Abstract
Sarcopenia, or age-related muscle decline, occurs in most organisms and burdens both human health and the healthcare system. As our population ages, additional options for treating sarcopenia are needed. Mitochondrial dysfunction is implicated in the onset of sarcopenia, so therapies directed at improving mitochondrial function in muscle should be considered. Many naturally-occurring compounds, derived from commonly consumed foods, possess anti-sarcopenic effects, such asnicotinamide riboside, tomatidine, and Urolithin A. These naturally-occurring compounds can improve mitochondrial health and efficiency by modulating mitochondrial biogenesis, cellular stress resistance, or mitophagy. Further research should assess whether compounds that improve mitochondrial health can attenuate sarcopenia in humans.
Keywords: Sarcopenia, aging, mitochondria, mitophagy, phytochemical, Nicotinamide adenine dinucleotide
1. INTRODUCTION
Aging is associated with a host of disorders that increase morbidity and mortality. With an increasing population of older individuals worldwide1, 2, it is important to assess common health issues that impact their quality of life. Sarcopenia is age associated loss of skeletal muscle strength and function3, though this definition is still under consideration4. Many age-associated disorders that diminish quality of life affect highly energetic cell types, such is the case with sarcopenia and skeletal muscle. Mitochondria are critical to these energetic cell types, and a mitochondrial perspective of sarcopenia may prove fruitful towards understanding the complex disease. High-energy skeletal muscle tissue relies upon mitochondria for proper energy production and contractile function, and mitochondria quantity has been known to decrease in older individuals5.The association between sarcopenia and mitochondria may also berelevant for finding new, effective treatments. This review will assess the mitochondrial perspective of sarcopenia, and introduce some naturally-occurring compounds with the potential to attenuate the onset of sarcopenia.
From a clinical perspective, sarcopenia is highly problematic for patients, and no current treatment acts as a silver-bullet for it. Patients with sarcopenia have an increased risk of falling and have a general increase in mortality6. Sarcopenia is associated witha series of socio-economic problems, including increased hospital stay and high economic burden for both patients and their families7, 8. These negative outcomes show the need for additional research on treatments for patients with sarcopenia. Currently, exercise is considered the most effective intervention for sarcopenia9, 10, yet elderly populations have issues meeting necessary physical activity guidelines11, showing that additional interventions could assist in treatment. Therefore, supplements for treating sarcopeniaare currently being investigated. Research on these supplements involves various laboratory model organisms, as most organisms experience age-related decline of muscle function that is similar to humans.
Laboratory animal models compliment limited human population studies for research on sarcopenia, and allows for analysis of the underlying mechanisms of sarcopenia. A common, high-throughput model organism to study aging and sarcopenia is the nematode C. elegans. C. elegans is used extensively due to its relatively short lifespan, low cost of maintenance, availability of genetic manipulation, and conservation of major known age-related signaling pathways seen in humans12, 13. The pharynx of the C. elegans is of great relevance since behavioral, morphological, and molecular assays can be used on this tissue to assess sarcopenia14, 15. C. elegans sarcomeres can be assessed through the muscle specific p-myo-3 driven mitochondrial GFP fluorescence. In wild-type C. elegans, body wall muscle cells mitochondria becomes increasingly fragmented as the organism age16 (Figure 1), which attests to decreased mitochondrial function as C. elegans age. Many of the studies on potentially anti-sarcopenic naturally-occurring compounds useC. elegans as a model organism. Importantly, a list of genes involved in sarcopenia in C. elegans has been discovered, and many of these genes are developmentally conserved, including sac1, as160, tbc1d1 and daf-1617. Studies in this organism is an important first step for transitioning potential treatments to human clinical trials.
Figure 1. C. elegans muscle specific mitochondrial fragmentation with age.
