ABSTRACT
Arenaviruses are negative-strand, enveloped RNA viruses that cause significant human disease. In particular, Junín mammarenavirus (JUNV) is the etiologic agent of Argentine hemorrhagic fever. At present, little is known about the cellular proteins that the arenavirus matrix protein (Z) hijacks to accomplish its various functions, including driving the process of virus release. Furthermore, there is little knowledge regarding host proteins incorporated into arenavirus particles and their importance for virion function. To address these deficiencies, we used mass spectrometry to identify human proteins that (i) interact with the JUNV matrix protein inside cells or within virus-like particles (VLPs) and/or (ii) are incorporated into bona fide JUNV strain Candid#1 particles. Bioinformatics analyses revealed that multiple classes of human proteins were overrepresented in the data sets, including ribosomal proteins, Ras superfamily proteins, and endosomal sorting complex required for transport (ESCRT) proteins. Several of these proteins were required for the propagation of JUNV (ADP ribosylation factor 1 [ARF1], ATPase, H+ transporting, lysosomal 38-kDa, V0 subunit d1 [ATP6V0D1], and peroxiredoxin 3 [PRDX3]), lymphocytic choriomeningitis mammarenavirus (LCMV) (Rab5c), or both viruses (ATP synthase, H+ transporting, mitochondrial F1 complex, beta polypeptide [ATP5B] and IMP dehydrogenase 2 [IMPDH2]). Furthermore, we show that the release of infectious JUNV particles, but not LCMV particles, requires a functional ESCRT pathway and that ATP5B and IMPDH2 are required for JUNV budding. In summary, we have provided a large-scale map of host machinery that associates with JUNV and identified key human proteins required for its propagation. This data set provides a resource for the field to guide antiviral target discovery and to better understand the biology of the arenavirus matrix protein and the importance of host proteins for virion function.
IMPORTANCE Arenaviruses are deadly human pathogens for which there are no U.S. Food and Drug Administration-approved vaccines and only limited treatment options. Little is known about the host proteins that are incorporated into arenavirus particles or that associate with its multifunctional matrix protein. Using Junín mammarenavirus (JUNV), the causative agent of Argentine hemorrhagic fever, as a model organism, we mapped the human proteins that are incorporated into JUNV particles or that associate with the JUNV matrix protein. Functional analysis revealed host machinery that is required for JUNV propagation, including the cellular ESCRT pathway. This study improves our understanding of critical arenavirus-host interactions and provides a data set that will guide future studies to better understand arenavirus pathogenesis and identify novel host proteins that can be therapeutically targeted.
KEYWORDS: Junín virus, mammarenavirus, matrix protein, Z, interactome, budding, proteomics, ESCRT, VLP, Rab5, arenavirus, lymphocytic choriomeningitis virus, matrix protein Z, protein-protein interactions, virus particle
INTRODUCTION
Arenaviruses are a family of enveloped, negative-strand RNA viruses that cause significant human disease. Mammarenaviruses are primarily maintained in rodent hosts, where they are thought to establish lifelong, asymptomatic infections (1–3). Viruses of the mammarenavirus genus are divided into two major subclasses based on the geographic distribution of each virus, namely, Old World and New World, which represent viruses primarily from Africa or the Americas, respectively (4). Several arenaviruses cause severe hemorrhagic fever disease in humans, with high morbidity and mortality. Lassa virus (LASV) causes ∼200,000 infections per year in western Africa (5), while Guanarito, Junín, Machupo, and Sabiá viruses cause hemorrhagic fever syndromes in South America (6). In particular, Junín virus (JUNV) is endemic to central Argentina and causes Argentine hemorrhagic fever in humans (7). The live, attenuated vaccine strain of JUNV, Candid#1 (C#1), has demonstrated efficacy in reducing cases of Argentine hemorrhagic fever in the region where it is endemic but has not been approved for use by the U.S. Food and Drug Administration (8). Furthermore, the only therapy with demonstrated efficacy against JUNV infection is immune plasma, and thus, there is a clear need for therapeutics to treat JUNV infection (8).
Arenaviruses have a negative-sense, bisegmented RNA genome comprised of two single-stranded segments with an ambisense coding strategy. The small (S) segment encodes the nucleoprotein (NP) and the glycoprotein (GP), while the large (L) segment encodes the RNA-dependent RNA polymerase (L) and the matrix protein Z (9–15). All four proteins are structural components of the virion. GP forms spikes on the exterior of the virion, NP and L together with the genome form the ribonucleoprotein complex, which is packaged inside virions, and Z forms a matrix layer on the interior side of the viral membrane (16–19). Arenaviruses are enveloped and enter cells through receptor-mediated endocytosis, undergo replication and assembly in the cytoplasm, and are released from the cell via budding from the plasma membrane (20). GP mediates cell entry by binding cell surface receptors, which triggers endocytosis of the bound virus into the endocytic pathway (21–26). Within endosomes, GP triggers fusion of the viral envelope with the endosomal membrane, releasing viral ribonucleoproteins into the cytoplasm (27). Viral replication occurs in the cytoplasm, where the genome is transcribed and replicated by the L polymerase but NP is also required (28, 29).
In the present study, we focused on the multifunctional matrix protein Z. A small protein, Z is largely comprised of a central zinc-binding really interesting new gene (RING) domain, and the Z proteins of most arenaviruses contain one or two late domains within their flexible C-terminal regions (12, 30). Z is responsible for a number of critical functions in the viral life cycle. Z regulates viral genome replication and transcription by antagonizing the viral L polymerase (31–33). Z can repress the translation of capped mRNAs by directly binding to and inhibiting the translation initiation factor eukaryotic initiation factor 4E (eIF4E) (33–36). The Z protein of pathogenic arenaviruses can also inhibit the innate immune response by binding to and inhibiting retinoic acid-inducible gene 1 (RIG-I)-like proteins (37, 38). Finally, the most studied role of Z is to drive the assembly, budding, and release of virions at the plasma membrane.
Z coordinates the assembly of virus particles at sites of budding by interacting with GP and the viral ribonucleoprotein complex (39, 40). The release of arenavirus virions has traditionally been thought to be mediated by Z's recruitment of the cellular endosomal sorting complex required for transport (ESCRT) through its C-terminal late domains, P(S/T)AP and/or PPXY (17, 41, 42). Accordingly, Z, in the absence of other viral proteins, can induce the formation of virus-like particles (VLPs) and, thus, is both necessary and sufficient for driving the budding process (17, 42, 43). However, our group recently reported that the prototypic Old World arenavirus, lymphocytic choriomeningitis virus (LCMV), requires both a functional PPXY late domain and ESCRT complex for the production of defective interfering (DI) particles, but not for infectious virus particles (44). We also identified a motif outside any known late domain in the LCMV Z protein that contributes to both infectious virus and DI particle production (45). Additionally, the New World arenavirus Tacaribe virus (TACV) lacks a functional late domain in Z, while a recombinant Pichinde virus (PICV) with mutations to its Z-encoded PSAP late domain is viable but attenuated (46, 47). Collectively, these findings highlight the considerable diversity displayed by different arenaviruses in completing the viral budding process.
Viruses are dependent upon the hosts they infect to complete their life cycle. While several functions of the arenavirus Z protein are known, the specific molecular mechanisms and corresponding host machinery hijacked by Z to drive these processes are not well described. Furthermore, the host proteins packaged into arenavirus particles and their importance for virion structure and function are largely unknown. In this study, we employed a proteomics approach to generate a large-scale map of human proteins that are incorporated into JUNV particles or that associate with the JUNV Z protein in cells or VLPs. Functional studies identified several host proteins that are required for arenavirus propagation. Notably, our studies revealed that a functional ESCRT complex is required for the production of infectious JUNV particles, unlike the case for its Old World arenavirus counterpart, LCMV (44).
RESULTS
Identification of host proteins that associate with the JUNV matrix protein.
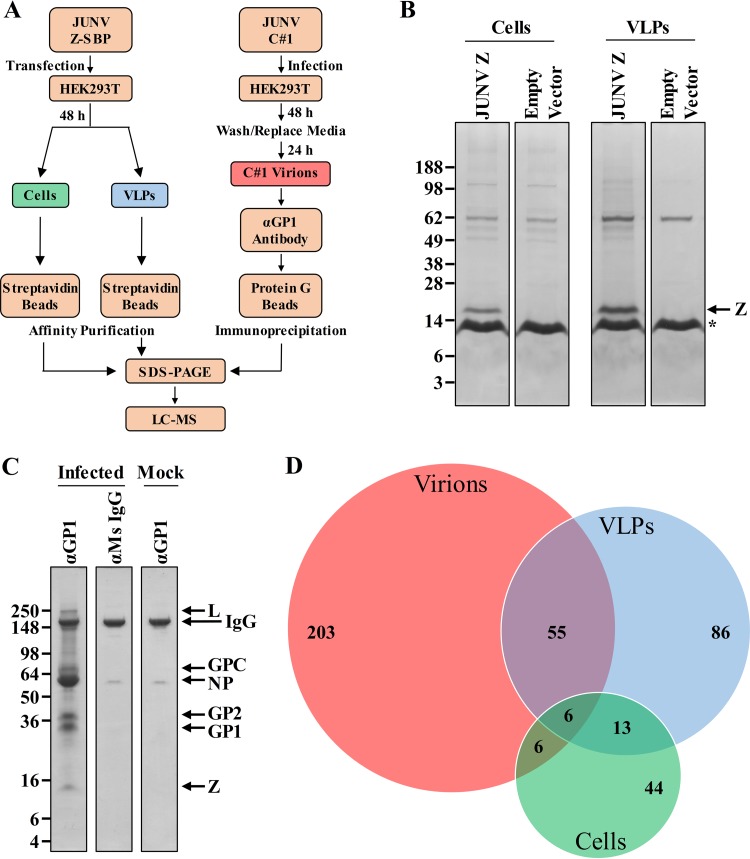
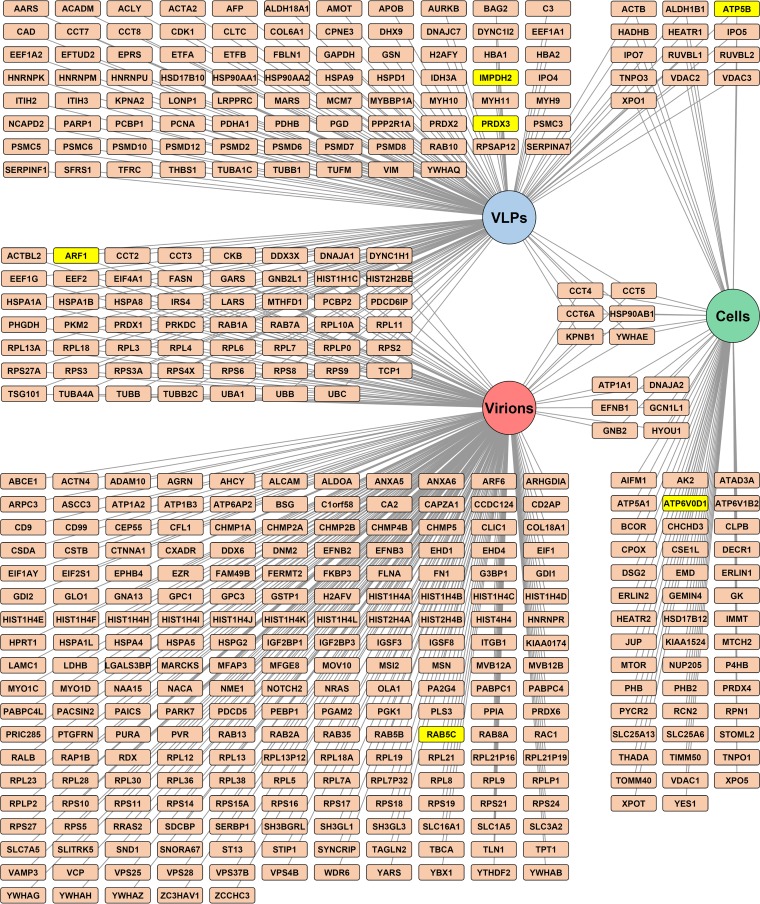
To identify host proteins that interact with the JUNV Z protein in cells or in VLPs, HEK293T cells were transfected with a plasmid encoding a streptavidin binding peptide (SBP)-tagged Z protein or, as a control, an empty vector. Two days later, streptavidin beads were used to affinity purify Z and its associated host protein partners from either whole-cell lysates or clarified supernatants that contained JUNV VLPs (Fig. 1A). The protein complexes captured were first separated by gel electrophoresis and then subjected to in-gel digestion (Fig. 1B). The resultant peptides were analyzed by liquid chromatography-tandem mass spectrometry. Using the stringency criteria described in Materials and Methods, we identified 262 host proteins as intracellular partners of Z in at least one experiment, while 69 of these proteins were identified in both runs (Fig. 1D; see Table S1 in the supplemental material). A total of 516 host proteins were identified as partners of Z in VLPs, of which 160 (31%) were found in both replicate experiments (Fig. 1D; Table S1).
FIG 1.
