ABSTRACT
Daurichromenic acid (DCA) is a meroterpenoid with anti-HIV activities that is isolated from Rhododendron dauricum L. We recently reported that DCA is biosynthesized and accumulated in the apoplast of glandular scales attached on the surface of young leaves of R. dauricum. In the present study, we confirmed that a cell suspension culture of R. dauricum could not produce DCA and its precursor grifolic acid even after elicitation with methyl jasmonate and β-cyclodextrin. In addition, exogenous supplementation of DCA and grifolic acid effectively induced cell death in the same culture, with apoptosis-associated phenomena such as cytoplasmic shrinkage, chromatin condensation, and genomic DNA degradation. These findings suggested that DCA and grifolic acid are phytotoxic metabolites that have to be sequestered in the apoplast to avoid self-poisoning.
KEYWORDS: Rhododendron dauricum, daurichromenic acid, grifolic acid, meroterpenoid, phytotoxic activity
Rhododendron dauricum L. (Ericaceae), distributed in northeastern Asia, produces unique secondary metabolites including daurichromenic acid (DCA) (Fig. 1), a novel meroterpenoid composed of orsellinic acid and sesquiterpene moieties.1 DCA has attracted considerable attention as a medicinal resource because this compound shows a potent anti-HIV activity.1 In addition, DCA might be a metabolite specialized for defense, because it has antibacterial and antifungal activities.2,3 Concerning the biosynthesis of DCA, we have recently reported that DCA is produced from grifolic acid by DCA synthase, a flavoprotein oxidase with a covalently attached flavin adenine dinucleotide (FAD) cofactor.4 DCA synthase catalyzes the oxidative cyclization of farnesyl moiety of grifolic acid, in a FAD- and oxygen-dependent manner, to produce DCA and hydrogen peroxide, as shown in Fig. 1. The unique feature of DCA biosynthesis is relative to its tissue distribution and localization; DCA synthase is distributed exclusively in the glandular scales, the multicellular epidermal tissues covering young leaves of R. dauricum.4 In addition, as DCA synthase is a secreted protein, the biosynthetic reaction presumably proceeds extracellularly, leading to the accumulation of DCA in the apoplast of glandular scales as we reported previously.4 With respect to this unique extracellular biosynthesis and accumulation of DCA, we proposed the possibility that DCA would serve as a chemical defense component outside of the scale cells, the outermost layer of plants. In addition, we suspected that DCA is excluded from cells because it might be toxic to plant cells, as in the case of structurally related meroterpenoids, such as cannabinoids.5 To confirm this possibility, in the present study, we analyzed whether DCA shows phytotoxic activity to newly established cell suspension cultures of R. dauricum.
Figure 1.
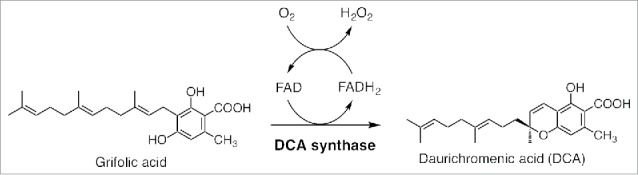
Biosynthesis of daurichromenic acid (DCA). DCA is synthesized by DCA synthase via stereoselective oxidocyclization of the farnesyl moiety of grifolic acid. Two electrons from the substrate are abstracted by enzyme-bound flavin adenine dinucleotide (FAD), and then transferred to molecular oxygen to release H2O2 as the by-product, as described previously.4
We first attempted to induce calli in cut stem pieces of young R. dauricum specimens. After an examination of various supplements, including plant growth regulators, actively growing yellowish calli were successfully obtained on a solidified Murashige–Skoog (MS) medium,6 which was supplemented with 1 mg/L of 2,4-dichlorophenoxyacetic acid (2,4-D) and 200 mg/L of KH2PO4. Interestingly, the addition of KH2PO4, as well as 2,4-D, was crucial for efficient callus induction and successive maintenance, indicating that phosphate ions promote cell division in R. dauricum, as is reported for tobacco BY-2 and Catharanthus roseus cell cultures.7,8 The rapidly growing cell suspension culture was then obtained in the aforementioned liquid medium. The culture consisted of cell aggregates of round, oval, and expanded oval-shaped cells, of which the diameter was in the range of 100–500 μm (Fig. 2A). As described, a cell culture of R. dauricum was successfully established using a simply modified MS medium.
Figure 2.
