Abstract
Objectives
To describe the change in the incidence rates of primary and secondary FSGS from 1994 to 2013 in Olmsted County and to identify the clinical and biopsy characteristics that can help distinguish primary from secondary FSGS.
Patients and Methods
Olmsted County adult residents with native kidney biopsy between January 1, 1994 and December 31, 2013 and FSGS as the only glomerulopathy were identified. The clinical and pathological charachterstics of primary and secondary FSGS were described and compared. Incidence rates of primary and secondary FSGS over period of 1994–2013 were calculated.
Results
Among 370 adults biopsied during this period, 281 had glomerular diseases, of which 46 (16%) had FSGS. From 1994–2003 to 2004–2013, there was a significant increase in kidney biopsy rates (14.7; 95% CI, 12.1–17.3 vs. 22.9; 95% CI, 20.0–25.7 per 100,000 person-years, 17% increase per 5 years, P<.001) and total FSGS rates (1.4; 95% CI, 0.6–2.2 vs. 3.2; 95% CI, 2.1–4.3 per 100,000 person-years, 41% increase per 5 years, P=.02). Compared to patients with limited foot process effacement (<80%), patients with diffuse effacement (≥80%) without an identifiable cause had lower serum albumin (−0.7 g/dl, P<.001), higher proteinuria (+4.5 g/day, P<.001) and were more likely to have nephrotic syndrome (100% vs 4%, P<.001). Patients with diffuse effacement without an identifiable cause were classified as primary FSGS, which accounted for 3/12 (25%) of cases during 1994–2003 and 9/34 (26%) of cases during 2004–2013.
Conclusion
While the incidence of FSGS has increased, the proportions of primary and secondary FSGS have remained stable.
INTRODUCTION
The incidence of focal segmental glomerulosclerosis (FSGS) in adults has increased over the past few decades. This temporal trend has been observed in both large metropolitan areas and in small rural communities, regardless of their racial and ethnic background.1–7(Table 1) FSGS now accounts for 20 to 40% of all biopsy-proven glomerular diseases in adults.3–8 However, the previous studies have important limitations. First, the majority of the studies reported trends in relative disease frequencies among biopsied patients rather than true population-based incidence rates of FSGS. This approach can result in misleading conclusions since a change in the proportion of one disease automatically affects the proportions of other diseases. Furthermore, as the referral population that undergoes kidney biopsy changes over time, so do the relative frequencies of different diseases. Another important limitation in the previous studies is the approach of reporting FSGS as a single disease entity.2–8 We now know that FSGS is a histological pattern of injury that characterizes a broad spectrum of diseases with different pathophysiologies. Primary FSGS is presumed to be due to a circulating permeability factor diffusely toxic to podocytes, which may respond to immunosuppressive treatment.9 On the other hand, secondary FSGS is a response to reduction in the number of functioning nephrons (e.g., unilateral renal agenesis), or from an abnormal stress on initially normal nephrons.9 The treatment is centered around unloading the pressure on glomeruli with renin-angiotensin-aldosterone system (RAAS) inhibition.
Table 1.
Summary of previous studies that evaluated focal segmental glomerulosclerosis (FSGS) frequency or incidence
| Study | Time Period | Location | Type of FSGS | Type of Study | Findings |
|---|---|---|---|---|---|
| Haas, M., et al. (1995)1 | 1974–1993 | Chicago, IL | Excluded cases with clear risk factors for secondary glomerulonephritis | Relative disease frequency | FSGS relative frequency increased from 4% during 1974–1979 to 12.2% during 1987–1993 |
| Korbet, S. M., et al. (1996)2 | 1975–1994 | Chicago, IL | Total FSGS | Relative disease frequency | FSGS accounted for 57% of glomerular lesions in blacks and 23% in whites. FSGS relative frequency increased from 39% in 1975–1984 to 64% in 1985–1994 among blacks. |
| Braden, G. L., et al. (2000)3 | 1974–1994 | Springfield, MA | Total FSGS | Relative disease frequency | Relative frequency of FSGS increased from 13.7% in 1975–1979 to 25% in 1990–1994. The increase was most notable in Blacks and Hispanics with only modest increase in Whites. |
| Swaminathan, S., et al. (2006)4 | 1974–2003 | Olmsted County, MN | Total FSGS | Population-based | Rate of FSGS increased from 0.1 per 100,000 person years in 1974–1983 to 1.8 per 100,000 person years in 1994–2003 |
| Sim, J. J., et al. (2016)6 | 2000–2011 | Southern California | Total FSGS | Population-based | FSFS was the most common diagnosis (38.9%) across all race and ethnic groups. Incidence rate increased from 1.6 per 100,000 person years in 2000 to 5.3 per 100,0000 person years in 2011 |
| Murugapandian, S., et al. (2016)5 | 2004–2014 | Tuscon, AZ | Total FSGS | Relative disease frequency | FSGS was the most common histopathological diagnosis (22%) |
| O'Shaughnessy, M. M., et al. (2017)7 | 1986–2015 | Chapel Hill, NC | Total FSGS | Relative disease frequency | Relative frequency of FSGS increased over three decades from 22.6% to 29.7% |
To better evaluate the incidence of FSGS, it is critical to distinguish primary FSGS from secondary forms within a population-based study. Olmsted County in Minnesota, USA is particularly well suited to perform a population-based study of glomerular diseases. The aim of this study was to describe the change in the incidence rates of primary and secondary FSGS from 1994 to 2013 in Olmsted County and to identify the clinical and biopsy characteristics that can help distinguish primary FSGS from secondary forms.
