Abstract
In jawed vertebrates, the adaptive immune system (AIS) cooperates with the innate immune system (IIS) to protect hosts from infections. Although targeting non-self-components, the AIS also generates self-reactive antibodies which, when inadequately counter-selected, can give rise to autoimmune diseases (ADs). ADs are on the rise in western countries. Why haven’t ADs been eliminated during the evolution of a ∼500 million-year old system? And why have they become more frequent in recent decades? Self-recognition is an attribute of the phylogenetically more ancient IIS and empirical data compellingly show that some self-reactive antibodies, which are classifiable as elements of the IIS rather then the AIS, may protect from (rather than cause) ADs. Here, we propose that the IIS’s self-recognition system originally fathered the AIS and, as a consequence of this relationship, its activity is dampened in hygienic environments. Rather than a mere breakdown or failure of the mechanisms of self-tolerance, ADs might thus arise from architectural constraints.
Keywords: adaptive immune system, innate immune system, natural autoantibodies, self-recognition, pregnancy, immune tolerance
INTRODUCTION
Jawed vertebrates are protected from invading pathogens or pathogenic compounds by two multilayered and synergistic mechanisms: the Innate Immune System (IIS) and the Adaptive Immune System (AIS) [1]. IIS and AIS provide incredibly high levels of protection against external threats. However, these systems may also bear disadvantages that can be life threatening. Healthy autologous or self-components such as DNA, cells or secreted proteins may become targets of antibodies (Abs) that are generated during an immune response thus giving rise to autoimmune diseases (ADs). Self-reactive Abs have long been considered as an unfortunate byproduct of the process of somatic recombination which must be coped with in return for a sophisticated defense system [2]. It has been proposed that mechanisms such as clonal deletion, receptor editing, and anergy (i.e. functional unresponsiveness) [3–6] have evolved to mitigate the negative impacts of self-reactive Abs. On the other hand, self-reactivity can also be non-pathological and crucial for the proper functioning of the AIS [7–10].
Herein we leverage and build on this knowledge to put forward a hypothesis for the emergence of AIS, which proposes that self-recognition was (i) a major driving force in the evolution of a system that would eventually give rise to the current AIS, and (ii) a significant player in imposing constraints on the present-day AIS. It is maintained that the AIS emerged subsequent to the weakening of existing immune defenses, a suboptimal condition that imposed selection pressures favoring further immune defense mechanisms. The proposed hypothesis is uniquely capable of concurrently integrating empirical findings such as the homeostatic function of B-1-derived natural self-reactive Abs and the immunological changes that occur during normal pregnancy with prominent theoretical arguments such as the ‘immunological homunculus’ [8], the ‘immune network theory’ [11–13], the ‘hygiene hypothesis’ [14], and the ‘2R hypothesis’ [15]. Our hypothetical scenario delineates specific changes in the population-genetic environment that occurred around the time when vertebrate lineages diverged from a common ancestor. It also makes testable predictions regarding the mechanistic relationships between the expression/activity of B-1 and B-2-derived Abs.
INNATE AND ADAPTIVE IMMUNITY
The IIS is a collection of defense measures that provide immediate protection against infections. Widespread across eukaryotes, the IIS acts against conserved pathogen-associated patterns (e.g. lipopolysaccharides of bacterial cell walls). It involves a diverse range of assets (e.g. macrophages, neutrophils, complement system etc.) and employs genetically encoded receptors (e.g. Toll-like receptors) and secreted proteins [1].
The AIS with its classical immunoglobulins (Igs) and molecules such as the major histocompatibility complex (MHC), on the other hand, is currently restricted to the group of jawed vertebrates. Dependent on IIS elements, the AIS is highly specific and generates a diverse repertoire of receptors during the process of germ-line-to-soma differentiation. When challenged by new pathogens or pathogenic elements, matching receptors are selectively amplified to target the threat and to mount an immune response wherein components of the IIS are also involved. During this process, some of the selected cells are stored as memory cells enabling an accelerated immune response should the pathogen be re-encountered [1].
The diversity of the acquired antibody repertoire is generated by V(D)J recombination [16, 17]. During this developmental process, immune cells known as B and T lymphocytes recombine one copy each of multiple V, (D) and J gene sections in the genomic receptor locus to form functional somatic genes, i.e. B-cell receptors (BCRs) and T-cell receptors (TCRs). Two proteins (RAG1 and RAG2) are required to activate V(D)J recombination [18, 19]. As multiple copies of V, (D) and J segments are present in the germ-line genome, >106 antibody variants can be yielded theoretically for human BCRs by combinatory possibilities, although some diversity restriction may be imposed by the non-random use of individual amino acids in the third complementarity region of the H chain or the biased composition of the antibody repertoire in fetus and adult [20]. This number may rise further as a consequence of the imprecise joining of the V, (D) and J segments [21, 22]. The considerable diversity of receptor variants that result from this process of diversification should provide adequate protection against any new pathogen encountered. However, there is a risk that it may target self-components and harm the host. Auto-reactive cells/receptors may be repressed or selected against at various points during the lymphocytes’ lifetime [23]. Elimination of self-reactive immune cells may not always be complete however. Additionally, self-reactive immune cells can normally be detected in physiological conditions (see section ‘The healthy aspect of self-recognition’ below).
ADAPTIVE IMMUNITY AND ‘THE CHICKEN AND THE EGG’ CONUNDRUM
Although the deletion of self-reactive BCRs and/or TCRs may make sense from a system viewpoint, the modern AIS’s organization raises questions about the sequence of evolutionary events that shaped it. The AIS requires finely tuned regulation to prevent autoimmunity and to secure functional interactions with the IIS. Although it is possible that the early AIS was unregulated and yet selected for its inherent fitness benefits (e.g. pathogen resistance outweighed the costs of autoimmunity), it seems unlikely that such a sophisticated system could have developed without regulatory mechanisms in place. But how can regulatory elements evolve in the absence of the system? A possible answer to this conundrum is that regulatory mechanisms were already present for a system that originally served (and may continue to serve) different functions than those that the AIS presently plays. In this hypothetical system, primordial lymphocyte cells produced germ-line encoded antibody-like proteins that were at least partly reactive towards self-components. Therefore, control mechanisms could evolve to prevent the emergence of pathogenic self-reactivity. The ability to efficiently recognize foreign antigens would have evolved later in the presence—if not owing to the existence—of this self-reactive cell-producing system. While both T and B cells may have played an equally central role in an evolutionary system where self-recognition is a key step towards the modern AIS, in this article we only focus on B cells and Abs/BCRs as possible key players in AIS evolution. This choice is based mainly on the striking properties of B cells, which are discussed further below.
THE HEALTHY ASPECT OF SELF-RECOGNITION
A traditional paradigm in immunology is that self-reactive Abs are negatively selected owing to the harm that they may cause the organism. This paradigm has been repeatedly challenged however. For example, it has been found that self-reactivity is a common attribute of T cells [24, 25]. Furthermore, self-reactive BCRs may be even necessary for B cells to develop properly and to form the diverse repertoire of BCRs [26, 27]. Finally, the discovery of naturally occurring autoantibodies or NAAbs challenges the notion that self-recognition is merely deleterious [28]. NAAbs closely resemble the primordial lymphocyte receptors that we envisage in our hypothesis. They are naturally found in the sera of healthy, non-immunized, humans and other vertebrates [29–34], and are also detected in animals raised in germ-free conditions [35, 36]. As opposed to somatically hypermutated Abs that result from active immunization, NAAbs are encoded by rearranged germ-line V(D)J gene segments that are unaltered or only minimally altered [37, 38]. They are typically polyreactive, have low binding affinity, and may recognize a broad spectrum of antigens including non-self and self-antigens (e.g. single-stranded DNA, carbohydrate epitopes) [39, 40]. NAAbs primarily belong to the IgM isotype—the most ancient of the antibody classes, also found in sharks in trans-membrane and secretory forms [41, 42]. It is worth noting that IgM NAAbs from sharks, humans, and other vertebrates show considerable levels of conservation in the overall 3D structure and in some framework regions such as the variable domains VH3-30 and VH3-23 [43]. Abs that retain such conserved regions are often expressed early in fetal development, when the ability to respond to specific antigens is low, and are a part of the repertoire directed against T-independent antigens [20, 44].
