Abstract
Wound care is a major healthcare expenditure. Treatment of burns, surgical and trauma wounds, diabetic lower limb ulcers and skin wounds is a major medical challenge with current therapies largely focused on supportive care measures. Successful wound repair requires a series of tightly coordinated steps including coagulation, inflammation, angiogenesis, new tissue formation and extracellular matrix remodelling. Zinc is an essential trace element (micronutrient) which plays important roles in human physiology. Zinc is a cofactor for many metalloenzymes required for cell membrane repair, cell proliferation, growth and immune system function. The pathological effects of zinc deficiency include the occurrence of skin lesions, growth retardation, impaired immune function and compromised would healing. Here, we discuss investigations on the cellular and molecular mechanisms of zinc in modulating the wound healing process. Knowledge gained from this body of research will help to translate these findings into future clinical management of wound healing.
Keywords: inflammation, immune response, anti-oxidant, tissue proliferation, matrix remodelling, TRIM family proteins, matrix metalloproteinase
1. Introduction
Wound healing is a physiological response to injury that is essential across all tissue systems. Wound care is a major public health issue. Based on an economic evaluation of 2014 Medicare expenses, the total annual costs of all wound types, such as pressure ulcer, venous ulcers, diabetic foot ulcer, surgical and traumatic wounds and infection, ranged from $USA28.1 to $USA96.8 billion in the United States [1]. The burden of wound treatment remains a significant challenge due to an aging population and increases in diabetes and obesity, conditions that contribute to aberrant wound healing. The underlying complexities of the wound healing process include membrane repair, coagulation, control of inflammation, angiogenesis, cell proliferation, tissue remodelling and scar formation. These cumulative functions are integral for restoring tissue architecture.
Zinc is an essential micronutrient, present at less than 50 mg/kg, in the human body. It is important for human health and disease due to its critical roles in growth and development, bone metabolism, the central nervous system, immune function and wound healing [2]. Zinc is a vital cofactor for the function of more than 10% of proteins encoded by the human genome (~3000 proteins/enzymes) [3,4]. Zinc-dependent proteins play numerous indispensable roles within cells, such as transcriptional regulation, DNA repair, apoptosis [5,6], metabolic processing [7], extracellular matrix (ECM) regulation [8] and antioxidant defence [9].
Low concentration of zinc is found intracellularly across all tissues as a divalent cation incapable of passive diffusion across cell membranes. As such, the cellular homeostasis and bioavailability of zinc is tightly controlled through transcriptional regulation, ion transporters and compartmental stores [10,11,12,13]. Metabolically active, labile zinc is present inside cells at pico- to nanomolar concentrations with extracellular concentrations in the sub-micromolar to micromolar range [14]. The zinc transporter protein (ZIP) family zinc uptake/transporter proteins, particularly ZIP4, are responsible for zinc uptake from the extracellular milieu or intracellular vesicles [15]. Cytosolic free zinc ions have recently been identified as secondary messengers, similar to calcium ion transients, capable of interacting with target proteins in order to regulate many signal transduction pathways [16,17]. In this regard, zinc availability and regulation constitute an important component in cell physiology.
A common cause of zinc deficiency is malnutrition. Dietary zinc is absorbed in the small intestines through a carrier-mediated mechanism. Illness/infection and high phytate-containing foods are shown to hinder zinc bioavailability by inhibiting uptake [18,19]. The vast majority of zinc found within the human body is stored in skeletal muscle (60%) but reserves are also found in bone (30%), skin and liver (5%) and other organs (2–3%) [13]. Due to the ubiquitous and multifaceted nature of zinc, the effects of zinc deficiency are widespread and impact many organ systems and tissues. Globally, zinc deficiency is a common plight. This may be the combined result of hereditary or dietary problems and can manifest clinically as many disorders including gastro-intestinal (GI) tract malabsorption syndromes [20], liver and renal diseases [21], aging [22], immune dysfunction [22,23], dermatitis [24], mental and growth retardation [25,26], hypogonadism [27] and impaired wound healing [28].
Studies dating back to 1970 and earlier have shown the importance of zinc concentrations towards healing wounds in patients with thermal injuries or exposure to surgical stress [29]. Zinc is especially important in skin [30]. Skin contains a relatively high (about 5% of body content) zinc content, primarily associated within the epidermis (50–70 μg/g dry weight) [31]. Due to its abundance in the epidermis, mild zinc deficiency is noted to lead to roughened skin and impaired wound healing [32]. Severe zinc deficiency was found to be related to Acrodermatitis enteropathica (AE), a potentially fatal genetic disorder in which individuals are unable to absorb sufficient dietary zinc as a result of mutations in the SLC39A4 gene which encodes hZIP4 [33]. Zinc deficiency associated with AE leads to dermatitis, diarrhoea, secondary bacterial/fungal infections and often mortality in untreated infants. AE patients’ symptoms can be dramatically improved via dietary zinc supplements [34].
Zinc deficiency has been linked to delayed wound healing [32,35] and low serum zinc levels have been reported in critically ill patients within Intensive Care Units [36]. Although the benefits of supplemental zinc have been documented in critically ill patients [37], severe burn injury [38,39], subcutaneous abscess, minor surgery [40] and pressure ulcers [41,42], the effects of zinc on wound healing has only been minimally reviewed [32,35].
Wound healing, inflammation and immune response are intimately associated with one another. Over the years zinc has been shown capable of modulating both innate and adaptive immune functions. Zinc alters immune responses in a multitude of ways ranging from myeloid-derived cells and inflammatory signalling to lymphocyte differentiation and antibody production. The ability of zinc to regulate immune homeostasis is a burgeoning field of study and has been extensively reviewed by others [23]. Here, we discuss the contributions of zinc within the wound repair processes with an emphasis on platelet cells and haemostasis, inflammation and host defence, granulation and re-epithelization, ECM remodelling via regulation of matrix metalloproteinases (MMPs) and its association with tripartite motif (TRIM) family proteins in membrane repair and wound healing [43,44]. By identifying roles by which zinc coordinates wound healing, we hope to stimulate further research in tissue repair and regeneration.
2. The Phases of Wound Healing and Zinc’s Impact
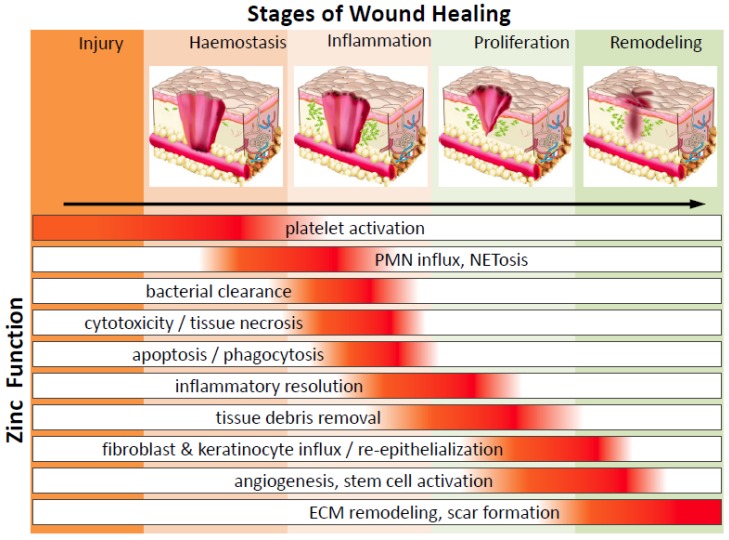
Wound healing is an intricate and dynamic process which can be subdivided into a series of phases including: (1) coagulating fibrin clot formation (haemostasis, occurs within seconds to 1 h), (2) inflammatory response (within minutes to days), (3) cell proliferation, re-epithelialization, granulation and angiogenesis (begins 18–24 h after wounding and lasts from days to weeks) and (4) matrix remodelling and scar formation (5–7 days after injury and could persist months to years) (Figure 1). As detailed below, these distinct but overlapping phases involve dynamic coordination of soluble mediators (reactive oxygen species (ROS), chemokines, cytokines and growth factors), ECM turnover and cellular cross-talk amongst platelets, infiltrating immune cells, resident keratinocytes, endothelial cells, fibroblasts, epithelial cells and stem cells. Within this section we will closely examine the impact of zinc regulation on wound healing.
Figure 1.
The function of zinc throughout the stages of wound healing. Zinc has a significant function in many cells over the entire process of wound repair. After injury wounded tissue must establish haemostasis via coagulation and clot formation. Injury is quickly followed by immune infiltration and inflammation as a means to clear the wound of damaged tissue and microbes, thus preventing infection and allowing room for granulation. Fibroblast, epithelial cells, keratinocytes and endothelial cells, will proliferate and migrate into wounds to deposit ECM and re-populate the injury site, facilitating wound closure. Finally, matrix deposition and clearance regulates the development of scar formation. PMN—polymorphonuclear leukocytes; NETosis—a novel form of programed neutrophil death that resulted in the formation of neutrophil extracellular traps (NETs); ECM—extracellular matrix.
2.1. Haemostasis and Platelets
Serious injury results in vasculature damage and it becomes imperative to stanch bleeding. The major mechanism by which haemostasis is accomplished is via activation and aggregation of platelet cells. Platelets are capable of responding to vascular damage by means of aggregating at the damage site to form an initial clot followed by fibrin deposition and polymerization to further strengthen the plug [45]. It has been understood for decades now that zinc is capable of enhancing platelet activity and aggregation [46,47]. Recently it was shown that the effect of zinc on platelets is mediated through Protein kinase C (PKC)-mediated tyrosine phosphorylation of platelet proteins [48,49]. Exogenous zinc treatment (at millimolar concentration range) induced zinc entry into the platelet cytosol and time-dependent (within 30 min) stimulation of tyrosine phosphorylation on certain high molecular weight proteins which could be blocked by PKC inhibitors. The intriguing role of zinc on pathophysiological thrombus formation during tissue injury is still largely unknown. Platelets are increasingly being recognized as immune cells capable of pathogen recognition and mediating an inflammatory response via cytokines and chemokines [50]. Platelets contain alpha-granules, which carry a plethora of proteins and factors, such as Chemokine (C-X-C motif) ligand 1 (CXCL1, GRO-α (growth regulated α protein), CXCL4, CXCL5 (ENA-78 ,epithelial-derived neutrophil-activating protein 78), CXCL7 (PPBP (Pro-Platelet basic protein), β-TG (Beta-Thromboglobulin), CTAP-III (connective tissue activating peptide III), NAP-2 (neutrophil-activating peptide-2)), CXCL8 (IL-8, interleukin-8), CXCL12 (SDF-1α, stromal cell-derived factor-1α), Chemokine (C-C motif) ligand 2 (CCL2, MCP-1(monocyte chemoattractant protein-1)), CCL3 (MIP-1α, macrophage inflammatory protein 1-α) and CCL5 (RANTES, regulated upon activation normal T cell expressed and secreted); factors capable of recruiting and activating innate immune cells to the wound site [51]. It has been shown that zinc induces alpha-granule release [48]. These studies suggest that platelets and zinc play an important role in initiating the inflammatory phase of wound healing.
