the presence of CAD and T2DM: control (non-CAD and non-T2DM), CAD (CAD and non-T2DM), T2DM (T2DM and non-CAD) and CAD+T2DM (CAD and T2DM).
Anthropometric measurement and laboratory assessment
The body mass index (BMI) was calculated by dividing the weight (kg) by height squared (m). The systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in seated patients after 5 min of rest using a standard sphygmomanometer. After the subject had fasted overnight, blood samples were collected and the fasting blood glucose (FBG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), alanine amino transferase (ALT), aspartate amino transferase (AST) and creatinine (Cr) were measured using commercially available kits (Pars Azmoon; Iran). Serum insulin levels were assessed by ELISA kit (Monobind; USA). Homeostasis model assessment for insulin resistance (HOMA-IR) was calculated using the formula (FBG [mg/dL]) × (fasting blood insulin [μU/ml])/405).
Measurement of circulating adipokines, cytokines and adhesion molecules
Serum levels of TNF-α (Cat #DTA00C), IL-6 (Cat #HS600B), ICAM-1 (Cat #DCD540), VCAM-1 (Cat #DVC00) and E-Selectin (Cat #DSLE00) were measured using ELISA kits (Quantikine; R&D Systems; USA). Minimum detectable doses of TNF-α and IL-6 were 1.6 and 0.7 pg/ml, respectively. The intra- and inter-assay coefficients of variation (CV) of ICAM-1, VCAM-1 and E-Selectin were <7%, <6.5% and <7.5%, respectively. Circulating adiponectin levels were measured by an ELISA kit (Adipogen; South Korea; Cat #AG-45A-0001YEK-KI01) with intra- and inter-assay variations of 4.6% and 4.4%, respectively. Serum levels of CTRP9 were measured using an ELISA kit (USCN Life Science; USA; Cat #SER877Hu) (Intra-assay: CV = 3.4%; Inter-assay: CV = 4.3%).
Statistical analysis
Categorical variables were presented as frequencies and percentages and compared using the chi-square test. Shapiro-Wilkes testing was conducted to determine the normal distribution of quantitative variables. Variables with normal distribution were reported as mean ± standard error of mean (SEM) and compared using student's t-test or one-way ANOVA followed by Bonferroni post hoc. Non-normally distributed variables were shown as median ± inter quartile range (IQR) and tested using Mann–Whitney U or Kruskal-Wallis tests with the Bonferroni correction. Analysis of covariance (ANCOVA) was performed to control potential covariates. Logarithmic transformation carried out for non-normally distributed data and correlations between continuous variables were determined by Pearson’s correlation test. Multinomial logistic regression was conducted to evaluate the association between serum CTRP9 levels and disease conditions. Multiple linear regression analysis was run to assess the relations between CTRP9 levels and correlated variables.
Results
Anthropometric and clinical characteristics of the study participants are presented in Table 1. No significant differences in age, sex or BMI were observed between groups. The number of smokers and individuals taking statins and antihypertensive medications were higher in the case groups than in the control. Anti-hyperglycemic medications were used only by T2DM patients (T2DM and CAD+T2DM groups). Details of medication use are given in S1 Table. Higher SBP, DBP and FBS values were found in the CAD+T2DM group compared to the control. The highest level of FBS and the lowest levels of insulin and the HOMA-IR index were observed in the control group. In addition, compared to T2DM and CAD+T2DM groups, CAD individuals had lower HOMA-IR indexes. Furthermore, the results of post hoc analysis showed a significant difference in circulating levels of TG, TC, LDL-C, HDL-C, ALT and AST between the case and control groups.
Table 1. Clinical and biochemical characteristics of study population.
