Summary
Infections with tick-transmitted Borreliella (Borrelia) burgdorferi, the cause of Lyme disease, represent an increasingly large public health problem in North America and Europe. The ability of these spirochetes to maintain themselves for extended periods of time in their tick vectors and vertebrate reservoirs is crucial for continuance of the enzootic cycle as well as for the increasing exposure of humans to them. The stringent response mediated by the alarmone (p)ppGpp has been determined to be a master regulator in B. burgdorferi. It modulates the expression of identified and unidentified open reading frames needed to deal with and overcome the many nutritional stresses and other challenges faced by the spirochete in ticks and animal reservoirs. The metabolic and morphologic changes resulting from activation of the stringent response in B. burgdorferi may also be involved in the recently described non-genetic phenotypic phenomenon of tolerance to otherwise lethal doses of antimicrobials and to other antimicrobial activities. It may thus constitute a linchpin in multiple aspects of infections with Lyme disease borrelia, providing a link between the micro-ecological challenges of its enzootic life-cycle and long-term residence in the tissues of its animal reservoirs, with the evolutionary side-effect of potential persistence in incidental human hosts.
Introduction
Borreliella (Borrelia) burgdorferi (Adeolu and Gupta, 2014; Barbour et al., 2017), the cause of Lyme disease, has a small but complex genome consisting of one large linear chromosome and multiple linear and circular plasmids, together comprising approximately 1,520 kbp with a G+C content of 28.6% (Fraser et al., 1997; Casjens et al., 2000). This spirochete has two two-component regulatory systems (TCS), three sigma factors, is totally dependent on anaerobic glycolysis to generate ATP (i.e., it has no enzymes of the tricarboxylic cycle), and as such, is unable to synthesize de novo amino acids, nucleotides and fatty acids (Fraser et al., 1997; Radolf et al., 2012; Corona and Schwartz, 2015). It is thus a fastidious auxotroph whose nutritional requirements are still undetermined (Gherardini et al., 2010; Corona and Schwartz, 2015).
Despite the paucity of TCS compared to other bacterial pathogens, B. burgdorferi is still able to overcome the challenges encountered in infecting, colonizing and surviving long-term in ticks and vertebrates (Radolf et al., 2012; Corona and Schwartz, 2015). The regulatory axis mediated by the histidine kinase 1 (Hk1)-response regulator 1 (Rrp1) TCS is involved in tick colonization (Rogers et al., 2009; Freedman et al., 2010; He et al., 2011; Pappas et al., 2011; Caimano et al., 2015). It generates the second messenger cyclic diguanylate monophosphate (c-di-GMP) and stimulates utilization of glycerol and other functions necessary for survival in ticks (Rogers et al., 2009; Freedman et al., 2010; He et al., 2011; Pappas et al., 2011; He et al., 2014; Novak et al., 2014). In contrast, the regulatory axis mediated by RpoN, RpoS and the Hk2-Rrp2 TCS modulates expression of genes essential for tick transmission and mammalian infection (Hübner et al., 2001; Caimano et al., 2004; Fisher et al., 2005; Caimano et al., 2007; Smith et al., 2007; Boardman et al., 2008; Ouyang et al., 2008; Ouyang et al., 2012). This second TCS regulatory axis also represses glycerol utilization and many other functions needed by B. burgdorferi for proliferation in ticks but not in the mammalian host where glucose, a preferred carbon source, is readily available (Caimano et al., 2007; Corona and Schwartz, 2015).
In many pathogens, the ancestral stringent response triggered by amino acid starvation and mediated by the alarmones guanosine tetraphosphate and guanosine pentaphosphate (collectively referred to as (p)ppGpp or “magic spots”) is involved in coordinated regulation of many genes and regulatory and metabolic pathways (Fig. 1) (Potrykus and Cashel, 2008; Dalebroux and Swanson, 2012; Boutte and Crosson, 2013; Hauryliuk et al., 2015; Liu et al., 2015; Steinchen and Bange, 2016). The stringent response links cell division, bacterial growth, intermediary metabolism, chemotaxis and motility, morphotypic transformations, and virulence properties necessary to survive environmental challenges. In some bacteria, (p)ppGpp is mainly synthesized and hydrolyzed by two enzymes, RelA and SpoT, in others chiefly by a single bifunctional enzyme, Rel or RSH (RelA/SpoT homolog), with both activities. Cytoplasmic redundant short alarmone synthetases and GTPases in some bacteria provide additional paths to (p)ppGpp regulation (Gaca et al., 2015a; Gaca et al., 2015b). The global changes in transcription seen with the stringent response are due to allosteric changes in RNA polymerase that modify its specificity for different promoters and are caused by the interaction of (p)ppGpp with the RNA polymerase β’ and ω subunits and with the small protein DksA (Mallik et al., 2006; Doniselli et al., 2015; Ross et al., 2016). In E. coli, the ability of (p)ppGpp to repress or trigger transcription of different promoters is provided by the presence of specific DNA sequences called discriminators (Potrykus and Cashel, 2008).
Fig. 1. The stringent response in B. burgdorferi.
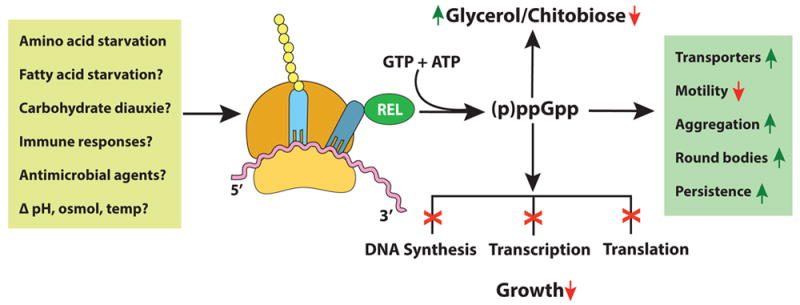
Potential triggers and consequences of the stringent response that could facilitate borrelial adaptation to microenvironmental challenges in the mammal and in the tick vector. Factors shown in the box on the left induce the activity of RelBbu, which converts ATP and GTP into the alarmone (p)ppGpp. The effects on the bacteria include altered rates of growth and motility, regulation of transport of metabolites, shifts in sugars (diauxie), amino acids and lipid utilization, and different morphotypes. Many of these changes enable persistence in ticks and mammals and the progression and maintenance of the enzootic cycle.
The limited number of TCS in B. burgdorferi suggests that alternative global regulators able to sense environmental conditions will be prominent in modulating gene expression in this organism (Radolf et al., 2012; Corona and Schwartz, 2015; Caimano et al., 2016). Extrapolations based on results obtained with E. coli and other bacteria can provide important clues to this analysis, with the understanding that the functions and interactions of borrelial orthologs might not always solely be determinable by extrapolation from these other organisms (Hyde et al., 2006; Caimano et al., 2007). We long ago suggested that the stringent response was likely to be involved in the ability of B. burgdorferi to survive and persist in its vector and vertebrate hosts (Godfrey et al., 2002). Several studies have subsequently confirmed that the stringent response is a global regulator in B. burgdorferi, and in fact is the only such regulator that can simultaneously modulate DNA replication, synthesis of stable RNAs (tRNA and rRNA), and synthesis and translation of mRNA in this organism (Fig. 1) (Bugrysheva et al., 2015; Drecktrah et al., 2015). We now review evidence revealing that the stringent response plays an important role in the perpetuation of the B. burgdorferi enzootic cycle, suggest where it may exert its functions in the enzootic cycle, and indicate how these might be recruited to pathological ends in hosts outside it (Figs. 2 and 3).
Fig. 2. Hypothetical role of the B. burgdorferi stringent response in the I. scapularis reservoir and vector.
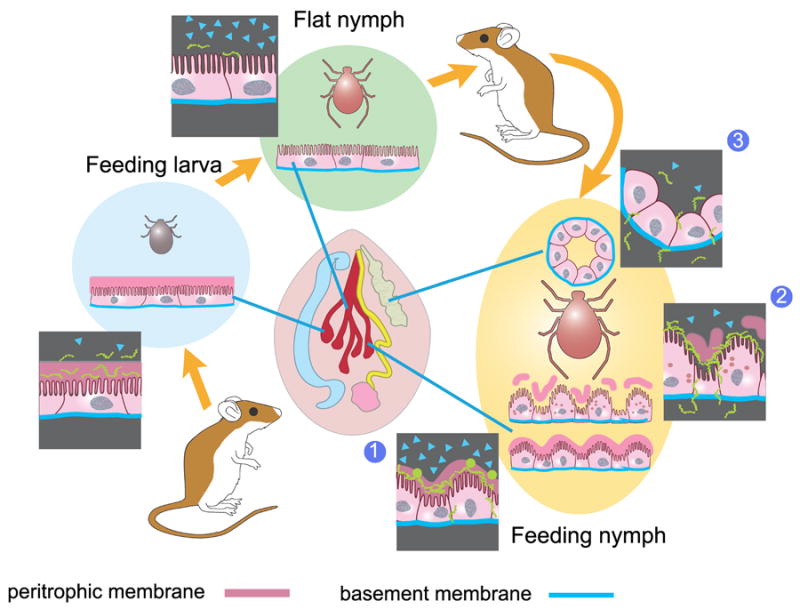
Modulation of bacterial growth mediated by the stringent response is crucial for its adaptation to nutritional challenges. Internal organs of the tick are shown in the central panel (dark red: gut; light green: salivary gland). In feeding larvae during acquisition of spirochetes (light blue circle), rapid growth in the gut (cell layer and magnified inset) results from attenuation of the stringent response within bacterial cells. In flat nymphs (bright green circle)., high levels of (p)ppGpp within the bacteria together with other regulatory molecules stimulate utilization of glycerol and decreased spirochete motility, and appearance of persister cells in the gut lumen via the stringent response. This state continues at the early transmission stage (yellow oval) (1) in the feeding nymph where the stringent response might be involved in spirochete bleb formation, generation of reversible epithelium-associated biofilm-like spirochete networks, round forms and persisters in the gut (cell layers and insets). Later (2), attenuation of the stringent response associated with irruption of blood into the tick gut activates spirochete motility at the basement membrane and migration to the haemocele and the salivary glands (3). Degradation of the peritrophic membrane, produced by enzymes from gut cells and the blood, generates chitobiose, the metabolism of which is derepressed by attenuation of the stringent response and low levels of (p)ppGpp. The shift from glycerol utilization to chitobiose utilization may also be a stimulus for the generation of persister cells. ▲ = (p)ppGpp in borrelia cells
Fig. 3. Hypothetical role of the B. burgdorferi stringent response in the P. leucopus reservoir.