Identification of human proteins that associate with the JUNV matrix protein or JUNV C#1 particles. (A) Overview of the experimental strategies used to identify host proteins that (i) associate with JUNV Z in cells or VLPs or (ii) are found in JUNV C#1 virions. (B and C) Coomassie-stained SDS-PAGE gels of affinity-purified Z protein from cells or VLPs with associated cellular protein partners (B) or purified JUNV C#1 virions with associated host proteins (C). (B) HEK293T cells were transfected with a plasmid encoding JUNV Z with a C-terminal streptavidin binding peptide (SBP) tag or, as a control, an empty vector. Two days later, cells and their corresponding VLP-containing supernatants were collected and lysed to liberate JUNV Z and its associated host protein partners. Z-host protein complexes from either cells or supernatant-derived VLPs were captured via affinity purification using magnetic streptavidin beads, eluted from the beads, and then run out on SDS-PAGE gels for subsequent tryptic digestion and proteomics analysis. The asterisk indicates monomeric streptavidin that is eluted off the streptavidin beads when boiled. (C) HEK293T cells were infected with JUNV C#1, and 72 h later, virus particles were immunoprecipitated using an antibody specific for the viral envelope glycoprotein (GP1). Control conditions included (i) immunoprecipitation of virus-containing supernatants with a nonspecific mouse (Ms) IgG antibody and (ii) immunoprecipitation of an uninfected (mock) supernatant with the GP1-specific antibody. Captured virions were lysed and then run out on an SDS-PAGE gel for subsequent tryptic digestion and proteomics analysis. (B and C) Molecular mass in kDa, viral proteins, and immunoglobulin are labeled. Each gel is representative of two independent experiments. (D) Venn diagram of host proteins identified in JUNV C#1 virions or as partners of JUNV Z in cells or VLPs. Only proteins identified in both replicate experiments for a given condition are listed.
Identification of host proteins in JUNV particles.
It is possible that plasmid-based expression of Z to identify its host protein partners may miss key interactions that occur during infection or, alternatively, identify artifactual associations. Furthermore, in the case of the Z VLP experiments whose results are shown in Fig. 1B, both VLPs and cellular exosomes are present in the supernatant at the time of lysis, making it possible that some of the host proteins found to associate with Z could have originated from exosomes instead of VLPs. To account for these possibilities and to confirm Z interactions in the context of VLPs, the host protein content of bona fide JUNV C#1 virions purified by immunoprecipitation was determined by mass spectrometry. An antibody specific to the surface glycoprotein (GP1) was used to immunoprecipitate cell-free virions from clarified supernatants that were collected 72 h postinfection (p.i.). Purifying JUNV C#1 virions with a GP-specific antibody serves as an additional control, as exosomes/cellular vesicles with similar densities (that might comigrate when purified by ultracentrifugation) would not be expected to bind to a virus-specific antibody. Two control conditions were used to further eliminate the detection of nonspecific host proteins: immunoprecipitation of an uninfected (mock) supernatant using the GP1-specific antibody, and immunoprecipitation of a JUNV C#1 virion-containing supernatant using a nonspecific antibody (Fig. 1C). Samples were subjected to protein gel electrophoresis and in-gel tryptic digestion, followed by liquid chromatography-mass spectrometry analysis (Fig. 1A and C). In-gel tryptic digestion of individual slices of each gel lane, while more laborious than techniques such as MudPIT, maximizes the identification of protein partners by first segregating each lane of the protein gel into multiple slices that are all analyzed independently by mass spectrometry. High-abundance bands on the gel, including the IgG band indicated in Fig. 1C, were specifically cut around to eliminate any potential masking of underlying proteins in adjacent regions of the gel lane. A total of 476 host proteins were identified in JUNV virions, and 270 (57%) were conserved across two replicate experiments (Table S1). Overall, there was a relatively high degree of overlap in host proteins that associated with Z in VLPs and were also found in JUNV virions (e.g., 38% of proteins found in Z VLPs were also found in virions) (Fig. 1D). In contrast, there was less overlap in host proteins that were intracellular partners of Z and those found either in virions or associated with Z in VLPs (Fig. 1D).
Bioinformatics analysis of host proteins in JUNV C#1 particles or that associate with JUNV Z in cells or VLPs.
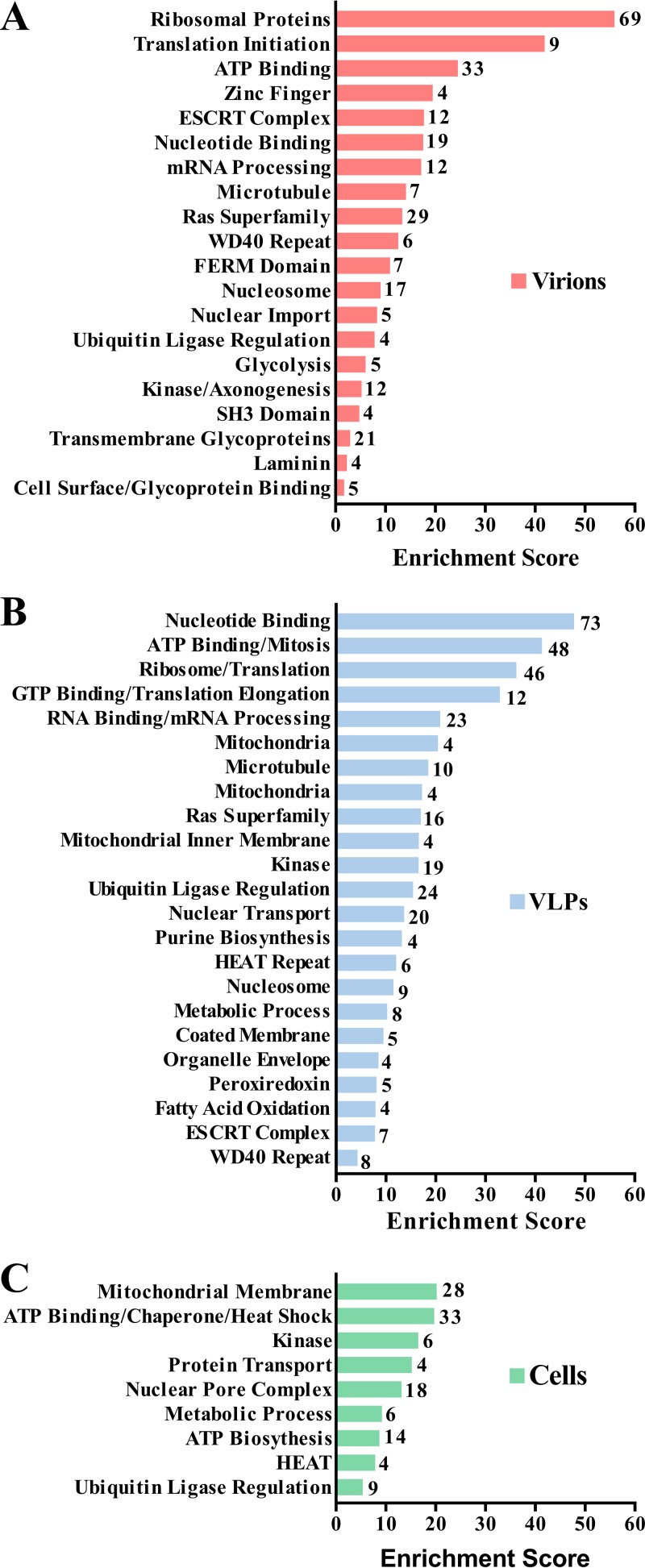
A total of 918 host proteins were identified in at least one replicate experiment as associating with JUNV Z in host cells or VLPs and/or with JUNV C#1 virions (Table S1). We used the DAVID version 6.7 functional gene classification tool (48, 49) to categorize functionally related proteins that were overrepresented under each of these conditions (Fig. 2A to C; Table S2A to C). Ribosomal proteins were highly enriched in both virions and Z VLPs (Fig. 2A and B). This finding is not unexpected, as arenaviruses are named for the Latin word arenosus, meaning “sandy,” for electron-dense granules, presumed to be ribosomes, that are observed in virions (50). Several specific structural domains were enriched in the data set, including WD40 repeats, HEAT repeats, and FERM domains (Fig. 2A to C). A variety of nuclear import or export factors were overrepresented in VLPs, as well as cells, which raises questions about whether Z executes any functions in the nucleus (Fig. 2B and C). The ESCRT complex and the Ras superfamily, both involved in intracellular vesicular trafficking, were enriched in virions as well (Fig. 2A and B). It should be noted that the ESCRT complex proteins CHMP1A and TSG101, as well as the ESCRT accessory protein PDCD6IP (also known as ALIX), were identified in this study (Fig. 3; Table S1), despite not being included in the ESCRT complex protein class generated by DAVID (Table S2A and B). Additionally, intracellular Z interacted with several ATP synthases and ATPases, as well as mitochondrial membrane proteins. Notably, Z associated with nearly the entire human peroxiredoxin family, particularly in the context of VLPs (Fig. 2B and 3; Table S1). Finally, several virion-associated host proteins were also found to associate with intracellular Z, suggesting that Z may play a role in recruiting these cellular proteins into virions.
FIG 2.
Bioinformatics analysis of host proteins in JUNV C#1 particles or that associate with JUNV Z in cells or VLPs. The gene functional classification tool in DAVID version 6.7 was used to identify the most highly enriched biological function categories among host proteins found in virions (A) or that associate with JUNV Z in VLPs (B) or cells (C) using the medium stringency setting. The enrichment score for each gene functional class is plotted, and the number of proteins identified in each class is listed adjacent to each bar.
FIG 3.
Interactome map of human proteins that associate with JUNV Z or JUNV C#1 particles. An interactome map of host proteins identified as components of JUNV C#1 virions or partners of JUNV Z in the context of cells or VLPs was generated using Cytoscape 3.3.0 software. Note that only host proteins identified in both replicate experiments are shown. Lines link each host protein to the condition (circular nodes) in which it was identified. Host proteins selected for further biochemical and functional analysis are highlighted in yellow.
Biochemical validation of select JUNV-host protein interactions.
To select proteins for functional screening, several criteria were considered, including whether a particular host protein (i) was identified in both replicate experiments for a particular condition (Fig. 3; Table S1) and (ii) belonged to an enriched functional class (Fig. 2; Table S2). In an effort to select host proteins that might be fundamentally required for a range of viruses and thus could serve as broad-spectrum antiviral targets, we also took into consideration whether a given host protein was required for the propagation of other viruses. To do this, the list of proteins identified in this study was cross-referenced with published data from genome-scale RNA interference (RNAi) screening for required host factors for different viruses, including human immunodeficiency virus (51–53), dengue virus (54, 55), hepatitis C virus (56, 57), influenza virus (58–61), and vesicular stomatitis virus (62). Based on these criteria, the following host proteins were selected for further study: ADP ribosylation factor 1 (ARF1), ATP synthase, H+ transporting, mitochondrial F1 complex, beta polypeptide (ATP5B), ATPase, H+ transporting, lysosomal 38-kDa, V0 subunit d1 (ATP6V0D1), IMP dehydrogenase 2 (IMPDH2), peroxiredoxin 3 (PRDX3), and Rab5c (Fig. 3 and Table 1).
TABLE 1.