| Variables | Control(n = 80) | CAD (n = 157) | T2DM (n = 37) | CAD+T2DM (n = 63) | p value |
|---|---|---|---|---|---|
| Sex [male (%)] | 58 (72.5) | 112 (71.3) | 21 (56.8) | 45 (71.4) | 0.318 |
| Age (year) | 57.03 ± 0.97 | 58.18 ± 0.62 | 58.51 ± 1.23 | 58.49 ± 1.13 | 0.661 |
| BMI (kg/m2)N | 25.8 ± 3.4 | 26.6 ± 3.9 | 26.7 ± 4.2 | 26.4 ± 4.1 | 0.435 |
| Smoker [n (%)] | 17 (21.2) | 66 (42) | 11 (29.7) | 31 (49.2) | <0.001 |
| SBP (mm Hg) | 128.5 ± 16.2 | 132.4 ± 18.3 | 131.8 ± 18.8 | 138.4 ± 19.1c** | 0.014 |
| DBP (mm Hg) | 79.5 ± 11.4 | 82.6 ± 13.0 | 81.2 ± 14.0 | 86.5 ± 13.6 c** | 0.015 |
| FBG (mg/dl) | 93.8 ± 11.7 | 95.2 ± 11.5 | 167.9 ± 23.7b**,d** | 156.0 ± 22.4c**,e**,f** | <0.001 |
| Insulin (μU/ml) | 3.2 (2.1–5.5) | 5.5 (2.8–8.6)a** | 10.9 (8.9–12.8) b**,d** | 9.4 (7.0–12.2) c**,e** | <0.001 |
| HOMA-IR | 0.78 (0.46–1.24) | 1.35 (0.64–2.17)a** | 4.15 (3.45–5.44)b**,d** | 3.53 (2.51–4.94)c**,e** | <0.001 |
| Triglyceride (mg/dl) | 121.8 ± 47.4 | 140.9 ± 48.9a* | 148.9 ± 38.9b* | 169.8 ± 63.6c**,e** | <0.001 |
| Total Cholesterol (mg/dl) | 170.4 ± 37.7 | 182.6 ± 46.0 | 181.4 ± 44.1 | 196.1 ± 45.7c** | 0.008 |
| LDL-C (mg/dl) | 102 ± 30.6 | 110.3 ± 33.5 | 108.5 ± 37.2 | 124.0 ±35.2c**,e* | 0.002 |
| HDL-C (mg/dl) | 45.9 ± 7.2 | 44.0 ± 10.1 | 42.2 ± 4.3 | 41.4 ± 6.0c** | 0.009 |
| Creatinine (mg/dl) | 1.13 ± .18 | 1.15 ± .18 | 1.20 ± .15 | 1.19 ± .15 | 0.116 |
| AST (U/l) | 18.2 ± 5.5 | 21.5 ± 6.7a* | 19.8 ± 5.4 | 19.7 ± 6.6 | 0.002 |
| ALT(U/l) | 18.2 ± 7.6 | 22.9 ± 8.4a* | 18.3 ± 7.4 | 21.8 ± 8.3 | <0.001 |
| Antihypertensive medication [n (%)] | 11 (13.8) | 56 (35.7) | 5 (13.5) | 33 (52.4) | <0.001 |
| Statin use [n (%)] | 23 (28.7) | 88 (56.1) | 13 (35.1) | 34 (54) | <0.001 |
| Oral hypoglycemic agent [n (%)] | 0 | 0 | 20 (54.1) | 42 (66.7) | <0.001 |
| Insulin ± Oral hypoglycemic agent [n (%)] | - | - | 11 (29.7) | 17 (27) | <0.001 |
a: Control and CAD groups were compared.
b: Control and T2DM groups were compared.
c: Control and T2DM+CAD groups were compared.
d: CAD and T2DM groups were compared.
e: CAD and T2DM+CAD groups were compared.
f: T2DM and CAD+T2DM groups were compared.
* P < 0.05
** P < 0.01.
Serum concentrations of CTRP9, cytokines and adhesion molecules
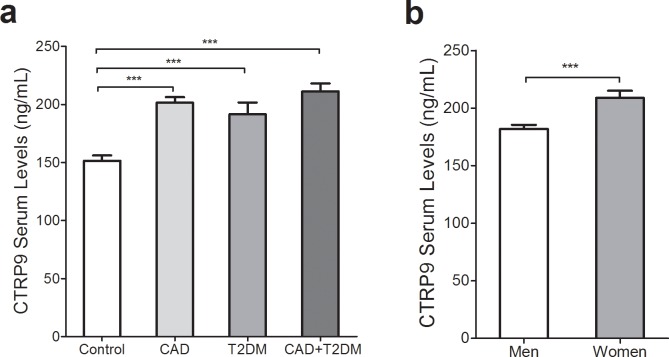
Circulating CTRP9 levels were significantly higher in CAD (202.03 ± 4.89), T2DM (191.38 ± 10.13) and CAD+T2DM (211.19 ± 6.81) patients (p < 0.001) compared to control individuals (148.7 ±4.0) (Fig 1A). The results remained significant even after adjusting for age, BMI, sex, medication and adiponectin (S2 Table). Likewise, the results of multiple logistic regression indicate that serum CTRP9 concentration was independently associated with the increased risk of CAD (OR [CI] = 1.018 [1.009–1.026]; p < 0.001), T2DM (OR [CI] = 1.015 [1.005–1.025]; p = 0.003) and CAD+T2DM (OR [CI] = 1.021 [1.012–1.031]; p < 0.001) after adjustment for age, sex, BMI, adiponectin, IL-6 and TNF-α (Table 2). Serum levels of CTRP9 were significantly higher in women (208.7 ± 6.3) than men (181.8 ± 3.7; p < 0.001; Fig 1B).