METHODS
Study population
The population of Olmsted County and their clinical care by nearly all providers are enumerated through the Rochester Epidemiology Project.10 This study included all adult residents of Olmsted County, Minnesota, USA who underwent native kidney biopsy between 1994 and 2013 at Mayo Clinic in Rochester, Minnesota. Mayo Clinic was the only regional center in Olmsted County that performed and read kidney biopsies in the study period.
Pathology characteristics
The following was abstracted from kidney biopsy reports: pathological diagnosis, the number of glomeruli and globally sclerotic glomeruli, arteriosclerosis, arteriolar hyalinosis, and interstitial fibrosis and tubular atrophy (IFTA). We identified patients with FSGS as the only glomerulopathy, defined as the presence of segmental sclerotic lesions on the biopsy. Patients with only focal global sclerosis were excluded. For each patient with FSGS, available light microscopy slides and electron micrographs were reviewed by two renal pathologists blinded to the clinical data (M.P.A. and S.S.). Each biopsy was assigned a Columbia Classification.11 Since the first histological manifestation of recurrent primary FSGS post kidney transplant is widespread foot process effacement (FPE), we initially divided the patients according to the degree of FPE. Patients were classified into having diffuse FPE (≥ 80%) vs limited FPE (<80%). The cut-off value of 80% was choosen based on previous work showing that patients with nephrotic syndrome, FSGS lesions and no identifiable risk factors for secondary FSGS had FPE in the range of 80–100%.12 The degree of FPE was evaluated on EM sections of at least two non-sclerosed glomeruli. FPE quantification was based on loops examined: 100%, all loops showed complete effacement; 90%, one of 10 loops did not show complete effacement; 80%, 2 of 10 loops did not show complete effacement. If foot processes were preserved, it was considered limited FPE. Arteriosclerosis and arteriolar hyalinosis were graded on a scale of 0–3 with 0=none, 1=mild, 2=moderate, and 3=severe. IFTA was classified into 0–5% (none to minimal), 6–25% (mild), and >25% (moderate to severe).13
Clinical characteristics
Baseline clinical characteristics were abstracted from the episode of care closest to the time of kidney biopsy and included age, sex, race, hypertension, diabetes mellitus, vascular disease (composite of coronary artery disease, stroke or peripheral arterial disease), dyslipidemia, use of ACEi, ARBs, statins, systolic and diastolic blood pressure, serum creatinine, serum albumin, total cholesterol, and 24 hour proteinuria. When timed urine collection was not available to quantify proteinuria, protein to creatinine or protein to osmolality ratio on spot urine sample was used to estimate daily proteinuria. Nephrotic syndrome at baseline was defined as proteinuria ≥ 3.5g/24h and serum albumin ≤ 3.5g/dl.14 Follow-up data included treatment initiated after the diagnosis of FSGS, serum creatinine, and proteinuria trends after the biopsy date, last visit date, and when applicable, death and development of end-stage renal disease (ESRD) dates.
FSGS classification
The identified FSGS cases were classified as having diffuse FPE (≥ 80%) or limited FPE (<80%). We further classified patients with diffuse FPE into those with identifiable causes for FPE and those without. Primary FSGS was defined as having diffuse FPE without an identifiable cause. Patients who had limited FPE, or had diffuse FPE but had an identifiable cause for FPE were classified as secondary FSGS. Patients who did not have electron micrographs to review were classified into primary vs secondary FSGS based on their clinical presentation.
Statistical analysis
The incidence rate and confidence intervals (per 100,000 person-years) for native kidney biopsies, FSGS, and other glomerulopathies were calculated for the entire study period as well as separately for the periods of 1994 to 2003 and 2004 to 2013. Rates were calculated using the exact method assuming a Poisson distribution and were adjusted for age and sex using the 2010 US Decennial Census. Poisson regression models were used to calculate the change in incidence rate per 5 years from 1994 to 2013. P-values were calculated using Fisher’s exact test for categorical variables and Wilcoxon Rank Sum test for continuous variables. Baseline characteristics, FSGS subtypes, and treatment approaches were described for patients with primary FSGS and secondary FSGS. Statistical analysis was done using SAS software and JMP® Pro, Version 10.0.0 (SAS Institute Inc., Cary, NC). P-values <.05 were considered statistically significant. This study was approved by the institutional review board at the Mayo Clinic in Rochester, Minnesota.
