Abstract
Staphylococcus aureus requires branched-chain amino acids (BCAAs; isoleucine, leucine, valine) for protein synthesis, branched-chain fatty acid synthesis, and environmental adaptation by responding to their availability via the global transcriptional regulator CodY. The importance of BCAAs for S. aureus physiology necessitates that it either synthesize them or scavenge them from the environment. Indeed S. aureus uses specialized transporters to scavenge BCAAs, however, its ability to synthesize them has remained conflicted by reports that it is auxotrophic for leucine and valine despite carrying an intact BCAA biosynthetic operon. In revisiting these findings, we have observed that S. aureus can engage in leucine and valine synthesis, but the level of BCAA synthesis is dependent on the BCAA it is deprived of, leading us to hypothesize that each BCAA differentially regulates the biosynthetic operon. Here we show that two mechanisms of transcriptional repression regulate the level of endogenous BCAA biosynthesis in response to specific BCAA availability. We identify a trans-acting mechanism involving isoleucine-dependent repression by the global transcriptional regulator CodY and a cis-acting leucine-responsive attenuator, uncovering how S. aureus regulates endogenous biosynthesis in response to exogenous BCAA availability. Moreover, given that isoleucine can dominate CodY-dependent regulation of BCAA biosynthesis, and that CodY is a global regulator of metabolism and virulence in S. aureus, we extend the importance of isoleucine availability for CodY-dependent regulation of other metabolic and virulence genes. These data resolve the previous conflicting observations regarding BCAA biosynthesis, and reveal the environmental signals that not only induce BCAA biosynthesis, but that could also have broader consequences on S. aureus environmental adaptation and virulence via CodY.
Author summary
To infect its human host, the bacterial pathogen Staphylococcus aureus must either take up nutrients from the surrounding environment or produce them itself. Previous studies have reported that S. aureus does not produce the amino acids leucine and valine, despite it possessing the genes to do so. In this study, we show that S. aureus does indeed produce leucine and valine, but only under certain nutritional conditions. We select for mutants of S. aureus able to grow in valine-depleted environments to uncover genetic variants that enable valine production. We discover genetic variants in a repressor protein and a region of non-coding DNA that both, when properly functioning, inhibit production of leucine and valine under nutrient-rich conditions. We further identify the nutritional conditions where the inhibition of leucine and valine production is relieved, thus revealing a previously overlooked role for another amino acid, isoleucine, in influencing nutrient metabolism. We show that isoleucine levels also influence expression of genes involved in the ability of S. aureus to cause disease. These findings help to reconcile conflicting reports regarding leucine and valine production in S. aureus and reveal nutritional cues that could influence its ability to cause infection.
Introduction
Staphylococcus aureus is a serious human pathogen capable of causing infections that range from mild skin and soft tissue infections, to severe infections of the bone, muscle, heart and lung [1–4]. To survive and thrive in such diverse host environments, S. aureus must maintain sufficient levels of metabolites and co-factors to support virulence determinant production and replication [5,6]. The branched-chain amino acids (BCAAs; Ile, Leu, Val) represent an important group of nutrients for S. aureus metabolism and virulence, as they are required for synthesis of proteins and membrane branched-chain fatty acids (BCFAs), which are important for S. aureus membrane homeostasis and environmental adaptation. In addition to their nutritional importance, the BCAAs are key regulatory molecules in low GC-content Gram-positive bacteria, as they are activators of the global transcriptional regulator CodY. CodY coordinates expression of nutrient scavenging and synthesis systems, as well as virulence genes, upon depletion of both BCAAs and GTP [7–13]. The requirement of BCAAs for both S. aureus replication and niche adaptation necessitates that it either synthesize these nutrients or acquire them from the environment. Indeed, both BCAA biosynthesis [14–18] and transport [19–22] have been linked to promoting the virulence of other important pathogens in host environments.
Bacteria acquire BCAAs via dedicated active transporters, including BrnQ (Gram-negative and–positive bacteria), BcaP (Gram-positive bacteria), and the high affinity ATP-Binding Cassette (ABC) transporter LIV-I (Gram-negative bacteria) [23–36]. S. aureus encodes three BrnQ homologs (BrnQ1, BrnQ2, BrnQ3), and BcaP. BrnQ1 and BcaP transport all three BCAAs, with BrnQ1 playing a predominant role, and BrnQ2 is an Ile-dedicated transporter [24,25]. No appreciable BCAA transport function is associated with BrnQ3 [24]. Despite encoding the BCAA biosynthetic operon, S. aureus relies on the acquisition of BCAAs, most importantly Leu and Val, for rapid growth in media with excess or limiting concentrations of BCAAs, indicating that BCAA biosynthesis is typically repressed [24,25]. Paradoxically, biosynthesis remains repressed even in the absence of an exogenous source of Leu or Val, with growth of S. aureus observed only after a prolonged period, likely explaining why previous studies have been misled to conclude that S. aureus is auxotrophic for Leu and Val [37,38]. The molecular explanation for this phenotype in S. aureus has remained elusive.
Both Gram-positive and Gram-negative bacteria repress BCAA biosynthesis when intracellular levels are sufficient to support growth. In the Gram-negative bacteria Escherichia coli and Salmonella enterica sv. Typhimurium, this is regulated by transcriptional attenuation, which couples translation of a BCAA-rich peptide upstream of the biosynthetic genes with transcriptional termination, such that high levels of BCAAs prevent transcription of the biosynthetic genes [39–44]. In Gram-positive bacteria, including Bacillus subtilis, Listeria monocytogenes, and S. aureus, CodY represses transcription of the biosynthetic genes by binding to a CodY box and inhibiting binding of RNA polymerase [8,9,45–49]. Additional levels of regulation of the ilv-leu operon in the Gram-positive bacterium B. subtilis include activation by CcpA in response to glucose and repression by TnrA in response to nitrogen levels [50]. Additional fine-turning of the operon in this species is mediated by a Leu-responsive T-box riboswitch [50–53], as well as mRNA processing [54].
The BCAA biosynthetic genes in S. aureus, encoded by the ilvDBNCleuABCDilvA operon (ilv-leu), and ilvE are similarly repressed by CodY; this regulator binds to two regions upstream of ilvD proximal to the transcriptional start site and two regions within the operon, proximal to ilvC and leuC (Fig 1) [7–9]. Repression is also mediated by the essential genes gcp and yeaZ through an unknown mechanism [55,56]. Given that CodY transcriptional repression should be alleviated in the absence of BCAAs, it is unclear why in the case of Leu and Val specifically, growth remains inhibited when either of these two amino acids is absent from the growth medium. We therefore investigated the mechanisms governing these phenotypes in S. aureus to resolve this paradox and to identify the signals required to induce synthesis. Here, we unravel the complex regulation of BCAA biosynthesis in S. aureus, by demonstrating that control is mediated by both trans and cis acting mechanisms of repression. We identify the metabolic cues regulating each mechanism, therefore revealing how S. aureus controls its preference for exogenous BCAAs, and the conditions under which endogenous synthesis is induced. In doing so, we uncover an unappreciated role for Ile in CodY-dependent regulation, demonstrating that it is this BCAA that plays a dominant role in controlling the expression of genes involved in BCAA synthesis and transport. Moreover, since we show that Ile can dominate CodY-dependent gene expression, we highlight an important role for Ile limitation in virulence gene expression, where the absence of this BCAA can induce expression of nuclease, a known CodY-dependent virulence factor.
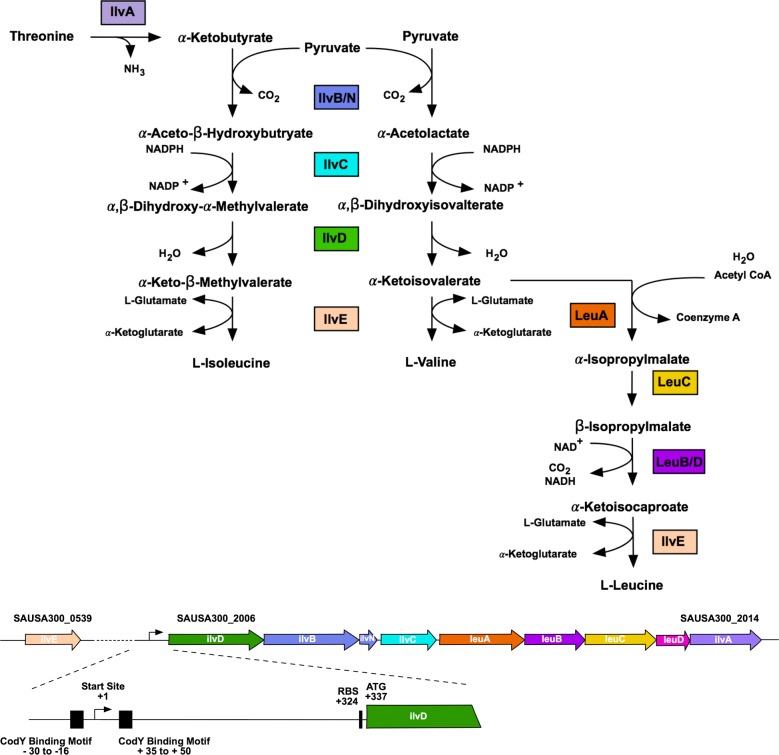
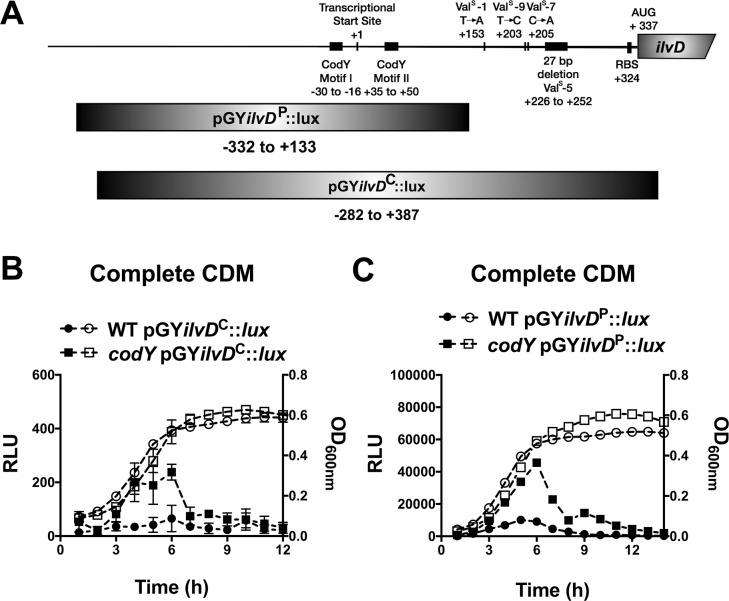
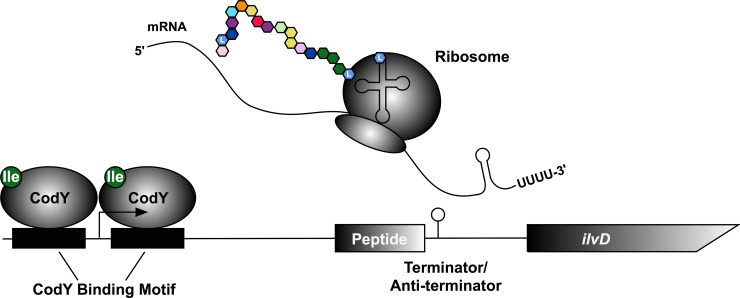
Fig 1. Organization of the ilv-leu operon in S. aureus.
Top diagram is a schematic of the BCAA biosynthetic pathway in S. aureus. Bottom diagram depicts the genomic context of the BCAA biosynthetic genes in the USA300 FPR3757 genome. Regulatory features and their coordinates relative to the transcription start site are depicted, including the canonical CodY binding motifs [57,97] and the ribosome binding site (RBS).
Results
Growth of S. aureus in response to BCAA deprivation
S. aureus has previously been reported as auxotrophic for Leu and Val [37,38], despite possessing a complete BCAA biosynthetic operon. In contrast to these reports, we have observed that S. aureus is indeed able to grow in the absence of Leu and Val following an extended growth period [24], which might in part explain the discrepancy in these observations. Curiously, when investigating the kinetics of S. aureus growth in response to deprivation of each individual BCAA, we found differing growth phenotypes, even though all enzymes required for the synthesis of each of the BCAAs are encoded from the same biosynthetic loci. For example, when grown in chemically-defined media (CDM) lacking Leu, S. aureus exhibited a growth lag of 6–8 h, and when grown in CDM lacking Val S. aureus exhibited a growth lag of ~ 20 h, relative to its growth in complete CDM (Fig 2A). In contrast, growth of S. aureus in CDM lacking Ile was comparable to growth in complete CDM (Fig 2A). These observations were particularly perplexing given the known mechanism, via CodY, regulating BCAA biosynthesis. CodY represses the BCAA biosynthetic operon, such that inactivation of codY results in growth of S. aureus in media lacking either Ile, Leu, or Val (Fig 2B). Given that all three BCAAs have been reported to individually activate CodY DNA binding activity in vitro [13,57,58], it was surprising to observe the differences in growth upon omission of the individual BCAAs from the growth medium, and how different it was from that of WT S. aureus and a codY mutant (compare panels A and B in Fig 2). We therefore hypothesized that the individual BCAAs differentially regulate CodY activity during growth, and that at least one additional mechanism regulates BCAA biosynthesis.
Fig 2. Growth of S. aureus upon BCAA depletion.