NAAbs are produced primarily by self-replenishing B-1a cells (NAAbB-1a), a subgroup of B-cell lymphocytes that could be classified as part of the IIS [45, 46]. Although responding to probably innate immune signals [47], B-1a cells do not appear to develop into memory B cells [48, 49], which are crucial for the adaptive immune response. Moreover, after being produced chiefly during the fetal and neonatal period, they persist in the individual and, with increasing age, may be complemented by bone marrow-derived B-1 cells [50, 51]. On the other hand, the majority of B-cell lymphocytes are made of follicular B-2 cells that develop later in life and produce non-self-reactive Abs with high-binding affinities [37, 52]. Interestingly, ‘early’ B-2 cells produce Abs that resemble NAAbB-1a in being positively selected for their ability to bind to self-antigens [26]. On the other hand, when the regulatory mechanisms underperform, developing B-2 cells which undergo negative selection for self-reactivity may also produce class switched and affinity matured self-reactive Abs with pathogenic properties. Hereinafter we will refer to B-2 cell-related self-reactive Abs as SR-AbB-2, where SR stands for self-reactive. Unlike B-2 cells, which undergo negative selection at a later stage of development [23] to guarantee the proper production of non-self reactive Abs, autoreactive B-1a cells persist [53–55]. Thus, not only may self-reactive cells not be purged, they may also be deliberately produced and, in the case of the B-1a cells, preserved.
It is unlikely, as argued in the immunological homunculus theory [8, 56], that the presence of NAAbB-1a indicates a failure of the mechanisms of self-tolerance or that they are a threat to the organisms. Rather, the production and conservation of NAAbB-1a is presumably linked to their unique functions: they are major contributors to tissue homeostasis. Among the functions that have been ascribed to NAAbB-1a are: (i) acting as first line of defense against bacteria, viruses and other pathogenic agents, (ii) participation in the clearance of apoptotic cells as well as tumor and senescent cells, (iii) modulation of the inflammatory response and (iv) reduction of the risk of tissue damage during an immune response [32, 57–68]. B-1a cell-derived NAAbs may also be involved in the maintenance and regulation of the commensal microbiota [69] and contribute to the enhancement of antigene-specific responses e.g. they can stimulate and regulate T-cell responses [51] as well as modulate specific T-cell functions like cytokine secretion and chemotaxis in certain allograft contexts [70].
NAAbB-1a can also protect from AD development [71–73]. For example, the abundance of IgM-NAAbB-1a generally correlates negatively with the severity of systemic lupus erythematosus, whereas the deficiency of IgM-NAAbB-1a can help accelerate this disease [69, 74]. In addition, IgM-NAAbB-1a treatment reduces atherosclerosis [75, 76]. In contrast, IgM-NAAbB-1a deficiency may lead to atherosclerosis [77]. Last, mice that are deficient in serum IgM-NAAbB-1a display an increased and pathological response to self-components [77, 78]. The protective properties of IgM autoAbs against autoimmunity may lie principally in their ability to inhibit inflammatory responses by recognizing and removing apoptotic cells [60]. NAAbB-1a may also protect from autoimmunity by functionally masking antigenic epitopes [79]. By reducing the amount of unengaged antigen, this operation would lessen the need for the AIS to mount a response.
FROM NAABB-1A TO THE ORIGIN OF THE AIS
The findings that are outlined above suggest that in addition to acting as the first line of defense against pathogens, NAAbB-1a may also be instrumental for the deployment of Abs against foreign antigens [27, 80, 81]. These observations align with the accepted view that IIS and AIS act synergistically to mediate host responses to infection and tissue injury. They also hint that a system that has spawned self-reactive Abs in the ancestor of jawed vertebrates may have predated the emergence of a system of non-self targeting Abs, in a similar vein to previously proposed hypotheses [37, 82–87]. Below, we elaborate further on this idea and put forward a three-step evolutionary scenario for the origin of the AIS. In this scenario, the proliferation of distinct Ig-domain containing receptors facilitated the birth (or the amplification) of a regulatory system that controlled the interactions between these Ab-like receptors and their ligand(s) in early vertebrates. Measures for guaranteeing or optimizing the clearance of antigen-bound Abs evolved subsequently and linked the existing innate immunity circuit to the emergent system of Ab-like proteins. Lastly, the AIS emerged from the system producing non-self-reactive Abs. Although targeting non-self-antigens, the AIS could (and continues to) target occasionally self-components, as testimony to its evolutionary origins. Population-genetic environments where the efficiency of natural selection is reduced may have facilitated these evolutionary innovations by favoring the accumulation of mildly deleterious mutations and the preservation of gene duplicates [88].
THE BIRTH OF AN IMMUNE REGULATORY SYSTEM
Genes homologous to recombinases that are essential for adaptive immunity (RAG1 and RAG2) were present well before the emergence of the AIS-bearing jawed vertebrates [89]. It is likely then that RAG1 and RAG2 were domesticated for BCR and/or TCR assembly in primordial lymphocytes sometime after the split between Hyperoartia (e.g. lamprey) and Gnathostomata (jawed vertebrates).
With the RAG genes in place, Ig genes that contained a set of primordial V, (D) and J-like modules may have yielded distinct Ab-like proteins. Some of these Ab-like proteins would have binding affinity toward self-components (e.g. cytokines), as many modern Ig domain-containing proteins do. This polyreactivity may have facilitated the emergence of a powerful regulatory system. As is exquisitely illustrated in immune network theory [11–13], Abs can also function as antigens and as such they may stimulate the production of second-class Abs—where ‘class’ refers to a set of Abs with a certain idiotype—if some threshold concentration is exceeded. Second-class Abs could in turn stimulate the production of third-class Abs and so on. In this chain of reaction Ig domain-containing proteins reversibly bind and thereby block each other. Thus, even if present, Ab-like proteins may operate at minimal levels or may lie dormant, leaving their ligands unbound. In cases when e.g. third-class Abs are stimulated and block second-class Abs, first class Abs could efficiently respond to their ligands [11–13]. It is worth noting that if we consider how widespread Ig domain-containing proteins are in nature, it is possible that a similar regulatory system predated the evolution of jawed vertebrates.
The Ab-like proteins that are described above would have features found in existing NAAbB-1a. Moreover, the expression of a diverse repertoire of Ab-like proteins may enable a broad range of interactions between Abs and self-components and provokes the emergence (or the amplification) of a system that regulates these interactions.
LINKING ANTIBODY RESPONSE TO INNATE IMMUNITY
The emergence (or the optimization) of a system for the clearance of Abs-antigen complexes would bring us a step further toward the modern AIS. In enabling the removal of Abs that are bound to e.g. remnants of apoptotic or necrotic cells or conserved patterns of commensal microbiota, this system would link the components of the IIS to the low-affinity Abs-producing system. B-1 (but not B-2) cells are able to phagocytose large particles and bacteria, though not as extensively as macrophages [90–92]. Thus, a clearance system might have already been in place. Alternatively, Fc receptors, which interact with the constant region of Abs and appeared at the base of the bony fishes [93], might have played a significant role in this clearance system.
LINKING THE PRODUCTION OF NAABS TO THE PRODUCTION OF ANTIGEN-SPECIFIC ABS
The interactions described thus far involve Ab-like proteins that (i) are partly self-reactive, (ii) are encoded by germ-line, unmutated or minimally mutated V(D)J gene sequences and (iii) have low affinity for their antigens. We postulate that the regulated production of non-self-reactive and high-affinity Abs emerged from this immune environment, subsequent to the whole genome duplication (WGD) that occurred before the radiation of jawed vertebrates [15, 94–97] (Fig. 1). The process of somatic hypermutation by the evolution of activation-induced cytidine deaminase from RNA-editing enzymes, affinity maturation and clonal selection would be paramount for achieving higher levels of Ab affinity. Post-WGD neofunctionalization of one or both copies of duplicated genes and/or events of subfunctionalization (i.e. partition of functions between copies of duplicated genes) could have facilitated the emergence of these AIS components, even in the absence of adaptive benefits [98–100]. This hypothetical scenario predicts that members of duplicate-gene pairs might display distinct patterns of expression in B-1a and B-2 cells. Although waiting for this prediction to be formally tested, we note that several mouse genes with duplicated copies [101] are expressed differentially between B-1 cells and B-2 cells [102].
Figure 1.