Fig 1. Serum levels of CTRP9 in control, CAD, T2DM and CAD+T2DM category.
a) Serum CTRP9 levels were lower in controls (148.7 ± 4.0) than CAD (202.0 ± 4.9), T2DM (191.4 ± 10.1) and CAD+T2DM (211.2 ± 6.8). (all, p<0.001). b) Serum concentration of CTRP9 was higher in women (208.8 ± 6.2) compared to men (181.8 ± 3.7) (p<0.001).
Table 2. Odds ratios for development of CAD, T2DM and CAD+T2DM based on serumCTRP9 levels.
| CTRP9 serum levels | ||
|---|---|---|
| OR (95% Cl) | P value | |
| CAD group | ||
| Unadjusted model | 1.022 (1.015–1.029) | <0.001 |
| Model 1 | 1.018 (1.009–1.026) | <0.001 |
| T2DM group | ||
| Unadjusted model | 1.018 (1.010–1.027) | <0.001 |
| Model 1 | 1.015 (1.005–1.025) | 0.003 |
| CAD+T2DM group | ||
| Unadjusted model | 1.025 (1.017–1.032) | <0.001 |
| Model 1 | 1.021 (1.012–1.031) | <0.001 |
Model 1: Adjusted for age, sex, BMI, adiponectin, IL-6 and TNF-α
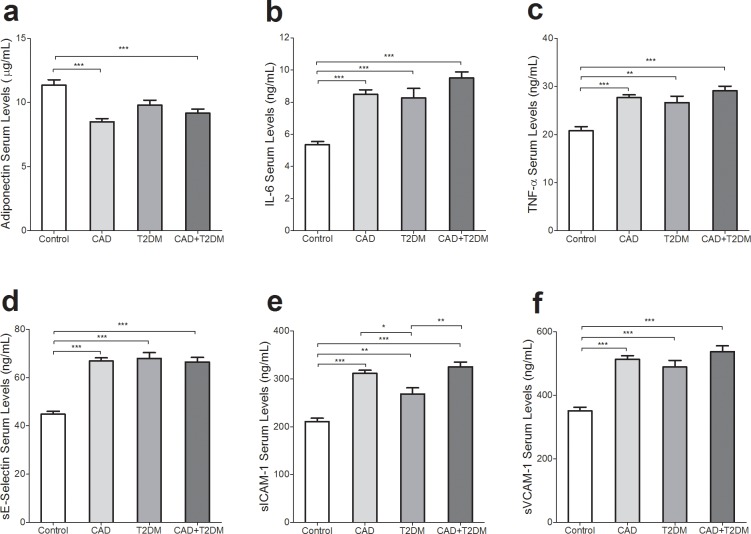
Circulating levels of adiponectin were lower in CAD (8.51 ± 0.24) and CAD+T2DM (9.17 ± 0.33) than in the control category (11.36 ± 0.42; p < 0.001; Fig 2A). Serum IL-6 was higher in concentration in CAD (8.5 ± 0.3), T2DM (8.3 ± 0.6) and CAD+T2DM (9.5 ± 0.4) than in the controls (5.3 ± 0.2; p < 0.001; Fig 2B). Circulating levels of TNF-α were lower in control subjects (20.82 ± 0.87) than in those with CAD (27.74 ± 0.54), T2DM (26.65 ± 01.32) and CAD+T2DM (29.12 ± 0.94) (p < 0.001, p < 0.01 and p < 0.001, respectively; Fig 2C). Serum sE-Selectin levels were also higher in CAD (66.9 ± 1.3), T2DM (68.0 ± 2.3) and CAD+T2DM (66.5 ± 1.9) patients compared to normal participants (44.9 ± 1.2; p < 0.001; Fig 2D). Compared with the CAD (311.5 ± 6.4), T2DM (268.0 ± 13.1) and CAD+T2DM (324.9 ±9.9) groups, a lower concentration of serum sICAM-1 was observed in the controls (210.7 ± 6.9; p < 0.001). Serum sICAM-1 was higher in the CAD+T2DM and CAD groups than in subjects with T2DM (p < 0.01 and p < 0.05, respectively; Fig 2E). Compared to normal subjects (352.5 ± 11.2), the CAD (513.5 ± 10.4), T2DM (489.2 ± 20.6) and CAD+T2DM (537.3 ± 18.0) subjects recorded higher concentrations of sVCAM-1 (p < 0.001; Fig 2F).