Host proteins selected for biochemical validation and functional analysis
| Gene symbol | Entrez ID | Description of gene product | Associates with JUNV: |
Other virus(es) in which protein is: |
|||
|---|---|---|---|---|---|---|---|
| Z protein in: |
C#1 virions | Found in virions | Required for propagation | ||||
| Cells | VLPs | ||||||
| ARF1 | 375 | ADP ribosylation factor 1 | x | x | Filovirus (92), herpes simplex virus (106), HIV-1 (107), vaccinia virus (108) | HIV (51), vesicular stomatitis virus (62), LCMV (62), coxsackievirus B (109), poliovirus (109) | |
| ATP5B | 506 | ATP synthase, H+ transporting, mitochondrial F1 complex, beta polypeptide | x | x | HIV-1 (107) | HIV (53) | |
| ATP6V0D1 | 9114 | ATPase, H+ transporting, lysosomal 38 kDa, V0 subunit d1 | x | HIV-1 (107), Moloney murine leukemia virus (110) | Dengue virus (54, 55), Hendra virus (111), influenza A virus (59, 61, 65), LCMV (62), vesicular stomatitis virus (62), West Nile virus (54), yellow fever virus (66) | ||
| IMPDH2 | 3615 | IMP dehydrogenase 2 | x | Hendra virus (111) | |||
| PRDX3 | 10935 | Peroxiredoxin 3 | x | Vaccinia virus (112) | |||
| RAB5C | 5878 | RAB5C, member RAS oncogene family | x | Filovirus (92), herpes simplex virus (106), HIV-1 (107), vaccinia virus (113) | Dengue virus (114), LCMV (62), vaccinia virus (112), vesicular stomatitis virus (62) | ||
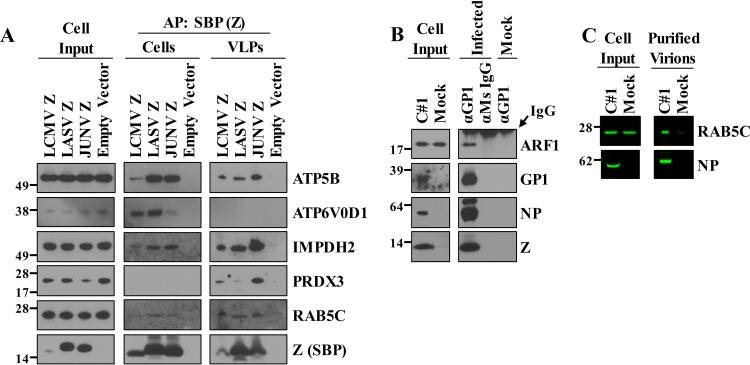
To validate that the selected host proteins interact with JUNV, we affinity purified cell-free JUNV C#1 particles or JUNV Z from cells or VLPs and then used Western blot analysis of the purified protein complexes to verify the presence of specific host proteins. This analysis was extended to LASV and LCMV Z proteins to determine whether the interactions might be conserved among Old World arenaviruses. The results from this Western blot screen largely recapitulated the mass spectrometry data but afforded greater sensitivity for certain proteins (Fig. 4A). For example, IMPDH2, which was only detected in VLPs by mass spectrometry, associated with all 3 arenavirus Z proteins in the context of both cells and VLPs. Mass spectrometry, despite its ability to identify proteins in a high-throughput manner, may fail to consistently detect proteins in complex samples. Likewise, a total of 15 Rab family proteins were identified as JUNV Z protein partners or in JUNV C#1 virions in at least one replicate mass spectrometry experiment (Table S1), and yet, only three of the Rab proteins (Rab1a, Rab7a, and Rab10) were identified in both replicate experiments for Z protein partners. Additionally, Rab5c, which was only detected in JUNV C#1 virions by mass spectrometry, was found to interact with LASV and JUNV Z proteins in cells and all 3 Z proteins in the context of VLPs. Thus, host proteins identified under only one or two of the conditions may not be exclusive to that condition. Importantly, Z protein contact with host proteins identified in VLPs or virions is likely initiated inside cells, and with a more sensitive assay, some of these protein-protein interactions might also be detected in cells. The LCMV Z expression levels in the experiment whose results are shown in Fig. 4A were lower than the levels of LASV Z or JUNV Z, giving the appearance that the interaction of ATP5B or IMPDH2 with LCMV Z may be weaker than the interaction with LASV Z or JUNV Z. However, the lower levels of ATP5B and IMPDH2 detected with LCMV Z are more likely due to the smaller quantity of LCMV Z available to be captured during affinity purification. Finally, to validate the presence of ARF1 and Rab5c in virions, we immunoprecipitated C#1 virions and probed for these proteins by Western blotting (Fig. 4B). We were able to confirm ARF1 as a component of the virus particles (Fig. 4B), but Western blot validation of ATP5B was hindered by the comigrating heavy chain IgG band from the antibody used to immunoprecipitate virions. To circumvent this problem, we concentrated JUNV virions using polyethylene glycol (PEG) precipitation and high-speed centrifugation, followed by gradient centrifugation and fractionation. A fraction containing peak levels of the viral nucleoprotein was probed for Rab5c by Western blotting, which showed that Rab5c was enriched in virus particles (Fig. 4C).
FIG 4.
Biochemical validation of JUNV-host protein interactions. (A) HEK293T cells were transfected with a plasmid encoding the SBP-tagged Z protein of lymphocytic choriomeningitis virus (LCMV), Lassa virus (LASV), or JUNV or an empty vector to serve as a control. Two days later, cells and their VLP-containing supernatants were collected and lysed to liberate each respective Z protein and its host protein partners. Z-host protein complexes were affinity purified (AP) with streptavidin beads, separated by SDS-PAGE, and screened for the presence of Z (bait) and the indicated host proteins (prey) via Western blot analysis using antibodies specific to each host protein or the SBP tag on Z. (B) HEK293T cells were infected with JUNV C#1, and 72 h later, virus particles were immunoprecipitated using an antibody specific for the viral envelope glycoprotein (GP1). Controls conditions included (i) immunoprecipitation of virus-containing supernatants with a nonspecific mouse (Ms) IgG antibody and (ii) immunoprecipitation of an uninfected (mock) supernatant with the GP1-specific antibody. Captured virions were lysed and then run out on an SDS-PAGE gel for subsequent Western blot analysis using antibodies specific for the JUNV proteins GP1, NP, and Z or the host protein ARF1. (A and B) All proteins were detected with horseradish peroxidase-conjugated secondary antibodies and chemiluminescence-based detection with standard film. The Western blots shown are representative of at least 2 independent experiments. (C) Clarified cell culture medium from JUNV C#1-infected or mock-infected HEK293T cells was subjected to polyethylene glycol precipitation, and the resulting material was resuspended in TNE buffer, layered onto a discontinuous sucrose gradient, and then subjected to ultracentrifugation. The centrifuged material from each condition was collected in 1-ml fractions using a peristaltic pump, and then the levels of viral NP and Rab5c were determined by fluorescent Western blotting. Molecular mass in kDa is shown to the left of each gel.
In summary, the data from these experiments validate and extend the mass spectrometry findings and suggest that the selected panel of host proteins have highly conserved interactions among Old and New World arenaviruses.
Identification of proviral host factors.
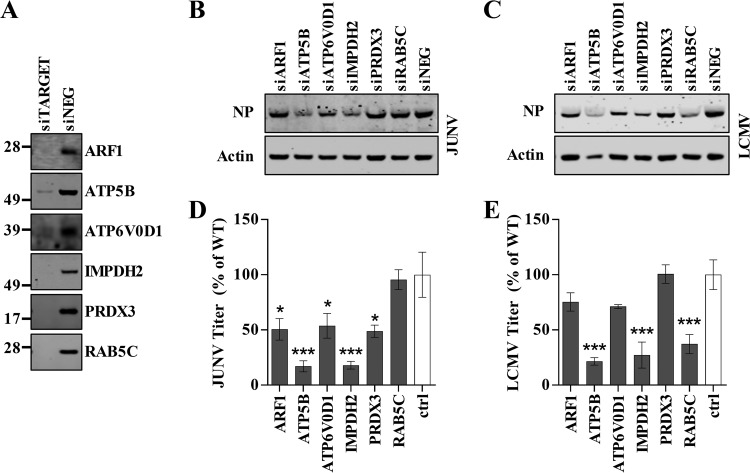
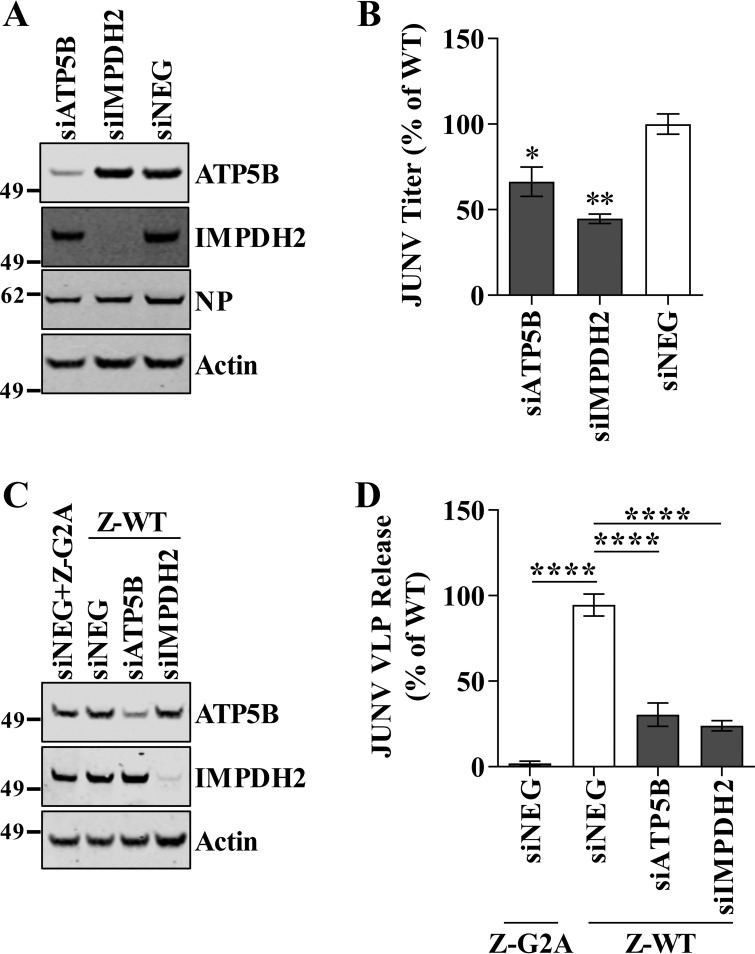
We next wished to determine whether the selected host proteins (listed in Table 1) were functionally required for JUNV C#1 or LCMV replication. This was accomplished by knocking down the expression of each host protein using silencing RNAs (small interfering RNAs [siRNAs]) and measuring how the loss of a particular protein impacted infectious virus production compared to that in cells that received a scrambled, nonspecific control siRNA. The protein levels of each targeted host protein were greatly reduced in cells that received a gene-specific siRNA compared to their levels in cells that received the nonspecific control siRNA (Fig. 5A). Furthermore, the levels of cellular actin across the various siRNA transfections did not change, indicating that the transfections did not adversely impact overall cell viability (Fig. 5B and C). Loss of the expression of ARF1, ATP5B, ATP6V0D1, IMPDH2, or PRDX3 significantly impaired JUNV C#1 propagation, while loss of Rab5c expression did not (Fig. 5D). LCMV also required ATP5B and IMPDH2 for efficient propagation, but not ARF1, ATP6V0D1, or PRDX3 (Fig. 5E). Unlike JUNV, LCMV required Rab5c for growth (Fig. 5D and E), consistent with a previous finding by Panda et al. (62). The requirement for ATP5B and IMPDH2 during JUNV infection was confirmed using a second set of siRNAs targeting these proteins (Fig. 6A and B). Finally, in order to determine whether ATP5B and IMPDH2 block JUNV infection at the stage of virus release, we utilized siRNA and a VLP release assay. VLP release was reduced 69% following ATP5B knockdown and 76% with IMPDH2 knockdown, indicating that both of these proteins impact the release of JUNV.
FIG 5.
Identification of host factors required for arenavirus propagation. (A to E) Human lung carcinoma (A549) cells were reverse transfected with a nontargeting siRNA (siNEG) or with the indicated gene-specific siRNAs (siTARGET). Two days following transfection, cells were infected with either JUNV C#1 or LCMV, and supernatants and cellular protein lysates were collected at 2 days p.i. (A) Host protein expression in siRNA-transfected cells was visualized by Western blotting to confirm knockdown. Molecular mass in kDa is shown to the left. (B and C) Cell lysates were probed for cellular actin and either JUNV C#1 NP (B) or LCMV NP (C) by Western blotting using fluorescent detection. (D and E) The quantities of infectious JUNV C#1 (D) or LCMV (E) in the supernatants were determined by plaque assay, and values are shown as percentages of the virus titer under the nontargeting control siRNA condition. The Western blot images shown are representative of 3 independent experiments, while the infectious titers represent the mean values ± standard errors of the means (SEM) of 3 independent experiments. Mean values were compared using a one-way ANOVA with Holm-Sidak's test for multiple comparisons. *, P < 0.05; ***, P < 0.001.
FIG 6.
ATP5B and IMPDH2 are required for efficient JUNV budding. A second set of siRNAs targeting ATP5B and IMPDH2 (unique from those used in the experiments whose results are shown in Fig. 5) was used to confirm the requirement of these proteins in JUNV infection. A549 cells were infected with JUNV C#1 2 days after reverse transfection with siRNA, and the cells and supernatants were collected 48 h later. (A) Expression of targeted host proteins and viral NP were determined by fluorescent Western blotting. (B) Virus titers were determined by plaque assay, and the values are shown as percentages of the virus titer under the nontargeting siRNA control condition. (C and D) HEK293T cells were reverse transfected with an siRNA targeting ATP5B or IMPDH2 or a nontargeting control siRNA. Two days later, the cells were transfected with plasmids encoding either WT or G2A mutant JUNV Z protein. Z protein quantities in cells and virus-like particle (VLP)-containing supernatants were determined via quantitative fluorescent Western blotting. The VLP release efficiency was calculated as the amount of Z in the supernatants divided by the quantity of intracellular Z. (A and C) Molecular mass in kDa is shown to the left of each gel. (B and D) The virus titers and VLP release values represent the mean values ± SEM of 3 independent experiments each with 2 technical replicates. The ROUT method for outlier identification was used to identify and justify the removal of one technical replicate from the results of the experiment shown in panel D. Mean values were compared using one-way ANOVA with Holm-Sidak's test for multiple comparisons. *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
JUNV requires a functional ESCRT pathway for infectious virus production.