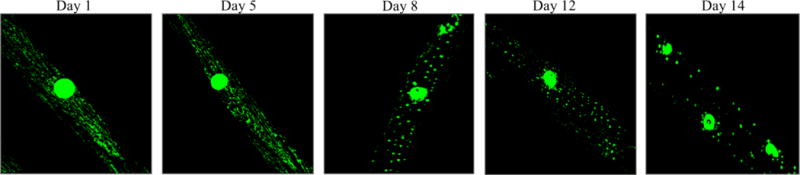
Both the nucleus and the mitochondria within a muscle cell of C. elegans are seen here. The quality of mitochondria can be assessed by the relative morphology and connectivity.In healthy organisms, mitochondria are maintained as a network in muscle cells and other cells, which increases efficiency. This network can be compromised as the organism naturally ages and in premature aging diseases (progeria). This fragmentation of mitochondrial network is associated with decreased healthspan, and is likely tied to mitophagy and other mitochondrial quality control processes. Compounds that alleviate this excessive fragmentation may improve muscle health. (I realy like this images, so clear. I think, it will be nice to include also some with e.g. tomatodin or NR treatment for confirming this reviews contents, if it’s possible)
2. A MITOCHONDRIA PERSPECTIVE OF SARCOPENIA
The etiologies of sarcopenia are not fully understood, but mitochondrial dysfunction has been strongly implicated in muscular health through metabolism, reactive oxygen species (ROS), or mitochondrial dysfunction and maintenance. Impaired metabolism in muscle cells is implicated in sarcopenia, and decreased catabolism or increasing anabolism may alleviate this by increasing bulk muscle mass18,19, 20.These include ingesting an assortment of vitamins, amino acids, or creatine. The clinical supplements mentioned have broad effects on the human body20, but mechanistic insight has been gained recently. For example, supplementation of the amino acid leucine is a necessity for amino acid-dependent attenuation of sarcopenia in elderly adults, but not necessary for attenuating muscle decline in younger adults19. This suggests that sarcopenia is related to, but dissimilar from, normalmuscle decline.
Reactive oxygen species (ROS) play a complex role in etiology of sarcopenia21, 22. In aged organisms, increased ROS is associated with higher decline of muscle function due to inactivity23. Also, as organisms age, oxidative stress is increased, in parallel with decreased anti-oxidative activity. Yet it is unknown whether ROS is the causative agent or a byproduct of sarcopenia24. Counter evidence for ROS exacerbating sarcopenia have been offered. A highly potent anti-sarcopenia intervention is exercise, its known to increase ROS production25.While supplementation with antioxidants in antioxidant-deficient aged mice improved muscle function and quality26, the effectiveness of antioxidant supplementation in humans with normal physiological levels of antioxidants is inconclusive27, 28.Accumulating mechanistic and intervention studies using multiple model organisms suggest a different perspective for ROS.ROS can be advantageous for cells, as it acts as a signaling molecule for regulating hypoxia, innate immunity, and mitophagy responses29, 30. ROS may act through a hormetic mechanism, in which physiological levels or moderate increases in ROS (like through exercise and fasting) are beneficial for health by activating essential cellular signaling pathways that protect against oxidative, metabolic and proteotoxic stress29, 31, 32. However, persistently disproportionate or supraphysiological levels of ROS induces lethal damage to DNA, proteins, and cells, prompting chronic diseases and aging33–36. Future studies should distinguish ROS quantity when assessing its effect on sarcopenia, as this may be the difference between benefit and detriment. The connection of ROS levels, anti-oxidative ability, and sarcopenia in human populations needs further elucidation.
Progression of sarcopenia has been linked with mitochondrial dysfunction, as well as changes in mitochondrial mass and morphology. There is an age-dependent reduction of mitochondrial mass, possibly due to dysfunction of major regulators of mitochondrial biogenesis including AMPK and PGC-1α37, 38. In addition to decreased mass, there is age-dependent change of mitochondrial morphology, indicating imbalance between mitochondrial fusion and fission39. Since mitochondria constantly undergo damage, partly through normal aging, maintenance of a healthy mitochondrial pool is extremely important and is regulated by a cellular self-clearance system, mitochondrial autophagy (termed mitophagy)40, 41. Mitophagy is tightly regulated by an inter-collected complex network with major known executors including PINK1, Parkin, NDP52,optineurin, and NIX/BNIP3L (DCT-1 in C. elegans)40, 42, 43. The importance of mitophagy in longevity and healthy aging, which includes maintenance of muscle performance, has been investigated in both mice and C. elegans40, 43, 44. For instance, specifically older mice with dysfunctional mitophagy have increased fatigue and decreased grip strength, which is an indicator of sarcopenia45. In C. elegans, there is age-dependent decline of mitophagy, which leads to mitochondrial dysfunction and increased susceptibility to external stressors43.However, the relationship between mitophagy and muscle function in humans has not been assessed. Taken together, these findings suggest mitochondrial dysfunction plays a significant role in the etiology of sarcopenia in laboratory animal models.
3. PROSPECTIVE INTERVENTIONAL STRATEGIES FOR SARCOPENIA
Based on evidence that mitochondrial dysfunction contributes to sarcopenia, novel strategies for disease intervention have been proposed. These include using bioactive phytochemicals, their derivatives, metabolites, or other synthetic small compounds to target a series of mitochondrial pathways. Since sarcopenia is an age-related disorder, naturally-occurring compounds with strong anti-aging effects have been assessed for anti-sarcopenic properties. These compounds modulate mitochondrial health in various ways, including the NAD+/SIRT1 pathway, mitochondrial stress pathways, or upregulating mitophagy.