2.2. Inflammation and Immune Defence
During and after haemostasis, wounded tissue undergoes an inflammatory stage. This is an elaborate process involving coordination amongst a diversity of cell types. Innate immune cells migrate to and infiltrate injured tissue whereby they can rid the environment of cellular debris and infectious microbes. In experimental human studies of mild zinc deficiency, the affected elderly subjects (ages 55–85 years) had increased inflammatory cytokines and oxidative stress production [52]. Zinc supplementation in elderly subjects reduced plasma levels of oxidative stress markers, decreased ex vivo production of inflammatory cytokines like C-Reactive Protein (CRP) and IL-6, reduced chemokines such as monocyte chemoattractant protein-1 (MCP-1) and reduced secretary cell adhesion molecules (soluble vascular cell adhesion molecule-1 (sVCAM-1), soluble intercellular adhesion molecule-1 (sICAM-1) and s-E-selectin) which represent important biomarkers of cell damage-associated inflammation in endothelium and platelets [18,52,53]. Inflammation-associated vascular dysfunction could be due to peroxisome proliferator-activated receptors (PPAR) dysregulation during zinc deficiency [54].
Neutrophils are granular polymorphonuclear leukocytes (PMN), which often act as one of the first responders to tissue injury and bacterial infection. The migration of neutrophils and other leukocytes to infected/damaged sites is accomplished via increasing gradients of chemokines and cytokines, a process known as chemotaxis. In rhesus monkeys, zinc deficiency obstructs PMN chemotaxis while supplementation reverses the effects [55]. Upon arrival to wounds neutrophils are capable of secreting inflammatory cytokines and phagocytizing pathogens in order to prevent infection. In a study of zinc-deficient acquired immune deficiency syndrome (AIDS) patients, zinc supplementation was observed to enhance neutrophilic phagocytosis of opsonized zymosan particles, a Toll-like receptor-2 (TLR2) agonist [56].
Phagocytes, such as neutrophils, are able to destroy intracellular pathogens via ROS. Enzymes important for the generation of ROS precursors and bacterial clearance, are nicotinamide adenine dinucleotide phosphate (NADPH)-oxidases. Interestingly excess zinc, as well as zinc-deficiency, can both inhibit NADPH-oxidases and thereby hinder microbial elimination [57,58]. A unique mechanism by which neutrophils are able to eliminate pathogens is via the release of neutrophil extracellular traps (NETs). NETs are extracellular matrices consisting of granule proteins, chromatin and DNA; capable of trapping and killing microbes [59]. Absence of zinc, via chelators, compromises NETosis [60]. The contents of NETs include heterodimeric proteins known as calprotectin [61]. Calprotectin is able to bind and sequester zinc ions [62]. By sequestering zinc, calprotectin deprives bacteria of essential metals and hinders bacterial survival and expansion. This method of microbial control falls under a category known as “Nutritional Immunity”, a process in which host cells sequester and limit the availability of essential trace elements thereby inhibiting pathogenic growth. Bacteria have developed their own response to combat strategies such as calprotectin. Some bacteria have evolved to express zinc uptake receptors with higher zinc-affinity than calprotectin, or even receptors that bind and repurpose calprotectin, thus allowing them to evade nutritional immunity [63,64].
Monocytes are pro-inflammatory innate immune cells. Monocytes respond to chemokines and migrate to affected tissue where they adhere to endothelial cells, infiltrate tissue and differentiate into macrophages capable of clearing pathogens/damaged tissue and propagating inflammation. It has been reported that zinc has conflicting roles on monocyte/endothelial adhesion. It has been shown that zinc-deficiency as well as zinc oxide treatment result in elevated monocyte adhesion [65,66]. Dierichs et al. recently reported that zinc could participate in modulation of monocyte differentiation into pro-inflammatory (M1) or immune-regulatory/wound healing (M2) macrophages [67]. M1 macrophages are important for early inflammation and microbial/debris clearance, while M2 macrophages are involved in immune suppression and later tissue remodelling/repair. Using the human monocyte cell line THP-1s (Tohoku Hospital Pediatrics-1), they discovered that both zinc deficiency and supplementation promote M1 phenotypes, while inhibiting M2 differentiation. Counterintuitively, they also report that zinc supplementation suppresses inducible nitric oxide synthases (iNOS) expression, an M1 hallmark associated with reactive nitrogen species production and pathogen clearance. Maintaining an appropriate balance between M1/M2 macrophage populations is complex and crucial during wound healing. Fully elucidating the effect of zinc on macrophage phenotypes and functions will aid in the advancement of wound healing treatments.
Macrophages eliminate inflammatory pathogens by phagocytosis, a process that microbes are taken up and trapped via membrane bound intracellular phagosomes. From there microbes can be killed by means of oxidation or lysosomal degradation. Phagosomes can fuse with lysosomes, whereby the protease-containing acidic environment can destroy bacteria. Many bacteria have developed strategies whereby they can live within intracellular phagosomes undetected and prevent lysosome fusion/degradation. Under these circumstances, similar to neutrophils, macrophages have developed their own forms of nutritional immunity. Through the use of zinc transporters, macrophages are able to shuttle zinc in or out of bacterial laden phagosomes. Depending on the microbes present, macrophages are able to deprive bacteria of zinc, essentially starving them, or alternatively poison bacteria with toxic levels of zinc and other heavy metals [68,69]. M. tuberculosis developed a defence to zinc toxicity however, via P-type ATPases, capable of exporting zinc and maintaining viable ion concentrations even within the toxic environment of phagosomes [70].
Macrophages and other immune cells, are major producers of cytokines. Many studies have shown that zinc modulation has an effect on cytokine production. Some of the major regulators of inflammatory cytokine production are T cell receptor (TLR) proteins. TLR proteins are capable of recognizing pathogen-specific molecular motifs and implement signalling cascades. Closely associated with TLRs is nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signalling. NF-κB is a potent transcriptional regulator involved in a plethora of cellular processes associated with wound healing including: inflammatory response, tissue remodelling, proliferation, apoptosis, cell adhesion, etc. [71]. Numerous reports have detailed the effects of zinc on TLR/NF-κB mediated inflammatory signalling, however studies have come to conflicting conclusions. Hasse et al. reported that lipopolysaccharide (LPS)/TLR4 mediated NF-κB signalling is dependent upon intracellular free zinc [72]. In their study sequestration of zinc via an intracellular membrane-permeable zinc ion chelator, TPEN (N,N,N′,N′-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine), completely abolishes NF-κB activation after LPS stimulus. In contrast, there is a growing body of evidence that zinc acts as an inhibitor of TLR/NF-κB signalling. Reports have shown that zinc is capable of negatively regulating NF-κB signalling via PPAR-α, A20, IκB kinase-β (IKKβ) and phosphodiesterase (PDE) [73,74,75,76].
Dynamic crosstalk exists between zinc homeostasis and inflammation. A negative feedback loop appears to exist within zinc/NF-κB signalling. It has been demonstrated that upon inflammatory stimulation NF-κB upregulates the zinc transporter ZIP8. ZIP8 translocates to the plasma membrane where it facilitates zinc uptake into the cell. Intracellular zinc is thereupon free to inhibit IKKβ and negatively regulate the inflammatory process [75]. In a similar negative feedback loop, IL-6 stimulus results in upregulation of ZIP14, which is also capable of attenuating inflammation in hepatocytes [77].
IL-1β and IL-18 are potent pro-inflammatory cytokines under the regulation of caspase 1 activation. Caspase-1 belongs to the pro-apoptotic endoprotease family of Caspases. Research has yielded conflicting results on the pro vs. anti-effects of zinc on apoptosis. While results have been ambiguous, zinc concentration appears to be an important factor [78]. Groups have shown that zinc has a direct inhibitory effect on the activity of caspases 3, 6, 7, 8 and 9 [79,80,81]. A similar study utilizing a zinc-containing compound, ziram, showed pro-caspase1, the inactive precursor of caspase1, degraded upon ziram treatment [82]. Degradation of the pro-inflammatory precursor (pro-caspase1) indicates a potential role for zinc in regulating caspase-mediated inflammation. This notion is supported by clinical studies showing higher levels of IL-1β expression in overweight patients with low dietary zinc intake, compared to patients with higher zinc intake [83].
As mentioned earlier, macrophages partake in the clearance of not only microbes but also damaged tissue. Lymphocytes and the adaptive immune system also play an important role in this element of wound healing. It has been shown that B-lymphocytes aid in wound clearance and repair [84,85]. Mature B-cells/plasma cells are capable of producing antibodies that detect injured tissue. These antibodies serve as signals by which macrophages recognize and phagocytize damaged cells. Zinc deficiency results in lowered populations of both precursor and mature B-cells and can reduce antibody production [86]. Diminishing B-cells populations and ergo circulating antibodies, would negatively affect phagocytosis causing hindered wound clearance and chronic wounds. In fact, it has been demonstrated in chronic diabetic skin lesions, that direct B-cell treatment accelerates wound healing [87].
2.3. Inflammatory Resolution and Tissue Growth (Proliferation) Stage
During wound healing, it is important to resolve inflammation and initiate re-epithelization, the process where epithelial cells proliferate and repopulate injured tissue for wound closure. M2 macrophages are one cell type that helps mitigate inflammation but there are numerous other immune cells that aid in this process. Zinc deficiency also has a significant impact on T lymphocyte populations [86,88]. Regulatory T lymphocytes (Tregs) regulate and suppress inflammation. Zinc supplementation is known to increase the number of Tregs in multiple circumstances: mixed lymphocyte cultures, in response to allergens and when combined with transforming growth factor-β (TGFβ) [89,90,91]. Cutaneous wound studies have shown that Tregs help resolve inflammation, promote re-epithelization and wound contraction [92]. It would be interesting to determine whether zinc supplementation is capable of upregulating a regulatory T-cell response in order to promote accelerated wound repair.
About two or three days after the wound occurs, fibroblasts begin to enter the wound site, marking the onset of the tissue proliferative phase. Fibroblast infiltration is associated with collagen/ECM deposition, which serves as a temporary scaffold for repair. Collagen serves as a bed enabling migration of epithelium, keratinocytes and microvasculature. One of the primary regulators of ECM deposition and fibrosis is TGFβ/SMAD (Mothers against decapentaplegic homolog protein) signalling. Zinc is a vital cofactor for SMAD signalling and thus plays a major role in formation of granulation tissue [91]. During re-epithelialization and wound closure, there is transient proliferation and migration of resident keratinocytes, fibroblasts, epithelial cells and endothelial cells with simultaneous activation and trans-differentiation of multiple epidermal stem cells populations [93]. The collaborative efforts of collagenases and plasminogen activators led to degradation of fibrin clots along with the zinc-dependent matrix metalloproteinase (MMP, discussed in more details below), which digest dermal basal membranes and ECM, allows room for cell growth, migration and angiogenesis.
