Abstract
Present study was designed to verify which or if any of plastome loci is a hotspot region for mutations and hence might be useful for molecular species identification in feather grasses. 21 newly sequenced complete plastid genomes representing 19 taxa from the genus of Stipa were analyzed in search of the most variable and the most discriminative loci within Stipa. The results showed that the problem with selecting a good barcode locus for feather grasses lies in the very low level of genetic diversity within its plastome. None of the single chloroplast loci is polymorphic enough to play a role of a barcode or a phylogenetic marker for Stipa. The biggest number of taxa was successfully identified by the analysis of 600 bp long DNA fragment comprising a part of rbcL gene, the complete rbcL-rpl23 spacer and a part of rpl23 gene. The effectiveness of multi-locus barcode composed of six best-performing loci for Stipa (ndhH, rpl23, ndhF-rpl32, rpl32-ccsA, psbK-psbI and petA-psbJ) didn’t reach 70% of analyzed taxa. The analysis of complete plastome sequences as a super-barcode for Stipa although much more effective, still didn’t allow for discrimination of all the analyzed taxa of feather grasses.
Introduction
Feather grass (Stipa L.) is a genus from the tribe of Stipeae (Poaceae), common or dominant in grasslands and steppes in worm temperate regions of the Old World. In narrow concept it comprises over 150 grass species native to Asia, Europe and north Africa1. Discrimination of species representing feather grasses is based on their morphological characters and geographical distribution. However, within particular section of Stipa, eg. sect. Smirnovia Tzvel., sect. Leiostipa Dumort., Barbatae A. Junge and sect. Stipa, there are several couple of taxa that are morphologically very similar to each other and differ in one to few characteristics connected with size of plant or their particular parts, type of indumentum, and/or geographical distribution. As an example of such situation can serve such couple of species like: Stipa richteriana Kar. & Kir. – S. jagnobica Ovcz. & Czuk.; S. arabica Trin. & Rupr. – S. hohenackeriana Trin. & Rupr., S. pennata L. – S. borysthenica Klok., S. krylovii Roshev. – S. sareptana A.K. Besser; S. macroglossa P.A. Smirn. – S. kungeica Golosk.; S. subsessiliflora (Rupr.) Roshev. – S. basiplumosa Munro ex Hook. f.2–6. Morphological similarity and variability of particular taxa was a case of taxonomic confusion and discussion within agrologists. Due to high phenotypic plasticity observed within Stipa, narrow species concept or taxonomic splitting may cause many difficulties in determination of species, but on the other hand, too broad species concept can also create problems in understanding patterns of diversity5,7–9. For precise delimitation of some feather grasses, there is a need to use molecular methods that can help to explain variation that is not masked by phenotypic plasticity and can put new light on hitherto unsolved problems and attempts to trace evolutional tendencies.
Molecular identification of species uses genetic based method called DNA barcoding. The method allows to rapidly identify specimens to species even from trace amounts or degraded sample tissue. DNA barcoding relies on the amplification of specific barcoding locus or multiple loci in the genomes of the target species10,11. Numerous loci are applied in plant barcoding with more or less success. Unfortunately, discrimination power of known barcodes is too low to work across all species, especially in higher plants, therefore there is no universal barcode loci neither for all plants nor for grasses12,13. To find more robust single- or multi-locus barcode which would increase the resolution in distinguishing among species within particular groups of plants, numerous researches are still ongoing14. Despite several researches on Stipa carried so far15–21, still there is lack of molecular marker which would allow for effective species delimitation within a significant part of Stipa representatives nor for resolving phylogenetic relationships within them. For this reason, phylogenetic inferences for Stipa are scarce16. Thus far researches using molecular methods proved distinctiveness of small group of Himalayan species comprising Stipa basiplumosa, S. capillacea Keng, S. penicillata Hand.-Mazz., S. purpurea Griseb., S. regeliana Hack. and S. roborowskyi Roshev. from remaining Stipa species representing almost all distinguished sections within the genus17,21. Therefore, finding a molecular marker suitable for species delimitation and for phylogenetic implications within the genus of Stipa is pending.
In previous studies on the tribe of Stipeae, involving representatives of Stipa s.l. a few nuclear markers and several plastid markers were applied. Among nuclear loci usefulness of internal transcribed spacers (ITS) was tested both as a complete ITS15,17,20 and separately as ITS1 and ITS219,21. Moreover, external transcribed spacer (ETS) was applied in one study19. Recently Krawczyk et al.22 showed that the nuclear rRNA intergenic spacer region (IGS), and especially its part adjacent to 26S nrDNA, is a molecular marker giving a real chance for a phylogeny reconstruction of Stipa. Due to extremely high rate of evolution within the part comprising inter-repeats, the IGS region is useful for phylogenetic analyses of Stipa at genus level or in shallower taxonomic scale. The region seems to be the most phylogenetically informative for Stipa from all the chloroplast and nuclear markers tested so far22.
Within cpDNA utility of both, coding (matK, ndhF, rpl16, rps3, rpoA, trnK) and non-coding regions (trnH-psbA, trnK-matK, trnL-trnF, trnT-trnL, rpl32-trnL, rps16-trnK and rps16 intron) was studied to date15–21. However, none of these molecular markers tested individually or as a set of loci revealed sufficient level of variability to illustrate genetic diversity within the genus of Stipa. Therefore, a question arises whether variability of Stipa representatives within plastid genome is very low, or is it concentrated in regions that had not been tested for feather grasses so far. It is also possible that the only method for species delimitation or phylogenetic inferences in Stipa is the use of the whole-plastid genome sequence supported by next-generation sequencing. This method, recently proposed by researches and called “super-barcoding” gives new prospects in molecular plant identification, especially in cases, where single- or multi-locus barcoding method is insufficient in discriminating closely related species12,23–26.
Through our research we wanted to answer the question, which or if any of plastome loci is a hotspot region for mutations and hence is useful for molecular species identification in feather grasses. For this purpose, we performed a comparative analysis of complete cpDNA sequences from 19 taxa of feather grasses to find within them the most variable DNA regions. Our analysis was conducted in two ways. In the first method, each of coding and non-coding regions was individually tested. In the second approach, we evaluated the variability of the sequence without considering its division into functional regions. We used for that a sliding window 600 bp in length moved by a 100 bp step. The length of analyzed fragment was chosen considering its potential use in DNA barcoding. In other words, we have chosen the length of a sequence that can be easily sequenced with Sanger method with a use of one pair of primers.