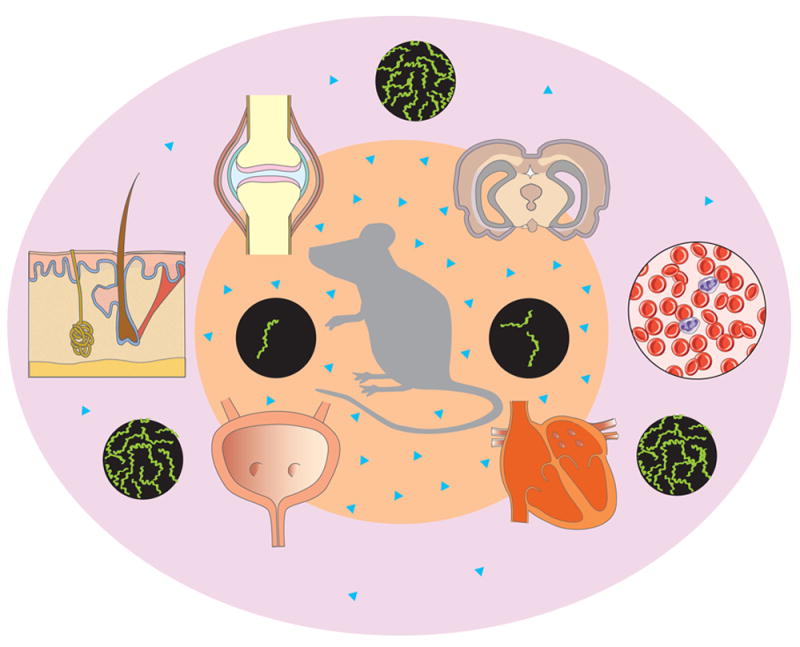
Transmission of B. burgdorferi into the dermis of mice, usually by I. scapularis nymphs, generates an acute infection. In the dermal environment with high levels of glucose and other nutrients, borrelia begin to display an attenuated stringent response with low levels of (p)ppGpp, which in turn enables rapid borrelial division, multiplication and motility. These rapidly dividing and motile bacteria subsequently invade adjacent areas of the dermis, bloodstream and various organs, reaching relatively high concentrations (outer pink circle, attenuated stringent response). After several weeks, as a result of the immune response, borrelia disappear from blood, and few remain in connective tissues. These low numbers of borrelia display an activated stringent response and high levels of (p)ppGpp in response to nutritional depletion and other stimuli, including the potential ability of the immune response to block the uptake of nutrients by borrelial transporters. These spirochetes will probably be slow moving, transcription competent and persisters (inner orange circle, high concentration of (p)ppGpp). ▲ = (p)ppGpp in borrelia cells
RelBbu and (p)ppGpp in B. burgdorferi
B. burgdorferi contains a single relBbu gene (BB0198) transcribed from its own σ70 promoter (Fraser et al., 1997). It encodes a bifunctional enzyme able to synthesize and hydrolyze (p)ppGpp and complement E. coli mutants unable to produce (p)ppGpp (Bugrysheva et al., 2003; Bugrysheva et al., 2005; Bugrysheva et al., 2015; Drecktrah et al., 2015). Null mutants of relBbu of non-infectious B. burgdorferi B31 and infectious N40 and B31-5A4 strains failed to generate (p)ppGpp, confirming its unique responsibility for the presence of the alarmone in this bacterium (Bugrysheva et al., 2005; Drecktrah et al., 2015). Growth of B. burgdorferi B31 in nutrient-limited RPMI media in the absence of rabbit sera and in the presence of tick saliva led to increases in the transcription of relBb and levels of (p)ppGpp, decreased synthesis of motility genes and the appearance of round forms (Alban et al., 2000; Concepcion and Nelson, 2003; Drecktrah et al., 2015). Experiments with wild-type infectious B. burgdorferi B31-5A4 and its null relBbu mutant and complemented derivatives confirmed the induction of (p)ppGpp under nutrient depletion and indicated that these markers decreased but did not totally disappear when B. burgdorferi cells were returned to an enriched media (Drecktrah et al., 2015). Moreover, both transcriptional and post-transcriptional expression of RelBbu was modulated by the host environment during growth in rat peritoneal chambers, in the presence of tick cells, and in ticks. Although regulation and the 5 levels of (p)ppGpp appeared to be strain dependent, (p)ppGpp was always present in B. burgdorferi B31 and N40 growing in BSK-H (Bugrysheva et al., 2002; Bugrysheva et al., 2003; Bugrysheva et al., 2005). Further experiments are needed to characterize the nutritional requirements of B. burgdorferi and the stimuli that trigger the stringent response in various environments.
Absence of (p)ppGpp in a B. burgdorferi 297 relBbu null mutant grown in BSK-H media was associated with a paradoxical growth deficit resulting from the slowing of cell division, most dramatically at the stationary phase (Bugrysheva et al., 2005). Transition to stationary phase from exponential phase in this mutant was associated with abnormally high levels of rRNA, similar to that seen during unbalanced growth of the relaxed E. coli mutant (Gallant and Cashel, 1967; Lazzarini et al., 1971; Bugrysheva et al., 2011). This suggests that the observed growth deficit in the stationary phase may be due to aberrant gene expression and may indicate that (p)ppGpp is an important regulator of balanced growth in B. burgdorferi (Fig. 1) (Bugrysheva et al., 2005; Potrykus et al., 2011; Bugrysheva et al., 2011). Furthermore, a B. burgdorferi B31-5A4 null relBbu derivative grown in RMPI media to stationary phase lost viability and exhibited a significant number of borrelial round bodies, thus confirming the presence of these structures in the life cycle of the organism (Brorson and Brorson, 1997; Alban et al., 2000; Dunham-Ems et al., 2012; Drecktrah et al., 2015).
The stringent response is a global regulator in B. burgdorferi
A global gene regulation pattern corresponding to a potential stringent response was observed in early microarray studies of wild type B. burgdorferi 297 exposed to in vitro growth conditions that mimicked those in ticks (Revel et al., 2002). Comparative microarray analysis of this strain and its relBbu null mutant found altered expression of many genes in both the exponential (6%) and stationary phases (20%) of growth (Bugrysheva et al., 2015). Analysis of similar mutant derivatives of B. burgdorferi B31-5A4 by RNA-seq showed the stringent response to be an important global regulator, especially in the stationary phase. In these latter studies, (p)ppGpp could shift gene expression both positively and negatively from that characteristic of stationary phase to that of starvation and recovery (Drecktrah et al., 2015). The B. burgdorferi stringent response modulated expression of genes mediating DNA synthesis and repair, proteins synthesis, cell division and cell envelope synthesis, motility and chemotaxis and intermediary metabolism (Bugrysheva et al., 2015; Drecktrah et al., 2015). Lipoprotein genes, including several adhesins and a decorin binding protein (dbpB) as well as the antigenic variation surface exposed lipoproteins (VslEs) were also modulated by (p)ppGpp in the stationary phase (Drecktrah et al., 2015).
The B. burgdorferi stringent response also modulates expression of regulatory and structural genes affecting important biochemical pathways such as carbon source and amino acid metabolism (Bugrysheva et al., 2015; Drecktrah et al., 2015). For example, lack of synthesis of (p)ppGpp was accompanied by increased transcription of the genes of the stringent response regulator DksA, σ70 and the Rrp1/Hk1 TCS. This suggests some repression of these global regulators during the stringent response (Bugrysheva et al., 2015). Transcription of genes encoding regulatory proteins CsrA, RpoS and BosR were also modulated by the borrelial stringent response, implying that (p)ppGpp may modulate gene expression indirectly through other global regulators (Bugrysheva et al., 2015; Drecktrah et al., 2015). Metabolic genes positively regulated during the stringent response included those involved in metabolism of glycerol, a sugar utilized by B. burgdorferi in ticks, and genes involved in oligopeptide transport, as would be expected as a response to lack of nutrients (Bugrysheva et al., 2015; Drecktrah et al., 2015). Genes negatively regulated by the stringent response in B. burgdorferi included those involved in the metabolism of chitobiose, an alternative sugar also present in ticks and utilized by B. burgdorferi and the mevalonate pathway genes hmg and mvaA involved in cell wall and membrane synthesis (Fig. 2) (Rhodes et al., 2009; Van Laar et al., 2012; Bugrysheva et al., 2015; Drecktrah et al., 2015).
The alarmone (p)ppGpp is thus an important global regulator of B. burgdorferi. It can modulate expression of approximately 30% of its genes during both exponential and stationary phases of growth and during shifts to and from starvation and repletion, and redirect borrelia metabolism accordingly (Bugrysheva et al., 2015; Drecktrah et al., 2015). Although more than half of regulated B. burgdorferi genes encode currently hypothetical proteins, the patterns of altered regulation of known genes in this organism generally correspond to those associated with the stringent response in other bacteria (Potrykus and Cashel, 2008; Dalebroux and Swanson, 2012; Boutte and Crosson, 2013; Hauryliuk et al., 2015; Liu et al., 2015). The short half-life of (p)ppGpp means that reversal of the B. burgdorferi stringent response triggered by starvation and other still uncharacterized stimuli is rapid once their stimuli subside which helps it quickly accommodate to the microenvironmental changes associated with borrelial residence in ticks and vertebrates (Bugrysheva et al., 2015; Drecktrah et al., 2015; Bergkessel et al., 2016). B. burgdorferi, like other bacteria, produces basal detectable levels of (p)ppGpp in the absence of nutritional stimuli (Bugrysheva et al., 2005; Drecktrah et al., 2015; Gaca et al., 2015a; Gaca et al., 2015b); the function of these basal levels of (p)ppGpp in global gene regulation deserves further investigation.
The stringent response during acquisition and transmission of B. burgdorferi by Ixodes larvae and nymphs
After feeding to repletion on an infected mammalian host, each larva contains about 500 B. burgdorferi per tick (Piesman et al., 1990; Soares et al., 2006). By 10 days, this rises to less than 3,000 just before molting into nymphs is completed and the reservoir blood is absorbed. Once molting is completed, the concentration of B. burgdorferi in the unfed nymphs is less than 100 organisms per tick, probably because of a lack of nutrients and exposure to the tick’s innate immune responses and microbiome-mediated antibacterial properties (Piesman et al., 1990; Soares et al., 2006; Narasimhan et al., 2014). In contrast, there is a much larger range in numbers of B. burgdorferi in feeding nymphs during transmission, with less than 100 spirochetes in the flat nymph at the start of feeding to close to 105 at repletion (Fig. 2) (Piesman et al., 1990). These changes appear to be tied to the increase and/or decrease of nutritional components provided by the blood meal, the blood meal’s antibacterial properties, and antibacterial components present in the tick (Piesman et al., 1990; Soares et al., 2006; Pal and Fikrig, 2010). Involvement of the stringent response in the ability of B. burgdorferi to traverse tick metamorphosis and transmission to a new host (Fig. 2) is suggested by its ability to regulate growth and modulate expression of genes involved in glycerol and chitobiose metabolism, both carbon sources crucial to its survival in larvae and nymphs (Fig. 1) (He et al., 2011; Pappas et al., 2011; Bugrysheva et al., 2015; Drecktrah et al., 2015). In fact, B. burgdorferi relBbu null mutants unable to produce (p)ppGpp acquired by nymphs from infected mice could not reach sufficiently high concentrations to permit transmission and completion of the enzootic cycle (Drecktrah et al., 2015).
We suggest that the B. burgdorferi stringent response is unlikely to be active in feeding larvae during acquisition because blood entering the tick gut contains amino acids, glucose, other sugars and fatty acids (Fig. 2) (Radolf et al., 2012; Corona and Schwartz, 2015). By the time larvae finish feeding and molting, the blood meal has been digested and its nutrients completely absorbed. This lack of nutrients stimulates the stringent response and results in non-dividing borrelia with inhibited growth and sluggish motility in the flat nymphs (Dunham-Ems et al., 2009; Pal and Fikrig, 2010; Corona and Schwartz, 2015). After the flat nymphs start to feed, we assume that the stringent response continues for a short period of time but is blunted by the blood entering the tick gut. The hypothesized active B. burgdorferi stringent response in the first 24-48 h of nymph feeding would coincide with observed borrelial cell blebs, transient round forms and formation of biofilm-like networks and aggregates, and expression of borrelial adhesins for tick epithelial cell receptors together with adhesion-mediated motility through the gut epithelial cells (Fig. 2) (Pal et al., 2004; Ferullo and Lovett, 2008; Traxler et al., 2008; Srivastava and de Silva, 2009; Dunham-Ems et al., 2009; Zhang et al., 2011; Dunham-Ems et al., 2012; Meriläinen et al., 2015; Gupta et al., 2016). It is plausible that the stringent response mediates these processes since the stringent response in B. burgdorferi (like that in other bacteria) modulates expression of genes of the mevalonate pathway involved in cell wall morphogenesis, inhibition of motility, and formation of aggregates, biofilms and quorum sensing (Potrykus and Cashel, 2008; Dalebroux and Swanson, 2012; Boutte and Crosson, 2013; Arnold et al., 2015; Bugrysheva et al., 2015; Drecktrah et al., 2015; Gupta et al., 2016). After 48 h of feeding, as nutrients from the blood meal begin to be utilized by tick gut epithelial cells and increased nutrients are available to the spirochetes, the stringent response will subside, and spirochetes will begin to divide and rapidly reach high concentrations (Piesman et al., 1990; Dunham-Ems et al., 2009). By the time spirochetes reach the basement membrane of the tick gut epithelia, motility has been reactivated, and a fraction of motile borrelia migrate from the gut to the haematocele and the salivary glands (Fig. 2) (Dunham-Ems et al., 2009; Dunham-Ems et al., 2012).