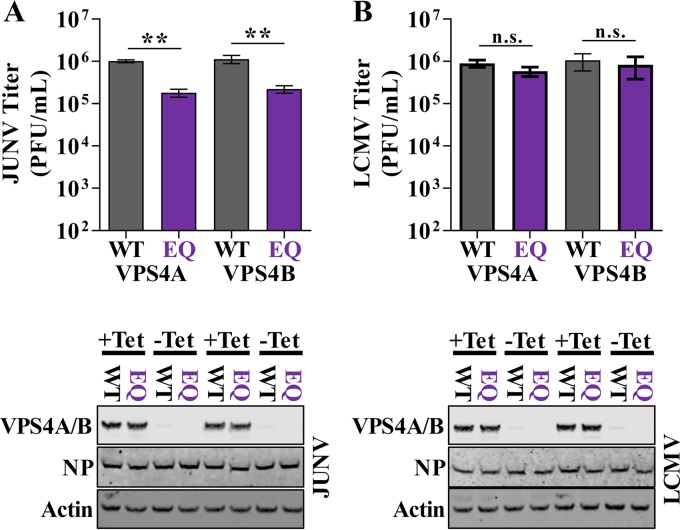
The importance of the cellular ESCRT pathway for infectious virus production by New World arenaviruses has not been addressed previously. In the current study, we identified 14 ESCRT complex or ESCRT-associated proteins in Z VLPs or JUNV C#1 virions under at least one experimental condition (Fig. 3; Table S1). To formally address the role of the ESCRT pathway in infectious JUNV particle release, cell lines stably transduced with wild-type (WT) or dominant-negative (EQ mutant) versions of the ESCRT accessory protein vacuolar protein sorting 4A (VPS4A) or VPS4B were infected with JUNV or, as a control, LCMV. To specifically measure the release of infectious virus particles and avoid measuring an impact of ESCRT inhibition on viral entry, the expression of VPS4 was not induced until 48 h after infection, when all cells in the culture were productively infected. Cells were washed 5 h later to remove virus produced prior to induction. Compared to cells expressing WT VPS4A or VPS4B, there was a significant impairment in the release of infectious JUNV particles in cells that expressed either VPSA EQ (82% reduction, P = 0.009) or VPS4B EQ (80% reduction, P = 0.006), respectively (Fig. 7A). In contrast, the release of infectious LCMV virions was not affected by VPS4A EQ or VPS4B EQ expression (P values of 0.98 or 0.98, respectively), which is in agreement with previous results (Fig. 7B) (44). Thus, our data suggest that JUNV requires a functional ESCRT pathway for the efficient release of infectious particles.
FIG 7.
JUNV requires a functional ESCRT pathway for infectious virus production. (A and B) T-Rex HEK293 cells stably transduced with vectors for tetracycline-based induction of GFP-tagged WT or dominant-negative mutant (EQ) vacuolar protein sorting 4A (VPS4A) or VPS4B were infected with JUNV C#1 at an MOI of 0.1 (A) or rLCMV WT at an MOI of 0.001 (B), and 2 days p.i., treated with tetracycline to induce expression of WT or dominant-negative VPS4A/B. Five hours after induction (53 h p.i.), the cells were washed and fresh tetracycline-containing medium was added. Fifteen hours later (68 h p.i.), supernatants were collected to measure infectious virus titer via plaque assay and cells were collected to measure expression of GFP-tagged VPS4A/B, JUNV or LCMV NP, or cellular actin via Western blot analysis. Western blots are representative of 3 independent experiments, and infectious titers represent the mean values ± SEM of 3 independent experiments. Mean values were compared using one-way ANOVA with Holm-Sidak's test for multiple comparisons. n.s., not significant; **, P < 0.01.
DISCUSSION
Viruses commonly hijack host machinery to complete the various stages of their life cycles. In this study, we employed a large-scale proteomics approach to identify human proteins that associate with the JUNV matrix protein or are incorporated into JUNV particles (Fig. 1 and 3; Table S1). Bioinformatics analysis of the resulting data set revealed enrichment of several classes of host proteins, including those of the ESCRT complex (Fig. 2; Table S2 in the supplemental material). Functional studies demonstrated that two of the host proteins identified (ATP5B and IMPDH2) were required for the propagation of both New and Old World arenaviruses (Fig. 5 and 6) and could specifically control JUNV release (Fig. 6C and D), while others were specific for the growth of JUNV (ARF1, ATP6V0D1, and PRDX3) or LCMV (Rab5c) (Fig. 5). Furthermore, for the first time, we show that the efficient release of infectious JUNV particles requires a functional ESCRT pathway, in contrast to the release of LCMV (Fig. 7) (44).
While several proviral host proteins were identified via siRNA screening (Fig. 5 and 6), additional lines of evidence exist to support their potential importance for viral growth and, in some cases, to suggest a potential mechanism by which they impact the viral life cycle. For example, IMPDH2, which was required for optimal replication of JUNV and LCMV (Fig. 5), normally functions to catalyze the rate-limiting step in guanine nucleotide biosynthesis. Interestingly, depletion of the intracellular GTP pool by mycophenolic acid (MPA) or 5-ethynyl-1-β-d-ribofuranosylimidazole-4-carboxamide (EICAR) treatment inhibits arenavirus production (63). This finding could indicate that arenaviruses recruit IMPDH2 as a means to support the processes of genome replication and transcription. However, this study also demonstrated that IMPDH2 depletion inhibits JUNV VLP release (Fig. 6C and D), suggesting that this protein may impact arenavirus infection at more than one stage of virus replication. Another example is ATP6V0D1, which is a component of the vacuolar ATPase complex that is responsible for the acidification of endosomes (64). We found ATP6V0D1 to be important for JUNV propagation (Fig. 5), while others have shown its importance for the replication of multiple RNA viruses, including LCMV (Table 1) (54, 55, 59, 61, 62, 64–66). The finding that bafilomycin treatment, which targets the vacuolar ATPase complex, restricts arenavirus entry further supports the potential importance of ATP6V0D1 for arenavirus propagation (64, 67, 68). Additionally, the absence of ATP6V0D1 in JUNV VLPs and virions (Table S1) suggests it may indeed act early in the viral life cycle. Last, we showed that depletion of ATP5B, which can function as an ATPase and localize to the plasma membrane, impaired JUNV and LCMV growth (Fig. 5 and 6) (69, 70). Depletion of ATP5B also resulted in a significant reduction in JUNV VLP release (Fig. 6C and D). The ATPase activity of ATP5B was recently shown to be an important factor in influenza A virus release and human immunodeficiency virus 1 (HIV-1) cell-to-cell transfer (70, 71). Our findings are consistent with a role for ATP5B in the energetics of virus release and suggest that this protein might be important for the release of a diverse range of viruses.
Several vesicular trafficking proteins, including members of the Ras superfamily, were enriched in Z VLPs and/or JUNV virions (Fig. 2A and B). Our studies show that ARF1, a regulator of intracellular trafficking, is required for JUNV propagation, but not that of LCMV (Fig. 5). However, a parallel siRNA screen found ARF1 necessary for optimal LCMV propagation, suggesting that it may be broadly important for arenavirus infection (62). ARF1 is required for the trafficking of the HIV-1 Gag protein to the plasma membrane (72). In particular, disruption of ARF1 function impairs the assembly and release of HIV-1 particles. Thus, ARF1 could be important for the trafficking of Z or additional viral proteins to sites of virus assembly. ARF1 is also an important factor in the secretory pathway and regulates cargo release from the endoplasmic reticulum (ER)-Golgi intermediate compartment (ERGIC) (73). Our laboratory has shown that a key component of the ERGIC, ERGIC-53 (alternately named LMAN1), is incorporated into JUNV particles and is required for their infectivity (74). This raises the possibility that trafficking of ERGIC-53 to sites of viral budding for incorporation into virions may be disrupted by ARF1 depletion. Additionally, trafficking of the mature viral glycoprotein to viral budding sites could be disrupted by ARF1 depletion as arenavirus glycoproteins undergo processing in the endoplasmic reticulum and Golgi apparatus (75, 76).
Fifteen Rab family proteins were identified in Z VLPs and JUNV virions (Table S1). Rab family GTPases are key mediators of intracellular vesicular trafficking and are exploited by a wide variety of viruses (77–79). In the case of arenaviruses, Rab5 has been implicated in JUNV and LASV entry, while Rab7 is required for efficient JUNV but not LASV entry (80, 81). The presence of Rab proteins in virions, however, suggests that some of these Rab proteins may have a role in late steps of the virus life cycle (i.e., assembly and budding) but, importantly, does not exclude that these protein-protein interactions are initiated in the cells. For example, Rab5c, which was identified by mass spectrometry only in C#1 virions (Fig. 3; Table S1), is also a protein partner of the Z protein both in cells and VLPs (Fig. 4A). Accordingly, Rab11 has been implicated in the assembly and budding of several viruses, including influenza A virus (79, 82–87). While the work here revealed that LCMV but not JUNV infection depended on Rab5c (Fig. 5), the overrepresentation of this family of proteins (Fig. 2A and B) suggests a functional role and that disparate arenaviruses may engage this host machinery in distinct ways. Indeed, we recently demonstrated that perinuclear aggregates of the LCMV genome (e.g., viral replication factories) specifically colocalize with Rab5c and the viral glycoprotein only late during the acute phase of infection (88). This finding suggests that LCMV may use Rab5c-positive endosomal membranes as a site for genome replication and, perhaps, preassembly of viral components prior to their trafficking to the plasma membrane for budding. Furthermore, Z may initiate the formation of this preassembly complex through its ability to associate with Rab5c (Fig. 3; Table S1) and recruit additional viral components via its capacity to directly bind them (39, 40, 43).
The arenavirus matrix protein drives the budding and release of virus particles at the plasma membrane, a process thought to be completed through the recruitment of ESCRT complexes (17, 41, 42). We identified, in JUNV Z VLPs or JUNV C#1 virions, host proteins representative of ESCRT-I (MVB12A/B, TSG101, VPS28, and VPS37B/C), ESCRT-II (VPS25), ESCRT-III (CHMP1A, CHMP2A/B, CHMP4, and CHMP5), and ESCRT accessory proteins (VPS4B and PDCD6IP [ALIX]), but not ESCRT-0 (Fig. 3; Table S1) (89). These data support a model whereby the JUNV Z protein utilizes its PTAP late domain to directly bind TSG101 for the recruitment of the ESCRT-I complex (90). The associated ESCRT-I proteins could then presumably facilitate the subsequent recruitment of ESCRT-II, ESCRT-III, and VPS4A/B to drive virus release (91). Indeed, ESCRT proteins, including the ESCRT-I proteins TSG101 and VPS28, as well as the ESCRT accessory protein VPS4B, have been identified in virions of other viruses containing the P(T/S)AP late domain, including Ebola virus and HIV-1, presumably because these proteins play a role in the final stages of virus release (92, 93). It is critical to note that, while all of the ESCRT proteins that were identified in this study were found only in either VLPs or virions, which may serve as an indicator of a protein's role late in the virus life cycle, these interactions between Z and ESCRT proteins presumably are initiated inside the cell, as a topological requirement.
The ESCRT complex is hypothesized to function in the release of infectious arenavirus particles, and yet, evidence for this model has been limited primarily to VLP assays. Knockdown of the ESCRT-I protein TSG101 results in decreased VLP production for LCMV and LASV (17, 42). Perturbation of the ESCRT accessory protein VPS4 reduces VLP production for Tacaribe virus and LASV but not Lujo virus (41, 47, 94). Recently, our laboratory has shown that LCMV utilizes the ESCRT complex specifically for defective interfering particle production but not the release of infectious virus (44). This indicates that VLP assays, while useful, may not always faithfully recapitulate all the facets of infection or, alternatively, may reflect the biology of budding for specific classes of virus particles. Accordingly, we assessed the ESCRT dependency of JUNV C#1 infectious particle release using VPS4-expressing cell lines. Importantly, the expression of either WT or dominant-negative (EQ mutant) VPS4A/B was induced only after all cells were productively infected to eliminate the potential impact that ESCRT inhibition could have on virus entry, as Old World arenaviruses utilize the ESCRT complex during viral entry (95). Efficient release of JUNV C#1 infectious virions did require a functional ESCRT pathway (Fig. 7A), while the release of infectious LCMV virions did not, in concordance with our previous results (Fig. 7B) (44). The requirement of ESCRT for JUNV C#1 is also supported by recent work which demonstrated that an inhibitor of the P(T/S)AP-TSG101 interaction reduced infectious JUNV production (90).
In conclusion, we have provided a detailed map of JUNV virus interactions with the human proteome and identified several host factors required for JUNV infection. This work, combined with our previous proteomics studies (74, 96), represents a crucial step in understanding the protein networks hijacked by arenaviruses to drive the virus life cycle. This study highlights the important role that the ESCRT pathway plays in driving infectious JUNV particle release, while further demonstrating the diversity exhibited among arenaviruses in their use of ESCRT machinery for the production of different classes of virus particles. Finally, the data set reported here will aid in the identification of novel host targets that can be therapeutically targeted.
MATERIALS AND METHODS
Cells, viruses, and plasmids.