Results
Characteristics of the Stipa plastid genome
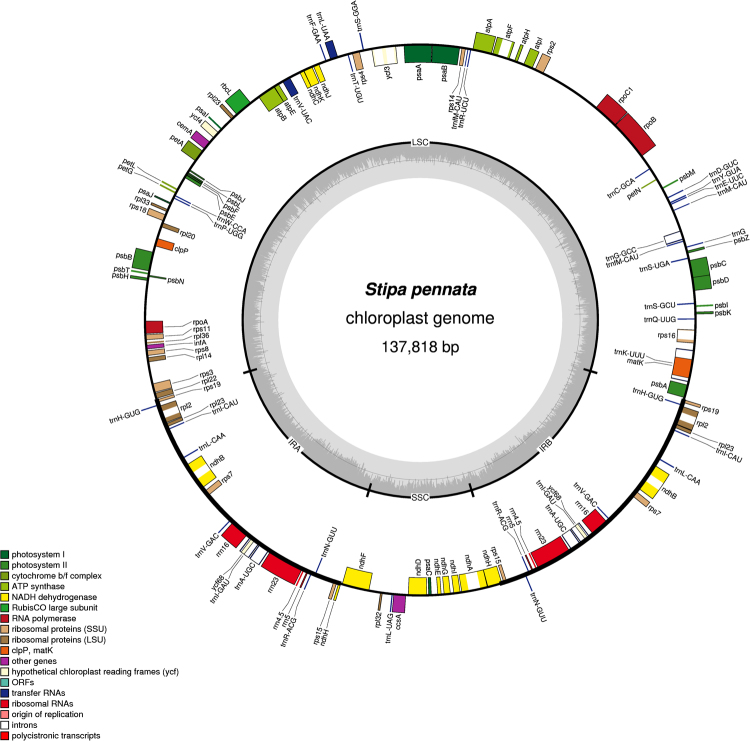
The plastome of Stipa was a circular molecule, comprising a large single copy (LSC) region ranging from 81,533 to 81,806 bp and a small single copy (SSC) region ranging from 12,836 to 12,837 bp, separated by two inverted repeat regions (IRs) of 21,616 bp (Fig. 1). It contained 127 genes, including 81 protein-coding genes, 8 ribosomal RNA genes and 38 tRNA genes. The genome contained 20 genes duplicated in the IRs (Fig. 1). Nine genes (atpF, ndhA, ndhB, rpl2, rps16, trnA-UGC, trnG-GCC, trnI-GAU, trnK-UUU) contained a single intron, while ycf3 harbored two introns. The base composition of the genome was the following: A (30.7%), C (19.3%), G (19.5%) and T (30.5%) with an overall GC content of 38.8% and the corresponding values of the LSC, SSC and IR regions reaching 36.9, 33.0 and 44.1%, respectively.
Figure 1.
Gene map of the Stipa pennata subsp. pennata chloroplast genome. Dashed area in the inner circle indicates the GC content.
Sequence variation
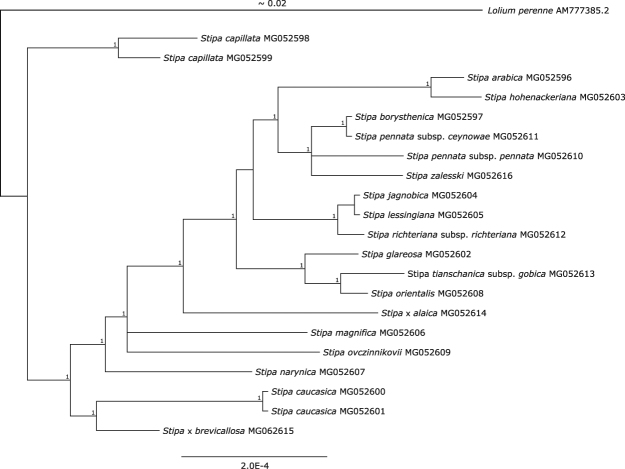
21 newly sequenced complete plastid genomes representing 19 taxa from the genus of Stipa were analyzed (Supplementary Table S1). Their length ranged from 137 602 bp in S. glareosa to 137 874 bp in S. ovczinnikovii. The alignment comprising the set of 21 plastomes was 138 077 bp in length and was characterized by 38.8% GC content and 99.9% pairwise identity. Sequence variability was due solely to the presence of single nucleotide polymorphism (SNP) and indels. No gene rearrangements of genome or differences in gene content were observed. The number of bases different between two compared sequences reached the highest value in the case of S. glareosa and S. ovczinnikovii and amounted to 402. At the same time, the analysis of differences between sequence pairs revealed plastomes of S. pennata subsp. ceynowae and S. borysthenica were identical. On a phylogram these taxa are grouped into one clade sister to S. pennata subsp. pennata and S. zalesskii (Fig. 2). Very few nucleotide differences were found between S. jagnobica and S. richteriana (11) as well as between S. arabica and S. hohenackeriana (17). Plastomes of two specimens representing S. caucasica differed in only two single indel mutations. One of them was localized in psbH-rpoA spacer and the second one in the ycf3 intron. Between cpDNA sequences obtained for two representatives of S. capillata, 28 base differences were found. 15 of them was caused by substitutions and 13 by indel mutations. Indels were in most cases single, only in two cases comprised two adjacent nucleotides. 20 of 28 mutations were localized within intergenic spacers and three within introns. Five mutations were found within coding regions (ndhC, rpl23, rps11, psbC and matK), however only one SNP within the psbC gene was nonsynonymous and resulted in the replacement of Leu with Phe. In spite of differences between the specimens of S. capillata, they form a 100% credible clade, significantly distinct from the other representatives of the genus tested in the study (Fig. 2).
Figure 2.
Plastome-based phylogram. The 70% majority-rule consensus phylogram derived from a Bayesian analysis of complete plastomes (excluding one Inverted Repeat region). Credibility values above 0.95 are given in the top line. For tree legibility the length of the branch of the outgroup has been shortened.
Species delimitation
Simple heuristic search performed within The Poisson Tree Processes (PTP) method grouped the analyzed individuals with the highest support into 20 species. The result of the analysis exceeded the number of species used in the study by one, because the two representatives of S. caucasica were separated into two cryptic species. However, the posterior delimitation probability for both of them amounted only to 0.194 The support exceeded 0.8 only in three cases: S. arabica (1.0), S. hohenackeriana (1.0) and S. × alaica (0.88) confirming separateness of these species with great certainty (Supplementary Table S2). Probability of delimitation for other taxa ranged from 0.247 (S. borysthenica and S. pennata subsp. ceynowae) to 0.795 (S. magnifica).