These hypothesized shifts in activity of the B. burgdorferi stringent response are consistent with experimentally observed changes in transcription of genes associated with sugar utilization (Pappas et al., 2011; He et al., 2011; Bugrysheva et al., 2015; Corona and Schwartz, 2015; Drecktrah et al., 2015). Borrelia preferentially utilize glucose provided in feeding ticks by ingested blood (von Lackum and Stevenson, 2005; Corona and Schwartz, 2015). The suggested activation of the stringent response in flat nymphs is associated with glycerol being used as a carbon source (He et al., 2011; Pappas et al., 2011). With the hypothesized blunting of the stringent response as feeding in nymphs is being completed, utilization will shift from glycerol to glucose and chitobiose (Fig. 2) (Traxler et al., 2006; Pappas et al., 2011; Corona and Schwartz, 2015). The latter sugars only become available at this stage because of the reorganization of the peritrophic membrane produced by the incoming blood and by the sloughing of tick gut intestinal cells (Fig. 2) (Zhu et al., 1991; Dunham-Ems et al., 2009; Pal and Fikrig, 2010; Pappas et al., 2011; Dunham-Ems et al., 2012; Corona and Schwartz, 2015).
Regulation of these metabolic shifts in B. burgdorferi by other global regulators including RpoS, BosR, BadR and c-di-GMP as well as by (p)ppGpp underlines their relevance for the bacterial life cycle in the tick (Hyde et al., 2006; Hyde et al., 2010; Freedman et al., 2010; He et al., 2011; Pappas et al., 2011; Sze et al., 2012; Miller et al., 2013; Sze et al., 2013; Corona and Schwartz, 2015; Caimano et al., 2015; Ouyang and Zhou, 2015; Caimano et al., 2016). The progressive variations described here, in cell division, growth, morphotypes, motility, carbon source utilization and potential tolerance to noxious stimuli all appear to be necessary for borrelia to complete the tick cycle (Fig.2) (Bugrysheva et al., 2015; Drecktrah et al., 2015). The stimuli responsible for the stringent response in their transit through the ticks, although currently undetermined, can be reasonably assumed (as in other bacteria) to result from a lack of amino acids required for protein synthesis (Potrykus and Cashel, 2008; Dalebroux and Swanson, 2012; Boutte and Crosson, 2013; Bugrysheva et al., 2015; Drecktrah et al., 2015). This supposition is supported by the fact that the stringent response positively regulates the Opp transporter systems needed for the transport of oligopeptides, the intracellular source of these amino acids (Wang et al., 2002; Medrano et al., 2007; Bugrysheva et al., 2015; Drecktrah et al., 2015). The stringent response could also be generated by as yet uncharacterized mechanisms including the availability, or lack of, other nutrients such as fatty acids, sugars, metal ions, oxidative stress and changes in osmolarity or pH (Revel et al., 2002; Potrykus and Cashel, 2008; Boutte and Crosson, 2013; Bontemps-Gallo et al., 2016).
The stringent response and B. burgdorferi residence in vertebrate reservoirs
The emergence and spread of B. burgdorferi infections depends on its ability to take up residence in its tick vectors and vertebrate hosts for extended periods of time (i.e., its permanence) in a fashion that ensures continuity of the enzootic cycle (Radolf et al., 2012; Schotthoefer and Frost, 2015; Steere et al., 2016). While the estimated numbers of spirochetes in the salivary glands of nymphs during transmission are approximately 60 per gland, only some of them are injected and only a fraction of those injected are infectious (Leuba-Garcia et al., 1998; Ohnishi et al., 2001; Lima et al., 2005). This suggests that borrelia undergo a proliferative burst after a low dose inoculation in the skin (Fig. 3) (Leuba-Garcia et al., 1998; O’Rourke et al., 2013; Stupica et al., 2015). This has been directly confirmed both in humans with Lyme disease and in Peromyscus and Mus musculus mice infected with B. burgdorferi by either feeding nymphs or needle injection (Piesman et al., 1987; Piesman, 1989; Barthold et al., 1991; Liveris et al., 2002; Barthold et al., 2010b; Li et al., 2011).
The few spirochetes inoculated in the mouse dermis are exposed to appreciable concentrations of tissue nutrients and glucose, their preferred carbon source (von Lackum and Stevenson, 2005; Corona and Schwartz, 2015), and the stringent response probably abates. The rate of cell division in the skin rapidly increases; concentrations of 1 × 104 to 1 × 105 per mg tissue are reached in a few days, with dissemination towards the periphery of the site of inoculation (Liveris et al., 2002; O’Rourke et al., 2013; Stupica et al., 2015). This growth is accompanied by active motility mediated by chemotaxis and hematogenous and lymphatic spread to distant organs (Barthold et al., 1991; Wang et al., 2001; Wang, 2002; Barthold et al., 2010a; Kumar et al., 2015). Another burst of cell division and growth occurs on colonization of organs a few days later, and densities of approximately 1 × 104 spirochetes per mg tissue can be reached in this phase of infection (Fig. 3) (Barthold et al., 1991; Wang et al., 2001; Wang, 2002; Barthold et al., 2010b).
In time, bacterial multiplication subsides, probably as result of antibacterial immune responses and nutrient limitations. Months after infection, only scattered borrelia are detected in some organ refugia by light microscopy and PCR (Fig. 3) (Barthold et al., 1991; Zeidner et al., 2001; Barthold et al., 2010a). In animal models of B. burgdorferi infection, there is a predilection of the spirochetes for collagenous tissues, which may be the result of borrelial adhesins for receptor molecules on the cells of these tissues (Coburn et al., 2013; Imai et al., 2013; Brissette and Gaultney, 2014; Caine and Coburn, 2015; Kumar et al., 2015; Wager et al., 2015; Zhi et al., 2015) and/or the presence of antibodies preventing multiplication and invasion of other tissues and organs (Barthold et al., 1991; Bockenstedt et al., 2001; Barthold et al., 2006; Barthold et al., 2010b).
In mice, resurgence in the number of B. burgdorferi can occur many months after infection as antibody levels begin to wane (Barthold et al., 1993; Barthold et al., 2010b). The sparse numbers of bacteria in these tissues display a quiescent state with low motility and no evidence of multiplication, but are probably viable as evidenced by their transcriptional competence that may permit their resurgence under some conditions (Barthold et al., 1993; Liang et al., 2004; Cabello et al., 2007; Barthold et al., 2010b; Imai et al., 2013). In this apparently quiescent state in the collagenous tissues, it is expected that the stringent response will be activated because of nutrient limitation, and transport mechanisms for amino acids and other molecules will be stimulated (Wang et al., 2002; Medrano et al., 2007; Potrykus and Cashel, 2008; Boutte and Crosson, 2013; Hauryliuk et al., 2015; Liu et al., 2015; Bugrysheva et al., 2015; Drecktrah et al., 2015). This quiescent state could have the side-effect of making the borrelia tolerant to antimicrobials and immune activity and lead to spirochetal persistence (Fig. 3) (Lusitani et al., 2003; Barbour, 2012; Feng et al., 2014; Caskey and Embers, 2015; Sharma et al., 2015; Feng et al., 2015a).
This pattern of infection in the mammalian host suggests that following transmission of B. burgdorferi into the dermis by feeding nymphs, the stringent response is turned off because sufficient levels of nutrients are available in the dermis, blood and other host tissues (Corona and Schwartz, 2015). This could possibly account for the finding that mice were not infected following injection of a low concentration (1 × 104 cells) of a B. burgdorferi 297 relBbu mutant unable to synthetize (p)ppGpp (Bugrysheva et al., 2005). However, a similar B. burgdorferi mutant of a different strain (B. burgdorferi B31-5A4) was infectious at higher doses of organisms (1 × 105 to 1 × 106) (Drecktrah et al., 2015). The potential relevance of relBbu and (p)ppGpp for B. burgdorferi infection and continued presence in the mouse reservoir may thus be dependent on both the inoculated dose and the B. burgdorferi strain involved (Bugrysheva et al., 2003; Bugrysheva et al., 2005; Drecktrah et al., 2015). The ability of the stringent response to coordinate the changes needed by borrelia to transition rapidly from actively multiplying to quiescent states and back in vertebrate reservoirs and to insure its persistence and availability for the vector during acquisition argues for its role in this stage of the enzootic cycle (Fig. 3) (Barthold et al., 1991; Bugrysheva et al., 2003; Bugrysheva et al., 2005; Bugrysheva et al., 2015; Drecktrah et al., 2015).
Could the borrelial stringent response mediate antimicrobial tolerance and persistence as an adaptation for the enzootic cycle?
While still a matter of dispute, there are numerous reports of antimicrobial treatment unable to completely eliminate B. burgdorferi from the tissues of experimentally infected rodents and non-human primates (Bockenstedt et al., 2002; Hodzic et al., 2008; Wormser and Schwartz, 2009; Barthold et al., 2010b; Barbour, 2012; Embers et al., 2012; Embers and Barthold, 2012; Wormser et al., 2012; Hodzic et al., 2013; Iyer et al., 2013; Hodzic et al., 2014). In some instances, bacteria could be rescued from treated animals by xenodiagnoses; these rescued bacteria were non-culturable and displayed a decreased infectious potential (Bockenstedt et al., 2002; Hodzic et al., 2008; Embers et al., 2012). They appeared to be transcriptionally active in the reservoir, could be transmitted transstadially in ticks after xenodiagnoses, were able to infect SCID mice and produce pathological lesions, and could be transmitted by transplanted tissue (Hodzic et al., 2008; Barthold et al., 2010b; Embers and Barthold, 2012). This suggests that antimicrobial-tolerant forms described in vitro may have relevance in explaining their occurrence in the mammalian reservoir (Feng et al., 2014; Caskey and Embers, 2015; Sharma et al., 2015; Feng et al., 2015a; Feng et al., 2016).
Borrelia may become phenotypically (non-heritably) tolerant to antimicrobials. For example, calprotectin, a human neutrophil antibacterial protein, both inhibits Borrelia growth in vitro and makes the organism tolerant to penicillin (Lusitani et al., 2003; Montgomery et al., 2006). Stationary phase B. burgdorferi cells in culture can also become phenotypically tolerant to antimicrobials used in treating Lyme borreliosis such as ceftriaxone, doxycycline, and amoxicillin (Feng et al., 2014; Caskey and Embers, 2015; Sharma et al., 2015; Feng et al., 2015b). This tolerance appears to be a function of both cell concentration and growth phase, since mathematical and experimental analyses show these antimicrobial-tolerant bacteria to represent slow-growing variants whose prevalence is increased in the stationary phase (Feng et al., 2014; Caskey and Embers, 2015; Sharma et al., 2015; Feng et al., 2015a). RNAseq analysis also suggests that borrelia, like other bacteria, probably have multiple and redundant mechanisms to draw on for the development of such tolerance to antimicrobials (Lewis, 2010; Mok et al., 2015; Feng et al., 2015a). Intriguingly, B. burgdorferi tolerant to doxycycline and amoxicillin appear to have a pattern of gene modulation similar to that seen with the stringent response (Feng et al., 2015a).