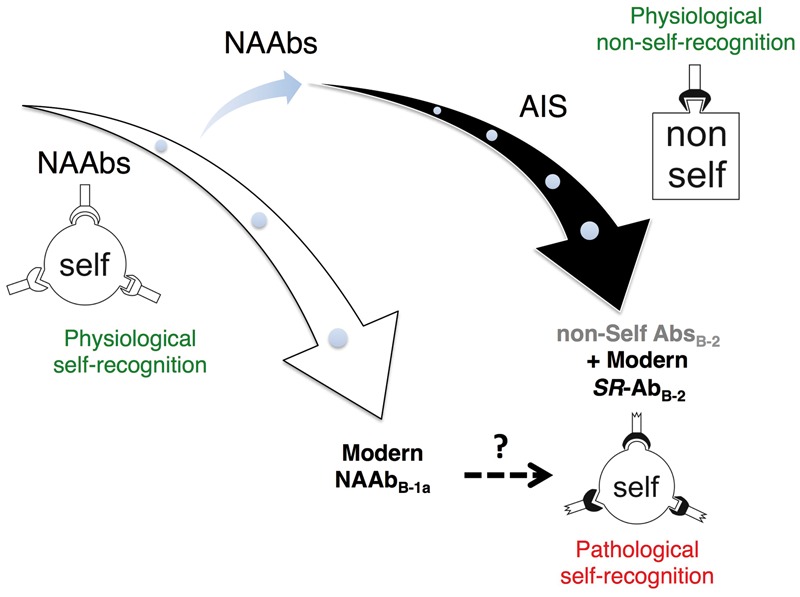
Hypothesis for the origin of the AIS in jawed vertebrates. Subsequent to the whole genome duplication that predated the radiation of jawed vertebrates (blue arrow), the AIS—a non-self-recognition system—gradually emerged from a regulated self-recognition system that is presently part of the IIS and produces natural autoantibodies via B-1a cells (NAAbB-1a). A population-genetic environment wherein the power of random genetic drift exceeds the power of selection might have favored the emergence of the AIS. NAAbB-1a are physiologically produced; they contribute to tissue homeostasis and protect from pathological self-reactivity. SR-AbB-2 are the AIS’s counterpart of NAAbB-1a. They can cause pathological self-reactivity and are normally counter-selected during the production of B-2 cell-derived non-self-targeting Abs (non-Self AbsB-2). It still remains unclear whether pathological self-reacting Abs result from misregulated B-1a cells, B-2 cells or subgroups thereof. Furthermore, the primary source of pathological self-reacting Abs may vary depending upon the types of AD
A central idea of our hypothesis is that SR-AbB-2 and non-self-recognizing Abs were evolutionarily generated not only from but also ‘at the expense’ of NAAbB-1a. This implies that the production of high-affinity non-self-recognizing Abs was (and might still be) facilitated in an environment where the production/activity of NAAbB-1a is reduced, i.e. an environment where the host suffers from a lowered ability to avoid autoimmunity and where the first line of defense against infections is weakened. This idea is consistent with observed hyperactivity of the AIS in diseases with autoimmune components [103] and with the observation that autoimmunity—a condition that can result from a low titer of NAAbB-1a—has enhancing effects on the fine-tuning of the adaptive immune responses [104]. Furthermore, this idea predicts that the enhanced production/activity of NAAbB-1a, e.g. in response to relatively heavy pathogen loads, protects from autoimmunity and inhibits or delays the production of SR-AbB-2 and antigen-specific Abs (Fig. 2). These predictions align well with the hygiene hypothesis, which posits that the lowered exposure to infectious agents (particularly in early life) is the basis for increasing incidence of both autoimmune and allergic diseases [14, 105–107]. Noteworthy, the mechanisms underlying the hygiene hypothesis center on the antagonizing effects of T-helper cells (Th1/Th17 and Th2) [108] The theoretical framework that we propose support and further extend these mechanisms, given the role that B1-cells play in stimulating and regulating T-cell responses [109].
Figure 2.
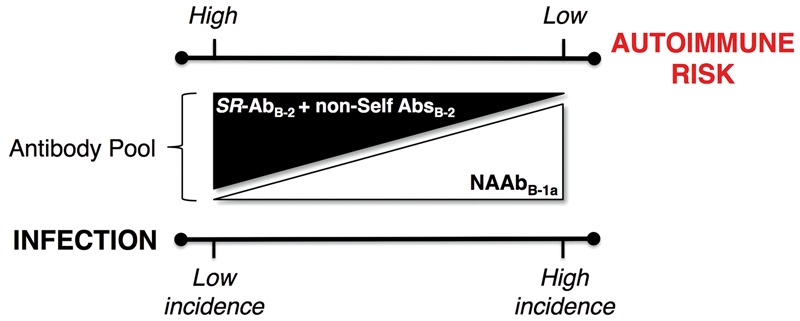
We propose (i) that SR-AbB-2 alongside non-Self AbsB-2 were originally produced from NAAbB-1a-like receptors and (ii) that the present-day production of B2-cell-derived Abs is enhanced in environments where NAAbB-1a’ production—chiefly taking place during fetal/neonatal period—and/or activity are reduced. Acting as first line of defense, the production of NAAbB-1a is enhanced in environments with a high incidence of infection. This excess of NAAbB-1a protects from ADs whereas the limited production of SR-AbB-2 reduces the risk for ADs. In environments with a low incidence of infection the relative excess of SR-AbB-2 alongside the reduction of NAAbB-1a enhance the risk of developing ADs
Which factors might have determined the suggested reduction in production/activity of early NAAbB-1a in the ancestor of jawed vertebrates? One factor could be gene duplication, which may be coupled with a substantial reduction in expression level of each copy compared to the progenitor gene [110, 111]. Another factor could be a population-genetic environment where the power of random genetic drift exceeds the power of selection. In this environment mildly deleterious mutations, which can fix in small populations and might be selected secondarily [112], are expected to accumulate and duplicated genes to be preserved more easily by subfunctionalization [98, 99]. These accrued mutations could have weakened the existing IIS—including the system that produced natural autoantibody-like receptors —thereby imposing selection pressures for novel defense mechanisms with fitness benefits that outweigh the costs of inadequate immune responses. These fitness effects are compatible with empirical studies in sheep, chicken, and human where autoantibody production—whether physiological or reflecting antibody responsiveness is difficult to conclude firmly—has been found to scale positively with survival [113–118]. In sum, it is conceivable that a drift-dominated population-genetic environment in the ancestor of jawed vertebrates could have facilitated (i) the preservation of duplicated genes, (ii) the weakening of the mechanisms that control self-recognition and/or regulated the production/activity of NAAb-like receptors and (iii) the emergence of the somatic hyper mutation machinery.
SELF- AND NON-SELF-RECOGNITION, THROUGH EVOLUTIONARY TIME
The arguments laid out until this point provide a reasonable and expandable framework which explains how the AIS could have gradually emerged in the ancestor of jawed vertebrates and may continue to operate today. Arguments that support or challenge aspects of our hypothesis may be established through an extended examination of existing accredited immunological models. Natural Abs can recognize self, altered self, and foreign antigens. This ability might be testimony to the proposed evolutionary link between self-reactivity and adaptive immunity. Self-antigens can be altered (e.g. due to oxidative stress [119]) and become immunogenic. Additionally, many natural Abs that react with altered self-antigen recognize epitopes expressed on pathogens. In this context, an examination of the altered-self antigen model [120, 121] might provide valuable insight on the early evolution of Abs and the regulation of self and non-self antigen recognition.