Hotspots of variation within protein coding regions
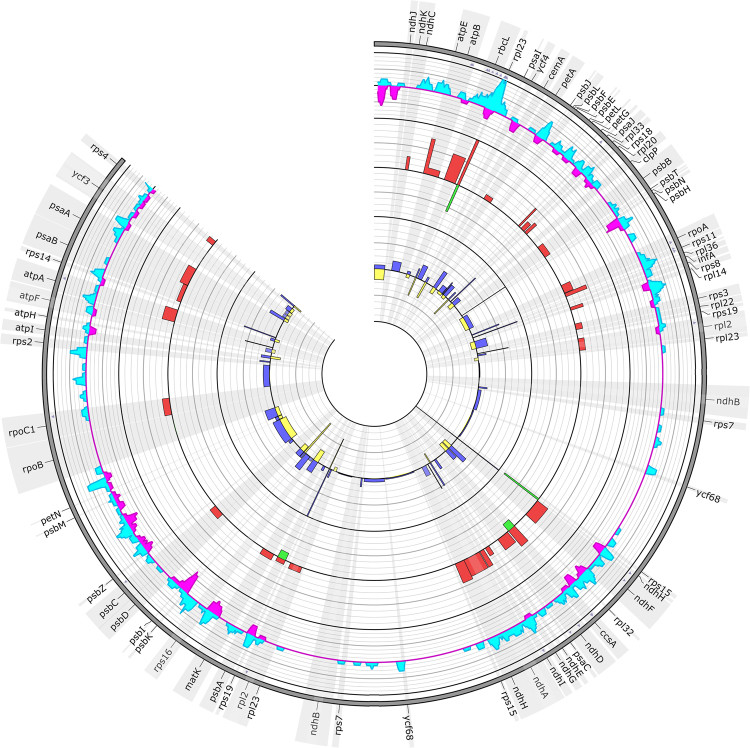
Analysis of the distribution of genetic variability within the plastome of Stipa revealed that the most variable protein-coding region characterized with 3.16 percent of nucleotide variation (PV) was one of three copies of rpl23 gene (Fig. 3, Table 1). The copy is located near rbcL gene in the Large Single Copy Unit (Fig. 1) is 285 bp long (286 bp in S. tianschanica subsp. gobica) and contains eight SNP sites and one indel. In the case of S. tianschanica subsp. gobica the indel resulted in a shift of a reading frame, appearance of six internal stop codons and a pseudogenization of the gene. In the other sequences, mutations were mostly nonsynonymous and caused replacement of amino acid only once in S. orientalis (Pro into Gln) and twice in S. × alaica (Tyr into His). Comparative analysis of the rpl23 gene allowed for identification of five out of 19 analyzed taxa of Stipa. The other two copies of the rpl23 gene were 282 bp long and did not contain any SNPs or indels.
Figure 3.
SNP and indel variation among plastomes of Stipa. Track A shows nonsynonymous SNP occurrence within genes. Track B and C represent identified SNPs (cyan histogram) and indels (magenta histogram) per 600 bp window size with 100 bp shift, respectively. Track D represents percent of SNPs per CDS length while track E represents percent of indels per CDS length. Track F represents percent of SNPs per noncoding region length while track G represents percent of indels per noncoding region length.
Table 1.
Hotspots of variation. The most polymorphic coding and non-coding cpDNA regions over 100 bp in length and containing at least three PICs.
| Region | Length [bp] | SNP | Indel | % of variation (PV) | π | Number of identified taxa | |
|---|---|---|---|---|---|---|---|
| coding | rpl23 | 285 | 8 | 1 | 3.16 | 0.003286 | 5 (26.32%) |
| atpE | 414 | 3 | 0 | 0.72 | 0.001104 | 2 (10.53%) | |
| rbcL | 1434 | 8 | 0 | 0.56 | 0.001999 | 3 (15.79%) | |
| ndhH | 1182 | 5 | 0 | 0.52 | 0.000471 | 6 (31.58%) | |
| ccsA | 975 | 4 | 1 | 0.51 | 0.000916 | 3 (15.79%) | |
| ndhH | 1182 | 5 | 0 | 0.42 | 0.548000 | 4 (21.05%) | |
| ndhA | 1089 | 4 | 0 | 0.37 | 0.000498 | 2 (10.53%) | |
| ndhD | 1503 | 4 | 0 | 0.27 | 0.000425 | 2 (10.53%) | |
| ndhF | 2220 | 6 | 0 | 0.27 | 0.000335 | 3 (15.79%) | |
| atpA | 1524 | 4 | 0 | 0.26 | 0.000625 | 3 (15.79%) | |
| non-coding | rps19-psbA | 160 | 4 | 2 | 3.75 | 0.016378 | 2 (10.53%) |
| psbK-psbI | 408 | 1 | 6 | 1.72 | 0.000241 | 6 (31.58%) | |
| ndhG-ndhI | 250 | 3 | 1 | 1.60 | 0.001157 | 3 (15.79%) | |
| rpl33-rps18 | 264 | 1 | 2 | 1.14 | 0.000364 | 1 (5.26%) | |
| rbcL-rpl23 | 274 | 1 | 2 | 1.09 | 0.003866 | 2 (10.53%) | |
| ndhF-rpl32 | 916 | 7 | 3 | 1.09 | 0.000735 | 7 (36.84%) | |
| petA-psbJ | 822 | 6 | 2 | 0.97 | 0.002869 | 5 (26.32%) | |
| matK-rps16 | 1289 | 6 | 6 | 0.93 | 0.000580 | 3 (15.79%) | |
| ycf4-cemA | 455 | 1 | 3 | 0.88 | 0.000244 | 1 (5.26%) | |
| psA-ycf3 | 631 | 4 | 1 | 0.79 | 0.000741 | 4 (21.05%) | |
| rpl32-ccsA | 895 | 4 | 3 | 0.78 | 0.000523 | 7 (36.84%) | |
Among coding regions higher resolving power than rpl23 had only the ndhH gene (PV = 0.52%; π = 0.000471), distinguishing six (31.58%) analyzed species. However, it should be noted that the ndhH gene is more than four times longer than rpl23 reaching 1182 bp in length and contains only five polymorphic sites. Even longer rbcL gene (1434 bp) carrying eight SNPs and characterized by PV = 0.56% and π = 0.001999, successfully identified only three (15.79%) from the set of analyzed taxa.