3.1 Small compounds targeting the NAD+/SIRT1 pathway
SIRT1 is a NAD+-dependent deacetylase which participates in multiple cellular processes such as cell survival, circadian rhythm, DNA repair, metabolism, and mitochondrial homeostasis46, 47. SIRT1 plays an important role in longevity in yeast (homolog Sir2), C. elegans (Sir2.1), and mice (SIRT1), and has been implicated in the prevention of age-related diseases, including type 2 diabetes, cancer, Alzheimer’s disease, and age-related muscle dysfunction38, 40, 46–48. Data from C. elegans and rodents suggest an age-dependent decrease of SIRT1 activity is due to NAD+ depletion49, 50, and thus reestablishing NAD+/SIRT1 activity through NAD+ precursor or SIRT1 allosteric activator supplementation is likely to have health benefits.
NAD+ is a critical co-factor involved in multiple cellular processes including metabolism, DNA repair, mitochondrial turnover and antioxidantresponses. Increasing NAD+ levels extends lifespan in laboratory animals through mitochondrial maintenance51–53. In mice, impaired intramuscular NAD+ synthesis decreases skeletal muscle mass and strength with age54. Exogenous NAD+ sources, including nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), can be derived from milk and other related food products55 (Figure 2).These exogenous NAD+ sources exhibit promising muscle protection activities. For example, 7-day NMN treatment (intraperitoneal injection, 500 mg NMN/kg body weight/day) on 22-month old wild type mice exhibited increased oxidative phosphorylation and ATP production in gastrocnemius muscle56. Improvement of muscle strength was not seen, probably due to the short-term treatment. Another NAD+ precursor, NR, also increased muscle function and lifespan extension in wild-type mice. Old C57BL/6J mice (22–24 months)fed regular chow diet supplemented with NR(400 mg/kg/day) had slight but statistically significant extension of lifespan, and improved muscle function57. Compared with controls, NR supplementation increased muscle ATP production, oxidative phosphorylation, and physical muscle performance (running distance and grip strength)57. Mechanistically, NR prevented muscle stem cell senescence in both wild-type mice and in the Mdx mouse model of muscular dystrophy through inducing the mitochondrial unfolded protein response (UPRmt) and upregulation of prohibitin proteins involved in stress response57.UPRmt compensates for imbalances in mitochondrial proteostasis by facilitating expression of both mitochondrial DNA and nuclear DNA derived proteins52.We recently showed that a 2-week NR supplementation (intraperitoneal injection, 500 mg NMN/kg body weight/day) significantly ameliorated mitochondrial dysfunction in two DNA-repair deficiency disease mouse models (XPA and what)49. Collectively, these data demonstrate the potential anti-aging and anti-sarcopenia benefits of exogenous NAD+.
Figure 2. Representativebioactive small compounds exhibit mitochondrial benefits.
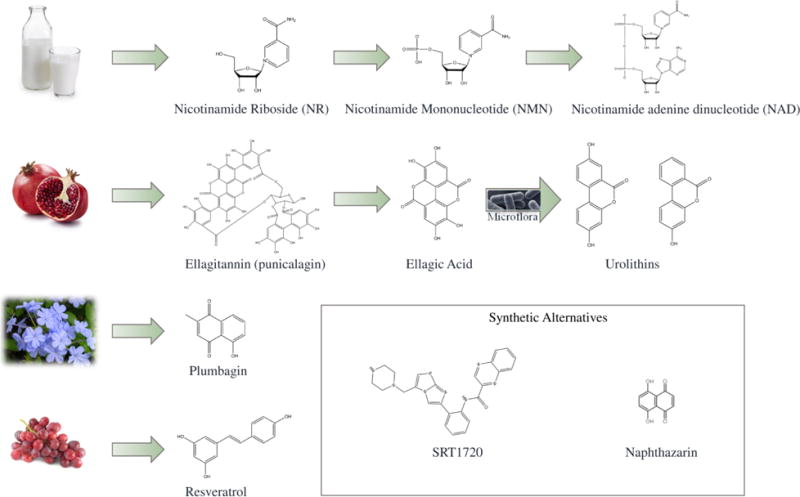
Many of these naturally-occurring compounds are derived from common foods, such as milk,pomegranate, plumbago and grapes. Phytochemicals can be innately synthesized by the plant, such as the case for plumbagin and resveratrol. They can also be metabolized in the body to produce the potential anti-sarcopenic compound, such as the metabolism of NR to NAD+, or the conversion of ellagitannins to urolithins with help of intestinal microflora. Synthetic derivatives of naturally-occurring compounds are also effective in modulating mitochondrial function through similar mechanisms, which is seen with the resveratrol-derived SRT1720 or the plumbagin-derived naphthazarin.