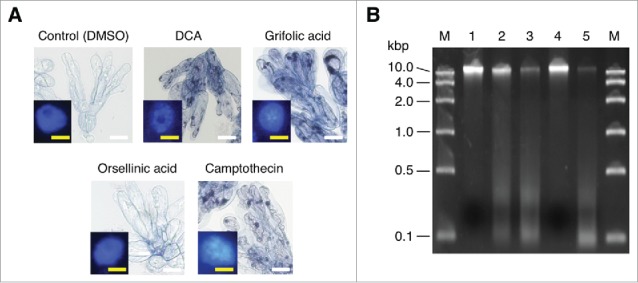
Phytotoxic effects of daurichromenic acid (DCA) and grifolic acid on R. dauricum cells. (A) Trypan blue staining to evaluate cell viability. Seven-day-old cell cultures were incubated with 100 µM of each indicated compound for 24 h, then stained with trypan blue. Control cells were treated with the solvent dimethyl sulfoxide (DMSO) only. White Bar, 100 µm. Inserted images are the fluorescent detection of nuclei after staining with 4′,6-diamidino-2-phenylindole (DAPI). Yellow bar, 10 µm. (B) Agarose gel electrophoresis of genomic DNA from R. dauricum cells treated as in A. DNA was separated on a 2% agarose gel and stained with ethidium bromide. Lane M, marker DNA with the indicated sizes; lane 1, control cells; lane 2, DCA-treated cells; lane 3, grifolic acid-treated cells; lane 4, orsellinic acid-treated cells; and lane 5, camptothecin-treated cells.
We conducted HPLC analyses to confirm the production of DCA in the cell suspension culture. As a result, peaks corresponding to DCA or grifolic acid were not detected in the cell extract nor the culture supernatant (Supplementary Fig. S1). The detected unknown peaks were analyzed by liquid chromatography-mass spectrometry as described previously,4 but there were no compounds likely to be DCA-related intermediates or metabolites (data not shown). Thus, we next tested the effects of elicitor treatments on DCA biosynthesis in the cultured cells using methyl jasmonate and β-cyclodextrin. However, DCA and grifolic acid were not detected, even after the combined addition of 100 μM of methyl jasmonate and 9 g/L of β-cyclodextrin (Fig. S1), which was previously reported to be highly effective for the production of meroterpenoid natural products, such as prenylated stilbenoids in Arachis hypogaea cell cultures.8 Therefore, we assumed that the artificial control of DCA biosynthesis in cell cultures is quite difficult to conduct. The present study of a R. dauricum cell culture evoked previous studies on cannabinoids in Cannabis sativa L. (Cannabaceae). Cannabinoids are a group of meroterpenoids structurally related to DCA, and could not be produced in C. sativa cell cultures, although various conditions were tested in a number of laboratories.10 Similarly to DCA, cannabinoids are accumulated in the extracellular compartment of specialized epidermal tissues; they are stored in the secretory cavities of glandular trichomes on the flowers and young leaves of C. sativa.5
As described, we could not produce DCA, a promising medicinal resource, in our cell culture, likely due to its phytotoxic properties making it difficult to be produced within non-differentiated plant cells. Thus, to confirm this possibility, DCA and grifolic acid were exogenously added to the cell suspension culture, and the viability of cells after the treatment of each compound was measured by trypan blue staining. Camptothecin was also used as a chemical inducer of programmed cell death.11 As was seen in the camptothecin treatment, 100 μM of DCA and grifolic acid induced almost 100% cell death in the culture within the 24 h treatment (Fig. 2A), whereas orsellinic acid, their polyketide precursor, had no significant effects on the cell viability, suggesting that the isoprenoid moieties are crucial for the phytotoxic activity of DCA and grifolic acid. In addition, it was of interest that apoptosis-related morphological properties, such as cytoplasmic shrinkage and chromatin condensation,12 were clearly observed for most of the meroterpenoids-treated cells, as well as camptothecin-treated cells (Fig. 2A). Chromatin condensation was studied using 4′,6-diamidino-2-phenylindole (DAPI) staining coupled with fluorescence microscopy, wherein dead cells could be identified by the glandular appearance of the chromatin. Furthermore, significant DNA degradation was also detectable in genomic DNA samples from meroterpenoids- and camptothecin-treated cells (Fig. 2B). These results clearly indicated that DCA and grifolic acid are phytotoxic meroterpenoids that induce cell death in their producer plant R. dauricum, probably via the apoptotic pathway, as is the case with camptothecin-mediated cell death.11
