Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) and the amiloride-sensitive epithelial sodium channels (ENaC) are located in the apical membranes of airway and alveolar epithelial cells. These transporters play an important role in the regulation of lung fluid balance across airway and alveolar epithelia by being the conduits for chloride (Cl−) and bicarbonate () secretion and sodium (Na+) ion absorption, respectively. The functional role of these channels in the respiratory tract is to maintain the optimum volume and ionic composition of the bronchial periciliary fluid (PCL) and alveolar lining fluid (ALF) layers. The PCL is required for proper mucociliary clearance of pathogens and debris, and the ALF is necessary for surfactant homeostasis and optimum gas exchange. Dysregulation of ion transport may lead to mucus accumulation, bacterial infections, inflammation, pulmonary edema, and compromised respiratory function. Influenza (or flu) in mammals is caused by influenza A and B viruses. Symptoms include dry cough, sore throat, and is often followed by secondary bacterial infections, accumulation of fluid in the alveolar spaces and acute lung injury. The underlying mechanisms of flu symptoms are not fully understood. This review summarizes our present knowledge of how influenza virus infections alter airway and alveolar epithelial cell CFTR and ENaC function in vivo and in vitro and the role of these changes in influenza pathogenesis.
Keywords: epithelial sodium channels, cystic fibrosis transmembrane conductance regulator, calcium-activated Cl− channels, Na+/K+-ATPase, M2 protein
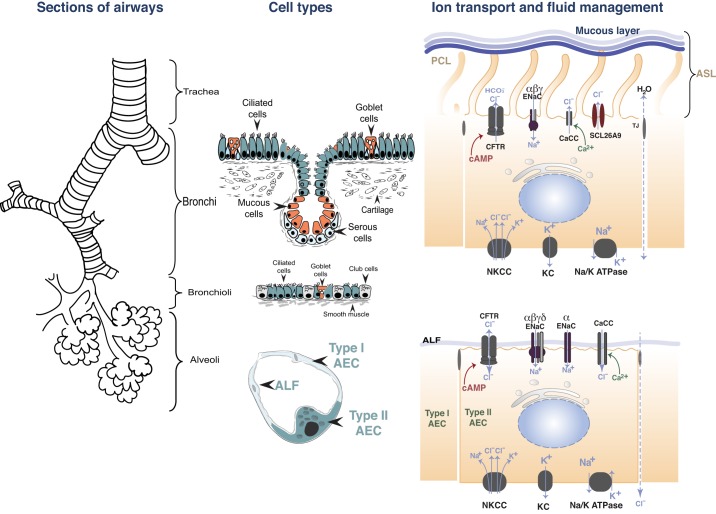
mucociliary clearance is a major first line of defense against respiratory pathogens. It is carried out by motile cilia on the surface of the airway epithelium and the overlying mucus, which consists of a superficial gel or mucous layer and the periciliary liquid (PCL) layer. The combination of the mucous and PLC layer is referred to as the airway surface fluid (ASL) layer. The mucous layer traps environmental debris and pathogens, whereas the PCL provides an environment in which cilia can freely beat to propel the overlying mucus and therefore regulates mucociliary clearance (108). In the distal respiratory tract, epithelial cells are covered by the alveolar lining fluid (ALF), with a volume of ~1 µl in mice and 1–10 ml in humans, and plays an important role in governing surfactant homeostasis (112). Maintenance of the PCL volume and composition requires the careful coordination of Na+ absorption, mainly through the amiloride-sensitive epithelial Na+ channel (ENaC), and Cl− and bicarbonate () secretion, through the cystic fibrosis transmembrane conductance regulator (CFTR), Ca2+-activated Cl− (5, 26, 40, 53), and SLC26A9 Cl− channels, present in the apical membranes of epithelial cells (82, 90). Cl− ions enter the basolateral membranes through Na+/K+/2 Cl− (NKCC) transporters (27). These processes are driven by a favorable electrochemical gradient generated the basolateral Na+/K+-ATPase, which exchanges 3 Na+ for 2 K+ ions (65) (Fig. 1).
Fig. 1.
The respiratory tract: segments of the airways, cell types, and ion transport in epithelial cells. Na+ ions enter the cytoplasm of ciliated airway cells and alveolar type I (ATI) and type II (ATII) cells, through channels, down an electrochemical gradient, created by the energy-consuming, ouabain-sensitive Na+/K+-ATPase located at the basolateral surface. Two different types of Na+ channels have been identified. Highly selective Na+ channels, consisting of at least 3 subunits (α, β, and γ), which are inhibited by micromolar concentrations of amiloride (epithelial Na channels, ENaC) and nonselective cation channels, consisting of the α-subunit. K+ ions exit the cells via basolateral K+ channels (KC). Ciliated cells secrete Cl− ions through cystic fibrosis transmembrane conductance regulator (CFTR), as well as Ca2+- activated Cl− channels (CaCC) and SCL26A9. Cl− ions enter the cells via the basolateral Na+/K+/2Cl− (NKCC) symporter, down an electrochemical gradient created by the Na+/K+-ATPase. In alveolar cells, Cl− transport across CFTR is likely bidirectional and depends on the concentration gradient. In ATII and ATI cells, Cl− enters cells through CFTR or crosses across the paracellular junctions to maintain electroneutrality. PCL, pericilary fluid; ASL, airway surface fluid; ALF, alveolar lining fluid; AEC, airway epithelial cell.
In utero, fetal lung epithelial cells also secrete Cl− by mechanisms similar to those of airway epithelial cells. The vectorial transport of NaCl generates an osmotic gradient, which contributes to fetal lung fluid formation, which fills the bronchial and alveolar spaces. Shortly before birth, Cl− secretion ceases, and Na+ absorption is initiated through the upregulated amiloride-sensitive ENaCs (68, 78, 101) (Fig. 1). Disturbances of Na+ reabsorption and Cl− secretion may have significant impact in airway fluid homeostasis and can lead to alveolar edema or dehydrated PCL in the conducting airways, excessive mucus accumulation, and infections by opportunistic pathogens (2, 92, 106). We review basic mechanisms of ion transport across airway and alveolar lung epithelia and discuss how influenza virus infection (30) may lead to significant alterations in ion transport and fluid homeostasis across the airways and alveoli, which may contribute to the clinical symptoms of influenza.
Ion Transport Across Lung Epithelial Cells
Early experiments demonstrated that inhibition of the lung epithelial Na+/K+-ATPase with ouabain blocks all active cation and anion transport across lung epithelia. In addition, inhibition of ENaC by amiloride or its structural analogs, benzamil and phenamil, blocked a significant fraction of reabsorption of alveolar lung fluid, especially following injury to the alveolar epithelium (68–70, 114, 115). ENaC is a highly selective unidirectional Na+ transporter, with a single-channel conductance of ~4–5 pS. It is composed of at least three subunits (α, β, and γ) (7) and expressed in the apical membrane of epithelial cells; an additional subunit (δ) has been identified in human alveolar epithelial cells (45, 116). The alveolar epithelium makes up 99% of the respiratory surface area of the lung with alveolar type I (ATI) cells, accounting for ~95% of the alveolar space but only 30% of the total alveolar cells. ATII cells constitute the remaining 5% of the surface area (69) and may act as progenitor cells that form a new epithelial surface following injury to ATI cells (50, 74) (Fig. 1).
Originally, it was thought that ion transport occurred only across ATII cells. However, the studies of Johnson et al. (47) and Lazrak et al. (55) showed that rodent ATI and ATII cells express similar channel densities of highly selective Na+ channels, with a unitary conductance of ~4 pS, most likely composed of the α-, β-, γ-ENaC subunits, and nonselective ENaC cation channels, with a conductance of 16–21 pS, composed of α-subunits only (42). It has also been reported that the nonselective channels consist of a combination of the α-ENaC subunit and one or more acid-sensing ion channel 1 (ASIC1a) proteins (103). Human alveolar cells also express ENaC (23) and actively transport Na+, albeit at rates lower than in rodent lungs (89). ENaCs represent the rate-limiting step in Na+ absorption as only a small fraction of the basolateral Na+/K+-ATPase activity necessary for normal Na+ transport across the alveolar epithelium; indeed, mice with a 50% decrease in Na+/K+-ATPase levels have normal levels of Na+-dependent lung fluid clearance, and, when exposed to hyperoxia, they do not develop more severe pulmonary edema than the wild-type controls (64).
In the alveolar spaces, Cl− is absorbed across the paracellular junctions or across epithelial cells through CFTR or calcium-activated Cl− channels to preserve electroneutrality (22, 67). Cl− secretion has also been demonstrated in vivo across alveolar epithelial cells (59) in the presence of a Cl− secretory gradient, after cAMP activation (75), or following injury to ENaC (94). In this case, Cl− ion secretion may contribute to the formation of alveolar edema. Interestingly, although a robust Cl− current is seen across confluent monolayers of ATII cells mounted in Ussing chambers (52), CFTR channels in ATII cells patched in the whole cell or cell-attached configurations following cAMP stimulation are seldom seen (47, 55, 117). This is most likely due to the small number of CFTR channels in the apical membranes of ATII or ATI cells, making CFTR difficult to detect in individual cells by electrophysiological measurements. CFTR is also the main conduit for Cl− secretion in submucosal glands and in proximal airway epithelial cells (3). In addition, CFTR is responsible for reduced glutathione secretion, which is crucial for redox status of the epithelial lining fluid (13).
A delicate balance between Na+ absorption and Cl− secretion is required for maintenance of the thin layer of liquid that coats the airways. Ciliary cells beat continuously within the PCL to move the mucin above it toward the pharynx, thus clearing the lungs of pathogens and debris. Normal mucociliary clearance requires that the PCL and the cilia are ~7 μm in height (60). Anything that interferes with the critical balance that influences PCL height and/or pH can directly affect mucus clearance. Mucus accumulation provides optimal condition to bacterial infections and may lead to activation of chronic inflammatory responses and subsequent lung damage (2, 5, 106). Prime examples of dysregulation of this process are clearly seen in cystic fibrosis, chronic bronchitis, chronic obstructive pulmonary disease (COPD), and influenza. In the next section, we discuss how influenza virus infection affects ion transport in the respiratory tract.