The term “bacterial persistence” is used to describe the ability of pathogenic bacteria (“persisters”) to survive in infected host tissues despite the presence of effective levels of antimicrobials and antibacterial cellular and humoral immunity (Lewis, 2010; Nguyen et al., 2011; Balaban et al., 2013; Amato et al., 2014; Conlon et al., 2015; Michiels et al., 2016). Though its applicability to B. burgdorferi has been controversial (Wormser and Schwartz, 2009; Wormser et al., 2012; Iyer et al., 2013), persistence is a widely-accepted phenomenon in microbiology which in some instances can have therapeutic implications (Dahl et al., 2003; Lewis, 2010; Amato et al., 2014; Zhang, 2014; Putrins et al., 2015; Brauner et al., 2016; Chuang et al., 2016; Corrigan et al., 2016). Persisters, while non-dividing, appear to be metabolically active (Michiels et al., 2016). They are thus similar to viable but non-culturable (VBNC) organisms but are present at a lower concentration than VBNC organisms, and potentially capable of being rescued by media without antimicrobials and generating colonies (Amato et al., 2013; Amato and Brynildsen, 2014; Amato et al., 2014; Ayrapetyan et al., 2015; Orman et al., 2016; Michiels et al., 2016). Persisters also seem to be different from what have been termed dormant bacteria with a decreased rate of metabolism (Kim and Wood, 2017). Clearly, much work needs to be done to clarify these phenotypic differences. It should be noted that although persistence is phenotypic, the presence of persisters can also facilitate emergence of genetically antimicrobial-resistant bacteria, e.g., by mutation (Levin-Reisman et al., 2017).
It is also important to note that bacterial persistence with tolerance to antimicrobials may be generated by multiple and redundant mechanisms involving both regulatory and non-regulatory genes (Lewis, 2010; Zhang, 2014 ; Amato and Brynildsen, 2015; Mok et al., 2015; Feng et al., 2015a; Brauner et al., 2016; Kaldalu et al., 2016; Michiels et al., 2016). In E. coli and in many other bacteria (e.g., Salmonella, Mycobacterium tuberculosis), toxin-antitoxin (TA) systems have been widely characterized as responsible for stringent response-mediated extracellular and intracellular persistence (Korch et al., 2003; Germain et al., 2013, 2015; Maisonneuve et al., 2013; Maisonneuve and Gerdes, 2014; Gerdes and Maisonneuve, 2015; Harms et al., 2016). Because B. burgdorferi does not appear to have conventional Type I and II TA systems (most probably as a result of gene loss associated with its small genome) (Fraser et al., 1997; Makarova et al., 2009; Harms et al., 2016), unbalanced synthesis and hydrolysis of (p)ppGpp might act in their place to promote a persister phenotype (Amato and Brynildsen, 2015).
Increased frequency of persisters in B. burgdorferi might result from increases in the levels of (p)ppGpp generated by spontaneous variations in the synthetic/hydrolytic activity of relBbu followed by slow growth and tolerance to antimicrobials (Terekhova et al., 2002; Bugrysheva et al., 2005; Kotte et al., 2014; Bugrysheva et al., 2015; Amato and Brynildsen, 2015; Drecktrah et al., 2015). These borrelia would be expected to have metabolic characteristics of classical bacterial persisters in utilizing glycerol and being tolerant to antimicrobial peptides, variations in osmolarity, and reactive oxygen and nitrogen species (Bugrysheva et al., 2005; Bugrysheva et al., 2015; Drecktrah et al., 2015). That such a mechanism might be at work is suggested by the observed increased frequency of persisters in stationary phase cultures of B. burgdorferi, since the observed levels of (p)ppGpp in B. burgdorferi are higher in this growth phase; possibilities for spontaneous variations in (p)ppGpp levels may also arise under these conditions (Fig. 1) (Bugrysheva et al., 2005; Bugrysheva et al., 2015; Caskey and Embers, 2015; Drecktrah et al., 2015; Feng et al., 2015a; Sharma et al., 2015). Alternatively, (p)ppGpp and DksA might play a role in Borrelia comparable to the one they play in E. coli where they are involved in mediating the increasing numbers of persisters generated during the diauxic shift from glycerol to trehalose utilization (Amato and Brynildsen, 2015; Bugrysheva et al., 2015; Drecktrah et al., 2015).
The ability to shift between different carbon sources (diauxie), changes in intermediary metabolism, metabolic challenges, and exposure to human sera all appear to play an important role in the evolution of persisters (Amato et al., 2013; Amato et al., 2014; Amato and Brynildsen, 2014; Amato and Brynildsen, 2015; Mok et al., 2015; Putrins et al., 2015; Ayrapetyan et al., 2015). For example, independent modulation of glycerol and trehalose metabolism in E. coli is related to the formation of persisters (Spoering et al., 2006; Kuczynska-Wisnok et al., 2015). Such metabolic changes in carbon utilization would be expected to occur in B. burgdorferi as it transits the enzootic cycle and would therefore be expected to stimulate both the stringent response and the formation of persisters (Fig. 2) (Tilly et al., 2001; von Lackum and Stevenson, 2005; Rhodes et al., 2009; He et al., 2011; Pappas et al., 2011; Bugrysheva et al., 2015; Corona and Schwartz, 2015; Drecktrah et al., 2015). The role these metabolic alterations may play in the formation of persisters in B. burgdorferi therefore deserves detailed examination (Amato and Bryldnilsen, 2015; Corona and Schwartz, 2015; Troy et al., 2016). In addition, B. burgdorferi CgtA is a GTPase of the Obg family involved in (p)ppGpp degradation. In other bacteria it is involved in persistence and might be involved in persistence in B. burgdorferi because its repression by the stringent response might increase levels of (p)ppGpp (Drecktrah et al., 2015; Verstraeten et al., 2015; Gaca et al., 2015a; Steinchen and Bange, 2016).
Nutritional fluctuations in vertebrate tissues during development of chronic infections might also trigger the stringent response (Fig. 3) and create a bi-stable heterogeneous population of transcriptionally competent borrelia growing at different rates, with slow growing bacteria becoming tolerant to antimicrobials and innate and adaptive immunity (Kotte et al., 2014; Bugrysheva et al., 2015; Drecktrah et al., 2015). In infected vertebrate hosts, such refugia could be found in collagenous and other avascular tissues where borrelia are not multiplying. These tissues would include the aortic root, tendons and entheses associated with joints, and synovial and spinal fluids (Fig. 3) (Barthold et al., 2010b; Barbour, 2012; Bockenstedt et al., 2012; Embers and Barthold, 2012). In these tissues, host antibodies might increase the nutritional stress of borrelia, for example, by blunting uptake of oligopeptides and other essential substrates by Opp and other unknown transporters whose genes are induced by the borrelial stringent response (Wang et al., 2002; Medrano et al., 2007; Barthold et al., 2010a; Barthold et al., 2010b; Raju et al., 2011; Hodzic et al., 2014; Bugrysheva et al., 2015; Drecktrah et al., 2015). The stringent response, by its ability to generate persisters, may thus be crucial for progression of B. burgdorferi through its enzootic cycle.
Conclusions and outstanding questions
The ability of B. burgdorferi to utilize the stringent response to mediate metabolic shifts during cycling between its tick vector and its vertebrate reservoir likely involves coordination between global regulators such as Rel, RpoS, BosR, c-di-GMP, BadR, CsrA, and DksA (Tilly et al., 2001; Miller et al., 2013; Novak et al., 2014; Bugrysheva et al., 2015; Drecktrah et al., 2015; Corona and Schwartz, 2015; Ouyang and Zhou, 2015; Caimano et al., 2016). The role played by c-di-GMP in the Hk1-Rrp1 pathway in motility and in glycerol and chitobiose metabolism suggests that it has perhaps parallel and coordinated functions to that of (p)ppGpp, albeit most probably in response to different stimuli (Rhodes et al., 2009; Sultan et al., 2010; He et al., 2011; Pappas et al., 2011; Sze et al., 2013; Corona and Schwartz, 2015; Caimano et al., 2016). In M. smegmatis, for example, increased levels of (p)ppGpp and c-di-GMP act coordinately to decrease motility, facilitate aggregation and biofilm formation, and increase tolerance to antimicrobials (Gupta et al., 2016). Similarly, since null mutants of relBbu and RpoS increase the frequency of round form morphotypes in vitro and in vivo (Dunham-Ems et al., 2012; Drecktrah et al., 2015), they might also act coordinately at some stage to generate various morphotypes during B. burgdorferi migration in nymphal ticks (Dunham-Ems et al., 2012; Harms et al., 2016).
The global regulator DksA is an important factor in persistence and virulence in a number of pathogens (Azriel et al., 2015; Amato and Brynildsen, 2015; Holley et al., 2015), and while the stringent response in B. burdorgferi modulates production of DksA, it is not known whether the regulation achieved by (p)ppGpp in borrelia is due to interactions with DksA and RNA polymerase, or whether, as in other bacteria, an independent alternative DksA regulon exists (Bugrysheva et al., 2015; Holley et al., 2015). Similarly, identification of sequences functionally homologous to discriminators in the promoters of B. burgdorferi need further study (Potrykus and Cashel, 2008). The stringent response, like other global regulators of B. burgdorferi, can regulate expression of a succession of genes between the different stages of infection in ticks and mammals, with many common genes expressed across different stages (Bugrysheva et al., 2015; Drecktrah et al., 2015; Iyer et al., 2015). This ability creates a continuum of gene regulation in response to nutritional and other challenges, and insures the successful perseverance of the bacteria in its very demanding and complex enzootic cycle. It is yet another example of the role the stringent response has in maintenance of the enzootic cycles of vector-transmitted pathogens (Charity et al., 2009; Sun et al., 2009).
Both CsrA and the stringent response can modulate borrelial motility (Sze et al., 2011; Bugrysheva et al., 2015; Drecktrah et al., 2015). In E. coli, CsrA is also involved in glucose utilization through the phosphotransferase system, a system also present in B. burgdorferi (Corona and Schwartz, 2015; Leng et al., 2016). Because the networks of these two global regulators are heavily interlinked in other bacteria (where they also regulate virulence) (Edwards et al., 2011; Vinella et al., 2012; Romeo et al., 2013; Vakulskas et al., 2015), future investigations of the interactions between CsrA and the borrelial stringent response would be of great interest. The relevance of BadR, the growth phase regulator that upregulates expression of Rel and downregulates RpoS expression in B. burgdorferi should also be explored given the centrality of the stringent response and its potential interconnections with the two well characterized B. burgdorferi TCS axes (Miller et al., 2013; Ouyang and Zhou, 2015; Iyer and Schwartz, 2016). As BadR and (p)ppGpp both repress expression of genes involved in chitobiose utilization, it would clearly be relevant to ascertain whether they do so independently or if they constitute an epistatic regulatory cascade (Miller et al., 2013; Bugrysheva et al., 2015; Ouyang and Zhou, 2015). In B. burgdorferi as in other bacteria, (p)ppGpp might be involved in global regulation by directly binding to proteins and modifying their function, and pppGpp and ppGpp may have different and independent regulatory roles (Rymer et al., 2012; Mechold et al., 2013; Liu et al., 2015). Global regulation in B. burgdorferi by (p)ppGpp could also be mediated by its ability to modulate RNA transcription initiation of promoters depending on nucleotide concentrations (Krasny and Gourse, 2004; Hauryliuk et al., 2015) as a function of its ability to modify the GTP/ATP ratio by consumption of GTP during its synthesis and its inhibition of GTPases such as CgtA as is the case with other bacteria (Kriel et al., 2014; Hauryliuk et al., 2015; Drecktrah et al., 2015; Verstraeten et al., 2015; Gaca et al., 2015a).
If the stringent response is utilized in the enzootic life cycle of borrelia as we and others have suggested (Godfrey et al., 2002; Bugrysheva et al., 2005; Bugrysheva et al., 2015; Drecktrah et al., 2015; Caimano et al., 2016), its mobilization in different developmental stages and tissue types in its arthropod hosts and primary mammalian reservoirs can be expected to have been under strong evolutionary pressure with respect to tissue tropism and timing (Radolf et al., 2012; Caimano et al., 2016; Steere et al., 2016). In dead-end mammalian hosts such as humans which are not critical to the spirochete’s propagation, the spirochete’s ability to evade immune responses and antimicrobial treatment and take up residence in refugia would, by implication, be a side-effect of selection on other traits (a “spandrel” in the terminology of Gould and Lewontin (1979)). Its incidental origin would make it no less of a potential clinical problem if it were found to be involved in manifestations of late Lyme disease such as arthritis and post-treatment Lyme disease syndrome (Steere et al., 1994; Chandra et al., 2011; Arvikar and Steere, 2015; Steere et al., 2016).
