Abstract
An emerging body of evidence supports the concept that the pituitary is a site for integration of multiple physiological and metabolic signals that inform and modulate endocrine pathways. Multiple endocrine mediators of energy balance and adiposity are known to impinge on the neuroendocrine axis regulating reproduction. Observations in humans show that obesity is correlated with decreased gonadotropin secretion, and studies have also suggested that pituitary sensitivity to stimulation by gonadotropin-releasing hormone (GnRH) is decreased in obese individuals. Free fatty acids are a potential mediator of adiposity and energy balance, but their impact as an endocrine modulator of pituitary function has not been closely examined. We evaluated the impact of free fatty acids on a pituitary gonadotrope cell line and in primary pituitary cultures of female mice. We show that increasing physiologically relevant doses of the monounsaturated ω-9 fatty acid oleate induces cellular stress and increases production of reactive oxygen species in a mouse gonadotrope cell line. In contrast, the unsaturated ω-3 α-linolenic and ω-6 linoleic fatty acids do not have this effect. Additionally, oleate can activate immediate-early gene expression independent of GnRH stimulation but has a negative impact on GnRH induction and expression of the gonadotropin subunit gene Lhb. Further, oleate suppresses gonadotropin secretion in response to pulsatile stimulation by GnRH. These results indicate that free fatty acids can directly alter gonadotropin gene expression and secretion in response to GnRH and may provide a link between energy sensing and reproduction.
Oleate induces oxidative stress and inhibits gene expression and secretion of the LH and FSH from female mouse pituitary, demonstrating a link between metabolic stress and reproductive control.
As mediators of energy balance and long-term energy stores, factors derived from or influenced by adipose are ideal candidates for reporters of energy balance. Adipose-associated changes in gonadotropin levels imply the presence of a sensing mechanism that reports energy status to the reproductive endocrine axis (1–9). Disorders of reproduction such as polycystic ovary syndrome (PCOS) and hypogonadotropic hypogonadism are associated with disorders of metabolism and obesity. However, not all metabolic signals associated with obesity explain the inverse relationship between adiposity and gonadotropin levels observed in men and women (1, 5, 10, 11).
In the pituitary, gonadotropin synthesis and release is a highly regulated process that involves coordinated gene expression and protein synthesis in conjunction with acute regulation of secretion (12). The gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are related heterodimeric glycoprotein hormones that share a common α-subunit encoded by Cga and are differentiated by their unique β-subunits encoded by Lhb and Fshb, respectively. LH secretion by gonadotropes is particularly sensitive to gonadotropin-releasing hormone (GnRH) stimulation and is regulated by pulse amplitude and/or frequency changes that are characteristic of the preovulatory surge (13). FSH secretion is less dependent on GnRH pulse regimen, although basal secretion is dependent on an active pulsatile stimulation of gonadotropes. Transcriptional regulation of the gonadotropin β-subunit genes is largely controlled by immediate-early transcription factors such as EGR1 for Lhb and AP1 for Fshb, although other factors such as NAB, SKIL, TGIF1, ICER, and β-catenin also play noteworthy roles (14–20). Activation of immediate-early genes by GnRH receptor signaling is dependent on mitogen-activated protein kinase (MAPK) signaling, particularly through MAPK1/3 [also extracellular signal-regulated kinase (ERK) 2/1], which also modulates the translational machinery to increase protein synthesis (12, 21, 22). Activation of MAPK1/3 by the GnRH receptor depends on reactive oxygen species (ROS) signaling intermediates produced by the reduced form of nicotinamide adenine dinucleotide phosphate oxidase/dual oxidase (NOX/DUOX) family of enzymes (23). Activation of MAPKs and, consequently, of immediate early and gonadotropin genes involves rapid activation and subsequent resolution of signaling cascades to maintain the capacity for interpretation of GnRH pulse patterns (24–30).
Free fatty acids (FFA) signal through both membrane receptors and metabolic intermediates, complicating interpretation of their action (31). Communication of energy balance between tissues and selection of glucose or FFA as fuel is controlled through processes initially outlined by Randle et al. (32) that rely on the antagonistic action of glucose and insulin toward FFA oxidation. FFA levels are elevated in obese women with PCOS, and these women exhibit the greatest adiposity-related suppression of LH (3, 4, 6). As a direct messenger of energy balance and a fuel source, lipids likely contribute to energy sensing by the hypothalamus (33). Increased adiposity contributes to circulating FFA and fatty acid (FA) flux, and this presents a number of mechanisms by which FFA can mediate the decline in gonadotropins noted in obese individuals. Direct mobilization of FFA into β-oxidative pathways inhibits glucose utilization and leads to generation of ROS, which, in excess, induces the unfolded protein response (UPR) (34). A consequence of UPR activation is reduced protein synthesis and transport through the endoplasmic reticulum (ER). Thus, secretory cells are particularly sensitive to UPR activation, and the inability to resolve UPR activation can lead to cell death.
FFA are also ligands for broadly expressed G-protein–coupled receptors (31, 35–37). GPR40 promotes secretion in pancreatic β-cells and induces calcium release from ER stores (38–40). This induces cell stress and contributes to UPR activation and translational inhibition. In contrast, the ω-3 FA receptor GPR120 is a key modulator of the macrophage inflammatory response and acts as an insulin sensitizer in obese mice (31). Thus, receptor-mediated FFA actions can have divergent affects, and individual cell responses are dependent on the receptor complement they express.
Biophysical effects of FFA are also relevant to cellular function. At high concentrations, FAs can disrupt membrane fluidity and induce apoptotic signaling (41). The saturated FFA palmitate can induce insulin resistance in cells through incorporation into ceramide, an active inhibitor of phosphatidylinositol-3 kinase (42). Further, ω-6 FA can also be shunted to inflammatory eicosanoid production and cause elevation of cellular inflammatory responses. FFA have a direct impact on gonadotropins in ruminant animals (43), and in rats, linoleic acid (LA) differentially stimulates and represses Lhb and Fshb expression, respectively, and inhibits SMAD signaling in the LβT2 gonadotrope cell line (44, 45). In cultured porcine pituitary, FFA inhibits GnRH-induced LH and growth hormone secretion (46). The FA receptors GPR40 and GPR120 have been identified in mouse LβT2 gonadotrope cells, which could influence cell signaling and gene expression through their activation by FFA (44). Finally, the Toll-like receptor (TLR) 2 and TLR4 can also be activated by FFA and present another signaling arm through which FFA can influence gonadotropin synthesis or secretion (47, 48). These signaling mechanisms can alter protein production or cellular signaling responses and suggest that one component of the body mass index (BMI)–associated decline in gonadotropin secretion may be related to the pituitary exposure to FFA.
Previous work has shown that activation of GnRH receptor signaling can lead to transient activation of the UPR (49). FFA are also known activators of the UPR, and it is possible that chronic UPR activation can lead to suppressed protein synthesis or possibly autophagy through unresolved activation of the ER-associated degradation pathway. The cellular response to ER stress is mediated through the action of a complex and broad transcriptional regulatory pathway largely controlled by the x-box binding protein XBP1 (50). A unique feature of Xbp1 gene expression is cytosolic splicing of a 26-bp intron by the ER-resident factor ERN1 during activation of the UPR. We have shown that translation of Lhb messenger RNA (mRNA) is inhibited by the UPR, and this correlates with induction of Xbp1 splicing (49). Therefore, Xbp1 splicing can serve as a proxy measurement of UPR activation, translational inhibition, and cellular stress.
To establish the possibility that FFA can suppress gonadotropins, we examined the impact of various FFA across physiological levels of exposure to identify a role in activation of the UPR, MAPK signaling, gonadotropin gene expression, or gonadotropin secretion. We report that, although FFA can independently activate immediate-early gene expression, secretion of LH is suppressed by exposure to physiological levels of the ω-9 monounsaturated FA oleate (OLA). This implicates the contribution of FFA to the BMI-associated decline in gonadotropin secretion.
Materials and Methods
LβT2 gonadotrope cell culture
The male mouse-derived LβT2 gonadotrope cell line (51) was maintained in high-glucose HEPES-buffered Dulbecco’s modified Eagle medium (DMEM) supplemented with penicillin/streptomycin and 10% fetal bovine serum (FBS). The cells were maintained at 37°C in a humidified atmosphere of 5% CO2. To test the potential impact of FFA on stress signaling in the pituitary gonadotrope, the LβT2 cells were pretreated with serum-free DMEM for 12 to 16 hours before FFA or hormone treatments.
Hormone and drug treatments
The LβT2 cells were plated at a density of 2 ×105 cells/cm2 in a six-well dish in high-glucose HEPES-buffered DMEM supplemented with penicillin/streptomycin and 10% FBS overnight for 24 to 30 hours before the medium was changed to serum-free DMEM medium and then incubated for an additional 12 to 16 hours. Cells were then pretreated with or without N-acetyl-L-cysteine (NAC; Sigma-Aldrich) for 30 minutes and then treated with indicated FAs at final concentrations as described in each figure (Sigma-Aldrich) for 3 hours with or without 10 nM GnRH in the final 30 minutes of treatment. Afterward, media was aspirated, the cells were washed with ice-cold phosphate-buffered saline, and then harvested for total RNA isolation or protein purification as described below.
RNA isolation and reverse transcription polymerase chain reaction
After hormone treatments, total RNA from LβT2 cells was isolated using TRIzol or TRIzol LS (Thermo Fisher Scientific). Complementary DNA was synthesized using qScript cDNA SuperMix per the manufacturer’s instructions (Quanta BioSciences). Reverse transcription polymerase chain reaction (RT-PCR) was performed using Xbp1 primers and AccuPower PCR PreMix kit containing Top DNA polymerase (Bioneer). PCR products were subjected to agarose gel electrophoresis in the presence of ethidium bromide. Primer sequences were designed against murine mRNA sequences as available through PubMed. The sequences of the primers used were as follows: Xbp1 forward, 5′-TCTGAAGAGCTTAGAGGTGC-3′ and Xbp1 reverse, 5′-TACGGGAGAAAACTCACG-3′.
