Abstract
Memories that have strong emotions associated with them are particularly resilient to forgetting. This is not necessarily problematic, however some aspects of memory can be. In particular, the involuntary expression of those memories, e.g. intrusive memories after trauma, are core to certain psychological disorders. Since the beginning of this century, research using animal models shows that it is possible to change the underlying memory, for example by interfering with its consolidation or reconsolidation. While the idea of targeting maladaptive memories is promising for the treatment of stress and anxiety disorders, a direct application of the procedures used in non-human animals to humans in clinical settings is not straightforward. In translational research, more attention needs to be paid to specifying what aspect of memory (i) can be modified and (ii) should be modified. This requires a clear conceptualization of what aspect of memory is being targeted, and how different memory expressions may map onto clinical symptoms. Furthermore, memory processes are dynamic, so procedural details concerning timing are crucial when implementing a treatment and when assessing its effectiveness. To target emotional memory in its full complexity, including its malleability, science cannot rely on a single method, species or paradigm. Rather, a constructive dialogue is needed between multiple levels of research, all the way ‘from mice to mental health’.
This article is part of a discussion meeting issue ‘Of mice and mental health: facilitating dialogue between basic and clinical neuroscientists'.
Keywords: intrusive memory, memory reconsolidation, translational science, aversive conditioning, post-traumatic stress disorder, psychological treatment
1. Introduction
Your memory is a monster; you forget - it doesn't. It simply files things away. It keeps things for you, or hides things from you - and summons them to your recall with a will of its own. You think you have a memory; but it has you!
From: ‘A prayer for Owen Meany: a novel’ [1].
After psychological trauma, people can report vividly seeing the event unfold again in their mind's eye. For example, a person who was mugged at gunpoint while working at the cash till in a shop might see in their mind's eye vividly the gun pointed at them, the sunlight glance off the metal, hearing the sound of the safety lock clicking. This sensori-perceptual retrieval of an episode brings back with it the intense emotional experience of fear and the thought their life is about to end (for further examples, see table 1). These types of episodic memories are known clinically as ‘intrusive memory’—a core clinical feature of post-traumatic stress disorder (PTSD) and described in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) as ‘recurrent, involuntary, and intrusive distressing memories of the traumatic event(s)’ ([3, PTSD criterion B1; p. 271]). A critical aspect of this form of memory is that it springs to mind unbidden—that is, against the person's will. Thus, in the experimental memory literature, intrusive memories are a type of episodic memory that has been retrieved ‘involuntarily’ [4].
Table 1.
Examples of intrusive memories from patients diagnosed with post-traumatic stress disorder. Examples taken from Holmes et al. [2].
| ‘The body between the tube and the platform’ |
| ‘Being pushed to floor, them saying “get down” and being tied up’ |
| ‘Gun put to head’ |
| ‘He runs off and I look back to my house to see my daughter crying and banging at door’ |
| ‘His face above me, laughing, laughing, laughing’ |
Involuntary retrieval is not restricted to episodic memories. Anxiety and stress-related disorders also involve automatic physiological responses, triggered either spontaneously or in response to (internal or external) threat-associated cues. For example, a person may experience increases in heart rate, muscle tone and perspiration when revisiting the location of an accident or assault, or when reminiscing about the event. Although one might not necessarily think of anxiety and stress-related disorders as ‘memory disorders’, this paper builds on the assumption that many of their symptoms are involuntary expressions of memory of previously experienced or imagined emotional events. Understanding what aspects of memory are maintaining the disorder may provide a way to progress much-needed treatment development.
To date, there is a good evidence base for the effectiveness of psychological treatments for both anxiety (e.g. obsessive compulsive disorder and social anxiety) and stress-related disorders (e.g. PTSD). Specifically, the types of psychological treatment recommended are forms of cognitive behavioural therapy [5–7] and, for PTSD, also eye movement desensitization and reprocessing (EMDR) therapy (e.g. [8]). While the UK national clinical guidelines [9–11] among others emphasize the aforementioned treatments, it is also noted that psychological treatment trial methodology has been a topic of debate [12]. Recently, some advances have been made with pharmacological treatments, specifically for anxiety disorders [13], although less so for PTSD [14]. It will be of interest to examine similarities and differences in mechanisms underlying effective treatments, regardless of modality (psychological or pharmacological) [12]. Overall, however, it is clear that even our best treatments need improvement: a substantial proportion of patients do not benefit from them, or experience a relapse after initially successful treatment (e.g. [15–17]). Furthermore, effective interventions to prevent and treat traumatic stress symptoms within the first hours and days after a traumatic event are lacking [18,19]. Bringing novel insights from basic science to the field of mental health, to understand mechanisms of change at a fundamental level, seems imperative to drive clinical treatment innovation [20]. Such insights may, furthermore, point at ways to make promising interventions more effective and widely applicable. Yet, translational science is challenging, in part because there is a ‘culture gap’ between basic and clinical science, with little interdisciplinary communication and collaboration, different language to describe the same phenomena, different journals to disseminate findings and thus limited knowledge of needs and discoveries in the other fields [20]. Aside from this gap, there are the methodological challenges associated with laboratory procedures, and boundary conditions, meaning that pioneering attempts to apply laboratory-developed interventions to clinical settings risk failure.
We believe that challenges to translational science arise, at least in part, from a lack of a shared vocabulary, which gives rise to a misunderstanding of clinically relevant targets at one end and insufficient appreciation of basic memory principles at the other end. We argue that, to successfully bridge findings ‘from mice to mental health’, it is essential to clarify (i) what aspects of emotional memory are clinical targets and (ii) when to assess and target these aspects.
We discuss the following questions:
— Why study emotional memory to understand anxiety and stress-related disorders?
— How to define emotional memory and targets for clinical interventions?
— How can the science of memory inform treatment innovation?
— What are the challenges of translational science from mice to mental health?
— How can neuroimaging in humans contribute to translational science?
— How could we facilitate the dialogue between basic and clinical science?
2. Why study emotional memory to understand anxiety and stress-related disorders?
Emotional memories are particularly resilient to forgetting [21,22]. This is not problematic per se. Long-lasting memories for aversive experiences can help us deal with similar situations in the future. However, aspects of emotion-laden memories can also form the basis of psychological disorders [23–25], particularly when memory retrieval is involuntary. ‘Intrusive memories’—typically of emotional events—are a form of involuntary recall and have been theorized to play a critical role in the development and maintenance of various psychological disorders [24–29], such as PTSD, and also including depression [30,31], bipolar disorder [32,33], social anxiety [34], agoraphobia [35], spider phobia [36] and health anxiety [37]. Involuntary memories may differ across disorders: intrusive memories in PTSD replay trauma content from an index episode; intrusions in agoraphobia have content related to agoraphobic themes such as being trapped—whether real or imagined; intrusive memories in bipolar disorder can have a future quality as if pre-playing a mania-inducing event. Although highly specific to the given disorder, what is common is their intrusive and imagery-based natures. Clearly, not all involuntary memories are pathological [38]—involuntarily recall can be useful at times—but when the intrusion contains highly aversive content, is unwanted and disrupts a person's functioning in daily life, then it is maladaptive and can enter the clinical domain. For example, Syrian refugees indicated that intrusive memories of traumatic experiences were linked to concentration problems and functional impairment on tasks associated with adaptation to the host country, such as language learning [39].
Here, we focus on memory in PTSD and, to a lesser degree, anxiety disorders. In PTSD, patients typically experience intrusive episodic memories of several distinct moments of the wider traumatic event [2]. That is, intrusive memories are not a replay of the whole event from beginning to end, but rather of a few fragments within it—known clinically as ‘hotspots’ [40]. Aside from these sensory episodic (predominantly visual) intrusive memories ([3, PTSD criterion B1; p. 271]), people with PTSD frequently exhibit increased heart rate, sweating and muscle tone in response to trauma-related cues, as well as impaired extinction of such responses. In the DSM-5, the diagnostic criterion B5 for ‘intrusion symptoms’ involves ‘marked physiological reactions to internal or external cues that symbolize or resemble an aspect of the traumatic event(s)’ ([3, PTSD criterion B5; p. 271]), while criterion E for hyperarousal symptoms involves ‘marked alterations in arousal and reactivity associated with the traumatic events(s) including hypervigilance (E3), exaggerated startle responses (E4) and problems with concentration (E5)’ ([3, PTSD criterion E; p. 272]). Both intrusive episodic memories and heightened physiological reactivity have been found to be predictive (among other factors) of the development of PTSD [41–43]. However, while general alterations in physiological reactivity are common across many disorders (e.g. most anxiety disorders), it is trauma-related intrusion symptoms (particularly criterion B1) that are the core clinical feature of PTSD (and acute stress disorder) and distinguish them from other disorders. Interestingly, evidence from experimental studies also suggests that, although physiological reactions in response to trauma reminders and intrusive episodic memories frequently co-occur, they do not seem to be associated with each other [44,45], which has implications for intervention development (see §3a).
As yet, it remains unclear whether it is better to focus on the ‘impact’ of a memory, or on the underlying memory itself. When an involuntary memory occurs, it can not only bring back the sensori-perceptual, emotional, psychophysiological and peri-traumatic cognitions described earlier (e.g. table 1), but it can trigger a cascade of other, more distal symptoms, such as further distress, non-specific hyperarousal symptoms, negative mood and cognitions, and unwanted avoidance. It is these cognitive and emotional reports of the immediate and more distal symptoms that are the typical focus of cognitive therapy. However, even exposure therapy procedures thought to focus more proximally on the trauma memory involve approaches that are thought to leave the original trauma memory unaltered [46], creating a new inhibitory memory trace instead.
Here, we argue that focusing on treatments that target the underlying memory more directly may be beneficial: controlling the expression of involuntary emotional memories may prevent the occurrence of the other symptoms altogether. Since the beginning of the century, a vast growing body of literature suggests that it is indeed possible to change the underlying memory trace. As explained below, the theory of ‘reconsolidation’ states that memories are not necessarily permanent [47–49], but can be updated, reduced or enhanced, under the right circumstances. As we shall discuss, this has inspired a whole new line of research looking at how the plasticity of memories can be used to modify clinically dysfunctional memories. But what exactly do we mean by ‘emotional memory’?
3. How to define emotional memory and targets for clinical interventions?
Studying memory is complicated by the fact that we cannot directly observe a memory trace, but have to infer it from the different ways it is expressed. In memory research, a variety of behavioural measures provide different ‘read-outs’ of information that has been stored, for example neural responses, peripheral physiology, actions or action tendencies, (subjective) verbal reports or explicit tests of memory. These responses are thought to be indices of some sort of underlying neural ‘engram’, the memory trace, which supports these responses. While in many situations different read-outs converge, below we argue that a distinction between multiple memory systems is useful and paramount to making translational progress.