RESULTS
A total of 370 adult patients underwent native kidney biopsy between 1994 and 2013, of which 281 had glomerular diseases. Among those with glomerular diseases, 46 (16%) had FSGS as the only glomerulopathy (Supplemental Figure 1). Twelve cases of FSGS occurred in the first decade (1994–2003) and 34 in the second decade (2004–2013). Avilable electron micrographs were reviwed to classify patients according to degree of FPE. Four patients did not have electron micrographs to review. For the remaining 42 patients; 11 had diffuse FPE without an identifiable cause, 27 had limited FPE, and 4 had diffuse FPE due to an identifiable cause which included pre-eclampsia (N=1), syndromic presentation suspicious for genetic FSGS (N=1) and the presence of ischemic glomeruli only on electron micrography (N=2). Table 2 and Table 3 compare the clinical and biopsy characteristics of patients with diffuse FPE without identifiable cause and patients with limited FPE. Compared to patients with limited FPE, patients with diffuse FPE without identifiable cause had lower serum albumin (−0.7 g/dl, P<.001), higher proteinuria (+4.5 g/day, P<.001), were more likely to have nephrotic syndrome (NS) on presentation (100% vs 4%, P<.001). There was a single patient with limited FPE who approached criteria for NS. That patient had morbid obesity, severe uncontrolled hypertension, had serum albumin at the lower limit of normality (3.5 g/dl), proteinuria of 5 g/d, and 10% FPE. Although diffuse FPE was highly associated with NS, proteinuria alone had limited correlation with the degree of FPE (Pearson’s correlation coefficient r=0.41, P=.008)(Supplemental Figure 2)
Table 2.
Clinical characteristics of patients with focal segmental glomerulosclerosis by foot process effacement status
| Characteristic | Foot Process Effacement ≥ 80% without an identifiable cause N=11 | Foot Process Effacement <80%N=27 | P value |
|---|---|---|---|
|
| |||
| Mean ± SD or N(%) | Mean ± SD or N(%) | ||
| Demographics | |||
| Age, yr | 53 ± 20.1 | 53 ± 17.4 | .88 |
| Male | 6 (55%) | 15 (56%) | .9 |
| White | 9 (82%)a | 20 (74%)b | .9 |
| Clinical characteristics at time of biopsy | |||
| SBP at time of biopsy, mmHg | 132 ± 18.3 | 137 ± 24.6 | .62 |
| DBP at time of biopsy, mmHg | 74 ± 11.7 | 79 ± 15.9 | .27 |
| BMI, kg/m2 | 31.6 ± 6.4 | 32.2 ± 7.8 | .74 |
| Comorbidities | |||
| HTN | 8 (73%) | 23 (85%) | .39 |
| DM | 0 | 7 (26%) | .08 |
| Vascular diseasec | 2 (18%) | 8 (32%) | .69 |
| Dyslipidemia | 4 (36%) | 13 (48%) | .72 |
| BMI > 30 kg/m2 | 6 (55%) | 16 (59%) | .9 |
| Medications at time of biopsy | |||
| ACEi/ARB | 5 (45%) | 20 (59%) | .28 |
| Statins | 4 (36%) | 17 (50%) | .48 |
| Laboratory data at time of biopsy | |||
| Serum creatinine mg/dl | |||
| Median (IQR) | 1.4 (1.3–2.7) | 1.4 (1.1–2) | .83 |
| Albumin, g/dl | 3.3 ± 0.3 | 4.0 ± 0.3 | <.001 |
| Proteinuria, g/day | 7.7 ± 3.2 | 3.2 (2.7) | <.001 |
| Total cholesterol, mg/dl | 244 ± 50.3 | 215 (64.0) | .09 |
| Nephrotic syndrome | 11 (100%) | 1 (4%) | <.001 |
Abbreviations: ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate using creatinine-based CKD-EPI equation; HTN, hypertension; SBP, systolic blood pressure
Remaining 2 patients did not report race.
Remaining patients included 3(11%) African American, 1(4%) Asian, and 3 (11%) unknown/other
Vascular disease is composite of coronary artery disease, stroke or peripheral arterial disease
Table 3.