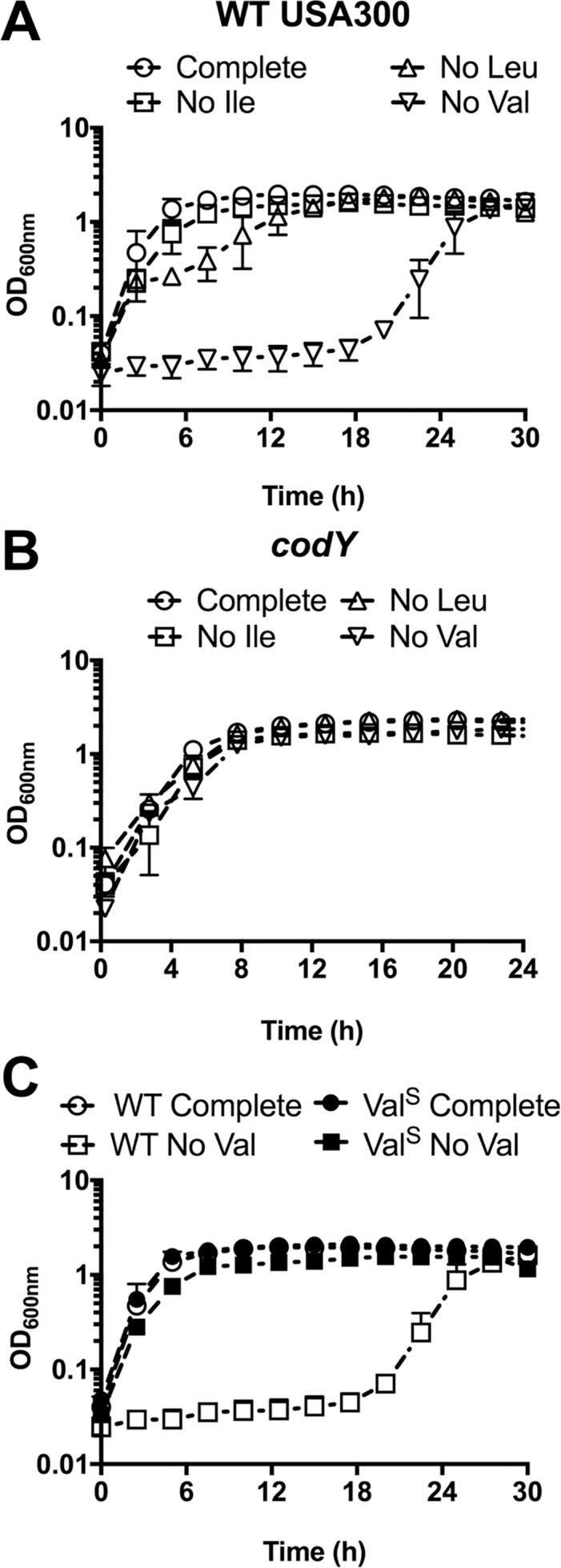
A) WT USA300 was pre-grown in complete CDM to mid-exponential phase and then sub-cultured into either complete CDM or CDM with BCAAs omitted, as indicated. B) USA300 with a transposon insertion in codY (codY::ϕNΣ) was pre-grown in complete CDM to mid-exponential phase, and then sub-cultured into either complete CDM or CDM with BCAAs omitted, as indicated. C) Cells recovered from the CDM with no Val in panel A were plated. A single colony was selected (ValSup (abbreviated ValS) —filled symbols) and subjected to growth in complete CDM and CDM with no Val. Growth was compared to the parental WT strain (open symbols) in the same conditions. Data are the mean +/- SD of three biological replicates.
To uncover the molecular mechanisms governing these phenotypes, we first questioned whether the absence of Leu or Val selects for mutations that enable growth in the absence of these BCAAs. To address this question, we recovered cells that had grown up following the growth lag in media lacking Leu (CDM-Leu) or Val (CDM-Val), and then sub-cultured these isolates back into the same medium from which they were recovered. Cells recovered from CDM-Leu medium exhibited the same growth delay upon sub-culture into the same medium, indicating that this condition does not select for mutations. Conversely, cells recovered from CDM-Val medium grew readily when re-inoculated into CDM-Val, suggesting that they were synthesizing Val (-Val suppressors, referred to as ValSup) (Fig 2C). These results suggest that growth in the absence of exogenous Leu requires a regulatory adaptation, whereas growth in the absence of Val selects for a heritable mutation. We hypothesized that identification of the genetic mutations permitting growth of S. aureus in the absence of exogenous Val would reveal important regulators of the BCAA biosynthetic operon and, in-turn, would help reveal the mechanisms behind the BCAA-specific growth phenotypes.
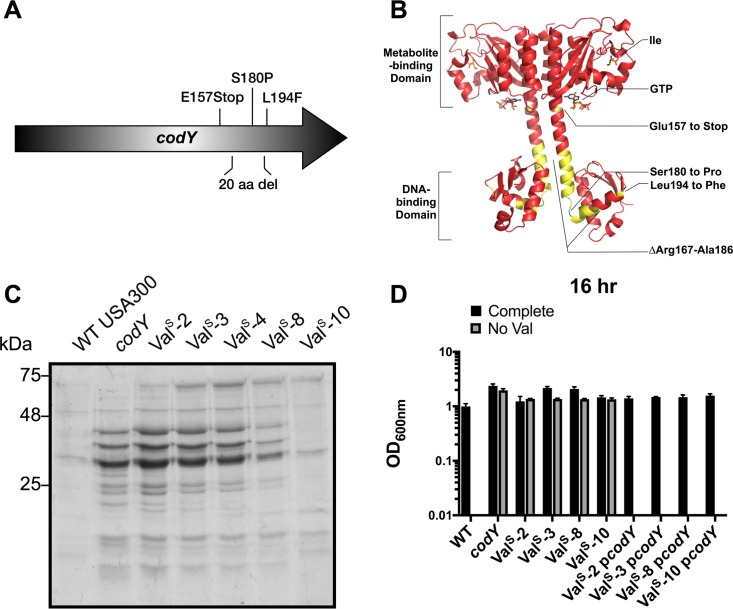
Growth in media lacking Val selects for mutations in codY
Since a codY mutant synthesizes BCAAs and is, thus, capable of growth in the absence of BCAAs, we reasoned that the absence of exogenous Val may select for mutations in codY. We again grew cells in the absence of Val, isolated mutants from twelve independent cultures (ValSup mutants) and amplified the codY gene by PCR. Five out of the twelve mutants contained mutations in codY; one had a point mutation resulting in a premature stop codon, two had a 60-bp deletion, and two had independent point mutations resulting in nonsynonymous mutations (Table 1) and (Fig 3A). We then mapped the mutations to identify their position within the CodY protein structure (PDB ID:5EY0) [59]. All mutations occurred in the linker region between the metabolite sensing domain and the DNA-binding domain (Fig 3B). We used secreted protein profiles as a read-out of CodY function, since CodY represses many secreted proteins [60] and therefore the secreted protein profile of a codY mutant differs substantially from WT. The secreted protein profiles of the ValSup mutants with confirmed mutations in codY resembled the protein profile of the codY mutant, except for ValSup-10 mutant (Fig 3C), indicating that all but one of our codY mutations result in an inactive CodY protein, at least insofar as its ability to repress synthesis of secreted proteins. The growth phenotype for each of the unique ValSup strains with confirmed mutations in codY could be reverted to WT-like growth in CDM-Val through complementation with an intact copy of the codY gene in trans (Fig 3D). These results confirm that codY inhibits Val synthesis, and that all of the ValSup codY mutants, along with the codY insertion mutant (codY::ϕNΣ), alleviate this inhibition.
Table 1. Mutations identified in CodY.
| Mutants | Positiona | Genetic Mutation | Protein Mutation |
|---|---|---|---|
| ValSup-2 | 1260149–1260208 | 60 bp deletion | ΔArg167-Ala186 |
| ValSup-3 | 1260119 | G to T | Glu157 to Stop |
| ValSup-4 | 1260149–1260208 | 60 bp deletion | ΔArg167-Ala186 |
| ValSup-8 | 1260188 | T to C | Ser180 to Pro |
| ValSup-10 | 1260230 | C to T | Leu194 to Phe |
aPosition in the USA300 FPR3757 genome (NC_007793.1)
Fig 3. The absence of exogenous valine selects for mutations that inactivate CodY.
A) Schematic representation of the mutations identified in CodY. B) Mutations identified are indicated on the CodY structure (PDB ID:5EY0) in yellow, except for the Ser180 to Pro mutation, which is indicated in green. CodY ligands, Ile and GTP, are coloured based on atomic composition. C) Strains were pre-grown in TSB to mid-exponential phase, then sub-cultured into TSB for 16 hr. Supernatants were collected and proteins were precipitated using TCA. Protein samples were normalized to the equivalent of 5 ODs and run on a 12% SDS-PAGE gel. D) Strains with unique mutations in codY (ValSup-2 carries an identical mutation to ValSup-4) were pre-grown in complete CDM to mid-exponential phase, then sub-cultured into either complete CDM or CDM with Val omitted. OD600nm was read after 16 hr of growth. USA300 with a transposon insertion in codY (codY::ϕNΣ) was used for comparison. ValSup is abbreviated to ValS. Data are the mean +/- SD of three biological replicates.
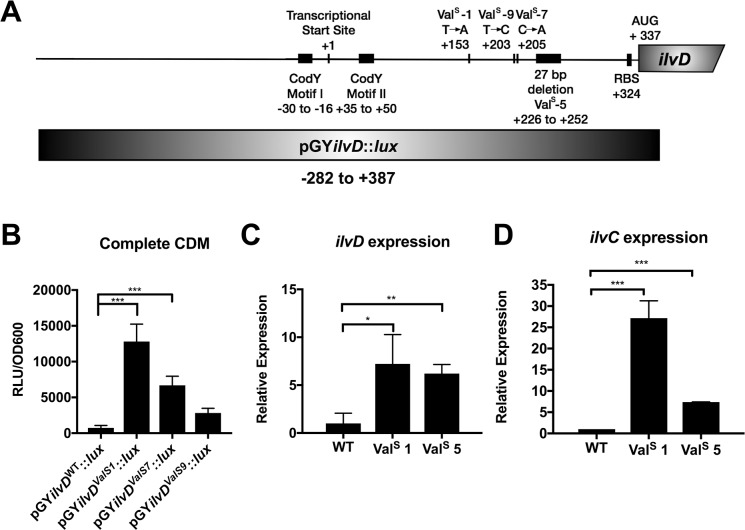
Growth in media lacking Val selects for mutations in the 5’UTR of ilvD
The remaining seven ValSup mutants that did not have mutations in the codY gene may have acquired other mutations that indirectly affect the ability of CodY to regulate the ilv-leu operon (e.g. mutations in GTP synthesis). To address this possibility, we assessed the secreted protein profiles of these mutants and all seven were found to exhibit profiles comparable to the WT strain (S1 Fig). These results suggest the mutations occurring within these ValSup strains affect a CodY-independent mechanism of BCAA synthesis regulation. We therefore performed whole genome sequencing to identify the nature of these mutations. This revealed that all seven of these ValSup strains had mutations in the 5’ untranslated region (UTR) upstream of ilvD, with a total of three unique point mutations and one 27-bp deletion (Table 2). The mutations did not overlap with the known promoter features upstream of the ilvD gene (i.e. the CodY binding motifs) (Fig 4A). To confirm that mutations in the 5’UTR of ilvD result in an increase in expression of the ilv-leu operon, which would yield the phenotype of growth in CDM-Val without delay (Fig 2, panel C), we generated a luminescence reporter of the ilvD promoter by cloning the 5’UTR of ilvD into the pGY::lux vector (Fig 4A). Within this reporter construct, we then mutated the 5’UTR to contain the three point mutations identified from genome sequencing of the mutants (ValSup-1, ValSup-7, and ValSup-9). Two of the mutant sequences (ValSup-1 and ValSup-7) resulted in a statistically significant increase in ilvD promoter activity, and the third mutant sequence (ValSup-9) resulted in a trend towards increased promoter activity, although not significant (Fig 4B). Furthermore, using qPCR, we observed that levels of ilvD and ilvC transcripts were elevated when we examined two of the mutants compared to the WT strain (Fig 4C and 4D). Together, these data suggest that the mutations in the 5’UTR of ilvD relieve repression of the ilv-leu operon.
Table 2. Mutations identified in the 5’ UTR of ilvD.
| Mutants | Positiona | Position relative to ilvD transcriptional start site | Mutation |
|---|---|---|---|
| ValSup-1 | 2164689 | +153 | T to A |
| ValSup-5 | 2164762–2164788 | +226 to +252 | 27 bp deletion |
| ValSup-6 | 2164762–2164788 | +226 to +252 | 27 bp deletion |
| ValSup-7 | 2164741 | +205 | C to A |
| ValSup-9 | 2164739 | +203 | T to C |
| ValSup-11 | 2164762–2164788 | +226 to +252 | 27 bp deletion |
| ValSup-12 | 2164739 | +203 | T to C |
aPosition in the USA300 FPR3757 genome (NC_007793.1)
Fig 4. Mutations in the ilvD promoter result in an increase in promoter activity and ilv-leu operon expression.
A) Position map of the mutations identified in the ilvD promoter relative to the transcriptional start site, the canonical CodY binding motifs, and the region used for cloning in the promoter:reporter construct. B) WT USA300 with the pGY::lux plasmid containing either the WT ilvD promoter, or the mutant ilvD promoter was grown in complete CDM to mid-exponential phase and then sub-cultured into complete CDM. Luminescence was read at mid-exponential phase and normalized to the OD600nm. Data are the mean +/- SD of three biological replicates. Data were analyzed by one-way ANOVA with Dunnet’s multiple comparison test. *** P < 0.001. C, D) Strains were grown in complete CDM to mid-exponential phase and then sub-cultured into complete CDM. Cells were harvested at mid-exponential phase and RNA was isolated. Expression of ilvD and ilvC was normalized to expression of rpoB. ValSup is abbreviated to ValS. Data are the mean +/- SD of three biological replicates. Data were analyzed by an Student’s unpaired t-test. *** P < 0.001, ** P < 0.01, * P < 0.05.