(a). Multiple memory systems
It is possible to show evidence of remembering in the absence of awareness of that remembering. For example, the Swiss neurologist Claparède [50] concealed a sharp pin between his fingers while greeting one of his amnesic patients with a handshake. Even though the patient reacted with surprise and anger, she forgot the encounter within minutes. However, when the neurologist tried to reintroduce himself shortly thereafter, the patient resolutely refused to shake his hand. She explained her reaction by stating that she was afraid that, perhaps, a pin was hidden in his hand, but even after repeated questioning could not remember that she herself had been stuck with it. While her brain was apparently able to form this association between a neutral handshake and a painful consequence, her brain was not able to voluntarily retrieve the event. The episodic part of the memory had not been stored.
Clinical cases like these, substantiated by abundant scientific evidence (e.g. [51–54]), teach us that our mind is made up of a constellation of agents that are, in principle, separable and rely on semi-independent circuits. In cognitive psychology, a classic distinction is made between ‘explicit’ or ‘declarative’ memory (what we can report, including episodic memory for events and semantic memory for facts) and ‘implicit’ or ‘non-declarative’ memory (including priming, reflexes and procedural memory such as aversive conditioning, motor skills and habits) (for a review, see [55]). Declarative memory is mostly subserved by the medial temporal lobe; most forms of non-declarative memory are subserved by subcortical areas such as the amygdala (aversive conditioning) and the striatum (skills and habits) [55]. These memory systems can be independently targeted [56–61] or damaged [62–65].
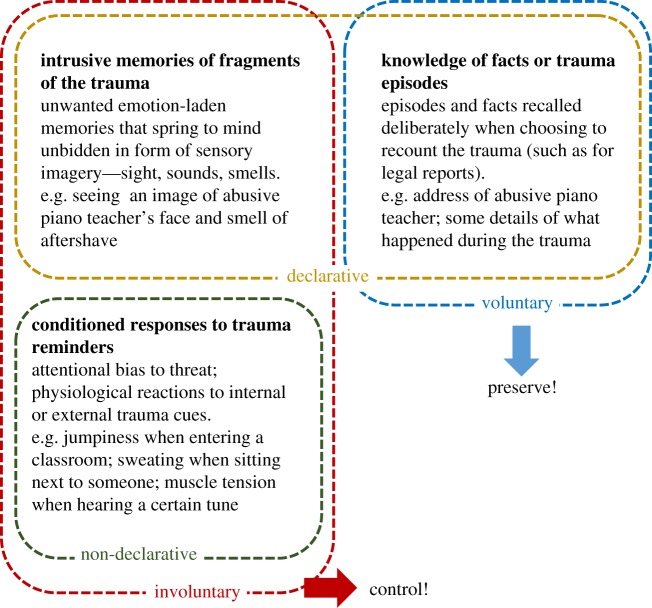
The idea that memory is composed of different systems is not new, but has now been widely accepted [66,67]. Furthermore, work in both non-human animals and humans has highlighted that certain types of memory can be modified, while preserving other types. Yet, when translating these findings to develop treatments for psychological disorders, relatively little attention has been paid to specifying what type of memory (i) can be modified and (ii) should be modified. We argue that rather than the traditional declarative versus non-declarative memory system distinction, the clinical focus should be on the intrusive nature of an aversive memory [68], which could, in principle, stem from both systems. A clinical intervention should thus seek to target the involuntary expressions of the memory (both declarative and non-declarative), without compromising voluntary attempts to access the same information or skill (figure 1). Voluntary forms of memory need to be preserved, e.g. for legal reasons a person may need to recount details of a trauma (eye witness testimonies and asylum reports). The simultaneous consideration of involuntary and voluntary retrieval of the same events should be a more central feature of traumatic stress research.
Figure 1.
Diagram depicting how different memory systems may represent different aspects of the traumatic event(s), for example sexual abuse by a piano teacher. In general, clinically beneficial interventions should aim to target the maladaptive involuntary expression of trauma memories (e.g. intrusive memories), while preserving its voluntary recall (e.g. ability to testify in court). (Online version in colour.)
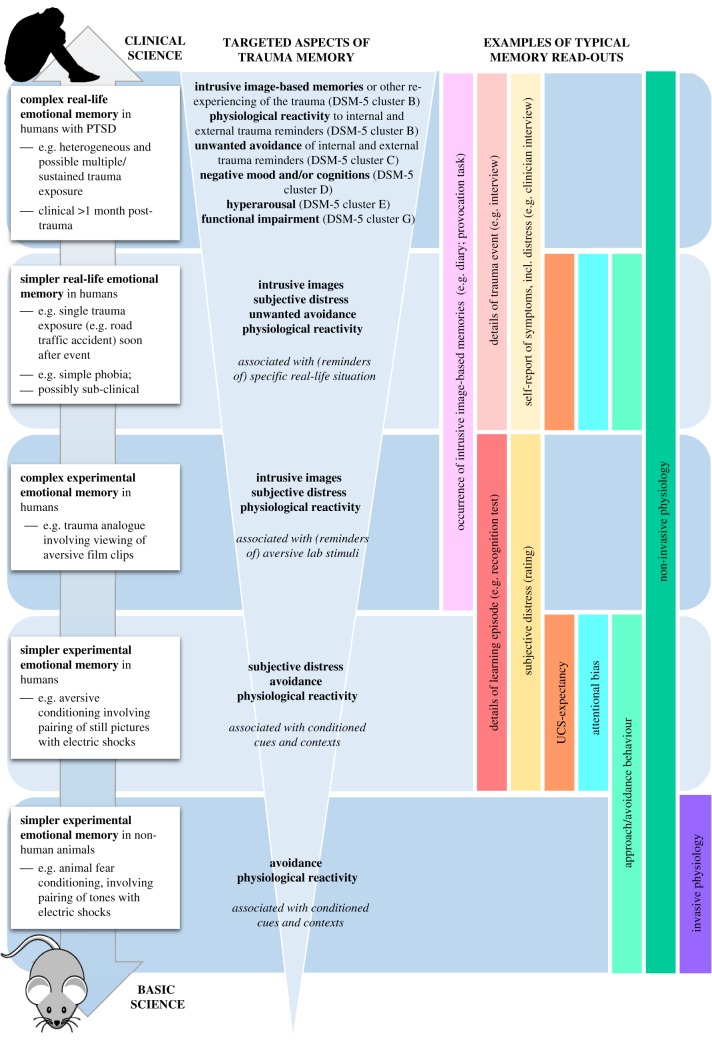
Successful translation of insights from basic science to inform clinical interventions (figure 2), depends on a clear conceptualization of what type of memory is being targeted, and how to model this. A widely used experimental model of traumatic stress responses and other types of aversive learning has been Pavlovian conditioning (box 1). Research using this paradigm in rodents shows that it is possible to selectively update one (aspect of) memory, without affecting another [72–74], and one response system (e.g. reducing freezing to a conditioned stimulus), but not another (alleviating suppressed reward seeking after conditioning) [75]. Similarly, many interventions based on this paradigm in humans show effects on one type of memory read-out (e.g. reduced startle reflexes in response to conditioned pictures), but not on other types (e.g. expectancy ratings of the likelihood of an aversive consequence) (e.g. [58,76,77]). Thus, it appears possible that rather than the whole memory, involuntary non-declarative aspects can be selectively targeted. Such dissociations are crucial when designing interventions for disorders such as PTSD: which aspects of memory can we modify, and is the aspect that can be modified, in fact, clinically meaningful?
Figure 2.
Examples of different aspects of trauma memory that can be targeted and associated research approaches along the translational research pathway(s) from mice to mental health. The left column shows the different levels at which trauma can be modelled. The middle column indicates which aspect of the (modelled) trauma may be relevant to target by an intervention, i.e. from a clinical perspective. The right vertical column highlights some of the memory read-outs that can be assessed at each level, with measures of declarative memory and subject reports being restricted to human research, and invasive physiology measures (such as structural at the synapse) being restricted to non-human animals. By and large most of the knowledge about timing and conditions for memory updating comes from the lower level, i.e. experimental research in non-human animals. Note that voluntary memory recall (e.g. details of the trauma) can be measured in humans, but is not the key clinical target of a treatment. DSM-5, Diagnostic and Statistical Manual of Mental Disorders [3]; UCS, unconditioned stimulus.
Box 1. Aversive conditioning paradigm: modelling involuntary and voluntary memory of threat associations in non-human animals and humans.
The classic model to study simple associative learning and memory is Pavlovian conditioning [69], which is well suited for research across species [70,71]. In this paradigm, an initially neutral stimulus (conditioned stimulus, CS+; e.g. a triangle) is repeatedly paired with an intrinsically aversive stimulus (unconditioned stimulus, UCS; e.g. an electric shock), while another conditioned stimulus (CS−; e.g. a circle) is never paired with the UCS. With sufficient CS+/UCS pairings, the CS+ acquires the same aversive qualities as the UCS and will elicit a conditioned defensive response (CR) on its own. After repeated presentations of the CS+ without the UCS, the defensive response usually diminishes, a process that is referred to as ‘extinction learning’. In non-human animals, defensive responses are typically measured by assessing the amount of freezing in response to the CS, or avoidance behaviour; in humans, common measures include skin conductance responses, acoustic startle responses, heart rate, pupil dilation responses, action tendencies, UCS expectancies and subjective distress (figure 2).
Although aversive conditioning very precisely models the formation of associations between environmental stimuli and allows research across species, it does not model one of the core symptoms of PTSD: intrusive memories of the traumatic event. Involuntary retrieval of an intrusive memory is not the same as a conditioned physiological response: it (also) involves consciously retrieving a memory, i.e. drawing from the declarative memory system. Relatedly, physiological responses to trauma reminders are listed as a separate symptom from intrusive memories in the DSM-5 [3]; see earlier. Although a defensive response may very well be part of an intrusive memory, it does not account for its image-based episodic nature. Surprisingly, prominent neural circuitry models of PTSD based on aversive conditioning findings do not include mental imagery (e.g. [78]), which is a defining feature of episodic recall of an event. This conditioning literature, therefore, does not directly speak to the dissociation between involuntary and voluntary declarative memories.
An alternative experimental model with ecological validity for traumatic stress is the trauma film paradigm, which can model intrusive memories in response to viewing experimental material with traumatic content under controlled settings (for a review, see [79]; box 2). Emerging experimental interventions based on this paradigm have shown effects on one type of memory read-out (e.g. frequency of intrusive memories), but not on other types (e.g. recognition memory test) (e.g. [57]). Thus, it also appears possible that, rather than the whole memory, involuntary declarative aspects (e.g. frequency of intrusions) can be selectively targeted.
Box 2. The trauma film paradigm: modelling involuntary and voluntary memory of psychological trauma in humans.
The trauma film paradigm [79,80] has emerged as a well-established methodology to study intrusive memories of trauma, a core symptom of post-traumatic stress and one that can be distressing in its own right. It uses film stimuli in the laboratory, which contain traumatic content that can bring about intrusive memories subsequently in daily life. These memories are typically recorded in a diary, allowing for a frequency count of intrusive memories. Aside from intrusive memories, physiological reactivity and neural activity as well as subjective distress can be measured during and immediately after film viewing. This is similar to what is measured in conditioning paradigms (box 1; figure 2). Additional measures of voluntary memory include free recall and recognition of details of the film. Therefore, this paradigm enables the study of memory for more ecologically valid stimuli, but still within a laboratory setting, conferring additional experimental control.