Kidney biopsy characteristics of patients with focal segmental glomerulosclerosis by foot process effacement status
| Characteristic | Foot Process Effacement ≥ 80% without identifiable cause N=11 | Foot Process Effacement < 80% N=27 | Pvalue |
|---|---|---|---|
|
| |||
| Mean ± SD or N(%) | Mean ± SD or N(%) | ||
| Number of glomeruli | 19 ± 22.3 | 14 ± 11.9 | .62 |
| % Globally sclerotic glomeruli | 39% ± 27 | 40% ± 24 | .95 |
| Globally sclerotic glomeruli abnormal for age | 6 (55%) | 18 (67%) | .71 |
| Interstitial fibrosis >5% | 9 (82%) | 24 (89%) | .62 |
| Interstitial fibrosis >25% | 5 (45%) | 10 (37%) | .72 |
| Arteriosclerosis 0–3a | 1.3 ± 1.0 | 1.2 ±1.0 | .80 |
| Arteriolar hyalinosis 0–3a | 0.5 ± 0.7 | 1.2 ±1.1 | .12 |
| Foot process effacement | |||
| Median (range) | 100% (80–100) | 30% (0–70%) | <.001 |
| FSGS subtypesb | |||
| Non otherwise specified (NOS) | 5 (46%) | 23 (85%) | … |
| Perihilar | 2 (18%) | 3 (11%) | … |
| Collapsing | 2 (18%) | 1 (4%) | … |
| Tip lesion | 2 (18%) | 0 | … |
scale 0–3 with 0=none, 1=mild, 2=moderate, 3=severe
Per Columbia classification11
Based on these results, we classified the 11 patients who presented with diffuse FPE without identifiable cause and NS as primary FSGS. The remaining 31 patients with limited FPE or diffuse FPE due to an identifiable cause were classified as secondary FSGS. This left 4 patients who did not have electron micrographs to review. These 4 patients were classified based on their clinical presentation: one patient presented with sudden onset severe NS, treated with prednisone without significant reduction in proteinuria and progressed to ESRD. This patient was classified as primary FSGS. The other three patients did not have NS on presentation and had risk factors for secondary FSGS (reflux nephropathy, long standing HTN, and long-term use of lithium) and were classified as secondary FSGS. Thus, further analysis was based on 12 patients with primary FSGS and 34 patients with secondary FSGS. (Supplemental Figure 1).
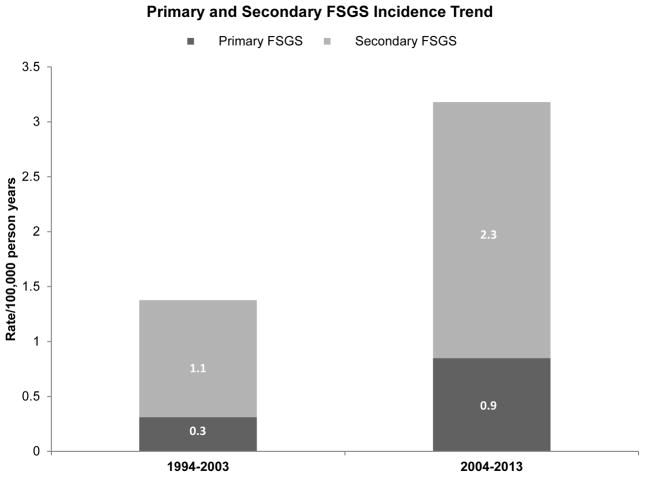
Table 4 summarizes the incidence rates of kidney biopsy, glomerulopathies, and FSGS over the study period. Estimated native kidney biopsy incidence rates increased significantly from 1994–2003 to 2004–2013 (14.7; 95% CI, 12.1–17.3 vs. 22.9; 95% CI, 20.0–25.7 per 100,000 person-years, 17% increase per 5 years, P<.001), so did total FSGS incidence rates (1.4; 95% CI, 0.6–2.2 vs. 3.2; 95% CI, 2.1–4.3 per 100,000 person-years, 41% increase per 5 years, P=0.02).(Supplemental Figure 3). Incidence rates of all glomerular diseases also increased over the same time period, but not to a statistically significant degree (12.4; 95% CI, 10.0–14.7 vs 16.2; 95% CI, 13.8–18.6 per 100,000 person-years, 11% increase per 5 years, P=.05); the increase was also not statistically significant for non-FSGS glomerular diseases. The subtypes of glomerular diseases are shown in Supplemental Figure 4. Although the rate of total FSGS increased over the study period, the proportion of primary FSGS remained relatively stable around 25% (Figure 1). Among all biopsied patients, serum creatinine and proteinuria at the time of biopsy were not significantly different across the two decades (median biopsy creatinine of 1.6 vs 1.8 mg/dl, P=.48, median proteinuria 2.1 vs 1.6 g/day, P=.17).
Table 4.
Incidence rates of renal biopsies and glomerulopathies in Olmsted County from 1994 to 2013a
| 1994–2003 | 2004–2013 | Change in incidence rate per 5 yearsb | |||
|---|---|---|---|---|---|
|
| |||||
| n | Rate (95% CI) | n | Rate (95% CI) | ||
| Native kidney biopsy | 126 | 14.7 (12.1, 17.3) | 244 | 22.9 (20.0, 25.7) | 17% increase, P<.001 |
| All glomerulopathies | 107 | 12.4 (10.0, 14.7) | 174 | 16.2 (13.8, 18.6) | 11% increase, P=.05 |
| Total FSGS | 12 | 1.4 (0.6, 2.2) | 34 | 3.2 (2.1, 4.3) | 41% increase, P=.02 |
| Primaryc | 3 | 0.3 (<.001, 0.7) | 9 | 0.9 (0.3, 1.4) | … |
| Secondaryc | 9 | 1.1 (0.4, 1.8) | 25 | 2.3 (1.4, 3.3) | … |
Abbreviations: FSGS, Focal segmental glomerulosclerosis
Adjusted rates calculated using the age and sex distribution of the US 2010 census. Incidence rate per 100,000 person years
Poisson regression models were used to calculate the change in incidence rate per 5 years from 1994 to 2013
Patients with FSGS who had diffuse foot process effacement without an identifiable cause and had nephrotic syndrome were classified as primary FSGS. Patients with FSGS who had limited foot process effacement, no nephrotic syndrome, or had diffuse effacement but had an identifiable cause for effacement were classified as secondary FSGS.