Fig 2. Circulating levels of adiponectin, inflammatory cytokines and soluble adhesion molecules in control, CAD, T2DM and CAD+T2DM category.
a) A higher levels of serum adiponectin was demonstrated in controls compared to persons with CAD and CAD+T2DM (both, p<0.001). b) Serum concentration of IL-6 was higher in patients groups compared to controls (all, p<0.001). c) TNF-α concentration were higher in CAD, T2DM and CAD+T2DM category compared to control (all, p<0.001). d) Those with CAD, T2DM, and CAD+T2DM showed a higher serum levels of E-selectin compared to control group (all, p<0.001). e) sICAM-1 was higher in case groups compared to control (all, p<0.01). Also, higher serum levels of sICAM-1 were shown in CAD (p<0.05) and CAD+T2DM patients (p<0.01) compared to T2DM group. f) Serum level of sVCAM-1 had higher levels in patients compared to healthy individuals (all, p<0.001).
Association of CTRP9 with biochemical and metabolic parameters
The results of the Pearson correlation of CTRP9 with anthropometric and metabolic parameters are shown in Table 3. Significant correlations were found between serum CTRP9 levels, BMI and glucose metabolism parameters (FBG, insulin and HOMA-IR) in the control group. The results of multiple stepwise linear regression showed that BMI (β [SE] = 2.9 [1.1]; p = 0.001) and HOMA-IR (β [SE] = 40.7[13.8]; p = 0.004) were independently associated with serum CTRP9 levels in normal subjects. In the CAD group, CTRP9 was significantly and positively correlated with inflammatory cytokines (IL-6 and TNF-α), adhesion molecules (sE-selectin, sICAM-1 and sVCAM-1) and was negatively associated with adiponectin (p < 0.01). The results of multiple stepwise linear regression analysis revealed that TNF-α (β [SE] = 83.8 [38.7]; p = 0.32), sE-Selectin (β [SE] = 0.794 [0.264]; p = 0.003), sICAM-1 (β [SE] = 0.138 [0.058]; p = 0.018), sVCAM-1 (β [SE] = 0.106 [0.035]; p = 0.003) and adiponectin (β [SE] = -4.822 [1.38]; p = 0.001) were independently associated with serum CTRP9 levels. In the T2DM group, CTRP9 showed significant positive correlations with BMI, FBG, insulin, HOMA-IR, sE-Selectin, sVCAM-1 and sICAM-1 (p < 0.05) and a negative association with adiponectin (p < 0.05); however; the correlations between CTRP9 and BMI and insulin and sICAM-1 disappeared after adjustment for adiponectin in the T2DM group. A multiple linear regression model demonstrated the independent association of CTRP9 with HOMA-IR (β [SE] = 97.67 [44.1]; p = 0.035). In CAD+T2DM individuals, CTRP9 was positively correlated with insulin, HOMA-IR, TNF-α, IL-6, sE-Selectin, sVCAM-1 and sICAM-1 (p < 0.01) and negatively correlated with adiponectin (p < 0.05); however, a correlation between CTRP9 and IL-6 disappeared after further adjustment for adiponectin.
