Abstract
A number of studies in the past 20 years have shown that perturbation of activity of the nervous system leads to compensatory changes in synaptic strength that serve to return network activity to its original level. This response has been termed homeostatic synaptic plasticity. Despite the intense interest in homeostatic synaptic plasticity, little attention has been paid to its role in the prototypic synaptic disease: myasthenia gravis. In this review, we discuss mechanisms that have been shown to mediate homeostatic synaptic plasticity at the mammalian neuromuscular junction. A subset of these mechanisms have been shown to occur in myasthenia gravis. The homeostatic changes occurring in myasthenia gravis appear to involve the presynaptic nerve terminal and may even involve changes in the excitability of motor neurons within the spinal cord. The finding of presynaptic homeostatic synaptic plasticity in myasthenia gravis leads us to propose that changes in the motor unit in myasthenia gravis may be more widespread than previously appreciated.
Keywords: activity, acetylcholine receptor, endplate, action potential, synapse, transmission
In myasthenia gravis (MG), there is an immune attack on postsynaptic acetylcholine (ACh) receptors (AChRs) that disrupts synaptic transmission. Here, we discuss the synaptic plasticity triggered by this immune attack. Before considering how the neuromuscular junction (NMJ) responds to an immune attack, we discuss the advantages of studying synaptic plasticity at the NMJ. The detailed study of what happens at this synapse following a perturbation in function is not possible at most other synapses.
Advantages of using the NMJ to study synaptic plasticity
The neuromuscular junction is an ideal synapse at which to study synaptic plasticity. There is only one axon entering the adult NMJ such that changes in synaptic strength cannot be due to gain or loss of inputs. Additionally, at the adult NMJ, release of Ach from the presynaptic terminal binds and opens a single type of nicotinic AChR containing two identical α subunits, a β, a δ, and an ε subunit.1 The presence of a uniform population of AChRs simplifies interpretation of experiments in which plasticity of the synapse is studied in response to partial blockage of receptors.
One goal of studies of synaptic plasticity is to determine whether changes in synaptic strength are due to mechanisms located within the presynaptic terminal or mechanisms localized to the postsynaptic cell. We attempt this by measuring the synaptic current generated in response to an action potential in the presynaptic terminal (evoked release) and comparing it to the amplitude of the spontaneously occurring synaptic currents (spontaneous release). By dividing the amplitude of the evoked current by the amplitude of the spontaneous current, one can determine the number of synaptic vesicles released (quantal content (QC)).2 As QC is a purely presynaptic property, changes in this number locate the plasticity to the presynaptic terminal. A key assumption in this calculation is that the vesicles responsible for spontaneous synaptic currents are the same vesicles responsible for evoked currents. Recently, the generality of this assumption has been called into question.3, 4 If the vesicles released spontaneously and those released during stimulation come from different pools of vesicles, calculation of QC by dividing the amplitude of the evoked current by the amplitude of spontaneous currents will lead to an erroneous estimate of the number of vesicles released.
Localizing changes in synaptic strength to the postsynaptic cell is more difficult. It is believed that spontaneous synaptic currents are due to the release of ACh from the spontaneous fusion of a single presynaptic vesicle containing Ach.2, 5 The amplitude of spontaneous synaptic currents is determined by the amount of ACh released, the rate of breakdown of ACh by acetylcholinesterase within the synaptic cleft, and the number of postsynaptic AChRs activated.6 Despite the possibility that several different factors might alter the amplitude of spontaneous synaptic currents, it is often assumed that reduction in their amplitude represents a change in the number of postsynaptic receptors activated. One can measure the density of postsynaptic AChRs by measuring the brightness of fluorescently tagged α-bungarotoxin (BTX) that binds the receptors. However, as quantitation of fluorescence suffers from a number of artifacts that can lead to incorrect measurements, we developed a technique in which the slope of the change in brightness is plotted against the illumination intensity.6 We confirmed that this technique was accurate by mixing different ratios of red- and green-tagged BTX and quantifying the density of AChRs labeled with one of the colors. As there is only one type of AChR at the NMJ, it thus becomes possible to determine whether changes in synaptic current amplitude are due to a change in AChR density at the NMJ.