Quantitative real-time PCR
For quantitative real-time PCR analysis of mRNA from LβT2 cells, quantitative PCR (qPCR) was carried out using the MyIQ Single-Color Real-Time Detection System (Bio-Rad). Quantitative real-time PCR was performed with KAPA SYBR Green PCR kit (Kapa Biosystems) supplemented with 200 nM transcript-specific primers. The cycling conditions used were those recommended by the reagent manufacturer. A melt curve was performed after each PCR run to ensure that a single product was amplified, and the sizes of the products were verified using agarose gel electrophoresis. At least three independent determinations of mRNA content were made, and the relative transcript levels were determined using the 2−ΔΔCT method with Gapdh as an endogenous control. Primer sequences were designed against murine mRNA sequences as available through PubMed. The primer sequences were as follows: Egr1 forward, 5′-ATTTTTCCTGAGCCCCAAAGC-3′ and Egr1 reverse, 5′-ATGGGAACCTGGAAACCACC-3′; c-fos forward, 5′-GGCAAAGTAGAGCAGCTATCTCCT-3′ and c-fos reverse, 5′-TCAGCTCCCTCCTCCGATTC-3′; Lhb forward, 5′-GCAACTCTGGCCGCAGAGAATG-3′ and Lhb reverse, 5′-CAGCTGAGGGCTACAGGAAAGG-3′; and Gapdh forward, 5′-TGCACCACCACCTGCTTAG-3′ and Gapdh reverse, 5′-GGATGCAGGGATGATGTTC-3′.
All experimental reactions were performed in triplicate. Duplicate reactions were performed for standard curve and reaction efficiency determination. The mass values for each transcript were extrapolated from the standard curve. The Pfaffl method (52) was used to calculate relative mass levels of qPCR products with respect to Gapdh as an internal control.
Transient transfection
For transient transfection assays, LβT2 cells were plated at a density of 2.75 × 105 cells/cm2 in HEPES-buffered DMEM supplemented with 10% FBS and incubated for 24 hours. Cells were then changed into serum-free HEPES-buffered DMEM and transfected with the previously described 1.8-kb rat Lhβ promoter–driven reporter plasmid pGL3-1.8 rLHβ-luc and the internal control pGL3-CMV-βGal reporter plasmids (21) using Fugene 6 (Promega) according to the manufacturer’s instructions. After incubation for 12 to 16 hours, cells were treated with vehicle or 500 µM OLA for 4 hours and either vehicle or 10 nM GnRH during the final 30 minutes of incubation. The cells were harvested with phosphate-buffered saline/0.1% Triton X-100, and lysates were assayed using the Luciferase Assay Kit (Promega) for luciferase and Galacto-Light Plus Kit (Thermo Fisher Scientific) for β-galactosidase activity, respectively. Luminescence was measured in a Veritas microplate luminometer (Turner BioSystems).
Western blotting
After hormone treatments, the cells were harvested and lysed in radioimmunoprecipitation assay/Nonidet P-40 buffer supplemented with protease and phosphatase inhibitors (Roche). For confirmation of the phosphorylation status of ERK, all samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis on 10% gels and transferred onto polyvinyl difluoride membranes (Millipore). Membranes were blocked in 5% bovine serum albumin (BSA; Vector Laboratories) for 1 hour at room temperature and incubated with anti–phospho-ERK (E-4) mouse monoclonal immunoglobulin G (IgG) 2a antibody [SC-7383; Santa Cruz Biotechnology; Research Resource Identifier (RRID): AB_627545] in 1:1000 dilution in 1% BSA/Tris-buffered saline with Tween 20 overnight at 4°C and then in a 1:2000 dilution of biotinylated secondary antibody (SC-2005; Santa Cruz Biotechnology; RRID: AB_631736) at room temperature for 2 hours. Blots were visualized by chemiluminescence using a 1:5000 dilution of horseradish peroxidase–conjugated avidin-biotin complex (Vector Laboratories) and imaged using a FluorChem Q (ProteinSimple) or PXi (Syngene) system. After imaging, blots were stripped in Restore Western Blot Stripping Buffer (Thermo Fisher Scientific) for 15 minutes and reblotted with anti-ERK1 (K-23) rabbit polyclonal antibody (SC-94; Santa Cruz Biotechnology; RRID: AB_2140110) in 1:1000 dilution in 1% BSA/Tris-buffered saline with Tween 20 at room temperature for 1 hour and then in 1:2000 dilution in biotinylated secondary antibody (SC-2004; Santa Cruz Biotechnology; RRID: AB_631746) at room temperature for 2 hours to control for loading. Quantification of MAPK1/3 phosphorylation was performed relative to total MAPK1/3 in the same lane for consistency. Quantitative densitometry was performed by using AlphaView Q (ProteinSimple) or GeneTools (Syngene) software. Data from at least three independent experiments were normalized to total MAPK1/3 and reported as fold change relative to the control.
ROS measurement
LβT2 cells plated on glass-bottom dishes (MatTek) coated with poly-D-lysine at a density of 2 × 105 cells/cm2 were treated with 500 μM FFA for 3 hours and then with 5 μM CellROX Green oxidative stress reagent (Life Technologies) and 1 μg/mL (∼1.6 μM) Hoechst 33342 (Thermo Fisher Scientific) for 15 minutes. Wide-field fluorescence images were obtained using a TE2000-U microscope (Nikon Instruments) equipped with an X-Cite 120PC collimated light source (Lumen Dynamics Group) and DAPI-1160A and either a GFP-3035B or FITC-5050A filter set (Semrock) using a CoolSNAP EZ monochrome camera (Photometrics). Living cell images were captured and analyzed using Nikon NIS-Elements BR software (Nikon Instruments). Fluorescence intensity measurements and object counting were obtained every 5 minutes through six experimental repeats and normalized to control vehicle-treated cells.
Primary pituitary culture
Pituitaries were dissected from 8- to 10-week-old cohoused C57/Bl6 female mice. Pituitaries were pooled, dispersed, and the cellular equivalent of five pituitaries were cultured on 1-mL bed volume of Cytodex 3 microcarrier beads (GE Healthcare) per treatment group, as previously described (23, 49). Cell suspensions cultured in 10 mL 10% FBS DMEM were changed to serum-free DMEM with antibiotics for 16 hours, resulting in 1% serum. Cultures were then loaded into perifusion columns and equilibrated for 40 minutes in serum-free DMEM alone or supplemented with 500 μM OLA at a flow rate of 200 μL/min. Subsequently, cells were pulsed for 2 minutes to achieve a 10 nM GnRH peak pulse concentration with a 58-minute interpulse interval for 4 hours. Perifusion culture and pulse methodology were described previously (16). Five-minute fractions of ∼1 mL were collected. LH and FSH levels were measured by a MILLIPLEX MAP Kit (Mouse Pituitary Magnetic Bead Panel, catalog no. MPTMAG-49K; EMD Millipore) following the manufacturer’s instructions. The Institutional Animal Care and Use Committee for the University of California, San Diego approved all animal procedures performed in this study.
Statistics and data analysis
All experiments were repeated at least three times independently, and reported values are presented as the means ± standard error of the mean. Statistical analysis was conducted using JMP software (SAS Institute) on raw or normalized values or values optimally Box-Cox transformed to correct for heteroscedasticity. Data were evaluated by Student t test or multifactor analysis of variance (ANOVA) as indicated and testing with the indicated post hoc comparison tests. A value of P < 0.05 was considered significant for all tests.
Results
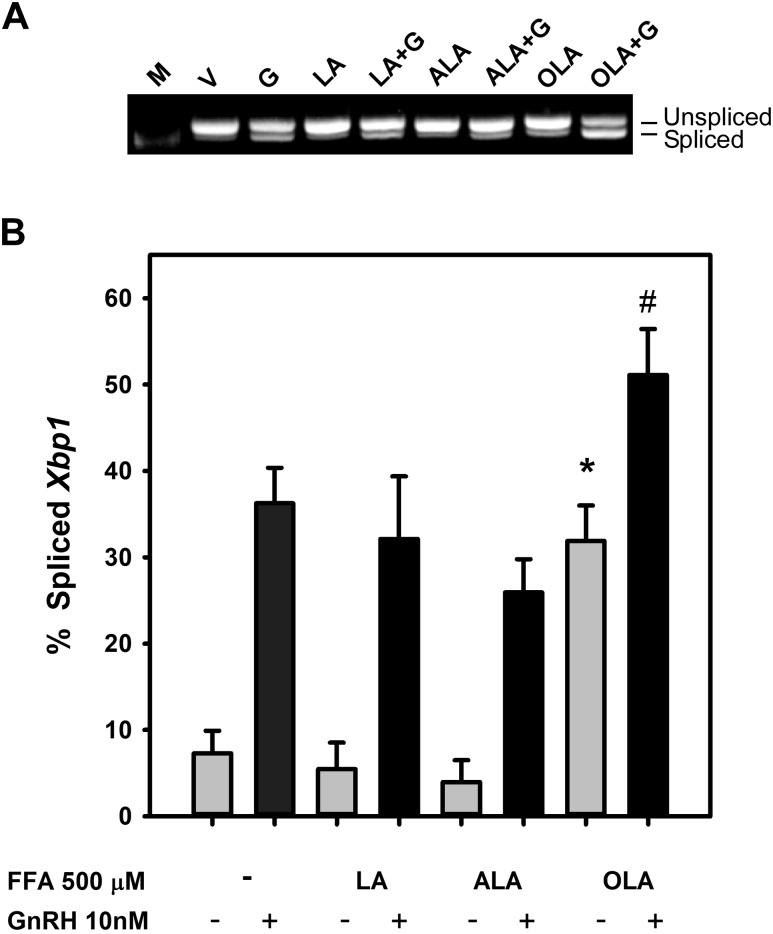
To examine the potential activation of the UPR and cellular stress by FFA, we monitored the splicing of Xbp1 mRNA in the gonadotrope cell line LβT2 in response to exposure to a variety of FFA. This included the ω-6 FA LA and the ω-3 FA α-linolenic acid (ALA) as well as OLA. FFA can impact cell signaling through a number of mechanisms to induce the UPR, including activation of the FA G-protein–coupled receptor GP120 or the TLR TLR4, which are both expressed in the LβT2 cell line and pituitary (48, 53), or through mitochondrial oxidative phosphorylation. We examined the extent of Xbp1 mRNA splicing after exposure of LβT2 gonadotrope cells to 500 μM ALA, LA, and OLA for 3 hours (Fig. 1) either alone or in combination with exposure to 10 nM GnRH in the final 30 minutes. As shown in Fig. 1A and quantified in the histogram in Fig. 1B, incubation in serum-free medium alone, with LA, or with ALA induced Xbp1 splicing nor did they impact GnRH-mediated activation of Xbp1 splicing. In comparison, 500 μM OLA did induce Xbp1 splicing ∼40-fold, and this was further exaggerated by exposure to 10 nM GnRH in the final 30 minutes of treatment.