Because the DCA synthase reaction is accompanied by the production of hydrogen peroxide (H2O2) as illustrated in Fig. 1, the effects of H2O2 on the R. dauricum cell culture were also examined. As expected, H2O2 also induced apotosis-related reactions leading to cell death in the culture, however, higher concentrations (>10 mM) were required to effectively induce cell death compared to DCA and grifolic acid. A previous study has similarly demonstrated that more than 10 mM of H2O2 is required for apoptosis induction in tobacco BY-2 cell cultures,13 although H2O2 is generally regarded as a potent cell death mediator in plants.14 We supposed that, because H2O2 is highly reactive, exogenously added H2O2 might be decomposed considerably through interactions with medium components such as metal ions.15 In addition, secreted class III peroxidases,16 commonly distributed in the plant kingdom, would participate in detoxification of exogenously added H2O2 in the R. dauricum cell culture. Nevertheless, because DCA is highly accumulated in plants,4 a large amount of H2O2 would also be produced as a by-product during DCA biosynthesis. Thus, it is reasonable that DCA synthase reactions take place extracellularly in specialized tissues to avoid cellular damage by H2O2 as well as DCA. Additionally, the production of H2O2 in the apoplastic space might also be a self-defense mechanism, as H2O2 has antimicrobial properties.17
It is well known that plants produce a vast array of specialized metabolites for various purposes, including self-defense and communication with other organisms in their environments.18 These specialized metabolites are often toxic, even for their host plants, and therefore plants elaborate this process to circumvent self-poisoning, for example, by sequestering them into extracellular compartments such as the storage cavities of glandular trichomes.19,20 Meroterpenoids are hybrid natural products that harbor isoprenoid moieties in their molecules, many of which have characteristic biological activities.4 It has been reported that a number of meroterpenoids, such as prenylated flavonoids,21 stilbenoids,9 and furanocoumarins,22 are secreted into apoplastic compartments after their biosynthesis to avoid self-poisoning, and probably to serve as self-defense. This process can be seen in cannabinoids, as we reported previously: they possess phytotoxic properties and are localized in the storage cavities of glandular trichomes.5 DCA and grifolic acid, structurally related to cannabinoids, were found to be similar examples in this study.
In summary, we herein demonstrated that DCA is toxic to the cultured cells of R. dauricum, and therefore has to be stored outside of the scale cells. In addition, the precursor grifolic acid was also phytotoxic, whereas orsellinic acid was not. Thus, grifolic acid has to be effectively secreted after biosynthesis via the prenylation of orsellinic acid. Generally, prenylation is a key diversification step to changing the biological activity of specialized metabolites dramatically,23 and this holds true for the phytotoxicity of grifolic acid. The molecular mechanism to secrete grifolic acid, along with the unidentified aromatic prenyltransferase to produce it, is of significant research interest, because few mechanisms involved in the secretion of plant specialized metabolites have been identified and characterized, even in the recent -omics era.24
Methods
In this study, all chemicals were purchased from Wako Pure Chemical Industries (Osaka, Japan), unless otherwise stated.
R. dauricum plants were cultivated at the Experimental Station for Medicinal Plant Research, University of Toyama. Young stems were cut into ∼1 cm pieces, which were surface sterilized with 1% sodium hypochlorite for 15 min. The sterilized stems were placed on a MS agar plate, supplemented with 30 g/L of sucrose, 100 mg/L of inositol, 200 mg/L of K2HPO4, and 1 mg/L of 2,4-D, and were cultured in the dark at 25°C for two months to induce the forming of calli. A liquid suspension culture was then generated by transferring fresh calli into 30 ml liquid mediums of the same composition in 100-ml conical flasks. The flasks were shaken at 130 rpm in the dark at 25°C. Subculture was conducted by adding 3 mL of full-growth culture into fresh medium every two weeks.
Elicitor treatments were performed with 100 μM of methyl jasmonate and/or 9 g/L of β-cyclodextrin on seven-day old cultures, and incubated for 96 h prior to analyses. The cellular extract was prepared from cultured cells by homogenization with methanol, and analyzed with a reversed-phase HPLC system,25 together with culture supernatant. Elution was performed isocratically with 75% aqueous acetonitrile containing 0.1% formic acid at a flow rate of 1.0 mL/min, and constituents were detected by absorption at 254 nm.
