Abstract
Cross-sex hormone therapy (XHT) is widely used by transgender people to alter secondary sex characteristics to match their desired gender presentation. Here, we investigate the long-term effects of XHT on bone health using a murine model. Female mice underwent ovariectomy at either 6 or 10 wk and began weekly testosterone or vehicle injections. Dual-energy X-ray absorptiometry (DXA) was performed (20 wk) to measure bone mineral density (BMD), and microcomputed tomography was performed to compare femoral cortical and trabecular bone architecture. The 6-wk testosterone group had comparable BMD with controls by DXA but reduced bone volume fraction, trabecular number, and cortical area fraction and increased trabecular separation by microcomputed tomography. Ten-week ovariectomy/XHT maintained microarchitecture, suggesting that estrogen is critical for bone acquisition during adolescence and that late, but not early, estrogen loss can be sufficiently replaced by testosterone alone. Given these findings, we then compared effects of testosterone with effects of weekly estrogen or combined testosterone/low-dose estrogen treatment after a 6-wk ovariectomy. Estrogen treatment increased spine BMD and microarchitecture, including bone volume fraction, trabecular number, trabecular thickness, and connectivity density, and decreased trabecular separation. Combined testosterone-estrogen therapy caused similar increases in femur and spine BMD and improved architecture (increased bone volume fraction, trabecular number, trabecular thickness, and connectivity density) to estrogen therapy and were superior compared with mice treated with testosterone only. These results demonstrate estradiol is critical for bone acquisition and suggest a new cross-sex hormone therapy adding estrogens to testosterone treatments with potential future clinical implications for treating transgender youth or men with estrogen deficiency.
Keywords: hormone therapy, cross-sex hormone therapy, transgender, female-to-male, osteoporosis, testosterone
transgender people have gender identities that do not match the sex assigned at birth and make up an estimated 0.6% of the U.S. population (~1,400,000 people; Ref. 24). Cross-sex hormone therapy (here termed XHT) is often prescribed for transgender people to align secondary sexual characteristics with the desired gender presentation. For female-to-male transgender patients, this involves lowering endogenous sex hormone levels (often by oophorectomy) and substituting testosterone at typical endogenous cisgender (nontransgender) male levels to induce body and facial hair growth, voice deepening, and alterations in fat distribution among other changes (42). XHT can be very important for improving transgender individuals’ quality of life by improving psychological health and reducing gender dysphoria (34, 48).
Once prescribed, XHT is generally chronic and administered indefinitely. Since many physiological systems are both sex-steroid-responsive and sexually dimorphic, the biological ramifications of XHT extend beyond the reproductive tract and alter other biological processes (32). Indeed, the potential negative health effects of XHT include increased incidence of type 2 diabetes in both female-to-male (FtM) and male-to-female (MtF) transgender people (89), osteoporosis in MtF patients (52, 90), and cardiovascular disease in MtF patients (33). However, these correlations are based on limited observational studies that lack both controls and long-term data (particularly for FtM individuals). Observational studies have found higher mortality among transgender people (3), but that increased risk has not been disentangled from social factors, such as social stressors and risk of assault, which transgender individuals have been shown to experience more frequently than cisgender peers (9, 65), as well as reduced socioeconomic status and lack of access to medical care (62).
Despite the prevalence of XHT use, very little research has investigated the effects of prolonged exogenous cross-sex hormone exposure on bone health, and no preclinical models have yet been investigated. Since gonadal steroid hormones are implicated in bone health (80), osteoporosis risk is of particular concern in transgender individuals. In one study, FtM individuals who transitioned as adults were not found to be at an increased risk for osteoporosis (67). However, that investigation was of a small scope, had a relatively short-term follow-up, and did not consider the effects of transitioning earlier in life. Estrogen deficiency leads to significant bone loss in postmenopausal women (1), ovariectomized mouse models (40), and individuals with Turner syndrome (35, 63), suggesting that estrogen deficiency could affect long-term outcomes for XHT patients following oophorectomy. Indeed, mean bone mineral density (BMD) in FtM transgender people after oophorectomy and 2–12 yr of testosterone therapy was significantly lower than that of FtM transgender people who had not undergone surgery or XHT (30, 82, 83), suggesting that after oophorectomy, testosterone-based XHT does not eliminate the risk of bone loss. More importantly, osteoporosis and osteopenia result from deterioration in trabecular architecture, not just a decrease in BMD, and prior studies on XHT have not considered bone architecture. A recent study demonstrated that among FtM individuals who received gonadotropin-releasing hormone (GnRH) antagonists to delay puberty with subsequent XHT, those who began treatment before age 14 yr had lower BMD than those who began treatment at an older age (86). Prior research has shown that estrogens and androgens play distinct roles in bone metabolism before and after sexual maturity (60), suggesting research is needed to delineate the effects of undergoing oophorectomy and beginning XHT during vs. after puberty.
Investigating physiological risks of XHT is difficult due to numerous confounding factors in clinical studies. For example, bone density is lower in MtF patients than in cisgender males before beginning XHT, impeding efforts to isolate the possible effects of XHT on osteoporosis risk (81). However, although some have hypothesized that transgender people have atypical hormone levels before undergoing XHT, there is not yet replicable evidence supporting that theory (31, 68). Some neurological studies have suggested distinct responses to hormones compared with cisgender people of the same natal sex (51, 84), yet those studies have had highly variant findings and have not been widely reproduced (74). Regardless, larger physiological implications of those potential differences have not been shown in the currently available literature. Therefore, although an imperfect endocrinological model for humans, rodent models such as mice provide a preclinical opportunity to understand the mechanisms underlying the health effects of XHT. Specifically, a mouse model for the effects of masculinization on female mice provides a model for evaluating the long-term bone outcomes of XHT. Being able to do so using a randomized controlled trial design without confounding socioeconomic factors is a definitive strength of this model, since such research is not possible in transgender humans.
The purpose of this study is to understand the mechanisms underlying preservation of bone in transgender individuals undergoing gender-affirming hormonal treatment. Here, we focus on the implications of XHT for FtM humans by considering the long-term effects of testosterone-based XHT after ovariectomy (OVX) in female mice, comparing the effects of transitioning before vs. after puberty on bone development and maintenance. We also explore the potential benefits of adding low-dose estrogen to testosterone therapy for improving bone density and architecture, mimicking the skeletal effects of estrogen replacement therapy.
MATERIALS AND METHODS
Animals.
This study was prepared according to ARRIVE guidelines (50). All animal experiments were conducted using randomly assigned wild-type female C57BL/6 mice (n = 31) obtained from The Jackson Laboratory (Bar Harbor, ME) in accordance with an approved Yale University Animal Care Committee protocol. Each animal was considered an experimental unit. Food consisted of normal chow, and consumption was ad libitum for all animals. In one set of experiments, four groups of mice (n = 5 in each group) underwent OVX at either 6 wk (early) or 10 wk (late) and at the time of the surgery began weekly subcutaneous injections of testosterone (groups T6 and T10) or sesame oil vehicle control (C6 and C10) continuing through 20 wk of age. The late groups received weekly injections of sesame oil for weeks 6–9 until beginning XHT injections at the time of OVX. The early groups model an adolescent transition, whereas the late groups model transitioning during early adulthood. In another experiment, conducted in response to those findings, one group of mice (n = 5) received estrogen replacement injections of estradiol benzoate after 6-wk OVX and another group (n = 6) had OVX at 6 wk and began weekly injections of testosterone and estradiol benzoate (T+E 6). Testosterone and estradiol benzoate were obtained from Sigma-Aldrich (St. Louis, MO).
Bilateral ovariectomy was performed ventrally. All surgeries were conducted in the morning using isoflurane anesthetic and meloxicam analgesic in the laboratory. All injections were 100 µl and delivered without analgesic or anesthetic in the laboratory in sesame oil subcutaneously on a weekly schedule during mornings. This route, vehicle, and schedule of administration mimicked human XHT. Testosterone groups received the same compound (testosterone) and amount as used for human XHT adjusted to average adult C57BL/6 body weight (31 µg/wk; Refs. 5, 14, 17, 19, 95). Estrogen add-back controls received a previously characterized dose used as a model for postmenopausal hormone replacement therapy in female mice after OVX adjusted for a weekly treatment schedule and scaled to average adult C57BL/6 body weight, which was 6.4 μg/wk estradiol benzoate per mouse (53). The T+E 6 group received testosterone at the previously described dose and estradiol benzoate at a dose matching estrogen levels in cisgender males, adjusted to average adult C57BL/6 body weight (0.8 µg/wk). Dual-energy X-ray absorptiometry (DXA; GE Medical Systems, Lunar Division, Madison, WI) scans were performed in a blinded manner on all 31 mice at 20 wk of age to measure bone mineral density and content at the spine, femur, and total body as well as fat mass, lean mass, and total mass. Percent body fat was calculated by dividing fat mass by total mass and multiplying by 100. Mice were killed at 21 wk of age, plasma was collected by cardiac puncture, and femurs were dissected and frozen at −20°C until analysis.
Plasma analysis.
Estradiol and testosterone levels were determined using standard commercial ELISA kits according to the manufacturer's protocol [Calbiotech, Spring Valley, CA, and Immuno-Biological Laboratories (IBL America), Minneapolis, MN, respectively].
Microcomputed tomography.
To assess trabecular and cortical bone morphology, mouse femurs were placed in a 19-mm-diameter specimen holder and scanned using microcomputed tomography (microCT; μCT 100; SCANCO Medical, Bassersdorf, Switzerland). Scan parameters were 70 kVp, 114 μA, 0.5-mm aluminum filter, and 500-ms integration time. The metaphysis of the distal femur and the midshaft femur were scanned using an 18-µm isotropic voxel size. Analyses were performed using SCANCO evaluation software with fixed global thresholds of 18% (180 on a grayscale of 0–1,000) for trabecular bone and 30% (300 on a grayscale of 0–1,000) for cortical bone to segment bone from nonbone.