Although the trauma film paradigm can only be used in humans, there are putative parallels with other types of memory read-out at ‘lower’ translational levels (e.g. distress ratings or psychophysiological measures in the conditioning paradigms; figure 2), which tentatively allow for translation of findings from basic neuroscience to simple real-life trauma. Yet, this is an understudied area. Given that assessment of intrusive memory frequency in humans typically involves ambulatory recordings over prolonged periods of time, research that directly compares intrusive memory frequency with other read-outs is sparse. The few studies that have assessed both physiology and intrusions in a laboratory setting indicate, however, a dissociation: trauma film reminder cues relative to control cues elicited both intrusive memories and heightened physiological reactions (e.g. skin conductance level) in one study [44], but the two measures were not associated [44]. Another study found more intrusions to film reminder cues, without heightened physiological reactivity (skin conductance and heart rate). Moreover, individual differences in cue-elicited physiological responses were not predictive of subsequent intrusive memory frequency in everyday life [45]. To translate findings from one memory read-out to another one in humans, future research should systematically examine under which conditions different read-outs converge or diverge.
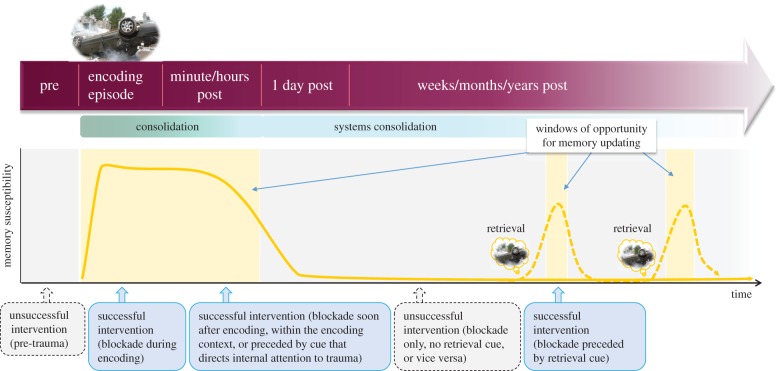
Not only is memory for a traumatic event likely to rely on different independent systems, but its nature also appears to change over time. In that light, the timing of any intervention is crucial. That is, it is important to appreciate that memory processes are dynamic, and that procedural details concerning timing are crucial when implementing a treatment, and again later when assessing its effectiveness (figure 3).
Figure 3.
In the hours after an experience, memories are believed to go through an initial labile phase, before being stored into stable long-term memory, i.e. consolidation. The purple arrow depicts different time intervals with respect to the encoding of an aversive episode. Green gradients below indicate the putative processes of memory encoding and consolidation that occur during these different intervals, with systems consolidation referring to process by which memories become less hippocampus-dependent and integrated into a wider semantic network. Recent insights from studies in non-human animals suggest that (certain aspects of) memories are not necessarily permanent. Instead, they may become transiently malleable upon their reactivation, rendering them susceptible to interference or updating before returning to a fixed state, a process referred to as ‘reconsolidation’. This offers a second window of opportunity to interfere with consolidated memory (shown by yellow background shaded areas). Successful interventions (blue arrows) need to be timed such that the blockade interferes with memory when it is in an active, susceptible state (indicated by the dotted yellow line)—either in the first hours after an experience, or at later time intervals after a retrieval procedure (e.g. reactivation through reminder cues). In the first hours after an experience, blockade procedures may also need to be preceded by cues that orient attentional resources to the event in order for procedures to successfully interfere with it, for example when the intervention is delivered in a context other than the one in which the trauma occurred. Unsuccessful interventions, timed when memories do not yet exist, or are in a fixed state (i.e. not recently retrieved), are indicated by grey arrows with dotted outlines.
(b). Multiple time points
The mind is not a video camera, automatically storing everything that occurs in front of the lens in a way that is easy to retrieve. To come back to the amnesic patient described by Claparède, she may be perfectly capable of remembering her neurologist's name and recognize his face in the short term, providing this information is actively rehearsed. Her failure to explicitly remember the incident with the pin does not indicate that she did not successfully encode the information. Rather, the information appeared not to be successfully consolidated in long-term storage, or lost in storage and therefore not successfully retrieved. Many people may respond with terror during a highly aversive experience, and will experience intrusions for a few days after, but only a few of them develop a disorder like PTSD where intrusions appear to continue for months or years.
A first time point to consider for memory modification is the ‘consolidation’ period: memory does not reach its final form during, or immediately after, encoding (unlike a video recording), but is still malleable for some time after the event. If altered during this malleable phase, the effects become apparent not initially, but at a later time points, after the memory has had a chance to consolidate [81]. The concept of memory consolidation has been most convincingly illustrated by experiments in which pharmacological manipulations administered immediately after encoding left short-term (4 h) retrieval intact, but induced full amnesia in the long term (24 h) [81,82]. Post-encoding processes account for this dissociation, as they induce the synaptic changes, including long-term potentiation (LTP), that are necessary for the stabilization of a memory trace in the hours after its acquisition [83,84]. This means that memory is initially malleable, i.e. there is the possibility to interfere with it.
While the duration of the consolidation window is typically estimated to be in the order of hours [85], another form of consolidation may continue indefinitely, with memories becoming more and more integrated into cortical networks [86,87]. However, this so-called systems consolidation happens mostly offline, when memory is in a stable state and not susceptible to interference (figure 3).
A second time point to consider for altering memories therapeutically is inspired by the theory of memory ‘reconsolidation’: findings from basic neuroscience indicate that consolidated memories can—upon their retrieval—enter a transient labile state, i.e. become malleable again [47–49] (figure 3). During this window, a memory can be updated, that is, new information can be integrated with a reactivated memory to modify future retrievals. For example, extinction learning within this window may diminish conditioned defensive responses more persistently than normal extinction learning, as the safety information has become part of the original fear memory [88]. The re-stabilization of a labile memory relies on a postulated process called ‘reconsolidation’. This process is dependent on de novo protein synthesis, and may be disrupted through pharmacological interventions (e.g. noradrenergic blockade [89]) or behavioural procedures that take away resources necessary for storage of certain elements of the memory (e.g. a competing visuospatial task [57]). To our knowledge, the precise time window of memory (re)consolidation has not been systematically investigated in non-human animals nor in humans, though is assumed to be in the order of hours [86]. The duration may depend on memory system and protocol used, and systematic work (e.g. using optogenetic manipulations to reactivate memory networks, followed by biochemical assays of plasticity markers) is needed to determine when exactly the window opens and closes. Again, the effects become apparent not initially (i.e. post-reactivation short-term memory is intact), but at a later time points after the (updated) memory has had a chance to reconsolidate [48,90,91].
The success of interventions that aim to harness principles of memory plasticity is dependent on their ability to target a memory when it is in an active, labile state. We have discussed two main time points during which memories can be altered: one that draws on theories of memory consolidation and one that draws on theories of memory reconsolidation. Inherent in this is the importance of a third type of time point: the outcome of memory modification cannot be observed until the memory has (re)turned to a stable state. This is important to consider when assessing the effectiveness of an intervention. Time has even further critical consequences: for example and fourth, a time gap of a few minutes may be needed between a retrieval cue and a blockade (figure 3), to allow a memory to destabilize (e.g. [88]). Fifth, pharmacological interventions require a precise understanding of temporal profiles of drug actions to target the memory when it is labile [92,93]. Research is clearly needed to define optimal time points for intervention delivery, which may be key to the success of a treatment.
4. How can the science of memory inform treatment innovation?
(a). Successful translations
The principles of consolidation and reconsolidation imply that memory is malleable, either directly after an experience or upon reactivation [47–49]. This has inspired exciting lines of research aimed at modifying emotion-laden memory in humans, with the hope to develop relatively simple-to-deliver strategies that have the potential to interfere with involuntary recall of memories once they are established, i.e. hours, days, months or even years after the trauma (figure 3).
The first studies that translated findings in non-human animals [48,88,89] to humans used classical aversive conditioning to induce a simple emotional memory [94,95]. These showed that conditioned physiological responses could be eliminated by presenting a single reminder cue (the CS+) and administering a pharmacological agent (the beta-adrenergic antagonist propranolol [95]) or a behavioural intervention (an extinction procedure [94]) within the putative reconsolidation window. These findings have been replicated a number of times, using either the noradrenergic blockade [56,58,76,77,96–98] or a behavioural extinction procedure [99–103], with effects persisting for over a year (e.g. [58]) (for a discussion of non-replications, see section 4b ‘Challenges to translation’). Notably, these procedures only eliminated the automatic reflexive fear response (involuntary recall) and subjective feelings of fear, while leaving declarative knowledge about the aversive associations intact (figure 1).
Another line of research, which has drawn on theories of consolidation or reconsolidation (depending on the time window), uses the trauma film paradigm (box 2) to mimic complex emotional memory in non-clinical volunteers (figure 2). This has shown that the number of intrusive memories can be reduced, for example, by directly interfering with the molecular processes underlying memory consolidation at the receptor level. Nitrous oxide, also known as ‘laughing gas’, blocks N-methyl-d-aspartate (NMDA) receptors which are important for LTP. Individuals who inhaled nitrous oxide for 30 min immediately after trauma film viewing, compared with medical air (control condition), showed a steeper decline in the number of intrusive memories over the course of one week [104]. This suggests that across species, memory stabilization may rely on similar molecular processes (e.g. [90]).
Interestingly, a variety of interventions (whether pharmacological or behavioural/psychological or both) could, in principle, interfere with memory stabilization. For example, the frequency of intrusive memories can also be reduced by a relatively simple behavioural approach—by performing visuospatial tasks (e.g. Tetris; complex finger tapping), during a time period when memories are assumed to be labile (figure 3), i.e. either during trauma film viewing [105], within minutes to hours after film viewing [106–109], or upon their reactivation 24 h after encoding [57]. As expected, the task was not effective when administered before film viewing [110], or when only the task was given without retrieving the memory first [57]. The rationale behind this procedure is that the task taxes visuospatial working memory resources which are thought necessary to (re)store intrusive memories with strong visual components [108], acting as a ‘cognitive blockade’. In line with this notion, non-visuospatial tasks appear to be less effective or have no effect ([107,109], but see [111]). Crucially, when using a visuospatial task, across several studies the cognitive blockade selectively reduced intrusion frequency (involuntary recall), while leaving verbal and visual recognition (voluntary memory) intact (figure 1) (for a review, see [79]). As mentioned before, that a task could selectively reduce the number of intrusive memories while sparing voluntary memory of a traumatic event is desirable from a clinical perspective: someone would still be able to recall the factual course of events that constitute the trauma (e.g. recognize the perpetrator and testify in court), without having to fear being overtaken by unwanted intrusions of the trauma in their everyday life. Yet, the mechanisms behind this are still not fully understood and require further investigation [112].