Identification of a putative attenuator and terminator
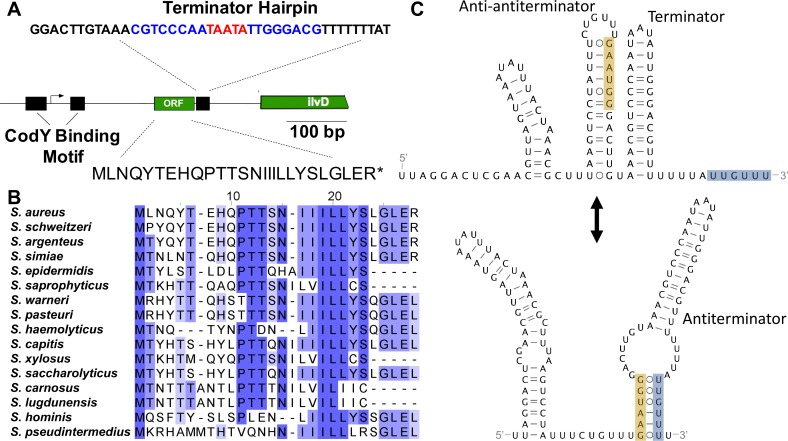
We next investigated whether the 5’UTR of ilvD contained a cis-regulatory element, initially considering a T-box riboswitch, since the ilv-leu operon in B. subtilis is regulated by a tRNALeu-responsive T-box riboswitch [51,53]. Predictive structure analysis and sequence comparison of the ilvD 5’UTR to known T-box riboswitch sequences revealed that although the S. aureus ilvD 5’UTR contains some features that loosely resemble T-box riboswitches (S2 Fig), it lacks the conserved Stem 1 motifs and structures essential to tRNA anchoring and decoding [61]. We next considered translation-dependent transcriptional regulation (i.e. attenuation), since BCAA-rich leader peptides have been found to regulate BCAA biosynthetic genes in E. coli [42] and S. typhimurium [43,44], and are predicted to regulate BCAA synthesis in Lactococcus lactis sp. lactis [62], Corynebacterium glutamicum [63,64], and Streptococcus spp. [65]. A search for open reading frames (ORFs) in the ilvD leader sequence revealed a short coding region that would be predicted to encode a 26-aa peptide. The predicted peptide contains a string of three Ile codons followed by two Leu codons, and an additional three interspersed Leu codons (Fig 5A). A putative ribosome binding site was also identified 9 nucleotides (nts) upstream of the start codon and a putative terminator hairpin structure is located 52 nts downstream from the peptide stop codon, consistent with transcription termination (S3 Fig). We found that the Ile and Leu codons in the peptide were highly conserved across the staphylococci (Fig 5B), as was the predicted terminator stem loop structure (Fig 5C and S3 Fig), suggesting that these features are biologically relevant. Secondary structure predictions revealed an alternative mRNA structure could also form that sequesters the terminator poly-U within an antiterminator (Fig 5C). In the antiterminator fold, the terminator stem-loop is intact, but two upstream stem-loops refold into a new, long stem-loop shifted further upstream. This rearrangement frees a 5’-GAAUGG-3’ motif to pair with 5’-UUGUUU-3’ in the terminator poly-U tail (Fig 5C). Ribosome pausing within these regions could foreseeably disrupt folding to favor antiterminator formation and drive transcription of the ilv-leu operon.
Fig 5. Attenuator features upstream of ilvD.
A) The position and sequence of the terminator hairpin is shown for the WT USA300 strain and the ValSup-5 mutant strain. The terminator sequence is in bold, with the stem highlighted in blue and the loop highlighted in red, followed by a poly-U tail. The putative open reading frame (ORF) and the corresponding translated attenuator peptide is shown for the WT USA300 strain and the ValSup-1 mutant. The positions of the features are indicated relative to the transcriptional start site identified in Majerczyk et al., 2010. B) Multiple sequence alignments of the homologous peptide found in the ilvD leader from other staphylococcal species. C) Alternate RNA secondary structures of a terminator and antiterminator predicted in the ilvD leader using the Mfold webserver. The alternatively paired segments in the antiterminator stem are highlighted in orange and gray. Base pairing is shown using Leontis-Westhof notation. ValSup is abbreviated to ValS.
When we considered how the mutations that were selected for in media lacking Val might disrupt these features, we found that the 27-bp deletion in ValSup-5/6/11 deletes the predicted terminator stem-loop and the T to A mutation in ValSup-1 changes a Leu codon in the leader peptide to a stop codon (Fig 5A). These mutations would therefore be predicted to relieve repression of transcription, supporting that these are biologically relevant features. The T to C mutation in ValSup-9/12 and the C to A mutation in ValSup-7 occur in predicted secondary structural elements that stabilize terminator formation, and could also relieve repression. We therefore hypothesize that expression of the BCAA biosynthesis operon is regulated by Leu-dependent attenuation in S. aureus. We predict that, in conditions of high Leu availability, translation of the attenuator peptide promotes formation of the terminator hairpin and subsequently transcriptional termination. In conditions of low Leu availability, the ribosome stalls during translation of the attenuator peptide, promoting formation of the antiterminator hairpin and leading to transcriptional read-through. This region, upstream of ilvD, is henceforth referred to as the attenuator sequence and not the 5’UTR.
Ile and Leu regulate the ilv operon via trans- and cis-acting mechanisms, respectively
The in vitro selection experiment revealed two mechanisms involved in repression of the ilv-leu operon, and yet the growth data (see Fig 2A) suggest that the operon is fully repressed only under conditions of Val deprivation and not Ile or Leu deprivation. We therefore continued to investigate how these mechanisms respond to deprivation of the individual BCAAs to explain these unique growth phenotypes. To test our hypothesis that Leu availability regulates expression of the ilv-leu operon via attenuation, we used our luminescence reporter construct containing the attenuator sequence cloned into the pGY::lux vector to examine how it responds to Leu deprivation. We were also interested to investigate how BCAA availability regulates CodY regulation of the ilv-leu operon, since the growth phenotypes of S. aureus in the absence of Leu or Val suggests that depletion of these nutrients alone is not sufficient to relieve CodY-dependent repression (compare Fig 2A and 2B). To study CodY-dependent promoter activity in isolation of attenuation, we generated a second reporter construct (partial promoter; pGYilvDP::lux) that lacked the attenuator sequence and contained only the CodY binding sequence and compared this to the original construct (complete promoter; pGYilvDC::lux) that contained both regulatory elements (Fig 6A). We first confirmed that both constructs responded to CodY and, indeed, observed higher promoter activity in the codY mutant compared to the WT strain that peaked during mid-exponential growth (Fig 6B and 6C). All endpoint pGY::lux experiments from this point on are therefore the luminescence normalized to the optical density of mid-exponential phase cells (RLU/OD600). We note that, generally, the partial promoter fusion has higher activity than the complete promoter fusion, likely due to omitting the attenuator sequence.
Fig 6. Characterization of cis and trans regulation of ilvD expression.
A) Diagram of the regions cloned into the pGY::lux vector to create pGYilvDP::lux and pGYilvDC::lux. B,C) Strains were pre-grown in complete CDM to mid-exponential phase and then sub-cultured into complete CDM in a 96-well plate. Luminescence (left axis, filled shapes) and OD600nm (right axis, open shapes) were read hourly. ValSup is abbreviated to ValS. Data are the mean +/- SD of three biological replicates.
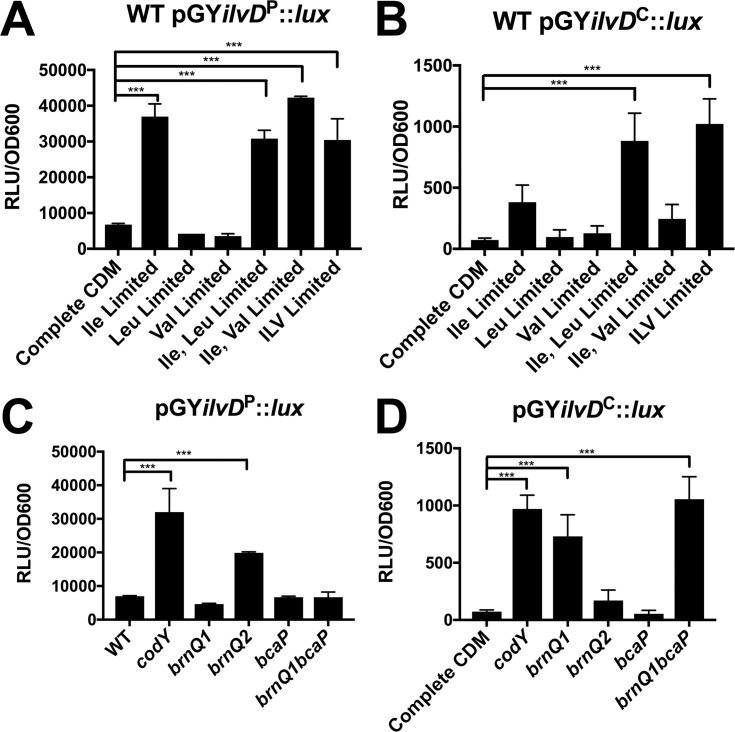
We next assessed promoter activity in response to depletion of each BCAA. Since complete omission of Leu and Val from the growth medium significantly attenuates S. aureus growth, we instead limited their concentrations to 10% of that in complete CDM to minimize differences in growth. We first examined CodY-dependent promoter activity using the pGYilvDP::lux construct. Promoter activity increased to levels comparable to the codY mutant only upon Ile limitation, and limitation of Leu or Val in combination with Ile did not alter ilvD promoter activity any further (Fig 7A), indicating a predominant role of Ile in regulating CodY activity on the ilvD promoter. We next examined the effect of BCAA limitation on attenuator-dependent regulation. ilvD promoter activity of the pGYilvDC::lux construct also increased upon Ile limitation, however, we also observed a further increase in promoter activity when Leu and Ile were limited simultaneously (Fig 7B). These data suggest that the attenuator sequence, which is unique to the pGYilvDC::lux construct, responds to Leu availability. This is consistent with our hypothesis that the attenuator represses the BCAA operon in response to Leu.
Fig 7. Ile is the predominant BCAA to regulate CodY activity on the ilvD promoter.
WT S. aureus containing the lux reporter vector with either A) the partial ilvD promoter region (pGYilvDP::lux) or B) the complete ilvD promoter region (pGYilvDC::lux) was pre-grown in complete CDM to mid-exponential phase and then sub-cultured into either complete CDM or CDM with limiting concentrations of BCAAs, as indicated. Concentrations of Ile, Leu, and Val in complete CDM are 228 μM, 684 μM, 684 μM, respectively. Concentrations of Ile, Leu, and Val in limited media are 23 μM, 68 μM, and 68 μM, respectively. Luminescence values were read when cells reached mid-exponential phase and were normalized to the OD600nm. Data are the mean of three biological replicates +/- SD. S. aureus strains with mutations in either codY (codY::ϕNΣ) or BCAA transporters and containing either C) the partial ilvD promoter region (pGYilvDP::lux) or D) the complete ilvD promoter region (pGYilvDC::lux) were pre-grown in complete CDM to mid-exponential phase and then sub-cultured into complete CDM. Luminescence values were read when cells reached mid-exponential phase and were normalized to the OD600nm. Data are the mean of three biological replicates +/- SD. Data were analyzed by one-way ANOVA with Dunnet’s multiple comparisons test. *** P < 0.001.
We previously identified mechanisms of BCAA transport in S. aureus, including BrnQ1 and BcaP, which transport Ile, Leu and Val, and BrnQ2, a dedicated Ile transporter [24,25]. To determine the contribution of each of these transporters to either CodY-dependent or attenuator-dependent regulation of BCAA biosynthesis, we assessed ilvD promoter activity in various BCAA transporter mutants. ilvD promoter activity of the pGYilvDP::lux construct increased only in the brnQ2 mutant (Fig 7C), whereas the pGYilvDC::lux increased in the brnQ1 and brnQ1bcaP mutants (Fig 7D). These data indicate that BrnQ2-dependent Ile transport is linked to CodY activity and BrnQ1/BcaP-dependent Leu transport is linked to attenuation. Notably, using the complete ilvD promoter region, we did not observe a change in promoter activity in the brnQ2 mutant. We have previously shown that brnQ1 is upregulated in a brnQ2 mutant and consequently a brnQ2 mutant takes up more Leu and Val permitting enhanced growth in media limited for these BCAAs [24]. We thus postulate that in a brnQ2 mutant, the increased Leu uptake causes repression of ilvD promoter activity via the attenuator and overrides the CodY-dependent Ile response.