Insights offered by appropriate systems may be leveraged to further assess other aspects of our hypothesis. Pregnancy may be one such system. Given that pregnancy evolved much later than the AIS (probably <∼250 My ago [122, 123]), it may provide minimal new knowledge about the early evolution of AIS. However, because of the intimate relationship between pregnancy and immunity, pregnancy presents itself as a powerful system for scrutinizing the hypothesis that the present-day production of high-affinity non-self-recognizing Abs and the production/activity of NAAbB-1a are interconnected. Because the genomes of the placenta and fetus are partially of paternal origin, immune tolerance—which bears on the responses to self- and non-self antigens [124]—is absolutely required for normal pregnancy to unfold. When these peculiar immunological circumstances are in place, pregnancy provides a natural laboratory for studying the relationships between IIS and AIS as well as these systems’ plasticity. Given that the AIS response is weakened during pregnancy whereas the IIS response is boosted [125–130], our hypothesis specifically predicts that the production/activity of NAAbB-1a is enhanced during the course of a normal pregnancy, in line with previous studies [128, 131]. We expect that this relative excess of NAAbB-1a (i) mitigates autoimmunity and (ii) inhibits/delays the production/activity of non-self-recognizing Abs and hence of potentially harmful SR-AbB-2. Indeed, ADs may regress during normal pregnancy only to reappear in the post-partum phase [132]. For example, rheumatoid arthritis and multiple sclerosis often attenuate during normal pregnancy, only to re-aggravate after childbirth [133–136]. Pregnant women, on the other hand, seem to be relatively more susceptible to some infectious diseases, such as listeriosis and influenza [137–139]. We also expect that in women with an abnormally strong adaptive immune response against fetal and placental antigens (e.g. in egg-donation pregnancies [140])—note that in these conditions our hypothesis predicts an intensified production of SR-AbB-2 alongside a lower-than-normal production/activity of NAAbB-1a—the likelihood of pregnancy-related pathologies with autoimmune components, such as recurrent pregnancy loss, preeclampsia and preterm delivery, should increase as is indeed observed [141–145]. Finally, because physiological high levels of progesterone may inhibit the antigen-presentation function of B cells, an aspect that should facilitate implantation and pregnancy [146], we expect that artificially enhancing the levels of progesterone in the maternal body could dually hinder the adaptive immune response [146] and help treat high-risk pregnancies with autoimmune components, in line with previous observations [147–150]. In sum, the maternal immune system does not simply shut down to promote the tolerance of the fetus. Rather, it adopts a peculiar state. In this new state, the relationships between the self- and non-self-recognition systems are largely consistent with the theoretical framework that we propose.
A FRAMEWORK FOR TESTABLE PREDICTIONS
Our hypothesis provides other testable predictions, some of which (listed below) may provide a mechanistic explanation of a number of observations.
First, intravenous immunoglobulins (IVIg) are commercial soluble preparations obtained from human sera pooled from a broad number of healthy donors. B-1a cell-derived NAAbs constitute a considerable part of Ig in humans and are part of these preparations [151]. Provided that NAAbB-1a truly down-regulate the production of high-affinity antigen-specific Abs (and thus SR-AbB-2), IVIg should help counter inflammation and autoimmune disorders. In line with this idea, the therapeutic preparation of IVIg often has positive effects on individuals who suffer from autoimmunity [152]. With regard to semi alloimmunity (i.e. the condition of the fetus presenting only partially foreign (paternal) antigens) and pregnancy, we may further expect that experimental protocols based on IVIg application alleviate diseases with possible autoimmune components such as recurrent abortion, preterm delivery and preeclampsia [153].
Second, if B-1a cell-derived NAAbs have a homeostatic function that predates the emergence of the AIS, homeostatic perturbations might be detected in Ig-deficient jawed vertebrates such as severe combined immunodeficiency (SCID) mice. The altered expression of cytokines in the serum of SCID-mice compared with wild-type mice, for example, may be one of these perturbations [154]. In contrast, the loss of somatic hypermutation in an otherwise functional AIS background should not exhibit such homeostatic alterations. Additionally, specific alterations of the immune repertoire in animals such as the axolotl, a salamander that has a diverse antibody repertoire but fails to mount efficient adaptive immune responses [155], could shed light on alternative functions of NAAbB-1a.
Third and last, it has been found that dysbiosis may activate the AIS [69]. Based on our hypothesis, this finding implies that the production/activity of NAAbB-1a may decrease in hosts with altered microbiota. Thus, dysbiosis might increase the risk for developing ADs. Enhancing the production/activity of NAAbB-1a in hosts with altered microbiota should offset the hyperactive AIS and ward off or mitigate existing ADs. The administration of probiotics, which are proposed to elevate the production of NAAbB-1a [156], might be beneficial in this regard. Likewise the exposure to sufficiently large pathogen loads should, in theory, also be advantageous. This latter prediction is in line with the rationale for and the proposed beneficial effects of the helminth therapy [157].
AN EXPANDABLE HYPOTHESIS
The conceptual framework summarized here makes fair assumptions about how current data and theories can be interpreted and integrated coherently towards a better understanding of AIS evolution. We believe that our propositions may be useful to further expand current views on the origins and the evolution of the innate and AISs. Further efforts are still required, however, to account for the role of other immunological players such as TCRs and the MHC, which is involved in antigen presentation and regulation of B- and T-cell activity during immune responses, or Ig-isotypes (IgM, IgG, IgA etc.), whose different roles at specific life stages have not been extensively discussed here. Self-reactive Abs may be beneficial, merely decorative or pathogenic and self-reactivity needs to be considered through the prism of functional outcome. In addition, ADs are often a compilation of symptoms with potentially different ontologies, which makes it difficult to ascribe effects of a particular molecular agent on a specific disease. Finally, published observations largely stem from studies of mammals, with little knowledge yielded on other clades of vertebrates. All these knowledge gaps and limitations will be at least partly overcome in the future and new developments would serve to assess the validity of our hypothesis.
CONCLUSIONS
We have presented a hypothesis for the origins and the evolution of the AIS in jawed vertebrates, where a self-recognition system alongside mechanisms that prevent pathogenic self-reactivity and do not elicit T-dependent responses predated and were instrumental for the emergence of a non-self-recognition system. Our hypothesis leverages and extends previous arguments and experimental observations concerning a number of evolutionary and medical aspects that have been published in the past few decades. In addition to making specific predictions, our hypothesis coherently integrates propositions about the evolutionary origin of the AIS with (i) current knowledge about NAAbs, (ii) concepts drawn from the ‘immune network’ and the ‘immunological homunculus’ theories, (iii) the 2 R hypothesis, (iv) mechanisms of gene duplicate preservation, (v) the ‘hygiene hypothesis’ and (vi) clinical observations and characteristics of pregnancy at the same time. Our simple hypothesis reconciles and bridges experimental and theoretical arguments that for long time have lived apart. It provides a framework for interpreting recent and less recent observations and offers potentially useful guidelines for future experiments.
ACKNOWLEDGEMENTS
We thank Joachim Kurtz, Florian Horn, Nina Kranke, Andrea L. Graham and two anonymous reviewers for their valuable comments. This study was carried out within the Deutsche Forschungsgemeinschaft (DFG) Research Training Group 2220 ‘Evolutionary Processes in Adaptation and Disease’ at the University of Münster.
Conflict of interest: None declared.