Table 3. Pearson correlation of CTRP9 with anthropometric and metabolic parameters.
| Control (n = 80) | CAD (n = 157) | T2DM (n = 37) | T2DM+CAD (n = 63) | |
|---|---|---|---|---|
| Age | .108 | -.070 | -.194 | -.134 |
| BMI | .331* | .141 | .345* | .210 |
| SBP | .169 | .005 | -.124 | -.004 |
| DBP | .062 | -.035 | -.027 | .020 |
| FBG | .242* | .039 | .420** | .235 |
| Insulina | .353** | -.112 | .253* | .380** |
| HOMA-IRa | .378** | -.103 | .336* | .420** |
| TG | .134 | -.011 | .057 | .184 |
| TC | -.050 | .018 | .250 | -.063 |
| LDL-C | -.119 | .001 | .297 | -.029 |
| HDL-C | -.167 | .125 | .124 | -.002 |
| Creatinine | -.137 | .048 | .098 | -.156 |
| AST | .058 | -.094 | -.036 | .124 |
| ALT | .134 | -.137 | .057 | .129 |
| IL-6 | .076 | .273** | -.024 | .325** |
| TNF-αa | -.041 | .250** | .153 | .365** |
| E-Selectin | .088 | .382** | .420** | .339** |
| ICAM-1 | .206 | .403** | .362* | .421** |
| VCAM-1 | .044 | .392** | .388* | .381** |
| Adiponectin | -.068 | -.347** | -.266* | -.262* |
* Correlation is significant at the 0.05 level (2-tailed).
** Correlation is significant at the 0.01 level (2-tailed).
a Logarithmic transformation was performed
Independent associations of CTRP9 with sICAM-1 (β [SE] = 0.196 [0.08]; p = 0.012), HOMA-IR (β [SE] = 74.64 [24.7]; p = 0.004) and TNF-α (β [SE] = 2.05[0.8]; p = 0.010) were shown by multiple linear regression analysis. Strikingly, in CAD patients (CAD and CAD+T2DM groups), CTRP9 was found to be significantly associated with BMI (β [SE] = 1.64 [0.815], p = 0.045), adiponectin (β [SE] = -4.37 [1.13]; p < 0.001), sE-Selectin (β [SE] = 0.757 [0.216]; p = 0.001), sICAM-1 (β [SE] = 0.142 [0.05); p = 0.003), sVCAM-1 (β [SE] = 0.091 [0.03]; p = 0.001) and TNF-α (β [SE] = 1.192 [0.53]; p = 0.026). Finally, results of multiple linear regression analysis in patients with T2DM (T2DM and CAD+T2DM groups) revealed the independent association of CTRP9 with BMI (β [SE] = 2.76 [1.17]; p = 0.021), HOMA-IR(β [SE] = 61.71 [20.54]; p = 0.003), sE-Selectin (β [SE] = 0.737 [0.34]; p = 0.033) and sVCAM-1(β [SE] = 0.084 [0.04]; p = 0.041).
Discussion
The findings of the present study revealed that circulating CTRP9 levels were associated with an increased risk of T2DM and CAD. The associations between CTRP family members and cardio-metabolic abnormalities have been documented by previous studies [24–26]. Interestingly, we found an independent association of CTRP9 levels with soluble adhesion molecules in patients with CAD and T2DM. These results indicate that serum levels of CTRP9 were elevated in CAD and T2DM patients. Preceding studies showed increased levels of serum CTRP9 in obesity and its attendant health risks [8,18–20], while decreased serum levels of CTRP9 was reported in CAD patients [16]. These observed discrepancies may have resulted from differences such as non-CAD subject selection/exclusion criteria and ethnicities between studies.
In the current study, non-CAD subjects were selected from individuals who underwent angiography and had normal coronary arteries. Moreover, a PPAR-agonist could affect the expression of CTRP9 [27] and, therefore, patients treated with this drug (agonist) were excluded from the study. The results of this study were in line with those of Chang Hee Jung et al., who reported a positive association of CTRP9 levels with BMI and arterial stiffness [19] and a study by Asada et al. in which a positive association between serum CTRP9 levels and atherosclerosis in T2DM patients was demonstrated [18]. In contrast, decreased and increased levels of circulating CTRP9 after bariatric surgery and in patients with impaired fasting glucose were shown, respectively [20], as well as increased serum CTRP9 levels in newly diagnosed T2DM patients [28].
It has been proposed that elevated circulating CTRP9 levels in T2DM and CAD patients might be a compensatory response to insulin resistance and atherogenic milieu [19]. Notably, the current study found higher levels of CTRP9 in women than men, which is in agreement with the results of previous studies reporting a sexually dimorphic pattern of circulating CTRP9 levels [8,19]. In addition, discrepancies in the concentration range of CTRP9 levels among previous studies [19–21,29,30] may be related to the performance of various ELISA kits and the basis of their design.