Readily detectable borrelia tolerant to antimicrobials in suspension cultures and biofilms in vitro (Terekhova et al., 2002; Caskey and Embers, 2015; Sharma et al., 2015; Feng et al., 2015a), detection of B. burgdorferi gene expression in tick stages (Iyer et al., 2015), development of models of infection for vertebrate reservoirs, including potential refugia for persister spirochetes (Akins et al., 1998; Zambrano et al., 2004; Cabello et al., 2007; Iyer et al., 2015), and the appearance of apparently quiescent round forms under several kinds of environmental stresses (Brorson and Brorson, 1997; Alban et al., 2000; Dunham-Ems et al., 2012; Drecktrah et al., 2015; Meriläinen et al., 2015; Feng et al., 2016) suggest that mechanisms of these potentially clinically relevant phenomena may eventually be discovered (Bockenstedt et al., 2012; Marques et al., 2014; Steere et al., 2016). For example, the genetic and metabolic make-up of non-culturable B. burgdorferi tolerant to antimicrobials rescued from animals by xenodiagnoses could provide insight into the relationship between tolerance and resistance to these compounds (Bockenstedt et al., 2002; Embers et al., 2012; Marques et al., 2014). The use of recently developed genomic tools (Lybecker et al., 2014; Arnold et al., 2016; Wright et al., 2016; Adams et al., 2017), should permit ready isolation of multiple mutants of B. burgdorferi global regulators including relBbu that will allow assessment of their hierarchic order in gene regulation by epistasis and their role in borrelial permanence in the enzootic cycle and borrelial persistence in vitro and in vivo (Avery and Wasserman, 1992; Phillips, 2008). Dissection of these networks of interactions among global regulators of B. burgdorferi is essential for understanding the ability of this organism to persist in its hosts, its vectors and its enzootic cycle (Corona and Schwartz, 2015; Iyer et al., 2015), and will be critical to informing the design of relevant vaccines and antimicrobials (Wexselblatt et al., 2013; Syal et al., 2017).
Acknowledgments
This work was supported by NIH grant R01 AI48856 to F.C.C. We acknowledge discussions, comments and suggestions by Drs. Ira Schwartz, Nyles Charon, Monica Morici-Embers, Brian Stevenson, Radha Iyer, Denis Liveris and Sandra Aedo, and thank two anonymous reviewers for their insightful criticism and comments. We apologize to the many authors whose research could not be cited and discussed due to space limitations.
References
- Adams PP, Flores AC, Popitsch N, Bilusic I, Schroeder R, Lybecker M, Jewett MW. In vivo expression technology and 5’ end mapping of the Borrelia burgdorferi transcriptome identify novel RNAs expressed during mammalian infection. Nucleic Acids Res. 2017;45:775–792. doi: 10.1093/nar/gkw1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeolu M, Gupta RS. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: the emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex) Antonie van Leeuwenhoek. 2014;105:1049–1072. doi: 10.1007/s10482-014-0164-x. [DOI] [PubMed] [Google Scholar]
- Akins DR, Bourell KW, Caimano MJ, Norgard MV, Radolf JD. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Invest. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alban PS, Johnson PW, Nelson DR. Serum-starvation-induced changes in protein synthesis and morphology of Borrelia burgdorferi. Microbiology. 2000;146:119–127. doi: 10.1099/00221287-146-1-119. [DOI] [PubMed] [Google Scholar]
- Amato SM, Orman MA, Brynildsen MP. Metabolic control of persister formation in Escherichia coli. Mol Cell. 2013;50:475–487. doi: 10.1016/j.molcel.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Amato SM, Brynildsen MP. Nutrient transitions are a source of persisters in Escherichia coli biofilms. PLoS One. 2014;9:e93110. doi: 10.1371/journal.pone.0093110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato SM, Fazen CH, Henry TC, Mok WW, Orman MA, Sandvik EL, et al. The role of metabolism in bacterial persistence. Front Microbiol. 2014;5:70. doi: 10.3389/fmicb.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato SM, Brynildsen MP. Persister heterogeneity arising from a single metabolic. 2015 doi: 10.1016/j.cub.2015.06.034. [DOI] [PubMed] [Google Scholar]
- Arnold WK, Savage CR, Antonicello AD, Stevenson B. Apparent role for Borrelia burgdorferi LuxS during mammalian infection. Infect Immun. 2015;83:1347–1353. doi: 10.1128/IAI.00032-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold WK, Savage CR, Brissette CA, Seshu J, Livny J, Stevenson B. RNA-seq of Borrelia burgdorferi in multiple phases of growth reveals insights into the dynamics of gene expression, transcriptome architecture, and noncoding RNAs. PLoS One. 2016;11:e0164165. doi: 10.1371/journal.pone.0164165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, Wasserman S. Ordering gene function: the interpretation of epistasis in regulatory hierarchies. Trends Genet. 1992;8:312–316. doi: 10.1016/0168-9525(92)90263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. 2015;29:269–280. doi: 10.1016/j.idc.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayrapetyan M, Williams TC, Baxter R, Oliver JD. Viable but nonculturable and persister cells coexist stochastically and are Induced by human serum. Infect Immun. 2015;83:4194–4203. doi: 10.1128/IAI.00404-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azriel S, Goren A, Rahav G, Gal-Mor O. The stringent response regulator DksA is required for Salmonella enterica serovar Typhimurium growth in minimal medium, motility, biofilm formation, and intestinal colonization. Infect Immun. 2015;84:375–384. doi: 10.1128/IAI.01135-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban NQ, Gerdes K, Lewis K, McKinney JD. A problem of persistence: still more questions than answers? Nat Rev Microbiol. 2013;11:587–591. doi: 10.1038/nrmicro3076. [DOI] [PubMed] [Google Scholar]
- Barbour A. Remains of infection. J Clin Invest. 2012;122:2344–2346. doi: 10.1172/JCI63975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Aeolu M, Gupta RS. Division of the genus Borrelia into two genera (corresponding to Lyme disease and relapsing fever groups) reflects their genetic and phenotypic distinctiveness and will lead to a better understanding of these two groups of microbes (Margos et al. (2016) There is inadequate evidence to support the division of the genus Borrelia. Int J Syst Evol Microbiol. 2017;67:2058–2067. doi: 10.1099/ijsem.0.001815. [DOI] [PubMed] [Google Scholar]
- Barthold SW, Persing DH, Armstrong AL, Peeples RA. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, De Souza MS, Janotka JL, Smith AL, Persing DH. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, Hodzic E, Tunev S, Feng S. Antibody-mediated disease remission in the mouse model of Lyme borreliosis. Infect Immun. 2006;74:4817–4825. doi: 10.1128/IAI.00469-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, Cadavid D, Philipp M. Animal models of borreliosis. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010a. pp. 359–411. [Google Scholar]
- Barthold SW, Hodzic E, Imai DM, Feng S, Yang X, Luft BJ. Ineffectiveness of tigecycline against persistent Borrelia burgdorferi. Antimicrob Agents Chemother. 2010b;54:643–651. doi: 10.1128/AAC.00788-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergkessel M, Basta DW, Newman DK. The physiology of growth arrest: uniting molecular and environmental microbiology. Nat Rev Microbiol. 2016;14:549–562. doi: 10.1038/nrmicro.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman BK, He M, Ouyang Z, Xu H, Pang X, Yang XF. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect Immun. 2008;76:3844–3853. doi: 10.1128/IAI.00467-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenstedt LK, Kang I, Chang C, Persing D, Hayday A, Barthold SW. CD4+ T helper 1 cells facilitate regression of murine Lyme carditis. Infect Immun. 2001;69:5264–5269. doi: 10.1128/IAI.69.9.5264-5269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenstedt LK, Mao J, Hodzic E, Barthold SW, Fish D. Detection of attenuated, noninfectious spirochetes in Borrelia burgdorferi-infected mice after antibiotic treatment. J Infect Dis. 2002;186:1430–1437. doi: 10.1086/345284. [DOI] [PubMed] [Google Scholar]
- Bockenstedt LK, Gonzalez DG, Haberman AM, Belperron AA. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J Clin Invest. 2012;122:2652–2660. doi: 10.1172/JCI58813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps-Gallo S, Lawrence K, Gherardini FC. Two different virulence-related regulatory pathways in Borrelia burgdorferi are directly affected by osmotic fluxes in the blood meal of feeding Ixodes ticks. PLoS Pathog. 2016;12:e1005791. doi: 10.1371/journal.ppat.1005791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutte CC, Crosson S. Bacterial lifestyle shapes stringent response activation. Trends Microbiol. 2013;21:174–180. doi: 10.1016/j.tim.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner A, Fridman O, Gefen O, Balaban NQ. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol. 2016;14:320–330. doi: 10.1038/nrmicro.2016.34. [DOI] [PubMed] [Google Scholar]
- Brissette CA, Gaultney RA. That’s my story, and I’m sticking to it--an update on B. burgdorferi adhesins. Front Cell Infect Microbiol. 2014;4:41. doi: 10.3389/fcimb.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brorson O, Brorson SH. Transformation of cystic forms of Borrelia burgdorferi to normal, mobile spirochetes. Infection. 1997;25:240–246. doi: 10.1007/BF01713153. [DOI] [PubMed] [Google Scholar]
- Bugrysheva JV, Dobrikova EY, Godfrey HP, Sartakova ML, Cabello FC. Modulation of Borrelia burgdorferi stringent response and gene expression during extracellular growth with tick cells. Infect Immun. 2002;70:3061–3067. doi: 10.1128/IAI.70.6.3061-3067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugrysheva JV, Dobrikova EY, Sartakova ML, Caimano MJ, Daniels TJ, Radolf JD, et al. Characterization of the stringent response and relBbu expression in Borrelia burgdorferi. J Bacteriol. 2003;185:957–965. doi: 10.1128/JB.185.3.957-965.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugrysheva JV, Bryksin AV, Godfrey HP, Cabello FC. Borrelia burgdorferi rel is responsible for generation of guanosine-3’-diphosphate-5’-triphosphate and growth control. Infect Immun. 2005;73:4972–4981. doi: 10.1128/IAI.73.8.4972-4981.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugrysheva JV, Godfrey HP, Schwartz I, Cabello FC. Patterns and regulation of ribosomal RNA transcription in Borrelia burgdorferi. BMC Microbiol. 2011;11:17. doi: 10.1186/1471-2180-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugrysheva JV, Pappas CJ, Terekhova DA, Iyer R, Godfrey HP, Schwartz I, et al. Characterization of the RelBbu regulon in Borrelia burgdorferi reveals modulation of glycerol metabolism by (p)ppGpp. PLoS One. 2015;10:e0118063. doi: 10.1371/journal.pone.0118063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello FC, Godfrey HP, Newman SA. Hidden in plain sight: Borrelia burgdorferi and the extracellular matrix. Trends Microbiol. 2007;15:350–354. doi: 10.1016/j.tim.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Caimano MJ, Eggers CH, Hazlett KR, Radolf JD. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect Immun. 2004;72:6433–6445. doi: 10.1128/IAI.72.11.6433-6445.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, et al. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol. 2007;65:1193–1217. doi: 10.1111/j.1365-2958.2007.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Dunham-Ems S, Allard AM, Cassera MB, Kenedy M, Radolf JD. Cyclic di-GMP modulates gene expression in Lyme disease spirochetes at the tick-mammal interface to promote spirochete survival during the blood meal and tick-to-mammal transmission. Infect Immun. 2015;83:3043–3060. doi: 10.1128/IAI.00315-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Drecktrah D, Kung F, Samuels DS. Interaction of the Lyme disease spirochete with its tick vector. Cell Microbiol. 2016;18:919–927. doi: 10.1111/cmi.