Evidence for presynaptic changes in myasthenia gravis
Since the discovery that MG is caused by an autoimmune attack on postsynaptic AChRs, the focus of the field has appropriately been on stopping the attack and lessening its effects on neuromuscular transmission. However, there has been evidence that the postsynaptic attack on AChRs may have presynaptic effects, such that MG may cause more widespread dysfunction of the motor unit than generally recognized. It should be pointed out that the notion of presynaptic involvement in MG is not a new one.7–10
One of the most direct pieces of evidence that the presynaptic motor terminal of the NMJ is affected by the postsynaptic attack on AChRs came from a study in which individual NMJs were imaged in vivo in mice before and 1 week after application of anti-AChR antibody. The attack on postsynaptic regions of AChRs was surprisingly patchy. Some regions of postsynaptic AChRs in individual NMJs were completely eliminated, while nearby regions were largely unscathed.11 The nerve terminal overlying the destroyed region of the NMJ had withdrawn, suggesting that presynaptic plasticity was triggered by the postsynaptic attack. The most likely explanation for the withdrawal of the overlying nerve terminal is the loss of functional AChRs underlying the regions of nerve that had withdrawn. This interpretation is based on a study in which it was shown that blockage of AChRs with BTX in only part of a single NMJ triggers withdrawal of the overlying nerve terminal, while nearby regions of nerve terminal remain in place.12 This suggests an ongoing trophic relationship between AChRs and the overlying nerve terminal that is interrupted by blockage/removal of AChRs. For an in-depth review of molecules and signaling pathways that might be involved in retrograde signaling at the NMJ in MG, the reader is referred to an excellent recent review on synaptic homeostasis in MG.13
If blockage of AChRs at the NMJ is prolonged, the changes in presynaptic properties can extend all the way back to the motor neuron soma. Following injury to the motor nerve, motor neuron excitability increases such that the minimum current necessary to trigger action potentials (rheobase current) decreases.14, 15 The signaling at the NMJ that leads to the trophic regulation of motor neuron excitability does not require evoked release of Ach, as motor neuron excitability recovers following reinnervation even when action potential propagation down the axon is blocked.16 The increase in motor neuron excitability can be triggered by injection of BTX into muscle to block AChRs.17 This indicates that the increase in excitability is not due to nerve injury, but rather to interruption of a trophic signal from the NMJ. Given that the signaling can occur in the absence of evoked release, it appears that the spontaneous release of ACh from the NMJ activates enough AChRs to operate the trophic signal.
In addition to the changes in motor neuron excitability following prolonged blockage of AChRs at the NMJ, there is a functional change in the nerve terminal at the NMJ. Measurements of synaptic function suggest there is greater release of ACh from the nerve terminal of patients with MG.18, 19 As is discussed below, the increase in ACh release appears to be triggered by reduction in the number of functional postsynaptic AChRs. It appears that the increase in ACh release serves to counter the blockage of AChRs. It has been found in a number of systems that synapses and neurons respond to perturbations of activity in ways that serve to restore activity.13, 20–22 This type of plasticity of synapses has been termed homeostatic synaptic plasticity.
Homeostatic synaptic plasticity at the NMJ
At the NMJ, synaptic strength is regulated in a homeostatic manner to ensure the safety of motor neuron–muscle transmission under a variety of conditions.13, 20, 23 There are at least two distinct forms of homeostatic upregulation of QC at the mouse NMJ. The first is triggered by blockage of action potentials entering the presynaptic nerve terminal and the second is by blockage of postsynaptic AChRs, as occurs in MG.24, 25
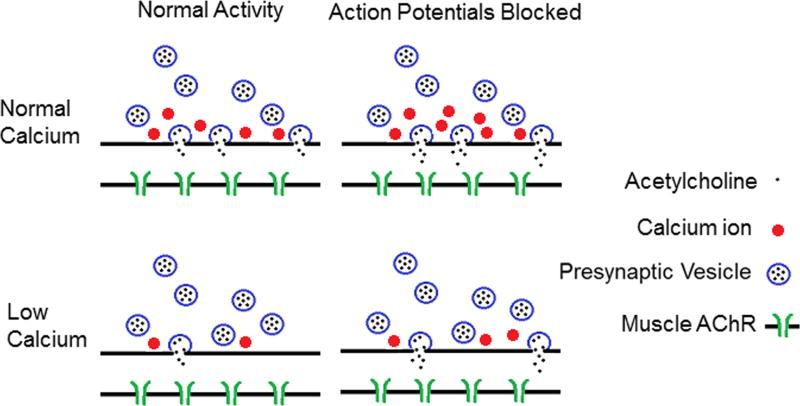
We will first discuss homeostatic plasticity triggered by blockage of action potentials (Fig. 1). Action potentials can be blocked in mice in vivo by placing a cuff around the sciatic nerve that continuously releases tetrodotoxin, a toxin that blocks Na channels in the nerve. The blockage of Na channels prevents action potentials from propagating down the axon into the presynaptic terminal of NMJs. This manipulation silences both nerve and muscle. When action potentials were blocker for a week, there was an increase in the amplitude of spontaneous synaptic currents.6 We examined whether blocking presynaptic or postsynaptic action potentials was involved in triggering the increase in spontaneous synaptic currents. This was done by placing a TTX cuff on the sciatic nerve of mice with myotonia congenita. These mice harbor a loss-of-function mutation in the muscle chloride channel such that they have spontaneous firing of the muscle. In awake behaving mice with myotonia congenita, there is continued muscle activity when nerve activity is blocked by a TTX cuff.6 The amplitude of spontaneous events in these mice was large following blockage of nerve activity, despite the continued muscle activity. These data suggest that blocking presyanptic action potentials triggers an increase in release of ACh from individual synaptic vesicles.6 Our studies suggest that the increase in amplitude of spotaneous synaptic currents may be due to increased presynaptic loading of ACh into vesicles that is mediated by the synaptic vesicle protein Rab-3A.26
Figure 1.