For trabecular bone, we assessed the secondary spongiosa in a 1.08-mm region of interest beginning 180 µm proximal to the distal femur growth plate. Outcomes were bone volume fraction, trabecular thickness, trabecular separation, trabecular number, and connectivity density.
For cortical bone, we obtained transverse computed tomography slices in a 540-µm region of interest at the femoral midshaft to obtain total cross-sectional area, cortical bone area, and medullary area, cortical thickness, and bone area fraction. All methods followed American Society for Bone and Mineral Research guidelines (6). Authors performing the microCT analysis were blinded to treatment group.
Statistical analysis.
All statistical analyses were performed on GraphPad Prism for Mac. Unpaired one-tailed t-tests were used to compare plasma testosterone in T6 mice with C6 mice and T10 mice with C10 mice, chosen based on clear directionality of hypothesis, two-tailed t-tests were used for equivalent comparisons for estradiol levels, and unpaired one-tailed t-tests were used to compare E6 and T+E 6 groups with T6 mice for testosterone and estradiol levels. Unpaired two-tailed t-tests were used to compare the T6 group with the C6 group and to compare the T10 group with the C10 for both DXA and microCT data to consider the outcome of treatment after OVX compared with OVX without subsequent hormone injections. We restricted our analysis to our primary outcome, considering how testosterone treatment after OVX compared with the outcomes of controls. One-way ANOVA with a Šidák correction for multiple comparisons was used for comparing the T+E 6 and E6 groups with the T6 group. The primary outcome for the first DXA experiment was femur BMD to allow consistent comparison with our microCT experiments analyzing femur architecture, and other outcomes were secondary. The primary outcome for the first microCT experiment was bone volume fraction because of the connection between osteoporosis and trabecular architecture (12, 15, 58), and other outcomes were secondary. Similarly, the primary outcome for the second DXA experiment was femur BMD, and other outcomes were secondary, and the primary outcome for the second microCT experiment was bone volume fraction, and other outcomes were secondary. No mice were excluded from analysis.
RESULTS
Hormone levels, body composition, and bone mineral density.
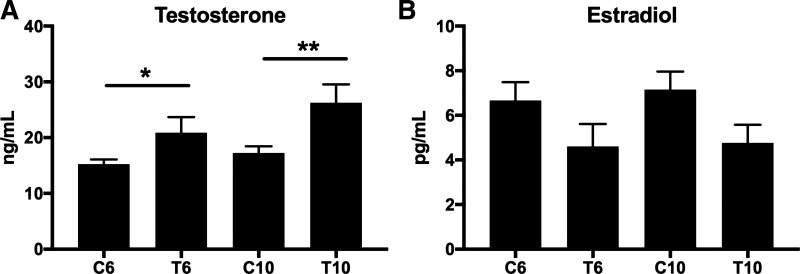
Testosterone-treated mice had increased testosterone levels compared with OVX controls (C6 vs. T6: +35%, P = 0.05; C10 vs. T10: +50%, P = 0.02; Fig. 1A). Estradiol levels were not significantly different (Fig. 1B). Mice were not different in size, and there were no significant differences in total body mass, percent fat, total lean tissue mass, or total fat mass (Fig. 2, A–D) between ovariectomized testosterone-treated mice and controls in either the early (T6 vs. C6) or late (T10 vs. C10) groups.
Fig. 1.
Early and late XHT testosterone and estradiol levels. Testosterone (A) and estradiol (B) levels are shown comparing early (6 wk: C6 vs. T6) and late (10 wk: C10 vs. T10) transition groups and controls. All groups were n = 5. Significance: *P = 0.05, **P = 0.02.
Fig. 2.
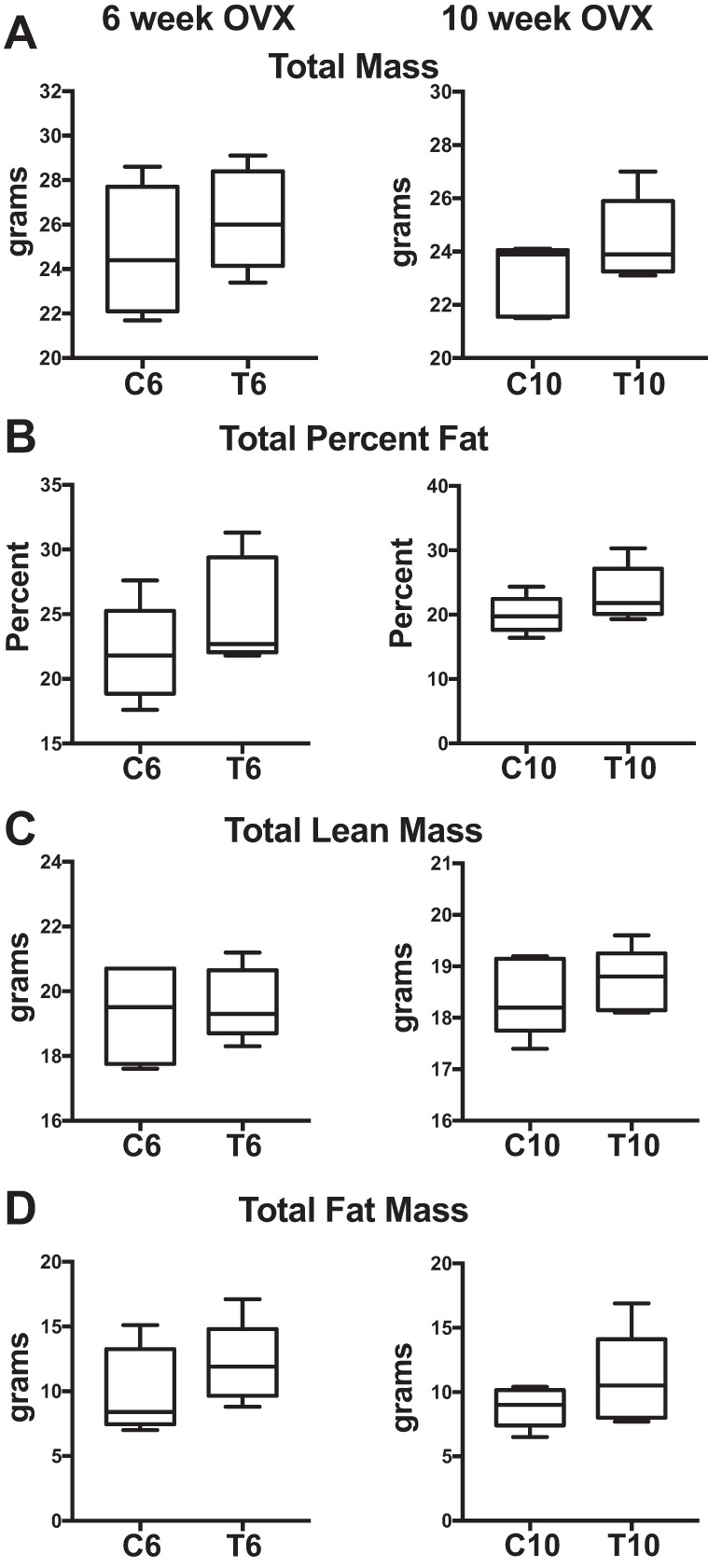
Early and late XHT DXA body composition. General body composition obtained by DXA is shown comparing early (6 wk: C6 vs. T6) and late (10 wk: C10 vs. T10) transition groups and controls. All groups were n = 5. A: total mass. B: total percent fat. C: total lean mass. D: total fat mass. No comparisons are significant.
According to DXA, there were no differences between the early-transition groups (T6 vs. C6) in BMD or bone mineral content (BMC) of the total body, femur, or spine (Figs. 3, A–F). The late-transition (T10) group had greater total BMD compared with the respective control (C10) group (+7%; P = 0.03; Fig. 3E), but no other parameters differed (Fig. 3, A–D and F).
Fig. 3.
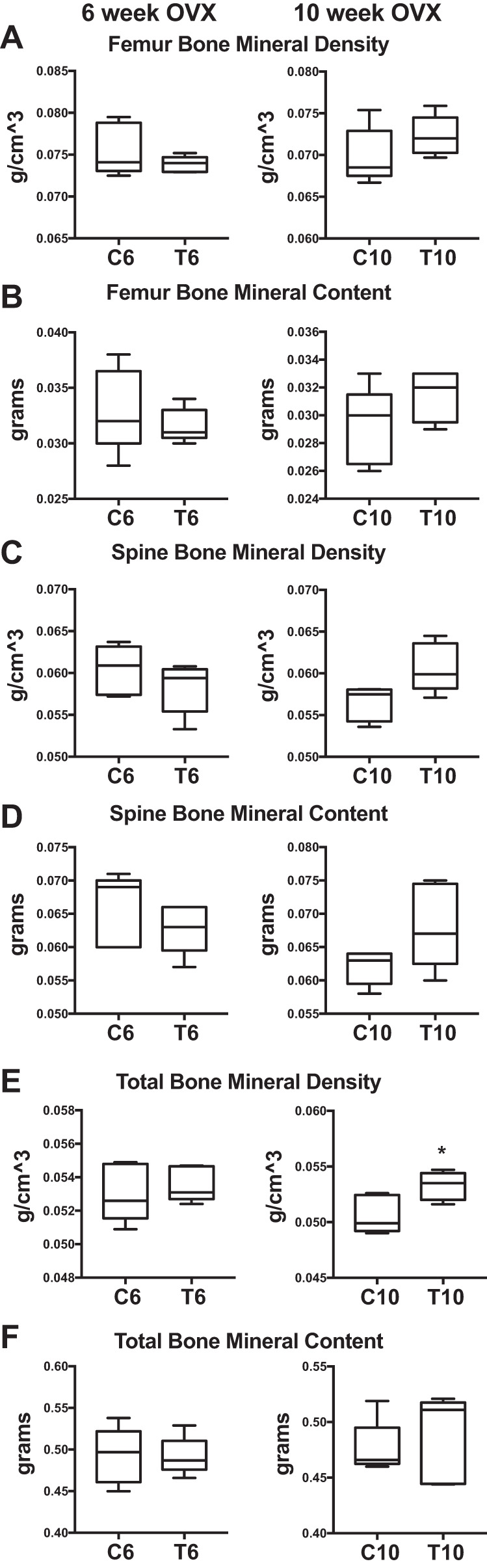
Early and late XHT DXA bone. DXA results are shown comparing early (6 wk: C6 vs. T6) and late (10 wk: C10 vs. T10) OVX transition groups and controls. All groups were n = 5. A: femur bone mineral density. B: femur bone mineral content. C: spine bone mineral density. D: spine bone mineral content. E: total bone mineral density. F: total bone mineral content. Significance: *P = 0.03.