For images used here, Milk: Krans, B. (2014). “Almond Milk vs. Cow Milk vs. Soy Milk vs. Rice Milk”; Promagranate: TrimDownClub (2015). “10 Things We Love About Pomegranates (Plus Pomegranate Recipes)”; Plumbago: brewbooks (2005). Plumbagoauriculata. Wikimedia Commons; Grapes: Council, C. N. C. (2012). “Chicken With Grapes and Rosemary Recipe.”
In addition to NAD+ supplementation, an alternative strategy to increase SIRT1 activity is using sirtuin-activating compounds (STACs). Currently, there are three generations of STACs, all of which act on a common allosteric mechanism to stimulate sirtuin activity46. Some first generation STACs are naturally-occurring compounds, with resveratrol being the best described. Resveratrol is a natural phenol found in grapes and berries58, 59. Studies from mice suggest that resveratrol can improve mitochondrial health through activation of the NAD+/SIRT1-PGC-1α axis38, 60. Middle aged mice (approximatelyone year) treated with resveratrol (0.04% in chow, approximately 22 mg/kg/day) for 6 months had increased survival and muscle performance through rotarod analysis38. While a high fat diet induced higher levels of glycolytic muscle fibers (type IIb), resveratrol normalized fat-diet induced glycolytic muscle fibers to wild type levels, and increased oxidative fast twitch fibers (type IIa and IIx)60. Interestingly, administration of resveratrol (0.05% resveratrol in chow for 18 months) to middle-aged C57BL/6 mice for 10 months did not appear to eliminate sarcopenia, but increased anti-oxidative activity through enhanced mitochondrial SOD2 activity and preserved fast-twitch fiber contractile function61. Based on these studies, resveratrol can have a significant benefit on mice if administered at an early age, though this benefit may be compromised if administration begins later. Consistent with this hypothesis, the mechanical stretch of muscle can induce expression of SIRT1 and its downstream proteins (like SOD2) in an early growth factor 1 (EGR1)-dependent manner62. This activation is lost in aged animals due to loss of EGR1. Second generation synthetic STACs may bestow various health benefits as well, ranging from neuroprotection to improved muscle function and extension of lifespan63, 64. The second generation STAC SRT1720 (100 mg/kg body weight/day) was given to young male C57BL/6J mice for the entirety of their life. In addition to extension of mean lifespan (8.8%) by SRT1720 compared with control, SRT1720 also increased mitochondrial biogenesis and improved muscle performance (rotarod analysis)63. Another synthetic STAC, SRT2104, fed to male mice (200 mg/kg body weight/day) for four weeks prior to hind limb suspension had increased trabecular bone quality and attenuated muscle loss due to disuse65.In summary, STACs are likely able to improve mitochondrial function, and may improve sarcopenia, though further investigation is needed.
3.2 Compounds that enhance cellular stress resistance
Regulation of cellular stress-related pathways may prevent age-related physiological decline. A major cellular stress response pathway is the nuclear erythroid 2-related factor 2/antioxidant response element (Nrf2/ARE) pathway which participates in anti-oxidative response66. Activation of Nrf2 causes many downstream effects, including upregulating anti-oxidative stress67, 68, anti-apoptotic pathways69, anti-inflammatory proteins70, and mitochondrial biogenesis43, 71, 72. Phytochemical plumbagin, derived from the Plumbago genus of flowering plants (Figure 2), directly activates Nrf2/ARE and increases neuronal resistance to damage by oxidative stress73 and excitotoxicity74. Plumbagin and its synthetic derivatives, such as naphthazarin, were shown to increase maximal lifespan in C. elegans through an Nrf2-dependent mechanism (C. elegans homolog SKN-1)75.Sulforaphane, found in broccoli and other similar foods, is another naturally-occurring Nrf2 activator with some mitochondrial effect76 and may be effective in ameliorating muscular dystrophy, a disease with muscle wasting phenotypes77. Repression of the Nrf2 pathway contributes to Hutchinson-Gilford progeria syndrome (HGPS). HGPS is etiologically related to mutations of nuclear architectural proteins lamin A and C. HGPS patients show profound growth delays and premature aging phenotypes, including cardiac and skeletal muscle pathologies66, 78. It has been suggested that inhibition of Nrf2/ARE activity contributes to premature aging in experimental models of HGPS by increasing chronic oxidative stress. Reestablishment of the Nrf2/ARE pathway using small compound Nrf2 activators ameliorates disease phenotypes66. Amyotrophic lateral sclerosis (ALS) is a progressively debilitating disease with motor neuron degeneration and irreversible muscle atrophy. Studies examining ALS mice muscles suggest a retrograde Nrf2/ARE activity in disease progression79. In line with these findings, aged Nrf2−/− mice exhibited impaired antioxidant activity and accelerated muscle cell death compared with wild-type mice80. The direct role for the Nrf2/ARE pathway in preserving muscle function warrants further investigation.