Influenza and Influenza Viruses
Influenza type A and B virus infections in humans results in an estimated 150,000 to 200,000 hospitalizations and 30,000 to 50,000 deaths in the United States (US) and 250,000 to 500,000 deaths worldwide annually (30). In addition, the annual cost in the US surpasses $11 billion in direct medical costs and $88 billion per year including indirect costs, due to loss of life and productivity (77). Influenza viruses typically infect the upper respiratory airways and cause fever, headache, myalgia, malaise, sore throat, nonproductive cough, sneezing, and nasal drainage. Pulmonary complications of influenza include pneumonia (viral and bacterial), croup, asthma, bronchitis, and acute respiratory distress syndrome (ARDS) (30, 56, 93, 110). Opportunistic bacterial pneumonia following influenza infection is a leading cause of death worldwide (56, 71).
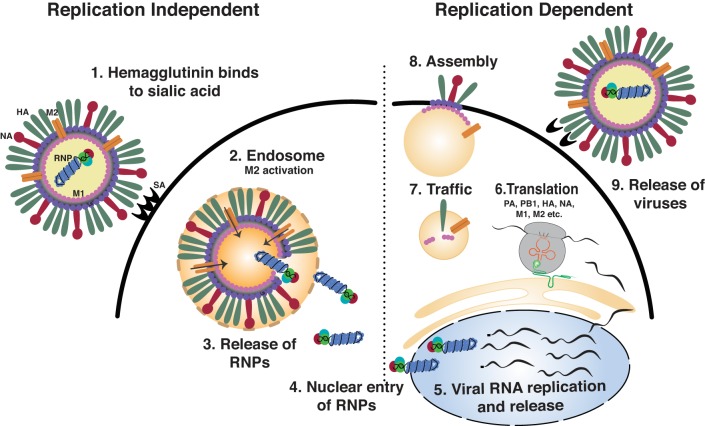
There are three types of influenza viruses, A, B and C, with influenza A and B regularly infecting humans and being responsible for the influenza epidemics that occur annually. Influenza A is a negative-sense RNA virus that contains eight gene segments that code for ~13 proteins (77). Three subtypes of hemagglutinin (H1, H2, and H3) and two subtypes of neuraminidases (N1 and N2) are found in influenza A virus strains that regularly infect humans. Zoonotic reservoirs of influenza A virus drastically enhance its potential to cause major pandemics. Avian influenza virus strains H5, H7, and H9 hemagglutinin and the N7 and N9 neuraminidase variants periodically cross over into humans, who have little exposure and protection from these viral strains, and lead to high morbidity and mortality rates. During infection, the influenza virus hemagglutinin binds to the sialic acid residues on the surface of the lung epithelium (76, 77) and is then internalized through a cytochalasin D-dependent mechanism. After internalization of the virus, the low pH in endocytic vesicles initiates hemagglutinin fusion with the endosomal membrane. Simultaneously, the influenza virus M2 protein, located at the viral membrane, transports protons into the virion, causing the virus to uncoat and release of viral ribonucleoproteins (RNPs) into the cytosol (98). Viral RNPs are then transported into the nucleus, where viral replication occurs. Viral cRNA is produced and serves as a template for synthesizing vRNA for new virion production. Viral mRNA encodes proteins that aid in replication (nonstructural), and proteins are incorporated into the new virion (structural). Viral mRNA is trafficked into the cytosol and translated by the host cell machinery. Viral proteins and RNA assemble at the plasma membrane, where budding and neuraminidase-mediated cleavage facilitates influenza virus particle release (Fig. 2). Influenza B viruses are not divided into subtypes but can be further broken down into lineages and strains. Presently circulating influenza B viruses belong to one of two lineages: B/Yamagata and B/Victoria (77).
Fig. 2.
Influenza virus replication cycle (1). Hemagglutinin binds to sialic acid residues at the plasma membrane (2). Influenza virus is internalized and tracked to the endosomes. Low pH in the endosome activates M2, which transports protons to the interior of the virion, causing viral ribonucleoprotein (RNP) to dissociate from the virion. Simultaneously, fusion of the hemagglutinin to the endosomal membrane, also initiated by low pH, allows the virions to track into the cytosol (3). Viral RNPs are released (4). Viral RNP is tracked to the nucleus (5). Viral RNA produces cRNA, which encodes new viral genome (vRNA) and mRNA (6). Viral mRNA is translated in the cytosol using host machinery (7). vRNA and viral proteins are tracked to the cell surface (8). Viral assembly and budding from the cell surface occurs (9). Neuraminidase cleaves sialic acid residues from the surface of the cell to allow the virus to detach and infect new host cells.
Interactions of Influenza Viruses with Respiratory Epithelial Cells
The pseudostratified, ciliated, columnar epithelium of the upper respiratory tract is the primary site of influenza virus infection in mammals (100). However, nonciliated club cells and mucous cells can be infected experimentally (32). Influenza virus infection of the pulmonary mucosa can have important influence on airway functions, and, although poorly understood, the cytopathology of replicating influenza virus in the airway epithelium can disrupt normal cellular architecture and morphology. Differentiated, polarized monolayers of mammalian and particularly human airway epithelial cells exhibit exclusive viral entry and viral release through their apical surface (104, 105), and the sheer numbers of progeny virions released from the apical membrane can undoubtedly alter the normal composition of the apical membrane. As enveloped viruses, influenza viruses use the epithelial cell membrane as a coat, and the appropriation of significant membrane segments likely alters both the function as well as subsequent viral infection of host cells in the airway. Despite the cytopathology of influenza viruses in standardized propagation cultures, such as the use of Madin-Darby canine kidney cells, the replication and release of influenza viruses by epithelial cells are not immediately lethal to the host (32, 72), and thus the altered function of the infected epithelium may contribute to the pathogenesis of influenza disease. Aberrant cilia mucociliary and rheological properties of influenza-infected airways and other important cellular functions that are critical for lung fluid balance are well recognized both experimentally and during the course of the disease (8, 28, 36, 38, 84).
Alteration of Ion Transport by Influenza Virus Infection
Rhinorrhea, accumulation of fluid in the Eustachian tubes or middle ear and in the respiratory tract, are commonly described symptoms of flu in humans and animals as well (19, 49, 97). Severe infections by influenza viruses also target the distal lung epithelial cells and damage the pulmonary surfactant that can lead to pneumonia, pulmonary edema, and ARDS (38, 41, 110).
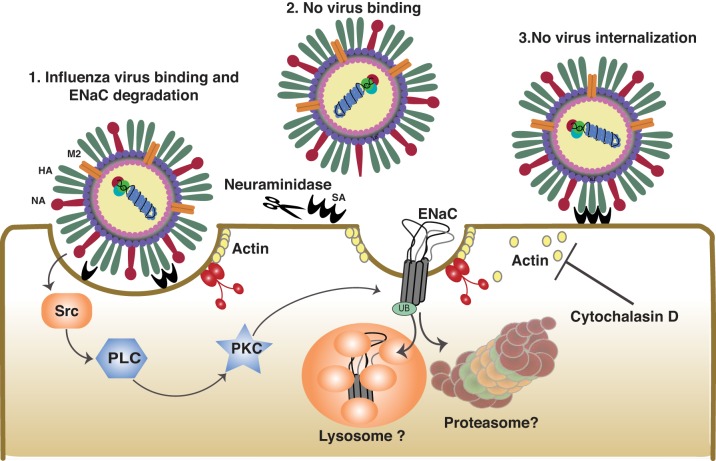
The first experiments examining the alteration of ion transport across lung epithelial cells infected with influenza viruses were conducted by Kunzelmann and colleagues (51). In these studies, apical membranes of mouse tracheal epithelium, colonic epithelium, and kidney cortical collecting ducts were exposed to A/WSN/33 (WSN33; H1N1) and A/PR/8/34 (PR8; H1N1) mouse-adapted influenza A viruses, and ion transport was measured in Ussing chambers. One hour after infection with influenza virus, there was decreased transepithelial potential difference, reduced total short-circuit currents, and diminished amiloride-sensitive currents (a measure of ENaC function). Inhibitors of viral internalization, such as cytochalasin D, had no effect on ENaC inhibition. Only the prevention of virus binding by cleaving surface sialic acid residues or the targeting of influenza virus hemagglutinin with a blocking antibody prevented the influenza virus-mediated alteration of ENaC activity. In addition, a split virus preparations that retained hemagglutinin activity but lacked the replicative capacity could also decrease amiloride-sensitive currents. Furthermore, incubating cells with the lectin concanavalin A, which mimics viral hemagglutinin, also inhibited amiloride-sensitive fluid clearance (51). Protein kinase C (PKC) was activated shortly following viral attachment and was responsible for reduced amiloride-sensitive currents in infected mice. This was also the first demonstration of PKC-mediated ENaC inhibition, a now widely recognized mechanism of Na+ transport regulation (51).
In agreement with earlier studies in the tracheal epithelium, studies by Chen et al. (12) confirmed that, in ATII cells, virus binding, but not internalization, decreased ENaC activity. Infection of ATII cells with influenza virus inhibited the amiloride-sensitive, inwardly rectifying cation channel conductances, characteristic of α- and β-containing canonical ENaC (43). The authors suggested that changes in the function of the Src cytosolic tyrosine kinase, which governs an early step of the clathrin-mediated internalization process, was responsible for ENaC inhibition during influenza viral infection of ATII cells (12) (Fig. 3).
Fig. 3.
Inhibition of epithelial sodium channels (ENaC) by virus binding. Influenza virus hemagglutinin binding to sialic acid residues at the cell surface and activates Src kinase. Src activates PLC, which activates PKC. PKC downregulates ENaC expression and activity. Neuraminidase cleaves sialic acid residues, preventing virus binding and ENaC inhibition. Cytochalasin D allows the virus to bind but prevents virus internalization. Blocking internalization with cytochalasin D does not prevent ENaC inhibition. This is based on conclusions published previously (12, 51).