All three BCAAs activate CodY DNA-binding activity
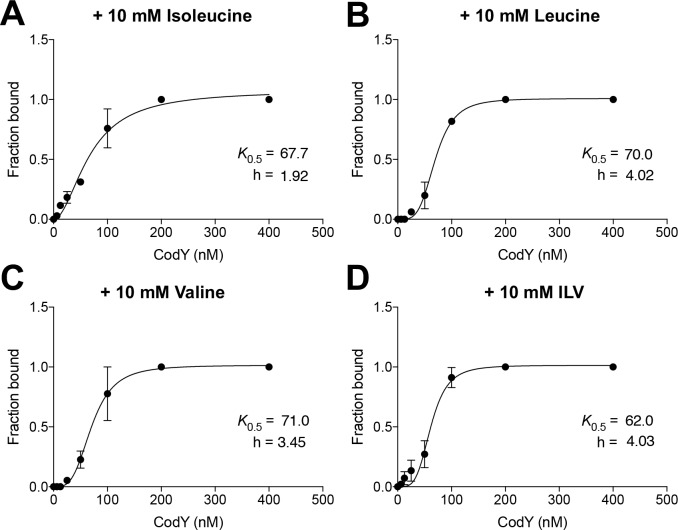
We next revisited the DNA binding activity of CodY at the ilvD promoter in the presence of each BCAA to compare CodY activity in vitro vs during growth. To test whether Ile activates CodY to bind DNA more efficiently than Leu or Val, we analyzed the interaction of CodY with a fluorescently-labeled DNA fragment (ilvD266p+) containing the annotated CodY regulatory region of ilvD [9]. Ile-activated CodY formed DNA:CodY complexes with as little as 6.5 nM CodY monomer, whereas Leu- and Val-activated CodY formed similar, multiple DNA:protein complexes as Ile-activated CodY, but required ~4-fold more CodY protein (S4A–S4C Fig). However, band densitometry analysis and fitting the data to a Hill equation revealed that the apparent binding constant values were essentially identical for all ligands tested (Fig 8). We did not observe an additive effect of all three BCAAs on CodY binding activity (S4D Fig). Thus, CodY binds all three amino acids in vitro.
Fig 8. CodY binds all three branched-chain amino acids in vitro.
Fraction of bound ilvD266p+ fragment was analyzed in EMSAs containing GTP and A) isoleucine, B) leucine, C) valine, or D) all three amino acids (ILV). The binding constants (K0.5) and Hill coefficients (h) were determined by fitting the data to the Hill equation [98]. Data points indicate the mean +/- SEM of two independent experiments. Error bars were plotted for all points; in some cases, the error bars are too small to see.
Absence of exogenous Ile restores growth in media lacking Leu, but not Val
Thus far, our data provide insight into the molecular mechanisms governing the BCAA-specific growth phenotypes of S. aureus observed in panel A of Fig 2. In complete CDM, the ilv-leu operon is repressed in an Ile-dependent manner via CodY and in a Leu-dependent manner via the attenuator peptide. Omission of Ile from the growth medium relieves CodY repression of the ilv-leu operon, resulting in Ile synthesis, which supports rapid growth in the absence of an exogenous Ile source. In media lacking Val, CodY remains active and the presence of Leu triggers transcriptional termination of the ilv-leu operon via the attenuator peptide; thus S. aureus is unable to synthesize Val and consequently unable to grow unless either of the aforementioned mechanisms is mutated. Omission of Leu from the growth medium relieves attenuator-dependent repression, however CodY remains active, and consequently, Leu synthesis is only partially relieved, resulting in a reduced growth rate. It therefore follows that simultaneous omission of Ile and Leu or Val should permit growth of S. aureus due to de-repression of CodY. Indeed, we found that the growth of S. aureus in CDM lacking Ile and Leu initiated more rapidly than growth in CDM lacking Leu alone, indicating that the reduced growth rate in CDM–Leu is due to Ile-dependent CodY repression (Fig 9A). Unexpectedly, S. aureus grown in CDM lacking Ile and Val resembled growth of S. aureus in media lacking Val alone (Fig 9B). Since the presence of Leu also contributes to repression of the operon, we further examined growth of S. aureus in CDM lacking all three BCAAs, however the growth of S. aureus remained attenuated, with no observable growth until a prolonged period of ~ 16 hr (Fig 9C). These data are curious given that the immediate precursor of Val is also a precursor of Leu, and the aminotransferase (IlvE) that converts ketoisovalerate to Val also produces Ile and Leu (Fig 1). Since our promoter:reporter data demonstrate that the ilvD promoter is active in media limited for all three BCAAs (Fig 7A and 7B) and thus the operon is presumed derepressed, we postulate that the growth impairment in media lacking all three BCAAs is related to enzymatic activity of the biosynthetic enzymes, whereby either the aminotransferase exhibits substrate bias towards Ile or Leu synthesis, or there is negative or positive cross-regulation between the pathways. For example, the threonine deaminase (IlvA) required only for Ile synthesis (Fig 1) is activated by Val in E. coli and B. subtilis [66,67]. Therefore, it is possible that in the absence of Val, Ile synthesis is reduced, contributing to the growth impairment in media lacking both Ile and Val.
Fig 9. Omission of Ile from CDM restores growth in media lacking Leu, but not Val.
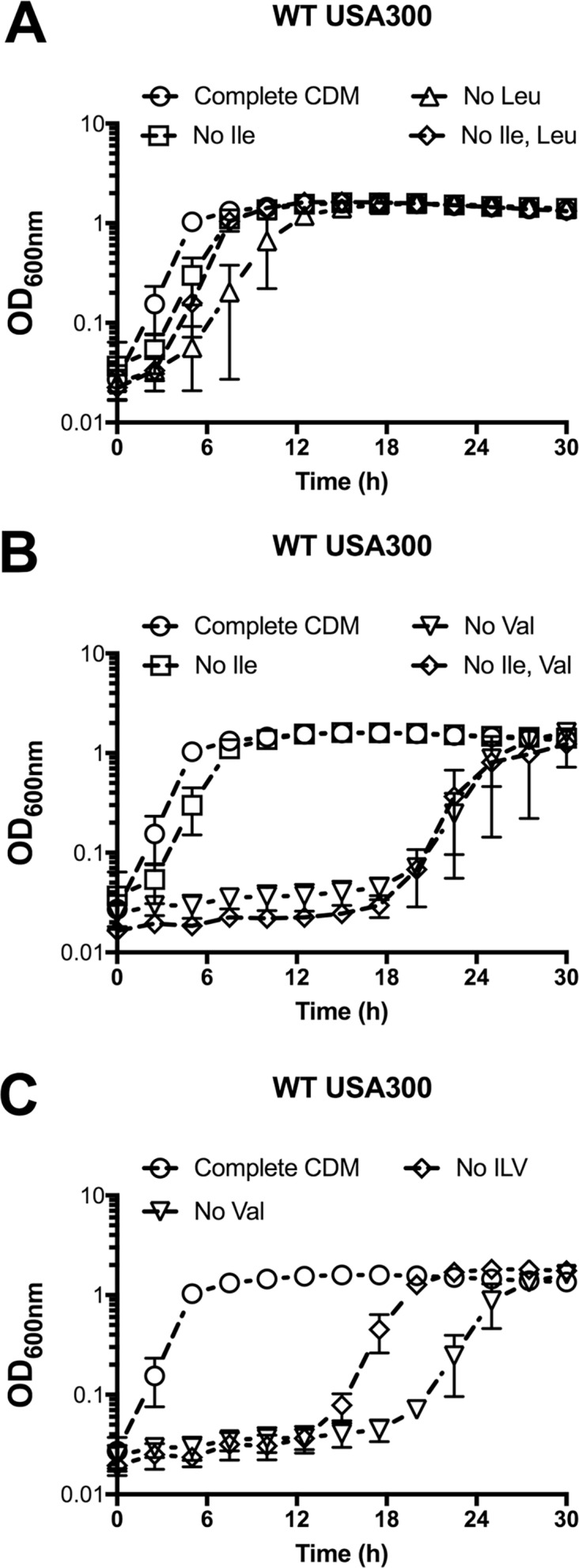
A-C) WT USA300 was pre-grown in complete CDM to mid-exponential phase and then sub-cultured into either complete CDM or CDM with amino acids omitted, as indicated. Data are the mean +/- SD of three biological replicates.
We were curious to investigate whether mutations in the promoter region of ilvD arise in the environment, reasoning that S. aureus might encounter Val-limited environments that impair growth and therefore select for mutations in the regulatory mechanisms involved in repression. We compared the nucleotide sequence of the ilvD promoter region from USA300 FPR3757 to all complete genome sequences of S. aureus. Overall, there was high sequence conservation, however, several variants were identified in the putative regulatory regions (S5 Fig). Intriguingly, two variants occur in the putative ORF upstream of ilvD and both alter the number of Leu codons in the peptide (located at +151 and +162 in S5 Fig). Several sequence variants also occur in the first CodY binding region. Ongoing studies will investigate the consequence of these mutations on the level ilv-leu expression and subsequent BCAA biosynthesis in these strains.
Ile deprivation induces expression of a nutrient transporter and nuclease
Our data revealed an unexpected role for Ile, and not Leu or Val, in regulating CodY activity on the ilvD promoter. The predominant role for Ile could have important implications for S. aureus physiology and virulence given that CodY is considered a master regulator of metabolism and virulence gene expression in S. aureus [7–9,49,60]. It was therefore of high interest to us to investigate whether the predominant role of Ile in regulating CodY activity was unique to ilvD or if it extended to other CodY-regulated genes.
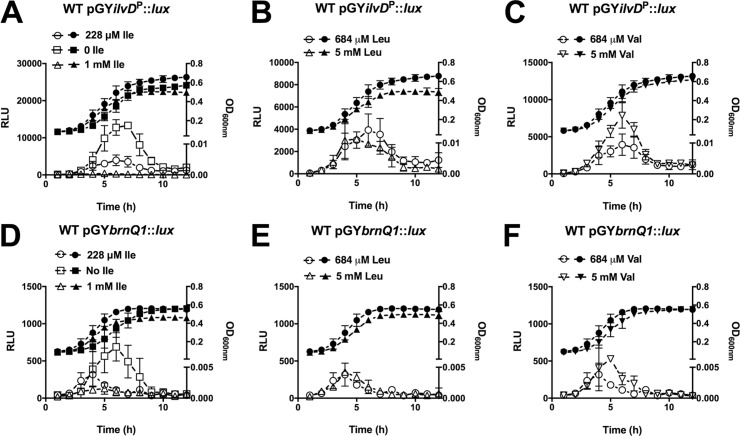
We selected the CodY-regulated brnQ1 gene as a representative metabolic gene [7–9], and we modified the luminescent reporter experiment slightly, such that instead of limiting BCAAs, which can alter growth, we added back excess BCAAs and examined whether the addition of excess BCAAs has repressive effects on CodY target gene expression. We first confirmed the effect of excess BCAAs on the ilvD promoter. Indeed, we observed that excess amounts of Ile in the growth medium repressed ilvD promoter activity (Fig 10A), whereas excess Leu had no effect (Fig 10B) and, intriguingly, excess Val had the opposite effect of increasing promoter activity (Fig 10C). We repeated this experiment with a lux promoter:reporter containing the brnQ1 promoter. Consistent with the ilvD promoter:reporter, we observed excess Ile, but not Leu or Val, to have a repressive effect on promoter activity (Fig 10D–10F).
Fig 10. Ile is the predominant BCAA to regulate CodY activity on the brnQ1 promoter.
A-F) WT S. aureus containing the lux reporter vector with either A-C) the partial ilvD promoter region (pGYilvDP::lux) or B) the complete brnQ1 promoter region (pGYbrnQ1::lux) was pre-grown in complete CDM to mid-exponential phase and then sub-cultured into either complete CDM (284 μM, 684 μM Leu, 684 μM Val) or CDM with limiting/excess concentrations of BCAAs, as indicated. Luminescence (left axis, open shapes) and OD600nm (right axis, filled shapes) were read hourly. Data are the mean +/- SD of three biological replicates.
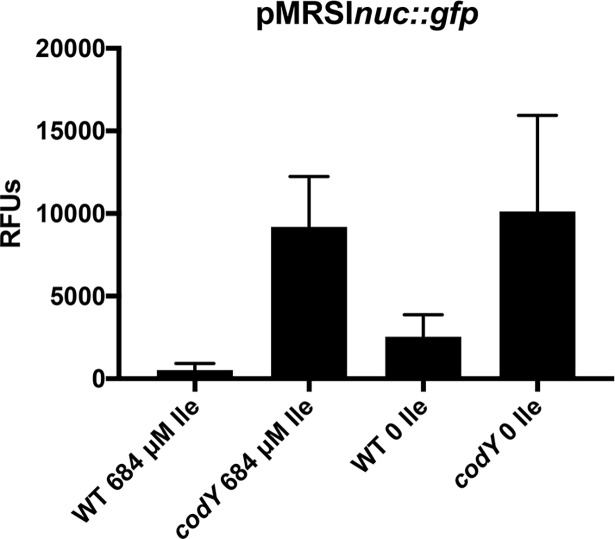
We next investigated whether Ile limitation results in relief of CodY-mediated repression of virulence gene expression, specifically, the secreted factor nuclease [7,9,68]. To do this, we took advantage of a previously constructed nuc-gfp reporter [7] and measured fluorescence during mid-exponential phase. In agreement with past results, we measured relatively low nuc-gfp fluorescence when WT cells were cultured in complete CDM; the fusion was derepressed ~17-fold in codY null mutant cells in the same medium (Fig 11). When WT cells were grown in CDM lacking Ile, nuc-gfp fluorescence increased ~5-fold over that observed in WT cells grown in CDM with excess Ile. Compared with complete CDM, we measured essentially the same amount of nuc-gfp fusion fluorescence in codY null mutant cells when Ile was omitted from the medium. Thus, Ile limitation results in a partial derepression of nuc-gfp in a CodY-dependent manner. Together, these data suggest that the role of Ile in regulating CodY activity is not unique to the ilvD promoter.
Fig 11. Isoleucine limitation induces nuc expression.
Strains were pre-grown in complete CDM to exponential phase and then sub-cultured into either complete CDM or CDM lacking Ile, as indicated. Fluorescence values were read when cells achieved mid-exponential phase and were normalized to OD600nm. Data are the mean of three biological replicates +/- SEM.