REFERENCES
- 1. Murphy K, Travers P, Walport M. et al. 2012. Janeway’s Immunobiology, 8th ed. New York: Garland Science. [Google Scholar]
- 2. Burnet FM. 1959. The Clonal Selection Theory of Aquired Immunity. Cambridge: The University Press. [Google Scholar]
- 3. Goodnow CC, Crosbie J, Adelstein S. et al. Altered Immunoglobulin expression and functional silencing of self-reactive lymphocytes-B in transgenic mice. Nature 1988;334:676–82.http://dx.doi.org/10.1038/334676a0 [DOI] [PubMed] [Google Scholar]
- 4. Nemazee D, Buerki K.. Clonal deletion of autoreactive lymphocytes-B in bone-marrow chimeras. Proc Natl Acad Sci U S A 1989;86:8039–43.http://dx.doi.org/10.1073/pnas.86.20.8039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tiegs SL, Russell DM, Nemazee D.. Receptor editing in self-reactive bone-marrow B-cells. J Exp Med 1993;177:1009–20.http://dx.doi.org/10.1084/jem.177.4.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gay D, Saunders T, Camper S. et al. Receptor editing - an approach by autoreactive B-cells to escape tolerance. J Exp Med 1993;177:999–1008.http://dx.doi.org/10.1084/jem.177.4.999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Avrameas S. Natural autoantibodies: from ‘horror autotoxicus’ to ‘gnothi seauton’. Immunol Today 1991;12:154–9. [DOI] [PubMed] [Google Scholar]
- 8. Cohen IR, Young DB.. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today 1991;12:105–10.http://dx.doi.org/10.1016/0167-5699(91)90093-9 [DOI] [PubMed] [Google Scholar]
- 9. Lacroix-Desmazes S, Kaveri SV, Mouthon L. et al. Self-reactive antibodies (natural autoantibodies) in healthy individuals. J Immunol Methods 1998;216:117–37.http://dx.doi.org/10.1016/S0022-1759(98)00074-X [DOI] [PubMed] [Google Scholar]
- 10. Lobo PI. Role of natural autoantibodies and natural IgM anti-leucocyte autoantibodies in health and disease. Front Immunol 2016;7:198.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoffmann GW. Immune Network Theory, 2008. http://www.phas.ubc.ca/~hoffmann/ni.html (January 2018, date last accessed). [Google Scholar]
- 12. Jerne NK. Towards a network theory of the immune system. Ann Immunol (Paris) 1974;125C:373–89. [PubMed] [Google Scholar]
- 13. Richter PH. A network theory of the immune system. Eur J Immunol 1975; 5:350–4.http://dx.doi.org/10.1002/eji.1830050511 [DOI] [PubMed] [Google Scholar]
- 14. Strachan DP. Hay-fever, hygiene, and household size. Br Med J 1989;299:1259–60.http://dx.doi.org/10.1136/bmj.299.6710.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohno S. Evolution by Gene Duplication. New York: Springer, 1970. [Google Scholar]
- 16. Brack C, Hirama M, Lenhard-Schuller R. et al. A complete immunoglobulin gene is created by somatic recombination. Cell 1978;15:1–14.http://dx.doi.org/10.1016/0092-8674(78)90078-8 [DOI] [PubMed] [Google Scholar]
- 17. Tonegawa S. Somatic generation of antibody diversity. Nature 1983;302:575–81.http://dx.doi.org/10.1038/302575a0 [DOI] [PubMed] [Google Scholar]
- 18. Huang SF, Tao X, Yuan SC. et al. Discovery of an active RAG transposon illuminates the origins of V(D)J recombination. Cell 2016;166:102–14.http://dx.doi.org/10.1016/j.cell.2016.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gellert M. V(D)J recombination: RAG proteins, repair factors, and regulation. Annu Rev Biochem 2002;71:101–32.http://dx.doi.org/10.1146/annurev.biochem.71.090501.150203 [DOI] [PubMed] [Google Scholar]
- 20. Schroeder HW., Jr. Similarity and divergence in the development and expression of the mouse and human antibody repertoires. Dev Comp Immunol 2006;30:119–35.http://dx.doi.org/10.1016/j.dci.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 21. Desiderio SV, Yancopoulos GD, Paskind M. et al. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature 1984;311:752–5.http://dx.doi.org/10.1038/311752a0 [DOI] [PubMed] [Google Scholar]
- 22. Lafaille JJ, DeCloux A, Bonneville M. et al. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell 1989;59:859–70.http://dx.doi.org/10.1016/0092-8674(89)90609-0 [DOI] [PubMed] [Google Scholar]
- 23. Wardemann H, Yurasov S, Schaefer A. et al. Predominant autoantibody production by early human B cell precursors. Science 2003;301:1374–7.http://dx.doi.org/10.1126/science.1086907 [DOI] [PubMed] [Google Scholar]
- 24. Wooldridge L, Ekeruche-Makinde J, van den Berg HA. et al. A single autoimmune T cell receptor recognizes more than a million different peptides. J Biol Chem 2012;287:1168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martin B, Auffray C, Delpoux A. et al. Highly self-reactive naive CD4 T cells are prone to differentiate into regulatory T cells. Nat Commun 2013;4:2209.. [DOI] [PubMed] [Google Scholar]
- 26. Eschbach C, Bach MP, Fidler I. et al. Efficient generation of B lymphocytes by recognition of self-antigens. Eur J Immunol 2011;41:2397–403.http://dx.doi.org/10.1002/eji.201041344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kohler F, Hug E, Eschbach C. et al. Autoreactive B cell receptors mimic autonomous pre-B cell receptor signaling and induce proliferation of early B cells. Immunity 2008;29:912–21.http://dx.doi.org/10.1016/j.immuni.2008.10.013 [DOI] [PubMed] [Google Scholar]
- 28. Lutz HU. Naturally Occurring Antibodies (NAbs). New York: Springer, 2012. [Google Scholar]
- 29. Hunter KW Jr., Dupre SA, Sharp T. et al. Western blot can distinguish natural and acquired antibodies to Mycoplasma agassizii in the desert tortoise (Gopherus agassizii). J Microbiol Methods 2008;75:464–71.http://dx.doi.org/10.1016/j.mimet.2008.07.022 [DOI] [PubMed] [Google Scholar]
- 30. Gonzalez R, Charlemagne J, Mahana W. et al. Specificity of natural serum antibodies present in phylogenetically distinct fish species. Immunology 1988;63:31–6. [PMC free article] [PubMed] [Google Scholar]
- 31. Adelman MK, Schluter SF, Marchalonis JJ.. The natural antibody repertoire of sharks and humans recognizes the potential universe of antigens. Protein J 2004;23:103–18.http://dx.doi.org/10.1023/B:JOPC.0000020077.73751.76 [DOI] [PubMed] [Google Scholar]
- 32. Avrameas S, Selmi C.. Natural autoantibodies in the physiology and pathophysiology of the immune system. J Autoimmun 2013;41:46–9.http://dx.doi.org/10.1016/j.jaut.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 33. Merbl Y, Zucker-Toledano M, Quintana FJ. et al. Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. J Clin Invest 2007; 117:712–8.http://dx.doi.org/10.1172/JCI29943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dooley H, Flajnik MF.. Shark immunity bites back: affinity maturation and memory response in the nurse shark, Ginglymostoma cirratum. Eur J Immunol 2005;35:936–45.http://dx.doi.org/10.1002/eji.200425760 [DOI] [PubMed] [Google Scholar]
- 35. Haury M, Sundblad A, Grandien A. et al. The repertoire of serum IgM in normal mice is largely independent of external antigenic contact. Eur J Immunol 1997;27:1557–63.http://dx.doi.org/10.1002/eji.1830270635 [DOI] [PubMed] [Google Scholar]
- 36. Bos NA, Kimura H, Meeuwsen CG. et al. Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur J Immunol 1989;19:2335–9.http://dx.doi.org/10.1002/eji.1830191223 [DOI] [PubMed] [Google Scholar]
- 37. Baumgarth N, Tung JW, Herzenberg LA.. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol 2005;26:347–62.http://dx.doi.org/10.1007/s00281-004-0182-2 [DOI] [PubMed] [Google Scholar]
- 38. Casali P, Schettino EW.. Structure and function of natural antibodies. Curr Top Microbiol Immunol 1996;210:167–79. [DOI] [PubMed] [Google Scholar]
- 39. Lutz HU, Binder CJ, Kaveri S.. Naturally occurring auto-antibodies in homeostasis and disease. Trends Immunol 2009;30:43–51.http://dx.doi.org/10.1016/j.it.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 40. Avrameas S. Autopolyreactivity confers a holistic role in the immune system. Scand J Immunol 2016;83:227–34.http://dx.doi.org/10.1111/sji.12414 [DOI] [PubMed] [Google Scholar]
- 41. Flajnik MF. Comparative analyses of immunoglobulin genes: surprises and portents. Nat Rev Immunol 2002;2:688–98.http://dx.doi.org/10.1038/nri889 [DOI] [PubMed] [Google Scholar]
- 42. Rumfelt LL, Diaz M, Lohr RL. et al. Unprecedented multiplicity of Ig transmembrane and secretory mRNA forms in the cartilaginous fish. J Immunol 2004;173:1129–39.http://dx.doi.org/10.4049/jimmunol.173.2.1129 [DOI] [PubMed] [Google Scholar]