Inhibition of ENaC and CFTR by Influenza Virus M2 Protein
The previously described findings of Kunzelmann and Chen advanced the hypothesis that ENaC downregulation by influenza virus infection was due almost exclusively to the binding of virus to airway and alveolar epithelial cells. However, later studies showed that viral protein synthesis might also play a role in this process. The influenza virus genome encodes ~13 proteins that are continuously expressed at the later stages of infection. On the basis of the hypothesis that a virally expressed ion channel may alter the activity of resident ion channels, Lazrak and colleagues (54) undertook a series of studies examining how the influenza ion channel M2 protein alters the expression and activity of ENaC.
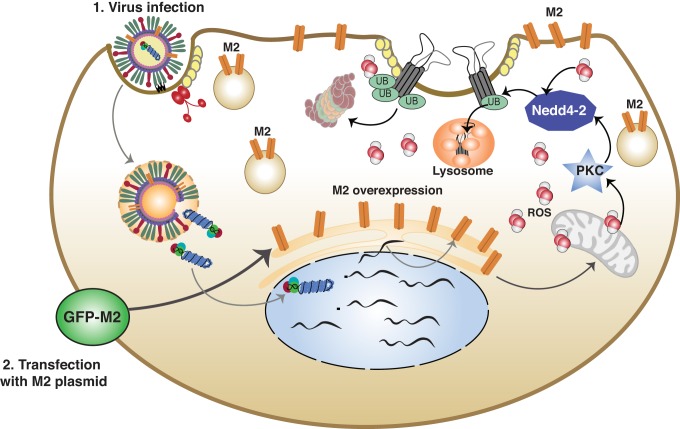
The influenza virus gene segment 7 codes for both matrix protein 1 and matrix protein 2 through alternative RNA splicing. The influenza A virus matrix-2 (M2) protein is a 97-amino acid (AA), type I, single-pass membrane protein consisting of a 22-AA NH2-terminal external domain, 21-AA membrane-spanning domain, and a 54-AA COOH-terminal internal domain (83). M2 is a homotetrameric proton-conducting transmembrane ion channel. The HXXXW motif serves as the core of this conduction pathway and is conserved in both influenza A virus and influenza B. The histidine residue serves as a selectivity filter, allowing only the passage of protons. The pore-lining residues are also responsible for susceptibility to the channel blocker amantadine. Lazrak et al. (54) determined that the influenza virus M2 protein inhibits amiloride-sensitive currents in both Xenopus oocytes, injected with cRNA encoding the α-, β-, and γ-subunits of ENaC and in the club cell-like human airway epithelial cell line H441 transfected with green fluorescent protein (GFP)-tagged M2. Measurements of single-channel conductances confirmed ENaC inhibition. In contrast, expression of M1 protein had no effect on ENaC currents (54). Expression of all three ENaC subunits was decreased in M2 coinjected oocytes compared with controls and was consistent with the decreases in ENaC activity. ENaC activity was restored in M2-injected oocytes by inhibition of proteasomal protein degradation. To determine how ENaC is targeted for degradation, the authors coexpressed ENaC subunits with mutations that are present in Liddle syndrome and M2. Liddle mutant ENaC channels cannot be ubiquitinated, and therefore they are insensitive to proteasomal degradation. M2 coexpression had no effect on Liddle-mutant ENaC currents, suggesting that reduced ENaC activity was due to ubiquitin-mediated proteasomal degradation of the protein. They also determined that inhibiting the PKC-ζ isoform specifically rescued ENaC activity. Interestingly, severe acute respiratory syndrome coronavirus (SARS-CoV) structural proteins S and E also inhibit ENaC activity via activation of PKC-α/β1 and PKC-ζ (44).
Reactive oxidants have also been shown to alter ENaC activity by either posttranslational modifications (such as oxidation, nitration, nitrosylation, and glutathionylation of critical residues) or through activation of signal transduction pathways (96). Furthermore, oxidized glutathione has been shown to inhibit ENaC activity in primary alveolar epithelial cells (18, 33). Lazrak et al. (54) measured the induction of reactive oxygen species (ROS) by M2 overexpression. The transfection of A549 cells, an adenocarcinoma cell line, with M2 resulted in increased cytosolic and mitochondrial ROS (54). Supplementation with a cell-permeable glutathione ethyl ester (GSH), which increases the concentration of reduced thiols, significantly attenuated ROS levels and the ENaC inhibition by M2 (54) (Fig. 4). Peters et al. (81) also reported that TGF-β inhibited ENaC activity via a mechanism involving increased production of ROS from NADPH oxidase 4 (NOX4), which caused internalization of β-ENaC. In other studies, ROS-reactive nitrogen species were shown to downregulate amiloride-sensitive currents in Xenopus oocytes, injected with ENaC cRNAs, by oxidizing critical thiol groups in aENaC (20), as well as confluent monolayers of ATII cells mounted in Ussing chambers (31).
Fig. 4.
Expression of influenza virus M2 inhibits epithelial sodium channel (ENaC) activity. Influenza M2 expression increases cellular reactive oxygen species (ROS) either through mitochondrial dysfunction or through activation of the NADPH oxidases. ROS activates PKC, which increases ENaC ubiquitination via the E3 ubiquitin ligase Nedd4-2. Ubiquitinated ENaC is proteasomally degraded, leading to decreased ENaC-mediated sodium transport. This is based on conclusions published previously (54).
In addition to decreasing ENaC levels and function, Londino and colleagues (61) showed that CFTR activity was also inhibited by M2 in 1) Xenopus oocytes injected with human CFTR, 2) epithelial cells (HEK-293) stably transfected with CFTR, and 3) a human bronchial epithelial cell line (16HBE14o), expressing CFTR endogenously. Interestingly, although endoplasmic reticulum (ER) core-glycosylated CFTR protein expression was enhanced in M2-injected oocytes, the maturely glycosylated form responsible for cell surface ion transport was significantly reduced, suggesting a protein-trafficking defect. Importantly, the effects of M2 were not indiscriminant because M2 did not inhibit the activity of another ion channel, TRPV5, in Xenopus oocytes.
The mechanisms by which M2 may alter CFTR trafficking in the post-ER compartments are likely related to its biological function. During transport through the Golgi and other acidic vesicular organelles, M2 ion channel activity raises the pH by acting as a counter-ion transporter to the vacuolar ATPase. The influenza virus M2-mediated increase in pH is essential in protecting the influenza virus hemagglutinin from premature conversion to its low pH form (35, 88). En route through the secretory pathway, influenza virus M2 has been shown to alter glycosylation patterns, slow protein trafficking, and decrease plasma membrane protein recycling (34). These effects can be reversed by inhibition of M2 ion transport with amantadine.
Because the maturation of CFTR is dependent on proper function of the cellular trafficking machinery, it is possible that the M2 proton channel activity is responsible for reduced CFTR maturation and/or recycling at the cell surface. Consistent with this hypothesis, inhibition of M2 function with amantadine rescued mature CFTR expression levels and activity. M2 mutant proteins with attenuated proton transport, as well as an M2 mutant that was retained in the ER, did not decrease CFTR activity. Londino and colleagues (61) also showed that, when in Xenopus oocytes incubated with agents that raised the pH of the secretory pathway (ammonium chloride or concanamycin A), CFTR activity was inhibited in a dose-dependent manner. There data suggest that raising the pH of acidified organelles by M2 may have contributed to the M2 CFTR inhibition. Finally, when ubiquitination was inhibited using an E1 ubiquitin-activating enzyme inhibitor, CFTR activity was rescued in M2-coinjected oocytes. These results are consistent with the idea that, in the presence of active M2, cell surface CFTR is ubiquitinated and targeted for early degradation by the lysosome from the cell surface, resulting in reduced mature CFTR levels compared with controls and diminished activity.
Influenza Virus M2 is Necessary for the Inhibition of CFTR Function During Viral Infection
To examine the role of M2 in viral infection, Londino and colleagues (62) conducted a series of experiments examining the consequences of influenza virus infection in Ussing chambers. Infection of normal human bronchial epithelial cells and murine nasal epithelial cells with various strains of influenza A virus significantly reduced ENaC and CFTR short-circuit currents, transepithelial resistance, and CFTR protein levels at 18 h postinfection. To exclude the possibility that the decrease of ENaC and CFTR short-circuit currents resulted from the previously demonstrated reduction of Na+/K+-ATPase activity following viral infection (80), they infected CFTR-expressing HEK-293 cells with a GFP-tagged influenza virus and performed patch clamp on GFP-expressing and nonexpressing cells in the whole cell recording mode. GFP-expressing cells (i.e., those infected with virus and expressing M2 protein) had significantly reduced CFTR currents and smaller amounts of surface CFTR. On the other hand, adjacent, noninfected cells (identified by the lack of GFP) had normal currents and CFTR levels. These data suggest that there is a profound dysregulation of both CFTR expression and activity after virus infection, rather than simply a disruption of epithelial functions in general. These results also argue against the concept that influenza virus inhibition of CFTR is due to a paracrine effect; instead, they suggest that a viral intermediate may play a role in the suppression of ion transport (61).
To test whether M2 was necessary and sufficient for the influenza virus inhibition of CFTR, Londino et al. (62) knocked down virally expressed M2 protein with siRNA or inhibited its activity with amantadine. Inhibiting M2 prevented the decrease in CFTR expression and function, confirming in combination with the previously described M2 overexpression studies that M2 was responsible for the decrease in CFTR activity and protein levels. Because the degradation of CFTR by M2 is inhibited by blocking ubiquitination in Xenopus oocytes, Londino et al. (62) next examined the role of the ubiquitin system in CFTR degradation during whole virus infection. During transit through the ER, improperly folded proteins, including some CFTR mutants and posttranslational modified CFTR, are polyubiquitinated through the linkage of lysine 48 or 29 (K48, K29) of the ubiquitin molecules and subsequently degraded by the proteasome. Upon exiting the ER, recycling and degradation of fully glycosylated plasma membrane CFTR are primarily regulated by polyubiquitination through the linkage between lysine 63, 11, or 6 (K63, K11, and K6) of the ubiquitin molecules, followed by lysosomal degradation (63). Several E3 ligases have been implicated in the degradation of CFTR including Nedd4–2, c-cbl, CHIP, MARCH2, and RMA1/RNF5 (24, 79). Consistent with the idea that influenza virus enhances CFTR degradation by the lysosome, CFTR was stabilized by the lysosomal inhibitor bafilomycin A1 but not by the specific proteasome inhibitor lactacystin. Bafilomycin A1, added after infection, had no effect on viral protein expression. Importantly, there were enhanced levels of K63-linked polyubiquitinated CFTR but not K48-linked polyubiquitinated CFTR in cells infected with influenza virus (62).