Discussion
In this study, we sought to determine the mechanisms by which each BCAA regulates expression of the ilv-leu operon to explain the unique growth phenotypes of S. aureus upon depletion of each of Ile, Leu and Val. By selecting for genetic variants of S. aureus that grew rapidly in the absence of an exogenous source of Val, we characterized two classes of mutations that relieve repression of the ilv-leu operon; mutations in the transcriptional repressor CodY and mutations in the region upstream of ilvD, the first gene in the ILV biosynthetic operon. We demonstrate that CodY activity is predominantly regulated by Ile availability during growth, an unexpected finding given that all three BCAAs activate CodY:DNA binding in vitro (S4 Fig). Bioinformatic analysis revealed that the region upstream of the ilvD coding sequence contains a highly-conserved attenuator peptide that is rich in Leu codons and, therefore, presumably controls transcriptional read-through in response to Leu availability. This is supported by experimental evidence demonstrating that ilvD promoter activity increases in response to i) Leu depletion, and ii) mutations in the attenuator peptide. Therefore, Ile and Leu each regulate expression of the ilv-leu operon through unique mechanisms (summarized in Fig 12).
Fig 12. Model of mechanisms regulating ilv-leu expression.
Transcription of the ilv-leu operon is repressed by CodY. In the presence of Ile, CodY binds and represses transcription. As Ile is depleted, CodY becomes inactive and expression of the operon is induced. As the operon is transcribed, the ribosome beings translating the open reading frame (ORF) upstream of ilvD. The ORF is rich in Ile and Leu and will stall if cells are depleted of either tRNA. Stalling of the ribosome prevents formation of the terminator hairpin, allowing transcription of the operon to proceed. When there is sufficient Ile/Leu tRNA, the ORF is translated and the terminator hairpin forms, terminating transcription.
The primary reservoir of S. aureus is the anterior nares. That Ile was not detected in human nasal secretions [69] lends support to the idea that Ile deprivation is perceived by S. aureus in vivo and is a signal, via CodY, to upregulate ILV synthesis that would presumably aid in bacterial survival in at least this niche. A predominant role for Ile in regulating CodY activity during growth has also been observed in another S. aureus strain [8], as well as other species, including B. subtilis [12], L. lactis [57], and L. monocytogenes [70]. Since CodY has been linked with virulence factor expression in S. aureus [7,8,71–75], including nuc as demonstrated in this study, it will be important to determine whether Ile is the predominant BCAA to modulate CodY activity on additional target genes, including virulence genes. It is also noteworthy that we demonstrated an important link between the BrnQ2 transporter, but not BrnQ1 or BcaP, and Ile availability to CodY activity. BrnQ transporters exist in other organisms, yet none function, as BrnQ2 does, as a dedicated Ile-transporter [32,34,35]. This suggests that BrnQ2 could provide an advantage to the adaptation of S. aureus to Ile-depleted environments. The fact that mutations in CodY are selected for when S. aureus is grown in the absence of exogenous Val supports the notion that Val contributes minimally to regulating CodY activity, at least on the ilvD and brnQ1 promoters, during growth.
In addition to trans regulation, via CodY, of ilv-leu operon expression in S. aureus, we identified a cis-dependent mode of regulation of operon expression, via an attenuator. Attenuation regulates BCAA biosynthesis in E. coli and S. enterica [42–44], and predicted attenuators can be found in various Gram-negative and Gram-positive bacteria [62–64,76]. In support of attenuation regulation of ilv-leu in S. aureus, one of the mutations we identified in our screen occurs in the predicted leader peptide and changes a Leu codon to a stop codon, which we predict would reduce Leu-dependent repression. The leader peptide also contains three Ile, suggesting that the level of uncharged tRNAIle also regulates ilv-leu expression. In support of this, expression of the ilv-leu operon is increased upon exposure to mupirocin, an antibiotic that binds to isoleucyl-tRNA synthetase and blocks the charging of tRNAIle [77]. CodY appears to be the dominant mechanism of repression, since S. aureus exhibits a growth delay in media lacking Leu, but not Ile, suggesting that Leu deprivation alone is not sufficient to fully relieve repression. Two additional CodY binding regions have been identified in the ilv-leu operon [9], and therefore transcription of downstream genes in the operon would occur in a codY mutant, bypassing transcriptional termination at the ilvD leader. Alternatively, CodY repression could block further transcription upon relief of attenuation, resulting in shorter transcripts. Notably, these conditions did not select for mutations in other previously described regulators of the ilv-leu operon, such as Gcp and YeaZ [55,56]. Since Gcp and YeaZ are essential genes in S. aureus, we did not expect to isolate mutations in these genes, however, we cannot rule out the possibility that the mutations in the attenuator region upstream of the ilvD coding region reduce binding of YeaZ [56].
Our model predicts that expression of the ilv-leu operon would be de-repressed upon Ile and Leu deprivation, yet S. aureus exhibits a significant growth lag in the absence of all three BCAAs. One possible explanation for this observation is potential allosteric regulation of the BCAA biosynthetic enzymes. The last gene in the ilv-leu operon, ilvA, encodes a threonine deaminase (TD), which catalyzes the first step in Ile synthesis by converting threonine to α-ketobuytrate. The E. coli TD enzyme is inhibited by Ile and activated by Val [78–80,66]. The B. subtilis TD enzyme is inhibited by Ile and it is proposed that Val activates TD in the presence of Ile and inhibits TD at high concentrations [67]. If TD activity in S. aureus is most efficient in the presence of Val, it follows that Ile synthesis would be reduced in the absence of Val. This could explain the absence of growth in media lacking Ile and Val. It would be of interest to investigate whether TD in S. aureus is similarly subject to allosteric regulation. Given the multiple physiological roles of BCAAs, another possibility is that simultaneous removal of all three BCAAs imparts enhanced stress on S. aureus compared to when the amino acids are omitted alone. In support of this, we demonstrated that in the absence of Leu and Val acquisition, the S. aureus membrane lacks Leu- and Val-derived iso-fatty acids, but this loss is compensated for by higher incorporation of Ile-derived iso-fatty acids [25]. Perhaps in the absence of all three BCAAs, such compensatory mechanisms are not achievable. Whatever the mechanism, it is evident that environments where Val is limited or absent poses a challenge to S. aureus and suggests that Val transport is critical for its growth. Indeed, we have previously shown that BCAA transporters BrnQ1 and BcaP, the only S. aureus transporters for Val, are required for S. aureus growth in vivo [24,25].
Altogether, this study details the molecular mechanisms regulating BCAA biosynthesis in S. aureus and uncovers environments where S. aureus engages in BCAA biosynthesis. In doing so, we reveal a predominant role for Ile in regulating CodY activity on the ilvD and brnQ1 promoter. Given the role of CodY in additionally regulating virulence genes, our data support the hypothesis that environmental availability of Ile is an important regulatory cue for S. aureus adaptation to nutrient limitation and virulence gene expression.
Materials and methods
Growth conditions
All strains and plasmids used in this study are described in Table 3. Methicillin-resistant S. aureus (MRSA) pulsed-field gel electrophoresis type USA300 LAC that has been cured of pUSA03, a plasmid conferring macrolide and lincosamide resistance, was used in all experiments as the wild-type (WT) strain. S. aureus strains were grown in either tryptic soy broth (TSB) (EMD Millipore, Billerica, MA) or in a chemically defined medium (CDM), described previously [24]. Final concentrations of Ile, Leu and Val in complete CDM were 228 μM, 684 μM, and 684 μM, respectively. Final concentrations were adjusted to 10% of their concentration in complete CDM in some experiments, as indicated. For growth experiments in TSB, S. aureus strains were pre-cultured in TSB until mid-exponential phase was reached, and then sub-cultured into fresh TSB to a starting optical density (OD600) of 0.01. For growth experiments in CDM, S. aureus strains were pre-cultured in CDM until mid-exponential phase was reached, and then sub-cultured into fresh CDM to a starting OD600 of 0.05 in either complete CDM or CDM where BCAA concentrations were limited or omitted, as indicated. Growth curves were performed in 100-well plates containing 200 μL/well of media and were read using the Bioscreen C visible spectrophotometer (Growth Curves USA; Piscataway, NJ). End point growth assays were performed in tubes with a 7:1 v/v tube:media ratio. All growth experiments were performed at 37°C with shaking. Growth media were supplemented with chloramphenicol (10 μg mL-1), ampicillin (100 μg mL-1), or erythromycin (3 μg mL-1), where required.
Table 3. Strains and plasmids.
| Strain or Plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| S. aureus | ||
| USA300 | USA300 LAC cured of antibiotic resistance plasmid | Heinrichs lab stock |
| RN4220 | rK- mK+; capable of accepting foreign DNA | [81] |
| H3001 | USA300 codY::ϕNΣ; EmR | [82] |
| H2568 | USA300 ΔbrnQ1 | [24] |
| H2563 | USA300 ΔbrnQ2 | [24] |
| H3386 | USA300 ΔbcaP | This study |
| H3584 | USA300 ΔbrnQ1ΔbcaP | This study |
| SRB687 | USA300 LAC cured of antibiotic resistance plasmid | A. Horswill |
| SRB746 | USA300 ΔcodY::ermC | [7] |
| SRB837 | USA300 /pRMS1-nuc bla cat nuc-gfp | [83] |
| SRB838 | USA300 ΔcodY::ermC /pRMS1-nuc bla cat nuc-gfp | [83] |
| E. coli | ||
| DH5α | F- ϕ80dlacZΔM15 recA1 endA1 gyrA96 thi-1 hsdR17(rK- mK-) supE44 relA1 deoR Δ(lacZyA-argF)U169 phoA | Promega |
| Plasmids | ||
| pRMC2 | Anhydrotetracycline-inducible expression vector; ApR in E. coli; Cmr in S. aureus | [84] |
| pcodY | pRMC2 containing codY; CmR | This study |
| pGYlux | Vector harboring promoterless luxABCDE operon; CmR | [85] |
| pGYilvDWT::lux | Lux reporter vector with ilvD promoter from WT USA300; CmR | This study |
| pGYilvDValS1::lux | Lux reporter vector with ilvD promoter mutated to contain the ValSup-1 SNP; CmR | This study |
| pGYilvDValS7::lux | Lux reporter vector with ilvD promoter mutated to contain the ValSup-7 SNP; CmR | This study |
| pGYilvDValS9::lux | Lux reporter vector with ilvD promoter mutated to contain the ValSup-9 SNP; CmR | This study |
| pGYilvDP::lux | Lux reporter vector with only the CodY binding motifs in the ilvD promoter; CmR | This study |
| pGYilvDC::lux | Lux reporter vector with the ilvD promoter from WT USA300; CmR | This study |
| pRMS1-nuc | GFP reporter vector with nuc promoter from WT UAMS-1; CmR | [7] |
aAbbreviations: EmR, ApR, CmR, designate resistance to erythromycin, ampicillin and chloramphenicol respectively.
Mutagenesis and construction of plasmids
Deletion of bcaP was constructed using the pKOR1 plasmid as described previously [24]. Primer sequences were based on the published USA300 FPR3757 genome and are displayed in Table 4. The bcaP deletion was introduced into the markerless brnQ1 deletion mutant, described previously [24]. The pGYlux vectors were constructed using primers described in Table 4. The pGYlux plasmid is derived from a low copy plasmid (5 copies/cell) [85], and we estimated 20 copies/cell in our experiments. lux plasmids were further used as templates for site-directed mutagenesis, using primers described in Table 4. Briefly, PCR reactions containing the Phusion High-Fidelity DNA Polymerase (ThermoFisher, Waltham, MA) were set up such that half of the reaction mixture contained the forward primer and the remaining half contained the reverse primer. These reactions proceeded for 3 cycles of 98°C for 10 s, 60°C for 30 s, and 72°C for 12 min. After 3 cycles, the forward and reverse primer reactions were mixed together and the reactions proceeded for an additional 17 cycles. Plasmids were treated with DpnI (New England Biolabs, Ipswich MA) for 1 hr at 37°C and were then transformed into E. coli DH5α. Mutations were confirmed by PCR. All plasmids were first constructed in E. coli DH5α and subsequently electroporated into the restriction-defective S. aureus strain, RN4220, prior to electroporation into the desired strain.
Table 4. Oligonucleotides used in this study.
| Oligonucleotides a | Sequence (5’-3’) |
|---|---|
|
bcaP Ups F bcaP Ups R |
GGGGACAAGTTTGTACAAAAAAGCAGGCTCAGTCTTCGTATTCACCTGC CTTCCCATAAACTTTCCTCC For generating upstream arm for bcaP deletion |
|
bcaP Dwn F bcaP Dwn R |
5’ /Phos/ ACGTAGCTGAATACCACCC GGGGACCACTTTGTACAAGAAAGCTGGGTTGTACCTGCTGACGAAGTAG For generating downstream arm for bcaP deletion |
|
ilvDC F ilvDC R |
GATCCCCGGGACCTGCTCCTAAATCTCCG GATCGTCGACACTTCTTGCTGGTGCTTGG For cloning ilvD 5’UTR into pGYlux |
|
ilvDP F ilvDPR |
GATCCCCGGGGTACGTCTTACACCAAG GATCGTCGACAGTTGTCGGTTGATGTTC For cloning partial ilvD 5’UTR into pGYlux |
|
ilvDValS-1 F ilvDValS-1 R |
CAA ATA TTA TTA TTT TAT aAT ACT CTT TAG GAC TCG CGA GTC CTA AAG AGT ATt ATA AAA TAA TAA TAT TTG For site directed mutagenesis of pGYlux::ilvD |
|
ilvDValS-7 F ilvDValS-7 R |
CTA AAC GCT TTA AGT CaT ATT TCT GTT TGA ATG CAT TCA AAC AGA AAT AtG ACT TAA AGC GTT TAG For site directed mutagenesis of pGYlux::ilvD |
|
ilvDValS-9 F ilvDValS-9 R |
CTA AAC GCT TTA AGc CCT ATT TCT GTT TG CAA ACA GAA ATA GGg CTT AAA GCG TTT AG For site directed mutagenesis of pGYlux::ilvD |
|
codY F codY R |
GATCGGTACCCCGAATGCAGTTGTAGATATTACC GATCGAGGCTCTTATGTCCCAGACTCATCGAC For cloning codY into pRMC2 |
|
codY Seq F codY Seq R |
GCAATTACTCGCTTAGCTGAG GTGTGTATTGGCTTTATAGCCG For target directed sequencing of codY |
|
ilvD qPCR F ilvD qPCR R |
GCTATCTTTTGCTCTGGTGG AGGGCAGGCATTTTGTTCC For qPCR of ilvD |
|
ilvC qPCR F ilvC qPCR R |
CAAGATGTAAAAACGGACGC GTCAAAAGAACGACCTGGG For qPCR of ilvC |
| oAK031 | 6-FAM/ATCCATTGTTCAATCGTATC |
| oNW025 | GAAGTTGTCGGTTGATGTTC generate ilvD266p for EMSA |
a All primer sequences, except oAK031 and oNW025 (based on UAMS-1), are based on the USA300 FPR3757 genome; restriction sites are underlined; nucleotide mutated in site directed mutagenesis is indicate in lower case.