Hotspots of variation within non-coding regions
Among the non-coding regions, the highest frequency of polymorphism was found in 95bp-long ndhH-ndhF spacer (Supplementary Table S1) and in the rps19-psbA spacer (Table 1). Within 160 bp-long rps19-psbA region four SNPs and two indels were identified, which represents 3.75% of sites and is the highest value among analyzed non-coding regions over 100 bp in length. The region had also the highest π value for non-coding regions equal to 0.016378. However, the rps19-psbA spacer allowed to identify only two (10.53%) taxa out of the analyzed set of 19. The biggest number of taxa was successfully identified analyzing variability of ndhF-rpl32 and rpl32-ccsA spacers. The percent of polymorphic sites and π score within these regions amounted to: PV = 1.09%; π = 0.000735 and PV = 0.78%; π = 0.000523, respectively. Relatively high variability was also characteristic for psbK-psbI spacer (PV = 1.72%; π = 0.000241) which identified six (31.58%) taxa.
Multi-locus barcode
Six plastome loci characterized by the highest discriminative power within Stipa: ndhH, rpl23, ndhF-rpl32, rpl32-ccsA, psbK-psbI and petA-psbJ were tested as one multi-locus barcode. It was 4508 bp long and contained 46 PICs (31 SNPs and 15 indels). Frequency of polymorphism of this barcode amounted to PV = 1.44%, π score was equal to 0.001131, while the success of taxon identification reached 68.4% (13 taxa).
Hotspots of variation within 600 bp sliding-window fragments
The analysis of 600 bp nucleotide fragments of plastome, carried out apart from their biological functions, showed that although fragments determined in this way are not characterized by the highest frequency of polymorphism, they have higher resolving power within the analyzed set of species than the regions discussed above. The biggest concentration of polymorphic sites was located in a sequence fragment comprising rpl23 gene and adjacent regions (Fig. 3). The biggest number of taxa (10) was successfully identified by the analysis of DNA fragment comprising a part of rbcL gene, the complete rbcL-rpl23 spacer and a part of rpl23 gene (Table 2). The fragment was characterized with PV = 1.67% and π = 0,003489. The percent of variation within the analyzed reading frame can be increased up to 1.83% or 2.17%, by moving the frame about 100, 200, 300 or 500 bp downwards (in 3′ direction) and by this including into analysis part of rpl23-psaI spacer, however discriminative power of a fragment will then decrease to nine or eight taxa (Table 2). Among the remaining 600bp-long fragments, relatively high variability was observed for a part of a ndhF-rpl32 spacer (PV = 1.33%; π = 0.000646), which identified six (31,58%) taxa. The same number of taxa was identified by the analysis of the fragment comprising psbK gene and adjacent parts of trnQ-psbK and psbK-psbI spacers (PV = 1.33%; π = 0.000324).
Table 2.
The most polymorphic 600 bp long regions. The number of nucleotides covered by the reading frame is given in brackets. The regions covered completely by a reading frame are given in bold.
| Comprised regions | Range | SNP | Indel | % of variation (P V ) | π | Number of identified taxa |
|---|---|---|---|---|---|---|
| rbcL (16), rbcL-rpl23 (274), rpl23 (286), rpl23-psaI (24) | 10200–10800 | 10 | 3 | 2.17 | 0.004107 | 9 (47.37%) |
| rbcL-rpl23 (190), rpl23 (286), rpl23-psaI (124) | 10300–10900 | 10 | 3 | 2.17 | 0.003489 | 8 (42.10%) |
| rbcL (116), rbcL-rpl23 (274), rpl23 (210) | 10100–10700 | 8 | 3 | 1.83 | 0.003794 | 9 (47.37%) |
| rpl23 (276), rpl23-psaI (324) | 10500–11100 | 10 | 1 | 1.83 | 0.001879 | 8 (42.10%) |
| rbcL (216), rbcL-rpl23 (274), rpl23 (110) | 10000–10600 | 7 | 3 | 1.67 | 0.003489 | 10 (52.63%) |
| rbcL-rpl23 (90), rpl23 (286), rpl23-psaI (224) | 10400–11000 | 9 | 1 | 1.67 | 0.001720 | 5 (26.32%) |
| petA-psbJ (600) | 15300–15900 | 6 | 2 | 1.33 | 0.003934 | 5 (26.32%) |
| petA-psbJ (596), psbJ (4) | 15400–16000 | 6 | 2 | 1.33 | 0.003934 | 5 (26.32%) |
| rpl23 (176), rpl23-psaI (424) | 10600–11200 | 8 | 0 | 1.33 | 0.001556 | 3 (15.79%) |
| trnK (41), trnK-rps16 (551), rps16 (8) | 95200–95800 | 5 | 3 | 1.33 | 0.001087 | 2 (10.53%) |
| trnK-rps16 (492), rps16 (108) | 95300–95900 | 5 | 3 | 1.33 | 0.001087 | 2 (10.53%) |
| trnK (341), trnK-rps16 (259) | 94900–95500 | 4 | 4 | 1.33 | 0.000932 | 5 (26.32%) |
| trnK (241), trnK-rps16 (359) | 95000–95600 | 4 | 4 | 1.33 | 0.000932 | 5 (26.32%) |
| ndhF-rpl32 (600) | 59100–59700 | 5 | 3 | 1.33 | 0.000646 | 6 (31.58%) |
| trnT-trnL (588), trnL (12) | 300–900 | 2 | 6 | 1.33 | 0.000327 | 4 (21.05%) |
| trnT-trnL (488), trnL (112) | 400–1000 | 2 | 6 | 1.33 | 0.000327 | 4 (21.05%) |
| trnQ-psbK (113), psbK (186), psbK-psbI (301) | 98000–98600 | 2 | 6 | 1.33 | 0.000324 | 6 (31.58%) |
| psbK-psbI (407), psbI (111), psbI-trnS (82) | 98300–98900 | 1 | 7 | 1.33 | 0.000163 | 5 (26.32%) |
Discussion
Although examined here members of Stipa represents five the richest in species and well distinguished sections of the genus3, similarity of complete plastome sequences between pairs of species amounted from 99.7% to 100%, indicating the very low variability of the analyzed genomes. The high conservativity of the chloroplast genome in Poaceae at the generic level has previously been observed for Bambusa, where 18 analyzed plastomes representing different species shared 99.8% sequence similarity27. Slightly lower level of genome conservation reaching 96.9%-99.5% was revealed by genome-wide comparison of the Lolium-Festuca species complex28. Higher genetic variability was observed within the plastomes of dicotyledonous plants. For example, in the genus of Daucus complete cpDNA sequence similarity amounted to 97%29. In turn, in the genus Ipomoea, only the number of parsimony informative sites reached 3%30.