Human embryonic kidney cells (HEK-293T/17) (CRL-11268; American Type Culture Collection, Manassas, VA) (referred to as HEK293T cells in the manuscript) were maintained in Dulbecco's modified Eagle's medium (DMEM) (catalog number 11965-092) supplemented with 10% fetal bovine serum (FBS) (catalog number 16140-071), 1% penicillin-streptomycin (catalog number 15140-122), 1% minimal essential medium (MEM)–nonessential amino acids solution (catalog number 11140-050), 1% HEPES buffer solution (catalog number 15630-130), and 1% GlutaMAX (catalog number 35050-061) purchased from Invitrogen (Carlsbad, CA). Stably transduced T-Rex HEK293 cells expressing WT or the dominant-negative EQ mutant of vacuolar protein sorting 4A (VPS4A) or VPS4B under a tetracycline-inducible promoter described in references 97 to 99 were kindly provided by M. Kielian (Albert Einstein College of Medicine, Bronx, NY) and were maintained in the same medium as HEK293T cells but were also supplemented with 100 μg/ml Zeocin (R250-01, Invitrogen). African green monkey kidney cells (Vero E6) were kindly provided by J. L. Whitton (The Scripps Research Institute, La Jolla, CA) and grown in DMEM supplemented with 10% FBS, 1% penicillin-streptomycin, and 1% HEPES buffer solution. Human lung carcinoma cells (A549) (CCL-185; American Type Culture Collection) were maintained in 50:50 DMEM–F-12 medium (catalog number 11330-032; Invitrogen) supplemented with 10% FBS and 1% penicillin-streptomycin. All cell lines were grown at 37°C in a humidified incubator containing 5% CO2. LCMV strain Armstrong 53b was kindly provided by J. L. Whitton. Recombinant LCMV (rLCMV) WT, based on the Armstrong 53b strain and used in VPS4 assays, was generated as described previously (44, 100). The candidate vaccine strain of JUNV, C#1, an attenuated strain derived from virulent JUNV strain XJ as described in references 101 and 102, was kindly provided by M. Buchmeier (University of California, Irvine) and R. Tesh (The University of Texas Medical Branch at Galveston). Working stocks of these viruses were generated, and infectious titers were measured via plaque assay using Vero E6 cells.
Plasmids pLCMV-Z, pLASV-Z, and pJUNV-Z contain a Z gene fused to the streptavidin binding peptide (SBP) sequence through an 18-bp linker at the C terminus. Z was amplified by PCR from existing constructs using forward primer LCMVZf (5′-ACAAGTTTGTACAAAAAAGCAGGCTGATATCGCCACCATGGGTCAAGGCAAGTCCAGA-3′), LASVZf (5′-ACAAGTTTGTACAAAAAAGCAGGCTGATATCGCCACCATGGGAAACAAGCAAGCCAAA-3′), or JUNVZf (5′-ACAAGTTTGTACAAAAAAGCAGGCTGATATCGCCACCATGGGCAACTGCAACGGGGCA-3′), each of which has a 5′ overhang containing AttB1 and Kozak sequences, and reverse primer LCMVZr (5′-ACCTCCACCTCCAGCTGCCTCTTCGTAGGGAGGTGGAGA-3′), LASVZr (5′-ACCTCCACCTCCAGCTGCGGGACTGTAGGGTGGGGGTCT-3′), or JUNVZr (5′-ACCTCCACCTCCAGCTGCTGGTGGTGGTGCTGTTGGCTC-3′), each of which has an overhang containing the linker sequence. The SBP sequence was amplified by PCR from pT7-FLAG-SBP-1 (Sigma-Aldrich) using forward primer SBPf (5′-GCAGCTGGAGGTGGAGGTATGGACGAAAAAACCACCGGT-3′), which has a 5′ overhang containing the linker sequence, and reverse primer SBPr (5′-ACCACTTTGTACAAGAAAGCTGGGTCTTACGGTTCACGCTGACCCTGCGG-3′), containing a 3′ overhang with a stop codon preceding the AttB2 sequence. The two products were fused by PCR using the appropriate Z forward primer and the SBPr primer. Each cassette was subcloned, using the Gateway cloning system (Invitrogen) following the manufacturer's instructions, into a modified pCAGGS vector containing AttB sites that was previously described (74, 103). The JUNV Z G2A gene in the pJUNV-Z-G2A plasmid contains an alanine in place of glycine at position 2 and was synthesized and subcloned by Biobasic, Inc. (Markham, ON, Canada).
Affinity purification and immunoprecipitation.
For Z mass spectrometry experiments and subsequent Western blot validation, 5 × 105 HEK293T cells were seeded in each well of two 6-well plates per condition. One day later, cells were transfected with 100 μl DMEM containing 2 μg of the SBP-tagged JUNV Z construct or an empty vector (p0) and 8 μg of 1-mg/ml polyethyleninimine (catalog number 23966; Polysciences, Inc., Warrington, PA) per well. VLPs and cells were harvested 48 h after transfection. The VLP-containing cell culture medium was clarified by centrifugation, and the cells were scraped into phosphate-buffered saline (PBS) and pelleted by centrifugation. VLPs and cells were lysed on ice for 20 min using Triton lysis buffer (final concentration of 0.5% NP-40, 1% Triton X-100 [Acros], 140 mM NaCl, 25 mM Tris HCl) with protease inhibitor cocktail (product number 04693159001; Roche, Indianapolis, IN) and PhosphoStop phosphatase inhibitor cocktail (product number 4906837001; Roche). SBP-tagged Z protein in complex with host proteins was purified by incubation with Dynabeads MyOne streptavidin T1 beads (catalog number 65602; Invitrogen) for 2 h at 4°C on a rotating platform. The protein-bound beads were washed 4 times with Triton lysis buffer and then eluted in Laemmli sample buffer (62.5 mM Tris-HCl, 10% glycerol, 2% sodium dodecyl sulfate [SDS], and 0.01% bromophenol blue) containing 5% 2-mercaptoethanol by heating at 100°C for 10 min.
For JUNV C#1 virion mass spectrometry experiments and subsequent Western blot validation, 6 × 106 HEK293T cells were seeded in 5 T-150 tissue culture flasks per condition 24 h prior to infection. Cells were infected with JUNV C#1 at a multiplicity of infection of 0.1 PFU per cell (PFU/cell). Two days later, the medium was aspirated and the cells were washed with warmed PBS and then overlaid with 8 ml of Pro293 medium (12-764Q, Lonza) supplemented with 1% penicillin-streptomycin (15140-122), 1% MEM–nonessential amino acids solution (catalog number 11140-050), 1% HEPES buffer solution (catalog number 15630-130), and 1% GlutaMAX (catalog number 35050-061) purchased from Invitrogen (Carlsbad, CA). One day later (at 72 h p.i.), cell culture medium was pooled and clarified by centrifugation at 1,200 rpm. To the virus-containing medium, calcium chloride to a final concentration of 1 mM and 1 protease inhibitor cocktail tablet (product number 04693159001; Roche) per 15 ml were added, and then 68 μl of magnetic, protein G-conjugated beads (catalog number 10004D; Invitrogen) was added to each tube and the mixture incubated for 15 min on ice. The precleared supernatant was transferred to a new tube and incubated with 34 μg of mouse monoclonal glycoprotein 1 (GP1)-specific antibody clone QC03-BF11 (NR-2566; BEI Resources) or 34 μg of mouse IgG1 (MAB002; R&D Biosystems) for 2 h, and then 600 μl of protein G magnetic beads was added and the mixture incubated for an additional hour. The virion-bound beads were washed 4 times with PBS containing 0.1% bovine serum albumin (catalog number BP1600-100; Fisher Scientific) and then resuspended in 2×-concentrated Laemmli sample buffer (125 mM Tris-HCl, 20% glycerol, 4% sodium dodecyl sulfate, and 0.02% bromophenol blue). Virion protein content was eluted from the beads by boiling the resuspended beads at 100°C for 10 min with 5% 2-mercaptoethanol or without it (for Western blotting with anti-GP1 antibody).
Identification of host proteins by mass spectrometry.
Protein precipitates were separated using NuPAGE 4 to 12% Bis-Tris or 4 to 20% Tris-glycine gels (Invitrogen). Following electrophoresis, each gel was stained with Coomassie blue stain (40% methanol, 20% acetic acid, and 0.1% brilliant blue R [Sigma-Aldrich]) overnight, and then excess stain was removed with a solution of 30% methanol and 10% acetic acid and the gel imaged using a Canon Canoscan 8800F scanner. Each sample lane was cut into 16 (Z) or 12 (virion) slices, which were processed with chemicals from Fisher Scientific as follows. Each slice was rinsed with high-performance liquid chromatography (HPLC)-grade water and then incubated with destain solution (50 mM ammonium bicarbonate and 50% acetonitrile) for 30 min at 37°C. The destain solution was removed, and the gel slices were dehydrated by incubating with 100% acetonitrile for 5 min twice. Samples were reduced with 25 mM dithiothreitol in 50 mM ammonium bicarbonate for 30 min at 55°C and then allowed to cool for 10 min at room temperature. The solution was removed, and the slices were partially dehydrated by incubating with 100% acetonitrile for 5 min and then alkylated with 10 mM iodoacetamide in 50 mM ammonium bicarbonate for 45 min in the dark at room temperature. The samples were washed two times by incubating with destain solution for 5 min, dehydrating with 100% acetonitrile, and then rehydrating with water for 10 min. The gel slices were then completely dehydrated by incubating two times with 100% acetonitrile for 5 min and dried at room temperature. The gel slices were rehydrated with a solution of 12.5 ng/μl sequencing-grade modified trypsin (Promega) in 50 mM ammonium bicarbonate on ice for 30 min and then digested overnight at 37°C. Peptides were extracted with a solution of 2.5% formic acid in 50% acetonitrile while spinning in a microcentrifuge at 13,000 rpm for 10 min. The supernatant was collected, and the gel slices were dehydrated by twice incubating with 100% acetonitrile and collecting the extract. All solvent was removed from the extracts using a vacuum centrifuge at 37°C. The peptides were resuspended in 2.5% acetonitrile and 2.5% formic acid and then separated in a microcapillary column packed with 12 cm of Magic C18, 200-Å, 5-μm material (catalog number PM5/66100/00; Michrom Bioresources, Auburn, CA) using a MicroAS autosampler (Thermo Scientific, Pittsburgh, PA). The peptides were separated and eluted with a 5-to-35% acetonitrile (0.15% formic acid) gradient using a Surveyor pump plus HPLC instrument (Thermo Scientific) over 40 min, after a 15-min isocratic loading at 2.5% acetonitrile and 0.15% formic acid. Mass spectra were acquired in an LTQ XL linear ion trap mass spectrometer (Thermo Scientific) using 10 tandem mass spectrometry scans following each survey scan over the entire run. The human IPI forward and reverse concatenated databases were queried with SEQUEST software, requiring tryptic peptide matches with a 2-Da mass tolerance. For spectral analysis, the following precursor mass differences were allowed: for serine, threonine, and tyrosine residues, +79.96633 Da; for methionine residues, +15.99492 Da; and for cysteine residues, +57.02146 Da or +71.0371 Da. Proteins identified from the database were further filtered by excluding peptides with XCorr scores of less than 2.0. Furthermore, proteins with only one unique peptide sequence identified were subtracted. Proteins that were identified under the Z protein or JUNV C#1-infected conditions were excluded if they were also identified in the empty vector or mock-infected controls, unless there were at least 5-fold more total peptide sequences identified in the Z protein or JUNV C#1-infected conditions.
SDS-PAGE and Western blotting.
Protein samples diluted in Laemmli buffer with 5% 2-mercaptoethanol were subjected to polyacrylamide gel electrophoresis on NuPAGE 4 to 12% Bis-Tris gels with morpholineethanesulfonic acid (MES) buffer. Western blotting was carried out using nitrocellulose iBlot gel transfer stacks (catalog number IB301001; Invitrogen) and the Invitrogen iBlot transfer apparatus. Protein transfer was confirmed by staining membranes with a solution containing 0.1% Ponceau S (product number P3504; Sigma-Aldrich) and 5% acetic acid, which was subsequently removed by washing with water. Membranes were blocked for 1 h using PBS containing 5% nonfat milk (fluorescent detection) or 5% nonfat milk, 0.05% Igepal CA-630 (product number 198596; MP Biomedicals, Solon, OH) in PBS (chemiluminescent detection). Primary antibodies were diluted in PBS containing 5% nonfat milk and 0.2% Tween 20 (Fisher Scientific) (fluorescent detection) or in 5% nonfat milk, 0.05% Igepal CA-630, and 3% fetal bovine serum (chemiluminescent detection) and were incubated overnight at room temperature. The membranes were washed 5 times for 5 min with Western wash solution (0.05% Igepal CA-630 [product number 198596; MP Biomedicals, Solon, OH] in PBS) and then incubated for 1 h at room temperature with secondary antibodies diluted in PBS containing 5% milk, 0.2% Tween 20 (catalog number BP337; Fisher Scientific), and 0.02% sodium dodecyl sulfate (fluorescent detection) or incubated for 2 h with secondary antibodies diluted in 5% nonfat milk, 0.05% Igepal CA-630, and 3% fetal bovine serum (chemiluminescent detection). The membranes were then washed 5 times for 5 min with Western wash buffer. For fluorescence detection, the membranes were washed one time for 5 min with PBS and then imaged using a Licor Odyssey CLx imaging system. For fluorescence detection of proteins in the experiments whose results are shown in Fig. 4C, 6, and 7, the iBind flex Western device (catalog number SLF2000; Thermo Scientific) and the corresponding iBind fluorescence detection solution (catalog number SLF2019; Thermo Scientific) were used. For chemiluminescence detection, membranes were developed using chemiluminescence substrate (SuperSignal West Pico [catalog number 34080] or Femto [catalog number 34096]; Thermo Scientific) and detected with film.