Shown is a cartoon of homeostatic synaptic plasticity at the mouse NMJ triggered by prolonged blockage of nerve action potentials. In the top row, synaptic function is shown in solution containing normal external Ca. Following blockage of action potentials, there are two changes in synaptic function. The first is an increase in the release of acetylcholine (ACh) during fusion of individual synaptic vesicles (illustrated as increased ACh in the synaptic cleft). This is likely responsible for the increase in the amplitude of spontaneous synaptic currents (miniature endplate currents (MEPCs)). The second change is an increase in entry of Ca into the presynaptic terminal during an action potential. However, when extracellular Ca is normal, this has no significant effect on the number of synaptic vesicles released (quantal content (QC)), as each releasable vesicle is already released with each action potential. The bottom row shows the situation when extracellular Ca is lowered. In this case, Ca entry during the action potential limits QC. When Ca entry is increased following blockage of action potentials, even a small increase in Ca entry triggers a significant increase in QC. In addition, the increase in ACh release from each vesicle also occurs. This combines with the increase in QC to cause a dramatic increase in synaptic strength. This is illustrated in the cartoon as an increase from two to eight ACh molecules in the synaptic cleft. AChR, acetylcholine receptor.
A second change in synaptic function that is triggered following blockage of action potentials is an increase in QC. QC is determined by the probabalistic release of individual vesicles from a pool of vesicles that are primed for release (docked vesicles). The number of vesicles that are ready for release is represented by the binomial parameter n. Each vesicle has a set probability of release following a presynaptic action potential that is represented by the binomial parameter p.27, 28 There are thus two ways to increase the number of vesicles released. The first is to have more releaseable vesicles (an increase in n). The second is to increase the probability that any individual vesicle is released in response to a presynaptic action potential (an increase in p). An assumption of binomial analysis is that the probability of release is uniform among all releasable vesicles. The generality of this assumption has been called into question.28–31 Studies in which mouse NMJs expressing synapto-pHluorin were imaged during stimulation suggest that, during repetitive stimulation, there are hotspots of vesicle release that may be due to a subset of vesicles with a higher probability of release.32 However, when stimulation rates are lower, release is more spatially uniform,33 suggesting that release probabilities are similar across release sites such that binomial analysis is valid.
We analyzed variance of endplate current amplitude to estimate p and n as previously described30, 34, 35 and found that the increase in vesicle release following a blockage of action potentials was due to an increase in the probability of release.36 Because the probability of release is so high at the NMJ in normal physiologic solution, the increase had no effect on the number of vesicles releases in normal solution.36 The only way to determine that synaptic plasticity had occurred was to lower extracellular Ca such that probability of vesicle release was low. In this setting, the increase in probability of release led to a doubling in the number of vesicles released.24, 36 We hypothesize that the increase in probability of release is due to increased Ca entry into the nerve terminal during action potentials (Fig. 1). In normal physiologic solution, the increase in Ca entry following blockage of activity may not trigger an increase in vesicle release owing to saturation of Ca-dependent processes.
A second form of homeostatic regulation at NMJs is triggered by blocking postsynpatic receptors rather than action potentials. The most extensive studies of this phenomenon have been carried out at the Drosophila NMJ (reviewed in Refs. 22 and 37). For discussion of the similarities and differences between the Drosophila and mouse NMJ in this form of homeostatic synaptic plasticity, see Refs. 13 and 25. Here, we focus on what has been shown in the mammalian NMJ, as it is a cholinergic synapse (the Drosophila NMJ is glutaminergic) and thus is more relevant to understanding plasticity of the NMJ in patients with MG. The current review complements a recent review on this topic.13
The first thing to consider is what happens to the amplitude of spontaneous syanptic currents in MG. Owing to blockage of AChRs as well as an immune attack on AChRs, there is a marked decrease in the amplitude of spontaneous synaptic currents.38 It would be interesting to determine whether the Rab-3A–mediated increase in ACh release from individual vesicles described above occurs during MG. Unfortunately, because the loss of AChRs decreases the amplitude of spontaneous events relative to control, we cannot determine whether there has been Rab-3A–mediated compensation in amplitude of spontaneous synaptic currents.