Figure 1. Trend in the incidence rates of primary and secondary focal segmental glomerulosclerosis (FSGS) over the period of 1994–2013.
In the first decade of 1994–2003, out of the 12 cases of FSGS, 3 (25%) were primary FSGS. In the second decade of 2004–2013, out of 34 cases of FSGS, 9 (26%) were primary FSGS.
Among patients with secondary FSGS, a risk factor for adaptive FSGS could be identified in 13 of the 34 (35%) patients. Risk factors included reflux and obstructive nephropathy (N=3), unilateral nephrectomy or dysplastic kidney (N=2), renal artery stenosis (N=1), pre-eclampsia (N=2), pamidronate (N=1), and chronic lithium use (N=1). Underlying genetic disease was diagnosed in 3 patients: INF2 mutation (N=1) and thin basement membrane disease (N=2). There were no significant differences in clinical and biopsy characteristics of patients with identifiable secondary FSGS risk factors and patients without identifiable secondary risk factors (Supplemental Table 1).
Among the 12 patients with primary FSGS, 4 were treated with immunosuppression (3 with steroids alone and 1 with steroids followed by cyclosporin after NS relapsed) (Supplemental Table 2). The reasons for not treating the other 8 patients with immunosuppression included improvement in proteinuria with RAAS blockade (N=3), treating physician impression that FSGS was secondary (N=3) and low baseline eGFR (N=2). Eventually, 3 patients (25%) with primary FSGS progressed to ESRD over an average time of 6 months from the biopsy date. This included 1 patient treated with prednisone but who continued to have NS and two patients treated conservatively. Among the 34 patients with secondary FSGS, 2 were treated with immunosuppression. One patient had proteinuria of 1.2 g/d after treatment with ACEi and the treating physician elected a trial of prednisone for 3 months, after which proteinuria stabilized around 0.6 g/day. The other patient had an initial improvement in proteinuria after starting ACEi but then it increased back to 3.4 g/day. Steroid trial for 3 months followed by cyclosporin led to proteinuria persisting around 2 g/day. Eventually, 11 patients (34%) with secondary FSGS progressed to ESRD over an average time of 4.5 years from biopsy date. Only a small number of patients underwent kidney transplantion. Two patients with primary FSGS received kidney transplant, had an allograft survival > 10 years, with no FSGS recurrence. Two patients with secondary FSGS received kidney transplant within the last 3 years and did not have evidence of FSGS recurrence on allograft biopsies.
DISCUSSION
The salient observation of this population-based study is that the incidence rates of combined primary and secondary FSGS increased over the past two decades, while the proportions of primary and secondary FSGS remained stable.
The first question is whether this is a true rise in disease incidence or simply better and earlier identification of patients. The rate of native kidney biopsy adjusted for the change in the population increased by 56% during the study period. This increase is likely a reflection of improved biopsy techniques and lower complication rates making nephrologist more comfortable proceeding with a kidney biopsy.15 Although this increase in biopsies may contribute to better identification of patients, the incidence rate of FSGS increased by more than 130% over the same time period. Furthermore, serum creatinine and proteinuria at the time of biopsy were not significantly different over the study period, which is contrary to the notion that nephrologists are biopsying patients with milder forms of disease.
The increasing incidence of FSGS has been a consistent observation in older and recent biopsy studies.1–7 In some studies, this increase was observed in black patients, in particular,2,3 while other studies revealed an increased incidence of FSGS across all races. We report a significant rise in the incidence of FSGS in a population that is predominantly white, indicating that factors other than race are responsible. While changes in lifestyle and diet could explain a rise in FSGS caused by obesity, the rise in primary FSGS remains unexplained.