Microcomputed tomography.
By microCT, both trabecular and cortical bone properties were markedly lower in T6 mice compared with C6. In trabecular bone, the T6 group exhibited dramatically decreased bone volume fraction (−35%; P = 0.05; Fig. 4A) as well as decreased trabecular number (−11%; P = 0.05; Fig. 4B) and increased trabecular separation (+13%; P = 0.04; Fig. 4D). These changes indicate substantial trabecular bone loss after OVX and testosterone treatment compared with OVX alone. The late-transition group did not exhibit this significant bone loss, signifying that undergoing early OVX followed by testosterone-based XHT results in a negative effect on trabecular architecture, whereas later OVX followed by XHT does not.
Fig. 4.
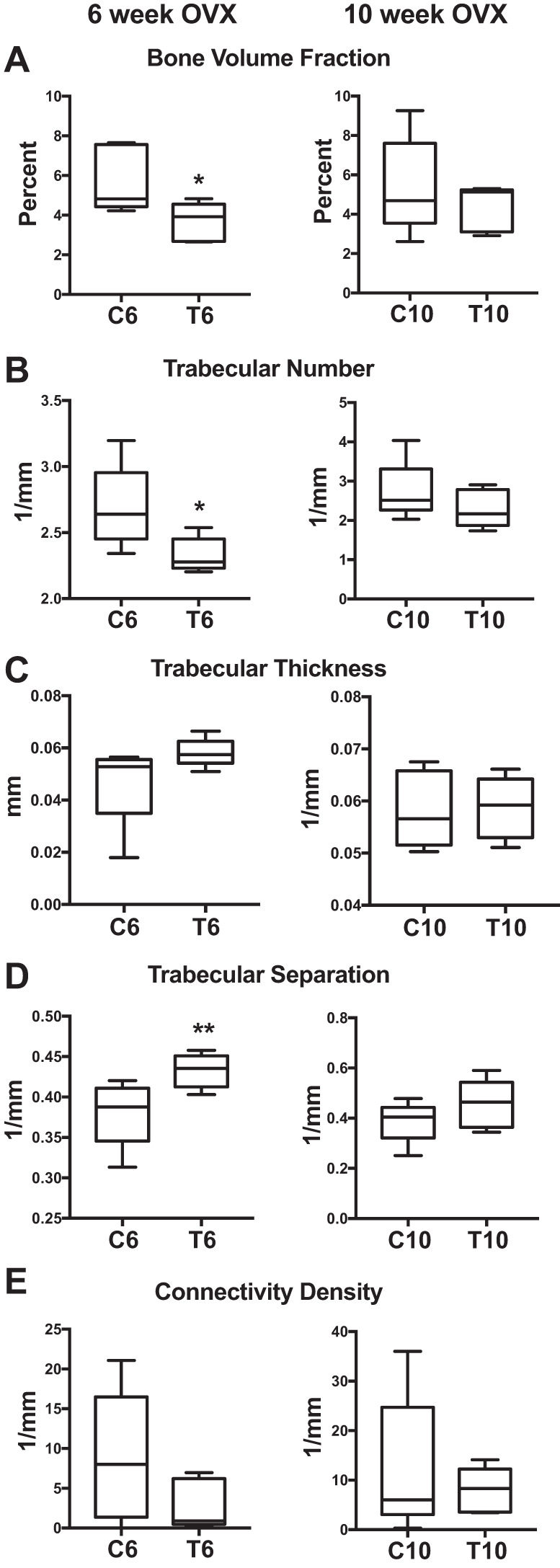
Early and late microCT trabecular. MicroCT results are shown comparing early (6 wk: C6 vs. T6) and late (10 wk: C10 vs. T10) transition groups and controls. All groups were n = 5. A: bone volume fraction. B: trabecular number. C: trabecular thickness. D: trabecular separation. E: connectivity density. Significance: *P = 0.05, **P = 0.04.
Cortical bone data also suggest that early testosterone treatments are insufficient to preserve bone architecture following OVX. Bone area increased (+11%; P = 0.01; Fig. 5B) in T6 mice compared with C6. However, cortical area fraction decreased (−5%; P = 0.01; Fig. 5C) and medullary area increased (+7%; P = 0.03; Fig. 5E) in T6 vs. C6. For the late-transition group, total area increased (+9%; P = 0.01; Fig. 5A), but medullary area also increased (+10%; P = 0.02; Fig. 5E). These data demonstrate that mice treated with testosterone after OVX exhibited decreased cortical properties compared with OVX alone, and although testosterone treatment after OVX impaired bone architecture at both ages, the mice that had late OVX (and longer endogenous estrogen exposure) fared better than the early-transition group.
Fig. 5.
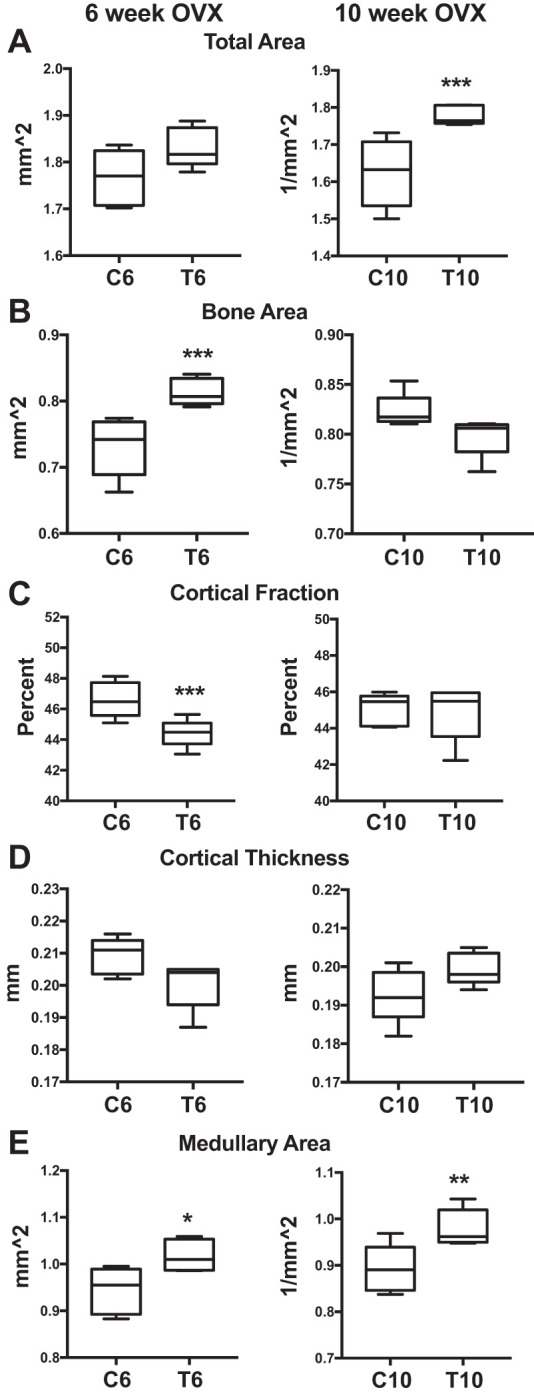
Early and late microCT cortical. MicroCT results are shown comparing early (6 wk: C6 vs. T6) and late (10 wk: C10 vs. T10) transition groups and controls. All groups were n = 5. A: total area. B: bone area. C: cortical area fraction. D: cortical thickness. E: medullary area. Significance: *P = 0.03, **P = 0.02, ***P = 0.01.
Testosterone and estrogen combined treatment.
Having demonstrated that mice treated with testosterone alone fared worse than controls for bone architecture development and maintenance, we wanted to explore a possible, novel alternative treatment to see whether adding a low dose of estrogen to cross-sex testosterone therapy improved long-term outcomes. Since treating mice with testosterone alone following OVX was insufficient to preserve bone architecture and estrogen is suggested to promote bone formation (80), we hypothesized that supplementing testosterone XHT with a low dose of estrogen at the level found endogenously in cisgender men would improve long-term bone development. We investigated this in our early-transition model (6-wk OVX and subsequent weekly hormone injections) in which OVX followed by testosterone led to the greatest bone loss. As a reference, we also tested the effects of estrogen alone, which has previously been shown to promote bone acquisition (80).