Presynaptic properties are easier to study in the setting of MG, as they do not depend on the amplitude of the postsynaptic response to a single vesicle. Higher QC was detected in patients with MG.18, 19 A similar increase occurs in a rat model of MG in which postsynaptic AChRs are blocked for weeks with repeated injection of BTX.8, 39, 40 Furthermore, it has been shown in rat hemidiaphragm in vitro that evoked ACh release is increased by blocking AChRs with BTX for hours.9 More recently, a similar increase in QC was shown in mice 5 days after blocking AChRs with a single injection of BTX.36 Studies of the time course of the increase in QC determined that it occurs very rapidly. Observations of a rapid increase in QC have been made at the NMJs of cats,41 frogs,42 rats,10, 43–45 and mice.46–48 While the majority of studies have found a rapid increase in QC in response to blockage of AChRs, there are some studies in which a rapid increase did not appear to occur.49–51
The increase in QC triggered by partial blockage of AChRs is dependent on both external Ca/Mg concentration and the frequency with which the nerve is stimulated. In studies in which high Mg/low Ca concentrations were used to prevent muscle twitch in response to nerve stimulation, no increase in QC was detected following either chronic8, 40, 52 or acute blockage of AChRs.25, 43, 47, 53 However, in studies in which extracellular Ca and Mg were normal, large increases in QC were detected.25, 36, 39, 40, 54 These findings suggest that there must either be sufficient resting Ca or sufficient Ca entry during presynaptic action potentials to enable the triggering of synaptic plasticity following blockage of AChRs.
To determine whether elevation of resting Ca is the trigger for increased QC, one can measure the frequency of spontaneous synaptic currents. This frequency is thought to be dependent on the resting level of presynaptic Ca. In support of this possibility, it has been shown that, at the NMJ of mice lacking a Na/Ca exchanger, spontaneous event frequency remains elevated for a longer period after repetitive stimulation.55 When spontaneous synaptic current frequency was measured following partial blockage of AChRs, no increase was detected at a time when QC was increased.56 This suggests the increase in QC is not due to an increase in the level of resting Ca in the presynaptic nerve terminal. The dependence on sufficient Ca influx during action potentials to trigger upregulation of QC was tested by application of 3,4-diaminopyridine in the setting of low extracellular Ca. 3,4-Diaminopyridine blocks voltage-gated K channels in the presynaptic terminal. This causes the action potential to become widened and thus increases Ca entry.57 We found that when QC was increased by 3,4-diaminopyridine in the setting of low extracellular Ca, the increase in QC following blockage of AChRs was restored.25 We conclude that low extracellular Ca may prevent the increase in QC owing to reduction of Ca entry during action potentials.
The other feature of the increase in QC triggered by partial blockage of AChRs is that it is highly dependent on stimulation frequency. The increase in QC was only present during low-frequency stimulation or during the first few pulses of a train of action potentials.10, 45–48, 58 The rundown of synaptic current during high-frequency repetitive stimulation was shown not to be due to reduction of postsynaptic sensitivity to Ach44, 58 but to be due to reduction in presynaptic transmitter release.10, 46, 58
To determine why the increase in QC was only present during low-frequency stimulation, we estimated the size of the readily releasable vesicle pool at the NMJ before and after partial blockage of AChRs.25 This pool of vesicles is thought to represent the number of vesicles that are primed for release in response to action potentials. Other vesicles present at the NMJ are thought to not be ready for immediate release, but instead have to be primed by some change in either proteins associated with the vesicle or mobilization to active zones. One way to estimate the size of the readily releasable pool is with high-frequency repetitive stimulation.59 The idea is that not enough time is given for mobilization of the reserve pools of synaptic vesicles. This technique has been validated with imaging for high rates of stimulation at the mouse NMJ.60 When we used repetitive stimulation to estimate the size of the readily releasable pool, we found an increase following partial blockage of AChRs.25
We also estimated the size of the pool by blocking refilling of synaptic vesicles with ACh following release of their contents. This was done by incubating the NMJs with vesamicol, a vesicular ACh transporter (VAChT) inhibitor that does not alter the quantal size of preformed vesicles (reviewed in Ref. 61) and has no detectable postsynaptic effect at the dose used.62, 63 Normally, the increase in QC can be repeatedly triggered by repeated application and washing out the blocker. However, in the presence of vesamicol, partial blockage of AChRs could trigger an increase in QC only once.25 We conclude that homeostatic upregulation of QC is accomplished by mobilizing a small pool of unique synaptic vesicles that are able to recycle during slow trains of stimuli but cannot do so during high-frequency stimulation.25 This pool of vesicles recycles rapidly, and thus continual refilling of vesicles is necessary for function of the pool (Fig. 2).