The naturally-occurring compound tomatidine exhibits anti-sarcopenia activity in mice possibly through the regulation of cellular stress81, 82. Tomatidine is a metabolite of α-tomatine, which is abundant in unripe green tomatoes, and elicits broad effects on cells, possessing antibiotic, anti-inflammatory, and anti-carcinogenic properties83, 84. Interestingly, recent studies in mice showed that tomatidine can improve muscular strength and decrease adiposity through inhibition of activating transcription factor 4 (ATF-4)81,82.Two-month supplementation with tomatidine (0.05%)in 22 months old male C57BL/6 mice significantly reduced age-dependent decline of skeletal muscle mass, strength, and quality. At a molecular level, tomatidine inhibited the activity of ATF-4, a bZIP transcription factor subunit regulating oxidative and other stress responses85. Consistent with this finding, mice with muscle-specific knock out of ATF4 showed reduced age-related muscle atrophy82. Ursolic acid, a pentacyclic triterpenoid found in apples, may also exhibit anti-sarcopenic activity in aged mice through modulation of stress responses86, 87. The specific mechanism by which these compounds improve muscle function requires further elucidation. Many phytochemicals were shown to exhibit beneficial effects on cells and organisms through a hormetic mechanism88, 89.A compelling avenue of research would be to address if the anti-sarcopenic properties of tomatidine and ursolic acid are due to a similar hormetic mechanism.
3.3 Mitophagy-inducing compounds
Mitophagy plays a significant role in mitochondrial maintenance in muscles, and mitophagy-inducing agents may hold anti-sarcopenic functions90. Urolithin A is an end-product of a group of naturally-occurring compounds, ellagitannins (ETs), which are found in pomegranates44 (Figure 2). A recent study in C. elegans and mice suggests that urolithin A is a mitophagy-inducing compound which increases muscle function44. In aged C. elegans, urolithin A was able to maintain a healthy mitochondrial pool through both mitochondrial biogenesis and cleavage of damaged mitochondria via mitophagy, which contributed improvement of healthspan and lifespan. Interestingly, in younger C. elegans, mitophagy was induced without mitochondrial biogenesis, and yielded lower mitochondrial content and lower respiration44. Mechanistic insight into how urolithin A induces mitophagy is still limited.
4. Conclusions and future perspectives
Improving the quality of life in older individuals will alleviate pressure on various socio-economic systems, especially health care91. Skeletal muscle atrophy and dysfunction disrupts older individuals from living a healthy lifestyle, and administration of bioactive compounds to preserve muscle function may address this problem. Studies from laboratory animal models suggest the aforementioned naturally-occurringcompounds target mitochondrial biogenesis, cellular stress response, and mitophagy, andhave promising anti-sarcopenic function. Supplementation of these naturally-occurring compounds may ameliorate sarcopenia in older individuals, especially when clinically assessed with other medical treatment options. However, further clinical trials are warranted. Importantly, some of these compounds are currently in clinical trials, including NR and urolithin A (https://clinicaltrials.gov/). Major tasks in the field include a) further elucidation of the molecular mechanisms of sarcopenia, which will facilitate drug design; and b) studies on the optimal pharmacokinetic conditions of these compounds in humans. In conclusion, pharmacological intervention of muscle atrophy and other aging-related phenotypes holds promise to improving human healthspan.
Acknowledgments
We acknowledge the valuable work of the many investigators whose published articles we were unable to cite owing to space limitations. We thank Wendy Iser for worm image preparation. We thank Drs. Sarah J. Mitchell and Tyler G. Demarest for critical reading of the manuscript. This research was supported by the Intramural Research Program of the National Institute on Aging.