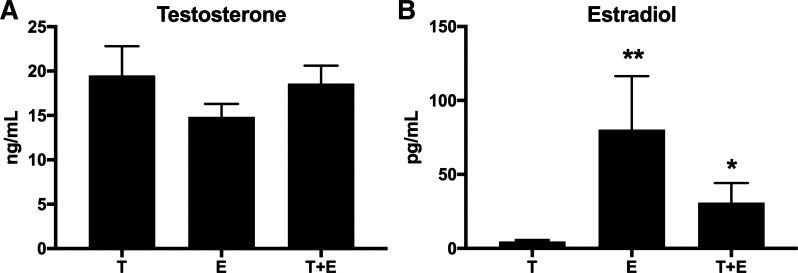
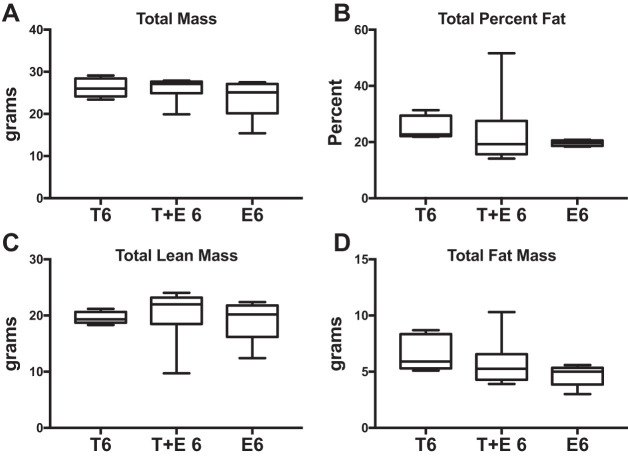
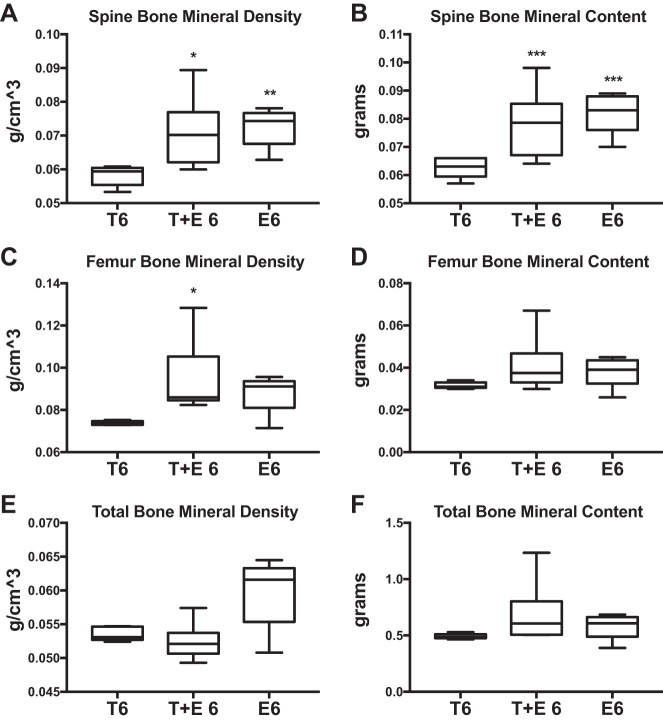
Testosterone levels did not differ in combined therapy-treated mice or estradiol-treated mice compared with those treated with testosterone alone after 6-wk OVX (Fig. 6A), but estradiol levels were increased (T6 vs. T+E 6: 500%, P = 0.05; T6 vs. E6: +1,500%, P = 0.04; Fig. 6B) Mice treated with testosterone and estrogen (T+E 6) did not differ in size and did not exhibit any significant differences in total body mass, percent fat, total lean tissue mass, or total fat mass compared with the T6 treatment group (Fig. 7, A–D). However, we found significantly greater femur BMD (+28%; P = 0.03; Fig. 8C) as well as increased spine BMD (+22%; P = 0.03; Fig. 8A) and spine BMC (+24%; P = 0.01; Fig. 8B) for T+E 6 mice compared with T6 mice. Femur BMC, total BMD, and total BMC were not significantly different in T+E 6 mice compared with the T6 group (Fig. 8, D–F). These results demonstrated that adding low-dose estrogen to testosterone XHT following OVX during puberty increased bone accrual without altering other aspects of body composition.
Fig. 6.
Combined testosterone + estrogen XHT testosterone and estradiol levels. Testosterone (A) and estradiol (B) levels are shown comparing the effects of combined testosterone and estradiol treatment after 6-wk OVX (T+E 6) with testosterone alone after 6-wk OVX (T6). T6 and E6 groups were n = 5, and T+E 6 was n = 6. Significance compared with T6 group: *P = 0.05, **P = 0.04.
Fig. 7.
Combined testosterone + estrogen XHT DXA body composition. DXA results are shown comparing the effects of combined testosterone and estrogen treatment after 6-wk OVX (T+E 6) with testosterone alone after 6-wk OVX (T6). T6 and E6 groups were n = 5, and T+E 6 was n = 6. A: total mass. B: total percent fat. C: total lean mass. D: total fat mass. Estrogen-only treatment after 6-wk OVX (E6) is provided as a reference to compare the effects of testosterone after OVX with standard care. There are no significant differences.
Fig. 8.
Combined testosterone + estrogen XHT DXA bone. DXA results are shown comparing the effects of combined testosterone and estrogen treatment after 6-wk OVX (T+E 6) with testosterone alone after 6-wk OVX (T6). T6 and E6 groups were n = 5, and T+E 6 was n = 6. A: spine bone mineral density. B: spine bone mineral content. C: femur bone mineral density. D: femur bone mineral content. E: total bone mineral density. F: total bone mineral content. Estrogen-only treatment after 6-wk OVX (E6) is provided as a reference to compare the effects of testosterone after OVX with standard care. Significance compared with T6 group: *P = 0.03, **P = 0.02, ***P = 0.01.
Mice treated with estrogen alone (E6) had significantly greater spine BMD (+25%; P = 0.02; Fig. 8A) and BMC (+24%; P = 0.03; Fig. 8B) vs. T6. The E6 treatment group did not differ from T6 mice for percent fat, total body mass, total lean tissue mass, or total fat mass (Fig. 7, A–D). Thus, in adolescent mice, estrogen therapy promoted a higher BMD than testosterone alone, and combined testosterone and low-dose estrogen treatment mimicked this effect.
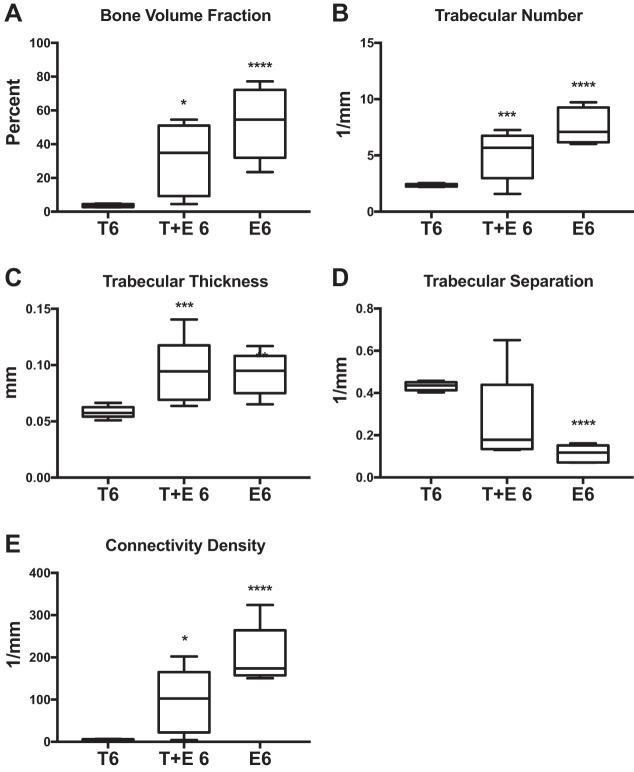
Trabecular properties were also improved in T+E 6 mice compared with T6 mice, including higher bone volume fraction (+750%; P = 0.05; Fig. 9A), trabecular number (+120%; P = 0.03; Fig. 9B), trabecular thickness (+62%; P = 0.03; Fig. 9C), and connectivity density (+3,300%; P = 0.05; Fig. 9E). Cortical properties did not differ in T+E 6 vs. T6 mice (Fig. 10). These changes demonstrate that adding low-dose estrogen to testosterone treatment greatly improves trabecular microarchitecture after OVX compared with testosterone treatment alone in adolescent mice.
Fig. 9.
Combined testosterone + estrogen XHT microCT trabecular. MicroCT results are shown comparing the effects of combined testosterone and estrogen treatment after 6-wk OVX (T+E 6) with testosterone alone after 6-wk OVX (T6). T6 and E6 groups were n = 5, and T+E 6 was n = 6. A: bone volume fraction. B: trabecular number. C: trabecular thickness. D: trabecular separation. E: connectivity density. Estrogen-only treatment after 6-wk OVX (E6) is provided as a reference to compare the effects of testosterone after OVX with standard care. Significance compared with the T6 group: *P = 0.05, **P = 0.04, ***P = 0.03, ****P = 0.01.