Figure 2.
Shown is a cartoon of NMJ homeostatic synaptic plasticity following blockage or destruction of AChRs, as occurs in MG. In the top row, synaptic function is shown in solution containing normal external Ca. Two types of synaptic vesicles are present. The blue vesicles represent normal vesicles that participate in synaptic transmission at baseline. The red vesicles represent a special pool of synaptic vesicles that normally do not play an important role in synaptic transmission. A few vesicles in this pool are released late in the course of the synaptic current (indicated by the long dotted red arrow). Following partial blockage of AChRs, the special pool of synaptic vesicles is released more rapidly (synchronous with the normal pool). In addition, the special pool of vesicles rapidly recycles (indicated by the curved red arrow) to increase QC. Because the special pool of vesicles is so small, it is rapidly depleted during repetitive stimulation. In the bottom row is shown the situation when extracellular Ca is lowered. When extracellular Ca is lowered, the special pool of synaptic vesicles does not participate in synaptic transmission (thus, the pool is not shown in the cartoon). This suggests that this pool requires high levels of Ca entry in order to be mobilized for release. Because this pool does not participate in release, blockage of AChRs does not trigger an increase in QC and thus there is functionally no synaptic plasticity.
The role of homeostatic synaptic plasticity in failure of synaptic transmission in MG
The result of homeostatic mobilization of vesicles following partial blockage of AChRs is that the first synaptic current is larger than it would otherwise be. This increases synaptic strength and thus serves to counter the reduction in postsynaptic AChRs caused by MG. However, as the pool of mobilized vesicles rapidly depletes, there is greater depression of the synaptic current during repetitive stimulation.25 At the adult mouse NMJ, repetitive stimulation at high rates (20–50 Hz) causes depression of synaptic current. Normally, because of the safety factor of neuromuscular transmission, this depression does not cause weakness.64 However, in MG, the safety factor is decreased owing to reduction of postsynaptic sensitivity to ACh. The idea is that the normal synaptic depression then causes the muscle depolarization in response to a presynaptic action potential to become insufficient to trigger a postsynaptic action potential.64 In this setting, an increase in QC is helpful in that it will tend to lessen the failure of neuromuscular transmission.
The finding that the synaptic current depresses to a greater degree following partial blockage of AChRs has led us to rethink our understanding of depression of the compound muscle action potential during repetitive stimulation in MG. Depression of the compound muscle action potential is a measure of the number of muscle fibers in which the action potential–induced depolarization of the muscle fiber falls below threshold such that there is failure of synaptic transmission.64 We propose that the depression during repetitive stimulation in MG is due to two factors. The first is the widely appreciated reduction in the safety factor for synaptic transmission during repetitive stimulation mentioned above. The second is the greater depression of synaptic current during repetitive stimulation of NMJs in MG. This second contributor is due to depletion of the pool of vesicles that was mobilized in response to blockage/loss of AChRs.
In summary, the attack on postsynaptic AChRs in MG triggers homeostatic synaptic plasticity resulting in increased release of synaptic vesicles from the nerve terminal. However, as the pool of vesicles mobilized is small, they are rapidly depleted, leading to greater depression during repetitive stimulation. This depression may contribute to the finding of decrement of the compound muscle action potential during repetitive stimulation in patients with MG.