- 43. Marchalonis JJ, Adelman MK, Schluter SF. et al. The antibody repertoire in evolution: chance, selection, and continuity. Dev Comp Immunol 2006;30:223–47.http://dx.doi.org/10.1016/j.dci.2005.06.011 [DOI] [PubMed] [Google Scholar]
- 44. Hoffman W, Lakkis FG, Chalasani G.. B cells, antibodies, and more. Clin J Am Soc Nephrol 2016;11:137–54.http://dx.doi.org/10.2215/CJN.09430915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Martin F, Kearney JF.. B1 cells: similarities and differences with other B cell subsets. Curr Opin Immunol 2001;13:195–201.http://dx.doi.org/10.1016/S0952-7915(00)00204-1 [DOI] [PubMed] [Google Scholar]
- 46. Berland R, Wortis HH.. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol 2002;20:253–300.http://dx.doi.org/10.1146/annurev.immunol.20.100301.064833 [DOI] [PubMed] [Google Scholar]
- 47. Choi YS, Baumgarth N.. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med 2008;205:3053–64.http://dx.doi.org/10.1084/jem.20080979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matejuk A, Beardall M, Xu Y. et al. Exclusion of natural autoantibody-producing B cells from IgG memory B cell compartment during T cell-dependent immune responses. J Immunol 2009;182:7634–43.http://dx.doi.org/10.4049/jimmunol.0801562 [DOI] [PubMed] [Google Scholar]
- 49. Pugh-Bernard AE, Silverman GJ, Cappione AJ. et al. Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J Clin Invest 2001;108:1061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Holodick NE, Repetny K, Zhong X. et al. Adult BM generates CD5+ B1 cells containing abundant N-region additions. Eur J Immunol 2009;39:2383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rothstein TL, Griffin DO, Holodick NE. et al. Human B-1 cells take the stage. Ann N Y Acad Sci 2013;1285:97–114.http://dx.doi.org/10.1111/nyas.12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lund FE, Randall TD.. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol 2010;10:236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Qian Y, Santiago C, Borrero M. et al. Lupus-specific antiribonucleoprotein B cell tolerance in nonautoimmune mice is maintained by differentiation to B-1 and governed by B cell receptor signaling thresholds . J Immunol 2001;166:2412–9.http://dx.doi.org/10.4049/jimmunol.166.4.2412 [DOI] [PubMed] [Google Scholar]
- 54. Hayakawa K, Asano M, Shinton SA. et al. Positive selection of natural autoreactive B cells. Science 1999;285:113–6.http://dx.doi.org/10.1126/science.285.5424.113 [DOI] [PubMed] [Google Scholar]
- 55. Hayakawa K, Asano M, Shinton SA. et al. Positive selection of anti-thy-1 autoreactive B-1 cells and natural serum autoantibody production independent from bone marrow B cell development. J Exp Med 2003;197:87–99.http://dx.doi.org/10.1084/jem.20021459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cohen IR. Biomarkers, self-antigens and the immunological homunculus. J Autoimmun 2007;29:246–9.http://dx.doi.org/10.1016/j.jaut.2007.07.016 [DOI] [PubMed] [Google Scholar]
- 57. Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol 2011;11:34–46.http://dx.doi.org/10.1038/nri2901 [DOI] [PubMed] [Google Scholar]
- 58. Notley CA, Brown MA, Wright GP. et al. Natural IgM is required for suppression of inflammatory arthritis by apoptotic cells. J Immunol 2011;186:4967–72.http://dx.doi.org/10.4049/jimmunol.1003021 [DOI] [PubMed] [Google Scholar]
- 59. Nagata S, Hanayama R, Kawane K.. Autoimmunity and the clearance of dead cells. Cell 2010;140:619–30.http://dx.doi.org/10.1016/j.cell.2010.02.014 [DOI] [PubMed] [Google Scholar]
- 60. Gronwall C, Akhter E, Oh C. et al. IgM autoantibodies to distinct apoptosis-associated antigens correlate with protection from cardiovascular events and renal disease in patients with SLE. Clin Immunol 2012;142:390–8.http://dx.doi.org/10.1016/j.clim.2012.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhou ZH, Wild T, Xiong Y. et al. Polyreactive antibodies plus complement enhance the phagocytosis of cells made apoptotic by UV-light or HIV. Sci Rep 2013;3:2271..http://dx.doi.org/10.1038/srep02271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chou MY, Fogelstrand L, Hartvigsen K. et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest 2009;119:1335–49.http://dx.doi.org/10.1172/JCI36800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hardy RR, Hayakawa K.. Development of B cells producing natural autoantibodies to thymocytes and senescent erythrocytes. Springer Semin Immunopathol 2005;26:363–75.http://dx.doi.org/10.1007/s00281-004-0183-1 [DOI] [PubMed] [Google Scholar]
- 64. Ochsenbein AF, Fehr T, Lutz C. et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science 1999;286:2156–9.http://dx.doi.org/10.1126/science.286.5447.2156 [DOI] [PubMed] [Google Scholar]
- 65. Litvack ML, Djiadeu P, Renganathan SD. et al. Natural IgM and innate immune collectin SP-D bind to late apoptotic cells and enhance their clearance by alveolar macrophages in vivo. Mol Immunol 2010;48:37–47.http://dx.doi.org/10.1016/j.molimm.2010.09.014 [DOI] [PubMed] [Google Scholar]
- 66. Boes M, Esau C, Fischer MB. et al. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol 1998;160:4776–87. [PubMed] [Google Scholar]
- 67. Tuominen A, Miller YI, Hansen LF. et al. A natural antibody to oxidized cardiolipin binds to oxidized low-density lipoprotein, apoptotic cells, and atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2006;26:2096–102.http://dx.doi.org/10.1161/01.ATV.0000233333.07991.4a [DOI] [PubMed] [Google Scholar]
- 68. Hosseini H, Li Y, Kanellakis P. et al. Phosphatidylserine liposomes mimic apoptotic cells to attenuate atherosclerosis by expanding polyreactive IgM producing B1a lymphocytes. Cardiovasc Res 2015;106:443–52.http://dx.doi.org/10.1093/cvr/cvv037 [DOI] [PubMed] [Google Scholar]
- 69. Lopez P, de Paz B, Rodriguez-Carrio J. et al. Th17 responses and natural IgM antibodies are related to gut microbiota composition in systemic lupus erythematosus patients. Sci Rep 2016;6:24072..http://dx.doi.org/10.1038/srep24072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lobo PI, Schlegal KH, Vengal J. et al. Naturally occurring IgM anti-leukocyte autoantibodies inhibit T-cell activation and chemotaxis. J Clin Immunol 2010;30:S31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chen Y, Khanna S, Goodyear CS. et al. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J Immunol 2009;183:1346–59.http://dx.doi.org/10.4049/jimmunol.0900948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Silverman GJ, Gronwall C, Vas J. et al. Natural autoantibodies to apoptotic cell membranes regulate fundamental innate immune functions and suppress inflammation. Discov Med 2009;8:151–6. [PubMed] [Google Scholar]
- 73. Fillatreau S, Sweenie CH, McGeachy MJ. et al. B cells regulate autoimmunity by provision of IL-10. Nat Immunol 2002;3:944–50.http://dx.doi.org/10.1038/ni833 [DOI] [PubMed] [Google Scholar]
- 74. Su J, Hua X, Concha H. et al. Natural antibodies against phosphorylcholine as potential protective factors in SLE. Rheumatology (Oxford) 2008;47:1144–50.http://dx.doi.org/10.1093/rheumatology/ken120 [DOI] [PubMed] [Google Scholar]
- 75. Cesena FH, Dimayuga PC, Yano J. et al. Immune-modulation by polyclonal IgM treatment reduces atherosclerosis in hypercholesterolemic apoE-/- mice. Atherosclerosis 2012;220:59–65.http://dx.doi.org/10.1016/j.atherosclerosis.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 76. Su J, Georgiades A, Wu R. et al. Antibodies of IgM subclass to phosphorylcholine and oxidized LDL are protective factors for atherosclerosis in patients with hypertension. Atherosclerosis 2006;188:160–6.http://dx.doi.org/10.1016/j.atherosclerosis.2005.10.017 [DOI] [PubMed] [Google Scholar]
- 77. Ehrenstein MR, Cook HT, Neuberger MS.. Deficiency in serum immunoglobulin (Ig)M predisposes to development of IgG autoantibodies. J Exp Med 2000;191:1253–8.http://dx.doi.org/10.1084/jem.191.7.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Boes M, Schmidt T, Linkemann K. et al. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci U S A 2000;97:1184–9.http://dx.doi.org/10.1073/pnas.97.3.1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hurez V, Kazatchkine MD, Vassilev T. et al. Pooled normal human polyspecific IgM contains neutralizing anti-idiotypes to IgG autoantibodies of autoimmune patients and protects from experimental autoimmune disease. Blood 1997;90:4004–13. [PubMed] [Google Scholar]