Figure 1.
FFA induction of Xbp1 mRNA splicing in LβT2 gonadotropes. LβT2 cells were serum-starved for 12 to 16 hours prior to treatment with vehicle (V) or 500 µM LA, ALA, or OLA for 3 hours with or without 10 nM GnRH (G) for the final 30 minutes. Total RNA was isolated, and Xbp1 was analyzed by RT-PCR using primers that cross the 26-bp intron that is spliced in response to activation of the UPR by the ER-resident RNAse ERN1. Bands are visualized in comparison to 500-bp DNA ladder (M). (A) Agarose gel electrophoresis of RT-PCR products showing the presence of unspliced (top band) and spliced (bottom band) Xbp1 mRNA. (B) Summary of densitometric quantification of digitally acquired agarose gel images from three independent experiments showing Xbp1 splicing induced by OLA or GnRH, but not LA, or ALA. Bars represent the percentage of spliced form of Xbp1 relative to total detected. Error bars denote standard error of the mean of at least three independent experiments. *Significant difference from vehicle control; #significant difference from GnRH-treated control by ANOVA and Tukey post hoc multiple-comparison test. All GnRH-treated groups are significantly increased from their respective untreated control.
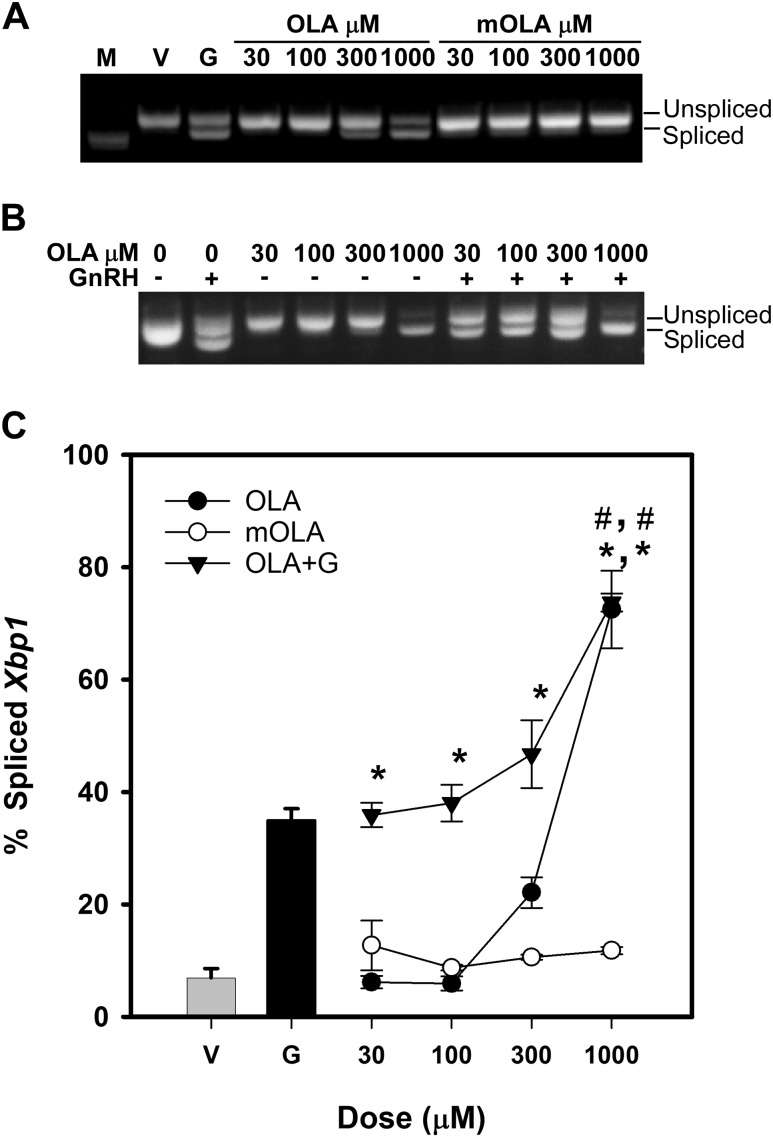
We have demonstrated that OLA induces mitochondrial ROS production in LβT2 gonadotropes (54). However, FFA can affect cell signaling through a number of mechanisms to induce the UPR, including activation of the FA G-protein–coupled receptor GP120 or the TLR TLR4, which are both expressed in the LβT2 cell line and pituitary, or through mitochondrial oxidative phosphorylation. This can be differentiated by testing the ability of the metabolic-resistant analog methyl oleate (mOLA). mOLA is capable of GP120 activation and can induce the UPR through downstream receptor signaling cascades but does not participate in β-oxidative metabolism. To examine the potential for induction of stress by either OLA or mOLA across physiological concentrations of FFA, we conducted dose-response tests of treatment-induced Xbp1 splicing across concentrations of 30 to 1000 μM. LβT2 cells incubated overnight in serum-free medium were treated with increasing doses of OLA for 3 hours with additional treatment with vehicle or 10 nM GnRH for the final 30 minutes. Afterward, mRNA was harvested, Xbp1 splicing was determined as shown in Fig. 2A, and relative splicing of Xbp1 in multiple experiments was quantified and summarized in the chart in Fig. 2C. Splicing was detected at physiological concentrations from 300 μM to 1000 μM with a half-maximal effect occurring at ∼580 μM. Overall activation of Xbp1 splicing induced by OLA was about twice that induced by 10 nM GnRH for 30 minutes, the maximal stimulation observed with GnRH induction of the UPR. These results indicate that OLA can induce cellular stress at high physiological concentrations as indicated by induction of the UPR and Xbp1 splicing. Treatment with mOLA did not induce Xbp1 splicing, suggesting that Xbp1 splicing could be induced through incomplete oxidative phosphorylation and metabolism of OLA rather than through activation of extracellular FA receptors.
Figure 2.
Dose-dependent activation of Xbp1 splicing by OLA. LβT2 cells were serum starved for 12 to 16 hours prior to treatment with vehicle (V) or increasing doses of OLA or mOLA for 3 hours or with OLA for 3 hours with 10 nM GnRH (G) during the final 30 minutes. Cells were harvested and analyzed for Xbp1 splicing as described in Fig. 1. Bands are visualized in comparison to 500-bp DNA ladder (M). (A) Representative agarose gel electrophoresis image of RT-PCR products showing OLA-induced and OLA plus GnRH-induced splicing of Xbp1 mRNA indicating activation of the UPR. (B) Representative agarose gel electrophoresis image of RT-PCR products showing OLA, but not mOLA, induced splicing of Xbp1 mRNA indicating activation of the UPR by OLA. (C) Summary of densitometric analysis of digitally acquired images from independent experiments showing a dose-dependent activation of Xbp1 splicing induced by OLA and OLA + GnRH. Error bars denote standard error of the mean of at least three independent experiments. *Significant difference from vehicle-treated control as determined by ANOVA and Dunnett post hoc comparison with control test; #significant difference from GnRH treatment alone as determined by Tukey post hoc multiple-comparison test.
The potential interaction of GnRH and oleate induction of Xbp1 splicing is of interest due to the long-term impact that chronic UPR activation may have on protein synthesis and gene expression. Unresolved Xbp1 activation can lead to autophagy (55), and it is possible that mild, chronic activation can lead to decreased protein synthesis or secretion of gonadotropins due to reduced endoplasmic reticulum function. Examination of the oleate dose response in the presence and absence of 10 nM GnRH was conducted to evaluate whether GnRH and OLA are independent or interactive stimulants of Xbp1 splicing and, by inference, of UPR activation. As above, LβT2 cells were treated with increasing doses of OLA for 3 hours with an additional treatment with vehicle or 10 nM GnRH for the final 30 minutes. Afterward, mRNA was harvested, Xbp1 splicing was determined as shown in Fig. 2B, and relative splicing of Xbp1 in multiple experiments was quantified and summarized in Fig. 2C. At the highest dose of 1000 μM, OLA induced 72.49 ± 6.88% Xbp1 splicing compared with GnRH alone at 34.96 ± 2.10%. At maximal doses of OLA, cotreatment with GnRH did not induce further splicing, indicating that splicing and, by inference, activation of the UPR is maximally stimulated. Overall levels were elevated compared with OLA and GnRH alone, and a significant interaction between OLA and GnRH was determined by two-factor ANOVA with interaction (P < 0.05).