Acknowledgments
This work was supported by National Institutes of Health Grants P01NS057228 (M.M.R.) and R01NS082354 (M.M.R.).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Auerbach A. Activation of endplate nicotinic acetylcholine receptors by agonists. Biochem Pharmacol. 2015;97:601–608. doi: 10.1016/j.bcp.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Castillo J, Katz B. Quantal components of the end-plate potential. J Physiol. 1954;124:560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kavalali ET. The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci. 2015;16:5–16. doi: 10.1038/nrn3875. [DOI] [PubMed] [Google Scholar]
- 4.Peled ES, Newman ZL, Isacoff EY. Evoked and spontaneous transmission favored by distinct sets of synapses. Curr Biol. 2014;24:484–493. doi: 10.1016/j.cub.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heuser JE, et al. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979;81:275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, et al. Activity-dependent presynaptic regulation of quantal size at the mammalian neuromuscular junction in vivo. J Neurosci. 2005;25:343–351. doi: 10.1523/JNEUROSCI.3252-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desmedt JE. Presynaptic mechanisms in myasthenia gravis. Ann N Y Acad Sci. 1966;135:209–246. doi: 10.1111/j.1749-6632.1966.tb45474.x. [DOI] [PubMed] [Google Scholar]
- 8.Molenaar PC, et al. A non-immunogenic myasthenia gravis model and its application in a study of transsynaptic regulation at the neuromuscular junction. Eur J Pharmacol. 1991;196:93–101. doi: 10.1016/0014-2999(91)90413-k. [DOI] [PubMed] [Google Scholar]
- 9.Miledi R, Molenaar PC, Polak RL. Alpha-Bungarotoxin enhances transmitter "released" at the neuromuscular junction. Nature. 1978;272:641–643. doi: 10.1038/272641a0. [DOI] [PubMed] [Google Scholar]
- 10.Wilson DF. Influence of presynaptic receptors on neuromuscular transmission in rat. American Journal of Physiology. 1982;242:C366–372. doi: 10.1152/ajpcell.1982.242.5.C366. [DOI] [PubMed] [Google Scholar]
- 11.Rich MM, Colman H, Lichtman JW. In vivo imaging shows loss of synaptic sites from neuromuscular junctions in a model of myasthenia gravis. Neurology. 1994;44:2138–2145. doi: 10.1212/wnl.44.11.2138. [DOI] [PubMed] [Google Scholar]
- 12.Balice-Gordon RJ, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372:519–524. doi: 10.1038/372519a0. [DOI] [PubMed] [Google Scholar]
- 13.Plomp JJ. Trans-synaptic homeostasis at the myasthenic neuromuscular junction. Front Biosci (Landmark Ed) 2017;22:1033–1051. doi: 10.2741/4532. [DOI] [PubMed] [Google Scholar]
- 14.Pinter MJ, Vanden Noven S. Effects of preventing reinnervation on axotomized spinal motoneurons in the cat. I. Motoneuron electrical properties. J Neurophysiol. 1989;62:311–324. doi: 10.1152/jn.1989.62.2.311. [DOI] [PubMed] [Google Scholar]
- 15.Pinter MJ, et al. Axotomy-like changes in cat motoneuron electrical properties elicited by botulinum toxin depend on the complete elimination of neuromuscular transmission. J Neurosci. 1991;11:657–666. doi: 10.1523/JNEUROSCI.11-03-00657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bichler EK, et al. Rat motoneuron properties recover following reinnervation in the absence of muscle activity and evoked acetylcholine release. J Physiol. 2007;585:47–56. doi: 10.1113/jphysiol.2007.135541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakanishi ST, et al. Regulation of motoneuron excitability via motor endplate acetylcholine receptor activation. J Neurosci. 2005;25:2226–2232. doi: 10.1523/JNEUROSCI.5065-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cull-Candy SG, et al. On the release of transmitter at normal, myasthenia gravis and myasthenic syndrome affected human end-plates. J Physiol. 1980;299:621–638. doi: 10.1113/jphysiol.1980.sp013145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molenaar PC, et al. Acetylcholine in intercostal muscle from myasthenia gravis patients and in rat diaphragm after blockade of acetylcholine receptors. Prog Brain Res. 1979;49:449–458. doi: 10.1016/S0079-6123(08)64657-9. [DOI] [PubMed] [Google Scholar]
- 20.Rich MM, Wenner P. Sensing and expressing homeostatic synaptic plasticity. Trends Neurosci. 2007;30:119–125. doi: 10.1016/j.tins.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol. 2012;4:a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis GW, Muller M. Homeostatic control of presynaptic neurotransmitter release. Annu Rev Physiol. 2015;77:251–270. doi: 10.1146/annurev-physiol-021014-071740. [DOI] [PubMed] [Google Scholar]