- 80. Herzog S, Jumaa H.. Self-recognition and clonal selection: autoreactivity drives the generation of B cells. Curr Opin Immunol 2012;24:166–72.http://dx.doi.org/10.1016/j.coi.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 81. Ubelhart R, Jumaa H.. Autoreactivity and the positive selection of B cells. Eur J Immunol 2015;45:2971–7.http://dx.doi.org/10.1002/eji.201444622 [DOI] [PubMed] [Google Scholar]
- 82. Klein J, Nikolaidis N.. The descent of the antibody-based immune system by gradual evolution. Proc Natl Acad Sci U S A 2005;102:169–74.http://dx.doi.org/10.1073/pnas.0408480102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Stewart J. Immunoglobulins did not arise in evolution to fight infection. Immunol Today 1992;13:396–9. discussion 399–400. [DOI] [PubMed] [Google Scholar]
- 84. Bouvet JP, Dighiero G.. From natural polyreactive autoantibodies to a la carte monoreactive antibodies to infectious agents: is it a small world after all? Infect Immun 1998;66:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Du Pasquier L. Innate immunity in early chordates and the appearance of adaptive immunity. C R Biol 2004;327:591–601.http://dx.doi.org/10.1016/j.crvi.2004.04.004 [DOI] [PubMed] [Google Scholar]
- 86. Herzenberg LA, Kantor AB, Herzenberg LA.. Layered evolution in the immune system. A model for the ontogeny and development of multiple lymphocyte lineages. Ann N Y Acad Sci 1992;651:1–9. [DOI] [PubMed] [Google Scholar]
- 87. Koonin EV, Krupovic M.. Evolution of adaptive immunity from transposable elements combined with innate immune systems. Nat Rev Genet 2014;16:184–92.http://dx.doi.org/10.1038/nrg3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lynch M. The frailty of adaptive hypotheses for the origins of organismal complexity. Proc Natl Acad Sci U S A 2007;104(Suppl 1):8597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fugmann SD. The origins of the Rag genes–from transposition to V(D)J recombination. Semin Immunol 2010;22:10–6.http://dx.doi.org/10.1016/j.smim.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Li J, Barreda DR, Zhang YA. et al. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol 2006;7:1116–24. [DOI] [PubMed] [Google Scholar]
- 91. Parra D, Rieger AM, Li J. et al. Pivotal advance: peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J Leukoc Biol 2012;91:525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mills CD. Anatomy of a discovery: m1 and m2 macrophages. Front Immunol 2015;6:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Akula S, Mohammadamin S, Hellman L, Nikolaidis N.. Fc receptors for immunoglobulins and their appearance during vertebrate evolution. PLoS One 2014;9:e96903.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Furlong RF, Holland PW.. Were vertebrates octoploid? Philos Trans R Soc Lond B Biol Sci 2002;357:531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Van de Peer Y, Maere S, Meyer A.. The evolutionary significance of ancient genome duplications. Nat Rev Genet 2009;10:725–32.http://dx.doi.org/10.1038/nrg2600 [DOI] [PubMed] [Google Scholar]
- 96. Kasahara M. The 2R hypothesis: an update. Curr Opin Immunol 2007;19:547–52.http://dx.doi.org/10.1016/j.coi.2007.07.009 [DOI] [PubMed] [Google Scholar]
- 97. Flajnik MF, Kasahara M.. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat Rev Genet 2010;11:47–59.http://dx.doi.org/10.1038/nrg2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lynch M, Force A.. The probability of duplicate gene preservation by subfunctionalization. Genetics 2000;154:459–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Force A, Lynch M, Pickett FB. et al. Preservation of duplicate genes by complementary, degenerative mutations. Genetics 1999;151:1531–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Stoltzfus A. On the possibility of constructive neutral evolution. J Mol Evol 1999;49:169–81.http://dx.doi.org/10.1007/PL00006540 [DOI] [PubMed] [Google Scholar]
- 101. Ouedraogo M, Bettembourg C, Bretaudeau A. et al. The duplicated genes database: identification and functional annotation of co-localised duplicated genes across genomes. PLoS One 2012;7:e50653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mabbott NA, Gray D.. Identification of co-expressed gene signatures in mouse B1, marginal zone and B2 B-cell populations. Immunology 2014;141:79–95.http://dx.doi.org/10.1111/imm.12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tsai S, Clemente-Casares X, Revelo XS. et al. Are obesity-related insulin resistance and type 2 diabetes autoimmune diseases? Diabetes 2015;64:1886–97. [DOI] [PubMed] [Google Scholar]
- 104. Kaur K, Zheng NY, Smith K. et al. High affinity antibodies against influenza characterize the plasmablast response in SLE patients after vaccination. PLoS One 2015;10:e0125618.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bach JF. Mechanisms of disease: The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002;347:911–20.http://dx.doi.org/10.1056/NEJMra020100 [DOI] [PubMed] [Google Scholar]
- 106. Greenwood BM. Autoimmune diseases in Nigerians. Lancet 1968;2:573..http://dx.doi.org/10.1016/S0140-6736(68)92439-2 [DOI] [PubMed] [Google Scholar]
- 107. Okada H, Kuhn C, Feillet H. et al. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol 2010;160:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Versini M, Jeandel PY, Bashi T. et al. Unraveling the Hygiene Hypothesis of helminthes and autoimmunity: origins, pathophysiology, and clinical applications. BMC Med 2015;13:81..http://dx.doi.org/10.1186/s12916-015-0306-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhong X, Gao W, Degauque N. et al. Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur J Immunol 2007;37:2400–4.http://dx.doi.org/10.1002/eji.200737296 [DOI] [PubMed] [Google Scholar]
- 110. Qian W, Liao BY, Chang AY. et al. Maintenance of duplicate genes and their functional redundancy by reduced expression. Trends Genet 2010;26:425–30.http://dx.doi.org/10.1016/j.tig.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gout JF, Lynch M.. Maintenance and loss of duplicated genes by dosage subfunctionalization. Mol Biol Evol 2015;32:2141–8.http://dx.doi.org/10.1093/molbev/msv095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lynch M, Bobay LM, Catania F. et al. The repatterning of eukaryotic genomes by random genetic drift. Annu Rev Genomics Hum Genet 2011;12:347–66.http://dx.doi.org/10.1146/annurev-genom-082410-101412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Graham AL, Hayward AD, Watt KA. et al. Fitness correlates of heritable variation in antibody responsiveness in a wild mammal. Science 2010;330:662–5.http://dx.doi.org/10.1126/science.1194878 [DOI] [PubMed] [Google Scholar]
- 114. Nussey DH, Watt KA, Clark A. et al. Multivariate immune defences and fitness in the wild: complex but ecologically important associations among plasma antibodies, health and survival. Proc Biol Sci 2014;281:20132931..http://dx.doi.org/10.1098/rspb.2013.2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Watson RL, McNeilly TN, Watt KA. et al. Cellular and humoral immunity in a wild mammal: Variation with age & sex and association with overwinter survival. Ecol Evol 2016;6:8695–705.http://dx.doi.org/10.1002/ece3.2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sun Y, Parmentier HK, Frankena K. et al. Natural antibody isotypes as predictors of survival in laying hens. Poult Sci 2011;90:2263–74.http://dx.doi.org/10.3382/ps.2011-01613 [DOI] [PubMed] [Google Scholar]
- 117. Star L, Frankena K, Kemp B. et al. Natural humoral immune competence and survival in layers. Poult Sci 2007;86:1090–9. [DOI] [PubMed] [Google Scholar]
- 118. Albertus DL, Seder CW, Chen G. et al. AZGP1 autoantibody predicts survival and histone deacetylase inhibitors increase expression in lung adenocarcinoma. J Thorac Oncol 2008;3:1236–44.http://dx.doi.org/10.1097/JTO.0b013e318189f5ec [DOI] [PubMed] [Google Scholar]
- 119. Binder CJ, Shaw PX, Chang MK. et al. The role of natural antibodies in atherogenesis. J Lipid Res 2005;46:1353–63. [DOI] [PubMed] [Google Scholar]