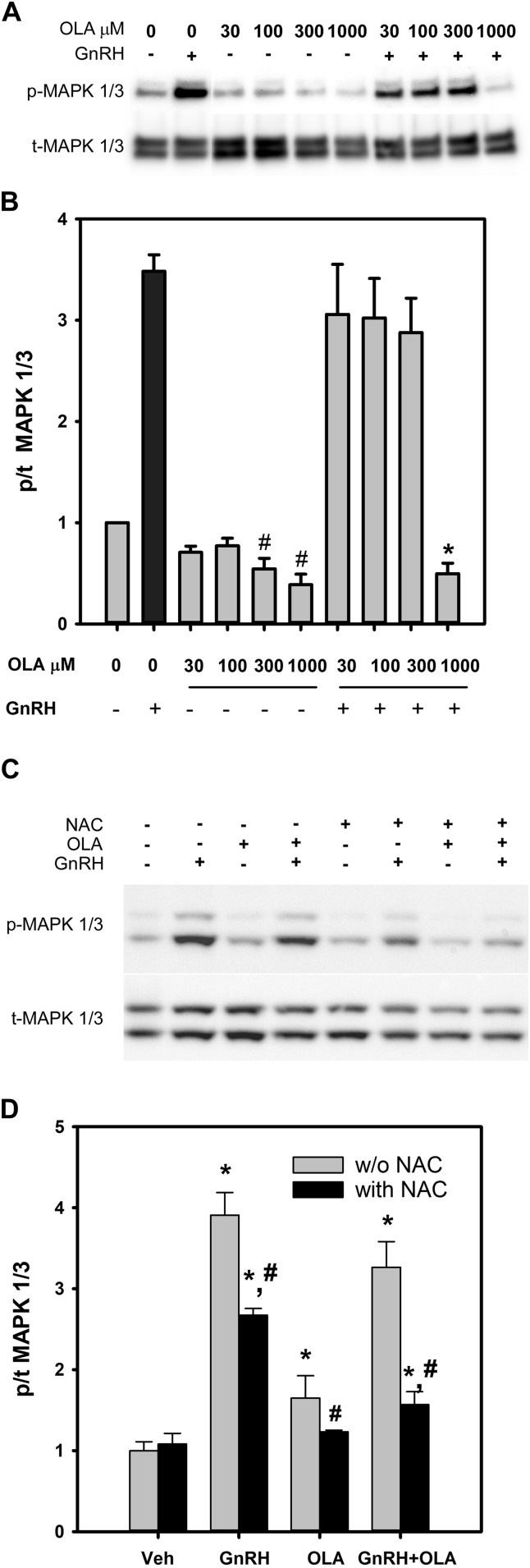
GnRH targets MAPK signaling cascades regulating transcription and translation factors that promote gonadotropin subunit gene expression and protein synthesis. In LβT2 cells, MAPK1/3 is robustly and transiently activated by GnRH (24). To examine the integrity of MAPK signaling in response to GnRH stimulation, LβT2 cells were incubated overnight in serum-free medium and treated with increasing doses of OLA alone or with the addition of 10 nM GnRH in the last 30 minutes of incubation. Cells were harvested, protein was subjected to western blotting and quantitative chemiluminescent imaging as shown in Fig. 3A, and multiple experiments are summarized in the histogram in Fig. 3B as the ratio of phosphorylated to total MAPK1/3 detected. OLA treatment led to a substantial suppression of baseline MAPK1/3 activity at the 300-μM and 1000-μM doses. Overall phosphorylation of MAPK1/3 was not significantly changed in the presence of OLA in comparison with GnRH treatment alone, except at the highest 1000-μM dose, in which it was completely abolished. Activation of MAPK1/3 by GnRH requires enzymatically derived ROS (23). In contrast, FFA metabolism results in mitochondrial ROS production (54). It is possible that MAPK1/3 may be activated by ROS from either source. We examined the potential for a common linkage to MAPK1/3 activation via ROS by examining the MAPK1/3 activation in response to GnRH and OLA exposure alone or in the presence of the ROS scavenger NAC. Preincubation of LβT2 cells with NAC limits both GnRH- and OLA-induced MAPK1/3 activation (Fig. 3C and 3D), showing that MAPK1/3 and downstream targets can be linked by ROS, and there is a potential for signaling interaction.
Figure 3.
Dose-dependent inhibition of basal and GnRH-induced MAPK1/3 activation by OLA. LβT2 cells were serum starved for 12 to 16 hours, treated with vehicle or OLA for 3 hours, and then stimulated with 10 nM GnRH for the final 15 minutes. Cells were harvested after incubation and protein extracts were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis and western blot for phosphorylated and total MAPK1/3. (A) Representative chemiluminescent image of phosphorylated (p-) and total (t-)MAPK1/3. (B) Summary of six independent experiments of OLA- and GnRH-induced MAPK1/3 phosphorylation reported as fold change from vehicle-treated controls. Bars represent the ratio of p-MAPK1/3 to t-MAPK1/3 normalized to vehicle control. *Significant difference from positive control GnRH treatment alone as determined by ANOVA and Dunnett post hoc test; #significant difference from normalized vehicle control as determined by Student t test. Results indicate that OLA reduces basal activation of MAPK1/3 and abolishes the stimulatory effect of GnRH on MAPK1/3 phosphorylation at the 1000-μM dose. To assess the dependence of activation on ROS, LβT2 cells were treated as in A with vehicle or 500 μM OLA for 3 hours with the addition of vehicle or 1 mM NAC 30 minutes prior to the addition of OLA. Cells were subsequently treated with vehicle or 10 nM GnRH in the final 15 minutes and evaluated after harvest for MAPK1/3 activation as above. (C) Representative chemiluminescent image of p- and t-MAPK1/3. (D) Summary of six independent experiments showing NAC-sensitive activation of MAPK1/3 by GnRH and OLA. Bars represent the ratio of p- to total MAPK1/3. Error bars denote standard error of the mean of at least three independent experiments. *Significant difference from vehicle control as determined by ANOVA and Dunnett post hoc test; #significant difference of NAC-treated group from the corresponding untreated group as determined by Student t test. w/o, without.
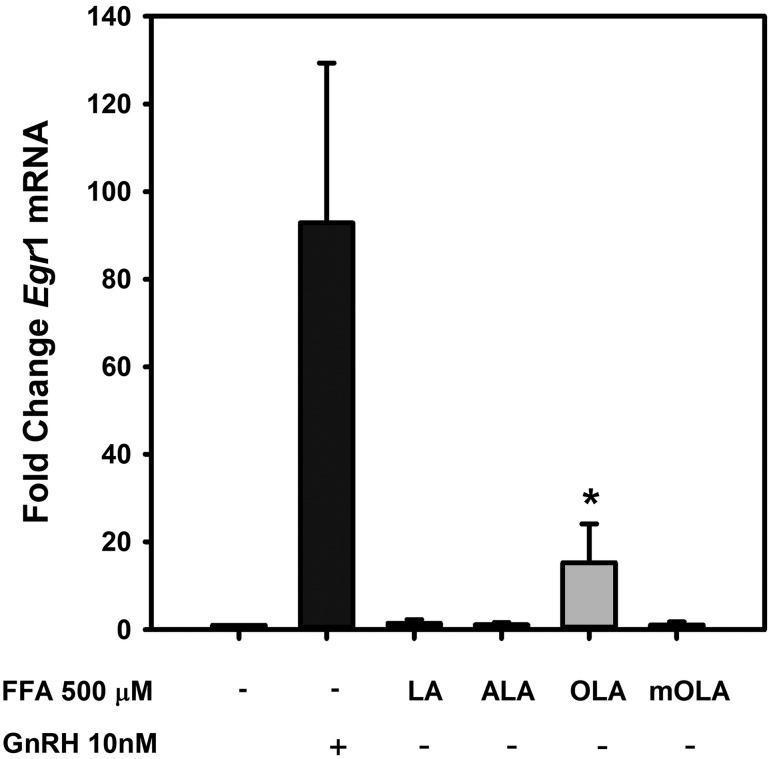
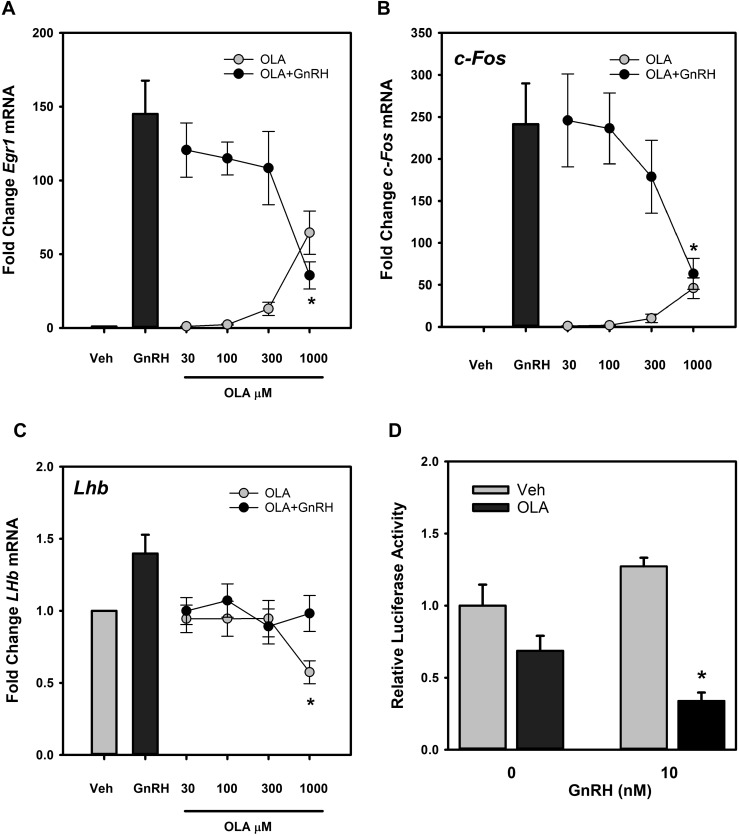
The rapid decline in activity at the highest OLA concentrations suggests that downstream targets of MAPK1/3 activation may also be limited in response to GnRH in the presence of elevated OLA exposure. The most prominent target of MAPK1/3 is the transcription of the immediate-early transcription factor Egr1, which is highly responsive to MAPK1/3 activation. EGR1 is a direct transcriptional regulator of the LH β-subunit gene Lhb. To determine any impact on Egr1 activation by OLA, we compared baseline and GnRH-induced Egr1 mRNA levels in the presence of 500 μM LA, ALA, OLA, and mOLA for 3 hours. Cells were harvested, RNA was subjected to real-time qPCR, and expression of Egr1 mRNA relative to untreated control cells was examined (Fig. 4). We observed that modest elevation of Egr1 mRNA occurs in response to OLA, consistent with modest activation of MAPK1/3 observed in Fig. 3. In the presence of GnRH, no notable differences are observed (Supplemental Fig. 1 (24.2MB, tif) ).