- 23.Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, et al. Decreased synaptic activity shifts the calcium dependence of release at the mammalian neuromuscular junction in vivo. J Neurosci. 2004;24:10687–10692. doi: 10.1523/JNEUROSCI.2755-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Pinter MJ, Rich MM. Reversible Recruitment of a Homeostatic Reserve Pool of Synaptic Vesicles Underlies Rapid Homeostatic Plasticity of Quantal Content. J Neurosci. 2016;36:828–836. doi: 10.1523/JNEUROSCI.3786-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, et al. Regulation of quantal shape by Rab3A: evidence for a fusion pore-dependent mechanism. J Physiol. 2008;586:3949–3962. doi: 10.1113/jphysiol.2008.151191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyamoto MD. Binomial analysis of quantal transmitter release at glycerol treated frog neuromuscular junctions. Journal of Physiology. 1975;250:121–142. doi: 10.1113/jphysiol.1975.sp011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Provan SD, Miyamoto MD. Unbiased estimates of quantal release parameters and spatial variation in the probability of neurosecretion. Am J Physiol. 1993;264:C1051–1060. doi: 10.1152/ajpcell.1993.264.4.C1051. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto MD. Probability of quantal transmitter release from nerve terminals: theoretical considerations in the determination of spatial variation. J Theor Biol. 1986;123:289–304. doi: 10.1016/s0022-5193(86)80244-2. [DOI] [PubMed] [Google Scholar]
- 30.Silver RA. Estimation of nonuniform quantal parameters with multiple-probability fluctuation analysis: theory, application and limitations. J Neurosci Methods. 2003;130:127–141. doi: 10.1016/j.jneumeth.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Pinter MJ, Rich MM. Ca2+ dependence of the binomial parameters p and n at the mouse neuromuscular junction. J Neurophysiol. 2010;103:659–666. doi: 10.1152/jn.00708.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyatt RM, Balice-Gordon RJ. Heterogeneity in synaptic vesicle release at neuromuscular synapses of mice expressing synaptopHluorin. J Neurosci. 2008;28:325–335. doi: 10.1523/JNEUROSCI.3544-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaffield MA, Tabares L, Betz WJ. The spatial pattern of exocytosis and post-exocytic mobility of synaptopHluorin in mouse motor nerve terminals. J Physiol. 2009;587:1187–1200. doi: 10.1113/jphysiol.2008.166728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clements JD. Variance-mean analysis: a simple and reliable approach for investigating synaptic transmission and modulation. J Neurosci Methods. 2003;130:115–125. doi: 10.1016/j.jneumeth.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Clements JD, Silver RA. Unveiling synaptic plasticity: a new graphical and analytical approach. Trends Neurosci. 2000;23:105–113. doi: 10.1016/s0166-2236(99)01520-9. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, et al. Activity-dependent regulation of the binomial parameters p and n at the mouse neuromuscular junction in vivo. J Neurophysiol. 2010;104:2352–2358. doi: 10.1152/jn.00460.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frank CA. Homeostatic plasticity at the Drosophila neuromuscular junction. Neuropharmacology. 2014;78:63–74. doi: 10.1016/j.neuropharm.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lambert EH, Lindstrom JM, Lennon VA. End-plate potentials in experimental autoimmune myasthenia gravis in rats. Ann N Y Acad Sci. 1976;274:300–318. doi: 10.1111/j.1749-6632.1976.tb47694.x. [DOI] [PubMed] [Google Scholar]
- 39.Plomp JJ, van Kempen GT, Molenaar PC. Adaptation of quantal content to decreased postsynaptic sensitivity at single endplates in alpha-bungarotoxin-treated rats. J Physiol. 1992;458:487–499. doi: 10.1113/jphysiol.1992.sp019429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plomp JJ, van Kempen GT, Molenaar PC. The upregulation of acetylcholine release at endplates of alpha-bungarotoxin-treated rats: its dependency on calcium. J Physiol. 1994;478(Pt 1):125–136. doi: 10.1113/jphysiol.1994.sp020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blaber LC. The mechanism of the facilitatory action of edrophonium in cat skeletal muscle. Br J Pharmacol. 1972;46:498–507. doi: 10.1111/j.1476-5381.1972.tb08147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katz B, Miledi R. A re-examination of curare action at the motor endplate. Proc R Soc Lond B Biol Sci. 1978;203:119–133. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- 43.Wilson DF, West AE, Lin Y. Inhibitory action of nicotinic antagonists on transmitter release at the neuromuscular junction of the rat. Neurosci Lett. 1995;186:29–32. doi: 10.1016/0304-3940(95)11274-z. [DOI] [PubMed] [Google Scholar]