Selection of ValSup mutants and whole genome sequencing
To select for genetic mutations that permit adaptation to growth in media lacking Val, twelve independent colonies of WT S. aureus were grown in complete CDM to mid-exponential phase and sub-cultured into CDM lacking Val. Recovered cells were harvested and plated onto TSB agar and grown overnight at 37°C. Isolated colonies were grown in complete CDM to mid-exponential phase and sub-cultured into CDM lacking Val to confirm the occurrence of a heritable mutation. Genomic DNA was isolated from all twelve mutants, referred to as ValSup-mutants, as well as from two biological replicates of our laboratory WT USA300, using the Invitrogen PureLink Genomic DNA Preparation Kit (ThermoFisher Scientific, Boston MA) per the manufacturer’s instructions. Primers used for the targeting sequencing of the ilvD promoter and the codY gene are listed in Table 4. Samples were sent to the London Regional Genomics Center for sequencing on the MiSeq platform. Libraries were prepared using the Nextera XT DNA Library Preparation kit (Illumina, San Diego, CA). 150 bp reads were mapped to the USA300 FPR3757 (NC_007793.1) genome using the BWA-MEM aligner [86] and variants were determined using SAMtools [87].
TCA precipitation of proteins and SDS-PAGE
Strains were pre-grown in TSB to mid-exponential phase and then sub-cultured into TSB to a starting OD600 of 0.01 and grown overnight. The OD600 of stationary phase cultures were determined and a supernatant volume equivalent to 5 OD units was harvested and incubated with trichloroacetic acid (TCA) (Sigma-Aldrich, St. Louis, MO) at a final concentration of 20% overnight at 4°C. Precipitated protein samples were dissolved, run on a 12% acrylamide gel and stained with Coomassie-Blue.
lux reporter assays
Kinetic lux reporter experiments were performed in flat, clear-bottom 96-well white plates (Thermo Fisher Scientific) and read using a BioTek Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek Instruments Inc, Winooski, VT). Pre-cultures were inoculated into either complete or limited CDM to a starting OD600 of 0.01 in 200 μL/well. Luminescence and OD600 values were read at hourly intervals. For end-point lux reporter experiments, pre-cultures were sub-cultured into either complete or limited CDM to a starting OD600 of 0.05 in tubes with a 7:1 tube:media ratio. At hourly intervals, aliquots of 200 μL were transferred to flat, clear-bottom 96-well white plates (Thermo Fisher Scientific) and luminescence and OD600 values were read. Samples of strains containing the lux construct with the complete ilvD promoter were supplemented with 0.1% (v/v) decanal in 40% ethanol and luminescence was measured immediately. Data presented are the relative light unit (RLU) values normalized to the OD600 of the sample when the cultures reached mid-exponential phase (OD600 0.6–0.8). Data were analyzed by one-way ANOVA with Dunnet’s multiple comparison test relative to the control sample in GraphPad Prism Version 7.0b.
RT-qPCR
RNA was isolated from cells grown to mid-exponential phase (OD600 of 0.6–0.8) in complete CDM using the Aurum Total RNA Mini Kit (Bio-Rad; Hercules, CA) per the manufacturer’s instructions. RNA (500 ng) was reverse transcribed using SuperScript II (Invitrogen, Carlsbad, CA) per the manufacturer’s instructions using 500 μg mL-1 of random hexamers. cDNA was PCR-amplified using SensiFAST SYBR No-ROX Kit (Bioline, Taunton, MA). Data were normalized to expression of the rpoB reference gene, and analyzed by an unpaired student’s t-test in GraphPad Prism Version 7.0b. Primers used are listed in Table 4.
Cloning, expression, and purification of recombinant codY protein
The codY ORF (QV15_05910) was amplified from S. aureus strain UAMS-1 using oligonucleotides oKM1 and oSRB410. The PCR fragment was purified and subjected to a second round of PCR using oKM1 and oSRB411 to append a Tobacco Etch Virus (TEV) protease cleavage sequence followed by six histidine (CAT) codons and a TAA stop codon. The 830-nt fragment was digested with SacI/SphI and ligated to the same sites of pBAD30 [88]. The resulting plasmid was introduced into E. coli DH5α. CodY-His6 was overproduced by growing the strain carrying the plasmid in LB at 37°C until mid-exponential phase (OD600 ~0.3). At this time, L-(+)-arabinose was added to a final concentration of 0.2% (w/v). After four hours of induction at 37°C, cells were pelleted by centrifugation (8,610 x g at 4°C) and frozen at -80°C. The cells were thawed, resuspended in Buffer A (20 mM Tris-Cl [pH 7.9], 500 mM NaCl, 5 mM imidazole, 5% [v/v] glycerol) supplemented with 0.1% (v/v) nonidet P-40 and 1 mM phenylmethylsulfonyl fluoride (PMSF), and lysed by sonication. CodY-His6 protein was purified from clarified soluble extracts using a computer-controlled ÄKTAPrime plus FPLC system equipped with a His-Trap FF column (GE Healthcare Life Sciences) using a linear gradient elution with Buffer B (20 mM Tris-Cl [pH 7.9], 500 mM NaCl, 685 mM imidazole, 5% [v/v] glycerol). Fractions containing CodY-His6 protein were pooled and supplemented with glycerol to 50% (v/v) and stored at -20°C.
Electrophoretic mobility shift assays (EMSAs)
A 266-bp fragment (ilvD266p+) spanning -131 to +134 relative to the annotated ilvD transcriptional start site in S. aureus UAMS-1 [9] was synthesized by PCR using primers oNW025 and oAK031 (Table 4), simultaneously incorporating a 6-carboxyfluorescein (FAM)-label. EMSAs were performed with purified CodY-His6 protein and FAM-labeled ilvD266p+ fragment in binding buffer (20 mM Tris-Cl [pH 8.0], 50 mM KCl, 2 mM MgCl2, 5% [v/v] glycerol, 0.05% [v/v] Nonidet P-40, 1 mM dithiothritol [DTT], 0.025 mg ml-1 salmon sperm DNA). Samples (20 μl) containing various amounts of CodY-His6, 200 fmol of 6-FAM-labeled DNA fragment, 2 mM GTP, and 10 mM of the indicated BCAA(s) were incubated for 20 min at 25°C in a thermomixer (Eppendorf) with moderate agitation (250 rpm). The samples were separated on 8% non-denaturing 35 mM HEPES (pH 7.4)-43 mM imidazole-10 mM BCAA polyacrylamide gels for 40 minutes at 200 V. Fluorescent DNA fragments were detected and quantified using a computer-controlled ImageQuant LAS 4000 biomolecular imager (GE Healthcare Life Sciences) using a SYBR filter set. Quantitative analysis of CodY binding to ilvD266p+ was performed using ImageJ software [89]. Since the binding curves appeared to have a sigmoidal shape, the data from two independent experiments were fitted to the Hill equation Θ = Ch/(Ch + K0.5h) using Prism (ver. 7; GraphPad Software). In this equation, Θ is the fraction of bound DNA, C is the concentration of CodY, K0.5 is the binding constant, and h is the Hill coefficient. K0.5 and h shown are from fitted data where r2 > 0.96.
nuc-gfp reporter assays
Strains were grown overnight in CDM complete, then sub-cultured the next morning in CDM complete to a starting OD600 of 0.05 in 125 ml DeLong shake flasks (5:1 flask:medium ratio). Incubation was performed in an Innovo orbital shaking water bath (New Brunswick) with vigorous agitation (280 rpm). At an OD600 of 0.8, cells were pelleted and resuspended in either CDM complete or CDM lacking isoleucine to an initial OD600 of ~0.05. When cells reached mid-exponential phase (OD600 of 0.4–0.5), a 1-mL sample was removed, washed once with phosphate buffered saline (PBS), and resuspended in PBS to minimize background fluorescence from the medium. Fluorescence was measured using a computer-controlled Tecan Infinite F200 Pro plate reader equipped with 485 nm excitation and 535 nm emission filters. GFP signal acquisition parameters were kept constant throughout the experiment (gain, 49%; flash number, 10; integration time, 40 μs; lag time, 0 μs; settle time, 0 ms). Data are presented as relative fluorescence units (RFUs) after subtracting the fluorescence from USA300 LAC (lacking the GFP reporter plasmid) and dividing by OD600 to correct for cell density.
Bioinformatics
Putative terminator structures in the ilvD 5’UTR were identified using the predictive software RibEx [90] and RNAfold [91]. Mfold [92] was used to identify putative antiterminator sequences. RNAfold and Mfold were used to search for conserved T-box riboswitch features. T-box riboswitch multiple sequence alignments were generated with predicted T-box riboswitch sequences in S. aureus subsp. aureus N315 (NC_002745.2) that were annotated in the Rfam database [93] and the ilvB T-box riboswitch from B. subtilis (NC_000964.3) using MUSCLE [94] with default parameters. The alignments were manually adjusted in JalView [95] with insight gained from experimentally characterized S. aureus T-box leaders: glyS, ileS and metI. The putative S. aureus ilvD leader was then added to the finished alignment using MAFFT [96]. The peptide multiple sequence alignments were generated by extracting the top 15 BLAST results for ilvD leaders from different staphylococci, translating the ORFs, and aligning the peptide sequences using MUSCLE [94]. The ilvD 5’UTR from USA300 FPR3757 was aligned to 168 S. aureus complete genomes using BLAST.
Supporting information
Strains were pre-grown in TSB to mid-exponential phase, then sub-cultured into TSB for 16 hr. Supernatants were collected and proteins were precipitated using TCA. Protein samples were normalized to the equivalent of 5 ODs and run on a 12% SDS-PAGE gel.
(TIF)
Sequences of all annotated S. aureus T-boxes (based on the S. aureus subsp. aureus N315 genome NC_002745.2) and the B. subtilis ilvB T-box (NC_000964.3), labelled by regulated gene, were analyzed. Key features analyzed and annotated above the alignment are the AG Bulge (AGVGA-box), Distal Loop (GNUG-box); and the Specifier Loop (GAA…XXXA) where XXX represents the tRNA codon.
(TIF)
(A) Multiple sequence alignments of the top 15 hits from a BLAST search using the S. aureus ilvD promoter region were extracted and aligned as described in the methods. Dark blue shading represents conservation above 80%. Coding regions and key structures are labeled above and below the alignment. (B) Secondary structure predictions for the region aligned in the top panel. The start and stop codons are highlighted in green and red, respectively and the alternative pairing regions in the terminator/antiterminator are highlighted in yellow and blue.
(TIF)
6-FAM-labeled ilvD266p+ DNA fragment was incubated with increasing amounts of S. aureus CodY protein in the presence of GTP and A) isoleucine, B) leucine, C) valine, or all three amino acids (ILV). Concentrations of CodY used (nM of monomer) are indicated below each lane. Unbound DNA fragments are indicated by the right-pointing open arrowheads; CodY:ilvD266p+ complexes are indicated by the right-pointing closed arrowheads. Data are representative of at least two independent experiments.
(TIF)
The ilvD promoter region was aligned across 168 complete S. aureus genomes. In (A), the location of the mutations is indicated relative to the transcription start site identified by Majercyzk et al., 2010 [9]. The number of strains containing the mutation is indicated in brackets. Mutations in green are the SNPs identified in this study. Shown in (B) are the CodY binding sites identified by Majercyzk et al., 2010 [9], with the canonical CodY motif in bold and italicized, along with the predicted anti-terminator and terminator sequences. In (C) is listed the currently available strains for which genomes have the identified SNPs that are shown in panel A.
(TIF)
Acknowledgments
The authors wish to thank Kevin Mlynek for help with strain construction.