Recently, findings from conditioning studies in non-clinical volunteers have been translated to emotional memory for real-life events in sub-clinical populations (figure 2). Three studies suggested that phobic responses could be decreased via reconsolidation-update mechanisms ([59], snake or spider phobia; [113], spider phobia; [114]). The procedure involved brief exposure to the object of fear (long enough to reactivate the memory, but short enough to prevent extinction; see below, section 4b ‘Challenges to translation’), followed by either a pharmacological manipulation (i.e. the administration of 40 mg propranolol [59]) or a behavioural manipulation (i.e. extinction training [113,114]). The manipulation changed avoidance behaviour into approach behaviour [59,113,114], with effects lasting up until three-month and 1-year follow-up. Importantly, the placebo group and the propranolol group without memory reactivation (i.e. no brief exposure to the spider) did not improve [59]. Also, in this case, different memory read-outs of the fear memory diverged (figure 2): initially, no differences were observed in subjective fear reports, but after three months, the reactivation + propranolol group scored in the normal range [59]. This suggests that effects of memory modification may require time to transfer to more distal symptoms, possibly via mechanisms other than memory reconsolidation (e.g. sufficient exposure to the previously avoided stimulus without the concurrent physiological and behavioural fear responses, leading to cognitive reappraisal of threat).
Findings from studies using the trauma film paradigm with non-clinical volunteers and cognitive task interference [106–109] have recently been translated to hospital settings, testing the modification of real-life emotional memory in the first few hours after a road traffic accident [115], and after traumatic child birth [116]—i.e. within the putative consolidation time window. Both early phase (proof of concept) clinical studies aimed to prevent the occurrence of intrusive memories of trauma, by having patients play the computer game Tetris soon after the event, as a way to interfere with the consolidation of the image-based components of the memory. In the road traffic accident study [115], a brief reminder cue was given prior to the cognitive task to orient the patient's memory to the accident, because patients were now in a hospital setting, whereas the accident had occurred in another context. In the traumatic child birth study [116], a reminder cue was deemed not to be necessary—mothers were in the same hospital setting context in which the trauma occurred as for the delivery of the intervention—and often with the baby. Both studies showed, as predicted based on studies with experimental trauma which used the same outcome measure [106–109], that the intervention group recorded significantly fewer intrusions in a diary that they kept in the week following the traumatic event compared with control groups.
Targeting simple phobias and recent real-life trauma memory (i.e. a prevention approach because this is before a clinical diagnosis of PTSD can be made) may be regarded as an early step of intermediate clinical complexity, compared with laboratory research in healthy individuals on the one hand, and the more severe anxiety disorders and PTSD on the other (figure 2). The more complex the memory becomes (e.g. older, or multiple events), the more important it is to define what aspect of the memory is being targeted and what read-out is used to assess whether it is effective (figure 2), and to ensure the timing of the intervention uses or induces periods of memory malleability (figure 3). Real trauma memories are typically stronger and broader than aversive memories formed in the laboratory. Case studies [117] and pilot studies [118], which combined a memory reactivation procedure and propranolol administration to block reconsolidation of trauma memories in patients with PTSD, seem to be promising, though many more are needed to understand potential limitations and non-replications (see next section). Such small-scale work helps to get the right ingredients to develop a new treatment. To test effectiveness of a treatment once it is there, various types of randomized controlled trials (RCTs) are necessary, but as argued below, at present more mechanistic insights may be required before investing in ‘expensive and time-consuming RCTs' [117].
(b). Challenges to translation
While discoveries of memory plasticity fuelled excitement about the potential to offer brief interventions for mental health disorders with long-lasting effects, there has been considerable scepticism as well, especially among clinicians [6].
A first criticism that has been put forward is that the effects of the reconsolidation-update manipulation are usually restricted to one response system (for a review, see [6]). However, we argue to the contrary that this can be an asset therapeutically. While it is true that this means that an intervention does not wipe out an entire memory or miraculously ‘cures’ someone of a full disorder from one day to another, from a clinical point of view this does not mean it is not worth investing in. The elimination of one debilitating symptom may improve quality of life and functioning, and open up resources to tackle (or prevent) other symptoms, as well as have distal effects after time (e.g. [59]), and it may be possible to target different aspects of the memory one at a time. Moreover, as argued above, for trauma it is an advantage that manipulations are usually restricted to one response system—permanent erasure of all aspects of memory for a traumatic event is not the aim of evidence-based clinical interventions (figure 1) and could, in fact, bring about profound legal, ethical and clinical consequences of concern [119].
More broadly, specifying clear, precise clinical targets and memory read-outs allows one to systematically test and optimize interventions along the translational pathway (figure 2), which is impractical if one tries to treat an entire psychiatric syndrome at once. It is well recognized that the current classification system for mental health disorders, DSM-5 [3], poses challenges for research owing to ongoing debate about the precise symptoms that constitute a given disorder and the overlapping symptoms across different disorders [120–122]. Thus, a ‘precision focus’, focusing on one symptom rather than a whole disorder, may be more helpful when considering the aetiology of disorders. Indeed, arguably, it is the confusion that arises when talking about syndromes rather than symptoms that can stagnate scientific progress across various areas of mental health.
Second, scepticism has been fuelled by the fact that initial attempts to apply procedures in the clinic have been difficult to replicate. For example, the initial finding that trauma cue evoked physiological responding could be reduced following a pharmacological procedure that was postulated to tap into reconsolidation mechanisms [118,123] was not replicated in a follow-up study [124]. It should be noted that both studies had several important limitations, such as small sample sizes [118,124] and lack of control groups for either the effects of reactivation [118] or general drug effects [124]. It is important to follow this up and delineate boundary conditions that arise during the complexity of clinical translation. Failed replications are not unique to clinical settings: pharmacological [125] and extinction procedures within the reconsolidation window [97,126–129] have sometimes not replicated—though note that there are more successful replications than non-replications. Furthermore, there is evidence that the procedure does not work strongly enough to eliminate all involuntary recall for everyone [115,130]. Occasional (or partial) non-replications are to be expected at this early stage and yield important clues to direct future lines of research.
An important question is whether studies that failed to find an effect managed to actually trigger reconsolidation. Experimental studies have highlighted so-called boundary conditions—circumstances under which memory updating cannot take place (for a review, see [6]). The first one is cue and context specificity: the reminder situation that is used to reactivate the memory may need to be identical to the encoding situation [73], which may be difficult to achieve in clinical practice. However, other experimental studies have found effects of memory modification extending from one reactivated element to other elements of a compound memory [74], to similar—but not identical—stimuli, including imagined threat events [76,77], and to persist across contexts [77,88]. Furthermore, the finding that interventions in sub-clinical populations were successful in contexts other than the original encoding contexts [59,115] suggests that this is not such an issue, as long as the targeted memory system is sufficiently engaged. Another boundary condition is the finding that reactivation of a memory is necessary, but not sufficient, to destabilize a memory. Whether or not a memory trace is destabilized may, at least partly, depend on whether there is something to be learned, i.e. whether novel or conflicting information requires an update [56,96,98,131,132], highlighting a potential evolutionary function of reconsolidation mechanisms [86,133]. This may be governed by prediction error, i.e. a mismatch between what is expected and what occurs (for a computational account of when and how prediction may play a role in memory updating, see [134]). For example, one study [56] showed that in the case that a certain stimulus (CS) always predicted a shock (UCS), a single unreinforced presentation of the CS led to a violation of predictions and triggered memory destabilization. However, in the case of uncertainty (e.g. the stimulus was only reinforced in 50% of the trials during encoding), more unreinforced trials were needed to create a mismatch with what was expected [56]. Similarly, older and stronger memories may require additional retrieval procedures for updating to take place (e.g. [135,136]), as do individual differences such as exposure to chronic stress and high trait anxiety [130,137].
Thus, the optimal timings (see the earlier section (3b) ‘Multiple time points') and duration of the reactivation procedure are likely to depend on the precise encoding history, with more ambiguous memories possibly requiring more conflicting information, longer durations or possibly multiple retrieval instances. Yet, longer and repeated retrievals of a memory bear the risk of initiating extinction learning (which involves the creation of a new, inhibitory memory trace [46]) instead of memory destabilization. When this happens, no updating appears to take place [98,138–140]. Some clinical pilot studies [118,123,124] have used script-driven imagery for the reactivation of the trauma memory, similar to EMDR-like treatments: the question is whether the method of retrieval used in these treatments (prolonged, and with no obvious violation of expectancy) would have triggered reconsolidation, or instead extinction learning. Furthermore, memory read-outs during an intervention should not be mistaken for clinical targets. For example, a clinician may be inclined to continue a reactivation procedure until a decline in physiological arousal or subjective distress in response to trauma cues is observed. Yet, such a focus on acute fear relief may be counterproductive, and by inducing extinction learning, in fact prevent modification of the original trauma memory [98,138–140]. This would have enormous implications for clinical practice, suggesting that therapists should not merely rely on seeing direct within-session change, but should prioritize capturing longer-term effects, i.e. after their therapy session has ended.
This touches on an important point: we do not currently have an index for memory destabilization or reconsolidation, other than inferring it retrospectively from the strength of memory recall. But this is essentially circular: if we observe a behavioural change, we infer that the memory was modified; if not, we conclude that some of the criteria for updating were not met. While, in an experimental setting, overt expectancies have been used to indicate at a group level whether memory destabilization was achieved [56], this index may not be sensitive enough to apply in real time on an individual level, as patients may not always be able to voice what they fear and what event violates their expectation. What we need is a memory read-out for offline processes that underlie memory plasticity.
5. How can neuroimaging in humans contribute to translational science?
Neuroimaging—especially (functional) magnetic resonance imaging (fMRI)—has been frequently used for studying memory processes because of the possibility to predict which elements of an encoding episode will later be remembered and which will later be forgotten. So-called subsequent memory paradigms [141] have yielded several markers that predicted the later voluntary recollection of information [142], or the involuntary retrieval of experimental trauma [143–145]. Recent conditioning studies showed that patterns of neural activation during encoding can also be used to predict the long-term expression of involuntary non-declarative memory (i.e. physiological responses to conditioned stimuli) [146,147]. Furthermore, neuroimaging has been used to study memory reactivation and offline consolidation processes directly, without having to interfere with them [148–152]. Perhaps, in time, techniques such as fMRI could help us capture critical time frames for memory updating—a process for which no other read-out exists. Such a read-out may not be feasible for use in clinical practice (yet), but it would allow us to test out procedures in real time that trigger memory destabilization, without having to retrospectively infer it from behaviour.
Another way that neuroimaging could advance translational science is by checking whether neural circuitries underlying a certain read-out of memory (e.g. startle responses) are comparable across species, or across non-clinical and clinical human populations. If so, there is stronger theoretical reason to translate interventions that target that read-out in non-human animals to humans, and eventually to clinical populations. If not, it may be necessary to go back a step and focus on a different read-out, until these ‘intermediate phenotypes' yield sufficient overlap to proceed. By covarying different memory read-outs within and across different translational levels (figure 2), we may be able—in a ‘Sudoku’ way—to bridge gaps across paradigms and species. Neuroimaging could provide a valuable tool to build these bridges.