CTRP9 is predominantly secreted by adipose tissue and the CTRP9 gene is up-regulated in the adipose tissue of obese mice [8]. Serum CTRP9 levels were found to be inversely correlated with BMI in individuals with T2DM [19] and reduced CTRP9 levels were observed in obese patients following bariatric surgery [20]. In the current study, circulating CTRP9 levels were independently correlated to BMI. These findings suggest that CTRP9 is up-regulated in the adipose tissue of normal and T2DM subjects compared to CAD patients, because no correlation was found between CTRP9 and BMI in CAD individuals. The favorable effects of CTRP9 on glucose metabolism and insulin sensitivity have been previously demonstrated [9,10]. Targeted deletion of CTRP9 has been shown to decrease insulin sensitivity in mice [10] and a positive association of serum CTRP9 levels with glucose metabolism parameters has been shown in human studies [19,20].
It has been suggested that CTRP9 activates Akt, AMPK and p42/44 MAPK and increases glucose uptake [31]. The current study found that CTRP9 levels are positively correlated with glucose metabolism parameters in healthy subjects and patients with T2DM (T2DM and T2DM+CAD groups), suggesting a compensatory increase in CTRP9 in insulin-resistant conditions; however, more studies are needed to prove this concept. Positive correlations between CTRP9 and inflammatory cytokines (IL-6 and TNF-α) in CAD individuals (CAD and CAD+T2DM groups) were observed in the results, indicating a compensatory response to inflammatory milieu in patients with CAD. CTRP9 inhibits inflammatory responses in macrophages and was shown to improve plaque stability by reducing inflammatory cytokine secretions in mice [31,32].
CTRP9 plays a protective role in endothelium functions as well as vasorelaxation, modulation of vascular smooth muscle cell proliferation, regulation of arterial stiffness and neointimal hyperplasia for both in vitro and in vivo models [12,14,15,19]. Moreover, CTRP9 stimulates AMP-activated protein kinase, which could inhibit expression of adhesion molecules (ICAM-1 and VCAM-1) in endothelial cells [33]. Notably, the current results revealed a positive association between CTRP9 and soluble adhesion molecules in CAD and T2DM patients. To the best of our knowledge, this is the first in vivo evidence for association of cell adhesion molecules with CTRP9.
It was also observed that serum levels of CTRP9 were negatively related to adiponectin in CAD patients (CAD and T2DM+CAD group). Adiponectin has anti-atherogenic properties and forms a heterodimer with CTRP9 [8]. The observed correlations between CTRP9 and outcomes of interest were significant, even after adjustment for adiponectin level, with the exception of BMI, insulin and sICAM-1 in the T2DM group and IL-6 in the CAD+T2DM group. These findings suggest that increased levels of CTRP9 may be a compensatory response to decreased adiponectin levels, insulin resistance and inflammatory milieu in CAD and T2DM patients.
In conclusion, the present study showed elevated levels of CTRP9 in T2DM and CAD patients as well as positive correlations of CTRP9 with BMI, glucose metabolism parameters, inflammatory markers and adhesion molecules and a negative correlation with adiponectin. The results of this study suggest that pathological conditions such as insulin resistance in T2DM are accompanied by a lack of sensitivity to CTRP9; yet this hypothesis should be investigated with future longitudinal, cellular and molecular biology studies.