The viability of the cultured cells 24 h after the addition of the chemicals was measured as previously described5 using trypan blue staining. For the analysis of nuclear morphology, DAPI staining was performed following the manufacturer's instruction (Bio-Rad Laboratories, Hercules, CA, USA). Cells were visualized using a BX-50 microscope equipped with a BX-FLA fluorescent unit (Olympus, Tokyo, Japan), using a DAPI filter set. Genomic DNA extraction and electrophoresis were conducted as described previously.5
Abbreviations
- 2,4-D
2,4-dichlorophenoxyacetic acid
- DAPI
4′,6-diamidino-2-phenylindole
- DCA
daurichromenic acid
- DMSO
dimethyl sulfoxide
- FAD
flavin adenine dinucleotide
- MS
Murashige–Skoog
Supplementary Material
Funding Statement
This work was supported by JSPS/MEXT KAKENHI (grant nos. 15K07994 and 17H05436 to F.T.), a Grant-in-Aid for JSPS Fellows (no. 17J10178 to M.I.), and JSPS Core-to-Core Program, B. Asia-Africa Science Platforms (F.T. is one of the recipients).
Disclosure of potential conflict of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Hiroharu Fujino, Yoshiaki Tatsuo, Yasumasa Takao, and Yoshiya Murakami, at the Experimental Station for Medicinal Plant Research of University of Toyama, for breeding R. dauricum plants.
References
- 1.Kashiwada Y, Yamazaki K, Ikeshiro Y, Yamagishi T, Fujioka T, Mihashi K, Mizuki K, Cosentino LM, Fowke K, Morris-Natschke SL, et al. Isolation of rhododaurichromanic acid B and the anti-HIV principles rhododaurichromanic acid A and rhododaurichromenic acid from Rhododendron dauricum. Tetrahedron. 2001;57:1559–63. doi: 10.1016/S0040-4020(00)01144-3. [DOI] [Google Scholar]
- 2.Hashimoto T, Quang DN, Nukada M, Asakawa Y. Isolation, synthesis and biological activity of grifolic acid derivatives from the inedible mushroom Albatrellus dispansus. Heterocycles. 2005;65:2431–39. doi: 10.3987/COM-05-10501. [DOI] [Google Scholar]
- 3.Okada M, Saito K, Wong PC, Li C, Wang D, Iijima M, Taura F, Kurosaki F, Awakawa T, Abe I. Combinatorial biosynthesis of (+)-daurichromenic acid and its halogenated analogue. Org Lett. 2017;19:3183–86. doi: 10.1021/acs.orglett.7b01288. [DOI] [PubMed] [Google Scholar]
- 4.Iijima M, Munakata R, Takahashi H, Kenmoku H, Nakagawa R, Kodama T, Asakawa Y, Abe I, Yazaki K, Kurosaki F, et al. Identification and characterization of daurichromenic acid synthase active in anti-HIV biosynthesis. Plant Physiol. 2017;174:2213–30. doi: 10.1104/pp.17.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sirikantaramas S, Taura F, Tanaka Y, Ishikawa Y, Morimoto S, Shoyama Y. Tetrahydrocannabinolic acid synthase, the enzyme controlling marijuana psychoactivity, is secreted into the storage cavity of the glandular trichomes. Plant Cell Physiol. 2005;46:1578–82. doi: 10.1093/pcp/pci166. [DOI] [PubMed] [Google Scholar]
- 6.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–97. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 7.Sano T, Kuraya Y, Amino S, Nagata T. Phosphate as a limiting factor for the cell division of tobacco BY-2 cells. Plant Cell Physiol. 1999;40:1–8. doi: 10.1093/oxfordjournals.pcp.a029464. [DOI] [PubMed] [Google Scholar]
- 8.Amino S, Fujimura T, Komamine A. Synchrony induced by double phosphate starvation in a suspension culture of Catharanthus roseus. Physiol Plant. 1983;59:393–96. doi: 10.1111/j.1399-3054.1983.tb04220.x. [DOI] [Google Scholar]
- 9.Yang T, Fang L, Nopo-Olazabal C, Condori J, Nopo-Olazabal L, Balmaceda C, Medina-Bolivar F. Enhanced production of resveratrol, piceatannol, arachidin-1, and arachidin-3 in hairy root cultures of peanut co-treated with methyl jasmonate and cyclodextrin. J Agric Food Chem. 2015;22:3942–50. doi: 10.1021/jf5050266. [DOI] [PubMed] [Google Scholar]