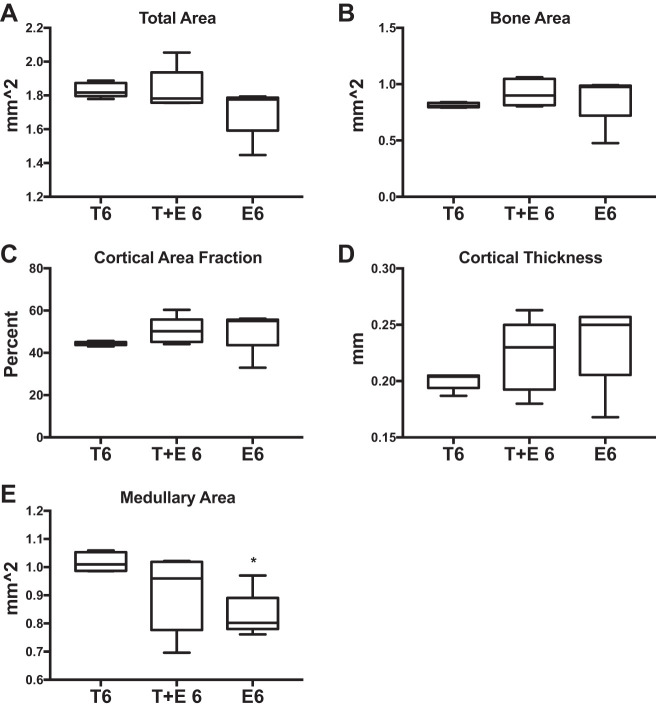
Fig. 10.
Combined testosterone + estrogen XHT microCT cortical. MicroCT results are shown comparing the effects of combined testosterone and estrogen treatment after 6-wk OVX (T+E 6) with testosterone alone after 6-wk OVX (T6). T6 and E6 groups were n = 5, and T+E 6 was n = 6. A: total area. B: bone area. C: cortical area fraction. D: cortical thickness. E: medullary area. Estrogen-only treatment after 6-wk OVX (E6) is provided as a reference to compare the effects of testosterone after OVX with standard care. Significance compared with the T6 group: *P = 0.01.
The effects of combined testosterone and estrogen therapy were very similar to effects of estrogen alone. The E6 group also exhibited improved trabecular bone properties compared with T6 mice, including increased bone volume fraction (+800%; P = 0.01; Fig. 9A), trabecular number (+180%; P = 0.01; Fig. 9B), trabecular thickness (+100%; P = 0.04; Fig. 9C), and connectivity density (+2,100%; P = 0.01; Fig. 9E) and decreased trabecular separation (−70%; P = 0.01; Fig. 9D). There were no significant differences between E6 and T6 in cortical bone with the exception of a decrease in medullary area (−19%; P = 0.01; Fig. 10E). These improvements in bone microarchitecture signify that the benefits of adding low-dose estrogen to testosterone XHT after OVX in adolescent mice are similar to the established best standard of care for bone preservation after OVX.
DISCUSSION
In this study, we assessed the skeletal effects of testosterone treatment following ovariectomy in mice as a model for cross-sex testosterone therapy following oophorectomy during female-to-male gender-affirmative treatment in humans. We demonstrated that OVX and cross-sex testosterone therapy led to decreased trabecular bone architecture in mice if performed during puberty but not if done well after puberty. These findings suggest that testosterone cannot substitute for estrogens in early-life bone acquisition. Adding a low dose of estrogen to testosterone XHT increased skeletal acquisition in mice transitioning early, similar to the effects of estrogen replacement alone, eliminating the adverse skeletal consequences without significantly affecting body composition.
Comparison with prior studies of gonadectomy and hormone replacement in rodent models.
Consistent with our findings, many prior studies have demonstrated bone loss in rodents following ovariectomy due to loss of endogenous estrogen (4, 7, 8, 46, 55, 79, 92–94) and improved trabecular and cortical bone properties with estrogen treatment after ovariectomy (76, 97). Surprisingly, however, although estrogen is readily aromatized from testosterone, we found that testosterone alone was insufficient to preserve bone mass in female mice following ovariectomy. These results are consistent with prior reports in men suggesting that serum estrogen levels are much better predictors of BMD than testosterone levels (49, 72). Aromatization is variable and sexually dimorphic, with men aromatizing twice as much testosterone to estradiol as women (0.4 vs. 0.2%, respectively; Ref. 57). Perhaps cisgender men have sufficient aromatization to promote healthy bone, whereas our findings suggest that females treated with cross-sex hormone therapy after gonad removal do not. Additionally, bone development in response to testosterone and estrogen after gonadectomy is sexually dimorphic in rodent models (64), as male skeletal acquisition requires and is stimulated by androgens (85, 88), whereas testosterone does not elicit similar skeletal acquisition in females, even for those masculinized in early life (88), nor comparable protections against bone loss after gonadectomy (96). Second, estrogen and testosterone have different mechanisms of action in bone. Both testosterone and estrogen directly inhibit osteoclast formation and bone resorption, preventing bone loss, whereas estrogen additionally induces osteoclast apoptosis, further suppresses bone loss, and stimulates osteoblasts to promote bone accrual (41, 45, 47, 61, 69, 80). Therefore, particularly in females, testosterone treatment might be sufficient for protecting bone that has already formed but insufficient to promote bone accrual in younger mice, which would explain the age-dependent response to testosterone XHT after OVX. Estrogen receptor has specifically been shown to be necessary for trabecular bone formation in both male and female mice and is unable to be replaced sufficiently by androgen receptor (70, 91). Those results, in conjunction with the potential for testosterone treatment to inhibit estrogen production, could explain our finding that testosterone treatment was insufficient to preserve trabecular architecture in female mice. Indeed, combined dihydrotestosterone-estrogen treatments improve bone microarchitecture in ovariectomized female rats, consistent with our findings (16, 78).
Comparison with prior studies of hormone replacement therapy in humans.
In humans, estrogen is essential for normal skeletal development in both men and women, including the adolescent growth spurt, epiphyseal fusion, and peak bone mass acquisition (18, 36, 73). In a man with aromatase mutations, estrogen therapy improves skeletal growth and bone maturation, but testosterone therapy does not (13, 66). Additionally, combined testosterone-estrogen treatments have been effective for promoting bone density (10) and bone metabolism markers (25) in postmenopausal women, supporting the potential clinical use of a low dose of estrogen along with testosterone XHT for FtM transgender patients to improve bone outcomes.
Our data suggest that estrogen is of particular importance during adolescence, consistent with prior clinical research in individuals with Turner syndrome, in whom estrogen therapy in adolescence produced improved outcomes compared with later treatment (75). Clinical studies have shown that FtM individuals on testosterone therapy do not lose bone while the ovaries remain intact (39), which suggests that estrogen can maintain bone during XHT. These prior studies support our conclusions that estrogen promotes and protects bone development in a way that testosterone alone cannot in natal females. FtM individuals who received aromatase inhibitors after oophorectomy had significantly reduced serum estradiol levels, which could further exacerbate these effects (11). Since long-term FtM XHT generally involves undergoing oophorectomy and/or aromatase inhibitors, supplementing cross-sex testosterone treatments with estrogen, as we explored here, could be beneficial for such patients. Peak bone mass is attained around age 25 yr (87), implying that FtM transgender individuals beginning testosterone XHT before age 25 yr, as currently prescribed, might achieve a reduced peak bone mass and consequently be at a greater risk for osteoporosis compared with those who transition later in life.
Finally, although it is well-known that postmenopausal estrogen replacement protects women from bone loss (21–23, 26, 29, 44, 71), prior work has also demonstrated bone benefits of testosterone therapy for postmenopausal women (20). However, these studies focused solely on BMD and BMC, not trabecular or cortical architecture. In keeping with these studies, we also observed improved BMD in the postpubertal period transition group compared with controls and did not find differences in BMD in the adolescent transition group compared with controls. Furthermore, the reductions we observed in trabecular architecture were only in the pubertal period transition group, and prior studies have not investigated the implications of testosterone treatment after oophorectomy for bone health in female adolescents. Whereas previous rodent work has similarly not found decreased bone quality after postpuberty OVX and testosterone (78), the reduction in bone quality we observed in pubertal period transition mice is also consistent with the bone loss seen clinically in FtM individuals after XHT (30, 82, 83). The FtM patients in those clinical studies were younger than those of the postmenopausal hormone therapy trials, which could reflect a distinct age-dependent response similar to what we observed for mice that received XHT after OVX.
It is also essential to note that, in our study, the impaired bone architecture with testosterone therapy after ovariectomy was not detectable with gross measurements. This suggests that future research on bone quality in transgender individuals after gonadectomy and XHT should separately consider different metrics of trabecular and cortical bone quality in addition to regional BMD. For example, using DXA with subdivided regions or high-resolution imaging for specific visualization of trabecular bone architecture, as proposed for monitoring osteoporosis (56), could be more effective for detecting clinically relevant changes in trabecular architecture. Future studies are needed to determine the most effective clinical methods for monitoring bone in transgender patients.
Limitations and future directions.
Although this work is an important first step, animal models are not perfect corollaries for human patients, and consequently there are limitations to our data. Mice lack sex hormone-binding globulin (2) and consequently metabolize hormones differently from how humans do, which could confound our results. We chose to follow the established paradigm for rodents of weekly subcutaneous testosterone and estradiol benzoate injections to model human hormone therapy (including as a replacement of endogenous hormones for males; Refs. 27, 28, 37, 38, 43, 54, 59, 77). This approach mimics human XHT in treatment route and schedule as closely as possible to account for the potential metabolic influence of fluctuations due to weekly testosterone dosing. It is also possible that the testosterone dose used in this study was too low, such that our findings could be due to a dose effect, but previous work has shown that much higher doses of testosterone are similarly incapable of matching the bone benefits of estrogen or a combined testosterone and estrogen therapy (16), consistent with our data. Similarly, although mice have low endogenous estrogen levels, the dose used here is based on published postmenopausal estrogen replacement paradigms in female mice (53). This study was additionally limited in sample size. It is possible that, with a larger study, possible trends to increased bone density and decreased bone quality in the late-transition group would become more distinct. Further work is needed to validate these doses and our treatment model. Finally, although testosterone can be converted to estradiol by aromatase within bone tissue, both our findings and prior research (82, 83) suggest that this aromatization is insufficient to sustain estrogen levels.