- 120. Zinkernagel RM, Doherty PC.. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature 1974;251:547–8.http://dx.doi.org/10.1038/251547a0 [DOI] [PubMed] [Google Scholar]
- 121. Ostrov DA, Grant BJ, Pompeu YA. et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc Natl Acad Sci U S A 2012;109:9959–64.http://dx.doi.org/10.1073/pnas.1207934109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ji Q, Luo ZX, Yuan CX. et al. The earliest known eutherian mammal. Nature 2002;416:816–22.http://dx.doi.org/10.1038/416816a [DOI] [PubMed] [Google Scholar]
- 123. Brawand D, Wahli W, Kaessmann H.. Loss of egg yolk genes in mammals and the origin of lactation and placentation. PLoS Biol 2008;6:e63.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Trowsdale J, Betz AG.. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol 2006;7:241–6. [DOI] [PubMed] [Google Scholar]
- 125. Zoller AL, Schnell FJ, Kersh GJ.. Murine pregnancy leads to reduced proliferation of maternal thymocytes and decreased thymic emigration. Immunology 2007;121:207–15.http://dx.doi.org/10.1111/j.1365-2567.2006.02559.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kraus TA, Engel SM, Sperling RS. et al. Characterizing the pregnancy immune phenotype: results of the viral immunity and pregnancy (VIP) study. J Clin Immunol 2012;32:300–11.http://dx.doi.org/10.1007/s10875-011-9627-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Birkeland SA, Kristoffersen K.. Lymphocyte transformation with mitogens and antigens during normal human pregnancy: a longitudinal study. Scand J Immunol 1980;11:321–5.http://dx.doi.org/10.1111/j.1365-3083.1980.tb00240.x [DOI] [PubMed] [Google Scholar]
- 128. Lima J, Martins C, Leandro MJ. et al. Characterization of B cells in healthy pregnant women from late pregnancy to post-partum: a prospective observational study. BMC Pregnancy Childbirth 2016;16:139..http://dx.doi.org/10.1186/s12884-016-0927-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Medina KL, Smithson G, Kincade PW.. Suppression of B lymphopoiesis during normal pregnancy. J Exp Med 1993;178:1507–15.http://dx.doi.org/10.1084/jem.178.5.1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Muzzio DO, Soldati R, Ehrhardt J. et al. B cell development undergoes profound modifications and adaptations during pregnancy in mice. Biol Reprod 2014;91:1–11. [DOI] [PubMed] [Google Scholar]
- 131. Gleicher N. Autoantibodies in normal and abnormal pregnancy. Am J Reprod Immunol 1992;28:269–73.http://dx.doi.org/10.1111/j.1600-0897.1992.tb00812.x [DOI] [PubMed] [Google Scholar]
- 132. Markle JG, Fish EN.. SeXX matters in immunity. Trends Immunol 2014;35:97–104.http://dx.doi.org/10.1016/j.it.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 133. de Man YA, Dolhain RJ, van de Geijn FE. et al. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum 2008;59:1241–8.http://dx.doi.org/10.1002/art.24003 [DOI] [PubMed] [Google Scholar]
- 134. Barrett JH, Brennan P, Fiddler M. et al. Does rheumatoid arthritis remit during pregnancy and relapse postpartum? Results from a nationwide study in the United Kingdom performed prospectively from late pregnancy. Arthritis Rheum 1999;42:1219–27.http://dx.doi.org/10.1002/1529-0131(199906)42:6<1219::AID-ANR19>3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- 135. Ostensen M, Villiger PM.. The remission of rheumatoid arthritis during pregnancy. Semin Immunopathol 2007;29:185–91.http://dx.doi.org/10.1007/s00281-007-0072-5 [DOI] [PubMed] [Google Scholar]
- 136. Confavreux C, Hutchinson M, Hours MM. et al. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med 1998;339:285–91. [DOI] [PubMed] [Google Scholar]
- 137. Gellin BG, Broome CV, Bibb WF. et al. The epidemiology of listeriosis in the United States–1986. Listeriosis Study Group. Am J Epidemiol 1991;133:392–401. [DOI] [PubMed] [Google Scholar]
- 138. Siston AM, Rasmussen SA, Honein MA. et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. Jama 2010;303:1517–25.http://dx.doi.org/10.1001/jama.2010.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Robinson DP, Klein SL.. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav 2012;62:263–71.http://dx.doi.org/10.1016/j.yhbeh.2012.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Letur H, Peigne M, Ohl J. et al. Hypertensive pathologies and egg donation pregnancies: Results of a large comparative cohort study. Fertil Steril 2016;106:284–90.http://dx.doi.org/10.1016/j.fertnstert.2016.03.031 [DOI] [PubMed] [Google Scholar]
- 141. Muzzio DO, Soldati R, Rolle L. et al. B-1a B cells regulate T cell differentiation associated with pregnancy disturbances. Front Immunol 2014;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Carp HJ, Selmi C, Shoenfeld Y.. The autoimmune bases of infertility and pregnancy loss. J Autoimmun 2012;38:J266–74. [DOI] [PubMed] [Google Scholar]
- 143. Capece A, Vasieva O, Meher S. et al. Pathway analysis of genetic factors associated with spontaneous preterm birth and pre-labor preterm rupture of membranes. PLoS One 2014;9:e108578.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Korevaar TI, Schalekamp-Timmermans S, de Rijke YB. et al. Hypothyroxinemia and TPO-antibody positivity are risk factors for premature delivery: the generation R study. J Clin Endocrinol Metab 2013;98:4382–90. [DOI] [PubMed] [Google Scholar]
- 145. Kajino T. Polyclonal activation of IgM antibodies to phospholipids in patients with idiopathic fetal growth retardation. Am J Reprod Immunol 1991;25:28–34.http://dx.doi.org/10.1111/j.1600-0897.1991.tb01060.x [DOI] [PubMed] [Google Scholar]
- 146. Zhang L, Chang KK, Li MQ. et al. Mouse endometrial stromal cells and progesterone inhibit the activation and regulate the differentiation and antibody secretion of mouse B cells. Int J Clin Exp Pathol 2014;7:123–33. [PMC free article] [PubMed] [Google Scholar]
- 147. Manuck TA, Esplin MS, Biggio J. et al. Predictors of response to 17-alpha hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth. Am J Obstet Gynecol 2016;214:376 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Amaral LM, Cornelius DC, Harmon A. et al. 17-hydroxyprogesterone caproate significantly improves clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension 2015;65:225–31.http://dx.doi.org/10.1161/HYPERTENSIONAHA.114.04484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Carpentier PA, Stanford JB, Boyle PC.. Progesterone in Women with Recurrent Miscarriages. N Engl J Med 2016;374:894..http://dx.doi.org/10.1056/NEJMc1600491 [DOI] [PubMed] [Google Scholar]
- 150. Coomarasamy A, Williams H, Truchanowicz E. et al. A randomized trial of progesterone in women with recurrent miscarriages. N Engl J Med 2015;373:2141–8.http://dx.doi.org/10.1056/NEJMoa1504927 [DOI] [PubMed] [Google Scholar]
- 151. Mackay IR, Rosen FS, Kazatchkine MD, Kaveri SV.. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med 2001;345:747–55. [DOI] [PubMed] [Google Scholar]
- 152. Simon HU, Spath PJ.. IVIG–mechanisms of action. Allergy 2003;58:543–52.http://dx.doi.org/10.1034/j.1398-9995.2003.00239.x [DOI] [PubMed] [Google Scholar]
- 153. Kotlan B, Padanyi A, Batorfi J. et al. Alloimmune and autoimmune background in recurrent pregnancy loss - successful immunotherapy by intravenous immunoglobulin. Am J Reprod Immunol 2006;55:331–40.http://dx.doi.org/10.1111/j.1600-0897.2006.00368.x [DOI] [PubMed] [Google Scholar]
- 154. Bronsart LL, Contag CH.. A role of the adaptive immune system in glucose homeostasis. BMJ Open Diabetes Res Care 2016;4:e000136.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Tournefier A, Laurens V, Chapusot C. et al. Structure of MHC class I and class II cDNAs and possible immunodeficiency linked to class II expression in the Mexican axolotl. Immunol Rev 1998;166:259–77.http://dx.doi.org/10.1111/j.1600-065X.1998.tb01268.x [DOI] [PubMed] [Google Scholar]
- 156. Haghighi HR, Gong J, Gyles CL. et al. Probiotics stimulate production of natural antibodies in chickens. Clin Vaccine Immunol 2006;13:975–80.http://dx.doi.org/10.1128/CVI.00161-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Weinstock JV. Autoimmunity: The worm returns. Nature 2012;491:183–5http://dx.doi.org/10.1038/491183a [DOI] [PMC free article] [PubMed] [Google Scholar]