6. How could we facilitate the dialogue between basic and clinical science?
Harnessing the science of memory plasticity seems promising for the innovation of treatments to control the expression of unwanted emotional memories. Yet, to capture emotional memory in its full complexity, including its malleability and its context-dependent activation, science cannot rely on a single method, species or paradigm. Indeed, work on memory updating, including reconsolidation, has been conducted in different species (e.g. crabs, mice, rats and humans), different paradigms (e.g. classical context and cue conditioning, trauma film paradigm, as discussed here, but also operant conditioning, motor tasks) and different modalities (vision, audition and olfaction). A constructive dialogue between different levels of research is difficult as long as clinicians do not familiarize themselves with basic science, and scientists do not go beyond formulating the implications of their findings as merely ‘offering opportunities for the treatment of disorder ‘X’’. Both sides need to communicate and bear the challenges that will ensue in order to make progress [20]. Here, we end with two recommendations.
1. Across translational steps, it is important to have a clear conceptualization of what aspect of memory is being targeted (or not) and how different memory systems map onto core clinical symptom(s).
Scientists need to be clear what memory system they are assessing and manipulating in their experiments, and how it links to clinical phenomena (figure 2). Clinicians, in turn, need to feedback which clinical phenomena are most relevant to target and are core to a given disorder. For precision, it can be an advantage to focus on one symptom instead of a whole disorder, because it is virtually impossible to model a complete syndrome in the laboratory, especially when there is less clinical consensus than desirable about how symptoms should be clustered in the first place.
2. It is important to appreciate that memory encoding and retrieval refer to dynamic constructs and that many aspects of timing are crucial, both when designing and implementing an intervention, and when assessing its effectiveness.
Memory is not permanent, but can be updated (figure 3). A range of factors determine whether updating will actually take place. Factors that need to be taken into consideration for treatment include the time since the traumatic event (e.g. immediately post-trauma or later), the time of diagnosis/symptom assessment relative to the intervention (memory read-out), the duration of the consolidation window as well as the reconsolidation window, and the similarities between encoding situations and retrieval cues. To systematically establish timing parameters for an intervention and avoid circular reasoning, an independent (real-time) read-out of memory susceptibility is urgently required. Neuroimaging may be a promising tool to provide such a read-out and validate translational steps across different levels (figure 2).
With the challenges raised here, one can imagine that it is demanding during the treatment of established emotional memories to define various key parameters of an intervention, including (i) what retrieval cue represents the key aspect of the trauma memory that needs to be changed, (ii) how to present this retrieval cue to destabilize a memory, (iii) how to select an intervention to interfere with a destabilized memory, (iv) how to find the optimal timings to deliver such an intervention, and (v) how to tailor treatment to an individual, i.e. taking into account that the stimulus or procedure that triggers memory plasticity in some individuals may not be optimal for others. It should be noted that some of these challenges (e.g. selecting appropriate retrieval/reminder cues) do not exclusively apply to (re)consolidation-based treatments, but also apply to existing psychological treatments that seek to reduce established trauma symptoms (e.g. exposure therapy and imagery rescripting). Rather than setting challenges aside as definite boundary conditions, we are actually encouraged by the rapid accumulation of knowledge that is advancing our insights about necessary and optimal conditions for effective memory modification. In turn, ‘back-translation’ studies of effective procedures, in practice, may yield invaluable cues for basic research. We think that further progress can be made by investing in frameworks that reduce obstacles in the translational path from mice to mental health.
An effective dialogue to facilitate translational research is dependent on regular meetings and funding opportunities that allow memory scientists to consult with clinicians and vice versa. Scientists often seem unaware that evidence-based psychological treatments target processes of emotional learning and memory. Similarly, experimental work in the laboratory seems abstract and remote to clinicians working with patients with complex trauma. A constructive dialogue is not just important for the field of PTSD and anxiety—maladaptive emotional memory is core to many other psychological disorders. A focus on transdiagnostic processes and single symptoms opens up new opportunities for precision compared with a focus on full disorders. For example, intrusive memories occur frequently in depression [30] and in substance-use disorders (albeit with different content), while cue-induced responses (e.g. craving, approach tendencies evoked by drug paraphernalia) can be reduced by similar reconsolidation-update manipulations as those used in phobias [153,154].
If memory is like a monster carrying traumatic information, it may be time to tame it. A first step is to familiarize ourselves with its many heads, each responding to its own triggers and each producing its own sights and sounds. We may come to realize that not all of the heads are ugly and not all of the sounds are terrifying. We can live with the monster, as long as it is not jumping out unexpectedly, carrying the negative emotions, physical stress and sensori-perceptual fragments that impair functioning.
Data accessibility
This article has no additional data.
Author contributions
All authors contributed actively to the writing of this paper and have approved the final version of the manuscript.
Competing interests
E.A.H. is an Honorary Professor of Clinical Psychology at the University of Oxford, Department of Psychiatry, holds an honorary contract at the Medical Research Council Cognition and Brain Sciences Unit, University of Cambridge, UK and serves on the Board of the Charity ‘MQ; transforming mental health’; she receives no remuneration for these roles. R.M.V., R.N.H. and A.L.-Z. declare no competing interests.
Funding
We are grateful to the Royal Society for their support of the costs of attending this meeting ‘Of mice and mental health: facilitating dialogue between basic and clinical neuroscientists' convened by Amy L. Milton and Emily A. Holmes. R.M.V. is supported by the European Union's Horizon 2020 research and innovation programme under grant agreement no. 705641 (SUAI/023/RG92025). A.L.-Z. was supported by a Cambridge International Scholarship awarded by the Cambridge Commonwealth, European and International Trust. R.N.H. is supported by the UK Medical Research Council Programme (SUAI/010/ RG91365). E.A.H. receives support from the Karolinska Institutet and the Lupina Foundation. Funding to pay the Open Access publication charges for this article was provided by the UK Medical Research Council (SUAI/013/ RG91365).
References
- 1.Irving J. 1989. A prayer for Owen Meany: a novel, 1st edn. New York, NY: William Morrow. [Google Scholar]
- 2.Holmes EA, Grey N, Young KAD. 2005. Intrusive images and ‘hotspots’ of trauma memories in posttraumatic stress disorder: an exploratory investigation of emotions and cognitive themes. J. Behav. Ther. Exp. Psychiatry 36, 3–17. ( 10.1016/j.jbtep.2004.11.002) [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders, 5th edn. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- 4.Richardson-Klavehn A, Bjork RA. 1988. Measures of memory. Annu. Rev. Psychol. 39, 475–543. ( 10.1146/annurev.ps.39.020188.002355) [DOI] [Google Scholar]
- 5.Butler AC, Chapman JE, Forman EM, Beck AT. 2006. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin. Psychol. Rev. 26, 17–31. ( 10.1016/j.cpr.2005.07.003) [DOI] [PubMed] [Google Scholar]
- 6.Treanor M, Brown LA, Rissman J, Craske MG. 2017. Can memories of traumatic experiences or addiction be erased or modified? A critical review of research on the disruption of memory reconsolidation and its application. Perspect. Psychol. Sci. 12, 290–305. ( 10.1177/1745691616664725) [DOI] [PubMed] [Google Scholar]
- 7.Mayo-Wilson E, Dias S, Mavranezouli I, Kew K, Clark DM, Ades AE, Pilling S. 2014. Psychological and pharmacological interventions for social anxiety disorder in adults: a systematic review and network meta-analysis. Lancet Psychiatry 1, 368–376. ( 10.1016/S2215-0366(14)70329-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bisson J, Ehlers A, Matthews R, Pilling S, Richards D, Turner S. 2007. Psychological treatments for chronic post-traumatic stress disorder: systematic review and meta-analysis. Br. J. Psychiatry 190, 97–104. ( 10.1192/bjp.bp.106.021402) [DOI] [PubMed] [Google Scholar]
- 9.NICE. 2013. Social anxiety disorder: recognition, assessment and treatment. Clinical guideline 159. London: National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/cg159. [Google Scholar]
- 10.NICE. 2005. Post-traumatic stress disorder (PTSD): the management of PTSD in adults and children in primary and secondary care. Clinical guideline 26. London: National Institute for Health and Clinical Excellence. https://www.nice.org.uk/guidance/cg26. [Google Scholar]
- 11.NICE. 2005. b Obsessive-compulsive disorder: core interventions in the treatment of obsessive-compulsive disorder and body dysmorphic disorder. Clinical guideline 31. London: National Institute for Health and Clinical Excellence. https://www.nice.org.uk/guidance/cg31 [Google Scholar]
- 12.Holmes EA, et al. In press. Psychological treatments research in tomorrow's science: seeing further. Lancet Psychiatry. ( 10.1016/S2215-0366(17)30513-8) [DOI] [PubMed]
- 13.Baldwin DS, et al. 2014. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J. Psychopharmacol. 28, 403–439. ( 10.1177/0269881114525674) [DOI] [PubMed] [Google Scholar]
- 14.Krystal JH, et al. 2017. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol. Psychiatry 82, e51–e59. ( 10.1016/j.biopsych.2017.03.007) [DOI] [PubMed] [Google Scholar]