Conclusions.
With this study, we investigated potential adverse impacts on bone health of current transgender testosterone hormone therapy, and our findings suggest a potentially improved treatment model for long-term bone health of FtM transgender youth and for cisgender males with estrogen deficiency. Our findings also reinforce the importance of measuring both architecture and density to track bone development, since whole body DXA scans were not able to recognize the adverse effects on bone architecture that were apparent using microCT scans. These results are of additional importance because this project represents the first preclinical model of research on transgender XHT utilizing a randomized and controlled experimental design. If confirmed in humans, these findings would have profound implications for offering transgender youth gender-affirming treatment while preserving bone health.
GRANTS
This study was funded by National Institute of Child Health and Human Development Grant R01-HD-076422 and by the Bruce L. Cohen Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M. and H.S.T. conceived and designed research; L.G.G., R.M., M.J.D., A.E.R., and M.M.-Z. performed experiments; L.G.G., R.M., and H.S.T. analyzed data; R.M. and H.S.T. interpreted results of experiments; L.G.G. prepared figures; L.G.G. drafted manuscript; R.M. and H.S.T. edited and revised manuscript; R.M. and H.S.T. approved final version of manuscript.
REFERENCES
- 1.Almeida M, Laurent MR, Dubois V, Claessens F, O’Brien CA, Bouillon R, Vanderschueren D, Manolagas SC. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev 97: 135–187, 2017. doi: 10.1152/physrev.00033.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson DC. Sex-hormone-binding globulin. Clin Endocrinol (Oxf) 3: 69–96, 1974. doi: 10.1111/j.1365-2265.1974.tb03298.x. [DOI] [PubMed] [Google Scholar]
- 3.Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol 164: 635–642, 2011. doi: 10.1530/EJE-10-1038. [DOI] [PubMed] [Google Scholar]
- 4.Bain SD, Bailey MC, Celino DL, Lantry MM, Edwards MW. High-dose estrogen inhibits bone resorption and stimulates bone formation in the ovariectomized mouse. J Bone Miner Res 8: 435–442, 1993. doi: 10.1002/jbmr.5650080407. [DOI] [PubMed] [Google Scholar]
- 5.Benten WP, Ulrich P, Kühn-Velten WN, Vohr HW, Wunderlich F. Testosterone-induced susceptibility to Plasmodium chabaudi malaria: persistence after withdrawal of testosterone. J Endocrinol 153: 275–281, 1997. doi: 10.1677/joe.0.1530275. [DOI] [PubMed] [Google Scholar]
- 6.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25: 1468–1486, 2010. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 7.Bouxsein ML, Devlin MJ, Glatt V, Dhillon H, Pierroz DD, Ferrari SL. Mice lacking β-adrenergic receptors have increased bone mass but are not protected from deleterious skeletal effects of ovariectomy. Endocrinology 150: 144–152, 2009. doi: 10.1210/en.2008-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouxsein ML, Myers KS, Shultz KL, Donahue LR, Rosen CJ, Beamer WG. Ovariectomy-induced bone loss varies among inbred strains of mice. J Bone Miner Res 20: 1085–1092, 2005. doi: 10.1359/JBMR.050307. [DOI] [PubMed] [Google Scholar]
- 9.Bradford J, Reisner SL, Honnold JA, Xavier J. Experiences of transgender-related discrimination and implications for health: results from the Virginia Transgender Health Initiative Study. Am J Public Health 103: 1820–1829, 2013. doi: 10.2105/AJPH.2012.300796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Britto R, Araújo L, Barbosa I, Silva L, Rocha S, Valente AP. Hormonal therapy with estradiol and testosterone implants: bone protection? Gynecol Endocrinol 27: 96–100, 2011. doi: 10.3109/09513590.2010.489131. [DOI] [PubMed] [Google Scholar]
- 11.Bunck MC, Toorians AW, Lips P, Gooren LJ. The effects of the aromatase inhibitor anastrozole on bone metabolism and cardiovascular risk indices in ovariectomized, androgen-treated female-to-male transsexuals. Eur J Endocrinol 154: 569–575, 2006. doi: 10.1530/eje.1.02126. [DOI] [PubMed] [Google Scholar]
- 12.Burkhardt R, Kettner G, Böhm W, Schmidmeier M, Schlag R, Frisch B, Mallmann B, Eisenmenger W, Gilg T. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone 8: 157–164, 1987. doi: 10.1016/8756-3282(87)90015-9. [DOI] [PubMed] [Google Scholar]
- 13.Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 337: 91–95, 1997. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 14.Center of Excellence for Transgender Health UCSF Hormone Administration (Online). http://transhealth.ucsf.edu/trans?page=protocol-hormones. 2016.
- 15.Chappard D, Baslé MF, Legrand E, Audran M. Trabecular bone microarchitecture: a review. Morphologie 92: 162–170, 2008. doi: 10.1016/j.morpho.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Coxam V, Bowman BM, Mecham M, Roth CM, Miller MA, Miller SC. Effects of dihydrotestosterone alone and combined with estrogen on bone mineral density, bone growth, and formation rates in ovariectomized rats. Bone 19: 107–114, 1996. doi: 10.1016/8756-3282(96)00135-4. [DOI] [PubMed] [Google Scholar]
- 17.Delić D, Grosser C, Dkhil M, Al-Quraishy S, Wunderlich F. Testosterone-induced upregulation of miRNAs in the female mouse liver. Steroids 75: 998–1004, 2010. doi: 10.1016/j.steroids.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Devlin MJ. Estrogen, exercise, and the skeleton. Evol Anthropol 20: 54–61, 2011. doi: 10.1002/evan.20299. [DOI] [PubMed] [Google Scholar]
- 19.Dkhil MA, Al-Quraishy S, Abdel-Baki AA, Ghanjati F, Arauzo-Bravo MJ, Delic D, Wunderlich F. Epigenetic modifications of gene promoter DNA in the liver of adult female mice masculinized by testosterone. J Steroid Biochem Mol Biol 145: 121–130, 2015. doi: 10.1016/j.jsbmb.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Elraiyah T, Sonbol MB, Wang Z, Khairalseed T, Asi N, Undavalli C, Nabhan M, Firwana B, Altayar O, Prokop L, Montori VM, Murad MH. The benefits and harms of systemic testosterone therapy in postmenopausal women with normal adrenal function: a systematic review and meta-analysis. J Clin Endocrinol Metab 99: 3543–3550, 2014. doi: 10.1210/jc.2014-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ettinger B, Genant HK, Cann CE. Long-term estrogen replacement therapy prevents bone loss and fractures. Ann Intern Med 102: 319–324, 1985. doi: 10.7326/0003-4819-102-3-319. [DOI] [PubMed] [Google Scholar]
- 22.Ettinger B, Genant HK, Cann CE. Postmenopausal bone loss is prevented by treatment with low-dosage estrogen with calcium. Ann Intern Med 106: 40–45, 1987. doi: 10.7326/0003-4819-106-1-40. [DOI] [PubMed] [Google Scholar]
- 23.Farr JN, Drake MT, Amin S, Melton LJ 3rd, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res 29: 787–795, 2014. doi: 10.1002/jbmr.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flores AR, Herman JL, Gates GJ, Brown TN. How Many Adults Identify as Transgender in the United States. Los Angeles, CA: The Williams Institute, 2016. [Google Scholar]
- 25.Flöter A, Nathorst-Böös J, Carlström K, Ohlsson C, Ringertz H, Schoultz B. Effects of combined estrogen/testosterone therapy on bone and body composition in oophorectomized women. Gynecol Endocrinol 20: 155–160, 2005. doi: 10.1080/09513590400021193. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher JC, Kable WT, Goldgar D. Effect of progestin therapy on cortical and trabecular bone: comparison with estrogen. Am J Med 90: 171–178, 1991. doi: 10.1016/0002-9343(91)80156-G. [DOI] [PubMed] [Google Scholar]
- 27.Gardner WU. Ovarian and lymphoid tumors in female mice subsequent to roentgen-ray irradiation and hormone treatment. Proc Soc Exp Biol Med 75: 434–436, 1950. doi: 10.3181/00379727-75-18222. [DOI] [PubMed] [Google Scholar]
- 28.Gardner W, Allen E, Smith G, Strong L. Carcinoma of the cervix of mice receiving estrogens. J Am Med Assoc 110: 1182–1183, 1938. doi: 10.1001/jama.1938.62790150003010a. [DOI] [Google Scholar]
- 29.Genant HK, Baylink DJ, Gallagher JC. Estrogens in the prevention of osteoporosis in postmenopausal women. Am J Obstet Gynecol 161: 1842–1846, 1989. doi: 10.1016/S0002-9378(89)80004-3. [DOI] [PubMed] [Google Scholar]
- 30.Goh HH, Ratnam SS. Effects of hormone deficiency, androgen therapy and calcium supplementation on bone mineral density in female transsexuals. Maturitas 26: 45–52, 1997. doi: 10.1016/S0378-5122(96)01073-0. [DOI] [PubMed] [Google Scholar]
- 31.Gooren L. The endocrinology of transsexualism: a review and commentary. Psychoneuroendocrinology 15: 3–14, 1990. doi: 10.1016/0306-4530(90)90041-7. [DOI] [PubMed] [Google Scholar]