Within the epidermis, apoptotic and necrotic cells stimulate epidermal cells proliferation and migration over granulation tissue in order to repair the epidermis. Topical zinc application enhanced re-epithelialization in porcine skin wound healing model [94]. Moreover, a study testing galvanic Cu/zinc (Zn) microparticles on wound closure reports ROS-mediated enhancement of human dermal fibroblast migration [95]. Similarly, zinc was shown to increase keratinocyte migration and participate in re-epithelialization of the epidermis [96]. Concurrent with re-epithelialization, endothelial cells migrate and proliferate into wound sites to establish new blood vessels in a process called neovascularization, or angiogenesis, thus supplying essential oxygen and nutrients for the growth of cells in the wound bed. Zinc has been shown effective for angiogenesis in vivo [97]. However, studies analysing the effect of micronutrient trace elements on vascular biology report contradictory findings in which zinc acts as an anti-angiogenic agent altering important genes and growth factors [98]. Further research and characterization is needed regarding zinc and this phase of tissue healing and regeneration.
2.4. Wound Resolution and Matrix Remodelling
ECM is composed of a complex mixture of insoluble molecules such as collagens, laminins, fibronectin, integrin and heparin sulphate proteoglycans and provide a solid supportive matrix scaffold for cells. The ECM network also serves as a reservoir of a plethora of cytokines, growth factors and molecular cues, both active and latent, to promote cellular migration, epithelialization, adhesion and wound contraction [99]. Among the many proteins essential for epidermal wound repair are the ECM-remodelling matrix metalloproteinase (MMPs) family proteins. MMPs are secreted by distinct cell types such as inflammatory cells, keratinocytes, endothelial cells and fibroblasts. MMPs are zinc-dependent endopeptidases which act to modulate growth factor activation, cleavage, degradation and composition of ECM, processing of cell-cell junctional adhesion molecules, cytokines and cell surface receptors and cell-matrix signalling during different stages of wound healing [100,101,102,103].
MMPs are synthesized as inactive zymogens (proMMPs) and their functions are modulated in four ways—namely gene expression, compartmentalization, proMMPs activation and MMPs enzymatic inactivation. The structures of MMPs are composed of four domains, the pro-peptide domain with a conserved cysteine switch motif PRCGXPD, the zinc-binding catalytic domain, a hinge region and the C-terminal hemopexin-like domain. Activation of MMPs is dependent on a “cysteine switch” mechanism [104]. The pro-peptide cysteine interacts with the catalytically important zinc ion in the active site to keep proMMPs in an enzymatic inactive state to prevent their interaction and cleavage of substrates. The C-terminal hemopexin-like domains determine substrate specificity and their interaction with MMP inhibitors, the tissue inhibitor of metalloproteinases (TIMPs). Many stimuli such as tissue injury [105], oxidative stress upon UV exposure [106], inflammatory cytokines such as TNFα or IL-1α [107] and certain growth factors like vascular endothelial growth factor (VEGF) trigger the activation of MMPs [108]. Secreted or cell surface localized TIMPs also play important roles in wound repair. TIMPs process a wide range of extracellular substrates such as ECM proteins, cytokines and their receptors through their regulations of metalloproteinase activities [109]. These intricate activation mechanisms keep MMPs activities under tight control and in dynamic homeostasis under different wound healing demands [110].
Coordinated MMP function is pivotal for ECM degradation, migration of keratinocytes over ECM (wound re-epithelialization) [111], neo-angiogenesis [112], cell proliferation, fibroblast activation/migration and collagen deposition and fibrous tissue (fibroplasia and scar) formation during tissue remodelling to restore epidermal tissue integrity [113]. Prolonged ECM deposition leads to tissue fibrosis, whereas MMPs dysfunction is linked to defective wound closure [100,114]. Zinc and calcium ions are required for optimal MMPs function in vitro [115], however, the molecular mechanism by how zinc regulates MMPs function in vivo is still not fully understood due to the complexity of their regulation and the numerous MMPs involved in wound healing.
3. Zinc Is an Antioxidant Micronutrient
Several studies have shown that zinc deficiency increases oxidative stress [22,116,117]. It is well accepted that oxidative damage is a major cause of tissue injury and redox regulation plays a prominent role in wound repair [118,119,120]. Superoxide radicals such as reactive oxygen species (ROS) and reactive nitrogen free radical species (RNS) are by-products of electron leakage during mitochondrial electron transfer and constitute various forms of oxidative stress [121,122]. Superoxide radicals can cause oxidative damage to biomolecules, such as DNA, protein and lipids, thus impairing their bioactivity.
In biology, zinc is redox-inert and opposes the absorption and activity of redox-active transition metals like copper and iron, which catalyse the production of highly reactive oxidative stress [123,124]. Metallothionein (MT) is a family of low molecular weight, cysteine-rich (cysteine constitutes 30% of MT amino acids) proteins capable of binding heavy metals through their sulfhydryl (-SH)-rich cysteine residues. A large percentage (nearly 20%) of intracellular zinc is bound to MTs and can be rapidly released according to physiological needs. MTs are redox-sensitive antioxidant proteins, crucial for zinc regulation and protection against heavy-metal toxicity and oxidative stress. Interestingly, synthesis of MTs is dependent on availability of the dietary zinc, copper and selenium [125]. Zinc exhibits acute antioxidant properties by means of binding to and thus protecting redox-sensitive sulfhydryl groups of MTs [124,125]. Zinc supplementation induces MT expression and has been shown to protect against UV immunosuppression [126]. Oxidative stress associated with long term zinc deficiencies can be attributed to a reduction in the synthesis of MTs and a decline in the antioxidant activity of zinc-containing Cu, Zn superoxide dismutase (SOD1) [127].
4. TRIM Proteins in Membrane Repair and Wound Healing
Tripartite motif family (TRIM) proteins are characterized by the presence of N-terminal Ring (Really Interesting New Gene)-finger domain, zinc-finger B-box domains and a coil-coil domain. There are over 80 identified human TRIM proteins which play important roles in regulating cellular processes such as protein degradation, innate immunity, cell survival/death, oncogenesis, development and intracellular signalling. TRIM proteins exhibit a wide breadth of diversity along their C-termini, resulting in 12 different subfamilies/classifications. Most TRIM proteins contain E3 ubiquitin ligase activity, a class of enzymes which catalyze the final step (E3 step) in the ubiquitination cascade to form an ubiquitin covalent bond with a substrate lysine. TRIM proteins have been widely studied and reviewed within the context of immunology, cell death/survival and cancer [128,129,130,131], however an emerging role in wound healing has developed.
Maintaining cell membrane integrity is vital to normal cell physiology and function [132,133,134]. Repair of damaged cellular membranes requires intracellular vesicle trafficking which leads to an accumulation of vesicles close to the plasma membrane [135]. Subsequent resealing of the cell membrane is imperative for the survival and long-term viability of cells. Disruption of membrane repair contributes to pathophysiological conditions such as dystrophic muscle [136], diabetes [137], poor wound healing, chronic ulcer and scarring [133,134].
We have previously identified a novel TRIM protein, Mitsugumin 53 (MG53) or TRIM72, as an essential component of the cell membrane repair machinery [138,140] and is highly expressed in muscle. MG53 is capable of sensing oxidative stress associated with cell membrane damage and responds by binding to phosphatidylserine containing intracellular vesicles and then shuttles pre-assembled sub-membrane/intracellular vesicles to patch plasma membrane disruptions caused by mechanical or chemical injury [138,139,140]. Furthermore, MG53 is capable of protecting against ROS-mediated cellular stress, as seen with ischemic reperfusion injuries and metabolic disorders and prevents cell death [141]. MG53 ablation in mice (MG53−/−) results in defective membrane repair with progressive pathological consequences in skeletal and cardiac muscle and enhanced susceptibility to injuries in lungs, kidneys and skin [43,142,143,144,145,146,147,148]. The protective repair function of endogenous and recombinant human MG53 (rhMG53) protein have been reported in multiple tissue types ranging from skeletal muscle [136,144,149], heart [141,145,146,150], kidney [142], lung [143,151], skin [43,44] and brain [147].
The binding of zinc to TRIM family proteins is critical to their E3 ligase activities [152]. While membrane repair mechanisms can be context dependent, we have shown that zinc serves as a molecular switch linking oxidative stress and the membrane reseal function of MG53 [153]. MG53 consists of two zinc binding domains in the Ring-finger and B-box motifs [138,153]. Zinc binding appears to be essential for MG53-mediated membrane repair, as mutation of either zinc binding residue compromises the membrane resealing ability of MG53. Simultaneous disruption of both zinc binding motifs in double mutants further exacerbates this effect [153]. Moreover, defective MG53-mediated vesicular transport and membrane repair was observed in the absence of extracellular zinc via a biochemical zinc chelator. These results suggest that MG53 acts as an acceptor for zinc during cell membrane repair.
Current reports support the findings that TGFβ plays a central role throughout wound healing [154,155,156]. Excessive TGFβ signalling can lead to maladaptive repair conditions and tissue fibrosis [157]. Recently, we showed MG53 is a vital component of wound healing and topical application of rhMG53 protein promotes wound healing with reduced scar formation [43,44]. MG53 deficiency, in mice, leads to delayed wound healing with aberrant scar formation, while therapeutic treatment with rhMG53 accelerates healing and prevents scarring. In vitro assays revealed that rhMG53 induces fibroblast migration while simultaneously suppressing myofibroblast differentiation and production of fibrotic proteins. The underlying molecular mechanisms of MG53-mediated protection against dermal wounding involves membrane repair, modulation of cell migration and suppression of ECM protein synthesis via inhibition of TGFβ/SMAD signalling (Figure 2).
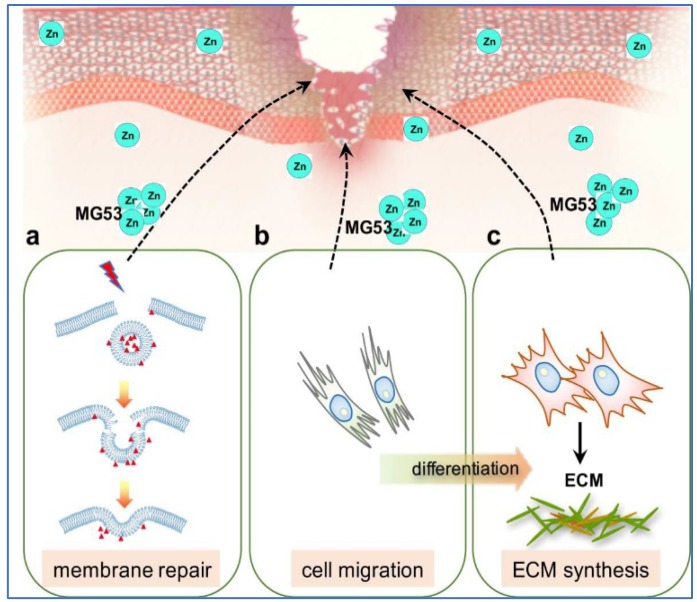
Figure 2.