The cause of enhanced cell surface, K63-linked polyubiquitination of CFTR in cells infected with influenza virus remains to be elucidated. Earlier studies have demonstrated that M2 increased NEDD4-2, E3-ligase-mediated ubiquitination of ENaC (54). In contrast, it has been reported that NEDD4-2 does not ubiquitinate CFTR in airway epithelia under basal conditions (48). Importantly, it is not clear what types of posttranslational modifications lead to enhanced cell surface polyubiquitination of CFTR, and the E3 ligase(s) that mediate this process has yet to be identified. What we know is that, in contrast to ENaC, antioxidants did not prevent the M2-mediated reduction in mature CFTR levels and function, suggesting that reactive species were not involved (61, 62) contrary to previous reports showing that ROS-reactive nitrogen species intermediates may either upregulate or downregulate CFTR protein levels and function depending on their concentrations (4, 9, 10, 46). In addition, inhibition of influenza virus M2-mediated proton transport rescued CFTR expression and activity but did not prevent ENaC decrease. It is possible that the M2-mediated pH increase in the secretory pathway modifies CFTR structure, leading to polyubiquitination. Another possibility is that structural changes in CFTR, caused by infection with influenza virus, result in reduced recognition by deubiquitination enzyme, such as USP10, as reported previously following Pseudomonas aeriguinosa infection (6). Determining the specific molecular mechanisms by which influenza virus enhances CFTR and ENaC degradation from the cell surface may yield novel targets for therapeutics.
Although the inhibition of CFTR expression and function by influenza infection have not been demonstrated previously, several other viruses have been shown to modify CFTR activity. Specifically, respiratory syncytial virus (RSV) was also shown to inhibit CFTR activity of mouse trachea and in epithelial cells in primary culture (11). Furthermore, Tarran et al. (99) showed that infection of ciliated airway cells from human donors with RSV decreases the PCL height, which was attributed to the inhibition of CFTR. RSV-mediated inhibition of ENaC in epithelial cells was mediated via upregulation of inducible nitric oxide synthase and UTP and was also seen in infected cells (95).
Cytokines and Other Danger-Associated Molecular Patterns Alter Ion Transport
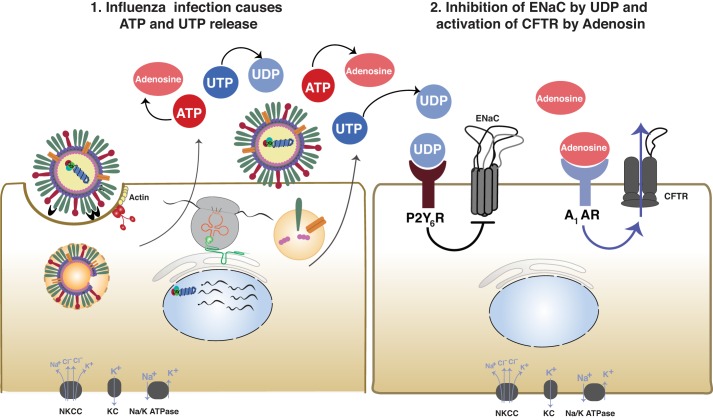
In contrast to studies implying a direct role of the virus in the inhibition of ion transport discussed above, several studies have implicated secondary mediators of viral infection, including the release of cytokines and other molecular danger-associated molecular patterns (DAMPs), such as ATP and UTP. In earliest in vivo studies, influenza viruses were instilled intratracheally, and lung wet-dry ratios, a marker of alveolar flooding, were measured. Lung weights were increased at 2 h postinstillation, far too early for significant epithelial damage to have occurred. Inhibition of ENaC with amiloride had no additive effect on the wet-dry ratios, suggesting that influenza virus effects were due to ENaC inhibition (12). Although fluid clearance in the intact lung is known to be controlled almost exclusively by active ion transport, fluid deposition in the influenza virus-infected lung may also be enhanced by increased passive fluid transport through the compromised epithelium and tight junctions (73). To compensate for the increased fluid leakage in influenza-infected mouse lungs, Wolk and colleagues (109) corrected for murine albumin leakage into the alveolar epithelium by comparing its levels to instilled albumin. Using this system, they determined that the active Na+ transport-driven alveolar fluid clearance (AFC) was maximally inhibited in infected lungs ~2 days after infection and remained compromised as late as day 18. Most of the observed decreases in AFC were due to the decreased ENaC activity (109). Gotts and colleagues (28) reported that infection of mice with influenza virus results in significant and sustained injury of the alveolar epithelium that peaks at 1 wk postinfection. Although UV-inactivated virus, which is capable of binding but not replicating, inhibited amiloride-sensitive fluid clearance, the effect was muted compared with the wild-type virus (51). Instead, it appeared that the inhibition of ENaC was dependent on the release of both ATP and UTP in the bronchoalveolar lavage (BAL) fluid (Fig. 5). Levels of ATP/UTP significantly increased in lung BAL fluid at 1 and 2 days after viral infection and decreased by day 4. Inhibition of de novo pyrimidine synthesis and blocking UTP export into the alveolar space prevented the influenza virus-mediated decrease in fluid transport. This is consistent with previous studies showing that increased UTP release mediates the inhibition of ENaC during RSV infection (15–17).
Fig. 5.
Influenza virus alteration of cystic fibrosis transmembrane conductance regulator (CFTR) and epithelial sodium channel (ENaC) activity through the release of ATP/UTP. After influenza infection, both ATP and UTP were significantly increased 1 and 2 days postinfection due to viral infection of the lung epithelium. UTP is hydrolyzed to UDP and acts on the P2Y6R receptor, leading to decreased amiloride-sensitive ion transport. Simultaneously, ATP is hydrolyzed to adenosine, which acts on the A1-AR. Activation of the A1-AR increases Cl− secretion through CFTR. The combination of decreased Na+ absorption and increased Cl− secretion leads to increased airway surface fluid and pulmonary edema. This is based on conclusions previously published (109). NKCC, Na+/K+/2Cl−; KC, K+ channels.
In addition to decreased AFC by ENaC inhibition, 2 days after the instillation of WSN influenza virus into the airways, increased Cl− transport through CFTR significantly contributed to fluid accumulation in the alveolar space. Blocking CFTR activity with specific inhibitors significantly enhanced fluid clearance (i.e., decreased fluid accumulation). This is contrary to patch-clamp and Ussing chamber studies showing that influenza virus inhibits both ENaC and CFTR activities (54, 61, 62).
Unlike ENaC, which conducts Na+ in one direction into the cell, CFTR is capable of bidirectional transport of Cl−. Because the movement of Cl− through CFTR may be necessary to some degree for basal Na+ reabsorption across alveolar cells and necessary for the β-agonist increase in AFC (21), it appears that a switch from Cl− absorption to secretion occurs in the lungs infected with influenza virus (109). This switch has been observed under other pathological conditions. During the onset of hydrostatic pulmonary edema, increased hydrostatic pulmonary pressure decreased active Na+ transport acutely and reversibly with little change in lung permeability and increased Cl− secretion through CFTR (63, 94) that promoted fluid accumulation in the lung. Solymosi et al. (94) proposed that inhibition of apical Na+ transport through ENaC changes the electrochemical gradient that may result in Cl− secretion through CFTR. Consistent with this theory, when amiloride was added to the lung instillate to block ENaC activity at normal arterial pressure, CFTR Cl− transport shifted from absorptive to secretory.
Cytokines released during viral infection have also been demonstrated to alter ion transport across alveolar epithelial cells. Chan and colleagues (8) infected primary human alveolar epithelial cells cultured on Transwell supports. Infection led to the release of inflammatory cytokines that resulted in decreased paracellular permeability and decreased fluid clearance. The inhibition of ion transport appeared to be caused by decreased mRNA expression of multiple subunits of the Na+/K+-ATPase, α-, β-, and γ-ENaC, and CFTR and was only observed during inflection with H5N1 and not the H1N1 virus. Given the low MOI (0.1, considerably lower than most previous studies) and high pathology of the H5N1 virus, it is plausible that the induction of cytokines may be necessary for the inhibition of ion transport under certain conditions and at certain time points postinfection. Another recent paper by Peteranderl et al. (80) supports the role of inflammatory cytokines in the suppression of ion transport. In their experiments, IFN-α released from alveolar epithelial cells led to both the direct and indirect inhibition of the Na+/K+-ATPase through the recruitment of macrophages, which released the TNF-related apoptosis-inducing ligand (TRAIL). Adoptive transfer of TRAIL-deficient macrophages as well a therapeutic blockade of the IFN/TRAIL network by neutralizing antibodies resulted in significantly improved AFC, suggesting an essential role for macrophages in the alteration of fluid clearance by influenza virus. Interestingly, TNF-α increases ENaC activity (14, 25) although, as previously mentioned, ROS may alter both ENaC and CFTR activity, depending on their concentration levels (66).
Role of Ion Transport in Influenza Pathology
Infection of the lung epithelium by influenza virus has been consistently demonstrated to alter ion transport by modifying ENaC, CFTR, and the Na+/K+-ATPase. Dysregulation of ion transport has also been demonstrated to have detrimental effects in many pathological conditions. The alteration of ENaC and CFTR by influenza virus is probably responsible for or exacerbates several symptoms described during influenza infection.
A number of studies have demonstrated the relevance of ENaC-mediated Na+ transport across distal lung epithelia in vivo. In experimental mouse models, knockout of α-ENaC is lethal because of the inability to clear lung fluid at birth (39). In the adult mouse lung, inhibition of α-ENaC by siRNA decreased basal fluid clearance, whereas terbutaline, a β2-adrenergic receptor agonist, stimulated clearance (58). In humans, Na+ transport through ENaC is also essential for the resolution of high-altitude edema (91). Patients hospitalized for ARDS that exhibit inhibited amiloride-sensitive fluid clearance have a higher incidence of mortality (107). Impairment of ENaC during influenza virus infection in humans would likely lead to both alveolar edema, as well as the commonly observed upper airway symptoms, such as rhinorrhea, observed during less severe infections.