The following primary antibodies (at the indicated concentrations) were used for Western blotting: mouse anti-β-actin (A5441; Sigma-Aldrich) (1:2,500) rabbit anti-actin (A2066; Sigma-Aldrich) (1:2,500), mouse anti-ARF1 (MA4-060; Thermo Scientific) (1:1,000), mouse anti-ATP5B (sc-166443; Santa Cruz Biotechnology, Dallas, TX) (1:100), anti-ATP6V0D1 (sc-81887; Santa Cruz Biotechnology) (1:500), mouse anti-green fluorescent protein (GFP) (632380; Clontech, Mountain View, CA) (1:1,000), rabbit anti-IMPDH2 (ab131158; Abcam, Cambridge, MA), rabbit anti-LCMV nucleoprotein (2165) (1:5,000), mouse anti-JUNV glycoprotein 1 (GP1) (NR-2564; BEI Resources, Manassas, VA) (1:250), mouse anti-JUNV nucleoprotein antibody clone NA05-AG12 (NR-2582; BEI Resources) (1:200), rabbit anti-JUNV Z protein (generously provided by Sandra Goñi and described in reference 104) (1:1,000), mouse anti-PRDX3 (LF-MA0044; AbFrontier, Seoul, South Korea) (1:1,000), mouse anti-Rab5c (sc-365667; Santa Cruz Biotechnology) (1:500), and anti-streptavidin binding peptide (SBP) (MAB10764; EMD Millipore, Billerica, MA) (1:10,000) antibodies. The LCMV nucleoprotein antibody, 2165, was generously provided by M. J. Buchmeier (University of California, Irvine). JUNV Z protein antibody was generously provided by Sandra Goñi. The following secondary antibodies (at the indicated concentrations) were used for Western blotting: goat anti-mouse IRDye 680LT antibody (926-68020; LI-COR) (1:20,000), goat anti-rabbit IRDye 800CW antibody (926-32211) (1:20,000), goat anti-mouse horseradish peroxidase (HRP) antibody (71045; EMD Millipore) (1:5,000), goat anti-mouse IgG Fcγ-specific HRP (115-035-164; Jackson ImmunoResearch Laboratories, West Grove, PA) (1:50,000), goat anti-mouse light chain-specific HRP antibody (AP200P; EMD Millipore), and goat anti-rabbit HRP antibody (111-035-045, Jackson ImmunoResearch Laboratories).
RNA interference virus challenges.
For RNA interference experiments, 1.2 μl of RNAiMax (catalog number 13778075; Life Technologies) and a 10 nM final concentration of Silencer select siRNA (catalog number 4390824; Life Technologies) targeting ARF1 (assay identification number [ID] s1551; CCAUAGGCUUCAACGUGGA), ATP5B (assay ID s1774; GGCAGAAUCAUGAAUGUCA), ATP6V0D1 (assay ID s17395; CGGUGUCAGUCAUCGAUGA), IMPDH2 (assay ID s7416; GGAUCCGGCUAAAGAAAUA), PRDX3 (s21509; GUGACAAAGCUAACGAAUU), or Rab5c (assay ID s11710; GCAAUGAACGUGAACGAAA) or a nontargeting control siRNA (catalog number 4390843) in Opti-MEM (catalog number 31985070; Thermo Fisher Scientific) was added to each well of a 12-well plate and then combined with 40,000 A549 cells per well in normal growth medium. Two days after reverse transfection, the cells were infected with JUNV C#1 at a multiplicity of infection (MOI) of 0.1 PFU/cell or with LCMV Armstrong at an MOI of 0.001 PFU/cell. One hour later, the virus inoculum was removed and fresh medium was added. Two days after infection, the virus-containing supernatant and the cells were collected. Viral titers were determined by standard plaque assay, the values for each independent experiment were normalized to the sum of all the values within each experiment (normalization by summation), and then each value was normalized to the mean of the WT values and the mean of the WT was set to 100% as described in reference 105. Host protein knockdown, as well as viral nucleoprotein and cellular actin levels, were determined by Western blotting. For the RNA interference assays whose results are shown in Fig. 6, Silencer select siRNAs (catalog number 4390824; Life Technologies) targeting ATP5B (assay ID s1773; GGACUACCACCAAUUCUAA) and IMPDH2 (s7417; CCAAGAAAAUCACUCUUAA) were used.
Z protein VLP release assay.
To determine the impact of host proteins on the Z protein's ability to drive the release of particles, 1 × 105 HEK293T cells were reverse transfected with 10 nM final concentration of siRNA targeting ATP5B (assay ID s1773) or IMPDH2 (assay ID s7417) with 1.2 μl of RNAiMax in poly-d-lysine-treated 12-well plates. Two days after reverse transfection, 0.8 μg of pJUNV-Z-G2A or pJUNV-Z was transfected with 3.2 μg of 1-mg/ml polyethyleninimine per well. Twenty-four hours after plasmid transfection, the cells and clarified virus-like particle (VLP)-containing medium were lysed with Triton lysis buffer and the Z protein was detected via quantitative Western blot analysis.
Centrifugation-based virus purification.
Thirty 150-cm2 tissue culture flasks were seeded with 6 × 106 HEK293T cells per flask. The next day, 15 of the flasks were infected with JUNV C#1 at an MOI of 0.1 and 15 were treated identically but were not infected (mock). The virus-containing medium was collected and clarified by centrifugation 72 h after infection. Thirty-five milliliters of clarified medium was added to each of 3 50-ml conical tubes per condition, each containing 2.8 g of polyethylene glycol 8000 (product number 81268; Sigma-Aldrich) and were mixed on a rotating platform for 2 h at 4°C. The tubes were then centrifuged at 10,000 rpm for 30 min in a Thermo Scientific Sorval Legend RT+ centrifuge with a Sorval Fiberlite F15-8x50cy rotor. The supernatant was removed, and the pellet resuspended in 20 ml total TNE buffer (10 mM Tris base, 1 mM EDTA, 0.2 M NaCl), pH 7.4, per condition. Fifteen milliliters of the sample from each condition was layered onto a discontinuous sucrose gradient (from bottom to top, 4 ml of 60%, 5 ml of 45%, and 6 ml of 25% sucrose) and centrifuged for 10 h at 4°C in a Thermo-Scientific Sorval WX ultra 80 ultracentrifuge with a Sorval SureSpin 630 rotor. Sixteen 1-ml fractions from each condition were then collected from the bottom of the gradient using a New Era NE-9000G programmable peristaltic pump.
Virus challenges in VPS4A- and VPS4B-transduced cells.
VPS4A or VPS4B WT or dominant-negative (EQ mutant) T-Rex HEK293 cells were used to assess the dependency of JUNV C#1 on the ESCRT pathway for infectious virus release. For these experiments, 2.5 × 105 cells were seeded in poly d-lysine-treated 6-well plates and infected 24 h later with JUNV C#1 at an MOI of 0.1 PFU/cell or with LCMV Armstrong at an MOI of 0.001 PFU/cell. Forty-eight hours after infection, when all cells were productively infected, the expression of transduced VPS4A or VPS4B was induced with 1 μg/ml tetracycline or medium only as a control. Five hours later, the cells were washed with PBS, and fresh medium containing 1 μg/ml tetracycline or medium alone was added. Fifteen hours later, the cells and virus-containing supernatants were collected. Infectious virus titers were determined via plaque assay, and the expression of exogenous GFP-tagged VPS4A or VPS4B was determined by Western blotting.
Statistics.
GraphPad Prism software was used to perform all statistical analyses. One-way analysis of variance (ANOVA) with Holm-Sidak's test for multiple comparisons was used to analyze the viral titers in the siRNA experiments whose results are shown in Fig. 5D and E and 6B and D, as well as the VPS4A and VPS4B challenges whose results are shown in Fig. 7A and B. Outlying data points were removed if identified as such by the robust regression of outliers (ROUT) method with a Q coefficient of 1%.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge NIH grants T32 AI055402 (C.M.Z.), T32 HL076122 (B.R.K. and C.M.Z.), R21 AI088059 (J.B.), and P20RR021905 and P30GM118228 (Immunobiology and Infectious Disease COBRE awards) (J.B.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The mass spectrometry analysis was supported by the Vermont Genetics Network through NIH grant 8P20GM103449 from the INBRE program and of the NIGMS.
We thank the UVM Immunobiology group for insightful discussions and are grateful to Michael Buchmeier, Sandra Goñi, Margaret Kielian, Bob Tesh, and Lindsay Whitton for providing critical reagents described in Materials and Methods.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.01565-17.
REFERENCES
- 1.Salazar-Bravo J, Ruedas LA, Yates TL. 2002. Mammalian reservoirs of arenaviruses. Curr Top Microbiol Immunol 262:25–63. [DOI] [PubMed] [Google Scholar]
- 2.Childs JE, Glass GE, Korch GW, Ksiazek TG, Leduc JW. 1992. Lymphocytic choriomeningitis virus infection and house mouse (Mus Musculus) distribution in urban Baltimore. Am J Trop Med Hyg 47:27–34. doi: 10.4269/ajtmh.1992.47.27. [DOI] [PubMed] [Google Scholar]
- 3.Keenlyside RA, McCormick JB, Webb PA, Smith E, Elliott L, Johnson KM. 1983. Case-control study of Mastomys natalensis and humans in Lassa virus-infected households in Sierra Leone. Am J Trop Med Hyg 32:829–837. doi: 10.4269/ajtmh.1983.32.829. [DOI] [PubMed] [Google Scholar]
- 4.Charrel RN, de Lamballerie X, Emonet S. 2008. Phylogeny of the genus Arenavirus. Curr Opin Microbiol 11:362–368. doi: 10.1016/j.mib.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 5.McCormick JB, Webb PA, Krebs JW, Johnson KM, Smith ES. 1987. A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis 155:437–444. doi: 10.1093/infdis/155.3.437. [DOI] [PubMed] [Google Scholar]
- 6.Charrel RN, de Lamballerie X. 2003. Arenaviruses other than Lassa virus. Antiviral Res 57:89–100. doi: 10.1016/S0166-3542(02)00202-4. [DOI] [PubMed] [Google Scholar]
- 7.Maiztegui JI. 1975. Clinical and epidemiological patterns of Argentine haemorrhagic fever. Bull World Health Organ 52:567–575. [PMC free article] [PubMed] [Google Scholar]
- 8.Enria DA, Briggiler AM, Sánchez Z. 2008. Treatment of Argentine hemorrhagic fever. Antiviral Res 78:132–139. doi: 10.1016/j.antiviral.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riviere Y, Ahmed R, Southern PJ, Buchmeier MJ, Dutko FJ, Oldstone MB. 1985. The S RNA segment of lymphocytic choriomeningitis virus codes for the nucleoprotein and glycoproteins 1 and 2. J Virol 53:966–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clegg JCS, Wilson SM, Oram JD. 1991. Nucleotide sequence of the S RNA of Lassa virus (Nigerian strain) and comparative analysis of arenavirus gene products. Virus Res 18:151–164. doi: 10.1016/0168-1702(91)90015-N. [DOI] [PubMed] [Google Scholar]
- 11.Auperin DD, Sasso DR, McCormick JB. 1986. Nucleotide sequence of the glycoprotein gene and intergenic region of the Lassa virus S genome RNA. Virology 154:155–167. doi: 10.1016/0042-6822(86)90438-1. [DOI] [PubMed] [Google Scholar]
- 12.Djavani M, Lukashevich IS, Sanchez A, Nichol ST, Salvato MS. 1997. Completion of the Lassa fever virus sequence and identification of a RING finger open reading frame at the L RNA 5′ end. Virology 235:414–418. doi: 10.1006/viro.1997.8722. [DOI] [PubMed] [Google Scholar]
- 13.Lukashevich IS, Djavani M, Shapiro K, Sanchez A, Ravkov E, Nichol ST, Salvato MS. 1997. The Lassa fever virus L gene: nucleotide sequence, comparison, and precipitation of a predicted 250 kDa protein with monospecific antiserum. J Gen Virol 78:547–551. doi: 10.1099/0022-1317-78-3-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salvato MS, Shimomaye EM. 1989. The completed sequence of lymphocytic choriomeningitis virus reveals a unique RNA structure and a gene for a zinc finger protein. Virology 173:1–10. doi: 10.1016/0042-6822(89)90216-X. [DOI] [PubMed] [Google Scholar]
- 15.Singh MK, Fuller-Pace FV, Buchmeier MJ, Southern PJ. 1987. Analysis of the genomic l RNA segment from lymphocytic choriomeningitis virus. Virology 161:448–456. doi: 10.1016/0042-6822(87)90138-3. [DOI] [PubMed] [Google Scholar]
- 16.Neuman BW, Adair BD, Burns JW, Milligan RA, Buchmeier MJ, Yeager M. 2005. Complementarity in the supramolecular design of arenaviruses and retroviruses revealed by electron cryomicroscopy and image analysis. J Virol 79:3822–3830. doi: 10.1128/JVI.79.6.3822-3830.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strecker T, Eichler R, ter Meulen J, Weissenhorn W, Klenk DH, Garten W, Lenz O. 2003. Lassa virus Z protein is a matrix protein sufficient for the release of virus-like particles. J Virol 77:10700–10705. doi: 10.1128/JVI.77.19.10700-10705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salvato MS, Schweighofer KJ, Burns J, Shimomaye EM. 1992. Biochemical and immunological evidence that the 11 kDa zinc-binding protein of lymphocytic choriomeningitis virus is a structural component of the virus. Virus Res 22:185–198. doi: 10.1016/0168-1702(92)90050-J. [DOI] [PubMed] [Google Scholar]
- 19.Lee KJ, de la Torre JC. 2002. Reverse genetics of arenaviruses. Curr Top Microbiol Immunol 262:175–193. [DOI] [PubMed] [Google Scholar]