Distinguishing primary from secondary FSGS remains a big challenge. The common denominator in all types of FSGS is the presence of podocyte injury as the initiating event. Podocytes can be damaged by a variety of mechanisms; from a non-mechanical insult (e.g., immunologic), to mechanical stress, to genetic mutations disrupting endogenous components. Whatever the type of stress, the podocytes initially respond with loss of the interdigitating foot process pattern, termed foot process effacement (FPE). Whether FPE is a coordinated process to increase the chances of cell survival, or merely a sign of derangement of a highly organized system remains controversial.16 When the podocyte injury is initiated by intensified mechanical stress due to glomerular hyperfiltration and hypertrophy, secondary FSGS result. In this situation, FPE is typically focal due to the fact that shear stress is unevenly distributed along the glomerular capillaries, decreasing towards the end of the network. 17 These patients typically do not develop NS although proteinuria may be in the nephrotic range.18,19 On the other hand, in primary FSGS a putative circulating permeability factor causes generalized podocyte dysfunction and the resulting cytoskeletal dysregulation ensues in diffuse FPE. The evidence supporting this presumptive toxic circulating factor includes the rapid recurrence of primary FSGS in transplanted kidneys that can be treated with early initiation of plasmapheresis,20–23 and the observation that explanting and retransplanting kidneys with recurrent primary FSGS into patients without FSGS lead to resolution of proteinuria and histological pattern of injury to podocytes.24,25 Transient proteinuria in infants born to mothers with primary FSGS has been reported, also suggesting the transfer of permeability factors across the placenta.26 Finally, the central feature of primary FSGS is the diffuse FPE that if left untreated can progress to NS in the majority of patients. This diffuse pattern supports the notion that systemic rather than local factors drive podocyte injury. Unlike in secondary FSGS, RAAS blockade is ineffective in reducing proteinuria in primary FSGS.27.
Taken together, from a pathophysiological point of view it can be derived that the extent of FPE is determined by the underlying mechanism of podocyte injury. We therefore classified patients according to the degree of FPE in this study. A morphometric analysis of foot process width excluding patients with familial forms of FSGS found broader foot process in patients with primary FSGS as compared to those with secondary FSGS.28 As a consequence, the degree of FPE can be used as a tool to help distinguish primary from secondary FSGS. If this is true, patients with widespread FPE should have significant proteinuria and hypoalbuminemia due to the severe and diffuse impairment in the filtration barrier. Indeed, we found an almost perfect correlation between the presence of diffuse FPE and having NS. Since electron microscopy may not be routinely available to all practicing nephrologists, we propose the use of NS a surrogate marker for diffuse FPE. Indeed, AUC for Receiver operating characteristic curve using the presence of nephrotic syndrome to predict presence of FPP ≥ 80% was 0.85. However, this is only applicable to patients not treated with immunosuppressive therapy, since such treatment can alter FPE degree and NS if instituted prior to obtaining the biopsy. Although the presence of NS was commensurate with diffuse FPE, the correlation between proteinuria alone and the degree FPE was less robust. Another study also revealed a poor correlation between FPE and proteinuria in patients with minimal change disease and IgA nephropathy.29
Genetic cases of FSGS pose a challenge to this approach. It is established that infants and children with genetic FSGS usually present with explosive NS,30 but this is not the case in adults.31 Some adult patients with genetic FSGS may present with diffuse FPE and NS, while others may have focal FPE. In fact, in our center, we have seen siblings expressing the same mutation but presenting with variable degrees of FPE and proteinuria. Other external factors may modulate the response of the podocytes to the underlying mutation. Thus, it is important to consider a genetic mutation when patients with presumed primary FSGS appear to be resistant to immunosuppressive therapy or when patients present in unusual way (for example, diffuse FPE but no NS).9 Genetic mutations should also be considered in patients with secondary FSGS without identifiable risk factors. Current diagnostic tools failed to identify a specific risk factor for secondary FSGS in a substantial number of patients (>50%) despite having histopathological and clinical features suggestive of secondary FSGS. Some of these patients may have undiagnosed genetic forms of FSGS, which may be uncovered by using next-generation sequencing.32
It is also important to point out that light microscopy features and Columbia classification does not help distinguish primary from secondary FSGS with certainity. In our experience, even though perihilar segmental sclerosis is more likely to be seen in secondary FSGS, it is also noted in primary FSGS.12 A perihilar FSGS does not exclude a primary FSGS.
This study has important strengths. To our knowledge, this is the first population-based study that reported incidence rates of primary and secondary FSGS separately, rather than reporting relative disease frequencies or reporting FSGS as a single disease entity. Although we had small number of patients, we had complete clinical data and we were able to review the biopsy slides and electron micrographs to quantify FPE and excluded patients with focal global glomerulosclerosis only. Alhough the incidence of FSGS continues to increase over the last two decades, the majority of cases are secondary FSGS, while primary FSGS is uncommon. The comprehensive medical records linkage system has allowed Olmsted County to contribute to some of the best epidemiological studies published to date.
Our study has limitations. The Olmsted County population is largely White, limiting generalizability of the results to other races. Given the retrospective nature of this study, we had limited data about potential risk factors for developing FSGS, including medications and infections. Also, genetic testing was not routinely performed. Genetic FSGS may be mistakenly classified as primary FSGS given the degree of proteinuria and diffuse FPE associated with certain genetic defects. Nevertheless, current guidelines do not recommend routine screening of adult patients without family history of renal disease.33
CONCLUSION
In conclusion, while the incidence rate of FSGS is increasing, the ratio of primary and secondary FSGS has remained stable over the last two decades. The increasing rate of kidney biopsy may contribute to this observed increase in the incidence of FSGS, but does not explain the whole picture. Distinguishing primary from secondary FSGS remains a challenge in the absence of a serological marker. Until a specific gold standard biomarker test for diagnosing primary FSGS is developed, using a combination of clinical features (nephrotic syndrome) and pathological features (diffuse FPE), in the absence of any identifiable cause, provides the best approach to distinguishing primary from secondary FSGS.