- 44.Gibb AJ, Marshall IG. Pre-and post-junctional effects of tubocurarine and other nicotinic antagonists during repetitive stimulation in the rat. J Physiol. 1984;351:275–297. doi: 10.1113/jphysiol.1984.sp015245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson DF, Thomsen RH. Nicotinic receptors on the rat phrenic nerve: evidence for negative feedback. Neurosci Lett. 1991;132:163–166. doi: 10.1016/0304-3940(91)90292-2. [DOI] [PubMed] [Google Scholar]
- 46.Tian L, et al. Nicotinic antagonist-produced frequency-dependent changes in acetylcholine release from rat motor nerve terminals. J Physiol. 1994;476:517–529. doi: 10.1113/jphysiol.1994.sp020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian L, et al. Hexamethonium- and methyllycaconitine-induced changes in acetylcholine release from rat motor nerve terminals. Br J Pharmacol. 1997;122:1025–1034. doi: 10.1038/sj.bjp.0701481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magleby KL, Pallotta BS, Terrar DA. The effect of (+)-tubocurarine on neuromuscular transmission during repetitive stimulation in the rat, mouse, and frog. J Physiol. 1981;312:97–113. doi: 10.1113/jphysiol.1981.sp013618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harborne AJ, Bowman WC, Marshall IG. Effects of tubocurarine on end-plate current rundown and quantal content during rapid nerve stimulation in the snake. Clin Exp Pharmacol Physiol. 1988;15:479–490. doi: 10.1111/j.1440-1681.1988.tb01104.x. [DOI] [PubMed] [Google Scholar]
- 50.Glavinovic MI. Presynaptic action of curare. J Physiol. 1979;290:499–506. doi: 10.1113/jphysiol.1979.sp012786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wessler I, et al. Presynaptic nicotine receptors mediating a positive feed-back on transmitter release from the rat phrenic nerve. Naunyn Schmiedebergs Arch Pharmacol. 1986;334:365–372. doi: 10.1007/BF00569371. [DOI] [PubMed] [Google Scholar]
- 52.Gallant PE. The relationship between anti-acetylcholine receptor antibody levels and neuromuscular function in chronically myasthenic rats. J Neurol Sci. 1982;54:129–141. doi: 10.1016/0022-510x(82)90225-8. [DOI] [PubMed] [Google Scholar]
- 53.Matzner H, Parnas H, Parnas I. Presynaptic effects of d-tubocurarine on neurotransmitter release at the neuromuscular junction of the frog. J Physiol. 1988;398:109–121. doi: 10.1113/jphysiol.1988.sp017032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plomp JJ, et al. Acetylcholine release in myasthenia gravis: regulation at single end-plate level. Ann Neurol. 1995;37:627–636. doi: 10.1002/ana.410370513. [DOI] [PubMed] [Google Scholar]
- 55.Sokolow S, et al. Impaired neuromuscular transmission and skeletal muscle fiber necrosis in mice lacking Na/Ca exchanger 3. J Clin Invest. 2004;113:265–273. doi: 10.1172/JCI18688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angleson JK, Betz WJ. Intraterminal Ca(2+) and spontaneous transmitter release at the frog neuromuscular junction. J Neurophysiol. 2001;85:287–294. doi: 10.1152/jn.2001.85.1.287. [DOI] [PubMed] [Google Scholar]
- 57.Thomsen RH, Wilson DF. Effects of 4-aminopyridine and 3,4-diaminopyridine on transmitter release at the neuromuscular junction. J Pharmacol Exp Ther. 1983;227:260–265. [PubMed] [Google Scholar]
- 58.Hong SJ, Chang CC. Run-down of neuromuscular transmission during repetitive nerve activity by nicotinic antagonists is not due to desensitization of the postsynaptic receptor. Br J Pharmacol. 1991;102:817–822. doi: 10.1111/j.1476-5381.1991.tb12258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneggenburger R, Meyer AC, Neher E. Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron. 1999;23:399–409. doi: 10.1016/s0896-6273(00)80789-8. [DOI] [PubMed] [Google Scholar]
- 60.Ruiz R, et al. Active zones and the readily releasable pool of synaptic vesicles at the neuromuscular junction of the mouse. J Neurosci. 2011;31:2000–2008. doi: 10.1523/JNEUROSCI.4663-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van der Kloot W. Loading and recycling of synaptic vesicles in the Torpedo electric organ and the vertebrate neuromuscular junction. Progress in Neurobiology. 2003;71:269–303. doi: 10.1016/j.pneurobio.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Searl T, Prior C, Marshall IG. Acetylcholine recycling and release at rat motor nerve terminals studied using (-)-vesamicol and troxpyrrolium. J Physiol. 1991;444:99–116. doi: 10.1113/jphysiol.1991.sp018868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Enomoto K. Post- and presynaptic effects of vesamicol (AH5183) on the frog neuromuscular junction. Eur J Pharmacol. 1988;147:209–215. doi: 10.1016/0014-2999(88)90779-0. [DOI] [PubMed] [Google Scholar]
- 64.Rich MM. The control of neuromuscular transmission in health and disease. Neuroscientist. 2006;12:134–142. doi: 10.1177/1073858405281898. [DOI] [PubMed] [Google Scholar]