- 20.Urata S, Yasuda J. 2012. Molecular mechanism of arenavirus assembly and budding. Viruses 4:2049–2079. doi: 10.3390/v4102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, Nichol ST, Compans RW, Campbell KP, Oldstone MBA. 1998. Identification of α-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 22.Spiropoulou CF, Kunz S, Rollin PE, Campbell KP, Oldstone MBA. 2002. New World arenavirus clade C, but not clade A and B viruses, utilizes α-dystroglycan as its major receptor. J Virol 76:5140–5146. doi: 10.1128/JVI.76.10.5140-5146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunz S. 2009. Receptor binding and cell entry of Old World arenaviruses reveal novel aspects of virus–host interaction. Virology 387:245–249. doi: 10.1016/j.virol.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 24.Quirin K, Eschli B, Scheu I, Poort L, Kartenbeck J, Helenius A. 2008. Lymphocytic choriomeningitis virus uses a novel endocytic pathway for infectious entry via late endosomes. Virology 378:21–33. doi: 10.1016/j.virol.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 25.Borrow P, Oldstone MBA. 1994. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology 198:1–9. doi: 10.1006/viro.1994.1001. [DOI] [PubMed] [Google Scholar]
- 26.Martinez MG, Cordo SM, Candurra NA. 2007. Characterization of Junín arenavirus cell entry. J Gen Virol 88:1776–1784. doi: 10.1099/vir.0.82808-0. [DOI] [PubMed] [Google Scholar]
- 27.Klewitz C, Klenk H-D, ter Meulen J. 2007. Amino acids from both N-terminal hydrophobic regions of the Lassa virus envelope glycoprotein GP-2 are critical for pH-dependent membrane fusion and infectivity. J Gen Virol 88:2320–2328. doi: 10.1099/vir.0.82950-0. [DOI] [PubMed] [Google Scholar]
- 28.Lee KJ, Novella IS, Teng MN, Oldstone MBA, de la Torre JC. 2000. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA Analogs. J Virol 74:3470–3477. doi: 10.1128/JVI.74.8.3470-3477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez N, Jacamo R, Franze-Fernandez MT. 2001. Transcription and RNA replication of Tacaribe virus genome and antigenome analogs require N and L proteins: Z protein is an inhibitor of these processes. J Virol 75:12241–12251. doi: 10.1128/JVI.75.24.12241-12251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fehling S, Lennartz F, Strecker T. 2012. Multifunctional nature of the arenavirus RING finger protein Z. Viruses 4:2973–3011. doi: 10.3390/v4112973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornu TI, de la Torre JC. 2001. RING finger Z protein of lymphocytic choriomeningitis virus (LCMV) inhibits transcription and RNA replication of an LCMV S-segment minigenome. J Virol 75:9415–9426. doi: 10.1128/JVI.75.19.9415-9426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jácamo R, López N, Wilda M, Franze-Fernández MT. 2003. Tacaribe virus Z protein interacts with the L polymerase protein to inhibit viral RNA synthesis. J Virol 77:10383–10393. doi: 10.1128/JVI.77.19.10383-10393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kranzusch PJ, Whelan SPJ. 2011. Arenavirus Z protein controls viral RNA synthesis by locking a polymerase–promoter complex. Proc Natl Acad Sci U S A 108:19743–19748. doi: 10.1073/pnas.1112742108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell Dwyer EJ, Lai H, MacDonald RC, Salvato MS, Borden KLB. 2000. The lymphocytic choriomeningitis virus RING protein Z associates with eukaryotic initiation factor 4E and selectively represses translation in a RING-dependent manner. J Virol 74:3293–3300. doi: 10.1128/JVI.74.7.3293-3300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kentsis A, Dwyer EC, Perez JM, Sharma M, Chen A, Pan ZQ, Borden KLB. 2001. The RING domains of the promyelocytic leukemia protein PML and the arenaviral protein Z repress translation by directly inhibiting translation initiation factor eIF4E. J Mol Biol 312:609–623. doi: 10.1006/jmbi.2001.5003. [DOI] [PubMed] [Google Scholar]
- 36.Volpon L, Osborne MJ, Capul AA, de la Torre JC, Borden KLB. 2010. Structural characterization of the Z RING-eIF4E complex reveals a distinct mode of control for eIF4E. Proc Natl Acad Sci U S A 107:5441–5446. doi: 10.1073/pnas.0909877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xing J, Ly H, Liang Y. 2015. The Z proteins of pathogenic but not nonpathogenic arenaviruses inhibit RIG-i-like receptor-dependent interferon production. J Virol 89:2944–2955. doi: 10.1128/JVI.03349-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan L, Briese T, Lipkin WI. 2010. Z proteins of New World arenaviruses bind RIG-I and interfere with type I interferon induction. J Virol 84:1785–1791. doi: 10.1128/JVI.01362-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlie K, Maisa A, Freiberg F, Groseth A, Strecker T, Garten W. 2010. Viral protein determinants of Lassa virus entry and release from polarized epithelial cells. J Virol 84:3178–3188. doi: 10.1128/JVI.02240-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Capul AA, Perez M, Burke E, Kunz S, Buchmeier MJ, de la Torre JC. 2007. Arenavirus Z-glycoprotein association requires Z myristoylation but not functional RING or late domains. J Virol 81:9451–9460. doi: 10.1128/JVI.00499-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urata S, Noda T, Kawaoka Y, Yokosawa H, Yasuda J. 2006. Cellular factors required for Lassa virus budding. J Virol 80:4191–4195. doi: 10.1128/JVI.80.8.4191-4195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez M, Craven RC, de la Torre JC. 2003. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proc Natl Acad Sci U S A 100:12978–12983. doi: 10.1073/pnas.2133782100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eichler R, Strecker T, Kolesnikova L, ter Meulen J, Weissenhorn W, Becker S, Klenk HD, Garten W, Lenz O. 2004. Characterization of the Lassa virus matrix protein Z: electron microscopic study of virus-like particles and interaction with the nucleoprotein (NP). Virus Res 100:249–255. doi: 10.1016/j.virusres.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 44.Ziegler CM, Eisenhauer P, Bruce EA, Weir ME, King BR, Klaus JP, Krementsov DN, Shirley DJ, Ballif BA, Botten J. 2016. The lymphocytic choriomeningitis virus matrix protein PPXY late domain drives the production of defective interfering particles. PLoS Pathog 12:e1005501. doi: 10.1371/journal.ppat.1005501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziegler CM, Eisenhauer P, Bruce EA, Beganovic V, King BR, Weir ME, Ballif BA, Botten J. 2016. A novel phosphoserine motif in the LCMV matrix protein Z regulates the release of infectious virus and defective interfering particles. J Gen Virol 97:2084–2089. doi: 10.1099/jgv.0.000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Danzy S, Kumar N, Ly H, Liang Y. 2012. Biological roles and functional mechanisms of arenavirus Z protein in viral replication. J Virol 86:9794–9801. doi: 10.1128/JVI.00385-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urata S, Yasuda J, de la Torre JC. 2009. The Z protein of the New World arenavirus Tacaribe virus has bona fide budding activity that does not depend on known late domain motifs. J Virol 83:12651–12655. doi: 10.1128/JVI.01012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang DW, Sherman BT, Lempicki RA. 2009. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang DW, Sherman BT, Lempicki RA. 2008. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 50.Rowe WP, Murphy FA, Bergold GH, Casals J, Hotchin J, Johnson KM, Lehmann-Grube F, Mims CA, Traub E, Webb PA. 1970. Arenoviruses: proposed name for a newly defined virus group. J Virol 5:651–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, Lieberman J, Elledge SJ. 2008. Identification of host proteins required for HIV infection through a functional genomic screen. Science 319:921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- 52.König R, Zhou Y, Elleder D, Diamond TL, Bonamy GMC, Irelan JT, Chiang C-Y, Tu BP, De Jesus PD, Lilley CE, Seidel S, Opaluch AM, Caldwell JS, Weitzman MD, Kuhen KL, Bandyopadhyay S, Ideker T, Orth AP, Miraglia LJ, Bushman FD, Young JA, Chanda SK. 2008. Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell 135:49–60. doi: 10.1016/j.cell.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou H, Xu M, Huang Q, Gates AT, Zhang XD, Castle JC, Stec E, Ferrer M, Strulovici B, Hazuda DJ, Espeseth AS. 2008. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe 4:495–504. doi: 10.1016/j.chom.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Krishnan MN, Ng A, Sukumaran B, Gilfoy FD, Uchil PD, Sultana H, Brass AL, Adametz R, Tsui M, Qian F, Montgomery RR, Lev S, Mason PW, Koski RA, Elledge SJ, Xavier RJ, Agaisse H, Fikrig E. 2008. RNA interference screen for human genes associated with West Nile virus infection. Nature 455:242–245. doi: 10.1038/nature07207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sessions OM, Barrows NJ, Souza-Neto JA, Robinson TJ, Hershey CL, Rodgers MA, Ramirez JL, Dimopoulos G, Yang PL, Pearson JL, Garcia-Blanco MA. 2009. Discovery of insect and human dengue virus host factors. Nature 458:1047–1050. doi: 10.1038/nature07967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Q, Brass AL, Ng A, Hu Z, Xavier RJ, Liang TJ, Elledge SJ. 2009. A genome-wide genetic screen for host factors required for hepatitis C virus propagation. Proc Natl Acad Sci USA 106:16410–16415. doi: 10.1073/pnas.0907439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tai AW, Benita Y, Peng LF, Kim S-S, Sakamoto N, Xavier RJ, Chung RT. 2009. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe 5:298–307. doi: 10.1016/j.chom.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brass AL, Huang IC, Benita Y, John SP, Krishnan MN, Feeley EM, Ryan BJ, Weyer JL, van der Weyden L, Fikrig E, Adams DJ, Xavier RJ, Farzan M, Elledge SJ. 2009. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell 139:1243–1254. doi: 10.1016/j.cell.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karlas A, Machuy N, Shin Y, Pleissner K-P, Artarini A, Heuer D, Becker D, Khalil H, Ogilvie LA, Hess S, Maurer AP, Muller E, Wolff T, Rudel T, Meyer TF. 2010. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature 463:818–822. doi: 10.1038/nature08760. [DOI] [PubMed] [Google Scholar]
- 60.Shapira SD, Gat-Viks I, Shum BOV, Dricot A, de Grace MM, Wu L, Gupta PB, Hao T, Silver SJ, Root DE, Hill DE, Regev A, Hacohen N. 2009. A physical and regulatory map of host-influenza interactions reveals pathways in H1N1 infection. Cell 139:1255–1267. doi: 10.1016/j.cell.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Konig R, Stertz S, Zhou Y, Inoue A, Hoffmann HH, Bhattacharyya S, Alamares JG, Tscherne DM, Ortigoza MB, Liang Y, Gao Q, Andrews SE, Bandyopadhyay S, De Jesus P, Tu BP, Pache L, Shih C, Orth A, Bonamy G, Miraglia L, Ideker T, Garcia-Sastre A, Young JAT, Palese P, Shaw ML, Chanda SK. 2010. Human host factors required for influenza virus replication. Nature 463:813–817. doi: 10.1038/nature08699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Panda D, Das A, Dinh PX, Subramaniam S, Nayak D, Barrows NJ, Pearson JL, Thompson J, Kelly DL, Ladunga I, Pattnaik AK. 2011. RNAi screening reveals requirement for host cell secretory pathway in infection by diverse families of negative-strand RNA viruses. Proc Natl Acad Sci U S A 108:19036–19041. doi: 10.1073/pnas.1113643108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Linero FN, Sepúlveda CS, Giovannoni F, Castilla V, García CC, Scolaro LA, Damonte EB. 2012. Host cell factors as antiviral targets in arenavirus infection. Viruses 4:1569. doi: 10.3390/v4091569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forgac M. 2007. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 65.Hao L, Sakurai A, Watanabe T, Sorensen E, Nidom CA, Newton MA, Ahlquist P, Kawaoka Y. 2008. Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature 454:890–893. doi: 10.1038/nature07151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Sommer C, Barrows NJ, Bradrick SS, Pearson JL, Garcia-Blanco MA. 2012. G protein-coupled receptor kinase 2 promotes flaviviridae entry and replication. PLoS Negl Trop Dis 6:e1820. doi: 10.1371/journal.pntd.0001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castilla V, Palermo ML, Coto EC. 2001. Involvement of vacuolar proton ATPase in Junin virus multiplication. Arch Virol 146:251–263. doi: 10.1007/s007050170173. [DOI] [PubMed] [Google Scholar]