Supplementary Material
Acknowledgments
Financial Support:
None
This study was made possible by the Rochester Epidemiology Project (grant number R01-AG034676). Kharmen Bharucha was supported by NIH grant R25-DK101405 (PI: Michael F. Romero).
Abbreviations
- ACEi
angiotensin-converting-enzyme inhibitor
- ARBs
angiotensin receptor blockers
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- FPE
foot process effacement
- FSGS
focal segmental glomerulosclerosis
- HTN
hypertension
- IFTA
interstitial fibrosis and tubular atrophy
- NS
nephrotic syndrome
- RAAS
renin-angiotensin-aldosterone system
Footnotes
Disclosure:
The authors declare no financial conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haas M, Spargo BH, Coventry S. Increasing incidence of focal-segmental glomerulosclerosis among adult nephropathies: a 20-year renal biopsy study. Am J Kidney Dis. 1995;26(5):740–750. doi: 10.1016/0272-6386(95)90437-9. [DOI] [PubMed] [Google Scholar]
- 2.Korbet SM, Genchi RM, Borok RZ, Schwartz MM. The racial prevalence of glomerular lesions in nephrotic adults. Am J Kidney Dis. 1996;27(5):647–651. doi: 10.1016/s0272-6386(96)90098-0. [DOI] [PubMed] [Google Scholar]
- 3.Braden GL, Mulhern JG, O'Shea MH, Nash SV, Ucci AA, Jr, Germain MJ. Changing incidence of glomerular diseases in adults. Am J Kidney Dis. 2000;35(5):878–883. doi: 10.1016/s0272-6386(00)70258-7. [DOI] [PubMed] [Google Scholar]
- 4.Swaminathan S, Leung N, Lager DJ, et al. Changing incidence of glomerular disease in Olmsted County, Minnesota: a 30-year renal biopsy study. Clin J Am Soc Nephrol. 2006;1(3):483–487. doi: 10.2215/CJN.00710805. [DOI] [PubMed] [Google Scholar]
- 5.Murugapandian S, Mansour I, Hudeeb M, et al. Epidemiology of Glomerular Disease in Southern Arizona: Review of 10-Year Renal Biopsy Data. Medicine (Baltimore) 2016;95(18):e3633. doi: 10.1097/MD.0000000000003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sim JJ, Batech M, Hever A, et al. Distribution of Biopsy-Proven Presumed Primary Glomerulonephropathies in 2000–2011 Among a Racially and Ethnically Diverse US Population. Am J Kidney Dis. 2016;68(4):533–544. doi: 10.1053/j.ajkd.2016.03.416. [DOI] [PubMed] [Google Scholar]
- 7.O'Shaughnessy MM, Hogan SL, Poulton CJ, et al. Temporal and Demographic Trends in Glomerular Disease Epidemiology in the Southeastern United States, 1986–2015. Clin J Am Soc Nephrol. 2017;12(4):614–623. doi: 10.2215/CJN.10871016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gipson DS, Troost JP, Lafayette RA, et al. Complete Remission in the Nephrotic Syndrome Study Network. Clin J Am Soc Nephrol. 2016;11(1):81–89. doi: 10.2215/CJN.02560315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethi S, Glassock RJ, Fervenza FC. Focal segmental glomerulosclerosis: towards a better understanding for the practicing nephrologist. Nephrol Dial Transplant. 2015;30(3):375–384. doi: 10.1093/ndt/gfu035. [DOI] [PubMed] [Google Scholar]
- 10.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Agati VD, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43(2):368–382. doi: 10.1053/j.ajkd.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 12.Sethi S, Zand L, Nasr SH, Glassock RJ, Fervenza FC. Focal and segmental glomerulosclerosis: clinical and kidney biopsy correlations. Clin Kidney J. 2014;7(6):531–537. doi: 10.1093/ckj/sfu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sethi S, D'Agati VD, Nast CC, et al. A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int. 2017;91(4):787–789. doi: 10.1016/j.kint.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Glassock RJ, Fervenza FC, Hebert L, Cameron JS. Nephrotic syndrome redux. Nephrol Dial Transplant. 2015;30(1):12–17. doi: 10.1093/ndt/gfu077. [DOI] [PubMed] [Google Scholar]
- 15.Soares SM, Fervenza FC, Lager DJ, Gertz MA, Cosio FG, Leung N. Bleeding complications after transcutaneous kidney biopsy in patients with systemic amyloidosis: single-center experience in 101 patients. Am J Kidney Dis. 2008;52(6):1079–1083. doi: 10.1053/j.ajkd.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV. The podocyte's response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol. 2013;304(4):F333–347. doi: 10.1152/ajprenal.00478.2012. [DOI] [PubMed] [Google Scholar]