- 10.Andre CM, Hausman JF, Guerriero G. Cannabis sativa: The plant of the thousand and one molecules. Front Plant Sci. 2016;7:19. doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Jong AJ, Hoeberichts FA, Yakimova ET, Maximova E, Woltering EJ. Chemical-induced apoptotic cell death in tomato cells: involvement of caspase-like proteases. Planta. 2000;211:656–62. doi: 10.1007/s004250000341. [DOI] [PubMed] [Google Scholar]
- 12.Latrasse D, Benhamed M, Bergounioux C, Raynaud C, Delarue M. Plant programmed cell death from a chromatin point of view. J Exp Bot. 2016;67:5887–5900. doi: 10.1093/jxb/erw329. [DOI] [PubMed] [Google Scholar]
- 13.Houot V, Etienne P, Petitot AS, Barbier S, Blein JP, Suty L. Hydrogen peroxide induces programmed cell death features in cultured tobacco BY-2 cells, in a dose-dependent manner. J Exp Bot. 2001;52:1721–30. doi: 10.1093/jexbot/52.361.1721. [DOI] [PubMed] [Google Scholar]
- 14.Gechev TS, Hille J. Hydrogen peroxide as a signal controlling plant programmed cell death. J Cell Biol. 2005;168:17–20. doi: 10.1083/jcb.200409170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haber F, Weiss J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc R Soc Lond A Matter. 1934;147:332–351. doi: 10.1098/rspa.1934.0221. [DOI] [Google Scholar]
- 16.Cosio C, Dunand C. Specific functions of individual class III peroxidase genes. J Exp Bot. 2009;60:391–408. doi: 10.1093/jxb/ern318. [DOI] [PubMed] [Google Scholar]
- 17.Custers JH, Harrison SJ, Sela-Buurlage MB, Deventer E van, Lageweg W, Howe PW, Meijs PJ van der, Ponstein AS, Simons BH, Melchers LS, et al. Isolation and characterisation of a class of carbohydrate oxidases from higher plants, with a role in active defence. Plant J. 2004;39:147–60. doi: 10.1111/j.1365-313X.2004.02117.x. [DOI] [PubMed] [Google Scholar]
- 18.Bennett RN, Wallsgrove RM. Secondary metabolites in plant defence mechanisms. New Phytologist. 1994;127:617–33. doi: 10.1111/j.1469-8137.1994.tb02968.x. [DOI] [PubMed] [Google Scholar]
- 19.Sirikantaramas S, Yamazaki M, Saito K. Mechanisms of resistance to self-produced toxic secondary metabolites in plants. Phytochem Rev. 2008;7:467–77. doi: 10.1007/s11101-007-9080-2. [DOI] [Google Scholar]
- 20.Shitan N. Secondary metabolites in plants: transport and self-tolerance mechanisms. Biosci Biotechnol Biochem. 2016;80:1283–93. doi: 10.1080/09168451.2016.1151344. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto H, Yamaguchi M, Inoue K. Absorption and increase in the production of prenylated flavanones in Sophora flavescens cell suspension cultures by cork pieces. Phytochemistry. 1996;43:603–08. doi: 10.1016/0031-9422(96)00321-4. [DOI] [Google Scholar]
- 22.Voo SS, Grimes HD, Lange BM. Assessing the biosynthetic capabilities of secretory glands in citrus peel. Plant Physiol. 2012;159:81–94. doi: 10.1104/pp.112.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yazaki K, Sasaki K, Tsurumaru Y. Prenylation of aromatic compounds, a key diversification of plant secondary metabolites. Phytochemistry. 2009;70:1739–45. doi: 10.1016/j.phytochem.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Tatsumi K, Yano M, Kaminade K, Sugiyama A, Sato M, Toyooka K, Aoyama T, Sato F, Yazaki K. Characterization of shikonin derivative secretion in Lithospermum erythrorhizon hairy roots as a model of lipid-soluble metabolite secretion from plants. Front Plant Sci. 2016;7:1066. doi: 10.3389/fpls.2016.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taura F, Iijima M, Lee JB, Hashimoto T, Asakawa Y, Kurosaki F. Daurichromenic acid-producing oxidocyclase in the young leaves of Rhododendron dauricum. Nat Prod Commun. 2014;9:1329–32. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