Analyzing the distribution of genetic variation within particular regions of plastome in Stipa, it can be seen that cpDNA loci previously considered in studies on this genus, do not belong to the most variable ones. Our research shows that the most polymorphic protein coding region, applied in research on Stipa so far16,17 is the ndhF gene. Although it is one of ten most variable genes in plastome of Stipa, it discriminates less than 16% of analyzed taxa. Another previously tested locus is the trnT-trnL spacer18,19. As a complete, separate region it was not characterized by a high level of variability, but under the use of 600 bp long reading frame and including part of sequence coding for trnL, it was among the 18 most variable and informative sequence fragments in Stipa (Table 2). However, our research clearly shows that neither the ndhF gene nor the trnT-trnL spacer were the most variable hotspots in plastome of the genus. Therefore, phylogenetic analyses based on them did not give satisfactory results and did not allowed for reliable phylogenetic implications.
Even lower level of genetic variability (PV = 0.13%) was found within the matK gene (Supplementary Table S1), which is recommended as one of two core DNA barcodes for plants31. The matK region is widely used both in DNA barcoding and as a phylogenetic marker in studies on various groups of plants12,32,33 and was previously applied in research on Stipa15–17. The second one of the recommended and commonly used barcode loci, the rbcL gene31,34,35, although was one of three most variable coding regions in Stipa, its ability to discriminate between species at the level of less than 16% is highly insufficient.
The trnL, matK and rbcL genes were widely sequenced to develop a database of barcodes for xerothermic plants from central Europe36. However, a presence of only single representative of Stipa in this database does not allow to assess usefulness of these loci in barcoding of Stipa.
Another widely used as a single-loci or a component of two- or multi-locus plant barcode is trnH-psbA11,12,37. This non-coding intergenic chloroplast region exhibits extreme sequence divergence and has high rates of indels35. These attributes make this locus highly suitable for species discrimination35,38. The trnH-psbA spacer is especially effective barcode in pteridophytes39 but also in some genera of angiosperms such as Hydrocotyle40 and Dendrobium41, where the region discriminates almost all the species. Distinctive feature of the trnH-psbA spacer is its considerable length variation from less than 100 bp in bryophytes42 to more than 1000 bp in some conifers and monocots33,43. In the plastome of Stipa the trnH-psbA region ranged from 545 to 574 bp and contained the rps19 gene. All the PICs (four SNPs and two indels) identified within trnH-psbA were located in the spacer between rps19 and psbA and enabled identification only of two examined species which is very low score for such a usually variable region.
In recent years attention has been paid to high variability of the ycf1 gene in seed plants14,44. Especially two regions within ycf1 called ycf1a and ycf1b, present in the SSC region, have been predicted to have the highest nucleotide diversity (π) at the species level within angiosperm plastid genomes44. It has been showed that ycf1b generally performed better than any of the matK, rbcL and trnH-psbA applied individually and even was slightly better than the two-locus combination of matK and rbcL14. In the case of Stipa we were not able to assess the utility of this promising marker, because the ycf1 locus was absent in the analyzed plastomes of feather grasses.
Analyzing the distribution of genetic diversity in cpDNA of Stipa and referring results of our research to the literature data, we noted that location of hot-spots for mutations within non-coding regions largely corresponds with the results obtained for monocots by Shaw et al.45, where the biggest share of PICs among non-coding regions was found in the ndhF-rpl32 spacer. In the case of Stipa the region was characterized by relatively high diversity and was one of the two most successful non-coding regions in species discrimination. Among 13 most polymorphic spacers presented by Shaw et al.45 there was also the trnT-trnL region, discussed above, petA-psbJ and trnK2-rps16, which in Stipa were also in a group of most variable non-coding regions.
Spacers and introns are generally more variable than protein coding regions46. However, the high variability of the rpl23 gene present in the LSC unit in Stipa ranks it in the third place among the most polymorphic loci just behind ndhH-ndhF and rps19-psbA spacers. The hypervariability of rpl23 is a common phenomenon in grasses and is associated with pseudogenization of this locus. In most Poaceae there is a mutational hotspot in the region between rbcL and psaI47,48 containing a pseudogenized copy of rpl23 that ranges from 40 to 243 bp in length49. It is supposed that ψrpl23 locus is a result of nonreciprocal translocation of this gene from one copy present in the inverted region50,51. Considering that in Stipa rpl23 present in LSC differs in length and amino acid composition from its copies present in IRs, we can assume that the loci is also pseudogenized, and not only in S. tianschanica subsp. gobica where internal stop codons are present, but in all the studied representatives of the genus.
The results of our research indicate that the rbcL-psaI region together with a partial rbcL sequence is the best candidate barcode loci for Stipa. Unfortunately, even this most discriminative of the analyzed cpDNA fragments allows for identification barely 53% of taxa surveyed. The level of variation of this one, as well as any other candidate barcode region tested in this study, is too low to successfully discriminate species and to work as an effective phylogenetic marker in Stipa. The application of multi-locus barcode composed of six most discriminative loci allowed for identification of 68.4% of analyzed taxa, what taking into account the length of a sequence as well as the effort and cost needed for its sequencing, it is not a satisfactory result.
The problem with selecting a good barcode locus for Stipa results from the very low level of genetic diversity within its plastome. An extreme example is here the complete absence of nucleotide differences between cpDNA of S. pennata subsp. ceynowae and S. borysthenica as well as very small differences reaching 0.009% for S. jagnobica and S. richteriana and 0.012% between S. arabica and S. hohenackeriana, despite of the fact that all of the aforementioned species are morphologically well separated and easily identifiable3,5,52. The comparative analysis of complete cpDNA sequences as super-barcodes in the case of Stipa was much more effective than a traditional barcoding approach. The application of super-barcodes enabled discrimination 18 of 19 (94.74%) analyzed taxa. However, it should be noted that due to the high interspecies similarity and at the same time high variability between two representatives of S. capillata, the identification in some cases was based on several PICs. High conspecies variability, in some cases exceeding the congeneric variability is an unfavorable phenomenon for molecular species identification. Therefore, the species delimitation probability for S. capillata was low (0.296) and only slightly exceeded the support calculated for its two representatives (0.249). The analysis of dataset covered by our study provides no basis for a broader discussion on the phenomenon of barcoding gap. However, the example of S. capillata plastome and its in-depth comparison with cpDNA of other feather grasses shows that due to genetic diversity between its two analyzed representatives, a significant part of PICs is not specific for this species and is useless in species discrimination. It should therefore be expected that extending the dataset with multiple representatives of each species would greatly reduce the effectiveness of barcoding within Stipa.