- 15.Durham RC, Higgins C, Chambers JA, Swan JS, Dow MGT. 2012. Long-term outcome of eight clinical trials of CBT for anxiety disorders: symptom profile of sustained recovery and treatment-resistant groups. J. Affect. Disord. 136, 875–881. ( 10.1016/j.jad.2011.09.017) [DOI] [PubMed] [Google Scholar]
- 16.Hofmann SG, Smits JAJ. 2008. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo controlled trials. J. Clin. Psychiatry 69, 621–632. ( 10.4088/JCP.v69n0415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loerinc AG, Meuret AE, Twohig MP, Rosenfield D, Bluett EJ, Craske MG. 2015. Response rates for CBT for anxiety disorders: need for standardized criteria. Clin. Psychol. Rev. 42, 72–82. ( 10.1016/j.cpr.2015.08.004) [DOI] [PubMed] [Google Scholar]
- 18.Sijbrandij M, Kleiboer A, Bisson JI, Barbui C, Cuijpers P. 2015. Pharmacological prevention of post-traumatic stress disorder and acute stress disorder: a systematic review and meta-analysis. Lancet Psychiatry 2, 413–421. ( 10.1016/S2215-0366(14)00121-7) [DOI] [PubMed] [Google Scholar]
- 19.Roberts NP, Kitchiner NJ, Kenardy J, Bisson JI. 2009. Multiple session early psychological interventions for the prevention of post-traumatic stress disorder. Cochrane Database Syst. Rev., CD006869(3). ( 10.1002/14651858.CD006869.pub2) [DOI] [PubMed] [Google Scholar]
- 20.Holmes EA, Craske MG, Graybiel AM. 2014. Psychological treatments: a call for mental-health science. Clinicians and neuroscientists must work together to understand and improve psychological treatments. Nature 511, 287–289. ( 10.1038/511287a) [DOI] [PubMed] [Google Scholar]
- 21.Bradley MM, Greenwald MK, Petry MC, Lang PJ. 1992. Remembering pictures: pleasure and arousal in memory. J. Exp. Psychol. Learn. Mem. Cogn. 18, 379–390. ( 10.1037/0278-7393.18.2.379) [DOI] [PubMed] [Google Scholar]
- 22.Hamann S. 2001. Cognitive and neural mechanisms in emotional memory. Trends Cogn. Neurosci. 5, 394–400. ( 10.1016/S1364-6613(00)01707-1) [DOI] [PubMed] [Google Scholar]
- 23.Brewin CR, Dalgleish T, Joseph S. 1996. A dual representation theory of posttraumatic stress disorder. Psychol. Rev. 103, 670–686. ( 10.1037/0033-295X.103.4.670) [DOI] [PubMed] [Google Scholar]
- 24.Ehlers A, Clark DM. 2000. A cognitive model of posttraumatic stress disorder. Behav. Res. Ther. 38, 319–345. ( 10.1016/S0005-7967(99)00123-0) [DOI] [PubMed] [Google Scholar]
- 25.Holmes EA, Mathews A. 2010. Mental imagery in emotion and emotional disorders. Clin. Psychol. Rev. 30, 349–362. ( 10.1016/j.cpr.2010.01.001) [DOI] [PubMed] [Google Scholar]
- 26.Brewin CR, Gregory JD, Lipton M, Burgess N. 2010. Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychol. Rev. 117, 210–232. ( 10.1037/a0018113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalgleish T. 2004. Cognitive approaches to posttraumatic stress disorder: the evolution of multirepresentational theorizing. Psychol. Bull. 130, 228–260. ( 10.1037/0033-2909.130.2.228) [DOI] [PubMed] [Google Scholar]
- 28.Foa EB, Kozak MJ. 1986. Emotional processing of fear: exposure to corrective information. Psychol. Bull. 99, 20–35. ( 10.1037/0033-2909.99.1.20) [DOI] [PubMed] [Google Scholar]
- 29.Hackmann A, Holmes EA. 2004. Reflecting on imagery: a clinical perspective and overview of the special issue of Memory on mental imagery and memory in psychopathology. Memory 12, 389–402. ( 10.1080/09658210444000133) [DOI] [PubMed] [Google Scholar]
- 30.Holmes EA, Blackwell SE, Burnett Heyes S, Renner F, Raes F. 2016. Mental imagery in depression: phenomenology, potential mechanisms, and treatment implications. Annu. Rev. Clin. Psychol. 12, 249–280. ( 10.1146/annurev-clinpsy-021815-092925) [DOI] [PubMed] [Google Scholar]
- 31.Patel T, Brewin CR, Wheatley J, Wells A, Fisher P, Myers S. 2007. Intrusive images and memories in major depression. Behav. Res. Ther. 45, 2573–2580. ( 10.1016/j.brat.2007.06.004) [DOI] [PubMed] [Google Scholar]
- 32.Gregory JD, Brewin CR, Mansell W, Donaldson C. 2010. Intrusive memories and images in bipolar disorder. Behav. Res. Ther. 48, 698–703. ( 10.1016/j.brat.2010.04.005) [DOI] [PubMed] [Google Scholar]
- 33.Hales SA, Deeprose C, Goodwin GM, Holmes EA. 2011. Cognitions in bipolar disorder versus unipolar depression: imagining suicide. Bipolar Disord. 13, 651–661. ( 10.1111/j.1399-5618.2011.00954.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wild J, Hackmann A, Clark DM. 2008. Rescripting early memories linked to negative images in social phobia: a pilot study. Behav. Ther. 39, 47–56. ( 10.1016/j.beth.2007.04.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Day SJ, Holmes EA, Hackmann A. 2004. Occurrence of imagery and its link with early memories in agoraphobia. Memory 12, 416–427. ( 10.1080/09658210444000034) [DOI] [PubMed] [Google Scholar]
- 36.Pratt D, Cooper MJ, Hackmann A. 2004. Imagery and its characteristics in people who are anxious about spiders. Behav. Cogn. Psychother. 32, 165–176. ( 10.1017/S1352465804001158) [DOI] [Google Scholar]
- 37.Muse K, McManus F, Hackmann A, Williams M. 2010. Intrusive imagery in severe health anxiety: prevalence, nature and links with memories and maintenance cycles. Behav. Res. Ther. 48, 792–798. ( 10.1016/j.brat.2010.05.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berntsen D, Rubin DC. 2002. Emotionally charged autobiographical memories across the life span: the recall of happy, sad, traumatic, and involuntary memories. Psychol. Aging 17, 636–652. ( 10.1037/0882-7974.17.4.636) [DOI] [PubMed] [Google Scholar]
- 39.Holmes EA, Ghaderi A, Eriksson E, Lauri KO, Kukacka OM, Mamish M, James EL, Visser RM. 2017. ‘I can't concentrate’: a feasibility study with young refugees in Sweden on developing science-driven interventions for intrusive memories related to trauma. Behav. Cogn. Psychother. 45, 97–109. ( 10.1017/S135246581600062X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grey N, Holmes EA. 2008. ‘Hotspots’ in trauma memories in the treatment of post-traumatic stress disorder: a replication. Memory 16, 788–796. ( 10.1080/09658210802266446) [DOI] [PubMed] [Google Scholar]
- 41.Pole N. 2007. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol. Bull. 133, 725–746.. ( 10.1037/0033-2909.133.5.725) [DOI] [PubMed] [Google Scholar]
- 42.Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. 2012. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 13, 769–787. ( 10.1038/nrn3339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galatzer-Levy IR, Karstoft KI, Statnikov A, Shalev AY. 2014. Quantitative forecasting of PTSD from early trauma responses: a machine learning application. J. Psychiatr. Res. 59, 68–76. ( 10.1016/j.jpsychires.2014.08.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wegerer M, Blechert J, Kerschbaum H, Wilhelm FH. 2013. Relationship between fear conditioning and aversive memories: evidence from a novel conditioned-intrusion paradigm. PLoS ONE 8, e79025 ( 10.1371/journal.pone.0079025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Streb M, Conway MA, Michael T. 2017. Conditioned responses to trauma reminders: how durable are they over time and does memory integration reduce them? J. Behav. Ther. Exp. Psychiatry 57, 88–95. ( 10.1016/j.jbtep.2017.04.005) [DOI] [PubMed] [Google Scholar]
- 46.Bouton ME. 2000. A learning theory perspective on lapse, relapse, and the maintenance of behavior change. Health Psychol. 19, 57 ( 10.1037/0278-6133.19.Suppl1.57) [DOI] [PubMed] [Google Scholar]
- 47.Misanin JR, Miller RR, Lewis DJ. 1968. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science 160, 554–555. ( 10.1126/science.160.3827.554) [DOI] [PubMed] [Google Scholar]
- 48.Nader K, Schafe GE, LeDoux JE. 2000. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406, 722–726. ( 10.1038/35021052) [DOI] [PubMed] [Google Scholar]
- 49.Sara SJ. 2000. Review. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn. Mem. 7, 73–84. ( 10.1101/lm.7.2.73) [DOI] [PubMed] [Google Scholar]
- 50.Feinstein JS, Duff MC, Tranel D. 2010. Sustained experience of emotion after loss of memory in patients with amnesia. Proc. Natl Acad. Sci. USA 107, 7674–7679. ( 10.1073/pnas.0914054107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bradley MM, Miccoli L, Escrig MA, Lang PJ. 2008. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology 45, 602–607. ( 10.1111/j.1469-8986.2008.00654.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamm AO, Vaitl D. 1996. Affective learning: awareness and aversion. Psychophysiology 33, 698–710. ( 10.1111/j.1469-8986.1996.tb02366.x) [DOI] [PubMed] [Google Scholar]
- 53.Mauss IB, Robinson MD. 2009. Measures of emotion: a review. Cogn. Emot. 23, 209–237. ( 10.1080/02699930802204677) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weike AI, Schupp HT, Hamm AO. 2007. Fear acquisition requires awareness in trace but not delay conditioning. Psychophysiology 44, 170–180. ( 10.1111/j.1469-8986.2006.00469.x) [DOI] [PubMed] [Google Scholar]
- 55.Squire LR. 2004. Memory systems of the brain: a brief history and current perspective. Neurobiol. Learn. Mem. 82, 171–177. ( 10.1016/j.nlm.2004.06.005) [DOI] [PubMed] [Google Scholar]
- 56.Sevenster D, Beckers T, Kindt M. 2013. Prediction error governs pharmacological induced amnesia for learned fear. Science 339, 830–833. ( 10.1126/science.1231357) [DOI] [PubMed] [Google Scholar]
- 57.James EL, Bonsall MB, Hoppitt L, Tunbridge EM, Geddes JR, Milton AL, Holmes EA. 2015. Computer game play reduces intrusive memories of experimental trauma via reconsolidation update mechanisms. Psychol. Sci. 26, 1201–2015. ( 10.1177/0956797615583071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soeter M, Kindt M. 2010. Dissociating response systems: erasing fear from memory. Neurobiol. Learn. Mem. 94, 30–41. ( 10.1016/j.nlm.2010.03.004) [DOI] [PubMed] [Google Scholar]
- 59.Soeter M, Kindt M. 2015. An abrupt transformation of phobic behavior after a post-retrieval amnesic agent. Biol. Psychiatry 78, 880–886. ( 10.1016/j.biopsych.2015.04.006) [DOI] [PubMed] [Google Scholar]
- 60.Sevenster D, Beckers T, Kindt M. 2014. Fear conditioning of SCR but not the startle reflex requires conscious discrimination of threat and safety. Front. Behav. Neurosci. 8, 32 ( 10.3389/fnbeh.2014.00032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sevenster D, Beckers T, Kindt M. 2012. Instructed extinction differentially affects the emotional and cognitive expression of associative fear memory. Psychophysiology 49, 1426–1435. ( 10.1111/j.1469-8986.2012.01450.x) [DOI] [PubMed] [Google Scholar]
- 62.Adolphs R, Tranel D, Buchanan TW. 2005. Amygdala damage impairs emotional memory for gist but not details of complex stimuli. Nat. Neurosci. 8, 512–518. ( 10.1038/nn1413) [DOI] [PubMed] [Google Scholar]