Figure 4.
FFA induction of Egr1 expression in LβT2 gonadotropes. LβT2 cells were serum-starved for 12 to 16 hours and then treated for 3 hours with vehicle or 500 µM LA, ALA, OLA, or mOLA with or without treatment with 10 nM GnRH for the final 30 minutes of incubation. At the end of incubation, cells were harvested, and total RNA was prepared from cell extracts. Egr1 mRNA expression was analyzed by quantitative real-time PCR using Gapdh as an internal reference control. Bars summarize the fold change of Egr1 mRNA after treatment relative to untreated vehicle control from three independent experiments. The corresponding GnRH treatment groups are provided in Supplemental Fig. 1 (24.2MB, tif) . The results show that both GnRH and OLA treatment increases Egr1 mRNA. Cotreatment with OLA and GnRH showed no additive or synergistic effects, and treatment with LA, ALA, and mOLA either alone or with GnRH treatment had no effect (Supplemental Fig. 1 (24.2MB, tif) ). Error bars denote standard error of the mean of at least three independent experiments. *Significant difference from untreated control as determined by ANOVA and post hoc testing with Dunnett comparison with control test.
The correspondence between MAPK1/3 activation and induction of the immediate-early gene Egr1 mRNA by OLA in the absence of GnRH stimulation suggests a connection between MAPK1/3 activation and Egr1 gene expression. To determine if OLA alone can induce immediate-early gene expression in LβT2 gonadotropes, cells were exposed to increasing OLA concentrations and changes in Egr1, as well as c-Fos, a component of the AP1 transcription factor that directly regulates transcription of the FSH β-subunit gene Fshb were measured. OLA-induced change in Lhb gene expression was also examined, and results are shown in Fig. 5A–5C. Both Egr1 (Fig. 5A) and c-Fos (Fig. 5B) were significantly induced in a dose-dependent manner by 300 μM and 1000 μM OLA, but GnRH induction of Egr1 and c-Fos were suppressed by OLA. GnRH induction of Lhb mRNA in vivo is mild at ∼1.4-fold (56). In this study, a similar induction by 10 nM GnRH is also observed, but substantial impact of OLA was observed only at 1000 μM (Fig. 5C). Although mRNA levels are only mildly affected, OLA significantly inhibits GnRH activation of reporter gene expression under the control of the rat 1.8-kbp Lhb promoter (Fig. 5D). Coincubation of transfected cells with 500 μM OLA with 10 nM GnRH limits activation of the reporter gene in comparison with cells treated with GnRH alone. These results suggest that the limitation of Egr1 activation may result in limited activation of Lhb transcription by GnRH.
Figure 5.
OLA induces a dose-dependent increase in Egr1 and c-Fos mRNA but has no effect on Lhb mRNA. LβT2 cells were serum starved for 12 to 16 hours and subsequently treated for 3 hours with vehicle (Veh) or increasing doses of OLA alone or with the addition of 10 nM GnRH for the final 30 minutes and harvested. Total RNA was prepared and levels of Egr1, c-Fos, and Lhb mRNA were determined relative to Gapdh as an internal reference control. Values from three independent experiments are summarized in the histograms as fold change relative to untreated vehicle control samples. The results show that GnRH and OLA increase (A) Egr1 and (B) c-Fos, but cotreatment results in a dose-dependent suppression of mRNA relative to GnRH treatment alone. (C) OLA suppresses Lhb mRNA levels at the 1000-μM dose and limits responsiveness to GnRH. *Significant difference from untreated control as determined by Student t test. (D) OLA suppresses Lhb promoter activity in LβT2 gonadotropes. Cells transfected with a 1.8-kbp rat Lhb promoter-driven luciferase reporter gene and a cytomegalovirus promoter–based β-galactosidase reporter gene as an internal control were treated with vehicle or 500 μM OLA for 30 minutes and then stimulated with the addition of 10 nM GnRH for 4 hours. Cells were harvested, and luciferase activity was measured and normalized to β-galactosidase activity. The chart represents the ratio of luciferase to β-galactosidase activity. Error bars denote standard error of the mean of at least three independent experiments. *Represents significant difference between OLA and corresponding vehicle-treated controls.
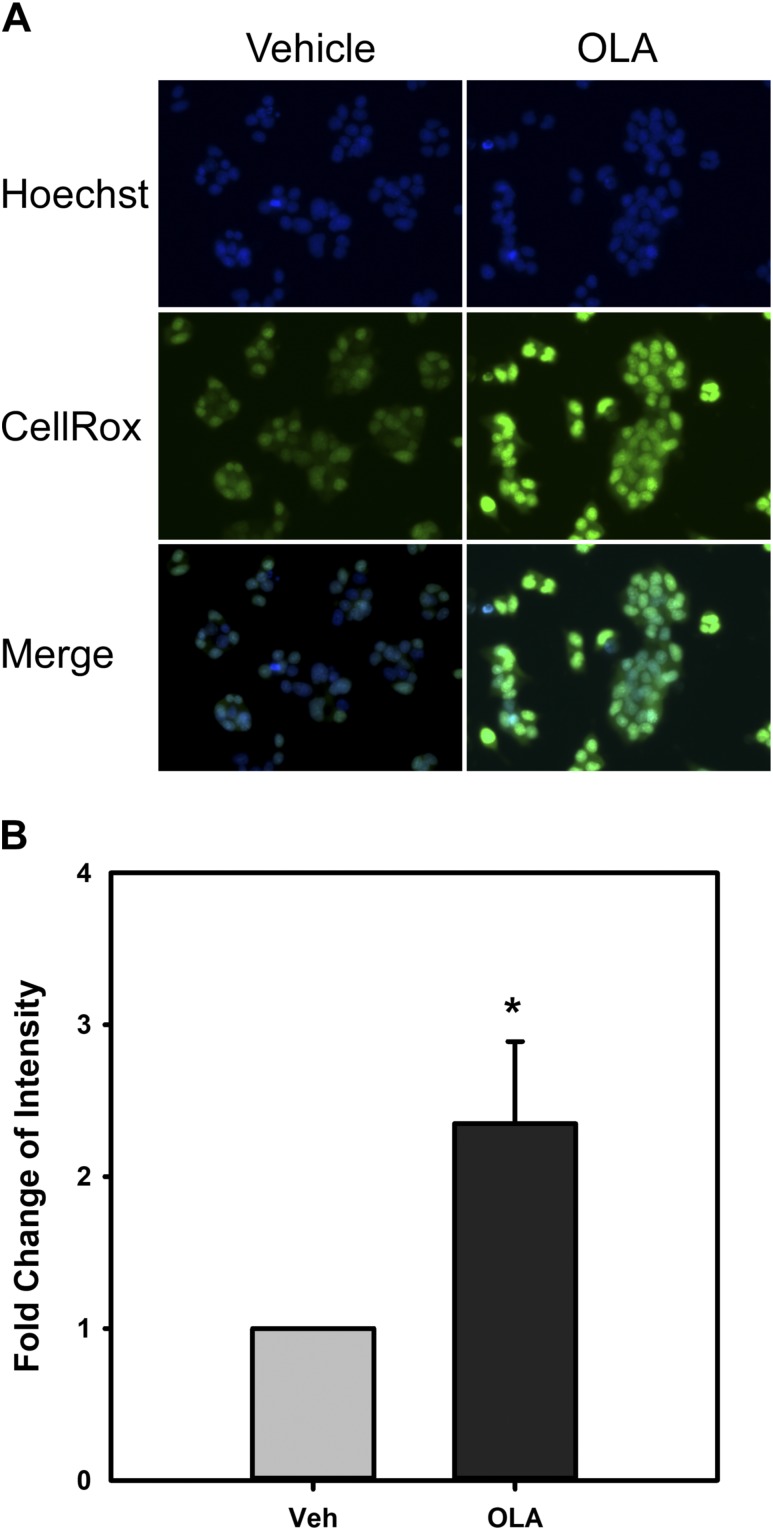
Unsaturated FAs can induce mitochondrial ROS production, and this is associated with altered cellular Ca2+ homeostasis, ultimately resulting in ER stress and activation of the UPR (57). Previous studies have shown that GnRH transiently induces the UPR and that GnRH signaling is dependent on ROS produced by the NOX/DUOX family of reduced form of nicotinamide adenine dinucleotide phosphate oxidases (23, 49). Our observations previously that OLA induces Xbp1 splicing suggest that this may occur in LβT2 gonadotrope cells. To confirm that OLA can induce ROS production in LβT2 cells, we monitored oxidative conversion of the fluorogenic dye CellROX in the presence of 300 μM OLA or vehicle. Cells incubated for 3 hours with OLA or vehicle were labeled with CellROX green and Hoechst 33342 and monitored over time for blue and green fluorescence using wide-field microscopy. Maximum green fluorescence in OLA was observed at 15 minutes after application, as shown in Fig. 6A. Densitometric analysis of fluorescence intensity was performed, and the summary of multiple trials is presented in Fig. 6B. Rapid fluorogenic conversion of CellROX in the presence of OLA in comparison with vehicle-treated cells is consistent with increased ROS production. The different sources of ROS from NOX/DUOX by GnRH (23) or mitochondrial stress may partly explain the disparity between GnRH- and OLA-induced activation of MAPK1/3 and immediate-early gene expression.