Proposed roles of zinc in MG53 mediated wound healing process. MG53 exists as intrinsic intracellular vesicles which could be secreted into blood circulation as a myokine to mediate wound healing. In response to dermal injury, MG53 could exert the following functions. (a) MG53-containing vesicles nucleate the cell membrane repair machinery and trafficking to damaged cell membrane to protect against acute membrane injury of keratinocytes and fibroblasts; (b) MG53 mediates cytoskeletal stress fibre remodelling to promote fibroblasts migration to the wound sites; and (c) MG53 regulates TGFβ signalling to modulate trans-differentiation of fibroblasts into myofibroblasts and suppresses deposition of ECM proteins during tissue remodelling stage of wound healing. Zinc is proposed to bind to MG53 on its two zinc-binding sites and modulates MG53-mediated wound healing process (Modified from JBC 290(40): 24592). MG53: Mitsugumin 53; ECM: extracellular matrix; TGFβ: transforming growth factor-β.
MG53 is a versatile molecule with function associated with various signalling pathways like reperfusion injury salvage kinase (RISK) [145], glycogen synthase kinase-3β (GSK3β) [136,145,158], cell survival kinase AKT, TGFβ [43] and modulation of inflammatory mediators [143]. These findings are promising with regards to MG53s translational potential in wound healing and tissue regeneration. However, how zinc impacts MG53 E3-ubiquitin ligase activity and the identities of the E3-ligase substrates involved in MG53-mediated wound healing remain elusive and requires further investigation.
Two other TRIM proteins have been identified that might play a role in wound healing. Beer et al. reported that TRIM16, also known as oestrogen-responsive B box protein (EBBP), is a regulator of keratinocyte differentiation. TRIM16 expression is downregulated in skin wounds and hyper-thickened epithelium [159]. Over expression of TRIM16 enhances early differentiation of keratinocytes and is believed to regulate the differentiation capacity of cells. TRIM16 has extensively been studied in the field of cancer but little attention as has been directed toward wound healing. Interestingly, a study on ovarian cancer metastasis showed that overexpression of TRIM16 inhibited expression of MMP2 and MMP9 [160]. It is possible that TRIM16 is downregulated during wound healing in order to allow for tissue granulation, re-epithelialization and remodelling.
Recently the function of TRIM28 in endothelium was investigated by Wang et al. They show that TRIM28 helps mediate not only inflammation but also angiogenesis in a VEGFR2 dependent manner. Scratch wound assay showed endothelial cell migration was significantly reduced, after TRIM28 siRNA treatment [161]. These findings have significant implications for inflammation and wound healing. While both TRIM16 and TRIM28 have promising potential for wound repair, they are yet to be directly applied to such a study. Additionally, characterizing the impact of zinc on these proteins’ functions would be beneficial. Further research into zinc regulation of TRIM protein expression/function during wound healing would provide new and exciting insight on wound repair.
5. Zinc in Wound Healing—Clinical Perspectives
The role zinc plays in wound healing can be viewed from two perspectives: first, the impact of zinc deficiency and second, the effect of zinc supplementation (topical/local or systemic) on wound repair. The association between zinc deficiency and delayed wound healing has been described [32,35]. Treating zinc deficiency results in improved wound healing compared to those with zinc deficiency. For example, in patients considered to be at risk of refeeding syndrome, it may be appropriate to give a loading dose of 10–30 mg of Zn, followed by the daily maintenance dose of 2.5–5 mg [4]. However, the impact of zinc supplementation on wound healing in patients without zinc deficiency is less well known. There are very few well-done clinical studies on the topic and what little information currently available is inconsistent. For example, the Cochrane database reports 6 small studies on patients with arterial or venous ulcers and found that oral zinc supplementation did not improve wound healing [162]. Contrarily, another meta-analysis of topical zinc therapy with zinc oxide paste-medicated dressing containing zinc concentration between 6–15% for chronic venous leg ulcers showed improved healing, though the authors point out that the studies were small and of sub-optimal quality [163]. Cereda and co-authors performed a randomized, prospective trial in malnourished patients with chronic pressure ulcers [164]. They found a significant reduction in the size of the ulcers after 12 weeks of supplementation with a high calorie, high protein oral formula that was also supplemented with arginine, anti-oxidants and zinc (either orally or tube fed with 18–20 mg zinc daily). It is unknown how significant of a role the zinc supplementation played in this study. Attia and colleagues reported on 90 non-diabetic patients with uncomplicated wounds who were topically treated with one of two zinc-containing fluids (regular crystalline insulin or aqueous zinc chloride solutions at 0.2 mg/100 mL per 10 cm2 wound) versus a control of 0.9% normal saline. The groups treated with the zinc-containing fluids had significantly improved healing [165]. On the other hand, a study of 42 patients with pressure ulcers treated with oral l-carnosine versus zinc containing polaprezinc (at 34 mg per day) showed no difference in healing [166]. It is clear that more studies utilizing more stringent controls will be necessary to fully understand the clinical potential of zinc supplementation.
Due to the loss of zinc during injury, zinc therapy has been used in wound care to enhance healing in zinc-deficient patients [32,167]. Topical zinc sulphate (ZnSO4) application, usually at an optimal 3% concentration, has been widely used in wound healing for its antioxidant effect [30]. Other forms of application include 1% ZnCl2 or the largely insoluble zinc oxide (ZnO). ZnO provides prolonged supply of zinc to wounds and enhance its healing ability. Additionally, ZnO increases collagen degradation in necrotic wounds [168]. It has been shown that topical zinc application induces mRNA expression of metallothionein, which could account for its anti-UV photoprotective effect [30,169]. A standard regimen for severe burn care includes regular daily dietary zinc supplementation equivalent or exceeding 22 mg [39,170]. Moreover, recent advances in drug delivery with zinc oxide nanoparticle (ZnO-NPs) technology has received considerable attention for the treatment of wounds due to their effective cell penetration, immunomodulation and antimicrobial capacity [171,172]. However, in-depth pharmacodynamics and toxicology studies are still needed prior to widespread applications [173].
6. Concluding Remarks and Future Perspectives
Zinc is a micronutrient that is essential to human health. Zinc plays a major role in regulating every phase of the wound healing process; ranging from membrane repair, oxidative stress, coagulation, inflammation and immune defence, tissue re-epithelialization, angiogenesis, to fibrosis/scar formation. With huge demands for improved wound care, we need a more thorough in-depth understanding of the molecular mechanisms in which zinc functions. A more thorough comprehension of the cellular cross-talks and MMP regulation would go a long way in progressing the field wound healing. The biological function and therapeutic potential of TRIM proteins, such as MG53, in wound repair is emerging. Understanding the precise mechanisms by which zinc regulates their activity remains unexplored, yet is crucial. Further inquiry into the mechanisms of zinc and the proteins for which it serves as a cofactor, will greatly advance the treatment and care of difficult-to-heal wounds.
Acknowledgments
Grant supports are provided to J.M. by the NIH (AR061385, AG056919, AR070752 and DK106394); to P.-H.L. by an OSU intramural Lockwood Research Fund.
Abbreviations
| AE | Acrodermatitis enteropathica |
| CAM | Cell adhesion molecules |
| CXCL | Chemokine (C-X-C motif) ligand |
| CCL | Chemokine (C-C motif) ligand |
| CRP | C-reactive protein |
| ECM | Extracellular matrix |
| ILs | Interleukins |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MMP | Matrix metalloproteinase |
| MG53 | Mitsugumin 53 |
| MT | Metallothioneins |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PPARα | Peroxisome proliferator activated receptors-a |
| PMN | Polymorphonuclear leukocytes |
| NET | Neutrophil extracellular traps |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| ROS | Reactive oxygen species |
| TIMP | Tissue inhibitor of metalloproteinases |
| TLR | Toll-like receptor |
| TRIM | Tripartite motif |
| TGFβ | Transforming Growth Factor-β |
| VEGF | Vascular endothelial growth factor |
| ZIPs | Zinc transporter proteins |
Author Contributions
P.-H.L., M.S. and S.M.S. wrote manuscript. H.L., P.H.U.L. and J.M. contributed idea and edited manuscript.
Conflicts of Interest
J.M. is a founder of TRIM-edicine, Inc., a biotechnology company developing rhMG53 as a therapeutic protein. All other authors declare no competing financial interests.