In conclusion, none of the single chloroplast loci is polymorphic enough to play a role of a barcode or a phylogenetic marker for Stipa. Also, the effectiveness of multi-locus barcode composed of best-performing loci for Stipa (ndhH, rpl23, ndhF-rpl32, rpl32-ccsA, psbK-psbI and petA-psbJ) didn’t reach 70% of analyzed taxa. Complete plastome sequences, although applied as a super-barcode for Stipa are not effective in 100%, would be valuable for further study of these genus and also for a broader understanding of Poaceae plastome evolution. For molecular species identification and for phylogenetic implications within the Stipa genus it seems necessary to apply nuclear loci in the studies.
Materials and Methods
Plant material
Plant material of Stipa taxa used in the study was collected during the field studies in 2010–2016 (Supplementary Table S1). 21 individuals representing 19 taxa from the genus of Stipa were analyzed. Only in few cases material (dry leaves) for molecular studies were taken from older herbarium specimens. Samples of all the taxa used in the analyses are preserved at KRA (Herbarium of the Institute of Botany, Jagiellonian University).
Plastid genome sequencing and assembly
A genomic library for MiSeq sequencer was developed with the use of the Nextera XT Kit (Illumina, San Diego, CA, USA) according to the manufacturer protocol. The libraries were quantified and normalized using Kappa Library Quantification Kit for Illumina (Kapa, Wilmington, MA, USA) and sequenced using the MiSeq. 600v2 cartridge that enable 2x300 bp pair-end reads. The details on library preparation, validation, quantification and sequencing are given in Myszczyński et al.53 and in Sawicki et al.54. The remained libraries were prepared using TruSeq Nano DNA Library Preparation Kit (Illumina) and sequenced using HiSeq. 2500 sequencer (Illumina) by Macrogen Inc. (Korea).
The obtained raw reads were cleaned by removing low quality and short (<than 50 bp) reads. The remained reads were assembled de novo using Velvet assembler as implemented in the Geneious R8 software (Biomatters, Auckland, New Zealand).
The flow chart for the sin silico reconstruction of the sequenced Stipa plastomes was the same as previously published55.
Confirmation of plastome structure through nanopore sequencing
The junctions between single-copy and inverted repeats regions were confirmed by long-read nanopore sequencing. Genomic library was prepared using Rapid Sequencing Kit R9 Version SQK RAD001 (Oxford Nanopore Technologies, UK). The library was sequenced using SQK-MAP005 Nanopore Sequencing Kit, Spot-ON Flow Cell Mk1 and MinION Mk1B device (Oxford Nanopore Technologies, UK). The reads were assembled using Canu software56.
Annotation and construction of a physical map of the plastome
The annotation of chloroplast genome was conducted using the Geneious R9 software (Biomatters, Auckland, New Zealand) by comparing with the genome of Stipa lipskyi (GenBank accession no. KT692644)53. A physical map of the plastome was generated using OGDRAW 1.2 (http://ogdraw.mpimp-golm.mpg.de)57.
Variation analyses
Chloroplast genomes of 21 taxa of Stipa genus were aligned using the MAFFT genome aligner58. Afterwards based on alignment of genomes polymorphism analyses were conducted separately for each coding sequence, intron and intergenic spacer. Every variation within aforementioned regions was identified as single nucleotide polymorphism (SNP) or insertion/deletion (indel) and counted using custom Python script. Each SNP within coding sequence was tested if it affects the protein sequence and defined as synonymous or nonsynonymous SNP. Finally, variations were visualized using Circos software59 combined with custom Python script.
Phylogenetic analyses
Phylogenetic analyses were performed using 21 aforementioned Stipa taxa and Lolium perenne L. as a root species. First, PartitionFinder260 was used to determine the best partitioning schemes and corresponding nucleotide substitution models. The data-set blocks were predefined a priori based on protein coding genes (CDS) and intergenic spacers as well as for first, second and third position for each of CDS. The Bayesian information criterion (BIC) and the ‘greedy’ algorithm with branch lengths estimated as unlinked were used to search for the best-fit scheme. Phylogenetic analyses were conducted using BI method. Bayesian analysis (BI) was conducted using MrBayes 3.25961, and the MCMC algorithm was run for 20,000,000 generations (sampling every 1,000) with four incrementally heated chains (starting from random trees). The first 1000 trees were discarded as burn-in, and the remaining trees were used to develop a Bayesian consensus tree.
Species delimitation
The Poisson Tree Processes method was applied to delimitate species boundaries62. The analysis was performed using a rooted tree, the MCMC algorithm was run for 500 000 generations, with 100 thinning and 0.2 burn-in.
Electronic supplementary material
Supplementary Table S1 and Supplementary Table S2
Acknowledgements
Financial support for this study came from the National Science Center, Poland (DEC-2013/09/B/NZ8/03287 and partially 2014/15/N/NZ8/00340).
Author Contributions
Conception and design: K.K., J.S. Collection and determination of plant material: M.N., E.K. Analysis of NGS data: J.S. Bioinformatic analyses: K.M., K.K. Interpretations of results: K.K., M.N. Drafting of manuscript and final approval of manuscript: All authors.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20399-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nobis M. Taxonomic revision of the Central Asiatic Stipa tianschanica complex (Poaceae) with particular reference to the epidermal micromorphology of the lemma. Folia Geobot. 2014;49(2):283–308. doi: 10.1007/s12224-013-9164-2. [DOI] [Google Scholar]
- 2.Roshevitz, R.Yu. Stipa L. In: Flora SSSR, 2. (ed. Komarov, V. L.) 79–112 & 740–741 (Akademiia nauk SSSR, 1934).
- 3.Tzvelev, N. N. Zlaki SSSR [Grasses of the Soviet Union] 1–778 (Nauka, 1976).
- 4.Martinovský, J. O. (1980) Stipa L. In: FloraEuropaea, 5 (ed. Tutin, T. G. et al.) 247–252 (Cambridge University Press, 1980).