References
- 1.Ortman JM, Velkoff VA, Hogan H. An Aging Nation: The Older Population in the United States. US Census Bureau. 2014 [Google Scholar]
- 2.United Nations, D.o.E.a.S.A. Population Division World Population: Ageing 2015. ST/ESA/SER.A/390. 2015 [Google Scholar]
- 3.Cruz-Jentoft AJ, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014;43:748–759. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7:28–36. doi: 10.1002/jcsm.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Short KR, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatr Med. 2011;27:387–399. doi: 10.1016/j.cger.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Sousa AS, et al. Financial impact of sarcopenia on hospitalization costs. Eur J Clin Nutr. 2016 doi: 10.1038/ejcn.2016.73. [DOI] [PubMed] [Google Scholar]
- 8.Sousa AS, Guerra RS, Fonseca I, Pichel F, Amaral TF. Sarcopenia and length of hospital stay. Eur J Clin Nutr. 2016;70:595–601. doi: 10.1038/ejcn.2015.207. [DOI] [PubMed] [Google Scholar]
- 9.Rygiel KA, Picard M, Turnbull DM. The ageing neuromuscular system and sarcopenia - A mitochondrial perspective. J Physiol. 2016 doi: 10.1113/JP271212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witard OC, McGlory C, Hamilton DL, Phillips SM. Growing older with health and vitality: a nexus of physical activity, exercise and nutrition. Biogerontology. 2016;17:529–546. doi: 10.1007/s10522-016-9637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.While AE, Norman JI, Sun F. Physical activity in older people: a systematic review. BMC Public Health. 2013;13 doi: 10.1186/1471-2458-13-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- 13.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 14.Chow DK, Glenn CF, Johnston JL, Goldberg IG, Wolkow CA. Sarcopenia in the Caenorhabditis elegans pharynx correlates with muscle contraction rate over lifespan. Exp Gerontol. 2006;41:252–260. doi: 10.1016/j.exger.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herndon LA, et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419 doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- 16.Regmi SG, Rolland SG, Conradt B. Age-dependent changes in mitochondrial morphology and volume are not predictors of lifespan. Aging (Albany NY) 2014;6:118–130. doi: 10.18632/aging.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashyap L, Perera S, Fisher AL. Identification of novel genes involved in sarcopenia through RNAi screening in Caenorhabditis elegans. The journals of gerontology Series A, Biological sciences and medical sciences. 2012;67:56–65. doi: 10.1093/gerona/glr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morley JE. Sarcopenia in the elderly. Fam Pract. 2012;29(Suppl 1):i44–i48. doi: 10.1093/fampra/cmr063. [DOI] [PubMed] [Google Scholar]
- 19.Moro T, Ebert SM, Adams CM, Rasmussen BB. Amino Acid Sensing in Skeletal Muscle. Trends Endocrinol Metab. 2016 doi: 10.1016/j.tem.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denison HJ, Cooper C, Sayer AA, Robinson SM. Prevention and optimal management of sarcopenia: a review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin Interv Aging. 2015;10:859–869. doi: 10.2147/CIA.S55842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010;11:1509–1526. doi: 10.3390/ijms11041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fulle S, et al. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Experimental Gerontology. 2004;39:17–24. doi: 10.1016/j.exger.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Siu PM, Pistilli EE, Alway SE. Age-dependent increase in oxidative stress in gastrocnemius muscle with unloading. J Appl Physiol (1985) 2008;105:1695–1705. doi: 10.1152/japplphysiol.90800.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powers SK, Smuder AJ, Judge AR. Oxidative stress and disuse muscle atrophy: cause or consequence? Curr Opin Clin Nutr Metab Care. 2012;15:240–245. doi: 10.1097/MCO.0b013e328352b4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinbacher P, Eckl P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules. 2015;5:356–377. doi: 10.3390/biom5020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dijk MV, et al. Improved muscle function and quality after diet intervention with leucine-enriched whey and antioxidants in antioxidant deficient aged mice. Oncotarget. 2016;7:17338–17355. doi: 10.18632/oncotarget.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fusco D, Colloca G, Monaco MRL, Cesari M. Effects of antioxidant supplementation on the aging process. Clin Interv Aging. 2007;2:377–387. [PMC free article] [PubMed] [Google Scholar]
- 28.Lee IM, et al. Vitamin E in the Primary Prevention of Cardiovascular Disease and Cancer The Women’s Health Study: A Randomized Controlled Trial. JAMA. 2016;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 29.Yun J, Finkel T. Mitohormesis. Cell Metab. 2014;19:757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finkel T. Signal transduction by mitochondrial oxidants. J Biol Chem. 2012;287:4434–4440. doi: 10.1074/jbc.R111.271999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell metabolism. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ristow M, Schmeisser K. Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS) Dose Response. 2014;12:288–341. doi: 10.2203/dose-response.13-035.Ristow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nature medicine. 2014;20:709–711. doi: 10.1038/nm.3624. [DOI] [PubMed] [Google Scholar]