- 63.Bechara A, Tranel D, Damasio H, Adolphs R. 1995. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 269, 1115 ( 10.1126/science.7652558) [DOI] [PubMed] [Google Scholar]
- 64.LaBar KS, LeDoux JE, Spencer DD, Phelps EA. 1995. Impaired fear conditioning following unilateral temporal lobectomy in humans. J. Neurosci. 15, 6846–6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weike AI, Hamm AO, Schupp HT, Runge U, Schroeder HW, Kessler C. 2005. Fear conditioning following unilateral temporal lobectomy: dissociation of conditioned startle potentiation and autonomic learning. J. Neurosci. 25, 11 117–11 124. ( 10.1523/JNEUROSCI.2032-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eichenbaum H, Cohen NJ. 2004. From conditioning to conscious recollection: memory systems of the brain. Oxford, UK: Oxford University Press. [Google Scholar]
- 67.Schacter DL, Wagner AD, Buckner RL. 2000. Memory systems of 1999. In The Oxford handbook of memory (eds Tulving E, Craikpp FIM), pp. 627–643. New York: Oxford University Press. [Google Scholar]
- 68.Holmes EA, Sandberg A, Iyadurai L. 2010. Erasing trauma memories: is this what the science suggests or even what we want to do? [Letter to the editor]. Br. J. Psychiatry Online 190, 81a ( 10.1192/bjp.197.5.414b) [DOI] [PubMed] [Google Scholar]
- 69.Pavlov IP. 1927. Conditioned reflexes. An investigation of the physiological activity of the cerebral cortex (ed. Anrep GV.). London, UK: Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.LeDoux JE. 2003. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol. 23, 727–738. ( 10.1023/A:1025048802629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rescorla RA, Holland PC. 1982. Behavioral studies of associative learning in animals. Annu. Rev. Psychol. 33, 265–308. ( 10.1146/annurev.ps.33.020182.001405) [DOI] [Google Scholar]
- 72.Doyere V, Dębiec J, Monfils M-H, Schafe GE, Ledoux JE. 2007. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat. Neurosci. 10, 414–416. ( 10.1038/nn1871) [DOI] [PubMed] [Google Scholar]
- 73.Dębiec J, Doyère V, Nader K, LeDoux JE. 2006. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc. Natl Acad. Sci. USA 103, 3428–3433. ( 10.1073/pnas.0507168103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones CE, Ringuet S, Monfils M. 2013. Learned together, extinguished apart: reducing fear to complex stimuli. Learn. Mem. 20, 674–685. ( 10.1101/lm.031740.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shumake J, Monfils MH. 2015. Assessing fear following retrieval+extinction through suppression of baseline reward seeking vs. freezing. Front. Behav. Neurosci. 9, 355 ( 10.3389/fnbeh.2015.00355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soeter M, Kindt M. 2012. Stimulation of the noradrenergic system during memory formation impairs extinction learning but not the disruption of reconsolidation. Neuropsychopharmacology 37, 1204–1215. ( 10.1038/npp.2011.307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soeter M, Kindt M. 2012. Erasing fear for an imagined threat event. Psychoneuroendocrinology 37, 1769–1779. ( 10.1016/j.psyneuen.2012.03.011) [DOI] [PubMed] [Google Scholar]
- 78.Liberzon I, Martis B. 2006. Neuroimaging studies of emotional responses in PTSD. Ann. NY Acad. Sci. 1071, 87–109. ( 10.1196/annals.1364.009) [DOI] [PubMed] [Google Scholar]
- 79.James EL, Lau-Zhu A, Clark IA, Visser RM, Hagenaars MA, Holmes EA. 2016. The trauma film paradigm as an experimental psychopathology model of psychological trauma: intrusive memories and beyond. Clin. Psychol. Rev. 47, 106–142. ( 10.1016/j.cpr.2016.04.010) [DOI] [PubMed] [Google Scholar]
- 80.Horowitz MJ. 1969. Psychic trauma. Return of images after a stressful film. Arch. Gen. Psychiatry 20, 552–559. ( 10.1001/archpsyc.1969.01740170056008) [DOI] [PubMed] [Google Scholar]
- 81.Schafe GE, LeDoux JE. 2000. Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J. Neurosci. 20, RC96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miserendino MJ, Sananes CB, Melia KR, Davis M. 1990. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature 345, 716–718. ( 10.1038/345716a0) [DOI] [PubMed] [Google Scholar]
- 83.McGaugh JL. 1966. Time-dependent processes in memory storage. Science 153, 1351–1358. ( 10.1126/science.153.3742.1351) [DOI] [PubMed] [Google Scholar]
- 84.Pape H-C, Pare D. 2010. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 90, 419–463. ( 10.1152/physrev.00037.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schafe GE, Nader K, Blair HT, LeDoux JE. 2001. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 24, 540–546. ( 10.1016/S0166-2236(00)01969-X) [DOI] [PubMed] [Google Scholar]
- 86.Dudai Y. 2012. The restless engram: consolidations never end. Annu. Rev. Neurosci. 35, 227–247. ( 10.1146/annurev-neuro-062111-150500) [DOI] [PubMed] [Google Scholar]
- 87.Winocur G, Moscovitch M. 2011. Memory transformation and systems consolidation. J. Int. Neuropsychol. Soc. 17, 766–780. ( 10.1017/S1355617711000683) [DOI] [PubMed] [Google Scholar]
- 88.Monfils M, Cowansage KK, Klann E, LeDoux JE. 2009. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science 324, 951–955. ( 10.1126/science.1167975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dębiec J, Ledoux JE. 2004. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 129, 267–272. ( 10.1016/j.neuroscience.2004.08.018) [DOI] [PubMed] [Google Scholar]
- 90.Rodríguez M, Campos J, Forcato C, Leiguarda R, Maldonado H, Molina V, Pedreira ME. 2013. Enhancing a declarative memory in humans: the effect of clonazepam on reconsolidation. Neuropharmacology 64, 432–442. ( 10.1016/j.neuropharm.2012.06.059) [DOI] [PubMed] [Google Scholar]
- 91.Sandrini M, Censor N, Mishoe J, Cohen LG. 2013. Causal role of prefrontal cortex in strengthening of episodic memories through reconsolidation. Curr. Biol. 23, 2181–2184. ( 10.1016/j.cub.2013.08.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Finnie PSB, Nader K. 2012. The role of metaplasticity mechanisms in regulating memory destabilization and reconsolidation. Neurosci. Biobehav. Rev. 36, 1667–1707. ( 10.1016/j.neubiorev.2012.03.008) [DOI] [PubMed] [Google Scholar]
- 93.Thomas É, Saumier D, Pitman RK, Tremblay J, Brunet A. 2017. Consolidation and reconsolidation are impaired by oral propranolol administered before but not after memory (re)activation in humans. Neurobiol. Learn. Mem. 142, 118–125. ( 10.1016/j.nlm.2016.12.010) [DOI] [PubMed] [Google Scholar]
- 94.Schiller D, Monfils M, Raio C, Johnson DC, LeDoux JE, Phelps EA. 2010. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463, 49–53. ( 10.1038/nature08637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kindt M, Soeter M, Vervliet B. 2009. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat. Neurosci. 12, 256–258. ( 10.1038/nn.2271) [DOI] [PubMed] [Google Scholar]
- 96.Sevenster D, Beckers T, Kindt M. 2012. Retrieval per se is not sufficient to trigger reconsolidation of human fear memory. Neurobiol. Learn. Mem. 97, 338–345. ( 10.1016/j.nlm.2012.01.009) [DOI] [PubMed] [Google Scholar]
- 97.Soeter M, Kindt M. 2011. Disrupting reconsolidation: pharmacological and behavioural manipulations. Learn. Mem. 18, 357–366. ( 10.1101/lm.2148511) [DOI] [PubMed] [Google Scholar]
- 98.Sevenster D, Beckers T, Kindt M. 2014. Prediction error demarcates the transition from retrieval, to reconsolidation, to new learning. Learn. Mem. 21, 580–584. ( 10.1101/lm.035493.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Golkar A, Tjaden C, Kindt M. 2017. Vicarious extinction learning during reconsolidation neutralizes fear memory. Behav. Res. Ther. 92, 87–93. ( 10.1016/j.brat.2017.02.004) [DOI] [PubMed] [Google Scholar]
- 100.Björkstrand J, Agren T, Åhs F, Frick A, Larsson E-M, Hjorth O, Furmark T, Fredrikson M. 2017. Think twice, it's all right: long lasting effects of disrupted reconsolidation on brain and behavior in human long-term fear. Behav. Brain Res. 324, 125–129. ( 10.1016/j.bbr.2017.02.016) [DOI] [PubMed] [Google Scholar]
- 101.Björkstrand J, Agren T, Frick A, Engman J, Larsson E-M, Furmark T, Fredrikson M. 2015. Disruption of memory reconsolidation erases a fear memory trace in the human amygdala: an 18-month follow-up. PLoS ONE 10, e0129393 ( 10.1371/journal.pone.0129393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Agren T, Engman J, Frick A, Bjorkstrand J, Larsson E-M, Furmark T, Fredrikson M. 2012. Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science 337, 1550–1552. ( 10.1126/science.1223006) [DOI] [PubMed] [Google Scholar]
- 103.Oyarzun JP, lopez-Barroso D, Fuentemilla L, Cucurell D, Pedraza C, Rodriguez-Fornells A, de Diego-Balaguer R. 2012. Updating fearful memories with extinction training during reconsolidation: a human study using auditory aversive stimuli. PLoS ONE 7, 38849 ( 10.1371/journal.pone.0038849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Das RK, Tamman A, Nikolova V, Freeman TP, Bisby JA, Lazzarino AI, Kamboj SK. 2016. Nitrous oxide speeds the reduction of distressing intrusive memories in an experimental model of psychological trauma. Psychol. Med. 46, 1749–1759. ( 10.1017/S003329171600026X) [DOI] [PubMed] [Google Scholar]
- 105.Holmes EA, Brewin CR, Hennessy RG. 2004. Trauma films, information processing, and intrusive memory development. J. Exp. Psychol. Gen. 133, 3–22. ( 10.1037/0096-3445.133.1.3) [DOI] [PubMed] [Google Scholar]
- 106.Bourne C, Frasquilho F, Roth AD, Holmes EA. 2010. Is it mere distraction? Peri-traumatic verbal tasks can increase analogue flashbacks but reduce voluntary memory performance. J. Behav. Ther. Exp. Psychiatry 41, 316–324. ( 10.1016/j.jbtep.2010.03.001) [DOI] [PubMed] [Google Scholar]
- 107.Deeprose C, Zhang S, Dejong H, Dalgleish T, Holmes EA. 2012. Imagery in the aftermath of viewing a traumatic film: using cognitive tasks to modulate the development of involuntary memory. J. Behav. Ther. Exp. Psychiatry 43, 758–764. ( 10.1016/j.jbtep.2011.10.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Holmes EA, James EL, Coode-Bate T, Deeprose C. 2009. Can playing the computer game ‘Tetris’ reduce the build-up of flashbacks for trauma? A proposal from cognitive science. PLoS ONE 4, e4153 ( 10.1371/journal.pone.0004153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Holmes EA, James EL, Kilford EJ, Deeprose C. 2010. Key steps in developing a cognitive vaccine against traumatic flashbacks: visuospatial Tetris versus verbal Pub Quiz. PLoS ONE 5, e13706 ( 10.1371/journal.pone.0013706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.James EL, Lau-Zhu A, Tickle H, Horsch A, Holmes EA. 2016. Playing the computer game Tetris prior to viewing traumatic film material and subsequent intrusive memories: examining proactive interference. J. Behav. Ther. Exp. Psychiatry 53, 25–33. ( 10.1016/j.jbtep.2015.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Krans J, Näring G, Becker ES. 2009. Count out your intrusions: effects of verbal encoding on intrusive memories. Memory 17, 809–815. ( 10.1080/09658210903130780) [DOI] [PubMed] [Google Scholar]