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine JA, Coburn J. A short-term Borrelia burgdorferi infection model identifies tissue tropisms and bloodstream survival conferred by adesion proteins. Infect Immun. 2015;83:3184–3194. doi: 10.1128/IAI.00349-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, Rosa P, et al. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- Caskey JR, Embers ME. Persister development by Borrelia burgdorferi populations in vitro. Antimicrob Agents Chemother. 2015;59:6288–6295. doi: 10.1128/AAC.00883-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A, Wormser GP, Marques AR, Latov N, Alaedini A. Anti-Borrelia burgdorferi antibody profile in post-Lyme disease syndrome. Clin Vaccine Immunol. 2011;18:767–771. doi: 10.1128/CVI.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charity JC, Blalock LT, Costante-Hamm MM, Kasper DL, Dove SL. Small molecule control of virulence gene expression in Francisella tularensis. PLoS Pathog. 2009;5:e1000641. doi: 10.1371/journal.ppat.1000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YM, Dutta NK, Hung CF, Wu TC, Rubin H, Karakousis PC. Stringent response factors PPX1 and PPK2 play an important role in Mycobacterium tuberculosis metabolism, biofilm formation, and sensitivity to isoniazid in vivo. Antimicrob Agents Chemother. 2016;60:6460–6470. doi: 10.1128/AAC.01139-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn J, Leong J, Chaconas G. Illuminating the roles of the Borrelia burgdorferi adhesins. Trends Microbiol. 2013;21:372–379. doi: 10.1016/j.tim.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concepcion MB, Nelson DR. Expression of spoT in Borrelia burgdorferi during serum starvation. J Bacteriol. 2003;185:444–452. doi: 10.1128/JB.185.2.444-452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon BP, Rowe SE, Lewis K. Persister cells in biofilm associated infections. Adv Exp Med Biol. 2015;831:1–9. doi: 10.1007/978-3-319-09782-4_1. [DOI] [PubMed] [Google Scholar]
- Corona A, Schwartz I. Borrelia burgdorferi: carbon metabolism and the tick-mammal enzootic cycle. Microbiol Spectr. 2015;3:10–2014. doi: 10.1128/microbiolspec.MBP-0011-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan RM, Bellows LE, Wood A, Grundling A. ppGpp negatively impacts ribosome assembly affecting growth and antimicrobial tolerance in Gram-positive bacteria. Proc Natl Acad Sci U S A. 2016;113:E1710–E1719. doi: 10.1073/pnas.1522179113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl JL, Kraus CN, Boshoff HI, Doan B, Foley K, Avarbock D, et al. The role of RelMtb-mediated adaptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc Natl Acad Sci U S A. 2003;100:10026–10031. doi: 10.1073/pnas.1631248100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalebroux ZD, Swanson MS. ppGpp: magic beyond RNA polymerase. Nat Rev Microbiol. 2012;10:203–212. doi: 10.1038/nrmicro2720. [DOI] [PubMed] [Google Scholar]
- Doniselli N, Rodriguez-Aliaga P, Amidani D, Bardales JA, Bustamante C, Guerra DG, et al. New insights into the regulatory mechanisms of ppGpp and DksA on Escherichia coli RNA polymerase-promoter complex. Nucleic Acids Res. 2015;43:5249–5262. doi: 10.1093/nar/gkv391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drecktrah D, Lybecker M, Popitsch N, Rescheneder P, Hall LS, Samuels DS. The Borrelia burgdorferi RelA/SpoT homolog and stringent response regulate survival in the tick vector and global gene expression during starvation. PLoS Pathog. 2015;11:e1005160. doi: 10.1371/journal.ppat.1005160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham-Ems SM, Caimano MJ, Pal U, Wolgemuth CW, Eggers CH, Balic A, et al. Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J Clin Invest. 2009;119:3652–3665. doi: 10.1172/JCI39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham-Ems SM, Caimano MJ, Eggers CH, Radolf JD. Borrelia burgdorferi requires the alternative sigma factor RpoS for dissemination within the vector during tick-to-mammal transmission. PLoS Pathog. 2012;8:e1002532. doi: 10.1371/journal.ppat.1002532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AN, Patterson-Fortin LM, Vakulskas CA, Mercante JW, Potrykus K, Vinella D, et al. Circuitry linking the Csr and stringent response global regulatory systems. Mo Microbiol. 2011;80:1561–1580. doi: 10.1111/j.1365-2958.2011.07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embers ME, Barthold SW. Borrelia burgdorferi persistence post-antibiotic treatment. In: Embers ME, editor. The Pathogenic Spirochetes: Strategies for Evasion of Host Immunity and Persistence. New York: Springer Science+Business Media; 2012. pp. 229–257. [Google Scholar]
- Embers ME, Barthold SW, Borda JT, Bowers L, Doyle L, Hodzic E, et al. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS One. 2012;7:e29914. doi: 10.1371/journal.pone.0029914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Wang T, Shi W, Zhang S, Sullivan D, Auwaerter PG, Zhang Y. Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emerging Microbes Infections. 2014;3:e49. doi: 10.1038/emi.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Shi W, Zhang S, Zhang Y. Persister mechanisms in Borrelia burgdorferi: implications for improved intervention. Emerg Microbes Infect. 2015a;4:e51. doi: 10.1038/emi.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Weitner M, Shi W, Zhang S, Sullivan D, Zhang Y. Identification of additional anti-persister activity against Borrelia burgdorferi from an FDA drug library. Antibiotics (Basel) 2015b;4:397–410. doi: 10.3390/antibiotics4030397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Shi W, Zhang S, Sullivan D, Auwaerter PG, Zhang Y. A drug combination screen identifies drugs active against amoxicillin-induced round bodies of in vitro Borrelia burgdorferi persisters from an FDA drug library. Front Microbiol. 2016;7:743. doi: 10.3389/fmicb.2016.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferullo DJ, Lovett ST. The stringent response and cell cycle arrest in Escherichia coli. PLoS Genet. 2008;4:e1000300. doi: 10.1371/journal.pgen.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MA, Grimm D, Henion AK, Elias AF, Stewart PE, Rosa PA, et al. Borrelia burgdorferi σ54 is required for mammalian infection and vector transmission but not for tick colonization. Proc Natl Acad Sci U S A. 2005;102:5162–5167. doi: 10.1073/pnas.0408536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- Freedman JC, Rogers EA, Kostick JL, Zhang H, Iyer R, Schwartz I, et al. Identification and molecular characterization of a cyclic-di-GMP effector protein, PlzA (BB0733): additional evidence for the existence of a functional cyclic-di-GMP regulatory network in the Lyme disease spirochete, Borrelia burgdorferi. FEMS Immunol Med Microbiol. 2010;58:285–294. doi: 10.1111/j.1574-695X.2009.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaca AO, Colomer-Winter C, Lemos JA. Many means to a common end: the intricacies of (p)ppGpp metabolism and its control of bacterial homeostasis. J Bacteriol. 2015a;197:1146–1156. doi: 10.1128/JB.02577-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaca AO, Kudrin P, Colomer-Winter C, Beljantseva J, Liu K, Anderson B, et al. From (p)ppGpp to (pp)pGpp: characterization of regulatory effects of pGpp synthesized by the small alarmone synthetase of Enterococcus faecalis. J Bacteriol. 2015b;197:2908–2919. doi: 10.1128/JB.00324-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J, Cashel M. On the mechanism of amino acid control of ribonucleic acid biosynthesis. J Mol Biol. 1967;25:545–553. doi: 10.1016/0022-2836(67)90205-7. [DOI] [PubMed] [Google Scholar]
- Gerdes K, Maisonneuve E. Remarkable functional convergence: alarmone ppGpp mediates persistence by activating Type I and II toxin-antitoxins. Mol Cell. 2015;59:1–3. doi: 10.1016/j.molcel.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Germain E, Castro-Roa D, Zenkin N, Gerdes K. Molecular mechanism of bacterial persistence by HipA. Mol Cell. 2013;52:248–254. doi: 10.1016/j.molcel.2013.08.045. [DOI] [PubMed] [Google Scholar]
- Germain E, Roghanian M, Gerdes K, Maisonneuve E. Stochastic induction of persister cells by HipA through (p)ppGpp-mediated activation of mRNA endonucleases. Proc Natl Acad Sci U S A. 2015;112:5171–5176. doi: 10.1073/pnas.1423536112. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gherardini F, Boylan JA, Lawrence K, Skare J. Metabolism and physiology of Borrelia. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Norfold, UK: Caister Academic Press; 2010. pp. 103–138. [Google Scholar]
- Godfrey HP, Bugrysheva JV, Cabello FC. The role of the stringent response in the pathogenesis of bacterial infections. Trends Microbiol. 2002;10:349–351. doi: 10.1016/s0966-842x(02)02403-4. [DOI] [PubMed] [Google Scholar]
- Gould SJ, Lewontin RC. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc R Soc Lond B Biol Sci. 1979;205:581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- Gupta KR, Baloni P, Indi SS, Chatterji D. Regulation of growth, cell shape, cell division, and gene expression by second messengers (p)ppGpp and cyclic di-GMP in Mycobacterium smegmatis. J Bacteriol. 2016;198:1414–1422. doi: 10.1128/JB.00126-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms A, Maisonneuve E, Gerdes K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science. 2016;354 doi: 10.1126/science.aaf4268. [DOI] [PubMed] [Google Scholar]
- Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, Gerdes K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat Rev Microbiol. 2015;13:298–309. doi: 10.1038/nrmicro3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Ouyang Z, Troxell B, Xu H, Moh A, Piesman J, et al. Cyclic di-GMP is essential for the survival of the Lyme disease spirochete in ticks. PLoS Pathog. 2011;7:e1002133. doi: 10.1371/journal.ppat.1002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Zhang JJ, Ye M, Lou Y, Yang XF. Cyclic Di-GMP receptor PlzA controls virulence gene expression through RpoS in Borrelia burgdorferi. Infect Immun. 2014;82:445–452. doi: 10.1128/IAI.01238-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic E, Feng S, Holden K, Freet KJ, Barthold SW. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob Agents Chemother. 2008;52:1728–1736. doi: 10.1128/AAC.01050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic E, Feng S, Barthold SW. Assessment of transcriptional activity of Borrelia burgdorferi and host cytokine genes during early and late infection in a mouse model. Vector Borne Zoonotic Dis. 2013;13:694–711. doi: 10.1089/vbz.2012.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic E, Imai D, Feng S, Barthold SW. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS One. 2014;9:e86907. doi: 10.1371/journal.pone.0086907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley CL, Zhang X, Fortney KR, Ellinger S, Johnson P, Baker B, et al. DksA and (p)ppGpp have unique and overlapping contributions to Haemophilus ducreyi pathogenesis in humans. Infect Immun. 2015;83:3281–3292. doi: 10.1128/IAI.00692-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner A, Yang X, Nolen DM, Popova TG, Cabello FC, Norgard MV. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc Natl Acad Sci U S A. 2001;98:12724–12729. doi: 10.1073/pnas.231442498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JA, Seshu J, Skare JT. Transcriptional profiling of Borrelia burgdorferi containing a unique bosR allele identifies a putative oxidative stress regulon. Microbiology. 2006;152:2599–2609. doi: 10.1099/mic.0.28996-0. [DOI] [PubMed] [Google Scholar]