- 34.Sun N, Youle RJ, Finkel T. The Mitochondrial Basis of Aging. Molecular cell. 2016;61:654–666. doi: 10.1016/j.molcel.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheibye-Knudsen M, Fang EF, Croteau DL, Wilson DM, 3rd, Bohr VA. Protecting the mitochondrial powerhouse. Trends in cell biology. 2014 doi: 10.1016/j.tcb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maynard S, Fang EF, Scheibye-Knudsen M, Croteau DL, Bohr VA. DNA Damage, DNA Repair, Aging, and Neurodegeneration. Cold Spring Harbor perspectives in medicine. 2015 doi: 10.1101/cshperspect.a025130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji LL, Kang C. Role of PGC-1alpha in sarcopenia: etiology and potential intervention - a mini-review. Gerontology. 2015;61:139–148. doi: 10.1159/000365947. [DOI] [PubMed] [Google Scholar]
- 38.Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leduc-Gaudet JP, et al. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget. 2015;6:17923–17937. doi: 10.18632/oncotarget.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang EF, et al. Nuclear DNA damage signalling to mitochondria in ageing. Nature reviews Molecular cell biology. 2016;17:308–321. doi: 10.1038/nrm.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheibye-Knudsen M, Fang EF, Croteau DL, Bohr VA. Contribution of defective mitophagy to the neurodegeneration in DNA repair-deficient disorders. Autophagy. 2014;10:1468–1469. doi: 10.4161/auto.29321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazarou M, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palikaras K, Lionaki E, Tavernarakis N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature. 2015;521:525–528. doi: 10.1038/nature14300. [DOI] [PubMed] [Google Scholar]
- 44.Ryu D, et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nature medicine. 2016 doi: 10.1038/nm.4132. [DOI] [PubMed] [Google Scholar]
- 45.Sebastian D, et al. Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J. 2016 doi: 10.15252/embj.201593084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hubbard BP, et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends in cell biology. 2014;24:464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinclair DA, Guarente L. Small-molecule allosteric activators of sirtuins. Annual review of pharmacology and toxicology. 2014;54:363–380. doi: 10.1146/annurev-pharmtox-010611-134657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang EF, et al. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell. 2014;157:882–896. doi: 10.1016/j.cell.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mouchiroud L, et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang EF, et al. Nuclear DNA damage signalling to mitochondria in ageing. Nature reviews Molecular cell biology. 2016 doi: 10.1038/nrm.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quiros PM, Mottis A, Auwerx J. Mitonuclear communication in homeostasis and stress. Nat Rev Mol Cell Biol. 2016;17:213–226. doi: 10.1038/nrm.2016.23. [DOI] [PubMed] [Google Scholar]
- 53.Verdin E. NAD(+) in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–1213. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]
- 54.Frederick DW, et al. Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell metabolism. 2016;24:269–282. doi: 10.1016/j.cmet.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trammell SA, Yu L, Redpath P, Migaud ME, Brenner C. Nicotinamide Riboside Is a Major NAD+ Precursor Vitamin in Cow Milk. J Nutr. 2016;146:957–963. doi: 10.3945/jn.116.230078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomes AP, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H, et al. NAD+repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352 doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 58.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 59.Rocha-Gonzalez HI, Ambriz-Tututi M, Granados-Soto V. Resveratrol: a natural compound with pharmacological potential in neurodegenerative diseases. CNS Neurosci Ther. 2008;14:234–247. doi: 10.1111/j.1755-5949.2008.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Price NL, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jackson JR, Ryan MJ, Alway SE. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66:751–764. doi: 10.1093/gerona/glr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pardo PS, Boriek AM. The physiological roles of Sirt1 in skeletal muscle. Aging. 2011;3:430–437. doi: 10.18632/aging.100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mitchell SJ, et al. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell reports. 2014;6:836–843. doi: 10.1016/j.celrep.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Graff J, et al. A dietary regimen of caloric restriction or pharmacological activation of SIRT1 to delay the onset of neurodegeneration. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:8951–8960. doi: 10.1523/JNEUROSCI.5657-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mercken EM, et al. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell. 2014;13:787–796. doi: 10.1111/acel.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kubben N, et al. Repression of the Antioxidant NRF2 Pathway in Premature Aging. Cell. 2016;165:1361–1374. doi: 10.1016/j.cell.