Inhibition of CFTR function during viral infection could also lead to numerous detrimental effects. Inhibition of CFTR has been demonstrated to lead directly to PCL dehydration. In addition, decreased levels of CFTR may cause ENaC upregulation (55), which would further exacerbate the dehydration of the PCL. Indeed, administration of amiloride and amiloride analogs has been proposed as a therapy for cystic fibrosis and chronic bronchitis (37, 102). In cystic fibrosis, PCL dehydration is thought to be a primary driver of chronic bacterial infections in the respiratory tract that lead to airway obstruction, bronchiectasis, and respiratory failure (56, 85, 113). Single-cell, free-living bacteria, such as Pseudomonas aeruginosa, form colonies or biofilms under these conditions and become resistant to clearance by the immune response and antibiotics. Quantitative studies in cystic fibrosis airways demonstrated that the center of infection is localized in the mucous gel layer, with virtually no bacteria detected adherent to, or within, epithelial cells (86). Treatments resulting in hypersecretion of mucin in animal models did not show significantly enhanced airway obstruction, or increased during infections, indicating that mucin per se was not responsible for these effects (87). In contrast, decreasing the ASL volume through overexpression of the β-ENaC led to reduced mucociliary transport, increased neutrophilic inflammation, poor bacterial clearance, and enhanced airway obstruction (86). Compromised ion transport during viral infection, through dysregulation of ENaC, CFTR, Na+/K+-ATPase and other ion transporters, may partially explain the pathology in COPD exacerbations that are known to be induced by rhinoviruses, paramyxoviruses, and coronaviruses, as well as influenza and other viruses.
In addition to direct effects on PCL volume, studies by Pezzulo and colleagues (82) showed that the inhibition of CFTR altered the pH of the PCL because of lack of transport, which led to an acidic environment, resulting in decreased bacterial killing. CFTR has also been suggested to regulate antioxidant signaling (29), decrease tight junction permeability (57), and may modify the innate immune response. A study of influenza virus infection in normal and cystic fibrosis primary airway epithelium found that more genes are altered (both activated and inhibited) in normal lung epithelial cells than in cystic fibrosis cells. Early gene responses were particularly impacted (111), including IFN-β production and apoptotic responses. IL-8 gene response to influenza virus infection was also significantly increased in cystic fibrosis cells (111). These results are especially compelling because CFTR had independently been shown to inhibit IL-8 production in response to inflammatory mediators. This is consistent with a recent study demonstrating that CFTR heterozygous (wild-type/Δf508) mice are less susceptible to influenza injury than their homozygous wild-type mice although patients with cystic fibrosis are more susceptible to infections with influenza virus (87). Robust early inflammatory responses normally observed in wild-type animals early in infection are dampened in CFTR heterozygote mice, whereas the cytokine profile was altered later in infection (1, 110). These observed immune defects attributable to locally impaired CFTR activity could have profound effects on viral propagation and disease progression.
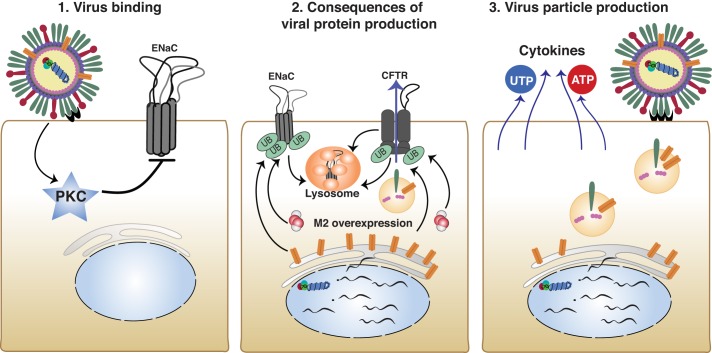
How the modification of ion transport results in influenza pathology is likely to depend on the location, timing, and the severity of infection. Local viral infection alters the activity and possibly the levels of ion channel proteins in many cells. This is followed by the production and release of viral particles, viral cytokines, and DAMPs, which may compound the injury (Fig. 6). Inhibition of ion transport manifests itself differently depending on the region of the respiratory system that is most affected by virus infection. For example, damage to the alveolar epithelial Na+ channels by viral proteins and cytokines and chemokines may lead to activation of Cl− secretion through CFTR or Ca2+-activated Cl− channels (94), which in turn may result in pulmonary edema (109). On the other hand, in airways, inhibition of ENaC and activation of CFTR may lead to increased PCL volume and rhinorrhea. Alterations in ENaC and CFTR activities in airway epithelial cells at the late stages of virus infection, when bacterial infections occur, warrant closer examination. Further research on the dysregulation of ion transport during viral infections will lead to increased understanding of the mechanism of viral pathology and better therapeutics for intervention.
Fig. 6.
Modification of ion transport by influenza virus at cellular and tissue level. Mechanisms underlie ion transport regulation by influenza virus infection. 1: Influenza virus hemagglutinin binds sialic acid residues, leading to PKC activation and epithelial sodium channel (ENaC) inhibition. 2: After internalization, influenza produces viral proteins. Influenza M2 leads to enhanced ubiquitination and degradation of both ENaC and cystic fibrosis transmembrane conductance regulator (CFTR). 3: Influenza replication assembly and budding result in the release of cytokines and UTP/ATP. Reinfection propagates earlier signaling pathways.
Conclusion
Infection with influenza viruses alters ion transport through binding to different cells in the respiratory tract, expression of viral proteins, and stimulation of cytokine release and other DAMPs (some of which remain to be identified). Dysregulation of ion transport aggravates respiratory symptoms by inducing pulmonary edema, leading to impaired gas exchange when ENaC is inhibited. In contrast, CFTR inhibition results in ASL dehydration, altered innate immune signaling, and enhanced bacterial colonization. The overall effect of influenza virus infection on ion transport depends on the strain of virus and the severity of infection and may vary through the course of the infection. Future studies are necessary to elucidate these mechanisms with an intention to identify therapeutic targets to reduce morbidity and mortality.
GRANTS
This research was supported by the CounterACT Program, NIH, Office of the Director, the National Institute of Environmental Health Sciences, Grant Numbers 5U01ES026458 02 (S. Matalon), 1 U01 ES027697 01 (S. Matalon), 17POST33410945 (J. Londino), and AI111475 (K. Harrod).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.L., A.L., and S.M. conceived and designed research; J.D.L., A.L., and S.M. performed experiments; J.D.L., A.L., and S.M. analyzed data; J.D.L., A.L., and S.M. interpreted results of experiments; J.D.L., Z.B., and S.M. prepared figures; J.D.L., J.F.C., Z.B., K.S.H., and S.M. drafted manuscript; J.D.L., A.L., J.F.C., Z.B., K.S.H., and S.M. edited and revised manuscript; J.D.L., A.L., J.F.C., Z.B., K.S.H., and S.M. approved final version of manuscript.
REFERENCES
- 1.Aeffner F, Abdulrahman B, Hickman-Davis JM, Janssen PM, Amer A, Bedwell DM, Sorscher EJ, Davis IC. Heterozygosity for the F508del mutation in the cystic fibrosis transmembrane conductance regulator anion channel attenuates influenza severity. J Infect Dis 208: 780–789, 2013. doi: 10.1093/infdis/jit251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Åstrand AB, Hemmerling M, Root J, Wingren C, Pesic J, Johansson E, Garland AL, Ghosh A, Tarran R. Linking increased airway hydration, ciliary beating, and mucociliary clearance through ENaC inhibition. Am J Physiol Lung Cell Mol Physiol 308: L22–L32, 2015. doi: 10.1152/ajplung.00163.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballard ST, Trout L, Bebök Z, Sorscher EJ, Crews A. CFTR involvement in chloride, bicarbonate, and liquid secretion by airway submucosal glands. Am J Physiol 277: L694–L699, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Bebok Z, Varga K, Hicks JK, Venglarik CJ, Kovacs T, Chen L, Hardiman KM, Collawn JF, Sorscher EJ, Matalon S. Reactive oxygen nitrogen species decrease cystic fibrosis transmembrane conductance regulator expression and cAMP-mediated Cl− secretion in airway epithelia. J Biol Chem 277: 43041–43049, 2002. doi: 10.1074/jbc.M203154200. [DOI] [PubMed] [Google Scholar]
- 5.Birket SE, Chu KK, Houser GH, Liu L, Fernandez CM, Solomon GM, Lin V, Shastry S, Mazur M, Sloane PA, Hanes J, Grizzle WE, Sorscher EJ, Tearney GJ, Rowe SM. Combination therapy with cystic fibrosis transmembrane conductance regulator modulators augment the airway functional microanatomy. Am J Physiol Lung Cell Mol Physiol 310: L928–L939, 2016. doi: 10.1152/ajplung.00395.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bomberger JM, Ye S, Maceachran DP, Koeppen K, Barnaby RL, O’Toole GA, Stanton BA. A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system. PLoS Pathog 7: e1001325, 2011. doi: 10.1371/journal.ppat.1001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canessa CM, Horisberger JD, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration Nature 361: 467–470, 1993. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- 8.Chan MC, Kuok DI, Leung CY, Hui KP, Valkenburg SA, Lau EH, Nicholls JM, Fang X, Guan Y, Lee JW, Chan RW, Webster RG, Matthay MA, Peiris JS. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci USA 113: 3621–3626, 2016. doi: 10.1073/pnas.1601911113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Bosworth CA, Pico T, Collawn JF, Varga K, Gao Z, Clancy JP, Fortenberry JA, Lancaster JR Jr, Matalon S. DETANO and nitrated lipids increase chloride secretion across lung airway cells. Am J Respir Cell Mol Biol 39: 150–162, 2008. doi: 10.1165/rcmb.2008-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L, Patel RP, Teng X, Bosworth CA, Lancaster JR Jr, Matalon S. Mechanisms of cystic fibrosis transmembrane conductance regulator activation by S-nitrosoglutathione. J Biol Chem 281: 9190–9199, 2006. doi: 10.1074/jbc.M513231200. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Song W, Davis IC, Shrestha K, Schwiebert E, Sullender WM, Matalon S. Inhibition of Na+ transport in lung epithelial cells by respiratory syncytial virus infection. Am J Respir Cell Mol Biol 40: 588–600, 2009. doi: 10.1165/rcmb.2008-0034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen XJ, Seth S, Yue G, Kamat P, Compans RW, Guidot D, Brown LA, Eaton DC, Jain L. Influenza virus inhibits ENaC and lung fluid clearance. Am J Physiol Lung Cell Mol Physiol 287: L366–L373, 2004. doi: 10.1152/ajplung.00011.2004. [DOI] [PubMed] [Google Scholar]