Data Availability
Sequence data are deposited in NCBI under accession SRP126843 (https://www.ncbi.nlm.nih.gov/sra/SRP126843). All other relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Discovery Grant 2016-05047 to DEH from the Natural Sciences and Engineering Research Council of Canada (NSERC). SRB was supported by Pathway to Independence Award (GM099893) from the National Institutes of Health, and Georgetown University Startup funds. MEPM was supported by Research Grant MOP-49597 from the Canadian Institutes of Health Research. DRE was supported by Discovery Grant 2015-04800 from NSERC. JCK was supported by an RGE Murray award from the Department of Microbiology and Immunology, University of Western Ontario. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.King MD, Humphrey BJ, Wang YF, Kourbatova E V, Ray SM. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144: 309–318. [DOI] [PubMed] [Google Scholar]
- 2.Gillet Y, Issartel B, Vanhems P, Fournet J-CC, Lina G, Bes M, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359: 753–759. doi: 10.1016/S0140-6736(02)07877-7 [DOI] [PubMed] [Google Scholar]
- 3.Miller LG, Perdreau-Remington F, Rieg G, Mehdi S, Perlroth J, Bayer AS, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352: 1445–53. doi: 10.1056/NEJMoa042683 [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez BE, Martinez-Aguilar G, Hulten KG, Hammerman WA, Coss-Bu J, Avalos-Mishaan A, et al. Severe staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics. 2005;115: 642–648. doi: 10.1542/peds.2004-2300 [DOI] [PubMed] [Google Scholar]
- 5.Mei JM, Nourbakhsh F, Ford CW, Holden DW. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol Microbiol. 1997;26: 399–407. doi: 10.1046/j.1365-2958.1997.5911966.x [DOI] [PubMed] [Google Scholar]
- 6.Coulter SN, Schwan WR, Ng EY, Langhorne MH, Ritchie HD, Westbrock-Wadman S, et al. Staphylococcus aureus genetic loci impacting growth and survival in multiple infection environments. Mol Microbiol. 1998;30: 393–404. doi: 10.1046/j.1365-2958.1998.01075.x [DOI] [PubMed] [Google Scholar]
- 7.Waters NR, Samuels DJ, Behera RK, Livny J, Rhee KY, Sadykov MR, et al. A spectrum of CodY activities drives metabolic reorganization and virulence gene expression in Staphylococcus aureus. Mol Microbiol. 2016;101: 495–514. doi: 10.1111/mmi.13404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pohl K, Francois P, Stenz L, Schlink F, Geiger T, Herbert S, et al. CodY in Staphylococcus aureus: a regulatory link between metabolism and virulence gene expression. J Bacteriol. 2009;191: 2953–2963. doi: 10.1128/JB.01492-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majerczyk CD, Dunman PM, Luong TT, Lee CY, Sadykov MR, Somerville GA, et al. Direct targets of CodY in Staphylococcus aureus. J Bacteriol. 2010;192: 2861–2877. doi: 10.1128/JB.00220-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratnayake-Lecamwasam M, Serror P, Wong KW, Sonenshein AL. Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev. 2001;15: 1093–1103. doi: 10.1101/gad.874201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guédon E, Serror P, Ehrlich SD, Renault P, Delorme C. Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol Microbiol. 2001;40: 1227–1239. mmi2470 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Shivers RP, Sonenshein AL. Activation of the Bacillus subtilis global regulator CodY by direct interaction with branched-chain amino acids. Mol Microbiol. 2004;53: 599–611. doi: 10.1111/j.1365-2958.2004.04135.x [DOI] [PubMed] [Google Scholar]
- 13.Handke LD, Shivers RP, Sonenshein AL. Interaction of Bacillus subtilis CodY with GTP. J Bacteriol. 2008;190: 798–806. doi: 10.1128/JB.01115-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkins T, Prior RG, Mack K, Russell P, Nelson M, Oyston PCF, et al. A mutant of Burkholderia pseudomallei, auxotrophic in the branched-chain amino acid biosynthetic pathway, is attenuated and protective in a murine model of melioidosis. Infect Immun. 2002;70: 5290–5294. doi: 10.1128/IAI.70.9.5290-5294.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAdam R, Weisbrod TR, Martin J, Scuderi JD, Brown AM, Cirillo JD, et al. In vivo growth characteristics of leucine and methionine auxotrophic mutants of Mycobacterium bovis BCG generated by transposon mutagenesis. Infect Immun. 1995;63: 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bange FC, Brown AM, Jacobs WR. Leucine auxotrophy restricts growth of Mycobacterium bovis BCG in macrophages. Infect Immun. American Society for Microbiology; 1996;64: 1794–9. Available: http://www.ncbi.nlm.nih.gov/pubmed/8613393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awasthy D, Gaonkar S, Shandil RK, Yadav R, Bharath S, Marcel N, et al. Inactivation of the ilvB1 gene in Mycobacterium tuberculosis leads to branched-chain amino acid auxotrophy and attenuation of virulence in mice. Microbiology. Microbiology Society; 2009;155: 2978–2987. doi: 10.1099/mic.0.029884-0 [DOI] [PubMed] [Google Scholar]
- 18.Joseph B, Przybilla K, Stühler C, Schauer K, Slaghuis J, Fuchs TM, et al. Identification of Listeria monocytogenes genes contributing to intracellular replication by expression profiling and mutant screening. J Bacteriol. American Society for Microbiology; 2006;188: 556–68. doi: 10.1128/JB.188.2.556-568.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benton BM, Zhang JP, Bond S, Pope C, Christian T, Lee L, et al. Large-scale identification of genes required for full virulence of Staphylococcus aureus. J Bacteriol. 2004;186: 8478–89. doi: 10.1128/JB.186.24.8478-8489.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palace SG, Proulx MK, Lu S, Baker RE, Goguen JD. Genome-wide mutant fitness profiling identifies nutritional requirements for optimal growth of Yersinia pestis in deep tissue. MBio. American Society for Microbiology; 2014;5: e01385–14. doi: 10.1128/mBio.01385-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molzen TE, Burghout P, Bootsma HJ, Brandt CT, van der Gaast-de Jongh CE, Eleveld MJ, et al. Genome-wide identification of Streptococcus pneumoniae genes essential for bacterial replication during experimental meningitis. Infect Immun. American Society for Microbiology; 2011;79: 288–97. doi: 10.1128/IAI.00631-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basavanna S, Khandavilli S, Yuste J, Cohen JM, Hosie AHF, Webb AJ, et al. Screening of Streptococcus pneumoniae ABC transporter mutants demonstrates that LivJHMGF, a branched-chain amino acid ABC transporter, is necessary for disease pathogenesis. Infect Immun. 2009;77: 3412–23. doi: 10.1128/IAI.01543-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams MD, Wagner LM, Graddis TJ, Landick R, Antonucci TK, Gibson AL, et al. Nucleotide sequence and genetic characterization reveal six essential genes for the LIV-I and LS transport systems of Escherichia coli. J Biol Chem. 1990;265: 11436–43. Available: http://www.ncbi.nlm.nih.gov/pubmed/2195019 [PubMed] [Google Scholar]
- 24.Kaiser JC, Omer S, Sheldon JR, Welch I, Heinrichs DE. Role of BrnQ1 and BrnQ2 in branched-chain amino acid transport and virulence in Staphylococcus aureus. Infect Immun. 2015;83: 1019–1029. doi: 10.1128/IAI.02542-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaiser JC, Sen S, Sinha A, Wilkinson BJ, Heinrichs DE. The role of two branched-chain amino acid transporters in Staphylococcus aureus growth, membrane fatty acid composition and virulence. Mol Microbiol. 2016;102: 850–864. doi: 10.1111/mmi.13495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braun PR, Al-Younes H, Gussmann J, Klein J, Schneider E, Meyer TF. Competitive inhibition of amino acid uptake suppresses chlamydial growth: involvement of the chlamydial amino acid transporter BrnQ. J Bacteriol. American Society for Microbiology; 2008;190: 1822–30. doi: 10.1128/JB.01240-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoshino T, Kageyama M. Sodium-dependent transport of L-leucine in membrane vesicles prepared from Pseudomonas aeruginosa. J Bacteriol. American Society for Microbiology; 1979;137: 73–81. Available: http://www.ncbi.nlm.nih.gov/pubmed/83991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoshino T, Kose K. Cloning, nucleotide sequences, and identification of products of the Pseudomonas aeruginosa PAO bra genes, which encode the high-affinity branched-chain amino acid transport system. J Bacteriol. 1990;172: 5531–5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uratani Y, Tsuchiya T, Akamatsu Y, Hoshino T. Na+(Li+)/Branched-chain amino acid cotransport in Pseudomonas aeruginosa. J Membr Biol. Springer-Verlag; 1989;107: 57–62. doi: 10.1007/BF01871083 [DOI] [PubMed] [Google Scholar]
- 30.Matsubara K, Ohnishi K, Kiritani K. The third general transport system for branched-chain amino acids in Salmonella typhimurium. J Gen Appl Microbiol. Applied Microbiology, Molecular and Cellular Biosciences Research Foundation; 1988;34: 183–189. doi: 10.2323/jgam.34.183 [Google Scholar]
- 31.Ohnishi K, Hasegawa A, Matsubara K, Date T, Okada T, Kiritani K. Cloning and nucleotide sequence of the brnQ gene, the structural gene for a membrane-associated component of the LIV-II transport system for branched-chain amino acids in Salmonella typhimurium. Japanese J Genet. 1988;63: 343–357. [DOI] [PubMed] [Google Scholar]
- 32.Belitsky BR. Role of branched-chain amino acid transport in Bacillus subtilis CodY activity. J Bacteriol. 2015;197: 1330–8. doi: 10.1128/JB.02563-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tauch A, Hermann T, Burkovski A, Kramer R, Puhler A, Kalinowski J, et al. Isoleucine uptake in Corynebacterium glutamicum ATCC 13032 is directed by the brnQ gene product. Arch Microbiol. 1998;169: 303–312. doi: 10.1007/s002030050576 [DOI] [PubMed] [Google Scholar]
- 34.Stucky K, Hagting A, Klein JR, Matern H, Henrich B, Konings WN, et al. Cloning and characterization of brnQ, a gene encoding a low-affinity, branched-chain amino acid carrier in Lactobacillus delbrückii subsp. lactis DSM7290. Mol Gen Genet. 1995;249: 682–690. [DOI] [PubMed] [Google Scholar]
- 35.den Hengst CD, Groeneveld M, Kuipers OP, Kok J. Identification and functional characterization of the Lactococcus lactis CodY-regulated branched-chain amino acid permease BcaP (CtrA). J Bacteriol. 2006;188: 3280 doi: 10.1128/JB.188.9.3280-3289.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trip H, Mulder NL, Lolkema JS. Cloning, expression, and functional characterization of secondary amino acid transporters of Lactococcus lactis. J Bacteriol. 2013;195: 340–50. doi: 10.1128/JB.01948-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lincoln RA, Leigh JA, Jones NC. The amino acid requirements of Staphylococcus aureus isolated from cases of bovine mastitis. Vet Microbiol. 1995;45: 275–279. Available: http://www.sciencedirect.com/science/article/pii/0378113595000418 [DOI] [PubMed] [Google Scholar]
- 38.Onoue Y, Mori M. Amino acid requirements for the growth and enterotoxin production by Staphylococcus aureus in chemically defined media. Int J Food Microbiol. 1997;36: 77–82. doi: 10.1016/S0168-1605(97)01250-6 [DOI] [PubMed] [Google Scholar]
- 39.Lawther RP, Wek RC, Lopes JM, Pereira R, Taillon BE, Hatfield GW. The complete nucleotide sequence of the ilvGMEDA operon of Escherichia coli K-12. Nucleic Acids Res. 1987;15: 2137–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nargang FE, Subrahmanyam CS, Umbarger HE. Nucleotide sequence of ilvGEDA operon attenuator region of Escherichia coli. Proc Natl Acad Sci. 1980;77: 1823–7. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=348600&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friden P, Newman T, Freundlich M. Nucleotide sequence of the ilvB promoter-regulatory region: a biosynthetic operon controlled by attenuation and cyclic AMP. Proc Natl Acad Sci. 1982;79: 6156–6160. doi: 10.1073/pnas.79.20.6156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wessler SR, Calvo JM. Control of leu operon expression in Escherichia coli by a transcription attenuation mechanism. J Mol Biol. 1981;149: 579–597. doi: 10.1016/0022-2836(81)90348-X [DOI] [PubMed] [Google Scholar]
- 43.Gemmill RM, Wessler SR, Keller EB, Calvo JM. leu operon of Salmonella typhimurium is controlled by an attenuation mechanism. Proc Natl Acad Sci. 1979;76: 4941–4945. Available: http://www.pnas.org/content/76/10/4941.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Searles LL, Wessler SR, Calvo JM. Transcription attenuation is the major mechanism by which the leu operon of Salmonella typhimurium is controlled. J Mol Biol. 1983;163: 377–394. doi: 10.1016/0022-2836(83)90064-5 [DOI] [PubMed] [Google Scholar]
- 45.Bennett HJ, Pearce DM, Glenn S, Taylor CM, Kuhn M, Sonenshein AL, et al. Characterization of relA and codY mutants of Listeria monocytogenes: identification of the CodY regulon and its role in virulence. Mol Microbiol. Blackwell Publishing Ltd; 2007;63: 1453–1467. doi: 10.1111/j.1365-2958.2007.05597.x [DOI] [PubMed] [Google Scholar]
- 46.Dineen SS, McBride SM, Sonenshein AL. Integration of metabolism and virulence by Clostridium difficile CodY. J Bacteriol. American Society for Microbiology; 2010;192: 5350–62. doi: 10.1128/JB.00341-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindbäck T, Mols M, Basset C, Granum PE, Kuipers OP, Kovács ÁT. CodY, a pleiotropic regulator, influences multicellular behaviour and efficient production of virulence factors in Bacillus cereus. Environ Microbiol. Blackwell Publishing Ltd; 2012;14: 2233–2246. doi: 10.1111/j.1462-2920.2012.02766.x [DOI] [PubMed] [Google Scholar]
- 48.Hendriksen WT, Bootsma HJ, Estevão S, Hoogenboezem T, De Jong A, De Groot R, et al. CodY of Streptococcus pneumoniae: Link between nutritional gene regulation and colonization. J Bacteriol. 2008;190: 590–601. doi: 10.1128/JB.00917-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brinsmade SR. CodY, a master integrator of metabolism and virulence in Gram-positive bacteria. Curr Genet. Springer Berlin Heidelberg; 2017;63: 417–425. doi: 10.1007/s00294-016-0656-5 [DOI] [PubMed] [Google Scholar]
- 50.Shivers RP, Sonenshein AL. Bacillus subtilis ilvB operon: an intersection of global regulons. Mol Microbiol. Blackwell Science Ltd; 2005;56: 1549–1559. doi: 10.1111/j.1365-2958.2005.04634.x [DOI] [PubMed] [Google Scholar]