Figure 6.
OLA induces ROS formation in LβT2 cells. LβT2 cells plated on poly-D-lysine–coated glass-bottom dishes were serum starved for 12 to 16 hours and then treated with vehicle or 500 μM OLA for 3 hours prior to loading with 5 μM CellROX Green reagent and Hoechst 33342. Cells were incubated for a further 15 minutes. Live cell images were captured as described in Materials and Methods. (A) Hoechst (blue) and CellROX Green (green) images individually and merged for both vehicle- and OLA-treated cells. (B) OLA treatment results in increased ROS production as measured by the relative intensity ratio between green and blue and normalized to vehicle (Veh) control. Error bars denote standard error of the mean of at least three independent experiments. *Significant difference from control by Student t test.
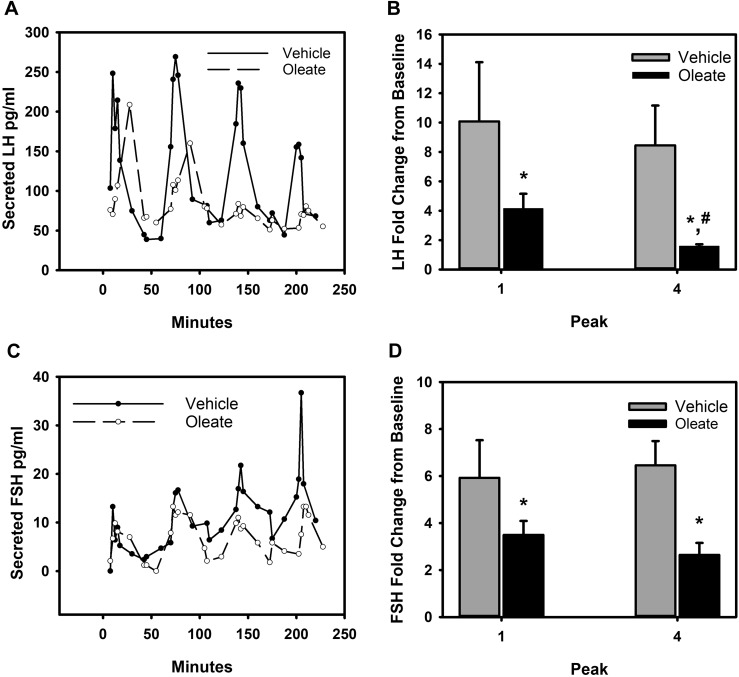
In humans, elevated BMI is associated with decreased gonadotropin output, and some studies have suggested that in obese men and women, the pituitary exhibits reduced sensitivity to GnRH challenge (5, 8, 44, 58). Many metabolic and endocrine factors can contribute to decreased sensitivity. Our in vitro studies previously indicate that although MAPK1/3 activation was reduced in the presence of mid to high physiological doses of OLA, immediate-early gene expression was activated. To determine the impact of elevated OLA exposure on gonadotropin secretion, we examined the secretory response of primary pituitary cultures of 8- to 10-week-old female mice to pulsatile GnRH stimulation alone or in the presence of OLA. Using cell perifusion culture (16), we exposed primary pituitary cultures to vehicle or 300 μM oleate while administering hourly 10-nM peak GnRH pulses. Perifusion culture media fractions were collected every 5 minutes over the course of the incubation and assayed for LH and FSH content. Resultant LH levels are charted in Fig. 7A, and a summary of fold change in peak LH relative to the baseline in each of four independent experiments is shown in the histogram in Fig. 7B. Over the course of a 4-hour perfusion, vehicle-treated cultures experienced a mild but nonsignificant decline in LH secretion. In comparison, perifusion cultures exposed to 300 μM OLA exhibited a reduced initial secretion of LH into the perifusate, and this continued to decline significantly throughout the time course, experiencing an ∼50% reduction in LH secretion by the fourth pulse. Interestingly, although LH secretion declined with OLA exposure, FSH secretion in those same cultures was maintained in the presence of OLA, although some decline in baseline level was evident (Fig. 7C and charted in Fig. 7D). However, the overall FSH excursion from baseline level was reduced significantly in the presence of OLA. It is of interest that, unlike peak LH secretion, which declined throughout the incubation, peak FSH secretion remained consistent. Taken together, these results suggest that some loss of GnRH sensitivity occurs, but that LH secretion is more dramatically affected and that chronic exposure to even moderate OLA levels reduces gonadotropin secretion.
Figure 7.
Suppression of GnRH-induced LH secretion by OLA. Primary pituitary cultures from 8- to 10-week-old female mice established on cytodex 3 microcarrier beads prepared as described in Materials and Methods were loaded into columns and perifused with serum-free DMEM. Columns were pretreated with vehicle or 300 mM OLA for 30 minutes prior to stimulation with hourly pulses of GnRH at peak values of ∼10 nM for 4 hours. Fractions collected from the perifusate were analyzed and charted for (A) LH and (C) FSH. Fold excursion from baseline values for each peak are summarized from four independent experiments and charted for LH in (B) and for FSH in (D). OLA treatment results in suppressed basal secretion levels of both LH and FSH. LH secretion is progressively suppressed by OLA, as evidenced by the loss of GnRH response by the fourth pulse. Baseline and excursion from baseline FSH secretion occurs in the presence of OLA but does not progressively decline in response to OLA, indicating a selective progressive suppression of LH secretion by OLA. Error bars denote standard error of the mean of at least three independent experiments. *Significant difference of peak 4 excursion from baseline in comparison with peak 1 values; #significant decrease in peak 1 values between vehicle- and OLA-treated groups as determined by Student t test.
Discussion
Observations of the age-independent relationship between body weight and menarche led to the development of the critical fat hypothesis, which has been further refined to state that an optimal level of body fat is required for initiation and maintenance of the ovulatory cycle. Movement below or above this level, ∼22%, alternately inhibits or permits normal cycling. Although this hypothesis generated considerable debate since its proposal, the elucidation of adipose and neuroendocrine mechanisms regulating the onset of puberty and modulating reproduction during adulthood coupled with improved measurement and understanding of various fat depots have led to the general acceptance of the hypothesis. Mechanisms thought to contribute to the regulation of the reproductive endocrine axis include leptin stimulation of the GnRH neurons of the hypothalamus and pituitary that would contribute to increased activity of the reproductive endocrine axis as adiposity and leptin levels increase or increased sex steroid synthesis in adipose or other alternative sites that prime the neuroendocrine reproductive axis. However, as adiposity increases beyond the optimum, suppression of the reproductive endocrine axis occurs, and fertility is reduced. Reduction of circulating gonadotropins is observed both in normal women and women with PCOS as adiposity increases (5, 9, 10), and suppression of gonadotropin secretion in response to GnRH challenge is observed in models of acute hyperinsulinemia or lipid infusion in both men and women (10, 59), suggesting that adipose-derived signals are not entirely necessary for the observed decline in neuroendocrine function. Our observations reported in this study suggest that the direct mobilization of FFAs into β-oxidative pathways in gonadotropes may contribute to the decline in GnRH sensitivity and reduced gonadotropin secretion through ROS production and activation of the UPR. Thus, the pituitary can serve as an independent sensor of metabolic status or energy balance and contribute to obesity-associated hypogonadism.
The regulation of reproduction is necessarily tied to energy sensing. The critical fat hypothesis is based on observations made under conditions of negative energy balance or low BMI. Some data suggest that FFA have a direct impact on gonadotropes in ruminant animals (43, 46). The role of FFA in energy sensing and suppression of the reproductive neuroendocrine axis is plausible under both conditions of negative and positive energy balance. Under negative balance, lipid stores are mobilized as fuel, and FFA may be an indicator of reduced caloric intake, conditions under which gestation is less desired. Under balanced conditions, lipid mobilization is reduced, and gonadotropes may be free to operate unrestrained. Under conditions of positive or extreme positive balance such as is present with high BMI or metabolic derangement, confounding signals of elevated FFA, hyperglycemia, and insulin resistance are present. The mutually antagonistic relationship between glucose and FFA for use as fuel and consequent inefficient β-oxidation of FFA may lead to elevated mitochondrial ROS production and activation of the UPR. Excess ROS can have the additional impact of gonadotrope desensitization due to the prominent role of transient ROS production in activation of the GnRH receptor signaling cascade via NOX/DUOX (23). Chronic activation or elevated negative feedback induced by oxidative stress may contribute to desensitization of GnRH signaling cascades, and reduced protein synthesis can be attributed to activation of the UPR. It is notable that, unlike Fshb mRNA, Lhb mRNA is acutely sensitive to translational inhibition by activation of the UPR (49, 60). Consistent with that observation, we report in this study that in primary pituitary, cultures in perifusion with OLA exhibit progressively reduced LH secretion in response to pulsatile GnRH stimulation, but FSH secretion is decreased but consistent. The mild increase of gonadotropin subunit transcription induced by OLA may not be sufficient to overcome the translational block imposed by UPR activation.