- 32.Gooren LJ, Kreukels B, Lapauw B, Giltay EJ. (Patho)physiology of cross-sex hormone administration to transsexual people: the potential impact of male-female genetic differences. Andrologia 47: 5–19, 2015. doi: 10.1111/and.12389. [DOI] [PubMed] [Google Scholar]
- 33.Gooren LJ, Wierckx K, Giltay EJ. Cardiovascular disease in transsexual persons treated with cross-sex hormones: reversal of the traditional sex difference in cardiovascular disease pattern. Eur J Endocrinol 170: 809–819, 2014. doi: 10.1530/EJE-14-0011. [DOI] [PubMed] [Google Scholar]
- 34.Gorin-Lazard A, Baumstarck K, Boyer L, Maquigneau A, Gebleux S, Penochet JC, Pringuey D, Albarel F, Morange I, Loundou A, Berbis J, Auquier P, Lançon C, Bonierbale M. Is hormonal therapy associated with better quality of life in transsexuals? A cross-sectional study. J Sex Med 9: 531–541, 2012. doi: 10.1111/j.1743-6109.2011.02564.x. [DOI] [PubMed] [Google Scholar]
- 35.Gravholt CH, Juul S, Naeraa RW, Hansen J. Morbidity in Turner syndrome. J Clin Epidemiol 51: 147–158, 1998. doi: 10.1016/S0895-4356(97)00237-0. [DOI] [PubMed] [Google Scholar]
- 36.Grumbach MM. Estrogen, bone, growth and sex: a sea change in conventional wisdom. J Pediatr Endocrinol Metab 13, Suppl 6: 1439–1455, 2000. doi: 10.1515/jpem-2000-s619. [DOI] [PubMed] [Google Scholar]
- 37.Guo W, Bachman E, Vogel J, Li M, Peng L, Pencina K, Serra C, Sandor NL, Jasuja R, Montano M, Basaria S, Gassmann M, Bhasin S. The effects of short-term and long-term testosterone supplementation on blood viscosity and erythrocyte deformability in healthy adult mice. Endocrinology 156: 1623–1629, 2015. doi: 10.1210/en.2014-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo W, Li M, Bhasin S. Testosterone supplementation improves anemia in aging male mice. J Gerontol A Biol Sci Med Sci 69: 505–513, 2014. doi: 10.1093/gerona/glt127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haraldsen IR, Haug E, Falch J, Egeland T, Opjordsmoen S. Cross-sex pattern of bone mineral density in early onset gender identity disorder. Horm Behav 52: 334–343, 2007. doi: 10.1016/j.yhbeh.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 40.He YX, Zhang G, Pan XH, Liu Z, Zheng LZ, Chan CW, Lee KM, Cao YP, Li G, Wei L, Hung LK, Leung KS, Qin L. Impaired bone healing pattern in mice with ovariectomy-induced osteoporosis: a drill-hole defect model. Bone 48: 1388–1400, 2011. doi: 10.1016/j.bone.2011.03.720. [DOI] [PubMed] [Google Scholar]
- 41.Heino TJ, Hentunen TA, Väänänen HK. Osteocytes inhibit osteoclastic bone resorption through transforming growth factor-β: enhancement by estrogen. J Cell Biochem 85: 185–197, 2002. doi: 10.1002/jcb.10109. [DOI] [PubMed] [Google Scholar]
- 42.Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, Gooren LJ, Meyer WJ 3rd, Spack NP, Tangpricha V, Montori VM; Endocrine Society . Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 94: 3132–3154, 2009. doi: 10.1210/jc.2009-0345. [DOI] [PubMed] [Google Scholar]
- 43.Hooker CW, Pfeiffer CA. The morphology and development of testicular tumors in mice of the A strain receiving estrogens. Cancer Res 2: 759–769, 1942. [Google Scholar]
- 44.Hosking D, Chilvers CE, Christiansen C, Ravn P, Wasnich R, Ross P, McClung M, Balske A, Thompson D, Daley M, Yates AJ; Early Postmenopausal Intervention Cohort Study Group . Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. N Engl J Med 338: 485–492, 1998. doi: 10.1056/NEJM199802193380801. [DOI] [PubMed] [Google Scholar]
- 45.Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, Boyce B, Broxmeyer H, Manolagas SC. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 257: 88–91, 1992. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 46.Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner 15: 175–191, 1991. doi: 10.1016/0169-6009(91)90124-I. [DOI] [PubMed] [Google Scholar]
- 47.Kameda T, Mano H, Yuasa T, Mori Y, Miyazawa K, Shiokawa M, Nakamaru Y, Hiroi E, Hiura K, Kameda A, Yang NN, Hakeda Y, Kumegawa M. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med 186: 489–495, 1997. doi: 10.1084/jem.186.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keo-Meier CL, Herman LI, Reisner SL, Pardo ST, Sharp C, Babcock JC. Testosterone treatment and MMPI-2 improvement in transgender men: a prospective controlled study. J Consult Clin Psychol 83: 143–156, 2015. doi: 10.1037/a0037599. [DOI] [PubMed] [Google Scholar]
- 49.Khosla S, Melton LJ 3rd, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 83: 2266–2274, 1998. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 50.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG; NC3Rs Reporting Guidelines Working Group . Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579, 2010. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kreukels BP, Guillamon A. Neuroimaging studies in people with gender incongruence. Int Rev Psychiatry 28: 120–128, 2016. doi: 10.3109/09540261.2015.1113163. [DOI] [PubMed] [Google Scholar]
- 52.Lapauw B, Taes Y, Simoens S, Van Caenegem E, Weyers S, Goemaere S, Toye K, Kaufman JM, T’Sjoen GG. Body composition, volumetric and areal bone parameters in male-to-female transsexual persons. Bone 43: 1016–1021, 2008. doi: 10.1016/j.bone.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Li J, McMurray RW. Effects of cyclic versus sustained estrogen administration on peripheral immune functions in ovariectomized mice. Am J Reprod Immunol 63: 274–281, 2010. doi: 10.1111/j.1600-0897.2009.00784.x. [DOI] [PubMed] [Google Scholar]
- 54.Li MH, Gardner WU. Further studies on the pathogenesis of ovarian tumors in mice. Cancer Res 9: 35–41, 1949. [PubMed] [Google Scholar]
- 55.Lindberg MK, Weihua Z, Andersson N, Movérare S, Gao H, Vidal O, Erlandsson M, Windahl S, Andersson G, Lubahn DB, Carlsten H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J Endocrinol 174: 167–178, 2002. doi: 10.1677/joe.0.1740167. [DOI] [PubMed] [Google Scholar]
- 56.Link TM, Majumdar S, Grampp S, Guglielmi G, van Kuijk C, Imhof H, Glueer C, Adams JE. Imaging of trabecular bone structure in osteoporosis. Eur Radiol 9: 1781–1788, 1999. doi: 10.1007/s003300050922. [DOI] [PubMed] [Google Scholar]
- 57.Longcope C, Kato T, Horton R. Conversion of blood androgens to estrogens in normal adult men and women. J Clin Invest 48: 2191–2201, 1969. doi: 10.1172/JCI106185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Majumdar S, Genant HK, Grampp S, Newitt DC, Truong VH, Lin JC, Mathur A. Correlation of trabecular bone structure with age, bone mineral density, and osteoporotic status: in vivo studies in the distal radius using high resolution magnetic resonance imaging. J Bone Miner Res 12: 111–118, 1997. doi: 10.1359/jbmr.1997.12.1.111. [DOI] [PubMed] [Google Scholar]
- 59.Mariotti R, Fattoretti P, Malatesta M, Nicolato E, Sandri M, Zancanaro C. Forced mild physical training improves blood volume in the motor and hippocampal cortex of old mice. J Nutr Health Aging 18: 178–183, 2014. doi: 10.1007/s12603-013-0384-1. [DOI] [PubMed] [Google Scholar]
- 60.Matsumoto C, Inada M, Toda K, Miyaura C. Estrogen and androgen play distinct roles in bone turnover in male mice before and after reaching sexual maturity. Bone 38: 220–226, 2006. doi: 10.1016/j.bone.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 61.Michael H, Härkönen PL, Väänänen HK, Hentunen TA. Estrogen and testosterone use different cellular pathways to inhibit osteoclastogenesis and bone resorption. J Bone Miner Res 20: 2224–2232, 2005. doi: 10.1359/JBMR.050803. [DOI] [PubMed] [Google Scholar]
- 62.Motmans J, Meier P, Ponnet K, T’Sjoen G. Female and male transgender quality of life: socioeconomic and medical differences. J Sex Med 9: 743–750, 2012. doi: 10.1111/j.1743-6109.2011.02569.x. [DOI] [PubMed] [Google Scholar]
- 63.Naeraa RW, Brixen K, Hansen RM, Hasling C, Mosekilde L, Andresen JH, Charles P, Nielsen J. Skeletal size and bone mineral content in Turner’s syndrome: relation to karyotype, estrogen treatment, physical fitness, and bone turnover. Calcif Tissue Int 49: 77–83, 1991. doi: 10.1007/BF02565125. [DOI] [PubMed] [Google Scholar]
- 64.Ornoy A, Giron S, Aner R, Goldstein M, Boyan BD, Schwartz Z. Gender dependent effects of testosterone and 17 β-estradiol on bone growth and modelling in young mice. Bone Miner 24: 43–58, 1994. doi: 10.1016/S0169-6009(08)80130-4. [DOI] [PubMed] [Google Scholar]