References
- 1.Nussbaum S.R., Carter M.J., Fife C.E., da Vanzo J., Haught R., Nusgart M., Cartwright D. An economic evaluation of the impact, cost, and medicare policy implications fo chronic nonhealing wounds. Value Health. 2017 doi: 10.1016/j.jval.2017.07.007. in press. [DOI] [PubMed] [Google Scholar]
- 2.Roohani N., Hurrell R., Kelishadi R., Schulin R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013;18:144–157. [PMC free article] [PubMed] [Google Scholar]
- 3.Freeland-Graves J.H., Sanjeevi N., Lee J.J. Global perspectives on trace element requirements. J. Trace Elem. Med. Biol. 2015;31:135–141. doi: 10.1016/j.jtemb.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Livingstone C. Zinc: Physiology, deficiency, and parenteral nutrition. Nutr. Clin. Pract. 2015;30:371–382. doi: 10.1177/0884533615570376. [DOI] [PubMed] [Google Scholar]
- 5.Zheng J., Lang Y., Zhang Q., Cui D., Sun H., Jiang L., Chen Z., Zhang R., Gao Y., Tian W., et al. Structure of human MDM2 complexed with RPL11 reveals the molecular basis of p53 activation. Genes Dev. 2015;29:1524–1534. doi: 10.1101/gad.261792.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho J.G., Park S., Lim C.H., Kim H.S., Song S.Y., Roh T.Y., Sung J.H., Suh W., Ham S.J., Lim K.H., et al. ZNF224, Kruppel like zinc finger protein, induces cell growth and apoptosis-resistance by down-regulation of p21 and p53 via miR-663a. Oncotarget. 2016;7:31177–31190. doi: 10.18632/oncotarget.8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cronin L., Walton P.H. Synthesis and structure of [Zn(OMe)(L)] × [Zn(OH)(L)] × 2(BPh4), L = cis,cis-1,3,5-tris[(E,E)-3-(2-furyl)acrylideneamino]cyclohexane: Structural models of carbonic anhydrase and liver alcohol dehydrogenase. Chem. Commun. (Camb. Engl.) 2003;13:1572–1573. doi: 10.1039/B302895J. [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson M.L., Garcia-Morales C., Abu-Elmagd M., Wheeler G.N. Three matrix metalloproteinases are required in vivo for macrophage migration during embryonic development. Mechan. Dev. 2008;125:1059–1070. doi: 10.1016/j.mod.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Pawlak K., Mysliwiec M., Pawlak D. The alteration in Cu/Zn superoxide dismutase and adhesion molecules concentrations in diabetic patients with chronic kidney disease: The effect of dialysis treatment. Diabetes Res. Clin. Pract. 2012;98:264–270. doi: 10.1016/j.diabres.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Choi S., Bird A.J. Zinc’ing sensibly: Controlling zinc homeostasis at the transcriptional level. Metallomics. 2014;6:1198–1215. doi: 10.1039/C4MT00064A. [DOI] [PubMed] [Google Scholar]
- 11.Colvin R.A., Holmes W.R., Fontaine C.P., Maret W. Cytosolic zinc buffering and muffling: Their role in intracellular zinc homeostasis. Metallomics. 2010;2:306–317. doi: 10.1039/b926662c. [DOI] [PubMed] [Google Scholar]
- 12.Hojyo S., Fukada T. Zinc transporters and signaling in physiology and pathogenesis. Arch. Biochem. Biophys. 2016;611:43–50. doi: 10.1016/j.abb.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Kambe T., Tsuji T., Hashimoto A., Itsumura N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015;95:749–784. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- 14.Bozym R.A., Chimienti F., Giblin L.J., Gross G.W., Korichneva I., Li Y., Libert S., Maret W., Parviz M., Frederickson C.J., et al. Free zinc ions outside a narrow concentration range are toxic to a variety of cells in vitro. Exp. Biol. Med. 2010;235:741–750. doi: 10.1258/ebm.2010.009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang T., Sui D., Hu J. Structural insights of ZIP4 extracellular domain critical for optimal zinc transport. Nat. Commun. 2016;7:11979. doi: 10.1038/ncomms11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reilly-O’Donnell B., Robertson G.B., Karumbi A., McIntyre C., Bal W., Nishi M., Takeshima H., Stewart A.J., Pitt S.J. Dysregulated Zn2+ homeostasis impairs cardiac type-2 ryanodine receptor and mitsugumin 23 functions, leading to sarcoplasmic reticulum Ca2+ leakage. J. Biol. Chem. 2017;292:13361–13373. doi: 10.1074/jbc.M117.781708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chappell R.L., Anastassov I., Lugo P., Ripps H. Zinc-mediated feedback at the synaptic terminals of vertebrate photoreceptors. Exp. Eye Res. 2008;87:394–397. doi: 10.1016/j.exer.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad A.S. Zinc is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front. Nutr. 2014;1:14. doi: 10.3389/fnut.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moynahan E.J. Letter: Acrodermatitis enteropathica: A lethal inherited human zinc-deficiency disorder. Lancet. 1974;2:399–400. doi: 10.1016/S0140-6736(74)91772-3. [DOI] [PubMed] [Google Scholar]
- 20.Tran C.D., Katsikeros R., Manton N., Krebs N.F., Hambidge K.M., Butler R.N., Davidson G.P. Zinc homeostasis and gut function in children with celiac disease. Am. J. Clin. Nutr. 2011;94:1026–1032. doi: 10.3945/ajcn.111.018093. [DOI] [PubMed] [Google Scholar]
- 21.Martinez S.S., Campa A., Li Y., Fleetwood C., Stewart T., Ramamoorthy V., Baum M.K. Low Plasma Zinc Is Associated with Higher Mitochondrial Oxidative Stress and Faster Liver Fibrosis Development in the Miami Adult Studies in HIV Cohort. J. Nutr. 2017;147:556–562. doi: 10.3945/jn.116.243832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mariani E., Mangialasche F., Feliziani F.T., Cecchetti R., Malavolta M., Bastiani P., Baglioni M., Dedoussis G., Fulop T., Herbein G., et al. Effects of zinc supplementation on antioxidant enzyme activities in healthy old subjects. Exp. Gerontol. 2008;43:445–451. doi: 10.1016/j.exger.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Gammoh N.Z., Rink L. Zinc in Infection and Inflammation. Nutrients. 2017;9:624. doi: 10.3390/nu9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammed J., Mehrotra S., Schulz H., Lim R. Severe Infant Rash Resistant to Therapy Due to Zinc Deficiency. Pediatr. Emerg. Care. 2017;33:582–584. doi: 10.1097/PEC.0000000000001218. [DOI] [PubMed] [Google Scholar]
- 25.Hagmeyer S., Haderspeck J.C., Grabrucker A.M. Behavioral impairments in animal models for zinc deficiency. Front. Behav. Neurosci. 2014;8:443. doi: 10.3389/fnbeh.2014.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boycott K.M., Beaulieu C.L., Kernohan K.D., Gebril O.H., Mhanni A., Chudley A.E., Redl D., Qin W., Hampson S., Kury S., et al. Autosomal-Recessive Intellectual Disability with Cerebellar Atrophy Syndrome Caused by Mutation of the Manganese and Zinc Transporter Gene SLC39A8. Am. J. Hum. Genet. 2015;97:886–893. doi: 10.1016/j.ajhg.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rotter I., Kosik-Bogacka D.I., Dolegowska B., Safranow K., Kuczynska M., Laszczynska M. Analysis of the relationship between the blood concentration of several metals, macro- and micronutrients and endocrine disorders associated with male aging. Environ. Geochem. Health. 2016;38:749–761. doi: 10.1007/s10653-015-9758-0. [DOI] [PubMed] [Google Scholar]
- 28.Zorrilla P., Gomez L.A., Salido J.A., Silva A., Lopez-Alonso A. Low serum zinc level as a predictive factor of delayed wound healing in total hip replacement. Wound Repair Regen. 2006;14:119–122. doi: 10.1111/j.1743-6109.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- 29.Henzel J.H., DeWeese M.S., Lichti E.L. Zinc concentrations within healing wounds. Significance of postoperative zincuria on availability and requirements during tissue repair. Arch. Surg. 1970;100:349–357. doi: 10.1001/archsurg.1970.01340220025005. [DOI] [PubMed] [Google Scholar]
- 30.Rostan E.F., DeBuys H.V., Madey D.L., Pinnell S.R. Evidence supporting zinc as an important antioxidant for skin. Int. J. Dermatol. 2002;41:606–611. doi: 10.1046/j.1365-4362.2002.01567.x. [DOI] [PubMed] [Google Scholar]
- 31.Gupta M., Mahajan V.K., Mehta K.S., Chauhan P.S. Zinc therapy in dermatology: A review. Dermatol. Res. Pract. 2014;2014:709152. doi: 10.1155/2014/709152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lansdown A.B., Mirastschijski U., Stubbs N., Scanlon E., Agren M.S. Zinc in wound healing: Theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007;15:2–16. doi: 10.1111/j.1524-475X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang K., Zhou B., Kuo Y.M., Zemansky J., Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am. J. Hum. Genet. 2002;71:66–73. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perafan-Riveros C., Franca L.F., Alves A.C., Sanches J.A., Jr. Acrodermatitis enteropathica: Case report and review of the literature. Pediatr. Dermatol. 2002;19:426–431. doi: 10.1046/j.1525-1470.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 35.Kogan S., Sood A., Garnick M.S. Zinc and Wound Healing: A Review of Zinc Physiology and Clinical Applications. Wounds Compend. Clin. Res. Pract. 2017;29:102–106. [PubMed] [Google Scholar]
- 36.Besecker B.Y., Exline M.C., Hollyfield J., Phillips G., Disilvestro R.A., Wewers M.D., Knoell D.L. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am. J. Clin. Nutr. 2011;93:1356–1364. doi: 10.3945/ajcn.110.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rech M., To L., Tovbin A., Smoot T., Mlynarek M. Heavy metal in the intensive care unit: A review of current literature on trace element supplementation in critically ill patients. Nutr. Clin. Pract. 2014;29:78–89. doi: 10.1177/0884533613515724. [DOI] [PubMed] [Google Scholar]
- 38.Kurmis R., Greenwood J., Aromataris E. Trace Element Supplementation Following Severe Burn Injury: A Systematic Review and Meta-Analysis. J. Burn Care Res. 2016;37:143–159. doi: 10.1097/BCR.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 39.Adjepong M., Agbenorku P., Brown P., Oduro I. The role of antioxidant micronutrients in the rate of recovery of burn patients: A systematic review. Burns Trauma. 2016;4:18. doi: 10.1186/s41038-016-0044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirastschijski U., Martin A., Jorgensen L.N., Sampson B., Agren M.S. Zinc, copper, and selenium tissue levels and their relation to subcutaneous abscess, minor surgery, and wound healing in humans. Biol. Trace Elem. Res. 2013;153:76–83. doi: 10.1007/s12011-013-9658-z. [DOI] [PubMed] [Google Scholar]
- 41.Posthauer M.E. Nutrition: Fuel for pressure ulcer prevention and healing. Nursing. 2014;44:67–69. doi: 10.1097/01.NURSE.0000456389.22724.ef. [DOI] [PubMed] [Google Scholar]
- 42.Sernekos L.A. Nutritional treatment of pressure ulcers: What is the evidence? J. Am. Assoc. Nurse Pract. 2013;25:281–288. doi: 10.1002/2327-6924.12025. [DOI] [PubMed] [Google Scholar]