- 13.Collawn JF, Matalon S. The role of CFTR in transepithelial liquid transport in pig alveolar epithelia. Am J Physiol Lung Cell Mol Physiol 303: L489–L491, 2012. doi: 10.1152/ajplung.00216.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czikora I, Alli A, Bao HF, Kaftan D, Sridhar S, Apell HJ, Gorshkov B, White R, Zimmermann A, Wendel A, Pauly-Evers M, Hamacher J, Garcia-Gabay I, Fischer B, Verin A, Bagi Z, Pittet JF, Shabbir W, Lemmens-Gruber R, Chakraborty T, Lazrak A, Matthay MA, Eaton DC, Lucas R. A novel tumor necrosis factor-mediated mechanism of direct epithelial sodium channel activation. Am J Respir Crit Care Med 190: 522–532, 2014. doi: 10.1164/rccm.201405-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis IC, Lazarowski ER, Chen FP, Hickman-Davis JM, Sullender WM, Matalon S. Post-infection A77-1726 blocks pathophysiologic sequelae of respiratory syncytial virus infection. Am J Respir Cell Mol Biol 37: 379–386, 2007. doi: 10.1165/rcmb.2007-0142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis IC, Lazarowski ER, Hickman-Davis JM, Fortenberry JA, Chen FP, Zhao X, Sorscher E, Graves LM, Sullender WM, Matalon S. Leflunomide prevents alveolar fluid clearance inhibition by respiratory syncytial virus. Am J Respir Crit Care Med 173: 673–682, 2006. doi: 10.1164/rccm.200508-1200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis IC, Sullender WM, Hickman-Davis JM, Lindsey JR, Matalon S. Nucleotide-mediated inhibition of alveolar fluid clearance in BALB/c mice after respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol 286: L112–L120, 2004. doi: 10.1152/ajplung.00218.2003. [DOI] [PubMed] [Google Scholar]
- 18.Downs CA, Kreiner L, Zhao XM, Trac P, Johnson NM, Hansen JM, Brown LA, Helms MN. Oxidized glutathione (GSSG) inhibits epithelial sodium channel activity in primary alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 308: L943–L952, 2015. doi: 10.1152/ajplung.00213.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doyle WJ, Skoner DP, Hayden F, Buchman CA, Seroky JT, Fireman P. Nasal and otologic effects of experimental influenza A virus infection. Ann Otol Rhinol Laryngol 103: 59–69, 1994. doi: 10.1177/000348949410300111. [DOI] [PubMed] [Google Scholar]
- 20.DuVall MD, Zhu S, Fuller CM, Matalon S. Peroxynitrite inhibits amiloride-sensitive Na+ currents in Xenopus oocytes expressing α β γ-rENaC. Am J Physiol 274: C1417–C1423, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Fang X, Fukuda N, Barbry P, Sartori C, Verkman AS, Matthay MA. Novel role for CFTR in fluid absorption from the distal airspaces of the lung. J Gen Physiol 119: 199–207, 2002. doi: 10.1085/jgp.119.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol 290: L242–L249, 2006. doi: 10.1152/ajplung.00178.2005. [DOI] [PubMed] [Google Scholar]
- 23.Fang X, Song Y, Zemans R, Hirsch J, Matthay MA. Fluid transport across cultured rat alveolar epithelial cells: a novel in vitro system. Am J Physiol Lung Cell Mol Physiol 287: L104–L110, 2004. doi: 10.1152/ajplung.00176.2003. [DOI] [PubMed] [Google Scholar]
- 24.Fu L, Rab A, Tang L, Bebok Z, Rowe SM, Bartoszewski R, Collawn JF. ΔF508 CFTR surface stability is regulated by DAB2 and CHIP-mediated ubiquitination in post-endocytic compartments. PLoS One 10: e0123131, 2015. doi: 10.1371/journal.pone.0123131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuda N, Jayr C, Lazrak A, Wang Y, Lucas R, Matalon S, Matthay MA. Mechanisms of TNF-α stimulation of amiloride-sensitive sodium transport across alveolar epithelium. Am J Physiol Lung Cell Mol Physiol 280: L1258–L1265, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Gallos G, Yocum GT, Siviski ME, Yim PD, Fu XW, Poe MM, Cook JM, Harrison N, Perez-Zoghbi J, Emala CW Sr. Selective targeting of the α5-subunit of GABAA receptors relaxes airway smooth muscle and inhibits cellular calcium handling. Am J Physiol Lung Cell Mol Physiol 308: L931–L942, 2015. doi: 10.1152/ajplung.00107.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillie DJ, Pace AJ, Coakley RJ, Koller BH, Barker PM. Liquid and ion transport by fetal airway and lung epithelia of mice deficient in sodium-potassium-2-chloride transporter. Am J Respir Cell Mol Biol 25: 14–20, 2001. doi: 10.1165/ajrcmb.25.1.4500. [DOI] [PubMed] [Google Scholar]
- 28.Gotts JE, Abbott J, Matthay MA. Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. Am J Physiol Lung Cell Mol Physiol 307: L395–L406, 2014. doi: 10.1152/ajplung.00110.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gould NS, Min E, Martin RJ, Day BJ. CFTR is the primary known apical glutathione transporter involved in cigarette smoke-induced adaptive responses in the lung. Free Radic Biol Med 52: 1201–1206, 2012. doi: 10.1016/j.freeradbiomed.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregory DJ, Kobzik L. Influenza lung injury: mechanisms and therapeutic opportunities. Am J Physiol Lung Cell Mol Physiol 309: L1041–L1046, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Y, DuVall MD, Crow JP, Matalon S. Nitric oxide inhibits Na+ absorption across cultured alveolar type II monolayers. Am J Physiol 274: L369–L377, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Heaton NS, Langlois RA, Sachs D, Lim JK, Palese P, tenOever BR. Long-term survival of influenza virus infected club cells drives immunopathology. J Exp Med 211: 1707–1714, 2014. doi: 10.1084/jem.20140488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helms MN, Jain L, Self JL, Eaton DC. Redox regulation of epithelial sodium channels examined in alveolar type 1 and 2 cells patch-clamped in lung slice tissue. J Biol Chem 283: 22875–22883, 2008. doi: 10.1074/jbc.M801363200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henkel JR, Apodaca G, Altschuler Y, Hardy S, Weisz OA. Selective perturbation of apical membrane traffic by expression of influenza M2, an acid-activated ion channel, in polarized madin-darby canine kidney cells. Mol Biol Cell 9: 2477–2490, 1998. doi: 10.1091/mbc.9.9.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henkel JR, Popovich JL, Gibson GA, Watkins SC, Weisz OA. Selective perturbation of early endosome and/or trans-Golgi network pH but not lysosome pH by dose-dependent expression of influenza M2 protein. J Biol Chem 274: 9854–9860, 1999. doi: 10.1074/jbc.274.14.9854. [DOI] [PubMed] [Google Scholar]
- 36.Henry PJ, Rigby PJ, Mackenzie JS, Goldie RG. Effect of respiratory tract viral infection on murine airway beta-adrenoceptor function, distribution and density. Br J Pharmacol 104: 914–921, 1991. doi: 10.1111/j.1476-5381.1991.tb12526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirsh AJ, Zhang J, Zamurs A, Fleegle J, Thelin WR, Caldwell RA, Sabater JR, Abraham WM, Donowitz M, Cha B, Johnson KB, St George JA, Johnson MR, Boucher RC. Pharmacological properties of N-(3,5-diamino-6-chloropyrazine-2-carbonyl)-N′-4-[4-(2,3-dihydroxypropoxy)phenyl]butyl-guanidine methanesulfonate (552-02), a novel epithelial sodium channel blocker with potential clinical efficacy for cystic fibrosis lung disease. J Pharmacol Exp Ther 325: 77–88, 2008. doi: 10.1124/jpet.107.130443. [DOI] [PubMed] [Google Scholar]
- 38.Hofer CC, Woods PS, Davis IC. Infection of mice with influenza A/WSN/33 (H1N1) virus alters alveolar type II cell phenotype. Am J Physiol Lung Cell Mol Physiol 308: L628–L638, 2015. doi: 10.1152/ajplung.00373.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet 12: 325–328, 1996. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- 40.Infield DT, Cui G, Kuang C, McCarty NA. Positioning of extracellular loop 1 affects pore gating of the cystic fibrosis transmembrane conductance regulator. Am J Physiol Lung Cell Mol Physiol 310: L403–L414, 2016. doi: 10.1152/ajplung.00259.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito Y, Correll K, Zemans RL, Leslie CC, Murphy RC, Mason RJ. Influenza induces IL-8 and GM-CSF secretion by human alveolar epithelial cells through HGF/c-Met and TGF-α/EGFR signaling. Am J Physiol Lung Cell Mol Physiol 308: L1178–L1188, 2015. doi: 10.1152/ajplung.00290.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain L, Chen XJ, Malik B, Al-Khalili O, Eaton DC. Antisense oligonucleotides against the α-subunit of ENaC decrease lung epithelial cation-channel activity. Am J Physiol 276: L1046–L1051, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Jain L, Chen XJ, Ramosevac S, Brown LA, Eaton DC. Expression of highly selective sodium channels in alveolar type II cells is determined by culture conditions. Am J Physiol Lung Cell Mol Physiol 280: L646–L658, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Ji HL, Song W, Gao Z, Su XF, Nie HG, Jiang Y, Peng JB, He YX, Liao Y, Zhou YJ, Tousson A, Matalon S. SARS-CoV proteins decrease levels and activity of human ENaC via activation of distinct PKC isoforms. Am J Physiol Lung Cell Mol Physiol 296: L372–L383, 2009. doi: 10.1152/ajplung.90437.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji HL, Zhao RZ, Chen ZX, Shetty S, Idell S, Matalon S. δ ENaC: a novel divergent amiloride-inhibitable sodium channel. Am J Physiol Lung Cell Mol Physiol 303: L1013–L1026, 2012. doi: 10.1152/ajplung.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jilling T, Haddad IY, Cheng SH, Matalon S. Nitric oxide inhibits heterologous CFTR expression in polarized epithelial cells. Am J Physiol 277: L89–L96, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci USA 103: 4964–4969, 2006. doi: 10.1073/pnas.0600855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koeppen K, Chapline C, Sato JD, Stanton BA. Nedd4-2 does not regulate wt-CFTR in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 303: L720–L727, 2012. doi: 10.1152/ajplung.00409.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kudlacz EM, Baugh LE, Porter WP, Kenny MT, Farrell AM. A time-course study of airway hyperresponsiveness in conscious parainfluenza virus type 3-infected guinea pigs. Lab Anim Sci 43: 445–453, 1993. [PubMed] [Google Scholar]