- Hyde JA, Shaw DK, Smith R, III, Trzeciakowski JP, Skare JT. Characterization of a conditional bosR mutant in Borrelia burgdorferi. Infect Immun. 2010;78:265–274. doi: 10.1128/IAI.01018-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai DM, Feng S, Hodzic E, Barthold SW. Dynamics of connective-tissue localization during chronic Borrelia burgdorferi infection. Lab Invest. 2013;93:900–910. doi: 10.1038/labinvest.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Mukherjee P, Wang K, Simons J, Wormser GP, Schwartz I. Detection of Borrelia burgdorferi nucleic acids after antibiotic treatment does not confirm viability. J Clin Microbiol. 2013;51:857–862. doi: 10.1128/JCM.02785-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Caimano MJ, Luthra A, Axline D, Jr, Corona A, Iacobas DA, et al. Stage-specific global alterations in the transcriptomes of Lyme disease spirochetes during tick feeding and following mammalian host adaptation. Mol Microbiol. 2015;95:509–538. doi: 10.1111/mmi.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Schwartz I. Microarray-based comparative genomic and transcriptome analysis of Borrelia burgdorferi. Microarrays (Basel) 2016;5:E9. doi: 10.3390/microarrays5020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldalu N, Hauryliuk V, Tenson T. Persisters-as elusive as ever. Appl Microbiol Biotechnol. 2016;100:6545–6553. doi: 10.1007/s00253-016-7648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Wood TK. Tolerant, growing cells from nutrient shifts are not persister cells. mBio. 2017;8:e00354–17. doi: 10.1128/mBio.00354-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korch SB, Henderson TA, Hill TM. Characterization of the hipA7 allele of Escherichia coli and evidence that high persistence is governed by (p)ppGpp synthesis. Mol Microbiol. 2003;50:1199–1213. doi: 10.1046/j.1365-2958.2003.03779.x. [DOI] [PubMed] [Google Scholar]
- Kotte O, Volkmer B, Radzikowski JL, Heinemann M. Phenotypic bistability in Escherichia coli’s central carbon metabolism. Mol Syst Biol. 2014;10:736. doi: 10.15252/msb.20135022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasny L, Gourse RL. An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation. EMBO J. 2004;23:4473–4483. doi: 10.1038/sj.emboj.7600423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriel A, Brinsmade SR, Tse JL, Tehranchi AK, Bittner AN, Sonenshein AL, et al. GTP dysregulation in Bacillus subtilis cells lacking (p)ppGpp results in phenotypic amino acid auxotrophy and failure to adapt to nutrient downshift and regulate biosynthesis genes. J Bacteriol. 2014;196:189–201. doi: 10.1128/JB.00918-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczynska-Wisnik D, Stojowska K, Matuszewska E, Leszczynska D, Algara MM, Augustynowicz M, et al. Lack of intracellular trehalose affects formation of Escherichia coli persister cells. Microbiology. 2015;161:786–796. doi: 10.1099/mic.0.000012. [DOI] [PubMed] [Google Scholar]
- Kumar D, Ristow LC, Shi M, Mukherjee P, Caine JA, Lee WY, et al. Intravital imaging of vascular transmigration by the Lyme spirochete: requirement for the integrin binding residues of the B. burgdorferi P66 protein. PLoS Pathog. 2015;11:e1005333. doi: 10.1371/journal.ppat.1005333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini RA, Cashel M, Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971;246:4381–4385. [PubMed] [Google Scholar]
- Leng Y, Vakulskas CA, Zere TR, Pickering BS, Watnick PI, Babitzke P, et al. Regulation of CsrB/C sRNA decay by EIIA(Glc) of the phosphoenolpyruvate: carbohydrate phosphotransferase system. Mol Microbiol. 2016;99:627–639. doi: 10.1111/mmi.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuba-Garcia S, Martinez R, Gern L. Expression of outer surface proteins A and C of Borrelia afzelii in Ixodes ricinus ticks and in the skin of mice. Zentralbl Bakteriol. 1998;287:475–484. doi: 10.1016/s0934-8840(98)80187-4. [DOI] [PubMed] [Google Scholar]
- Levin-Reisman I, Ronin I, Gefen O, Braniss I, Shoresh N, Balaban NQ. Antibiotic tolerance facilitates the evolution of resistance. Science. 2017;355:826–830. doi: 10.1126/science.aaj2191. [DOI] [PubMed] [Google Scholar]
- Lewis K. Persister cells. Annu Rev Microbiol. 2010;64:357–72. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- Li X, McHugh GA, Damle N, Sikand VK, Glickstein L, Steere AC. Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema migrans or Lyme arthritis. Arthritis Rheum. 2011;63:2238–2247. doi: 10.1002/art.30384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FT, Brown EL, Wang T, Iozzo RV, Fikrig E. Protective niche for Borrelia burgdorferi to evade humoral immunity. Am J Pathol. 2004;165:977–985. doi: 10.1016/S0002-9440(10)63359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima CM, Zeidner NS, Beard CB, Soares CA, Dolan MC, Dietrich G, et al. Differential infectivity of the Lyme disease spirochete Borrelia burgdorferi derived from Ixodes scapularis salivary glands and midgut. J Med Entomol. 2005;42:506–510. doi: 10.1093/jmedent/42.3.506. [DOI] [PubMed] [Google Scholar]
- Liu K, Bittner AN, Wang JD. Diversity in (p)ppGpp metabolism and effectors. Curr Opin Microbiol. 2015;24:72–79. doi: 10.1016/j.mib.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liveris D, Wang G, Girao G, Byrne DW, Nowakowski J, McKenna D, et al. Quantitative detection of Borrelia burgdorferi in 2-millimeter skin samples of erythema migrans lesions: correlation of results with clinical and laboratory findings. J Clin Microbiol. 2002;40:1249–1253. doi: 10.1128/JCM.40.4.1249-1253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusitani D, Malawista SE, Montgomery RR. Calprotectin, an abundant cytosolic protein from human polymorphonuclear leukocytes, inhibits the growth of Borrelia burgdorferi. Infect Immun. 2003;71:4711–4716. doi: 10.1128/IAI.71.8.4711-4716.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lybecker M, Bilusic I, Raghavan R. Pervasive transcription: detecting functional RNAs in bacteria. Transcription. 2014;5:e944039. doi: 10.4161/21541272.2014.944039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve E, Castro-Camargo M, Gerdes K. (p)ppGpp controls bacterial persistence by stochastic induction of toxin-antitoxin activity. Cell. 2013;154:1140–1150. doi: 10.1016/j.cell.2013.07.048. [DOI] [PubMed] [Google Scholar]
- Maisonneuve E, Gerdes K. Molecular mechanisms underlying bacterial persisters. Cell. 2014;157:539–548. doi: 10.1016/j.cell.2014.02.050. [DOI] [PubMed] [Google Scholar]
- Makarova KS, Wolf YI, Koonin EV. Comprehensive comparative-genomic analysis of type 2 toxin-antitoxin systems and related mobile stress response systems in prokaryotes. Biol Direct. 2009;4:19. doi: 10.1186/1745-6150-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallik P, Paul BJ, Rutherford ST, Gourse RL, Osuna R. DksA is required for growth phase-dependent regulation, growth rate-dependent control, and stringent control of fis expression in Escherichia coli. J Bacteriol. 2006;188:5775–5782. doi: 10.1128/JB.00276-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques A, Telford SR, III, Turk SP, Chung E, Williams C, Dardick K, et al. Xenodiagnosis to detect Borrelia burgdorferi infection: a first-in-human study. Clin Infect Dis. 2014;58:937–945. doi: 10.1093/cid/cit939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechold U, Potrykus K, Murphy H, Murakami KS, Cashel M. Differential regulation by ppGpp versus pppGpp in Escherichia coli. Nucleic Acids Res. 2013;41:6175–6189. doi: 10.1093/nar/gkt302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano MS, Ding Y, Wang XG, Lu P, Coburn J, Hu LT. Regulators of expression of the oligopeptide permease A proteins of Borrelia burgdorferi. J Bacteriol. 2007;189:2653–2659. doi: 10.1128/JB.01760-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriläinen L, Herranen A, Schwarzbach A, Gilbert L. Morphological and biochemical features of Borrelia burgdorferi pleomorphic forms. Microbiology. 2015;161:516–527. doi: 10.1099/mic.0.000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels JE, Van den Bergh B, Verstraeten N, Michiels J. Molecular mechanisms and clinical implications of bacterial persistence. Drug Resist Updat. 2016;29:76–89. doi: 10.1016/j.drup.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Miller CL, Karna SL, Seshu J. Borrelia host adaptation regulator (BadR) regulates rpoS to modulate host adaptation and virulence factors in Borrelia burgdorferi. Mol Microbiol. 2013;88:105–124. doi: 10.1111/mmi.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok WW, Orman MA, Brynildsen MP. Impacts of global transcriptional regulators on persister metabolism. Antimicrob Agents Chemother. 2015;59:2713–2719. doi: 10.1128/AAC.04908-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RR, Schreck K, Wang X, Malawista SE. Human neutrophil calprotectin reduces the susceptibility of Borrelia burgdorferi to penicillin. Infect Immun. 2006;74:2468–2472. doi: 10.1128/IAI.74.4.2468-2472.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Rajeevan N, Liu L, Zhao YO, Heisig J, Pan J, et al. Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host Microbe. 2014;15:58–71. doi: 10.1016/j.chom.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, et al. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science. 2011;334:982–986. doi: 10.1126/science.1211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak EA, Sultan SZ, Motaleb MA. The cyclic-di-GMP signaling pathway in the Lyme disease spirochete, Borrelia burgdorferi. Front Cell Infect Microbiol. 2014;4:56. doi: 10.3389/fcimb.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke M, Traweger A, Lusa L, Stupica D, Maraspin V, Barrett PN, et al. Quantitative detection of Borrelia burgdorferi sensu lato in erythema migrans skin lesions using internally controlled duplex real time PCR. PLoS One. 2013;8:e63968. doi: 10.1371/journal.pone.0063968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi J, Piesman J, de Silva AM. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc Natl Acad Sci U S A. 2001;98:670–675. doi: 10.1073/pnas.98.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orman MA, Henry TC, DeCoste CJ, Brynildsen MP. Analyzing persister physiology with fluorescence-activated cell sorting. Methods Mol Biol. 2016;1333:83–100. doi: 10.1007/978-1-4939-2854-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Z, Blevins JS, Norgard MV. Transcriptional interplay among the regulators Rrp2, RpoN and RpoS in Borrelia burgdorferi. Microbiology. 2008;154:2641–2658. doi: 10.1099/mic.0.2008/019992-0. [DOI] [PubMed] [Google Scholar]
- Ouyang Z, Narasimhan S, Neelakanta G, Kumar M, Pal U, Fikrig E, et al. Activation of the RpoN-RpoS regulatory pathway during the enzootic life cycle of Borrelia burgdorferi. BMC Microbiol. 2012;12:44. doi: 10.1186/1471-2180-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Z, Zhou J. BadR (BB0693) controls growth phase-dependent induction of rpoS and bosR in Borrelia burgdorferi via recognizing TAAAATAT motifs. Mol Microbiol. 2015;98:1147–1167. doi: 10.1111/mmi.13206. [DOI] [PubMed] [Google Scholar]
- Pal U, Li X, Wang T, Montgomery RR, Ramamoorthi N, de Silva AM, et al. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell. 2004;119:457–468. doi: 10.1016/j.cell.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Pal U, Fikrig E. Tick interactions. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 299–331. [Google Scholar]
- Pappas CJ, Iyer R, Petzke MM, Caimano MJ, Radolf JD, Schwartz I. Borrelia burgdorferi requires glycerol for maximum fitness during the tick phase of the enzootic cycle. PLoS Pathog. 2011;7:e1002102. doi: 10.1371/journal.ppat.1002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PC. Epistasis – the essential role of gene interactions in the structure and evolution of genetic systems. Nat Rev Genet. 2008;9:855–867. doi: 10.1038/nrg2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, Mather TN, Sinsky RJ, Spielman A. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557–558. doi: 10.1128/jcm.25.3.557-558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J. Transmission of Lyme disease spirochetes (Borrelia burgdorferi) Exp Appl Acarol. 1989;7:71–80. doi: 10.1007/BF01200454. [DOI] [PubMed] [Google Scholar]
- Piesman J, Oliver JR, Sinsky RJ. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini) Am J Trop Med Hyg. 1990;42:352–357. doi: 10.4269/ajtmh.1990.42.352. [DOI] [PubMed] [Google Scholar]