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bruns DR, et al. Nrf2 Signaling and the Slowed Aging Phenotype: Evidence from Long-Lived Models. Oxid Med Cell Longev. 2015;2015:732596. doi: 10.1155/2015/732596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Niture SK, Jaiswal AK. Nrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistance. Free Radic Biol Med. 2013;57:119–131. doi: 10.1016/j.freeradbiomed.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee IS, et al. Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. Neurochem Int. 2011;58:153–160. doi: 10.1016/j.neuint.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 71.Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free radical biology & medicine. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Merry TL, Ristow M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and antioxidant response in mice. J Physiol. 2016 doi: 10.1113/JP271957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Son TG, et al. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J Neurochem. 2010;112:1316–1326. doi: 10.1111/j.1471-4159.2009.06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Son TG, et al. Naphthazarin protects against glutamate-induced neuronal death via activation of the Nrf2/ARE pathway. Biochemical and biophysical research communications. 2013;433:602–606. doi: 10.1016/j.bbrc.2013.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hunt PR, et al. Extension of lifespan in C. elegans by naphthoquinones that act through stress hormesis mechanisms. PLoS One. 2011;6:e21922. doi: 10.1371/journal.pone.0021922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Denzer I, Munch G, Friedland K. Modulation of mitochondrial dysfunction in neurodegenerative diseases via activation of nuclear factor erythroid-2-related factor 2 by food-derived compounds. Pharmacol Res. 2016;103:80–94. doi: 10.1016/j.phrs.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 77.Sun C, et al. Sulforaphane alleviates muscular dystrophy in mdx mice by activation of Nrf2. J Appl Physiol (1985) 2015;118:224–237. doi: 10.1152/japplphysiol.00744.2014. [DOI] [PubMed] [Google Scholar]
- 78.Halaschek-Wiener J, Brooks-Wilson A. Progeria of stem cells: stem cell exhaustion in Hutchinson-Gilford progeria syndrome. The journals of gerontology Series A, Biological sciences and medical sciences. 2007;62:3–8. doi: 10.1093/gerona/62.1.3. [DOI] [PubMed] [Google Scholar]
- 79.Kraft AD, Resch JM, Johnson DA, Johnson JA. Activation of the Nrf2-ARE pathway in muscle and spinal cord during ALS-like pathology in mice expressing mutant SOD1. Experimental neurology. 2007;207:107–117. doi: 10.1016/j.expneurol.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller CJ, et al. Disruption of Nrf2/ARE signaling impairs antioxidant mechanisms and promotes cell degradation pathways in aged skeletal muscle. Biochimica et biophysica acta. 2012;1822:1038–1050. doi: 10.1016/j.bbadis.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 81.Dyle MC, et al. Systems-based discovery of tomatidine as a natural small molecule inhibitor of skeletal muscle atrophy. The Journal of biological chemistry. 2014;289:14913–14924. doi: 10.1074/jbc.M114.556241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ebert SM, et al. Identification and Small Molecule Inhibition of an Activating Transcription Factor 4 (ATF4)-dependent Pathway to Age-related Skeletal Muscle Weakness and Atrophy. The Journal of biological chemistry. 2015;290:25497–25511. doi: 10.1074/jbc.M115.681445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Friedman M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, alpha-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J Agric Food Chem. 2013;61:9534–9550. doi: 10.1021/jf402654e. [DOI] [PubMed] [Google Scholar]
- 84.Friedman M, et al. Tomatine-containing green tomato extracts inhibit growth of human breast, colon, liver, and stomach cancer cells. J Agric Food Chem. 2009;57:5727–5733. doi: 10.1021/jf900364j. [DOI] [PubMed] [Google Scholar]
- 85.Kaufman RJ, Rutkowski DT. All Roads Lead to ATF4. Developmental Cell. 2003;4:422–444. doi: 10.1016/s1534-5807(03)00100-x. [DOI] [PubMed] [Google Scholar]
- 86.Kunkel SD, et al. Ursolic acid increases skeletal muscle and brown fat and decreases diet-induced obesity, glucose intolerance and fatty liver disease. PLoS One. 2012;7:e39332. doi: 10.1371/journal.pone.0039332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu J, et al. Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by D-galactose. Biochem Pharmacol. 2007;74:1078–1090. doi: 10.1016/j.bcp.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 88.Mattson MP. What Doesn’t Kill You. Scientific American. 2015;313:40–45. doi: 10.1038/scientificamerican0715-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mattson MP. Toxic Chemicals in Fruits and Vegetables Are What Give Them Their Health Benefits. Scientific American. 2015;313 [Google Scholar]
- 90.Romanello V, Sandri M. Mitochondrial Quality Control and Muscle Mass Maintenance. Front Physiol. 2015;6:422. doi: 10.3389/fphys.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fang EF, et al. A research agenda for aging in China in the 21st century. Ageing Res Rev. 2015 doi: 10.1016/j.arr.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