- 17.Kriz W, Lemley KV. Mechanical challenges to the glomerular filtration barrier: adaptations and pathway to sclerosis. Pediatr Nephrol. 2017;32(3):405–417. doi: 10.1007/s00467-016-3358-9. [DOI] [PubMed] [Google Scholar]
- 18.Praga M, Morales E, Herrero JC, et al. Absence of hypoalbuminemia despite massive proteinuria in focal segmental glomerulosclerosis secondary to hyperfiltration. Am J Kidney Dis. 1999;33(1):52–58. doi: 10.1016/s0272-6386(99)70257-x. [DOI] [PubMed] [Google Scholar]
- 19.D'Agati VD, Chagnac A, de Vries AP, et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12(8):453–471. doi: 10.1038/nrneph.2016.75. [DOI] [PubMed] [Google Scholar]
- 20.Chang JW, Pardo V, Sageshima J, et al. Podocyte foot process effacement in postreperfusion allograft biopsies correlates with early recurrence of proteinuria in focal segmental glomerulosclerosis. Transplantation. 2012;93(12):1238–1244. doi: 10.1097/TP.0b013e318250234a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artero M, Biava C, Amend W, Tomlanovich S, Vincenti F. Recurrent focal glomerulosclerosis: natural history and response to therapy. Am J Med. 1992;92(4):375–383. doi: 10.1016/0002-9343(92)90267-f. [DOI] [PubMed] [Google Scholar]
- 22.Deegens JK, Andresdottir MB, Croockewit S, Wetzels JF. Plasma exchange improves graft survival in patients with recurrent focal glomerulosclerosis after renal transplant. Transplant international : official journal of the European Society for Organ Transplantation. 2004;17(3):151–157. doi: 10.1007/s00147-003-0679-y. [DOI] [PubMed] [Google Scholar]
- 23.Kashgary A, Sontrop JM, Li L, et al. The role of plasma exchange in treating post-transplant focal segmental glomerulosclerosis: A systematic review and meta-analysis of 77 case-reports and case-series. BMC Nephrol. 2016;17(1):104. doi: 10.1186/s12882-016-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rea R, Smith C, Sandhu K, Kwan J, Tomson C. Successful transplant of a kidney with focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2001;16(2):416–417. doi: 10.1093/ndt/16.2.416. [DOI] [PubMed] [Google Scholar]
- 25.Gallon L, Leventhal J, Skaro A, Kanwar Y, Alvarado A. Resolution of recurrent focal segmental glomerulosclerosis after retransplantation. N Engl J Med. 2012;366(17):1648–1649. doi: 10.1056/NEJMc1202500. [DOI] [PubMed] [Google Scholar]
- 26.Kemper MJ, Wolf G, Muller-Wiefel DE. Transmission of glomerular permeability factor from a mother to her child. N Engl J Med. 2001;344(5):386–387. doi: 10.1056/NEJM200102013440517. [DOI] [PubMed] [Google Scholar]
- 27.Praga M, Hernandez E, Montoyo C, Andres A, Ruilope LM, Rodicio JL. Long-term beneficial effects of angiotensin-converting enzyme inhibition in patients with nephrotic proteinuria. Am J Kidney Dis. 1992;20(3):240–248. doi: 10.1016/s0272-6386(12)80696-2. [DOI] [PubMed] [Google Scholar]
- 28.Deegens JK, Dijkman HB, Borm GF, et al. Podocyte foot process effacement as a diagnostic tool in focal segmental glomerulosclerosis. Kidney Int. 2008;74(12):1568–1576. doi: 10.1038/ki.2008.413. [DOI] [PubMed] [Google Scholar]
- 29.van den Berg JG, van den Bergh Weerman MA, Assmann KJ, Weening JJ, Florquin S. Podocyte foot process effacement is not correlated with the level of proteinuria in human glomerulopathies. Kidney Int. 2004;66(5):1901–1906. doi: 10.1111/j.1523-1755.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- 30.Hinkes BG, Mucha B, Vlangos CN, et al. Nephrotic syndrome in the first year of life: two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2) Pediatrics. 2007;119(4):e907–919. doi: 10.1542/peds.2006-2164. [DOI] [PubMed] [Google Scholar]
- 31.Guery B, Choukroun G, Noel LH, et al. The spectrum of systemic involvement in adults presenting with renal lesion and mitochondrial tRNA(Leu) gene mutation. Journal of the American Society of Nephrology : JASN. 2003;14(8):2099–2108. doi: 10.1097/01.asn.0000080180.51098.02. [DOI] [PubMed] [Google Scholar]
- 32.Brown EJ, Pollak MR, Barua M. Genetic testing for nephrotic syndrome and FSGS in the era of next-generation sequencing. Kidney Int. 2014;85(5):1030–1038. doi: 10.1038/ki.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rood IM, Deegens JK, Wetzels JF. Genetic causes of focal segmental glomerulosclerosis: implications for clinical practice. Nephrol Dial Transplant. 2012;27(3):882–890. doi: 10.1093/ndt/gfr771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.