It is of interest that OLA did not similarly compromise FSH secretion in this perifusion model system. FSH secretion occurs through both regulated and unregulated pathways, whereas LH secretion occurs through a highly regulated pathway, and partitioning is tied to protein structural motifs (61, 62). An alternative explanation of the selective impact of OLA on LH secretion via reduced protein synthesis is that FFA or ROS alters components of the secretory pathway that are distinct between these mechanisms. It can be suggested that the role of FSH maintenance of follicular reserve, oocyte selection, and maturation is distinct from the role of LH in precipitating ovulation and is not dependent on energy balance to the extent of LH. However, there are key differences between rodents and humans in response to FFA. In mice, diet-induced obesity is associated with a decline in FSH in males and suppression of the proestrus LH and FSH surges in females, and there is an inflammatory response-like role of TLRs in mediating changes in gene expression (48). In humans, FSH does not appear to be subject to BMI-associated decline, although increased inflammatory markers are characteristic of elevated adiposity. Adipose-derived endocrine molecules such as leptin and adiponectin and inflammatory signals such as tumor necrosis factor-α and interleukin-6 may act individually or in concert to mediate the observed suppression of gonadotropin secretion, but to date, this has not been demonstrated, and their known impact on the reproductive neuroendocrine axis is not consistent with the observed inhibition. Our results presented in this study show that, independent of these factors, FFA alone can mediate the observed effect of progressively reduced LH secretion in response to repeated GnRH stimulation.
In summary, we demonstrate that the monounsaturated long-chain FA OLA can invoke the UPR in gonadotropes, increase ROS production, and suppress GnRH-induced secretion of gonadotropins. We hypothesize that this contributes to the decline in gonadotropins and decreased LH secretion in response to GnRH associated with increased adiposity. We also suggest that lipid sensing in gonadotropes independently monitors energy balance and can contribute to the overall control of fertility through modulation of reproductive hormone synthesis and secretion.
Appendix.
Antibodies Used
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., RRID | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| Phospho-MAPK1/3 | p-ERK (E-4) | Santa Cruz Biotechnology, sc-7383, RRID: AB_627545 | Mouse; monoclonal | 1:1000 | |
| Total MAPK1/3 | ERK1 | Santa Cruz Biotechnology, sc-94, RRID: AB_2140110 | Rabbit; polyclonal | 1:1000 | |
| Goat anti-mouse IgG-HRP | Goat anti-mouse IgG-HRP | Santa Cruz Biotechnology, sc-2005, RRID: AB_631736 | Goat; polyclonal | 1:2000 | |
| Goat anti-rabbit IgG-HRP | Goat anti-rabbit IgG-HRP | Santa Cruz Biotechnology, sc-2004, RRID: AB_631746 | Goat; polyclonal | 1:2000 |
Abbreviation: HRP, horseradish peroxidase.
Acknowledgments
We thank Tae-Shin Kim and Terri Stoner for helpful discussions and comments.
Financial Support: This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants R01 HD037568, R01 HD082567, and P50 HD012303 (to M.A.L.). E.F.M. and D.T.J. were supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant T32 HD007203. D.T.J. was supported by a fellowship from the National Academy of Sciences and the Ford Foundation. T.T. was supported by the Uehara Memorial Foundation Postdoctoral Fellowship.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ALA
- α-linolenic acid
- ANOVA
- analysis of variance
- BMI
- body mass index
- DMEM
- Dulbecco’s modified Eagle medium
- DUOX
- dual oxidase
- ER
- endoplasmic reticulum
- ERK
- extracellular signal-regulated kinase
- FA
- fatty acid
- FBS
- fetal bovine serum
- FFA
- free fatty acids
- FSH
- follicle-stimulating hormone
- GnRH
- gonadotropin-releasing hormone
- IgG
- immunoglobulin G
- LA
- linoleic acid
- LH
- luteinizing hormone
- MAPK
- mitogen-activated protein kinase
- mOLA
- methyl oleate
- mRNA
- messenger RNA
- NAC
- N-acetyl-l-cysteine
- NOX
- nicotinamide adenine dinucleotide phosphate oxidase
- OLA
- ω-9 monounsaturated fatty acid oleate
- PCOS
- polycystic ovary syndrome
- qPCR
- quantitative polymerase chain reaction
- ROS
- reactive oxygen species
- RRID
- Research Resource Identifier
- RT-PCR
- reverse transcription polymerase chain reaction
- TLR
- Toll-like receptor
- UPR
- unfolded protein response.
References
- 1.Arroyo A, Laughlin GA, Morales AJ, Yen SS. Inappropriate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity. J Clin Endocrinol Metab. 1997;82(11):3728–3733. [DOI] [PubMed] [Google Scholar]
- 2.Fu JF, Liang JF, Zhou XL, Prasad HC, Jin JH, Dong GP, Rose SR. Impact of BMI on gonadorelin-stimulated LH peak in premenarcheal girls with idiopathic central precocious puberty. Obesity (Silver Spring). 2015;23(3):637–643. [DOI] [PubMed] [Google Scholar]
- 3.Holte J, Bergh T, Berne C, Lithell H. Serum lipoprotein lipid profile in women with the polycystic ovary syndrome: relation to anthropometric, endocrine and metabolic variables. Clin Endocrinol (Oxf). 1994;41(4):463–471. [DOI] [PubMed] [Google Scholar]
- 4.Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14(2):95–109. [DOI] [PubMed] [Google Scholar]
- 5.Pagán YL, Srouji SS, Jimenez Y, Emerson A, Gill S, Hall JE. Inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome: investigation of hypothalamic and pituitary contributions. J Clin Endocrinol Metab. 2006;91(4):1309–1316. [DOI] [PubMed] [Google Scholar]
- 6.Pirwany IR, Fleming R, Greer IA, Packard CJ, Sattar N. Lipids and lipoprotein subfractions in women with PCOS: relationship to metabolic and endocrine parameters. Clin Endocrinol (Oxf). 2001;54(4):447–453. [DOI] [PubMed] [Google Scholar]
- 7.Sánchez-Garrido MA, Ruiz-Pino F, Manfredi-Lozano M, Leon S, Garcia-Galiano D, Castaño JP, Luque RM, Romero-Ruiz A, Castellano JM, Diéguez C, Pinilla L, Tena-Sempere M. Obesity-induced hypogonadism in the male: premature reproductive neuroendocrine senescence and contribution of Kiss1-mediated mechanisms. Endocrinology. 2014;155(3):1067–1079. [DOI] [PubMed] [Google Scholar]
- 8.Veldhuis JD, Liu PY, Keenan DM, Takahashi PY. Older men exhibit reduced efficacy of and heightened potency downregulation by intravenous pulses of recombinant human LH: a study in 92 healthy men. Am J Physiol Endocrinol Metab. 2012;302(1):E117–E122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain A, Polotsky AJ, Rochester D, Berga SL, Loucks T, Zeitlian G, Gibbs K, Polotsky HN, Feng S, Isaac B, Santoro N. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468–2473. [DOI] [PubMed] [Google Scholar]
- 10.Lawson MA, Jain S, Sun S, Patel K, Malcolm PJ, Chang RJ. Evidence for insulin suppression of baseline luteinizing hormone in women with polycystic ovarian syndrome and normal women. J Clin Endocrinol Metab. 2008;93(6):2089–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srouji SS, Pagán YL, D’Amato F, Dabela A, Jimenez Y, Supko JG, Hall JE. Pharmacokinetic factors contribute to the inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome. J Clin Endocrinol Metab. 2007;92(4):1347–1352. [DOI] [PubMed] [Google Scholar]
- 12.Kim T, Do MH, Lawson MA. Translational control of gene expression in the gonadotrope. Mol Cell Endocrinol. 2014;385(1-2):78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109(2):376–385. [DOI] [PubMed] [Google Scholar]
- 14.Ciccone NA, Xu S, Lacza CT, Carroll RS, Kaiser UB. Frequency-dependent regulation of follicle-stimulating hormone beta by pulsatile gonadotropin-releasing hormone is mediated by functional antagonism of bZIP transcription factors. Mol Cell Biol. 2010;30(4):1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coss D, Jacobs SB, Bender CE, Mellon PL. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone beta gene by gonadotropin-releasing hormone. J Biol Chem. 2004;279(1):152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawson MA, Tsutsumi R, Zhang H, Talukdar I, Butler BK, Santos SJ, Mellon PL, Webster NJ. Pulse sensitivity of the luteinizing hormone beta promoter is determined by a negative feedback loop Involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol. 2007;21(5):1175–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mistry DS, Tsutsumi R, Fernandez M, Sharma S, Cardenas SA, Lawson MA, Webster NJ. Gonadotropin-releasing hormone pulse sensitivity of follicle-stimulating hormone-beta gene is mediated by differential expression of positive regulatory activator protein 1 factors and corepressors SKIL and TGIF1. Mol Endocrinol. 2011;25(8):1387–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy GR, Xie C, Lindaman LL, Coss D. GnRH increases c-Fos half-life contributing to higher FSHβ induction. Mol Endocrinol. 2013;27(2):253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson IR, Ciccone NA, Zhou Q, Xu S, Khogeer A, Carroll RS, Kaiser UB. GnRH pulse frequency control of Fshb gene expression is mediated via ERK1/2 regulation of ICER. Mol Endocrinol. 2016;30(3):348–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Chikina M, Zaslavsky E, Pincas H, Sealfon SC. β-catenin regulates GnRH-induced FSHβ gene expression. Mol Endocrinol. 2013;27(2):224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen KA, Santos SJ, Kreidel MK, Diaz AL, Rey R, Lawson MA. Acute regulation of translation initiation by gonadotropin-releasing hormone in the gonadotrope cell line LbetaT2. Mol Endocrinol. 2004;18(5):1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sosnowski R, Mellon PL, Lawson MA. Activation of translation in pituitary gonadotrope cells by gonadotropin-releasing hormone. Mol Endocrinol. 2000;14(11):1811–1819. [DOI] [PubMed] [Google Scholar]