- 5.Freitag H. The genus Stipa (Gramineae) in southwest and southAsia. Notes from the Royal Botanical Garden, Edinburgh. 1985;42:355–489. [Google Scholar]
- 6.Nobis M, et al. Stipa dickorei sp. nov. (Poaceae), three new records and a checklist of feather grasses of China. Phytotaxa. 2016;267(1):29–39. doi: 10.11646/phytotaxa.267.1.3. [DOI] [Google Scholar]
- 7.Kotukhov Y. Konspekt kovylei (Stipa L.) i kovylechkov (Ptilagrostis Griseb.) vostochnogo Kazakhstana (Kazakhstanskii Altai, Zaisanskaya kotlovina i Prialtaiskie khrebty) Bot Issl Sibiri i Kazakhstana. 2002;8:3–16. [Google Scholar]
- 8.Tzvelev NN. (2012) Notes on the tribe Stipeae Dumort. (Poaceae) Novosti Sistematiki Vysshikh Rastenii. 2012;43:20–29. [Google Scholar]
- 9.Gonzalo R, Aedo C, García MÁ. Taxonomic revision of the Eurasian Stipa subsections Stipa and Tirsae (Poaceae) Syst. Botany. 2013;38(2):344–378. doi: 10.1600/036364413X666615. [DOI] [Google Scholar]
- 10.Hebert PD, Cywinska A, Ball SL. Biological identifications through DNA barcodes. Proc. R. Soc. London Ser. B. 2003;270(1512):313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kress WJ, Wurdack KJ, Zimmer EA, Weigt LA, Janzen DH. Use of DNA barcodes to identify flowering plants. Proc. Natl Acad. Sci. USA. 2005;102(23):8369–8374. doi: 10.1073/pnas.0503123102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, et al. Plant DNA barcoding: from gene to genome. Biol. Reviews. 2015;90(1):157–166. doi: 10.1111/brv.12104. [DOI] [PubMed] [Google Scholar]
- 13.Wang A, Gopurenko D, Wu H, Lepschi B. Evaluation of six candidate DNA barcode loci for identification of five important invasive grasses in eastern Australia. PloS one. 2017;12(4):e0175338. doi: 10.1371/journal.pone.0175338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong W, et al. ycf1, the most promising plastid DNA barcode of land plants. Sci. Rep. 2015;5:8348. doi: 10.1038/srep08348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romaschenko K, et al. Molecular phylogenetic analysis of the American Stipeae (Poaceae) resolves Jarava sensu lato polyphyletic: evidence for a new genus. Pappostipa. J. Bot. Res. Inst. Texas. 2008;2(1):165–192. [Google Scholar]
- 16.Romaschenko, K., Peterson, P. M., Soreng, R. J., Garcia-Jacas, N. & Susanna, A. Phylogenetics of Stipeae (Poaceae: Pooideae) based on plastid and nuclear DNA sequences. Diversity, Phylogeny, and Evolution in Monocotyledons, 511–537 (2010).
- 17.Romaschenko K, et al. Systematics and evolution of the needle grasses (Poaceae: Pooideae: Stipeae) based on analysis of multiple chloroplast loci, ITS, and lemma micromorphology. Taxon. 2012;61(1):18–44. [Google Scholar]
- 18.Cialdella AM, et al. Phylogeny of New World Stipeae (Poaceae): an evaluation of the monophyly of Aciachne and Amelichloa. Cladistics. 2010;26(6):563–578. doi: 10.1111/j.1096-0031.2010.00310.x. [DOI] [PubMed] [Google Scholar]
- 19.Cialdella AM, et al. Phylogeny of Nassella (Stipeae, Pooideae, Poaceae) based on analyses of chloroplast and nuclear ribosomal DNA and morphology. Syst. Botany. 2014;39(3):814–828. doi: 10.1600/036364414X681419. [DOI] [Google Scholar]
- 20.Sclovich SE, Giussani LM, Cialdella AM, Sede SM. Phylogenetic analysis of Jarava (Poaceae, Pooideae, Stipeae) and related genera: testing the value of the awn indumentum in the circumscription of Jarava. Plant Syst. Evol. 2015;301(6):1625–1641. doi: 10.1007/s00606-014-1175-9. [DOI] [Google Scholar]
- 21.Hamasha HR, von Hagen KB, Röser M. Stipa (Poaceae) and allies in the Old World: molecular phylogenetics realigns genus circumscription and gives evidence on the origin of American and Australian lineages. Plant Syst. Evol. 2012;298(2):351–367. doi: 10.1007/s00606-011-0549-5. [DOI] [Google Scholar]
- 22.Krawczyk K, Nobis M, Nowak A, Szczecińska M, Sawicki J. Phylogenetic implications of nuclear rRNA IGS variation in Stipa L. (Poaceae) Sci. Rep. 2017;7(11506):1–11. doi: 10.1038/s41598-017-11804-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erickson DL, Spouge J, Resch A, Weigt LA, Kress JW. DNA barcoding in land plants: developing standards to quantify and maximize success. Taxon. 2008;57(4):1304–1316. [PMC free article] [PubMed] [Google Scholar]
- 24.Sucher NJ, Carles MC. Genome-based approaches to the authentication of medicinal plants. Planta Medica. 2008;74(6):603–623. doi: 10.1055/s-2008-1074517. [DOI] [PubMed] [Google Scholar]
- 25.Parks M, Cronn R, Liston A. Increasing phylogenetic resolution at low taxonomic levels using massively parallel sequencing of chloroplast genomes. BMC Biology. 2009;7(1):84–100. doi: 10.1186/1741-7007-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nock CJ, et al. Chloroplast genome sequences from total DNA for plant identification. Plant Biotech. J. 2011;9:328–333. doi: 10.1111/j.1467-7652.2010.00558.x. [DOI] [PubMed] [Google Scholar]
- 27.Wysocki WP, Clark LG, Attigala L, Ruiz-Sanchez E, Duvall MR. Evolution of the bamboos (Bambusoideae; Poaceae): a full plastome phylogenomic analysis. BMC Evol. Biol. 2015;15(1):50. doi: 10.1186/s12862-015-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hand ML, Spangenberg GC, Forster JW, Cogan NO. Plastome sequence determination and comparative analysis for members of the Lolium-Festuca grass species complex. G3: Genes, Genomes. Genetics. 2013;3(4):607–616. doi: 10.1534/g3.112.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spooner DM, Ruess H, Iorizzo M, Senalik D, Simon P. Entire plastid phylogeny of the carrot genus (Daucus, Apiaceae): Concordance with nuclear data and mitochondrial and nuclear DNA insertions to the plastid. Am. J. Bot. 2017;104(2):296–312. doi: 10.3732/ajb.1600415. [DOI] [PubMed] [Google Scholar]
- 30.Eserman LA, Tiley GP, Jarret RL, Leebens-Mack JH, Miller RE. Phylogenetics and diversification of morning glories (tribe Ipomoeeae, Convolvulaceae) based on whole plastome sequences. Am. J. Bot. 2014;101(1):92–103. doi: 10.3732/ajb.1300207. [DOI] [PubMed] [Google Scholar]
- 31.CBOL Plant Working Group A DNA barcode for land plants. Proc. Natl Acad. Sci. USA. 2009;106:12794–12797. doi: 10.1073/pnas.0905845106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford CS, et al. Selection of candidate coding DNA barcoding regions for use on land plants. Bot. J. Linn. Soc. 2009;159(1):1–11. doi: 10.1111/j.1095-8339.2008.00938.x. [DOI] [Google Scholar]