- 43.Li H., Duann P., Lin P.H., Zhao L., Fan Z., Tan T., Zhou X., Sun M., Fu M., Orange M., et al. Modulation of wound healing and scar formation by MG53 protein-mediated cell membrane repair. J. Biol. Chem. 2015;290:24592–24603. doi: 10.1074/jbc.M115.680074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li M., Li H., Li X., Zhu H., Xu Z., Liu L., Ma J., Zhang M. A Bioinspired Alginate-Gum Arabic Hydrogel with Micro-/Nanoscale Structures for Controlled Drug Release in Chronic Wound Healing. ACS Appl. Mater. Interfaces. 2017;9:22160–22175. doi: 10.1021/acsami.7b04428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Didar U., Sekhon S., Gupta A.S. Platelets and Platelet-Inspired Biomaterials Technologies in Wound Healing Applications. ACS Biomater. Sci. Eng. 2017 doi: 10.1021/acsbiomaterials.7b00013. [DOI] [PubMed] [Google Scholar]
- 46.Heyns Adu P., Eldor A., Yarom R., Marx G. Zinc-induced platelet aggregation is mediated by the fibrinogen receptor and is not accompanied by release or by thromboxane synthesis. Blood. 1985;66:213–219. [PubMed] [Google Scholar]
- 47.Marx G., Krugliak J., Shaklai M. Nutritional zinc increases platelet reactivity. Am. J. Hematol. 1991;38:161–165. doi: 10.1002/ajh.2830380302. [DOI] [PubMed] [Google Scholar]
- 48.Taylor K.A., Pugh N. The contribution of zinc to platelet behaviour during haemostasis and thrombosis. Metallomics Integr. Biomet. Sci. 2016;8:144–155. doi: 10.1039/C5MT00251F. [DOI] [PubMed] [Google Scholar]
- 49.Watson B.R., White N.A., Taylor K.A., Howes J.M., Malcor J.D., Bihan D., Sage S.O., Farndale R.W., Pugh N. Zinc is a transmembrane agonist that induces platelet activation in a tyrosine phosphorylation-dependent manner. Metallomics Integr. Biomet. Sci. 2016;8:91–100. doi: 10.1039/C5MT00064E. [DOI] [PubMed] [Google Scholar]
- 50.Morrell C.N., Aggrey A.A., Chapman L.M., Modjeski K.L. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123:2759–2767. doi: 10.1182/blood-2013-11-462432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blair P., Flaumenhaft R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009;23:177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bao B., Prasad A.S., Beck F.W., Fitzgerald J.T., Snell D., Bao G.W., Singh T., Cardozo L.J. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: A potential implication of zinc as an atheroprotective agent. Am. J. Clin. Nutr. 2010;91:1634–1641. doi: 10.3945/ajcn.2009.28836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prasad A.S. Zinc: An antioxidant and anti-inflammatory agent: Role of zinc in degenerative disorders of aging. J. Trace Elem. Med. Biol. 2014;28:364–371. doi: 10.1016/j.jtemb.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 54.Shen H., Oesterling E., Stromberg A., Toborek M., MacDonald R., Hennig B. Zinc deficiency induces vascular pro-inflammatory parameters associated with NF-kappaB and PPAR signaling. J. Am. Coll. Nutr. 2008;27:577–587. doi: 10.1080/07315724.2008.10719741. [DOI] [PubMed] [Google Scholar]
- 55.Vruwink K.G., Fletcher M.P., Keen C.L., Golub M.S., Hendrickx A.G., Gershwin M.E. Moderate zinc deficiency in rhesus monkeys. An intrinsic defect of neutrophil chemotaxis corrected by zinc repletion. J. Immunol. 1991;146:244–249. [PubMed] [Google Scholar]
- 56.Zazzo J.F., Rouveix B., Rajagopalon P., Levacher M., Girard P.M. Effect of zinc on the immune status of zinc-depleted AIDS related complex patients. Clin. Nutr. 1989;8:259–261. doi: 10.1016/0261-5614(89)90036-8. [DOI] [PubMed] [Google Scholar]
- 57.DeCoursey T.E., Morgan D., Cherny V.V. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–534. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]
- 58.Hasegawa H., Suzuki K., Suzuki K., Nakaji S., Sugawara K. Effects of zinc on the reactive oxygen species generating capacity of human neutrophils and on the serum opsonic activity in vitro. Lumin. J. Biol. Chem. Lumin. 2000;15:321–327. doi: 10.1002/1522-7243(200009/10)15:5321::AID-BIO6053.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 59.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 60.Hasan R., Rink L., Haase H. Zinc signals in neutrophil granulocytes are required for the formation of neutrophil extracellular traps. Innate Immun. 2013;19:253–264. doi: 10.1177/1753425912458815. [DOI] [PubMed] [Google Scholar]
- 61.Li A., Lu G., Qi J., Wu L., Tian K., Luo T., Shi Y., Yan J., Gao G.F. Structural basis of nectin-1 recognition by pseudorabies virus glycoprotein D. PLoS Pathog. 2017;13:e1006314. doi: 10.1371/journal.ppat.1006314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sohnle P.G., Collins-Lech C., Wiessner J.H. The zinc-reversible antimicrobial activity of neutrophil lysates and abscess fluid supernatants. J. Infect. Dis. 1991;164:137–142. doi: 10.1093/infdis/164.1.137. [DOI] [PubMed] [Google Scholar]
- 63.Lappann M., Danhof S., Guenther F., Olivares-Florez S., Mordhorst I.L., Vogel U. In vitro resistance mechanisms of Neisseria meningitidis against neutrophil extracellular traps. Mol. Microbiol. 2013;89:433–449. doi: 10.1111/mmi.12288. [DOI] [PubMed] [Google Scholar]
- 64.Stork M., Grijpstra J., Bos M.P., Manas Torres C., Devos N., Poolman J.T., Chazin W.J., Tommassen J. Zinc piracy as a mechanism of Neisseria meningitidis for evasion of nutritional immunity. PLoS Pathog. 2013;9:e1003733. doi: 10.1371/journal.ppat.1003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee S., Eskin S.G., Shah A.K., Schildmeyer L.A., McIntire L.V. Effect of zinc and nitric oxide on monocyte adhesion to endothelial cells under shear stress. Ann. Biomed. Eng. 2012;40:697–706. doi: 10.1007/s10439-011-0434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suzuki Y., Tada-Oikawa S., Ichihara G., Yabata M., Izuoka K., Suzuki M., Sakai K., Ichihara S. Zinc oxide nanoparticles induce migration and adhesion of monocytes to endothelial cells and accelerate foam cell formation. Toxicol. Appl. Pharmacol. 2014;278:16–25. doi: 10.1016/j.taap.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 67.Dierichs L., Kloubert V., Rink L. Cellular zinc homeostasis modulates polarization of THP-1-derived macrophages. Eur. J. Nutr. 2017 doi: 10.1007/s00394-017-1491-2. [DOI] [PubMed] [Google Scholar]
- 68.Subramanian Vignesh K., Landero Figueroa J.A., Porollo A., Caruso J.A., Deepe G.S., Jr. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity. 2013;39:697–710. doi: 10.1016/j.immuni.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Botella H., Stadthagen G., Lugo-Villarino G., de Chastellier C., Neyrolles O. Metallobiology of host-pathogen interactions: An intoxicating new insight. Trends Microbiol. 2012;20:106–112. doi: 10.1016/j.tim.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 70.Botella H., Peyron P., Levillain F., Poincloux R., Poquet Y., Brandli I., Wang C., Tailleux L., Tilleul S., Charriere G.M., et al. Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe. 2011;10:248–259. doi: 10.1016/j.chom.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tabruyn S.P., Griffioen A.W. A new role for NF-kappaB in angiogenesis inhibition. Cell Death Differ. 2007;14:1393–1397. doi: 10.1038/sj.cdd.4402156. [DOI] [PubMed] [Google Scholar]
- 72.Haase H., Ober-Blobaum J.L., Engelhardt G., Hebel S., Heit A., Heine H., Rink L. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J. Immunol. 2008;181:6491–6502. doi: 10.4049/jimmunol.181.9.6491. [DOI] [PubMed] [Google Scholar]
- 73.Reiterer G., Toborek M., Hennig B. Peroxisome proliferator activated receptors alpha and gamma require zinc for their anti-inflammatory properties in porcine vascular endothelial cells. J. Nutr. 2004;134:1711–1715. doi: 10.1093/jn/134.7.1711. [DOI] [PubMed] [Google Scholar]
- 74.Prasad A.S., Bao B., Beck F.W., Kucuk O., Sarkar F.H. Antioxidant effect of zinc in humans. Free Radic. Biol. Med. 2004;37:1182–1190. doi: 10.1016/j.freeradbiomed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 75.Liu M.J., Bao S., Galvez-Peralta M., Pyle C.J., Rudawsky A.C., Pavlovicz R.E., Killilea D.W., Li C., Nebert D.W., Wewers M.D., et al. ZIP8 regulates host defense through zinc-mediated inhibition of NF-kappaB. Cell Rep. 2013;3:386–400. doi: 10.1016/j.celrep.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Von Bulow V., Dubben S., Engelhardt G., Hebel S., Plumakers B., Heine H., Rink L., Haase H. Zinc-dependent suppression of TNF-alpha production is mediated by protein kinase A-induced inhibition of Raf-1, I kappa B kinase beta, and NF-kappa B. J. Immunol. 2007;179:4180–4186. doi: 10.4049/jimmunol.179.6.4180. [DOI] [PubMed] [Google Scholar]
- 77.Aydemir T.B., Chang S.M., Guthrie G.J., Maki A.B., Ryu M.S., Karabiyik A., Cousins R.J. Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (endotoxemia) PLoS ONE. 2012;7:e48679. doi: 10.1371/journal.pone.0048679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cummings J.E., Kovacic J.P. The ubiquitous role of zinc in health and disease. J. Vet. Emerg. Crit. Care. 2009;19:215–240. doi: 10.1111/j.1476-4431.2009.00418.x. [DOI] [PubMed] [Google Scholar]
- 79.Stennicke H.R., Salvesen G.S. Biochemical characteristics of caspases-3, -6, -7, and -8. J. Biol. Chem. 1997;272:25719–25723. doi: 10.1074/jbc.272.41.25719. [DOI] [PubMed] [Google Scholar]
- 80.Huber K.L., Hardy J.A. Mechanism of zinc-mediated inhibition of caspase-9. Protein Sci. Publ. Protein Soc. 2012;21:1056–1065. doi: 10.1002/pro.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Velazquez-Delgado E.M., Hardy J.A. Zinc-mediated allosteric inhibition of caspase-6. J. Biol. Chem. 2012;287:36000–36011. doi: 10.1074/jbc.M112.397752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muroi M., Tanamoto K. Zinc- and oxidative property-dependent degradation of pro-caspase-1 and NLRP3 by ziram in mouse macrophages. Toxicol. Lett. 2015;235:199–205. doi: 10.1016/j.toxlet.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 83.Costarelli L., Muti E., Malavolta M., Cipriano C., Giacconi R., Tesei S., Piacenza F., Pierpaoli S., Gasparini N., Faloia E., et al. Distinctive modulation of inflammatory and metabolic parameters in relation to zinc nutritional status in adult overweight/obese subjects. J. Nutr. Biochem. 2010;21:432–437. doi: 10.1016/j.jnutbio.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 84.Nishio N., Ito S., Suzuki H., Isobe K. Antibodies to wounded tissue enhance cutaneous wound healing. Immunology. 2009;128:369–380. doi: 10.1111/j.1365-2567.2009.03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iwata Y., Yoshizaki A., Komura K., Shimizu K., Ogawa F., Hara T., Muroi E., Bae S., Takenaka M., Yukami T., et al. CD19, a response regulator of B lymphocytes, regulates wound healing through hyaluronan-induced TLR4 signaling. Am. J. Pathol. 2009;175:649–660. doi: 10.2353/ajpath.2009.080355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fraker P.J., King L.E. Reprogramming of the immune system during zinc deficiency. Ann. Rev. Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- 87.Sirbulescu R.F., Boehm C.K., Soon E., Wilks M.Q., Ilies I., Yuan H., Maxner B., Chronos N., Kaittanis C., Normandin M.D., et al. Mature B cells accelerate wound healing after acute and chronic diabetic skin lesions. Wound Repair Regen. 2017 doi: 10.1111/wrr.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.King L.E., Frentzel J.W., Mann J.J., Fraker P.J. Chronic zinc deficiency in mice disrupted T cell lymphopoiesis and erythropoiesis while B cell lymphopoiesis and myelopoiesis were maintained. J. Am. Coll. Nutr. 2005;24:494–502. doi: 10.1080/07315724.2005.10719495. [DOI] [PubMed] [Google Scholar]