- 50.Kulkarni T, de Andrade J, Zhou Y, Luckhardt T, Thannickal VJ. Alveolar epithelial disintegrity in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 311: L185–L191, 2016. doi: 10.1152/ajplung.00115.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kunzelmann K, Beesley AH, King NJ, Karupiah G, Young JA, Cook DI. Influenza virus inhibits amiloride-sensitive Na+ channels in respiratory epithelia. Proc Natl Acad Sci USA 97: 10282–10287, 2000. doi: 10.1073/pnas.160041997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazrak A, Chen L, Jurkuvenaite A, Doran SF, Liu G, Li Q, Lancaster JR Jr, Matalon S. Regulation of alveolar epithelial Na+ channels by ERK1/2 in chlorine-breathing mice. Am J Respir Cell Mol Biol 46: 342–354, 2012. doi: 10.1165/rcmb.2011-0309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lazrak A, Creighton JR, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW Sr., Stober VP, Trempus CS, Garantziotis S and Matalon S. Hyaluronan mediates airway hyper-responsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol Physiol 308: L891–L903, 2015. doi: 10.1152/ajplung.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lazrak A, Iles KE, Liu G, Noah DL, Noah JW, Matalon S. Influenza virus M2 protein inhibits epithelial sodium channels by increasing reactive oxygen species. FASEB J 23: 3829–3842, 2009. doi: 10.1096/fj.09-135590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lazrak A, Jurkuvenaite A, Chen L, Keeling KM, Collawn JF, Bedwell DM, Matalon S. Enhancement of alveolar epithelial sodium channel activity with decreased cystic fibrosis transmembrane conductance regulator expression in mouse lung. Am J Physiol Lung Cell Mol Physiol 301: L557–L567, 2011. doi: 10.1152/ajplung.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee B, Robinson KM, McHugh KJ, Scheller EV, Mandalapu S, Chen C, Di YP, Clay ME, Enelow RI, Dubin PJ, Alcorn JF. Influenza-induced type I interferon enhances susceptibility to gram-negative and gram-positive bacterial pneumonia in mice. Am J Physiol Lung Cell Mol Physiol 309: L158–L167, 2015. doi: 10.1152/ajplung.00338.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.LeSimple P, Liao J, Robert R, Gruenert DC, Hanrahan JW. Cystic fibrosis transmembrane conductance regulator trafficking modulates the barrier function of airway epithelial cell monolayers. J Physiol 588: 1195–1209, 2010. doi: 10.1113/jphysiol.2009.182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li T, Folkesson HG. RNA interference for α-ENaC inhibits rat lung fluid absorption in vivo. Am J Physiol Lung Cell Mol Physiol 290: L649–L660, 2006. doi: 10.1152/ajplung.00205.2005. [DOI] [PubMed] [Google Scholar]
- 59.Lindert J, Perlman CE, Parthasarathi K, Bhattacharya J. Chloride-dependent secretion of alveolar wall liquid determined by optical-sectioning microscopy. Am J Respir Cell Mol Biol 36: 688–696, 2007. doi: 10.1165/rcmb.2006-0347OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Livraghi A, Randell SH. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol Pathol 35: 116–129, 2007. doi: 10.1080/01926230601060025. [DOI] [PubMed] [Google Scholar]
- 61.Londino JD, Lazrak A, Jurkuvenaite A, Collawn JF, Noah JW, Matalon S. Influenza matrix protein 2 alters CFTR expression and function through its ion channel activity. Am J Physiol Lung Cell Mol Physiol 304: L582–L592, 2013. doi: 10.1152/ajplung.00314.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Londino JD, Lazrak A, Noah JW, Aggarwal S, Bali V, Woodworth BA, Bebok Z, Matalon S. Influenza virus M2 targets cystic fibrosis transmembrane conductance regulator for lysosomal degradation during viral infection. FASEB J 29: 2712–2725, 2015. doi: 10.1096/fj.14-268755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Londino JD, Matalon S. Chloride secretion across adult alveolar epithelial cells contributes to cardiogenic edema. Proc Natl Acad Sci USA 110: 10055–10056, 2013. doi: 10.1073/pnas.1307480110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Looney MR, Sartori C, Chakraborty S, James PF, Lingrel JB, Matthay MA. Decreased expression of both the α1- and α2-subunits of the Na-K-ATPase reduces maximal alveolar epithelial fluid clearance. Am J Physiol Lung Cell Mol Physiol 289: L104–L110, 2005. doi: 10.1152/ajplung.00464.2004. [DOI] [PubMed] [Google Scholar]
- 65.Matalon S, Bartoszewski R, Collawn JF. Role of epithelial sodium channels in the regulation of lung fluid homeostasis. Am J Physiol Lung Cell Mol Physiol 309: L1229–L1238, 2015. doi: 10.1152/ajplung.00319.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matalon S, Hardiman KM, Jain L, Eaton DC, Kotlikoff M, Eu JP, Sun J, Meissner G, Stamler JS. Regulation of ion channel structure and function by reactive oxygen-nitrogen species. Am J Physiol Lung Cell Mol Physiol 285: L1184–L1189, 2003. doi: 10.1152/ajplung.00281.2003. [DOI] [PubMed] [Google Scholar]
- 67.Matalon S, Lazrak A, Jain L, Eaton DC. Invited review: biophysical properties of sodium channels in lung alveolar epithelial cells. J Appl Physiol (1985) 93: 1852–1859, 2002. doi: 10.1152/japplphysiol.01241.2001. [DOI] [PubMed] [Google Scholar]
- 68.Matalon S, O’Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol 61: 627–661, 1999. doi: 10.1146/annurev.physiol.61.1.627. [DOI] [PubMed] [Google Scholar]
- 69.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 82: 569–600, 2002. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 70.Matthay MA, Landolt CC, Staub NC. Differential liquid and protein clearance from the alveoli of anesthetized sheep. J Appl Physiol Respir Environ Exerc Physiol 53: 96–104, 1982. [DOI] [PubMed] [Google Scholar]
- 71.McCullers JA. Effect of antiviral treatment on the outcome of secondary bacterial pneumonia after influenza. J Infect Dis 190: 519–526, 2004. doi: 10.1086/421525. [DOI] [PubMed] [Google Scholar]
- 72.Mitchell H, Levin D, Forrest S, Beauchemin CA, Tipper J, Knight J, Donart N, Layton RC, Pyles J, Gao P, Harrod KS, Perelson AS, Koster F. Higher level of replication efficiency of 2009 (H1N1) pandemic influenza virus than those of seasonal and avian strains: kinetics from epithelial cell culture and computational modeling. J Virol 85: 1125–1135, 2011. doi: 10.1128/JVI.01722-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mori I, Komatsu T, Takeuchi K, Nakakuki K, Sudo M, Kimura Y. In vivo induction of apoptosis by influenza virus. J Gen Virol 76: 2869–2873, 1995. doi: 10.1099/0022-1317-76-11-2869. [DOI] [PubMed] [Google Scholar]
- 74.Nguyen H, Uhal BD. The unfolded protein response controls ER stress-induced apoptosis of lung epithelial cells through angiotensin generation. Am J Physiol Lung Cell Mol Physiol 311: L846–L854, 2016. doi: 10.1152/ajplung.00449.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nielsen VG, Duvall MD, Baird MS, Matalon S. cAMP activation of chloride and fluid secretion across the rabbit alveolar epithelium. Am J Physiol 275: L1127–L1133, 1998. [DOI] [PubMed] [Google Scholar]
- 76.Noah DL, Twu KY, Krug RM. Cellular antiviral responses against influenza A virus are countered at the posttranscriptional level by the viral NS1A protein via its binding to a cellular protein required for the 3′ end processing of cellular pre-mRNAS. Virology 307: 386–395, 2003. doi: 10.1016/S0042-6822(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 77.Noah JW, Noah DL, Matalon S. Influenza exerts continued pressure in an era of modern medicine. Am J Respir Cell Mol Biol 41: 3–7, 2009. doi: 10.1165/rcmb.2009-0158ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’Brodovich H, Canessa C, Ueda J, Rafii B, Rossier BC, Edelson J. Expression of the epithelial Na+ channel in the developing rat lung. Am J Physiol 265: C491–C496, 1993. [DOI] [PubMed] [Google Scholar]