- 23.Kim T, Lawson MA. GnRH regulates gonadotropin gene expression through NADPH/dual oxidase-derived reactive oxygen species. Endocrinology. 2015;156(6):2185–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen KA, Intriago RE, Upadhyay HC, Santos SJ, Webster NJ, Lawson MA. Modulation of gonadotropin-releasing hormone-induced extracellular signal-regulated kinase activation by dual-specificity protein phosphatase 1 in LbetaT2 gonadotropes. Endocrinology. 2010;151(10):4882–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caunt CJ, Armstrong SP, Rivers CA, Norman MR, McArdle CA. Spatiotemporal regulation of ERK2 by dual specificity phosphatases. J Biol Chem. 2008;283(39):26612–26623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garner KL, Perrett RM, Voliotis M, Bowsher C, Pope GR, Pham T, Caunt CJ, Tsaneva-Atanasova K, McArdle CA. Information transfer in gonadotropin-releasing hormone (GnRH) signaling: extracellular signal-regulated kinase (ERK)-mediated feedback loops control hormone sensing. J Biol Chem. 2016;291(5):2246–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perrett RM, McArdle CA. Molecular mechanisms of gonadotropin-releasing hormone signaling: integrating cyclic nucleotides into the network. Front Endocrinol (Lausanne). 2013;4:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perrett RM, Voliotis M, Armstrong SP, Fowkes RC, Pope GR, Tsaneva-Atanasova K, McArdle CA. Pulsatile hormonal signaling to extracellular signal-regulated kinase: exploring system sensitivity to gonadotropin-releasing hormone pulse frequency and width. J Biol Chem. 2014;289(11):7873–7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pratap A, Garner KL, Voliotis M, Tsaneva-Atanasova K, McArdle CA. Mathematical modeling of gonadotropin-releasing hormone signaling. Mol Cell Endocrinol. 2017;449:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern E, Ruf-Zamojski F, Zalepa-King L, Pincas H, Choi SG, Peskin CS, Hayot F, Turgeon JL, Sealfon SC. Modeling and high-throughput experimental data uncover the mechanisms underlying Fshb gene sensitivity to gonadotropin-releasing hormone pulse frequency. J Biol Chem. 2017;292(23):9815–9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talukdar S, Olefsky JM, Osborn O. Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends Pharmacol Sci. 2011;32(9):543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Randle PJ, Garland PB, Newsholme EA, Hales CN. The glucose fatty acid cycle in obesity and maturity onset diabetes mellitus. Ann N Y Acad Sci. 1965;131(1):324–333. [DOI] [PubMed] [Google Scholar]
- 33.Diéguez C, Fruhbeck G, López M. Hypothalamic lipids and the regulation of energy homeostasis. Obes Facts. 2009;2(2):126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29(3):317–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR Jr, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278(13):11303–11311. [DOI] [PubMed] [Google Scholar]
- 36.Covington DK, Briscoe CA, Brown AJ, Jayawickreme CK. The G-protein-coupled receptor 40 family (GPR40-GPR43) and its role in nutrient sensing. Biochem Soc Trans. 2006;34(Pt 5):770–773. [DOI] [PubMed] [Google Scholar]
- 37.Schiöth HB. G protein-coupled receptors in regulation of body weight. CNS Neurol Disord Drug Targets. 2006;5(3):241–249. [DOI] [PubMed] [Google Scholar]
- 38.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422(6928):173–176. [DOI] [PubMed] [Google Scholar]
- 39.Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes. 2006;55(Suppl 2):S16–S23. [DOI] [PubMed] [Google Scholar]
- 40.Schnell S, Schaefer M, Schöfl C. Free fatty acids increase cytosolic free calcium and stimulate insulin secretion from beta-cells through activation of GPR40. Mol Cell Endocrinol. 2007;263(1-2):173–180. [DOI] [PubMed] [Google Scholar]
- 41.Silva LC, de Almeida RF, Castro BM, Fedorov A, Prieto M. Ceramide-domain formation and collapse in lipid rafts: membrane reorganization by an apoptotic lipid. Biophys J. 2007;92(2):502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nogueira TC, Anhê GF, Carvalho CR, Curi R, Bordin S, Carpinelli AR. Involvement of phosphatidylinositol-3 kinase/AKT/PKCzeta/lambda pathway in the effect of palmitate on glucose-induced insulin secretion. Pancreas. 2008;37(3):309–315. [DOI] [PubMed] [Google Scholar]
- 43.Mattos R, Staples CR, Thatcher WW. Effects of dietary fatty acids on reproduction in ruminants. Rev Reprod. 2000;5(1):38–45. [DOI] [PubMed] [Google Scholar]
- 44.Garrel G, Simon V, Denoyelle C, Cruciani-Guglielmacci C, Migrenne S, Counis R, Magnan C, Cohen-Tannoudji J. Unsaturated fatty acids stimulate LH secretion via novel PKCepsilon and -theta in gonadotrope cells and inhibit GnRH-induced LH release. Endocrinology. 2011;152(10):3905–3916. [DOI] [PubMed] [Google Scholar]
- 45.Garrel G, Simon V, Denoyelle C, Ishaq M, Rouch C, Dairou J, Magnan C, Migrenne S, Cruciani-Guglielmacci C, Cohen-Tannoudji J. Unsaturated fatty acids disrupt Smad signaling in gonadotrope cells leading to inhibition of FSHβ gene expression. Endocrinology. 2014;155(2):592–604. [DOI] [PubMed] [Google Scholar]
- 46.Barb CR, Kraeling RR, Rampacek GB. Glucose and free fatty acid modulation of growth hormone and luteinizing hormone secretion by cultured porcine pituitary cells. J Anim Sci. 1995;73(5):1416–1423. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen MT, Satoh H, Favelyukis S, Babendure JL, Imamura T, Sbodio JI, Zalevsky J, Dahiyat BI, Chi NW, Olefsky JM. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280(42):35361–35371. [DOI] [PubMed] [Google Scholar]
- 48.Sharma S, Morinaga H, Hwang V, Fan W, Fernandez MO, Varki N, Olefsky JM, Webster NJ. Free fatty acids induce Lhb mRNA but suppress Fshb mRNA in pituitary LβT2 gonadotropes and diet-induced obesity reduces FSH levels in male mice and disrupts the proestrous LH/FSH surge in female mice. Endocrinology. 2013;154(6):2188–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Do MH, Santos SJ, Lawson MA. GNRH induces the unfolded protein response in the LbetaT2 pituitary gonadotrope cell line. Mol Endocrinol. 2009;23(1):100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shoulders MD, Ryno LM, Genereux JC, Moresco JJ, Tu PG, Wu C, Yates JR III, Su AI, Kelly JW, Wiseman RL. Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Reports. 2013;3(4):1279–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122(10):3319–3329. [DOI] [PubMed] [Google Scholar]
- 52.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moriyama R, Deura C, Imoto S, Nose K, Fukushima N. Expression of the long-chain fatty acid receptor GPR120 in the gonadotropes of the mouse anterior pituitary gland. Histochem Cell Biol. 2015;143(1):21–27. [DOI] [PubMed] [Google Scholar]
- 54.Terasaka T, Adakama ME, Li S, Kim T, Terasaka E, Li D, Lawson MA. Reactive oxygen species link gonadotropin-releasing hormone receptor signaling cascades in the gonadotrope. Front Endocrinol (Lausanne). 2017;8:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Margariti A, Li H, Chen T, Martin D, Vizcay-Barrena G, Alam S, Karamariti E, Xiao Q, Zampetaki A, Zhang Z, Wang W, Jiang Z, Gao C, Ma B, Chen YG, Cockerill G, Hu Y, Xu Q, Zeng L. XBP1 mRNA splicing triggers an autophagic response in endothelial cells through BECLIN-1 transcriptional activation. J Biol Chem. 2013;288(2):859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burger LL, Dalkin AC, Aylor KW, Haisenleder DJ, Marshall JC. GnRH pulse frequency modulation of gonadotropin subunit gene transcription in normal gonadotropes-assessment by primary transcript assay provides evidence for roles of GnRH and follistatin. Endocrinology. 2002;143(9):3243–3249. [DOI] [PubMed] [Google Scholar]
- 57.Ly LD, Xu S, Choi SK, Ha CM, Thoudam T, Cha SK, Wiederkehr A, Wollheim CB, Lee IK, Park KS. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp Mol Med. 2017;49(2):e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee HS, Yoon JS, Hwang JS. Luteinizing hormone secretion during gonadotropin-releasing hormone stimulation tests in obese girls with central precocious puberty. J Clin Res Pediatr Endocrinol. 2016;8(4):392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chosich J, Bradford AP, Allshouse AA, Reusch JEB, Santoro N, Schauer IE. Acute recapitulation of the hyperinsulinemia and hyperlipidemia characteristic of metabolic syndrome suppresses gonadotropins. Obesity (Silver Spring). 2017;25(3):553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Do MH, Kim T, He F, Dave H, Intriago RE, Astorga UA, Jain S, Lawson MA. Polyribosome and ribonucleoprotein complex redistribution of mRNA induced by GnRH involves both EIF2AK3 and MAPK signaling. Mol Cell Endocrinol. 2014;382(1):346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farnworth PG. Gonadotrophin secretion revisited. How many ways can FSH leave a gonadotroph? J Endocrinol. 1995;145(3):387–395. [DOI] [PubMed] [Google Scholar]
- 62.Pearl CA, Jablonka-Shariff A, Boime I. Rerouting of a follicle-stimulating hormone analog to the regulated secretory pathway. Endocrinology. 2010;151(1):388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]