- 51.Grandoni JA, Zahler SA, Calvo JM. Transcriptional regulation of the ilv-leu operon of Bacillus subtilis. J Bacteriol. American Society for Microbiology; 1992;174: 3212–9. doi: 10.1128/JB.174.10.3212–3219.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grandoni JA, Fulmer SB, Brizzio V, Zahler SA, Calvo JM. Regions of the Bacillus subtilis ilv-leu operon involved in regulation by leucine. J Bacteriol. American Society for Microbiology; 1993;175: 7581–93. doi: 10.1128/JB.175.23.7581–7593.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marta PT, Ladner RD, Grandoni JA. A CUC triplet confers leucine-dependent regulation of the Bacillus subtilis ilv-leu operon. J Bacteriol. American Society for Microbiology; 1996;178: 2150–3. doi: 10.1128/JB.178.7.2150–2153.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mäder U, Hennig S, Hecker M, Homuth G. Transcriptional organization and posttranscriptional regulation of the Bacillus subtilis branched-chain amino acid biosynthesis genes. J Bacteriol. 2004;186: 2240–2252. doi: 10.1128/JB.186.8.2240-2252.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lei T, Yang J, Zheng L, Markowski T, Witthuhn BA, Ji Y. The essentiality of staphylococcal Gcp is independent of its repression of branched-chain amino acids biosynthesis. PLoS One. 2012;7: e46836 doi: 10.1371/journal.pone.0046836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lei T, Yang J, Ji Y. Determination of essentiality and regulatory function of staphylococcal YeaZ in branched-chain amino acid biosynthesis. Virulence. Taylor & Francis; 2014;6: 75–84. doi: 10.4161/21505594.2014.986415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.den Hengst CD, Curley P, Larsen R, Nauta A, Sinderen D Van, Kuipers OP, et al. Probing direct interactions between CodY and the oppD promoter of Lactococcus lactis. J Bacteriol. 2005;187: 512–521. doi: 10.1128/JB.187.2.512-521.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villapakkam AC, Handke LD, Belitsky BR, Levdikov VM, Wilkinson AJ, Sonenshein AL. Genetic and biochemical analysis of the interaction of Bacillus subtilis CodY with branched-chain amino acids. J Bacteriol. 2009;191: 6865–76. doi: 10.1128/JB.00818-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han AR, Kang H-RR, Son J, Kwon DH, Kim S, Lee WC, et al. The structure of the pleiotropic transcription regulator CodY provides insight into its GTP-sensing mechanism. Nucleic Acids Res. Oxford University Press; 2016;44: 9483–9493. doi: 10.1093/nar/gkw775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Majerczyk CD, Sadykov MR, Luong TT, Lee C, Somerville GA, Sonenshein AL. Staphylococcus aureus CodY negatively regulates virulence gene expression. J Bacteriol. 2008;190: 2257–2265. doi: 10.1128/JB.01545-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grigg JC, Ke A. Sequence, structure, and stacking: Specifics of tRNA anchoring to the T box riboswitch. RNA Biol. Taylor & Francis; 2013;10: 1761–1764. doi: 10.4161/rna.26996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Godon JJ, Chopin MC, Ehrlich SD. Branched-chain amino acid biosynthesis genes in Lactococcus lactis subsp. lactis. J Bacteriol. American Society for Microbiology; 1992;174: 6580–9. doi: 10.1128/JB.174.20.6580–6589.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keilhauer C, Eggeling L, Sahm H. Isoleucine synthesis in Corynebacterium glutamicum: molecular analysis of the ilvB-ilvN-ilvC operon. J Bacteriol. American Society for Microbiology; 1993;175: 5595–603. doi: 10.1128/JB.175.17.5595–5603.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pátek M, Krumbach K, Eggeling L, Sahm H. Leucine synthesis in Corynebacterium glutamicum: enzyme activities, structure of leuA, and effect of leuA inactivation on lysine synthesis. Appl Environ Microbiol. American Society for Microbiology; 1994;60: 133–40. Available: http://www.ncbi.nlm.nih.gov/pubmed/8117072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Millman A, Dar D, Shamir M, Sorek R. Computational prediction of regulatory, premature transcription termination in bacteria. Nucleic Acids Res. Oxford University Press; 2017;45: 886–893. doi: 10.1093/nar/gkw749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eisensteins E. Cloning, Expression, Purification, and Characterization of Biosynthetic Threonine Deaminase from Escherichia coli. 1991;266: 5801–5807. Available: http://www.jbc.org/content/266/9/5801.full.pdf [PubMed] [Google Scholar]
- 67.Shulman A, Zalyapin E, Vyazmensky M, Yifrach O, Barak Z, Chipman DM. Allosteric regulation of Bacillus subtilis threonine deaminase, a biosynthetic threonine deaminase with a single regulatory domain. Biochemistry. American Chemical Society; 2008;47: 11783–11792. doi: 10.1021/bi800901n [DOI] [PubMed] [Google Scholar]
- 68.Olson ME, Nygaard TK, Ackermann L, Watkins RL, Zurek OW, Pallister KB, et al. Staphylococcus aureus nuclease is an SaeRS-dependent virulence factor. Infect Immun. American Society for Microbiology; 2013;81: 1316–24. doi: 10.1128/IAI.01242-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krismer B, Liebeke M, Janek D, Nega M, Rautenberg M, Hornig G, et al. Nutrient limitation governs Staphylococcus aureus metabolism and niche adaptation in the human nose. Gilmore MS, editor. PLoS Pathog. 2014;10: e1003862 doi: 10.1371/journal.ppat.1003862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lobel L, Sigal N, Borovok I, Ruppin E, Herskovits AA. Integrative genomic analysis identifies isoleucine and CodY as regulators of Listeria monocytogenes virulence. PLoS Genet. 2012;8: e1002887 doi: 10.1371/journal.pgen.1002887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stenz L, Francois P, Whiteson K, Wolz C, Linder P, Schrenzel J, et al. The CodY pleiotropic repressor controls virulence in gram-positive pathogens. FEMS Immunol Med Microbiol. Oxford University Press; 2011;62: 123–139. doi: 10.1111/j.1574-695X.2011.00812.x [DOI] [PubMed] [Google Scholar]
- 72.Ibberson CB, Jones CL, Singh S, Wise MC, Hart ME, Zurawski D V., et al. Staphylococcus aureus hyaluronidase is a CodY-regulated virulence factor. Infect Immun. 2014;82: 4253–4264. doi: 10.1128/IAI.01710-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roux A, Todd D a., Velázquez J V., Cech NB, Sonenshein AL. Cody-mediated regulation of the Staphylococcus aureus Agr system integrates nutritional and population density signals. J Bacteriol. 2014;196: 1184–1196. doi: 10.1128/JB.00128-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Montgomery CP, Boyle-Vavra S, Roux A, Ebine K, Sonenshein AL, Daum RS. CodY deletion enhances in vivo virulence of community-associated methicillin-resistant Staphylococcus aureus clone USA300. Infect Immun. 2012;80: 2382–9. doi: 10.1128/IAI.06172-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rivera FE, Miller HK, Kolar SL, Stevens SM. J, Shaw LN. The impact of CodY on virulence determinant production in community-associated methicillin-resistant Staphylococcus aureus. Proteomics. 2012;12: 263–8. doi: 10.1002/pmic.201100298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vitreschak AG, Lyubetskaya E V., Shirshin MA, Gelfand MS, Lyubetsky VA. Attenuation regulation of amino acid biosynthetic operons in proteobacteria: comparative genomics analysis. FEMS Microbiol Lett. Oxford University Press; 2004;234: 357–370. doi: 10.1016/j.femsle.2004.04.005 [DOI] [PubMed] [Google Scholar]
- 77.Reiß S, Pané-Farré J, Fuchs S, François P, Liebeke M, Schrenzel J, et al. Global analysis of the Staphylococcus aureus response to mupirocin. Antimicrob Agents Chemother. 2012;56: 787–804. doi: 10.1128/AAC.05363-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Umbarger H. Evidence for a negative-feedback mechanism in the biosynthesis of isoleucine. Science. 1956;123: 848 Available: http://science.sciencemag.org/content/123/3202/848.1 [DOI] [PubMed] [Google Scholar]
- 79.Umbargar H, Brown B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. American Society for Microbiology (ASM); 1957;73: 105–12. Available: http://www.ncbi.nlm.nih.gov/pubmed/13405870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Umbarger H, Brown B. Isoleucine and valine metabolism in Escherichia coli. J Biol Chem. 1958;233: 415–420. [PubMed] [Google Scholar]
- 81.Kreiswirth BN, Löfdahl S, Betley MJ, O’Reilly M, Schlievert PM, Bergdoll MS, et al. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305: 709–712. [DOI] [PubMed] [Google Scholar]
- 82.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, et al. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. MBio. 2013;4: e00537–12. doi: 10.1128/mBio.00537-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mlynek KD, Sause WE, Moormeier DE, Sadykov MR, Hill KR, Bayles KW, Torres VJ, et al. Nutritional regulation of the Sae two component system by CodY in Staphylococcus aureus. Journal of Bacteriology. 2018; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Corrigan RM, Foster TJ. An improved tetracycline-inducible expression vector for Staphylococcus aureus. Plasmid. 2009;61: 126–129. doi: 10.1016/j.plasmid.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 85.Mesak LR, Yim G, Davies J. Improved lux reporters for use in Staphylococcus aureus. Plasmid. 2009;61: 182–187. doi: 10.1016/j.plasmid.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 86.Li H, Durbin R. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Bioinformatics. 2013;arXiv:1303. [Google Scholar]
- 87.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinformatics. Oxford University Press; 2009;25: 2078–9. doi: 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. American Society for Microbiology; 1995;177: 4121–30. doi: 10.1128/JB.177.14.4121–4130.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9: 671–675. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abreu-Goodger C, Merino E. RibEx: A web server for locating riboswitches and other conserved bacterial regulatory elements. Nucleic Acids Res. 2005;33 doi: 10.1093/nar/gki445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gruber AR, Lorenz R, Bernhart SH, Neuböck R, Hofacker IL. The Vienna RNA websuite. Nucleic Acids Res. 2008;36 doi: 10.1093/nar/gkn188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31: 3406–3415. doi: 10.1093/nar/gkg595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Griffiths-Jones S, Bateman A, Marshall M, Khanna A, Eddy SR. Rfam: an RNA family database. Nucleic Acids Res. Oxford University Press; 2003;31: 439–441. doi: 10.1093/nar/gkg006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. Oxford University Press; 2004;32: 1792–1797. doi: 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics. Oxford University Press; 2009;25: 1189–1191. doi: 10.1093/bioinformatics/btp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Katoh K, Frith MC. Adding unaligned sequences into an existing alignment using MAFFT and LAST. Bioinformatics. Oxford University Press; 2012;28: 3144–6. doi: 10.1093/bioinformatics/bts578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guédon E, Sperandio B, Pons N, Ehrlich SD, Renault P. Overall control of nitrogen metabolism in Lactococcus lactis by CodY, and possible models for CodY regulation in Firmicutes. Microbiology. 2005;151: 3895–909. doi: 10.1099/mic.0.28186-0 [DOI] [PubMed] [Google Scholar]
- 98.Wray L V, Fisher SH. Bacillus subtilis CodY operators contain overlapping CodY binding sites. J Bacteriol. 2011;193: 4841–8. doi: 10.1128/JB.05258-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Strains were pre-grown in TSB to mid-exponential phase, then sub-cultured into TSB for 16 hr. Supernatants were collected and proteins were precipitated using TCA. Protein samples were normalized to the equivalent of 5 ODs and run on a 12% SDS-PAGE gel.
(TIF)
Sequences of all annotated S. aureus T-boxes (based on the S. aureus subsp. aureus N315 genome NC_002745.2) and the B. subtilis ilvB T-box (NC_000964.3), labelled by regulated gene, were analyzed. Key features analyzed and annotated above the alignment are the AG Bulge (AGVGA-box), Distal Loop (GNUG-box); and the Specifier Loop (GAA…XXXA) where XXX represents the tRNA codon.
(TIF)
(A) Multiple sequence alignments of the top 15 hits from a BLAST search using the S. aureus ilvD promoter region were extracted and aligned as described in the methods. Dark blue shading represents conservation above 80%. Coding regions and key structures are labeled above and below the alignment. (B) Secondary structure predictions for the region aligned in the top panel. The start and stop codons are highlighted in green and red, respectively and the alternative pairing regions in the terminator/antiterminator are highlighted in yellow and blue.
(TIF)
6-FAM-labeled ilvD266p+ DNA fragment was incubated with increasing amounts of S. aureus CodY protein in the presence of GTP and A) isoleucine, B) leucine, C) valine, or all three amino acids (ILV). Concentrations of CodY used (nM of monomer) are indicated below each lane. Unbound DNA fragments are indicated by the right-pointing open arrowheads; CodY:ilvD266p+ complexes are indicated by the right-pointing closed arrowheads. Data are representative of at least two independent experiments.
(TIF)
The ilvD promoter region was aligned across 168 complete S. aureus genomes. In (A), the location of the mutations is indicated relative to the transcription start site identified by Majercyzk et al., 2010 [9]. The number of strains containing the mutation is indicated in brackets. Mutations in green are the SNPs identified in this study. Shown in (B) are the CodY binding sites identified by Majercyzk et al., 2010 [9], with the canonical CodY motif in bold and italicized, along with the predicted anti-terminator and terminator sequences. In (C) is listed the currently available strains for which genomes have the identified SNPs that are shown in panel A.
(TIF)
Data Availability Statement
Sequence data are deposited in NCBI under accession SRP126843 (https://www.ncbi.nlm.nih.gov/sra/SRP126843). All other relevant data are within the paper and its Supporting Information files.