- 79.Okiyoneda T, Barrière H, Bagdány M, Rabeh WM, Du K, Höhfeld J, Young JC, Lukacs GL. Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science 329: 805–810, 2010. doi: 10.1126/science.1191542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peteranderl C, Morales-Nebreda L, Selvakumar B, Lecuona E, Vadász I, Morty RE, Schmoldt C, Bespalowa J, Wolff T, Pleschka S, Mayer K, Gattenloehner S, Fink L, Lohmeyer J, Seeger W, Sznajder JI, Mutlu GM, Budinger GR, Herold S. Macrophage-epithelial paracrine crosstalk inhibits lung edema clearance during influenza infection. J Clin Invest 126: 1566–1580, 2016. doi: 10.1172/JCI83931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peters DM, Vadász I, Wujak L, Wygrecka M, Olschewski A, Becker C, Herold S, Papp R, Mayer K, Rummel S, Brandes RP, Günther A, Waldegger S, Eickelberg O, Seeger W, Morty RE. TGF-β directs trafficking of the epithelial sodium channel ENaC which has implications for ion and fluid transport in acute lung injury. Proc Natl Acad Sci USA 111: E374–E383, 2014. doi: 10.1073/pnas.1306798111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, Bánfi B, Horswill AR, Stoltz DA, McCray PB Jr, Welsh MJ, Zabner J. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487: 109–113, 2012. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pinto LH, Lamb RA. The M2 proton channels of influenza A and B viruses. J Biol Chem 281: 8997–9000, 2006. doi: 10.1074/jbc.R500020200. [DOI] [PubMed] [Google Scholar]
- 84.Pittet LA, Hall-Stoodley L, Rutkowski MR, Harmsen AG. Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae. Am J Respir Cell Mol Biol 42: 450–460, 2010. doi: 10.1165/rcmb.2007-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Podsiad A, Standiford TJ, Ballinger MN, Eakin R, Park P, Kunkel SL, Moore BB, Bhan U. MicroRNA-155 regulates host immune response to postviral bacterial pneumonia via IL-23/IL-17 pathway. Am J Physiol Lung Cell Mol Physiol 310: L465–L475, 2016. doi: 10.1152/ajplung.00224.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Randell SH, Boucher RC; University of North Carolina Virtual Lung Group . Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol 35: 20–28, 2006. doi: 10.1165/rcmb.2006-0082SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Renk H, Regamey N, Hartl D. Influenza A(H1N1)pdm09 and cystic fibrosis lung disease: a systematic meta-analysis. PLoS One 9: e78583, 2014. doi: 10.1371/journal.pone.0078583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sakaguchi T, Leser GP, Lamb RA. The ion channel activity of the influenza virus M2 protein affects transport through the Golgi apparatus. J Cell Biol 133: 733–747, 1996. doi: 10.1083/jcb.133.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sakuma T, Gu X, Wang Z, Maeda S, Sugita M, Sagawa M, Osanai K, Toga H, Ware LB, Folkesson G, Matthay MA. Stimulation of alveolar epithelial fluid clearance in human lungs by exogenous epinephrine. Crit Care Med 34: 676–681, 2006. doi: 10.1097/01.CCM.0000201403.70636.0F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salomon JJ, Spahn S, Wang X, Füllekrug J, Bertrand CA, Mall MA. Generation and functional characterization of epithelial cells with stable expression of SLC26A9 Cl− channels. Am J Physiol Lung Cell Mol Physiol 310: L593–L602, 2016. doi: 10.1152/ajplung.00321.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scherrer U, Sartori C, Lepori M, Allemann Y, Duplain H, Trueb L Nicod P.. High-altitude pulmonary edema: from exaggerated pulmonary hypertension to a defect in transepithelial sodium transport. Adv Exp Med Biol 474: 93–107, 1999. [DOI] [PubMed] [Google Scholar]
- 92.Sears PR, Yin WN, Ostrowski LE. Continuous mucociliary transport by primary human airway epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 309: L99–L108, 2015. doi: 10.1152/ajplung.00024.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Short KR, Kroeze EJBV, Fouchier RAM, Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect Dis 14: 57–69, 2014. doi: 10.1016/S1473-3099(13)70286-X. [DOI] [PubMed] [Google Scholar]
- 94.Solymosi EA, Kaestle-Gembardt SM, Vadász I, Wang L, Neye N, Chupin CJ, Rozowsky S, Ruehl R, Tabuchi A, Schulz H, Kapus A, Morty RE, Kuebler WM. Chloride transport-driven alveolar fluid secretion is a major contributor to cardiogenic lung edema. Proc Natl Acad Sci USA 110: E2308–E2316, 2013. doi: 10.1073/pnas.1216382110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Song W, Liu G, Bosworth CA, Walker JR, Megaw GA, Lazrak A, Abraham E, Sullender WM, Matalon S. Respiratory syncytial virus inhibits lung epithelial Na+ channels by up-regulating inducible nitric-oxide synthase. J Biol Chem 284: 7294–7306, 2009. doi: 10.1074/jbc.M806816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song W, Wei S, Zhou Y, Lazrak A, Liu G, Londino JD, Squadrito GL, Matalon S. Inhibition of lung fluid clearance and epithelial Na+ channels by chlorine, hypochlorous acid, and chloramines. J Biol Chem 285: 9716–9728, 2010. doi: 10.1074/jbc.M109.073981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suarez DL, Perdue ML, Cox N, Rowe T, Bender C, Huang J, Swayne DE. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J Virol 72: 6678–6688, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takeda M, Pekosz A, Shuck K, Pinto LH, Lamb RA. Influenza a virus M2 ion channel activity is essential for efficient replication in tissue culture. J Virol 76: 1391–1399, 2002. doi: 10.1128/JVI.76.3.1391-1399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, Boucher RC. Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J Biol Chem 280: 35751–35759, 2005. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taubenberger JK, Morens DM. The pathology of influenza virus infections. Annu Rev Pathol 3: 499–522, 2008. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tingay DG, Rajapaksa A, Zannin E, Pereira-Fantini PM, Dellaca RL, Perkins EJ, Zonneveld CE, Adler A, Black D, Frerichs I, Lavizzari A, Sourial M, Grychtol B, Mosca F, Davis PG. Effectiveness of individualized lung recruitment strategies at birth: an experimental study in preterm lambs. Am J Physiol Lung Cell Mol Physiol 312: L32–L41, 2017. doi: 10.1152/ajplung.00416.2016. [DOI] [PubMed] [Google Scholar]
- 102.Tomkiewicz RP, App EM, Zayas JG, Ramirez O, Church N, Boucher RC, Knowles MR, King M. Amiloride inhalation therapy in cystic fibrosis. Influence on ion content, hydration, and rheology of sputum Am Rev Respir Dis 148: 1002–1007, 1993. doi: 10.1164/ajrccm/148.4_Pt_1.1002. [DOI] [PubMed] [Google Scholar]
- 103.Trac PT, Thai TL, Linck V, Zou L, Greenlee M, Yue Q, Al-Khalili O, Alli AA, Eaton AF, Eaton DC. Alveolar nonselective channels are ASIC1a/α-ENaC channels and contribute to AFC. Am J Physiol Lung Cell Mol Physiol 312: L797–L811, 2017. doi: 10.1152/ajplung.00379.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tucker SP, Compans RW. Virus infection of polarized epithelial cells. Adv Virus Res 42: 187–247, 1993. doi: 10.1016/S0065-3527(08)60086-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tucker SP, Thornton CL, Wimmer E, Compans RW. Vectorial release of poliovirus from polarized human intestinal epithelial cells. J Virol 67: 4274–4282, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tyrrell J, Qian X, Freire J, Tarran R. Roflumilast combined with adenosine increases mucosal hydration in human airway epithelial cultures after cigarette smoke exposure. Am J Physiol Lung Cell Mol Physiol 308: L1068–L1077, 2015. doi: 10.1152/ajplung.00395.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1376–1383, 2001. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 108.Widdicombe JH, Wine JJ. Airway gland structure and function. Physiol Rev 95: 1241–1319, 2015. doi: 10.1152/physrev.00039.2014. [DOI] [PubMed] [Google Scholar]
- 109.Wolk KE, Lazarowski ER, Traylor ZP, Yu EN, Jewell NA, Durbin RK, Durbin JE, Davis IC. Influenza A virus inhibits alveolar fluid clearance in BALB/c mice. Am J Respir Crit Care Med 178: 969–976, 2008. doi: 10.1164/rccm.200803-455OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Woods PS, Tazi MF, Chesarino NM, Amer AO, Davis IC. TGF-β-induced IL-6 prevents development of acute lung injury in influenza A virus-infected F508del CFTR-heterozygous mice. Am J Physiol Lung Cell Mol Physiol 308: L1136–L1144, 2015. doi: 10.1152/ajplung.00078.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu W, Zheng S, Goggans TM, Kiser P, Quinones-Mateu ME, Janocha AJ, Comhair SA, Slee R, Williams BR, Erzurum SC. Cystic fibrosis and normal human airway epithelial cell response to influenza a viral infection. J Interferon Cytokine Res 26: 609–627, 2006. doi: 10.1089/jir.2006.26.609. [DOI] [PubMed] [Google Scholar]
- 112.Yadav AK, Bracher A, Doran SF, Leustik M, Squadrito GL, Postlethwait EM, Matalon S. Mechanisms and modification of chlorine-induced lung injury in animals. Proc Am Thorac Soc 7: 278–283, 2010. doi: 10.1513/pats.201001-009SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang Z, Chiou TT, Stossel TP, Kobzik L. Plasma gelsolin improves lung host defense against pneumonia by enhancing macrophage NOS3 function. Am J Physiol Lung Cell Mol Physiol 309: L11–L16, 2015. doi: 10.1152/ajplung.00094.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yue G, Matalon S. Mechanisms and sequelae of increased alveolar fluid clearance in hyperoxic rats. Am J Physiol 272: L407–L412, 1997. [DOI] [PubMed] [Google Scholar]
- 115.Yue G, Russell WJ, Benos DJ, Jackson RM, Olman MA, Matalon S. Increased expression and activity of sodium channels in alveolar type II cells of hyperoxic rats. Proc Natl Acad Sci USA 92: 8418–8422, 1995. doi: 10.1073/pnas.92.18.8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao RZ, Nie HG, Su XF, Han DY, Lee A, Huang Y, Chang Y, Matalon S, Ji HL. Characterization of a novel splice variant of δ ENaC subunit in human lungs. Am J Physiol Lung Cell Mol Physiol 302: L1262–L1272, 2012. doi: 10.1152/ajplung.00331.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu S, Yue G, Shoemaker RL, Matalon S. Adult alveolar type II cells lack cAMP and Ca2+-activated Cl−channels. Biochem Biophys Res Commun 218: 302–308, 1996. doi: 10.1006/bbrc.1996.0053. [DOI] [PubMed] [Google Scholar]