The strength of this study lies in the selection of CAD and non-CAD patients using coronary angiographic findings as the gold standard and investigating the relation between adhesion molecules, indicators of inflammation and serum CTRP9 levels. Some limitations also should be addressed. These include the relatively small sample size, especially in the T2DM group, and a case-control study design in which the cause-and-effect relationship could not be determined.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
This work was supported by a grant (94-04-30-26498) from Iran University of Medical Science.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a grant (94-04-30-26498) from Iran University of Medical Science. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89: 2548–2556. doi: 10.1210/jc.2004-0395 [DOI] [PubMed] [Google Scholar]
- 2.Rasouli N, Kern PA (2008) Adipocytokines and the Metabolic Complications of Obesity. The Journal of Clinical Endocrinology and Metabolism 93: S64–S73. doi: 10.1210/jc.2008-1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fadaei R, Parvaz E, Emamgholipour S, Moradi N, Vatannejad A, Najafi M, et al. (2016) The mRNA Expression and Circulating Levels of Visfatin and Their Correlation with Coronary Artery Disease Severity and 25-Hydroxyvitamin D. Horm Metab Res 48: 269–274. doi: 10.1055/s-0035-1564133 [DOI] [PubMed] [Google Scholar]
- 4.Ouchi N, Parker JL, Lugus JJ, Walsh K (2011) Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11: 85–97. doi: 10.1038/nri2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahmoudi M, Aslani S, Fadaei R, Jamshidi AR (2017) New insights to the mechanisms underlying atherosclerosis in rheumatoid arthritis. Int J Rheum Dis 20: 287–297. doi: 10.1111/1756-185X.12999 [DOI] [PubMed] [Google Scholar]
- 6.Wong GW, Wang J, Hug C, Tsao TS, Lodish HF (2004) A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci U S A 101: 10302–10307. doi: 10.1073/pnas.0403760101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seldin MM, Tan SY, Wong GW (2014) Metabolic function of the CTRP family of hormones. Reviews in endocrine & metabolic disorders 15: 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C, et al. (2009) Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. Faseb j 23: 241–258. doi: 10.1096/fj.08-114991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson JM, Wei Z, Seldin MM, Byerly MS, Aja S, Wong GW (2013) CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei Z, Lei X, Petersen PS, Aja S, Wong GW (2014) Targeted deletion of C1q/TNF-related protein 9 increases food intake, decreases insulin sensitivity, and promotes hepatic steatosis in mice. Am J Physiol Endocrinol Metab 306: E779–790. doi: 10.1152/ajpendo.00593.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su H, Yuan Y, Wang X-M, Lau WB, Wang Y, Wang X, et al. (2012) Inhibition of CTRP9, a novel and cardiac-abundantly expressed cell survival molecule, by TNFα-initiated oxidative signaling contributes to exacerbated cardiac injury in diabetic mice. Basic Research in Cardiology 108: 315 doi: 10.1007/s00395-012-0315-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Q, Yuan Y, Yi W, Lau WB, Wang Y, Wang X, et al. (2011) C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler Thromb Vasc Biol 31: 2616–2623. doi: 10.1161/ATVBAHA.111.231050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Yi W, Yuan Y, Lau WB, Yi D, Wang X, et al. (2013) C1q/tumor necrosis factor-related protein-9, a novel adipocyte-derived cytokine, attenuates adverse remodeling in the ischemic mouse heart via protein kinase A activation. Circulation 128: S113–120. doi: 10.1161/CIRCULATIONAHA.112.000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Geng X, Wang H, Cheng G, Xu S (2016) CTRP9 Ameliorates Pulmonary Arterial Hypertension Through Attenuating Inflammation and Improving Endothelial Cell Survival and Function. J Cardiovasc Pharmacol 67: 394–401. doi: 10.1097/FJC.0000000000000364 [DOI] [PubMed] [Google Scholar]