- 89.Rosenkranz E., Metz C.H., Maywald M., Hilgers R.D., Wessels I., Senff T., Haase H., Jager M., Ott M., Aspinall R., et al. Zinc supplementation induces regulatory T cells by inhibition of Sirt-1 deacetylase in mixed lymphocyte cultures. Mol. Nutr. Food Res. 2016;60:661–671. doi: 10.1002/mnfr.201500524. [DOI] [PubMed] [Google Scholar]
- 90.Rosenkranz E., Hilgers R.D., Uciechowski P., Petersen A., Plumakers B., Rink L. Zinc enhances the number of regulatory T cells in allergen-stimulated cells from atopic subjects. Eur. J. Nutr. 2017;56:557–567. doi: 10.1007/s00394-015-1100-1. [DOI] [PubMed] [Google Scholar]
- 91.Maywald M., Meurer S.K., Weiskirchen R., Rink L. Zinc supplementation augments TGF-beta1-dependent regulatory T cell induction. Mol. Nutr. Food Res. 2017;61:3. doi: 10.1002/mnfr.201600493. [DOI] [PubMed] [Google Scholar]
- 92.Nosbaum A., Prevel N., Truong H.A., Mehta P., Ettinger M., Scharschmidt T.C., Ali N.H., Pauli M.L., Abbas A.K., Rosenblum M.D. Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. J. Immunol. 2016;196:2010–2014. doi: 10.4049/jimmunol.1502139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arwert E.N., Hoste E., Watt F.M. Epithelial stem cells, wound healing and cancer. Nat. Rev. Cancer. 2012;12:170–180. doi: 10.1038/nrc3217. [DOI] [PubMed] [Google Scholar]
- 94.Agren M.S., Chvapil M., Franzen L. Enhancement of re-epithelialization with topical zinc oxide in porcine partial-thickness wounds. J. Surg. Res. 1991;50:101–105. doi: 10.1016/0022-4804(91)90230-J. [DOI] [PubMed] [Google Scholar]
- 95.Tandon N., Cimetta E., Villasante A., Kupferstein N., Southall M.D., Fassih A., Xie J., Sun Y., Vunjak-Novakovic G. Galvanic microparticles increase migration of human dermal fibroblasts in a wound-healing model via reactive oxygen species pathway. Exp. Cell Res. 2014;320:79–91. doi: 10.1016/j.yexcr.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tenaud I., Leroy S., Chebassier N., Dreno B. Zinc, copper and manganese enhanced keratinocyte migration through a functional modulation of keratinocyte integrins. Exp. Dermatol. 2000;9:407–416. doi: 10.1034/j.1600-0625.2000.009006407.x. [DOI] [PubMed] [Google Scholar]
- 97.Li H., Chang J. Bioactive silicate materials stimulate angiogenesis in fibroblast and endothelial cell co-culture system through paracrine effect. Acta Biomater. 2013;9:6981–6991. doi: 10.1016/j.actbio.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 98.Saghiri M.A., Asatourian A., Orangi J., Sorenson C.M., Sheibani N. Functional role of inorganic trace elements in angiogenesis-Part II: Cr, Si, Zn, Cu, and S. Crit. Rev. Oncol. Hematol. 2015;96:143–155. doi: 10.1016/j.critrevonc.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 99.Tracy L.E., Minasian R.A., Caterson E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care. 2016;5:119–136. doi: 10.1089/wound.2014.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McCarty S.M., Cochrane C.A., Clegg P.D., Percival S.L. The role of endogenous and exogenous enzymes in chronic wounds: A focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair Regen. 2012;20:125–136. doi: 10.1111/j.1524-475X.2012.00763.x. [DOI] [PubMed] [Google Scholar]
- 101.Martins V.L., Caley M., O’Toole E.A. Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res. 2013;351:255–268. doi: 10.1007/s00441-012-1410-z. [DOI] [PubMed] [Google Scholar]
- 102.Caley M.P., Martins V.L., O’Toole E.A. Metalloproteinases and Wound Healing. Adv. Wound Care. 2015;4:225–234. doi: 10.1089/wound.2014.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xue M., Le N.T., Jackson C.J. Targeting matrix metalloproteases to improve cutaneous wound healing. Expert Opin. Ther. Targets. 2006;10:143–155. doi: 10.1517/14728222.10.1.143. [DOI] [PubMed] [Google Scholar]
- 104.Van Wart H.E., Birkedal-Hansen H. The cysteine switch: A principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. USA. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jansen P.L., Rosch R., Jansen M., Binnebosel M., Junge K., Alfonso-Jaume A., Klinge U., Lovett D.H., Mertens P.R. Regulation of MMP-2 gene transcription in dermal wounds. J. Investig. Dermatol. 2007;127:1762–1767. doi: 10.1038/sj.jid.5700765. [DOI] [PubMed] [Google Scholar]
- 106.Venditti E., Bruge F., Astolfi P., Kochevar I., Damiani E. Nitroxides and a nitroxide-based UV filter have the potential to photoprotect UVA-irradiated human skin fibroblasts against oxidative damage. J. Dermatol. Sci. 2011;63:55–61. doi: 10.1016/j.jdermsci.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 107.Zhou L., Yan C., Gieling R.G., Kida Y., Garner W., Li W., Han Y.P. Tumor necrosis factor-alpha induced expression of matrix metalloproteinase-9 through p21-activated kinase-1. BMC Immunol. 2009;10:15. doi: 10.1186/1471-2172-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim C.H., Lee J.H., Won J.H., Cho M.K. Mesenchymal stem cells improve wound healing in vivo via early activation of matrix metalloproteinase-9 and vascular endothelial growth factor. J. Korean Med. Sci. 2011;26:726–733. doi: 10.3346/jkms.2011.26.6.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gill S.E., Parks W.C. Metalloproteinases and their inhibitors: Regulators of wound healing. Int. J. Biochem. Cell Biol. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen Q., Jin M., Yang F., Zhu J., Xiao Q., Zhang L. Matrix metalloproteinases: Inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediat. Inflamm. 2013;2013:928315. doi: 10.1155/2013/928315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kyriakides T.R., Wulsin D., Skokos E.A., Fleckman P., Pirrone A., Shipley J.M., Senior R.M., Bornstein P. Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis. Matrix Biol. J. Int. Soc. Matrix Biol. 2009;28:65–73. doi: 10.1016/j.matbio.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zigrino P., Ayachi O., Schild A., Kaltenberg J., Zamek J., Nischt R., Koch M., Mauch C. Loss of epidermal MMP-14 expression interferes with angiogenesis but not with re-epithelialization. Eur. J. Cell Biol. 2012;91:748–756. doi: 10.1016/j.ejcb.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 113.Gawronska-Kozak B. Scarless skin wound healing in FOXN1 deficient (nude) mice is associated with distinctive matrix metalloproteinase expression. Matrix Biol. J. Int. Soc. Matrix Biol. 2011;30:290–300. doi: 10.1016/j.matbio.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Binnebosel M., Junge K., Schwab R., Antony A., Schumpelick V., Klinge U. Delayed wound healing in sacrococcygeal pilonidal sinus coincides with an altered collagen composition. World J. Surg. 2009;33:130–136. doi: 10.1007/s00268-008-9748-9. [DOI] [PubMed] [Google Scholar]
- 115.Tezvergil-Mutluay A., Agee K.A., Hoshika T., Carrilho M., Breschi L., Tjaderhane L., Nishitani Y., Carvalho R.M., Looney S., Tay F.R., et al. The requirement of zinc and calcium ions for functional MMP activity in demineralized dentin matrices. Dent. Mater. 2010;26:1059–1067. doi: 10.1016/j.dental.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sun Q., Zhong W., Zhang W., Zhou Z. Defect of mitochondrial respiratory chain is a mechanism of ROS overproduction in a rat model of alcoholic liver disease: Role of zinc deficiency. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;310:G205–G214. doi: 10.1152/ajpgi.00270.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Slepchenko K.G., Lu Q., Li Y.V. Cross talk between increased intracellular zinc (Zn2+) and accumulation of reactive oxygen species in chemical ischemia. Am. J. Physiol. Cell Physiol. 2017;313:C448–C459. doi: 10.1152/ajpcell.00048.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sen C.K. The general case for redox control of wound repair. Wound Repair Regen. 2003;11:431–438. doi: 10.1046/j.1524-475X.2003.11607.x. [DOI] [PubMed] [Google Scholar]
- 119.Sen C.K., Khanna S., Gordillo G., Bagchi D., Bagchi M., Roy S. Oxygen, oxidants, and antioxidants in wound healing: An emerging paradigm. Ann. N. Y. Acad. Sci. 2002;957:239–249. doi: 10.1111/j.1749-6632.2002.tb02920.x. [DOI] [PubMed] [Google Scholar]
- 120.Kivisaari J., Vihersaari T., Renvall S., Niinikoski J. Energy metabolism of experimental wounds at various oxygen environments. Ann. Surg. 1975;181:823–828. doi: 10.1097/00000658-197506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Drose S., Brandt U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv. Exp. Med. Biol. 2012;748:145–169. doi: 10.1007/978-1-4614-3573-0_6. [DOI] [PubMed] [Google Scholar]
- 122.Henderson J.R., Swalwell H., Boulton S., Manning P., McNeil C.J., Birch-Machin M.A. Direct, real-time monitoring of superoxide generation in isolated mitochondria. Free Radic. Res. 2009;43:796–802. doi: 10.1080/10715760903062895. [DOI] [PubMed] [Google Scholar]
- 123.Kloubert V., Rink L. Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct. 2015;6:3195–3204. doi: 10.1039/C5FO00630A. [DOI] [PubMed] [Google Scholar]
- 124.Valko M., Jomova K., Rhodes C.J., Kuca K., Musilek K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016;90:1–37. doi: 10.1007/s00204-015-1579-5. [DOI] [PubMed] [Google Scholar]
- 125.Ruttkay-Nedecky B., Nejdl L., Gumulec J., Zitka O., Masarik M., Eckschlager T., Stiborova M., Adam V., Kizek R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013;14:6044–6066. doi: 10.3390/ijms14036044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hanada K., Sawamura D., Hashimoto I., Kida K., Naganuma A. Epidermal proliferation of the skin in metallothionein-null mice. J. Investig. Dermatol. 1998;110:259–262. doi: 10.1046/j.1523-1747.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- 127.Penkowa M., Giralt M., Thomsen P.S., Carrasco J., Hidalgo J. Zinc or copper deficiency-induced impaired inflammatory response to brain trauma may be caused by the concomitant metallothionein changes. J. Neurotrauma. 2001;18:447–463. doi: 10.1089/089771501750171056. [DOI] [PubMed] [Google Scholar]