- 65.Reisner SL, White JM, Bradford JB, Mimiaga MJ. Transgender health disparities: comparing full cohort and nested matched-pair study designs in a community health center. LGBT Health 1: 177–184, 2014. doi: 10.1089/lgbt.2014.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rochira V, Zirilli L, Madeo B, Aranda C, Caffagni G, Fabre B, Montangero VE, Roldan EJ, Maffei L, Carani C. Skeletal effects of long-term estrogen and testosterone replacement treatment in a man with congenital aromatase deficiency: evidences of a priming effect of estrogen for sex steroids action on bone. Bone 40: 1662–1668, 2007. doi: 10.1016/j.bone.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 67.Ruetsche AG, Kneubuehl R, Birkhaeuser MH, Lippuner K. Cortical and trabecular bone mineral density in transsexuals after long-term cross-sex hormonal treatment: a cross-sectional study. Osteoporos Int 16: 791–798, 2005. doi: 10.1007/s00198-004-1754-7. [DOI] [PubMed] [Google Scholar]
- 68.Saraswat A, Weinand JD, Safer JD. Evidence supporting the biologic nature of gender identity. Endocr Pract 21: 199–204, 2015. doi: 10.4158/EP14351.RA. [DOI] [PubMed] [Google Scholar]
- 69.Shevde NK, Bendixen AC, Dienger KM, Pike JW. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci USA 97: 7829–7834, 2000. doi: 10.1073/pnas.130200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sims NA, Clément-Lacroix P, Minet D, Fraslon-Vanhulle C, Gaillard-Kelly M, Resche-Rigon M, Baron R. A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J Clin Invest 111: 1319–1327, 2003. doi: 10.1172/JCI200317246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 286: 2815–2822, 2001. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 72.Slemenda CW, Longcope C, Zhou L, Hui SL, Peacock M, Johnston CC. Sex steroids and bone mass in older men. Positive associations with serum estrogens and negative associations with androgens. J Clin Invest 100: 1755–1759, 1997. doi: 10.1172/JCI119701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331: 1056–1061, 1994. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 74.Smith ES, Junger J, Derntl B, Habel U. The transsexual brain–a review of findings on the neural basis of transsexualism. Neurosci Biobehav Rev 59: 251–266, 2015. doi: 10.1016/j.neubiorev.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 75.Sowińska-Przepiera E, Andrysiak-Mamos E, Friebe Z, Kapczuk K, Pilarska K. [The effect of primary lack of estrogens and the influence of the age at the beginning of estrogen therapy on bone mineral density in patients with Turner’s syndrome]. Endokrynol Pol 56: 145–153, 2005. [PubMed] [Google Scholar]
- 76.Syed FA, Mödder UI, Roforth M, Hensen I, Fraser DG, Peterson JM, Oursler MJ, Khosla S. Effects of chronic estrogen treatment on modulating age-related bone loss in female mice. J Bone Miner Res 25: 2438–2446, 2010. doi: 10.1002/jbmr.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thompson JS, Crawford MK, Reilly RW, Severson CD. The effect of estrogenic hormones on immune responses in normal and irradiated mice. J Immunol 98: 331–335, 1967. [PubMed] [Google Scholar]
- 78.Tivesten A, Movérare-Skrtic S, Chagin A, Venken K, Salmon P, Vanderschueren D, Sävendahl L, Holmäng A, Ohlsson C. Additive protective effects of estrogen and androgen treatment on trabecular bone in ovariectomized rats. J Bone Miner Res 19: 1833–1839, 2004. doi: 10.1359/JBMR.040819. [DOI] [PubMed] [Google Scholar]
- 79.Turner RT, Vandersteenhoven JJ, Bell NH. The effects of ovariectomy and 17 β-estradiol on cortical bone histomorphometry in growing rats. J Bone Miner Res 2: 115–122, 1987. doi: 10.1002/jbmr.5650020206. [DOI] [PubMed] [Google Scholar]
- 80.Väänänen HK, Härkönen PL. Estrogen and bone metabolism. Maturitas 23, Suppl: S65–S69, 1996. doi: 10.1016/0378-5122(96)01015-8. [DOI] [PubMed] [Google Scholar]
- 81.Van Caenegem E, TʼSjoen G. Bone in trans persons. Curr Opin Endocrinol Diabetes Obes 22: 459–466, 2015. doi: 10.1097/MED.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 82.Van Caenegem E, Wierckx K, Taes Y, Dedecker D, Van de Peer F, Toye K, Kaufman JM, T’Sjoen G. Bone mass, bone geometry, and body composition in female-to-male transsexual persons after long-term cross-sex hormonal therapy. J Clin Endocrinol Metab 97: 2503–2511, 2012. doi: 10.1210/jc.2012-1187. [DOI] [PubMed] [Google Scholar]
- 83.van Kesteren P, Lips P, Gooren LJ, Asscheman H, Megens J. Long-term follow-up of bone mineral density and bone metabolism in transsexuals treated with cross-sex hormones. Clin Endocrinol (Oxf) 48: 347–354, 1998. doi: 10.1046/j.1365-2265.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- 84.Veale JF, Clarke DE, Lomax TC. Biological and psychosocial correlates of adult gender-variant identities: a review. Pers Individ Dif 48: 357–366, 2010. doi: 10.1016/j.paid.2009.09.018. [DOI] [Google Scholar]
- 85.Venken K, De Gendt K, Boonen S, Ophoff J, Bouillon R, Swinnen JV, Verhoeven G, Vanderschueren D. Relative impact of androgen and estrogen receptor activation in the effects of androgens on trabecular and cortical bone in growing male mice: a study in the androgen receptor knockout mouse model. J Bone Miner Res 21: 576–585, 2006. doi: 10.1359/jbmr.060103. [DOI] [PubMed] [Google Scholar]
- 86.Vlot MC, Klink DT, den Heijer M, Blankenstein MA, Rotteveel J, Heijboer AC. Effect of pubertal suppression and cross-sex hormone therapy on bone turnover markers and bone mineral apparent density (BMAD) in transgender adolescents. Bone 95: 11–19, 2017. doi: 10.1016/j.bone.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 87.Walsh JS, Henry YM, Fatayerji D, Eastell R. Lumbar spine peak bone mass and bone turnover in men and women: a longitudinal study. Osteoporos Int 20: 355–362, 2009. doi: 10.1007/s00198-008-0672-5. [DOI] [PubMed] [Google Scholar]
- 88.Weisman Y, Cassorla F, Malozowski S, Krieg RJ Jr, Goldray D, Kaye AM, Sömjen D. Sex-specific response of bone cells to gonadal steroids: modulation in perinatally androgenized females and in testicular feminized male rats. Steroids 58: 126–133, 1993. doi: 10.1016/0039-128X(93)90049-S. [DOI] [PubMed] [Google Scholar]
- 89.Wierckx K, Elaut E, Declercq E, Heylens G, De Cuypere G, Taes Y, Kaufman JM, T’Sjoen G. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: a case-control study. Eur J Endocrinol 169: 471–478, 2013. doi: 10.1530/EJE-13-0493. [DOI] [PubMed] [Google Scholar]
- 90.Wierckx K, Mueller S, Weyers S, Van Caenegem E, Roef G, Heylens G, T’Sjoen G. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med 9: 2641–2651, 2012. doi: 10.1111/j.1743-6109.2012.02876.x. [DOI] [PubMed] [Google Scholar]
- 91.Windahl SH, Börjesson AE, Farman HH, Engdahl C, Movérare-Skrtic S, Sjögren K, Lagerquist MK, Kindblom JM, Koskela A, Tuukkanen J, Divieti Pajevic P, Feng JQ, Dahlman-Wright K, Antonson P, Gustafsson JA, Ohlsson C. Estrogen receptor-α in osteocytes is important for trabecular bone formation in male mice. Proc Natl Acad Sci USA 110: 2294–2299, 2013. doi: 10.1073/pnas.1220811110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wright LE, Christian PJ, Rivera Z, Van Alstine WG, Funk JL, Bouxsein ML, Hoyer PB. Comparison of skeletal effects of ovariectomy versus chemically induced ovarian failure in mice. J Bone Miner Res 23: 1296–1303, 2008. doi: 10.1359/jbmr.080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wronski TJ, Dann LM, Qi H, Yen CF. Skeletal effects of withdrawal of estrogen and diphosphonate treatment in ovariectomized rats. Calcif Tissue Int 53: 210–216, 1993. doi: 10.1007/BF01321840. [DOI] [PubMed] [Google Scholar]
- 94.Wronski TJ, Dann LM, Scott KS, Cintrón M. Long-term effects of ovariectomy and aging on the rat skeleton. Calcif Tissue Int 45: 360–366, 1989. doi: 10.1007/BF02556007. [DOI] [PubMed] [Google Scholar]
- 95.Wunderlich F, Mossmann H, Helwig M, Schillinger G. Resistance to Plasmodium chabaudi in B10 mice: influence of the H-2 complex and testosterone. Infect Immun 56: 2400–2406, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yarrow JF, Conover CF, Purandare AV, Bhakta AM, Zheng N, Conrad B, Altman MK, Franz SE, Wronski TJ, Borst SE. Supraphysiological testosterone enanthate administration prevents bone loss and augments bone strength in gonadectomized male and female rats. Am J Physiol Endocrinol Metab 295: E1213–E1222, 2008. doi: 10.1152/ajpendo.90640.2008. [DOI] [PubMed] [Google Scholar]
- 97.Zhao Q, Liu X, Zhang L, Shen X, Qi J, Wang J, Qian N, Deng L. Bone selective protective effect of a novel bone-seeking estrogen on trabecular bone in ovariectomized rats. Calcif Tissue Int 93: 172–183, 2013. doi: 10.1007/s00223-013-9739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]