- 15.Uemura Y, Shibata R, Ohashi K, Enomoto T, Kambara T, Yamamoto T, et al. (2013) Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. Faseb j 27: 25–33. doi: 10.1096/fj.12-213744 [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Hang T, Cheng XM, Li DM, Zhang QG, Wang LJ, et al. (2015) Associations of C1q/TNF-Related Protein-9 Levels in Serum and Epicardial Adipose Tissue with Coronary Atherosclerosis in Humans. Biomed Res Int 2015: 971683 doi: 10.1155/2015/971683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Hang T, Cheng X-m, Li D-m, Zhang Q-g, Wang L-j, et al. (2015) Associations of C1q/TNF-Related Protein-9 Levels in Serum and Epicardial Adipose Tissue with Coronary Atherosclerosis in Humans. BioMed Research International 2015: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asada M, Morioka T, Yamazaki Y, Kakutani Y, Kawarabayashi R, Motoyama K, et al. (2016) Plasma C1q/TNF-Related Protein-9 Levels Are Associated with Atherosclerosis in Patients with Type 2 Diabetes without Renal Dysfunction. Journal of Diabetes Research 2016: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung CH, Lee MJ, Kang YM, Jang JE, Leem J, Lee YL, et al. (2014) Association of serum C1q/TNF-related protein-9 concentration with arterial stiffness in subjects with type 2 diabetes. J Clin Endocrinol Metab 99: E2477–2484. doi: 10.1210/jc.2014-2524 [DOI] [PubMed] [Google Scholar]
- 20.Wolf RM, Steele KE, Peterson LA, Zeng X, Jaffe AE, Schweitzer MA, et al. (2016) C1q/TNF-Related Protein-9 (CTRP9) Levels Are Associated With Obesity and Decrease Following Weight Loss Surgery. J Clin Endocrinol Metab 101: 2211–2217. doi: 10.1210/jc.2016-1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang YC, Woo Oh S, Park SW, Park CY (2014) Association of serum C1q/TNF-Related Protein-9 (CTRP9) concentration with visceral adiposity and metabolic syndrome in humans. Int J Obes (Lond) 38: 1207–1212. [DOI] [PubMed] [Google Scholar]
- 22.Jung CH, Lee MJ, Kang YM, Lee YL, Seol SM, Yoon HK, et al. (2016) C1q/TNF-related protein-9 inhibits cytokine-induced vascular inflammation and leukocyte adhesiveness via AMP-activated protein kinase activation in endothelial cells. Mol Cell Endocrinol 419: 235–243. doi: 10.1016/j.mce.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 23.(2015) 2. Classification and Diagnosis of Diabetes. Diabetes Care 38: S8–S16. [DOI] [PubMed] [Google Scholar]
- 24.Fadaei R, Moradi N, Baratchian M, Aghajani H, Malek M, Fazaeli AA, et al. (2016) Association of C1q/TNF-Related Protein-3 (CTRP3) and CTRP13 Serum Levels with Coronary Artery Disease in Subjects with and without Type 2 Diabetes Mellitus. PLOS ONE 11: e0168773 doi: 10.1371/journal.pone.0168773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan X, Lu T, Wu F, Jin L, Zhang Y, Shi L (2014) Circulating complement-C1q TNF-related protein 1 levels are increased in patients with type 2 diabetes and are associated with insulin sensitivity in Chinese subjects. PLoS One 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanaki M, Fadaei R, Moradi N, Emamgholipour S, Poustchi H (2016) The Circulating CTRP13 in Type 2 Diabetes and Non-Alcoholic Fatty Liver Patients. PLOS ONE 11: e0168082 doi: 10.1371/journal.pone.0168082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seldin MM, Tan SY, Wong GW (2014) Metabolic function of the CTRP family of hormones. Rev Endocr Metab Disord 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia Y, Luo X, Ji Y, Xie J, Jiang H, Fu M, et al. (2017) Circulating CTRP9 levels are increased in patients with newly diagnosed type 2 diabetes and correlated with insulin resistance. Diabetes Res Clin Pract 131: 116–123. doi: 10.1016/j.diabres.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 29.Forouhi N, Saedisomeolia A, Djalali M, Eshraghian MR, Morshedzadeh N, Zabetian-Targhi F, et al. (2016) Serum C1q and tumor necrosis factor (TNF)-related protein 9 in women with Polycystic Ovary Syndrome. Diabetes Metab Syndr 10: S131–134. doi: 10.1016/j.dsx.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 30.Bai B, Ban B, Liu Z, Zhang MM, Tan BK, Chen J (2017) Circulating C1q complement/TNF-related protein (CTRP) 1, CTRP9, CTRP12 and CTRP13 concentrations in Type 2 diabetes mellitus: In vivo regulation by glucose. PLoS One 12: e0172271 doi: 10.1371/journal.pone.0172271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang P, Huang C, Li J, Li T, Guo H, Liu T, et al. (2016) Globular CTRP9 inhibits oxLDL-induced inflammatory response in RAW 264.7 macrophages via AMPK activation. Mol Cell Biochem 417: 67–74. doi: 10.1007/s11010-016-2714-1 [DOI] [PubMed] [Google Scholar]
- 32.Li J, Zhang P, Li T, Liu Y, Zhu Q, Chen T, et al. (2015) CTRP9 enhances carotid plaque stability by reducing pro-inflammatory cytokines in macrophages. Biochemical and biophysical research communications 458: 890–895. doi: 10.1016/j.bbrc.2015.02.054 [DOI] [PubMed] [Google Scholar]
- 33.Jung CH, Lee MJ, Kang YM, Lee YL, Seol SM, Yoon HK, et al. (2016) C1q/TNF-related protein-9 inhibits cytokine-induced vascular inflammation and leukocyte adhesiveness via AMP-activated protein kinase activation in endothelial cells. Molecular and Cellular Endocrinology 419: 235–243. doi: 10.1016/j.mce.2015.10.023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.