- 112.Brewin CR. 2014. Episodic memory, perceptual memory, and their interaction: foundations for a theory of posttraumatic stress disorder. Psychol. Bull. 140, 69 ( 10.1037/a0033722) [DOI] [PubMed] [Google Scholar]
- 113.Telch MJ, York J, Lancaster CL, Monfils MH. 2017. Use of a brief fear memory reactivation procedure for enhancing exposure therapy. Clin. Psychol. Sci. 5, 367–378. ( 10.1177/2167702617690151) [DOI] [Google Scholar]
- 114.Björkstrand J, Agren T, Ahs F, Frick A, Larsson E-M, Hjorth O, Furmark T, Fredrikson M. 2016. Disrupting reconsolidation attenuates long-term fear memory in the human amygdala and facilitates approach behavior. Curr. Biol. 26, 2690–2695. ( 10.1016/j.cub.2016.08.022) [DOI] [PubMed] [Google Scholar]
- 115.Iyadurai L, Blackwell SB, Meiser-Stedman R, Watson PC, Bonsall MB, Geddes JR, Nobre AC, Holmes EA. In press. Preventing intrusive memories after trauma via a brief intervention involving Tetris computer game play in the emergency department: a proof-of-concept randomized controlled trial. Mol. Psychiatry. ( 10.1038/mp.2017.23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Horsch A, Vial Y, Favrod C, Harari MM, Blackwell SE, Watson P, Iyadurai L, Bonsall MB, Holmes EA. 2017. Reducing intrusive traumatic memories after emergency caesarean section: a proof-of-principle randomized controlled study. Behav. Res. Ther. 94, 36–47. ( 10.1016/j.brat.2017.03.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kindt M, van Emmerik A. 2016. New avenues for treating emotional memory disorders: towards a reconsolidation intervention for posttraumatic stress disorder. Ther. Adv. Psychopharmacol. 6, 283–295. ( 10.1177/2045125316644541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. 2008. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J. Psychiatr. Res. 42, 503–506. ( 10.1016/j.jpsychires.2007.05.006) [DOI] [PubMed] [Google Scholar]
- 119.Liao SM, Sandberg A. 2008. The normativity of memory modification. Neuroethics 1, 85–99. ( 10.1007/s12152-008-9009-5) [DOI] [Google Scholar]
- 120.Regier DA, Kuhl EA, Kupfer DJ. 2013. The DSM-5: classification and criteria changes. World Psychiatry 12, 92–98. ( 10.1002/wps.20050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kupfer DJ, Regier DA. 2011. Neuroscience, clinical evidence, and the future of psychiatric classification in DSM-5. Am. J. Psychiatry 168, 172–174. ( 10.1176/appi.ajp.2011.11020219) [DOI] [PubMed] [Google Scholar]
- 122.Wakefield JC. 2016. Diagnostic issues and controversies in DSM-5: return of the false positives problem. Annu. Rev. Clin. Psychol. 12, 105–132. ( 10.1146/annurev-clinpsy-032814-112800) [DOI] [PubMed] [Google Scholar]
- 123.Brunet A, Poundja J, Tremblay J, Bui É, Thomas É, Orr S, Azzoug A, Birmes P, Pitman RK. 2011. Trauma reactivation under the influence of propranolol decreases posttraumatic stress symptoms and disorder: 3 open-label trials. J. Clin. Psychopharmacol. 31, 547–550. ( 10.1097/JCP.0b013e318222f360) [DOI] [PubMed] [Google Scholar]
- 124.Wood NE, et al. 2015. Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: three negative psychophysiological studies. Psychiatry Res. 225, 31–39. ( 10.1016/j.psychres.2014.09.005) [DOI] [PubMed] [Google Scholar]
- 125.Bos MGN, Beckers T, Kindt M. 2014. Noradrenergic blockade of memory reconsolidation: a failure to reduce conditioned fear responding. Front. Behav. Neurosci. 8, 412 ( 10.3389/fnbeh.2014.00412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kindt M, Soeter M. 2013. Reconsolidation in a human fear conditioning study: a test of extinction as updating mechanism. Biol. Psychol. 92, 43–50. ( 10.1016/j.biopsycho.2011.09.016) [DOI] [PubMed] [Google Scholar]
- 127.Golkar A, Bellander M, Olsson A, Öhman A. 2012. Are fear memories erasable? Reconsolidation of learned fear with fear-relevant and fear-irrelevant stimuli. Front. Behav. Neurosci. 6, 80 ( 10.3389/fnbeh.2012.00080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Klucken T, Kruse O, Schweckendiek J, Kuepper Y, Mueller EM, Hennig J, Stark R. 2016. No evidence for blocking the return of fear by disrupting reconsolidation prior to extinction learning. Cortex 79, 112–122. ( 10.1016/j.cortex.2016.03.015) [DOI] [PubMed] [Google Scholar]
- 129.Kredlow MA, Unger LD, Otto MW. 2016. Harnessing reconsolidation to weaken fear and appetitive memories: a meta-analysis of post-retrieval extinction effects. Psychol. Bull. 142, 314 ( 10.1037/bul0000034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Soeter M, Kindt M. 2013. High trait anxiety: a challenge for disrupting fear memory reconsolidation. PLoS ONE 8, e75239 ( 10.1371/journal.pone.0075239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pedreira ME, Pérez-Cuesta LM, Maldonado H. 2004. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learn. Mem. 11, 579–585. ( 10.1101/lm.76904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morris RG, Inglis J, Ainge JA, Olverman HJ, Tulloch J, Dudai Y, Kelly PAT. 2006. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron 50, 479–489. ( 10.1016/j.neuron.2006.04.012) [DOI] [PubMed] [Google Scholar]
- 133.Lee JLC. 2009. Reconsolidation: maintaining memory relevance. Trends Neurosci. 32, 413–420. ( 10.1016/j.tins.2009.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gershman SJ, Monfils M-H, Norman KA, Niv Y. 2017. The computational nature of memory modification. eLife 6, e23763 ( 10.7554/eLife.23763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Inda MC, Muravieva EV, Alberini CM. 2011. Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. J. Neurosci. 31, 1635–1643. ( 10.1523/JNEUROSCI.4736-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Frankland PW, Ding H-K, Takahashi E, Suzuki A, Kida S, Silva AJ. 2006. Stability of recent and remote contextual fear memory. Learn. Mem. 13, 451–457. ( 10.1101/lm.183406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hoffman AN, Parga A, Paode PR, Watterson LR, Nikulina EM, Hammer RP, Conrad CD. 2015. Chronic stress enhanced fear memories are associated with increased amygdala zif268 mRNA expression and are resistant to reconsolidation. Neurobiol. Learn. Mem. 120, 61–68. ( 10.1016/j.nlm.2015.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pedreira ME, Maldonado H. 2003. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron 38, 863–869. ( 10.1016/S0896-6273(03)00352-0) [DOI] [PubMed] [Google Scholar]
- 139.Merlo E, Milton AL, Goozee ZY, Theobald DE, Everitt BJ. 2014. Reconsolidation and extinction are dissociable and mutually exclusive processes: behavioral and molecular evidence. J. Neurosci. 4, 2422–2431. ( 10.1523/JNEUROSCI.4001-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. 2004. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 24, 4787–4795. ( 10.1523/JNEUROSCI.5491-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Paller KA, Wagner AD. 2002. Observing the transformation of experience into memory. Trends Cogn. Sci. 6, 93–102. ( 10.1016/S1364-6613(00)01845-3) [DOI] [PubMed] [Google Scholar]
- 142.Rissman J, Wagner AD. 2012. Distributed representations in memory: insights from functional brain imaging. Annu. Rev. Psychol. 63, 101–128. ( 10.1146/annurev-psych-120710-100344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Clark IA, Mackay CE, Woolrich MW, Holmes EA. 2016. Intrusive memories to traumatic footage: the neural basis of their encoding and involuntary recall. Psychol. Med. 46, 505–518. ( 10.1017/S0033291715002007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Clark IA, Niehaus KE, Duff EP, Di Simplicio MC, Clifford GD, Smith SM, Mackay CE, Woolrich MW, Holmes EA. 2014. First steps in using machine learning on fMRI data to predict intrusive memories of traumatic film footage. Behav. Res. Ther. 62, 37–46. ( 10.1016/j.brat.2014.07.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bourne C, Mackay CE, Holmes EA. 2013. The neural basis of flashback formation: the impact of viewing trauma. Psychol. Med. 43, 1521–1533. ( 10.1017/S0033291712002358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Visser RM, Kunze AE, Westhoff B, Scholte HS, Kindt M. 2015. Representational similarity analysis offers a preview of the noradrenergic modulation of long-term fear memory at the time of encoding. Psychoneuroendocrinology 55, 8–20. ( 10.1016/j.psyneuen.2015.01.021) [DOI] [PubMed] [Google Scholar]
- 147.Visser RM, Scholte HS, Beemsterboer T, Kindt M. 2013. Neural pattern similarity predicts long-term fear memory. Nat. Neurosci. 16, 388–390. ( 10.1038/nn.3345) [DOI] [PubMed] [Google Scholar]
- 148.Hermans EJ, Kanen JW, Tambini A, Fernández G, Davachi L, Phelps EA. 2016. Persistence of amygdala–hippocampal connectivity and multi-voxel correlation structures during awake rest after fear learning predicts long-term expression of fear. Cereb. Cortex 27, 3028–3041. ( 10.1093/cercor/bhw145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Staresina BP, Alink A, Kriegeskorte N, Henson RN. 2013. Awake reactivation predicts memory in humans. Proc. Natl Acad. Sci. USA 110, 21 159–21 164. ( 10.1073/pnas.1311989110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Polyn SM, Natu VS, Cohen JD, Norman KA. 2005. Category-specific cortical activity precedes retrieval during memory search. Science 310, 1963–1966. ( 10.1126/science.1117645) [DOI] [PubMed] [Google Scholar]
- 151.Wimber M, Alink A, Charest I, Kriegeskorte N, Anderson MC. 2015. Retrieval induces adaptive forgetting of competing memories via cortical pattern suppression. Nat. Neurosci. 18, 582–589. ( 10.1038/nn.3973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Henson R. 2005. What can functional neuroimaging tell the experimental psychologist? Q. J. Exp. Psychol. A 58, 193–233. ( 10.1080/02724980443000502) [DOI] [PubMed] [Google Scholar]
- 153.Xue Y-X, et al. 2012. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science 336, 241–245. ( 10.1126/science.1215070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Milton AL, Everitt BJ. 2012. The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neurosci. Biobehav. Rev. 36, 1119–1139. ( 10.1016/j.neubiorev.2012.01.002) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.