- 33.Hollingsworth ML, et al. Selecting barcoding loci for plants: evaluation of seven candidate loci with species‐level sampling in three divergent groups of land plants. Mol. Ecol. Res. 2009;9(2):439–457. doi: 10.1111/j.1755-0998.2008.02439.x. [DOI] [PubMed] [Google Scholar]
- 34.Chase MW, et al. Land plants and DNA barcodes: short-term and long-term goals. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2005;360(1462):1889–1895. doi: 10.1098/rstb.2005.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kress WJ, Erickson DL. A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS one. 2007;2(6):e508. doi: 10.1371/journal.pone.0000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heise W, Babik W, Kubisz D, Kajtoch Ł. A three-marker DNA barcoding approach for ecological studies of xerothermic plants and herbivorous insects from central Europe. Bot. J. Linn. Soc. 2015;177(4):576–592. doi: 10.1111/boj.12261. [DOI] [Google Scholar]
- 37.Ferri G, et al. Forensic botany II, DNA barcode for land plants: Which markers after the international agreement? Forensic Science International: Genetics. 2015;15:131–136. doi: 10.1016/j.fsigen.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Shaw J, et al. The tortoise and the hare II: relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am. J. Bot. 2005;92:142–166. doi: 10.3732/ajb.92.1.142. [DOI] [PubMed] [Google Scholar]
- 39.Ma XY, et al. Species identification of medicinal pteridophytes by a DNA barcode marker, the chloroplast psbA-trnH intergenic region. Biol. Pharm. Bull. 2010;33:1919–1924. doi: 10.1248/bpb.33.1919. [DOI] [PubMed] [Google Scholar]
- 40.Van De Wiel CCM, Van Der Schoot J, Van Valkenburg JLCH, Duistermaat H, Smulders MJM. DNA barcoding discriminates the noxious invasive plant species, floating pennywort (Hydrocotyle ranunculoides L.f.), from non‐invasive relatives. Mol. Ecol. Res. 2009;9(4):1086–1091. doi: 10.1111/j.1755-0998.2009.02547.x. [DOI] [PubMed] [Google Scholar]
- 41.Yao H, et al. Identification of Dendrobium species by a candidate DNA barcode sequence: the chloroplast psbA-trnH intergenic region. Planta Medica. 2009;75:667–669. doi: 10.1055/s-0029-1185385. [DOI] [PubMed] [Google Scholar]
- 42.Stech M, Quandt D. 20,000 species and five key markers: the status of molecular bryophyte phylogenetics. Phytotaxa. 2010;9(1):196–228. doi: 10.11646/phytotaxa.9.1.11. [DOI] [Google Scholar]
- 43.Chase MW, et al. A proposal for a standardised protocol to barcode all land plants. Taxon. 2007;56(2):295–299. [Google Scholar]
- 44.Dong W, Liu J, Yu J, Wang L, Zhou S. Highly variable chloroplast markers for evaluating plant phylogeny at low taxonomic levels and for DNA barcoding. PloS one. 2012;7(4):e35071. doi: 10.1371/journal.pone.0035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw J, et al. Chloroplast DNA sequence utility for the lowest phylogenetic and phylogeographic inferences in angiosperms: the tortoise and the hare IV. Am. J. Bot. 2014;101(11):1987–2004. doi: 10.3732/ajb.1400398. [DOI] [PubMed] [Google Scholar]
- 46.Kimura, M. The Neutral Theory of Molecular Evolution. 1–369 (Cambridge University Press 1983)
- 47.Morton BR, Clegg MT. A chloroplast DNA mutational hotspot and gene conversion in a noncoding region near rbcL in the grass family (Poaceae) Current Genet. 1993;24(4):357–365. doi: 10.1007/BF00336789. [DOI] [PubMed] [Google Scholar]
- 48.Clegg MT, Gaut BS, Learn GH, Morton BR. Rates and patterns of chloroplast DNAevolution. Proc. Natl Acad. Sci. USA. 1994;91(15):6795–6801. doi: 10.1073/pnas.91.15.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris LM, Duvall MR. (2010). The chloroplast genome of Anomochloa marantoidea (Anomochlooideae; Poaceae) comprises a mixture of grass-like and unique features. Am. J. Bot. 2010;97(4):620–627. doi: 10.3732/ajb.0900226. [DOI] [PubMed] [Google Scholar]
- 50.Shimada H, Sugiura M. Pseudogenes and short repeated sequences in the rice chloroplast genome. Current Genet. 1989;16(4):293–301. doi: 10.1007/BF00422116. [DOI] [PubMed] [Google Scholar]
- 51.Katayama H, Ogihara Y. Phylogenetic affinities of the grasses to other monocots as revealed by molecular analysis of chloroplast DNA. Current Genet. 1996;29(6):572–581. doi: 10.1007/BF02426962. [DOI] [PubMed] [Google Scholar]
- 52.Klichowska E, Nobis M. Stipa pennata subsp. ceynowae (Poaceae, Pooideae), a new taxon from CentralEurope. PhytoKeys. 2016;83:75–92. doi: 10.3897/phytokeys.83.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myszczyński K, Nobis M, Szczecińska M, Sawicki J, Nowak A. The complete plastid genome of the middle Asian endemic of Stipa lipskyi (Poaceae) Mitochondrial DNA Part A. 2016;27(6):4661–4662. doi: 10.3109/19401736.2015.1106491. [DOI] [PubMed] [Google Scholar]
- 54.Sawicki, J. et al. Mitogenomic analyses support the recent division of the genus Orthotrichum (Orthotrichaceae, Bryophyta). Sci. Rep. 7 (2017). [DOI] [PMC free article] [PubMed]
- 55.Szczecińska M, Gomolińska A, Szkudlarz P, Sawicki J. Plastid and nuclear genomic resources of a relict and endangered plant species: Chamaedaphne calyculata (L.) Moench (Ericaceae) Turk. J. Bot. 2014;38(6):1229–1238. doi: 10.3906/bot-1405-80. [DOI] [Google Scholar]
- 56.Koren S, et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017;27(5):722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lohse M, Drechsel O, Kahlau S, Bock R. OrganellarGenomeDRAW—a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucl. Acids Res. 2013;41(W1):W575–W581. doi: 10.1093/nar/gkt289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krzywinski M, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2016;34(3):772–773. doi: 10.1093/molbev/msw260. [DOI] [PubMed] [Google Scholar]
- 61.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17(8):754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J, Kapli P, Pavlidis P, Stamatakis A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics. 2013;29(22):2869–2876. doi: 10.1093/bioinformatics/btt499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1 and Supplementary Table S2