- Potrykus K, Cashel M. (p)ppGpp: still magical? Annu Rev Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- Potrykus K, Murphy H, Philippe N, Cashel M. ppGpp is the major source of growth rate control in E. coli. Environ Microbiol. 2011;13:563–575. doi: 10.1111/j.1462-2920.2010.02357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putrins M, Kogermann K, Lukk E, Lippus M, Varik V, Tenson T. Phenotypic heterogeneity enables uropathogenic Escherichia coli to evade killing by antibiotics and serum complement. Infect Immun. 2015;83:1056–1067. doi: 10.1128/IAI.02725-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju BV, Esteve-Gassent MD, Karna SL, Miller CL, Van Laar TA, Seshu J. Oligopeptide permease A5 modulates vertebrate host-specific adaptation of Borrelia burgdorferi. Infect Immun. 2011;79:3407–3420. doi: 10.1128/IAI.05234-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel AT, Talaat AM, Norgard MV. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc Natl Acad Sci U S A. 2002;99:1562–1567. doi: 10.1073/pnas.032667699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes RG, Coy W, Nelson DR. Chitobiose utilization in Borrelia burgdorferi is dually regulated by RpoD and RpoS. BMC Microbiol. 2009;9:108. doi: 10.1186/1471-2180-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EA, Terekhova D, Zhang HM, Hovis KM, Schwartz I, Marconi RT. Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol Microbiol. 2009;71:1551–1573. doi: 10.1111/j.1365-2958.2009.06621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo T, Vakulskas CA, Babitzke P. Post-transcriptional regulation on a global scale: form and function of Csr/Rsm systems. Environ Microbiol. 2013;15:313–324. doi: 10.1111/j.1462-2920.2012.02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W, Sanchez-Vazquez P, Chen AY, Lee JH, Burgos HL, Gourse RL. ppGpp binding to a site at the RNAP-DksA interface accounts for its dramatic effects on transcription initiation during the stringent response. Mol Cell. 2016;62:811–823. doi: 10.1016/j.molcel.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymer RU, Solorio FA, Tehranchi AK, Chu C, Corn JE, Keck JL, et al. Binding mechanism of metal-NTP substrates and stringent-response alarmones to bacterial DnaG-type primases. Structure. 2012;20:1478–1489. doi: 10.1016/j.str.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotthoefer AM, Frost HM. Ecology and epidemiology of Lyme borreliosis. Clin Lab Med. 2015;35:723–743. doi: 10.1016/j.cll.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Sharma B, Brown AV, Matluck NE, Hu LT, Lewis K. Borrelia burgdorferi, the causative agent of Lyme Disease, forms drug-tolerant persister cells. Antimicrob Agents Chemother. 2015;59:4616–4624. doi: 10.1128/AAC.00864-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Blevins JS, Bachlani GN, Yang XF, Norgard MV. Evidence that RpoS (σS) in Borrelia burgdorferi is controlled directly by RpoN (σ54/σN) J Bacteriol. 2007;189:2139–2144. doi: 10.1128/JB.01653-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares CA, Zeidner NS, Beard CB, Dolan MC, Dietrich G, Piesman J. Kinetics of Borrelia burgdorferi infection in larvae of refractory and competent tick vectors. J Med Entomol. 2006;43:61–67. doi: 10.1093/jmedent/43.1.61. [DOI] [PubMed] [Google Scholar]
- Spoering AL, Vulic M, Lewis K. GlpD and PlsB participate in persister cell formation in Escherichia coli. J Bacteriol. 2006;188:5136–5144. doi: 10.1128/JB.00369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava SY, de Silva AM. Characterization of Borrelia burgdorferi aggregates. Vector Borne Zoonotic Dis. 2009;9:323–329. doi: 10.1089/vbz.2008.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AC, Levin RE, Molloy PJ, Kalish RA, Abraham JH, 3rd, Liu NY, et al. Treatment of Lyme arthritis. Arthritis Rheum. 1994;37:878–888. doi: 10.1002/art.1780370616. [DOI] [PubMed] [Google Scholar]
- Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, et al. Lyme borreliosis. Nat Rev Dis Primers. 2016;2:16090. doi: 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinchen W, Bange G. The magic dance of the alarmones (p)ppGpp. Mol Microbiol. 2016;101:531–544. doi: 10.1111/mmi.13412. [DOI] [PubMed] [Google Scholar]
- Stupica D, Lusa L, Maraspin V, Bogovic P, Vidmar D, O’Rourke M, et al. Correlation of culture positivity, PCR positivity, and burden of Borrelia burgdorferi sensu lato in skin samples of erythema migrans patients with clinical findings. PLoS One. 2015;10:e0136600. doi: 10.1371/journal.pone.0136600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan SZ, Pitzer JE, Miller MR, Motaleb MA. Analysis of a Borrelia burgdorferi phosphodiesterase demonstrates a role for cyclic-di-guanosine monophosphate in motility and virulence. Mol Microbiol. 2010;77:128–142. doi: 10.1111/j.1365-2958.2010.07191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Roland KL, Branger CG, Kuang X, Curtiss R., III The role of relA and spoT in Yersinia pestis KIM5 pathogenicity. PLoS One. 2009;4:e6720. doi: 10.1371/journal.pone.0006720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syal K, Flentie K, Bhardwaj N, Maiti K, Jayaraman N, Stallings CL, et al. Synthetic (p)ppGpp analogue Is an inhibitor of stringent response in Mycobacteria. Antimicrob Agents Chemother. 2017;61:e00443–17. doi: 10.1128/AAC.00443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze CW, Morado DR, Liu J, Charon NW, Xu H, Li C. Carbon storage regulator A (CsrABb) is a repressor of Borrelia burgdorferi flagellin protein FlaB. Mol Microbiol. 2011;82:851–864. doi: 10.1111/j.1365-2958.2011.07853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze CW, Zhang K, Kariu T, Pal U, Li C. Borrelia burgdorferi needs chemotaxis to establish infection in mammals and to accomplish its enzootic cycle. Infect Immun. 2012;80:2485–2492. doi: 10.1128/IAI.00145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze CW, Smith A, Choi YH, Yang X, Pal U, Yu A, et al. Study of the response regulator Rrp1 reveals its regulatory role in chitobiose utilization and virulence of Borrelia burgdorferi. Infect Immun. 2013;81:1775–1787. doi: 10.1128/IAI.00050-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terekhova D, Sartakova ML, Wormser GP, Schwartz I, Cabello FC. Erythromycin resistance in Borrelia burgdorferi. Antimicrob Agents Chemother. 2002;46:3637–3640. doi: 10.1128/AAC.46.11.3637-3640.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K, Elias AF, Errett J, Fischer E, Iyer R, Schwartz I, et al. Genetics and regulation of chitobiose utilization in Borrelia burgdorferi. J Bacteriol. 2001;183:5544–5553. doi: 10.1128/JB.183.19.5544-5553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxler MF, Chang DE, Conway T. Guanosine 3’,5’-bispyrophosphate coordinates global gene expression during glucose-lactose diauxie in Escherichia coli. Proc Natl Acad Sci U S A. 2006;103:2374–2379. doi: 10.1073/pnas.0510995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxler MF, Summers SM, Nguyen HT, Zacharia VM, Hightower GA, Smith JT, et al. The global, ppGpp-mediated stringent response to amino acid starvation in Escherichia coli. Mol Microbiol. 2008;68:1128–1148. doi: 10.1111/j.1365-2958.2008.06229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy EB, Lin T, Gao L, Lazinski DW, Lundt M, Camilli A, et al. Global Tn-seq analysis of carbohydrate utilization and vertebrate infectivity of Borrelia burgdorferi. Mol Microbiol. 2016;101:1003–1023. doi: 10.1111/mmi.13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakulskas CA, Potts AH, Babitzke P, Ahmer BM, Romeo T. Regulation of bacterial virulence by Csr (Rsm) systems. Microbiol Mol Biol Rev. 2015;79:193–224. doi: 10.1128/MMBR.00052-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laar TA, Lin YH, Miller CL, Karna SL, Chambers JP, Seshu J. Effect of levels of acetate on the mevalonate pathway of Borrelia burgdorferi. PLoS One. 2012;7:e38171. doi: 10.1371/journal.pone.0038171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraeten N, Knapen WJ, Kint CI, Liebens V, Van den Bergh B, Dewachter L, et al. Obg and membrane depolarization are part of a microbial bet-hedging strategy that leads to antibiotic tolerance. Mol Cell. 2015;59:9–21. doi: 10.1016/j.molcel.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Vinella D, Potrykus K, Murphy H, Cashel M. Effects on growth by changes of the balance between GreA, GreB, and DksA suggest mutual competition and functional redundancy in Escherichia coli. J Bacteriol. 2012;194:261–273. doi: 10.1128/JB.06238-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lackum K, Stevenson B. Carbohydrate utilization by the Lyme borreliosis spirochete, Borrelia burgdorferi. FEMS Microbiol Lett. 2005;243:173–179. doi: 10.1016/j.femsle.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Wager B, Shaw DK, Groshong AM, Blevins JS, Skare JT. BB0744 affects tissue tropism and spatial distribution of Borrelia burgdorferi. Infect Immun. 2015;83:3693–3703. doi: 10.1128/IAI.00828-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Ojaimi C, Iyer R, Saksenberg V, McClain SA, Wormser GP, et al. Impact of genotypic variation of Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infect Immun. 2001;69:4303–4312. doi: 10.1128/IAI.69.7.4303-4312.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. Direct detection methods for Lyme Borrelia, including the use of quantitative assays. Vector Borne Zoonotic Dis. 2002;2:223–231. doi: 10.1089/153036602321653806. [DOI] [PubMed] [Google Scholar]
- Wang XG, Lin B, Kidder JM, Telford S, Hu LT. Effects of environmental changes on expression of the oligopeptide permease (opp) genes of Borrelia burgdorferi. J Bacteriol. 2002;184:6198–6206. doi: 10.1128/JB.184.22.6198-6206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexselblatt E, Kaspy I, Glaser G, Katzhendler J, Yavin E. Design, synthesis and structure-activity relationship of novel Relacin analogs as inhibitors of Rel proteins. Eur J Med Chem. 2013;70:497–504. doi: 10.1016/j.ejmech.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Wormser GP, Schwartz I. Antibiotic treatment of animals infected with Borrelia burgdorferi. Clin Microbiol Rev. 2009;22:387–395. doi: 10.1128/CMR.00004-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormser GP, Baker PJ, O’Connell S, Pachner AR, Schwartz I, Shapiro ED. Critical analysis of treatment trials of rhesus macaques infected with Borrelia burgdorferi reveals important flaws in experimental design. Vector Borne Zoonotic Dis. 2012;12:535–538. doi: 10.1089/vbz.2012.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AV, Nunez JK, Doudna JA. Biology and Applications of CRISPR Systems: Harnessing Nature’s Toolbox for Genome Engineering. Cell. 2016;164:29–44. doi: 10.1016/j.cell.2015.12.035. [DOI] [PubMed] [Google Scholar]
- Zambrano MC, Beklemisheva AA, Bryksin AV, Newman SA, Cabello FC. Borrelia burgdorferi binds to, invades, and colonizes native type I collagen lattices. Infect Immun. 2004;72:3138–3146. doi: 10.1128/IAI.72.6.3138-3146.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidner NS, Schneider BS, Dolan MC, Piesman J. An analysis of spirochete load, strain, and pathology in a model of tick-transmitted Lyme borreliosis. Vector Borne Zoonotic Dis. 2001;1:35–44. doi: 10.1089/153036601750137642. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang Y, Adusumilli S, Liu L, Narasimhan S, Dai J, et al. Molecular interactions that enable movement of the Lyme disease agent from the tick gut into the hemolymph. PLoS Pathog. 2011;7:e1002079. doi: 10.1371/journal.ppat.1002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Persisters, persistent infections and the Yin-Yang model. Emerg Microbes Infect. 2014;3:e3. doi: 10.1038/emi.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi H, Weening EH, Barbu EM, Hyde JA, Höök M, Skare JT. The BBA33 lipoprotein binds collagen and impacts Borrelia burgdorferi pathogenesis. Mol Microbiol. 2015;96:68–83. doi: 10.1111/mmi.12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Gern L, Aeschlimann A. The peritrophic membrane of Ixodes ricinus. Parasitol Res. 1991;77:635–641. doi: 10.1007/BF00931028. [DOI] [PubMed] [Google Scholar]