- 68.Cosset F-L, Marianneau P, Verney G, Gallais F, Tordo N, Pécheur E-I, ter Meulen J, Deubel V, Bartosch B. 2009. Characterization of Lassa virus cell entry and neutralization with Lassa virus pseudoparticles. J Virol 83:3228–3237. doi: 10.1128/JVI.01711-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jonckheere AI, Smeitink JAM, Rodenburg RJT. 2012. Mitochondrial ATP synthase: architecture, function and pathology. J Inherit Metab Dis 35:211–225. doi: 10.1007/s10545-011-9382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gorai T, Goto H, Noda T, Watanabe T, Kozuka-Hata H, Oyama M, Takano R, Neumann G, Watanabe S, Kawaoka Y. 2012. F1Fo-ATPase, F-type proton-translocating ATPase, at the plasma membrane is critical for efficient influenza virus budding. Proc Natl Acad Sci USA 109:4615–4620. doi: 10.1073/pnas.1114728109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yavlovich A, Viard M, Zhou M, Veenstra TD, Wang JM, Gong W, Heldman E, Blumenthal R, Raviv Y. 2012. Ectopic ATP synthase facilitates transfer of HIV-1 from antigen-presenting cells to CD4+ target cells. Blood 120:1246–1253. doi: 10.1182/blood-2011-12-399063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joshi A, Garg H, Nagashima K, Bonifacino JS, Freed EO. 2008. GGA and Arf proteins modulate retrovirus assembly and release. Mol Cell 30:227–238. doi: 10.1016/j.molcel.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Donaldson JG, Jackson CL. 2011. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol 12:362–375. doi: 10.1038/nrm3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klaus Joseph P, Eisenhauer P, Russo J, Mason AB, Do D, King B, Taatjes D, Cornillez-Ty C, Boyson JE, Thali M, Zheng C, Liao L, Yates JR III, Zhang B, Ballif BA, Botten JW. 2013. The intracellular cargo receptor ERGIC-53 is required for the production of infectious arenavirus, coronavirus, and filovirus particles. Cell Host Microbe 14:522–534. doi: 10.1016/j.chom.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lenz O, ter Meulen J, Klenk H-D, Seidah NG, Garten W. 2001. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc Natl Acad Sci U S A 98:12701–12705. doi: 10.1073/pnas.221447598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wright KE, Spiro RC, Burns JW, Buchmeier MJ. 1990. Post-translational processing of the glycoproteins of lymphocytic choriomeningitis virus. Virology 177:175–183. doi: 10.1016/0042-6822(90)90471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alenquer M, Amorim MJ. 2015. Exosome biogenesis, regulation, and function in viral infection. Viruses 7:5066–5083. doi: 10.3390/v7092862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stenmark H. 2009. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 79.Bruce Emily A, Stuart A, McCaffrey Mary W, Digard P. 2012. Role of the Rab11 pathway in negative-strand virus assembly. Biochem Soc Trans 40:1409–1415. doi: 10.1042/BST20120166. [DOI] [PubMed] [Google Scholar]
- 80.Martinez MG, Forlenza MB, Candurra NA. 2009. Involvement of cellular proteins in Junin arenavirus entry. Biotechnol J 4:866–870. doi: 10.1002/biot.200800357. [DOI] [PubMed] [Google Scholar]
- 81.Rojek JM, Sanchez AB, Nguyen NT, de la Torre J-C, Kunz S. 2008. Different mechanisms of cell entry by human-pathogenic Old World and New World arenaviruses. J Virol 82:7677–7687. doi: 10.1128/JVI.00560-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bruce EA, Digard P, Stuart AD. 2010. The Rab11 pathway is required for influenza A virus budding and filament formation. J Virol 84:5848–5859. doi: 10.1128/JVI.00307-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Momose F, Sekimoto T, Ohkura T, Jo S, Kawaguchi A, Nagata K, Morikawa Y. 2011. Apical transport of influenza A virus ribonucleoprotein requires Rab11-positive recycling endosome. PLoS One 6:e21123. doi: 10.1371/journal.pone.0021123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eisfeld AJ, Kawakami E, Watanabe T, Neumann G, Kawaoka Y. 2011. RAB11A is essential for transport of the influenza virus genome to the plasma membrane. J Virol 85:6117–6126. doi: 10.1128/JVI.00378-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rowe RK, Suszko JW, Pekosz A. 2008. Roles for the recycling endosome, Rab8, and Rab11 in hantavirus release from epithelial cells. Virology 382:239–249. doi: 10.1016/j.virol.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Utley TJ, Ducharme NA, Varthakavi V, Shepherd BE, Santangelo PJ, Lindquist ME, Goldenring JR, Crowe JE. 2008. Respiratory syncytial virus uses a Vps4-independent budding mechanism controlled by Rab11-FIP2. Proc Natl Acad Sci U S A 105:10209–10214. doi: 10.1073/pnas.0712144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Amorim MJ, Bruce EA, Read EKC, Foeglein Á, Mahen R, Stuart AD, Digard P. 2011. A Rab11- and microtubule-dependent mechanism for cytoplasmic transport of influenza A virus viral RNA. J Virol 85:4143–4156. doi: 10.1128/JVI.02606-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.King BR, Kellner S, Eisenhauer PL, Bruce EA, Ziegler CM, Zenklusen D, Botten JW. 2017. Visualization of the lymphocytic choriomeningitis mammarenavirus (LCMV) genome reveals the early endosome as a possible site for genome replication and viral particle pre-assembly. J Gen Virol 98:2454–2460. doi: 10.1099/jgv.0.000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmidt O, Teis D. 2012. The ESCRT machinery. Curr Biol 22:R116–R120. doi: 10.1016/j.cub.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu J, Han Z, Liu Y, Liu W, Lee MS, Olson MA, Ruthel G, Freedman BD, Harty RN. 2014. A host-oriented inhibitor of Junin Argentine hemorrhagic fever virus egress. J Virol 88:4736–4743. doi: 10.1128/JVI.03757-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Votteler J, Sundquist Wesley I. 2013. Virus budding and the ESCRT pathway. Cell Host Microbe 14:232–241. doi: 10.1016/j.chom.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spurgers KB, Alefantis T, Peyser BD, Ruthel GT, Bergeron AA, Costantino JA, Enterlein S, Kota KP, Boltz RCD, Aman MJ, DelVecchio VG, Bavari S. 2010. Identification of essential filovirion-associated host factors by serial proteomic analysis and RNAi screen. Mol Cell Proteomics 9:2690–2703. doi: 10.1074/mcp.M110.003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cantin R, Méthot S, Tremblay MJ. 2005. Plunder and stowaways: incorporation of cellular proteins by enveloped viruses. J Virol 79:6577–6587. doi: 10.1128/JVI.79.11.6577-6587.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Urata S, Weyer J, Storm N, Miyazaki Y, van Vuren PJ, Paweska JT, Yasuda J. 2016. Analysis of assembly and budding of Lujo virus. J Virol 90:3257–3261. doi: 10.1128/JVI.03198-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pasqual G, Rojek JM, Masin M, Chatton J-Y, Kunz S. 2011. Old World arenaviruses enter the host cell via the multivesicular body and depend on the endosomal sorting complex required for transport. PLoS Pathog 7:e1002232. doi: 10.1371/journal.ppat.1002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.King BR, Hershkowitz D, Eisenhauer PL, Weir ME, Ziegler CM, Russo J, Bruce EA, Ballif BA, Botten J. 2017. A map of the arenavirus nucleoprotein-host protein interactome reveals that Junín virus selectively impairs the antiviral activity of double-stranded RNA-activated protein kinase (PKR). J Virol 91:e00763-. doi: 10.1128/JVI.00763-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin Y, Kimpler LA, Naismith TV, Lauer JM, Hanson PI. 2005. Interaction of the mammalian endosomal sorting complex required for transport (ESCRT) III protein hSnf7-1 with itself, membranes, and the AAA+ ATPase SKD1. J Biol Chem 280:12799–12809. doi: 10.1074/jbc.M413968200. [DOI] [PubMed] [Google Scholar]
- 98.Dalal S, Rosser MFN, Cyr DM, Hanson PI. 2004. Distinct roles for the AAA ATPases NSF and p97 in the secretory pathway. Mol Biol Cell 15:637–648. doi: 10.1091/mbc.E03-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Taylor GM, Hanson PI, Kielian M. 2007. Ubiquitin depletion and dominant-negative VPS4 inhibit rhabdovirus budding without affecting alphavirus budding. J Virol 81:13631–13639. doi: 10.1128/JVI.01688-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Flatz L, Bergthaler A, de la Torre JC, Pinschewer DD. 2006. Recovery of an arenavirus entirely from RNA polymerase I/II-driven cDNA. Proc Natl Acad Sci U S A 103:4663–4668. doi: 10.1073/pnas.0600652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goñi SE, Iserte JA, Ambrosio AM, Romanowski V, Ghiringhelli PD, Lozano ME. 2006. Genomic features of attenuated Junín virus vaccine strain candidate. Virus Genes 32:37–41. doi: 10.1007/s11262-005-5843-2. [DOI] [PubMed] [Google Scholar]
- 102.US Department of Health and Human Services. 2009. Biosafety in microbiological and biomedical laboratories, 5th ed US Department of Health and Human Services, Washington, DC. [Google Scholar]
- 103.Cornillez-Ty CT, Liao L, Yates JR, Kuhn P, Buchmeier MJ. 2009. Severe acute respiratory syndrome coronavirus nonstructural protein 2 interacts with a host protein complex involved in mitochondrial biogenesis and intracellular signaling. J Virol 83:10314–10318. doi: 10.1128/JVI.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goñi SE, Borio CS, Romano FB, Rota RP, Pilloff MG, Iserte JA, Tortorici MA, Stephan BI, Bilen MF, Ghiringhelli PD, Lozano ME. 2010. Expression and purification of Z protein from Junín virus. J Biomed Biotechnol 2010:970491. doi: 10.1155/2010/970491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Degasperi A, Birtwistle MR, Volinsky N, Rauch J, Kolch W, Kholodenko BN. 2014. Evaluating strategies to normalise biological replicates of Western blot data. PLoS One 9:e87293. doi: 10.1371/journal.pone.0087293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lippé R. 2012. Deciphering novel host-herpesvirus interactions by proteomics. Front Microbiol 3:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chertova E, Chertov O, Coren LV, Roser JD, Trubey CM, Bess JW, Sowder RC, Barsov E, Hood BL, Fisher RJ, Nagashima K, Conrads TP, Veenstra TD, Lifson JD, Ott DE. 2006. Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J Virol 80:9039–9052. doi: 10.1128/JVI.01013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chung C-S, Chen C-H, Ho M-Y, Huang C-Y, Liao C-L, Chang W. 2006. Vaccinia virus proteome: identification of proteins in vaccinia virus intracellular mature virion particles. J Virol 80:2127–2140. doi: 10.1128/JVI.80.5.2127-2140.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coyne CB, Bozym R, Morosky SA, Hanna SL, Mukherjee A, Tudor M, Kim KS, Cherry S. 2011. Comparative RNAi screening reveals host factors involved in enterovirus infection of polarized endothelial monolayers. Cell Host Microbe 9:70–82. doi: 10.1016/j.chom.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Segura MM, Garnier A, Di Falco MR, Whissell G, Meneses-Acosta A, Arcand N, Kamen A. 2008. Identification of host proteins associated with retroviral vector particles by proteomic analysis of highly purified vector preparations. J Virol 82:1107–1117. doi: 10.1128/JVI.01909-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Deffrasnes C, Marsh GA, Foo CH, Rootes CL, Gould CM, Grusovin J, Monaghan P, Lo MK, Tompkins SM, Adams TE, Lowenthal JW, Simpson KJ, Stewart CR, Bean AGD, Wang L-F. 2016. Genome-wide siRNA screening at biosafety level 4 reveals a crucial role for fibrillarin in henipavirus infection. PLoS Pathog 12:e1005478. doi: 10.1371/journal.ppat.1005478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sivan G, Martin SE, Myers TG, Buehler E, Szymczyk KH, Ormanoglu P, Moss B. 2013. Human genome-wide RNAi screen reveals a role for nuclear pore proteins in poxvirus morphogenesis. Proc Natl Acad Sci U S A 110:3519–3524. doi: 10.1073/pnas.1300708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Resch W, Hixson KK, Moore RJ, Lipton MS, Moss B. 2007. Protein composition of the vaccinia virus mature virion. Virology 358:233–247. doi: 10.1016/j.virol.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 114.Savidis G, McDougall WM, Meraner P, Perreira JM, Portmann JM, Trincucci G, John SP, Aker AM, Renzette N, Robbins DR, Guo Z, Green S, Kowalik TF, Brass AL. 2016. Identification of Zika virus and dengue virus dependency factors using functional genomics. Cell Rep 16:232–246. doi: 10.1016/j.celrep.2016.06.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.