- 128.Hatakeyama S. TRIM Family Proteins: Roles in Autophagy, Immunity, and Carcinogenesis. Trends Biochem. Sci. 2017;42:297–311. doi: 10.1016/j.tibs.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 129.Ozato K., Shin D.M., Chang T.H., Morse H.C., 3rd TRIM family proteins and their emerging roles in innate immunity. Nat. Rev. Immunol. 2008;8:849–860. doi: 10.1038/nri2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rajsbaum R., Garcia-Sastre A., Versteeg G.A. TRIMmunity: The roles of the TRIM E3-ubiquitin ligase family in innate antiviral immunity. J. Mol. Biol. 2014;426:1265–1284. doi: 10.1016/j.jmb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Versteeg G.A., Benke S., Garcia-Sastre A., Rajsbaum R. InTRIMsic immunity: Positive and negative regulation of immune signaling by tripartite motif proteins. Cytokine Growth Factor Rev. 2014;25:563–576. doi: 10.1016/j.cytogfr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cooper S.T., McNeil P.L. Membrane Repair: Mechanisms and Pathophysiology. Physiol. Rev. 2015;95:1205–1240. doi: 10.1152/physrev.00037.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Demonbreun A.R., McNally E.M. Plasma Membrane Repair in Health and Disease. Curr. Top. Membr. 2016;77:67–96. doi: 10.1016/bs.ctm.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tan T., Ko Y.G., Ma J. Dual function of MG53 in membrane repair and insulin signaling. BMB Rep. 2016;49:414–423. doi: 10.5483/BMBRep.2016.49.8.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McNeil P.L., Kirchhausen T. An emergency response team for membrane repair. Nat. Rev. Mol. Cell Biol. 2005;6:499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- 136.He B., Tang R.H., Weisleder N., Xiao B., Yuan Z., Cai C., Zhu H., Lin P., Qiao C., Li J., et al. Enhancing muscle membrane repair by gene delivery of MG53 ameliorates muscular dystrophy and heart failure in delta-Sarcoglycan-deficient hamsters. Mol. Ther. 2012;20:727–735. doi: 10.1038/mt.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Howard A.C., McNeil A.K., Xiong F., Xiong W.C., McNeil P.L. A novel cellular defect in diabetes: Membrane repair failure. Diabetes. 2011;60:3034–3043. doi: 10.2337/db11-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cai C., Masumiya H., Weisleder N., Matsuda N., Nishi M., Hwang M., Ko J.K., Lin P., Thornton A., Zhao X., et al. MG53 nucleates assembly of cell membrane repair machinery. Nat. Cell Biol. 2009;11:56–64. doi: 10.1038/ncb1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cai C., Weisleder N., Ko J.K., Komazaki S., Sunada Y., Nishi M., Takeshima H., Ma J. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J. Biol. Chem. 2009;284:15894–15902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Demonbreun A.R., Quattrocelli M., Barefield D.Y., Allen M.V., Swanson K.E., McNally E.M. An actin-dependent annexin complex mediates plasma membrane repair in muscle. J. Cell Biol. 2016;213:705–718. doi: 10.1083/jcb.201512022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cao C.M., Zhang Y., Weisleder N., Ferrante C., Wang X., Lv F., Song R., Hwang M., Jin L., Guo J., et al. MG53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation. 2010;121:2565–2574. doi: 10.1161/CIRCULATIONAHA.110.954628. [DOI] [PubMed] [Google Scholar]
- 142.Duann P., Li H., Lin P., Tan T., Wang Z., Chen K., Zhou X., Gumpper K., Zhu H., Ludwig T., et al. MG53-mediated cell membrane repair protects against acute kidney injury. Sci. Transl. Med. 2015;7:279ra236. doi: 10.1126/scitranslmed.3010755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jia Y., Chen K., Lin P., Lieber G., Nishi M., Yan R., Wang Z., Yao Y., Li Y., Whitson B.A., et al. Treatment of acute lung injury by targeting MG53-mediated cell membrane repair. Nat. Commun. 2014;5:4387. doi: 10.1038/ncomms5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Weisleder N., Takizawa N., Lin P., Wang X., Cao C., Zhang Y., Tan T., Ferrante C., Zhu H., Chen P.J., et al. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci. Transl. Med. 2012;4:139ra185. doi: 10.1126/scitranslmed.3003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang Y., Lv F., Jin L., Peng W., Song R., Ma J., Cao C.M., Xiao R.P. MG53 participates in ischaemic postconditioning through the RISK signalling pathway. Cardiovasc. Res. 2011;91:108–115. doi: 10.1093/cvr/cvr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu J., Zhu H., Zheng Y., Xu Z., Li L., Tan T., Park K.H., Hou J., Zhang C., Li D., et al. Cardioprotection of recombinant human MG53 protein in a porcine model of ischemia and reperfusion injury. J. Mol. Cell. Cardiol. 2015;80:10–19. doi: 10.1016/j.yjmcc.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yao Y., Zhang B., Zhu H., Li H., Han Y., Chen K., Wang Z., Zeng J., Liu Y., Wang X., et al. MG53 permeates through blood-brain barrier to protect ischemic brain injury. Oncotarget. 2016;7:22474–22485. doi: 10.18632/oncotarget.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang C., Wang H., Wu D., Hu J., Wu W., Zhang Y., Peng X. A novel perspective for burn-induced myopathy: Membrane repair defect. Sci. Rep. 2016;6:31409. doi: 10.1038/srep31409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gushchina L.V., Bhattacharya S., McElhanon K.E., Choi J.H., Manring H., Beck E.X., Alloush J., Weisleder N. Treatment with Recombinant Human MG53 Protein Increases Membrane Integrity in a Mouse Model of Limb Girdle Muscular Dystrophy 2B. Mol. Ther. J. Am. Soc. Gene Ther. 2017;25:2360–2371. doi: 10.1016/j.ymthe.2017.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang C., Chen B., Wang Y., Guo A., Tang Y., Khataei T., Shi Y., Kutschke W.J., Zimmerman K., Weiss R.M., et al. MG53 is dispensable for T-tubule maturation but critical for maintaining T-tubule integrity following cardiac stress. J. Mol. Cell. Cardiol. 2017;112:123–130. doi: 10.1016/j.yjmcc.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nagre N., Wang S., Kellett T., Kanagasabai R., Deng J., Nishi M., Shilo K., Oeckler R.A., Yalowich J.C., Takeshima H., et al. TRIM72 modulates caveolar endocytosis in repair of lung cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016;310:L452–L464. doi: 10.1152/ajplung.00089.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lipkowitz S., Weissman A.M. RINGs of good and evil: RING finger ubiquitin ligases at the crossroads of tumour suppression and oncogenesis. Nat. Rev. Cancer. 2011;11:629–643. doi: 10.1038/nrc3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cai C., Lin P., Zhu H., Ko J.K., Hwang M., Tan T., Pan Z., Korichneva I., Ma J. Zinc Binding to MG53 Protein Facilitates Repair of Injury to Cell Membranes. J. Biol. Chem. 2015;290:13830–13839. doi: 10.1074/jbc.M114.620690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pakyari M., Farrokhi A., Maharlooei M.K., Ghahary A. Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing. Adv. Wound Care. 2013;2:215–224. doi: 10.1089/wound.2012.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hameedaldeen A., Liu J., Batres A., Graves G.S., Graves D.T. FOXO1, TGF-beta regulation and wound healing. Int. J. Mol. Sci. 2014;15:16257–16269. doi: 10.3390/ijms150916257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Morikawa M., Derynck R., Miyazono K. TGF-beta and the TGF-beta Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016;8:a021873. doi: 10.1101/cshperspect.a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Boo S., Dagnino L. Integrins as Modulators of Transforming Growth Factor Beta Signaling in Dermal Fibroblasts During Skin Regeneration After Injury. Adv. Wound Care. 2013;2:238–246. doi: 10.1089/wound.2012.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ma H., Liu J., Bian Z., Cui Y., Zhou X., Zhang B., Adesanya T.M., Yi F., Park K.H., Tan T., et al. Effect of metabolic syndrome on mitsugumin 53 expression and function. PLoS ONE. 2015;10:e0124128. doi: 10.1371/journal.pone.0124128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Beer H.D., Munding C., Dubois N., Mamie C., Hohl D., Werner S. The estrogen-responsive B box protein: A novel regulator of keratinocyte differentiation. J. Biol. Chem. 2002;277:20740–20749. doi: 10.1074/jbc.M111233200. [DOI] [PubMed] [Google Scholar]
- 160.Tan H., Qi J., Chu G., Liu Z. Tripartite Motif 16 Inhibits the Migration and Invasion in Ovarian Cancer Cells. Oncol. Res. 2017;25:551–558. doi: 10.3727/096504016X14758370595285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang Y., Li J., Huang Y., Dai X., Liu Y., Liu Z., Wang Y., Wang N., Zhang P. Tripartite motif-containing 28 bridges endothelial inflammation and angiogenic activity by retaining expression of TNFR-1 and -2 and VEGFR2 in endothelial cells. FASEB J. 2017;31:2026–2036. doi: 10.1096/fj.201600988RR. [DOI] [PubMed] [Google Scholar]
- 162.Wilkinson E.A. Oral zinc for arterial and venous leg ulcers. Cochrane Database Syst. Rev. 2012;8:CD001273. doi: 10.1002/14651858.CD001273.pub2. [DOI] [PubMed] [Google Scholar]
- 163.O’Connor S., Murphy S. Chronic venous leg ulcers: Is topical zinc the answer? A review of the literature. Adv. Skin Wound Care. 2014;27:35–44. doi: 10.1097/01.ASW.0000439173.79541.96. [DOI] [PubMed] [Google Scholar]
- 164.Cereda E., Gini A., Pedrolli C., Vanotti A. Disease-specific, versus standard, nutritional support for the treatment of pressure ulcers in institutionalized older adults: A randomized controlled trial. J. Am. Geriatr. Soc. 2009;57:1395–1402. doi: 10.1111/j.1532-5415.2009.02351.x. [DOI] [PubMed] [Google Scholar]
- 165.Attia E.A., Belal D.M., El Samahy M.H., El Hamamsy M.H. A pilot trial using topical regular crystalline insulin vs. aqueous zinc solution for uncomplicated cutaneous wound healing: Impact on quality of life. Wound Repair Regen. 2014;22:52–57. doi: 10.1111/wrr.12122. [DOI] [PubMed] [Google Scholar]
- 166.Sakae K., Agata T., Kamide R., Yanagisawa H. Effects of L-carnosine and its zinc complex (Polaprezinc) on pressure ulcer healing. Nutr. Clin. Pract. 2013;28:609–616. doi: 10.1177/0884533613493333. [DOI] [PubMed] [Google Scholar]
- 167.Lansdown A.B. Zinc in the healing wound. Lancet. 1996;347:706–707. doi: 10.1016/S0140-6736(96)90072-0. [DOI] [PubMed] [Google Scholar]
- 168.Agren M.S. Zinc oxide increases degradation of collagen in necrotic wound tissue. Br. J. Dermatol. 1993;129:221. doi: 10.1111/j.1365-2133.1993.tb03533.x. [DOI] [PubMed] [Google Scholar]
- 169.Pinnell S.R. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J. Am. Acad. Dermatol. 2003;48:1–19. doi: 10.1067/mjd.2003.16. [DOI] [PubMed] [Google Scholar]
- 170.Berger M.M., Baines M., Raffoul W., Benathan M., Chiolero R.L., Reeves C., Revelly J.P., Cayeux M.C., Senechaud I., Shenkin A. Trace element supplementation after major burns modulates antioxidant status and clinical course by way of increased tissue trace element concentrations. Am. J. Clin. Nutr. 2007;85:1293–1300. doi: 10.1093/ajcn/85.5.1293. [DOI] [PubMed] [Google Scholar]
- 171.Xiong H.M. ZnO nanoparticles applied to bioimaging and drug delivery. Adv. Mater. 2013;25:5329–5335. doi: 10.1002/adma.201301732. [DOI] [PubMed] [Google Scholar]
- 172.Oyarzun-Ampuero F., Vidal A., Concha M., Morales J., Orellana S., Moreno-Villoslada I. Nanoparticles for the Treatment of Wounds. Curr. Pharm. Des. 2015;21:4329–4341. doi: 10.2174/1381612821666150901104601. [DOI] [PubMed] [Google Scholar]
- 173.Pati R., Das I., Mehta R.K., Sahu R., Sonawane A. Zinc-Oxide Nanoparticles Exhibit Genotoxic, Clastogenic, Cytotoxic and Actin Depolymerization Effects by Inducing Oxidative Stress Responses in Macrophages and Adult Mice. Toxicol. Sci. 2016;150:454–472. doi: 10.1093/toxsci/kfw010. [DOI] [PubMed] [Google Scholar]